Abstract
Sulfonated (SO3H-bearing) activated carbon (AC-SO3H) was synthesized by an aryl diazonium salt reduction process. The obtained material had a SO3H density of 0.64 mmol·g−1 and a specific surface area of 602 m2·g−1. The catalytic properties of AC-SO3H were compared with that of two commercial solid acid catalysts, Nafion NR50 and Amberlyst-15. In a 10-h esterification reaction of acetic acid with ethanol, the acid conversion with AC-SO3H (78%) was lower than that of Amberlyst-15 (86%), which could be attributed to the fact that the SO3H density of the sulfonated carbon was lower than that of Amberlyst-15 (4.60 mmol·g−1). However, AC-SO3H exhibited comparable and even much higher catalytic activities than the commercial catalysts in the esterification of aliphatic acids with longer carbon chains such as hexanoic acid and decanoic acid, which may be due to the large specific surface area and mesoporous structures of the activated carbon. The disadvantage of AC-SO3H is the leaching of SO3H group during the reactions.
Keywords: sulfonate, activated carbon, solid acid, catalysts
1. Introduction
Sulfonated (SO3H-bearing) carbon materials have been reported to act as strong solid acid catalysts. Hara’s group first prepared sulfonated carbon catalysts via the sulfonation and carbonization of polycyclic aromatic hydrocarbons [1]. These catalysts showed excellent activity in a series of acid-catalyzed reactions, although, they were not stable enough and the aromatic molecules leached out above 100 ºC [2]. The problem could be overcome by selecting saccharides as carbon precursor [2,3,4,5,6,7]. The controlled carbonization and sulfonation of these materials resulted in stable carbon structures with a high SO3H group density. Carbon-based solid acid catalysts could also be obtained by sulfonation of carbon nanotubes [8] or mesoporous carbon materials [9,10,11].
Activated carbon (AC) is widely used as a catalyst support in a variety of industrial and environmental applications for its chemical stability, high specific surface area, and low cost [12]. Studies on the sulfonation of AC are however quite limited [13,14,15]. An early experimental result from Hara’s group showed that heating AC in H2SO4 only produced a carbon material with a very low SO3H group density (less than 0.15 mmol·g−1), a fact attributed to the chemical inertness of AC [1]. Onda et al. reported the generation of a sulfonated carbon material with a SO3H density of 0.44 mmol·g−1 by treatment of AC with concentrated H2SO4 [13,14]. The obtained carbon material in this case was quite stable and showed evident catalytic activity for the hydrolysis of cellulose. Recently, another paper from Hara’s group described the preparation of a porous sulfonated carbon catalyst with high specific surface area by carbonization and sulfonation of ZnCl2-impregnated wood powders [15].
Besides the H2SO4 treatment method, chemical reduction of aryl diazonium salts has also been proved to be quite efficient for the functionalization of carbon materials [16]. Feng et al. reported the preparation of carbon-based solid acid catalysts by covalent attachment of SO3H radicals on the surface of ordered mesoporous carbon in a chemical reduction process [17,18]. However, so far there have been no reports on sulfonation of AC using the chemical reduction method. Here we report the functionalization of AC with SO3H-containing group by reacting AC with 4-benzenediazonium sulfonate using hypophosphorous acid (H3PO2) as reducing agent. The structure and catalytic properties of the obtained material were also examined.
2. Results and Discussion
2.1. Material characteristics
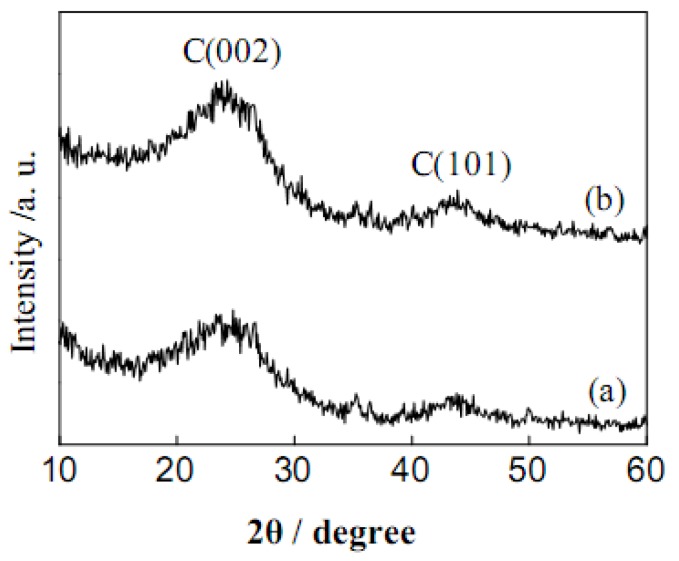
Figure 1 shows the XRD patterns of AC and the sulfonated carbon material (AC-SO3H). The broad C (002) diffraction peak (2θ = 15-30º) can be attributed to the amorphous carbon structures. The weak and broad C (101) diffraction peak (2θ = 40-50º) is due to the a axis of the graphite structure [1,2,3,4,5,6]. There is no noticeable difference in the XRD patterns between AC and AC-SO3H, suggesting that the chemical reduction process does not affect the microstructure of carbon materials.
Figure 1.
XRD patterns for activated carbon (a) before and (b) after sulfonation.
The SO3H density of sulfonated carbon material was determined by combining the S elemental analysis and the total acid site density results. As shown in Table 1, the S contents in AC before and after sulfonation were 0.26 and 0.90 mmol·g−1, respectively. The total acid density of AC increased from 0.30 to 1.01 mmol·g−1 via the sulfonation.
Table 1.
The textural properties of the materials.
| Samples | N2 adsorption(a) | S content (mmol g−1) |
Total acid density (mmol H+g−1) |
SO3H density (mmol g−1) |
||
|---|---|---|---|---|---|---|
| S. A. | P. V. | P. D. | ||||
| AC | 751 | 0.47 | 2.5 | 0.26 | 0.30 | 0 |
| AC-SO3H | 602 | 0.38 | 2.4 | 0.90 | 1.01 | 0.64 |
| Nafion NR50 | <1 | 0.45 | 0.49 | 0.45 | ||
| Amberlyst-15 | 45 | 4.60 | 4.66 | 4.60 | ||
(a) BET surface area (S. A.) in m2 g−1, pore volume (P. V.) in cm3 g−1, pore diameter (P. D.) in nm.
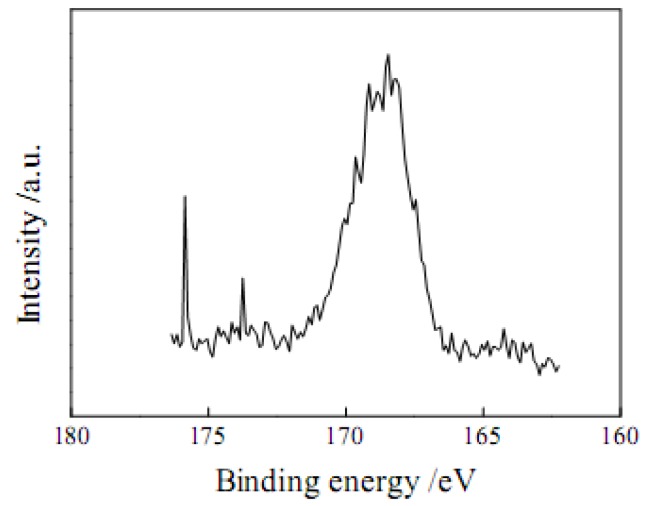
These results showed that the increment of S content (0.64 mmol·g−1) was close to that of the total acid density (0.71 mmol·g−1) in the sulfonation process. Therefore the SO3H density of sulfonated AC is about 0.64 mmol·g−1. X-ray photoelectron spectroscopy (XPS) analysis was conducted to further verify the chemical state of sulfur in AC-SO3H. As shown in Figure 2, a strong S 2p peak appears at about 168 eV, which can be denoted as SO3H group [3].
Figure 2.
S 2p XPS spectrum of AC-SO3H.
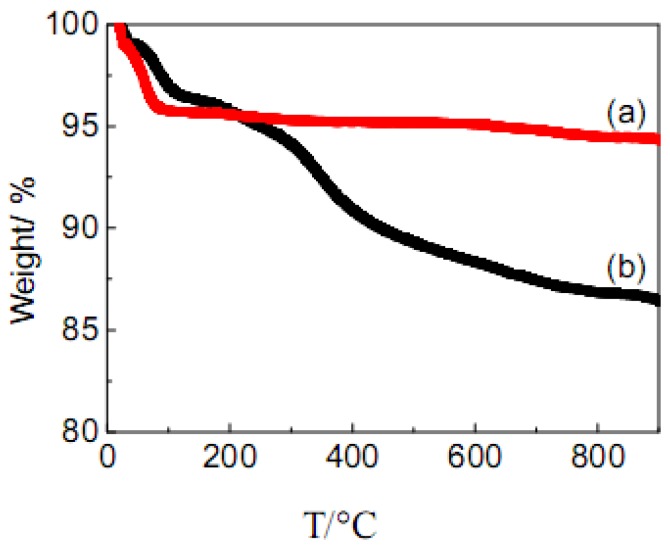
The thermal stability of AC-SO3H was studied by TGA results (under flowing N2). As shown in Figure 3, AC showed a slight weight loss (about 5%) below 150 ºC, which was mainly due to the loss of a small amount of adsorbed water. The weight loss process of AC-SO3H below 150 ºC was similar to that of AC. However, the thermogravimetric curve of AC-SO3H at higher temperatures showed a more significant weight loss compared to that of AC, which is mainly due to the gradual desorption and thermal decomposition process of PhSO3H group.
Figure 3.
Thermogravimetric profiles for (a) AC, (b) AC-SO3H.
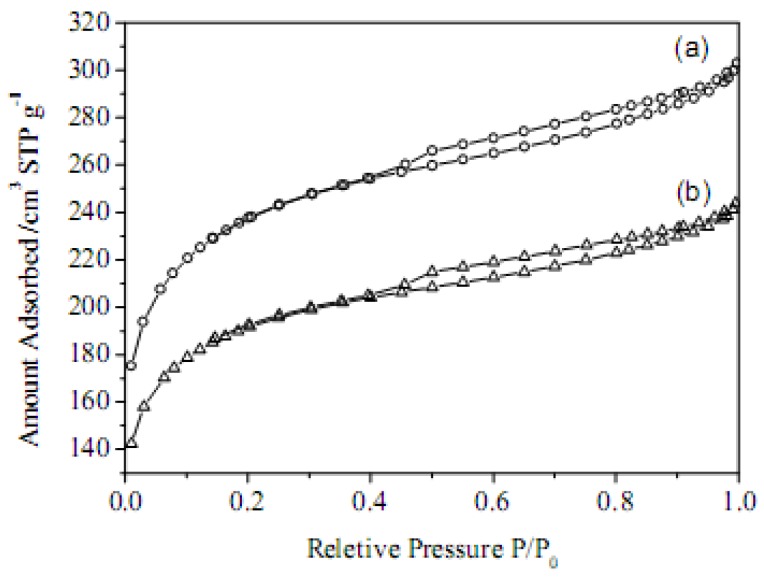
Figure 4 shows the N2 adsorption isotherms of the carbon materials. Both AC and AC-SO3H exhibit similar isotherms with evident hysteresis loops in the relative pressure range of about 0.4-0.9. The surface properties of the carbon materials are summarized in Table 1. After sulfonation, the surface area and pore volume of the carbon materials decrease from 751 m2·g−1 and 0.47 cm3·g−1 to 602 m2·g−1 and 0.38 cm3·g−1, suggesting that a small part of the pore space has been occupied by the grafted SO3H groups. The pore diameter does not change during sulfonation (from 2.5 to 2.4 nm).
Figure 4.
N2 adsorption isotherms of (a) AC and (b) AC-SO3H.
2.2. Catalytic properties
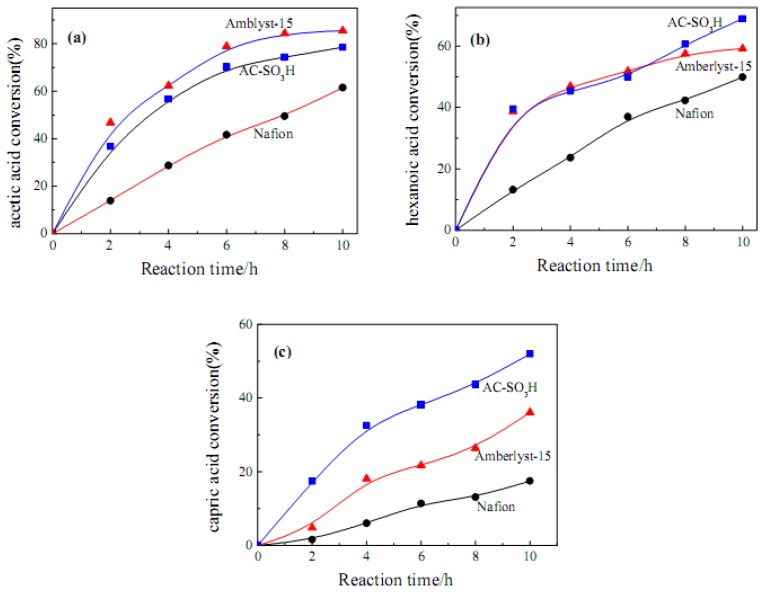
The catalytic activity of AC-SO3H has been investigated in the esterification of three kinds of aliphatic acids with different carbon chain lengths (acetic, hexanoic and decanoic acid), as shown in Figure 5. The results for two commercial solid acid catalysts such as Amberlyst-15 and protonated Nafion NR50 are also shown for comparison. From Figure 5(a), the highest acetic acid conversion (86%) is obtained with Amberlyst-15 after 10 h of reaction. AC-SO3H gives an acid conversion of 78%. The conversion with Nafion is 61%. As shown in Table 1, the SO3H density of Amberlyst-15 (4.60 mmol·g−1) is much higher than that of AC-SO3H (0.64 mmol·g−1) and Nafion (0.45 mmol·g−1). Therefore, it may be conferred that the catalytic activity for acetic acid esterification is mainly dependent on the acid density of the catalyst.
Figure 5.
Acid catalyzed esterification of several aliphatic acids with ethanol: (a) acetic acid, (b) hexanoic acid, and (c) decanoic acid.
Figure 5(b) shows the esterification results of hexanoic acid with ethanol. The catalytic activity of AC-SO3H is comparable in this case with that of Amberlyst-15. The advantage of our carbon catalyst becomes more evident in the esterification of decanoic acid, as shown in Figure 5(c). The decanoic acid conversion with AC-SO3H (52%) is much higher than that with Amberlyst-15 (36%) and with Nafion (17%) after 10 h of reaction. The results in Figure 5(b) and Figure 5(c) suggest that the catalytic activity for esterification of large aliphatic acid molecules is not solely dependent on the acid density. The surface properties of catalysts such as specific surface area and pore diameters are also quite important. As shown in Table 1, AC-SO3H has a large surface area (602 m2·g−1) and high density of mesopores, which may be more convenient for larger aliphatic acid molecules to adsorb on the catalyst surface and approach to the active acid sites. Therefore, our sulfonated carbon materials may have potential applications in the acid-catalyzed reactions of large organic molecules.
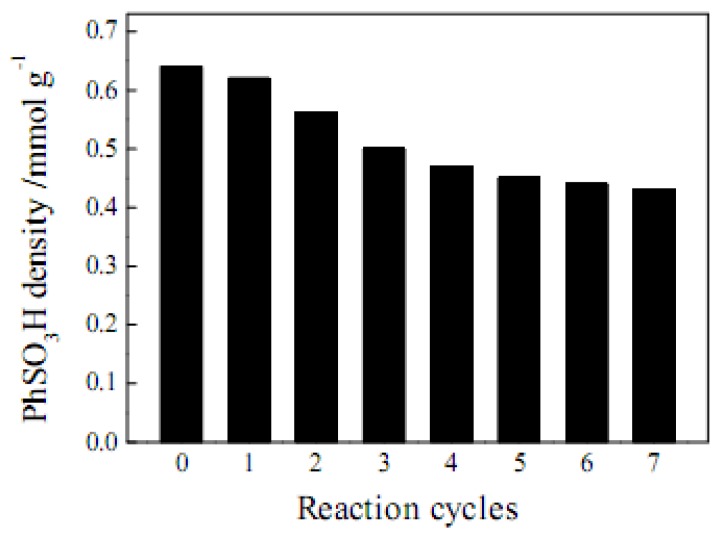
To investigate the stability of SO3H group on the carbon catalyst, AC-SO3H was reused in a series of acetic acid esterification reaction cycles. Before each cycle, AC-SO3H was washed with 1 mol·L−1 H2SO4, and then washed repeatedly with deionized water at least seven times until no SO42− ions were detected in the washed water [11].
Figure 6 shows the variation of SO3H density in the reaction cycles. The fresh catalyst has a SO3H density of 0.64 mmol·g−1. The value decreases evidently in the beginning four reaction cycles and then remains stable at 0.42 mmol·g−1 in the following three cycles. The results indicate that about 34% SO3H group (0.22 mmol·g−1) of the fresh catalyst will leach out during the reactions, while 64% SO3H is preserved. It has been pointed out elsewhere that the leaching of SO3H groups is a common problem for sulfonated catalysts [7]. More efforts should be made in the future to enhance the stability of SO3H groups.
Figure 6.
Variation of the SO3H density on the carbon catalysts in a series of 2-h acetic acid esterification reaction cycles.
3. Experimental Section
3.1. Materials and Reagents
Activated carbon was purchased from the Taiyuan Xinhua Activated Carbon Company (Taiyuan, Shanxi Province, P.R. China). Commercial solid acid catalysts, Amberlyst-15 and protonated Nafion NR50, were purchased from Alfa Aesar (Tianjin, China). All other reagents were in reagent grade and used without any further treatment.
3.2. Catalyst preparation
The sulfonated carbon material was obtained by the reaction of 4-benzene-diazonium sulfonate with activated carbon using hypophosphorous acid (H3PO2) as the reducing agent [17,18]. 4-Benzene-diazonium sulfonate was synthesized by diazotization of 4-aminobenzenesulfonic acid [17]. In a typical process, 4-aminobenzenesulfonic acid (13.0 g) was dispersed in 1M HCl (75 mL) in a three-necked flask. An ice-water bath was used to control the temperature at 0-5 ºC. Then NaNO2 (83 mL, 1 M) was added dropwise into the flask. The mixture was stirred for 50 min. The obtained white precipitate was filtered and washed with cold water.
To functionalize AC, distilled water (100 mL), ethanol (100 mL), diazonium salt (12.0 g) and AC (2.0 g) were added in a three-necked flask. Then 50 wt % H3PO2 (100 mL) was added and the temperature was controlled at 0-5 ºC. After stirring for 30 min, another portion of 50 wt % H3PO2 (100 mL) was added and the mixture was stirred for another 30 min. The sulfonated AC is filtered and washed with distilled water at least seven times and then with acetone, and dried at 80 ºC.
3.3. Catalyst characterization
The sulfur content of carbon materials and commercial catalysts (Amberlyst-15 and Nafion NR50) was determined by a Sulfur Analyzer (KZDL-4) [19]. The total acid site density of the samples was obtained using acid-base back titration [7]. Typically, the sample (0.1 g) was mixed with NaOH aqueous solution (50 mL, 0.004 M) while stirring for 1 h at room temperature. Then excess NaOH was neutralized with HCl (0.02 M). The obtained carbon material was characterized by XRD (Rigaku D/MAX-2400), XPS (Thermo Scientific K-Alpha), N2 adsorption analysis (Micromeritics ASAP300), and TGA (Netzsch STA 449C).
3.4. Catalytic reactions
The catalytic properties of the sulfonated carbon material were examined through the esterification of a series of aliphatic acids (acetic acid, hexanoic acid, and decanoic acid) with ethanol at 70 ºC. Typically, aliphatic acid (0.1 mol), ethanol (1.0 mol), and the carbon catalyst (or the commercial catalysts) (0.2 g) were used in the reactions. The liquid reaction mixtures were analyzed by gas chromatography (Agilent GC 7890).
4. Conclusions
A porous carbon-based solid acid catalyst possessing a SO3H density of 0.64 mmol·g−1 and a specific surface area of 602 m2·g−1 was synthesized by sulfonating activated carbon in an aryl diazonium salt reduction process. In the esterification reaction of acetic acid with ethanol, AC-SO3H showed inferior catalytic activity to the commercial catalyst, Amberlyst-15, because the former’s SO3H density is much lower than the latter’s (4.60 mmol·g−1). However, AC-SO3H possessed evident advantages in the esterification of decanoic acid, which was probably due to the high specific surface area and a large number of mesopores of the activated carbon. Our study results suggest that AC-SO3H may have potential applications in the acid-catalyzed reactions of large organic molecules. The main disadvantage of AC-SO3H is the leaching of SO3H groups during the reactions.
Acknowledgments
This work was financially supported by Chinese Universities Scientific Fund (QN2009050) and Opening Fund of State Key Laboratory of Coal Conversion (10-11-905).
Footnotes
Sample Availability: Samples of the AC-SO3H material are available from the authors.
References
- 1.Hara M., Yoshida T., Takagaki A., Takata T., Kondo J.N., Hayashi S., Domen K. A carbon material as a strong protonic acid. Angew. Chem. Int. Ed. 2004;43:2955–2958. doi: 10.1002/anie.200453947. [DOI] [PubMed] [Google Scholar]
- 2.Toda M., Takagaki A., Okamura M., Kondo J.N., Hayashi S., Domen K., Hara M. Biodiesel made with sugar catalyst. Nature. 2005;438:178. doi: 10.1038/438178a. [DOI] [PubMed] [Google Scholar]
- 3.Okamura M., Takagaki A., Toda M., Kondo J.N., Domen K., Tatsumi T., Hara M., Hayashi S. Acid-catalyzed reactions on flexible polycylic aromatic carbon in amorphous carbon. Chem. Mater. 2006;18:3039–3045. doi: 10.1021/cm0605623. [DOI] [Google Scholar]
- 4.Takagaki A., Toda M., Okamura M., Kondo J.N., Hayashi S., Domen K., Hara M. Esterrification of higer fatty acids by a novel strong solid acid. Catal. Today. 2006;116:157–161. doi: 10.1016/j.cattod.2006.01.037. [DOI] [Google Scholar]
- 5.Suganuma S., Nakajima K., Kitano M., Yamaguchi D., Kato H., Hayashi S., Hara M. Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups. J. Am. Chem. Soc. 2008;130:12787–12793. doi: 10.1021/ja803983h. [DOI] [PubMed] [Google Scholar]
- 6.Zong M.H., Duan Z.Q., Lou W.Y., Smith T.J., Wu H. Preparation of a sugar catalyst and its use for highly efficient production of biodiesel. Green Chem. 2007;9:434–437. doi: 10.1039/b615447f. [DOI] [Google Scholar]
- 7.Mo X., Lopez D.E., Suwannakarn K., Liu Y., Lotero E., Goodwin J.G., Lu C. Activation and deactivation characterisitics of sulfonated carbon catalysts. J. Catal. 2008;254:332–338. doi: 10.1016/j.jcat.2008.01.011. [DOI] [Google Scholar]
- 8.Peng F., Zhang L., Wang H., Lv P., Yu H. Sulfonated carbon nanotubes as a strong protonic acid catalyst. Carbon. 2005;43:2405–2408. doi: 10.1016/j.carbon.2005.04.004. [DOI] [Google Scholar]
- 9.Budarin V.L., Luque R., Macquarrie D.J., Clark J.H. Towards a bio-based industry: benign catalytic esterification of succinic acid in the presence of water. Chem. Eur. J. 2007;13:6914–6919. doi: 10.1002/chem.200700037. [DOI] [PubMed] [Google Scholar]
- 10.Budarin V.L., Clark J.H., Luque R., Macquarrie D.J., Koutinas A., Webb C. Tunable mesoporous materials optimised for aqueous phase esterifications. Green Chem. 2007;9:992–995. doi: 10.1039/b704055e. [DOI] [Google Scholar]
- 11.Xing R., Liu Y., Wang Y., Chen L., Wu H., Jiang Y., He M., Wu P. Active solid acid catalysts prepared by sulfonation of carbonization-controlled mesoporous carbon materials. Microporous Mesoporous Mat. 2007;105:41–48. doi: 10.1016/j.micromeso.2007.06.043. [DOI] [Google Scholar]
- 12.Rodriguez-Reinoso F. The role of carbon materials in heterogeneous catalysis. Carbon. 1998;36:159–175. doi: 10.1016/S0008-6223(97)00173-5. [DOI] [Google Scholar]
- 13.Onda A., Ochi T., Yanagisawa K. Selective hydrolysis of cellulose into glucose over solid acid catalysts. Green Chem. 2008;10:1033–1037. doi: 10.1039/b808471h. [DOI] [Google Scholar]
- 14.Onda A., Ochi T., Yanagisawa K. Hydrolysis of cellulose selectively into glucose over sulfonated activated-carbon catalyst under hydrothermal conditions. Topic. Catalysis. 2009;52:801–807. doi: 10.1007/s11244-009-9237-x. [DOI] [Google Scholar]
- 15.Kitano M., Arai K., Kodama A., Kousaka T., Nakajima K., Hayashi S., Hara M. Preparation of a sulfonated porous carbon catalyst with high specific surface area. Catal. Lett. 2009;131:242–249. doi: 10.1007/s10562-009-0062-4. [DOI] [Google Scholar]
- 16.Pandurangappa M., Lawrence N.S., Compton R.G. Homogeneous chemical derivatisation of carbon particles: a novel method for functionalizing carbon surfaces. Analyst. 2002;127:1568–1571. doi: 10.1039/b209711g. [DOI] [PubMed] [Google Scholar]
- 17.Wang X., Liu R., Waje M.M., Chen Z., Yan Y., Bozhilov K.N., Feng P. Sulfonated ordered mesoporous carbon as a stable and highly active protonic acid catalyst. Chem. Mater. 2007;19:2395–2397. doi: 10.1021/cm070278r. [DOI] [Google Scholar]
- 18.Liu R., Wang X., Zhao X., Feng P. Sulfonated ordered mesoporous carbon for catalytic preparation of biodiesel. Carbon. 2008;46:1664–1669. doi: 10.1016/j.carbon.2008.07.016. [DOI] [Google Scholar]
- 19.Ma H.L., Wang Y.Y., Jin G.Q., Guo X.Y. Structural changes in carbon produced by a sulfur-aided catalytic chemical vapor deposition. New Carbon Mater. 2009;24:13–17. doi: 10.1016/S1872-5805(08)60031-1. [DOI] [Google Scholar]