Abstract
The differentiation of the hormone-producing cell lineages of the anterior pituitary represents an informative model of mammalian cell fate determination. The generation and maintenance of two of these lineages, the GH-producing somatotropes and prolactin (PRL)-producing lactotropes, are dependent on the pituitary-specific transcription factor POU1F1. Whereas POU1F1 is expressed in both cell types, and plays a direct role in the activation of both the Gh and Prl genes, GH expression is restricted to somatotropes and PRL expression is restricted to lactotropes. These observations imply the existence of additional, cell type–enriched factors that contribute to the somatotrope and lactotrope cell identities. In this study, we use transgenic mouse models to facilitate sorting of somatotrope and lactotrope populations based on the expression of fluorescent markers expressed under Gh and Prl gene transcriptional controls. The transcriptomic analyses reveal a concordance of gene expression profiles in the two populations. The limited number of divergent mRNAs between the two populations includes a set of transcription factors that may have roles in pituitary lineage divergence and/or in regulating expression of cell type–specific genes after differentiation. Four of these factors were validated for lineage enrichment at the level of protein expression, two somatotrope enriched and two lactotrope enriched. Three of these four factors were shown to have corresponding activities in appropriate enhancement or repression of landmark genes in a cell culture model system. These studies identify novel regulators of the somatotropes and lactotropes, and they establish a useful database for further study of these lineages in the anterior pituitary.
The differentiation of multiple cell types from a common precursor cell and the subsequent maintenance of distinct cell identities throughout adult life are processes central to most developmental systems (1, 2). A key question in such processes is how a common precursor cell can give rise to multiple cell types with discrete transcriptomic profiles. Cell types differentiating from a common precursor cell must not only activate transcription of genes necessary for the appropriate cell fate, but also repress expression of genes that drive alternate cell identities. The coordination of these regulatory processes requires cell type–specific mechanisms of transcriptional control. Additionally, terminally differentiated cells must be subsequently maintained in the differentiated state, necessitating sustained mechanisms and pathways for maintaining cell fate. The establishment and maintenance of cell fate may be regulated by transcription factors that are expressed during development and/or by factors that are induced and sustained during the lifespan of the organism (3, 4).
The anterior pituitary is an ideal system for studying mechanisms of lineage establishment and maintenance due to the terminal differentiation and divergence of somatotropes and lactotropes. In mice, the pituitary-specific master regulatory transcription factor POU1F1 is expressed in a subset of developing pituitary cells beginning at embryonic day (e)13.5 (6–8). According to a frequently cited model, these POU1F1+ cells differentiate into the TSH-producing thyrotropes and the GH/prolactin (PRL) dual-expressing somatolactotropes at e16.5 (6, 9). The somatolactotrope precursor then gives rise to the terminally differentiated somatotropes and lactotropes, which produce high levels of GH and PRL protein, respectively (10). Although small populations of somatolactotropes remain present in the pituitaries of adult mice, comprising ∼1% of all cells in the anterior pituitary, their function remains poorly understood (11, 12). Additionally, previous studies have found evidence that a subset of lactotropes may derive from a cell with a previous somatotrope identity (13, 14). These findings bring into question the role of the somatolactotrope cells in generating these two terminal lineages and highlight the need to better understand the mechanisms that regulate the somatotrope and lactotrope populations. Despite some questions about the pathway of somatotrope and lactotrope divergence and terminal differentiation, all current models of this pathway agree that expression of Pou1f1 is essential to the establishment of both populations of cells and that the somatotrope and lactotrope cells are closely related. In addition to being a master regulator of the somatotrope and lactotrope lineages, POU1F1 is also a direct activator of both the Gh and Prl genes, and it remains expressed in terminally differentiated somatotropes and lactotropes, driving robust hormone expression throughout adult life (15–18). Despite this expression of Pou1f1 in both cell types, the expression of the GH and PRL proteins remains primarily restricted to the somatotropes and lactotropes, respectively (6, 19). This observation suggests the existence of unknown somatotrope- and lactotrope-specific factors that contribute to the divergence and corresponding specificity of gene expression in the two lineages.
Several genes have been previously identified as lineage enriched and crucial to the ability of somatotropes and lactotropes to respond to the appropriate physiological signals to secrete their respective hormones. GHRHR and DRD2 are examples of known receptors expressed on the surfaces of somatotrope and lactotrope cells, respectively. These cell surface receptors regulate the expression and secretion of GH and PRL in response to physiological cues that trigger signaling from the hypothalamus (20–22). However, the currently known sets of somatotrope and lactotrope genes are insufficient to adequately explain the mechanisms by which the somatotrope and lactotrope cell identities are established and maintained, as POU1F1 remains the only known transcription factor that has been demonstrated to directly activate the Gh and Prl genes (23, 24). Taken together, these observations suggest that there exists a set of currently unidentified transcription factors that contribute to the respective somatotrope and/or lactotrope expression profiles. The identification of these factors is essential to a detailed understanding of the function and pathology of these two predominant cell types in the anterior pituitary.
In this study, we have used a set of transgenic mouse lines to facilitate the isolation of primary somatotrope and lactotrope populations from the adult mouse pituitary. Transcriptomic profiling of these two cell populations reveals a noteworthy similarity in their respective transcriptomes. Despite this transcriptomic similarity, the analysis identified ∼300 genes that are significantly enriched (log2 fold change >2 and a P value of <0.05) in each of the two cell populations. These enriched genes included a subset of transcription factors. Six of these transcription factors were selected for in-depth analysis based on their putative functions. Four of these factors were confirmed for selective enrichment in primary somatotrope and lactotrope cells at the level of protein expression, and they were assessed for functional roles in gene regulation and for occupancy at chromatin sites encompassing the Gh and Prl gene promoters. One of the lactotrope-enriched factors was found to bind adjacent to POU1F1 at the Prl gene promoter and to synergize with POU1F1 in stimulation of Prl gene transcription. Our data establish a transcriptomic resource for analysis of somatotrope and lactotrope gene expression and cellular function, and they identify a set of novel cell type–enriched transcription factors that have potential roles in regulating the expression of landmark hormone genes.
Materials and Methods
Cell and transgenic mouse lines
Mice carrying the previously described Gh-GFP and Prl-DsRed transgenes were mated to generate compound transgenic mice carrying both reporter constructs (GFP+/DsRed+) (25, 26). All mice were of a hybrid CD1 × C57BL/6J background and were aged to 6 to 8 weeks before use in experiments to allow for the complete maturation of the pituitary gland. All mice used in these studies were virgin females. All mouse studies were reviewed and approved by the University of Pennsylvania Laboratory Animal Use and Care Committee. The Pit-1/Triple and Pit-1/0 cell lines, previously described (5), were cultured in DMEM (Life Technologies) plus 10% fetal bovine serum along with 1× antibiotic-antimycotic solution (Invitrogen). Cells were removed from plates for the purposes of passaging and for harvesting using a 0.05% trypsin/EDTA solution (Life Technologies).
Fluorescence-activated cell sorting
Pituitaries from female GFP+/DsRed+ mice were harvested at 6 to 8 weeks and dissociated mechanically in enzyme-free cell dissociation buffer (Life Technologies) before being passed through a 40-μm filter into DMEM plus 10% fetal bovine serum culture media and centrifuged at 1000g for 5 minutes. After centrifugation, cells were resuspended in PBS plus 0.1% BSA and layered onto a PBS plus 4% BSA cushion and centrifuged a second time at 100g for 5 minutes to remove cell debris. Cell pellets were resuspended in PBS plus 0.1% BSA, 1 μg of 4′,6-diamidino-2-phenylindole (DAPI) was added directly to the samples, and the cells were sorted on a FACSAria II platform (BD Biosciences) directly into TRIzol LS reagent (Thermo Fisher Scientific). The cell sorting was performed by the Flow Cytometry and Cell Sorting Facility at the University of Pennsylvania.
RNA isolation and RNA sequencing
RNA from fluorescence-activated cell sorting (FACS)–sorted cells was isolated (TRIzol LS protocol; Thermo Fisher Scientific) using 10 µg of GlycoBlue coprecipitant (Thermo Fisher Scientific) as a carrier. RNA quality and concentration were assayed using the RNA 6000 Pico kit (Agilent) with a 2100 BioAnalyzer (Agilent) platform. High-quality RNA samples, defined by RNA integrity number scores of >7, were submitted to the University of Pennsylvania Next Generation Sequencing Core for analysis. Each sample was amplified using the Ovation ultralow RNA sequencing (RNA-seq) library system (NuGEN), and libraries were sequenced on the HiSeq 2500 platform (Illumina). Sequencing data were mapped and analyzed by the University of Pennsylvania Next Generation Sequencing Core. The GEO accession number for sequencing data is GSE118863.
Cell transfection assays
The cDNA of Nupr1 was amplified from mouse pituitary cDNA. cDNAs for the other studied transcription factors were obtained from OriGene or Addgene and subcloned into an internal ribosome entry site (IRES) GFP vector (Addgene plasmid no. 51406). The expression of the cDNA and GFP in this vector is controlled by the cytomegalovirus promoter. Pit-1/Triple and Pit-1/0 cells were transfected with 10 μg of plasmid using the TransIT-293 transfection reagent (Mirus Bio). In cotransfection assays, cells were transfected with 5 µg of each plasmid to maintain the DNA/TransIT-293 ratio as recommended by the manufacturer. Two days after transfection, GFP+ cells were sorted using the method and platform described previously. RNA was isolated from the GFP+ cells as described previously, and cDNA was generated using the SuperScript III reverse transcription kit (Life Technologies). Quantitative reverse transcription PCR (qRT-PCR) was performed using the Fast SYBR Green Master Mix kit (Applied Biosystems) using a QuantStudio 7 Flex (Applied Biosystems) platform. qRT-PCR assays were done in biological triplicate, and all samples were normalized to the housekeeping gene Gapdh and compared with cells transfected with an empty vector by the ΔΔCt method (27). All transfections were performed using ∼1.6 million cells per sample, grown to 80% confluence in 10-cm plates. Amplification primer sequences are available upon request.
Immunofluorescence microscopy
Pituitaries were disaggregated and prepared using the same process as noted previously for FACS. The immunofluorescent staining was performed as previously described (28). Briefly, the disaggregated cells were placed on poly-l-lysine–coated slides and incubated at room temperature for 10 minutes to allow the cells to adhere to the slide. After attaching to the slides, cells were fixed in 4% formaldehyde for 10 minutes, then washed three times with PBS for 5 minutes per wash. Cells were permeabilized in a solution of 0.5% Triton X-100 and 0.5% saponin for 10 minutes. The cells were washed with PBS before being incubated in a blocking buffer of 4× SSC, 2% BSA, 0.1% Tween 20, and 15% donkey serum for 20 minutes at room temperature. After blocking, slides were incubated with antibodies specific to proteins of interest for 1 hour at room temperature, washed three times in PBS, and then incubated for one 1 at room temperature with secondary antibodies conjugated to fluorophores for detection. After incubation with secondary antibodies, the slides were washed a final time, incubated for 1 minute in 1 mg/mL DAPI, and coverslips were attached. Slides were imaged with a Leica SP8 confocal microscope platform. Images were analyzed using Fiji software (29). Antibodies used for each transcription factor are as follows: NUPR1 [Thermo-Fisher PA5-65826, RRID: AB_2662159 (30)], RXRG [Santa Cruz SC-514134, RRID: AB_2737293 (31)], TBX19 [Santa Cruz SC-22656, RRID: AB_2200381 (32)], PPARG [Abcam 55296, RRID: AB_944767 (33)], NR4A2 [Abcam ab41917, RRID: AB_776887 (34)], and POU4F1 [Abcam ab81213, RRID: AB_1640222 (35)].
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) assays were performed as previously described (36). Chromatin was isolated from 200,000 FACS-sorted transfected cells per sample and chromatin from 100,000 cells was used per ChIP reaction. For the purposes of performing ChIP assays, Pit-1/0 cells were transfected with 20 µg of plasmid DNA using Lipofectamine 3000 (Thermo Scientific) per the manufacturer’s directions to achieve higher transfection efficiency. Antibodies specific for NR4A2 [Abcam; ab41917; RRID: AB_776887 (34)] and POU4F1 [Abcam; ab81213; RRID: AB_1640222 (35)] were used in the respective assays. H3K27-acetylated ChIP was performed using antibody ab4279 from Abcam [RRID: AB_2118291 (37)]. A rabbit antibody specific for POU1F1 was generated by Alpha Diagnostics [RRID: AB_2732812 (38)] using the antigen sequence: PLLAEDPAASEFKQELRRKSKL. This affinity-purified antibody was demonstrated to be specific for POU1F1 by Western blot analysis (39). Normal rabbit IgG (Millipore) was used as a control in all ChIP assays. Input and bound DNAs were amplified by qRT-PCR using the same QuantStudio 7 Flex platform noted previously. A serial dilution of the input chromatin was used to determine the linear range of the amplification, and the enrichment of the bound DNA was calculated as a percentage of the input. ChIP of primary mouse pituitary cells was performed using the same protocol, with cells being collected by FACS as noted previously prior to isolation of chromatin. Amplification primer sequences are available upon request.
Results
The transcriptomes of the somatotropes and lactotropes are concordant and reveal selective enrichments of a limited number of transcription factors
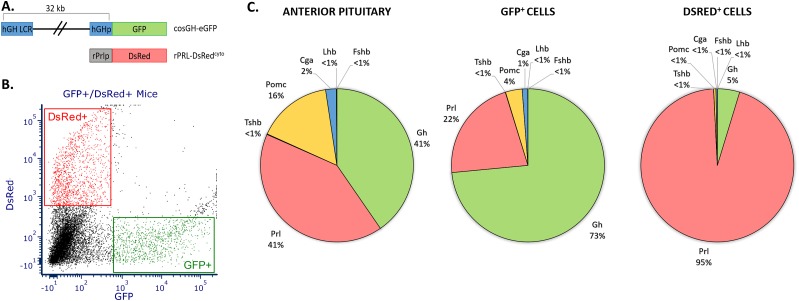
The goal of this study was to identify novel transcription factors involved in the maintenance of cell identity in the terminally differentiated somatotropes and lactotropes. This goal was approached by marking primary cell populations in the adult mouse pituitary with transgene-encoded fluorescent proteins. Somatotrope cells were tagged with a GFP reporter under the control of the human growth hormone promoter and its upstream locus control region (40), whereas lactotrope cells were tagged by a DsRed reporter regulated by the rat Prl promoter and upstream sequences (Fig. 1A). These regulatory elements have been previously demonstrated as sufficient to define somatotrope- and lactotrope-specific expression in mice (25, 26), and the somatotrope specificity of the Gh-GFP line was further validated by immunofluorescence microscopy (39). Virgin female compound transgenic mice (6 to 8 weeks old) carrying the Gh-GFP and Prl-DsRed transgenes were generated for analysis. The use of compound transgenic mice permits the isolation of somatotropes and lactotropes from the same mouse pituitaries simultaneously, allowing the direct comparison of somatotropes and lactotropes with a minimization of experimental variables. The use of females ensured appreciable numbers of lactotropes for subsequent analysis, and the mice were aged to 6 to 8 weeks to allow for full maturation of the pituitary and the resident cell lineages (41, 42). Five to six pituitaries were collected for each RNA-seq analysis, and these pituitaries were pooled and FACS sorted into GFP+ and DsRed+ populations (Fig. 1B).
Figure 1.
FACS of primary somatotropes and lactotropes from the mouse pituitary. (A) Schematic of the two transgenes used for selective isolation of somatotropes and lactotropes. (B) Representative FACS of pituitary cells harvested from cosGH-eGFP/rPrl-DsRedcyto compound transgenic mice. The x-axis represents GFP expression and the y-axis represents DsRed expression. Gating used for sorting is represented by rectangles encompassing the sorted populations. (C) Relative expression levels of mRNAs encoding the full array of anterior pituitary hormones in the whole pituitary and from the two FACS populations. Normalized reads (fragments per kilobase of transcript per million mapped reads) are shown for the anterior pituitary hormone genes in total pituitary (left), FACS-sorted GFP+ cells (middle), and FACS-sorted DsRed+ cells (right). All samples used for transcriptomic analyses were generated from 6- to 8-wk-old virgin female mice.
The study was carried out in biologic triplicate, beginning each independent study with a fresh set of compound transgenic mice. The RNA-seq data from the three sets of analyses were then pooled and the composite transcriptome data set was used to establish the RNA profiles for the GFP and DsRed cell sorts. The use of a composite transcriptomic data set allowed for statistical analyses that aided the process of selecting genes of interest from the data set based on the robustness of their enrichment in each of the replicate samples. RNA was extracted from each of the two flow-sorted cell populations for transcriptome analysis (see “Materials and Methods”). The enrichment of somatotropes or lactotropes in the flow-sorted cell populations was confirmed by comparing the relative levels of anterior pituitary hormone gene expression in the GFP+ and DsRed+ populations to the expression of these hormone genes in total pituitaries from virgin female, age-matched controls (Fig. 1C). These analyses included all hormone-encoding genes of the anterior pituitary, including proopiomelanocortin (Pomc), the β subunits for TSH (Tshb), LH (Lhb), and FSH (Fshb), as well as the shared heterodimerizing α subunit (Cga). Combined with Gh and Prl, these genes represent the full set of anterior pituitary hormones and serve as markers for each of the cell lineages of the anterior pituitary. Gh expression was enriched in the GFP+ samples whereas the expression of all other hormone genes was depleted as compared with total pituitary. Similarly, Prl expression was enriched in DsRed+ samples over the Prl expression observed in total pituitary. These data demonstrate that the described FACS procedure yields cell pools that are highly enriched for the somatotrope and lactotrope cell populations.
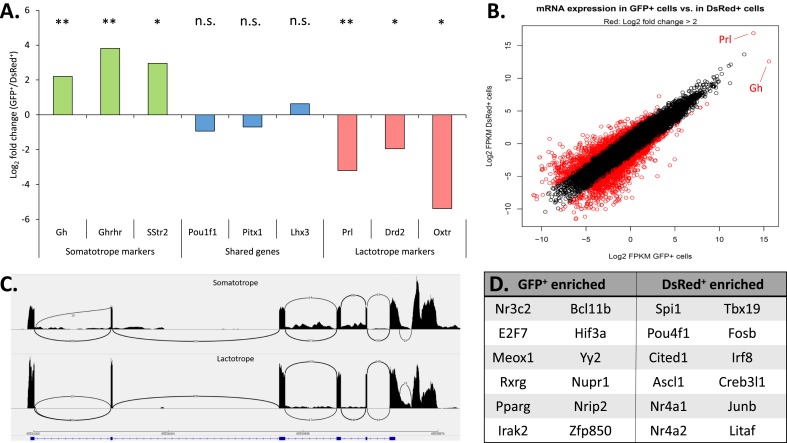
The two flow-sorted populations were further assessed for their relative expression of sets of well-characterized marker genes that define the somatotrope and lactotrope lineages. A plot of three known markers for each cell type as well as five genes expected to be expressed at similar levels in both cell types (Fig. 2A) revealed that each of the somatotrope and lactotrope marker genes was appropriately enriched in its respective GFP+ or DsRed+ cell pool whereas genes known to be shared in the somatotrope and lactotrope lineages were expressed at approximately equal levels in both populations. GFP and DsRed reads were also appropriately enriched in each population, confirming proper FACS sorting by the fluorescent reporter (39). Taken together, these data indicate that the transcriptomic data set is consistent with the assignment of somatotrope and lactotrope cell identities to the GFP+- and DsRed+-labeled populations, respectively (6, 43–47).
Figure 2.
RNA-seq analysis of flow-sorted GFP+ and DsRed+ cell populations. (A) Enrichment (log2 fold change) of mRNAs in the GFP+ and DsRed+ populations encoding defined markers for somatotropes (green), lactotropes (red), and for markers shared between somatotropes and lactotropes (blue). The designation of each respective marker gene is noted at the bottom of the figure. A positive value on the y-axis indicates enrichment in the GFP+ population whereas a negative value indicates enrichment in the DsRed+ population. Significance values relate to the enrichment in mRNA expression between the two flow sorted populations. *P < 0.05; **P < 0.01. (B) Correlation plot of the RNA-seq analysis of GFP+ cells vs DsRed+ cells. The log2 fragments per kilobase of transcript per million mapped reads values for all mRNAs in the GFP+ (x-axis) and DsRed+ (y-axis) samples were plotted, and mRNAs surpassing a threshold of fourfold enrichment or greater are highlighted in red. These enriched mRNAs were then further filtered to remove low expressers, leaving only genes that were significantly enriched (P ≤ 0.05). The positions of Gh and Prl are noted in this plot. (C) Representative Sashimi plot track showing raw mapped mRNA reads at the Pou1f1 locus in GFP+ and DsRed+ cell populations. The identity of the two Sashimi plots indicates identical splicing patterns of the Pou1f1 transcript in somatotropes and lactotropes. (D) List of transcription factors enriched in GFP+ vs DsRed+ cell populations were identified by taking the GFP/DsRed specific genes identified by differential expression analysis and using PANTHER to identify genes with known gene ontology terms for transcription factor activity. n.s., not significant.
Comparison of the transcriptomes of the flow-sorted GFP+ and DsRed+ cell pools reveals a striking level of concordance in gene expression (Fig. 2B). The overall Pearson correlation value for somatotropes vs lactotropes was calculated as 0.42. However, the expression of Gh and Prl in these cells is so robust (note their extreme outlier positions on a log2 scale in Fig. 2B) that these two transcripts markedly skew the Pearson r value and distort the overall transcriptomic comparison. Removing Gh and Prl from the correlation calculations yields a Pearson r value of 0.94. The significance of these values is twofold. First, the change from 0.42 to 0.94 observed in the Pearson value when Gh and Prl are excluded demonstrates the dominance of these two transcripts in the transcriptomes of the somatotropes and lactotropes, with Gh and Prl reads totaling ∼25% to 33% of the mRNA reads in both cell types. Second, the Pearson value of 0.94 after exclusion of Gh and Prl highlights the overall similarity of the transcriptomes of these two cell types, except for their hormone expression levels. This overall similarity at the transcriptomic level is consistent with a close developmental relationship of these two cell types and suggests that the somatotrope and lactotrope identities are likely to be driven and maintained by a limited number of lineage-enriched factors (6, 48, 49). These transcriptomic data further demonstrate that the structure of the Pou1f1 mRNA is identical in the two pools, ruling out a contribution by alternative splicing of the Pou1f1 transcript (50) to cell type–specific functions (Fig. 2C). Genes that surpassed a threshold log2 fold change of 2 (Fig. 2B, highlighted in red) were then further filtered by significance (P < 0.05) and expression level to remove genes that are expressed at low levels and not consistently enriched across all replicates. This differential expression analysis yielded ∼300 lineage-enriched genes for each cell type. Using gene ontology terms to guide the filtering of these 300 lineage-enriched genes produced a list of differentially expressed genes with annotated transcription factor activity (Fig. 2D). Thus, these transcriptomic analyses identified a set of lineage-enriched transcription factors that may be important to the distinct attributes of the somatotrope and lactotrope lineages.
Validation of transcription factor differential expression in somatotropes and lactotropes by immunofluorescence analysis of primary pituitary cells
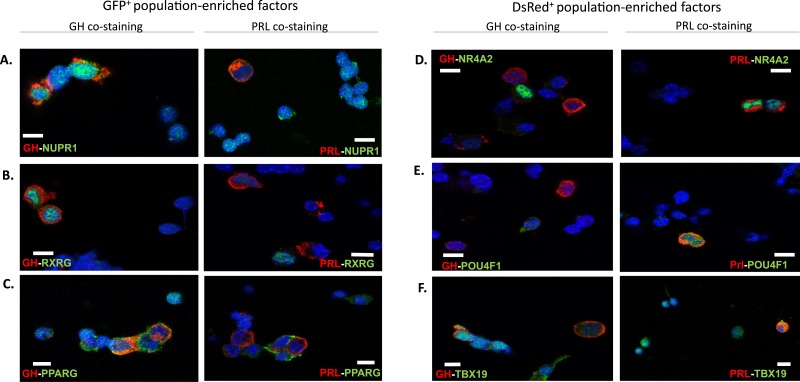
To maximize the effectiveness of downstream studies on the identified lineage-enriched transcription factors, we prioritized for further study a subset of six lineage-enriched transcription factors—three enriched in somatotropes and three enriched in lactotropes—that have reported functions consistent with a role that could be related to pituitary gene regulation. We next attempted to validate their respective cell type specificities at the level of protein expression. Wild-type adult female mouse pituitaries were disaggregated and assessed by immunofluorescence microscopy for each of the six factors in parallel with immunostaining for GH and PRL proteins. NUPR1, a somatotrope-enriched factor chosen based on its role in a defined pituitary cell differentiation process centered on the gonadotrope lineage (23, 51), was enriched in the nuclei of somatotrope (GH+) cells, consistent with the enrichment of the respective mRNA in the GFP+ cells (Fig. 3A). Of note, NUPR1, although enriched in somatotropes vs lactotropes, was also detected in additional pituitary cells that are both GH− and PRL−. This observation is consistent with reports that NUPR1 plays a role in the development of GH−/PRL− cells such as gonadotropes (51). A second somatotrope-enriched factor, RXRG, was selected for study owing to the stimulating effects of its ligand, retinoic acid, on GH production, as well as on its reported heterodimerization with the thyroid hormone receptor, which is known to bind at the Gh promoter (52–55). RXRG protein was also observed to be enriched in the nuclei of somatotrope cells, identifying it as a gene of interest for further study (Fig. 3B). The third somatotrope candidate factor, PPARG, has been shown in previous studies to be frequently expressed in GH-secreting pituitary adenomas, suggesting a role in regulating the somatotrope lineage (56). Immunofluorescence microscopy of PPARG protein revealed that it was enriched in somatotropes as expected (Fig. 3C), but it was also present in 48% of lactotropes (detailed cell counts for all immunofluorescence studies are available in Table 1). Therefore, although Pparg may have a role in the regulation of these lineages, we excluded Pparg from further study and focused on Nupr1 and Rxrg owing to their robust somatotrope enrichment.
Figure 3.
Immunofluorescence microscopy of selected transcription factors found to be enriched in either the GFP+ or the DsRed+ cell populations. (A–F) Cells disaggregated from wild-type pituitaries (female) were stained for each of six transcription factors whose mRNAs were enriched in (A–C) the GFP+ cell population and (D–F) the DsRed+ cell population (as in Fig. 2D). Each transcription factor is stained in green, and cells were costained red with antibodies to identify GH or PRL as a means of identifying somatotropes and lactotropes, respectively. (A) NUPR1. (B) RXRG. (C) PPARG. (D) NR4A2. (E) POU4F1. (F) TBX19. All cells were stained with DAPI (blue) to mark nuclei. Scale bars, 5 μm.
Table 1.
Cell Counts for Immunofluorescence Assays
| Transcription Factor | Number of Positive Somatotropes (% of All Somatotropes) | Number of Positive Lactotropes (% of All Lactotropes) |
|---|---|---|
| Nup1 | 176 (88) | 18 (9) |
| Rxrg | 183 (91.5) | 11 (5.5) |
| Pparg | 148 (74) | 96 (48) |
| Nr4a2 | 4 (2) | 186 (93) |
| Pou4f1 | 9 (4.5) | 193 (96.5) |
| Tbx19 | 174 (87) | 188 (94) |
Cell counts for immunofluorescence microscopy analysis of selected transcription factors. Columns list the number of GH+ or PRL+ cells examined (200 GH+ and 200 PRL+ cells counted per study) expressing the given transcription factor, as well as the percentage of total somatotropes/lactotropes observed expressing the protein in parentheses.
Among the candidate transcription factors selected from the lactotrope cell population, NR4A2 has been demonstrated to induce transcription of the PRL gene in extrapituitary tissue, making it a gene of interest for its potential role in the regulation of the lactotrope lineage (57). The NR4A2 protein was enriched in the nuclei of lactotrope (PRL+) cells (Fig. 3D), consistent with transcriptomic data. POU4F1, a member of the POU family of transcription factors, was selected for study owing to its role in stimulating aggressive proliferation of pituitary adenomas (58), and on its multiple regulatory roles in development throughout the neuroendocrine system (59, 60). We observed that the POU4F1 protein was enriched in the nuclei of lactotrope cells in mouse pituitary, suggesting a potential for regulating the lactotrope lineage (Fig. 3E). TBX19 was selected for this study because it is known to be crucial for pituitary development and establishment of cellular differentiation in the anterior lobe and intermediate lobe in pituitary, with its primary known function being in regulating the corticotrope lineage where it has been reported to be specifically expressed (61–63). Immunofluorescence microscopy of the remaining factor, TBX19, revealed expression in both somatotropes and lactotropes, as well as in additional cell types that were not further characterized (Fig. 3F). Thus, although Tbx19 may be of interest to the regulation of lactotropes, we chose to limit our current studies to Nr4a2, Rxrg, Pou4f1, and Nupr1 owing to their robust cell type enrichment and their restriction to the nuclei of cells, observations consistent with their identification as lineage-enriched transcription factors in our transcriptomic analyses. In conclusion, the immunofluorescence microscopy studies of these four transcription factors validated the RNA-seq data generated from primary somatotropes and lactotropes, and suggested that they may play a role in somatotrope vs lactotrope differentiation and/or specification of cellular function.
Functional assessment of candidate transcription factor impact on expression of landmark somatotrope and lactotrope genes
To determine the functional impact of the four candidate factors on somatotrope vs lactotrope lineage identity, we expressed each factor in a murine pituitary-derived cell line, Pit-1/Triple (5). Pit-1/Triple cells, isolated by T-antigen immortalization of mouse pituitary cells, express POU1F1 [also referred to as PIT-1 (64)] as well as the three POU1F1 dependent hormones: GH, PRL, and TSH. The Pit1/Triple cells express robust levels of Pou1f1 and express each of the Gh, Prl, and Tshb mRNAs at levels substantially lower than those detected in whole adult mouse pituitary (26). These Pit-1/Triple cells appear to represent cells at an early stage in pituitary development, immediately following the activation of Pou1f1 but prior to terminal differentiation of the individual hormone-expressing lineages (5). Transcriptomic analyses of the Pit-1/Triple cells revealed that each of the four candidate factors are either not expressed or expressed at low levels. We hypothesized that forced expression of each of the factors would have a specific impact on genes that in some manner define lactotrope or somatotrope differentiation. To test this hypothesis, each candidate factor was ectopically expressed in Pit-1/Triple cells from a bicistronic expression vector containing the transcription factor cDNA and a GFP cDNA behind an IRES (see details in “Materials and Methods”). Forty-eight hours after transfection, GFP+ Pit-1/Triple cells were isolated by FACS. RNA was isolated and assayed by qRT-PCR to assess impacts on the expression of somatotrope and lactotrope marker genes. The expression of each target gene was compared with the corresponding mRNA levels in GFP+ cells isolated from a control transfection with an empty (GFP-only) vector.
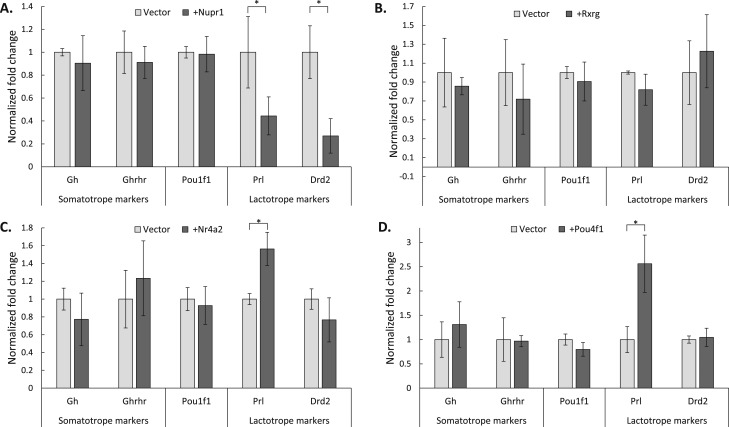
A panel of five lineage marker genes was assayed in each qRT-PCR assay. Gh and the GH-releasing hormone receptor (Ghrhr) were assayed as markers of the somatotrope lineage whereas Prl and the dopamine receptor (Drd2) were assayed as markers of the lactotrope lineage. Pou1f1 was included in the panel of assayed genes as a gene that is common to both lineages. This panel of genes, although not a complete set of somatotrope/lactotrope markers, includes the most prominent known markers and serves as a useful metric for assaying changes in the expression of genes crucial to these two lineages. Expression of the somatotrope candidate factor, Nupr1, failed to impact expression of Gh or Ghrhr, but it did cause a significant repression of both Prl and Drd2 (Fig. 4A). This impact of Nupr1 is consistent with a role in selectively repressing the expression of lactotrope genes in the somatotrope lineage. Expression of Rxrg did not cause a significant change in the expression of any of the assayed genes, even in the presence of its ligand, retinoic acid (39, 65, 66), and so Rxrg was excluded from further study (Fig. 4B). Expression of the lactotrope candidate factors, Nr4a2 and Pou4f1, both resulted in a significant increase in Prl expression and no significant change in the other assayed genes (Fig. 4C and 4D). Collectively, the data from these cell transfection studies support a role for the candidate factors in either activating lactotrope-specific genes linked to lactotrope identity (Nr4a2 and Pou4f1), or repressing lactotrope-specific genes to maintain the somatotrope identity (Nupr1).
Figure 4.
Impact of lineage-enriched transcription factors on expression of markers genes in Pit-1/Triple cells. Pit-1/Triple cells (5) were transfected with expression constructs encoding each noted transcription factor linked by an IRES to a GFP reporter open reading frame (see “Materials and Methods”). Transfected cells were isolated by GFP FACS and their RNA content was assayed for specific marker gene expression by qRT-PCR. (A) Nupr1. (B) Rxrg. (C) Nr4a2. (D) Pou4f1. In all assays, expression of the recombinant transcription factor was confirmed by targeted qRT-PCR (data not shown). Gene expression was normalized to an internal Gapdh control, and the fold change over empty vector controls was calculated using the ΔΔCt method (see “Materials and Methods”). Gapdh expression levels were stable across all assayed conditions, with no significant changes in Gapdh expression in any of the transfected samples. Error bars indicate 1 SD. n = 5 for all experiments. *P < 0.05, by t test.
Characterization of transcriptional regulatory mechanisms of the lineage-enriched transcription factors
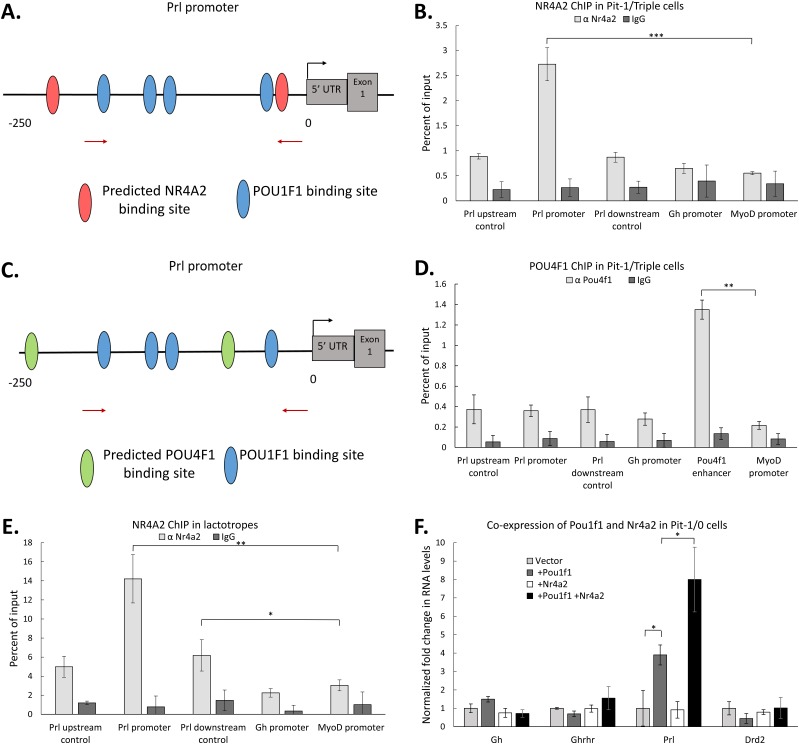
The mechanism of action by which a transcription factor affects the expression of a gene can be direct or indirect. One indication of direct action is the occupancy of the factor on regulatory elements linked to the gene of interest. Nr4a2 and Pou4f1 both enhance Prl expression when expressed in Pit-1/Triple cells (Fig. 4C and 4D). Two binding sites for both NR4A2 and POU4F1 are predicted within a 250-bp region of the Prl promoter by JASPAR analysis (67), and these sites are intermingled with four known POU1F1 binding sites (15, 67). Of particular interest was the observation that one of the NR4A2 predicted binding sites is located immediately adjacent to the POU1F1 binding site most proximal to the Prl start codon (Fig. 5A). Pit-1/Triple cells expressing recombinant NR4A2 or POU4F1 were generated and enriched as noted previously, and chromatin was assayed for factor occupancy at the Prl promoter. The MyoD promoter was assayed as a control for nonspecific binding, and the Gh promoter was assayed as a specificity control. Additional sites 500 bp upstream and downstream of the Prl promoter were also assayed to confirm that any binding at the promoter was promoter specific. The NR4A2 ChIP revealed significant and specific binding at the Prl promoter (Fig. 5B), whereas a parallel ChIP assay for POU4F1 failed to reveal evidence of significant binding in this region (Fig. 5C and 5D). To confirm that NR4A2 binds to the Prl promoter in vivo, NR4A2 ChIP was performed on chromatin isolated from FACS-sorted mouse lactotropes (Fig. 5E). The data from the analysis of these primary mouse cells demonstrated selective in vivo binding of NR4A2 at the Prl promoter. Thus, these ChIP studies reveal that NR4A2 is recruited to the Prl promoter and enhances Prl expression. In contrast, POU4F1 does not bind the Prl promoter and thus enhances Prl expression either by binding to an uncharacterized regulatory element beyond the proximal Prl promoter, or through an indirect mechanism.
Figure 5.
NR4A2 acts in conjunction with POU1F1 at the Prl promoter to enhance Prl expression. (A) Schematic of the mPrl gene promoter indicating the relative positions of known POU1F1 binding sites [blue ovals (15)] as well as predicted binding sites for NR4A2 (red ovals) (identified by JASPAR). (B) NR4A2 ChIP. Pit-1/Triple cells were transfected with an expression vector encoding NR4A2, and chromatin isolated from GFP+ cells (as in Fig. 4) was assayed by NR4A2 ChIP. Levels of transcription factor occupancy at specific sites within the Prl promoter were quantified by qRT-PCR of immunoprecipitated samples. Parallel controls included quantitation of occupancy at the Gh promoter and assessment of binding in regions 500 bp upstream and downstream of the Prl promoter. The MyoD promoter served as a negative control in all studies. (C) Schematic of the mPrl gene promoter indicating the relative positioning of known POU1F1 binding sites (blue ovals) as well as predicted binding sites for POU4F1 (green ovals) (identified by JASPAR). (D) POU4F1 ChIP. Pit-1/Triple cells were transfected with an expression vector encoding POU4F1 and chromatin isolated from GFP+ cells [as in (B)] was assayed by ChIP. The Pou4f1 enhancer was assayed in this study as a positive control for the POU4F1 ChIP. (E) Nr4a2 ChIP performed in FACS-sorted mouse lactotropes. (F) Impact of NR4A2 on expression of markers genes in Pit-1/0 cells. Pit-1/0 cells were transfected with expression vectors encoding Pou1f1, Nr4a2, or both linked by IRES to GFP, and GFP+ cells were collected by FACS. qRT-PCR analysis was performed to measure changes in mRNA expression of somatotrope and lactotrope marker genes. n = 3 for all experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
The lactotrope-enriched transcription factor, NR4A2, acts in conjunction with POU1F1 to enhance Prl gene expression
The binding of NR4A2 adjacent to a POU1F1 binding site within the Prl promoter suggested a model in which NR4A2 works in concert with POU1F1 to support Prl gene expression. This model was tested by transfections of the two factors either alone or in combination into the Pit-1/0 cell line (5). Pit-1/0 cells, generated by SV40 T-antigen–mediated transformation of mouse pituitary cells (as with the Pit1/Triple cells), represent an early stage of anterior pituitary development in which Pou1f1 is not yet expressed at significant levels, and in which none of the three POU1F1-dependent hormone genes (Gh, Prl, and Tshb) has yet been activated (5). Pit-1/0 cells were transfected with plasmids encoding either Pou1f1 or Nr4a2 alone, or with a 1:1 mix of the two expression vectors. Cells transfected with the Pou1f1 expression vector alone exhibited a fourfold increase in Prl expression over a vector control (Fig. 5F). Whereas a parallel transfection with Nr4a2 plasmid alone displayed no activation of Prl expression, cotransfection of Nr4a2 plasmid with the Pou1f1 plasmid stimulated Prl expression eightfold over the vector control. There was no apparent impact of the factor transfections, either alone or in combination, on the other marker genes in the assay panel. These data suggest that NR4A2 synergizes with POU1F1 to enhance the expression of the Prl gene.
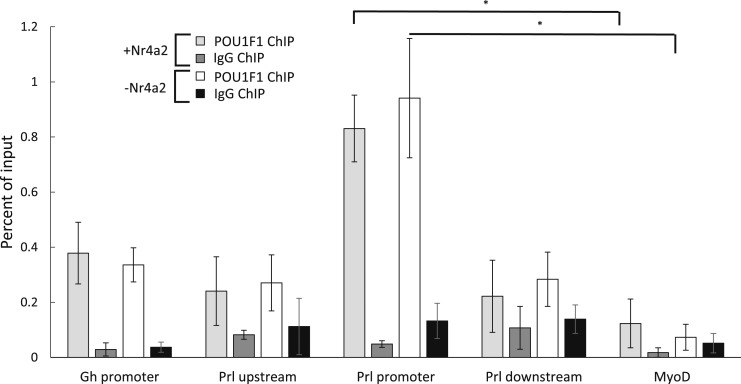
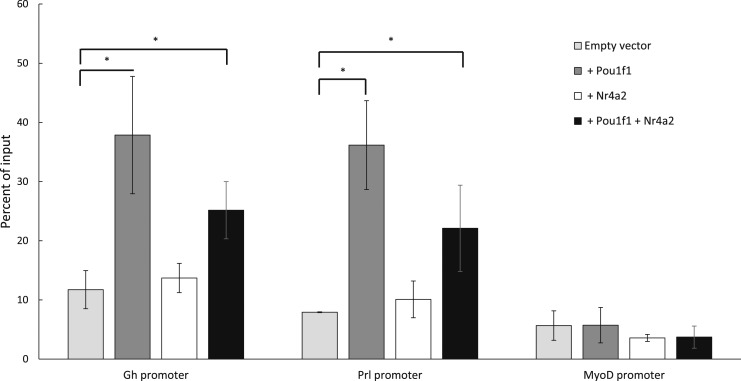
One potential mechanism by which NR4A2 may work with POU1F1 to enhance Prl expression is by facilitating recruitment of POU1F1 to the Prl promoter. To test this hypothesis, POU1F1 occupancy was assayed by ChIP in Pit-1/Triple cells expressing exogenous NR4A2 and compared with POU1F1 occupancy in Pit-1/Triple cells not expressing NR4A2 (Fig. 6). This experiment revealed no changes in POU1F1 occupancy at the Prl promoter in the presence of NR4A2. Thus, the functional interaction of NR4A2 with POU1F1 at the Prl promoter does not rely on increasing POU1F1 recruitment to the Prl promoter. Having observed that NR4A2 binds the Prl promoter adjacent to a known POU1F1 binding site and that NR4A2 does not enhance the binding of POU1F1, we next hypothesized that NR4A2 may play a role in histone modifications at the Prl promoter prior to transcription. Histone acetylation constitutes an early step toward initiating transcription (68, 69), and H3K27 acetylation has been identified as a mark that strongly correlates with active transcription (70, 71). To test our hypothesis, we assayed changes in H3K27 acetylation at both the Gh and Prl promoters in Pit-1/0 cells transfected with Pou1f1 or Nr4a2 alone, or transfected with a 1:1 mix of both factors. Pit-1/0 cells were used in this assay because the Gh, Prl, and Pou1f1 genes are inactive, making these cells a useful model for the process of transcriptional activation and histone acetylation. Transfection with Pou1f1 alone caused a significant increase in H3K27 acetylation at both the Gh and Prl promoters, consistent with the known roles of POU1F1 in activating both of these genes (Fig. 7). In contrast, transfection with Nr4a2 alone did not cause an increase in H3K27 acetylation over an empty vector control, and cells transfected with both Pou1f1 and Nr4a2 had levels of H3K27 acetylation similar to those of cells transfected with Pou1f1 alone. These data indicate that POU1F1 is sufficient for H3K27 acetylation at the Gh and Prl promoters and that NR4A2 enhances Prl expression via a mechanism unrelated to H3K27 acetylation. Taken together, these data led us to conclude that the lactotrope-enriched factor, NR4A2, binds adjacent to POU1F1 at the Prl promoter and synergizes with POU1F1 to enhance Prl gene expression.
Figure 6.
ChIP of POU1F1 in Pit-1/Triple cells expressing Nr4a2 vs Pit-1/Triple cells that do not express Nr4a2. Pit-1/Triple cells, which do not express Nr4a2, were transfected with the same Nr4a2-IRES-GFP plasmid used in assays presented in Fig. 4C, and control cells were transfected with the empty IRES-GFP vector. GFP+ cells were sorted by FACS, chromatin was isolated, and ChIP was performed using an antibody that recognizes POU1F1. Following immunoprecipitation, qRT-PCR was used to measure the relative levels of POU1F1 binding in Pit-1/Triple cells that had been transfected with Nr4a2 plasmid (+Nr4a2), as well as those transfected with empty vector. Immunoprecipitations using IgG served as a negative control. *P < 0.05.
Figure 7.
Immunoprecipitation of H3K27-acetylated (H3K27ac) chromatin in Pit-1/0 cells expressing Nr4a2 and/or Pou1f1. Pit-1/0 cells, which express neither Nr4a2 nor significant levels of Pou1f1, were transfected with Nr4a2-IRES-GFP, Pou1f1-IRES-GFP, or a 1:1 mixture of both expression vectors, and control cells were transfected with an empty IRES-GFP vector. Cells transfected with the 1:1 mixture of the Pou1f1 and Nr4a2 expression vectors express lower levels of each protein due to the concentration of each expression vector being halved during transfection to keep the total amount of DNA constant in each transfection. GFP+ cells were isolated by FACS and chromatin was isolated. ChIP was performed using an antibody that recognizes H3K27ac. Following ChIP, qRT-PCR was performed to assay the levels of H327 acetylation at the Gh promoter, the Prl promoter, and the MyoD promoter as a negative control. IgG controls (data not shown) were also included in this assay for all samples. In all cases, ChIP performed with IgG yielded <1% of input. *P < 0.05.
Discussion
The establishment and maintenance of cell lineages within the anterior pituitary represents a robust model of cell type specification (72, 73). In addition to providing insight into general mechanisms of cell fate maintenance that are likely to be of interest in systems beyond the pituitary, delineating the controls of these pathways is critical to understanding normal and pathologic pathways of pituitary functions as they relate to mammalian growth, reproduction, stress response, and metabolic functions. Identification of these pathways and their driving factors has the potential to provide novel targets for diagnosis and therapy of abnormalities in hormone expression and function. As an illustration of the potential benefits of improving understanding of these lineages, most PRL-secreting adenomas (prolactinomas) can be treated medically rather than surgically by administration of dopamine agonists, which bind to the DRD2 receptor and selectively inhibit lactotrope proliferation and PRL expression (74). Although this medical approach is widely employed in clinical practice, a subset of prolactinomas does not respond to such therapy, necessitating more invasive treatment due to the lack of known treatment targets beyond the dopamine receptor. This gap in therapeutic efficacy highlights the unmet need for better understanding these lineages and their underlying control pathways (75, 76).
Extensive genetic studies in humans and mice have identified a number of transcription factors that are essential for pituitary development and function in the mammalian embryo. However, the identification and functional characterizations of factors that drive specification of the two primary lineages in the anterior pituitary, the somatotropes and lactotropes, remain remarkably limited (5, 22). According to current models, these lineages are hypothesized to diverge from a common “somatolactotrope” precursor late in embryogenesis (6), although there is some evidence that a subset of lactotropes emerges directly from somatotrope cells (14). What is clear is that the pathways of cell expansion and terminal differentiation that generate and maintain both somatotropes and lactotropes are dependent on the functions of the pituitary master regulatory transcription factor, POU1F1. Although both of these lineages depend on POU1F1 for their expansion and hormonal expression, it is evident that additional factors must be involved in driving their functional divergence. The identification of these factors has been hampered in most studies owing to their focus on the limited number of known marker proteins or their encoding mRNAs. Thus, substantial advances will depend on the use of more broad-based discovery approaches.
In the present study, we have established and compared the transcriptomes of the somatotrope and lactotrope lineages isolated from the adult mouse pituitary. These transcriptomic databases were derived by RNA-seq analysis of cell populations isolated on the basis of fluorescent markers (GFP and DsRed) driven by the Gh or the Prl transcription regulatory elements. The validity of this approach was supported by a high level of enrichment of the expected hormone mRNAs in each RNA-seq data set (Fig. 1C) and by the enrichment of other known marker genes (Fig. 2A). Analysis of these data sets allowed us to arrive at several fundamental observations. First, the direct comparison of the transcriptomes of the two cell types, after removing Gh and Prl from the analysis, revealed a striking overall correlation (Pearson r = 0.94; Fig. 2B). This high level of concordance affirms the close developmental and functional relationships of these two lineages. The second observation is that the two cell populations partially overlap in the expression of their key hormone genes, Gh and Prl. Although we find that Gh mRNA is appropriately enriched in the GFP+ cell pool and Prl mRNA is appropriately enriched in the DsRed+ cell pool (Fig. 2A), there are appreciable levels of Gh mRNA in the lactotrope (DsRed+) population and a reciprocal representation of Prl mRNA in the somatotrope (GFP+) population (Fig. 1C). Although it is likely that small amounts of cross-contamination inherent in techniques such as FACS contribute in part to this result, these data are consistent with the established observations that both genes are activated by Pou1f1, which is expressed in both somatotropes and lactotropes, raising the possibility of a basal level of transcription of the reciprocal hormone in each lineage. This observation that a detectable amount of Gh and Prl mRNAs are produced in the reciprocal lineages supports a model in which somatotropes and lactotropes repress but do not entirely silence the Prl and Gh genes, respectively. The third observation is that, despite the overall similarities in the transcriptomes of the somatotropes and lactotropes noted previously, a set of ∼300 genes, including multiple transcription factors, is significantly enriched in each lineage. Taken together, these observations suggest that the transcriptomic differences between the somatotrope and lactotrope lineages are quite limited, yet clearly crucial; differential expression of a small set of genes is able to drive noteworthy differences in cell identity and function.
The transcriptome data generated in the current study provide insights into aspects of the somatotrope and lactotrope lineages beyond lineage-enriched transcription factors. Although the scope of the current study centers on these transcription factors, other aspects of the transcriptomes of the somatotropes and lactotropes may be of interest for future studies. One mechanism that has been hypothesized to contribute to differential regulation in the somatotrope and lactotrope lineages is alternative splicing of Pou1f1. There are two known isoforms of Pou1f1: the major isoform, known simply as Pou1f1, and the alternative isoform Pou1f1β, formed by alternative splicing extending the second exon, which has been reported previously to have a repressive function in regulating gene expression in the POU1F1-dependent lineages of the pituitary (50, 77, 78). Our data reveal that the levels of Pou1f1 mRNA and its exonic structure (Fig. 2C) are equivalent in the somatotrope and lactotrope populations. This observation supports the hypothesis that the divergence of lactotropes and somatotropes is based on the functions of a set of somatotrope- and lactotrope-specific factors that may work independently, or in conjunction with POU1F1, rather than differences in the levels or structure of POU1F1 itself.
Among the sets of differentially expressed genes, we identified a set of lineage-enriched transcription factors, most of which have not been previously linked to somatotrope or lactotrope identity and function (Fig. 2D). Six differentially expressed transcription factors were chosen for detailed study. This selection was based on reports of these six factors having mechanisms of action or functions that would be consistent with their suspected roles in the somatotrope and lactotrope lineages. In an initial screen, immunofluorescence analysis confirmed that four of these factors were robustly enriched in the nuclei of the cell type predicted by their differential mRNA expression (Fig. 3). The remaining two factors were not similarly enriched and were therefore eliminated from further study. The four factors with appropriate localization and enrichment by immunofluorescence analysis were subsequently assessed for functions relevant to lineage specification by expression of the recombinant proteins in the Pit-1/Triple cell line. Recombinant expression of the somatotrope-enriched factor, Nupr1, caused a decrease in the two most prominent lactotrope gene markers, Prl and Drd2. This action suggests that Nupr1 exerts a repressive action on lactotrope-specific genes in the somatotrope lineage (Fig. 4A). Notably, Nupr1−/− mice have been reported to be fertile and of normal size (51). The normal size of these Nupr1−/− mice is consistent with our observations that Nupr1 expression does not impact Gh levels. Based on the repressive effect of Nupr1 on Prl expression in the Pit-1/Triple cells, we would predict that Nupr1−/− mice will display elevated levels of Prl expression. The observation that Nupr1−/− mice are fertile suggests that the loss of Prl repression in somatotropes is not of an extent sufficient to result in fertility problems. Such conclusions must, however, await additional studies in vivo. Of further interest is our additional observation that NUPR1 is expressed in approximately half (54%) of somatolactotropes (39). These data suggest that NUPR1 alone is not sufficient to completely repress Prl expression. Taken together, our data and previous reports of Nupr1 activity suggest that Nupr1 does not impact Gh expression and that its repression of Prl in somatotropes is not total, indicating that there are likely other factors involved in repressing Prl expression in the somatotrope lineage, either additively or synergistically with Nupr1. Although the current study did not identify a corresponding transcription factor expressed in lactotropes that represses somatotrope genes, we hypothesize that such factors are likely to exist and future studies will aim to identify them.
Expression of the second somatotrope-enriched factor, Rxrg, in Pit-1/Triple cells did not significantly alter the expression of the assayed genes (Fig. 4B) even after the addition of retinoic acid, a known ligand of RXRG, to the tissue culture medium during transfection (39, 65). Although this result does not preclude a function for Rxrg in regulating the expression of other somatotrope genes beyond the markers assayed in this experimental model, Rxrg was excluded from further study to focus on transcription factors that impact the expression of the landmark somatotrope and lactotrope genes.
Both of the lactotrope-enriched factors, Pou4f1 or Nr4a2, were notable for a significant enhancement in Prl expression when expressed in Pit-1/Triple cells (Fig. 4C and 4D). The lack of a significant change in Drd2 expression when these factors were expressed suggested that their functions were targeted specifically toward expression of the Prl gene, rather than reflecting a broad effect on multiple genes in the lactotrope transcriptional program. Taken together, our transcription factor data reveal transcription factor actions that either enhance expression of a lineage-defining gene or repress expression of genes from a competing lineage. These observations further support a model in which these cells must actively repress the transcriptional program of the reciprocal lineage to maintain their cell identities and functions.
The function of the transcription factor NR4A2 was further explored by a combination of ChIP and cell transfection assays. NR4A2 ChIP analysis revealed direct binding to the Prl promoter in Pit-1/Triple cells (Fig. 5B). A parallel ChIP assay conducted using a primary cell pool enriched for lactotropes (Fig. 5E) confirmed that this same binding to the Prl promoter occurs in vivo. This suggests a mechanistic basis for NR4A2 activation of Prl gene expression via a direct interaction with the Prl promoter, and the concordance of the NR4A2 ChIP assays in both primary lactotropes and the Pit-1/Triple cell line supports the validity of using the Pit-1/Triple cell line as an experimental model. qRT-PCR assays performed on Pit-1/0 cells expressing Pou1f1, Nr4a2, or both factors together provided further insight into the mechanism of action for Nr4a2 (Fig. 5F). Expression of Pou1f1 activated Prl whereas the expression of Nr4a2 alone had no effect. However, coexpression of Nr4a2 with Pou1f1 resulted in a significant enhancement of Prl expression over that seen with Pou1f1 alone. These data suggest that NR4A2 is not sufficient to activate Prl expression on its own, but that it acts synergistically when POU1F1 is present at the Prl promoter to enhance Prl expression.
The positioning of an NR4A2 binding site in the Prl promoter adjacent to a functionally defined POU1F1 binding site suggests that its functions may involve enhancement of POU1F1 and occupancy. However, a ChIP analysis of POU1F1 in the presence and absence of exogenous NR4A2 failed to reveal a significant impact on POU1F1 occupancy at the Prl promoter (Fig. 6). Additionally, a ChIP analysis of the Gh and Prl promoters in Pit-1/0 cells transfected with Pou1f1, Nr4a2, or both factors revealed that POU1F1 establishes H3K27 acetylation whereas NR4A2 has no apparent effect on its own, nor does it enhance the modifications seen with Pou1f1 alone (Fig. 7). From these data, we conclude that NR4A2 does not enhance the binding of POU1F1 at the Prl promoter, and that POU1F1 is sufficient to establish histone acetylation at both the Gh and Prl promoters. Based on these observations we conclude that NR4A2 enhancement of Prl expression relates to a synergistic interaction between the two bound factors at the Prl promoter rather than reflecting enhancements of POU1F1 occupancy or a role in promoting histone H3K27 acetylation at the target promoter. Although the mechanism of action for Nr4a2 and other members of the Nr4a family remain poorly understood, reports from other systems indicate that this family of transcription factors is able to heterodimerize with a wide range of transcription factors and drive transcription (79–81), suggesting a possible mechanism for NR4A2 to heterodimerize with POU1F1 or with other, currently unknown transcription factors at the Prl promoter that will be of interest to future studies.
The observation of a novel cell type–enriched transcription factor binding to the promoter of the Prl gene and acting in conjunction with POU1F1 offers a potential explanation to the dilemma of Pou1f1 being expressed in both somatotropes and lactotropes, and being a known activator of Gh and Prl in experimental systems, yet selectively driving the production of one hormone protein per cell type. Although POU1F1 binding at the Gh and Prl promoters may be ubiquitous in somatotropes and lactotropes, and prime the respective promoters via targeted histone acetylation, the presence or absence of additional, lineage-enriched factors such as NR4A2 may be the crucial determinant of which hormone gene is activated by POU1F1 in a cell. It is also noteworthy that some factors, such as POU4F1, have effects on the expression of lineage markers such as Prl, yet are not observed to directly bind the promoters of either hormone gene (Fig. 5D). As a positive control in this ChIP study, the Pou4f1 autoregulatory enhancer was also assayed. The robust binding of POU4F1 to the Pou4f1 autoregulatory element was consistent with prior studies (82, 83), substantiating the negative data at the Prl promoter. These data suggest a mechanism in which POU4F1 acts on other, currently unidentified, regulatory elements to alter the expression of genes critical to cell identity such as Prl.
In conclusion, the analysis of the somatotrope and lactotrope transcriptomes supports a model in which cell type–enriched transcription factors are induced during the transition from somatolactotrope into a somatotrope or lactotrope. These factors then enhance the expression of genes crucial to cell identity or suppress the genes that drive the reciprocal lineage. These factors continue to be expressed in adult pituitary cells, and are likely needed for the maintenance of cell fate. Mechanistically, these transcription factors can act directly at the promoters of their target genes, or indirectly via additional mediators. The newly identified transcription factors presented in the present study are likely to represent only a subset of the factors that play important roles in the maintenance of somatotrope and lactotrope cell identities. The identification of additional lineage-defining determinants is now possible through the transcriptomic profiles of both cell types.
Acknowledgments
We acknowledge the assistance of the following Core facilities at the Perelman School of Medicine in this study: Dr. Jonathan Schug of the Next Generation Sequencing Core facility, Dr. Andrea Stout of the Microscopy Core in the Department of Cell and Development Biology, Dr. Jean Richa of the Transgenic and Chimeric Mouse Facility in the Department of Genetics, and Thomas Williams and members of the Flow Cytometry and Cell Sorting Facility Department of Pathology. We also acknowledge Dr. Paul Le Tissier (University of Edinburgh) for his gift of the rPrl-DsRed transgenic mouse line.
Financial Support: This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant DK107453.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ChIP
chromatin immunoprecipitation
- DAPI
4′,6-diamidino-2-phenylindole
- e
embryonic day
- FACS
fluorescence-activated cell sorting
- IRES
internal ribosome entry site
- PRL
prolactin
- qRT-PCR
quantitative reverse transcription PCR
- RNA-seq
RNA sequencing
References
- 1. Jabaudon D. Fate and freedom in developing neocortical circuits. Nat Commun. 2017;8:16042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132(4):631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao X, Wu J, Zheng M, Gao F, Ju G. Specification and maintenance of oligodendrocyte precursor cells from neural progenitor cells: involvement of microRNA-7a. Mol Biol Cell. 2012;23(15):2867–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M, Diefenbach A. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37(4):634–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sizova D, Ho Y, Cooke NE, Liebhaber SA. Research resource: T-antigen transformation of pituitary cells captures three novel cell lines in the Pit-1 lineage. Mol Endocrinol. 2010;24(11):2232–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu X, Gleiberman AS, Rosenfeld MG. Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev. 2007;87(3):933–963. [DOI] [PubMed] [Google Scholar]
- 7. Zhu X, Wang J, Ju BG, Rosenfeld MG. Signaling and epigenetic regulation of pituitary development. Curr Opin Cell Biol. 2007;19(6):605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andersen B, Rosenfeld MG. Pit-1 determines cell types during development of the anterior pituitary gland. A model for transcriptional regulation of cell phenotypes in mammalian organogenesis. J Biol Chem. 1994;269(47):29335–29338. [PubMed] [Google Scholar]
- 9. Vankelecom H, Chen J. Pituitary stem cells: where do we stand? Mol Cell Endocrinol. 2014;385(1–2):2–17. [DOI] [PubMed] [Google Scholar]
- 10. Rizzoti K. Adult pituitary progenitors/stem cells: from in vitro characterization to in vivo function. Eur J Neurosci. 2010;32(12):2053–2062. [DOI] [PubMed] [Google Scholar]
- 11. Núñez L, Villalobos C, Senovilla L, García-Sancho J. Multifunctional cells of mouse anterior pituitary reveal a striking sexual dimorphism. J Physiol. 2003;549(Pt 3):835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frawley LS, Boockfor FR. Mammosomatotropes: presence and functions in normal and neoplastic pituitary tissue. Endocr Rev. 1991;12(4):337–355. [DOI] [PubMed] [Google Scholar]
- 13. Castrique E, Fernandez-Fuente M, Le Tissier P, Herman A, Levy A. Use of a prolactin-Cre/ROSA-YFP transgenic mouse provides no evidence for lactotroph transdifferentiation after weaning, or increase in lactotroph/somatotroph proportion in lactation. J Endocrinol. 2010;205(1):49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luque RM, Amargo G, Ishii S, Lobe C, Franks R, Kiyokawa H, Kineman RD. Reporter expression, induced by a growth hormone promoter-driven Cre recombinase (rGHp-Cre) transgene, questions the developmental relationship between somatotropes and lactotropes in the adult mouse pituitary gland. Endocrinology. 2007;148(5):1946–1953. [DOI] [PubMed] [Google Scholar]
- 15. Mangalam HJ, Albert VR, Ingraham HA, Kapiloff M, Wilson L, Nelson C, Elsholtz H, Rosenfeld MG. A pituitary POU domain protein, Pit-1, activates both growth hormone and prolactin promoters transcriptionally. Genes Dev. 1989;3(7):946–958. [DOI] [PubMed] [Google Scholar]
- 16. Cohen LE, Wondisford FE, Radovick S. Role of Pit-1 in the gene expression of growth hormone, prolactin, and thyrotropin. Endocrinol Metab Clin North Am. 1996;25(3):523–540. [DOI] [PubMed] [Google Scholar]
- 17. Tatsumi K, Miyai K, Notomi T, Kaibe K, Amino N, Mizuno Y, Kohno H. Cretinism with combined hormone deficiency caused by a mutation in the PIT1 gene. Nat Genet. 1992;1(1):56–58. [DOI] [PubMed] [Google Scholar]
- 18. Nelson C, Albert VR, Elsholtz HP, Lu LI, Rosenfeld MG. Activation of cell-specific expression of rat growth hormone and prolactin genes by a common transcription factor. Science. 1988;239(4846):1400–1405. [DOI] [PubMed] [Google Scholar]
- 19. Nakane PK. Classifications of anterior pituitary cell types with immunoenzyme histochemistry. J Histochem Cytochem. 1970;18(1):9–20. [DOI] [PubMed] [Google Scholar]
- 20. Mayo KE, Miller T, DeAlmeida V, Godfrey P, Zheng J, Cunha SR. Regulation of the pituitary somatotroph cell by GHRH and its receptor. Recent Prog Horm Res. 2000;55:237–266. [PubMed]
- 21. Ramirez MC, Ornstein AM, Luque GM, Perez Millan MI, Garcia-Tornadu I, Rubinstein M, Becu-Villalobos D. Pituitary and brain dopamine D2 receptors regulate liver gene sexual dimorphism. Endocrinology. 2015;156(3):1040–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pellegrini-Bouiller I, Morange-Ramos I, Barlier A, Gunz G, Figarella-Branger D, Cortet-Rudelli C, Grisoli F, Jaquet P, Enjalbert A. Pit-1 gene expression in human lactotroph and somatotroph pituitary adenomas is correlated to D2 receptor gene expression. J Clin Endocrinol Metab. 1996;81(9):3390–3396. [DOI] [PubMed] [Google Scholar]
- 23. Davis SW, Castinetti F, Carvalho LR, Ellsworth BS, Potok MA, Lyons RH, Brinkmeier ML, Raetzman LT, Carninci P, Mortensen AH, Hayashizaki Y, Arnhold IJ, Mendonça BB, Brue T, Camper SA. Molecular mechanisms of pituitary organogenesis: In search of novel regulatory genes. Mol Cell Endocrinol. 2010;323(1):4–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brinkmeier ML, Davis SW, Carninci P, MacDonald JW, Kawai J, Ghosh D, Hayashizaki Y, Lyons RH, Camper SA. Discovery of transcriptional regulators and signaling pathways in the developing pituitary gland by bioinformatic and genomic approaches. Genomics. 2009;93(5):449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Magoulas C, McGuinness L, Balthasar N, Carmignac DF, Sesay AK, Mathers KE, Christian H, Candeil L, Bonnefont X, Mollard P, Robinson IC. A secreted fluorescent reporter targeted to pituitary growth hormone cells in transgenic mice. Endocrinology. 2000;141(12):4681–4689. [DOI] [PubMed] [Google Scholar]
- 26. He Z, Fernandez-Fuente M, Strom M, Cheung L, Robinson IC, Le Tissier P. Continuous on-line monitoring of secretion from rodent pituitary endocrine cells using fluorescent protein surrogate markers. J Neuroendocrinol. 2011;23(3):197–207. [DOI] [PubMed] [Google Scholar]
- 27. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 28. Ho Y, Liebhaber SA, Cooke NE. The role of the hGH locus control region in somatotrope restriction of hGH-N gene expression. Mol Endocrinol. 2011;25(5):877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.RRID:AB_2662159.
- 31.RRID:AB_2737293.
- 32.RRID:AB_2200381.
- 33.RRID:AB_944767.
- 34.RRID:AB_776887.
- 35.RRID:AB_1640222.
- 36. Shewchuk BM, Ho Y, Liebhaber SA, Cooke NE. A single base difference between Pit-1 binding sites at the hGH promoter and locus control region specifies distinct Pit-1 conformations and functions. Mol Cell Biol. 2006;26(17):6535–6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.RRID:AB_2118291.
- 38.RRID:AB_2732812.
- 39. Peel MT, Ho Y, Liebhaber SA. Data from: Transcriptome analyses of female somatotropes and lactotropes reveal novel regulators of cell identity in the pituitary. figshare 2018. Deposited 23 August 2018. https://doi.org/10.6084/m9.figshare.c.4206764.v2. [DOI] [PMC free article] [PubMed]
- 40. Jones BK, Monks BR, Liebhaber SA, Cooke NE. The human growth hormone gene is regulated by a multicomponent locus control region. Mol Cell Biol. 1995;15(12):7010–7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. González-Parra S, Argente J, García-Segura LM, Chowen JA. Cellular composition of the adult rat anterior pituitary is influenced by the neonatal sex steroid environment. Neuroendocrinology. 1998;68(3):152–162. [DOI] [PubMed] [Google Scholar]
- 42. Sasaki F, Iwama Y. Sex difference in prolactin and growth hormone cells in mouse adenohypophysis: stereological, morphometric, and immunohistochemical studies by light and electron microscopy. Endocrinology. 1988;123(2):905–912. [DOI] [PubMed] [Google Scholar]
- 43. Aspé-Sánchez M, Moreno M, Rivera MI, Rossi A, Ewer J. Oxytocin and vasopressin receptor gene polymorphisms: role in social and psychiatric traits. Front Neurosci. 2016;9:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Radl D, De Mei C, Chen E, Lee H, Borrelli E. Each individual isoform of the dopamine D2 receptor protects from lactotroph hyperplasia. Mol Endocrinol. 2013;27(6):953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Murabe H, Shimatsu A, Ihara C, Mizuta H, Nakamura Y, Nagata I, Kikuchi H, Nakao K. Expression of somatostatin receptor (SSTR) subtypes in pituitary adenomas: quantitative analysis of SSTR2 mRNA by reverse transcription-polymerase chain reaction. J Neuroendocrinol. 1996;8(8):605–610. [PubMed] [Google Scholar]
- 46. Danila DC, Haidar JN, Zhang X, Katznelson L, Culler MD, Klibanski A. Somatostatin receptor-specific analogs: effects on cell proliferation and growth hormone secretion in human somatotroph tumors. J Clin Endocrinol Metab. 2001;86(7):2976–2981. [DOI] [PubMed] [Google Scholar]
- 47. Nasonkin IO, Potok MA, Camper SA. Cre-mediated recombination in pituitary somatotropes. Genesis. 2009;47(1):55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vankelecom H. Pituitary stem cells: quest for hidden functions. In: Pfaff D, Christen Y, eds. Stem Cells in Neuroendocrinology [Internet]. Cham, Switzerland: Springer; 2016. Available at: https://www.ncbi.nlm.nih.gov/books/NBK435801/. [PubMed]
- 49. Fu Q, Vankelecom H. Regenerative capacity of the adult pituitary: multiple mechanisms of lactotrope restoration after transgenic ablation. Stem Cells Dev. 2012;21(18):3245–3257. [DOI] [PubMed] [Google Scholar]
- 50. Konzak KE, Moore DD. Functional isoforms of Pit-1 generated by alternative messenger RNA splicing. Mol Endocrinol. 1992;6(2):241–247. [DOI] [PubMed] [Google Scholar]
- 51. Million Passe CM, White CR, King MW, Quirk PL, Iovanna JL, Quirk CC. Loss of the protein NUPR1 (p8) leads to delayed LHB expression, delayed ovarian maturation, and testicular development of a Sertoli-cell-only syndrome-like phenotype in mice. Biol Reprod. 2008;79(4):598–607. [DOI] [PubMed] [Google Scholar]
- 52. Maliza R, Fujiwara K, Tsukada T, Azuma M, Kikuchi M, Yashiro T. Effects of retinoic acid on growth hormone-releasing hormone receptor, growth hormone secretagogue receptor gene expression and growth hormone secretion in rat anterior pituitary cells. Endocr J. 2016;63(6):555–561. [DOI] [PubMed] [Google Scholar]
- 53. Guibourdenche J, Djakouré C, Porquet D, Pagésy P, Rochette-Egly C, Peillon F, Li JY, Evain-Brion D. Retinoic acid stimulates growth hormone synthesis in human somatotropic adenoma cells: characterization of its nuclear receptors. J Cell Biochem. 1997;65(1):25–31. [DOI] [PubMed] [Google Scholar]
- 54. Koenig RJ, Brent GA, Warne RL, Larsen PR, Moore DD. Thyroid hormone receptor binds to a site in the rat growth hormone promoter required for induction by thyroid hormone. Proc Natl Acad Sci USA. 1987;84(16):5670–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu Y, Xu B, Koenig RJ. Thyroid hormone response element sequence and the recruitment of retinoid X receptors for thyroid hormone responsiveness. J Biol Chem. 2001;276(6):3929–3936. [DOI] [PubMed] [Google Scholar]
- 56. Winczyk K, Pawlikowski M. Immunohistochemical detection of PPARγ receptors in the human pituitary adenomas: correlation with PCNA. Folia Histochem Cytobiol. 2005;43(3):137–141. [PubMed] [Google Scholar]
- 57. McCoy JM, Walkenhorst DE, McCauley KS, Elaasar H, Everett JR, Mix KS. Orphan nuclear receptor NR4A2 induces transcription of the immunomodulatory peptide hormone prolactin. J Inflamm (Lond). 2015;12(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Leblond-Francillard M, Picon A, Bertagna X, de Keyzer Y. High expression of the POU factor Brn3a in aggressive neuroendocrine tumors. J Clin Endocrinol Metab. 1997;82(1):89–94. [DOI] [PubMed] [Google Scholar]
- 59. He X, Treacy MN, Simmons DM, Ingraham HA, Swanson LW, Rosenfeld MG. Expression of a large family of POU-domain regulatory genes in mammalian brain development [published correction appears in Nature. 1989;340(6235):662]. Nature. 1989;340(6228):35–42. [DOI] [PubMed] [Google Scholar]
- 60. Ingraham HA, Albert VR, Chen RP, Crenshaw EB III, Elsholtz HP, He X, Kapiloff MS, Mangalam HJ, Swanson LW, Treacy MN, Rosenfeld MG. A family of POU-domain and Pit-1 tissue-specific transcription factors in pituitary and neuroendocrine development. Annu Rev Physiol. 1990;52(1):773–791. [DOI] [PubMed] [Google Scholar]
- 61. Liu J, Lin C, Gleiberman A, Ohgi KA, Herman T, Huang HP, Tsai MJ, Rosenfeld MG. Tbx19, a tissue-selective regulator of POMC gene expression. Proc Natl Acad Sci USA. 2001;98(15):8674–8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Akcan N, Serakıncı N, Turkgenc B, Bundak R, Bahceciler N, Temel SG. A novel TBX19 gene mutation in a case of congenital isolated adrenocorticotropic hormone deficiency presenting with recurrent respiratory tract infections. Front Endocrinol (Lausanne). 2017;8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lamolet B, Pulichino AM, Lamonerie T, Gauthier Y, Brue T, Enjalbert A, Drouin J. A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell. 2001;104(6):849–859. [DOI] [PubMed] [Google Scholar]
- 64. Ingraham HA, Chen RP, Mangalam HJ, Elsholtz HP, Flynn SE, Lin CR, Simmons DM, Swanson L, Rosenfeld MG. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988;55(3):519–529. [DOI] [PubMed] [Google Scholar]
- 65. Cohen LE, Zanger K, Brue T, Wondisford FE, Radovick S. Defective retinoic acid regulation of the Pit-1 gene enhancer: a novel mechanism of combined pituitary hormone deficiency. Mol Endocrinol. 1999;13(3):476–484. [DOI] [PubMed] [Google Scholar]
- 66. Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43(11):1773–1808. [DOI] [PubMed] [Google Scholar]
- 67. Khan A, Fornes O, Stigliani A, Gheorghe M, Castro-Mondragon JA, van der Lee R, Bessy A, Chèneby J, Kulkarni SR, Tan G, Baranasic D, Arenillas DJ, Sandelin A, Vandepoele K, Lenhard B, Ballester B, Wasserman WW, Parcy F, Mathelier A. JASPAR 2018: update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res. 2018;46(D1):D1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12(5):599–606. [DOI] [PubMed] [Google Scholar]
- 69. Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389(6649):349–352. [DOI] [PubMed] [Google Scholar]
- 70. Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, Hanna J, Lodato MA, Frampton GM, Sharp PA, Boyer LA, Young RA, Jaenisch R. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci USA. 2010;107(50):21931–21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pradeepa MM. Causal role of histone acetylations in enhancer function. Transcription. 2017;8(1):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Booth A, Trudeau T, Gomez C, Lucia MS, Gutierrez-Hartmann A. Persistent ERK/MAPK activation promotes lactotrope differentiation and diminishes tumorigenic phenotype. Mol Endocrinol. 2014;28(12):1999–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Syro LV, Sundsbak JL, Scheithauer BW, Toledo RA, Camargo M, Heyer CM, Sekiya T, Uribe H, Escobar JI, Vasquez M, Rotondo F, Toledo SP, Kovacs K, Horvath E, Babovic-Vuksanovic D, Harris PC. Somatotroph pituitary adenoma with acromegaly and autosomal dominant polycystic kidney disease: SSTR5 polymorphism and PKD1 mutation. Pituitary. 2012;15(3):342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Molitch ME, Elton RL, Blackwell RE, Caldwell B, Chang RJ, Jaffe R, Joplin G, Robbins RJ, Tyson J, Thorner MO. Bromocriptine as primary therapy for prolactin-secreting macroadenomas: results of a prospective multicenter study. J Clin Endocrinol Metab. 1985;60(4):698–705. [DOI] [PubMed] [Google Scholar]
- 75. Whitelaw BC, Dworakowska D, Thomas NW, Barazi S, Riordan-Eva P, King AP, Hampton T, Landau DB, Lipscomb D, Buchanan CR, Gilbert JA, Aylwin SJ. Temozolomide in the management of dopamine agonist-resistant prolactinomas. Clin Endocrinol (Oxf). 2012;76(6):877–886. [DOI] [PubMed] [Google Scholar]
- 76. Oh MC, Aghi MK. Dopamine agonist-resistant prolactinomas. J Neurosurg. 2011;114(5):1369–1379. [DOI] [PubMed] [Google Scholar]
- 77. Haugen BR, Gordon DF, Nelson AR, Wood WM, Ridgway EC. The combination of Pit-1 and Pit-1T have a synergistic stimulatory effect on the thyrotropin beta-subunit promoter but not the growth hormone or prolactin promoters. Mol Endocrinol. 1994;8(11):1574–1582. [DOI] [PubMed] [Google Scholar]
- 78. Diamond SE, Gutierrez-Hartmann A. The Pit-1β domain dictates active repression and alteration of histone acetylation of the proximal prolactin promoter. J Biol Chem. 2000;275(40):30977–30986. [DOI] [PubMed] [Google Scholar]
- 79. Zhao Y, Bruemmer D. NR4A orphan nuclear receptors: transcriptional regulators of gene expression in metabolism and vascular biology. Arterioscler Thromb Vasc Biol. 2010;30(8):1535–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mahajan S, Saini A, Chandra V, Nanduri R, Kalra R, Bhagyaraj E, Khatri N, Gupta P. Nuclear receptor Nr4a2 promotes alternative polarization of macrophages and confers protection in sepsis. J Biol Chem. 2015;290(30):18304–18314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Han YF, Cao GW. Role of nuclear receptor NR4A2 in gastrointestinal inflammation and cancers. World J Gastroenterol. 2012;18(47):6865–6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Trieu M, Ma A, Eng SR, Fedtsova N, Turner EE. Direct autoregulation and gene dosage compensation by POU-domain transcription factor Brn3a. Development. 2003;130(1):111–121. [DOI] [PubMed] [Google Scholar]
- 83. Lanier J, Quina LA, Eng SR, Cox E, Turner EE. Brn3a target gene recognition in embryonic sensory neurons. Dev Biol. 2007;302(2):703–716. [DOI] [PMC free article] [PubMed] [Google Scholar]