Abstract
Background
The oldest-old (subjects aged 90 years and older) population represents the fastest growing segment of society and shows a high dementia prevalence rate of up to 40%. Only a few studies have investigated protective factors for cognitive impairment in the oldest-old. The EMIF-AD 90+ Study aims to identify factors associated with resilience to cognitive impairment in the oldest-old. In this paper we reviewed previous studies on cognitive resilience in the oldest-old and described the design of the EMIF-AD 90+ Study.
Methods
The EMIF-AD 90+ Study aimed to enroll 80 cognitively normal subjects and 40 subjects with cognitive impairment aged 90 years or older. Cognitive impairment was operationalized as amnestic mild cognitive impairment (aMCI), or possible or probable Alzheimer’s Disease (AD). The study was part of the European Medical Information Framework for AD (EMIF-AD) and was conducted at the Amsterdam University Medical Centers (UMC) and at the University of Manchester. We will test whether cognitive resilience is associated with cognitive reserve, vascular comorbidities, mood, sleep, sensory system capacity, physical performance and capacity, genetic risk factors, hallmarks of ageing, and markers of neurodegeneration. Markers of neurodegeneration included an amyloid positron emission tomography, amyloid β and tau in cerebrospinal fluid/blood and neurophysiological measures.
Discussion
The EMIF-AD 90+ Study will extend our knowledge on resilience to cognitive impairment in the oldest-old by extensive phenotyping of the subjects and the measurement of a wide range of potential protective factors, hallmarks of aging and markers of neurodegeneration.
Trial registration
Nederlands Trial Register NTR5867. Registered 20 May 2016.
Electronic supplementary material
The online version of this article (10.1186/s12877-018-0984-z) contains supplementary material, which is available to authorized users.
Keywords: Alzheimer’s disease, Dementia, Cognitive impairment, Amnestic mild cognitive impairment, Resilience, Oldest-old, Amyloid, Positron emission tomography, Magnetoencephalography (MEG)
Background
Introduction
The oldest-old (subjects aged 90 years and older) population represents the fastest growing segment of society [1]. Worldwide, the number of oldest-old subjects is expected to increase to 71.2 million in 2050, a 5-fold increase of the current oldest-old population [2, 3]. The oldest-old have a high risk of developing dementia with a prevalence up to 40% [4]. The increasing number of oldest-old subjects with dementia will have major clinical and financial consequences for patients, their families and society as a whole [5].
Still a considerable number of subjects remain cognitively normal at high age, indicating the presence of protective factors for cognitive impairment in these subjects. Identification of these protective factors is crucial and will have implications for preventive strategies. In addition, identifying the neurodegenerative markers associated with cognitive impairment in the oldest-old, will enhance our understanding of the underlying pathophysiology in this specific age group.
The EMIF-AD 90+ study was set-up to investigate protective factors for cognitive impairment in the oldest-old. We will first provide an overview of the current status of research on this topic and then present the study outline of the EMIF-AD 90+ study.
Review on studies on cognitive impairment in the oldest-old
We searched for studies focusing on protective factors for cognitive impairment in nonagenarians, which gave us two results: The 90+ Study in the USA and the Danish Birth Cohort Studies [6, 7]. Broadening the search to studies that started inclusion from the age of 85 years or focused on successful aging resulted in eight more studies: the H85 Gothenburg study, Leiden 85-plus Study, Newcastle 85+ Study, NonaSantfeliu study, Octabaix study, Project of Longevity and Aging in Dujangyan (PLAD), Umeå study and Vantaa 85+ Study [8–15]. Table 1 shows the design characteristics of these ten studies.
Table 1.
Design characteristics of other 85+ and 90+ studies that include data about cognitive functioning
| Domain | Danish Birth Cohort Studies [6]a | H85 Gothen-burg study [12] | Leiden 85-plus Study [9] | Newcastle 85+ Study [10] | NonaSant-feliu study [8] | Octabaix study [13] | PLAD [15] | The 90+ Study, USA [7] | Umeå 85+ study [14] | Vantaa 85+ Study [11] |
|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive reserve | + | + | + | + | + | + | + | + | + | + |
| Vascular comorbidity | + | + | + | + | + | + | + | + | + | + |
| Mood and sleep | + | + | + | + | – | – | + | + | + | + |
| Sensory system | – | – | + | + | + | + | – | + | + | – |
| Physical performance and capacity | + | – | + | + | – | + | + | + | + | – |
| Genetics | + | + | + | + | – | – | + | + | – | + |
| Hallmarks of agingb | – | – | + | – | – | + | – | + | – | – |
| Markers of neurodegeneration | – | + | – | – | – | – | – | + | – | – |
PLAD Project of Longevity and Aging in Dujangyan
aIncluding the cohorts recruited in 1895, 1905, 1910 and 1915, data availability varies per cohort. bInflammation and senescence markers (for example p16, p53 and telomere associated foci)
Protective factors for cognitive impairment in the oldest-old
Table 2 summarizes the findings on the protective factors for cognitive impairment or dementia of the ten studies. A high level of education was found to be protective against dementia in the oldest-old and one study indicated that high cognitive activity, examined by looking at the time spend on reading, around age 90 years was related to resilience to dementia [4, 16–18]. The influence of vascular comorbidities on cognition has been studied quite extensively in this age group. Most studies did not find an association between cholesterol levels and cognition in the oldest-old [15, 17, 19–22]. Hypertension has mostly been found to be protective in the oldest-old, especially when hypertension is diagnosed after the age of 80 years [17, 19, 23–27]. This is in contrast to studies that have shown a higher dementia risk in the presence of midlife hypertension [28]. In addition, although midlife diabetes mellitus has been related to dementia in younger subjects [29], the influence of diabetes mellitus on cognition might be less evident in the oldest-old [11, 30, 31]. The protective effect related to the absence of stroke seemed to persist in the oldest-old [18, 32] and one study on atrial fibrillation and dementia did not find an association [32]. The absence of depressive symptoms seemed to be associated with resilience to cognitive impairment, which is consistent with findings in younger subjects [14, 33, 34]. One study related sleep quality to cognition and reported a higher sleep quality in subjects without cognitive impairment, which is in line with results in younger subjects [35, 36]. With regard to the sensory system, visual and auditory impairments have been associated with worse cognitive functioning in the oldest-old [37, 38] and although olfactory impairment has been associated with incident dementia in a younger age group [39], no studies were found studying this in the oldest-old.
Table 2.
Potential protective factors for cognitive impairment in the oldest-old
| Domain | Potential protective factor | Study | Agea | Sample size (N) | Outcome variable | Result |
|---|---|---|---|---|---|---|
| Cognitive reserve | High level of education | H85 Gothenburg study [18] | 85.7 (±0.05) | No dementia: 794 Dementia: 271 |
Dementia | Protective |
| The 90+ Study [4] | 94 (90–106) | No dementia: 536 Dementia: 375 |
Dementia | Protective | ||
| Vantaa 85+ Study [17] | 88.4 (85.0–104.0) | No incident dementia: 239 Incident dementia: 100 |
Incident dementia | Protective | ||
| High cognitive activity | The 90+ Study [16] | 93 (90–103) | No incident dementia: 319 Incident dementia: 268 |
Incident dementia | Equivocal | |
| Vascular comorbidity | Low total/LDL or high HDL cholesterol level | Leiden 85-plus Study [20] | 85 (85) | No dementia: 488 Dementia: 73 |
Cognition Dementia |
Equivocal |
| Newcastle 85+ Study [19] | 85 (85) | No dementia: 767 Dementia: 78 |
Cognition Cognitive decline |
Equivocal | ||
| NonaSantfeliu study [21] | 94.3 (±2.6) | 62, dementia status unknown | Cognition | No effect | ||
| Octabaix study [22] | 85 (85) | 321, dementia status unknown | Cognition | No effect | ||
| PLAD [15] | 93.6 (90–108) | No cognitive impairment: 300 Cognitive impairment: 409 |
Cognition | No effect | ||
| Vantaa 85+ Study [17] | 88.4 (85.0–104.0) | No incident dementia: 239 Incident dementia: 100 |
Incident dementia | No effect | ||
| Absence of hypertension | Leiden 85-plus Study [23] | 85 (85) | 572, dementia status unknown | Cognition Cognitive decline |
Risk | |
| Newcastle 85+ Study [19] | 85 (85) | No dementia: 767 Dementia: 78 |
Cognition Cognitive decline |
Equivocal | ||
| PLAD [27] | 93.6 (90–108) | No cognitive impairment: 317 Cognitive impairment: 465 |
Cognition | No effect | ||
| Umeå 85+ study [26] | 85, 90 and ≥ 95 | No dementia: 342 Dementia: 233 |
Cognition Dementia |
Protective | ||
| Umeå 85+ study [25] | 88.8 (±4.1) | No incident dementia: 136 Incident dementia: 69 |
Incident dementia | No effect | ||
| The 90+ Study [24] | 93.2 (90–103) | No incident dementia: 335 Incident dementia: 224 |
Incident dementia | Risk | ||
| Vantaa 85+ Study [17] | 88.4 (85.0–104.0) | No incident dementia: 239 Incident dementia: 100 |
Incident dementia | Equivocal | ||
| Absence of DM | Leiden 85-plus Study [30] | 85 (85) | 596, dementia status unknown | Cognition Cognitive decline |
Equivocal | |
| Octabaix study [31] | 85 (85) | 167, dementia status unknown | Cognition Cognitive decline |
No effect | ||
| Vantaa 85+ Study [11] | ≥85 | No incident dementia: 249 Incident dementia: 106 |
Incident dementia | Protective | ||
| Absence of stroke | H85 Gothenburg study [18] | 85.7 (±0.05) | No dementia: 794 Dementia: 271 |
Dementia | Protective | |
| Vantaa 85+ Study [32] | 88.4 (±2.9) | No dementia: 339 Dementia: 214 Incident dementia: 100 |
Dementia Incident dementia |
Protective | ||
| Absence of AF | Vantaa 85+ Study [32] | 88.4 (±2.9) | No dementia: 339 Dementia: 214 Incident dementia: 100 |
Dementia Incident dementia |
No effect | |
| Mood and sleep | No depression | Leiden 85-plus Study [34] | 85 (85) | 500, dementia status unknown | Cognition | Protective |
| Umeå 85+ study [14] | 85, 90 and 95–103 | No dementia: 173 Dementia: 69 |
Dementia | Protective | ||
| High sleep quality | PLAD [35] | 93.5 (±3.4) | No dementia: 251 Dementia: 409 |
Dementia Cognition |
Protective | |
| Sensory system | Absence of visual impairment | Leiden 85-plus Study [37] | 85 (85) | 459, dementia status unknown | Cognition | Protective |
| Newcastle 85+ Study [38] | 85 (85) | No dementia: 771 Dementia: 68 |
Cognition | Protective | ||
| Absence of glaucoma or cataract | Newcastle 85+ Study [105] | 85 (85) | No dementia: 771 Dementia: 68 |
Cognition | Equivocal | |
| Absence of hearing impairment | Leiden 85-plus Study [37] | 85 (85) | 459, dementia status unknown | Cognition | Equivocal | |
| Physical performance and capacity | Good physical performance | Leiden 85-plus Study [40] | 85 (85) | 555, dementia status unknown | Cognition | Protective |
| The 90+ Study [41] | 93.3 (±2.6) | No incident dementia: 366 Incident dementia: 212 |
Incident dementia | Protective | ||
| High physical activity | The 90+ Study [16] | 93 (90–103) | No incident dementia: 319 Incident dementia: 268 |
Incident dementia | No effect | |
| Genetics | Absence of APOEε4 and/or presence of APOEε2 | Danish 1905 birth cohort [42] | 93.1 (±0.3) | 1551, dementia status unknown | Cognition Cognitive decline |
No effect |
| Leiden 85-plus Study [43] | 89.0 (87.4–91.2)b | No dementia: 242 Dementia: 78 |
Dementia | Protective | ||
| The 90+ Study [44] | 93.7 (90–105) | No dementia: 566 Dementia: 236 Incident dementia: 188 |
Dementia Incident dementia |
Equivocal | ||
| Vantaa 85+ Study [45] | ≥85 | 313 without dementia 197 with dementia |
Dementia | Protective | ||
| Vantaa 85+ Study [46] | ≥85 | No incident dementia: 187 Incident dementia: 58 |
Incident dementia Cognitive decline |
No effect | ||
| MnSOD, GLRX, GSTP1, MT1A, NDUFV1, PRDX3, UQCRFS1, PICALM | Danish 1905 birth cohort [106–108] | 92-93c | 1089–1650, dementia status unknown | Cognition | Protective | |
| ACOX1 | Danish 1905 birth cohort [106] | 93.2 (92.7–93.8) | 1089, dementia status unknown | Cognition | Risk | |
| Cytokine genes, CLU | Danish 1905 birth cohort [108–110] | 92-93c | 1380–1651, dementia status unknown | Cognition Cognitive decline |
Equivocal | |
| MTHFR, MTR | Danish 1905 birth cohort [111] | 93.1 (±0.3) | 1651, dementia status unknown | Cognition Cognitive decline |
No effect | |
| KLOTHO | PLAD [112] | 93.5 (90–108) | No cognitive impairment: 236 Cognitive impairment: 470 |
Cognition | Protective | |
| PPAR-γ2 | PLAD [113] | 93.7 (90–108) | No cognitive impairment: 257 Cognitive impairment: 475 |
Cognition | No effect | |
| LRP, LPL, ACE | Vantaa 85+ Study [114] | ≥85 | No dementia: 203 Dementia (AD): 113 |
Dementia | No effect | |
| Hallmarks of ageing | Low level of inflammation markers | Leiden 85-plus Study [49] | 85 (85) | No dementia: 491 | Cognition Cognitive decline |
Equivocal |
| The 90+ Study [50] | 94.3 (90–105) | No dementia: 232 Dementia: 73 |
Dementia | Equivocal | ||
| The 90+ Study [51] | 93.9 (90–102) | No incident dementia: 145 Incident dementia: 82 |
Incident dementia | No effect | ||
| Low level of senescence markers | Leiden 85-plus Study [52] | 89.8 (85–101) | No dementia: 452 Dementia: 146 Incident dementia: unknown |
Cognition Dementia Incident dementia |
No effect | |
| Markers of neurodegeneration | Normal levels of Aβ and tau in CSF | H85 Gothenburg study [56] | 85 (85) | No incident dementia: 28 Incident dementia: 7 |
Incident dementia | Protective |
| Negative amyloid PET-scan | The 90+ Study [57] | 94.2 (90–99)d | No incident dementia: 10 Incident dementia: 3 |
Cognitive decline | Protective | |
| Less brain atrophy | H85 Gothenburg study [58] | 85 (85) | No dementia: 30 Dementia: 23 |
Dementia | Equivocal | |
| Less WMH | H85 Gothenburg study [59] | 85 (85) | No dementia: 133 Dementia: 103 |
Dementia | Protective | |
| High white matter integrity | The 90+ Study [60] | 94.6 (90–103) | Normal: 64 CIND: 30 |
CIND | No effect |
Aβ Amyloid β, AD Alzheimer’s disease, APOE Apolipoprotein E, CIND Cognitive Impairment, No Dementia, CSF cerebrospinal fluid, DM diabetes mellitus, HDL high-density lipoproteins, LDL low-density lipoproteins, MCI Mild Cognitive Impairment, MMSE Mini-Mental State Examination, N Number, PET positron emission tomography, PLAD Project of Longevity and Aging in Dujangyan, WMH white matter hyperintensities
aMean age (range, if available, or ± if standard deviation) in years at baseline, unless stated otherwise; bMedian age (interquartile range, IQR) in years; cMinimal and maximum mean age in years of the studies referred to; dMedian age (range) in years
Data about physical performance and activity have been collected in the Leiden 85-plus study and The 90+ Study. Good physical performance, measured with handgrip strength, 4 m walk or standing balance tests, was associated with better cognitive functioning and lower dementia incidence in the oldest-old but high physical activity did not seem to influence dementia incidence [16, 40, 41].
With regard to genetics, the Apolipoprotein E (APOE) genotype, a major risk factor for AD in younger subjects, has been extensively studied in the oldest-old, with mixed results regarding the relation to cognition and dementia [42–46]. The Danish 1905 birth cohort, PLAD and Vantaa 85+ Study also studied a number of other genotypes in the oldest-old and found some additional protective and risk genotypes which are described in Table 2.
Hallmarks of aging and cognition in the oldest-old
Hallmarks of aging [47], such as inflammation and cellular senescence [48], have been scarcely studied in relation to cognition in the oldest-old. The Leiden 85-plus Study and The 90+ Study related inflammation markers to cognition and dementia but showed mixed results [49–51]. In addition, telomere length measured in white blood cells were not associated with cognition, dementia prevalence or incident dementia [52].
Markers of neurodegeneration and cognition in the oldest-old
Limited information is available about the relation of markers of neurodegeneration, such as amyloid β and tau measured in cerebrospinal fluid (CSF) and/or with a positron emission tomography (PET) scan with cognitive impairment in the oldest-old. Postmortem studies have shown that the prevalence of amyloid aggregation increases with age in cognitively healthy subjects but decreases in the oldest-old subjects with dementia [1]. A similar trend can be seen with regard to amyloid β measured in CSF or on an amyloid PET scan [53, 54]. In subjects without dementia, greater amyloid load has been associated with poorer cognitive functioning and a higher rate of incident dementia, although the number of oldest-old subjects in these studies was limited [55–57]. There are a few studies that have related brain MRI measurements in the oldest-old to cognitive functioning. Less atrophy and fewer white matter hyperintensities were seen in subjects without dementia compared to subjects with dementia [58, 59] but white matter integrity was not related to cognition [60]. In younger subjects, neurophysiological measures on magnetoencephalography (MEG) have been related to dementia [61] but it is unknown whether this relationship persists in the oldest-old.
Aims and objectives of the EMIF-AD 90+ study
The EMIF-AD 90+ Study was set-up to investigate the protective factors for cognitive impairment in the oldest-old. The study was part of the Innovative Medicine Initiative (IMI) European Medical Information Framework for AD (EMIF-AD) project (http://www.emif.eu/about/emif-ad) on diagnostic markers, prognostic markers, and protective factors for AD. The EMIF-AD 90+ study focuses on the extreme phenotype of the cognitively normal oldest-old. The primary objectives of the EMIF-AD 90+ study are:
-
i)
To identify factors associated with resilience to cognitive impairment in the oldest-old.
-
ii)
To test the relationship between hallmarks of aging and cognitive impairment in the oldest-old.
-
iii)
To test the relationship between markers of neurodegeneration and cognitive impairment in the oldest-old.
This paper describes the design and protocol of the study.
Methods
Study subjects
We aimed to include 80 cognitively normal subjects and 40 subjects with cognitive impairment, both aged 90 years and older. Inclusion criteria for cognitively normal subjects were a global Clinical Dementia Rating (CDR) score of 0 [62] and a score ≥ 26 points on the Mini-Mental State Examination (MMSE) [63]. Inclusion criteria for subjects with cognitive impairment were a diagnosis of amnestic MCI (aMCI) [64] or a diagnosis of probable or possible AD [65] by a neurologist, geriatrician, or general practitioner, a global CDR score ≥ 0.5 point (s) and a MMSE score of 20–28 points (inclusive). Exclusion criteria were the physical inability to undergo the procedures, visual or hearing impairment which made neuropsychological testing impossible, severe depression (Geriatric Depression Scale (GDS) score ≥ 11 points [66]) and other comorbidities or medication that could impair cognition at the discretion of the investigator (e.g. stroke, epilepsy or use of lithium carbonate). During the inclusion period it turned out to be difficult to identify subjects of 90 years and older with aMCI or probable or possible AD; we therefore broadened the inclusion criteria in this group to subjects older than 85 years.
Subjects were recruited at two sites: the Amsterdam UMC, The Netherlands and The University of Manchester, United Kingdom. Cognitively normal subjects were recruited from general practitioners or via advertisements (Amsterdam) or from the Manchester and Newcastle Ageing Study (MNAS, Manchester). Subjects with cognitive impairment were only recruited in the Netherlands. They were recruited from the Alzheimer Center Amsterdam and the Center Of Geriatric medicine Amsterdam (COGA) at the Amsterdam UMC, geriatric departments of other hospitals in the surroundings of Amsterdam, other healthcare facilities (such as a care home), general practitioners or via advertisement. The sample collection started on the 1st of June 2016 and ended on the 30th of June 2018. Currently we are working on the first data analyses.
The Medical Ethics Review Committee of the Amsterdam UMC approved the study in Amsterdam and the National Research Ethics Service Committee North West - Greater Manchester South performed approval of the study in Manchester. The study was carried out in accordance with the ethical conduct and juridical laws of the Declaration of Helsinki 64th WMA General Assembly, Fortaleza, Brazil, October 2013, (www.wma.net), and in accordance with the Medical Research Involving Human Subjects Act (WMO). All subjects gave written informed consent.
Study design
The EMIF-AD 90+ Study is a case-control study in which we search for protective factors for cognitive impairment. Therefore, the cognitively normal subjects are described as cases and the subjects with cognitive impairment as controls.
Study procedures
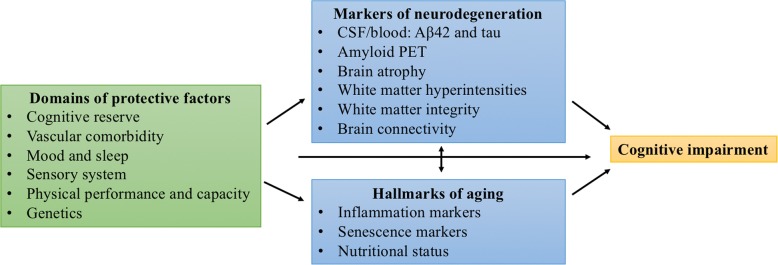
The study consisted of two home visits and one or two visits at the hospital/clinical research facility (CRF). During the first home visit, in- and exclusion criteria (MMSE, CDR, impression of physical ability to undergo the procedures, hearing and visual abilities) were verified, in addition to collection of first study data (Table 3, paragraphs 2.3.1, 2.3.2 and 2.3.4). The MMSE is a short cognitive screening test with a maximum score of 30 points [63]. The CDR is a scale for the severity of symptoms of dementia, which was assessed by interviews with the subject and, if available, study partner (somebody that is in regular contact with the subject) in combination with judgement by the researchers [62]. The second home visit consisted of a neuropsychological assessment performed by a neuropsychologist (paragraph 2.3.3). During the hospital/CRF visits several procedures were performed, which are listed in Table 3 and described in paragraphs 2.3.4. – 2.3.10. These procedures provided information on i) potential protective factors (classified in six different domains), ii) hallmarks of aging, and iii) markers of neurodegeneration (Fig. 1 and Table 3). For each domain, hallmark of aging or markers of neurodegeneration, we will test one or more parameters (Table 3). In most cases, all procedures were performed within three months from start of the inclusion. Any differences in study procedures between Amsterdam and Manchester are explicitly stated in this paper.
Table 3.
The domains of interest in the EMIF-AD 90+ Study
| Domain | Parameter | Procedure (measurement) | Schedule Amsterdam | Schedule Manchester |
|---|---|---|---|---|
| Cognitive reserve | Level of education | Interview | Home | Home |
| Cognitive activity | Cognitive abilities questionnaire | Home | Home | |
| Vascular comorbidity | Cholesterol level, hypertension, DM, stroke, AF | Blood collection | Hospital | WMIC |
| Medical history and medication use | Home | Home | ||
| Blood pressure | Hospital | CRF | ||
| Diagnostick/heart rate | Home | CRF | ||
| Ultrasound carotid artery | Hospital | CRF | ||
| Mood and sleep | Depressive symptoms | Geriatric Depression Scale | Home | Home |
| Sleep disorder | Berlin Questionnaire and MSQ | Home | Home | |
| Accelerometer (sleep quality) | Home | N/A | ||
| Sensory system | Visual acuity | ETDRS chart | Hospital | N/A |
| Retinal thickness | OCT | Hospital | N/A | |
| Auditory function | Digits-in-noise test | Home | N/A | |
| Olfactory function | Sniffin sticks | Hospital | N/A | |
| Physical performance and capacity | Physical performance | Grip strength | Home | CRF |
| Short Physical Performance Battery or 4-min walking test | Hospital | CRF | ||
| BIA (muscle mass) | Hospital | N/A | ||
| Physical activity | Accelerometer | Home | N/A | |
| Genetics | APOEε4 and APOEε2 | Blood collection | Hospital | WMIC |
| Hallmarks of ageing | Level of inflammation markers | Blood collection (i.a. PBMCs) | Hospital | WMIC |
| Level of senescence markers | Skin biopsy (senescence markers p16, p53 and telomere associated foci) | Hospital | N/A | |
| Nutritional status | BIA | Hospital | N/A | |
| Blood collection | Hospital | CRF | ||
| BMI | Hospital | CRF | ||
| MNA | Home | N/A | ||
| Markers of neurodegeneration | Aβ1–42 and tau in CSF and blood | CSF collection Blood collection |
Hospital | N/A |
| Amyloid PET scan | Amyloid PET scan | Hospital | WMIC | |
| Brain atrophy | Brain MRI scan or brain CT scan | Hospital | CRF | |
| WMH | Brain MRI scan or brain CT scan | Hospital | CRF | |
| White matter integrity | Brain MRI scan | Hospital | N/A | |
| Brain connectivity | Brain MRI scan | Hospital | CRF | |
| MEG | Hospital | N/A |
Aβ Amyloid β, AD Alzheimer’s disease, AF atrial fibrillation, APOE Apolipoprotein E, BIA Bioelectrical impedance analysis, BMI Body Mass Index, CRF Clinical Research Facility, CT Computerized Tomography, CSF cerebrospinal fluid, DM diabetes mellitus, ETDRS Early Treatment Diabetic Retinopathy Study, MEG magnetoencephalography, MNA Mini Nutritional Assessment, MRI Magnetic Resonance Imaging, MSQ Mayo Sleep Questionnaire, N/A not applicable, OCT Optical Coherence Tomography, PBMCs Peripheral Blood Mononuclear Cells, PET positron emission tomography, PLAD Project of Longevity and Aging in Dujangyan, WMH white matter hyperintensities, WMIC Wolfson Molecular Imaging Centre
Fig. 1.
Overview of the domains of interest in the EMIF-AD 90+ Study. Aβ Amyloid β, CSF cerebrospinal fluid, PET positron emission tomography
Interview
Data about the medical and family history, medication use, education and intoxications (alcohol use and smoking) were collected through a structured interview, in combination with information provided by the study partner (if available), general practitioner and/or medical specialist.
Questionnaires
In Amsterdam, subjects were asked to complete six questionnaires. Activities of daily living (ADL) were evaluated by use of the Katz ADL [67]. Functional health and wellbeing were evaluated by the Short form-12 Health-related Quality of Life (SF-12 HRQoL) questionnaire [68] and by the Cognitive Complaints Index (CCI) [69]. Nutrition was evaluated by the Mini Nutritional Assessment (MNA-long version) [70]. Sleep disorders were evaluated by use of the Berlin Questionnaire which identifies the risk of sleep disordered breathing [71]. Cognitive activity during life, such as reading books and playing games, was assessed with the cognitive abilities questionnaire [72]. Subjects with cognitive impairment filled in the questionnaires together with a study partner. The GDS was filled in together with the researcher [66].
In Amsterdam, the study partner was asked to complete five questionnaires: the AD8 (an 8-question test for the study partner to assess mild dementia) [73], the Amsterdam instrumental Activities of Daily Living (iADL) scale (a study partner based tool aimed at detecting iADL problems in early dementia) [74, 75], the Neuropsychiatric Inventory Questionnaire (NPI-Q, to assess the severity of behavioral symptoms in the subject and the distress these symptoms cause in the study partner) [76], the Mayo Sleep Questionnaire (MSQ, to screen for the presence of Rapid Eye Movement (REM) sleep disorders) [77], and finally the CCI [69].
In Manchester, subjects were asked to complete the SF-12 HRQoL questionnaire [68], the Physical Activity Scale for the Elderly (PASE) [78], the CCI [69] and the cognitive abilities questionnaire [72]. The study partner was asked to complete the AD8 [73], the Functional Activities Questionnaire (FAQ) [79] and the CCI [69].
Neuropsychological assessment
The neuropsychological assessment took approximately one and a half hours during which several cognitive domains were tested. Table 4 gives an overview of the different cognitive tests that were administered, which domain they examine and at which site they were performed.
Table 4.
Cognitive tests in the EMIF-AD 90+ Study
| Cognitive test | Cognitive domain | Site |
|---|---|---|
| CERAD 10 words test [115] Immediate recall Delayed recall after 10 min |
Memory | Ba |
| Logical Memory test [116] Immediate recall Delayed recall after 20–30 min |
Memory | A |
| Rey Auditory Verbal Learning Test [117] Immediate recall Delayed recall after 20 min |
Memory | M |
| Rey Complex Figure Test [118] Copy Delayed copy after 3 min |
Memory Visuoconstructive skills |
B |
| WAIS-III Digit span forward and backward [119, 120] | Executive functioning | B |
| Animal (2 min) and Letter fluency (1 min per letterb) [121] | Executive functioning | B |
| Clock Drawing Testc [122] | Executive functioning Visuospatial functioning |
A |
| Graded Naming Test [123] | Object-naming ability | B |
| Trail Making Test A and B [124] | Information processing speed Visual attention Task switching |
B |
| WAIS-R Digit Symbol Substitution Test [125] | Perceptual-motor speed Incidental learning |
B |
| Computerised Cambridge Neuropsychological Test Automated battery [126] | Paired associate learning Spatial-working memory Reaction time |
B |
| National Adult Reading Test [127] | Pre-morbid IQ | B |
| Visual Association Test [128] | Visuospatial association learning | A |
| Addenbrooke’s Cognitive Examination Revised battery [129] | Attention/orientation Memory Verbal fluency Language Visuospatial abilities |
M |
A administered only in Amsterdam, B administered in Amsterdam and Manchester, CERAD Consortium to Establish a Registry for Alzheimer’s Disease, M administered only in Manchester, min minute (s), WAIS (−R) Wechsler Adult Intelligence Scale (-Revised)
aIn Manchester only in the cognitively normal subjects. bIn Amsterdam using the letters D, A and T and in Manchester the letters F, A, and S. cThe subject will be asked to draw a clock showing the time “ten after eleven”. In total 14 points can be scored based on the presence and sequencing of the numbers and the positioning of the two hands
Physical examination
In Amsterdam, data on waist and hip circumference (cm), and hand grip strength (kg), as well as a standard neurologic screening examination were recorded during the first home visit. Hand grip strength was measured to estimate muscle strength and was performed with a hand dynamometer (Jamar hand dynamometer; Sammons Preston, Inc., Bolingbrook, IL., USA) [80]. In addition, a ‘Diagnostick’ was used to determine whether the subject had atrial fibrillation by measuring one derivative of an electrocardiogram [81]. At the end of the first home visit, the subject was asked to wear an accelerometer (DynaPort MoveMonitor, McRoberts B.V., The Hague, The Netherlands) for seven days to measure physical activity and sleep quality.
During the hospital visit in Amsterdam, continuous blood pressure measurements were performed non-invasively using a digital photoplethysmogram on the right middle finger (Nexfin®, BMEYE, Amsterdam, The Netherlands), resulting in beat-to-beat BP data. The Short Physical Performance Battery (SPPB) included balance tests, a 4 m walk to measure walking speed and the chair stand test [82]. Body composition, including the Body Mass Index (BMI), was measured using a Bioelectrical Impedance Analysis (BIA; InBody 770; Biospace Co., Ltd., Seoul, Korea).
In Manchester, waist and hip circumference (cm), hand grip strength (kg), BMI, resting blood pressure, heart rate, ankle/brachial pressure index [83] and a 4 min walking test were recorded at the clinical research facility.
Sensory system
Measurements of the sensory system were only performed in Amsterdam. With regard to visual functioning, best corrected visual acuity was tested with an Early Treatment Diabetic Retinopathy Study (ETDRS) chart. Intra-Ocular Pressure (IOP) and refraction data of all subjects were obtained, and all subjects underwent slit lamp examination and indirect fundoscopy. Pupils were dilated using tropicamide 0.5% and phenylephrine 5%. Peripapillary Retinal Nerve Fiber Layer (pRNFL) thickness and macular (layer) thickness were measured with Spectral Domain Optical Coherence Tomography (SD-OCT, Heidelberg Spectralis) using Heidelberg’s build-in software [84]. With enhanced depth imaging, the choroid was imaged and its thickness was (manually) measured. With fundus photography (Topcon TRC 50DX type IA), we acquired digital fundus images (50°). From these, seven Retinal Vascular Parameters (RVPs) were obtained using Singapore I Vessel Assessment (SIVA, version 3.0) [85].
For the auditory function, we used the digits-in-noise (DIN) test [86]. The DIN test is a speech-in-noise test using digit triplets as speech material. The digit triplets are presented against a constant level of stationary background noise. The test uses an adaptive procedure to determine the signal-to-noise ratio at which a listener understands 50% of the digit triplets correctly (i.e. the speech reception threshold (SRT) in noise). Olfactory function was measured using “Sniffin’ Sticks” (Burghart, Wedel, Germany). The test consists of pen-like odor dispensing devices with odors that are considered to be familiar. The smell test in the present study contained the odor identification part of the test [87].
Blood collection and skin biopsy
In both centers, blood samples were collected according to the biobanking pre-analytical Standard Operating Procedures (SOPs) of the Biomarkers for Alzheimer’s disease and Parkinson’s disease (BIOMARKAPD) project [88]. Blood samples were collected for DNA and RNA analysis, inflammation markers, proteomics, neurodegenerative markers (amyloid β, tau, neurofilament light), routine blood analysis (i.e. lipids and glucose), vitamin status (B12 and folic acid) and, in Amsterdam only, for Peripheral Blood Mononuclear Cells (PBMCs). Planned DNA analysis includes Single Nucleotide Polymorphisms (SNP) analysis of known genetic risk factors for AD or amyloid pathology [89–92]. DNA and RNA isolation will be performed by EMIF-AD partners. Remaining samples will be stored for future biomarker identification and validation studies.
In Amsterdam, four millimeter skin biopsies were taken from the inner upper medial arm and will be stained for senescence markers p16, p53 and telomere associated foci.
Cerebrospinal fluid collection
In Amsterdam, up to 20 mL CSF was obtained by lumbar puncture in Sarstedt polypropylene syringes using a Spinocan 25 Gauge needle in one of the intervertebral spaces between L3 and S1. A half mL CSF was immediately processed for leukocyte count, erythrocyte count, glucose, and total protein. The remaining CSF was mixed and centrifuged at 1300–2000 × g at 4 °C for ten minutes. Supernatants were stored in aliquots of 0.25–0.5 mL and frozen within two hours at − 80 °C and stored for future biomarker discovery studies. The processing and storing of CSF was performed according to the BIOMARKAPD SOP [88]. Amyloid β 1–42, total tau and phosphorylated tau 181 will be analyzed in a single batch. Remaining samples will be stored for future biomarker identification and validation studies.
Ultrasound carotid artery
At both sites, a duplex ultrasound scan of the carotid artery was performed. In Amsterdam, the right carotid artery was scanned to assess intima media thickness and distension using ArtLab software [93, 94]. In Manchester, left and right carotid arteries were scanned to determine velocity, vessel thickness, stenosis and plaques, rated according to the North American Symptomatic Carotid Endarterectomy Trial guidelines [95].
Brain MRI scan
Subjects underwent locally optimized brain MRI protocols including 3D-T1, fluid attenuated inversion recovery (FLAIR), susceptibility weighted imaging (SWI), diffusion tensor imaging (DTI) and resting state functional MRI (rs-fMRI). MRI scans were performed on Philips 3 T Achieva scanners. Additionally, in Manchester regional cerebral blood flow was measured by arterial spin labelling [96], but no DTI scan was acquired in Manchester. In Amsterdam, if a subject could not undergo the MRI scan, we considered a CT scan (Philips Ingenuity TF or Gemini TF camera). Scans will be analyzed locally and centrally by EMIF-AD partners using the Neugrid infrastructure if applicable (see Additional file 1).
Amyloid PET scan
[18F] Flutemetamol, a specific fibrillary amyloid β radiotracer, was used for the amyloid PET scans. In Amsterdam, [18F] flutemetamol was produced by General Electric (GE) Healthcare at the Cyclotron Research Center of the University of Liège (Liège, Belgium) and PET scans were performed using a Philips Ingenuity TF PET-MRI scanner (Philips Medical Systems, Cleveland, Ohio, USA) or, in case of a PET-CT scan, the Philips Ingenuity TF (Philips Medical Systems, Best, the Netherlands) or Gemini TF scanner (Philips Medical Systems, Best, the Netherlands). In Manchester, [18F] flutemetamol was produced at the Wolfson Molecular Imaging Centre (WMIC)‘s Good Manufacturing Practice radiochemistry facility using GE Healthcare’s FASTlab and cassettes and PET scans were performed using a High Resolution Research Tomograph (HRRT; Siemens/CTI, Knoxville, TN). In both centers, the emission scan was performed in two parts. First a 30 min dynamic emission scan was started simultaneously with a bolus intravenous injection of 185 MBq [18F] flutemetamol. The second part of the scan was performed from 90 to 110 min post injection. In Amsterdam, immediately before each part of the PET scan a T1-weighted gradient echo pulse MRI or low dose CT scan was obtained. This MRI or CT scan was used for attenuation correction of the PET scan. In Manchester, two seven minute transmission scans, one before the first emission scan and the other after the second emission scan, using a 137Cs point source were acquired for subsequent attenuation and scatter correction.
All [18F] flutemetamol scans were read visually as positive or negative. Additionally, we determined time activity curves for each region of interest with cerebellum grey matter as input function [97]. The dynamic data were analyzed on a voxel-by-voxel level using the Simplified Reference Tissue Model 2 (SRTM2) [98, 99]. Finally, we investigated tracer uptake by using a simplified method: the standardized uptake value ratio (SUVr, target to grey matter cerebellum SUV over 90–110 min pi) [100]. Variability in acquisition of amyloid PET scans were reduced by harmonizing acquisition protocols and will be reduced by adding it to the analyses as a covariate.
Neurophysiology
In Amsterdam, MEG was performed using a 306 channel whole-head system (Elekta Neuromag Oy, Helsinki, Finland). Eyes-closed and eyes-open resting-state MEG data were recorded with subjects in supine position inside a magnetically shielded room. We will use transformed time series [101] to extract spectral properties (relative band power and peak frequency) [102], and estimates of functional connectivity between brain regions, and metrics that characterize the topology of the functional brain networks [103, 104]. These analyses will be applied using Elekta’s beamformer software, and both in-house developed Matlab tools and BrainWave software (http://home.kpn.nl/stam7883/brainwave.html).
Planned statistical analyses
For each parameter listed in Table 3, we will test with logistic regression models whether it is associated with resilience to cognitive impairment. In addition, linear regression models will be used to associate the same parameters with cognitive functioning in the total sample. Potential additional analyses include the identification of protective factors for abnormal AD biomarkers in the subsample of cognitively normal subjects and the identification of protective factors for cognitive impairment in subjects with a high risk, for example APOE ε4 carriers.
Discussion
We described the design of the EMIF-AD 90+ Study that aims to unravel the factors associated with resilience to cognitive impairment in the oldest-old. An important additional value of the EMIF-AD 90+ Study compared to the previous studies is the extensive phenotyping of subjects, which includes data about cognitive reserve, vascular comorbidities, mood, sleep, sensory system capacity, physical performance and capacity and genetic risk factors. Furthermore, the EMIF-AD 90+ Study is one of the first studies that collects a broad range of markers of neurodegeneration in the oldest-old, including an amyloid PET scan, amyloid β and tau measured in CSF and blood and neurophysiological measures.
The EMIF-AD 90+ is the first study worldwide that combines data regarding the hallmarks of aging with markers of neurodegeneration. The process of aging and the incidence of aMCI and possible or probable AD are very much interrelated. Our study allows to test hypotheses such as that common risk factors and pathways drive both the aging process and development of cognitive impairment or AD. Another strength of the EMIF-AD 90+ study is that we use objective measures wherever possible, instead of using questionnaires. For example, physical activity and sleep quality were measured with an accelerometer in Amsterdam.
To conclude, the results of the EMIF-AD 90+ Study will provide an important contribution to the existing literature in many different ways. It will extend our knowledge on protective factors for cognitive impairment in the oldest-old and will determine how hallmarks of aging and markers of neurodegeneration relate to cognitive impairment in this specific age group.
Additional file
Table S1. Brain MRI scan analyses in the EMIF-AD 90+ Study. (DOCX 26 kb)
Acknowledgements
We very much acknowledge all subjects who participated in the EMIF-AD 90+ Study.
Funding
The EMIF-AD 90+ Study was funded by the EU/EFPIA Innovative Medicines Initiative Joint Undertaking EMIF grant agreement no. 115372. FB is supported by the NIHR UCLH biomedical research centre.
Availability of data and materials
Data collected in the EMIF-AD 90+ Study will be available through the EMIF-AD portal.
Abbreviations
- ACE-R
Addenbrooke’s Cognitive Examination Revised
- AD
Alzheimer’s disease
- ADL
Activities of daily living
- Amsterdam UMC
Amsterdam University Medical Centers
- APOE
Apolipoprotein E
- Aβ
Amyloid β
- BIA
Bioelectrical Impedance Analysis
- BIOMARKAPD
Biomarkers for Alzheimer’s disease and Parkinson’s disease
- BMI
Body Mass Index
- CANTAB
Cambridge Neuropsychological Test Automated battery
- CCI
Cognitive Complaints Index
- CDR
Clinical Dementia Rating
- CERAD
Consortium to Establish a Registry for Alzheimer’s Disease
- COGA
Center Of Geriatric medicine Amsterdam
- CSF
cerebrospinal fluid
- DIN test
digits-in-noise test
- DSST
Digit Symbol Substitution Test
- DTI
diffusion tensor imaging
- EMIF-AD
European Medical Information Framework for AD
- ETDRS
Early Treatment Diabetic Retinopathy Study
- FLAIR
fluid attenuated inversion recovery
- GDS
Geriatric Depression Scale
- GE
General Electric
- GNT
Graded Naming Test
- HRRT
High Resolution Research Tomograph
- iADL
instrumental Activities of Daily Living
- IMI
Innovative Medicine Initiative
- IOP
Intra-Ocular Pressure
- MEG
magnetoencephalography
- MMSE
Mini-Mental State Examination
- MNA
Mini Nutritional Assessment
- MNAS
Manchester and Newcastle Aging Study
- MSQ
Mayo Sleep Questionnaire
- N/A
not applicable
- NART
National Adult Reading Test
- NPI-Q
Neuropsychiatric Inventory Questionnaire
- OCT
Optical Coherence Tomography
- PASE
Physical Activity Scale for the Elderly
- PBMCs
Peripheral Blood Mononuclear Cells
- PET
positron emission tomography
- PLAD
Project of Longevity and Aging in Dujangyan
- pRNFL
peripapillary Retinal Nerve Fiber Layer
- RAVLT
Rey Auditory Verbal Learning Test
- RCFT
Rey Complex Figure Test
- REM
Rapid Eye Movement
- rs-fMRI
resting state functional MRI
- RVPs
Retinal Vascular Parameters
- SD-OCT
Spectral Domain Optical Coherence Tomography
- SF-12 HRQoL
Short form 12 Health-related Quality of Life
- SIVA
Singapore I vessel Assessment
- SNP
Single Nucleotide Polymorphisms
- SOP
Standard Operating Procedure
- SPPB
Short Physical Performance Battery
- SRT
speech reception threshold
- SRTM2
Simplified Reference Tissue Model 2
- SUVr
standardized uptake value ratio
- SWI
susceptibility weighted imaging
- TMT
Trail Making Test
- VAT
Visual Association Test
- WAIS
Wechsler Adult Intelligence Scale
- WMH
white matter hyperintensities
Ethics approval and consent to participate
The Medical Ethics Review Committee of the Amsterdam UMC approved the study in Amsterdam (reference number: 2015.374) and the National Research Ethics Service Committee North West - Greater Manchester South performed approval of the study in Manchester (reference number: 14/NW/0011). All subjects gave their written informed consent in accordance with the ethical conduct and juridical laws of the Declaration of Helsinki 64th WMA General Assembly, Fortaleza, Brazil, October 2013, (www.wma.net), and in accordance with the Medical Research Involving Human Subjects Act (WMO).
Consent for publication
Not applicable.
Competing interests
PJV is a Section Editor for BMC Geriatrics. None of the other authors reports any conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nienke Legdeur, Phone: +31 (0) 20 444 8023, Email: n.legdeur@vumc.nl.
Maryam Badissi, Email: m.badissi@vumc.nl.
Stephen F. Carter, Email: Stephen.F.Carter@manchester.ac.uk
Sophie de Crom, Email: sophie_decrom@hotmail.com.
Aleid van de Kreeke, Email: ja.vandekreeke@vumc.nl.
Ralph Vreeswijk, Email: r.vreeswijk@spaarnegasthuis.nl.
Marijke C. Trappenburg, Email: m.trappenburg@vumc.nl
Mardien L. Oudega, Email: M.Oudega@ggzingeest.nl
Huiberdina L. Koek, Email: H.L.Koek@umcutrecht.nl
Jos P. van Campen, Email: Jos.vanCampen@slz.nl, Email: Jos.vanCampen@slz.nl
Carolina J. P. W. Keijsers, Email: k.keijsers@jbz.nl
Chinenye Amadi, Email: chinenye.amadi@manchester.ac.uk.
Rainer Hinz, Email: Rainer.Hinz@manchester.ac.uk.
Mark F. Gordon, Email: mfgordonmd@optonline.net
Gerald Novak, Email: GNovak1@its.jnj.com.
Jana Podhorna, Email: jana.podhorna@boehringer-ingelheim.com.
Erik Serné, Email: E.Serne@vumc.nl.
Frank Verbraak, Email: f.verbraak@vumc.nl.
Maqsood Yaqub, Email: Maqsood.Yaqub@vumc.nl.
Arjan Hillebrand, Email: a.hillebrand@vumc.nl.
Alessandra Griffa, Email: alessandra.griffa@gmail.com.
Neil Pendleton, Email: neil.pendleton@manchester.ac.uk.
Sophia E. Kramer, Email: SE.Kramer@vumc.nl
Charlotte E. Teunissen, Email: c.teunissen@vumc.nl
Adriaan Lammertsma, Email: aa.lammertsma@vumc.nl.
Frederik Barkhof, Email: f.barkhof@vumc.nl.
Bart N. M. van Berckel, Email: B.Berckel@vumc.nl
Philip Scheltens, Email: p.scheltens@vumc.nl.
Majon Muller, Email: majon.muller@vumc.nl.
Andrea B. Maier, Email: Andrea.Maier@mh.org.au
Karl Herholz, Email: Karl.Herholz@manchester.ac.uk.
Pieter Jelle Visser, Email: PJ.Visser@vumc.nl.
References
- 1.Bullain SS, Corrada MM. Dementia in the oldest old. Continuum (Minneap Minn) 2013;19:457–469. doi: 10.1212/01.CON.0000429172.27815.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivoirard R, Chargari C, Trone J-C, Falk AT, Guy J-B, Eddekaoui H, et al. General management of nonagenarian patients: a review of the literature. Swiss Med Wkly. 2014;144:w14059. doi: 10.4414/smw.2014.14059. [DOI] [PubMed] [Google Scholar]
- 3.United Nations, Department of Economic and Social Affairs, Population Division, Population Estimates and Projections Section. World Population Prospects, the 2012 Revision. Population by Age Groups- Both Sexes. Medium-fertility variant, 2010–2100. n.d. http://esa.un.org/wpp/ExcelData/population.htm (accessed May 11, 2015).
- 4.Corrada M, Brookmeyer R, Berlau D, Paganini-Holl A, Kawas C. Prevalence of dementia after age 90 results from the 90+ study. Neurology. 2008;71:337–344. doi: 10.1212/01.wnl.0000310773.65918.cd. [DOI] [PubMed] [Google Scholar]
- 5.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen SH, Andersen-Ranberg K, Thinggaard M, Jeune B, Skytthe A, Christiansen L, et al. Cohort profile: the 1895, 1905, 1910 and 1915 Danish birth cohort studies - secular trends in the health and functioning of the very old. Int J Epidemiol. 2017;46(6):1746. doi: 10.1093/ije/dyx053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corrada MM, Berlau DJ, Kawas CH. A population-based clinicopathological study in the oldest-old: the 90+ study. Curr Alzheimer Res. 2012;9:709–717. doi: 10.2174/156720512801322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Formiga F, Ferrer A, Chivite D, Rubio-Rivas M, Cuerpo S, Pujol R. Predictors of long-term survival in nonagenarians: the NonaSantfeliu study. Age Ageing. 2011;40:111–116. doi: 10.1093/ageing/afq127. [DOI] [PubMed] [Google Scholar]
- 9.van Exel E, Gussekloo J, Houx P, de Craen AJM, Macfarlane PW, der Wiel AB, et al. Atherosclerosis and cognitive impairment are linked in the elderly. The Leiden 85-plus study. Atherosclerosis. 2002;165:353–359. doi: 10.1016/S0021-9150(02)00253-8. [DOI] [PubMed] [Google Scholar]
- 10.Collerton J, Barrass K, Bond J, Eccles M, Jagger C, James O, et al. The Newcastle 85+ study: biological, clinical and psychosocial factors associated with healthy ageing: study protocol. BMC Geriatr. 2007;7:14. doi: 10.1186/1471-2318-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, et al. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology. 2010;75:1195–1202. doi: 10.1212/WNL.0b013e3181f4d7f8. [DOI] [PubMed] [Google Scholar]
- 12.Skoog I, Nilsson L, Palmertz B, Andreasson L-A, Svanborg A. A population-based study of dementia in 85-years-olds. N Engl J Med. 1993;328:153–158. doi: 10.1056/NEJM199301213280301. [DOI] [PubMed] [Google Scholar]
- 13.Formiga F, Ferrer A, Megido MJ, Chivite D, Badia T, Pujol R. Low Co-Morbidity, Low Levels of Malnutrition, and Low Risk of Falls in a Community-Dwelling Sample of 85-Year-Olds Are Associated with Successful Aging: The Octabaix Study. Rejuvenation Res. 2011;14:309–314. doi: 10.1089/rej.2010.1131. [DOI] [PubMed] [Google Scholar]
- 14.Bergdahl E, Gustavsson JMC, Kallin K, Wågert PVH, Lundman B, Bucht G, et al. Depression among the oldest old: the Umea 85+ study. Int Psychogeriatrics. 2005;17:557–575. doi: 10.1017/S1041610205002267. [DOI] [PubMed] [Google Scholar]
- 15.Huang C-Q, Dong B-R, Wu H-M, Zhang Y-L, Wu J-H, Lu Z-C, et al. Association of cognitive impairment with serum lipid/lipoprotein among Chinese nonagenarians and centenarians. Dement Geriatr Cogn Disord. 2009;27:111–116. doi: 10.1159/000194660. [DOI] [PubMed] [Google Scholar]
- 16.Paganini-Hill A, Kawas CH, Corrada MM. Lifestyle factors and dementia in the oldest-old: the 90+ study. Alzheimer Dis Assoc Disord. 2016;30:21–26. doi: 10.1097/WAD.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rastas S, Pirttila T, Mattila K, Verkkoniemi A, Juva K, Niinisto L, et al. Vascular risk factors and dementia in the general population aged >85 years: prospective population-based study. Neurobiol Aging. 2010;31:1–7. doi: 10.1016/j.neurobiolaging.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 18.Skoog I, Börjesson-Hanson A, Kern S, Johansson L, Falk H, Sigström R, et al. Decreasing prevalence of dementia in 85-year olds examined 22 years apart: the influence of education and stroke. Sci Rep. 2017;7:1–8. doi: 10.1038/s41598-017-05022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison SL, Stephan BCM, Siervo M, Granic A, Davies K, Wesnes KA, et al. Is there an association between metabolic syndrome and cognitive function in very old adults? The Newcastle 85+ study. J Am Geriatr Soc. 2015;63:667–675. doi: 10.1111/jgs.13358. [DOI] [PubMed] [Google Scholar]
- 20.van Exel E, de Craen AJ, Gussekloo J, Houx P, Bootsma-van der Wiel A, Macfarlane PW, et al. Association between high-density lipoprotein and cognitive impairment in the oldest old. Ann Neurol. 2002;51:716–721. doi: 10.1002/ana.10220. [DOI] [PubMed] [Google Scholar]
- 21.Formiga F, Ferrer A, Chivite D, Pinto X, Cuerpo S, Pujol R. Serum high-density lipoprotein cholesterol levels, their relationship with baseline functional and cognitive status, and their utility in predicting mortality in nonagenarians. Geriatr Gerontol Int. 2011;11:358–364. doi: 10.1111/j.1447-0594.2010.00681.x. [DOI] [PubMed] [Google Scholar]
- 22.Formiga F, Ferrer A, Chivite D, Pinto X, Badia T, Padros G, et al. Serum high-density lipoprotein cholesterol levels correlate well with functional but not with cognitive status in 85-year-old subjects. J Nutr Health Aging. 2012;16:449–453. doi: 10.1007/s12603-012-0018-z. [DOI] [PubMed] [Google Scholar]
- 23.Sabayan B, Oleksik AM, Maier AB, van Buchem MA, Poortvliet RK, de Ruijter W, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus study. J Am Geriatr Soc. 2012;60:2014–2019. doi: 10.1111/j.1532-5415.2012.04203.x. [DOI] [PubMed] [Google Scholar]
- 24.Corrada MM, Hayden KM, Paganini-Hill A, Bullain SS, DeMoss J, Aguirre C, et al. Age of onset of hypertension and risk of dementia in the oldest-old: the 90+ study. Alzheimers Dement. 2017;10:1–8. doi: 10.1016/j.jalz.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molander L, Gustafson Y, Lovheim H. Longitudinal associations between blood pressure and dementia in the very old. Dement Geriatr Cogn Disord. 2010;30:269–276. doi: 10.1159/000320252. [DOI] [PubMed] [Google Scholar]
- 26.Molander L, Gustafson Y, Lövheim H. Low blood pressure is associated with cognitive impairment in very old people. Dement Geriatr Cogn Disord. 2010;29:335–341. doi: 10.1159/000289821. [DOI] [PubMed] [Google Scholar]
- 27.Huang C-Q, Dong B-R, Zhang Y-L, Wu H-M, Liu Q-X, Flaherty JH. Cognitive impairment and hypertension among Chinese nonagenarians and centenarians. Hypertens Res. 2009;32:554–558. doi: 10.1038/hr.2009.72. [DOI] [PubMed] [Google Scholar]
- 28.Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and dementia - a comprehensive review. Ther Adv Neurol Disord. 2009;2:241–260. doi: 10.1177/1756285609103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.Wnl.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 30.van den Berg E, de Craen AJ, Biessels GJ, Gussekloo J, Westendorp RG. The impact of diabetes mellitus on cognitive decline in the oldest of the old: a prospective population-based study. Diabetologia. 2006;49:2015–2023. doi: 10.1007/s00125-006-0333-1. [DOI] [PubMed] [Google Scholar]
- 31.Formiga F, Ferrer A, Padros G, Corbella X, Cos L, Sinclair AJ, et al. Diabetes mellitus as a risk factor for functional and cognitive decline in very old people: the Octabaix study. J Am Med Dir Assoc. 2014;15:924–928. doi: 10.1016/j.jamda.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Rastas S, Verkkoniemi A, Polvikoski T, Juva K, Niinisto L, Mattila K, et al. Atrial fibrillation, stroke, and cognition: a longitudinal population-based study of people aged 85 and older. Stroke. 2007;38:1454–1460. doi: 10.1161/STROKEAHA.106.477299. [DOI] [PubMed] [Google Scholar]
- 33.Diniz BS, Butters MA, Albert SM, Dew MA. Reynolds 3rd CF. late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stek ML, Gussekloo J, Beekman AT, Van Tilburg W, RGJ W. Prevalence, correlates and recognition of depression in the oldest old: the Leiden 85-plus study. J Affect Disord. 2004;78:193–200. doi: 10.1016/S0165-0327(02)00310-5. [DOI] [PubMed] [Google Scholar]
- 35.Jirong Y, Changquan H, Hongmei W, Bi-Rong D. Association of sleep quality and dementia among long-lived Chinese older adults. Age (Omaha) 2013;35:1423–1432. doi: 10.1007/s11357-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gildner TE, Liebert MA, Kowal P, Chatterji S, Snodgrass JJ. Associations between Sleep Duration , Sleep Quality , and Cognitive Test Performance among Older Adults from Six Middle Income Countries : Results from the Study on Global. J Clin Sleep Med. 2014;10:613–621. doi: 10.5664/jcsm.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gussekloo J, de Craen AJM, Oduber C, van Boxtel MPJ, Westendorp RGJ. Sensory impairment and cognitive functioning in oldest-old subjects: the Leiden 85+ study. Am J Geriatr Psychiatry. 2005;13:781–786. doi: 10.1097/00019442-200509000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Jefferis JM, Collerton J, Taylor JP, Jagger C, Kingston A, Davies K, et al. The impact of visual impairment on mini-mental state examination scores in the Newcastle 85+ study. Age Ageing. 2012;41:565–568. doi: 10.1093/ageing/afs042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts RO, Christianson TJH, Kremers WK, Mielke MM, Machulda MM, Vassilaki M, et al. Association between olfactory dysfunction andAmnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol. 2016;73:481. doi: 10.1001/jamaneurol.2015.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taekema DG, Gussekloo J, Maier AB, Westendorp RG, de Craen AJ. Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Ageing. 2010;39:331–337. doi: 10.1093/ageing/afq022. [DOI] [PubMed] [Google Scholar]
- 41.Bullain SS, Corrada MM, Perry SM, Kawas CH. Sound body sound mind? Physical performance and the risk of dementia in the oldest-old: the 90+ study. J Am Geriatr Soc. 2016;64:1408–1415. doi: 10.1111/jgs.14224. [DOI] [PubMed] [Google Scholar]
- 42.Bathum L, Christiansen L, Jeune B, Vaupel J, McGue M, Christensen K. Apolipoprotein E genotypes: relationship to cognitive functioning, cognitive decline, and survival in nonagenarians. J Am Geriatr Soc. 2006;54:654–658. doi: 10.1111/j.1532-5415.2005.53554.x. [DOI] [PubMed] [Google Scholar]
- 43.Heijmans BT, Slagboom PE, Gussekloo J, Droog S, Lagaay AM, Kluft C, et al. Association of APOE epsilon2/epsilon3/epsilon4 and promoter gene variants with dementia but not cardiovascular mortality in old age. Am J Med Genet. 2002;107:201–208. doi: 10.1002/ajmg.10142. [DOI] [PubMed] [Google Scholar]
- 44.Corrada MM, Paganini-Hill A, Berlau DJ, Kawas CH. APOE genotype, dementia and mortality in the oldest-old: the 90+ study. Alzheimers Dement. 2013;9:12–18. doi: 10.1016/j.jalz.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Juva K, Verkkoniemi A, Viramo P, Polvikoski T, Kainulainen K, Kontula K, et al. Apolipoprotein E, cognitive function, and dementia in a general population aged 85 years and over. Int Psychogeriatrics. 2000;72:379–387. doi: 10.1017/S1041610200006487. [DOI] [PubMed] [Google Scholar]
- 46.Juva K, Verkkoniemi A, Viramo P, Polvikoski T, Kainulainen K, Kontula K, et al. APOE epsilon4 does not predict mortality, cognitive decline, or dementia in the oldest old. Neurology. 2000;54:412–415. doi: 10.1212/WNL.54.2.412. [DOI] [PubMed] [Google Scholar]
- 47.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waaijer Mariëtte E.C., Parish William E., Strongitharm Barbara H., van Heemst Diana, Slagboom Pieternella E., de Craen Anton J.M., Sedivy John M., Westendorp Rudi G.J., Gunn David A., Maier Andrea B. The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell. 2012;11(4):722–725. doi: 10.1111/j.1474-9726.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schram MT, Euser SM, de Craen AJM, Witteman JC, Frolich M, Hofman A, et al. Systemic markers of inflammation and cognitive decline in old age. J Am Geriatr Soc. 2007;55:708–716. doi: 10.1111/j.1532-5415.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- 50.Kravitz BA, Corrada MM, Kawas CH. Elevated C-reactive protein levels are associated with prevalent dementia in the oldest-old. Alzheimers Dement. 2009;5:318–323. doi: 10.1016/j.jalz.2009.04.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kravitz BA, Corrada MM, Kawas CH. High levels of serum C-reactive protein are associated with greater risk of all-cause mortality, but not dementia, in the oldest-old: results from the 90+ study. J Am Geriatr Soc. 2009;57:641–646. doi: 10.1111/j.1532-5415.2009.02169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin-Ruiz CM, Gussekloo J, van Heemst D, von Zglinicki T, Westendorp RGJ. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study. Aging Cell. 2005;4:287–290. doi: 10.1111/j.1474-9726.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 53.Jansen WJ, Ossenkoppele R, Knol D, Al E. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ossenkoppele R, Jansen WJ, Rabinovici GD, Knol DL, van der Flier WM, van Berckel BN, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313:1939–1949. doi: 10.1001/jama.2015.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jansen WJ, Ossenkoppele R, Tijms BM, Fagan AM, Hansson O, Klunk WE, et al. Association of cerebral amyloid-β aggregation with cognitive functioning in persons without dementia. JAMA Psychiatry. 2018;75:84–95. doi: 10.1001/jamapsychiatry.2017.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skoog I, Davidsson P, Aevarsson O, Vanderstichele H, Vanmechelen E, Blennow K. Cerebrospinal fluid Beta-amyloid 42 is reduced before the onset of sporadic dementia: a population-based study in 85-year-olds. Dement Geriatr Cogn Disord. 2003;15:169–176. doi: 10.1159/000068478. [DOI] [PubMed] [Google Scholar]
- 57.Kawas CH, Greenia DE, Bullain SS, Clark CM, Pontecorvo MJ, Joshi AD, et al. Amyloid imaging and cognitive decline in nondemented oldest-old: the 90+ study. Alzheimers Dement. 2013;9:199–203. doi: 10.1016/j.jalz.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skoog I, Kern S, Zetterberg H, Östling S, Börjesson-Hanson A, Guo X, et al. Low cerebrospinal fluid Aβ42 and Aβ40 are related to white matter lesions in cognitively Normal elderly. J Alzheimers Dis. 2018;62:1877–1886. doi: 10.3233/JAD-170950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skoog I, Palmertz B, Andreasson LA. The prevalence of white-matter lesions on computed tomography of the brain in demented and nondemented 85-year-olds. J Geriatr Psychiatry Neurol. 1994;7:169–175. doi: 10.1177/089198879400700308. [DOI] [PubMed] [Google Scholar]
- 60.Bennett IJ, Greenia DE, Maillard P, Sajjadi SA, DeCarli C, Corrada MM, et al. Age-related white matter integrity differences in oldest-old without dementia. Neurobiol Aging. 2017;56:108–114. doi: 10.1016/j.neurobiolaging.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engels MMA, van der Flier WM, Stam CJ, Hillebrand A, Scheltens P, van Straaten ECW. Alzheimer’s disease: the state of the art in resting-state magnetoencephalography. Clin Neurophysiol. 2017;128:1426–1437. doi: 10.1016/j.clinph.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 62.Morris J. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/WNL.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 63.Folstein M, Robins L, Helzer J. The mini-mental state examination. Arch Gen Psychiatry. 1983;40:812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 64.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 65.McKhann G, Drachman D, Folstein M, Katzman R. Clinical diagnosis of Alzheimer’s disease: report of the MINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Forse on Alzheimer’s disease. Neurology. 1984;34:939. doi: 10.3233/JAD-122299. [DOI] [PubMed] [Google Scholar]
- 66.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 67.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of Adl: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 68.Jakobsson U. Using the 12-item short form health survey (SF-12) to measure quality of life among older people. Aging Clin Exp Res. 2007;19:457–464. doi: 10.1007/BF03324731. [DOI] [PubMed] [Google Scholar]
- 69.Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vellas B, Villars H, Abellan G, Soto ME, Rolland Y, Guigoz Y, et al. Overview of the MNA – It’s history and challenges. J Nutr. 2006;10:456–465. [PubMed] [Google Scholar]
- 71.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 72.Landau SM, Marks SM, Mormino EC, Rabinovici GD, Oh H, O’Neil JP, et al. Association of Lifetime Cognitive Engagement and low β-amyloid deposition. Arch Neurol. 2012;69:623–629. doi: 10.1001/archneurol.2011.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E, et al. The AD8: a brief informant interview to detect dementia. Neurol. 2005;65:559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 74.Sikkes SA, Knol DL, Pijnenburg YA, de Lange-de Klerk ES, Uitdehaag BM, Scheltens P. Validation of the Amsterdam IADL questionnaire©, a new tool to measure instrumental activities of daily living in dementia. Neuroepidemiology. 2013;41:35–41. doi: 10.1159/000346277. [DOI] [PubMed] [Google Scholar]
- 75.Jutten RJ, Peeters CFW, Leijdesdorff SMJ, Visser PJ, Maier AB, Terwee CB, et al. Detecting functional decline from normal aging to dementia: development and validation of a short version of the Amsterdam IADL questionnaire. Alzheimer’s Dement Diagnosis, Assess Dis Monit. 2017;8:26–35. doi: 10.1016/j.dadm.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 77.Boeve BF, Molano JR, Ferman TJ, Smith GE, Lin S-C, Bieniek K, et al. Validation of the Mayo sleep questionnaire to screen for REM sleep behavior disorder in an aging and dementia cohort. Sleep Med. 2011;12:445–453. doi: 10.1016/j.sleep.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 79.Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. Journals Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 80.Reijnierse EM, de Jong N, Trappenburg MC, Blauw GJ, Butler-Browne G, Gapeyeva H, et al. Assessment of maximal handgrip strength: how many attempts are needed? J Cachexia Sarcopenia Muscle. 2017;8:466–474. doi: 10.1002/jcsm.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tieleman RG, Plantinga Y, Rinkes D, Bartels GL, Posma JL, Cator R, et al. Validation and clinical use of a novel diagnostic device for screening of atrial fibrillation. Europace. 2014;16:1291–1295. doi: 10.1093/europace/euu057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–562. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al-Qaisi M, Nott DM, King DH, Kaddoura S. Ankle brachial pressure index (ABPI): an update for practitioners. Vasc Health Risk Manag. 2009;5:833–841. doi: 10.2147/VHRM.S6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mayer MA, Hornegger J, Mardin CY, Tornow RP. Retinal nerve fiber layer segmentation on FD-OCT scans of normal subjects and glaucoma patients. Biomed Opt Express. 2010;1:1358–1383. doi: 10.1364/BOE.1.001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheung CY, Hsu W, Lee ML, Wang JJ, Mitchell P, Lau QP, et al. A new method to measure peripheral retinal vascular caliber over an extended area. Microcirculation. 2010;17:495–503. doi: 10.1111/j.1549-8719.2010.00048.x. [DOI] [PubMed] [Google Scholar]
- 86.Smits C, Theo Goverts S, Festen JM. The digits-in-noise test: assessing auditory speech recognition abilities in noise. J Acoust Soc Am. 2013;133:1693–1706. doi: 10.1121/1.4789933. [DOI] [PubMed] [Google Scholar]
- 87.Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. “Sniffin” sticks’. Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22:39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- 88.Reijs BL, Teunissen CE, Goncharenko N, Betsou F, Blennow K, Baldeiras I, et al. The central biobank and virtual biobank of BIOMARKAPD: a resource for studies on neurodegenerative diseases. Front Neurol. 2015;6:216. doi: 10.3389/fneur.2015.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lambert J-C, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 91.Lambert J-C, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shulman JM, Chen K, Keenan BT, Chibnik LB, Fleisher A, Thiyyagura P, et al. Genetic susceptibility for Alzheimer’s disease Neuritic plaque pathology. JAMA Neurol. 2013;70:1150–1157. doi: 10.1001/jamaneurol.2013.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cardenas VA, Reed B, Chao LL, Chui H, Sanossian N, Decarli CC, et al. Associations among vascular risk factors, carotid atherosclerosis, and cortical volume and thickness in older adults. Stroke. 2012;43:2865–2870. doi: 10.1161/STROKEAHA.112.659722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Sloten TT, Schram MT, Van Den Hurk K, Dekker JM, Nijpels G, Henry RMA, et al. Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all-cause mortality: the Hoorn study. J Am Coll Cardiol. 2014;63:1739–1747. doi: 10.1016/j.jacc.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 95.Moneta GL, Edwards JM, Chitwood RW, Taylor LM, Lee RW, Cummings CA, et al. Correlation of north American symptomatic carotid endarterectomy trial (NASCET) angiographic definition of 70 to 99% internal carotid artery stenosis with duplex scanning. J Vasc Surg. 1993;17:152–159. doi: 10.1016/0741-5214(93)90019-I. [DOI] [PubMed] [Google Scholar]
- 96.Wierenga CE, Hays CC, Zlatar ZZ. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. J Alzheimers Dis. 2014;42(Suppl 4):S411–S419. doi: 10.3233/JAD-141467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 98.Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- 99.Wu Y, Carson RE. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab. 2002;22:1440–1452. doi: 10.1097/01.WCB.0000033967.83623.34. [DOI] [PubMed] [Google Scholar]
- 100.Vandenberghe R, Van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E, et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol. 2010;68:319–329. doi: 10.1002/ana.22068. [DOI] [PubMed] [Google Scholar]
- 101.Hillebrand A, Barnes GR, Bosboom JL, Berendse HW, Stam CJ. Frequency-dependent functional connectivity within resting-state networks: an atlas-based MEG beamformer solution. Neuroimage. 2012;59:3909–3921. doi: 10.1016/j.neuroimage.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fernández A, Hornero R, Mayo A, Poza J, Gil-Gregorio P, Ortiz T. MEG spectral profile in Alzheimer’s disease and mild cognitive impairment. Clin Neurophysiol. 2006;117:306–314. doi: 10.1016/j.clinph.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 103.de Haan W, van der Flier WM, Koene T, Smits LL, Scheltens P, Stam CJ. Disrupted modular brain dynamics reflect cognitive dysfunction in Alzheimer’s disease. Neuroimage. 2012;59:3085–3093. doi: 10.1016/j.neuroimage.2011.11.055. [DOI] [PubMed] [Google Scholar]
- 104.Stam CJ. Use of magnetoencephalography (MEG) to study functional brain networks in neurodegenerative disorders. J Neurol Sci. 2010;289:128–134. doi: 10.1016/j.jns.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 105.Jefferis JM, Taylor JP, Collerton J, Jagger C, Kingston A, Davies K, et al. The association between diagnosed glaucoma and cataract and cognitive performance in very old people: cross-sectional findings from the Newcastle 85+ study. Ophthalmic Epidemiol. 2013;20:82–88. doi: 10.3109/09286586.2012.757626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dato S, Soerensen M, Lagani V, Montesanto A, Passarino G, Christensen K, et al. Contribution of genetic polymorphisms on functional status at very old age: a gene-based analysis of 38 genes (311 SNPs) in the oxidative stress pathway. Exp Gerontol. 2014;52:23–29. doi: 10.1016/j.exger.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Soerensen M, Christensen K, Stevnsner T, Christiansen L. The Mn-superoxide dismutase single nucleotide polymorphism rs4880 and the glutathione peroxidase 1 single nucleotide polymorphism rs1050450 are associated with aging and longevity in the oldest old. Mech Ageing Dev. 2009;130:308–314. doi: 10.1016/j.mad.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mengel-From J, Christensen K, McGue M, Christiansen L. Genetic variations in the CLU and PICALM genes are associated with cognitive function in the oldest old. Neurobiol Aging. 2011;32:554 e7–554 11. doi: 10.1016/j.neurobiolaging.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dato S, Krabbe KS, Thinggaard M, Pedersen BK, Christensen K, Bruunsgaard H, et al. Commonly studied polymorphisms in inflammatory cytokine genes show only minor effects on mortality and related risk factors in nonagenarians. J Gerontol A Biol Sci Med Sci. 2010;65:225–235. doi: 10.1093/gerona/glp210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mengel-From J, Thinggaard M, Lindahl-Jacobsen R, McGue M, Christensen K, Christiansen L. CLU genetic variants and cognitive decline among elderly and oldest old. PLoS One. 2013;8:e79105. doi: 10.1371/journal.pone.0079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bathum L, von Bornemann HJ, Christiansen L, McGue M, Jeune B, Christensen K. Methylenetetrahydrofolate reductase 677C>T and methionine synthase 2756A>G mutations: no impact on survival, cognitive functioning, or cognitive decline in nonagenarians. J Gerontol A Biol Sci Med Sci. 2007;62:196–201. doi: 10.1093/gerona/62.2.196. [DOI] [PubMed] [Google Scholar]
- 112.Hao Q, Ding X, Gao L, Yang M, Dong B. G-395A polymorphism in the promoter region of the KLOTHO gene associates with reduced cognitive impairment among the oldest old. Age (Omaha) 2016;38:1–8. doi: 10.1007/s11357-015-9869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ji-Rong Y, Bi-Rong D, Chang-Quan H, Zhen-Chan L, Hong-Mei W, Yan-Ling Z. Pro12Ala polymorphism in PPAR-γ2 and dementia in Chinese nonagenarians/centenarians. Age (Omaha) 2010;32:397–404. doi: 10.1007/s11357-010-9132-1. [DOI] [Google Scholar]
- 114.Myllykangas L, Polvikoski T, Sulkava R, Verkkoniemi A, Tienari P, Niinisto L, et al. Cardiovascular risk factors and Alzheimer’s disease: a genetic association study in a population aged 85 or over. Neurosci Lett. 2000;292:195–198. doi: 10.1016/S0304-3940(00)01467-1. [DOI] [PubMed] [Google Scholar]
- 115.Morris J, Heyman A, Mohs R, Hughes J, van Belle G, Fillenbaum G, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD) Part I Clinical and neuropsychological assesment of Alzheimer’s disease. Neurology. 1989;39:1159. doi: 10.1212/WNL.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 116.Abikoff H, Alvir J, Hong G, Sukoff R, Orazio J, Solomon S, et al. Logical memory subtest of the Wechsler memory scale: age and education norms and alternate-form reliability of two scoring systems. J Clin Exp Neuropsychol. 1987;9:435–448. doi: 10.1080/01688638708405063. [DOI] [PubMed] [Google Scholar]
- 117.Rey A. L’examen clinique en psychologie (the clinical examination in psychology) Paris: Presses Universitaires de France; 1964. [Google Scholar]
- 118.Meyers JE, Bayless JD, Meyers KR. Rey complex figure: memory error patterns and functional abilities. Appl Neuropsychol. 1996;3:89–92. doi: 10.1207/s15324826an0302. [DOI] [PubMed] [Google Scholar]
- 119.Cronholm B, Viding G. Digit span as a test of immediate memory. Nord Med. 1956;56:1612–1614. [PubMed] [Google Scholar]
- 120.Wechsler D. The psychological corporation. TX: San Antonia; 1997. [Google Scholar]
- 121.Tombaugh T. Normative data stratified by age and education for two measures of verbal fluency FAS and animal naming. Arch Clin Neuropsychol. 1999;14:167–177. [PubMed] [Google Scholar]
- 122.Royall DR, Cordes JA, Polk M. CLOX: an executive clock drawing task. J Neurol Neurosurg Psychiatry. 1998;64:588–594. doi: 10.1136/jnnp.64.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McKenna PAT, Warrington EK. Testing for nominal dysphasia. J Neurol Neurosurg Psychiatry. 1980;43:781–788. doi: 10.1136/jnnp.43.9.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. doi: 10.2466/pms.1958.8.3.271. [DOI] [Google Scholar]
- 125.Wechsler D. Wechsler adult intelligence scale - revised manual. 1981. [Google Scholar]
- 126.Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge neuropsychological test automated battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- 127.Nelson HE, O’Connell A. Dementia: the estimation of premorbid intelligence levels using the new adult Reading test. Cortex. 1978;14:234–244. doi: 10.1016/S0010-9452(78)80049-5. [DOI] [PubMed] [Google Scholar]
- 128.Lindeboom J, Schmand B, Tulner L, Walstra G, Jonker C. Visual association test to detect early dementia of the Alzheimer type. J Neurol Neurosurg Psychiatry. 2002;73:126–133. doi: 10.1136/jnnp.73.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke’s cognitive examination revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry. 2006;21:1078–1085. doi: 10.1002/gps.1610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Brain MRI scan analyses in the EMIF-AD 90+ Study. (DOCX 26 kb)
Data Availability Statement
Data collected in the EMIF-AD 90+ Study will be available through the EMIF-AD portal.