Abstract
BACKGROUND
Mesenchymal stem cells (MSCs) are multipotent stem or stromal cells found in multiple tissues. Intravenous MSC injections have been used to treat various diseases with an inflammatory component in animals and humans. Inflammation is emerging as a key component of pathophysiology of intracranial aneurysms. Modulation of inflammation by MSCs may affect sustained inflammatory processes that lead to aneurysmal rupture.
OBJECTIVE
To assess the effect of MSCs on the development of aneurysm rupture using a mouse model.
METHODS
Intracranial aneurysms were induced with a combination of a single elastase injection into the cerebrospinal fluid and deoxycorticosterone acetate salt-induced hypertension in mice. We administered allogeneic bone marrow-derived MSCs or vehicle, 6 and 9 d after aneurysm induction.
RESULTS
MSC administration significantly reduced rupture rate (vehicle control vs MSCs, 90% vs 36%; P < .05). In cell culture experiments with an MSC and mast cell coculture, MSCs stabilized mast cells through cyclooxygenase-2 (COX-2)-dependent production of prostaglandin E2, thereby reducing the release of proinflammatory cytokines from mast cells. Pretreatment of MSCs with COX-2 inhibitor, NS-398, abolished the protective effect of MSCs against the development of aneurysm rupture.
CONCLUSION
Intravenous administration of MSCs after aneurysm formation prevented aneurysmal rupture in mice. The protective effect of MSCs against the development of aneurysm rupture appears to be mediated in part by the stabilization of mast cells by MSCs.
Keywords: Mesenchymal stem cells, Intracranial aneurysm, Stroke, Subarachnoid hemorrhage
ABBREVIATIONS
- MSC
mesenchymal stem cell
- COX-2
cyclooxygenase-2
- PGE2
prostaglandin E2
- TNF
tumor necrosis factor
Intracranial aneurysm is a common cerebrovascular lesion. Unruptured aneurysms may be detected in as much as 5% of the general population.1 Subarachnoid hemorrhage due to aneurysmal rupture causes significant adverse consequences with 30-d mortality approaching as high as 45%.1,2 Inflammation is emerging as a key component of pathophysiology of intracranial aneurysms.3-6 Higher degrees of infiltration of inflammatory cells including mast cells are associated with aneurysmal rupture in humans.7,8 Therefore, prevention of aneurysmal rupture by modulation of inflammation is considered as a potential therapeutic approach for unruptured aneurysms.4-6
Mesenchymal stem cells (MSCs) are multipotent progenitor cells, and they can differentiate into multiple cell types in the adult tissues.9 In addition, MSCs can exert systemic and local anti-inflammatory effects through various mechanisms.10-13 Previous studies proposed intravenous injections of MSCs as the potential treatment of diseases with an inflammatory component in animals and humans.11-13 Although a majority of intravenously administered MSCs can be initially trapped in the lungs, due to its size, they can reduce inflammation in peripheral tissues by secreting soluble factors that possess reparative properties.14 A small subset of the intravenously administered MSCs may home in on inflammatory sites and affect the local inflammatory milieu through the modulation of various inflammatory cells, including mast cells.15 The modulation of inflammation by intravenously injected MSCs may affect inflammatory processes that lead to aneurysmal rupture. Therefore, in this study, we assessed the effect of MSC treatment postaneurysm formation on the development of aneurysm rupture using a mouse model developed in our laboratory.3,16
METHODS
The study was approved by the Institutional Review Board and the animal care complied with the Guide for the Care and Use of Laboratory Animals.
Intracranial Aneurysm Model and MSC Treatment
Intracranial aneurysms were induced in C57BL/6J male mice (9 wk old; The Jackson Laboratory, Bar Harbor, Massachusetts) as previously described.3,5,16-18 To induce intracranial aneurysm in mice, we combined systemic hypertension with a single elastase injection into the cerebrospinal fluid.3,5,16-18 Deoxycorticosterone acetate-salt hypertension was used to induce systemic hypertension.3,17 A single dose of elastase (0.035U) was injected into the cerebrospinal fluid at the right basal cistern.3,17 The definition of aneurysm in this study was as follows: a localized outward bulging of the vascular wall with a diameter greater than the parental artery diameter.3,16 Two blinded observers conducted daily neurological examinations of mice to detect aneurysmal rupture, as we previously described.17
Our previous studies, including a study utilizing serial magnetic resonance imaging, found that aneurysm formation occurs during the first 6 to 7 d in this model; aneurysmal rupture begins approximately ∼7 d after aneurysm induction.6,17-19 Therefore, experimental treatments starting from 6 d after aneurysm induction can be used to test whether the experimental agents reduce the rupture rate.5,17,18 In the current study, MSCs (1×106 cells per 150 μL PBS) were injected intravenously 6 and 9 d after aneurysm induction, as shown in the experimental protocol (Figure 1A). Human bone marrow-derived MSCs were obtained from the Texas A & M Health Science Center College of Medicine, Institute for Regenerative Medicine (Temple, Texas), National Institutes of Health repository. The adult stem cells met the MSC criteria defined by the International Society of Cellular Therapy.20 We used human MSCs in this study for several reasons. First, human MSCs have been successfully used in multiple mouse disease models, and human MSCs exert similar therapeutic effects to mouse MSCs in mice.21-24 Second, human MSCs can evade clearance by the mouse immune system mostly because of low expression of major histocompatibility complex class II (MHCII) allowing xenogeneic transplantation.14 In addition, the use of human MSCs enables comparisons between our study and previous studies that utilized these cells.21-24
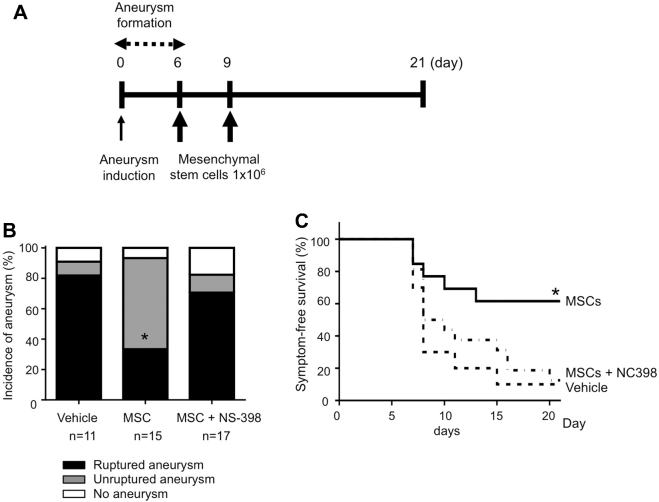
FIGURE 1.
A, Experimental protocol. Our previous studies showed that the ability of an experimental agent to reduce aneurysm rupture can be tested by treating mice with the agent from day 6 postaneurysm induction.6,17,18 Therefore, in this study, mesenchymal stem cells (MSCs; 1 × 106 cells in 150 μL PBS) were injected intravenously, 6 and 9 d after aneurysm induction through the jugular vein. B, Incidence of aneurysms and symptom-free survival curve. MSCs: mice received MSCs. NS398: cyclooxygenase-2 (COX-2) inhibitor. C, Symptom-free survival curve. As an exploratory analysis, survival analysis was performed using log-rank test. Mice that did not develop aneurysms were excluded from the survival analysis.
Cell Culture Studies
A mast cell line, LAD2, was donated by Dr. Arnold Kirshenbaum (NIAID (National Institute of Allergy and Infectious Diseases) and National Institutes of Health Office of Technology Development).25 The activation of LAD2 cells (human mast cell line) was achieved by the addition of a calcium ionophore (calcimycin, A23187, Sigma-Aldrich, St. Louis, Missouri), a lipid-soluble reagent.26 Activation and degranulation of mast cells were assessed by measuring the tumor necrosis factor-α (TNF-α) release following previous studies by others.15,27
Statistical Analysis
Fisher's exact was used to analyze rupture rate and the incidence of aneurysms. As an exploratory analysis, we analyzed symptom-free survival using the log-rank test. For the symptom-free survival analysis, we excluded mice that did not develop aneurysms. TNF-α concentrations in cell culture supernatants were compared using ANOVA, followed by Tukey's multiple comparison test. All results were expressed as the means ± standard deviation. Statistical significance was set at P < .05.
RESULTS
Effect of MSCs on the Development of Aneurysmal Rupture
As shown in Figure 1A, to test whether MSCs can protect against the development of aneurysm rupture, we treated mice with 1×106 MSCs or vehicle, twice after aneurysm formation. No difference in the overall incidence of aneurysm was observed between MSC-treated mice and vehicle-treated mice (Figure 1B). However, MSC treatment significantly reduced both the incidence of ruptured aneurysms (Figure 1B; vehicle control vs MSCs, 82% vs 33%; 9/11 vs 5/15; P < .05) and rupture rate (Figure 1B; vehicle control vs MSCs, 90% vs 36%; 9/10 vs 5/14; P < .05). Log-rank test of the symptom-free survival showed a significant reduction of aneurysmal rupture upon MSCs treatment (P < .05; Figure 1C). The treatments with MSCs did not significantly affect the blood pressure (Table).
TABLE.
Systolic Blood Pressure (mm Hg)
| –1 wk before | 1 wk after | 2 wk after | 2 wk after | |
|---|---|---|---|---|
| aneurysm induction | aneurysm induction | aneurysm induction | aneurysm induction | |
| Vehicle | 87.0 ± 8.9 | 119.9 ± 17.5* | 110.0 ± 14.0* | 127.0 ± 2.8* |
| MSC | 88.8 ± 9.1 (ns) | 121.3 ± 23.9* (ns) | 125.4 ± 26.7* (ns) | 126.8 ± 23.6* (ns) |
| MSC + NS-398 | 91.6 ± 7.9 (ns) | 133.5 ± 14.1* (ns) | 137.0 ± 10.7* (ns) | 132.0 ± 10.9* (ns) |
*P < .05 compared to the vehicle group. ns: no difference compared to the vehicle group. MSC: mice received MSCs. MSC + NS-398: mice received with MSCs pre-treated with NS-398.
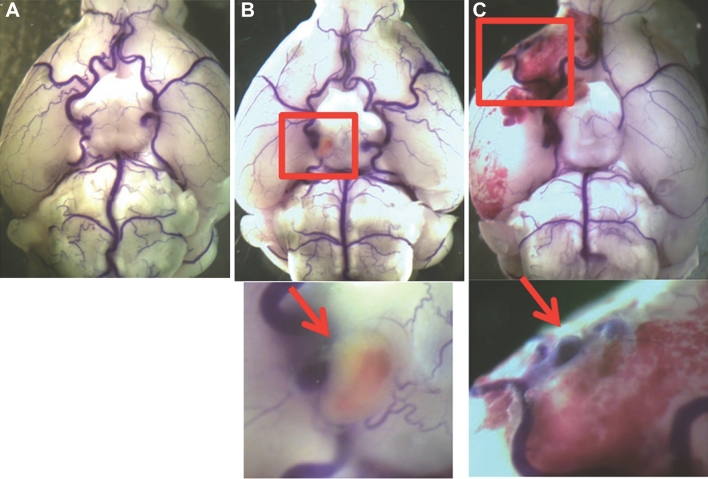
Figure 2 shows representative images of mouse normal cerebral arteries (Figure 2A), an unruptured aneurysm (Figure 2B), and a ruptured aneurysm from a mouse that developed neurological symptoms associated with aneurysmal rupture 8 d after aneurysm induction (Figure 2C).
FIGURE 2.
Intracranial aneurysms in the mouse model. Mouse cerebral arteries were visualized by bromophenol blue perfusion. A, No aneurysm. B, Unruptured aneurysm. C, Ruptured aneurysms and subarachnoid hemorrhage.
Effect of MSCs on Mast Cell Infiltration Into Aneurysms
Mast cells, classically known as key regulators of allergic reactions, are recently emerging as integral players in cardiovascular diseases.7,28 A higher degree of mast cell infiltration was found in ruptured aneurysms compared with unruptured aneurysms in humans.7 Therefore, we hypothesized that MSCs prevent aneurysm rupture through mast cell stabilization. As a first step, we assessed the effect of MSC treatment on the infiltration and activation of mast cells in cerebral arteries. The degree of mast cell activation was assessed by histological analysis of degranulated mast cells following the method previously described by others.29-31
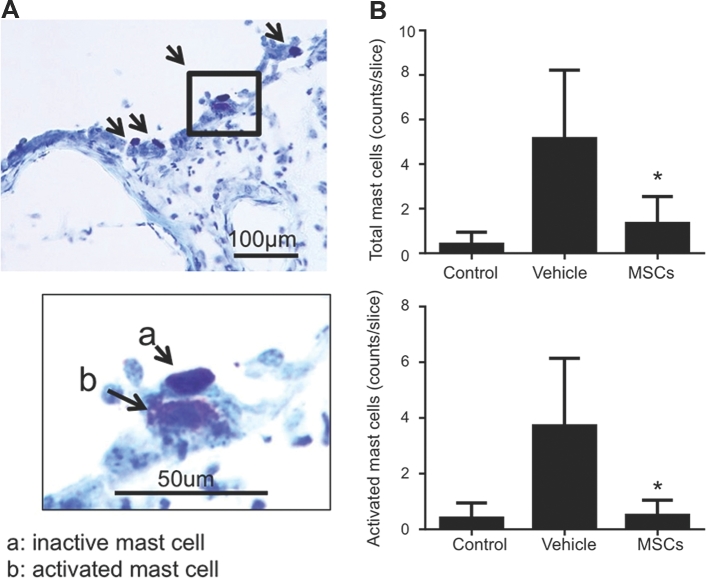
Representative toluidine staining of mast cells in the aneurysms and adjacent tissues in a mice after aneurysmal induction is shown in Figure 3A. As shown in Figure 3B, MSC treatment significantly reduced the overall mast cell infiltration into cerebral arteries (5.1 ± 1.2 vs 1.3 ± 0.5, P < .05). Moreover, MSC treatment significantly reduced the number of activated mast cells (3.7 ± 0.9 vs 0.5 ± 0.2; P < .05).
FIGURE 3.
Mast cell in intracranial aneurysm tissues from the mouse model. A, Representative toluidine staining of mast cells in the aneurysms and adjacent tissues in a mice after aneurysmal induction. B, Mast cell counting. Control: mice that did not receive aneurysm induction surgery or MSC treatment. Vehicle: mice that received aneurysm induction surgery and vehicle treatment. MSC: mice that received aneurysm induction surgery and MSC treatment.
Effect of MSCs on Mast Cell Activation in Coculture
Next, we investigated the potential mechanisms by which MSCs may stabilize mast cells and prevent their release of cytokines. Mast cells are a major source of TNF-α, an inflammatory cytokine that influences various aspects of inflammation.27 Previous studies indicated a key role of TNF-α in the pathophysiology of intracranial aneurysms4,32 TNF-α release from mast cells has been used to assess their activation status.15,27 Therefore, to evaluate mast cell activation in cell culture, we measure their TNF-α release, as previously described.15
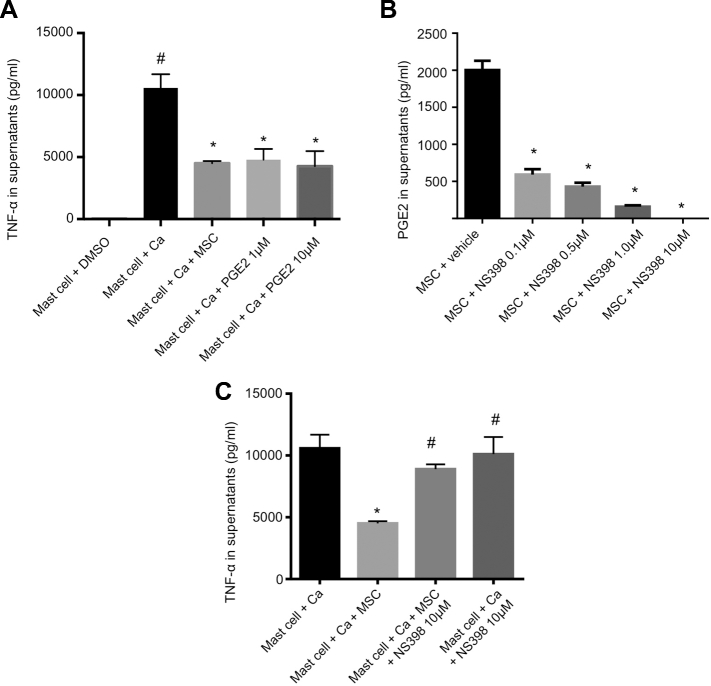
In the absence of MSCs, activation of mast cells by calcium ionophore resulted in a significant release of TNF-α (P < .05; Figure 4A). However, coculture with MSCs significantly reduced TNF-α release from mast cells, indicating suppression of mast cell activation by MSCs (2786 ± 66 vs 1121 ± 135 pg/mL, P < .05).
FIGURE 4.
Effect of MSCs on mast cells in coculture. A, Activation of mast cells assessed by TNF-α release. MSCs and prostaglandin E2 (PGE2) suppressed activation of mast cells. Ca: ionophore (calcimycin). *: P < .05 compared to “Mast cell + Ca.” #: P < .05 compared to “Mast cell + DMSO.” B, COX-2 inhibitor (NS398) suppressed the production of the COX-2 inhibitor (NS398) in a dose-dependent manner. *: P < .05 compared to “MSC + vehicle.” C, Effect of the COX-2 inhibitor treatment on the MSCs-induced stabilization of mast cells. MSCs stabilized mast cells and reduced TNF-α release. Treatment with COX-2 inhibitor (NS398) suppressed the stabilizing effect of MSCs. *: P < .05 compared to “Mast cell + Ca.” #: P < .05 compared to “Mast cell + Ca + MSC.”
Consistently with previous studies,15,33 prostaglandin E2 (PGE2) at 1 μM and 10 μM suppressed mast cell activation as evidenced by reduced release of TNF-α from mast cells (TNF-α: 10579 ± 1002 vs 4789 ± 789 pg/mL at 1 μM, P < .05 and 4264 ± 1114 pg/mL at 10 μM, P < .05; Figure 4A).
We then tested PGE2 release from mast cells and MSCs. Mast cells did not release PGE2 upon activation by calcium ionophore. However, the activation of MSCs by calcium ionophore induced the release of PGE2 (0 ± 0 [undetectable] vs 7937 ± 1325 pg/mL, P < .05; Figure 4B). The addition of mast cells to MSCs augmented the release of PGE2 (7937 ± 1325 vs 17234 ± 795 pg/mL, P < .05; Figure 4B).
Cyclooxygenase-2 (COX-2) is required for PGE2 production from arachidonic acid. Consistently with previous studies,15,33 NS-398 (COX-2 inhibitor) decreased PGE2 secretion by MSCs in a dose-dependent manner (vehicle vs 0.1, 0.5, 1.0, and 10 μM of NS-398, 2002 ± 103 vs 593 ± 59, 429 ± 43, 158 ± 16 pg/mL, and 0 pg/mL, respectively, P < .05; Figure 4B).
Next step, we tested whether COX-2 inhibitor abolishes the mast cell-stabilizing effect of MSCs. As shown in Figure 4C, the addition of MSCs significantly stabilized mast cells, as indicated by decreased TNF-α release from mast cells (activated mast cells only vs activated mast cells with MSCs, 10580 ± 1002 vs 4512 ± 140 pg/mL, P < .05). Treatment of MSCs with COX-2 inhibitor abrogated the stabilizing effect of MSCs on mast cells (MSCs vs MSCs pretreated with COX-2 inhibitor, 4512 ± 140 vs 8894 ± 358 pg/mL, P < .05).
Pretreatment of MSCs with COX-2 Inhibitor Abolished Their Protective Effect of MSCs
To test whether the protective effect of MSCs against the development of aneurysmal rupture is dependent on COX-2 in MSCs, we pretreated MSCs with COX-2 inhibitor in Vitro and then administered these pretreated MSCs to mice.
As shown in Figure 1B and C, pretreatment of MSCs with COX-2 inhibitor abolished their protective effect against the development of aneurysmal rupture, as evidenced by the finding that mice that received COX-2 inhibitor-pretreated MSCs had a significantly higher rupture rate than mice that received MSCs without COX-2 pretreatment (rupture rate: MSC vs COX-2 inhibitor treated MSC: 33% vs 71%; 5/14 vs 12/14; P < .05; incidence of ruptured aneurysms: MSC vs COX-2 inhibitor treated MSC: 36% vs 86%; 5/15 vs 12/17; P < .05).
DISCUSSION
Both clinical and animal studies strongly suggest the key role of inflammation in processes that lead to aneurysmal rupture.4,34,35 Analysis of human intracranial aneurysms revealed a higher degree of mast cell infiltration in ruptured intracranial aneurysms than in unruptured aneurysms,7 indicating a potentially significant role of mast cells in the development of aneurysmal rupture. Mast cells may therefore serve as a therapeutic target for the prevention of aneurysmal ruptures. In this study, we showed that the intravenous MSC administration after aneurysm formation reduced aneurysmal rupture in mice. MSC treatment reduced the number of activated mast cells in aneurysms. In addition, our cell culture experiments verified that MSCs stabilize mast cells and suppress the release of proinflammatory cytokines from mast cells, confirming previous observations by others.15,33 Furthermore, mast cell stabilization by MSC in cell culture was dependent on COX-2 mediated production of cytokines from MSCs. Finally, the pretreatment of MSCs with a selective COX-2 inhibitor abolished the protective effect of MSCs against the development of aneurysmal rupture. Intravenous MSC infusion may thus prevent aneurysmal rupture by stabilizing mast cells in a COX-2-dependent manner. This preliminary study provides basis for future investigations to further examine the administration of MSCs or MSC-derived factors as a potential therapeutic strategy for the prevention of aneurysmal rupture.
While this study indicates the involvement of COX-2 in MSCs’ protective effect, the exact mechanism is not clear. In this study, we did not investigate the fate of exogenously administered MSCs in this model. Previous studies have shown that more than 80% of exogenously administered MSCs initially accumulate in the lungs with a 24 h clearance half-life.36-38 Only a small fraction of MSCs (0.0005%) can reach the brain.39 MSCs may exert their effects after reaching to the aneurysm site, but it is more likely that effects are more systemic as previously postulated in other disease models.36-38 Anti-inflammatory cytokines secreted from MSCs trapped in the lungs, the spleen, and the liver may affect the inflammation of aneurysm wall in an endocrine fashion. Unfortunately, it is technically challenging to identify the exact time points of which MSCs can be detected in or near aneurysms.
In this study, MSCs were administered exogenously and, therefore, potential protective effects of MSCs may have been exaggerated in our model. However, it is possible that endogenous MSCs exert a similar protective effect. Unstable aneurysms that are prone to rupture show a higher degree of inflammation by magnetic resonance imaging.8,35 Stabilization of inflammation by MSCs may represents one of the intrinsic protective mechanisms that keep aneurysms stable preventing their rupture.
A previous study analyzing human intracranial aneurysm revealed the localization of COX-2 and microsomal prostaglandin E2 synthase-1 (mPGE2-S1), 2 enzymes that are required for PGE2 production, to the aneurysmal wall, albeit the exact cell type expressing COX-2 or microsomal PGE2 synthase type 1 (mPGE2-S1) has not been identified.40 Mice lacking mPGE2-S1 had a higher rupture rate and higher associated mortality than wild-type mice.41 The potential contribution of the pathways that involve PGE2 and COX-2 to the stabilization of aneurysms was further suggested by our current in Vitro and animal studies. Taken together, the findings from our study and previous investigations by other groups suggest that the COX-2-dependent production of PGE2 by MSCs may protect against the development of aneurysmal rupture by stabilizing mast cells.
Previous studies by our groups and others have shown that a majority of aneurysmal ruptures were detected during 6 to 12 d after elastase injection in this model.4,5,17,18,42 Intravenously administered MSCs disappear from the systemic circulation within 2 to 3 d.36,38 Therefore, to maximize therapeutic potential of MSCs for the prevention of aneurysmal rupture, MSCs were administered at day 6 and 9 post elastase injection. The same regimen of the 2 MSC injections was previously used in other disease models.43-46 We used the dose of MSCs (1 × 106 cells) based on the previous studies.33,43,46,47 More frequent dosing or a higher dose may further reduce the rupture rate. Alternatively, a single injection may be sufficient. Future studies should identify the optimal dosing strategy.
This study focused on the interaction between mast cells and MSCs as a potential mechanism for the protective effect of MSCs against the development of aneurysmal rupture. However, the exact mechanism may be more complex. Previous studies suggested that MSCs affect various types of inflammatory cells including lymphocytes and macrophages.48 The protective effect of MSCs against the development of aneurysmal rupture may therefore be mediated by the intricate interaction between MSCs and various inflammatory cell types.
Limitations
There are several limitations to the current study, as follows. (1) Despite multiple clinical trials demonstrating that MSC administration is safe and without immediate complications, the long-term risk of stem cell-based therapy (eg, iatrogenic tumor formation) is currently unknown.49 (2) More research is needed to determine the optimal dose, route, and timing of delivery as well as the source of MSCs, since MSCs can be harvested from multiple tissue sources including the bone marrow, fat, placenta, and other tissues.20 (3) In this study, we used only male mice. Our previous studies showed the roles of sex steroids in the pathophysiology of intracranial aneurysms. Effects of sex differences and menopausal state should be carefully studied in future studies. (4) MSCs treatment may affect the severity of subarachnoid hemorrhage, thereby potentially influencing outcomes after aneurysmal subarachnoid hemorrhage. Because of the small number of mice with aneurysmal subarachnoid hemorrhage in the MSC-treated group, we could not assess the effects of MSCs on the hemorrhage size. (5) There might be a trend for MSC treatment to increase blood pressure. Our sample size was too small to detect the statistical difference, if there was indeed a difference. However, it is more likely that MSC treatment causes hypotension as a result of the release of nitric oxide. Moreover, the higher blood pressure, if it happened, would promote more aneurysm ruptures as we described previously.18 (6) Perhaps, more importantly, little information is available regarding whether the effects of MSC administration are permanent or long lasting.
Although there is an increasing interest in utilizing MSCs in treating various diseases that are mediated by inflammation, stem cell-based therapy still carry some concerns including a risk for formation of iatrogenic tumor and growth of pre-existing tumor growth.50 As a possible alternative strategy, acellular microvesicles isolated from MSCs ex vivo may be used, avoiding the potential adverse effects related to the treatment using live cells.51,52 Microvesicles are anuclear membrane-bound fragments that can be released from MSCs as exosomes or as shedding vesicles arising from the plasma membrane.51 MSC-derived microvesicles can accumulate in tissues with inflammation, transfer biologically active proteins, and promote tissue repair and suppress inflammation.52 Future studies should examine the potential of MSCs-derived microvesicles in the prevention of aneurysmal rupture.
CONCLUSION
In conclusion, we demonstrated the potentially protective effect of MSCs against the development of aneurysmal rupture in a mouse model of intracranial aneurysm. MSCs that were administered after aneurysm formation prevented aneurysm rupture, suggesting that MSCs can affect the stability of matured aneurysms. Endogenous MSCs may affect the natural course of aneurysms (rupture vs stabilization) by modulating the inflammation of aneurysmal wall.
Stabilization of aneurysms may be facilitated by the administration of MSCs or pharmacological augmentation of MSCs.
Disclosures
This project was supported by R01NS055876 (TH) and R01NS082280 (TH) from the National Institute of Neurological Disorders and Stroke (NIH/NINDS), R01HL113022 (JWL) from the National Heart, Lung, and Blood Institute (NIH/NHLBI), and U54NS065705 (MTL) from the National Institute of Neurological Disorders and Stroke (NIH/NINDS). The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1. Connolly ES Jr, Rabinstein AA, Carhuapoma JR et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43(6):1711-1737. [DOI] [PubMed] [Google Scholar]
- 2. Shimada K, Furukawa H, Wada K et al. Angiotensin-(1-7) protects against the development of aneurysmal subarachnoid hemorrhage in mice. J Cereb Blood Flow Metab. 2015;35(7):1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanematsu Y, Kanematsu M, Kurihara C et al. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke. 2011;42(1):173-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Starke RM, Chalouhi N, Jabbour PM et al. Critical role of TNF-alpha in cerebral aneurysm formation and progression to rupture. J Neuroinflammation. 2014;11:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tada Y, Wada K, Shimada K et al. Estrogen protects against intracranial aneurysm rupture in ovariectomized mice. Hypertension. 2014;63(6):1339-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shimada K, Furukawa H, Wada K et al. Protective role of peroxisome proliferator-activated receptor-gamma in the development of intracranial aneurysm rupture. Stroke. 2015;46(6):1664-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hasan D, Chalouhi N, Jabbour P, Hashimoto T. Macrophage imbalance (M1 vs. M2) and upregulation of mast cells in wall of ruptured human cerebral aneurysms: preliminary results. J Neuroinflammation. 2012;9(222):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hasan DM, Mahaney KB, Magnotta VA et al. Macrophage imaging within human cerebral aneurysms wall using ferumoxytol-enhanced MRI: a pilot study. Arterioscler Thromb Vasc Biol. 2012;32(4):1032-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meyer EG, Buckley CT, Thorpe SD, Kelly DJ. Low oxygen tension is a more potent promoter of chondrogenic differentiation than dynamic compression. J Biomech. 2010;43(13):2516-2523. [DOI] [PubMed] [Google Scholar]
- 10. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71-74. [DOI] [PubMed] [Google Scholar]
- 11. Chen X, Armstrong MA, Li G. Mesenchymal stem cells in immunoregulation. Immunol Cell Biol. 2006;84(5):413-421. [DOI] [PubMed] [Google Scholar]
- 12. Nasef A, Ashammakhi N, Fouillard L. Immunomodulatory effect of mesenchymal stromal cells: possible mechanisms. Regen Med. 2008;3(4):531-546. [DOI] [PubMed] [Google Scholar]
- 13. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499-3506. [DOI] [PubMed] [Google Scholar]
- 14. Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: Mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29(6):913-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown JM, Nemeth K, Kushnir-Sukhov NM, Metcalfe DD, Mezey E. Bone marrow stromal cells inhibit mast cell function via a COX2-dependent mechanism. Clin Exp Allergy. 2011;41(4):526-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension. 2009;54(6):1337-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Makino H, Tada Y, Wada K et al. Pharmacological stabilization of intracranial aneurysms in mice: a feasibility study. Stroke. 2012;43(9):2450-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tada Y, Wada K, Shimada K et al. Roles of hypertension in the rupture of intracranial aneurysms. Stroke. 2014;45(2):579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Makino H, Hokamura K, Natsume T et al. Successful serial imaging of the mouse cerebral arteries using conventional 3-T magnetic resonance imaging. J Cereb Blood Flow Metab. 2015;35(9):1523-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. [DOI] [PubMed] [Google Scholar]
- 21. Chan MC, Kuok DI, Leung CY et al. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci USA. 2016;113(13):3621-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monsel A, Zhu YG, Gennai S et al. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192(3):324-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao Y, Gibb SL, Zhao J et al. Wnt3a, a protein secreted by mesenchymal stem cells is neuroprotective and promotes neurocognitive recovery following traumatic brain injury. Stem Cells. 2016;34(5):1263-1272. [DOI] [PubMed] [Google Scholar]
- 24. Pollock K, Dahlenburg H, Nelson H et al. Human mesenchymal stem cells genetically engineered to overexpress brain-derived neurotrophic factor improve outcomes in huntington's disease mouse models. Mol Ther. 2016;24(5):965-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kirshenbaum AS, Akin C, Wu Y et al. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leukemia Res. 2003;27(8):677-682. [DOI] [PubMed] [Google Scholar]
- 26. Johansen T. Mechanism of histamine release from rat mast cells induced by the ionophore A23187: effects of calcium and temperature. Br J Pharmacol. 1978;63(4):643-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakae S, Suto H, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci USA. 2005;102(18):6467-6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Swedenborg J, Mayranpaa MI, Kovanen PT. Mast cells: important players in the orchestrated pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2011;31(4):734-740. [DOI] [PubMed] [Google Scholar]
- 29. Wang J, Sjoberg S, Tia V et al. Pharmaceutical stabilization of mast cells attenuates experimental atherogenesis in low-density lipoprotein receptor-deficient mice. Atherosclerosis. 2013;229(2):304-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dimitriadou V, Lambracht-Hall M, Reichler J, Theoharides TC. Histochemical and ultrastructural characteristics of rat brain perivascular mast cells stimulated with compound 48/80 and carbachol. Neuroscience. 1990;39(1):209-224. [DOI] [PubMed] [Google Scholar]
- 31. Sun J, Sukhova GK, Yang M et al. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest. 2007;117(11):3359-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jayaraman T, Berenstein V, Li X et al. Tumor necrosis factor alpha is a key modulator of inflammation in cerebral aneurysms. Neurosurgery. 2005;57(3):558-564; discussion 558-564. [DOI] [PubMed] [Google Scholar]
- 33. Nemeth K, Leelahavanichkul A, Yuen PS et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hasan D, Chalouhi N, Jabbour P et al. Early change in ferumoxytol-enhanced magnetic resonance imaging signal suggests unstable human cerebral aneurysm: a pilot study. Stroke. 2012;43(12):3258-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Edjlali M, Gentric JC, Regent-Rodriguez C et al. Does aneurysmal wall enhancement on vessel wall MRI help to distinguish stable from unstable intracranial aneurysms? Stroke. 2014;45(12):3704-3706. [DOI] [PubMed] [Google Scholar]
- 36. Lee RH, Pulin AA, Seo MJ et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169(1):12-20. [DOI] [PubMed] [Google Scholar]
- 38. Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harting MT, Jimenez F, Xue H et al. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg. 2009;110(6):1189-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hasan D, Hashimoto T, Kung D, Macdonald RL, Winn HR, Heistad D. Upregulation of cyclooxygenase-2 (COX-2) and microsomal prostaglandin E2 synthase-1 (mPGES-1) in wall of ruptured human cerebral aneurysms: preliminary results. Stroke. 2012;43(7):1964-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pena Silva RA, Mitchell IJ, Kung DK et al. Paradoxical increase in mortality and rupture of intracranial aneurysms in microsomal prostaglandin E2 synthase type 1-deficient mice: attenuation by aspirin. Neurosurgery. 2015;77(4):613-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hasan DM, Starke RM, Gu H et al. Smooth muscle peroxisome proliferator-activated receptor gamma plays a critical role in formation and rupture of cerebral aneurysms in mice in vivo. Hypertension. 2015;66(1):211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jurewicz M, Yang S, Augello A et al. Congenic mesenchymal stem cell therapy reverses hyperglycemia in experimental type 1 diabetes. Diabetes. 2010;59(12):3139-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Choi SA, Lee JY, Wang KC et al. Human adipose tissue-derived mesenchymal stem cells: characteristics and therapeutic potential as cellular vehicles for prodrug gene therapy against brainstem gliomas. Eur J Cancer. 2012;48(1):129-137. [DOI] [PubMed] [Google Scholar]
- 45. Walker PA, Shah SK, Jimenez F et al. Intravenous multipotent adult progenitor cell therapy for traumatic brain injury: preserving the blood brain barrier via an interaction with splenocytes. Exp Neurol. 2010;225(2):341-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamawaki-Ogata A, Fu X, Hashizume R et al. Therapeutic potential of bone marrow-derived mesenchymal stem cells in formed aortic aneurysms of a mouse model. Eur J Cardiothorac Surg. 2014;45(5):e156-e165. [DOI] [PubMed] [Google Scholar]
- 47. Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39(2):573-576. [DOI] [PubMed] [Google Scholar]
- 48. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726-736. [DOI] [PubMed] [Google Scholar]
- 49. Lalu MM, McIntyre L, Pugliese C et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7(10):e47559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Prockop DJ, Brenner M, Fibbe WE et al. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy. 2010;12(5):576-578. [DOI] [PubMed] [Google Scholar]
- 51. Bruno S, Grange C, Deregibus MC et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20(5):1053-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu YG, Feng XM, Abbott J et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32(1):116-125. [DOI] [PMC free article] [PubMed] [Google Scholar]