Abstract
The essential oil in leaves of Polygonum minus Huds., a local aromatic plant, were identified by a pipeline of gas chromatography (GC) techniques coupled with mass-spectrometry (MS), flame ionization detector (FID) and two dimensional gas chromatography time of flight mass spectrometry (GC×GC–TOF MS). A total of 48 compounds with a good match and high probability values were identified using this technique. Meanwhile, 42 compounds were successfully identified in this study using GC-MS, a significantly larger number than in previous studies. GC-FID was used in determining the retention indices of chemical components in P. minus essential oil. The result also showed the efficiency and reliability were greatly improved when chemometric methods and retention indices were used in identification and quantification of chemical components in plant essential oil.
Keywords: comprehensive two-dimensional gas chromatography, volatile oil, TOF MS, Polygonum minus Huds, GC-MS, retention indices
1. Introduction
Polygonum minus Huds, commonly known as kesum, is widely used in Malaysian cooking, and several traditional practices utilise the leaves and stems of this plant [1]. Kesum is an aromatic plant that produces high levels of essential oil (72.54%) containing aliphatic aldehydes [2]. Yaacob [2] identified decanal (24.36%) and dodecanal (48.18%) as the two dominant aldehydes that contribute to the flavour of kesum. Apart from decanal and dodecanal, Yaacob also found that kesum contains 1-decanol (2.49%), 1-dodecanol (2.44%), undecanal (1.77%), tetradecanal (1.42%), 1-undecanol (1.41%), nonanal (0.86%), 1-nonanol (0.76%), and β-caryophyllene (0.18%). As a result, kesum is believed to have great potential as a natural source of aliphatic aldehydes, which could be useful as food additives and in the perfume industry.
With the development of botanical drugs, including traditional herbal medicines, analysis of their bioactive components is becoming more popular. Many botanical drugs have bioactive components in their essential oils, so characterization of plant essential oils it is an important and meaningful task. Gas chromatography (GC) or gas chromatography-mass spectroscopy (GC-MS) are used almost exclusively for the qualitative analysis of the volatiles [3].
Natural essential oils are usually mixtures of terpenoids (mainly monoterpenoids and sesquiterpenoids), aromatic compounds and aliphatic compounds. As mass spectra of these compounds are usually very similar, peak identification often becomes very difficult and sometimes impossible. In order to address the qualitative determination of composition of complex samples by GC-MS and to increase the reliability of the analytical results, it is necessary to utilize retention indices identities [4].
Meanwhile, comprehensive, two-dimensional gas chromatography (GC×GC) also has been extensively applied in the essential oil study [5]. This technique has also been successfully used in the industrial analysis of plant materials to improve component separation and identification. In addition, an analysis of Artemisia annua L. volatile oils using multi-dimensional gas chromatography has indicated that this technique can achieve the complete separation of a wide range of terpenes [6].
The objective of this study was to demonstrate different gas chromatography approaches to analyse the composition of the essential oils of kesum, with the hope that the improved component separation and identification would allow for a determination of unidentified minor components that may strongly influence the overall quality of the oil.
2. Results and Discussion
Using GC–MS, Yaacob detected only 10 components in kesum essential oil [2], with decanal and dodecanal being identified as marker compounds. According to the literature [7], a similarity and reverse number greater than 800 and a probability value greater than 1,000 indicate that an acquired mass spectrum is a good match with a library spectrum. Further identification information, including retention time, similarity, reverse, and probability values, greatly increases the reliability of this analysis. For a comparison study, we have also applied kesum essential oil on GC-MS. In our analysis we found 42 significant compounds in kesum essential oil, significantly more than the number reported by Yaacob [2], and all these compounds had similarity indexes or reverse similarities greater than 800 (Table 1). The retention indices for each compound are also presented in Table 1.
Table 1.
Compounds identified in the essential oil of kesum using gas chromatography mass spectrometry (GC-MS).
| No. | Rt | Compound | *Retention Indices | Formula | Similarity | R.Match | Probability(%) | Content (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.066 | Hexanal | 803 | C6H12O | 917 | 948 | 50 | 0.05 |
| 2 | 6.922 | 1-Hexanol | 868 | C6H14O | 888 | 895 | 39.1 | 0.09 |
| 3 | 8.932 | α-Pinene | 932 | C10H16 | 953 | 954 | 17.2 | 0.39 |
| 4 | 14.979 | Undecane | 1101 | C11H24 | 947 | 948 | 51.5 | 0.41 |
| 5 | 15.154 | Nonanal | 1106 | C9H18O | 916 | 933 | 78.2 | 0.15 |
| 6 | 17.581 | 1-Nonanol | 1173 | C9H20O | 887 | 944 | 45.1 | 0.05 |
| 7 | 18.857 | Decanal | 1209 | C10H20O | 950 | 950 | 74.3 | 16.263 |
| 8 | 21.113 | 1-Decanol | 1274 | C10H22O | 951 | 959 | 31.9 | 12.68 |
| 9 | 21.484 | Isobornyl acetate | 1285 | C12H20O2 | 858 | 879 | 18 | 2.39 |
| 10 | 22.289 | Undecanal | 1308 | C11H22O | 950 | 959 | 60.7 | 0.14 |
| 11 | 24.412 | n-Decanoic acid | 1373 | C10H20O2 | 918 | 920 | 57.7 | 0.52 |
| 12 | 24.495 | α-Cubebene | 1376 | C15H24 | 757 | 823 | 10.2 | 0.37 |
| 13 | 24.9 | Xanthorrhizol | 1388 | C15H22O | 876 | 923 | 59.9 | 0.1 |
| 14 | 25.709 | Dodecanal | 1413 | C12H24O | 955 | 972 | 56 | 43.47 |
| 15 | 25.926 | (E)-Caryophyllene | 1420 | C15H24 | 947 | 948 | 18.4 | 3.83 |
| 16 | 26.347 | trans-α-Bergamotene | 1434 | C15H24 | 938 | 955 | 54 | 0.49 |
| 17 | 26.63 | α-Bisabolol | 1443 | C15H26O | 818 | 836 | 11.4 | 0.06 |
| 18 | 26.947 | Farnesene | 1453 | C15H24 | 907 | 908 | 31.2 | 0.18 |
| 19 | 27.039 | α-Caryophyllene | 1456 | C15H24 | 939 | 942 | 54.6 | 1.02 |
| 20 | 27.64 | 1-Dodecanol | 1475 | C12H26O | 821 | 945 | 18 | 1.19 |
| 21 | 27.719 | β-Himachalene | 1478 | C15H24 | 787 | 850 | 42.8 | 0.48 |
| 22 | 27.907 | α-Selinene | 1484 | C15H24 | 851 | 860 | 6.6 | 0.15 |
| 23 | 28.073 | Valencene | 1489 | C15H24 | 828 | 896 | 7.8 | 0.32 |
| 24 | 28.294 | δ -Cadinine | 1496 | C15H24 | 835 | 854 | 10.7 | 0.19 |
| 25 | 28.507 | Alloaromadendrene | 1503 | C15H24 | 754 | 817 | 4.8 | 0.06 |
| 26 | 28.69 | α-Curcumene | 1510 | C15H22 | 861 | 904 | 17.1 | 0.18 |
| 27 | 28.974 | (-)-α-Panasinsene | 1519 | C15H24 | 886 | 889 | 27.4 | 0.27 |
| 28 | 29.145 | cis -Lanceol | 1525 | C15H24O | 824 | 845 | 34.4 | 0.27 |
| 29 | 29.708 | Farnesol | 1544 | C15H26O | 819 | 827 | 7.3 | 0.14 |
| 30 | 30.029 | Humulene | 1555 | C15H24 | 752 | 805 | 15.5 | 0.13 |
| 31 | 30.213 | Nerolidol | 1561 | C15H26O | 823 | 902 | 15.4 | 0.24 |
| 32 | 30.288 | Dodecanoic acid | 1564 | C12H24O2 | 847 | 854 | 49 | 0.23 |
| 33 | 30.826 | β-Caryophyllene oxide | 1582 | C15H24O | 883 | 885 | 50.1 | 0.35 |
| 34 | 31.526 | trans-α- (Z)-Bergamotol | 1606 | C15H24O | 856 | 865 | 71.2 | 0.13 |
| 35 | 31.739 | Tetradecanal | 1614 | C14H28O | 958 | 980 | 44.3 | 0.1 |
| 36 | 32.064 | Alloaromadendrene oxide-(1) | 1625 | C15H24O | 791 | 821 | 12.2 | 0.31 |
| 37 | 32.294 | trans- Longipinocarveol | 1634 | C15H24O | 828 | 851 | 8.1 | 0.28 |
| 38 | 32.448 | Neoisolongifolene, 8-bromo- | 1639 | C15H23Br | 790 | 849 | 11.7 | 3.09 |
| 39 | 35.117 | iso-Caryophyllene | 1737 | C15H24 | 842 | 901 | 10.2 | 0.08 |
| 40 | 35.997 | Drimenol | 1770 | C15H26O | 930 | 930 | 77.6 | 2.01 |
| 41 | 40.471 | Drimenin | 1941 | C15H22O2 | 835 | 938 | 81.8 | 0.28 |
| 42 | 44.25 | Phytol | - | C20H40O | 891 | 903 | 45.5 | 0.13 |
* Experimentally determined Kováts retention indices.
We also carried out GC-MS analysis by using multiple internal standards for quantification of compounds. The standard curve of standard mixtures was used to determine concentration of selected compounds in kesum essential oil. We found that the α-pinene content in kesum was 0.02 mg/mL. Meanwhile, drimenol was found at a concentration of 0.79 mg/mL, along with humulene (0.047 mg/mL), caryophyllene (0.031 mg/mL) and farnesol (0.030 mg/mL).
GCxGC-TOF MS analysis showed 48 significant compounds in kesum essential oil, six compounds more than detected by our GC-MS and all of these compounds had similarity values greater than 800 (Table 2).
Table 2.
Compounds identified in the essential oil of kesum using two-dimensional gas chromatography time-of-flight mass spectrometry GC×GC–TOF MS.
| No | t1R (s) | t2R (s) | Name | Formula | Similarity | Reverse | Probability | Content (%)a |
|---|---|---|---|---|---|---|---|---|
| 1 | 440 | 1.960 | 2-Hexenal | C6H10O | 861 | 861 | 5910 | 0.001 |
| 2 | 445 | 1.740 | cis-3-Hexenal | C6H10O | 882 | 882 | 6575 | 0.022 |
| 3 | 510 | 0.950 | Nonane | C9H20 | 907 | 907 | 4995 | 0.062 |
| 4 | 575 | 0.755 | 3-Carene | C10H16 | 829 | 837 | 1783 | 1.202 |
| 5 | 605 | 0.895 | Camphene | C10H16 | 903 | 903 | 3220 | 0.009 |
| 6 | 670 | 0.890 | Sabinene | C10H16 | 886 | 887 | 4388 | 0.013 |
| 7 | 975 | 0.905 | Undecane | C11H24 | 926 | 937 | 5688 | 2.286 |
| 8 | 995 | 1.500 | Nonanal | C9H18O | 896 | 896 | 8416 | 0.010 |
| 9 | 1270 | 1.055 | Cyclodecanol | C10H20O | 839 | 839 | 1534 | 5.691 |
| 10 | 1275 | 1.185 | Decanal | C10H20O | 951 | 951 | 8419 | 23.121 |
| 11 | 1445 | 1.115 | 2-Butyltetrahydrofuran | C8H16O | 925 | 925 | 4928 | 0.004 |
| 12 | 1445 | 1.260 | 1-Decanol | C10H22O | 938 | 938 | 3392 | 2.090 |
| 13 | 1450 | 1.860 | 1-Cyclopropylpentane | C8H16 | 850 | 865 | 1220 | 0.005 |
| 14 | 1465 | 1.085 | Isobornyl formate | C11H18O2 | 877 | 883 | 1980 | 0.071 |
| 16 | 1520 | 1.135 | Undecanal | C11H22O | 969 | 973 | 7142 | 0.990 |
| 17 | 1680 | 0.930 | α-Copaene | C15H24 | 865 | 865 | 4693 | 0.024 |
| 18 | 1695 | 1.650 | Octylcyclopropane | C11H22 | 908 | 908 | 2274 | 0.001 |
| 19 | 1720 | 1.055 | (Z,E)-α-Farnesene | C15H24 | 839 | 862 | 4954 | 0.928 |
| 20 | 1770 | 0.980 | α -Cedrene | C15H24 | 842 | 842 | 2894 | 0.012 |
| 21 | 1790 | 1.090 | 1-Dodecanal | C12H24O | 942 | 942 | 5140 | 4.785 |
| 22 | 1800 | 1.275 | Dodecanal | C12H24O | 963 | 974 | 6783 | 38.635 |
| 23 | 1805 | 0.895 | (E)-β-Caryophyllene | C15H24 | 898 | 898 | 4747 | 0.212 |
| 24 | 1830 | 0.875 | α-Bergamotene | C15H24 | 943 | 952 | 4035 | 0.801 |
| 25 | 1845 | 0.910 | γ -Gurjunene | C15H24 | 878 | 884 | 3364 | 0.095 |
| 26 | 1870 | 0.920 | α-Humulene | C15H24 | 898 | 898 | 8426 | 2.293 |
| 27 | 1885 | 0.945 | trans-β-Farnesene | C15H24 | 836 | 852 | 4383 | 0.907 |
| 28 | 1930 | 1.215 | 1-Dodecanol | C12H26O | 936 | 936 | 1769 | 1.380 |
| 29 | 1940 | 0.860 | 2-Isopropenyl-4a,8-dimethyl-1,2,3,4,4a,5, 6,7-Octahydro-naphthalene | C15H24 | 890 | 894 | 1897 | 0.697 |
| 30 | 1940 | 1.230 | α-Curcumene | C15H22 | 882 | 882 | 9172 | 0.080 |
| 31 | 1955 | 0.870 | Valencene | C15H24 | 869 | 896 | 1392 | 0.806 |
| 32 | 1985 | 0.950 | Alloaromadendrene | C15H24 | 859 | 860 | 1321 | 0.039 |
| 33 | 2000 | 1.150 | β-Bisabolene | C15H24 | 816 | 816 | 3899 | 0.014 |
| 34 | 2005 | 1.195 | α-Zingiberene | C15H24 | 796 | 827 | 2546 | 0.013 |
| 35 | 2020 | 0.870 | α-Panasinsene | C15H24 | 888 | 888 | 4332 | 0.563 |
| 36 | 2035 | 0.945 | δ-Cadinene | C15H24 | 870 | 875 | 5111 | 0.025 |
| 37 | 2095 | 1.215 | Patchulane | C15H26 | 804 | 807 | 1237 | 0.004 |
| 38 | 2130 | 1.320 | Nerolidol | C15H26O | 872 | 873 | 6205 | 0.075 |
| 39 | 2170 | 0.990 | Caryophyllene oxide | C15H24O | 914 | 915 | 6752 | 1.513 |
| 40 | 2200 | 1.140 | Ocimene | C10H16 | 808 | 871 | 2319 | 0.055 |
| 41 | 2235 | 1.090 | Tetradecanal | C14H28O | 875 | 931 | 2259 | 1.056 |
| 42 | 2280 | 0.955 | dehydro- Cyclolongifolene oxide | C15H22O | 810 | 812 | 3756 | 0.544 |
| 43 | 2280 | 1.130 | Acoradiene | C15H24 | 845 | 886 | 1125 | 0.079 |
| 44 | 2280 | 1.155 | 1,3,6,10-Dodeca-tetraene | C15H24 | 807 | 837 | 989 | 0.117 |
| 45 | 2290 | 1.260 | 4,4-Dimethyltetra-cyclo[6.3.2.0(2,5).0(1,8)]tridecan-9-ol | C15H24O | 847 | 847 | 4371 | 0.122 |
| 46 | 2550 | 1.205 | Drimenol | C15H26O | 930 | 930 | 8162 | 0.574 |
| 47 | 3200 | 1.465 | Phytol | C20H40O | 801 | 814 | 3655 | 0.003 |
| 48 | 3400 | 1.260 | Hexadecanal | C16H32O | 805 | 805 | 1312 | 0.004 |
a t1R and t2R retention times of peaks on first and second dimension, respectively; b Content is the peak volume percentage of compounds in the essential oil sample.
Compounds found both in GC-MS and GC×GC-TOF MS are shown in Table 3. Out of 42 compounds found in GC-MS analysis, only 21 compounds were also found in GC×GC–TOF MS. This may be due to the less sensitivity of GC-MS compared to GC×GC–TOF MS. The relative concentrations of several classes of volatile compounds in kesum are shown in Table 4.
Table 3.
The essential oil compounds found both in GC-MS and GC×GC–TOF MS.
| No | Name | Formula |
|---|---|---|
| 1 | Undecane | C11H24 |
| 2 | Nonanal | C9H18O |
| 3 | Decanal | C10H20O |
| 4 | 1-Decanol | C10H22O |
| 5 | Undecanal | C11H22O |
| 6 | Dodecanal | C12H24O |
| 7 | (E)-β-Caryophyllene | C15H24 |
| 8 | trans-α-Bergamotene | C15H24 |
| 9 | α-Humulene | C15H24 |
| 10 | trans-β-Farnesene | C15H24 |
| 11 | 1-Dodecanol | C12H26O |
| 12 | α-Curcumene | C15H22 |
| 13 | Valencene | C15H24 |
| 14 | Alloaromadendrene | C15H24 |
| 15 | α-Panasinsene | C15H24 |
| 16 | δ-Cadinene | C15H24 |
| 17 | Nerolidol | C15H26O |
| 18 | Caryophyllene oxide | C15H24O |
| 19 | Tetradecanal | C14H28O |
| 20 | Drimenol | C15H26O |
| 21 | Phytol | C20H40O |
Table 4.
Relative concentrations of several classes of volatile compounds in kesum.
| Chemical class of volatile compound | % Relative area |
|---|---|
| Esters | 0.071 |
| Furans | 0.004 |
| Alcohols | 9.857 |
| Aldehydes | 68.624 |
| Hydrocarbons and terpenes | 13.489 |
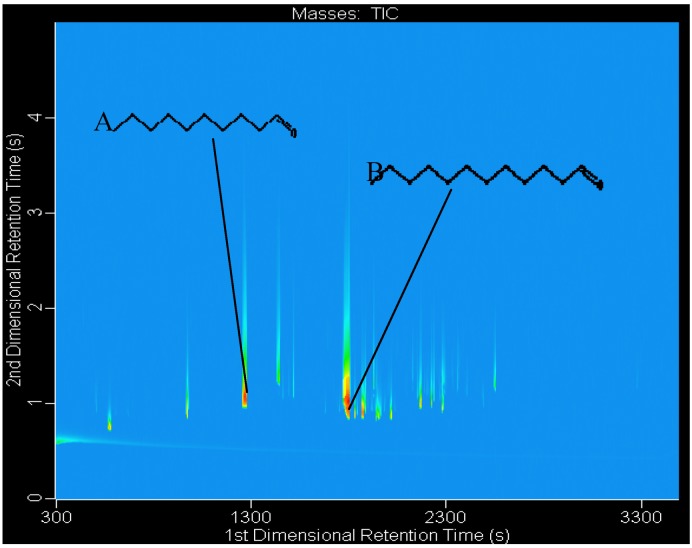
In GC×GC–TOF MS analysis, the 48 identified compounds were classified into groups, including one ester, one furan, five alcohols, nine aldehydes and 32 hydrocarbons. Therefore, the majority of the components found in the kesum volatile oil were terpene compounds. The number of terpenes found is far more than that reported by Yaacob [2], where only β-caryophyllene was observed and identified. Although decanal and dodecanal have been identified as the dominant components in the oil (Figure 1), we believe that the terpene group may also contribute strongly to the flavour of kesum. The presence of this group is shown in Table 2 and Table 4, and the significant components that exhibited a good match index with a compound in the NIST MS database are listed. This study demonstrates that GC×GC–TOF MS is a powerful separation and identification tool that allows for the identification of a much larger number of complex volatile oil components.
Figure 1.
2D-GC chromatogram contour plots of Polygonum minus Huds. volatile oil and structural pictures of (A) decanal and (B) dodecanal as the main constituents.
A probability value greater than 9,000 reflects that the mass spectrum is highly unique and could be the source of flavour and bioactive compounds in a mixture, identifying a compound that may be valuable for further pharmaceutical research. Based on our GC×GC–TOF MS result, we found that only α-curcumene had a probability value above 9,000. The sesqueterpenoid α-curcumene is produced as a major component in the essential oil of several plants, including Curcuma longa, and serves as an insect repellent and insect-feeding deterrent [8]. In our study, we tentatively identified α-curcumene in the essential oil of kesum (Figure 2).
Figure 2.
Details of the GC×GC contour plot chromatogram of α-curcumene, peak spectra of the sample, and peak spectra reported in the NIST library.
3. Experimental
3.1. Plant material
Fresh leaves of kesum were collected in January 2009 from the Genting Highland, Pahang, Malaysia (3° 25′ 22.43″ N, 101° 47′ 32.38″ E). Voucher specimens were deposited in the Herbarium of the Faculty of Science and Technology, Universiti Kebangsaan Malaysia, Bangi, Malaysia (UKMB).
3.2. Extraction procedure
Two hundred and fifty grams of kesum were subjected to hydrodistillation for 8 hours using a Clevenger-type apparatus [9]. The essential oils were collected over water, separated, dried over anhydrous sodium sulphate, and stored in the dark at 4 ºC prior to GC-FID, GC-MS and GC×GC–TOF MS analysis.
3.3. GC×GC–TOF MS analysis
The comprehensive two-dimensional gas chromatograph system employed consisted of an Agilent 6890N GC equipped with a flame ionisation detector (Agilent, Palo Alto, CA, USA) and filled with a cold-jet modulator KT-2007 retrofit prototype (Zoex Corporation, Lincoln, NE, USA). A time-of-flight mass spectrometer (Pegasus 4D, LECO Corporation, St. Joseph, MI, USA), equipped with an Agilent 6890N GC, was used to acquire mass spectral data. The MS parameters included a 70-eV electron impact ionisation value and a maximum spectral acquisition rate of 500 spectra per second. Two capillary columns were used, connected by a universal press-tight connector, and were installed in the same oven. The column sets used are listed in Table 5.
Table 5.
Features of the GC×GC column sets.
| Column 1 | Column 2 | |
|---|---|---|
| Length (m) | 30 | 1 |
| Diameter (mm) | 0.25 | 0.25 |
| Stationary phase | Rtx-5MS | DB-wax |
| Film thickness (μm) | 0.10 | 0.25 |
| Corporation | Restek Corporation, Bellefonte, PA | J&W Scientific, Folsom, CA |
Ultra-high purity (99.999%) helium was used in constant pressure mode as the carrier gas. The inlet pressure was 72.4 psi. An Agilent 7683B auto sampler was used to inject 1 μL of the sample with a splitless injector into the inlet of column 1 at 250 ºC. Column 1 was held at 45 ºC for 2 min, and then, the temperature was increased at a rate of 3 ºC/min until the column reached a final temperature of 200 ºC. Column 2 was set to be 15 ºC warmer than column 1. The mass spectrometer was operated at an acquisition rate of 50 spectrals. No mass spectra were collected during the first 3 min of the solvent delay. The modulation period was 5 s. The transfer line and the ion source temperature were 250 ºC and 200 ºC, respectively. The detector voltage was 1600 V, and the electron energy was -70 V. Mass spectra were collected from 50–400 m/z. The pressure inside the flight tube was approximately 1-7 torr. In the identification analysis, LECO® Software Version 3.34 was used to find all of the peaks in the raw chromatograms. The parameters, such as the similarity, reverse, and probability values of peaks identified through a library search using NIST/EPA/NIH Version 2.0, were combined into a single peak table.
3.4. GC-MS analysis
The essential oils were analysed using a Clarus 600 GC-MS system (Perkin Elmer, USA). The compounds were separated on 30 m × 0.25 mm × 0.25 μm Elite-5MS column (Perkin Elmer, USA). The column temperature was increased from 40 ºC to 220 ºC at a rate of 4 ºC/min; injector temperature, 250 ºC; injection volume, 1 μL; transfer temperature, 280 ºC. MS parameters were as follows: EI mode, with ionization voltage 70 eV, ion source temperature, 180 ºC; scan range, 50-600 Da. The peaks were tentatively identified based on library search using NIST and Wiley Registry 8 Edition. The identities of some components were confirmed by both mass spectral and retention data of the authentic chemicals obtained under identical GC-MS conditions. Internal standards were applied and concentration of selected compounds was determined based on standard calibration curve.
3.5. GC-FID analysis and n-Alkane standard solutions
In order to perform Kováts indices, the essential oil were analysed using a Hewlett Packard 5890 system GC-FID (Hewlett Packard, Palo Alto, CA, USA). The compounds were separated on 30 m × 0.25 mm × 0.1 µm DB-5HT column. The GC program was the same as those used for GC-MS analysis. n-alkane standard solutions C8-C20 (mixture no. 04070) and C21-C40 (mixture no. 04071) were purchased from Fluka Chemica. Retention indices of essential oil compounds was carried out according to standard method of Kováts Indices to support the identification of the compounds.
4. Conclusions
GC-MS can perform much more reliable qualitative and quantitative analysis of complex essential oils samples. Meanwhile, GC-FID eventually was a very basic chromatograph technique, but provides us more information on retention indices that are crucial in analytical chemistry. However, GC×GC-TOF MS system for the analysis of kesum essential oil identified five times more compounds than those reported from a previous study using GC-MS, and we found that the majority of these compounds were terpenes. We believe that the 10 major components in the essential oil of kesum detected by previous research exclude many minor components that should not be ignored, as they also strongly contribute to the overall qualities of the essential oil.
Acknowledgements
This research was supported by the Genomics and Molecular Biology Initiative of the Malaysia Genome Institute, Ministry of Science, Technology and Innovation, Malaysia (07-05-MGI-GMB 004). Authors would like to thank Khairunisa Khairudin and Ambar Yarmo for analytical help. We also thank Sahidin, Kamalrul Azlan Azizan, Syahmi Afiq Mustaza, Man Ghani and Lotus Saw (Perkin Elmer Malaysia) for technical assistance.
Footnotes
Sample Availability: Samples of the essential oil are available from the authors.
References and Notes
- 1.Burkill J.H. A Dictionary of Economic Products of the Malay Peninsula. Volume 2. Art Printing Works; Kuala Lumpur, Malaysia: 1966. p. 1823. [Google Scholar]
- 2.Yaacob K.B. Kesom oil: A natural source of aliphatic aldehydes. Perfumer Flavorist. 1987;12:27–30. [Google Scholar]
- 3.Zhao C.X., Liang Y.Z., Fang H.Z., Li X.N. Temperature-programmed retention indices for gas chromatography-mass spectroscopy analysis of plant essential oils. J. Chromatogr. A. 2005;1096:76–85. doi: 10.1016/j.chroma.2005.09.067. [DOI] [PubMed] [Google Scholar]
- 4.Wagner C., Sefkow M., Kopka J. Construction and application of a mass spectral and retention time index database generated from plant GC/EI-TOF-MS metabolite profiles. Phytochemistry. 2003;62:887–900. doi: 10.1016/S0031-9422(02)00703-3. [DOI] [PubMed] [Google Scholar]
- 5.Marriott P., Shellie R. Principles and applications of comprehensive two-dimensional gas chromatography. Trends Anal. Chem. 2002;21:573–583. doi: 10.1016/S0165-9936(02)00814-2. [DOI] [PubMed] [Google Scholar]
- 6.Ma C., Want H., Lu X., Li H., Liu B., Xu G. Analysis of Artemisia annua L. volatile oil by comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. J. Chromatogr. A. 2007;1150:50–53. doi: 10.1016/j.chroma.2006.08.080. [DOI] [PubMed] [Google Scholar]
- 7.Dallüge J., Beens J., Brinkman U.A.T. Comprehensive two dimensional gas chromatography: a powerful and versatile analytical tool. J. Chromatogr. A. 2003;1000:69–108. doi: 10.1016/S0021-9673(03)00242-5. [DOI] [PubMed] [Google Scholar]
- 8.Antonious G.F., Kochhar T.S. Zingiberene and curcumene in wild tomato. J. Environ. Sci. Health B. 2003;38:489–500. doi: 10.1081/PFC-120021668. [DOI] [PubMed] [Google Scholar]
- 9.Lucchesi M.E., Chemat F., Smadja J. Solvent-free microwave extraction of essential oil from aromatic herbs: comparison with conventional hydrodistillation. J. Chromatogr. A. 2004;1043:323–327. doi: 10.1016/j.chroma.2004.05.083. [DOI] [PubMed] [Google Scholar]