Abstract
Samples of unifloral sulla (Hedysarum coronarum L.) honey from Sardinia (Italy) were analysed. To investigate the chemical composition of the honey volatiles two solvent systems were used for ultrasonic solvent extraction (USE): 1) a 1:2 (v/v) pentane and diethyl ether mixture and 2) dichloromethane. All the extracts were analysed by GC and GC/MS. These procedures have permitted the identification of 56 compounds that include norisoprenoids, benzene derivatives, aliphatic compounds and Maillard reaction products. Norisoprenoids were the major compounds in both extracts, dominated by vomifoliol (5.3-11.2%; 9.6-14.0%) followed by minor percentages of other norisoprenoids such as α-isophorone, 4-ketoisophorone, 3-oxo-α-ionol or 3-oxo-α-ionone. Other abundant single compounds in the extracts were 3-hydroxy-4-phenylbutan-2-one (0.8-5.4%; 0.6-5.7%) and methyl syringate (3.0-5.7%; 2.2-4.1%). The composition of the volatiles and semi-volatiles in the obtained extracts suggests that sulla honey is quite distinctive relative to the other honeys that have been chemically studied by GC/MS, but no specific markers of the honey botanical origin were found.
Keywords: sulla (Hedysarum coronarium L.) honey, ultrasonic solvent extraction (USE), gas chromatography and mass spectrometry (GC and GC/MS), norisoprenoids
1. Introduction
Honeys produced from different floral sources may often have distinctly different aromas and tastes. During the last two decades, a lot of research has been done on the analysis of honey flavour volatile organic compounds. Some of these compounds derive from nectar, some are dependent on the physiology of the bee, and others arise during honey post-harvest processing as well as during storage. Methods of extracting honey volatiles may display a varying degree of selectivity and effectiveness, depending on the compounds involved [1,2,3,4]. Honey shake-flask liquid-liquid extraction with different solvents can be used for obtaining representative chemical composition of the volatiles without formation of thermal artefacts [4]. Ultrasonic solvent extraction (USE) has a major advantage over the previous method in significantly reducing extraction times [2,5]. There is mounting evidence that the organic solvent extractives of some unifloral honeys are chemically different from one another [6,7,8,9,10]. Different approaches in preparation of honey solvent extracts, prior to the GC and GC/MS analyses, have been reported: 1) a micro-scale simultaneous distillation-extraction (SDE) of the obtained solvent extract followed by the analysis of distillate [1]; 2) direct analysis of unmethylated extract [2,5]; 3) indirect analysis of methylated extract [7]. The chemical composition of honey solvent extracts obtained by 1) - 3) procedures revealed that the three main categories of natural volatiles are dominant in, or source specific for, honeys throughout the world: norisoprenoids, terpenes, and benzene derivatives [11]. Many researchers were focused on finding unique compounds to certify the botanical and geographical origin of different honeys and in many cases, such marker compounds seem to exist [11,12,13,14].
Sulla (Hedysarum coronarium L.) is a legume well adapted to semi-arid Mediterranean environments and represents an effective example of a multiple-uses species exploited for environmental protection, landscape enhancement and honey production [15]. Considerable amounts of sulla unifloral honey are produced in Southern and Central Italy. A few papers report general characteristics of sulla honey without detailed chemical analysis data. Honey from this species showed low invertase activity with an average value of 4.84 ± 2.26 SN [16]. In contrast, a high peroxide accumulation was obtained (45.25 ± 17.86 mg H2O2 g-1h-1). In addition, a chemometric approach to the discrimination of Italian honey samples from different floral origin [17] has been performed as a valuable classification tool to discriminate the botanical origin of six type of honey samples (chestnut, eucalyptus, heather, sulla, honeydew and wildflower).
Our preliminary research on sulla honey headspace by headspace solid-phase microextraction (HS-SPME) with fibers of different polarity as well as HPLC analyses of sulla honey ethyl acetate extracts did not reveal any useful data for characterization of this honey. Therefore, the scope of this work was to investigate the chemical composition of the organic extractives from unifloral sulla honey samples obtained by ultrasonic solvent extraction (USE) followed by GC and GC/MS analyses and to identify possible characteristic compounds for this honey type. Two extraction solvents were used: a pentane and diethyl ether (1:2 v/v) mixture and dichloromethane. To best of our knowledge, this is the first report on the honey volatiles composition of sulla.
2. Results and Discussion
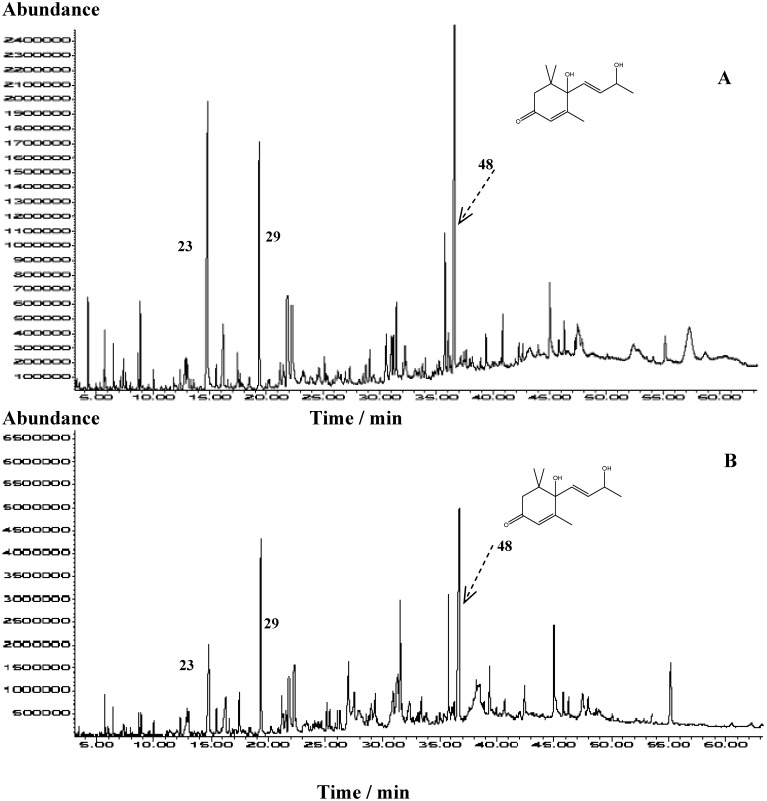
Ultrasonic solvent extraction was applied for sulla honey samples since it reproducibly extracts natural honey volatiles and semi-volatiles (high-boiling volatiles) with good recoveries and without application of heat [5]. Comparison with other isolation methods this method is particularly appropriate for the isolation of water-soluble honey compounds [2]. To obtain a more complete composition two extraction solvents were used: A – a pentane and diethyl ether mixture and B - dichloromethane. Selected TIC chromatograms obtained from GC/MS analyses of USE extract using two solvents are presented in Figure 1. Great qualitative and quantitative similarity is observed among the extracts. Identified compounds are listed in Table 1 in accordance to their elution order on HP-5MS column.
Figure 1.
Representative TIC chromatograms of sulla honey extracts obtained by USE: A - dichloromethane extract; B - pentane and diethyl ether (1:2 v/v) extract. Numbers refer to Table 1.
Table 1.
Sulla (Hedysarum coronarium L.) honey volatile organic composition obtained by USE with two solvents followed by GC and GC/MS analysis.
| No. | Compound | RI | Area percentage (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Solvent A | Solvent B | ||||||||||
| Min. | Max. | Av. | SD. | Min. | Max. | Av. | SD. | ||||
| 1. | 2-Furanmethanol | < 900 | 0.0 | 0.1 | 0.05 | 0.06 | - | - | - | - | |
| 2. | 2-Methylbutanoic acid | < 900 | 0.0 | 0.1 | 0.05 | 0.06 | - | - | - | - | |
| 3. | Hexan-1-ol | < 900 | 0.0 | 0.1 | 0.08 | 0.05 | - | - | - | - | |
| 4. | 1,4-Dimethylbenzene** | < 900 | 0.0 | 0.2 | 0.10 | 0.08 | - | - | - | - | |
| 5. | Benzaldehyde | 965 | 0.0 | 0.1 | 0.05 | 0.06 | 0.1 | 0.2 | 0.13 | 0.05 | |
| 6. | 2,4-Dimethyl-3,6-dihydro-2H-pyran | 973 | 0.0 | 0.1 | 0.05 | 0.06 | - | - | - | - | |
| 7. | 2-Methylfuran* | 988 | - | - | - | - | 0.2 | 2.3 | 0.98 | 0.93 | |
| 8. | 2-Hydroxy-3-methylcyclopent-2-en-1-one | 1036 | - | - | - | - | 0.0 | 0.1 | 0.05 | 0.06 | |
| 9. | Benzyl alcohol | 1037 | 0.1 | 0.3 | 0.18 | 0.09 | 0.0 | 0.3 | 0.15 | 0.13 | |
| 10. | Dihydro-3-hydroxy-4,4-dimethyl-2(3H)-furanone (Pantolactone) | 1044 | 0.1 | 0.2 | 0.15 | 0.06 | 0.3 | 0.6 | 0.43 | 0.15 | |
| 11. | Phenylacetaladehyde | 1048 | 0.0 | 0.2 | 0.13 | 0.10 | 0.3 | 0.5 | 0.28 | 0.17 | |
| 12. | 4,5-Dimethyl-2-formylfuran | 1078 | 0.4 | 0.8 | 0.40 | 0.29 | 0.3 | 0.7 | 0.26 | 0.26 | |
| 13. | Methyl 2-furoate | 1084 | 0.0 | 0.4 | 0.18 | 0.21 | 0.0 | 1.5 | 0.55 | 0.71 | |
| 14. | 1-(2-Furanyl)-2-hydroxyethanone | 1088 | 0.2 | 0.7 | 0.45 | 0.21 | 0.8 | 2.2 | 1.23 | 0.66 | |
| 15. | 2-Phenylethanol | 1116 | 0.2 | 0.4 | 0.30 | 0.12 | 0.2 | 0.5 | 0.35 | 0.13 | |
| 16. | 3,5,5-Trimethyl-cyclohex-3-en-1-one (α-Isophorone) | 1124 | 0.0 | 0.2 | 0.08 | 0.10 | 0.0 | 0.2 | 0.10 | 0.08 | |
| 17. | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | 1145 | 0.3 | 0.6 | 0.43 | 0.15 | 0.2 | 0.9 | 0.48 | 0.31 | |
| 18. | 3,5,5-Trimethyl-cyclohex-2-ene-1,4-dione (4-Ketoisophorone) | 1147 | 0.0 | 0.2 | 0.10 | 0.08 | 0.0 | 0.2 | 0.10 | 0.08 | |
| 19.. | Benzoic acid | 1162 | 0.4 | 4.6 | 1.73 | 1.95 | 0.4 | 3.9 | 1.48 | 1.67 | |
| 20. | 2,4-Dimethylphenol** | 1181 | 0.3 | 0.4 | 0.33 | 0.10 | 0.3 | 0.5 | 0.33 | 0.13 | |
| 21. | (E)-3,7-Dimethyl-octa-1,5-diene-3,7-diol | 1191 | 0.0 | 0.4 | 0.20 | 0.23 | 0.3 | 0.6 | 0.45 | 0.13 | |
| 22. | Decanal | 1207 | 0.0 | 0.2 | 0.08 | 0.10 | 0.0 | 0.1 | 0.05 | 0.06 | |
| 23. | 5-Hydroxymethylfurfural | 1230 | 2.4 | 9.3 | 4.78 | 3.08 | 6.5 | 22.8 | 13.03 | 6.91 | |
| 24. | 1,3-Bis(1,1-dimethylethyl)benzene | 1261 | 0.0 | 0.7 | 0.23 | 0.33 | - | - | - | - | |
| 25. | Phenylacetic acid | 1269 | 1.1 | 5.8 | 2.90 | 2.02 | 1.0 | 4.8 | 2.53 | 1.62 | |
| 26. | Nonanoic acid | 1273 | 0.0 | 0.2 | 0.08 | 0.10 | - | - | - | - | |
| 27. | 1-Methoxy-4-propylbenzene | 1305 | 0.0 | 0.5 | 0.18 | 0.24 | 0.0 | 0.8 | 0.40 | 0.34 | |
| 28. | 2,4,6-Trimethylphenol** | 1332 | 0.0 | 0.6 | 0.28 | 0.32 | 0.0 | 0.4 | 0.13 | 0.19 | |
| 29. | 3-Hydroxy-4-phenylbutan-2-one | 1354 | 0.8 | 5.4 | 3.73 | 2.02 | 0.6 | 5.7 | 3.43 | 2.15 | |
| 30. | 1-(4-Methoxyphenyl)-ethanone (4-Methoxyacetophenone) | 1360 | 0.0 | 0.2 | 0.08 | 0.10 | - | - | - | - | |
| 31. | (E)-8-Hydroxylinalool | 1367 | 0.0 | 1.1 | 0.33 | 0.53 | 0.2 | 0.3 | 0.23 | 0.05 | |
| 32. | Tetradecane | 1400 | 0.0 | 0.8 | 0.28 | 0.38 | - | - | - | - | |
| 33. | 4-Hydroxyphenyl ethanol | 1445 | 0.0 | 1.2 | 0.80 | 0.57 | 0.0 | 0.4 | 0.18 | 0.21 | |
| 34. | 4-Methoxybenzoic acid | 1451 | 0.0 | 0.7 | 0.23 | 0.33 | 0.0 | 0.6 | 0.33 | 0.25 | |
| 35. | 3-Phenylprop-2-enoic acid** (Cinnamic acid) | 1454 | 0.0 | 0.9 | 0.40 | 0.38 | 0.0 | 0.6 | 0.33 | 0.25 | |
| 36. | 4-Methoxyphenylacetic acid | 1496 | 0.0 | 2.6 | 0.85 | 1.23 | 0.0 | 2.2 | 0.73 | 1.04 | |
| 37. | Pentadecane | 1500 | 0.0 | 0.5 | 0.20 | 0.25 | - | - | - | - | |
| 38. | 4-Methyl-2,6-bis(1,1-dimethylethyl)-phenol | 1514 | 1.1 | 3.0 | 1.83 | 0.82 | 0.0 | 0.1 | 0.05 | 0.06 | |
| 39. | α-Hydroxyphenylpropanoic acid | 1545 | 0.0 | 15.7 | 5.25 | 7.40 | 0.0 | 1.4 | 0.48 | 0.66 | |
| 40. | 2-Hydroxydecanoic acid | 1557 | 0.0 | 2.0 | 0.78 | 0.86 | 0.0 | 0.6 | 0.33 | 0.25 | |
| 41. | 4-Hydroxybenzoic acid | 1558 | 0.2 | 1.2 | 0.55 | 0.55 | - | - | - | - | |
| 42. | cis-p-Menth-8-ene* | 1632 | 0.0 | 0.9 | 0.38 | 0.45 | 0.0 | 1.7 | 0.68 | 0.73 | |
| 43. | 3-Oxo-α-ionol | 1660 | 0.2 | 1.5 | 0.83 | 0.56 | 1.0 | 1.2 | 1.08 | 0.10 | |
| 44. | 3-Oxo-α-ionone | 1665 | 0.0 | 2.9 | 1.90 | 1.32 | 1.1 | 1.9 | 1.60 | 0.38 | |
| 45. | 3-Oxo-7,8-dihydro-α-ionone | 1682 | 0.0 | 0.6 | 0.38 | 0.26 | - | - | - | - | |
| 46. | 6,7-Dehydro-7,8-dihydro-3-oxo-α-ionol | 1720 | 0.0 | 0.6 | 0.25 | 0.30 | - | - | - | - | |
| 47. | Methyl syringate | 1744 | 3.0 | 5.7 | 4.38 | 1.15 | 2.2 | 4.1 | 3.28 | 0.79 | |
| 48. | 6-Hydroxy-3-oxo-α-ionol (Vomifoliol) | 1802 | 5.3 | 11.2 | 8.88 | 2.62 | 9.6 | 14.0 | 11.63 | 1.84 | |
| 49. | Hexadecan-1-ol | 1882 | 0.0 | 3.6 | 1.50 | 1.51 | 0.0 | 2.6 | 1.18 | 1.07 | |
| 50. | Nonadecane | 1900 | 0.0 | 0.7 | 0.33 | 0.38 | - | - | - | - | |
| 51. | Hexadecanoic acid | 1963 | 0.9 | 2.5 | 1.43 | 0.73 | 0.5 | 1.1 | 0.75 | 0.25 | |
| 52. | (Z)-Octadec-9-en-1-ol | 2060 | 1.0 | 4.9 | 2.55 | 1.95 | 1.5 | 3.6 | 2.70 | 0.98 | |
| 53. | Octadecan-1-ol | 2084 | 0.0 | 0.8 | 0.50 | 0.36 | - | - | - | - | |
| 54. | Heneicosane | 2100 | 0.0 | 0.6 | 0.20 | 0.28 | - | - | - | - | |
| 55. | (Z)-Octadec-9-enoic acid | 2147 | 0.0 | 2.4 | 1.30 | 1.10 | 0.0 | 1.2 | 0.58 | 0.53 | |
| 56. | Tetracosane | 2400 | 0.0 | 3.1 | 1.60 | 1.68 | 0.0 | 3.3 | 1.85 | 1.37 | |
RI = retention indices on HP-5MS column; A = USE with pentane and diethyl ether (1:2 v/v) mixture; B = USE with dichloromethane; - = not detected; * - tentatively identified; ** - correct isomer not identified.
Norisoprenoids were the major group of identified organic compounds in both extracts (Table 1), dominated by vomifoliol (5.3-11.2%; 9.6-14.0%) followed by minor percentages of 3-oxo-α-ionol (0.2-1.5%; 1.0-1.2%), 3-oxo-α-ionone (0.0-2.9%; 1.1-1.9%), 3-oxo-7,8-dihydro-α-ionone (0.0-0.6%; 0.0%), 4-ketoisophorone (0.0-0.2%; 0.0-0.2%), α-isophorone (0.0-0.2%; 0.0-0.2%) and 6,7-dehydro-7,8-dihydro-3-oxo-α-ionol (0.0-0.6%; 0.0%).
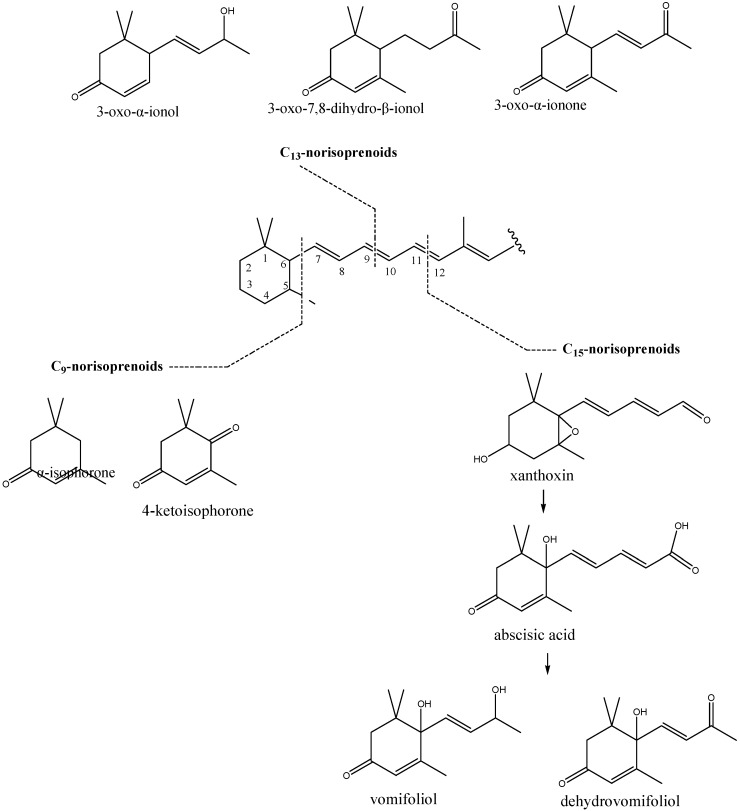
Norisoprenoids are degraded-carotenoid-like structures (3,5,5-trimethyl-cyclohex-2-ene and its derivatives) and identified structures are presented in Figure 2.
Figure 2.
Different classes of degraded carotenoids and identified compounds in sulla honey from the classes of C9, C13 and C15 norisoprenoids.
In addition to the most widespread C13-norisoprenoids, volatile carotenoid metabolites with C9, C10 or C11 are also frequently detected in Nature [18]. The cleavage of the carotenoid chain is generally considered to proceed on different chain double bonds. Cleavage of C11-C12 double bond produces C15 norisoprenoids via abscisic acid, a well-known growth hormone, formed after cleavage of C40 carotenoids. trans,cis-Abscisic acid along with trans,trans-abscisic acid were found in New Zealand nectar honeys [9]. Zeaxanthin is the first committed abscisic acid precursor. A series of enzyme-catalyzed epoxidations and isomerisations, and final cleavage of the C40 carotenoid by a dioxygenation reaction yields precursor, xanthoxin, which is then further oxidized to abscisic acid [19]. Vomifoliol, the compound found in our extracts, probably arises through degradation of abscisic acid. Cleavage of C9-C10 double bond generates C13-norisoprenoids (such as 3-oxo-7,8-dihydro-β-ionol, 3-oxo-α-ionone or 3-oxo-α-ionol). Identified C9-norisoprenoids were α-isophorone and 4-ketoisophorone. Degraded carotenoid-like structures were aslo found in different honey extracts from New Zealand [9,10]. Volatile norisoprenoids were found as markers of botanical origin of Sardinian strawberry-tree honey [20]. Among them, α-isophorone, β-isophorone and 4-oxoisophorone were recognized as specific floral origin markers.
3-Hydroxy-4-phenylbutan-2-one (0.8-5.4%; 0.6-5.7%) is other major single compound in both sulla honey extracts belonging to the class of benzene derivatives raised from shikimic biogenetic pathway. However, 3-hydroxy-4-phenylbutan-2-one is probably not specific for any type of floral honey since it was previously found in the extracts from a range of different honeys [7,21,22]. Due to its previous isolation from flowers, 3-hydroxy-4-phenylbutan-2-one appears to originate from the plant nectar, and may contribute to the honey aroma, even though it appears not to be useful for the sourcing of the honeys. Methyl syringate (3.0-5.7%; 2.2-4.1%) is an abundant benzene derivative in the samples and was already detected in robinia, rape, chestnut, clover, linden blossom, dandelion, sunflower, thyme, manuka and fir honeys [23], but only in asphodel honey it reached the highest level [24]. Additionally, the results in Table 1 indicate great variability of benzene derivatives among the minor constituents of the volatiles extracted from the honey samples: ubiquitous benzaldehyde (0.0-0.1%; 0.1-0.2%), benzyl alcohol (0.1-0.3%; 0.0-0.3%), phenylacetaldehyde (0.0-0.2%; 0.3-0.5%) and 2-phenylethanol (0.2-0.4%; 0.2-0.5%), phenols [such as 2,4-dimethylphenol (0.3-0.4%; 0.3-0.5%)], aromatic acids [such as benzoic acid (0.4-4.6%; 0.4-3.9%), phenylacetic acid (1.2-5.8%; 1.0-4.8%), 4-methoxybenzoic acid (0.0-0.7%; 0.0-0.6%), cinnamic acid (0.0-0.9%; 0.0-0.6%), 4-methoxyphenylacetic acid (0.0-2.6%; 0.0-2.2%), α-hydroxyphenylpropanoic acid (0.0-25.7%; 0.0-1.4%) or 4-hydroxybenzoic acid (0.2-1.2%; 0.0-0.0%)] and others.
Aliphatic compounds were also found in the extracts, being dominated by higher acids, alcohols and hydrocarbons such as hexadecanoic acid (0.9-2.5%; 0.5-1.1%), (Z)-octadec-9-en-1-ol (1.0-4.9%; 1.5-3.6%), hexadecan-1-ol (0.0-3.6%; 0.0-2.6%) or (Z)-octadec-9-enoic acid (0.0-2.4%; 0.0-1.2%). Detailed studies of these compounds in honey [10,25] indicate that they originate from beeswax and therefore are not useful as floral source descriptors.
Maillard reaction products (furan and pyran derivatives) were also found in minor percentages [such as 5-hydroxymethylfurfural (2.4–9.3%; 6.5-22.8%), 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (0.3-0.6%; 0.2-0.9%), 2-methylfuran (0.2-1.2%; 0.2-2.3%) or dihydro-3-hydroxy-4,4-dimethyl-2(3H)-furanone (0.1-0.2%; 0.3-0.6%)], not as the honey markers but as indicators of absence of heat treatment and appropriate storage conditions.
3. Experimental
3.1. Honey Samples
Fresh and unheated samples of sulla (Hedysarum coronarium L.) honey were collected from professional beekeepers in different areas of Sardinia (Italy). Sensory and melissopalynological analyses were used to attribute the floral origin and six samples were selected. Qualitative and quantitative melissopalynological analyses were carried out following the method of the International Commission of Bee Botany [26]. This involves the estimation of the absolute number of elements in the sediment, the identification of the most frequent elements, and the evaluation of the sulla pollen grains percentage.
3.2. Ultrasonic Solvent Extraction (USE)
Forty grams of each sample was diluted in distilled water (22 mL) in a 100-mL flask. Magnesium sulfate (1.5 g) was added and the flask was extensively vortexed. Two different extraction solvents were used: 1) mixture of pentane and diethyl ether (1:2 v/v) and 2) dichloromethane. The solvents were separately used for the extraction of each honey sample. Ultrasound-assisted solvent extraction (USE) was performed in an ultrasound cleaning bath (Elmasonic Typ S 30 H, Germany) by indirect sonication (sweep mode), at the frequency of 37 kHz at 25 ± 3 °C. Sonication was maintained for 30 min. After sonication, the organic layer was separated by centrifugation and filtered over anhydrous MgSO4. The aqueous layer was returned to the flask and another batch of the same extraction solvent (20 mL) was added and extracted by ultrasound for 30 min. The organic layer was separated in the same way as the previous one and filtered over anhydrous MgSO4, and the aqueous layer was sonicated a third time for 30 min with another batch (20 mL) of the extraction solvent. Joined organic extracts were concentrated to 0.2 mL by distillation with Vigreaux column, and 1 μL was used for GC and GC/MS analyses. For each sample, three replicates were obtained.
3.3. Gas Chromatography and Mass Spectrometry (GC, GC/MS)
Gas chromatography analyses were performed using an Agilent Technologies (Palo Alto, CA, USA) gas chromatograph model 7890A equipped with flame ionization detector, quadropol mass spectrometer model 5975C equipped with the capillary column HP-5MS ((5%-phenyl)-methylpolysiloxane Agilent J & W GC column, 30 m, 0.25 mm i.d., coating thickness 0.25 μm). Chromatographic conditions were as follows: helium was carrier gas at 1 mL·min−1, injector temperature was 250 °C, and FID detector temperature was 300 °C. The temperature used included the following settings: 70 °C isothermal for 2 min, and then increased to 200 °C at a rate of 3 °C·min−1 and held isothermal for 18 min. The injected volume was 1 μL and the split ratio was 1:50. Mass spectra were recorded in the electron ionization mode at 70 eV with ion source temperature 230 °C and scanning the 30-300 m/z range. The analyses were carried out in duplicate for each sample batch.
The individual peaks were identified by comparison of their retention indices (relative to C9-C25 n-alkanes for HP-5MS) to those of available authentic samples and literature [27], as well as by comparing their fragmentation pattern with the Wiley 275 MS library (Wiley, New York, NY, USA) and NIST02 (Gaithersburg, MD, USA) mass spectral database. The percentage composition of the samples was computed from the GC peak areas using the normalization method (without correction factors). The component percentages were calculated as mean values from duplicate GC and GC-MS analyses.
4. Conclusions
There is moderate diversity among the natural volatiles that are extractable from Sardinian sulla honey samples, including a variety of distinctive norisoprenoids, benzene derivatives, aliphatic compounds and Maillard reaction products, but only a few terpenes were found. Both extraction solvents revealed very similar chemical composition and therefore can both be used for the isolation of sulla honey volatiles. Sulla honey is characterized by high percentages of norisoprenoids, being dominated by vomifoliol. Other major compounds were 3-hydroxy-4-phenylbutan-2-one and methyl syringate. The composition of the volatiles and semi-volatiles in the obtained extracts suggests that sulla honey is quite distinctive relative to the other honeys that have been chemically studied by GC/MS, but no specific markers of the honey botanical origin were found. However the obtained results present good starting point for chemical characterization of the sulla honey, since no useful data were obtained by HPLC and HS-SPME in our preliminary study.
Acknowledgements
This research has been funded by UKF project 25/08 (Evaluation of Unifloral Honeys – Chemical Fingerprinting and Nutritional Properties) and supported by PIP d.o.o., KONCEPT-MEDIA d.o.o. and AlphaCrom d.o.o.
Supplementary Materials
Footnotes
Sample Availability: Contact the corresponding author.
References
- 1.Cuevas-Glory L.F., Pino J.A., Santiago L.S., Sauri-Duch E. A review of volatile analytical methods for determining the botanical origin of honey. Food Chem. 2007;103:1032–1043. doi: 10.1016/j.foodchem.2006.07.068. [DOI] [Google Scholar]
- 2.Jerković I., Mastelić J., Marijanović Z., Klein Ž., Jelić M. Comparison of hydrodistillation and ultrasonic solvent extraction for the isolation of volatile compounds from two unifloral honeys of Robinia pseudoacacia L. and Castanea sativa L. Ultrason. Sonochem. 2007;14:750–756. doi: 10.1016/j.ultsonch.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Alissandrakis E., Tarantilis P.A., Harizanis P.C., Polissiou M. Evaluation of four isolation techniques for honey aroma compounds. J. Sci. Food Agric. 2005;85:91–97. doi: 10.1002/jsfa.1934. [DOI] [Google Scholar]
- 4.Castro-Vázquez L., Pérez-Coello M.S., Cabezudo M.D. Analysis of volatile compounds of rosemary honey. Comparison of different extraction techniques. Chromatographia. 2003;57:227–233. doi: 10.1007/BF02491721. [DOI] [Google Scholar]
- 5.Alissandrakis E., Daferera D., Tarantilis P.A., Polissiou M., Harizanis P.C. Ultrasound-assisted extraction of volatile compounds from citrus flowers and citrus honey. Food Chem. 2003;82:575–582. doi: 10.1016/S0308-8146(03)00013-X. [DOI] [Google Scholar]
- 6.D’Arcy B.R., Rintoul G.B., Rowland C.Y., Blackman A.J. Composition of Australian honey extractives. 1. Norisoprenoids, monoterpenes, and other natural volatiles from blue gum (Eucalyptus leucoxylon) and yellow box (Eucalyptus melliodora) honeys. J. Agric. Food Chem. 1997;45:1834–1843. doi: 10.1021/jf960625+. [DOI] [Google Scholar]
- 7.Rowland C.Y., Blackman A.J., D’Arcy B.R., Rintoul G.B. Comparison of organic extractives found in leatherwood (Eucryphia lucida) honey and leatherwood flowers and leaves. J. Agric. Food Chem. 1995;43:753–763. doi: 10.1021/jf00051a036. [DOI] [Google Scholar]
- 8.Wilkins A.L., Tan S.-T., Molan P.C. Extractable organic substances from New Zealand unifloral vipers bugloss (Echium vulgare) honey. J. Apic. Res. 1995;34:73–78. doi: 10.1080/00218839.1995.11100890. [DOI] [Google Scholar]
- 9.Tan S.-T., Wilkins A.L., Holland P.T., McGhie T.K. Extractives from New Zealand unifloral honeys. 2. Degraded carotenoids and other substances from heather honey. J. Agric. Food Chem. 1989;37:1217–1221. doi: 10.1021/jf00089a004. [DOI] [Google Scholar]
- 10.Tan S.-T., Holland P.T., Wilkins A.L., Molan P.C. Extractives from New Zealand honeys. 1. White clover, manuka, and manuka unifloral honeys. J. Agric. Food Chem. 1988;36:453–460. doi: 10.1021/jf00081a012. [DOI] [Google Scholar]
- 11.De Maria C.A.B., Moreira R.F.A. Volatile compounds in floral honeys. Quim. Nova. 2003;26:90–96. doi: 10.1590/S0100-40422003000100016. [DOI] [Google Scholar]
- 12.de la Fuente E., Valencia-Barrera R.M., Martïnez-Castro I., Sanz J. Occurrence of 2-hydroxy-5-methyl-3-hexanone and 3-hydroxy-5-methyl-2-hexanone as indicators of botanic origin in eucalyptus honeys. Food Chem. 2007;103:1176–1180. doi: 10.1016/j.foodchem.2006.10.020. [DOI] [Google Scholar]
- 13.Guyot C., Bouseta A., Scheirman V., Collin S. Floral origin markers of chestnut and lime tree honeys. J. Agric. Food Chem. 1998;46:625–633. doi: 10.1021/jf970510l. [DOI] [PubMed] [Google Scholar]
- 14.Guyot C., Scheirman V., Collin S. Floral origin markers of heather honeys: Calluna vulgaris and Erica arborea. Food Chem. 1999;64:3–11. doi: 10.1016/S0308-8146(98)00122-8. [DOI] [Google Scholar]
- 15.Sulas L., Re G.A., Ledda L., Caredda S. The effect of utilization frequency on the forage production of sulla (Hedysarum coronarium L.) Ital. J. Agron. 1997;2:89–94. [Google Scholar]
- 16.Bonvehí J.S., Torrento M.S., Muntané Raich J. Invertase activity in fresh and processed honeys. J. Sci. Food Agric. 2000;80:507–512. doi: 10.1002/(SICI)1097-0010(200003)80:4<507::AID-JSFA558>3.0.CO;2-5. [DOI] [Google Scholar]
- 17.Marini F., Magrì A.L., Balestrieri F., Fabretti F., Marini D. Supervised pattern recognition applied to the discrimination of the floral origin of six types of Italian honey samples. Anal. Chim. Acta. 2004;515:117–125. doi: 10.1016/j.aca.2004.01.013. [DOI] [Google Scholar]
- 18.Winterhalter P., Russell L. Carotenoid-derived aroma compounds. American Chemical Society; Washington DC, USA: 2002. pp. 1–17. [Google Scholar]
- 19.Nambara E., Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi F., Careri M., Musci M. Volatile norisoprenoids as markers of botanical origin of Sardinian strawberry-tree (Arbutus unedo L.) honey: Characterisation of aroma compounds by dynamic headspace extraction and gas chromatography-mass spectrometry. Food Chem. 2005;89:527–532. doi: 10.1016/j.foodchem.2004.03.009. [DOI] [Google Scholar]
- 21.Graddon A.D., Morrison J.D., Smith J.F. Volatile constituents of some unifloral Australian honeys. J. Agric. Food Chem. 1979;27:832–837. doi: 10.1021/jf60224a046. [DOI] [Google Scholar]
- 22.Jerković I., Hegić G., Marijanović Z., Bubalo D. Organic extractives from Mentha spp. honey and the bee-stomach: methyl syringate, vomifoliol, terpenediol I, hotrienol and other compounds. Molecules. 2010;15:2911–2924. doi: 10.3390/molecules15042911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keeling C.I., Slessor K.N., Higo H.A., Winston M.L. Isolation and identification of new components of the honey bee (Apis mellifera L.) queen retinue pheromone. Proc. Nat. Acad. Sci. USA. 2003;100:4486–4491. doi: 10.1073/pnas.0836984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuberoso C.I.G., Bifulco E., Jerković I., Caboni P., Cabras P., Floris I. Methyl syringate: a chemical marker of asphodel (Asphodelus microcarpus Salzm. et Viv.) monofloral honey J. Agric. Food Chem. 2009;57:3895–3900. doi: 10.1021/jf803991j. [DOI] [PubMed] [Google Scholar]
- 25.Jerković I., Marijanović Z., Ljubičić I., Gugić M. Contribution of the bees and combs to honey volatiles: blank-trial probe for honey biodiversity chemical profiling. Chem. Biodivers. 2010;7:1217–1230. doi: 10.1002/cbdv.200900100. [DOI] [PubMed] [Google Scholar]
- 26.Louveaux J., Maurizio A., Vorwohl G. Methods of melissopalynology. Bee World. 1978;59:39–153. doi: 10.1080/0005772X.1978.11097714. [DOI] [Google Scholar]
- 27.El-Sayed A.M. The pherobase: database of insect pheromones and semiochemicals. [(accessed 21 June 2010)]; http://www.pherobase.com/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.