Abstract
Background: Olfaction is known to be impaired by aging. We hypothesized that insulin-like growth factor-1 (IGF-1) administered at an appropriate dose could prevent age-induced negative effects on olfactory receptor neurons (ORNs). We explored the effects of low- and high-dose administration of IGF-1 on the ORN cell system in aged mice and investigated the involvement of the cellular mechanisms of IGF-1 in the regeneration of ORNs in aged mice.
Methods: We subcutaneously administered recombinant human IGF-1 (rhIGF-1) to 16-month-old male mice over 56 days, and then examined the histological effects of rhGF-1 on cellular composition, cell proliferation, and cell death in the aged olfactory epithelium (OE), by comparing among saline-treated and low- and high-dose rhIGF-1-treated mice.
Results: Low-dose rhIGF-1 administration increased the numbers of olfactory progenitors, immature ORNs, and mature ORNs in the OE, despite an increase in Cas3+ apoptotic cells. Notably, high-dose rhIGF-1 administration increased the numbers of only immature ORNs, not olfactory progenitors and mature ORNs, with a concurrent increase in apoptotic cells.
Conclusion: Our data suggest that in aged mice, IGF-1 administered at an appropriate dose could increase the number of mature ORNs and further human studies may contribute to the development of treatments for aging-related olfactory impairment.
Keywords: olfactory receptor neurons, aging, insulin-like growth factor 1, dose effects, proliferation, apoptosis
Introduction
Olfaction is known to be impaired by aging. Aging negatively affects the ORN cell system in the olfactory neuroepithelium (OE) (Ueha et al., 2018a). The OE has a unique regenerative stem-cell system, which is maintained by the life-long replenishment of mature ORNs in the luminal layer from the progenitor cells in the basal layer (Costanzo, 1991; Schwob, 2002; Supplementary Figure S1). In steady-status neurogenesis, primitive olfactory progenitor cells differentiate into late progenitor cells and then differentiate into immature ORNs. A variety of stimuli, such as cytokines, neurotrophins, and growth factors, induce differentiation and maturation of immature ORNs (Buiakova et al., 1996; Nickell et al., 2012; Heron et al., 2013).
Neurotrophins and growth factors have important roles in neuronal differentiation, maturation, and maintenance in the steady-state condition (Fernandez and Torres-Aleman, 2012), and the dose-dependent differential effects of neurotrophins and growth factors have been reported (Tannemaat et al., 2008; Santos et al., 2016). Insulin-like growth factor-1 (IGF-1) is a growth factor that promotes neurogenesis and neuronal growth. Although IGF-1 is mainly produced in the liver and skeletal muscles, it is also generated in the central nervous system, where it plays an important role in the differentiation and maturation of neurons (Nishida et al., 2011; Nieto-Estevez et al., 2016; Pardo et al., 2016). As our previous study showed, reduction in ORN number and cell proliferation in the aged OE reduced gene expression of IGF-1, which may contribute to olfactory impairment during aging (Ueha et al., 2018a). Considering the previous studies, the balance of IGF-1 might be of importance in the regulation of OE homeostasis in the aging population.
We hypothesized that IGF-1 administered at an appropriate dose could restore the aging-induced reduction of ORNs. In the present study, we explored the effects of low-dose and high-dose IGF-1 administration on the ORN cell system in aged mice.
Materials and Methods
Mice
Sixteen-month-old male mice (n = 6 for each group) were used (C57BL/6, purchased from Saitama Experimental Animals, Saitama, Japan). The mice were maintained in a temperature-controlled (24 ± 1°C) environment under a 12-h light-dark cycle (light on from 09:00 to 21:00). Food and water were available ad libitum. All animal experiments were approved by the Animal Care and Use Committee of the University of Tokyo (Approval No. P17-010) and were conducted in accordance with institutional guidelines.
Mouse Model of IGF-1 Administration
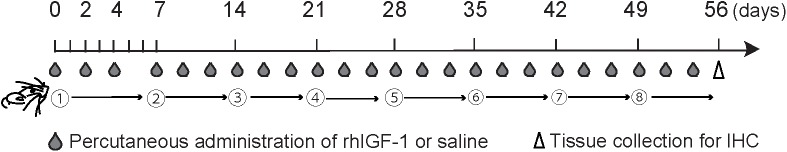
Mice were injected subcutaneously with 20 μg/kg (low-dose group) and 200 μg/kg (high-dose group) rhIGF-1 (rhIGF-1; Mecasermin, Orphan Pacific, Tokyo, Japan), which is also active in mice (Castro et al., 2014; Khwaja et al., 2014), dissolved in sterile saline, three times a week from days 0 to 53 (Castro et al., 2014; Khwaja et al., 2014; Ueha et al., 2016b; Figure 1). Control mice received saline solution subcutaneously on the same schedule as the rhIGF-1-treated mice. Both control and rhIGF-1-treated mice were sacrificed on day 56.
FIGURE 1.
Experimental timeline. Mice were injected subcutaneously with 20 μg/kg (low-dose group) and 200 μg/kg (high-dose group) recombinant human insulin-like growth factor-1 (rhIGF-1) or saline three times a week from days 0 to 53. Subsequently, the olfactory epithelium was collected for analysis by immunohistochemistry (IHC).
Tissue Preparation and Histological Analyses
The septal nasal mucosa was harvested and prepared for histological analyses as described previously (Ueha et al., 2014, 2016a,b, 2018a,b). In brief, all samples were cut at the level of the anterior end of the olfactory bulb. Four-micrometer-thick paraffin sections were deparaffinized and stained with hematoxylin and eosin or immunohistochemically. Antigen-retrieval and blocking of endogenous peroxidase activity were performed before immunostaining. Then, the sections were incubated in Blocking One (Nacalai Tesque, Kyoto, Japan) to block non-specific antibody binding. Primary antibodies against mouse SOX2 (SOX2; 1:300 dilution; rabbit monoclonal, Abcam clone EPR3131; Cambridge, United Kingdom), growth-associated protein 43 (GAP43; 1:1000 dilution; rabbit polyclonal, Novus #NB300-143B; Littleton, CO, United States), Ki-67 (1:200 dilution; rabbit monoclonal, Novus #NB600-1252), OMP (OMP; 1:8000 dilution, goat polyclonal, Wako, Osaka, Japan), and cleaved Cas3 (Cas3; 1:300 dilution; rabbit polyclonal, Cell Signaling #9661; Danvers, MA, United States) were detected with peroxidase-conjugated appropriate secondary antibodies and a diaminobenzidine substrate. In the OE, SOX2 is a transcription factor and expressed by proliferating progenitor cells in the basal layer. GAP43 and OMP are expressed by immature ORNs and mature ORNs, respectively. Ki-67 is a marker for cell proliferation and expressed in all active stages of the cell cycle, and Ki-67-positive cells are particularly detected in the basal layer (Ueha et al., 2018a,b). Caspases are crucial mediators of programmed cell death (apoptosis) and Cas3 is a well-defined activated death protease (Porter and Janicke, 1999).
Images of the bilateral septal OE were acquired using a digital microscope (BZ-X700; Keyence, Osaka, Japan) at 400× magnification. Using a previously reported method (Ueha et al., 2018a), the number of OMP+ ORNs, GAP43+ immature ORNs, and cleaved Cas3+ apoptotic cells per 1-mm length areas of the OE and the number of SOX2+ ORN progenitors and Ki-67+ cells on the basal layer per mm length of the OE were counted manually using digital imaging software (Photoshop CS6; Adobe, San Jose, CA, United States) in a blinded manner.
Statistical Analysis
Statistical comparisons among groups were performed by one-way analysis of variance with Tukey post hoc tests using the GraphPad Prism software version 6.0 (GraphPad Software Inc., San Diego, CA, United States1). P < 0.05 was considered to be statistically significant.
Results
We first investigated the influence of rhIGF-1 on the cellular composition of the aged OE by comparing the OE of saline-and rhIGF-1-treated mice (low IGF-1, high IGF-1). We found that the number of OMP+ mature ORNs was significantly higher in the low-dose rhIGF-1-treated group compared to the saline-treated and high-dose rhIGF-1-treated groups. The number of SOX2+ olfactory progenitors also increased only in the low-dose rhIGF-1-treated group. However, the number of GAP43+ immature ORNs significantly increased in both the low- and high-dose rhIGF-1-treated groups compared to the saline-treated group (Figures 2A–C and Supplementary Figure S1).
FIGURE 2.
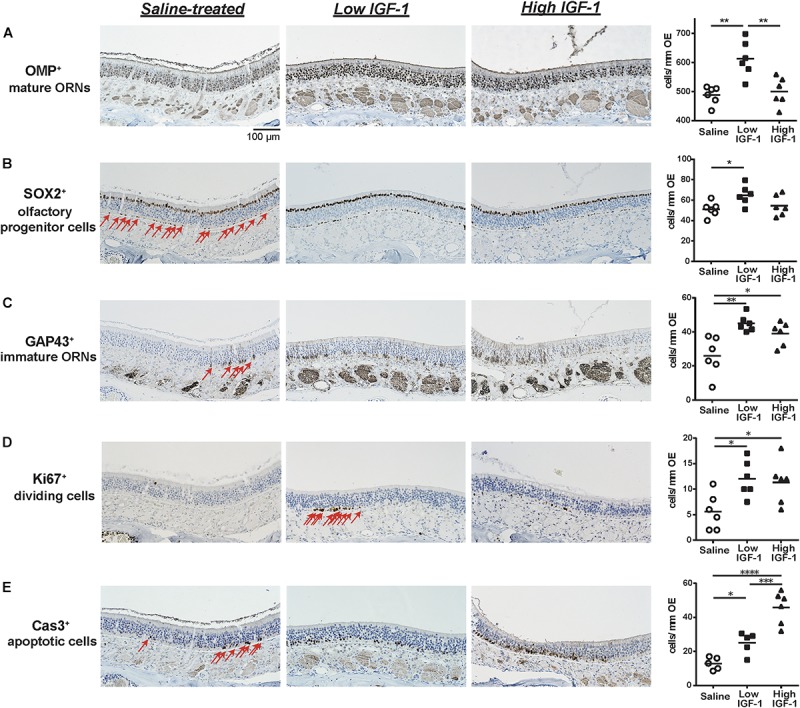
Representative images of immunohistological staining (brown) of OMP-positive (OMP+) cells (A), SOX2+ ORN progenitor cells (B), GAP43+ immature ORNs (C), Ki67+ proliferating cells (D), and cleaved Cas3+ apoptotic cells (E). Each cell except for many OMP+ cells is indicated by arrows. Tissue sections were counterstained with the nuclear dye hematoxylin (blue). Numbers of SOX2+ ORN progenitors and Ki67+ actively proliferating cells per mm of the basal layer and OMP+ mature ORNs, GAP43+ immature ORNs, and Cas3+ apoptotic cells per mm of the OE in saline or rhIGF-1-treated mice. Open circles, rectangles, and triangles represent the values for each mouse in the saline, low-IGF-1, and high-IGF-1 treated groups (each n = 6), respectively. The horizontal lines represent the mean value for each group. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; and ∗∗∗∗P < 0.0001 (one-way ANOVA).
We next examined the cellular mechanisms underlying the increase of mature and immature ORNs in rhIGF-1-treated mice. As the number of mature ORNs is determined by the balance between proliferation of the ORN precursors and cell death, we analyzed the number of Ki-67+ proliferating cells and Cas3+ apoptotic cells in the OE. Ki-67+ cells were mainly detected in and proximal to the basal layer, where olfactory progenitors and immature ORNs give rise to differentiated progenies. The number of Ki-67+ cells was significantly higher in both the low- and high-dose rhIGF-1-treated groups than in the saline-treated group. Interestingly, the number of Cas3+ apoptotic cells increased in the rhIGF-1-treated group compared to the saline-treated group, and this increase was more prominent in the high-dose rhIGF-1-treated group. Cas3+ cells were mainly detected in the basal and the intermediate layers, where immature ORNs are usually present (Figures 2D–E and Supplementary Table S1).
Discussion
Here, we demonstrated, for the first time, that low-dose rhIGF-1 administration increased the number of OMP+ mature ORNs in the aged OE, but high-dose rhIGF-1 did not have positive effects on the aged OE. These dose-dependent effects of rhIGF-1 on the aged OE may be important for developing treatments for aging-related olfactory dysfunction.
IGF-1 is a growth factor exerting trophic effects on neuronal development and regeneration in the central nervous system and peripheral nervous system (Ishii et al., 1994; Bianchi et al., 2017). IGF-1 activates protein synthesis in neurons, glia, oligodendrocytes, and Schwann cells; stimulates the survival of mature and immature neurons, and promotes the proliferation of neuronal precursors (Devesa et al., 2011; Bianchi et al., 2017), acting neuroprotectively after injury. Thus, IGF-1 controls tissue homeostasis throughout the lifespan via regulation of cell proliferation, survival, and cell death (Kooijman, 2006). Recently, IGF-1 was found to have contradictory effects on the regulation of apoptosis. IGF-1 reportedly prevents the expression of apoptosis-associated genes and inhibits apoptotic programs and pathways (Parrizas et al., 1997; Kang et al., 2003; Fernandez et al., 2004; Vincent et al., 2004), but on the other hand, it is considered to induce apoptosis (Granerus et al., 2001; Raile et al., 2003; Saile et al., 2004; Fu et al., 2007). These different effects may be influenced by the dose, administration route, number of administrations, and the interval between administrations (Santos et al., 2016; Bianchi et al., 2017). IGF-1 secretion decreases and the effects of IGF-1 on tissues alter with aging (Obermayr et al., 2005; Apel et al., 2010; Bianchi et al., 2017). Therefore, it is reasonable to consider that IGF-1 plays an important role in the prevention and treatment of many neurological diseases such as olfactory and neurodegenerative disorders and hearing loss and IGF-1 therapies at an appropriate dose could provide significant benefits for aged individuals. How IGF-1 acts on OE homeostasis remains unclear. However, considering that IGF-1 and the IGF-1 receptor (IGF-1R) are expressed in the OE and the olfactory bulb (Scolnick et al., 2008; Lee et al., 2018) and IGF-1 is transported to the olfactory bulb via the olfactory nerve-related nasal transport (Shiga et al., 2014), systemic administration of IGF-1 may directly and indirectly influence the OE.
The increase in the number of OMP+ mature ORNs after IGF-1 administration can be partly explained by the overwhelmingly higher cell proliferation of olfactory progenitors and immature ORNs compared to the increase in Cas3+ apoptotic cells. However, it should be noted that different doses could lead to different effects. We elucidated that high-dose rhIGF-1 administration increased the number of GAP43+ immature ORNs and accelerated apoptosis, but on the other hand, it did not cause an increase in the numbers of SOX2+ ORN progenitors and OMP+ mature ORNs. Collectively, these results indicate that the number of olfactory progenitors and immature and mature ORNs rises only by low-dose rhIGF-1 treatment and that high-dose rhIGF-1 administration did not increase the number of ORN progenitors and only promoted both proliferation and apoptosis of ORN progenitors/ immature ORNs in the aged OE. High-dose IGF-1 may saturate the IGF-1R, and free IGF-1 and the saturated IGF-1 – IGF-1R complexes could lead to an increase of apoptosis of ORN progenitors/ immature ORNs. Increased apoptosis is likely to be at least partly responsible for the unchanged ORN numbers in the high-dose rhIGF-1-treated mice.
Considering that the reduction in mature ORN numbers is associated with olfactory impairment in the aged population (Ueha et al., 2018b), IGF-1 administered at an appropriate dose could prevent aging-induced negative effects on ORNs. However, because our results were only obtained from mouse studies, care should be taken regarding IGF-1 application for the treatment of human olfactory impairment. Further studies are required to investigate the application of IGF-1 treatment to humans and to determine the appropriate dose of adequate intervals between IGF-1 administrations for the treatment of olfactory deterioration in aged individuals, and to assess the effects of IGF-1 administration on wellness and quality of life.
Low-dose, but not high-dose, rhIGF-1 administration increased the number of OMP+ mature ORNs in the aged OE. IGF-1 administration at an appropriate dose may be a key factor in treatments for aging-related olfactory dysfunction.
Data Availability
The data that support the findings of this study are available from the corresponding author, RU, upon reasonable request.
Author Contributions
RU, KK, and SU developed the concept, designed and performed the experiments, and analyzed the data. TY developed the concept and designed the experiments. All authors contributed to interpretation of the data and writing of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- Cas3
caspase-3
- GAP43
growth associated protein 43
- IGF-1
insulin-like growth factor-1
- IGF-1R
insulin-like growth factor-1 receptor
- OE
olfactory epithelium
- OMP
olfactory marker protein
- ORN
olfactory receptor neuron
- rhIGF-1
recombinant human IGF-1
- SOX2
sex determining region Y-box 2
Funding. This research was supported by a Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (Grant Nos. 24791749, 16KT0190) and by the Smoking Research Foundation (Tokyo, Japan).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2018.00385/full#supplementary-material
A,B: Representative images of the olfactory epithelium (OE) from untreated mice (hematoxylin and eosin stain; A, 40× magnification; B, 400× magnification). The box in A indicates the region of the OE shown in B,C: Diagram of cell lineage of olfactory receptor neurons (ORN).
Change rate of each cell number. Each change rate was calculated by dividing each cell number in the low and high-dose insulin-like growth factor-1 (IGF-1) groups by those in the saline-treated control group. ∗P < 0.05; ∗∗P < 0.01; and ∗∗∗∗P < 0.0001 (one-way ANOVA). Cas3, caspase-3; GAP43, growth associated protein 43; OMP, olfactory marker protein; ORN, olfactory receptor neuron; SOX2, sex determining region Y-box 2.
References
- Apel P. J., Ma J., Callahan M., Northam C. N., Alton T. B., Sonntag W. E., et al. (2010). Effect of locally delivered IGF-1 on nerve regeneration during aging: an experimental study in rats. Muscle Nerve 41 335–341. 10.1002/mus.21485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi V. E., Locatelli V., Rizzi L. (2017). Neurotrophic and neuro regenerative effects of GH/IGF1. Int. J. Mol. Sci. 18:E2441. 10.3390/ijms18112441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiakova O. I., Baker H., Scott J. W., Farbman A., Kream R., Grillo M., et al. (1996). Olfactory marker protein (OMP) gene deletion causes altered physiological activity of olfactory sensory neurons. Proc. Natl. Acad. Sci. U.S.A. 93 9858–9863. 10.1073/pnas.93.18.9858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J., Garcia R. I., Kwok S., Banerjee A., Petravicz J., Woodson J., et al. (2014). Functional recovery with recombinant human IGF1 treatment in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. U.S.A. 111 9941–9946. 10.1073/pnas.1311685111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo R. M. (1991). Regeneration of olfactory receptor cells. Ciba Found. Symp. 160 233–242; discussio n243–238. [DOI] [PubMed] [Google Scholar]
- Devesa J., Alonso B., Casteleiro N., Couto P., Castanon B., Zas E., et al. (2011). Effects of recombinant growth hormone (GH) replacement and psychomotor and cognitive stimulation in the neurodevelopment of GH-deficient (GHD) children with cerebral palsy: a pilot study. Ther. Clin. Risk Manag. 7 199–206. 10.2147/TCRM.S21403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A. M., Torres-Aleman I. (2012). The many faces of insulin-like peptide signalling in the brain. Nat. Rev. Neurosci. 13 225–239. 10.1038/nrn3209 [DOI] [PubMed] [Google Scholar]
- Fernandez M., Sanchez-Franco F., Palacios N., Sanchez I., Fernandez C., Cacicedo L. (2004). IGF-I inhibits apoptosis through the activation of the phosphatidylinositol 3-kinase/Akt pathway in pituitary cells. J. Mol. Endocrinol. 33 155–163. 10.1677/jme.0.0330155 [DOI] [PubMed] [Google Scholar]
- Fu P., Thompson J. A., Leeding K. S., Bach L. A. (2007). Insulin-like growth factors induce apoptosis as well as proliferation in LIM 1215 colon cancer cells. J. Cell. Biochem. 100 58–68. 10.1002/jcb.20984 [DOI] [PubMed] [Google Scholar]
- Granerus M., Johannisson A., Ekblom P., Engstrom W. (2001). Insulin-like growth factors I and II induce cell death in Wilms’s tumour cells. Mol. Pathol. 54 30–35. 10.1136/mp.54.1.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron P. M., Stromberg A. J., Breheny P., McClintock T. S. (2013). Molecular events in the cell types of the olfactory epithelium during adult neurogenesis. Mol. Brain 6:49. 10.1186/1756-6606-6-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii D. N., Glazner G. W., Pu S. F. (1994). Role of insulin-like growth factors in peripheral nerve regeneration. Pharmacol. Ther. 62 125–144. 10.1016/0163-7258(94)90007-8 [DOI] [PubMed] [Google Scholar]
- Kang B. P., Urbonas A., Baddoo A., Baskin S., Malhotra A., Meggs L. G. (2003). IGF-1 inhibits the mitochondrial apoptosis program in mesangial cells exposed to high glucose. Am. J. Physiol. Renal Physiol. 285 F1013–F1024. 10.1152/ajprenal.00209.2003 [DOI] [PubMed] [Google Scholar]
- Khwaja O. S., Ho E., Barnes K. V., O’Leary H. M., Pereira L. M., Finkelstein Y., et al. (2014). Safety, pharmacokinetics, and preliminary assessment of efficacy of mecasermin (recombinant human IGF-1) for the treatment of Rett syndrome. Proc. Natl. Acad. Sci. U.S.A. 111 4596–4601. 10.1073/pnas.1311141111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman R. (2006). Regulation of apoptosis by insulin-like growth factor (IGF)-I. Cytokine Growth Factor. Rev. 17 305–323. 10.1016/j.cytogfr.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Lee T. K., Chen B. H., Lee J. C., Shin M. C., Cho J. H., Lee H. A., et al. (2018). Age-dependent decreases in insulin-like growth factor-I and its receptor expressions in the gerbil olfactory bulb. Mol. Med. Rep. 17 8161–8166. 10.3892/mmr.2018.8886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickell M. D., Breheny P., Stromberg A. J., McClintock T. S. (2012). Genomics of mature and immature olfactory sensory neurons. J. Comp. Neurol. 520 2608–2629. 10.1002/cne.23052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Estevez V., Defterali C., Vicario-Abejon C. (2016). IGF-I: a key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front. Neurosci. 10:52. 10.3389/fnins.2016.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida F., Morel G. R., Herenu C. B., Schwerdt J. I., Goya R. G., Portiansky E. L. (2011). Restorative effect of intracerebroventricular insulin-like growth factor-I gene therapy on motor performance in aging rats. Neuroscience 177 195–206. 10.1016/j.neuroscience.2011.01.013 [DOI] [PubMed] [Google Scholar]
- Obermayr R. P., Mayerhofer L., Knechtelsdorfer M., Mersich N., Huber E. R., Geyer G., et al. (2005). The age-related down-regulation of the growth hormone/insulin-like growth factor-1 axis in the elderly male is reversed considerably by donepezil, a drug for Alzheimer’s disease. Exp. Gerontol. 40 157–163. 10.1016/j.exger.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Pardo J., Uriarte M., Console G. M., Reggiani P. C., Outeiro T. F., Morel G. R., et al. (2016). Insulin-like growth factor-I gene therapy increases hippocampal neurogenesis, astrocyte branching and improves spatial memory in female aging rats. Eur. J. Neurosci. 44 2120–2128. 10.1111/ejn.13278 [DOI] [PubMed] [Google Scholar]
- Parrizas M., Saltiel A. R., LeRoith D. (1997). Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3′-kinase and mitogen-activated protein kinase pathways. J. Biol. Chem. 272 154–161. 10.1074/jbc.272.1.154 [DOI] [PubMed] [Google Scholar]
- Porter A. G., Janicke R. U. (1999). Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 6 99–104. 10.1038/sj.cdd.4400476 [DOI] [PubMed] [Google Scholar]
- Raile K., Hille R., Laue S., Schulz A., Pfeifer G., Horn F., et al. (2003). Insulin-like growth factor I (IGF-I) stimulates proliferation but also increases caspase-3 activity, Annexin-V binding, and DNA-fragmentation in human MG63 osteosarcoma cells: co-activation of pro- and anti-apoptotic pathways by IGF-I. Horm. Metab. Res. 35 786–793. 10.1055/s-2004-814140 [DOI] [PubMed] [Google Scholar]
- Saile B., DiRocco P., Dudas J., El-Armouche H., Sebb H., Eisenbach C., et al. (2004). IGF-I induces DNA synthesis and apoptosis in rat liver hepatic stellate cells (HSC) but DNA synthesis and proliferation in rat liver myofibroblasts (rMF). Lab. Invest. 84 1037–1049. 10.1038/labinvest.3700116 [DOI] [PubMed] [Google Scholar]
- Santos D., Gonzalez-Perez F., Navarro X., Del Valle J. (2016). Dose-dependent differential effect of neurotrophic factors on in vitro and in vivo regeneration of motor and sensory neurons. Neural Plast. 2016 4969523. 10.1155/2016/4969523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob J. E. (2002). Neural regeneration and the peripheral olfactory system. Anat. Rec. 269 33–49. 10.1002/ar.10047 [DOI] [PubMed] [Google Scholar]
- Scolnick J. A., Cui K., Duggan C. D., Xuan S., Yuan X. B., Efstratiadis A., et al. (2008). Role of IGF signaling in olfactory sensory map formation and axon guidance. Neuron 57 847–857. 10.1016/j.neuron.2008.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiga H., Nagaoka M., Washiyama K., Yamamoto J., Yamada K., Noda T., et al. (2014). Reduced nasal transport of insulin-like growth factor-1 to the mouse cerebrum with olfactory bulb resection. Chem. Senses 39 595–599. 10.1093/chemse/bju032 [DOI] [PubMed] [Google Scholar]
- Tannemaat M. R., Eggers R., Hendriks W. T., de Ruiter G. C., van Heerikhuize J. J., Pool C. W., et al. (2008). Differential effects of lentiviral vector-mediated over expression of nerve growth factor and glial cell line-derived neurotrophic factor on regenerating sensory and motor axons in the transected peripheral nerve. Eur. J. Neurosci. 28 1467–1479. 10.1111/j.1460-9568.2008.06452.x [DOI] [PubMed] [Google Scholar]
- Ueha R., Mukherjee S., Ueha S., de Almeida Nagata D. E., Sakamoto T., Kondo K., et al. (2014). Viral disruption of olfactory progenitors is exacerbated in allergic mice. Int. Immunopharmacol. 22 242–247. 10.1016/j.intimp.2014.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueha R., Shichino S., Ueha S., Kondo K., Kikuta S., Nishijima H., et al. (2018a). Reduction of proliferating olfactory cells and low expression of extra cellular matrix genes are hallmarks of the aged olfactory mucosa. Front. Aging Neurosci. 10:86 10.3389/fnagi.2018.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueha R., Ueha S., Kondo K., Kikuta S., Yamasoba T. (2018b). Cigarette smoke-induced cell death causes persistent olfactory dysfunction in aged mice. Front. Aging Neurosci. 10:183. 10.3389/fnagi.2018.00183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueha R., Ueha S., Kondo K., Sakamoto T., Kikuta S., Kanaya K., et al. (2016a). Damage to olfactory progenitor cells is involved in cigarette smoke-induced olfactory dysfunction in mice. Am. J. Pathol. 186 579–586. 10.1016/j.ajpath.2015.11.009 [DOI] [PubMed] [Google Scholar]
- Ueha R., Ueha S., Sakamoto T., Kanaya K., Suzukawa K., Nishijima H., et al. (2016b). Cigarette smoke delays regeneration of the olfactory epithelium in mice. Neurotox. Res. 30 213–224. 10.1007/s12640-016-9617-9615 [DOI] [PubMed] [Google Scholar]
- Vincent A. M., Mobley B. C., Hiller A., Feldman E. L. (2004). IGF-I prevents glutamate-induced motor neuron programmed cell death. Neurobiol. Dis. 16 407–416. 10.1016/j.nbd.2004.03.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A,B: Representative images of the olfactory epithelium (OE) from untreated mice (hematoxylin and eosin stain; A, 40× magnification; B, 400× magnification). The box in A indicates the region of the OE shown in B,C: Diagram of cell lineage of olfactory receptor neurons (ORN).
Change rate of each cell number. Each change rate was calculated by dividing each cell number in the low and high-dose insulin-like growth factor-1 (IGF-1) groups by those in the saline-treated control group. ∗P < 0.05; ∗∗P < 0.01; and ∗∗∗∗P < 0.0001 (one-way ANOVA). Cas3, caspase-3; GAP43, growth associated protein 43; OMP, olfactory marker protein; ORN, olfactory receptor neuron; SOX2, sex determining region Y-box 2.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, RU, upon reasonable request.