Abstract
Protective immunity in tuberculosis (TB) is subject of debate in the TB research community, as this is key to fully understand TB pathogenesis and to develop new promising tools for TB diagnosis and prognosis as well as a more efficient TB vaccine. IFN-γ producing CD4+ T cells are key in TB control, but may not be sufficient to provide protection. Additional subsets have been identified that contribute to protection such as multifunctional and cytolytic T cell subsets, including classical and non-classical T cells as well as novel innate immune cell subsets resulting from trained immunity. However, to define protective immune responses against TB, the complexity of balancing TB immunity also has to be considered. In this review, insights in effector cell immunity and how this is modulated by regulatory cells, associated comorbidities and the host microbiome is discussed. We systematically map how different suppressive immune cell subsets may affect effector cell responses at the local site of infection. We also dissect how common co-morbidities such as HIV, helminthes and diabetes may bias protective TB immunity towards pathogenic and regulatory responses. Finally, also the composition and diversity of the microbiome in the lung and gut could affect host TB immunity. Understanding these various aspects of the immunological balance in the human host is fundamental to prevent TB infection and disease.
Keywords: Tuberculosis, immunity, pathogenesis, T-cell, co-morbidity
Introduction
Mycobacterium tuberculosis (Mtb) is one of the most successful pathogens, infecting one-fourth of the world population [1]. Although only ~5% of infected individuals do develop active tuberculosis (TB), the disease burden and transmission are major global health problems. So, what is required from the human immune system to combat persistent and inflammatory bacteria such as Mtb? Numerous attempts have been made to describe and map protective immunity in TB. Insights in protective mechanisms is required in order to develop new therapeutic strategies, a protective vaccine, and to be able to follow disease development as well as successful therapy. TB is a complex disease in that most Mtb-exposed individuals contain the infection in a latent state, meaning the bacteria are not cleared from infected sites but the host manages to mount an immune response efficient enough to contain the infection. Perturbations in this delicate balance of immune control may have detrimental effects and may be the result of many host factors, including changes in the microbiome, host metabolism and maybe even ageing, but also exposure to other pathogens as well as suppression mediated by regulatory T (Treg) cells or other immune cell subsets [2, 3]. Failure to control TB infection results in active disease, ranging from local Mtb infection in the lung or other organs, to disseminated and advanced disease including severe, irreversible immunopathology. Hence, TB immunity can be divided into early and late stages; from exposure to immunity in latent infection and progressive disease, and vaccine-induced immunity. Since Mtb is an intracellular bacterium, protective immunity is dependent on cell-mediated responses conducted by innate and adaptive cells, including macrophages and dendritic cells (DCs) and T-cells. Many different subsets amongst these cells have been identified and the heterogeneity in surface molecules as well as secreted effector and signaling molecules is large. Linking specific phenotypes and signaling pathways to function is key and to understand how these can change depending on the stage of infection, the Mtb strain, the local tissue environment and level of inflammation.
Mtb infection may already induce natural protection by itself, since a relatively low proportion of infected individuals will develop active TB disease during their life-time. Also Bacillus Calmette-Guerin (BCG), the only currently available vaccine against TB and the mostly distributed vaccine in the world, does protect infants and young children against severe forms of disease although BCG is less efficient in adults. The immunology of BCG vaccination has been discussed in detail recently [4], illustrating the complexity of BCG-induced immunity and even further illustrating our lack of understanding of protective responses. To complicate things further, the microbiota in the lung as well as the gut may interact with and affect the potency of Mtb-specific T-cell responses [5]. Likewise, concomitant infections, such as human immunodeficiency virus (HIV) and helminths, or other conditions including host metabolism, most extremely represented in patients with diabetes, could modify the immune responses and thereby reduce the host´s ability to fight Mtb infection [6]. Host immune responses have been analysed in various stages of Mtb infection, disease and upon vaccination. TB immunity may vary significantly depending on the time since infection or BCG vaccination. In this review, we discuss some of the current knowledge of protective immune responses in TB and how these are modulated (Figure 1).
Fig. 1.
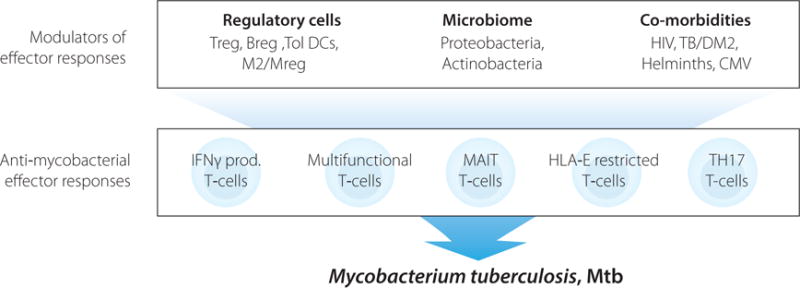
Anti-mycobacterial effector responses with protective functions in human TB involve both innate and adaptive immune cells capable of producing Th1 effector cytokines as well as cytolytic and antimicrobial effector molecules such as perforin and granulysin that could contribute to Mtb killing and disease control. Modulation of these effector responses by regulatory cells, the host microbiome and associated comorbidities could impair TB control and promote disease progression.
Effector cells subsets involved in protective TB immunity
Protective CD4+ Th1 cells
Although it is known that both CD4+ and CD8+ T-cells mediate protection against Mtb [7], the diverse phenotypes of protective T-cells are mostly undefined. The strongest evidence for a significant contribution of CD4+ T-cells in TB control derives from the HIV field [8, 9]. Rapid loss of memory CD4+ T-cells in simian immunodeficiency virus (SIV) infection enhanced reactivation of latent TB [10] and early HIV-mediated depletion of Mtb-specific T helper (Th)1 cells is also prominent in individuals with latent TB [11]. A gradual decline in cellular immunity increased the risk of poorly-organized TB granuloma formation and progression of TB disease in HIV-infected individuals [12]. Accordingly, both human data [13] and experimental animal models [14] suggest that interferon- γ (IFN- γ) signaling and CD4+ T-cells producing IFN- γ are of central importance in TB. A critical role of the type I cytokine pathway, comprised of IFN-γ and interleukin-12 (IL-12) signaling, in the defense against mycobacterial infections has been demonstrated in patients with defects in these pathways who succumb with unusual mycobacterial infections [15]. In addition, IFN- γ disrupted mice are highly susceptible to sub-lethal doses of M. bovis infection as shown by the absence of nitric oxide (NO)-production in BCG-infected macrophages [16]. Thus, IFN-γ production has been the hallmark of anti-mycobacterial immunity and has been analysed in great detail. IFN-γ producing cells have been enumerated extensively in individuals with latent TB, active TB disease and following BCG vaccination. Likewise, the significance of tumor necrosis factor-α (TNF- α) has been confirmed in mice [17] and in patients with autoimmune disorders who have a greatly enhanced risk to reactivate latent TB infection following anti-TNF therapy [18]. The widely accepted model is that particularly IFN-γ-producing CD4+ T-cells activate bactericidal effector mechanisms in Mtb-infected macrophages and other immune cells while TNF-α may be more involved in the structural organization and cellular recruitment to the TB granuloma [19]. In contrast, IL-4-producing CD4+ Th2 cells are typically involved in antibody-mediated immunity that contribute less to intracellular control of Mtb growth [20, 21].
From the other side of the coin, many studies report that IFN-γ-producing CD4+ T-cells are not reliable as immune correlates of protection in either murine [22, 23] or human TB [24]. Although IFN-γ positive cells are readily detected in TB patients, this may not correlate with protection against disease but rather indicate the presence of a mycobacterial infection or prior vaccination (Figure 2). Thus, even if Mtb can induce a strong T-cell response and critical production of IFN-γ, this may not be sufficient to execute protective responses. The quality of the response ie. co-expression with other imperative effector functions, is most likely also important. Production of multiple Th1 cytokines (IFN-γ, TNF-α, IL-2) by the same T-cells, so called multifunctional T-cells, were considered a better correlate of protective efficacy in TB [25]. Moreover, increased mortality in TB/HIV co-infected patients occurs independently of CD4+ T-cell counts [26], suggesting that protective immunity can also involve other players. Human [27-29] and non-human primate [30] studies support a more prominent role for CD8+ T-cells, which are capable of producing cytolytic and antimicrobial effector molecules such as perforin and granulysin [31]. But also non-classical CD8+ T-cells [32-34] and γδ T-cells [35] as well as natural killer (NK) cells [36] and natural killer T-cells (NKT) cells [37] may possess potent protective capacity in TB [38].
Fig. 2.
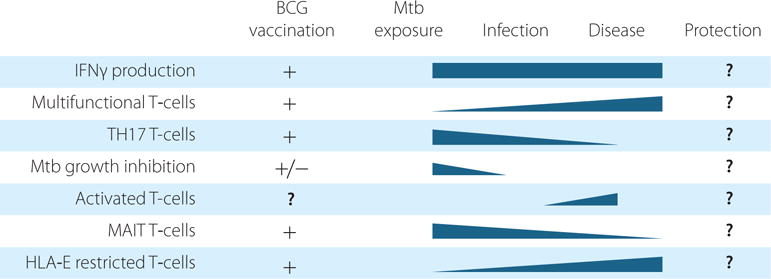
Dynamics of anti-mycobacterial effector responses potentially involved in protection, from Mtb exposure to Mtb infection and development of TB disease and how this balance is affected by BCG vaccination.
Although, multifunctional T-cells simultaneous producing IFN-γ, TNF-α and IL-2 have been associated with TB protection, and despite induction of these cells by multiple vaccines and vaccine candidates, these cells were also abundantly present in patients with pulmonary TB [39]. Moreover, no correlation between induction of multifunctional T-cells and BCG induced protection in young infants was observed [40]. In contrast, the risk to develop TB disease in these young infants was associated with an increased circulation of activated CD4+ T-cells, suggesting a possible contribution of early immune activation to disease susceptibility [41]. Intriguingly, stronger cellular responses were detected as pre-activated lymphocytes and increased Th1 cytokine production but impaired macrophage activation in bronchoalveolar lavage (BAL) from individuals with latent TB compared healthy exposed individuals [42]. The balance in protective versus pathogenic CD4+ T cells responses in different phases of TB infection and disease will certainly be an interesting subject for further exploration.
To understand how protective T-cell responses are induced, we also need to learn more about the interaction between effector T-cells and antigen presenting cells (APCs) at local sites of Mtb infection. While appropriate expression of cytokines and antimicrobial molecules is critical for T-cell function, efficient migration is also key to ensure trafficking and proper localization of protective cells into TB granulomas where Mtb-infected macrophages reside. Previous in vivo findings suggested that limited Mtb-antigen presentation and/or recognition within granulomas failed to support a high frequency of prolonged effector T-cell-APC interactions, which resulted in a muted T-cell response [43]. Moreover, it has been shown that the onset of protective CD4+ Th1 responses in vivo in the lung are delayed [44, 45]. Therefore, not only the quality, magnitude and localization, but also the timing of the T-cell response is important to secure effective early protection. Thus, we need to discover the nature of protective effector/memory T-cells and also how these cells are induced and regulated in the inflamed milieu.
Protective CD4+ Th17 cells
Th17 cells or CD4+ T-cells that secrete IL-17 are a pro-inflammatory subset frequently associated with autoimmune diseases but also associated with protective immunity in TB [46]. Genetic polymorphisms of IL-17 may be associated with genetic susceptibility to TB disease, but this also depends on the ethnicity of the individuals [47]. The requirement for IL-17 in early protective responses may also depend on the virulence of the Mtb strain [48]. Apart from Th17 cells, γδ T-cells [49] as well as mucosal-associated invariant T-cells (MAIT) cells [50] may contribute to production of IL-17 during TB protection. In patients with active TB disease, reduced frequencies of IL-17 producing CD4+ T-cells [51, 52] and also MAIT cells [50] were detected compared to healthy exposed individuals. Moreover, Th17 cells, nor IL-17, were hardly detected at the site of disease, suggesting that TB progression is associated with low Th17 responses [53]. Successful treatment of active TB patients resulted in increased frequencies of Mtb-specific Th17 cells [54]. Similarly, transfer of Th17 cells from BCG vaccinated mice to recombinase activating gene (RAG) −/− mice induced IFN-γ-independent protection against Mtb infection, although this effect was also associated with enhanced local neutrophil-mediated inflammation [55]. Contrary, IL-17A−/− mice showed increased bacterial burden and impaired granuloma formation in the lungs that correlated to reduced survival upon Mtb infection [49]. Deficient granuloma formation could be due to reduced IFN-γ and TNF-α in the chronic stage [49]. IL-17 may also ameliorate TB disease severity by limiting the development of hypoxic granulomas [56]. Others have also shown that IFN-γ produced by Th17 cells is involved in mediating protection and that localisation of the Th17 cells in close proximity with the macrophages is critical for protection [57]. Interestingly, the activity of Th17 cells may be down-regulated by Mtb-infected DCs recruiting IL-10-secreting neutrophils [58]. Although it has also been reported Mtb infected DCs are poor inducers of IFN-γ while enhancing IL17-producing CD4+ T cells [59], suggesting that Mtb targets DCs to skew the immune responses away from protective IFN-γ.
Vaccination studies in mice have shown that vaccines inducing stronger Th1 and Th17 responses also had a higher protective efficacy against Mtb challenge [60]. Accordingly, concomitant induction of IFN-γ and IL-17 was required to induce protection against Mtb infection in vivo, whereas induction of IFN-γ alone was not sufficient [61]. Likewise, balanced Th1 and Th17 responses were correlates of protective host responses in cynomolgous macaques [62]. In humans, concomitant infections with other pathogens such as hookworms, alter the frequency of protective Th17 cells that may promote disease progression [63]. Altogether, most of these data show that IL-17 producing T-cells contribute to the protective response against Mtb. However, a balanced Th17 response is critical to prevent excess neutrophil influx resulting in inflammation and pathology in the Mtb-infected lung. The debate is ongoing whether IFN-γ is involved in the IL-17-mediated protective responses, but this may depend on experimental variables such as the model system or Mtb strains used as well as timing of the response.
Protection mediated by unconventional T cell subsets
In addition to multifunctional cytokine producing T-cells, T-cells producing multiple cytolytic effector molecules have been described, so-called polycytotoxic T-cells. In particular, lipo-arabinomannan (LAM) responsive T-cells with a polycytotoxic phenotype correlated with the capacity to control mycobacterial growth in vitro [64]. LAM responsive T-cells are known as unconventionally restricted T-cells, since LAM is presented by CD1b and not by classical MHC molecules. Over the recent years multiple alternative antigen presentation molecules have been identified, and have been demonstrated to present Mtb-derived ligands to T-cells. Since these antigen presentation molecules are frequently non-polymorphic the responding T-cells are called ‘donor-unrestricted T-cells’ (DURT) or unconventional T-cells [65]. This family comprises CD1 restricted T-cells, mannose receptor (MR)1 restricted MAIT cells and HLA-E restricted T-cells that may play a role in early protective effector cell responses against mycobacterial infections, since these members can respond rapidly upon pathogen encounter [65].
MAIT cells are present at increased frequencies in the human airways and thought to have a role in the early defense against pathogens such as Mtb because of their innate-like pattern recognition based activation. Patients with severe (bacterial) infections had decreased circulating MAIT cell counts, which may contribute to their increased susceptibility to secondary infections [66]. Reduced circulating MAIT cells have also been detected in TB patients, while local frequencies in the lung were increased [67]. This may suggests that MAIT cells migrate from the peripheral blood to sites of Mtb infection. Thus, a decrease in MAIT cells observed in TB patients as well as patients infected with non-tuberculous mycobacteria, correlated with disease severity [68]. Moreover, MAIT cells from TB patients were not activated to produce cytokines such as IFN-γ upon in vitro stimulation, which indicated that MAIT cells may be functionally impaired in TB [68]. Studies in non-human primates also show that MAIT cells are activated in response to BCG vaccination as well as Mtb infection [69], however data on the relative contribution of MAIT cells to protective TB immunity in non-human primates is still lacking. In mice, Mtb infection induced heterogeneous MAIT responses [70]. In particular, Vα19i-Tg MAIT cells expressing CXCR3 and α4β1 were recruited to the lung and contributed to early protection [70], suggesting that MAIT cells may also have a protective role in murine TB. It would be of great interest to further explore MAITs as possible therapeutic targets in TB.
Another alternatively restricted subset of CD8+ T-cells are the HLA-E restricted T-cells. In contrast to the HLA class Ia genes, HLA-E is virtually non-polymorphic, with a single amino acid difference discriminating the major variants. Another interesting property of importance for potential future vaccine targeting, is the lack of down-regulation by HIV [71]. HLA-E typically presents signal sequences of HLA-A,B,C alleles to prevent NK mediated lysis of healthy cells [71]. Alternatively it can also present sequences from intracellular pathogens including Mtb [71-74]. Detailed characterization of these cells revealed a unique Th2 cytokine repertoire [75-77]. Compared to individuals with latent TB, patients with active TB disease had higher frequencies of Mtb specific HLA-E restricted cells that decreased over time during treatment [75-77]. Patients suffering from TB/HIV co-infection had the highest frequencies of HLA-E restricted cells [75]. Intriguingly, T-cell clones specific for several Mtb peptides had the capacity, not only to lyse Mtb-infected target cells, but also to reduce the outgrowth of intracellular Mtb [77]. The strong capacity of HLA-E restricted T-cells to restrict Mtb growth in infected cells suggests a protective role against TB. However, increased frequencies of HLA-E restricted T-cells were reported in patients with active TB compared to individuals with latent TB, but this may be the result of the enormous amount of antigen released during TB disease that evokes these responses. Mtb infection studies in mice have shown that the murine homologue of human HLA-E, Qa-1, is involved in protection, mostly through modulation of inflammation [78]. The induction of strong effector T-cells in combination with the low level of allelic variation and stable expression makes HLA-E a very interesting candidate for vaccine targeting.
Protection mediated by trained immunity
Initial priming of the immune system upon first mycobacterial contact seems increasingly important. Gene expression analysis revealed that BCG vaccination in young infants induced two dichotomous responses, likely involving differential monocyte activation [79]. Similarly, dichotomous responses to primary BCG vaccination were also observed in adults [80]. In mice, priming with live mycobacteria induced transient immunity in contrast to immunity induced with subunit vaccines, which was more long-lasting [81]. Characterization of the vaccine-induced T-cells, revealed accelerated differentiation and inferior homing to the lung parenchyma by T-cells activated by live mycobacteria [81]. This illustrates that an early response, probably mediated by the innate immune system, is critical in the activation and education of adaptive immunity. Thus, more research to investigate early priming of immune triggers is warranted. Transient alterations upon pathogenic exposure may also induce trained innate immunity [82-85]. Trained immunity reflects a state of temporary innate immune memory, in which cells, generally monocytes, are epigenetically reprogrammed [82-85]. This concept was originally described upon BCG vaccination of naïve adults and resulted in temporal reprogramming of innate immune cells [82]. Training of innate immune cells also augments cytokine production against non-related pathogens. A recent study demonstrated that a sub-group of BCG-vaccinated individuals responded to the vaccine by effectively reducing mycobacterial growth in vitro [86]. This effect was associated with a stable and robust differential DNA methylation pattern that was not observed in the BCG non-responders [86]. Studies in mice showed that BCG educated hematopoietic stem cells resulted in trained monocytes that efficiently combatted Mtb during subsequent infections [87]. Education at the level of hematopoietic stem cells is an interesting observation which may help explain the fact that training effects last longer than the life-span of individual monocytes or macrophages.
The process of training by live mycobacteria is unknown, but may significantly affect or even skew subsequent immune activation. Indeed, we observed signs of trained innate immunity upon recent Mtb exposure, but not in more established infection or disease [88]. Interestingly, not all donors that initially controlled BCG outgrowth also developed detectable adaptive immune responses against Mtb [88]. Together these data suggest that the initial activation of the immune system may be key in mounting the capacity to cope with mycobacteria later in life. Moreover, immune responses may vary considerably over time following infection or in the process to active disease. Longitudinal follow up of Mtb-infected adolescents revealed that TB disease development was associated with sequential modulation of immunological processes [89]. Early changes involved alterations in Type I/II interferon signaling and complement proteins, and repression of Th17 cells [89]. This illustrates the importance of kinetics and sequential events in the immune response against TB. Thus, it is important to consider the time elapsed since infection when selecting clinical samples for immunological characterization.
Functional read out of effector immune responses
Overall, analysis of CD4+ and CD8+ T-cells have not yielded satisfactorily markers or correlates of protective immunity, therefore functional approaches have received increasing attention. In vitro mycobacterial growth inhibition assays have been explored to determine the capacity of whole blood or PBMC samples to control the growth of live mycobacteria, mostly BCG [90, 91]. In these assays, samples collected after primary BCG vaccination had the capacity to control BCG outgrowth, but this effect was abrogated by a secondary BCG vaccination [92]. Also BCG vaccination of mice results in growth reduction of BCG after infection of bulk splenocytes in vitro [90, 93]. Mtb infection status did not influence the capacity of whole blood to control Mtb or BCG outgrowth, possibly due to the strong neutrophil activity in the whole blood based assay [94]. Recently it was shown that exposed individuals from contact investigations, had a remarkably well capacity to control BCG outgrowth [88]. Control of BCG outgrowth was mediated by a non-classical monocyte population producing CXCL10 and was the result of trained innate immunity [88]. These functional assays will be interesting candidates to monitor vaccine-induced protection in future vaccination trials. Moreover, all samples with strong control of BCG outgrowth will be essential to decipher the mechanism of growth inhibition.
Regulatory cell subsets involved in suppression of protective TB immunity
Regulatory T (Treg) cells and other pathogenic T cell subsets
Obviously, the ability of the host to mount protective TB immunity is a multifactorial event that is also dependent on the local induction and expansion of immune cells with regulatory properties (Table 1). Natural CD4+CD25+FoxP3+ Tregs (developed in the thymus) as well as induced CD4+FoxP3+ Tregs (generated outside the thymus) are the most well-characterized regulatory subsets [2]. CD4+CD25+ Treg cells were detected at a 3-fold increased level in blood from active TB patients and Tregs had the capacity to suppress Mtb-specific IFN-γ production [95]. IL-10-produicng CD4+CD25+FoxP3+ Treg cells have been shown to be elevated at the site of infection in the lung and to suppress Mtb-specific effector T-cell proliferation [96]. In addition, a recent study demonstrated that CD4+CD25+FoxP3+ Treg cells were elevated in the peripheral blood and in the granulomatous TB lesions of patients with extensively drug-resistant TB (XDR-TB) compared to drug-susceptible and latent TB [97], suggesting that expansion of Tregs may be an immune evasion mechanism exploited by virulent Mtb strains. Moreover, compartmentalization of TB immunity was previously shown to be characterized by few cytolytic CD8+ T-cells expressing perforin and granulysin but increased CD4+FoxP3+ Treg cells producing TGF-β in proximity of Mtb-infected macrophages inside human granulomas, which may prevent efficient killing of infected cells [98].
Table 1.
Regulatory immune cell subsets with a potential role in TB
| Cell subset | Suppressive mechanism(s) | Potential detrimental effects in TB disease |
|---|---|---|
| natural Treg (nTreg) induced Treg (iTreg) |
|
Suppress Th1 and Th17 effector cell responses early in the infection, either by mechanisms that target effector T and NK cells or that target different APCs. Migration of Tregs from the periphery and/or expansion at the site of infection may enhance bacterial growth in local lesions. Promotes the induction of Bregs, tolerogenic DCs and Mregs. |
| Regulatory B cells (Breg) |
|
Accumulation of Breg cells in lymphoid follicle-like structures in close proximity to TB lesions in the lung may counteract Th1, Th17 and macrophage functions and promote the recruitment of Treg and Mreg cells to the site of infection. IL-10 production induces iTregs cells. |
| Tolerogenic DCs |
|
Inhibits T cell proliferation and effector cell differentiation. May enhance expansion of Tregs at the local site of infection. |
| Alternatively activated macrophages (M2) |
|
Increased bacterial uptake promotes intracellular growth. Support immune polarization of Th2 responses that counteract Th1 immunity and increase tissue remodeling and repair in the infected lung. Arg-1 counteracts iNOS and result in decreased antimicrobial activity of macrophages. May induce Tregs at the local site of infection. |
| Regulatory macrophages (Mreg) |
|
Delete activated T cells and can suppress T cell proliferation using high levels of IL-10 and local induction of iNOS/NO or IDO. May promote the induction of both nTregs and iTregs. Enhanced suppressive activity in concert with myeloid-derived suppressor cells. |
While Tregs have an important role in TB, there are also other pathogenic T cell subsets and a complex network of both innate and adaptive regulatory cells and inhibitory pathway that contribute to the overall control of immune homeostasis and protective responses in TB. To mediate protection, T-cells should also be able to persist and maintain essential protective functions in the inflammatory environment in the lung. In this context, terminally differentiated T-cells expressing killer cell lectin-like receptor G1 (KLRG-1) have been suggested to be pathogenic in TB as these cells are usually associated with reduced proliferative [99] and lung migratory [100] capacities, including a higher sensitivity to the inflammatory environment in the lung parenchyma and more likely to be found in the vasculature [81, 101]. Similarly, elevated expression of IL-27 has been detected in patients with active TB and accordingly, IL-27R-expressing T-cells are associated with higher KLRG-1 and IFN-γ expression and consequently higher bacterial burden and reduced T-cell protection in Mtb-infected lungs [101]. KLRG-1-expressing CD4+ T-cells were recently demonstrated to have a multifunctional cytokine profile, which is consistent with the notion that multifunctionality may not always be protective [102]. In contrast, development of central memory CD4+KLRG1− T-cells producing IL-2, are associated with control of Mtb growth and prolonged protection [81, 103]. Other inhibitory receptors involved in reducing Th1 immunity in TB are programmed cell death protein-1 (PD-1) [99], lymphocyte activation gene-3 (LAG-3) [104], T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) [105] and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) [106]. Such immune checkpoint inhibitors may be targeted to amplify TB immunity, but may also contribute to pathology rather than protection [107].
Regulatory B (Breg) cells
Similar to T-cells, B-cells can have functionally and temporally distinct roles in the control of immune homeostasis. B-cells are also a major cellular component of TB granulomas in humans, non-human primates, and mice [108, 109]. In contrast to effector B-cells, which can secrete cytokines to support the differentiation of either Th1 or Th2 cells, regulatory B-cells (Breg cells) can suppress the inflammatory potential of effector T-cells and alter the activity of APCs [110]. Accordingly, human CD19+CD24hiCD38hi Breg cells have the ability to suppress the differentiation of both Th1 and Th17 cells, and to promote the expansion of FoxP3+ Tregs with suppressive functions [111]. CD19+CD1d+CD5+ Bregs cells have been shown to be elevated in patients with active TB and specifically suppress Th17 responses, which may have a negative impact on the clinical outcome of TB [112]. Such Breg cells are reduced upon successful anti-TB treatment, which restored Mtb-specific production of the Th17 cytokine IL-22 [113].
In contrast, a recent study failed to detect an increase in different subpopulations of Bregs in either active or latent TB, but instead observed an increased frequency of IgD−CD27− atypical B-cells that was normalized after successful anti-TB treatment [114]. Impaired B-cells correlated with poor cytokine production by T-cells, which could be mimicked by B-cell depletion in donors with latent TB infection. Therefore, functional B-cells may be required to induce a proper cytokine production and T-cell activation in human TB. Similarly, B-cell activation was restored upon anti-TB treatment including expression of IL-5RA and apoptosis-inducing FasL, which may suggest that B-cells with regulatory properties have a protective role in TB [115]. Further studies addressing the relevance of Bregs and atypical B-cells and their relative contribution to the pathogenesis of human TB will be of significant interest.
Tolerogenic DCs
Although DCs are the most potent APCs involved in triggering naive T-cell responses, tolerogenic DCs can support the development of an immunosuppressive environment. Virulent Mtb promotes tolerogenic DCs that produce suppressive cytokines such as IL-10, IL-13, or TGF-β but also inhibitory molecules including PDL1 and PDL2, CD103, TIM-3, arginase or Indoleamine 2,3-dioxygenase (IDO) [116], which effectively diminish Th1 activation and instead contributes to the generation of Tregs [117]. Mtb-treated human DCs have been shown to expand CD4+CD25+FoxP3+ Tregs via Toll like receptor 2 (TLR2)- and DC-SIGN–dependent induction of PD-L1 on the DCs [118]. Recently, a lot of attention has been devoted to the immunosuppressive enzyme IDO that degrades the essential amino acid tryptophan, causing tryptophan deprivation that may result in growth arrest of T-cells as well as decreased antigen uptake and down-regulation of costimulatory molecules on DCs [117]. Instead, an environment high in tryptophan metabolites favors expansion of CD4+FoxP3+ Tregs and thus IDO can cause immunosuppression both by growth factor starvation and by the direct action of the tryptophan metabolites. Increased levels of IDO have been detected in pleural fluid and serum from patients with TB pleuritis and pulmonary TB, respectively [119, 120]. Enhanced IDO activity in peripheral blood mononuclear cells from individuals vaccinated with the novel TB vaccine, modified vaccinia Ankara expressing antigen 85A (MVA85A), were found to be inversely correlated to vaccine-induced IFN-γ responses, suggesting that IDO activity may impair the generation of a CD4+ T-cell memory response [121]. The exact role of IDO and tolerogenic DCs in the regulation of TB infection remains to be determined.
Alternatively activated macrophages (M2)
The macrophage is one of the most central immune cells in the regulation of TB infection as this is the primary host cell to be infected by Mtb. Macrophages are versatile cells that can be polarized to a spectrum of functional programs in response to exogenous signals such as cytokine stimulation [122]. Although macrophage differentiation is not linear, the heterogeneity of macrophage activation is simplified by the division into classically activated or M1 macrophages (inflammatory) and alternatively activated or M2 macrophages (anti-inflammatory) as well as regulatory or Mreg macrophages (immunosuppressive) [123]. The phenotype and function of the different macrophages subsets have mostly been studied in mice, while this is less clear in humans [124]. While M1 macrophages typically express inducible nitric oxide synthase (iNOS) and trigger Th1 responses that is required in TB defense, M2 macrophages activate arginase (Arg1) and may be involved in the induction of Th2 and tissue repair responses [122]. Interestingly, iNOS and Arg1 uses the same cellular substrate, arginine, and thus directly competes for the total amount of arginine, making the relative expression of these enzymes an important determinant for macrophage-mediated killing of Mtb [125, 126]. IFN-γ and IL-4 are the main cytokines involved in M1 versus M2 activation, and therefore the Th1/Th2 balance at the site of Mtb infection may dictate the induction of local macrophage effector functions. Mycobacterial virulence factors may interfere with M1 polarization and instead promote polarization of alternatively activated M2 macrophages that maintain a poorly microbicidal state, which facilitate mycobacterial growth and intracellular persistence [127, 128]. Particularly Arg1-expression thwarts macrophage effector function and thus elimination of Arg1 decreased bacterial loads in TB [129]. An in vitro TB granuloma model has demonstrated that macrophage polarization changes from M1 to M2 over time following Mtb infection [130]. Accordingly, M2 macrophages were found to predominate both necrotic and non-necrotic granulomas from TB patients, while both M1 and M2 polarized macrophages were found in the non-granulomatous lung tissues [130]. It has been proposed that alveolar macrophages in the lung are inherently alternatively activated, offering a cell permissive for Mtb replication [131]. An overrepresentation of M2 macrophages in the TB infected lung, could fuel tissue remodeling and Mtb growth that results in reduced lung function observed in patients with untreated, progressive disease [126]. Thus, a balanced ratio of M1:M2 (iNOS/Arg1) macrophages present at the site of Mtb infection may be key to avoid subversion of immune control.
In addition to the direct permissive state of M2 macrophages, these can also contribute to the generation of CD25+FoxP3+ Treg cells [132], perhaps via production of IL-10 and TGF-β [122]. Moreover, the inhibitory molecule IDO can be expressed in Mtb-infected macrophages in vitro [133] and in the macrophage-rich areas of pulmonary TB granulomas in vivo [134]. While IDO-expression in murine macrophages failed to control Mtb infection in vivo [133], a recent study revealed that in vivo inhibition of IDO in non-human primates resulted in enhanced T-cell proliferation and bacterial control involving relocation of effector T-cells to the center of pulmonary granulomas [135]. A predominance of M2 or Mregs in the TB granuloma may promote local expansion of Treg cells and impair effector cell function and the host ability to control Mtb infection [136]. Thus, a shift in M1/Teffector cells towards M2(Mreg)/Treg cells could promote immunosuppressive pathways and bacterial persistence in local TB lesions [126].
Myeloid-derived suppressor cells (MDSC)
Protective T-cell responses in TB may also be regulated by local induction of novel innate monocyte-like cells called myeloid-derived suppressor cells (MDSC) [137]. These cells have been well-described in the cancer field, but data from their role in chronic infectious diseases such as TB has just started to emerge. MDSCs increase after Mtb infection and are able to suppress CD4+ T-cell proliferation and cytokine production, CD8+ T-cell function and T-cell migration [138]. MDSCs can phagocytose Mtb bacilli and accumulate at the site of Mtb infection in the inflamed lung together with neutrophils, where they interact with granuloma-residing cells and contribute to pathological inflammation [139, 140]. In vivo depletion of MDSCs reduced Mtb burden and ameliorated TB disease [139]. Increased frequencies of MDSCs in TB patients were described to be associated with elevated Arg1 activity and IDO protein levels in plasma samples [141], while IFN-γ could block MDSC activity by reducing expression of Arg1 [142]. CD14−CD11b+ MDSCs were reduced after anti-TB therapy, indicating these cells could be used as prognostic biomarkers in TB [143]. Identification of CD244hi MDSCs in patients with active TB have been shown to express high levels of iNOS that were inversely correlated with activation and effector function of CD4+ and CD8+ T-cells [144]. This may suggest that high local production of NO produced by MDSCs may inhibit Mtb-specific T-cell responses. The dual ability of MDSCs to induce both Arg1 as well as iNOS, may imply that also these cells can be divided into several subsets with different inhibitory functions.
Major co-morbidities associated with reactivation and progression of active TB
The risk to be infected with or reactivate TB is significantly enhanced by a number of disease conditions including HIV [145] and helminth infections [146] but also by metabolic disorders such as diabetes mellitus type 2 (DM2) [147]. An important risk factor for TB development is also common viral infections such as cytomegalovirus (CMV) [41]. Infants who develop TB in the first 2 years of life had increased IFN-γ production and CD8+ T-cell activation in response to CMV stimulation [148]. Viral infections may induce immune activation that was previously identified as a risk factor for TB disease development. However, the etiology and specificity of the interaction between viral infections and development of TB disease has to be determined.
TB/HIV co-infection
TB and HIV co-infection is well-known as the cursed duet as both pathogens are dependent on cellular immunity that is gradually destroyed in HIV infection. Clinical studies confirm that HIV increases the risk of TB and likewise, Mtb infection may exacerbate HIV infection [145]. Thus, HIV induces functional changes in all T-cells, including Mtb-specific T-cells and macrophages but Mtb can also modulate immune cell function that enhances HIV replication. Part of the vicious cycle is initiated by activation and up-regulation of the HIV co-receptor CCR5 on CD4+ T-cells by Mtb [149]. This results in enhanced viral spread and simultaneously a more rapid HIV-induced depletion of Mtb-specific effector memory CD4+CCR5+ T-cells from mucosal sites including the lung [150], which is associated with reactivation of latent TB infection [10, 151]. Remaining Mtb-specific CD4+ T-cells are preferentially retained in the lung and possess a reduced capacity to produce Th1 cytokines at the site of Mtb infection [152, 153]. Accordingly, Mtb-specific cytolytic T-cell activity is severely impaired in HIV-infected individuals resulting in reduced killing of mycobacteria-infected macrophages [154]. In addition, CD4+Foxp3+ Tregs expressing high levels of CCR5 and the survival factor Bcl-2, have been shown to be productively infected by HIV at the site of Mtb infection in the pleural cavity, providing CD4+FoxP3+ Tregs with a survival advantage compared to CD4+FoxP3− T-cells [155].
Within the granulomas, HIV has the ability to promote cellular dysfunctions on CD4+ and CD8+ T-cells and macrophages, causing profound necrosis, bacterial dissemination and TB reactivation [12, 145]. Reduced numbers of CD4+ T-cells have been found in granulomatous tissue of SIV/TB co-infected primates [10], which may partly explain deficient macrophage activation and enhanced intracellular Mtb growth. HIV-induced manipulation of Mtb-infected macrophages involves dampened Mtb-induced TNF-α production and less TNF-induced apoptosis of infected cells [156]. This may result in reduced cellular recruitment and lack of granuloma formation evident in HIV-infected patients with progressive disease [12]. On the other hand, Mtb can induce a proinflammatory environment at the site of infection that promotes HIV replication [157], which highlights the double-edged sword in TB/HIV co-infection. HIV may also contribute to Mtb pathogenesis by interfering with the DC-mediated immune control. HIV and Mtb co-infected human DCs showed reduced expression of co-stimulatory molecules CD40, CD80 and CD86 as well as pro-inflammatory IL-6, IL-1β and TNF-α, resulting in impairment to stimulate IFN-γ production by CD4+ T-cells [158]. In line with these findings, it was recently demonstrated that CD40-dependent co-stimulation of DCs was crucial to initiate protective Th17 responses in TB [159]. Qualitative changes in cytokine expression involving reduced Th1 responses have previously been demonstrated in PBMCs from TB/HIV co-infected patients [160, 161]. In contrast, transcriptional profiling of skin biopsies from the TST injection site, showed that TB/HIV co-infected patients with TST reactivity maintained Th1 responses but lacked immunoregulatory IL10-inducible responses that could promote pathological inflammation [162] such as IL-17-driven recruitment of neutrophils. Instead, a negative TST was associated with profound anergy of both innate and adaptive responses. Most likely, HIV-induced immune dysfunctions may be characterized by different mechanisms depending on the degree of HIV-associated immunosuppression [12].
TB/type 2 diabetes (DM2)
Host metabolism affects the functional capacities of the immune system, this is most exemplified in individuals with extreme metabolic dysregulation such as patients with type 2 diabetes. Patients with DM2 have a 3-fold increased risk to progress to TB disease [163]. Hyperglycemia and cellular insulinopenia may affect both macrophage and lymphocyte functions, leading to a diminished ability to contain Mtb [164]. Currently, there is an inconsistency regarding loss of immune control in the progression of TB in DM2 patients; some studies suggest too little inflammation and Th1 activation while other studies imply that TB/DM2 is associated with enhanced cell-mediated immunity. In a mouse model of diet-induced DM2, it was recently shown that an increased number of inflammatory lesions was associated with a reduced proinflammatory cytokine response and suppressed phagocytosis and bactericidal capacity of alveolar macrophages infected with mycobacteria [165]. Monocytes from TB/DM2 patients have also been suggested to have an altered phenotype that may enhance TB susceptibility, i.e. an enhanced expression of CCR2 that could prevent monocyte migration to the site of Mtb infection [166]. Consistently, an impaired and delayed Th1 response and a concomitant reduction in NO-expression is evident in TB infected mice with chemically induced type 2 DM [164, 167]. Moreover, diabetic TB patients have been shown to have a lower Th1 to Th2 cytokine ratio in peripheral blood [168]. Another study suggests that selective expansion of CD4+CD25+CD127− Treg cells in the lung of TB/DM2 patients compared to the peripheral blood, correlates to reduced IFN-g and increased IL-10 levels at the site of Mtb infection [169].
Impaired control of diabetes has also been associated with increased systemic levels of pro-inflammatory cytokines (IL-1β, IL-6 and IL-18) as well as anti-inflammatory IL-10 [170]. Cytokine and transcriptional profiling of plasma and whole blood samples support findings on elevated levels of proinflammatory cytokines and chemokines known to promote neutrophilic inflammation [171]. Likewise, enhanced Th1 and Th17 responses have been detected in TB/DM2 patients [172], while the cytolytic activity including expression of CD107a, perforin and granzyme B was defective in CD8+ T-cells [173]. Consistent with this finding, central memory CD4+ and CD8+ T-cell frequencies displayed a positive correlation with fasting blood glucose and HbA1c levels [174], whereas effector/memory T-cells that strongly expressing cytolytic effector molecules [175] were detected in reduced levels in TB/DM2 patients. Overall, these contradictory results warrant a more comprehensive analysis of aberrant lymphocyte as well as macrophage responses in TB/DM2 disease.
TB/helminth co-infection
The third most recognized TB associated co-morbidity is concomitant infection with helminths. Mtb and helminths induce Th1 and Th2 immunity, respectively, that are likely to have antagonistic effects in vivo. TB patients with concomitant helminth infections present with reduced frequencies of peripheral T-cells as well as more severe radiological findings of pulmonary TB disease [176]. Asymptomatic helminth infection was shown to be associated with increased Treg and Th2 responses and a lower rate of sputum smear positivity [177]. Although deworming with albendazole failed to improve clinical symptoms, there was a significant decline in eosinophils and IL-10 production along with weight gain observed in the deworming group compared to placebo three months post-treatment [178]. Accordingly, helminths induced an IL-10 mediated inhibition of multifunctional CD4+ T-cells responses with potential protective efficacy in TB [179]. Diminished Th1 and Th17 responses and increased Treg cells have also been observed in individuals with latent TB and hookworm infection [63]. Accumulation of alternatively activated macrophages in the lungs of co-infected mice was dependent on the IL-4Ra-signaling pathway, which resulted in a Th2 response that promoted intracellular persistence of Mtb [180]. Additionally, chronic worm infection reduced the immunogenicity of BCG as individuals dewormed prior to vaccination, possessed elevated IFN-γ and IL-12 production while untreated BCG-vaccinated subjects had an increased production of TGF-β upon restimulation with mycobacterial antigens in vitro [181]. Interestingly, the BCG-vaccinated cohort did not show enhanced classical Th2 immunity, suggesting that helminths could modulate protective TB immunity in other ways.
The role of the host microbiome in modulating protective TB immunity
Shaping the immune response
The linings of the human body are colonized with huge amounts of micro-organisms including the skin, intestine and airways, called the microbiome. A long-time these micro-organisms have been considered to be commensal, non-harmful but also non-meaningful. Although the contribution of various bacteria to degradation and absorption of nutrients in the gut was recognized, the effects on shaping the immune response were quite recently appreciated. Immune modulating capacities of the microbiome may also be relevant for the onset of TB but may also contribute to treatment efficacy. Gut bacteria fermentate fibres (large carbohydrates) into several short chain fatty acids (SCFA), including acetate (C2), proprionate (C3) and butyrate (C4). SCFA can modulate cellular processes, including gene expression, differentiation, proliferation and cell survival [182]. Differential expression of the receptors for SCFA on immune cells may explain the various effects on different cell types [182, 183]. Monocytes and macrophages exposed to SCFA generally have a more anti-inflammatory response, although opposing effects have also been published [182]. In general, SCFA induce a more tolerogenic T-cell profile, frequently with an augmented induction of Treg cells [182]. Since concentrations of SCFA are primary increased in the colon, this is largely reflecting increased Treg populations in the lamina propria [184]. Butyrate is the most powerful inducer of Tregs in the colon, likely through inhibition of histone deacetylases (HDAC) in the promotor region of FoxP3 [184].
SCFA not only mediate their effects in the intestinal system, several key components of SCFA metabolism have been shown to play a role in the lung as well. Receptors for SCFA are also expressed in the airways as well as on cells infiltrating the lungs and upper airways. Free fatty acid receptor 3 (FFAR3; GPR41) is the SCFA receptor that is key in regulating airway inflammation, and plays an important role in fibre-induced diminution of allergic asthma [183]. SCFA can induce increased IL-8 production by airway epithelial cells through FFAR3 [185]. Moreover, SCFA can reduce growth of pathogenic Pseudomonas in vitro [186]. Although the substrates for SCFA production in the lung are unclear, these data indicate that also the microbiome at the lung epithelial surface can contribute to locally increased concentrations of SCFA that modulate host immunity.
Microbiome and TB
A potential contribution of the host microbiome in the combat against TB has also been investigated. To obtain comparable results, it is critical to select appropriate clinical materials from particularly the control population, since in TB patient’s, samples from the oropharynx, but not nasal samples, mimicked sputum [187] and thus the former are the preferred sample. Although major phyla were not different between TB and controls, the relative abundances of particular taxa suggest disturbances in microbial communities [187]. Analysis of nasopharynx samples (induced sputa) of children with TB, revealed no significant differences in the microbiota of children with or without TB [188]. Others have analysed the microbial composition of sputum from pulmonary TB patients and indicated that these samples were more complicated, characterized by many foreign bacteria compared to respiratory secretions from healthy individuals [189]. Phylogenetic analysis revealed more Proteobacteria and Bacteroidetes in TB samples and more Firmicutes in controls with similar coughing symptoms [190]. In contrast, another study reported that the relative abundance of Firmicutes and Actinobacteria was significantly higher in TB samples [191]. The presence of certain bacteria and the disturbance of the lung microbiota may be associated with not only the onset of TB but also disease recurrence and treatment failure [192]. Assessment of microbiota in BAL from TB patients revealed specific differences, with Cupriavidus as dominant genus, in lower respiratory tracts [193]. The microbiome of individuals with detectable proprionate levels in BAL had a relative abundance of anaerobic bacteria [194], which is in line with the hypothesis that SCFA are produced locally in the lung. Together, these data indicate that the microbiome locally in the airways is disturbed during active TB disease, however, the differences in study populations (adults vs children, different ethnic origins including differences in diet), clinical samples (nasopharynx, sputum, BAL) and analysis methods make it impossible to draw any major conclusions on the alterations in microbiota composition during TB disease.
In addition to the local microbiome, also gut microbiomes have been investigated in patients with TB and in experimental animal models of TB. In mice, antibiotic disturbance of the gut microbiota increased survival and dissemination of Mtb, which could be restored with fecal transplantation [195]. Similarly, modified gut microbiota also resulted in failed immune control of Mtb [196], while pulmonary TB patients had increased diversity in their gut microbiome [197]. Feces from patients with recurrent TB was enriched with Actinobacteria and Proteobacteria, many of which are pathogenic [197]. In contrast, controls had more beneficial, commensal organisms of the Bacteroidetes phylum [197]. Furthermore, increased serum concentrations of SCFA, the products of the commensal bacteria, were associated with increased TB susceptibility in HIV-infected individuals [194]. Butyrate inhibited IFN-γ and IL-17 production in response to Mtb-antigen stimulation of PBMCs [194], in a HDAC dependent manner [198]. The host microbiome may not only influence host immunity through SCFA production and HDAC activation, recent data suggest also a contribution in the antigen repertoire of T-cells [199]. Antigen recognition of presumed Mtb epitopes was influenced by prior episodes of TB disease, but only in the relatively recent history (< 6 years) [199]. A specific set of epitopes was poorly recognized by CD4+ T-cells producing IFN-γ from donors with TB diagnosis and treatment in their recent history. These epitopes were poorly regulated in Mtb and had a very high degree of homology with epitopes in bacteria in the microbiome [199]. The authors claim that their recent TB antibiotic treatment has disturbed the composition of the microbiome such that these antigens are no longer presented to the immune system [199]. Indeed, several studies have demonstrated that anti-TB treatment affected the composition of the host microbiome, ie. chemotherapy depleted multiple immunologically significant commensal bacteria from stool [200]. The perturbation of the stool microbiome lasted for at least 1.2 years [200]. Experimental studies in mice revealed that each of the components of anti-TB therapy contributes to the observed dysbiosis, but rifampin and pyrazinamide have the largest effects [201].
In conclusion, the interplay between Mtb and the microbiome is interesting but highly complex. Firstly, not only the local, airway, microbiome but also the gut microbiome influence activation of immune responses against Mtb. Secondly, prior disturbances in the microbiome may influence the susceptibility to Mtb infection but may also alter protective TB immunity. Finally, since Mtb infection in experimental animals also altered the microbiome it renders a catch 22 on cause and consequence. Thus the interplay between the microbiome and TB deserves a more detailed, systematic investigation.
Concluding remarks
Effector responses against Mtb are diverse and multiple subsets contribute to the protective efficacy. Many different subsets have been associated with protection in experimental models of TB, however the lack of protective vaccines in humans limits validation in humans. Some key players are essential but not sufficient to execute TB protection such as IFN-γ. Recent data have suggested contributions of novel players in the combat against Mtb, including innate immune responses such as those resulting from trained innate immunity.
However, induction of effector responses as such, and as friends from the human host perspective, will not be the only factor determining the outcome in the combat against the bacillus. Infection with Mtb also results in activation of many regulatory or modulatory immune subsets, the foes of the human host, and the balance between all of these determines the outcome of the infection. Moreover, many circumstances associated with health or disease influence the magnitude and power of the effector responses. The specific host related conditions such as the microbiome lining the body surface will modify the capacity of effector cells to respond to infection. Also host metabolic status, in the extreme in DM2, will affect functioning of the immune system. Similar co-existing infections with viruses, such as CMV or HIV, or parasites, such as helminths, will also modify the power of the immune system to respond to infection. Timing may be a critical factor in particular with regard to the comorbidities as they may impact differentially during initial priming of the immune system as compared to recall stages of effector immunity.
Thus, solely the induction of effector immunity, although absolutely required, may not be sufficient to combat complex pathogens such as Mtb that have the capacity to modify many host processes. We will have to elucidate further on the modulation of host immunity by Mtb and other concomitant conditions in order to identify novel strategies to combat this world-threatening infection.
Acknowledgments
Funding
The authors are supported by funds from the Swedish Research Council (VR) (521-2014-3238) and the Swedish Heart and Lung Foundation (HLF) (20160470 and 20160815) (S. Brighenti) as well as the EC HORIZON2020 TBVAC2020 (643381); ECFP7 TANDEM (305279) and the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health (R21AI127133) (S.A. Joosten). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or any funder.
Abbreviations
- TB
tuberculosis
- Mtb
Mycobacterium tuberculosis
- Treg
regulatory T cell
- DC
dendritic cell
- BCG
Bacillus Calmette-Guerin
- HIV
human immunodeficiency virus
- SIV
simian immunodeficiency virus
- Th
T helper
- IFN- γ
interferon- γ
- IL
interleukin
- TNF-α
tumor necrosis factor-α
- NK
natural killer
- NKT
natural killer T-cells
- BAL
bronchoalveolar lavage
- APC
antigen presenting cell
- MAIT
mucosal-associated invariant T-cells
- RAG
recombinase activating gene
- LAM
lipo-arabinomannan
- DURT
donor-unrestricted T-cells
- MR1
mannose receptor 1
- HLA
human leukocyte antigen
- MDR-TB
multidrug-resistant TB
- XDR-TB
extensively drug-resistant TB
- TGF-β
transforming growth factor-β
- KLRG-1
killer cell lectin-like receptor G1
- PD-1
programmed cell death protein-1
- TIM-3
T-cell immunoglobulin and mucin-domain containing-3
- CTLA-4
cytotoxic T-lymphocyte associated protein 4
- Breg cells
regulatory B-cells
- IDO
Indoleamine 2,3-dioxygenase
- TLR2
Toll like receptor 2
- DC-SIGN
dendritic cell-specific ICAM-grabbing non-integrin
- MVA85A
modified vaccinia Ankara expressing antigen 85A
- Mreg
regulatory macrophages
- iNOS
inducible nitric oxide synthase
- Arg1
arginase
- MDSC
myeloid-derived suppressor cells
- DM2
diabetes mellitus type 2
- CMV
cytomegalovirus
- SCFA
short chain fatty acids
- HDAC
histone deacetylases
- FFAR3
free fatty acid receptor 3
Footnotes
DR. SUSANNA BRIGHENTI (Orcid ID : 0000-0001-8540-153X)
Conflict of interest statement
No conflict of interest to declare.
References
- 1.Houben RM, Dodd PJ. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016;13(10):e1002152. doi: 10.1371/journal.pmed.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brighenti S, Ordway DJ. Regulation of Immunity to Tuberculosis. Microbiol Spectr. 2016;4(6) doi: 10.1128/microbiolspec.TBTB2-0006-2016. [DOI] [PubMed] [Google Scholar]
- 3.Rajaram MV, Ni B, Dodd CE, Schlesinger LS. Macrophage immunoregulatory pathways in tuberculosis. Semin Immunol. 2014;26(6):471–85. doi: 10.1016/j.smim.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dockrell HM, Smith SG. What Have We Learnt about BCG Vaccination in the Last 20 Years? Front Immunol. 2017;8:1134. doi: 10.3389/fimmu.2017.01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood MR, Yu EA, Mehta S. The Human Microbiome in the Fight Against Tuberculosis. Am J Trop Med Hyg. 2017;96(6):1274–84. doi: 10.4269/ajtmh.16-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scriba TJ, Coussens AK, Fletcher HA. Human Immunology of Tuberculosis. Microbiol Spectr. 2016;4(5) doi: 10.1128/microbiolspec.TBTB2-0016-2016. [DOI] [PubMed] [Google Scholar]
- 7.Orme IM. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138(1):293–8. [PubMed] [Google Scholar]
- 8.Havlir DV, Barnes PF. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1999;340(5):367–73. doi: 10.1056/NEJM199902043400507. [DOI] [PubMed] [Google Scholar]
- 9.Geldmacher C, Zumla A, Hoelscher M. Interaction between HIV and Mycobacterium tuberculosis: HIV-1-induced CD4 T-cell depletion and the development of active tuberculosis. Curr Opin HIV AIDS. 2012;7(3):268–75. doi: 10.1097/COH.0b013e3283524e32. [DOI] [PubMed] [Google Scholar]
- 10.Diedrich CR, Mattila JT, Klein E, et al. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PLoS One. 2010;5(3):e9611. doi: 10.1371/journal.pone.0009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geldmacher C, Schuetz A, Ngwenyama N, et al. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J Infect Dis. 2008;198(11):1590–8. doi: 10.1086/593017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawn SD, Butera ST, Shinnick TM. Tuberculosis unleashed: the impact of human immunodeficiency virus infection on the host granulomatous response to Mycobacterium tuberculosis. Microbes Infect. 2002;4(6):635–46. doi: 10.1016/s1286-4579(02)01582-4. [DOI] [PubMed] [Google Scholar]
- 13.Newport MJ, Huxley CM, Huston S, et al. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335(26):1941–9. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 14.Chackerian AA, Perera TV, Behar SM. Gamma interferon-producing CD4+ T lymphocytes in the lung correlate with resistance to infection with Mycobacterium tuberculosis. Infect Immun. 2001;69(4):2666–74. doi: 10.1128/IAI.69.4.2666-2674.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ottenhoff TH, Verreck FA, Lichtenauer-Kaligis EG, Hoeve MA, Sanal O, van Dissel JT. Genetics, cytokines and human infectious disease: lessons from weakly pathogenic mycobacteria and salmonellae. Nat Genet. 2002;32(1):97–105. doi: 10.1038/ng0902-97. [DOI] [PubMed] [Google Scholar]
- 16.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259(5102):1739–42. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 17.Flynn JL, Goldstein MM, Chan J, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2(6):561–72. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 18.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345(15):1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 19.Bean AG, Roach DR, Briscoe H, et al. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162(6):3504–11. [PubMed] [Google Scholar]
- 20.Ashenafi S, Aderaye G, Bekele A, et al. Progression of clinical tuberculosis is associated with a Th2 immune response signature in combination with elevated levels of SOCS3. Clin Immunol. 2014;151(2):84–99. doi: 10.1016/j.clim.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Rook GA. Th2 cytokines in susceptibility to tuberculosis. Curr Mol Med. 2007;7(3):327–37. doi: 10.2174/156652407780598557. [DOI] [PubMed] [Google Scholar]
- 22.Mittrucker HW, Steinhoff U, Kohler A, et al. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc Natl Acad Sci U S A. 2007;104(30):12434–9. doi: 10.1073/pnas.0703510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakai S, Kauffman KD, Sallin MA, et al. CD4 T Cell-Derived IFN-gamma Plays a Minimal Role in Control of Pulmonary Mycobacterium tuberculosis Infection and Must Be Actively Repressed by PD-1 to Prevent Lethal Disease. PLoS Pathog. 2016;12(5):e1005667. doi: 10.1371/journal.ppat.1005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kagina BM, Abel B, Scriba TJ, et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. Am J Respir Crit Care Med. 2010;182(8):1073–9. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forbes EK, Sander C, Ronan EO, et al. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. JImmunol. 2008;181(7):4955–64. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151(1):129–35. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- 27.Bruns H, Meinken C, Schauenberg P, et al. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119(5):1167–77. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersson J, Samarina A, Fink J, Rahman S, Grundstrom S. Impaired expression of perforin and granulysin in CD8+ T cells at the site of infection in human chronic pulmonary tuberculosis. Infect Immun. 2007;75(11):5210–22. doi: 10.1128/IAI.00624-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lalvani A, Brookes R, Wilkinson RJ, et al. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1998;95(1):270–5. doi: 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CY, Huang D, Wang RC, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 2009;5(4):e1000392. doi: 10.1371/journal.ppat.1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stenger S, Hanson DA, Teitelbaum R, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282(5386):121–5. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 32.Heinzel AS, Grotzke JE, Lines RA, et al. HLA-E-dependent presentation of Mtb-derived antigen to human CD8+ T cells. J Exp Med. 2002;196(11):1473–81. doi: 10.1084/jem.20020609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gold MC, Cerri S, Smyk-Pearson S, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8(6):e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shang S, Siddiqui S, Bian Y, Zhao J, Wang CR. Nonclassical MHC Ib-restricted CD8+ T Cells Recognize Mycobacterium tuberculosis-Derived Protein Antigens and Contribute to Protection Against Infection. PLoS Pathog. 2016;12(6):e1005688. doi: 10.1371/journal.ppat.1005688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dieli F, Troye-Blomberg M, Ivanyi J, et al. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vgamma9/Vdelta2 T lymphocytes. J Infect Dis. 2001;184(8):1082–5. doi: 10.1086/323600. [DOI] [PubMed] [Google Scholar]
- 36.Lu CC, Wu TS, Hsu YJ, et al. NK cells kill mycobacteria directly by releasing perforin and granulysin. J Leukoc Biol. 2014;96(6):1119–29. doi: 10.1189/jlb.4A0713-363RR. [DOI] [PubMed] [Google Scholar]
- 37.Gansert JL, Kiessler V, Engele M, et al. Human NKT cells express granulysin and exhibit antimycobacterial activity. J Immunol. 2003;170(6):3154–61. doi: 10.4049/jimmunol.170.6.3154. [DOI] [PubMed] [Google Scholar]
- 38.Abebe F. Is interferon-gamma the right marker for bacille Calmette-Guerin-induced immune protection? The missing link in our understanding of tuberculosis immunology. Clin Exp Immunol. 2012;169(3):213–9. doi: 10.1111/j.1365-2249.2012.04614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caccamo N, Guggino G, Joosten SA, et al. Multifunctional CD4+T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol. 2010;40:2211–20. doi: 10.1002/eji.201040455. [DOI] [PubMed] [Google Scholar]
- 40.Kagina BM, Abel B, Scriba TJ, et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. AmJRespirCrit Care Med. 2010;182(8):1073–9. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fletcher HA, Snowden MA, Landry B, et al. T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun. 2016;7:11290. doi: 10.1038/ncomms11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herzmann C, Ernst M, Lange C, et al. Pulmonary immune responses to Mycobacterium tuberculosis in exposed individuals. PLoS One. 2017;12(11):e0187882. doi: 10.1371/journal.pone.0187882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egen JG, Rothfuchs AG, Feng CG, Horwitz MA, Sher A, Germain RN. Intravital imaging reveals limited antigen presentation and T cell effector function in mycobacterial granulomas. Immunity. 2011;34(5):807–19. doi: 10.1016/j.immuni.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallegos AM, Pamer EG, Glickman MS. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. J Exp Med. 2008;205(10):2359–68. doi: 10.1084/jem.20080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. 2011;4(3):288–93. doi: 10.1038/mi.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mourik BC, Lubberts E, de Steenwinkel JEM, Ottenhoff THM, Leenen PJM. Interactions between Type 1 Interferons and the Th17 Response in Tuberculosis: Lessons Learned from Autoimmune Diseases. Front Immunol. 2017;8:294. doi: 10.3389/fimmu.2017.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu ZG, Wang BZ, Li J, Ding ZL, Wang K. Association between interleukin-17 genetic polymorphisms and tuberculosis susceptibility: an updated meta-analysis. Int J Tuberc Lung Dis. 2017;21(12):1307–13. doi: 10.5588/ijtld.17.0345. [DOI] [PubMed] [Google Scholar]
- 48.Gopal R, Monin L, Slight S, et al. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoSPathog. 2014;10(5):e1004099. doi: 10.1371/journal.ppat.1004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Umemura M, Okamoto-Yoshida Y, Yahagi A, et al. Involvement of IL-17A-producing TCR gammadelta T cells in late protective immunity against pulmonary Mycobacterium tuberculosis infection. Immun Inflamm Dis. 2016;4(4):401–12. doi: 10.1002/iid3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coulter F, Parrish A, Manning D, et al. IL-17 Production from T Helper 17, Mucosal-Associated Invariant T, and gammadelta Cells in Tuberculosis Infection and Disease. Front Immunol. 2017;8:1252. doi: 10.3389/fimmu.2017.01252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scriba TJ, Kalsdorf B, Abrahams DA, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180(3):1962–70. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perreau M, Rozot V, Welles HC, et al. Lack of Mycobacterium tuberculosis-specific interleukin-17A-producing CD4+ T cells in active disease. Eur J Immunol. 2013;43(4):939–48. doi: 10.1002/eji.201243090. [DOI] [PubMed] [Google Scholar]
- 53.Matthews K, Wilkinson KA, Kalsdorf B, et al. Predominance of interleukin-22 over interleukin-17 at the site of disease in human tuberculosis. Tuberculosis(Edinb) 2011;91(6):587–93. doi: 10.1016/j.tube.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.da Silva MV, Massaro Junior VJ, Machado JR, et al. Expression pattern of transcription factors and intracellular cytokines reveals that clinically cured tuberculosis is accompanied by an increase in Mycobacterium-specific Th1, Th2, and Th17 cells. Biomed Res Int. 2015;2015:591237. doi: 10.1155/2015/591237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wozniak TM, Saunders BM, Ryan AA, Britton WJ. Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infect Immun. 2010;78(10):4187–94. doi: 10.1128/IAI.01392-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Domingo-Gonzalez R, Das S, Griffiths KL, et al. Interleukin-17 limits hypoxia-inducible factor 1alpha and development of hypoxic granulomas during tuberculosis. JCI Insight. 2017;2(19) doi: 10.1172/jci.insight.92973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monin L, Griffiths KL, Slight S, Lin Y, Rangel-Moreno J, Khader SA. Immune requirements for protective Th17 recall responses to Mycobacterium tuberculosis challenge. Mucosal Immunol. 2015;8(5):1099–109. doi: 10.1038/mi.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doz E, Lombard R, Carreras F, Buzoni-Gatel D, Winter N. Mycobacteria-infected dendritic cells attract neutrophils that produce IL-10 and specifically shut down Th17 CD4 T cells through their IL-10 receptor. J Immunol. 2013;191(7):3818–26. doi: 10.4049/jimmunol.1300527. [DOI] [PubMed] [Google Scholar]
- 59.Sondergaard JN, Laursen JM, Rosholm LB, Brix S. Mycobacterium tuberculosis promotes Th17 expansion via regulation of human dendritic cells toward a high CD14 and low IL-12p70 phenotype that reprograms upon exogenous IFN-gamma. Int Immunol. 2014;26(12):705–16. doi: 10.1093/intimm/dxu085. [DOI] [PubMed] [Google Scholar]
- 60.da Costa AC, Costa-Junior Ade O, de Oliveira FM, et al. A new recombinant BCG vaccine induces specific Th17 and Th1 effector cells with higher protective efficacy against tuberculosis. PLoS One. 2014;9(11):e112848. doi: 10.1371/journal.pone.0112848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verwaerde C, Debrie AS, Dombu C, et al. HBHA vaccination may require both Th1 and Th17 immune responses to protect mice against tuberculosis. Vaccine. 2014;32(47):6240–50. doi: 10.1016/j.vaccine.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 62.Wareham AS, Tree JA, Marsh PD, Butcher PD, Dennis M, Sharpe SA. Evidence for a role for interleukin-17, Th17 cells and iron homeostasis in protective immunity against tuberculosis in cynomolgus macaques. PLoSOne. 2014;9(2):e88149. doi: 10.1371/journal.pone.0088149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.George PJ, Anuradha R, Kumaran PP, Chandrasekaran V, Nutman TB, Babu S. Modulation of mycobacterial-specific Th1 and Th17 cells in latent tuberculosis by coincident hookworm infection. J Immunol. 2013;190(10):5161–8. doi: 10.4049/jimmunol.1203311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Busch M, Herzmann C, Kallert S, et al. Lipoarabinomannan-Responsive Polycytotoxic T Cells Are Associated with Protection in Human Tuberculosis. Am J Respir Crit Care Med. 2016;194(3):345–55. doi: 10.1164/rccm.201509-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. NatImmunol. 2015;16(11):1114–23. doi: 10.1038/ni.3298. [DOI] [PubMed] [Google Scholar]
- 66.Grimaldi D, Le Bourhis L, Sauneuf B, et al. Specific MAIT cell behaviour among innate-like T lymphocytes in critically ill patients with severe infections. Intensive Care Med. 2014;40(2):192–201. doi: 10.1007/s00134-013-3163-x. [DOI] [PubMed] [Google Scholar]
- 67.Gold MC, Cerri S, Smyk-Pearson S, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoSBiol. 2010;8(6):e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kwon YS, Cho YN, Kim MJ, et al. Mucosal-associated invariant T cells are numerically and functionally deficient in patients with mycobacterial infection and reflect disease activity. Tuberculosis (Edinb) 2015;95(3):267–74. doi: 10.1016/j.tube.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 69.Greene JM, Dash P, Roy S, et al. MR1-restricted mucosal-associated invariant T (MAIT) cells respond to mycobacterial vaccination and infection in nonhuman primates. Mucosal Immunol. 2017;10(3):802–13. doi: 10.1038/mi.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sakala IG, Kjer-Nielsen L, Eickhoff CS, et al. Functional Heterogeneity and Antimycobacterial Effects of Mouse Mucosal-Associated Invariant T Cells Specific for Riboflavin Metabolites. J Immunol. 2015;195(2):587–601. doi: 10.4049/jimmunol.1402545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joosten SA, Sullivan LC, Ottenhoff TH. Characteristics of HLA-E Restricted T-Cell Responses and Their Role in Infectious Diseases. J Immunol Res. 2016;2016:2695396. doi: 10.1155/2016/2695396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joosten SA, van Meijgaarden KE, van Weeren PC, et al. Mycobacterium tuberculosis peptides presented by HLA-E molecules are targets for human CD8 T-cells with cytotoxic as well as regulatory activity. PLoSPathog. 2010;6(2):e1000782. doi: 10.1371/journal.ppat.1000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grotzke JE, Harriff MJ, Siler AC, et al. The Mycobacterium tuberculosis phagosome is a HLA-I processing competent organelle. PLoSPathog. 2009;5(4):e1000374. doi: 10.1371/journal.ppat.1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heinzel AS, Grotzke JE, Lines RA, et al. HLA-E-dependent presentation of Mtb-derived antigen to human CD8+ T cells. JExpMed. 2002;196(11):1473–81. doi: 10.1084/jem.20020609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caccamo N, Pietra G, Sullivan LC, et al. Human CD8 T lymphocytes recognize Mycobacterium tuberculosis antigens presented by HLA-E during active tuberculosis and express type 2 cytokines. EurJImmunol. 2015;45(4):1069–81. doi: 10.1002/eji.201445193. [DOI] [PubMed] [Google Scholar]
- 76.Prezzemolo T, van Meijgaarden KE, Franken KL, et al. Detailed characterization of human Mycobacterium tuberculosis specific HLA-E restricted CD8+ T-cells. Eur J Immunol. 2017 doi: 10.1002/eji.201747184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Meijgaarden KE, Haks MC, Caccamo N, Dieli F, Ottenhoff TH, Joosten SA. Human CD8+ T-cells Recognizing Peptides from Mycobacterium tuberculosis (Mtb) Presented by HLA-E Have an Unorthodox Th2-like, Multifunctional, Mtb Inhibitory Phenotype and Represent a Novel Human T-cell Subset. PLoSPathog. 2015;11(3):e1004671. doi: 10.1371/journal.ppat.1004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bian Y, Shang S, Siddiqui S, et al. MHC Ib molecule Qa-1 presents Mycobacterium tuberculosis peptide antigens to CD8+ T cells and contributes to protection against infection. PLoS Pathog. 2017;13(5):e1006384. doi: 10.1371/journal.ppat.1006384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fletcher HA, Filali-Mouhim A, Nemes E, et al. Human newborn bacille Calmette-Guerin vaccination and risk of tuberculosis disease: a case-control study. BMC Med. 2016;14:76. doi: 10.1186/s12916-016-0617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boer MC, Prins C, van Meijgaarden KE, van Dissel JT, Ottenhoff TH, Joosten SA. Mycobacterium bovis BCG Vaccination Induces Divergent Proinflammatory or Regulatory T Cell Responses in Adults. ClinVaccine Immunol. 2015;22(7):778–88. doi: 10.1128/CVI.00162-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lindenstrom T, Moguche A, Damborg M, Agger EM, Urdahl K, Andersen P. T Cells Primed by Live Mycobacteria Versus a Tuberculosis Subunit Vaccine Exhibit Distinct Functional Properties. EBioMedicine. 2017 doi: 10.1016/j.ebiom.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kleinnijenhuis J, Quintin J, Preijers F, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109(43):17537–42. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kleinnijenhuis J, van Crevel R, Netea MG. Trained immunity: consequences for the heterologous effects of BCG vaccination. Trans R Soc Trop Med Hyg. 2015;109(1):29–35. doi: 10.1093/trstmh/tru168. [DOI] [PubMed] [Google Scholar]
- 84.Lerm M, Netea MG. Trained immunity: a new avenue for tuberculosis vaccine development. J Intern Med. 2016;279(4):337–46. doi: 10.1111/joim.12449. [DOI] [PubMed] [Google Scholar]
- 85.Netea MG, Joosten LA, Latz E, et al. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verma D, Parasa VR, Raffetseder J, et al. Anti-mycobacterial activity correlates with altered DNA methylation pattern in immune cells from BCG-vaccinated subjects. Sci Rep. 2017;7(1):12305. doi: 10.1038/s41598-017-12110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaufmann E, Sanz J, Dunn JL, et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell. 2018;172(1–2):176–90 e19. doi: 10.1016/j.cell.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 88.Joosten SA, Meijgaarden KEV, Arend SM, et al. Mycobacterial growth inhibition is associated with trained innate immunity. J Clin Invest. 2018 doi: 10.1172/JCI97508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scriba TJ, Penn-Nicholson A, Shankar S, et al. Sequential inflammatory processes define human progression from M. tuberculosis infection to tuberculosis disease. PLoS Pathog. 2017;13(11):e1006687. doi: 10.1371/journal.ppat.1006687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zelmer A, Tanner R, Stylianou E, et al. A new tool for tuberculosis vaccine screening: Ex vivo Mycobacterial Growth Inhibition Assay indicates BCG-mediated protection in a murine model of tuberculosis. BMC Infect Dis. 2016;16:412. doi: 10.1186/s12879-016-1751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tanner R, O’Shea MK, Fletcher HA, McShane H. In vitro mycobacterial growth inhibition assays: A tool for the assessment of protective immunity and evaluation of tuberculosis vaccine efficacy. Vaccine. 2016;34(39):4656–65. doi: 10.1016/j.vaccine.2016.07.058. [DOI] [PubMed] [Google Scholar]
- 92.Fletcher HA, Tanner R, Wallis RS, et al. Inhibition of mycobacterial growth in vitro following primary but not secondary vaccination with Mycobacterium bovis BCG. ClinVaccine Immunol. 2013;20(11):1683–9. doi: 10.1128/CVI.00427-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marsay L, Matsumiya M, Tanner R, et al. Mycobacterial growth inhibition in murine splenocytes as a surrogate for protection against Mycobacterium tuberculosis (M.tb) Tuberculosis (Edinb) 2013;93(5):551–7. doi: 10.1016/j.tube.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 94.Baguma R, Penn-Nicholson A, Smit E, et al. Application of a whole blood mycobacterial growth inhibition assay to study immunity against Mycobacterium tuberculosis in a high tuberculosis burden population. PLoS One. 2017;12(9):e0184563. doi: 10.1371/journal.pone.0184563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ribeiro-Rodrigues R, Resende Co T, Rojas R, et al. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006;144(1):25–34. doi: 10.1111/j.1365-2249.2006.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sharma PK, Saha PK, Singh A, Sharma SK, Ghosh B, Mitra DK. FoxP3+ regulatory T cells suppress effector T-cell function at pathologic site in miliary tuberculosis. Am J Respir Crit Care Med. 2009;179(11):1061–70. doi: 10.1164/rccm.200804-529OC. [DOI] [PubMed] [Google Scholar]
- 97.Davids M, Pooran AS, Pietersen E, et al. Regulatory T Cells Subvert Mycobacterial Containment in Patients Failing Extensively Drug-resistant TB Treatment. Am J Respir Crit Care Med. 2018 doi: 10.1164/rccm.201707-1441OC. [DOI] [PubMed] [Google Scholar]
- 98.Rahman S, Gudetta B, Fink J, et al. Compartmentalization of immune responses in human tuberculosis: few CD8+ effector T cells but elevated levels of FoxP3+ regulatory t cells in the granulomatous lesions. Am J Pathol. 2009;174(6):2211–24. doi: 10.2353/ajpath.2009.080941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boer MC, van Meijgaarden KE, Goletti D, et al. KLRG1 and PD-1 expression are increased on T-cells following tuberculosis-treatment and identify cells with different proliferative capacities in BCG-vaccinated adults. Tuberculosis (Edinb) 2016;97:163–71. doi: 10.1016/j.tube.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 100.Sakai S, Kauffman KD, Schenkel JM, et al. Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J Immunol. 2014;192(7):2965–9. doi: 10.4049/jimmunol.1400019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Torrado E, Fountain JJ, Liao M, et al. Interleukin 27R regulates CD4+ T cell phenotype and impacts protective immunity during Mycobacterium tuberculosis infection. J Exp Med. 2015;212(9):1449–63. doi: 10.1084/jem.20141520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu Z, Zhao HM, Li CL, et al. The role of KLRG1 in human CD4+ T cell immunity against tuberculosis. J Infect Dis. 2018 doi: 10.1093/infdis/jiy046. [DOI] [PubMed] [Google Scholar]
- 103.Lindenstrom T, Knudsen NP, Agger EM, Andersen P. Control of chronic mycobacterium tuberculosis infection by CD4 KLRG1- IL-2-secreting central memory cells. J Immunol. 2013;190(12):6311–9. doi: 10.4049/jimmunol.1300248. [DOI] [PubMed] [Google Scholar]
- 104.Phillips BL, Gautam US, Bucsan AN, et al. LAG-3 potentiates the survival of Mycobacterium tuberculosis in host phagocytes by modulating mitochondrial signaling in an in-vitro granuloma model. PLoS One. 2017;12(9):e0180413. doi: 10.1371/journal.pone.0180413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jayaraman P, Jacques MK, Zhu C, et al. TIM3 Mediates T Cell Exhaustion during Mycobacterium tuberculosis Infection. PLoS Pathog. 2016;12(3):e1005490. doi: 10.1371/journal.ppat.1005490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Merlo A, Saverino D, Tenca C, Grossi CE, Bruno S, Ciccone E. CD85/LIR-1/ILT2 and CD152 (cytotoxic T lymphocyte antigen 4) inhibitory molecules down-regulate the cytolytic activity of human CD4+ T-cell clones specific for Mycobacterium tuberculosis. Infect Immun. 2001;69(10):6022–9. doi: 10.1128/IAI.69.10.6022-6029.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thye T, Scarisbrick G, Browne EN, et al. CTLA4 autoimmunity-associated genotype contributes to severe pulmonary tuberculosis in an African population. PLoS One. 2009;4(7):e6307. doi: 10.1371/journal.pone.0006307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tsai MC, Chakravarty S, Zhu G, et al. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8(2):218–32. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 109.Ulrichs T, Kosmiadi GA, Trusov V, et al. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J Pathol. 2004;204(2):217–28. doi: 10.1002/path.1628. [DOI] [PubMed] [Google Scholar]
- 110.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol. 2010;10(4):236–47. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Flores-Borja F, Bosma A, Ng D, et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med. 2013;5(173):173ra23. doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- 112.Zhang M, Zheng X, Zhang J, et al. CD19(+)CD1d(+)CD5(+) B cell frequencies are increased in patients with tuberculosis and suppress Th17 responses. Cell Immunol. 2012;274(1–2):89–97. doi: 10.1016/j.cellimm.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 113.Zhang M, Zeng G, Yang Q, et al. Anti-tuberculosis treatment enhances the production of IL-22 through reducing the frequencies of regulatory B cell. Tuberculosis (Edinb) 2014;94(3):238–44. doi: 10.1016/j.tube.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 114.Joosten SA, van Meijgaarden KE, Del Nonno F, et al. Patients with Tuberculosis Have a Dysfunctional Circulating B-Cell Compartment, Which Normalizes following Successful Treatment. PLoS Pathog. 2016;12(6):e1005687. doi: 10.1371/journal.ppat.1005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van Rensburg IC, Wagman C, Stanley K, et al. Successful TB treatment induces B-cells expressing FASL and IL5RA mRNA. Oncotarget. 2017;8(2):2037–43. doi: 10.18632/oncotarget.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim H, Kwon KW, Kim WS, Shin SJ. Virulence-dependent induction of interleukin-10-producing-tolerogenic dendritic cells by Mycobacterium tuberculosis impedes optimal T helper type 1 proliferation. Immunology. 2017;151(2):177–90. doi: 10.1111/imm.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harden JL, Egilmez NK. Indoleamine 2,3-dioxygenase and dendritic cell tolerogenicity. Immunol Invest. 2012;41(6–7):738–64. doi: 10.3109/08820139.2012.676122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Trinath J, Maddur MS, Kaveri SV, Balaji KN, Bayry J. Mycobacterium tuberculosis promotes regulatory T-cell expansion via induction of programmed death-1 ligand 1 (PD-L1, CD274) on dendritic cells. J Infect Dis. 2012;205(4):694–6. doi: 10.1093/infdis/jir820. [DOI] [PubMed] [Google Scholar]
- 119.Suzuki Y, Miwa S, Akamatsu T, et al. Indoleamine 2,3-dioxygenase in the pathogenesis of tuberculous pleurisy. Int J Tuberc Lung Dis. 2013;17(11):1501–6. doi: 10.5588/ijtld.13.0082. [DOI] [PubMed] [Google Scholar]
- 120.Suzuki Y, Suda T, Asada K, et al. Serum indoleamine 2,3-dioxygenase activity predicts prognosis of pulmonary tuberculosis. Clin Vaccine Immunol. 2012;19(3):436–42. doi: 10.1128/CVI.05402-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tanner R, Kakalacheva K, Miller E, et al. Serum indoleamine 2,3-dioxygenase activity is associated with reduced immunogenicity following vaccination with MVA85A. BMC Infect Dis. 2014;14:660. doi: 10.1186/s12879-014-0660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 123.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thomas AC, Mattila JT. “Of mice and men”: arginine metabolism in macrophages. Front Immunol. 2014;5:479. doi: 10.3389/fimmu.2014.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mattila JT, Ojo OO, Kepka-Lenhart D, et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J Immunol. 2013;191(2):773–84. doi: 10.4049/jimmunol.1300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Flynn JL, Chan J, Lin PL. Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal Immunol. 2011;4(3):271–8. doi: 10.1038/mi.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Redente EF, Higgins DM, Dwyer-Nield LD, Orme IM, Gonzalez-Juarrero M, Malkinson AM. Differential polarization of alveolar macrophages and bone marrow-derived monocytes following chemically and pathogen-induced chronic lung inflammation. J Leukoc Biol. 2010;88(1):159–68. doi: 10.1189/jlb.0609378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kahnert A, Seiler P, Stein M, et al. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur J Immunol. 2006;36(3):631–47. doi: 10.1002/eji.200535496. [DOI] [PubMed] [Google Scholar]
- 129.El Kasmi KC, Qualls JE, Pesce JT, et al. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol. 2008;9(12):1399–406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang Z, Luo Q, Guo Y, et al. Mycobacterium tuberculosis-Induced Polarization of Human Macrophage Orchestrates the Formation and Development of Tuberculous Granulomas In Vitro. PLoS One. 2015;10(6):e0129744. doi: 10.1371/journal.pone.0129744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Day J, Friedman A, Schlesinger LS. Modeling the immune rheostat of macrophages in the lung in response to infection. Proc Natl Acad Sci U S A. 2009;106(27):11246–51. doi: 10.1073/pnas.0904846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Savage ND, de Boer T, Walburg KV, et al. Human anti-inflammatory macrophages induce Foxp3+ GITR+ CD25+ regulatory T cells, which suppress via membrane-bound TGFbeta-1. J Immunol. 2008;181(3):2220–6. doi: 10.4049/jimmunol.181.3.2220. [DOI] [PubMed] [Google Scholar]
- 133.Blumenthal A, Nagalingam G, Huch JH, et al. M. tuberculosis induces potent activation of IDO-1, but this is not essential for the immunological control of infection. PLoS One. 2012;7(5):e37314. doi: 10.1371/journal.pone.0037314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mehra S, Alvarez X, Didier PJ, et al. Granuloma correlates of protection against tuberculosis and mechanisms of immune modulation by Mycobacterium tuberculosis. J Infect Dis. 2013;207(7):1115–27. doi: 10.1093/infdis/jis778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gautam US, Foreman TW, Bucsan AN, et al. In vivo inhibition of tryptophan catabolism reorganizes the tuberculoma and augments immune-mediated control of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2018;115(1):E62–E71. doi: 10.1073/pnas.1711373114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lugo-Villarino G, Hudrisier D, Benard A, Neyrolles O. Emerging trends in the formation and function of tuberculosis granulomas. Front Immunol. 2012;3:405. doi: 10.3389/fimmu.2012.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dorhoi A, Du Plessis N. Monocytic Myeloid-Derived Suppressor Cells in Chronic Infections. Front Immunol. 2017;8:1895. doi: 10.3389/fimmu.2017.01895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.du Plessis N, Loebenberg L, Kriel M, et al. Increased frequency of myeloid-derived suppressor cells during active tuberculosis and after recent mycobacterium tuberculosis infection suppresses T-cell function. Am J Respir Crit Care Med. 2013;188(6):724–32. doi: 10.1164/rccm.201302-0249OC. [DOI] [PubMed] [Google Scholar]
- 139.Knaul JK, Jorg S, Oberbeck-Mueller D, et al. Lung-residing myeloid-derived suppressors display dual functionality in murine pulmonary tuberculosis. Am J Respir Crit Care Med. 2014;190(9):1053–66. doi: 10.1164/rccm.201405-0828OC. [DOI] [PubMed] [Google Scholar]
- 140.Dorhoi A, Kaufmann SH. Versatile myeloid cell subsets contribute to tuberculosis-associated inflammation. Eur J Immunol. 2015;45(8):2191–202. doi: 10.1002/eji.201545493. [DOI] [PubMed] [Google Scholar]
- 141.Du Plessis N, Jacobs R, Gutschmidt A, et al. Phenotypically resembling myeloid derived suppressor cells are increased in children with HIV and exposed/infected with Mycobacterium tuberculosis. Eur J Immunol. 2017;47(1):107–18. doi: 10.1002/eji.201646658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhan X, Fang Y, Hu S, et al. IFN-gamma differentially regulates subsets of Gr-1(+)CD11b(+) myeloid cells in chronic inflammation. Mol Immunol. 2015;66(2):451–62. doi: 10.1016/j.molimm.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 143.El Daker S, Sacchi A, Tempestilli M, et al. Granulocytic myeloid derived suppressor cells expansion during active pulmonary tuberculosis is associated with high nitric oxide plasma level. PLoS One. 2015;10(4):e0123772. doi: 10.1371/journal.pone.0123772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang B, Wang X, Jiang J, Zhai F, Cheng X. Identification of CD244-expressing myeloid-derived suppressor cells in patients with active tuberculosis. Immunol Lett. 2014;158(1–2):66–72. doi: 10.1016/j.imlet.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 145.Diedrich CR, Flynn JL. HIV-1/mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infect Immun. 2011;79(4):1407–17. doi: 10.1128/IAI.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Akuffo H, Britton S, Schon T. Worms and Humans: A Happy Divorce? For Immunopathol Dis Therap. 2015;6(1–2):27–32. doi: 10.1615/ForumImmunDisTher.2015015300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Restrepo BI, Schlesinger LS. Host-pathogen interactions in tuberculosis patients with type 2 diabetes mellitus. Tuberculosis (Edinb) 2013;93(Suppl):S10–4. doi: 10.1016/S1472-9792(13)70004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Muller J, Matsumiya M, Snowden MA, et al. Cytomegalovirus infection is a risk factor for TB disease in Infants. bioRxiv. 2017 doi: 10.1172/jci.insight.130090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Juffermans NP, Speelman P, Verbon A, et al. Patients with active tuberculosis have increased expression of HIV coreceptors CXCR4 and CCR5 on CD4(+) T cells. Clin Infect Dis. 2001;32(4):650–2. doi: 10.1086/318701. [DOI] [PubMed] [Google Scholar]
- 150.Veazey RS, Mansfield KG, Tham IC, et al. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J Virol. 2000;74(23):11001–7. doi: 10.1128/jvi.74.23.11001-11007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Day CL, Abrahams DA, Harris LD, et al. HIV-1 Infection Is Associated with Depletion and Functional Impairment of Mycobacterium tuberculosis-Specific CD4 T Cells in Individuals with Latent Tuberculosis Infection. J Immunol. 2017;199(6):2069–80. doi: 10.4049/jimmunol.1700558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jambo KC, Sepako E, Fullerton DG, et al. Bronchoalveolar CD4+ T cell responses to respiratory antigens are impaired in HIV-infected adults. Thorax. 2011;66(5):375–82. doi: 10.1136/thx.2010.153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kalsdorf B, Scriba TJ, Wood K, et al. HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. Am J Respir Crit Care Med. 2009;180(12):1262–70. doi: 10.1164/rccm.200907-1011OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ravn P, Pedersen BK. Mycobacterium avium and purified protein derivative-specific cytotoxicity mediated by CD4+ lymphocytes from healthy HIV-seropositive and-seronegative individuals. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12(5):433–41. doi: 10.1097/00042560-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 155.Hirsch CS, Baseke J, Kafuluma JL, Nserko M, Mayanja-Kizza H, Toossi Z. Expansion and productive HIV-1 infection of Foxp3 positive CD4 T cells at pleural sites of HIV/TB co-infection. J Clin Exp Immunol. 2016;1(1) [PMC free article] [PubMed] [Google Scholar]
- 156.Patel NR, Zhu J, Tachado SD, et al. HIV impairs TNF-alpha mediated macrophage apoptotic response to Mycobacterium tuberculosis. J Immunol. 2007;179(10):6973–80. doi: 10.4049/jimmunol.179.10.6973. [DOI] [PubMed] [Google Scholar]
- 157.Garrait V, Cadranel J, Esvant H, et al. Tuberculosis generates a microenvironment enhancing the productive infection of local lymphocytes by HIV. J Immunol. 1997;159(6):2824–30. [PubMed] [Google Scholar]
- 158.Singh SK, Andersson AM, Ellegard R, et al. HIV Interferes with Mycobacterium tuberculosis Antigen Presentation in Human Dendritic Cells. Am J Pathol. 2016;186(12):3083–93. doi: 10.1016/j.ajpath.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 159.Sia JK, Bizzell E, Madan-Lala R, Rengarajan J. Engaging the CD40-CD40L pathway augments T-helper cell responses and improves control of Mycobacterium tuberculosis infection. PLoS Pathog. 2017;13(8):e1006530. doi: 10.1371/journal.ppat.1006530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang M, Gong J, Iyer DV, Jones BE, Modlin RL, Barnes PF. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J Clin Invest. 1994;94(6):2435–42. doi: 10.1172/JCI117611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Klein SA, Dobmeyer JM, Dobmeyer TS, et al. Demonstration of the Th1 to Th2 cytokine shift during the course of HIV-1 infection using cytoplasmic cytokine detection on single cell level by flow cytometry. AIDS. 1997;11(9):1111–8. doi: 10.1097/00002030-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 162.Bell LC, Pollara G, Pascoe M, et al. In Vivo Molecular Dissection of the Effects of HIV-1 in Active Tuberculosis. PLoS Pathog. 2016;12(3):e1005469. doi: 10.1371/journal.ppat.1005469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5(7):e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Martens GW, Arikan MC, Lee J, Ren F, Greiner D, Kornfeld H. Tuberculosis susceptibility of diabetic mice. Am J Respir Cell Mol Biol. 2007;37(5):518–24. doi: 10.1165/rcmb.2006-0478OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Alim MA, Sikder S, Bridson TL, Rush CM, Govan BL, Ketheesan N. Anti-mycobacterial function of macrophages is impaired in a diet induced model of type 2 diabetes. Tuberculosis (Edinb) 2017;102:47–54. doi: 10.1016/j.tube.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 166.Stew SS, Martinez PJ, Schlesinger LS, Restrepo BI. Differential expression of monocyte surface markers among TB patients with diabetes co-morbidity. Tuberculosis (Edinb) 2013;93(Suppl):S78–82. doi: 10.1016/S1472-9792(13)70015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yamashiro S, Kawakami K, Uezu K, et al. Lower expression of Th1-related cytokines and inducible nitric oxide synthase in mice with streptozotocin-induced diabetes mellitus infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2005;139(1):57–64. doi: 10.1111/j.1365-2249.2005.02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Al-Attiyah RJ, Mustafa AS. Mycobacterial antigen-induced T helper type 1 (Th1) and Th2 reactivity of peripheral blood mononuclear cells from diabetic and non-diabetic tuberculosis patients and Mycobacterium bovis bacilli Calmette-Guerin (BCG)-vaccinated healthy subjects. Clin Exp Immunol. 2009;158(1):64–73. doi: 10.1111/j.1365-2249.2009.04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sun Q, Zhang Q, Xiao H, Cui H, Su B. Significance of the frequency of CD4+CD25+CD127- T-cells in patients with pulmonary tuberculosis and diabetes mellitus. Respirology. 2012;17(5):876–82. doi: 10.1111/j.1440-1843.2012.02184.x. [DOI] [PubMed] [Google Scholar]
- 170.Kumar NP, Sridhar R, Banurekha VV, et al. Type 2 diabetes mellitus coincident with pulmonary tuberculosis is associated with heightened systemic type 1, type 17, and other proinflammatory cytokines. Ann Am Thorac Soc. 2013;10(5):441–9. doi: 10.1513/AnnalsATS.201305-112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Prada-Medina CA, Fukutani KF, Pavan Kumar N, et al. Systems Immunology of Diabetes-Tuberculosis Comorbidity Reveals Signatures of Disease Complications. Sci Rep. 2017;7(1):1999. doi: 10.1038/s41598-017-01767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Nutman TB, Babu S. Expansion of pathogen-specific T-helper 1 and T-helper 17 cells in pulmonary tuberculosis with coincident type 2 diabetes mellitus. J Infect Dis. 2013;208(5):739–48. doi: 10.1093/infdis/jit241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kumar NP, Sridhar R, Nair D, Banurekha VV, Nutman TB, Babu S. Type 2 diabetes mellitus is associated with altered CD8(+) T and natural killer cell function in pulmonary tuberculosis. Immunology. 2015;144(4):677–86. doi: 10.1111/imm.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kumar NP, Moideen K, Viswanathan V, Kornfeld H, Babu S. Effect of standard tuberculosis treatment on naive, memory and regulatory T-cell homeostasis in tuberculosis-diabetes co-morbidity. Immunology. 2016;149(1):87–97. doi: 10.1111/imm.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Willinger T, Freeman T, Hasegawa H, McMichael AJ, Callan MF. Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets. J Immunol. 2005;175(9):5895–903. doi: 10.4049/jimmunol.175.9.5895. [DOI] [PubMed] [Google Scholar]
- 176.Resende Co T, Hirsch CS, Toossi Z, Dietze R, Ribeiro-Rodrigues R. Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin Exp Immunol. 2007;147(1):45–52. doi: 10.1111/j.1365-2249.2006.03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Abate E, Belayneh M, Idh J, et al. Asymptomatic Helminth Infection in Active Tuberculosis Is Associated with Increased Regulatory and Th-2 Responses and a Lower Sputum Smear Positivity. PLoS Negl Trop Dis. 2015;9(8):e0003994. doi: 10.1371/journal.pntd.0003994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Abate E, Elias D, Getachew A, et al. Effects of albendazole on the clinical outcome and immunological responses in helminth co-infected tuberculosis patients: a double blind randomised clinical trial. Int J Parasitol. 2015;45(2–3):133–40. doi: 10.1016/j.ijpara.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 179.George PJ, Anuradha R, Kumar NP, et al. Helminth infections coincident with active pulmonary tuberculosis inhibit mono- and multifunctional CD4+ and CD8+ T cell responses in a process dependent on IL-10. PLoS Pathog. 2014;10(9):e1004375. doi: 10.1371/journal.ppat.1004375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Potian JA, Rafi W, Bhatt K, McBride A, Gause WC, Salgame P. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J Exp Med. 2011;208(9):1863–74. doi: 10.1084/jem.20091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Elias D, Britton S, Aseffa A, Engers H, Akuffo H. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-beta production. Vaccine. 2008;26(31):3897–902. doi: 10.1016/j.vaccine.2008.04.083. [DOI] [PubMed] [Google Scholar]
- 182.Correa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology. 2016;5(4):e73. doi: 10.1038/cti.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Alvarez-Curto E, Milligan G. Metabolism meets immunity: The role of free fatty acid receptors in the immune system. Biochem Pharmacol. 2016;114:3–13. doi: 10.1016/j.bcp.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 184.Hoeppli RE, Wu D, Cook L, Levings MK. The environment of regulatory T cell biology: cytokines, metabolites, and the microbiome. Front Immunol. 2015;6:61. doi: 10.3389/fimmu.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mirkovic B, Murray MA, Lavelle GM, et al. The Role of Short-Chain Fatty Acids, Produced by Anaerobic Bacteria, in the Cystic Fibrosis Airway. Am J Respir Crit Care Med. 2015;192(11):1314–24. doi: 10.1164/rccm.201505-0943OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ghorbani P, Santhakumar P, Hu Q, et al. Short-chain fatty acids affect cystic fibrosis airway inflammation and bacterial growth. Eur Respir J. 2015;46(4):1033–45. doi: 10.1183/09031936.00143614. [DOI] [PubMed] [Google Scholar]
- 187.Botero LE, Delgado-Serrano L, Cepeda ML, et al. Respiratory tract clinical sample selection for microbiota analysis in patients with pulmonary tuberculosis. Microbiome. 2014;2:29. doi: 10.1186/2049-2618-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dube FS, Kaba M, Robberts FJ, et al. Respiratory microbes present in the nasopharynx of children hospitalised with suspected pulmonary tuberculosis in Cape Town, South Africa. BMC Infect Dis. 2016;16(1):597. doi: 10.1186/s12879-016-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cui Z, Zhou Y, Li H, et al. Complex sputum microbial composition in patients with pulmonary tuberculosis. BMC Microbiol. 2012;12:276. doi: 10.1186/1471-2180-12-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cheung MK, Lam WY, Fung WY, et al. Sputum microbiota in tuberculosis as revealed by 16S rRNA pyrosequencing. PLoS One. 2013;8(1):e54574. doi: 10.1371/journal.pone.0054574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Krishna P, Jain A, Bisen PS. Microbiome diversity in the sputum of patients with pulmonary tuberculosis. Eur J Clin Microbiol Infect Dis. 2016;35(7):1205–10. doi: 10.1007/s10096-016-2654-4. [DOI] [PubMed] [Google Scholar]
- 192.Wu J, Liu W, He L, et al. Sputum microbiota associated with new, recurrent and treatment failure tuberculosis. PLoS One. 2013;8(12):e83445. doi: 10.1371/journal.pone.0083445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhou Y, Lin F, Cui Z, et al. Correlation between Either Cupriavidus or Porphyromonas and Primary Pulmonary Tuberculosis Found by Analysing the Microbiota in Patients’ Bronchoalveolar Lavage Fluid. PLoS One. 2015;10(5):e0124194. doi: 10.1371/journal.pone.0124194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Segal LN, Clemente JC, Li Y, et al. Anaerobic Bacterial Fermentation Products Increase Tuberculosis Risk in Antiretroviral-Drug-Treated HIV Patients. Cell Host Microbe. 2017;21(4):530–7 e4. doi: 10.1016/j.chom.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Khan N, Vidyarthi A, Nadeem S, Negi S, Nair G, Agrewala JN. Alteration in the Gut Microbiota Provokes Susceptibility to Tuberculosis. Front Immunol. 2016;7:529. doi: 10.3389/fimmu.2016.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Majlessi L, Sayes F, Bureau JF, et al. Colonization with Helicobacter is concomitant with modified gut microbiota and drastic failure of the immune control of Mycobacterium tuberculosis. Mucosal Immunol. 2017;10(5):1178–89. doi: 10.1038/mi.2016.140. [DOI] [PubMed] [Google Scholar]
- 197.Luo M, Liu Y, Wu P, et al. Alternation of Gut Microbiota in Patients with Pulmonary Tuberculosis. Front Physiol. 2017;8:822. doi: 10.3389/fphys.2017.00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lachmandas E, van den Heuvel CN, Damen MS, Cleophas MC, Netea MG, van Crevel R. Diabetes Mellitus and Increased Tuberculosis Susceptibility: The Role of Short-Chain Fatty Acids. J Diabetes Res. 2016;2016:6014631. doi: 10.1155/2016/6014631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Scriba TJ, Carpenter C, Pro SC, et al. Differential Recognition of Mycobacterium tuberculosis-Specific Epitopes as a Function of Tuberculosis Disease History. Am J Respir Crit Care Med. 2017;196(6):772–81. doi: 10.1164/rccm.201706-1208OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wipperman MF, Fitzgerald DW, Juste MAJ, et al. Antibiotic treatment for Tuberculosis induces a profound dysbiosis of the microbiome that persists long after therapy is completed. Sci Rep. 2017;7(1):10767. doi: 10.1038/s41598-017-10346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Namasivayam S, Maiga M, Yuan W, et al. Longitudinal profiling reveals a persistent intestinal dysbiosis triggered by conventional anti-tuberculosis therapy. Microbiome. 2017;5(1):71. doi: 10.1186/s40168-017-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


