Abstract
Mid-life hypertension and cerebral hypoperfusion may be preclinical abnormalities in people who later develop Alzheimer’s disease. Although accumulation of amyloid-beta (Aβ) is characteristic of Alzheimer’s disease and is associated with upregulation of the vasoconstrictor peptide endothelin-1 within the brain, it is unclear how this affects systemic arterial pressure. We have investigated whether infusion of Aβ40 into ventricular cerebrospinal fluid modulates blood pressure in the Dahl salt-sensitive rat. The Dahl salt-sensitive rat develops hypertension if given a high-salt diet. Intracerebroventricular infusion of Aβ induced a progressive rise in blood pressure in rats with pre-existing hypertension produced by a high-salt diet (p < 0.0001), but no change in blood pressure in normotensive rats. The elevation in arterial pressure in high-salt rats was associated with an increase in low frequency spectral density in systolic blood pressure, suggesting autonomic imbalance, and reduced cardiac baroreflex gain. Our results demonstrate the potential for intracerebral Aβ to exacerbate hypertension, through modulation of autonomic activity. Present findings raise the possibility that mid-life hypertension in people who subsequently develop Alzheimer’s disease may in some cases be a physiological response to reduced cerebral perfusion complicating the accumulation of Aβ within the brain.
Keywords: Alzheimer disease, amyloid-beta peptides, baroreflex, Dahl salt-sensitive rats, hypertension
Introduction
Hypertension affects at least one-quarter of adults and more than half of those over 60 years.1 It is a major risk factor for cognitive decline and vascular dementia (VaD)2 and has been reported to increase the risk of developing Alzheimer’s disease (AD)3,4 but the mechanisms are unclear. Cerebrovascular changes in AD include disruption to the blood–brain barrier (BBB), endothelial dysfunction, arterial stiffness and cerebral amyloid angiopathy (CAA).5–7 Inadequate cerebral perfusion is an early-stage manifestation of AD and probably contributes to concomitant white matter pathology and cognitive decline.8–13 Recent studies in humans implicate cerebrovascular hypotension as a driver of systemic hypertension, the latter serving as a mechanism to preserve cerebral blood flow.14
The identification of mid-life hypertension and cerebral hypoperfusion as preclinical abnormalities in people who later develop AD provides a potential opportunity for intervention to delay or slow disease. However, it remains unclear how the vascular and neurodegenerative processes interact. Studies in animal models suggest that hypertension and associated non-amyloid small vessel disease (SVD) may cause Aβ to accumulate within the brain.15–17 Retrospective analysis showed Aβ plaque load to be higher in post-mortem brain tissue from AD patients with than without a history of hypertension,18 supporting findings in animal models.
However, the converse possibility should also be considered: that mid-life metabolic and structural cerebrovascular alterations in people who subsequently develop AD, may induce hypertension.14 In AD, intracerebral vascular tone, neurovascular coupling and autoregulation are disrupted by abnormalities that affect regulation of vascular calibre and reduce blood flow.9 These abnormalities include cholinergic deafferentation of cortical blood vessels,19 free radical-mediated cerebral vasoconstriction,20–23 Aβ-mediated upregulation of endothelin-1 (EDN1) production,8,24–26 and an increase in brain angiotensin II mediated at least partly by a rise in the activity of angiotensin-converting enzyme (ACE).27,28 Maintenance of cerebral perfusion in the face of these metabolic abnormalities would require an increase in cerebral perfusion pressure, manifesting as systemic hypertension.29–31
Ageing is associated with progressive decline in cardiovascular autonomic reflexes, particularly baroreflex sensitivity.32 The consequences can include increased BP variability, impaired responsiveness to challenges to BP, and an increased risk of sudden cardiac death. Altered baroreflex sensitivity is an independent risk factor for the development of memory problems in older people.33 There is evidence of autonomic dysfunction in most types of dementia. It is particularly common in Parkinson’s disease dementia (PDD) and dementia with Lewy bodies (DLB), reflecting disruption to central autonomic pathways in the brain stem and hypothalamus. In AD, neurodegenerative changes affect autonomic nervous system pathways in the anterior cingulate, insular cortex and amygdala.34 Patients with Lewy body diseases, AD and VaD have an increased prevalence of orthostatic hypotension.
Cardiovascular autonomic control mechanisms can be monitored by measurement of spontaneous fluctuations in heart rate and blood pressure, heart rate variability (HRV), systolic blood pressure variability (SBPV), and baroreflex gain (BRG) which provides information on the relative contribution of the baroreflex to control of heart rate (HR). Impaired BRG may indicate cardiovascular or neurological disease. The baroreflex is essential for the short-term control of BP but may also influence BP over the longer term, although this is more controversial.35
Aβ, in particular Aβ40, has been shown to induce vasoconstriction following topical application to isolated arteries36 or direct administration to mouse cortex.23 Application of Aβ40 but not Aβ42 to pial vessels impaired neurovascular coupling and reduced cerebral perfusion, replicating abnormalities of cerebral blood flow in a range of hAPP transgenic mouse models. Our aims in this study were to investigate whether intracerebroventricular (ICV) infusion of Aβ40 would induce hypertension, or augment hypertension in a hypertensive animal model. To manipulate these variables in vivo, we have used the Dahl salt-sensitive (DAHL/SS) rat in conjunction with ICV Aβ40 infusion. Aβ42 aggregates too readily for sustained delivery by infusion pump, whereas Aβ40 is more soluble, is the major species of Aβ in human cerebrospinal fluid (CSF) and, as noted above, is known to induce the constriction of pial vessels that we wanted to model by ICV infusion. Our hypotheses were that Aβ40 would exacerbate systemic hypertension, with more pronounced effects in the presence of pre-existing hypertension (high-salt DAHL/SS), and that the increase in blood pressure would be reflected in alterations in its neuro-humoral control.
Methods
Animals
All experimental procedures in this study were conducted in strict accordance with the UK Animals (Scientific Procedures) Act 1986 and approved by University of Bristol local ethical review committee. The study used male Dahl salt-sensitive 12–14-week-old rats (from Charles River, UK) with a mean initial weight of 360 g (range 303–416 g – for details see Table 1). DAHL/SS rats develop hypertension when fed a high-salt diet (8% NaCl), whereas DAHL/SS rats fed a low-salt diet (0.3% NaCl) have blood pressure within the normal range (systolic BP < 140 mmHg).37 DAHL/SS rats have other traits in common with many hypertensive humans, including insulin resistance, hyperlipidaemia with high serum cholesterol, and reduced renal function. Animals were fed a low-salt diet (0.3% NaCl) from weaning but half were subsequently preconditioned on a high-salt diet (8% NaCl) for three weeks prior to arrival. The two groups were fed these respective low- or high-salt diets throughout the study. Welfare assessments were conducted daily. The animals were housed at a constant 22℃ under a 14:10 light/dark cycle with free access to food and water. Animals were initially accommodated in groups, before transfer to individual cages after implantation of the telemetry device. All data were analysed and are reported in accordance with ARRIVE guidelines.38
Table 1.
Pre-infusion baseline telemetry data.
| Group | N | Weighta (g) | MBP (mmHg) | SBP (mmHg) | DBP (mmHg) | HR (bpm) | RR (breaths/min) |
|---|---|---|---|---|---|---|---|
| High salt | 12 | 361 ± 6 | 151 ± 6* | 169 ± 7* | 134 ± 5* | 373 ± 5 | 92 ± 4* |
| Low salt | 15 | 359 ± 9 | 114 ± 2 | 127 ± 2 | 103 ± 2 | 364 ± 4 | 82 ± 3 |
Note: Pre-infusion baseline telemetry data in high- and low-salt groups. Mean values ± SEM.
Weight prior to telemetry surgery at 12–14 weeks of age. Baseline MBP, SBP, DBP and RR were significantly higher in the high- than the low-salt group (*p < 0.0001, *p < 0.05).
MBP, SBP, DBP: mean, systolic and diastolic blood pressure; HR: heart rate; RR: respiratory rate.
Study design
Groups of 5 DAHL/SS rats were assigned to a low- or high-salt diet prior to arrival to our facility, according to a block randomization schedule determined by the supplier. Simple randomization was used to assign animals in each group to receive ICV infusion of saline or Aβ40. The Aβ infusion model does not fully reproduce the complex neuropathology of AD but does allow for the precise study of Aβ-specific, time course-dependent changes.39 A power calculation was performed to determine the minimum number (n = 6) of animals required for each experimental group; based on previous experience of similar studies, a conservative estimate of the standard deviation of mean blood pressure values (≤10%) was used, giving 80% power to detect changes of 12% or more at the p = 0.05 level and 90% power to detect differences of 15% or more. Male DAHL/SS were 12–14 weeks of age prior to telemetry surgery (see below), specific pathogen free (barrier-maintained) and experiment-naïve.
Experimental procedures
Telemetry device implantation
A telemetry system (Data Sciences International, MN, US) was used to record arterial pressure. The telemetry device was implanted according to the method of Waki et al.40 An aseptic laparotomy was performed under reversible general anaesthetic to expose the abdominal aorta. The catheter tip of a radio-telemetry device (PhysioTel® PA series transmitter model PA-C40) was implanted into the descending aorta and secured with tissue adhesive. The body of the device was placed in the abdominal cavity and secured to the abdominal wall during suture closure of the incision. After recovery from anaesthesia, rats were placed in individual cages on receiver platforms (PhysioTel® Receivers model RPC-1) connected to a CED1401 data acquisition interface and a computer running Spike2 software (both Cambridge Electronic Design, UK), enabling continuous acquisition of data. Baseline telemetry data collection was started five days after telemetry surgery and continued for five to seven days, prior to mini-osmotic pump surgery.
Mini-osmotic pump implantation
Ten to twelve days after implantation of the telemetry device, a mini-osmotic pump was inserted for ICV infusion of Aβ40 or saline (control).39,41 Prior to surgery, the mini-osmotic pump and vinyl catheter (Alzet® osmotic pump model 2004 and Alzet® brain infusion kit 1; Charles River) were filled under aseptic conditions with a solution containing either Aβ40 (1 mg/ml (231 µM) of stock solution prepared in 0.35% acetonitrile and diluted to the final concentration in sterile saline) or saline (with acetonitrile to the same final concentration). Under reversible general anaesthetic, the rat was placed in a stereotaxic frame and the skull exposed using aseptic surgical technique. The fine-gauge stainless steel cannula (brain infusion kit) was held in position by a clamp arm attached to the stereotaxic frame. The cannula was positioned and implanted below the skull surface into the right lateral ventricle (AP −1.0, L −1.6, D 5.0) and attached to the catheter and pump. The cannula was fixed in position and the mini-osmotic pump implanted subcutaneously. ICV osmotic infusion allowed continuous delivery of Aβ40 or saline at 0.25 µl per hour over a four-week period (equivalent to 0.006 mg of Aβ40 per day). The rate of CSF production and removal in the rat is about 3.6 ml/d.42 The physiological level of Aβ40 in rat CSF is approximately 0.3–0.4 nM, so the addition of 0.006 mg/d Aβ40 should elevate this by approximately 1000-fold to 385 nM. However, Aβ40 is eliminated much more rapidly than the CSF itself,43 so we estimate the mean level to have been raised by up to about 100-fold over the duration of the infusion.
Experimental outcomes
Telemetry monitoring and data analysis
Rats were fitted with a telemetry device capable of measuring arterial pulse pressure. HR, respiratory rate (RR), systolic (SBP), diastolic (DBP) and mean blood pressure (MBP) were derived from this waveform using Spike2 software. The telemetry device was switched on five days after implantation to record for five to seven days (baseline). Mean HR, RR, SBP, DBP and MBP were calculated for each 24-h period and an average baseline value for each parameter was determined. Following insertion of the ICV cannula, telemetry measurements were recorded at a digital sampling frequency of 100 Hz (low pass filter at 45 Hz) continuously from 3 to 28 days post-surgery and the average for each 24-h period was determined relative to mean baseline values.
HR and BP variability analysis
Spike2 software was used for spectral analysis of HR and SBP variability (HRV and SBPV). These indicators of autonomic function were analysed by separating the main peaks of the power spectrum in the frequency domain using a Fourier transform algorithm (Supplementary Figure 6). The three main components of the power spectrum were interpolated at specific frequencies: very low frequency (VLF) fluctuations at 0–0.26 Hz; low frequency (LF) fluctuations at 0.26–0.76 Hz; and high frequency (HF) fluctuations at 0.76–3.3 Hz. VLF, LF and HF measurements were made in absolute values of HR power (bpm2/Hz) or SBP power (bpm2/Hz).40
By use of Spike2 software, an index of baroreceptor sensitivity was derived from spontaneous fluctuations and pulse interval, according the time-series method of Oosting et al.:44 (1) blood pressure and pulse interval traces are smoothed to minimize respiratory fluctuations; (2) episodes in which blood pressure rises or falls over several consecutive heartbeats (ramps) were identified; (3) ramp changes in blood pressure versus changes in pulse interval are plotted after delays of three, four and five heartbeats; and (4) a linear best-fit of the relationship between changes in blood pressure and changes in pulse interval is calculated to estimate baroreceptor sensitivity, as described previously (Supplementary Figure 6).40 A mean r2 value >0.7 for the three linear fits was used as a goodness-of-fit threshold, thereby excluding ramps with a poor straight-line fit. In younger adults with normal baroreflex function, no more than about 30% of spontaneous SBP ramps are compensated for by a progressive reflex change in pulse interval.45 This physiological behaviour is explained by the fact that the sinus node is not under the exclusive control of the baroreflex.
Tissue collection and preparation
Brain tissue and plasma samples were collected post mortem. Tissue homogenates were separately prepared from anterior, intermediate and posterior regions of each cerebral hemisphere, yielding six homogenates from each brain. Tissue was transferred to 2 mL screw-cap tubes with ceramic beads and 1% SDS homogenization buffer (1% SDS, 10 mM Tris base pH 6, 0.1 mM NaCl, 1 µM PMSF, 1 µg/ml Aprotinin) at 20% w/v. Tissue was homogenized for 2 × 20 s at 6000 r/min in a Precellys® homogenizer (Bertin Technologies, from Stretton Scientific, UK) and centrifuged at 13,000 r/min for 15 min at 4℃. Homogenates were aliquoted and stored at −80℃. Blood samples were collected by cardiac puncture under terminal anaesthesia in EDTA tubes and centrifuged immediately at 6000 r/min for 5 min. The plasma supernatant was collected and stored in aliquots at −80℃. Total protein was quantified in tissue and plasma samples by Coomassie (Bradford) protein assay (Thermo Scientific, UK).
EDN1, Aβ40 and ECE-2 sandwich ELISAs
EDN1 in plasma and brain homogenates was measured by sandwich ELISA using the Endothelin Pan Specific DuoSet ELISA kit (R&D Systems, UK). The recommended protocol was followed, with samples (diluted 1:4 in reagent diluent, 1% BSA in PBS, pH 7.2), standards (2-fold serial dilutions of EDN1 standard, 0.98–250 pg/ml) and blanks (reagent diluent alone) added to the plate for 2 h. Aβ40 in plasma and brain homogenates was measured by sandwich ELISA, as described.46,47 Sample homogenates (total protein 0.05 mg/ml) or plasma (1:2 dilution), blanks and a 7-point standard curve of recombinant Aβ40 (0.2–12.5 nM; Sigma-Aldrich, UK), prepared in PBS containing 1% 1,10 phenanthroline. ECE-2 was measured by sandwich ELISA, modified from Palmer et al.24 Homogenate samples (1:2 dilution), blanks and seven serial dilutions of recombinant ECE-2 (R&D Systems) at 54.1–3460 ng/ml were added to wells in duplicate. For each assay, the bound antibody was detected with a 1:1 mixture of substrate solution (hydrogen peroxide and tetramethylbenzidine; R&D Systems) with 2N sulphuric acid to stop the reaction. The optical density was read at 450 nm (FLUOstar OPTIMA plate reader; BMG Labtech, Germany). To correct for any optical imperfections in the microplate, readings at 590 nm were subtracted from those at 450 nm. Sample concentrations of EDN1, Aβ40 and ECE-2 were interpolated from the respective standard curve. Each sample was assayed in duplicate.
ECE-1 Western blot
ECE-1 was measured by Western blot with a biotinylated ECE-1 antibody (BAF1784, R&D systems), as described.48 The antibody detects full-length ECE-1 at 120 kDa and a 75 kDa splice variant (ECE-1sv). Recombinant ECE-1 standard (1784ZN, R&D systems) was diluted to create a four-point standard curve (0.1–2.75 ng/µl) for each gel. Standards and samples of tissue homogenates (15 µg total protein) in SDS sample buffer were run on pre-cast gels before transfer to a nitrocellulose membrane. The primary antibody was added at 1:2000 in blocking buffer (3% BSA-TTBS) for 2 h. Streptavidin-conjugated peroxidase antibody was used for detection at 1:800 in blocking buffer. Each sample was measured in duplicate and the mean total ECE-1 + ECE-1sv concentration calculated.
ACE activity
ACE activity in brain homogenates and plasma was measured using the ACE1-specific fluorogenic substrate Abz-FRK(Dnp)-P (Biomol International) in the presence of the ACE-inhibitor, captopril (1 mM), as described by Miners et al.27,28 ACE capture antibody (MAB929), recombinant ACE standard (929-ZN, R&D Systems) and fluorogenic substrate (ES005) were all from R&D Systems. Fluorescence (320/405 nm) was measured after overnight incubation. ACE activity measurements were interpolated from the standard curve using a 4-parameter curve fit.
Statistical methods
Statistical analyses were performed in GraphPad Prism 6. All data sets were assessed for normality of distribution by the D'Agostino and Pearson test.
Baseline telemetry data (collected prior to mini-pump implantation) were log-transformed prior to analysis: the resultant parametric data were compared by unpaired samples t-test. Post-infusion telemetry data from each of the four sub-groups (low/high salt; Aβ/saline infusion) were compared by paired samples t-test to the respective baseline data. To compare the effects of Aβ and saline infusion, the telemetry data collected following infusion was adjusted relative to the corresponding sample pre-infusion baseline mean and analysed for each 24-h period; best-fit regression lines (for change in physiological parameter over time post-infusion) were compared by sum-of-squares F-test.
Biochemical data for each subgroup of animals were compared by t-test.
Results
Baseline data
Mean baseline data are presented in Table 1. DAHL/SS rats fed the high-salt diet had significant (p < 0.0001) hypertension at baseline (MBP (mean ± SEM): 151 ± 6 mmHg) compared to those on the low-salt diet (MBP: 114 ± 2 mmHg).
Outcomes and estimation
High salt DAHL/SS telemetry post-infusion
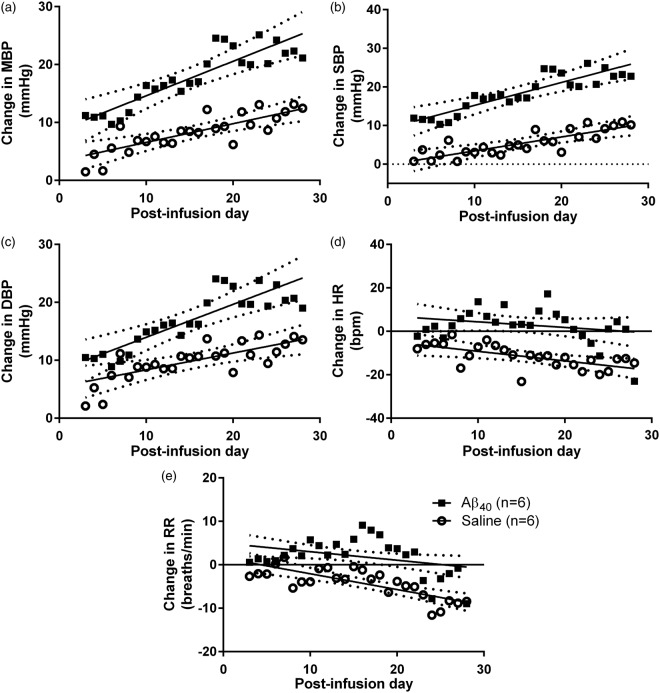
We compared the changes in HR, RR, MBP, SBP and DBP over baseline in Aβ- (n = 6) and saline- (n = 6) infused high-salt DAHL/SS rats from 3 to 28 days post-infusion (Supplementary Figure 1). BP increased over time in both saline- and Aβ-infused rats but the increase was markedly greater in the Aβ-infused animals. Comparison of best-fit regression lines by sum-of-squares F-test showed that the increases in MBP, SBP and DBP were much greater in Aβ-infused than saline-infused animals, and the decline in HR and RR less marked (p < 0.0001 for all five comparisons; Figure 1). The BP increase in the saline-infused group was accompanied by a drop in both HR and RR over time. The HR and RR of the Aβ-infused group did not change over the infusion period, despite the greater BP increase. In the final week following infusion (days 22–28 inclusive), MBP relative to pre-infusion baseline had increased by 21 ± 11 mmHg (mean ± SD) in the Aβ group (t (5) = 4.73, p = 0.005), compared to 11 ± 8 mmHg in the saline group (t (5) = 3.64, p = 0.015; Supplementary Figure 4(a)).
Figure 1.
Change in BP, HR and RR in High-Salt DAHL/SS. Change in BP, HR and RR in high salt DAHL/SS over 28 days of Aβ (n = 6) or saline (n = 6) infusion. Each data point represents the group mean value on a given day during infusion. Best-fit linear regression (solid) and 95% CI (dotted) lines are shown. A comparison (sum-of-squares F-test) of best-fit regression lines tested the null hypothesis (H0) that a single linear regression line could fit the combined data sets for the change in HR, RR, MBP, SBP and DBP, relative to baseline, after infusion of Aβ or saline. H0 was rejected for each comparison. There was a highly significant greater effect of Aβ infusion compared to control (saline) infusion on (a) MBP (F2,288 = 38.45, p < 0.0001), (b) SBP (F2,288 = 61.50, p < 0.0001), (c) DBP (F2,288 = 19.95, p < 0.0001), (d) HR (F2,288 = 23.94, p < 0.0001), (e) RR (F2,288 = 26.51, p < 0.0001). The increase in MBP, SBP and DBP was much greater in Aβ40- than saline-infused animals (P < 0.0001), and the decline in HR and RR less marked (P < 0.0001).
Low-salt DAHL/SS telemetry post-infusion
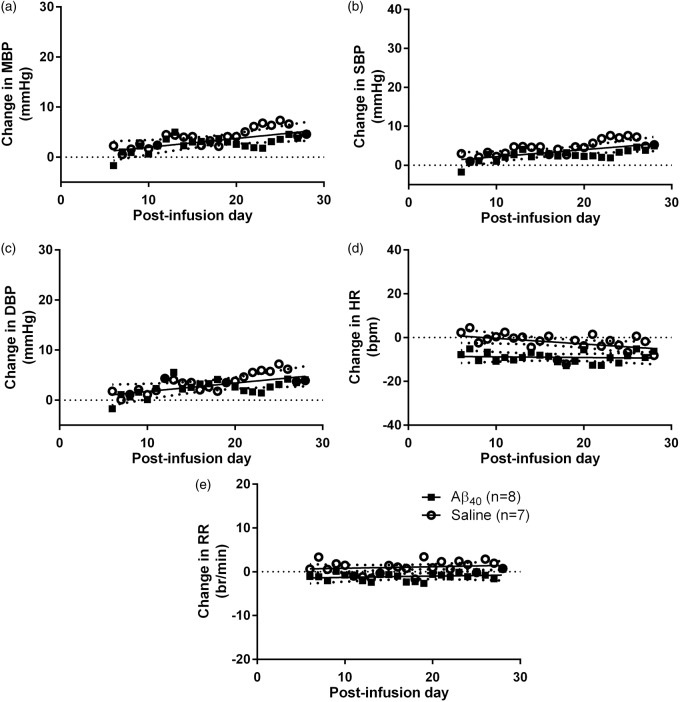
We compared the changes in HR, RR, MBP, SBP and DBP with respect to baseline mean in Aβ- (n = 8) and saline- (n = 7) infused low-salt DAHL/SS rats (Supplementary Figure 2). Comparison of best-fit regression lines by sum-of-squares F-test revealed no differences in MBP, SBP or DBP between the Aβ- and saline-infused groups. Small but significant reductions were found in both HR and RR (p < 0.0001 for both) in the Aβ- compared to the saline-infused animals (Figure 2). MBP was marginally elevated by 3 ± 3 mmHg following Aβ infusion (days 22–28 mean vs. baseline mean; t (5) = 2.95, p = 0.022), but did not differ significantly after saline infusion (Supplementary Figure 4(b)). This finding should be interpreted with caution as there was not a significant difference between Aβ- and saline-infusion by regression line comparison and the possibility of a type I statistical error cannot be excluded.
Figure 2.
Change in BP, HR and RR in low-salt DAHL/SS. Change in (a) MBP, (b) SBP, (c) DBP, (d) HR and (e) RR in Aβ- (n = 8) or saline-infused (n = 7) DAHL/SS rats on low-salt diet. A sum-of squares F-test comparison tested the null hypothesis (H0) that a single linear regression line could fit the combined data sets. H0 was accepted for MBP, SBP and DBP but rejected for HR and RR. There was no significant effect of Aβ infusion compared to control (saline) infusion on either MBP (F2,328 = 1.138, p > 0.05), SBP (F2,328 = 2.386, p > 0.05) or DBP (F2,328 = 0.632, ns). As the best-fit lines for blood pressure (MBP, SBP and DBP) did not differ significantly, a shared best-fit line is shown for the two infusion groups. There was a significant effect of Aβ infusion compared to control (saline) on both HR (F2,328 = 19.24, p < 0.0001) and RR (F2,325 = 11.59, p < 0.0001). Individual best-fit lines are shown for HR and RR.
HRV, SBPV and BRG in high-salt DAHL/SS post-infusion
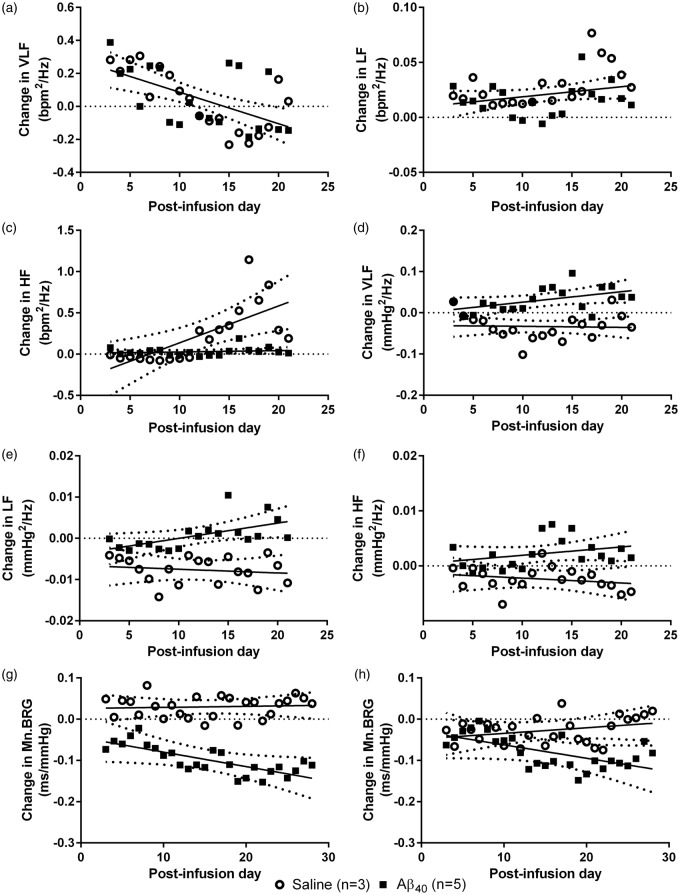
Data available for a subset of high-salt DAHL/SS (Aβ: n = 5; saline: n = 3) from 3 to 21 d post-infusion were analysed for HRV, SBPV and BRG (Supplementary Figure 3). A sum-of squares F-test comparison tested the null hypothesis (H0) that a single linear regression line could fit the combined data sets. We found no significant difference in the VLF (F2,148 = 0.120, ns) or LF component (F2,148 = 2.507, ns) of HRV (change in bpm2/Hz) between the saline- and Aβ-infused rats (H0 accepted; Figure 3(a) and (b)). The HF component of HRV, reflecting respiration, increased over time in the saline group but remained at baseline in the Aβ40-infusion group (F2,148 = 10.64, p < 0.0001; H0 rejected; Figure 3(c)). A comparison of best-fit lines for the VLF, LF and HF components (mmHg2/Hz) of SBPV demonstrated that each increased significantly in the Aβ- compared to the saline-infusion group (VLF F2,148 = 17.46, LF: F2,148 = 15.59 both p < 0.0001; HF: F2,148 = 17.46 p < 0.0005; H0 rejected; Figure 3(d) to (f)).
Figure 3.
Change in HRV, SBPV and BRG in High-Salt DAHL/SS. Change in HRV, SBPV and mean BRG in high salt DAHL/SS after Aβ (n = 5) or saline (n = 3) infusion. There were no significant effects of Aβ infusion on the (a) VLF (F2,148 = 0.120, ns) and (b) LF (F2,148 = 2.507, ns) components of HRV. There was a significant effect of Aβ infusion on (c) HF (F2,148 = 10.64, p < 0.0001). There was a significant effect of Aβ infusion on (d) VLF (F2,148 = 17.46, p < 0.0001), (e) LF (F2,148 = 15.59, p < 0.0001), and (f) HF (F2,148 = 17.46, p < 0.0005) components of SBPV. There was a significant difference in the change in mean BRG (Mn.BRG) (g) positive (F2,202 = 28.91, p < 0.0001) and (h) negative (F2,202 = 4.761, p < 0.001) ramps during Aβ compared with saline infusion. Data are presented as daily group means, with lines of best-fit (solid) and 95% CI (dotted). A combined best-fit line is shown for both infusion groups when the H0 was accepted (a–b). Separate lines for Aβ- and saline- infusion groups are shown when the H0 was rejected (c–h).
We compared the relative change in BRG over baseline in a subset of Aβ- (n = 5) and saline-infused (n = 3) high-salt DAHL/SS rats from 3 to 28 days post-infusion. There was a progressive decline in both positive (F2,202 = 28.91, p < 0.0001) and negative ramps (F2,202 = 4.761, p < 0.001) over the 28 days following Aβ40 infusion but little change after saline infusion (H0 rejected; Figure 3(g) and (h)). Comparison of best-fit regression lines showed that the data from the Aβ- and saline-infused groups differed significantly for both positive (p < 0.0001) and negative (p < 0.01) ramps, indicating a reduction in the sensitivity of the baroreflex response over time during Aβ infusion.
Aβ40 level in brain tissue homogenates and plasma
The level of Aβ40 (measured by sandwich ELISA) remaining in the brain after completion of the infusions was measured in homogenates of anterior, medial and posterior thirds of the left and right hemispheres. We found no consistent differences between groups, either when comparing individual brain regions or when the measurements were combined to indicate the mean for each case (Supplementary Figure 5(a)). Aβ40 is cleared quite rapidly from the CSF43,49 and although there is some penetration of the brain parenchyma39 it does not aggregate to any significant extent.50 The finding that Aβ40 was not significantly elevated in the brain tissue of the rats that had received ICV infusion of Aβ is in keeping with these previous studies and suggests that infused Aβ peptide which may have penetrated the brain had been largely cleared from the brain by the time that the tissue was collected, i.e. after completion of the four weeks of infusion.
We also measured Aβ40 in plasma samples. The levels of peptide for several cases fell below the detection limit of the sandwich ELISA. No significant differences were found between the Aβ-infused groups in either the high-salt or low-salt DAHL/SS (Supplementary Figure 5(b)).
EDN1, ECE-1, ECE-2 and ACE activity
We did not find significant differences between Aβ- and saline-infused rats in the level of EDN1, ECE-1, ECE-2 or ACE activity in brain homogenates, when either the mean values for the whole brain (Supplementary Figure 5(c) to (f)) or the values for anterior, intermediate and posterior regions of each cerebral hemisphere (data not shown) were compared between subgroups. We also found no significant difference in ACE activity in plasma between Aβ- and saline-infused groups (Supplementary Figure 5(g)).
Adverse events
One animal from the high salt/Aβ40 infused group experienced a late failure of the aortic telemetry implant, three weeks after infusion surgery.
Discussion
In this study, we investigated the physiological effects of ICV Aβ40 peptide in a rat model of conditional hypertension (dependent on dietary salt intake). Aβ ICV infusion induced a progressive, highly significantly rise in BP in rats with pre-existing hypertension (high-salt DAHL/SS) but no change in BP in rats that were normotensive (low-salt DAHL/SS) prior to infusion. Our results suggest that Aβ has the potential to increase BP, particularly in the context of pre-existing hypertension.
The hypertension induced by Aβ may be a protective response to cerebral hypoperfusion. In a series of 259 normotensive and hypertensive adults, Warnert et al.14 found that hypertension was associated with vertebral artery hypoplasia, an incomplete posterior circle of Willis, increased cerebral vascular resistance and reduced cerebral blood flow. There is a substantial literature suggesting that Aβ increases cerebral vascular resistance and reduces cerebral blood flow. Topical application of Aβ40 to isolated arteries or to mouse cortex in vivo was shown to induce vasoconstriction.23,36 In mice overexpressing mutant amyloid-β precursor protein (Tg2576), excess endogenous Aβ was shown to induce vasoconstriction and inhibit autoregulation and neurovascular coupling.20,21,23 Our results demonstrate the prolonged effects of long-term infusion with Aβ40 on BP, and differ somewhat from previous in vivo studies which had a short infusion period. For example, Sprague-Dawley rats given short-term intra-arterial infusion of Aβ40 showed a substantial increase of MBP but only in hypotensive animals, with no change in normotensive animals. In the same study, vehicle infusion in spontaneously hypertensive animals induced a significant reduction in BP which was abolished by the infusion of Aβ40.51 In another study, mice were given subcutaneous DOCA-salt implants to induce hypertension, followed by subcutaneous Aβ40 over 30–45 min; the increase in MBP was no different between animals that received Aβ40 and those given vehicle, but the former showed an attenuated response to ACh.52
Aβ has multiple cerebrovascular effects that influence the regulation of CBF (for review see Love and Miners7 and Miners and Love 9). Cerebral perfusion declines several years before the development of dementia, in both sporadic11 and familial AD,10 particularly in brain regions with early accumulation of Aβ,10 and this is associated with preclinical white matter damage (i.e. the fall in perfusion exceeds the decline in metabolic demand and causes tissue injury).12 Further evidence of a deficit in cerebral perfusion in AD comes from studies showing elevation of vascular endothelial growth factor, and significantly greater loss of the ischaemia-sensitive myelin-associated glycoprotein (MAG) than the relatively resistant proteolipid protein-1 (PLP-1).7–9,53–55 Hypoxic alterations in brain tissue in early AD correlate closely with Aβ accumulation.9 Aβ accumulation also correlates with BBB leakage, indicating involvement of multiple components of the neurovascular unit in AD.56 There is a complex relationship between Aβ, BBB dysfunction and cerebral hypoperfusion, whereby BBB dysfunction reduces blood flow and impairs Aβ clearance,57,58 and Aβ damages the BBB through pericytic57,59,60 and endothelial toxicity and increased monocyte adhesion.61 A sustained reduction in cerebral blood flow might be expected to induce systemic hypertension, as a homeostatic response to restore blood flow by raising perfusion pressure.29–31
Aβ infusion had sustained or progressive effects on multiple cardiovascular control mechanisms, as evidenced by the changes in HR and blood pressure variability, and in baroreflex responsiveness. These physiological alterations were most marked in the hypertensive (high-salt) animals and included elevation of VLF, LF and HF components of SBPV but not significant alterations in HRV. The interpretation of and physiological basis for SBPV are somewhat contentious, but there is agreement that the LF and VLF components involve sympathetic supply to arteries.62 The physiological data suggest strongly that elevated sympathetic activity contributed to the increase in total peripheral resistance underling the hypertensive effects of Aβ.
Both HRV and SBPV are altered in AD, with some changes evident before development of dementia. For example, patients with mild AD and MCI have altered HRV and SBPV in response to orthostatic stress.63 Lower cognitive performance was associated with altered HRV in AD, evidence of elevated cardiac sympathetic activity (LF:HF power ratio) and lower parasympathetic activity (HF power).64 Increased SBPV variability (adjusted for raised SBP average itself and monitored over medium to long periods) is associated more generally with negative clinical outcomes, cardiovascular events, mortality and stroke.65 In a study of 25,000 people at high risk of cardiovascular disease, the risk of developing cognitive impairment was elevated in patients with greater variability of visit-to-visit BP, independent of the absolute BP itself.66
Aβ infusion affected control of the baroreceptor reflex. This is also compromised in AD. Compared to age-matched control, AD patients had depressed baroreflex sensitivity.67 In a further study, baroreflex values were lower in AD than in controls or MCI, and differed significantly between MCI and controls.68 These changes presumably reflect alterations (functional or structural) to brain regions involved in the baroreflex regulation of sympathetic activity, for example adrenaline-synthesizing medullary neurons (around 30% of which are lost in AD) and noradrenergic neurons in the locus coeruleus, affected by neurofibrillary tangles and neurodegeneration in early AD.64,69 Baroreflex sensitivity in midlife has a positive association with regional perfusion to the hippocampus (hypoperfused in apparently healthy, middle-aged normotensive adults in whom baroreflex sensitivity is reduced).70 In older adults (65 ± 6 y) with and without MCI, stiffness of central arteries and depressed baroreflex sensitivity correlated independently with white matter lesions.71
Alterations to the activity of the sympathetic nervous system and baroreflex could be driving the hypertension observed in this study. Central administration of Aβ40 to the lateral ventricle may mediate the raised sympathetic activity and reduced BRG via NADPH oxidase-mediated production of reactive oxygen species (ROS) and/or downregulation of nitric oxide synthase (NOS). Oxidative stress plays a role in the generation and maintenance of arterial hypertension in human and several animal models, including the DAHL/SS rat. Oxidative stress in the brain is linked to hypertension pathogenesis via reductions to baroreflex sensitivity and activation of the sympathetic nervous system.72 The hypertensive effects of high-salt in DAHL/SS rats are partly mediated by increased activity of NADPH oxidase and the production of higher levels of ROS, and impaired vasodilation resulting from reduced activity of NOS. NADPH-derived ROS and associated reduction in bioavailability of nitric oxide probably contribute to the cerebrovascular dysfunction induced by Aβ.73
We investigated two potential mechanisms whereby Aβ might have increased cerebral vascular resistance and induced hypertension: upregulation of EDN1 production and elevation of ACE activity. We did not find evidence of significant changes in either pathway but it is difficult to interpret these end-stage analyses, as they were conducted after depletion of the Aβ infusate; according to the manufacturer, the delivery of infusate by the osmotic pumps is constant for approximately 28 days but then declines rapidly, and in keeping with this we found little residual Aβ40 in brain tissue homogenates after this period. It seems most likely that the peptide is cleared relatively quickly in this model. Rapid turnover of Aβ40 was previously demonstrated in APP transgenic mice, with a half-life of approximately 0.7 h for soluble, non-deposited forms of Aβ40.74 Limitations of the present study include the relatively small sample sizes for the measurements of some of the baroreflex and variability data, and our use of supra-physiological levels of Aβ40 to model the effects of chronic Aβ-induced cerebral hypoperfusion but over a much shorter period than would apply in human AD. In addition, we did not assess hypoperfusion by measurement of vascular endothelial growth factor or the MAG:PLP-1 ratio in this series of experiments but that would certainly be of interest in future studies.
In conclusion, we have shown that ICV infusion of Aβ40 over a four-week period alters autonomic control of cardiovascular function, causing progressive elevation in systemic blood pressure in rats with pre-existing hypertension. These findings raise the possibility that (i) mid-life hypertension may, in some cases, be a physiological response to reduced cerebral perfusion complicating the accumulation of Aβ within the brain, and (ii) cerebral accumulation of Aβ may be particularly likely to exacerbate pre-existing hypertension.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study was provided by the British Heart Foundation (grant number: PG/10/47/28285). Additional funding for equipment provided by BRACE and Alzheimer’s Research UK.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
All experiments were performed at the University of Bristol. The study was conceived by SL, and designed by SL, JCP and PK. JCP designed and optimized the surgical procedures. HMT and JCP executed and analysed the in vivo studies. HMT and TLT performed the telemetry analyses and biochemical experiments. HMT wrote the manuscript with feedback from JCP, SL, PGK and JFRP.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365: 217–223. [DOI] [PubMed] [Google Scholar]
- 2.Tzourio C. Hypertension, cognitive decline, and dementia: an epidemiological perspective. Dialogues Clin Neurosci 2007; 9: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Power MC, Weuve J, Gagne JJ, et al. The association between blood pressure and incident Alzheimer disease: a systematic review and meta-analysis. Epidemiology 2011; 22: 646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottesman RF, Schneider AL, Albert M, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol 2014; 71: 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer's disease – lessons from pathology. BMC Med 2014; 12: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalaria RN, Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord 1999; 13(Suppl 3): S115–S123. [DOI] [PubMed] [Google Scholar]
- 7.Love S, Miners JS. Cerebrovascular disease in ageing and Alzheimer's disease. Acta Neuropathol 2016; 131: 645–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miners JS, Palmer J, Love S. Pathophysiology of hypoperfusion of the precuneus in early Alzheimer’s disease. Brain Pathol 2016; 26: 533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miners JS, Love S. Cerebral hypoperfusion and the energy deficit in Alzheimer’s disease. Brain Pathol 2016; 26: 607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benzinger TL, Blazey T, Jack CR, Jr., et al. Regional variability of imaging biomarkers in autosomal dominant Alzheimer's disease. Proc Natl Acad Sci U S A 2013; 110: E4502–E4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binnewijzend MA, Kuijer JP, Benedictus MR, et al. Cerebral blood flow measured with 3D pseudocontinuous arterial spin-labeling MR imaging in Alzheimer disease and mild cognitive impairment: a marker for disease severity. Radiology 2013; 267: 221–230. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Viqar F, Zimmerman ME, et al. White matter hyperintensities are a core feature of Alzheimer's disease: evidence from the dominantly inherited Alzheimer network. Ann Neurol 2016; 79: 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarumi T, Dunsky DI, Khan MA, et al. Dynamic cerebral autoregulation and tissue oxygenation in amnestic mild cognitive impairment. J Alzheimers Dis 2014; 41: 765–778. [DOI] [PubMed] [Google Scholar]
- 14.Warnert EA, Rodrigues JC, Burchell AE, et al. Is high blood pressure self-protection for the brain? Circ Res 2016; 119: e140–e151. [DOI] [PubMed] [Google Scholar]
- 15.Bueche CZ, Hawkes C, Garz C, et al. Hypertension drives parenchymal β-amyloid accumulation in the brain parenchyma. Ann Clin Transl Neurol 2014; 1: 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carnevale D, Mascio G, Ajmone-Cat MA, et al. Role of neuroinflammation in hypertension-induced brain amyloid pathology. Neurobiol Aging 2012; 33: 205.e219–205.e229. [DOI] [PubMed] [Google Scholar]
- 17.Carnevale D, Mascio G, D'Andrea I, et al. Hypertension induces brain β-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension 2012; 60: 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashby EL, Miners JS, Kehoe PG, et al. Effects of hypertension and anti-hypertensive treatment on amyloid-β (Aβ) plaque load and Aβ-synthesizing and Aβ-degrading enzymes in frontal cortex. J Alzheimers Dis 2016; 50: 1191–1203. [DOI] [PubMed] [Google Scholar]
- 19.Tong XK, Hamel E. Regional cholinergic denervation of cortical microvessels and nitric oxide synthase-containing neurons in Alzheimer's disease. Neuroscience 1999; 92: 163–175. [DOI] [PubMed] [Google Scholar]
- 20.Niwa K, Carlson GA, Iadecola C. Exogenous Aβ1-40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J Cereb Blood Flow Metab 2000; 20: 1659–1668. [DOI] [PubMed] [Google Scholar]
- 21.Niwa K, Kazama K, Younkin SG, et al. Alterations in cerebral blood flow and glucose utilization in mice overexpressing the amyloid precursor protein. Neurobiol Dis 2002; 9: 61–68. [DOI] [PubMed] [Google Scholar]
- 22.Niwa K, Porter VA, Kazama K, et al. Aβ-peptides enhance vasoconstriction in cerebral circulation. Am J Physiol Heart Circ Physiol 2001; 281: H2417–H2424. [DOI] [PubMed] [Google Scholar]
- 23.Niwa K, Younkin L, Ebeling C, et al. Aβ1-40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci U S A 2000; 97: 9735–9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer JC, Baig S, Kehoe PG, et al. Endothelin-converting enzyme-2 is increased in Alzheimer's disease and up-regulated by Aβ. Am J Pathol 2009; 175: 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer JC, Barker R, Kehoe PG, et al. Endothelin-1 is elevated in Alzheimer's disease and upregulated by amyloid-β. J Alzheimers Dis 2012; 29: 853–861. [DOI] [PubMed] [Google Scholar]
- 26.Palmer JC, Tayler HM, Love S. Endothelin-converting enzyme-1 activity, endothelin-1 production, and free radical-dependent vasoconstriction in Alzheimer's disease. J Alzheimers Dis 2013; 36: 577–587. [DOI] [PubMed] [Google Scholar]
- 27.Miners JS, Ashby E, Van Helmond Z, et al. Angiotensin-converting enzyme (ACE) levels and activity in Alzheimer's disease, and relationship of perivascular ACE-1 to cerebral amyloid angiopathy. Neuropathol Appl Neurobiol 2008; 34: 181–193. [DOI] [PubMed] [Google Scholar]
- 28.Miners S, Ashby E, Baig S, et al. Angiotensin-converting enzyme levels and activity in Alzheimer's disease: differences in brain and CSF ACE and association with ACE1 genotypes. Am J Transl Res 2009; 1: 163–177. [PMC free article] [PubMed] [Google Scholar]
- 29.Warnert EA, Rodrigues JC, Burchell AE, et al. Is high blood pressure self-protection for the brain? Circ Res 2016; 119: e140–e151. [DOI] [PubMed] [Google Scholar]
- 30.Cates MJ, Steed PW, Abdala AP, et al. Elevated vertebrobasilar artery resistance in neonatal spontaneously hypertensive rats. J Appl Physiol 2011; 111: 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paton JF, Dickinson CJ, Mitchell G. Harvey Cushing and the regulation of blood pressure in giraffe, rat and man: introducing ‘Cushing's mechanism'. Exp Physiol 2009; 94: 11–17. [DOI] [PubMed] [Google Scholar]
- 32.Monahan KD. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol 2007; 293: R3–R12. [DOI] [PubMed] [Google Scholar]
- 33.Saint Martin M, Sforza E, Thomas-Anterion C, et al. Baroreflex sensitivity, vascular risk factors, and cognitive function in a healthy elderly population: the PROOF cohort. J Am Geriatr Soc 2013; 61: 2096–2102. [DOI] [PubMed] [Google Scholar]
- 34.Idiaquez J, Roman GC. Autonomic dysfunction in neurodegenerative dementias. J Neurol Sci 2011; 305: 22–27. [DOI] [PubMed] [Google Scholar]
- 35.La Rovere MT, Pinna GD, Raczak G. Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol 2008; 13: 191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crawford F, Suo Z, Fang C, et al. Characteristics of the in vitro vasoactivity of β-amyloid peptides. Exp Neurol 1998; 150: 159–168. [DOI] [PubMed] [Google Scholar]
- 37.Rapp JP. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension 1982; 4: 753–763. [DOI] [PubMed] [Google Scholar]
- 38.McGrath JC, Drummond GB, McLachlan EM, et al. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol 2010; 160: 1573–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nitta A, Itoh A, Hasegawa T, et al. β-Amyloid protein-induced Alzheimer's disease animal model. Neurosci Lett 1994; 170: 63–66. [DOI] [PubMed] [Google Scholar]
- 40.Waki H, Kasparov S, Katahira K, et al. Dynamic exercise attenuates spontaneous baroreceptor reflex sensitivity in conscious rats. Exp Physiol 2003; 88: 517–526. [DOI] [PubMed] [Google Scholar]
- 41.Srivareerat M, Tran TT, Alzoubi KH, et al. Chronic psychosocial stress exacerbates impairment of cognition and long-term potentiation in β-amyloid rat model of Alzheimer's disease. Biol Psychiatry 2009; 65: 918–926. [DOI] [PubMed] [Google Scholar]
- 42.Chiu C, Miller MC, Caralopoulos IN, et al. Temporal course of cerebrospinal fluid dynamics and amyloid accumulation in the aging rat brain from three to thirty months. Fluids Barriers CNS 2012; 9: 3–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujiyoshi M, Tachikawa M, Ohtsuki S, et al. Amyloid-beta peptide(1-40) elimination from cerebrospinal fluid involves low-density lipoprotein receptor-related protein 1 at the blood-cerebrospinal fluid barrier. J Neurochem 2011; 118: 407–415. [DOI] [PubMed] [Google Scholar]
- 44.Oosting J, Struijker-Boudier HA, Janssen BJ. Validation of a continuous baroreceptor reflex sensitivity index calculated from spontaneous fluctuations of blood pressure and pulse interval in rats. J Hypertens 1997; 15: 391–399. [DOI] [PubMed] [Google Scholar]
- 45.Di Rienzo M, Parati G, Castiglioni P, et al. Baroreflex effectiveness index: an additional measure of baroreflex control of heart rate in daily life. Am J Physiol Regul Integr Comp Physiol 2001; 280: R744–R751. [DOI] [PubMed] [Google Scholar]
- 46.Van Helmond Z, Miners JS, Kehoe PG, et al. Higher soluble amyloid β concentration in frontal cortex of young adults than in normal elderly or Alzheimer's disease. Brain Pathol 2010; 20: 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miners JS, Jones R, Love S. Differential changes in Aβ42 and Aβ40 with age. J Alzheimers Dis 2014; 40: 727–735. [DOI] [PubMed] [Google Scholar]
- 48.Palmer JC, Kehoe PG, Love S. Endothelin-converting enzyme-1 in Alzheimer's disease and vascular dementia. Neuropathol Appl Neurobiol 2010; 36: 487–497. [DOI] [PubMed] [Google Scholar]
- 49.Bateman RJ, Munsell LY, Morris JC, et al. Human amyloid-β synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med 2006; 12: 856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frautschy SA, Yang F, Calderon L, et al. Rodent models of Alzheimer's disease: rat Aβ infusion approaches to amyloid deposits. Neurobiol Aging 1996; 17: 311–321. [DOI] [PubMed] [Google Scholar]
- 51.Arendash GW, Su GC, Crawford FC, et al. Intravascular β-amyloid infusion increases blood pressure: implications for a vasoactive role of beta-amyloid in the pathogenesis of Alzheimer's disease. Neurosci Lett 1999; 268: 17–20. [DOI] [PubMed] [Google Scholar]
- 52.Faraco G, Park L, Zhou P, et al. Hypertension enhances Aβ-induced neurovascular dysfunction, promotes β-secretase activity, and leads to amyloidogenic processing of APP. J Cereb Blood Flow Metab 2016; 36: 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barker R, Ashby EL, Wellington D, et al. Pathophysiology of white matter perfusion in Alzheimer's disease and vascular dementia. Brain 2014; 137: 1524–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barker R, Wellington D, Esiri MM, et al. Assessing white matter ischemic damage in dementia patients by measurement of myelin proteins. J Cereb Blood Flow Metab 2013; 33: 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas T, Miners S, Love S. Post-mortem assessment of hypoperfusion of cerebral cortex in Alzheimer's disease and vascular dementia. Brain. 2015; 138: 1059–1069. [DOI] [PubMed] [Google Scholar]
- 56.van de Haar HJ, Jansen JF, van Osch MJ, et al. Neurovascular unit impairment in early Alzheimer's disease measured with magnetic resonance imaging. Neurobiol Aging 2016; 45: 190–196. [DOI] [PubMed] [Google Scholar]
- 57.Sagare AP, Bell RD, Zhao Z, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun 2013; 4: 2932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Sagare AP, Bell RD, Zlokovic BV. Neurovascular defects and faulty amyloid-β vascular clearance in Alzheimer's disease. J Alzheimers Dis 2013; 33(Suppl 1): S87–S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sagare AP, Sweeney MD, Makshanoff J, et al. Shedding of soluble platelet-derived growth factor receptor-β from human brain pericytes. Neurosci Lett 2015; 607: 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verbeek MM, Van Nostrand WE, Otte-Holler I, et al. Amyloid-β-induced degeneration of human brain pericytes is dependent on the apolipoprotein E genotype. Ann N Y Acad Sci 2000; 903: 187–199. [DOI] [PubMed] [Google Scholar]
- 61.Burgmans S, van de Haar HJ, Verhey FR, et al. Amyloid-β interacts with blood-brain barrier function in dementia: a systematic review. J Alzheimers Dis 2013; 35: 859–873. [DOI] [PubMed] [Google Scholar]
- 62.Malpas SC. Neural influences on cardiovascular variability: possibilities and pitfalls. Am J Physiol Heart Circ Physiol 2002; 282: H6–H20. [DOI] [PubMed] [Google Scholar]
- 63.Mellingsæter MR, Wyller TB, Ranhoff AH, et al. Reduced sympathetic response to head-up tilt in subjects with mild cognitive impairment or mild Alzheimer's dementia. Dement Geriatr Cogn Dis Extra 2015; 5: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nonogaki Z, Umegaki H, Makino T, et al. Relationship between cardiac autonomic function and cognitive function in Alzheimer's disease. Geriatr Gerontol Int 2015; 17: 92–98. [DOI] [PubMed] [Google Scholar]
- 65.Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ 2016; 354: i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Böhm M, Schumacher H, Leong D, et al. Systolic blood pressure variation and mean heart rate is associated with cognitive dysfunction in patients with high cardiovascular risk. Hypertension 2015; 65: 651–661. [DOI] [PubMed] [Google Scholar]
- 67.Szili-Török T, Kálmán J, Paprika D, et al. Depressed baroreflex sensitivity in patients with Alzheimer’s and Parkinson’s disease. Neurobiol Aging 2001; 22: 435–438. [DOI] [PubMed] [Google Scholar]
- 68.den Abeelen AS, Lagro J, van Beek AH, et al. Impaired cerebral autoregulation and vasomotor reactivity in sporadic Alzheimer's disease. Curr Alzheimer Res 2014; 11: 11–17. [DOI] [PubMed] [Google Scholar]
- 69.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82: 239–259. [DOI] [PubMed] [Google Scholar]
- 70.Laosiripisan J, Tarumi T, Gonzales MM, et al. Association between cardiovagal baroreflex sensitivity and baseline cerebral perfusion of the hippocampus. Clin Auton Res 2015; 25: 213–218. [DOI] [PubMed] [Google Scholar]
- 71.Tarumi T, de Jong DL, Zhu DC, et al. Central artery stiffness, baroreflex sensitivity, and brain white matter neuronal fiber integrity in older adults. Neuroimage 2015; 110: 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep 2006; 8: 232–241. [DOI] [PubMed] [Google Scholar]
- 73.Park L, Anrather J, Zhou P, et al. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid β peptide. J Neurosci 2005; 25: 1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abramowski D, Wiederhold K-H, Furrer U, et al. Dynamics of Aβ turnover and deposition in different β-amyloid precursor protein transgenic mouse models following γ-secretase inhibition. J Pharmacol Exp Ther 2008; 327: 411–424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.