Abstract
An efficient and improved procedure for the synthesis of 1,4-diazepine and 1,5-benzodiazepine derivatives via the reaction of ketimine intermediates with aldehydes in the presence of Keggin-type heteropolyacids (HPAs) was developed. High yields and short reaction times were obtained for both electron-releasing and electron-withdrawing substituted 1,4-diazepine and 1,5-benzodiazepines derivatives.
Keywords: 1,4-diazepine; 1,5-benzodiazepine; dehydroacetic acid; 1,3-aminomethyl propane; orthophenylene diamine; heteropolyacids
1. Introduction
Diazepines and benzodiazepines have various therapeutic applications. Many members of the diazepine family are widely used as anticonvulsants, antianxiolitics, analgesics, sedatives, antidepressives and hypnotic agents [1,2,3,4]. Benzodiazepine derivatives are used as dyes for acrylic fibers [5]. In addition, benzodiazepines are valuable intermediates for the synthesis of fused ring compounds such as triazolo-, oxadiazolo-, oxazino-, and furanobenzodiazepines [6,7,8,9,10,11].
Due to their wide range of pharmacological activity and industrial applications, the development of mild and efficient protocols for their preparation continues to be a challenging endeavor in the synthetic organic chemistry [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. The common procedure for the synthesis of these compounds is a one pot condensation between o-phenylenediamines and carbonyl compounds [29]. However, a large number of the modified methods reported in the literature, suffer from several drawbacks such as the use of a large amount of catalysts, unsatisfactory product yields and critical product isolation procedures. These disadvantages require a development of an efficient and practically useful process of preparation [30].
In recent years, the use of polyoxometalates as catalysts in homogeneous and heterogeneous systems has attracted great interest in organic synthesis because their acidic and redox properties can be controlled at the molecular or atomic level and they also offer economical and environmental benefits [31,32,33,34,35].
Among the different classes of polyoxometalates, the Keggin-type heteropolyacids (HPAs) offer a strong option for efficient and cleaner processing compared to polluting and corrosive liquid acid catalysts [36]. They have the particularity of possessing multi-functional properties as strong Brønsted acidity and strong redox power. Their use allows eductions of costs and waste generation through reuse and recycling [37,38].
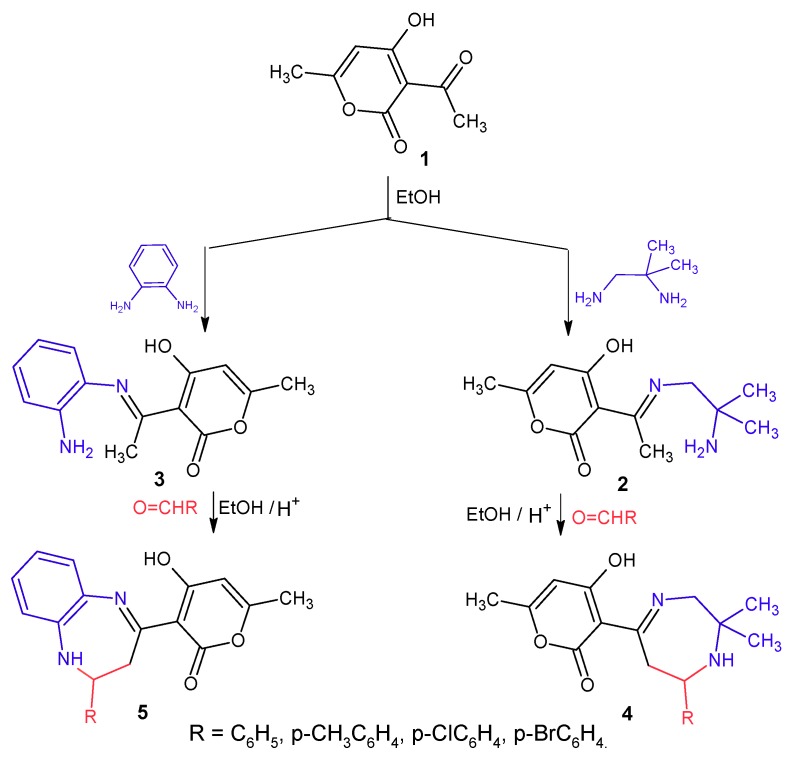
We report here for the first time the synthesis of 1,4-diazepines 4 from enaminones 2 in the presence of both CF3COOH and Keggin type HPA catalysts. 1,5-Benzodiazepines 5 have also been prepared, again in the presence of the HPA catalysts [29].
2. Results and Discussion
Previously, we have synthesized 1,5-benzodiazepine 5 products [29] from the reaction between product 3 and aromatic aldehydes in the presence of catalytic amounts of trifluroacetic acid in ethanol under reflux conditions. In continuation of this work on the synthesis of diazepine rings, we have now prepared a series of novel 1,4-diazepines 4 from derivatives 2 following to the same procedure (Scheme 1). The results (Table 1) show that the reaction is slow with unsatisfactory yields.
Scheme 1.
Synthesis of 1,4-benzodiazepines 4 and 1,5- benzodiazepines 5.
Table 1.
Synthesis of 1,4-diazepine and 1,5-benzodiazepine derivatives using CF3COOH as catalyst under reflux conditions.
| Compounds | R | Ratio of aldehyde | CF3COOH Times (min) / Yields (%) |
|---|---|---|---|
| 4a | C6H5 | 1.5 | 420/60 |
| 4b | p-CH3C6H5 | 1.5 | 420/55 |
| 4c | p-ClC6H5 | 2.5 | 360/64 |
| 4d | p-BrC6H5 | 2.5 | 360/69 |
| 5a | C6H5 | 1.5 | 360/79 |
| 5b | p-CH3C6H5 | 1.5 | 420/83 |
| 5c | p-ClC6H5 | 3 | 720/64 |
| 5d | p-BrC6H5 | 3 | 720/74 |
Due to strong Brønsted acidity and high oxidative power of Keggin type HPAs, that give them a bifunctional character, we decided to test the following series of HPAs in the preparation of derivatives 4 and 5: H3PW12O40 and H3+xPMo12-xVxO40, with x = 0-3, as catalysts. The obtained results are shown in Table 2.
Table 2.
Synthesis of 1,4-diazepine and 1,5-benzodiazepine derivatives using equimolar reactants in presence of various heteropolyacids under refluxing conditions.
| Compounds | R | H3PW12O40 | H3PMo12O40 | H4PMo11VO40 | H5PMo10V2O40 | H5PMo9V3O40 |
|---|---|---|---|---|---|---|
| Times (min) / Yields (%) | ||||||
| 4a | C6H5 | 600/72 | 300/69 | 180/70 | 30/85 | 60/83 |
| 4b | p-CH3C6H5 | 660/75 | 300/79 | 180/77 | 30/80 | 60/83 |
| 4c | p-ClC6H5 | 640/69 | 300/73 | 180/73 | 30/78 | 60/74 |
| 4d | p-BrC6H5 | 600/75 | 300/71 | 180/72 | 30/75 | 60/77 |
| 5a | C6H5 | 420/87 | 220/79 | 120/85 | 15/90 | 40/78 |
| 5b | p-CH3C6H5 | 390/82 | 220/83 | 120/82 | 15/88 | 40/89 |
| 5c | p-ClC6H5 | 360/70 | 220/77 | 120/75 | 15/93 | 40/87 |
| 5d | p-BrC6H5 | 360/76 | 220/73 | 120/74 | 15/91 | 40/94 |
The catalysts were used with equimolar amounts of reactants in refluxing ethanol; the reaction proceeded smoothly, affording the products in good to excellent yields. The results indicate that the catalytic activity depends on the composition of the catalyst. The substitution of one to three molybdenum atoms by vanadium atoms decreases the reaction time and increases the product yields. This is can be attributed to the character more oxidative and less acidic compared to those of H4PMo11VO40, H3PMo12O40 and H3PW12O40 [31,35,38]. As shown in Table 2, the order of efficiency follows the sequence: H5PMo10V2O40> H6PMo9V3O40> H4PMo11VO40 > H3PMo12O40 > H3PW12O40. Since the best results in term of yield and reaction time have been achieved with H5PMo10V2O40, this was selected for its better catalytic performance for the synthesis of diazepine rings.
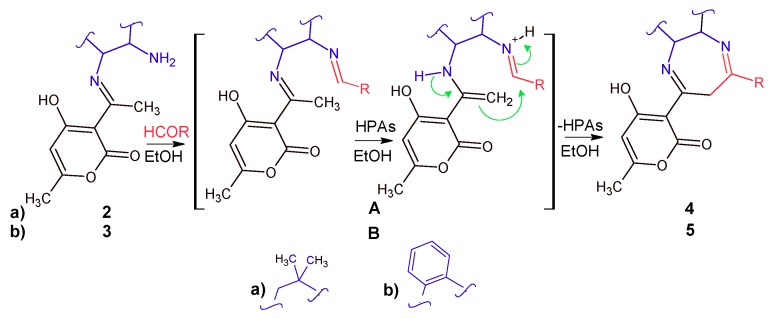
To optimize the reaction, temperature and molar ratio of H5PMo10V2O40 catalyst and reactants were checked. No reaction was detected at room temperature. Intramolecular cyclization of 2 or 3 via respectively intermediate A or B (Scheme 2) under reflux conditions in the presence of only 0.1 % of H5PMo10V2O40 catalyst is enough to drive the reaction forward; higher amounts of catalysts did not improve the results.
Scheme 2.
Synthetic route to compounds 4 and 5.
It is difficult to clarify how HPAs acts as a catalyst in this reaction. On the basis of a previously reported mechanism [29], it was suggested that the formation time of derivatives 5 depends on the nature of the substituent R on the aldehyde ring. Formation of 5 is particularly rapid when R is a strong donor group, whereas a withdrawing substituent inhibits the formation of compounds 5. Yields are also affected by electronic effects and they significantly increased by using an excess of aldehyde (Table 1). Similarly, intermediate A undergoes intramolecular cyclization under acidic conditions, according to the described conditions [29] leading to products 4. Yields and reaction times are very comparable to those obtained with intermediate B. These results can be explained by the nature of substituent R as shown in Table 1. When the optimized conditions (in presence of HPAs) are applied to substrates, high yields of 4 and 5 are obtained. These results show that the electronic nature of the substituent seems not to have a great effect on the reaction yields and that both acidic and redox properties of the heteropolyacids are involved during the synthesis.
The nature and the composition of the polyanion have an important effect on the catalytic properties of the HPAs. However, it is very difficult to clarify the difference between catalytic behaviour of these systems in this reaction. We believe that there is a complex relationship between the activity, the chemical composition and the position of the vanadium atoms in the Keggin polyanions.
3. Experimental
3.1. General
Melting points were measured on a Buchi 512 apparatus and were uncorrected. FTIR spectra were taken in Nujol on a Perkin –Elmer spectrometer. The 1H-NMR spectra (250 and 300 MHz) and 13C- NMR (63 MHz) were run on a Bruker spectrometer in CDCl3 using tetramethylsilane as internal standard. The impact ionisation (IE) mass spectra were recorded on a Nermag R-10-10C at 70 ev. Chemicals were purchased from Aldrich and Fluka. Compounds 2 and 3 were prepared according to the literature [39]. All the products were identified by comparison of analytical data (mp, NMR) with those reported (in the case of derivatives 5) or with authentic samples prepared by the conventional method using CF3COOH as catalyst (in the case of compounds 4).
3.2. Catalyst preparation
Phosphomolybdic and phosphotungstic acid samples, H3PMo12O40 and H3PW12O40 were obtained according to the method of Tsigdinos [40] by heating MoO3 (WO3) with a solution of diluted H3PO4. Synthesis of mono-, di- and tri-vanadium substituted phosphomolybdic acids, H3+x[PMo12−xVxO40] (x = 1–3) was carried out according to the procedure of Tsigdinos and Hallada [41] by acidifying with concentrated H2SO4 an appropriate mixture solutions of Na2HPO4, Na2MoO4 and NaVO3 in appropriate molar ratio. The HPAs formed were extracted with diethyl ether.
3.3. General procedure for the heteropolyacid-Keggin acid catalyzed synthesis of derivatives 4 and 5
A mixture of 2 or 3 (10 mmol) and aldehyde (10 mmol) in presence of Keggin acid catalyst (1% mmol) dissolved in ethanol (15 mL) was refluxed with stirring for the indicated time (see Table 2). The reaction was followed by TLC. When the reaction time was over, the organic solution was concentrated in vacuum. Then the compounds were filtered and washed twice with ethanol (2 × 10 mL). The catalyst was recovered after evaporating of ethanol.
3-(2,2-Dimethyl-7-phenyl-2,3,6,7-tetrahydro-1H-1,4-diazepin-5-yl)-4-hydroxy-6-methyl-2H-pyran-2-one (4a).Yellow solid, m.p. 186 ºC; 1H-NMR (CDCl3, 250 MHz): δ 1.45 (s, 3H, CH3), 1.48 (s, 3H, CH3), 2.13 (s, 3H, CH3), 3.58 (s, 2H, CH2-N=), 4.32 (t, 1H, J = 9 Hz, CH-Ar), 4.75 (dd, 2H, J = 9, 2 Hz, CH2-CH), 5.85 (s, 1H, =CH), 7.16-7.37 (m, 5H, H-Ar), 7.52 (s, 1H, NH), 13.80 (s, 1H, OH); 13C-NMR (CDCl3): δ 20, 22, 30, 52, 53, 55, 62, 96, 108, 128, 129, 132, 143, 163, 164, 179, 184; MS m/z (%): 326 ([M·]+, 6), 235 (19), 181 (60), 151 (27), 136 (14), 43 (37).
3-[2,2-Dimethyl-7-(4-methylphenyl)-3,6-dihydro-2H-1,4-diazepin-5-yl]-4-hydroxy-6-methyl-2H-pyran-2-one (4b).Yellow solid, m.p. 220 ºC; 1H-NMR (CDCl3, 250 MHz): δ 1.37 (s, 3H, CH3), 1.38 (s, 3H, CH3), 2.14 (s, 3H, CH3), 2.24 (s, 3H, CH3), 3.54 (s, 2H, CH2-N=), 4.17 (t, 1H, J = 9 Hz, CH-Ar), 4.70 (dd, 2H, J = 9, 2 Hz, CH2-CH), 5.75 (s, 1H, =CH), 7.00-7.10 (d, 2H, J = 8.3 Hz, H-Ar), 7.10-7.20 (d, 2H, J = 8.1 Hz, H-Ar), 7.80 (s, 1H, NH), 14.03 (s, 1H, OH); 13C-NMR (CDCl3): δ 20, 21, 32, 51, 53, 55, 61, 95, 108, 128 - 129, 134, 146, 163, 164, 178, 18; MS m/z (%): 340 ([M·]+, 2), 235 (16), 181 (40), 151 (4), 136 (1), 43 (70).
3-[7-(4-Chlorophenyl)-2,2-dimethyl-2,3,6,7-tetrahydro-1H-1,4-diazepin-5-yl]-4-hydroxy-6-methyl-2H-pyran-2-one (4c).Yellow solid, m.p. 223 ºC; 1H-NMR (CDCl3, 250 MHz): δ 1.38 (s, 3H, CH3), 1.39 (s, 3H, CH3), 2.15 (s, 3H, CH3), 3.65 (s, 2H, CH2-N=), 4.39 (t, 1H, J = 9 Hz, CH-Ar), 4.70 (dd, 2H, CH2, J = 9, 2 Hz, CH2-CH), 5.90 (s, 1H, =CH), 7.20-7.25 (m, 2H, H-Ar), 7.25-7.30 (m, 2H, H-Ar), 7.96 (s, 1H, NH), 13.82 (s, 1H, OH); 13C-NMR (CDCl3): δ 19, 20, 30, 50, 54, 55, 63, 96, 105, 129, 130, 133, 136, 165, 166, 177, 182; MS m/z (%): 360 ([M·]+, 24), 235 (14), 181 (100), 151 (8), 136 (8), 43 (28).
3-[7-(4-bromophenyl)-2,2-dimethyl-2,3,6,7-tetrahydro-1H-1,4-diazepin-5-yl]-4-hydroxy-6-methyl-2H-pyran-2-one (4d). Yellow solid, m.p. 245 ºC; 1H-NMR (CDCl3, 250 MHz): δ 1.44 (s, 3H, CH3), 1.47 (s, 3H, CH3), 2.15 (s, 3H, CH3), 3.62 (s, 2H, CH2-N=), 4.36 (t, 1H, J = 9 Hz, CH-Ar), 4.70 (dd, 2H, J = 9, 2 Hz, CH2-CH), 5.90 (s, 1H, =CH), 7.10-7.20 (m, 2H, H-Ar), 7.35-7.45 (m, 2H, H-Ar), 7.99 (s, 1H, NH), 13.79 (s, 1H, OH); 13C-NMR (CDCl3): δ 19, 20, 34, 53, 54, 56, 60, 95, 107, 130, 131, 135, 145, 163, 165, 178, 181; MS m/z (%): 371 ([M·]+, 0.5), 235 (18), 181 (20), 151 (3), 136 (5), 43 (66).
4. Conclusions
We present here the synthesis of a new series of 1,4-benzodiazepine derivatives 4 using two different catalyst classes, a conventional catalyst, CF3COOH, and HPAs. The results have shown the superior catalytic efficiency of the heteropolyacids. The principal features of the methodology using HPAs as catalyst are high-yields and shorter reaction times for both electron-releasing and electron-withdrawing substituted derivatives 4 and 5, substances with potentially interesting biological and medicinal properties.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- 1.Schutz H. Benzodiazepines. Springer; Heidelberg, Germany: 1982. [Google Scholar]
- 2.Landquist J.K. In: Comprehensive Heterocyclic Chemistry. Katritzky A.R., Rees C.W., editors. Volume 1. Pergamon; Oxford, UK: 1984. pp. 166–170. [Google Scholar]
- 3.Fryer R.I. Bicyclic Diazepines. In: Taylor E.C., editor. Comprehensive Heterocyclic Chemistry. Volume 50. Wiley; New York, NY, USA: 1991. Chapter ІІ. [Google Scholar]
- 4.Randall L.O., Kappel B., Garattini S., Musini E., Randall L.O., editors. Benzodiazepines. Raven Press; New York, NY, USA: 1973. p. 27. [Google Scholar]
- 5.Harris R.C., Straley J.M. Cationic polymethine dyes for acrylic fibers. 1,537,757. U.S. Patent. 1968 [Chem. Abstr.1970, 73, 100054w]
- 6.Bennamane N., Kaoua R., Hammal L., Nedjar-Kolli B. Synthesis of new amino-1,5-benzodiazepine and benzotriazole derivatives from dimedone. Org. Commun. 2008;1:62–68. [Google Scholar]
- 7.Hammal L., Bouzroura S., André C., Nedjar-Kolli B., Hoffmann P. Versatile Cyclization of Aminoanilino Lactones: Access to Benzotriazoles and Condensed Benzodiazepin-2-thiones. Synt. Commun. 2007;37:501–511. doi: 10.1080/00397910601039259. [DOI] [Google Scholar]
- 8.Aversa M.C., Ferlazzo A., Gionnetto P., Kohnke F.H. A convenient synthesis of novel [1,2,4]triazolo[4,3-a][1,5]benzodiazepine derivatives. Synthesis. 1986;3:230–231. doi: 10.1055/s-1986-31628. [DOI] [Google Scholar]
- 9.Essaber M., Hasnaoui A., Benharref A., Lavergne J.P. Synthesis of new tri- and tetraheterocyclic systems: 1,3-dipolar cycloaddition of nitrilimines on 2,7-dimethyl-4-phenyl-3H-1,5-benzodiazepin. Synth. Commun. 1998;28:4097–4104. doi: 10.1080/00397919809458689. [DOI] [Google Scholar]
- 10.El-Sayed A.M., Abdel-Ghany H., EI-Saghier A.M.M. A novel synthesis of pyrano(2,3-c)-, 1,3-oxazino(2,3 b)-,1,2,4-triazolo(3,4-b)-, oxazolo(2,3-b)-, furano(3,2-c)-, and 3-substituted-(1,5)benzodiazepin-2-ones. Synth Commun. 1999;29:3561–3572. doi: 10.1080/00397919908085990. [DOI] [Google Scholar]
- 11.Chimirri A., Grasso S., Ottana R., Romeo G., Zappala M. Synthesis and stereochemistry of novel [1,2,4]oxadiazolo[4,5-a][1,5]benzodiazepine derivatives. J. Heterocycl. Chem. 1990;27:371–374. doi: 10.1002/jhet.5570270250. [DOI] [Google Scholar]
- 12.Herbert J.A.L., Suschitzky H. Syntheses of heterocyclic compounds. Part XXIX. Substituted 2,3-dihydro-1H-1,5-benzodiazepines. J. Chem. Soc., Perkin Trans. 1974;1:2657–2661. doi: 10.1039/p19740002657. [DOI] [Google Scholar]
- 13.Morales H.R., Ulbarela B.A., Contreras R. New synthesis of dihydro- and tetrahydro-1,5-benzodiazepines by reductive condensation of o-phenylenediamine and ketones in the presence of sodium borohydride. Heterocycles. 1986;24:135–139. [Google Scholar]
- 14.Jung D.I., Choi T.W., Kim Y.Y., Kim I.S., Park Y.M., Lee Y.G., Jung D.H. Synthesis of 1,5-benzodiazepine derivatives. Synth. Commun. 1999;29:1941–1951. [Google Scholar]
- 15.Balakrishna M.S., Kaboudin B. A simple and new method for the synthesis of 1,5 benzodiazepine derivatives on a solid surface. Tetrahedron Lett. 2001;42:1127–1129. [Google Scholar]
- 16.Curini M., Epifano F., Marcotullio M.C., Rosati O. Corrigendum to Ytterbium triflate promoted synthesis of 1,5-benzodiazepine derivatives. Tetrahedron Lett. 2001;42:3193–3195. doi: 10.1016/S0040-4039(01)00413-0. [DOI] [Google Scholar]
- 17.Kaboudin B., Navaee K. Alumina/Phosphorus Pentoxide (App) as an Efficient Reagent for the Synthesis of 1,5-Benzodiazepines under Microwave Irradiation. Heterocycles. 2001;55:1443–1446. doi: 10.3987/COM-01-9253. [DOI] [Google Scholar]
- 18.Pozarentzi M., Stephanatou J.S., Tsoleridis C.A. An Efficient Method for the Synthesis of 1,5-Benzodiazepine Derivatives under Microwave Irradiation without Solvent. Tetrahedron Lett. 2002;43:1755–1758. doi: 10.1016/S0040-4039(02)00115-6. [DOI] [Google Scholar]
- 19.Yadav J.S., Reddy B.V.S., Eshwaraiah B., Anuradha K. Amberlyst-15: Anovel and recyclable reagent for the synthesis of 1,5-benzodiazepines anionic liquids. Green Chem. 2002;4:592–594. doi: 10.1039/B206558B. [DOI] [Google Scholar]
- 20.Sabitha G., Reddy G.S.K.K., Reddy K.B., Reddy N.M., Yadav J.S. A new, efficient and environmentally benign protocol for the synthesis of 1,5-benzodiazepines using cerium (III) chloride/sodium iodide supported on silica gel. Adv. Synth. Catal. 2004;346:921–923. doi: 10.1002/adsc.200303196. [DOI] [Google Scholar]
- 21.Yadav J.S., Reddy B.V.S., Kumar S.P., Nagaiah K. Indium(III) bromide: A novel and efficient reagent for the rapid synthesis of 1,5-benzodiazepines under solvent-free conditions. Synthesis. 2005:480–484. doi: 10.1055/s-2004-834939. [DOI] [Google Scholar]
- 22.Jarikote D.V., Siddiqui S.A., Rajagopal R., Daniel T., Lahoti R.J., Srinivasan K.V. Room temperature ionic liquid promoted synthesis of 1,5- benzodiazepine derivatives under ambient conditions. Tetrahedron Lett. 2003;44:1835–1838. doi: 10.1016/S0040-4039(03)00096-0. [DOI] [Google Scholar]
- 23.De S.K., Gibbs R.A. Scandium(III) triflate as an efficient and reusable catalyst for synthesis of 1,5-benzodiazepine derivatives. Tetrahedron Lett. 2005;46:1811–1813. doi: 10.1016/j.tetlet.2005.01.113. [DOI] [Google Scholar]
- 24.Varala R., Ramu E., Sreelatha N., Adapa S.R. Ceric ammonium nitrate (CAN) promoted efficient synthesis of 1,5-benzodiazepine derivatives. Synlett. 2006:1009–1014. doi: 10.1002/chin.200636159. [DOI] [Google Scholar]
- 25.Pasha M.A., Jayashankara V.P. Synthesis of 1,5-benzodiazepine derivatives catalyzed by zinc chloride. Heterocycles. 2006;68:1017–1023. doi: 10.3987/COM-05-10647. [DOI] [Google Scholar]
- 26.Kumar R., Chaudhary P., Nimesh S., Verma A.K., Chandra R. An efficient synthesis of 1,5-benzodiazepine derivatives catalyzed by silver nitrate. Green Chem. 2006;8:519–521. doi: 10.1039/b601993e. [DOI] [Google Scholar]
- 27.Reddy B.M., Sreekanth P.M., Lakshmanan P. Sulfated zirconia as an efficient catalyst for organic synthesis and transformation. J. Mol. Catal. A: Chem. 2005;237:93–100. doi: 10.1016/j.molcata.2005.04.039. [DOI] [Google Scholar]
- 28.Yadav J.S., Reddy B.V.S., Satheesh G., Srinivasulu G., Kunwar A.C. InCI3-catalyzed stereoselective synthesis of optically pure 1,5-benzodiazepines. Arkivoc. 2005;iii:221–227. [Google Scholar]
- 29.Fodili M., Amari M., Kolli B., Robert A., Baudy-Floch M., Le Grel P. An efficient Synthesis of New 2-pyronyl-1,5-benzodiazepine derivatives. Synthesis. 1999;5:811–814. doi: 10.1055/s-1999-3479. [DOI] [Google Scholar]
- 30.Heravi M.M., Sadjadi S., Oskooie H.A. An Efficient Synthesis of 3H-1,5-benzodiazepine Derivatives Catalyzed by Heteropolyacids as a Heterogeneous Recyclable Catalyst. J. Chin. Chem. Soc. 2008;55:842–845. doi: 10.1002/jccs.200800125. [DOI] [Google Scholar]
- 31.Khozhevnikov I.V. Catalysis by heteropolyacids and multicomponent polyoxometalates in liquid-phase reactions. Chem. Rev. 1998;98:171–198. doi: 10.1021/cr960400y. [DOI] [PubMed] [Google Scholar]
- 32.Ono Y., Thomas J.M., Zamaraev K.I., editors. Perspectives in Catalysis. Blackwell; London, UK: 1992. p. 341. [Google Scholar]
- 33.Khozhevnikov I.V., Matveev K.I. Homogeneous Catalysts Based on Heteropolyacids. Appl. Catal. 1983;5:135–150. doi: 10.1016/0166-9834(83)80128-6. [DOI] [Google Scholar]
- 34.Izumi Y., Urabe K., Onaka A. Zeolite, Clay and Heteropolyacids in Organic Chemistry. Kodansha, Tokyo-VCH; Weinheim, Germany: 1992. p. 99. [Google Scholar]
- 35.Khozhevnikov I.V. Heteropolyacids and related compounds as catalysts for fine chemical synthesis. Catal. Rev.-Sci. Eng. 1995;37:311–352. doi: 10.1080/01614949508007097. [DOI] [Google Scholar]
- 36.Heravi M.M., Derikvand F., Haeri A., Oskoie H.A., Bamoharram F.F. Heteropolyacids as Green and Reusable Catalysts for the Synthesis of Isoxazole Derivatives. Synth. Commun. 2008;38:135–140. doi: 10.1080/00397910701651326. [DOI] [Google Scholar]
- 37.Schwegler M.A., Bekkum H., Van Munck N. Heteropolyacids as catalysts for the production of phthalate diesters. Appl. Catal. 1991;74:191–204. [Google Scholar]
- 38.Mizuno N., Misono M. Studies of the activity of catalysts based on heteropolyacids. J. Mol. Catal. 1994;86:319–342. doi: 10.1016/0304-5102(93)E0155-A. [DOI] [Google Scholar]
- 39.Maiti B.C., Maitra S.K. Reaction of dehydroacetic acid with aliphatic, aromatic and heterocyclic amines. Indian J. Chem. 1998;37:710–712. [Google Scholar]
- 40.Tsigdinos G.A. Preparation and Characterization of 12-Molybdophosphoric and 12-Molybdosilicic Acids and Their Metal Salts. Ind. Eng. Chem., Prod. Res. Dev. 1974;13:267–274. doi: 10.1021/i360052a011. [DOI] [Google Scholar]
- 41.Tsigdinos G.A., Hallada C.J. Molybdovanadophosphoricacids and their salts (I) Investigation of methodsof preparation and characterization. Inorg. Chem. 1968;7:437–441. doi: 10.1021/ic50061a009. [DOI] [Google Scholar]