Abstract
By mediating estrogen synthesis and follicular growth in response to FSH, the ovarian FSH receptor (FSHR) is essential for female fertility. Indeed, ovarian stimulation via administration of FSH to women with infertility is part of the primary therapeutic intervention used in assisted reproductive technology. In physiological and therapeutic contexts, current dogma dictates that once ovulation has occurred, FSH/FSHR signaling is no longer required for successful pregnancy outcomes. However, a continued role for FSH during pregnancy is suggested by recent studies demonstrating extraovarian FSHR in the female reproductive tract. Furthermore, functional roles for FSHR in placenta and in uterine myometrium have now been demonstrated. In placenta, vascular endothelial FSHR of fetal vessels within the chorionic villi (human) or labyrinth (mouse) mediate angiogenesis, and it has further been shown that deletion of placental Fshr in mice has deleterious effects on pregnancy. In uterine myometrium, changes in the densities of FSHR in muscle fiber and stroma in the nonpregnant state, early pregnancy, and term pregnancy differentially regulate contractile activity, suggesting that signaling through myometrial FSHR may contribute to the quieting of contractile activity required for successful implantation and that the temporal upregulation of the FSHR at term pregnancy may be required for the appropriate timing of parturition. In addition, extraovarian expression of mRNAs encoding the glycoprotein hormone α subunit and the FSH β subunit has been demonstrated, suggesting that these novel aspects of extraovarian FSH/FSHR signaling during pregnancy may be mediated by locally synthesized FSH.
The FSH receptor (FSHR) is a G protein–coupled receptor whose expression has been thought for many years to be limited to the gonads. In females, FSHR expressed on granulosa cells of the ovary respond to pituitary FSH to mediate follicular growth and estrogen synthesis. The role of FSH in supporting pregnancy, therefore, has been viewed as limited to these actions occurring during the follicular phase of the ovarian cycle, negating a need for FSH once ovulation has occurred. In recent years, however, there have been several studies reporting the extragonadal expression of FSHR, suggesting that FSH may exert additional physiological functions (1–23). Interestingly, a recent study using an unbiased evolutionary genomic approach to identify novel proteins associated with the timing of birth in humans reported that the strongest association was with a haplotype of single-nucleotide polymorphisms in an intron of the FSHR gene (24, 25). An association of FSHR with the timing of birth cannot be reconciled with the known actions of ovarian FSHR. In light of this apparent paradox, our laboratory undertook a systematic examination of extraovarian FSHR in the female reproductive tract and has begun to evaluate the potential roles of extraovarian FSHR in pregnancy.
Expression of FSHR in Extraovarian Reproductive Tissues
Using a highly specific antibody to the human FSHR (8, 12, 13, 26), we observed FSHR protein expression in several extraovarian reproductive tissues of cycling and pregnant women (12). The immunohistochemical detection of FSHR in extraovarian tissues, however, required considerably longer exposure times than its detection in the ovary, suggesting relatively low levels of FSHR. Details and representative images depicting the immunostaining can be found in a study by Stilley et al. (12), a summary of which is presented in the following paragraphs. Table 1 summarizes detection of FSHR in nongonadal reproductive tissues, as reported by our laboratory and others.
Table 1.
Methods for Detecting FSH Receptor in Extraovarian Reproductive Tissues
| Tissue/Cell | FSHR Protein a | FSHR Protein b | FSHR mRNA c | 125I-hCG Binding | Functional Response (Hormone Addition or Fshr Deletion) |
|---|---|---|---|---|---|
| Cervix | Detectable (12) | Detectable (2) | Detectable (2) | Not done | Detectable (2) |
| Endometrium | Detectabled (12, 20–22) | Detectablee (23) | Detectable (4, 15, 21–23) | Not done | Detectable (21–23) |
| Decidua | Detectable (14) | Not done | Detectable (3) | Not done | Detectable (1) |
| Myometrium | Detectable (12, 14) | Not done | Detectable (12) | Not done | Detectable (6, 7, 14) |
| Placental chorionic villi | Detectable (14) | Not done | Not done | Not done | Detectable (12, 16) |
| Umbilical cord | Detectable (14) | Not done | Not done | Not done | Not done |
| Amnion | Detectable (14) | Not done | Not done | Not done | Not done |
| Vascular smooth muscle | Detectable (14) | Not done | Not done | Not done | Not done |
| Vascular endothelium | Detectable (8, 14) | Not donef | Not done | Not done | Detectable (16) |
| HUVECs | Detectable (13) | Not detectable | Detectable (13) | Detectable (13) | Detectable (13) |
Abbreviation: HUVEC, human umbilical vein endothelial cell.
Measured by immunohistochemistry or immunofluorescence.
Measured by Western blot.
Measured by Northern blots, quantitative PCR, nonquantitative PCR, or microarrays.
Specificity of the commercial antibody used by Sacchi et al. (22) was not independently validated.
Specificity of the commercial antibody used by James et al. (23) was not independently validated.
Although not done using vascular endothelium from reproductive tissues, Radu et al. (8) did detect FSHR protein by co-immunoprecipitation/Western blotting using vascular endothelium of tumor tissue.
In reproductive tissues from cycling women, FSHR protein was observed in vascular endothelium and arterial smooth muscle of all tissues examined. In addition, FSHR was detected in the following:
Cervix: in glandular epithelium and, to a lesser extent, the stroma (12). Bovine cervix expresses FSHR and responds to FSH with increased prostanoid synthesis (2).
Proliferative and secretory endometrium: in glandular epithelium and, to a lesser extent, the stroma (12). Other laboratories have also reported expression of FSHR in normal human endometrium (4, 15, 20–23), and two groups have demonstrated its expression in endometriotic lesions (20, 21). Notably, primary cultures of endometrial tissue responded to FSH with increased expression of CYP19A1 and estrogen production (21).
Myometrium: in muscle fibers and stroma, albeit at particularly low levels (12, 14). Earlier studies had shown that addition of FSH to nonpregnant rat myometrium suppressed myoelectric activity (6, 7). Studies from our laboratory demonstrating the physiological contributions of myometrial FSHR to the regulation of contractile activity (14) are discussed later in this article.
In reproductive tissue from pregnant women, FSHR protein was observed in vascular endothelium and arterial smooth muscle of all tissues examined. In addition, FSHR was detected in the following:
Placenta: in the stroma, but at much reduced levels relative to the vascular endothelium (12). Recent studies by our laboratory that established the physiological importance of vascular endothelial FSHR to placental angiogenesis are discussed later in this article.
Umbilical cord: in Wharton Jelly, but at much reduced levels relative to the vascular endothelium and arterial smooth muscle (14).
Amnion: in epithelial cells of the outer layer (14).
Decidua: in some, but not all, decidual cells (14). FSHR was observed in decidualized cells in a nonhomogeneous manner such that clustered subsets of cells stained more intensely for FSHR than other areas of the decidua. Interestingly, at 18 to 20 weeks of gestation, maternal spiral arteries undergoing remodeling were negative for FSHR, but they were surrounded by decidual cells that were intensely positive (14). Earlier studies had shown an upregulation of FSHR mRNA in human endometrial stromal cells that were decidualized (3) and an induction of decidualization when FSH was added to stromal cells isolated from proliferative human endometrium (1). These studies, together with our more recent findings describing the nature of FSHR expression in decidua (14), suggest that FSHR may play roles, as yet unexplored, in the implantation of the embryo and remodeling of maternal spiral arteries.
Myometrium: in muscle fibers and stroma (12, 14). Myometrial FSHR levels in women at term pregnancy are much higher than in nonpregnant women (12, 14). The significance of these findings as they relate to the regulation of uterine contractile activity is discussed later in this article.
In this article, we summarize recent studies specifically addressing the roles of FSHR in placental vascular endothelium and its role in promoting fetal vessel angiogenesis, FSHR in the myometrium and its role in regulating uterine contractile activity, and data suggesting the local synthesis of FSH in reproductive tissues.
FSHR in placental vascular endothelium and its role in promoting fetal vessel angiogenesis
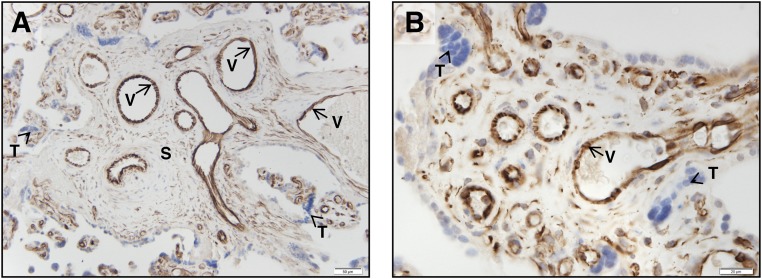
As reported by Radu et al. (8) and subsequently replicated by our laboratory (12), FSHR expression is detected on the endothelium of the fetal vasculature within the chorionic villi (Fig. 1) by 8 to 10 weeks of gestation (the earliest time point examined) and continuing throughout term. It is also present, albeit at much lower levels of expression, in stroma cells of the chorionic villi. No FSHR were detected in trophoblasts.
Figure 1.
Micrographs showing FSHR expression in human placental chorionic villi at 38 to 40 weeks’ gestation. Tissues were stained with antibody FSHR-323 IgG2a (brown) and counterstained with hematoxylin (blue). Images were taken at (A) ×200 and (B) ×600 magnification. Trophoblasts are indicated by arrowhead labeled T. Samples stained with nonimmune IgG2a did not exhibit staining. S, chorionic stromal core; V, endothelial cells of the villi vessels. [Adapted with permission from Stilley JA, Christensen DE, Dahlem KB, et al. FSH receptor (FSHR) expression in human extragonadal reproductive tissues and the developing placenta, and the impact of its deletion on pregnancy in mice. Biol Reprod. 2014;91:74.]
To address the actions of endothelial FSHR in response to FSH in vitro, we used primary cultures of human umbilical vein endothelial cells (HUVECs) as a model system (13). Using a saturating concentration of 125I-FSH, specific binding was detected on HUVECs but at extremely low levels that precluded the determination of binding affinity. Low levels of FSHR on HUVECs were also detected by immunofluorescence when using a sensitive setting. Using traditional PCR methods, FSHR mRNA in HUVECs was not detectable. However, FSHR mRNA was detectable using a nonquantitative method using gene-specific reverse transcription (13) first described by Robinson et al. (27) and Zhu et al. (28) for the detection of FSHR mRNA in osteoclasts. Using primers designed to amplify individual regions of the FSHR gene, HUVECs expressed a splice variant of FSHR lacking exon 9 (13) rather than the full-length mRNA. This is also the primary FSHR transcript found in osteoclasts (27, 28). Interestingly, but perhaps not surprisingly, given that the predominant FSHR form in HUVECs is not the full-length receptor, FSH addition to HUVECs did not stimulate cAMP production (13). Although a complete analysis of FSH-provoked signaling pathways activated in HUVECs was not undertaken, we did observe FSH-stimulated AKT activation (13). Notably, FSH addition to HUVECs provoked dose-dependent increases in tube formation and other cellular processes associated with angiogenesis (13). Similar to VEGF, FSH dose-response curves were bell shaped, with higher-than-optimal concentrations of the stimulus yielding suboptimal responses. Optimal concentrations of FSH or VEGF produced the same maximal response, indicating that FSH is as efficacious as VEGF in the stimulation of angiogenic processes in vitro.
In a subsequent publication, Stelmaszewska et al. (29) reported the inability to detect FSHR in HUVECs and a concomitant lack of stimulation of angiogenic processes in response to FSH. There are several factors that may account for the apparent discrepancies between the two studies, the primary one being the conditions used to culture the cells. Even in granulosa cells, which indisputably express FSHR, there is a time-dependent loss of Fshr mRNA when placed in culture (30), with our preliminary studies indicating a loss of >90% of Fshr mRNA in mouse granulosa cells after 5 days of culture. It is possible, therefore, that the culture conditions used by Stelmaszewska et al. (29), though suitable for maintaining the responsiveness of HUVECs to VEGF, may not have been appropriate for maintaining FSHR expression.
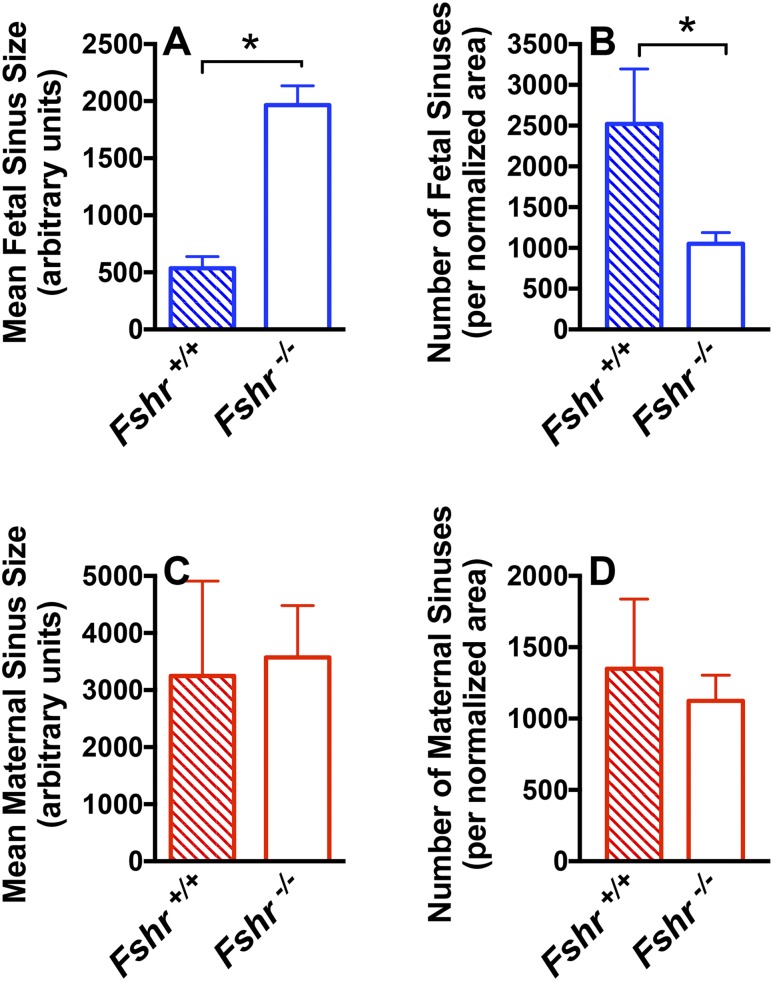
More recently, we undertook an in vivo approach using Fshr null mice (31) to unequivocally establish a role for vascular endothelial FSHR in mediating angiogenesis (16). In these experiments, we mated Fshr+/− mice with each other so that all pregnant dams were Fshr+/− but the fetoplacental units could be Fshr+/+, Fshr+/−, or Fshr−/−. Analyses of the Fshr+/+ and Fshr−/− fetoplacental units at midgestation revealed that the Fshr−/− placentas harbored a significantly decreased number and increased size of fetal vessels, with no change in maternal sinuses, revealing an important role for the Fshr in fetal vessel angiogenesis (Fig. 2). In addition, the proportion of the placenta composed of labyrinth was significantly reduced. These in vivo findings with Fshr null mice conclusively demonstrate that Fshr is required for placental angiogenesis and corroborate our in vitro studies indicating increased FSH-provoked angiogenic responses in HUVECs.
Figure 2.
Quantification of angiogenesis in the midgestational placental labyrinth of wild-type (Fshr+/+) and null (Fshr−/−) fetuses. The (A) number and (B) size of fetal sinuses, and the (C) number and (D) size of maternal sinuses in Fshr+/+ and Fshr−/− labyrinths of placentas 15 days post coitus, with number of sinuses normalized to overall labyrinth size. Data shown are the mean ± SEM of sinus quantifications of three Fshr+/+ and six Fshr−/− placentas. *P < 0.05. [Reproduced with permission from Stilley JAW, Segaloff DL. Deletion of fetoplacental Fshr inhibits fetal vessel angiogenesis in the mouse placenta. Mol Cell Endocrinol. 2018;476:79–83.]
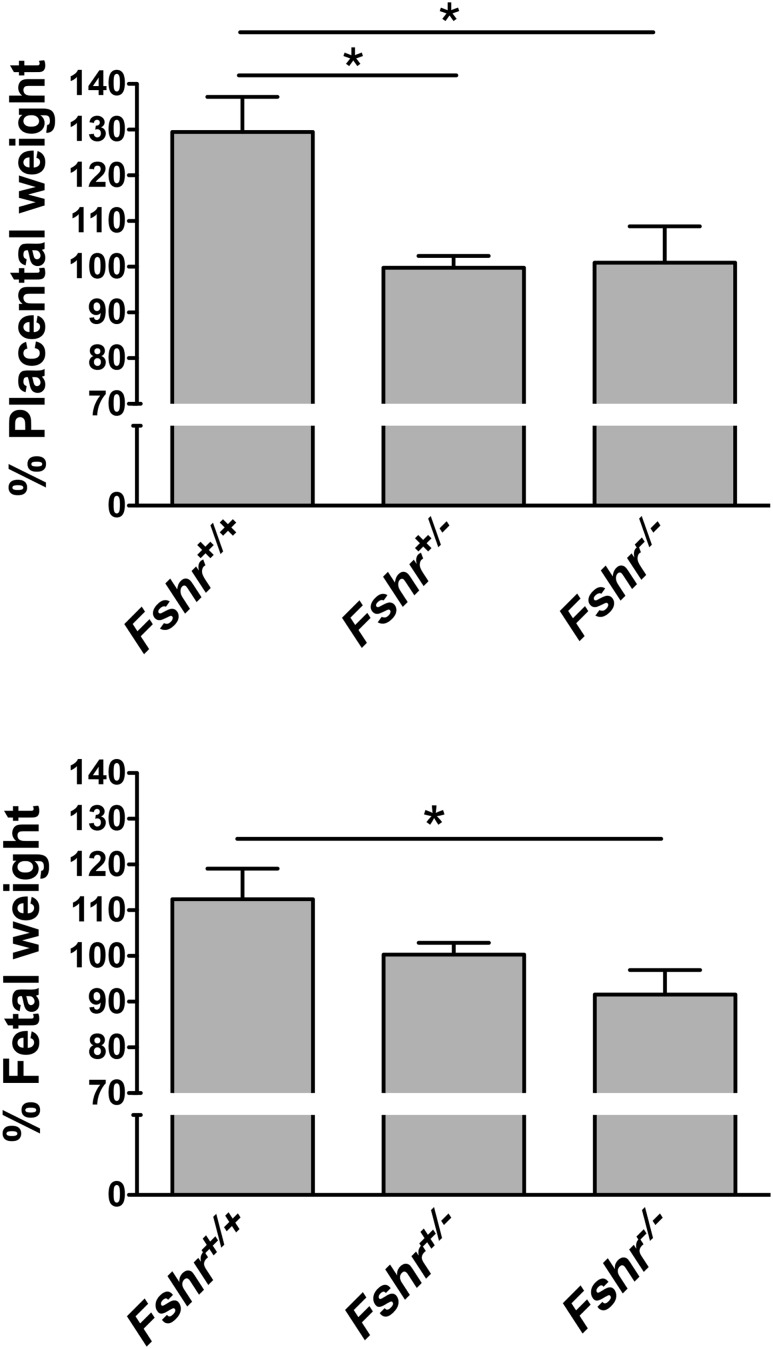
Using this in vivo approach, we also determined that midgestational weights of fetuses and placentas of Fshr+/− and Fshr−/− genotypes were reduced (12) (Fig. 3). We attribute these outcomes, at least in part, to decreased fetal vessel angiogenesis in the placental labyrinth, although we cannot formally exclude other potential contributions, including the ablation of potential FSH actions in the fetus. Interestingly, with respect to fetal and placental weights, Fshr haploinsufficiency appeared to have as great an impact as complete deletion of Fshr. It had been reported that female mice haploinsufficient for Fshr exhibited decreased fertility and decreased serum concentrations of estradiol and progesterone, suggesting that decreased ovarian function contributed at least in part to the subfertility (32, 33). That the impact of Fshr haploinsufficiency on the ovary resulted in a milder phenotype than Fshr haploinsufficiency Fshr in the placental vascular endothelium may be due to the much higher density of ovarian FSHR and, therefore, spare receptors on granulosa cells. Analysis of 170 live pups born to Fshr+/− mice (more than those included in our initial report (12)) revealed a lower-than-expected percentage of Fshr−/− pups (14% instead of the expected 25%), suggesting that deletion of the Fshr from the fetoplacental unit is detrimental to fetal viability.
Figure 3.
Deletion of fetoplacental Fshr reduces midgestational placental and fetal weights. (Upper graph) Placental weight as a function of fetal Fshr genotype. Data are expressed as a percentage of the mean weight of littermate Fshr+/− placentas. (Lower graph) Fetal weight as a function of fetal Fshr genotype. Data are expressed as a percentage of the mean weight of littermate Fshr+/− fetuses. Data are reported as the mean ± SEM for 54 pups from nine litters. *P < 0.05. [Reproduced with permission from Stilley JA, Christensen DE, Dahlem KB, et al. FSH receptor (FSHR) expression in human extragonadal reproductive tissues and the developing placenta, and the impact of its deletion on pregnancy in mice. Biol Reprod. 2014;91:74.]
A healthy pregnancy is predicated upon the normal development and function of the placenta. Impairments in placental development can result in placental insufficiency, fetal growth retardation, and fetal death. The ability of the placenta to mediate bidirectional maternal-fetal exchange of nutrients and respiratory gases requires extensive angiogenesis throughout its component tissues. Indeed, abnormalities in the placental vasculature account for the most common placental pathologies identified in various pregnancy complications (34–38). The appropriate timing and growth of the placental vasculature is finely regulated by proangiogenic and antiangiogenic factors, many of which are synthesized within the placenta. The studies discussed herein expand the repertoire of known hormones and growth factors regulating placental angiogenesis by demonstrating that signaling through fetal placental FSHR is essential for appropriate angiogenesis of placental fetal vessels and to the achievement of a healthy pregnancy.
FSHR in the myometrium and its role in regulating uterine contractile activity
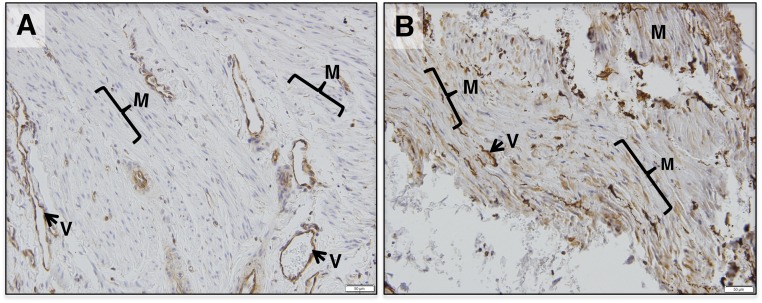
Myometrium of cycling and pregnant women expresses FSHR (12, 14). Although both exhibit similar FSHR expression in the vascular endothelium of blood vessels, the levels of FSHR in myometrial muscle fibers and stroma are significantly upregulated at term pregnancy (12, 14) (Fig. 4). Morphometric analyses of immunohistochemically stained sections reflected an approximately ninefold increase in FSHR protein levels in pregnant, term, nonlaboring myometrium (14), and quantitative PCR measurements indicated an approximately fivefold increase in FSHR mRNA (14).
Figure 4.
FSHR protein expression is higher in muscle fibers and stroma of human pregnant term myometrium relative to nonpregnant myometrium. Shown are representative micrographs of (A) human nonpregnant myometrium and (B) nonpathological pregnant term myometrium stained with antibody FSHR-323 IgG2a for FSHR (brown) and counterstained with hematoxylin (blue). Images are each representative of five samples and were taken at ×200 magnification; scale bars, 50 μm. Samples stained with nonimmune IgG2a did not exhibit staining (not shown). M, myometrial muscle fibers; V, endothelium of blood vessels. [Reproduced under a Creative Commons BY-NC 4.0 license from Stilley JA, Guan R, Santillan DA, et al. Differential Regulation of human and mouse myometrial contractile activity by FSH as a function of FSH receptor density. Biol Reprod. 2016;95:1–10.]
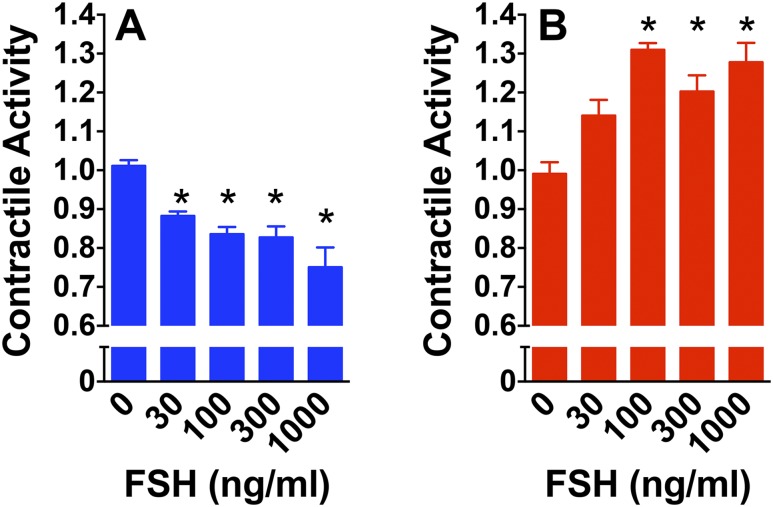
In contrast to endothelial FSHR mRNA, myometrial FSHR mRNA is the full-length form, identical to gonadal FSHR mRNA (12). Addition of gonadotropin hormone to gonadal cells or heterologous cells transfected with recombinant gonadotropin receptor leads to an increase in cAMP production at all densities of receptor expressed, but an increase in IP3 production is observed only at the higher densities of receptor (14, 39–42). Consequently, one would expect nonpregnant myometrium to respond to FSH with increased cAMP and pregnant term myometrium to respond with increased cAMP and IP3. Such a scenario would be predicted to differentially affect contractile activity in response to FSH, because cAMP quiets whereas IP3 stimulates myometrial contractile activity (43–47). Therefore, in cycling myometrium, when FSH would stimulate only cAMP, FSH would be expected to quiet contractions. In pregnant, term myometrium, the Gs/adenylyl cyclase signaling system is downregulated (48–51). Therefore, at term pregnancy, when FSHR is upregulated, FSH-stimulated IP3 would be expected to predominate and stimulate contractile activity. Remarkably, when isometric tension recordings were performed on strips of uterine myometrium, FSH indeed quieted contractile activity in samples from nonpregnant cycling women and stimulated contractile activity in samples from women at term pregnancy (14) (Fig. 5).
Figure 5.
FSH quiets contractile activity in human nonpregnant myometrium and stimulates contractile activity in pregnant term nonlaboring myometrium. Relative contractile activities of (A) strips of human nonpregnant myometrium or (B) human, pregnant, term, nonlaboring myometrium in response to increasing FSH concentrations were determined using isometric tension recordings as described by Stilley et al. (14). Data are reported as mean ± SEM from four independent experiments. *P < 0.05 from no FSH addition. [Reproduced under a Creative Commons BY-NC 4.0 license from Stilley JA, Guan R, Santillan DA, et al. Differential Regulation of human and mouse myometrial contractile activity by FSH as a function of FSH receptor density. Biol Reprod. 2016;95:1–10.]
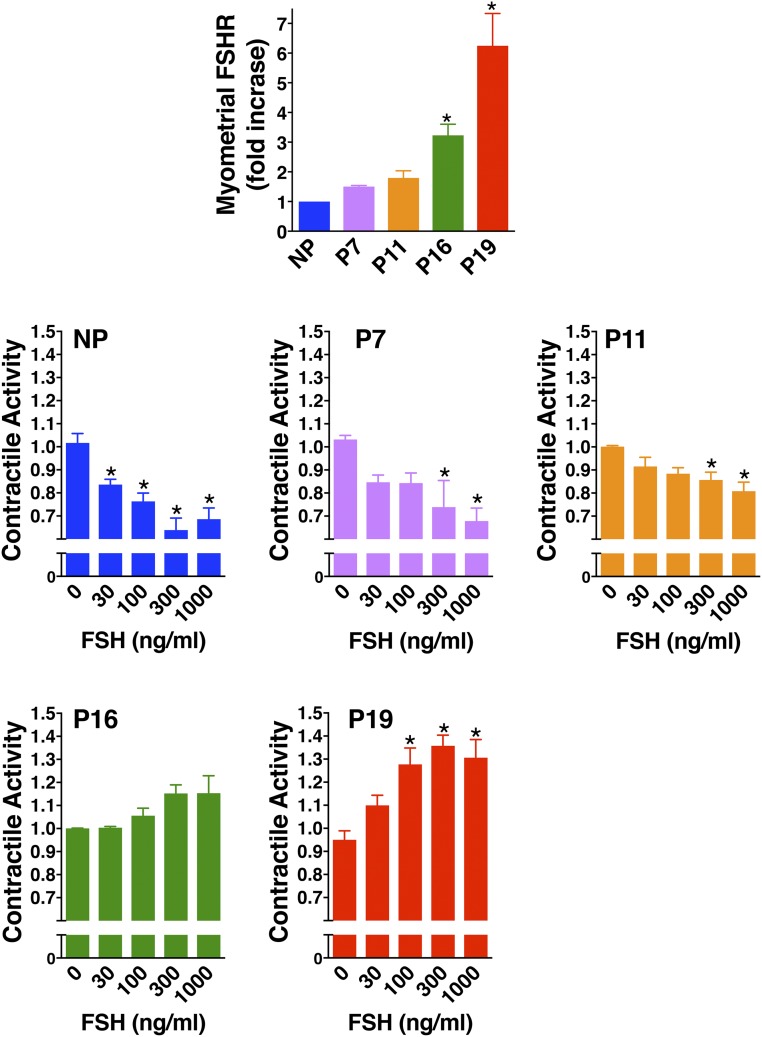
FSHR expression and FSH effects on contractile activity were further evaluated in mouse myometrium at various stages of pregnancy (14) (Fig. 6). During early and midpregnancy, FSHR expression remained low and FSH attenuated contractile activity. Later in pregnancy, FSHR levels were increased but were not sufficiently elevated to promote FSH-stimulated contractile activity. It is possible that at this stage, the relaxing effects of FSH-stimulated cAMP are counterbalanced by the stimulatory effects of FSH-provoked IP3. Finally, in pregnant, term, nonlaboring myometrium at P19 FSHR levels were further up-regulated and at this point significant dose-dependent increases in contractile activity were observed in response to FSH. Overall, these data suggest that in the nonpregnant state and in early to midpregnancy relatively low levels of myometrial FSHR mediate a quieting of contractile activity. In contrast, at term the up-regulated levels of myometrial FSHR mediate the stimulation of contractile activity. Preliminary data suggest that in the mouse, the concentration of Fshr mRNA in P19 myometrium, although upregulated approximately eightfold relative to nonpregnant mouse myometrium, is nonetheless expressed at much lower levels (∼400-fold lower) than ovarian Fshr mRNA from random cycling mice.
Figure 6.
In the mouse, FSH quiets myometrial contractile activity through midgestation, when FSHR remain low, and stimulates contractile activity at term, when FSHR is upregulated. (Top row) Staining of FSHR in mouse myometrium from six nonpregnant (NP), three P7, three P11, four P16, and three P19 samples was quantified as described by Stilley et al. (14) and data are reported as the mean ± SEM of the fold increase relative to nonpregnant myometrium. (Middle and bottom rows) Relative contractile activities of strips of mouse NP myometrium or mouse myometrium from different stages of gestation in response to increasing concentrations of FSH were determined using isometric tension recordings. Data are reported as mean ± SEM of three NP, three P7, five P11, three P16, and five P19 samples. *P < 0.05 from no FSH addition. [Adapted under a Creative Commons BY-NC 4.0 license from Stilley JA, Guan R, Santillan DA, et al. Differential Regulation of human and mouse myometrial contractile activity by FSH as a function of FSH receptor density. Biol Reprod. 2016;95:1–10.]
In future studies, it will be important to determine whether the quieting of contractive activity by low levels of FSHR in early and midgestation potentially facilitates implantation and the maintenance of pregnancy and whether the stimulation of contractile activity by upregulated FSHR at term contributes to parturition. In light of the observation that haplotypes in the noncoding regions of the FSHR are associated with the timing of birth in women (24, 25), it will also be critical to determine whether the appropriate temporal transcription upregulation of myometrial FSHR contributes to the timing of parturition.
Potential Synthesis of FSH in Reproductive Tissues
During pregnancy, serum FSH concentrations are quite low due to the inhibitory effects of the elevated concentrations of estrogen and progesterone on the hypothalamus and the pituitary gonadotropes. This begs the question of what the potential source(s) of FSH are that may engage extraovarian FSHR such as those in the placenta and myometrium during pregnancy. Considering that FSH may be synthesized locally, several tissues were examined for CGA mRNA, encoding the glycoprotein α subunit, and FSHB mRNA, encoding the β subunit of FSH. Neither mRNA was detected in HUVECs (12). However, both mRNAs were detected in placenta, decidua, and myometrium from human term pregnancy (12). These findings are in accord with microarray data available from National Center for Biotechnology Information Gene Expression Omnibus showing expression of CGA mRNA and FSHB mRNA in placental tissue from each trimester, the uterine endometrium, and the uterine myometrium (52–61). On these bases, we postulate that trophoblasts may be the placental source of FSH, where it acts in a paracrine manner to engage FSHR on placental endothelial cells. Confirmation of this, as well as identification of the cells synthesizing FSH in other extragonadal tissues in the reproductive tract, await further experimentation. Nonetheless, the FSHRB and CGA mRNA expression data suggest that locally synthesized FSH may be signaling through the low densities FSHR in extraovarian reproductive tissues via paracrine and/or autocrine mechanisms.
Clinical Perspectives on a Potential Role for FSH During Pregnancy
Results of several studies suggest that the risks of failed implantation, spontaneous miscarriage, and adverse perinatal outcomes in women using assisted reproductive technology (ART) approaches are not necessarily due to ART but may be due to maternal factors underlying infertility (62–70). For example, in women having spontaneous pregnancies, infants conceived after ≥12 months of attempting conception exhibit an increased risk of adverse perinatal outcomes relative to those conceived within 12 months (63–66, 68, 69). Also, a comparison of pregnancy outcomes in subfertile women who conceived naturally (i.e., long time to pregnancy) vs those of ART pregnancies revealed no differences in adverse outcomes (70). Notably, in a large population-based cohort study that compared the outcomes of two consecutive singleton pregnancies—one conceived after assisted fertilization and the other after spontaneously conception (i.e., the mothers served as the controls rather than a different population cohort)—adverse perinatal outcomes did not differ among infants of women who conceived both spontaneously and after assisted fertilization (67). These data suggest that some of the adverse outcomes of pregnancies aided by assisted fertilization when compared with those attained by spontaneous conception can be attributed to the factors leading to infertility rather than to factors related to reproductive technology. Decreased signaling through the FSHR of course can contribute to infertility or subfertility by virtue of the role of ovarian FSHR in promoting follicular growth and estrogen synthesis. In light of the findings discussed herein, it is reasonable to consider that if a woman with attenuated FSHR signaling were to achieve pregnancy, decreased FSHR signaling in extragonadal tissues may increase her risks for adverse pregnancy outcomes. As such, in addition to ovarian FSHR being critical for female fertility, extraovarian FSHR in the reproductive tract may contribute to the establishment of pregnancy, to the maintenance of a successful pregnancy, and to the appropriate timing of parturition.
Possibly arguing against a role for extraovarian FSHR in maintaining a healthy pregnancy is a clinical study demonstrating pregnancies in several women with ovarian failure due to an inactivating Ala189Val mutation of the FSHR who had undergone oocyte donation (71). Although most donations resulted in healthy pregnancies, complications were reported in some. Although these outcomes may have arisen because pregnancies arising from oocyte donations are associated with an increased rate of pregnancy complications (72–76), it is not possible to determine if the observed adverse pregnancy outcomes were attributable to the oocyte donation and/or to the mutant FSHR in extraovarian reproductive tissues. Importantly, any potential adverse effects due to decreased FSH signaling in the fetal portions of the placenta would not be observed in this clinical study, because the genotype of the placental labyrinth would be dictated by the fetus (who has wild-type FSHR) and not the mother (who has the mutant FSHR). Therefore, in this study, any potential contributions of the maternal mutant FSHR to the observed complications of pregnancies must have been though its expression in other reproductive tissues. The interpretation of the data from the clinical study becomes further complicated when one also considers that the Ala189Val mutation causes decreased, but not absent, cell surface FSHR expression (77, 78) and that the reduced numbers of mutant receptors at the cell surface exhibit an increased efficacy of hormone-stimulated signaling when compared with the same number of cell surface wild-type receptors (78).
In light of these considerations, a much more controlled means of experimentally determining the potential physiological significance of extraovarian FSHR in pregnancy would be to examine the potential impact of conditional deletions of the Fshr from targeted extraovarian tissues in genetically altered mice. As already discussed, the mating of mice heterozygous for the global deletion of the Fshr has enabled studies addressing the genetic deletion of Fshr from the fetoplacental unit, demonstrating decreased angiogenesis in the placental labyrinth, decreased placental and fetal weights, and increased rates of fetal demise (12, 16). To facilitate investigations into deletion of Fshr from selected mouse reproductive tissues, we recently generated a mouse in which the Fshr has been floxed.
The investigation of the impact of extraovarian FSHR in reproductive tissues on pregnancy is still in its early stages. We hope the findings obtained thus far will persuade other investigators of the potential significance of further advancing studies in this area. To that end, as well as for studies examining potential physiological roles of extragonadal FSHR in nonreproductive tissues, the floxed Fshr mouse we generated would be available.
Acknowledgments
We thank Drs. William Paradee (Genome Editing Core, University of Iowa), Kathryn G. Lamping (Department of Internal Medicine, The University of Iowa), Patricia A. Kirby (Department of Pathology, The University of Iowa) and Bryan F. Mitchell (Department of Obstetrics and Gynecology, University of Alberta) for their invaluable contributions to the studies discussed herein. We thank Dr. Mario Ascoli for critical review of the manuscript.
Financial Support: This work was supported in part by the National Institutes of Health [Grant T32DK007690 (to J.A.W.S.) and Grant HD1196566 to (D.L.S.)].
Current Affiliation: J.A.W. Stilley’s current affiliation is Department of Emergency Medicine, University of Missouri-Columbia, Columbia, Missouri 65212.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- FSHR
FSH receptor
- HUVEC
human umbilical vein endothelial cell
References
- 1. Tang B, Gurpide E. Direct effect of gonadotropins on decidualization of human endometrial stroma cells. J Steroid Biochem Mol Biol. 1993;47(1-6):115–121. [DOI] [PubMed] [Google Scholar]
- 2. Mizrachi D, Shemesh M. Follicle-stimulating hormone receptor and its messenger ribonucleic acid are present in the bovine cervix and can regulate cervical prostanoid synthesis. Biol Reprod. 1999;61(3):776–784. [DOI] [PubMed] [Google Scholar]
- 3. Popovici RM, Kao LC, Giudice LC. Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology. 2000;141(9):3510–3513. [DOI] [PubMed] [Google Scholar]
- 4. La Marca A, Carducci Artenisio A, Stabile G, Rivasi F, Volpe A. Evidence for cycle-dependent expression of follicle-stimulating hormone receptor in human endometrium. Gynecol Endocrinol. 2005;21(6):303–306. [DOI] [PubMed] [Google Scholar]
- 5. Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M. FSH directly regulates bone mass. Cell. 2006;125(2):247–260. [DOI] [PubMed] [Google Scholar]
- 6. Celik O, Tagluk ME, Hascalik S, Elter K, Celik N, Aydin NE. Spectrotemporal changes in electrical activity of myometrium due to recombinant follicle-stimulating hormone preparations follitropin alfa and beta. Fertil Steril. 2008;90(4, Suppl):1348–1356. [DOI] [PubMed] [Google Scholar]
- 7. Hascalik S, Celik O, Tagluk ME, Yildirim A, Aydin NE. Effects of highly purified urinary FSH and human menopausal FSH on uterine myoelectrical dynamics. Mol Hum Reprod. 2010;16(3):200–206. [DOI] [PubMed] [Google Scholar]
- 8. Radu A, Pichon C, Camparo P, Antoine M, Allory Y, Couvelard A, Fromont G, Hai MT, Ghinea N. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Engl J Med. 2010;363(17):1621–1630. [DOI] [PubMed] [Google Scholar]
- 9. Zhu LL, Blair H, Cao J, Yuen T, Latif R, Guo L, Tourkova IL, Li J, Davies TF, Sun L, Bian Z, Rosen C, Zallone A, New MI, Zaidi M. Blocking antibody to the β-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proc Natl Acad Sci USA. 2012;109(36):14574–14579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu P, Ji Y, Yuen T, Rendina-Ruedy E, DeMambro VE, Dhawan S, Abu-Amer W, Izadmehr S, Zhou B, Shin AC, Latif R, Thangeswaran P, Gupta A, Li J, Shnayder V, Robinson ST, Yu YE, Zhang X, Yang F, Lu P, Zhou Y, Zhu LL, Oberlin DJ, Davies TF, Reagan MR, Brown A, Kumar TR, Epstein S, Iqbal J, Avadhani NG, New MI, Molina H, van Klinken JB, Guo EX, Buettner C, Haider S, Bian Z, Sun L, Rosen CJ, Zaidi M. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017;546(7656):107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ji Y, Liu P, Yuen T, Haider S, He J, Romero R, Chen H, Bloch M, Kim SM, Lizneva D, Munshi L, Zhou C, Lu P, Iqbal J, Cheng Z, New MI, Hsueh AJ, Bian Z, Rosen CJ, Sun L, Zaidi M. Epitope-specific monoclonal antibodies to FSHβ increase bone mass. Proc Natl Acad Sci USA. 2018;115(9):2192–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stilley JA, Christensen DE, Dahlem KB, Guan R, Santillan DA, England SK, Al-Hendy A, Kirby PA, Segaloff DL. FSH receptor (FSHR) expression in human extragonadal reproductive tissues and the developing placenta, and the impact of its deletion on pregnancy in mice. Biol Reprod. 2014;91(3):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stilley JA, Guan R, Duffy DM, Segaloff DL. Signaling through FSH receptors on human umbilical vein endothelial cells promotes angiogenesis. J Clin Endocrinol Metab. 2014;99(5):E813–E820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stilley JA, Guan R, Santillan DA, Mitchell BF, Lamping KG, Segaloff DL. Differential regulation of human and mouse myometrial contractile activity by FSH as a function of FSH receptor density. Biol Reprod. 2016;95(2):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Altmäe S, Tamm-Rosenstein K, Esteban FJ, Simm J, Kolberg L, Peterson H, Metsis M, Haldre K, Horcajadas JA, Salumets A, Stavreus-Evers A. Endometrial transcriptome analysis indicates superiority of natural over artificial cycles in recurrent implantation failure patients undergoing frozen embryo transfer. Reprod Biomed Online. 2016;32(6):597–613. [DOI] [PubMed] [Google Scholar]
- 16. Stilley JAW, Segaloff DL. Deletion of fetoplacental Fshr inhibits fetal vessel angiogenesis in the mouse placenta. Mol Cell Endocrinol. 2018;476:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumar TR. Extragonadal actions of FSH: a critical need for novel genetic models. Endocrinology. 2018;159(1):2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zaidi M, Lizneva D, Kim SM, Sun L, Iqbal J, New MI, Rosen CJ, Yuen T. FSH, bone mass, body fat, and biological aging. Endocrinology. 2018;159(10):3503–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ghinea N. Vascular endothelial FSH receptor, a target of interest for cancer therapy. Endocrinology. 2018;159(9):3268–3274. [DOI] [PubMed] [Google Scholar]
- 20. Robin B, Planeix F, Sastre-Garau X, Pichon C, Olesen TK, Gogusev J, Ghinea N. Follicle-stimulating hormone receptor expression in endometriotic lesions and the associated vasculature: an immunohistochemical study. Reprod Sci. 2016;23(7):885–891. [DOI] [PubMed] [Google Scholar]
- 21. Ponikwicka-Tyszko D, Chrusciel M, Stelmaszewska J, Bernaczyk P, Sztachelska M, Sidorkiewicz I, Doroszko M, Tomaszewski J, Tapanainen JS, Huhtaniemi I, Wolczynski S, Rahman NA. Functional expression of FSH receptor in endometriotic lesions. J Clin Endocrinol Metab. 2016;101(7):2905–2914. [DOI] [PubMed] [Google Scholar]
- 22. Sacchi S, Sena P, Degli Esposti C, Lui J, La Marca A. Evidence for expression and functionality of FSH and LH/hCG receptors in human endometrium. J Assist Reprod Genet. 2018;35(9):1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. James K, Bhartiya D, Ganguly R, Kaushik A, Gala K, Singh P, Metkari SM. Gonadotropin and steroid hormones regulate pluripotent very small embryonic-like stem cells in adult mouse uterine endometrium. J Ovarian Res. 2018;11(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Plunkett J, Doniger S, Orabona G, Morgan T, Haataja R, Hallman M, Puttonen H, Menon R, Kuczynski E, Norwitz E, Snegovskikh V, Palotie A, Peltonen L, Fellman V, DeFranco EA, Chaudhari BP, McGregor TL, McElroy JJ, Oetjens MT, Teramo K, Borecki I, Fay J, Muglia L. An evolutionary genomic approach to identify genes involved in human birth timing. PLoS Genet. 2011;7(4):e1001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chun S, Plunkett J, Teramo K, Muglia LJ, Fay JC. Fine-mapping an association of FSHR with preterm birth in a Finnish population. PLoS One. 2013;8(10):e78032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vannier B, Loosfelt H, Meduri G, Pichon C, Milgrom E. Anti-human FSH receptor monoclonal antibodies: immunochemical and immunocytochemical characterization of the receptor. Biochemistry. 1996;35(5):1358–1366. [DOI] [PubMed] [Google Scholar]
- 27. Robinson LJ, Tourkova I, Wang Y, Sharrow AC, Landau MS, Yaroslavskiy BB, Sun L, Zaidi M, Blair HC. FSH-receptor isoforms and FSH-dependent gene transcription in human monocytes and osteoclasts. Biochem Biophys Res Commun. 2010;394(1):12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu LL, Tourkova I, Yuen T, Robinson LJ, Bian Z, Zaidi M, Blair HC. Blocking FSH action attenuates osteoclastogenesis. Biochem Biophys Res Commun. 2012;422(1):54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stelmaszewska J, Chrusciel M, Doroszko M, Akerfelt M, Ponikwicka-Tyszko D, Nees M, Frentsch M, Li X, Kero J, Huhtaniemi I, Wolczynski S, Rahman NA. Revisiting the expression and function of follicle-stimulation hormone receptor in human umbilical vein endothelial cells. Sci Rep. 2016;6:37095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tilly JL, LaPolt PS, Hsueh AJW. Hormonal regulation of follicle-stimulating hormone receptor messenger ribonucleic acid levels in cultured rat granulosa cells. Endocrinology. 1992;130(3):1296–1302. [DOI] [PubMed] [Google Scholar]
- 31. Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA. 1998;95(23):13612–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Danilovich N, Babu PS, Xing W, Gerdes M, Krishnamurthy H, Sairam MR. Estrogen deficiency, obesity, and skeletal abnormalities in follicle-stimulating hormone receptor knockout (FORKO) female mice. Endocrinology. 2000;141(11):4295–4308. [DOI] [PubMed] [Google Scholar]
- 33. Danilovich N, Sairam MR. Haploinsufficiency of the follicle-stimulating hormone receptor accelerates oocyte loss inducing early reproductive senescence and biological aging in mice. Biol Reprod. 2002;67(2):361–369. [DOI] [PubMed] [Google Scholar]
- 34. Macara L, Kingdom JC, Kaufmann P, Kohnen G, Hair J, More IA, Lyall F, Greer IA. Structural analysis of placental terminal villi from growth-restricted pregnancies with abnormal umbilical artery Doppler waveforms. Placenta. 1996;17(1):37–48. [DOI] [PubMed] [Google Scholar]
- 35. Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004;25(2-3):127–139. [DOI] [PubMed] [Google Scholar]
- 36. Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–1594. [DOI] [PubMed] [Google Scholar]
- 37. Reynolds LP, Caton JS, Redmer DA, Grazul-Bilska AT, Vonnahme KA, Borowicz PP, Luther JS, Wallace JM, Wu G, Spencer TE. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J Physiol. 2006;572(Pt 1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen DB, Zheng J. Regulation of placental angiogenesis. Microcirculation. 2014;21(1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gudermann T, Birnbaumer M, Birnbaumer L. Evidence for dual coupling of the murine luteinizing hormone receptor to adenylyl cyclase and phosphoinositide breakdown and Ca2+ mobilization. Studies with the cloned murine luteinizing hormone receptor expressed in L cells. J Biol Chem. 1992;267(7):4479–4488. [PubMed] [Google Scholar]
- 40. Zhu X, Gilbert S, Birnbaumer M, Birnbaumer L. Dual signaling potential is common among Gs-coupled receptors and dependent on receptor density. Mol Pharmacol. 1994;46(3):460–469. [PubMed] [Google Scholar]
- 41. Donadeu FX, Ascoli M. The differential effects of the gonadotropin receptors on aromatase expression in primary cultures of immature rat granulosa cells are highly dependent on the density of receptors expressed and the activation of the inositol phosphate cascade. Endocrinology. 2005;146(9):3907–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Allen MD, Neumann S, Gershengorn MC. Occupancy of both sites on the thyrotropin (TSH) receptor dimer is necessary for phosphoinositide signaling. FASEB J. 2011;25(10):3687–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sanborn BM, Yue C, Wang W, Dodge KL. G protein signalling pathways in myometrium: affecting the balance between contraction and relaxation. Rev Reprod. 1998;3(3):196–205. [DOI] [PubMed] [Google Scholar]
- 44. Price SA, Bernal AL. Uterine quiescence: the role of cyclic AMP. Exp Physiol. 2001;86(2):265–272. [DOI] [PubMed] [Google Scholar]
- 45. Hertelendy F, Zakar T. Regulation of myometrial smooth muscle functions. Curr Pharm Des. 2004;10(20):2499–2517. [DOI] [PubMed] [Google Scholar]
- 46. Aguilar HN, Mitchell BF. Physiological pathways and molecular mechanisms regulating uterine contractility. Hum Reprod Update. 2010;16(6):725–744. [DOI] [PubMed] [Google Scholar]
- 47. Mitchell BF, Aguilar HN, Mosher A, Wood S, Slater DM. The uterine myocyte as a target for prevention of preterm birth. Facts Views Vis ObGyn. 2013;5(1):72–81. [PMC free article] [PubMed] [Google Scholar]
- 48. Europe-Finner GN, Phaneuf S, Tolkovsky AM, Watson SP, López Bernal A. Down-regulation of G alpha s in human myometrium in term and preterm labor: a mechanism for parturition. J Clin Endocrinol Metab. 1994;79(6):1835–1839. [DOI] [PubMed] [Google Scholar]
- 49. López Bernal A, Rivera J, Europe-Finner GN, Phaneuf S, Asbóth G. Parturition: activation of stimulatory pathways or loss of uterine quiescence? Adv Exp Med Biol. 1995;395:435–451. [PubMed] [Google Scholar]
- 50. Grammatopoulos DK, Hillhouse EW. Activation of protein kinase C by oxytocin inhibits the biological activity of the human myometrial corticotropin-releasing hormone receptor at term. Endocrinology. 1999;140(2):585–594. [DOI] [PubMed] [Google Scholar]
- 51. Mhaouty-Kodja S, Bouet-Alard R, Limon-Boulez I, Maltier JP, Legrand C. Molecular diversity of adenylyl cyclases in human and rat myometrium. Correlation with global adenylyl cyclase activity during mid- and term pregnancy. J Biol Chem. 1997;272(49):31100–31106. [DOI] [PubMed] [Google Scholar]
- 52. Huuskonen P, Storvik M, Reinisalo M, Honkakoski P, Rysä J, Hakkola J, Pasanen M. Microarray analysis of the global alterations in the gene expression in the placentas from cigarette-smoking mothers. Clin Pharmacol Ther. 2008;83(4):542–550. [DOI] [PubMed] [Google Scholar]
- 53. Mikheev AM, Nabekura T, Kaddoumi A, Bammler TK, Govindarajan R, Hebert MF, Unadkat JD. Profiling gene expression in human placentae of different gestational ages: an OPRU Network and UW SCOR Study. Reprod Sci. 2008;15(9):866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Votavova H, Dostalova Merkerova M, Fejglova K, Vasikova A, Krejcik Z, Pastorkova A, Tabashidze N, Topinka J, Veleminsky M Jr, Sram RJ, Brdicka R. Transcriptome alterations in maternal and fetal cells induced by tobacco smoke. Placenta. 2011;32(10):763–770. [DOI] [PubMed] [Google Scholar]
- 55. Winn VD, Haimov-Kochman R, Paquet AC, Yang YJ, Madhusudhan MS, Gormley M, Feng KT, Bernlohr DA, McDonagh S, Pereira L, Sali A, Fisher SJ. Gene expression profiling of the human maternal-fetal interface reveals dramatic changes between midgestation and term. Endocrinology. 2007;148(3):1059–1079. [DOI] [PubMed] [Google Scholar]
- 56. Dezso Z, Nikolsky Y, Sviridov E, Shi W, Serebriyskaya T, Dosymbekov D, Bugrim A, Rakhmatulin E, Brennan RJ, Guryanov A, Li K, Blake J, Samaha RR, Nikolskaya T. A comprehensive functional analysis of tissue specificity of human gene expression. BMC Biol. 2008;6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Eyster KM, Klinkova O, Kennedy V, Hansen KA. Whole genome deoxyribonucleic acid microarray analysis of gene expression in ectopic versus eutopic endometrium. Fertil Steril. 2007;88(6):1505–1533. [DOI] [PubMed] [Google Scholar]
- 58. Hawkins SM, Creighton CJ, Han DY, Zariff A, Anderson ML, Gunaratne PH, Matzuk MM. Functional microRNA involved in endometriosis. Mol Endocrinol. 2011;25(5):821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mutter GL, Zahrieh D, Liu C, Neuberg D, Finkelstein D, Baker HE, Warrington JA. Comparison of frozen and RNALater solid tissue storage methods for use in RNA expression microarrays. BMC Genomics. 2004;5:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA. 2004;101(16):6062–6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147(3):1097–1121. [DOI] [PubMed] [Google Scholar]
- 62. Joffe M, Li Z. Association of time to pregnancy and the outcome of pregnancy. Fertil Steril. 1994;62(1):71–75. [DOI] [PubMed] [Google Scholar]
- 63. Henriksen TB, Baird DD, Olsen J, Hedegaard M, Secher NJ, Wilcox AJ. Time to pregnancy and preterm delivery. Obstet Gynecol. 1997;89(4):594–599. [DOI] [PubMed] [Google Scholar]
- 64. Basso O, Baird DD. Infertility and preterm delivery, birthweight, and caesarean section: a study within the Danish National Birth Cohort. Hum Reprod. 2003;18(11):2478–2484. [DOI] [PubMed] [Google Scholar]
- 65. Thomson F, Shanbhag S, Templeton A, Bhattacharya S. Obstetric outcome in women with subfertility. BJOG. 2005;112(5):632–637. [DOI] [PubMed] [Google Scholar]
- 66. Zhu JL, Obel C, Hammer Bech B, Olsen J, Basso O. Infertility, infertility treatment, and fetal growth restriction. Obstet Gynecol. 2007;110(6):1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Romundstad LB, Romundstad PR, Sunde A, von Düring V, Skjaerven R, Gunnell D, Vatten LJ. Effects of technology or maternal factors on perinatal outcome after assisted fertilisation: a population-based cohort study. Lancet. 2008;372(9640):737–743. [DOI] [PubMed] [Google Scholar]
- 68. Jaques AM, Amor DJ, Baker HW, Healy DL, Ukoumunne OC, Breheny S, Garrett C, Halliday JL. Adverse obstetric and perinatal outcomes in subfertile women conceiving without assisted reproductive technologies. Fertil Steril. 2010;94(7):2674–2679. [DOI] [PubMed] [Google Scholar]
- 69. Wisborg K, Ingerslev HJ, Henriksen TB. IVF and stillbirth: a prospective follow-up study. Hum Reprod. 2010;25(5):1312–1316. [DOI] [PubMed] [Google Scholar]
- 70. Raatikainen K, Kuivasaari-Pirinen P, Hippeläinen M, Heinonen S. Comparison of the pregnancy outcomes of subfertile women after infertility treatment and in naturally conceived pregnancies. Hum Reprod. 2012;27(4):1162–1169. [DOI] [PubMed] [Google Scholar]
- 71. Hovatta O, Söderström-Anttila V, Foudila T, Tuomivaara L, Juntunen K, Tiitinen A, Aittomäki K. Pregnancies after oocyte donation in women with ovarian failure caused by an inactivating mutation in the follicle stimulating hormone receptor. Hum Reprod. 2002;17(1):124–127. [DOI] [PubMed] [Google Scholar]
- 72. Abdalla HI, Baber R, Kirkland A, Leonard T, Power M, Studd JW. A report on 100 cycles of oocyte donation; factors affecting the outcome. Hum Reprod. 1990;5(8):1018–1022. [DOI] [PubMed] [Google Scholar]
- 73. Sauer MV, Paulson RJ. Human oocyte and preembryo donation: an evolving method for the treatment of infertility. Am J Obstet Gynecol. 1990;163(5 Pt 1):1421–1424. [DOI] [PubMed] [Google Scholar]
- 74. Pados G, Camus M, Van Waesberghe L, Liebaers I, Van Steirteghem A, Devroey P. Oocyte and embryo donation: evaluation of 412 consecutive trials. Hum Reprod. 1992;7(8):1111–1117. [DOI] [PubMed] [Google Scholar]
- 75. Söderström-Anttila V, Tiitinen A, Foudila T, Hovatta O. Obstetric and perinatal outcome after oocyte donation: comparison with in-vitro fertilization pregnancies. Hum Reprod. 1998;13(2):483–490. [DOI] [PubMed] [Google Scholar]
- 76. Söderström-Anttila V. Pregnancy and child outcome after oocyte donation. Hum Reprod Update. 2001;7(1):28–32. [DOI] [PubMed] [Google Scholar]
- 77. Rannikko A, Pakarinen P, Manna PR, Beau I, Misrahi M, Aittomäki K, Huhtaniemi I. Functional characterization of the human FSH receptor with an inactivating Ala189Val mutation. Mol Hum Reprod. 2002;8(4):311–317. [DOI] [PubMed] [Google Scholar]
- 78. Charmandari E, Guan R, Zhang M, Silveira LG, Fan QR, Chrousos GP, Sertedaki AC, Latronico AC, Segaloff DL. Misfolding ectodomain mutations of the lutropin receptor increase efficacy of hormone stimulation. Mol Endocrinol. 2016;30(1):62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]