Supplemental Digital Content is available in the text.
Keywords: carotid arteries, stroke, thrombectomy, tomography, tunica media
Abstract
Background and Purpose—
Previous studies suggest that intracranial carotid artery calcification (ICAC) volume might influence the clinical outcome of patients after endovascular treatment (EVT) for acute ischemic stroke. Importantly, ICAC can be subtyped into a medial or intimal pattern that may differentially influence the effect of EVT in patients with acute ischemic stroke.
Methods—
All 500 patients included in the MR CLEAN (Multicenter Randomized Clinical trial of Endovascular treatment for acute ischemic stroke in the Netherlands) were evaluated. Volume (mm3) and location pattern (tunica intima or tunica media) of ICAC could be determined on baseline noncontrast computed tomography in 344 patients. Functional outcome at 90 days was assessed with the modified Rankin Scale. Next, we investigated the association of ICAC volume and pattern with functional outcome using adjusted ordinal logistic regression models. Effect modification by EVT was assessed with an interaction term between treatment allocation and ICAC aspect.
Results—
We found evidence for treatment effect modification by ICAC pattern (P interaction=0.04). Patients with predominantly medial calcification had better functional outcome with EVT than without this treatment (adjusted common odds ratio, 2.32; 95% CI, 1.23–4.39), but we observed no effect of EVT in patients with predominantly intimal calcifications (adjusted common odds ratio, 0.82; 95% CI, 0.40–1.68). We did not find an association of ICAC volume with functional outcome (adjusted common odds ratio per unit increase ICAC volume 1.01 (95% CI, 0.89–1.13). Moreover, we found no evidence for effect modification by ICAC volume (P interaction=0.61).
Conclusions—
The benefit of EVT in acute ischemic stroke patients with a medial calcification pattern is larger than the benefit in patients with an intimal calcification pattern.
Clinical Trial Registration—
URL: http://www.trialregister.nl. Unique identifier: NTR1804. URL: http://www.isrctn.com. Unique identifier: ISRCTN10888758.
In the clinical management of acute ischemic stroke (AIS), endovascular treatment (EVT) has recently been established as an effective treatment for patients with a large vessel occlusion.1 Despite the large overall benefits, recent evidence shows that specific preprocedural patient characteristics may substantially influence the prognosis and absolute treatment benefit of the patient after EVT in terms of functional outcome. In particular, a larger amount of highly prevalent intracranial carotid artery calcification (ICAC) may be an indicator of poor functional outcome in AIS patient treated by EVT.2,3 One study reported an association between ICAC volume and poor recanalization status, which could not be explained by differences in procedural difficulties like accessibility of target occlusion, number of passes or periprocedural complications.2
Yet, given the retrospective design of previous studies, in combination with the older, less effective EVT techniques that were investigated, more evidence is required to establish whether ICAC influences functional outcome in AIS patients. Furthermore, because of the lack of control (non-EVT) groups in previous studies modification of treatment effect by ICAC volume could not be assessed.
In addition to the volume of ICAC, 2 distinct morphological patterns of ICAC were recently highlighted that likely represent 2 different pathological processes.4,5 In brief, one of the patterns is characterized by calcification in the tunica intima (intimal calcification pattern), whereas in the other pattern calcification is primarily present in the tunica media (medial calcification pattern).6–8 A recently published study observed differences in cardiovascular risk factor profile between patients with intimal and medial calcification patterns.9 The 2 types of calcification may relate differently to functional outcome and EVT effect in these patients.
Against this background, we performed a post hoc analysis of the MR CLEAN (Multicenter Randomized Clinical trial of Endovascular treatment of Acute ischemic stroke in the Netherlands) and investigated the effect of the volume and pattern of ICAC on functional outcome and on treatment effect.10 This knowledge may directly contribute to our insight into factors influencing the success of EVT in AIS patients.
Methods
Anonymized trial data and analytic methods that support our study findings are available from the principal investigator (Email mrclean@erasmusmc.nl) on reasonable request.
Patients
Data originated from the MR CLEAN trial which investigated the effectiveness of EVT in AIS patients.10 All patients had a radiographically confirmed proximal intracranial arterial occlusion and a minimal score of 2 on the National Institutes of Health Stroke Scale at baseline. Treatment had to be possible within 6 hours after symptom onset. Patients were randomized between EVT (intervention) or no EVT (control) along with usual medical care. Intravenous alteplase before randomization was allowed. Demographics, laboratory tests, and medical cardiovascular history were collected at baseline as previously described.11 Baseline imaging was performed with noncontrast computed tomography (NCCT) and CT angiography (CTA), evaluating the Alberta Stroke Program Early CT Score, the location of occlusion, and collateral status.12,13 Written informed consent before randomization was provided by all patients or their legal representatives. The study protocol was approved by a central medical ethics committee and the research board of each participating center. Funders of the original study and this post hoc study had no role in study design, data collection, data analysis, data interpretation, or writing of the article. All authors had full access to all the data in the study and approved the article for publication.
Assessment of ICAC
ICAC Volume
All study participants underwent NCCT before randomization, on which ICAC volumes were quantified (mm3) in the symptomatic intracranial internal carotid arteries separately. ICAC was evaluated from the horizontal part of the petrous (horizontal) segment of the artery till its top (circle of Willis). All segmentations of ICAC volume were done manually by 2 experienced observers (K.C.J. Compagne and P.R.D. Clephas) with a custom-made, reliable, and validated tool in ImageJ.14 The number of pixels with a Hounsfield unit ≥130 was multiplied by pixel-size and slice increment to obtain the volume of ICAC (mm3). Interobserver agreement has been published previously with an intraclass correlation coefficient of 0.99.14
ICAC Pattern
The pattern of ICAC was differentiated into intimal and medial ICAC according to a recently developed and validated scoring method15 (Figure 1). In short, this scoring method evaluates circularity, thickness, and morphology of the calcification using a specific weighting to determine whether calcification is predominantly intimal (<7 points) or medial (≥7 points). Two observers (K.C.J. Compagne and P.R.D. Clephas) independently graded all ICAC calcifications and were blinded to the symptomatic side during scoring. In case of disagreement, a consensus reading was performed between both observers.
Figure 1.
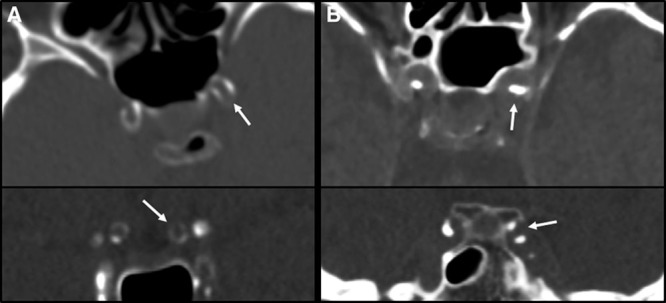
Patterns of medial and intimal intracranial carotid artery calcification on noncontrast computed tomography (CT). Medial calcification pattern is identified as a thin, continuous, and almost circular calcification patterns in axial viewing plane (A; upper) and coronal viewing plane (A; lower). Intimal calcification pattern is identified as a thick, irregular, and noncircular calcification patterns in axial viewing plane (B; upper) and coronal viewing plane (B; lower).
Outcome Assessment
Functional outcome at 90 days after the intervention was assessed with the modified Rankin Scale (mRS) by an independent research nurse who was blinded for treatment allocation.16 Recanalization status on follow-up CTA at 24 hours, evaluated by the modified arterial occlusive lesion score, was assessed by an independent core lab.17 Follow-up infarct volumes at 5 to 7 days follow-up were semiautomatically segmented on NCCT scans with the use of validated software.18 Safety end points were reported by local neurologists. Symptomatic intracranial hemorrhage was defined as neurological deterioration (an increase of 4 or more points on the National Institutes of Health Stroke Scale score) and evidence of intracranial hemorrhage on imaging studies.
Population for Analyses
Patients with NCCT scans with a slice thickness >3 mm, that could not be assessed reliably for ICAC, were excluded. Other reasons for excluding patients were movement artifacts, incomplete scans, inappropriate reconstruction, unavailable axial slices, or unavailable NCCT scan.
Statistical Analysis
Baseline characteristics of included patients between both intervention and control group were compared by means of the Mann-Whitney U test for continuous variables because of non-normal distributions, and χ2 test was used for categorical variables. The correlation between ICAC volume and age was evaluated by the Spearman rank correlation coefficient. Cohen’s kappa value (κ) and proportion of agreement were calculated to define the level of interobserver agreement in grading ICAC pattern.
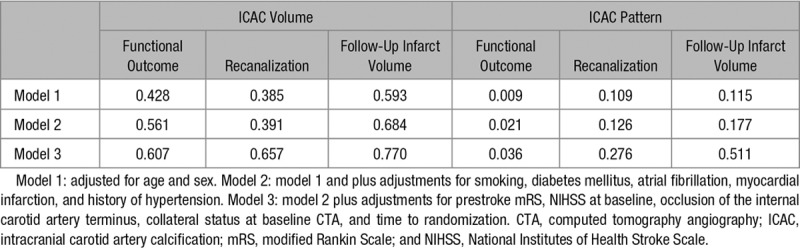
About ICAC volume, data were handled in 2 approaches. First, for continuous analyses, ICAC volumes were natural log-transformed after adding 1.0 mm3 to all volumes to deal with volumes of 0 mm3 (ln(ICAC volume +1.0)) because of the skewed distribution. Second, quartiles of ICAC volume of the total population were created. For illustration purposes, we compared the outcomes between the lower 3 quartiles with the upper (fourth) quartile; defined as severe ICAC. We assessed the association of ICAC volume and pattern (intimal calcification versus medial calcification pattern) with functional outcome (mRS score of 0–6) using ordinal logistic regression models (shift analysis). Relationships of ICAC volume or pattern with successful recanalization on follow-up CTA (modified Arterial Occlusive Lesion score) were assessed with adjusted ordinal logistic regression models. Linear regression was used to assess to association between ICAC volume and pattern with follow-up infarct volume. Modification of treatment effect by ICAC volume and pattern was tested with a multiplicative interaction term. In the first model, adjustments for age and sex only were made. In a second model, additional adjustments were made for cardiovascular risk factors: smoking, diabetes mellitus, atrial fibrillation, myocardial infarction, and history of hypertension according revised Framingham stroke risk profile.19 In the third model, adjustments were also made for prestroke mRS, National Institutes of Health Stroke Scale at baseline, occlusion of the internal carotid artery terminus, collateral status on baseline CTA, and time to randomization as proven predictors of outcome.20 P values ≤0.05 were considered as statistically significant. Analyses were performed with R statistical software (version 3.4.2) using packages foreign, rms, MASS, irr, and ggplot2.
Results
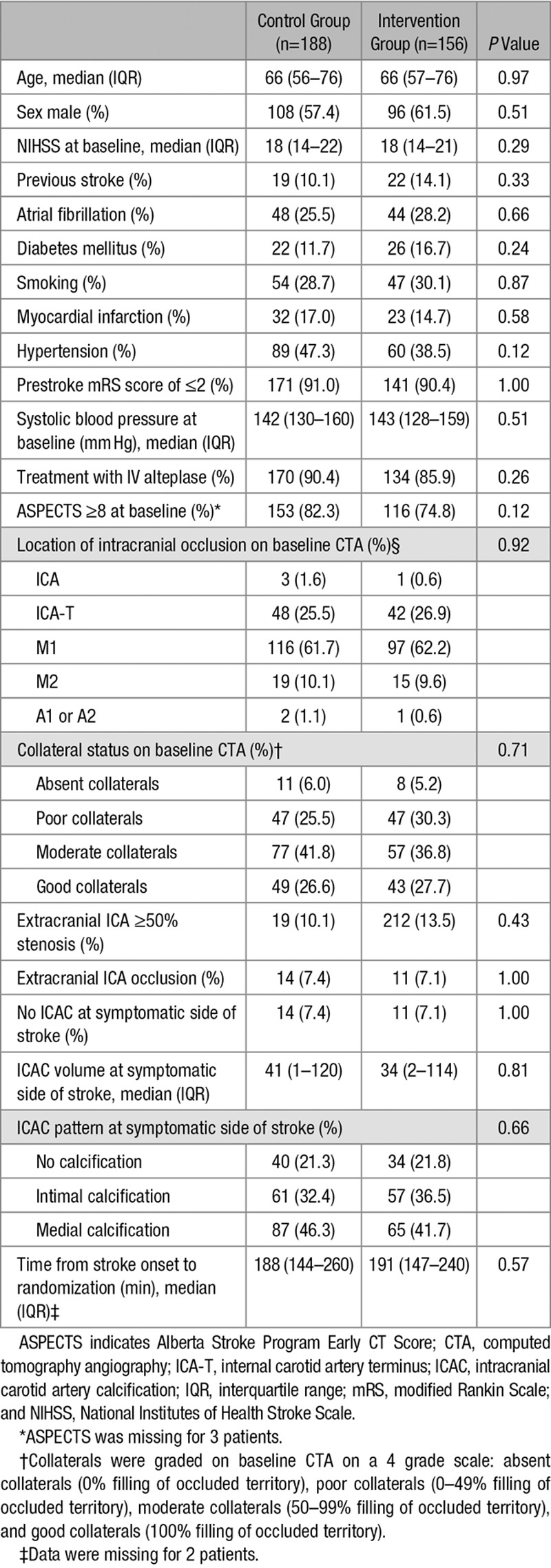
In total, 128 patients were excluded because of a slice thickness >3 mm on NCCT. Additional reasons for excluding patients were movement artifacts (n=20), incomplete scans (n=5), inappropriate reconstruction (n=1), unavailable axial slices (n=1), and unavailable NCCT scan (n=1). In total, 344 of the 500 patients (69%) in the MR CLEAN trial were included in this post hoc subgroup analysis (Table I in the online-only Data Supplement). Baseline characteristics of the study participants were equally distributed in the intervention and control group as shown in Table 1.
Table 1.
Baseline Characteristics of Analyzed Patients
ICAC Volume
ICAC in the symptomatic intracranial carotid artery of ischemic stroke (symptomatic ICAC) was present in 270 (78%) patients: 122/156 (78%) patients in the intervention group and 148/188 (79%) patients in the control group. Median ICAC volume in the symptomatic carotid artery was 69.8 mm3 (interquartile range, 19.2–171.1 mm3). A moderate correlation (ρ=0.6; P<0.001) between symptomatic ICAC volume and age was observed. There was no statistically significant difference in median volume of symptomatic ICAC between both treatment allocations (respectively, 65.5 versus 81.9 mm3; P=0.81).
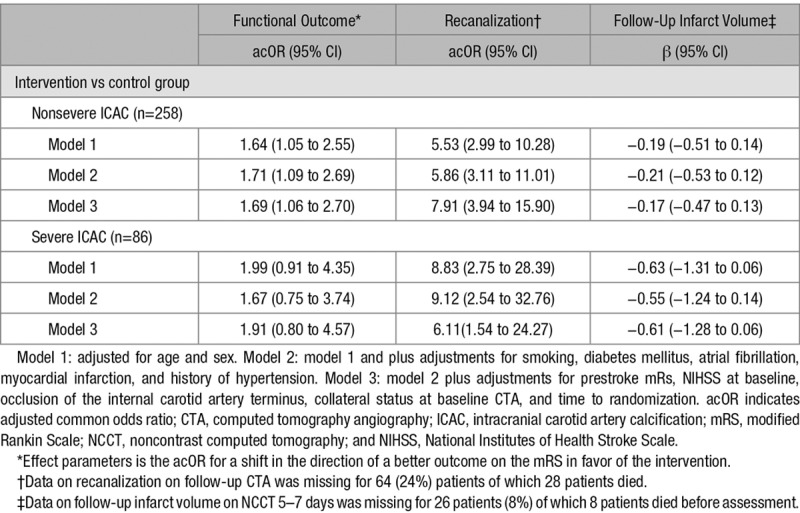
Overall, larger symptomatic ICAC volumes were not significantly associated with poorer functional outcome (adjusted common odds ratio [acOR] per unit increase in log-transformed ICAC volume 1.01; 95% CI, 0.89–1.13) in our first model. After additional adjustments, symptomatic ICAC volume was still not significantly associated with functional outcome (acOR, 0.99; 95% CI, 0.83–1.03). Treatment effects were similar in patients with severe ICAC volume (>119.2 mm3) and patients with nonsevere ICAC (Table 2) and no effect modification was observed (P interaction=0.61). Furthermore, the effect of treatment on final recanalization status and final infarct volume was comparable in patients with severe ICAC volume and patients with nonsevere ICAC volume without a significant effect modification (P interaction=0.66 and 0.77, respectively).
Table 2.
Association of Treatment Allocation With Functional Outcome,* Recanalization on CTA and Follow-Up Infarct Volume on CT According to Severity of Calcification Volume of the Intracranial Carotid Artery at the Symptomatic Side of Ischemic Stroke
ICAC Pattern
In the 270 patients with ICAC, we found 118 intimal calcification patterns and 152 medial calcification patterns in the symptomatic intracranial carotid artery (Figure 1). A good interobserver agreement was found in grading ICAC pattern (total agreement 93.9%; κ=0.88). No difference in distribution of ICAC pattern was observed between the intervention and control group (P=0.43). Patients with a medial ICAC pattern were in general older, more often female and had more often a history of diabetes mellitus, myocardial infarction, hypertension, and poorer collateral status on baseline CTA (Table II in the online-only Data Supplement).
In patients with ICAC, a medial calcification pattern was not associated with a shift to a poorer functional outcome (acOR, 0.62; 95% CI, 0.38–1.04). A significant EVT treatment effect was observed in patients with medial calcification pattern (acOR, 2.32; 95% CI, 1.23–4.39). This in contrast to patients with intimal calcification pattern, in whom we observed no treatment effect (acOR, 0.82; 95% CI, 0.40–1.68; Table 3; Figure 2). Consequently, a significant effect modification by ICAC pattern was noted (P interaction=0.04; Table 4). In EVT-treated patients, we observed a lower impact of reperfusion on functional outcome in patients with intimal ICAC pattern compared with patients with medical ICAC pattern (Table III in the online-only Data Supplement).
Table 3.
Association of Treatment Allocation With Functional Outcome,* Follow-Up Infarct Volume on CTA and Recanalization According to Calcification Pattern
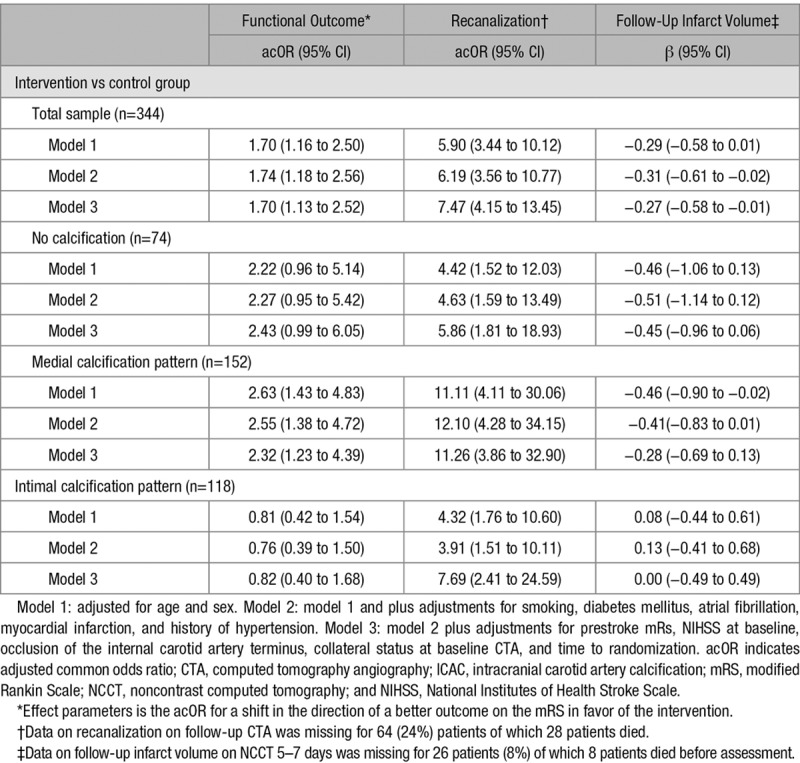
Figure 2.
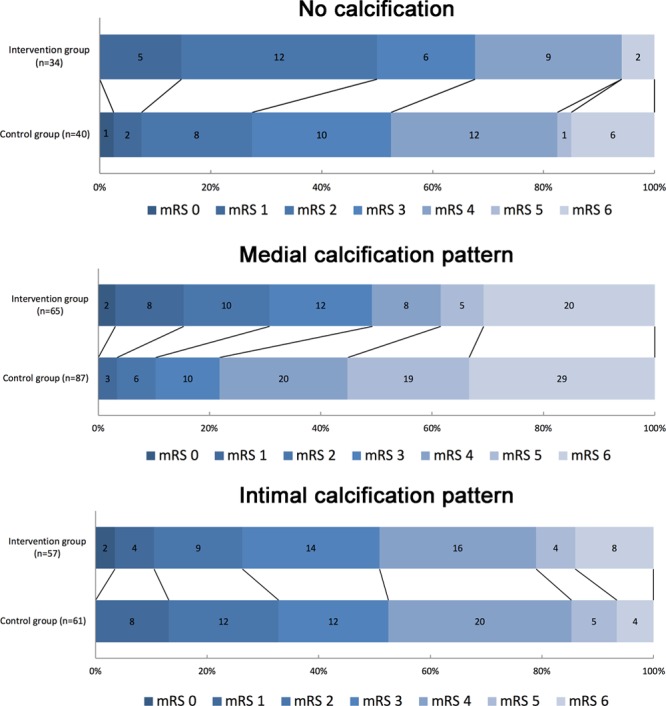
Distribution of modified Rankin Scale (mRS) scores at 90 days in patients with no calcification, medial, or intimal calcification pattern of the intracranial carotid artery at symptomatic side of ischemic stroke. A significant difference in the distribution of scores between both groups was observed in patients with medial calcification pattern but not in patients with intimal calcification pattern and no calcification. Numbers in bars are absolute numbers.
Recanalization grades on follow-up CTA were significantly higher in the intervention group in patients with medial and intimal calcification pattern (respectively, acOR, 11.26; 95% CI, 3.86–32.90 and acOR, 7.69; 95% CI, 2.41–24.59) with no observed significant treatment modification by ICAC pattern (P interaction=0.28).
In the control group, median follow-up infarct volumes were significantly larger in patients with medial calcification pattern compared with intimal calcification pattern (respectively, median volume 99.46 versus 69.52 mL; P=0.01). However, in the intervention group, infarct volumes did not differ between both calcification patterns (respectively, median volume 51.93 versus 55.12 mL; P=0.40). Compared with patients with intimal calcification pattern, patients with medial calcification pattern showed a larger effect on follow infarct volume. However, no significant effect modification was observed (P interaction=0.51).
There was no difference in the occurrence of serious adverse events or symptomatic intracerebral hemorrhage between patients with medial calcification pattern in the treatment and control groups (Table IV in the online-only Data Supplement). In patients with intimal calcification pattern, serious adverse events occurred more often in the intervention group. However, the absolute difference of 18% between was statistically nonsignificant (P=0.07).
Discussion
We did not find an association of ICAC volume with functional outcome after AIS because of large vessel occlusion nor modification of EVT treatment effect by ICAC volume. We found a trend towards a worse outcome in patients with medial ICAC pattern. Notwithstanding, patients with a medial ICAC pattern benefited from EVT in contrast to patients with intimal ICAC pattern.
In our analysis, the volume of ICAC was not associated with functional outcome as opposed to a recent observational study.2 This discrepancy may be explained by different study design, but selection of patients may have contributed to the findings. In that study, EVT was not yet standard care. Patients were only eligible for EVT if intravenous thrombolysis was contraindicated and specific clinical characteristics (National Institutes of Health Stroke Scale score of ≥6 and Alberta Stroke Program Early CT Score of >6) were present. Because of the pragmatic design of the randomized MR CLEAN trial, the patients included in our study reflect the population encountered in clinical practice. Another added value of our study compared with previous studies was the randomized controlled design which allowed us to investigate treatment effect modification by ICAC volume and pattern.
Two other studies that investigated effect of ICAC on revascularization and functional outcome assessed ICAC qualitatively using different evaluating approaches which did not differentiate between volume or pattern. One study included patients with a middle cerebral artery occlusion who received EVT and intravenous thrombolysis and found a significant association between high calcification burden and poor functional outcome (defined as mRS score of 5 or 6).3 However, the analysis was not stratified by type of treatment. Another study included EVT patients by perfusion imaging selection and found no association between total carotid siphon calcium score and successful reperfusion (Thrombolysis in Cerebral Infarction ≥2b) or good functional outcome (mRS score of ≤2).21 All 3 previous studies used a dichotomized mRS as primary outcome, and thus reported only the proportion of patients with a good functional outcome. Evaluating the entire mRS range with ordinal analysis, allows patients with suboptimal, but clinically important improvements to be captured and might outperform dichotomized outcomes used in other studies.22
In a recently published study, the risk factors of intimal and medial calcification patterns in patients with suspected ischemic stroke were investigated. Similar results to our observations were described with regard to clinical characteristics across the different ICAC patterns. Patients with medial calcification pattern were significantly older and less often male, suffered more often from diabetes mellitus and smoked less often.9 The poor functional outcome in patients with medial calcification pattern could be explained by arterial stiffening, characterized by an increasing pulse pressure, which causes impaired regulation of distal blood flow.23 This may lead to an impaired distal microvascular cerebral perfusion and thereby failure to improve microvascular function (Windkessel effect).24 Earlier studies investigated the pattern of calcifications in relation to clinical outcome in other cardiovascular diseases. They identified medial calcification as a risk factor for foot amputation in patients with diabetes mellitus compared with intimal calcifications, and it also appeared to be a strong prognostic marker for mortality in dialysis patients with end-stage renal disease.25–27
We found a significant treatment effect in favor of EVT in patients with medial calcification pattern but not in patients with an intimal calcification pattern. One could hypothesize that patients with medial calcifications, which is accompanied by arterial stiffening have already a compromised microcirculation and have developed already a microcollateral pathways. Another reason for our findings could be that the thrombus is different in patients with intimal and medial calcifications and that EVT is less effective in removing the thrombus in toto in patients with intimal calcification pattern. Furthermore, although infarct volumes in EVT-treated patients did not differ between the 2 ICAC patterns, the beneficial effect of EVT about prevention of infarct volume might be reduced in patients with intimal calcification pattern. Intimal calcifications are associated with local atherosclerotic plaques which might lead to plaque disruption and microemboli during stent retrieval. Besides, EVT might cause damage to the vascular endothelium as has been showed in different studies.28 It is known that atherosclerosis is also related to endothelial damage.29–31 It might be possible that endothelium in patients with intimal ICAC pattern is more prone to damage which may results in secondary injury of brain tissue.
A recent postmortem histopathologic correlation study showed that the pattern of ICAC can be reliably assessed on NCCT, and the developed scoring method was used in our study.15 Since NCCT is daily practice in AIS patients, determining the pattern of ICAC could be an interesting prognostic marker for selection of patients for EVT. Our experience is that this scoring method can be easily applied in clinical practice, as it can be executed quickly, and has a good interobserver agreement.
There are several limitations to our study. First, the quality of NCCT scans varied between patients because of different scanning protocols used in the participating centers of the MR CLEAN trial. Exclusion of patients in our analysis was slice thickness >3 mm which could have led to underestimation or overestimation of ICAC volume and misclassification of ICAC pattern. Therefore, our post hoc study included a limited number of patients, which contributed to the fairly wide CIs. Consequently, the results of the current study are rather hypothesis generating than definitive results that merit a change in imaging-based selection of AIS patients for EVT. Future studies dedicated to this topic must be performed to investigate whether these results hold. Second, significant observed baseline characteristics were observed between both ICAC patterns which may be important confounders. Although we adjusted for these confounding variables using covariable-adjusted regression analyses. However, residual confound might still be present. An important note with regard to these baseline differences is that these differences may also partly reflect the presence of the dominant ICAC patterns in these persons as recently published.9 About the observed differences in treatment effect, confounding will be marginal because of randomization between EVT and non-EVT. Third, our study used the recently published scoring method of Kockelkoren et al.15 Another possible scoring method is the modified Woodcock scale, which visually characterizes ICAC from 0 (absent) to 3 (thick, continuous calcification)32,33 combining volume and pattern in one score which is not desirable for our study. The score by Kockelkoren et al15 is developed to specifically determine the pattern of ICAC and it is histopathologically validated. Finally, we could not assess impaired cerebral microperfusion between both ICAC patterns in our study. In the MR CLEAN trial, CT or magnetic resonance imaging perfusion scans were not performed by the protocol. For future research, it would also be interesting to investigate the association between both degree of white matter hyperintensities and both ICAC patterns.
Further studies on patients from larger cohorts and randomized controlled trials are necessary to confirm our findings, but also to comprehend the underlying pathophysiological mechanism that determines the relation between ICAC pattern, treatment effect, and functional outcome.
Summary
The benefit of EVT in AIS patients with a medial calcification pattern is larger than the benefit in patients with an intimal calcification pattern.
Table 4.
P Interaction Values Between Treatment Groups (Intervention Versus Control) and ICAC Volume or Pattern
Sources of Funding
The MR CLEAN trial (Multicenter Randomized Clinical trial of Endovascular treatment for acute ischemic stroke in the Netherlands) was partly funded by the Dutch Heart Foundation and by unrestricted grants from AngioCare BV, Medtronic/Covidien/EV3, MEDAC GmbH/LAMEPRO, Penumbra Inc, Stryker, and Top Medical/Concentric. The MR CLEAN is registered under number NTR1804 in the Dutch trial register and under ISRCTN10888758 in the ISRCTN register.
Disclosures
Dr Majoie reports grants from CVON/Dutch Heart Foundation, during the conduct of the study (paid to institution); grants from TWIN foundation, grants from European Commission, grants from Stryker, outside the submitted work (paid to institution), is shareholder of Nico.lab, a company that focuses on the use of artificial intelligence for medical image analysis. Dr Roos reports a modest amount of shares in Nico-Lab. Dr Berkhemer reports other from Stryker, outside the submitted work. Dr van Zwam reports personal fees from Stryker, personal fees from Cerenovus (paid to institution). Dr Dippel reports grants from Dutch Heart Foundation, grants from the Brain Foundation Netherlands, grants from the Netherlands Organisation for Health Research and Development, grants from Health Holland Top Sector Life Sciences & Health, grants from AngioCare BV, grants from Medtronic/Covidien/EV3, grants from MEDAC Gmbh/LAMEPRO, grants from Penumbra Inc, grants from Top Medical/Concentric, grants from Stryker, grants from Thrombolytic Science during the conduct of the study; other from Stryker, other from Medtronic, other from Bracco Imaging, other from Servier, outside the submitted work. Dr van der Lugt reports grants from Dutch Heart Foundation, grants from AngioCare BV, Medtronic/Covidien/EV3, MEDAC Gmbh/LAMEPRO, Penumbra Inc, Stryker, and Top Medical/Concentric, during the conduct of the study; grants from Stryker, other from Stryker, outside the submitted work. The other authors report no conflicts.
Supplementary Material
Footnotes
A list of all MR CLEAN investigators are listed in the Appendix in the online-only Data Supplement.
Guest Editor for this article was Giuseppe Lanzino, MD.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.118.022400.
References
- 1.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. HERMES; Collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 2.Hernández-Pérez M, Bos D, Dorado L, Pellikaan K, Vernooij MW, López-Cancio E, et al. Intracranial carotid artery calcification relates to recanalization and clinical outcome after mechanical thrombectomy. Stroke. 2017;48:342–347. doi: 10.1161/STROKEAHA.116.015166. doi: 10.1161/STROKEAHA.116.015166. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Hong JM, Lee M, Huh K, Choi JW, Lee JS. Cerebral arterial calcification is an imaging prognostic marker for revascularization treatment of acute middle cerebral arterial occlusion. J Stroke. 2015;17:67–75. doi: 10.5853/jos.2015.17.1.67. doi: 10.5853/jos.2015.17.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovacic JC, Moreno P, Nabel EG, Hachinski V, Fuster V. Cellular senescence, vascular disease, and aging: part 2 of a 2-part review: clinical vascular disease in the elderly. Circulation. 2011;123:1900–1910. doi: 10.1161/CIRCULATIONAHA.110.009118. doi: 10.1161/CIRCULATIONAHA.110.009118. [DOI] [PubMed] [Google Scholar]
- 5.Persy V, D’Haese P. Vascular calcification and bone disease: the calcification paradox. Trends Mol Med. 2009;15:405–416. doi: 10.1016/j.molmed.2009.07.001. doi: 10.1016/j.molmed.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Thompson B, Towler DA. Arterial calcification and bone physiology: role of the bone-vascular axis. Nat Rev Endocrinol. 2012;8:529–543. doi: 10.1038/nrendo.2012.36. doi: 10.1038/nrendo.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vos A, Van Hecke W, Spliet WG, Goldschmeding R, Isgum I, Kockelkoren R, et al. Predominance of nonatherosclerotic internal elastic lamina calcification in the intracranial internal carotid artery. Stroke. 2016;47:221–223. doi: 10.1161/STROKEAHA.115.011196. doi: 10.1161/STROKEAHA.115.011196. [DOI] [PubMed] [Google Scholar]
- 8.Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, et al. Medial vascular calcification revisited: review and perspectives. Eur Heart J. 2014;35:1515–1525. doi: 10.1093/eurheartj/ehu163. doi: 10.1093/eurheartj/ehu163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vos A, Kockelkoren R, de Vis JB, van der Schouw YT, van der Schaaf IC, Velthuis BK, et al. DUST; Study; Group. Risk factors for atherosclerotic and medial arterial calcification of the intracranial internal carotid artery. Atherosclerosis. 2018;276:44–49. doi: 10.1016/j.atherosclerosis.2018.07.008. doi: 10.1016/j.atherosclerosis.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 11.Fransen PS, Beumer D, Berkhemer OA, van den Berg LA, Lingsma H, van der Lugt A, et al. MR CLEAN Investigators. MR CLEAN, a multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands: study protocol for a randomized controlled trial. Trials. 2014;15:343. doi: 10.1186/1745-6215-15-343. doi: 10.1186/1745-6215-15-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 13.Jansen IGH, Berkhemer OA, Yoo AJ, Vos JA, Lycklama À Nijeholt GJ, Sprengers MES, et al. MR CLEAN Investigators ( www.mrclean-trial.org); MR CLEAN Investigators ( www.mrclean-trial.org) Comparison of CTA- and DSA-based collateral flow assessment in patients with anterior circulation stroke. AJNR Am J Neuroradiol. 2016;37:2037–2042. doi: 10.3174/ajnr.A4878. doi: 10.3174/ajnr.A4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bos D, Ikram MA, Elias-Smale SE, Krestin GP, Hofman A, Witteman JC, et al. Calcification in major vessel beds relates to vascular brain disease. Arterioscler Thromb Vasc Biol. 2011;31:2331–2337. doi: 10.1161/ATVBAHA.111.232728. doi: 10.1161/ATVBAHA.111.232728. [DOI] [PubMed] [Google Scholar]
- 15.Kockelkoren R, Vos A, Van Hecke W, Vink A, Bleys RL, Verdoorn D, et al. Computed tomographic distinction of intimal and medial calcification in the intracranial internal carotid artery. PLoS One. 2017;12:e0168360. doi: 10.1371/journal.pone.0168360. doi: 10.1371/journal.pone.0168360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 17.Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T IMS-I Investigators. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke. 2005;36:2400–2403. doi: 10.1161/01.STR.0000185698.45720.58. doi: 10.1161/01.STR.0000185698.45720.58. [DOI] [PubMed] [Google Scholar]
- 18.Boers AM, Marquering HA, Jochem JJ, Besselink NJ, Berkhemer OA, van der Lugt A, et al. MR CLEAN; Investigators. Automated cerebral infarct volume measurement in follow-up noncontrast CT scans of patients with acute ischemic stroke. AJNR Am J Neuroradiol. 2013;34:1522–1527. doi: 10.3174/ajnr.A3463. doi: 10.3174/ajnr.A3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dufouil C, Beiser A, McLure LA, Wolf PA, Tzourio C, Howard VJ, et al. Revised Framingham stroke risk profile to reflect temporal trends. Circulation. 2017;135:1145–1159. doi: 10.1161/CIRCULATIONAHA.115.021275. doi: 10.1161/CIRCULATIONAHA.115.021275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venema E, Mulder MJHL, Roozenbeek B, Broderick JP, Yeatts SD, Khatri P, et al. Selection of patients for intra-arterial treatment for acute ischaemic stroke: development and validation of a clinical decision tool in two randomised trials. BMJ. 2017;357:j1710. doi: 10.1136/bmj.j1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haussen DC, Gaynor BG, Johnson JN, Peterson EC, Elhammady MS, Aziz-Sultan MA, et al. Carotid siphon calcification impact on revascularization and outcome in stroke intervention. Clin Neurol Neurosurg. 2014;120:73–77. doi: 10.1016/j.clineuro.2014.02.021. doi: 10.1016/j.clineuro.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Saver JL, Gornbein J. Treatment effects for which shift or binary analyses are advantageous in acute stroke trials. Neurology. 2009;72:1310–1315. doi: 10.1212/01.wnl.0000341308.73506.b7. doi: 10.1212/01.wnl.0000341308.73506.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985) 2008;105:1652–1660. doi: 10.1152/japplphysiol.90549.2008. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 25.London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 26.Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 27.Niskanen L, Siitonen O, Suhonen M, Uusitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care. 1994;17:1252–1256. doi: 10.2337/diacare.17.11.1252. [DOI] [PubMed] [Google Scholar]
- 28.Teng D, Pannell JS, Rennert RC, Li J, Li YS, Wong VW, et al. Endothelial trauma from mechanical thrombectomy in acute stroke: in vitro live-cell platform with animal validation. Stroke. 2015;46:1099–1106. doi: 10.1161/STROKEAHA.114.007494. doi: 10.1161/STROKEAHA.114.007494. [DOI] [PubMed] [Google Scholar]
- 29.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 30.Gory B, Bresson D, Kessler I, Perrin ML, Guillaudeau A, Durand K, et al. Histopathologic evaluation of arterial wall response to 5 neurovascular mechanical thrombectomy devices in a swine model. AJNR Am J Neuroradiol. 2013;34:2192–2198. doi: 10.3174/ajnr.A3531. doi: 10.3174/ajnr.A3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Power S, Matouk C, Casaubon LK, Silver FL, Krings T, Mikulis DJ, et al. Vessel wall magnetic resonance imaging in acute ischemic stroke: effects of embolism and mechanical thrombectomy on the arterial wall. Stroke. 2014;45:2330–2334. doi: 10.1161/STROKEAHA.114.005618. doi: 10.1161/STROKEAHA.114.005618. [DOI] [PubMed] [Google Scholar]
- 32.Woodcock RJ, Jr, Goldstein JH, Kallmes DF, Cloft HJ, Phillips CD. Angiographic correlation of CT calcification in the carotid siphon. AJNR Am J Neuroradiol. 1999;20:495–499. [PMC free article] [PubMed] [Google Scholar]
- 33.Subedi D, Zishan US, Chappell F, Gregoriades ML, Sudlow C, Sellar R, et al. Intracranial carotid calcification on cranial computed tomography: visual scoring methods, semiautomated scores, and volume measurements in patients with stroke. Stroke. 2015;46:2504–2509. doi: 10.1161/STROKEAHA.115.009716. doi: 10.1161/STROKEAHA.115.009716. [DOI] [PMC free article] [PubMed] [Google Scholar]


