Abstract
The essential oil composition of Peumus boldus and Drimys winterii was analyzed by means of capillary GC-FID and GC-MS. More than 96% of the total oil components (43 and 54 compounds, respectively) were identified, with ascaridole (51.17 ± 9.51), p-cymene (16.31 ± 2.52) and 1,8-cineole (14.45 ± 2.99) as the main compounds in P. boldus and γ-eudesmol (21.65 ± 0.41), followed of elemol (12.03 ± 0.34) and terpinen-4-ol (11.56 ± 1.06) in D. winterii. The herbicidal activity was tested against Amaranthus hybridus and Portulaca oleracea. P. boldus essential oil was the most phytotoxic against both weeds, inhibiting seed germination and seedling growth at all concentrations assayed (0.125–1 µL/mL). D. winterii essential oil did not show any effect on A. hybridus germination and only affected P. oleracea germination at the highest concentration. The results suggest the possible use of the essential oil from P. boldus as a natural herbicide.
Keywords: essential oils, phytotoxicity, germination, seedling growth, monoterpenes
1. Introduction
Weeds are a small group of different plants from the taxonomic point of view. They display a great adaptability to different habitats, taking advantage of the favorable conditions that occur in agricultural systems and competing with crops. Indiscriminate application of synthetic herbicides has contributed to increased resistance in weeds, also leading to gradual degradation of soil and the environment, and to hazards to human health. The secondary metabolites of plant species offer an excellent potential to develop new herbicide formulations, or as a guide towards identifying active components to obtain natural herbicides [1,2], thus numerous studies have been done with plant-derived compounds, in order to obtain synthetic herbicide substitutes for weed control [3].
Peumus boldus Mol. (Monimiaceae), is a native tree from the central region of Chile, being part of the sclerophyllous forests, characteristic of the Mediterranean climate. It is the source of the known crude drug Boldo folium, commonly used as a medicinal plant in Chile and reported as a herbal remedy in several pharmacopeias [4]. Infusion of boldo leaves is recommended for the treatment of gastrointestinal spasms, dyspectic and hepatobiliary disorders [5]. The main bioactive compounds of boldo leaves are flavonoids, alkaloids and essential oils. Several studies have demonstrated the antioxidant activity of flavonoids and alkaloids, particularly the aporphine alkaloid boldine [6,7,8]. The antimicrobial, fungicidal or antihelmintic effects of ascaridole, the main compound in the essential oil, is also reported [9,10], but to our knowledge, no assays of the herbicidal activity of the essential oil have been carried out so far.
Drimys winterii J.R.Forst. & G.Forst (Winteraceae) is a native tree of Chile that belongs to the Magallanian and Valdivian temperate rain forests. The indigenous Mapuches consider it a sacred tree with antiinflammatory, antitumoural, antibacterial and insecticidal properties. Its leaves contain flavonoids, tannins, terpenoids and also essential oil [11]. Thus, the essential oils of both native species could be promising sources of products to evaluate new activities such as the phytotoxic effect on seed germination and seedling growth of weeds.
Amaranthus hybridus and Portulaca oleracea are two annual weeds of tropical and subtropical crops with an extensive world distribution. They have become cosmopolitan weeds distributed in a wild range of soils and climates. In Mediterranean crops these weeds show summer phenology. The aims of the present study were to determine the composition of the essential oils from two endemic plants from Chile and to compare their phytotoxic activity against A. hybridus L. and. P. oleracea L with that of plants from the Mediterranean area that we had previously tested [12].
2. Results and Discussion
The phytotoxic effects of P. boldus and D. winterii essential oils obtained from Chilean plants have been investigated against A. hybridus and P. oleraceae, two major weeds in summer crops in the Mediterranean area,. Results obtained can be explained by the very different qualitative and quantitative chemical composition of both essential oils. The essential oil composition of P. boldus and D. winterii analyzed by GC-FID and GC-MS is shown in Table 1, where the identified compounds are classified by phytochemical groups and listed in order of their elution on a methylsilicone HP-1 column.
Table 1.
Constituents of the essential oils from Peumus boldus, and Drimys winterii by GC and GC-MS analysis.
| Compound | RI | Peumus boldus (mean ± s.e.) | Drimys winterii (mean ± s.e.) |
| Monoterpene hydrocarbons | 19.71 ± 2.94 | 16.74 ± 2.43 | |
| α-thujene | 930 | - | 0.05 ± 0.02 |
| α-pinene | 939 | 0.27 ± 0.04 | 0.93 ± 0.23 |
| camphene | 952 | 0.11 ± 0.01 | - |
| sabinene | 976 | 1.20 ± 0.25 | 2.97 ± 0.68 |
| β-pinene | 979 | 0.18 ± 0.02 | 2.68 ± 0.58 |
| myrcene | 991 | - | 0.99 ± 0.15 |
| α-phelladrene | 1005 | t | 0.25 ± 0.09 |
| δ-3-carene | 1011 | t | - |
| α-terpinene | 1019 | 0.30 ± 0.03 | 1.70 ± 0.19 |
| p-cymene | 1027 | 16.31 ± 2.52 | 0.05 ± 0.02 |
| limonene | 1032 | - | 2.46 ± 0.48 |
| β-phellandrene | 1033 | 0.31 ± 0.13 | - |
| cis-ocimene | 1042 | - | 0.47 ± 0.05 |
| γ-terpinene | 1062 | 0.42 ± 0.00 | 3.30 ± 0.20 |
| terpinolene | 1089 | - | 0.91 ± 0.04 |
| p-cymenene | 1090 | t | - |
| 1.3.8-p-menthatriene | 1112 | 0.64 ± 0.29 | - |
| Oxygenated monoterpenes | 74.77 ± 4.26 | 13.95 ± 1.23 | |
| dehydro-1,8-cineole | 992 | 0.09 ± 0.02 | - |
| 1,8-cineole | 1034 | 14.45 ± 2.99 | 0.13 ± 0.01 |
| cis-sabinene hydrate | 1070 | 0.56 ± 0.28 | t |
| fenchone | 1088 | t | - |
| trans-sabinene hydrate | 1097 | 0.51 ± 0.19 | t |
| linalool | 1100 | t | 0.07 ± 0.01 |
| dehydro-sabina ketone | 1121 | 0.83 ± 0.23 | - |
| cis-p-menth-2-en-1-ol | 1122 | - | t |
| trans-pinocarveol | 1140 | 1.50 ± 0.26 | - |
| trans-p-menth-2-en-1-ol | 1141 | - | 0.44 ± 0.06 |
| camphor | 1146 | 0.13 ± 0.02 | - |
| sabina ketone | 1160 | t | - |
| pinocarvone | 1169 | 0.30 ± 0.11 | - |
| δ-terpineol | 1169 | 0.64 ± 0.11 | - |
| terpinen-4-ol | 1179 | 2.15 ± 0.33 | 11.56 ± 1.06 |
| thuj-3-en-10-al | 1185 | t | - |
| cryptone | 1186 | t | - |
| α-terpineol | 1191 | 0.06 ± 0.01 | 1.61 ± 0.11 |
| myrtenal | 1195 | 0.09 ± 0.03 | - |
| cis-piperitol | 1196 | - | t |
| myrtenol | 1197 | 1.11 ± 1.33 | - |
| trans- piperitol | 1207 | - | 0.14 ± 0.01 |
| ascaridole | 1242 | 51.17 ± 9.51 | - |
| cis-piperitone epoxide | 1259 | 0.76 ± 0.20 | - |
| trans-piperitone epoxide | 1262 | t | - |
| bornyl acetate | 1288 | 0.08 ± 0.01 | - |
| thymol | 1293 | 0.06 ± 0.01 | - |
| p-cymen-7-ol | 1295 | 0.12 ± 0.02 | - |
| carvacrol | 1302 | 0.24 ± 0.15 | - |
| Sesquiterpene hydrocarbons | - | 2.89 ± 0.18 | |
| β-elemene | 1392 | - | 0.14 ± 0.02 |
| α-cedrene | 1411 | - | 0.30 ± 0.04 |
| β-caryophyllene | 1419 | - | 0.19 ± 0.00 |
| trans-β-farnesene | 1459 | - | 0.15 ± 0.04 |
| α-acoradiene | 1465 | - | t |
| γ-curcumene | 1481 | - | 1.91 ± 0.14 |
| α-curcumene | 1484 | - | 0.17 ± 0.03 |
| bicyclogermacrene | 1496 | - | 0.04 ± 0.01 |
| Oxygenated sesquiterpenes | 0.27 ± 0.05 | 57.82 ± 1.38 | |
| 4-epi-cis-dihydroagarofuran | 1499 | - | 0.34 ± 0.02 |
| italicene ether | 1536 | - | 0.12 ± 0.01 |
| elemol | 1555 | - | 12.03 ± 0.34 |
| E-nerolidol | 1566 | - | 0.36 ± 0.01 |
| spathulenol | 1578 | 0.12 ± 0.03 | t |
| rosifoliol | 1602 | - | 0.08 ± 0.00 |
| 5-epi-7-epi-α-eudesmol | 1607 | t | |
| β-oplopenone | 1609 | 0.15 ± 0.02 | - |
| epi-cedrol | 1614 | - | 0.75 ± 0.01 |
| 10-epi-γ-eudesmol | 1621 | - | 1.86 ± 0.06 |
| γ-eudesmol | 1639 | - | 21.65 ± 0.41 |
| β-eudesmol | 1656 | - | 7.27 ± 0.35 |
| α-eudesmol | 1659 | - | 7.35 ± 0.14 |
| 7-epi-α-eudesmol | 1662 | - | 0.12 ± 0.04 |
| β-bisabolol | 1674 | - | 4.61 ± 0.22 |
| α-bisabolol | 1687 | - | 0.07 ± 0.02 |
| drimenol | 1763 | - | 1.18 ± 0.04 |
| drimenin | 1951 | - | 0.06 ± 0.01 |
| Aromatic compounds (C6-C3) | 1.20 ± 0.18 | 4.83 ± 0.42 | |
| safrole | 1287 | - | 0.09 ± 0.01 |
| eugenol | 1360 | - | 1.09 ± 0.10 |
| methyl eugenol | 1406 | 1.20 ± 0.18 | t |
| myristicin | 1522 | - | 3.66 ± 0.32 |
| elemicin | 1560 | - | t |
| Others | 0.34 ± 0.03 | t | |
| 3-octanol | 993 | - | t |
| 1.4-dione-2-cyclohexene | 1015 | 0.06 ± 0.01 | - |
| 2-nonanone | 1093 | 0.07 ± 0.00 | - |
| 3-octanol acetate | 1128 | - | t |
| 2-undecanone | 1295 | 0.24 ± 0.03 | - |
| TOTAL IDENTIFIED | 96.90 ± 1.06 | 96.24 ± 0.28 | |
RI: retention index relative to C8-C32n-alkanes on the HP-1 column. Peak area percentages were calculated in GC analysis on an apolar HP-1 column. t: trace amounts <0.03. Values are means ± standard error of three samples.
P. boldus essential oil is rich in monoterpene compounds, which account for more than 95% of the total oil composition. The monoterpene hydrocarbons fraction (19.71 ± 2.94) is constituted mainly by p-cymene (16.31 ± 2.52). Of the twelve monoterpene hydrocarbons identified, only this compound and sabinene (1.20 ± 0.25) reached percentages higher than 1%. Twenty five compounds were identified in the oxygenated monoterpenes fraction (74.77 ± 4.26). The monoterpene endoperoxide ascaridole (51.17 ± 9.51) followed by 1,8-cineole (14.45 ± 2.99), terpinen-4-ol (2.15 ± 0.33) and trans-pinocarveol (1.50 ± 0.26) were the main compounds. Only two oxygenated sesquiterpenes, spathulenol (0.12 ± 0.03) and β-oplopenone (0.15 ± 0.02) have been identified in P. boldus essential oil. This essential oil is also characterized by the absence of sesquiterpene hydrocarbons. On the other hand, only methyl eugenol, that reached a percentage around 1%, was found among the aromatic compounds. The high herbicidal activity showed by P. boldus essential oil correlates with the fact that a high percentage of oxygenated monoterpenes is linked to a potent phytotoxic activity [13,14,15].
Nevertheless, it is interesting to note that according to previous results [12], not only the monoterpene compounds may be responsible for germination inhibition [16,17], because previous assays with Eucalyptus camaldulensis essential oil, rich in the oxygenated sesquiterpene spathulenol (41.46 ± 3.94), showed that it also completely inhibited A. hybridus and P. oleracea seed germination and seedling growth [12].
Fifty-four compounds were identified in D. winterii essential oil, accounting for 96% of the total oil composition. The monoterpene hydrocarbons with 12 identified compounds almost reached 17% (16.74 ± 2.42), among them p-cymene only reached 0.08 in one sample, being detected in the other two as trace amounts (<0.03%). The higher percentages corresponded to γ-terpinene (3.30 ± 0.20), sabinene (2.97 ± 0.68), β-pinene (2.68 ± 0.58), limonene (2.46 ± 0.48) and α-terpinene (1.70 ± 0.19). Substantial differences on oxygenated monoterpenes were observed between the two analyzed species. This fraction (13.95 ± 1.23), represented the second most important group on D. winterii essential oil, with terpinen-4-ol (11.56 ± 1.06) followed of α-terpineol (1.61 ± 0.11) as the main compounds. With the exception of 1,8-cineole, cis and trans-sabinene hydrate, linalool, terpinen-4-ol and α-terpineol, which were identified in both essential oils, the oxygenated monoterpenes found in P. boldus were not detected in D. winterii essential oil.
The greatest differences comparing both species show up in the sesquiterpene fraction. The eight sesquiterpene hydrocarbons found in D. winterii were absent in the three samples of P. boldus. With 17 identified compounds, oxygenated sesquiterpenes constituted the predominant group in D. winterii essential oil. This fraction (57.82 ± 1.38) contained mainly elemol (12.03 ± 0.34), β-bisabolol (4.61 ± 0.22) and eudesmol compounds: γ-eudesmol (21.65 ± 0.41), α-eudesmol (7.35 ± 0.14) and β-eudesmol (7.27 ± 0.35).
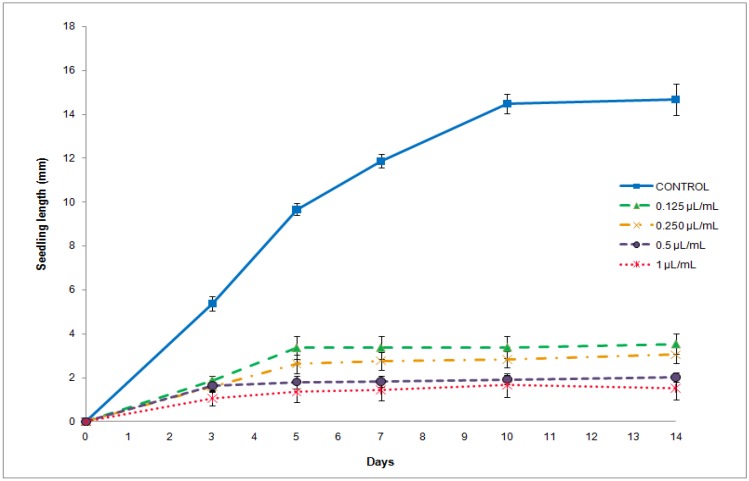
Finally, relative large amounts of myristicin (3.66 ± 0.32) and eugenol (1.09 ± 0.10), aromatic compounds (C6-C3) (4.83 ± 0.42) were found in D. winterii. From this fraction only methyl eugenol was detected in P. boldus essential oil. The essential oil of D.winterii, which also contained high percentages of oxygenated compounds was not active against A. hybridus germination and seedling growth. No significant differences were found between control and all concentrations applied. Nevertheless this essential oil was effective reducing P. oleracea germination at the higher concentrations assayed (Table 2). On the other hand D. winterii essential oil showed phytotoxic effects against P. oleraceae seedlings growth all concentrations assayed (Figure 1).
Table 2.
Effect of Peumus boldus and Drimys winterii essential oils on Portulaca oleracea seeds germination.
| Treatment | Peumus boldus essential oil | Drimys winterii essential oil |
|---|---|---|
| CONTROL | 71.0 ± 2.9 a | 71.0 ± 2.9 ab |
| 0.125 µL/mL | 0.0 ± 0.0 b | 74.0 ± 5.8 a |
| 0.250 µL/mL | 0.0 ± 0.0 b | 53.0 ± 6.0 b |
| 0.5 µL/mL | 0.0 ± 0.0 b | 30.0 ± 6.1 c |
| 1 µL/mL | 0.0 ± 0.0 b | 27.5 ± 9.9 c |
Values are means ± standard error of five replicates of 20 seeds each after 14 days of incubation. Within each species, different letters indicate that means are not equal at the 95% level of probability (Fisher’s least significant difference test, LSD).
Figure 1.
Seedling length (mm) (mean ± SE) measured for 14 days of P. oleracea control or treated with D. winterii essential oil at 0.125, 0.25, 0.5 and 1 µL/mL concentrations.
3. Experimental
3.1. Plant material
Leaves of wild Peumus boldus Molina and Drimys winterii J.R.Forst. & G.Forst. were collected in the winter of 2010 (May) at Comuna of Hualqui (36°57’36’’S; 72°55’48’’W, VIII Region, Chile) and in Quilpué (33°03’S; 71°27’W, V Region, Chile) respectively. Both areas are characterized by a temperate Mediterranean climate.
Mature seeds of the annual weeds Amaranthus hybridus L. and Portulaca oleracea L. were collected from parent plants growing in citrus orchards of the province of Valencia (Spain), in October 2005 and August 2008, respectively. The plants were dried during 15 days at room temperature, afterwards the seeds were extracted. Uniform healthy seeds were selected and stored at 4 ºC until germination tests.
3.2. Oil isolation
The fresh material was subjected to hydro-distillation for three hours in a Clevenger-type apparatus, yielding (v/w) % after three distillations 1.25 ± 0.20 for P. boldus essential oils and 0.22 ± 0.02 for D. winterii essential oils. All samples were stored at 4 °C until analysis, upon which they were either diluted to 1% (v/v) in dichloromethane or their herbicidal potential was tested.
3.3. GC and GC-MS analyses
Gas chromatography was performed using a Perkin-Elmer Clarus 500GC apparatus equipped with a flame ionization detector (FID), a Hewlett-Packard HP-1 (cross-linked methyl silicone) capillary column (30 m long and 0.2 mm i.d., with 0.33 μm film thickness). The column temperature program was 60 °C during 5 min, with 3 °C/min increases to 180 °C, then 20 °C/min increases to 280 °C, which was maintained for 10 min. The carrier gas was helium at a flow-rate of 1 mL/min. Both the FID detector and injector port temperature were maintained at 250 and 220 °C, respectively. Gas chromatography-mass spectrometry analysis were carried out with a Varian Saturn 2000 equipped with a Varian C.S VA-5MS capillary column (30 m long and 0.25 mm i.d. with 0.25 μm film thickness). The same working conditions used for GC and split mode injection (ratio 1:25) were employed. Mass spectra were taken over the m/z 28–400 range with an ionizing voltage of 70 eV. Kovat’s retention index were calculated using co-chromatographed standard hydrocarbons. The individual compounds were identified by MS [18] and their identity was confirmed by comparison of their RIs, relative to C8-C32 n-alkanes, and by comparing their mass spectra and retention times with those of authentic samples or with data already available in the NIST 98 library and in the literature.
3.4. Biological assay
Seed germination and growth seedling tests. Sets of 20 seeds each with five replicates per treatment were put in Petri dishes (9 cm diameter) between two layers of filter paper (Whatman No.1) wetted with 4 mL of distilled water for germination. Essential oils of P. boldus or D. winterii were added at volumes of 0 (control), 0.5, 1, 2, and 4 μL. Based on previous assays [12], A. hybridus and P. oleracea seeds were incubated alternating 30.0 ± 0.1 °C 16 hr in light and 20.0 ± 0.1 °C 8 hr in dark. To evaluate the allelopathic potential of the essential oils, germination and seedling length data were recorded after 3, 5, 7, 10 and 14 days.
3.5. Statistical analyses
Tests were conducted in a randomised complete design with five replications. Data were submitted to analysis of variance (ANOVA). Percentage values were arcsin transformed. The means were compared using Fisher’s least significant difference (LSD) test (P < 0.05).
4. Conclusions
The present study has examined the phytotoxic effects of the essential oils of P. boldus and D. winterii two medicinal tree from Chile, against A. hybridus and P. oleracea, two serious weeds in Mediterranean summer crops. The essential oil of P. boldus was the most effective, completely inhibiting both A. hybridus and P. oleraceae seed germination and seedling length at all the concentrations applied (0.125–1 µL/mL). D. winteri showed selective activity, depending on the weed assayed. Its essential oil was not active against A. hybridus germination and only significantly reduced P. oleracea germination (57.7 and 61.3%) at the highest concentrations (0.5 and 1 µL/mL). Both species provided essential oils rich in oxygenated compounds, P. boldus oxygenated monoterpenes (66.39–77.61%) and D. winterii oxygenated sesquiterpenes (55.65–60.37%). The results corroborated previous studies suggesting that a high percentage of oxygenated monoterpenes is correlated with potent phytotoxic activity. Our in vitro studies suggest a possible and new alternative use of P. boldus essential oil in herbicidal formulations, although further experiments involving field conditions are necessary to confirm the herbicidal potential of P. boldus.
Acknowledgements
Research performed under an International Cooperation Project (number A/024753/09), financed by Agencia Española de Cooperación Internacional para el Desarrollo (AECID).
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Duke S.O., Romagni J.G., Dayan F.E. Natural products as sources for new mechanisms of herbicidal action. Crop Prot. 2000;19:583–589. doi: 10.1016/S0261-2194(00)00076-4. [DOI] [Google Scholar]
- 2.Vyvyan J.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron. 2002;58:1631–1636. doi: 10.1016/S0040-4020(02)00052-2. [DOI] [Google Scholar]
- 3.Tehmina A., Stevenson P., Hall D., Bajwa R. Allelophatic potential of Helianthus annuus L. (sunflower) as natural herbicide; “Establishing the Scientific Base”, Proceedings of the Fourth World Congress on Allelopathy; Charles Sturt University: Wagga Wagga, NSW, Australia. 21–26 August 2005. [Google Scholar]
- 4.Speisky H., Cassel B.K. Boldo and boldine: An emerging case of natural drug development. Pharmacol. Res. 1994;29:1–10. doi: 10.1016/1043-6618(94)80093-6. [DOI] [PubMed] [Google Scholar]
- 5.Simirgiotis M.J., Schmeda-Hirschmann G. Direct identification of phenolic constituents in Boldo Folium (Peumus boldus Mol.) infusions by high-performance liquid chromatography with diode array detection and electrospray ionization tandem mass spectrometry. J. Cromatogr. A. 2010;1217:443–449. doi: 10.1016/j.chroma.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Silva E., Jopia M., Edwards A.M., Lemp E., de la Fuente J.R., Lissi E. Protective effect of boldo and tea infusions on the visible light-mediated pro-oxidant effects of vitamin B2, riboflavin. Photochem. Photobiol. 2002;75:585–590. doi: 10.1562/0031-8655(2002)075<0585:peobat>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Milián L., Estellés R., Abarca B., Ballesteros R., Sanz M.J., Blázquez M.A. Reactive oxygen species (ROS) generation inhibited by aporphine and phenanthrene alkaloids semi-synthesized from natural boldine. Chem. Pharm. Bull. 2004;52:696–699. doi: 10.1248/cpb.52.696. [DOI] [PubMed] [Google Scholar]
- 8.Quezada N., Asencio M., Del Valle J.M., Aguilera J.M., Gómez B. Antioxidant activity of crude extract, alkaloid fraction, and flavonoid fraction from Boldo (Peumus boldus Molina) leaves. J. Food Sci. 2004;69:371–376. [Google Scholar]
- 9.Vila R., Valenzuela L., Bello H., Cañigueral S., Montes M., Adzet T. Composition and antimicrobial activity of the essential oil of Peumus boldus leaves. Planta Med. 1999;65:178–179. doi: 10.1055/s-2006-960461. [DOI] [PubMed] [Google Scholar]
- 10.Bittner M., Aguilera M.A., Hernández V., Arbert C., Becerra J., Casanueva E. Fungistatic activity of essential oils extracted from Peumus boldus Mol., Laureliopsis philippiana (Looser) Schodde and Laurelia sempervirens (Ruix y Pav.) Tul (Chilean Monimiaceae) Chilean J. Agric. Res. 2009;69:30–37. [Google Scholar]
- 11.Muñoz-Concha D., Vogel H., Razmilic I. Variación de compuestos químicos en hojas de poblaciones de Drimys ssp (Magnoliophyta: Winteraceae) en Chile. Rev. Chil. Hist. Nat. 2004;77:43–50. [Google Scholar]
- 12.Verdeguer M., Blázquez M.A., Boira H. Phytotoxic effects of Lantana camara, Eucalyptus camaldulensis and Eriocephalus africanus essential oils in weeds of Mediterranean summer crops. Biochem. Syst. Ecol. 2009;37:362–369. doi: 10.1016/j.bse.2009.06.003. [DOI] [Google Scholar]
- 13.Vokou D., Douvli P., Blionis G.J., Halley J.M. Effects of monoterpenoids, acting alone or in pairs, on seed germination and subsequent seedling growth. J. Chem. Ecol. 2003;29:2281–2301. doi: 10.1023/A:1026274430898. [DOI] [PubMed] [Google Scholar]
- 14.Kotan R., Cakir A., Dadasoglu F., Aydin T., Cakmakci R., Ozer H., Kordali S., Mete E., Dikbas N. Antibacterial activities of essential oils and extracts of Turkish Achillea, Satureja and Thymus species against plant pathogenic bacteria. J. Sci. Food Agric. 2010;90:145–160. doi: 10.1002/jsfa.3799. [DOI] [PubMed] [Google Scholar]
- 15.Rolim de Almeida L.F., Frei F., Mancini E., De Martino L., De Feo V. Phytotoxic activities of Mediterranean essential oils. Molecules. 2010;15:4309–4323. doi: 10.3390/molecules15064309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudai N., Ben-Ami M., Chaimovich R., Chaimovitsh D. Essential oils as allelopathic agents: bioconversion of monoterpenes by germinating wheat seeds. Acta Hort. 2004;629:505–508. [Google Scholar]
- 17.Armirante F., De Falco E., De Feo V., De Martino L., Mancini E., Quarante E. Allelopathic activity of essential oils from Mediterranean Labiatae. Acta Hort. 2006;723:347–352. [Google Scholar]
- 18.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]