Abstract
The biologically active compounds in wine, especially phenolics, are responsible for reduced risk of developing chronic diseases (cardiovascular disrease, cancer, diabetes, etc.), due to their antioxidant activities. We determined the contents of total phenolics (TP) and total flavonoids (TF) in selected Serbian white wines by colorimetric methods. Total antioxidant activity (TAA) of the white wines was analyzed using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capacity assay. Međaš beli had the highest content of TP, TF and TAA. The radical scavenging capacity (RSC) and total antioxidant activity (TAA) of white wines were 15.30% and 1.055 mM Trolox equivalent, respectively. Total phenolic (TP) and total flavonoid (TF) contents in white wines ranged from 238.3 to 420.6 mg gallic acid equivalent per L of wines and 42.64 to 81.32 mg catechin equivalent per L of wines, respectively. A high and significant correlation between antioxidant activity and total phenolic content was determined in wines (R2 = 0.968, p < 0.01). For the individual polyphenols determination we used a high performance liquid chromatography (HPLC)-diode array detection (DAD) technique. The majority of white wine polyphenols was represent by four hydroxycinnamic acids (HCAs).
Keywords: white wines, total phenolics, total antioxidant activity, flavonoids, hydroxycinnamic acids
1. Introduction
Wine is a widely consumed beverage with thousands of years of tradition. The quality of a wine depends on its numerous constituents, with the presence/absence and amount of a given chemical playing a considerable role. Phenolic compounds are always present and they contribute markedly to the color, flavor, bitterness and astringency of the final product. In addition to their direct role, phenolic compounds may also contribute to the sensory and chemical qualities of wine because of their interaction with other compounds, namely proteins, polysaccharides or other polyphenols [1]. Moreover, interest in phenolic compounds in wine has increased in recent years because of their potential beneficial effects on human health [2].
The phenolic composition is deeply influenced by three sets of factors: the nature of the raw material (grape variety, its degree of maturation, the nature of the soil and the climate) [3], the vinification techniques [2,4] and evaluation of phenolic compounds during the ageing of wine [2]. White wines are usually made from the free running juice, without grape mash, having no contact with the grape skins. This was thought to be the main reason for relatively low polyphenol content and thus for the lower antioxidant activity of white wine in comparison to red wine.
The production of white wines involves a great effort to avoid extensive contact with oxygen, which might be deleterious in terse of color alteration (browning) and eventually determination of the overall quality and marketability. Browning in wines is the result of a complex series of oxidation reactions that take place during processing, ageing and storage, which give rise to a brown color that increases color intensity, decreases brightness and raises the browning index [5]. The most important polyphenolic constituents in white wines, both in term of quantity and ability to participate in redox reactions, are the hydroxycinnamates and flavanols. In particular, oxidation of ortho-dihydroxyphenolic compounds such as (+)-catechin, (-)-epicatechin, caffeic and other hydroxy-cinnamic acids leads to the formation of yellow or brown products due to the polymerization of ortho-quinones [6]. Other constituents of the wine, such as transition metal ions, the presence of SO2 and ascorbic acid are of equal importance in polyphenol oxidation [7]. Sulphur dioxide and ascorbic acid, when added to wine, are able to reduce the ortho-quinones, while metal ions can catalyze the oxidation reactions [7].
Serbia, with 82,000 ha of vine area and around 2 million hL of annual wine productions, is one of the middle-range wine producers in Europe. It has different regions, each with their specific climates, yield and distinct wine varieties. The main wine growing areas all lay along rivers: the Danube, all three Moravas, the Timok or the Nišava. There are several indigenous wines like the pale Smederevka or Slankamenka and the rosé Prokupac and Župljanka, that bear the names of the towns or regions where they originated. The aim of this study was to characterize the phenol components and antioxidant profile of white wines produced in the Zapadnomoravski and Banat wine regions of Serbia.
2. Results and Discussion
2.1. Total phenolic and flavonoid contents
Total phenolic contents of the 10 tested nwhite wines are presented in Table 1. Of all the white wines analysed and produced in 2007, Međaš Beli and Terra Lazarica-Chardonnay had the highest total phenolic content (420.6 ± 12.0 and 358.0 ± 12.1 mg of gallic acid equivalents/L of wine, respectively), followed by Banatski Riesling, Graševina, Chardonnay, Muscat Ottonel and Smederevka. The phenolic content of Međaš Beli was significantly different from the others (p < 0.05). Significant differences were found in total phenolic content when comparing between Terra Lazarica-Chardonnay and Graševina and Banatski Riesling and Muscat Ottonel (p < 0.05); however, no significant differences in total phenolic content were found between Graševina and Muscat Ottonel, between Terra Lazarica-Chardonnay and Banatski Riesling, among Chardonnay, Muscat Ottonel and Smederevka. Significant differences were also found in comparisons between Zupski Riesling and Car Constantin-Semillon, which were produced in 2003. In this study, there was a 1.76-fold difference in total phenolic content between the highest and lowest ranked varieties, Međaš Beli and Smederevka (p < 0.05). It is well known that genetic and agronomic or environmental factors play important roles in phenolic composition and thus nutritional quality of crops.
Table 1.
Total phenol (TP) and total flavonoid (TF) contents.
| Wine and vintage | TPa | TFb | TF/TP |
|---|---|---|---|
| (mg GAE/L) | (mg CE/L) | ||
| Zapadnomoravski wine region | |||
| Graševina (2007) | 268.8 ± 8.0 b | 46.50 ± 1.16 a | 0.17 |
| Smederevka (2007) | 238.3 ± 6.6 a | 45.30 ± 1.29 a | 0.19 |
| Chardonnay (2007) | 253.8 ± 7.8 ab | 46.29 ± 1.43 a | 0.18 |
| Terra Lazarica-Chardonnay (2007) | 358.0 ± 10.2 dc | 65.00 ± 1.86 c | 0.18 |
| Međaš beli (2007) | 420.6 ±12.0 e | 81.32 ± 2.36 d | 0.19 |
| Župski Riesling (2003) | 380.0 ± 12.1 d | 63.99 ± 1.92 c | 0.17 |
| Car Konstantin-Semillon (2003) | 261.5 ± 7.8 ab | 48.25 ± 1.38 a | 0.18 |
| Banat wine region | |||
| Traminac (2004) | 270.2 ± 7.9 b | 50.02 ± 1.55 a | 0.18 |
| Banatski Riesling (2007) | 330.2 ± 9.4 c | 56.29 ± 1.66 b | 0.17 |
| Muscat Ottonel (2007) | 252.0 ± 7.5 ab | 47.88 ± 1.35 a | 0.18 |
a The level of total phenolics is expressed as gallic acid equivalent (GAE) and data are reported as mean ± standard deviation (n = 3); b The level of total flavonoids is expressed as catechin equivalent (CE) and data are reported as mean ± standard deviation (n = 3); c-e Bars with no letters in common are significantly different (p < 0.05) in the same column.
2.2. Total flavonoid content
Total flavonoids of the 10 white wines were measured (Table 2). The Međaš Beli presented the highest flavonoid content (81.32 ± 2.36 mg of catechin equivalents/L of wines, (p < 0.05), followed by Terra Lazarica-Chardonnay, Župski Riesling, Banatski Riesling, Traminac, Car Konstantin-Semillon, Muscat Ottonel, Graševina, Chardonnay and Smederevka. The flavonoid content of Međaš Beli was significantly different from each others (p < 0.05) (except for Terra Lazarica-Chardonnay). Significant differences were found in total flavonoid content in comparisons between Banatski Riesling and Župski Riesling, Chardonnay and Terra Lazarica-Chardonnay, Graševina and Banatski Riesling and Terra Lazarica-Chardonnay and Muscat Ottonel. No significant differences in the total flavonoid content were found in comparisons between Terra Lazarica-Chardonnay and Župski Riesling, and among Traminac, Car Konstantin-Semillon, Muscat Ottonel, Graševina, Chardonnay and Smederevka (p < 0.05). An approximately 1.79-fold difference in total flavonoid content was found between the highst and lowest ranked varieties, Međaš Beli and Smederevka (p < 0.05). The average percent amount of flavonoids in total phenolics was 18 (16% in Croatian white wines) [8]. The mean concentration of the phenolic content of the Serbian white wines was 303.3 mg GAE/L. This is higher than the levels quoted in the literature for white wines grown in different countries (see Table 2).
Table 2.
Published values of phenolic content in different white wines.
| Country | TP (mg GAE/L) | Reference |
|---|---|---|
| Croatia | 161–431 (n = 15) | [8] |
| Croatia | 301–402 (n = 4) | [9] |
| Croatia | 231–273 (n = 2) | [25] |
| Croatia | 191–652 (n = 3) | [26] |
| Greece | 162–286 (n = 12) | [5] |
| Greece | 213–277 (n = 5) | [27] |
| Greece | 267 (n = 1) | [13] |
| Spain | 89–407 (n = 17) | [10] |
| Spain | 178–293(n = 5) | [28] |
| Italy | 170–260 (n = 16) | [29] |
| Italy | 96–146 (n = 3) | [30] |
| Chech Republic | 103–125 (n = 7) | [31] |
| South Africa | 242–292 (n = 4) | [13] |
2.3. Total antioxidant activity of wines using the DPPH scavenging assays
The radical scavenging capacity was evaluated by measuring the scavenging activity of examined wine samples on 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals. All antioxidant results are summarized in Table 3. A high phenolic content in the investigated white wines contributes to their increased antioxidant activity. The investigated white wines showed antioxidant behavior in the range from 19.05% to 12.7%. The percentage for Croatian white wines was 16.16–10.7 [8].
Table 3.
Radical scavenging capacity (RSC) and total antioxidant activity (TAA) in different white wines.
| Wine and vintage | RSC (%) | TAAa (mM TE/L) |
|---|---|---|
| Zapadnomoravski wine region | ||
| Graševina (2007) | 14.00 | 0.95 ± 0.02 a,b |
| Smederevka (2007) | 13.50 | 0.92 ± 0.03 a |
| Chardonnay (2007) | 12.73 | 0.87 ± 0.03 a |
| Terra Lazarica-Chardonnay (2007) | 16.30 | 1.16 ± 0.05 c,d |
| Međaš beli (2007) | 19.05 | 1.35 ± 0.06 e |
| Župski Riesling (2003) | 18.64 | 1.29 ± 0.03 d,e |
| Car Konstantin-Semillon (2003) | 12.83 | 0.88 ± 0.03 a |
| Banat wine region | ||
| Traminac (2004) | 13.82 | 0.94 ± 0.05 a |
| Banatski Riesling (2007) | 16.04 | 1.10 ± 0.04 b,c |
| Muscat Ottonel (2007) | 14.95 | 0.99 ± 0.06 a,b |
a The level of total antioxidant activity is expressed as Trolox equivalent (TE) and data are reported as mean ± standard deviation (n = 3).c-e Bars with no letters in common are significantly different (p < 0.05) in the same column.
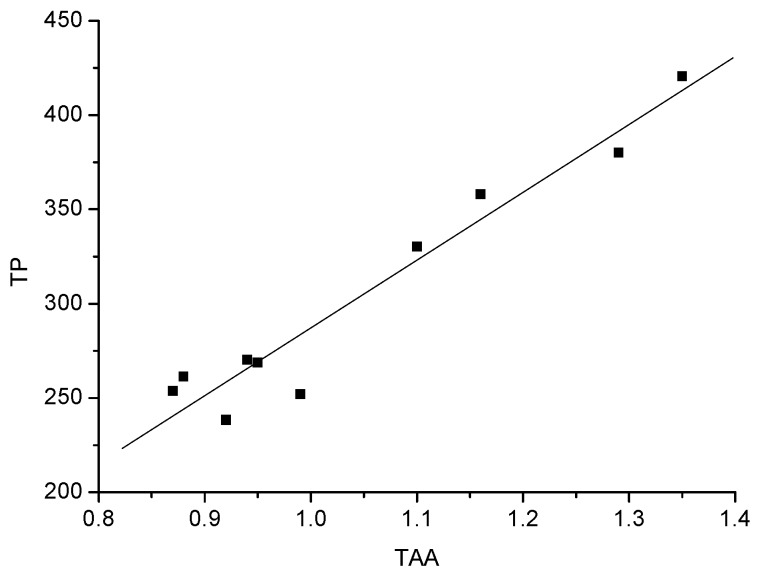
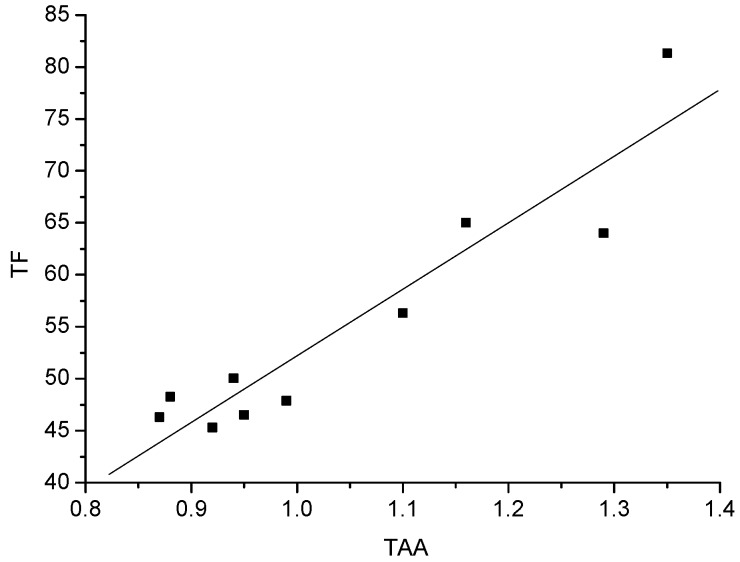
Total antioxidant activity (TAA) of the 10 white wines, expressed as millimoles (mmol) of Trolox equivalents per L of wine, is shown in Table 3. Međaš beli had the highest antioxidant activity, followed by Župski Riesling, Terra Lazarica-Chardonnay, Banatski Riesling, Muscat Ottonel, Graševina, Traminac, Smederevka, Car Konstantin-Semillon and Chardonnay (p < 0.05). A significant difference was found among Međaš beli, Terra Lazarica-Chardonnay and Chardonnay. The total antioxidant activities of Banatski Riesling and Muscat Ottonel were similar (p < 0.05), but lower (p < 0.05) than that of Međaš beli. No significant difference (p < 0.05) was found among Muscat Ottonel, Graševina, Traminac, Smederevka, Car Konstantin-Semillon and Chardonnay. The varieties containing high total phenolic contents had higher antioxidant activities. The present study reveals a very good correlation between total antioxidant activity and total phenolics (Figure 1) (R2 = 0.968). Also, the significant correlation between the total flavonoid content, and antioxidant activity (R2 = 0.938) of the tested white wine samples was confirmed (Figure 2).
Figure 1.
Correlation between total phenolics and total antioxidant activity.
Figure 2.
Correlation between total flavonoids and total antioxidant activity.
TAA values of the Serbian white wines analized in this study were in the range obtained for white wines from other wine-producing countries such as Spain (0.30–2.68 mM TE/L) [14] and 0.14–1.45 mM TE/L) [20] Portugal (1.4–2.9 mM TE/L) [21] and south African (0.54–0.72 mM TE/L) [19].
2.4. HPLC analysis
Tartaric esters of hydroxycinnamic acids represent the main non-flavonoid polyphenols in white grapes, mainly concentrated in the grapes fresh and thus in the wines made from white varieties. They represent 80% of all polyphenols of white grape juice [22]. They are involved in the browning reaction of must and wine, are precursors of volatile phenols and have antimicrobial and antioxidant activity. Their antioxidant properties may exert a positive health effect that is attributed to moderate wine consumption.
Hydroxycinnamic acids and their tartaric acid derivatives have been monitored at 320 nm, since that is their characteristic wavelength, together with GRP (grape reaction product or 2-S-glutathionyl-t-caftaric acid).GRP is the product of the reaction between caftaric acid and glutation, which, as can be observed, is only detectable in the wine and not in the grape skins [23].
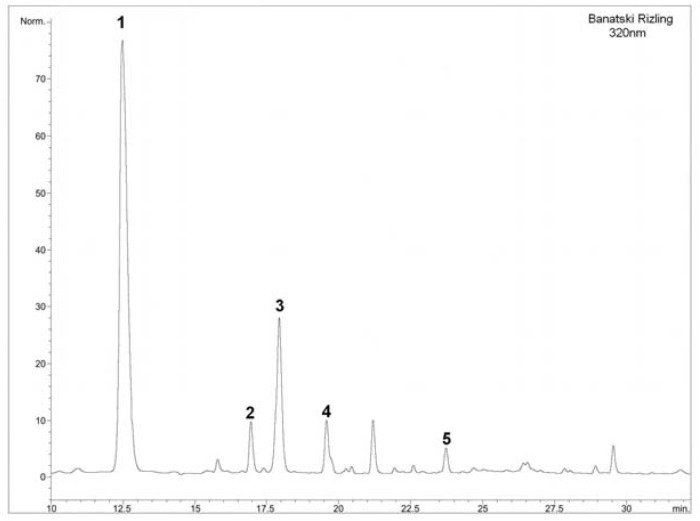
The chromatographic of some white wines monitored at 320 nm is given in Figure 3. The quantification of results for hydroxycinnamic acids in the analyzed samples is shown in Table 4. The predominant hydroxycinnamic acid was caftaric, followed by coutaric, caffeic and coumaric acid (Chardonny, 2007; Banatski Riesling, 2007; Graševina, 2007). The ratio of trans-caftaric acid (to all measured hydroxycinnamic acids) from Chardonnay, Banatski Riesling and Grasevina was on average 76.0%. Caftaric acid plays an important role in phenol oxidation and oxidative browning in must. The oxidized derivatives of coutaric and caftaric acid provide the yellowish-gold color in white wine. The values are within the range reported for white wines by other researchers [24,25,26,27].
Figure 3.
Chromatogram of a white wine. Identification of compounds: t-caftaric acid (1), GRP (2), trans-coutaric acid (3), caffeic acid (4), p-coumaric acid (5).
Table 4.
Hydroxycinnamic acids (mg/L) of white wines.
| Chardonnay | Banatski Riesling | Graševina | Traminac | Semillon | |
|---|---|---|---|---|---|
| (2007) | (2007) | (2007) | (2004) | (2003) | |
| Caffeic acid | 1.57 ± 0.18 | 3.39 ± 0.65 | 2.27 ± 0.82 | 7.70 ± 0.98 | 3.88 ± 0.80 |
| p-coumaric acid | nd | 0.62 ± 0.09 | 0.71 ± 0.08 | 2.02 ± 0.65 | 1.72 ± 0.06 |
| trans-caftaric acid | 14.71 ± 1.1 | 43.53 ± 1.5 | 21.96 ± 1.2 | 7.56 ± 0.85 | nd |
| trans-coutaric acid | 2.66 ± 0.74 | 5.74 ± 0.76 | 3.71 ± 0.90 | 1.36 ± 0.53 | 1.21 ± 0.22 |
| GRP | 1.13 ± 0.15 | 2.39 ± 0.35 | nd | 1.91 ± 0.48 | 4.14 ± 0.52 |
The data are reported as mean ± standard deviation (n = 3).
2.5. Principal component analysis
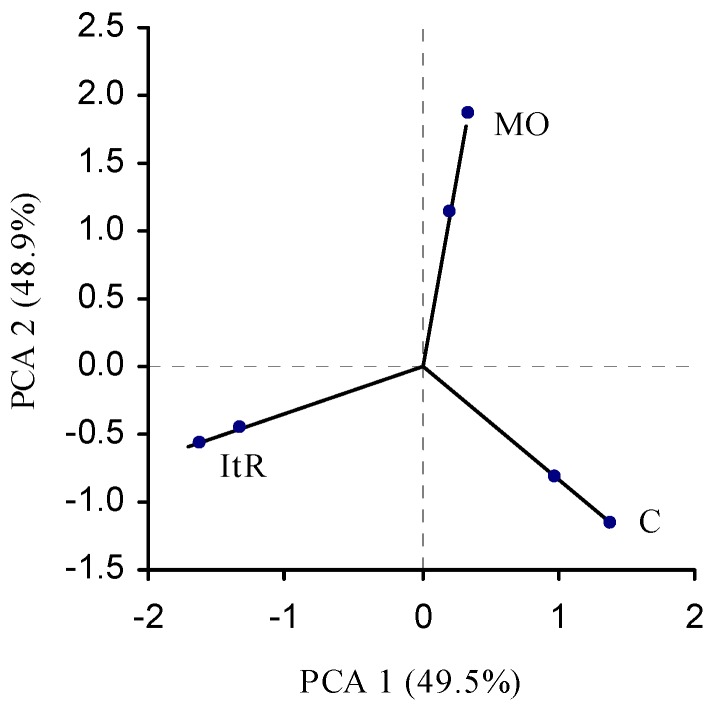
Principal component analysis (PCA) was applied in order to investigate the possible grouping of white wines (2007 vintage). From Table 5 and the correlation score plot in Figure 4, it can be seen that the first principal component (PC1) accounting for 49.544% of the variability and the second principal component (PC2) accounted for 48.853% of the variability, together PC1 and PC2 account for 98.397% of the total variance.
Table 5.
Explained variance and eigenvalues (PC1: first principal component, PC2: second principal component, PC3: third principal component).
| Value | PC1 | PC2 | PC3 |
|---|---|---|---|
| Eigenvalue | 1.486 | 1.330 | 0.048 |
| % of variance | 49.544 | 48.853 | 1.603 |
| Cumulative % | 49.544 | 98.397 | 100.000 |
Figure 4.
Principal component score plot (PC1 and PC2) of the studied white wines based on spectrophotometric data for the total phenols.
White wines from Muscat Ottonel (MO) grapes (Medjas Beli and Muscat Ottonel) are placed in the lower positive part of PC1, but high in PC2, while the white wines from Chardonnay (C) grapes (Chardonnay and Terra Lazarica-Chardonnay) are distributed in high positive part of PC1. White wines from Italian Riesling (ItR) grapes (Grasevina and Banatski Riesling) have a large negative contribution to PC1 and small negative contribution to PC2. This results suggest that the content of the phenolics in wine dependence on the grape varieties.
3. Experimental
3.1. Wine samples
10 commercially available wines made from different grape varieties were selected and then analyzed (June, 2009). A list of all wines analyzed in this study with their geographical origin and type is presented in Table 6. All samples were stored at 10 °C in the dark and analyzed shortly after opening. All wines analyzed are frequently consumed in Serbia.
Table 6.
Compositional factors determined in the white wines.
| Wine and vintage | Grape varieties | Alcohol Vol. (%) | Acidity a | pH |
|---|---|---|---|---|
| Zapadnomoravski wine region | ||||
| Graševina (2007) | Italien Riesling | 11.5 | 6.93 | 3.57 |
| Smederevka (2007) | Smederevka | 10.5 | 6.83 | 3.50 |
| Chardonay (2007) | Chardonnay | 11.0 | 6.70 | 3.40 |
| Terra Lazarica-Chardonnay (2007) | Chardonnay | 12.0 | 6.72 | 3.42 |
| Međaš beli (2007) | Chardonnay, Sauvignon, Muscat Ottonel | 11.5 | 6.38 | 3.57 |
| Župski Riesling (2003) | Italian Rieling | 10.7 | 6.12 | 3.81 |
| Car Konstantin-Semillon (2003) | Semillon | 11.5 | 6.35 | 3.62 |
| Banat wine region | ||||
| Traminac (2004) | Traminer | 12.5 | 6.30 | 3.24 |
| Banatski Riesling (2007) | Italian Riesling, Smederevka, Župljanka, Kreazer | 11.3 | 5.98 | 3.22 |
| Muscat Ottonel (2007) | Muscat Ottonel | 11.5 | 6.42 | 3.38 |
a Titritable acidity expressed as grams per liter tartaric acid.
3.2. Chemicals
All chemicals and reagents were of analytical grade and were obtained from Sigma Chemical Co. (St. Louis, MO, USA), Aldrich Chemical Co. (Steineheim, Germany), and Merck (Darmstadt, Germany). Calibration curves were obtained from triplicate injections of five concentrations.
3.3. Determination of total phenolic content (TP)
The total phenol content of the wines was measured spectrophotometrically at 760 nm after the reaction with Folin-Ciocalteu phenol reagent, according to the manual method described by Singleton et al. [28] using gallic acid as a calibration standard. Results are given as gallic acid equivalents (GAE, mg/L). All measurements were performed in triplicate.
3.4. Determination of total flavonoid content (TF)
The total flavonoid content in selected wine samples was determined spectrophotometrically [29], using a method based on the formation of complex flavonoid-aluminium. The absorbance was measured at 510 nm. The concentration of the total flavonoid compounds in the wines was expressed as catechin equivalent (mg/L). The results in every essay were obtained from three parallel determinations.
3.5. Measurement of the DPPH scavenging activity
The free radical scavenging activity of white wines was determined according to the method of Liu et al. [30]. 0.1 mL white wine sample (at the dilution of 1:10 with methanol) were added to 1.9 mL of a 0.1 mM methanolic solution of DPPH. The absorbance at 517 nm was measured after the solution had been allowed to stand in the dark for 30 min. Radical scavenging capacity (RSC), expressed as a percentage, was calculate by using the formula:
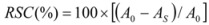 |
where A0 is the absorbance of the blank sample and As is the absorbance of the tested white wine sample. Using Trolox as a standard, total antioxidant activity (TAA) was expressed as milimoles of Trolox equivalents (TE) per L of white wine.
3.6. HPLC analysis of hydroxycinnamic acids
For determination of the hydroxycinnamic acids we used an HPLC Agilent-1200 series instrument equipped with a UV-Vis photodiode array (DAD) detector. The column was termostated at 30 °C. After injecting 5 µL of sample, separation was performed in an Agilent-Eclipse XDB C-18 4.6x150 mm column. Two solvents were used for the gradient elution: A-(H2O + 5%HCOOH) and B-(80%ACN + 5%HCOOH + H2O). The elution program used was as follows: from 0 to 28 min, 0.0% B, from 28 to 35 min, 25% B, from 35 to 40 min, 50% B, from 40 to 45 min, 80% B, and finally for last 10 min again 0% B. The different hydroxycinnamic acids analysed were tentatively identified according to their order of elution, retention times of pure compounds (caffeic, coumaric and ferulic acids) and the characteristics of the UV-Vis spectra published in different studies [23,31,32]. The calibration curves were obtained by injecting standards with different concentrations. The range of the linear calibration curves (R21,2,3 > 0.97) was 1.0 to 100.0 mg/L. Quantification of non-commercial compound was made using the calibration curves belonging to the most similar compound: caffeic acid for cis- and trans-caftaric acids and GRP; p-coumaric acid for cis- and trans-coutaric acids and ferulic acid for cis- and trans-fertaric acids. The calibration graphs were constructed for every standard by plotting each chromatographic peak area against its corresponding concentration.
3.7. Statistical analysis
Experiments were reported at least three times, and the data were analyzed statistically. All results are given as mean ± standard deviation (SD). Analysis of variance ANOVA tests were established for total phenolics, flavonoids and antioxidant activity in white wines.
4. Conclusions
This study shows that although all the analyzed white wines contained phenolic compounds and flavonoids, their contents are markedly different among grape varieties. All white wines varieties represent a potential source of natural antioxidants, but the Međaš Beli variety showed a better performance for wines which were produced in 2007. Also, the present data indicated differences in phenolic composition and antioxidant activity of different cultivar wines in spite of differences in agronomic factors and climatic conditions. In addition the total antioxidant activity of wines, correlated to their phenolic contents, but some individual phenilic compounds had higher antioxidant activity then others. Because of that, future research should include investigation of the effect of different individual phenolic compound on the antioxidant activity of wines.
Acknowledgements
This work was supported by the European Union, FP7-REG-POT-2007-3-01, KBBE: Food, Agriculture and Biotechnology project “CHROMOLAB-ANTIOXIDANT”, No 204756, and by the Serbian Ministry of Science and Environmental Protection (grant number 142015).
Footnotes
Sample Availability: Samples of the compounds are available from the authors
References
- 1.Silva R.L., Andrade P.B., Valentao P., Seabra R.M., Trujillo M.E., Velazquez E. Analysis of non-coloured phenolics in red wine: Effect of Dekkera bruxellensis yeast. Food Chem. 2005;89:185–189. doi: 10.1016/j.foodchem.2004.02.019. [DOI] [Google Scholar]
- 2.Zafrilla P., Morillas J., Mulero J., Cayuela J.M., Martinez-Cacha A., Pardo F., Nicolas J.M.L. Changes during storage in conventional and ecological wine: Phenolic content and antioxidant activity. J. Agric. Food Chem. 2003;51:4694–4700. doi: 10.1021/jf021251p. [DOI] [PubMed] [Google Scholar]
- 3.Andrade P.B., Oliveira B.M., Ferreira M.A., Ferreres F., Garcia-Viguera C. Analysis of phenolic compounds in Spanish Albarino and Portuguese Alvarinho and Loureiro wines by capillary zone electrophoresis and high-performance liquid chromatography. Electrophoresis. 2001;22:1568–1572. doi: 10.1002/1522-2683(200105)22:8<1568::AID-ELPS1568>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 4.Ramos R., Andrade P.B., Seabra R.M., Pereira C., Ferreira M.A., Faia M. A preliminary study of non-coloured phenolics in wines of vaeietal white grapes (codega, gouveio and malvasia fina): Effects of grape variety, grape maturation and technology of winemaking. Food Chem. 1999;67:39–44. doi: 10.1016/S0308-8146(99)00107-7. [DOI] [Google Scholar]
- 5.Salacha M.-I., Kallithraka S., Tzourou I. Browning of white wines: correlation with antioxidant characteristics, total polyphenolic composition and flavanol content. Int. J. Food Sci. Tech. 2008;43:1073–1077. doi: 10.1111/j.1365-2621.2007.01567.x. [DOI] [Google Scholar]
- 6.Guyot S., Vercauteren. J., Cheynier V. Structural determination of colourless and yellow dimers resulting from (+)-catechin coupling catalysed by grape polyphenoloxidase. Phytochemistry. 1996;42:1279–1288. doi: 10.1016/0031-9422(96)00127-6. [DOI] [Google Scholar]
- 7.Singleton V.L., Orthofer R., Lamuela-Raventos R.M. The phenolic cinnamates of white grapes and wine. J. Sci. Food Agric. 1987;29:403–410. [Google Scholar]
- 8.Katalinic V., Milos. M., Modun D., Music I., Boban M. Antioxidant effectiveness of selected wines in comparison with (+)-catechin. Food Chem. 2004;86:593–600. doi: 10.1016/j.foodchem.2003.10.007. [DOI] [Google Scholar]
- 9.Katalinic V., Ljubenkov I., Pezo I., Generalic I., Stricevic O., Milos M., Modun D., Boban N.M. Free resveratrol monomers in varietal red and white wines from Dalmatia (Croatia) Period. Biol. 2008;110:77–83. [Google Scholar]
- 10.Budic-Leto I., Lovric T. Identification of phenolic acids and changes in their content during fermentation and ageing of white wines Posip and Rukatac. Food Technol. Biotechnol. 2002;40:221–225. [Google Scholar]
- 11.Rastija V., Srecnik G., Medic-Saric M. Polyphenolic composition of Croatian wines with different geographical origin. Food Chem. 2009;115:54–60. doi: 10.1016/j.foodchem.2008.11.071. [DOI] [Google Scholar]
- 12.Roussis I.G., Lambropoulos I., Tzimas P., Gkoulioti A., Marinos V., Tsoupeis D., Boutaris I. Antioxidant activities of some Greek wines and wine phenolic extracts. J. Food Compos. Anal. 2008;21:614–621. doi: 10.1016/j.jfca.2008.02.011. [DOI] [Google Scholar]
- 13.Roussis I.G., Lambropoulos I., Soulti K. Scavenging Capacities of Some Wines and Wine Phenolic Extracts. Food Techol. Biotechnol. 2005;43:351–358. [Google Scholar]
- 14.Fernandez-Pachon M.S., Villano D., Troncoso A.M., Garcia-Parrilla M.C. (2006). Determination of phenolic composition of cherry and table white wines by liquid chromatography and their relation with antioxidant activity. Anal. Chim. Acta. 2006;563:101–108. doi: 10.1016/j.aca.2005.09.057. [DOI] [Google Scholar]
- 15.Sanchez-Moreno C., Larrauri J.A., Saura-Calixto F. Free radical scavenging capacity of selected red, rose and white wines. J. Sci. Food Agric. 1999;79:1301–1304. doi: 10.1002/(SICI)1097-0010(19990715)79:10<1301::AID-JSFA367>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 16.Stevanato R., Fabris S., Momo F. New enzymatic method for the determination of total phenolic content in tea and wine. J. Agric. Food Chem. 2004;52:6287–6293. doi: 10.1021/jf049898s. [DOI] [PubMed] [Google Scholar]
- 17.Simonetti P., Pietta P., Testolin G. Polyphenol content and total antioxidant potential of selected Italian wines. J. Agric. Food Chem. 1997;45:1152–1155. doi: 10.1021/jf960705d. [DOI] [Google Scholar]
- 18.Stratil P., Kuban V., Fojtova J. Comparison of the Phenolic Content and Total Antioxidant Activity in Wines as Determined by Spectrophotometric Methods. Czech. J. Food Sci. 2008;26:242–253. [Google Scholar]
- 19.De Beer T., Joubert E., Gelderblom W.C.A., Manley M. Antioxidant Activity of South African Red and White Cultivar Wines: Free Radical Scavenging. J. Agric. Food Chem. 2003;51:902–909. doi: 10.1021/jf026011o. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Pachon M.S., Villano D., Garcia-Parrilla M.C., Tronoso A.M. Antioxidant activity of wines and relation with their polyphenolic composition. Anal. Chim. Acta. 2004;513:113–118. doi: 10.1016/j.aca.2004.02.028. [DOI] [Google Scholar]
- 21.Pinto P.C.A.G., Saraiva M.L. M.F.S., Reis, Lima J.L.E.C. Automatic sequential determination of the hydrogen peroxide scavenging activity and evaluation of the antioxidant potential by the 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical cation assay in wines by sequential injection analysis. Anal. Chim. Acta. 2005;531:25–32. doi: 10.1016/j.aca.2004.10.005. [DOI] [Google Scholar]
- 22.Mozetič B., Tomažič I., Škvarč A., Trbše P. Determination of Polyphenpls in White Grape Berries cv. Rebula. Acta Chim. Slov. 2006;53:58–64. [Google Scholar]
- 23.Gomes-Alonso S., Garcia-Romero E., Hermosin-Gutierrez I. HPLC analysis of diverse grape and wine phenolics using direct injection and multidetection by DAD and fluorescence. J. Food Compos. Anal. 2007;20:618–626. doi: 10.1016/j.jfca.2007.03.002. [DOI] [Google Scholar]
- 24.Mayen M., Baron R., Merida J., Medina M. Changes in phenolic composition during accelerated browning in white wines from cv. Pedro Ximenez and cv. Baladi grapes. Food Chem. 1997;58:89–95. doi: 10.1016/S0308-8146(96)00218-X. [DOI] [Google Scholar]
- 25.Recamales A., Sayago A., Gonzalez-Miret M.L., Hernanz D. The effect of storage conditions on the phenolic composition and color of white wine. Food Res. Int. 2006;39:220–229. doi: 10.1016/j.foodres.2005.07.009. [DOI] [Google Scholar]
- 26.Sioumis N., Kallithraka S., Makris D., Kefalas P. Kinetics of browning onset in white wines: Influence of principal redox-active polyphenols and impact on the reducing capacity. Food Chem. 2006;94:98–104. doi: 10.1016/j.foodchem.2004.10.059. [DOI] [Google Scholar]
- 27.Kallithraka S., Salacha M.I., Tzourou I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 2009;113:500–505. doi: 10.1016/j.foodchem.2008.07.083. [DOI] [Google Scholar]
- 28.Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-ciocalteu reagent. Method Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 29.Zhishen J., Mengcheng T., Wu Jianming W. The determination of flavonoids content in mulberry and scavenging effect on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 30.Liu Q., Yao H. Antioxidant activities of barley seeds extracts. Food Chem. 2007;102:732–737. doi: 10.1016/j.foodchem.2006.06.051. [DOI] [Google Scholar]
- 31.Lamuela-Raventos R.M., Waterhous A.L. A direct HPLC separation of wine phenols. Am. J. Enol. Vitic. 1994;45:1–5. [Google Scholar]
- 32.Monagas M., Carmen Gómez-Cordovés C., Bartolomé B., Olga Laureano O., Da Silva J.M.R. Monomeric, Oligomeric, and Polymeric Flavan-3-ol Composition of Wines and Grapes from Vitis vinifera L. Cv. Graciano, Tempranillo, and Cabernet Sauvignon. J. Agric. Food Chem. 2003;51:6475–6481. doi: 10.1021/jf030325+. [DOI] [PubMed] [Google Scholar]