Abstract
Substrate stiffness has been recognized as an important regulator of cell fate and function, but an understanding of the full extent of processes affected by stiffness is lacking as its transcriptome-wide effects have not been mapped. This limited understanding has restricted the contexts in which engineers can employ stiffness as an engineering design parameter. To address these limitations, we performed RNA-seq on mesenchymal stem cells (MSCs) cultured in alginate hydrogels over a range of moduli to broadly map the transcriptome-wide changes associated with stiffness sensing. We found a large number of stiffness-sensitive genes, and that many genes respond to stiffness in nonlinear ways. Informed by these differential expression results, we explored a hypothesis related to current MSC clinical activity, and found that stiffness can regulate the expression of MSC immunomodulatory markers in response to cytokine stimulation. Overall, these results reveal previously unknown features of MSC stiffness response and demonstrate the value of coupling –omics approaches with biophysical experiments.
Keywords: mesenchymal stem cells, RNA-seq, systems biology, alginate, transcriptome, immune response
For almost two decades, engineers and biologists have studied the effects of adhesion substrate properties on cell phenotype[1, 2]. Over that time, approaches to control and study these features have evolved, revealing myriad ways in which cells sense and respond to their culture substrates[3]. For instance, Tekin et al found significant transcriptome-wide changes associated with 2D versus 3D tissue culture[4]. Within 3D cell culture, material characteristics such as adhesion ligand composition and density[5–7], time-dependent material properties[5, 8], nanoscale topography[9], and degradability[10], among others, have all been shown to affect phenotype, stiffness has perhaps been the most widely explored[11–13]. In both 2D and 3D cell culture, and recently in vivo, stiffness has been shown to affect cell morphology[2], proliferation[14], lineage commitment[15], cell-cell interactions[16], and tissue regeneration[11] in experiments that span a wide variety of cell types and materials systems. Mechanistic studies have uncovered biophysical assemblies underlying stiffness sensing[17] and a number of signal transduction modes that convert substrate sensing into changes in intracellular signaling pathways and gene expression[18].
The majority of studies on the role of substrate stiffness to date have been largely phenomenological, and a relatively narrow range of gene expression changes have been reported. The lack of an unbiased and broad exploration into these gene expression changes likely limits the field’s understanding of the number and extent of processes influenced by stiffness. These limitations have implications for biomaterial design in that they both restrict what processes have been engineered through stiffness, and specific stiffness conditions could unintentionally lead to broad and potentially off-target effects. This knowledge gap also hinders the ability to predict in vivo implications for stiffness in regulating physiology.
To address these limitations, we employed a hydrogel system with tunable stiffness along with RNA-seq to understand broad changes in the mouse MSC transcriptome as a function of stiffness. Alginate hydrogels were utilized due to the ability to easily and reliably control their mechanical properties, and their history of use in mechanotransduction studies[12, 19, 20]. Cell adhesion to these hydrogels was enabled by covalently decorating the polymer with fibronectin mimicking RGD peptides. MSCs are widely used today in various basic and clinical studies, with over 600 ongoing clinical trials[21], and here, a clonally-derived mouse MSC line was used to minimize the well described effects of cell-cell heterogeneity found in primary cultures[22]. Additionally, this MSC line has been shown to be responsive to substrate stiffness in similar alginate hydrogels using alternative, functional outputs[5, 12]. We uncovered wide-ranging changes in gene expression, and found that mMSCs respond to different stiffness ranges in varied ways. After generating and subsequently testing a notable hypothesis from our bioinformatics analysis of the RNA-seq data, we found that stiffness regulates the mMSC immune response under cytokine stimulation. Overall, our results expand on phenomenological explorations of substrate stiffness by providing a view into broad transcriptional changes in mMSCs induced by stiffness.
Results
The transcriptional response to stiffness was profiled in MSCs encapsulated in alginate hydrogels. Cells were cultured in hydrogels at three stiffnesses that span a range previously shown to influence MSC fate choice (Fig. 1a)[12, 13]. MSCs maintained high viability and were distributed uniformly throughout the hydrogels (Fig. 1b-c, 2a). The zonal mechanism of alginate crosslinking has been previously demonstrated to allow for the maintenance of hydrogel nanostructure even at different levels of crosslinking, minimizing differences in porosity and diffusion through the gels[5]. After 40 hours, we performed RNA-seq in order to, in a global and unbiased way, address the early events involved in MSC stiffness sensing and to generate hypotheses about the effects of stiffness sensing. At this time point, cultures had not undergone any significant cell proliferation, as measured by cell number (Fig. 1d), and adipogenic differentiation in all conditions was insignificant (Fig. S1). In stiff gels, a slight increase in the expression of alkaline phosphatase, an early marker of osteogenesis, was noted, consistent with previous work exploring the effects of stiffness on mMSCs[12] (Fig. S1).
Figure 1:
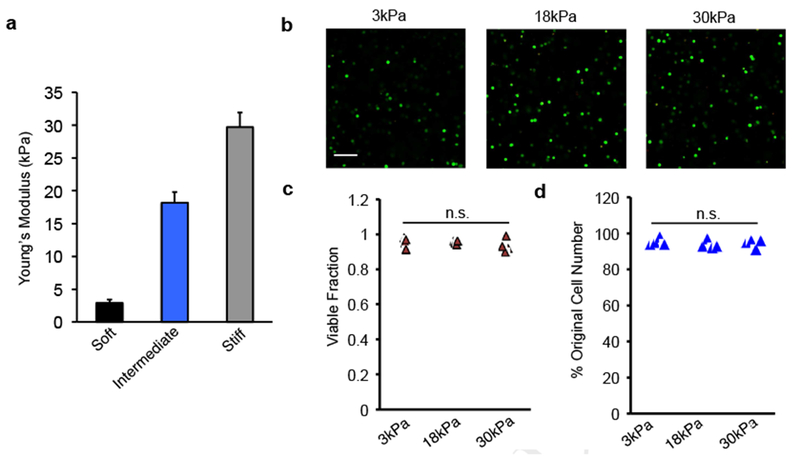
-
a)Young’s Moduli of alginate hydrogels used for mMSC culture. Mean + S.D.
-
b)Viability staining of mMSCs embedded in alginate hydrogels for the three gel moduli after 2 days in culture. Live cells stain green and dead cells stain red. Scale bar - 100μm.
-
c)Quantification of fraction of viable cells in hydrogels after 2 days in culture, computed from five representative fields of view for each gel. Triangles represent individual gels. Cells were segmented and counted using ImageJ. No statistically significant difference was found with ANOVA.
-
d)Percentage of original mMSC cell numbers after 2 days in culture in alginate hydrogels. Triangles represent individual gels. No statistically significant difference was found with ANOVA.
Figure 2:
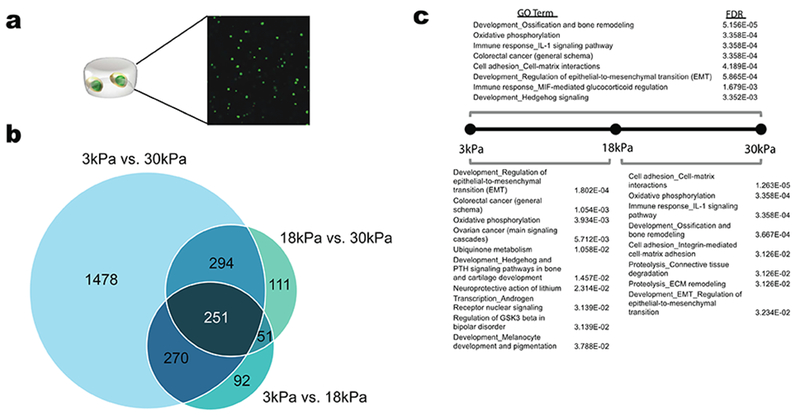
-
a)Schematic of MSCs encapsulated in alginate hydrogels with representative phalloidin stain showing actual encapsulated cell distribution.
-
b)Venn Diagram of DE genes across different stiffness comparisons. Differential expression was defined as at least a two-fold change and FDR<0.05.
-
c)Enrichment analysis of DE genes for each stiffness comparison.
Differential expression analysis revealed 1,637 differentially expressed (DE) genes between the 3kPa and 30kPa conditions, which included the majority, although not all of the differentially expressed genes from the intermediate stiffness comparisons (Fig. 2b). Enrichment analysis for processes run on the DE genes from each comparison showed significant enrichment for processes such as bone remodeling, cell-matrix adhesion, proteolysis, and IL-1 and androgen receptor signaling (Fig. 2c).
DE genes were next classified by the ranges of stiffness to which they responded and the direction of that response (Fig. 3). Binning genes by the stiffness response profiles and ranking by significance revealed a large number of genes that were up or downregulated across each stiffness range, and an even larger number of genes that were up or downregulated only across the extreme stiffness values, consistent with the Venn Diagram (Fig. 2). Intriguingly, a number of genes displayed non-monotonic profiles, either displaying a biphasic response to stiffness, or a response to only the lower or higher stiffness regime. Notable in the Up-Up profile were known mechanosensitive genes Sdc3, Wnt4, and Col1a1 along with the aforementioned Il1rl1, while the Down-Up profile featured genes known to be tangentially related to mechanics, such as Mmp9 and Fgfr3, and the epigenetic modifier Chd4. A large number of genes were only responsive to the extreme stiffness range, such as the actin binding protein Twf2 and proliferation related Triaf1, which were downregulated, and the kinase Src and the Rho-A responsive Dgkq, which were upregulated with the large increase in stiffness.
Fig. 3.
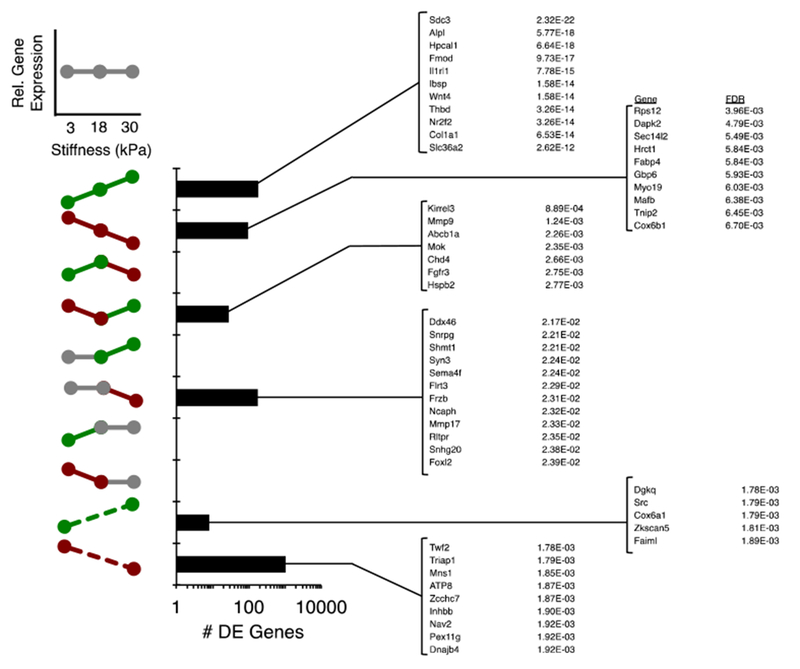
Classification of DE genes by stiffness response profile. The bar chart depicts the number of DE genes corresponding to each stiffness response profile. The most significantly DE genes as defined by FDR are listed, where the lowest FDR across any of the stiffness ranges is used for the ranking. Gray color-coding corresponds to a stiffness range with no statistically significant differential expression, while green and red color-coding correspond to positive and negative, statistically significant differential expression, respectively.
We next used the set of DE genes to infer transcription factors likely to display altered activity as a function of stiffness. After surveying the DE genes across the different stiffness pairwise comparisons and performing a transcription factor enrichment analysis on each of those, we noted the presence of the p65 subunit of NFkB, also known as RelA (Fig. 4a) in the 3kPa vs. 18kPa comparison. While the influence of substrate properties on MSC differentiation has been widely studied, an understanding of their influence on immunosuppression is lacking, but has significant relevance given the clinical use of MSC therapy for immune modulation[23]. The relevance of stiffness to immunomodulatory MSC function is additionally supported by the presence of immune-related processes enriched in our gene ontology analysis (Fig. 2c). We chose to focus on NF-kB, as it is responsive to substrate stiffness, acts as a signaling hub for immunosuppression[24, 25], and its enrichment in our inferred stiffness-responsive transcription factor list suggests that substrate stiffness could affect other NF-kB-related processes in MSCs. Stiffness was then compared by using 3kPa and 18kPa hydrogels and the MSCs were cultured for three days in media with or without the known NF-kB activators TNFa and IFNg, and with or without an NF-kB inhibitor peptide (Fig. 4b). Three days was chosen since previous studies on MSC immunostimulation had cultured cells on this timescale[26, 27]. No differences in proliferation, as indicated by total cell number, nor differentiation down the osteogenic or adipogenic pathways were noted with cytokine exposure over this time frame (Fig. S2). Analysis for activated NFkB-p65 showed an increase with stiffness and further increases with cytokine stimulation (Fig. 4c). NFkB-p65 was inhibited with the blocking peptide in all cases, although in the stiff condition with cytokines, the blockade was not complete. The downstream effects of the stiffness-induced enrichment were then explored by examining expression of MSC immunomodulatory markers IDO1, OPN, SDF-1, and COX-2. Stiffness was found to dominate IDO1 expression relative to cytokine stimulation (Fig. 4d). Cytokine stimulation upregulated COX-2 additively with stiffness, an effect that was only NFkB-p65 dependent in the soft condition (Fig. 4e). Cytokine stimulation inhibited OPN in soft conditions but stimulated it in stiff conditions; only the effects in the stiff conditions were NFkB dependent (Fig. 4f). SDF-1 was only diminished by cytokine stimulation in the stiff condition, which was also NFkB dependent (Fig. 4g).
Figure 4: Effects of substrate stiffness on MSC immunosuppressive gene products.
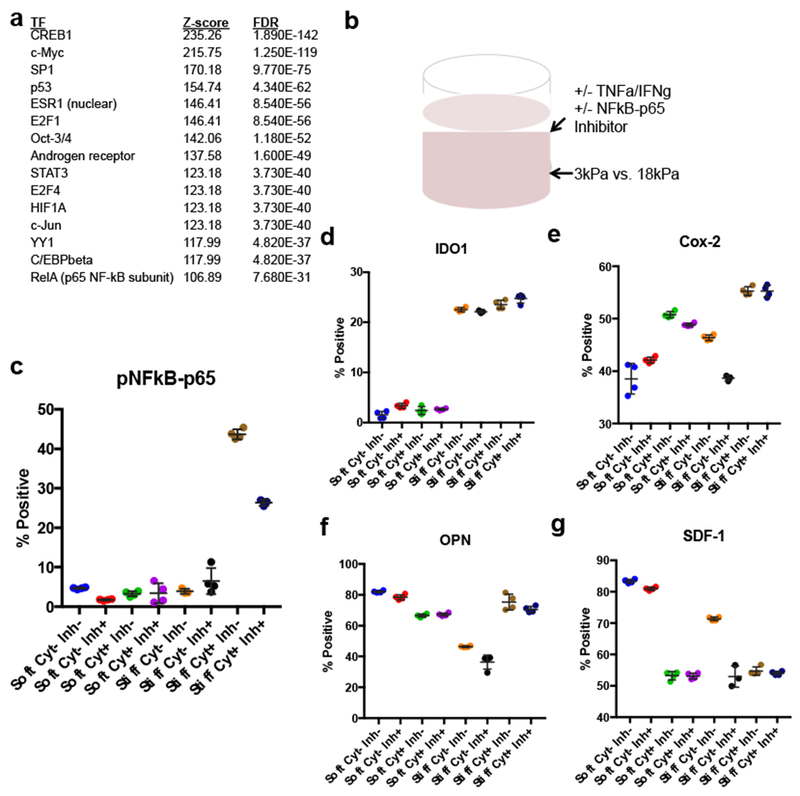
-
a)Significantly enriched inferred transcription factor hubs identified by Metacore after seeding with DE genes from RNA-seq data in 3 vs 18kPa hydrogels.
-
b)Experimental setup for MSC immunosuppression experiments. mMSCs were cultured in alginate hydrogels of 3 or 18kPa and cultured in all combinations of +/− IFNg and TNFa and +/− NFkB-p65 inhibitor.
-
c-g)Percentage of MSCs positive for pNFkB-p65 (c), IDO1 (d), Cox-2 (e), OPN (f), SDF-1 (g), respectively, after stimulation for 3 days in soft or stiff hydrogels. Cyt+ refers to culture with IFNg and TNFa, while cyt− refers to culture without these factors. Inh+ refers to culture with NFkB-p65 inhibitor and inh− refers to without NFkB-p65 inhibitor. Data represent mean ± S.D. Results are representative of at least two independent experiments. (One-way ANOVA, Tukey post-hoc test, for visual clarity given the large number of comparisons, summary statistics are given in Table S2).
Discussion
This work indicates that the transcriptome-wide response of MSCs to substrate stiffness is significant and varied. Substrate mechanical properties modulate MSC immunomodulatory behavior in response to pro-inflammatory cytokines, showing that a molecular signal can be contextualized by the material microenvironment of the cell. These results suggest a model in which persistent inputs from the adhesion substrate can bias certain network modules and transcription factor activity, setting a “material background” or boundary condition for the cell with which other cellular processes interact.
The RNA-seq analysis of the effects of substrate stiffness on MSCs encapsulated in 3-dimensional hydrogels generated a large number of differentially expressed genes. DE genes binned by their expression across the three different moduli displayed a variety of profiles. The presence of biphasic profiles is consistent with reported “optimal” moduli for MSC differentiation[12], but also suggests that similar biphasic responses exist for other processes. The response of certain genes to only low or high stiffness ranges is consistent with the quantitative nature of stiffness sensing and motivates the notion that stiffness could “switch” certain other processes on or off. Moreover, the composition of genes within these profiles was both consistent with previous phenomological studies and provided new insights. For instance, genes already associated with bone formation were upregulated with stiffness, consistent with their physiologic context; however, genes previously unassociated with substrate mechanics were found to display rich stiffness-dependent expression, such as Cox6a and Cox6b being oppositely affected by stiffness.
Examination of differentially expressed genes revealed a host of processes influenced by stiffness. The enrichment of processes such as bone remodeling[28, 29], cell-matrix adhesion[17], and proteolysis[10] are consistent with previous mechanotransduction studies; the enrichment of IL-1 and androgen receptor signaling suggests the mechanical regulation of diverse processes that have been less studied in mechanosensing. While consistent with previous studies concerning the role of substrate stiffness in regulating metabolism and the cell cycle[18], the identification of modular signaling hubs such as androgen receptor as well as promiscuous transcription factors such as E2F1 suggest that stiffness-sensing could not only be involved in crosstalk with a number of other cellular processes, but also could be an important epigenetic regulator. Stiffness-mediated epigenetic changes, such as histone status and the lamin A/lamin B ratio have been reported[30, 31], so these results suggest that the activity of broad-acting transcription factors could act as another layer of stiffness-mediated epigenetic regulation.
Additionally, the apparent effects of substrate stiffness on metabolic factors and processes are notable. With recent work emphasizing the importance of metabolic cues in determining stem cell fate choice[32, 33] such as shifting from glycolysis to oxidative phosphorlyation, the enrichment of metabolic processes with substrate stiffness presents a potential route through which substrate-mediated fate choice could act. Activation of early response genes represents one avenue to induce these metabolic changes, as their activation and subsequent MAPK activity (also seen in these results) has been noted in cells exposed to mechanical stretch[34].
The inferred enrichment of NFkB in stiffness sensing combined with previous work on MSC immunomodulation led to the hypothesis that the MSC response to pro-inflammatory cytokines could be altered by substrate stiffness. We found that the expression of several immunomodulatory markers was stiffness-dependent between 3kPa and 18kPa, and in several cases, mediated by a stiffness-dependence in NFkB-p65 activity. These results indicate that stiffness can act as a switch for the processing of an independent molecular input signal, and that at least in certain cases, this stiffness switch is transduced through NFkB-p65. A precedent for these results is found in the context-dependent regulation of transcriptional modules in immune cells[35]. Further, since tumors tend to display altered mechanical environments, altered stiffness in that microenvironment could potentially regulate the immunological role of MSCs in a tumor.
These results show that substrate stiffness regulates a diverse array of processes in mesenchymal stem cells and that genes respond to stiffness with a variety of different profiles. As this work expands on the molecular details of previous descriptions of stiffness sensing, we anticipate that similar approaches employing next generation sequencing will expand upon the molecular details of other biophysical properties, such as substrate viscoelasticity and composition, as well as their respective interactions.
Supplementary Material
Acknowledgements
The authors would like to acknowledge NIH/NIDCR (R01 DE013033) for funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Methods
Detailed methods are included in the supplementary information.
Data Accessibility
RNA-seq data can be found in the NCBI Short Read Archive under project PRJNA419311.
References
- 1.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294. [DOI] [PubMed] [Google Scholar]
- 2.Pelham RJ, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy WL, McDevitt TC, Engler AJ. Materials as stem cell regulators. Nat Mater 2014;13(6):547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tekin H, Simmons S, Cummings B, Gao L, Adiconis X, Hession CC, et al. Effects of 3D culturing conditions on the transcriptomic profile of stem-cell-derived neurons. Nature Biomedical Engineering. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater 2016;15(3):326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, et al. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat Mater 2014;13(10):979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J 2004;86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron AR, Frith JE, Cooper-White JJ. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials. 2011;32(26):5979–93. [DOI] [PubMed] [Google Scholar]
- 9.Dalby MJ, Gadegaard N, Oreffo ROC. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat Mater 2014;13(6):558–69. [DOI] [PubMed] [Google Scholar]
- 10.Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater 2013;12(5):458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huebsch N, Lippens E, Lee K, Mehta M, Koshy ST, Darnell MC, et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat Mater 2015;advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater 2010;9(6):518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126. [DOI] [PubMed] [Google Scholar]
- 14.Shin J-W, Mooney DJ. Extracellular matrix stiffness causes systematic variations in proliferation and chemosensitivity in myeloid leukemias. Proceedings of the National Academy of Sciences. 2016; 113(43):12126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, Kallivretaki M, et al. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur Cells Mater 2009;18. [DOI] [PubMed] [Google Scholar]
- 16.Das A, Fischer RS, Pan D, Waterman CM. YAP Nuclear Localization in the Absence of Cell-Cell Contact Is Mediated by a Filamentous Actin-dependent, Myosin II- and Phospho-YAP-independent Pathway during Extracellular Matrix Mechanosensing. Journal of Biological Chemistry. 2016;291(12):6096–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz MA, Simone DW. Cell Adhesion Receptors in Mechanotransduction. Current opinion in cell biology. 2008;20(5):551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mammoto A, Mammoto T, Ingber DE. Mechanosensitive mechanisms in transcriptional regulation. Journal of Cell Science. 2012;125(13):3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ovijit Chaudhuri LG, Klumpers Darinka, Darnell Max Bencherif Sidi A., Weaver James C. NH, Lee Hong-pyo, Lippens Evi, Duda Georg N. , Mooney David J.. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Materials. 2015;Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaudhuri O, Gu L, Darnell M, Klumpers D, Bencherif SA, Weaver JC, et al. Substrate stress relaxation regulates cell spreading. Nat Commun 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Health USNIo. ClinicalTrails.gov [cited 2016]. Available from: http://www.clinicaltrials.gov.
- 22.Diduch DR, Coe MR, Joyner C, Owen ME, Balian G. Two cell lines from bone marrow that differ in terms of collagen synthesis, osteogenic characteristics, and matrix mineralization. The Journal of Bone & Joint Surgery. 1993;75(1):92. [DOI] [PubMed] [Google Scholar]
- 23.Kim N, Cho S-G. Clinical applications of mesenchymal stem cells. The Korean Journal of Internal Medicine. 2013;28(4):387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nam J, Aguda BD, Rath B, Agarwal S. Biomechanical Thresholds Regulate Inflammation through the NF-κB Pathway: Experiments and Modeling. PLoS ONE. 2009;4(4):e5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young SRL, Gerard-O’Riley R, Harrington M, Pavalko FM. Activation of NF-κB by Fluid Shear Stress, but not TNF-α, Requires Focal Adhesion Kinase in Osteoblasts. Bone. 2010;47(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guilloton F, Caron G, Ménard C, Pangault C, Amé-Thomas P, Dulong J, et al. Mesenchymal stromal cells orchestrate follicular lymphoma cell niche through the CCL2-dependent recruitment and polarization of monocytes. Blood. 2012;119(11):2556. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Min X-H, Wang Q-Y, Leung FW, Shi L, Zhou Y, et al. Pre-activation of mesenchymal stem cells with TNF-α, IL-1β and nitric oxide enhances its paracrine effects on radiation-induced intestinal injury. Scientific Reports. 2015;5:8718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darnell M, Young S, Gu L, Shah N, Lippens E, Weaver J, et al. Substrate Stress-Relaxation Regulates Scaffold Remodeling and Bone Formation In Vivo. Advanced Healthcare Materials. 2017;6(1):1601185-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robling AG, Turner CH. Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene Expr 2009;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PCDP, Pinter J, et al. Nuclear Lamin-A Scales with Tissue Stiffness and Enhances Matrix-Directed Differentiation. Science (New York, NY). 2013;341(6149):1240104-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Downing TL, Soto J, Morez C, Houssin T, Fritz A, Yuan F, et al. Biophysical regulation of epigenetic state and cell reprogramming. Nat Mater 2013;12(12):1154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meleshina AV, Dudenkova VV, Shirmanova MV, Shcheslavskiy VI, Becker W, Bystrova AS, et al. Probing metabolic states of differentiating stem cells using two-photon FLIM. Scientific Reports. 2016;6:21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reyes JMG, Fermanian S, Yang F, Zhou S-Y, Herretes S, Murphy DB, et al. Metabolic Changes in Mesenchymal Stem Cells in Osteogenic Medium Measured by Autofluorescence Spectroscopy. STEM CELLS. 2006;24(5):1213–7. [DOI] [PubMed] [Google Scholar]
- 34.Copland IB, Post M. Stretch-activated signaling pathways responsible for early response gene expression in fetal lung epithelial cells. Journal of Cellular Physiology. 2007;210(1):133–43. [DOI] [PubMed] [Google Scholar]
- 35.Jojic V, Shay T, Sylvia K, Zuk O, Sun X, Kang J, et al. Identification of transcriptional regulators in the mouse immune system. Nat Immunol 2013;14(6):633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alsberg E, Anderson KW, Albeiruti A, Franceschi RT, Mooney DJ. Cell-interactive Alginate Hydrogels for Bone Tissue Engineering. Journal of Dental Research. 2001;80(11):2025–9. [DOI] [PubMed] [Google Scholar]
- 37.Liao Y, Smyth GK, Shi W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Research. 2013;41(10):e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general-purpose read summarization program. Bioinformatics 2013. [DOI] [PubMed] [Google Scholar]
- 39.Law CW, Chen Y, Shi W, Smyth GK. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biology. 2014;15(2):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research. 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


