Abstract
The placenta is critical for all aspects of fetal development. Bisphenol A (BPA) and phthalates are endocrine disruptors with ubiquitous exposure in pregnant women—their effects on the placenta is an area of growing research interest. Therefore, our objectives were to (1) summarize research related to the effects BPA or phthalates on placental outcomes in animal and cell models, and (2) evaluate the challenges for using such models to study the impacts of these chemicals on placental endpoints. Overall, studies in cells and animal models suggest that BPA and phthalates impact placental hormones, some epigenetic endpoints, increase inflammation and oxidative stress, and decrease cell viability and nutrient transfer. However, few animal or cell studies have assessed these outcomes at concentrations relevant to humans. Furthermore, it is unclear whether effects of BPA/phthalates on the placenta in animal models mediate fetal outcomes, as most studies have dosed after the earliest stages of placental and fetal development. It is also unclear whether effects of these chemicals are sex-specific, as few studies have considered placental sex. Finally, while there is substantial evidence for effects of mono-(2-ethylhexyl) phthalate (the major metabolite of di-(2-ethylhexyl) phthalate), on placental endpoints in cells, little is currently known about effects of other phthalates to which pregnant women are exposed. Moving forward, these limitations will need to be addressed to help us understand the precise mechanisms of action of these chemicals within the placenta, and how these reported perturbations impact fetal health.
Keywords: BPA, endocrine disrupting chemicals, phthalates, placenta
Bisphenol A (BPA) and phthalates are endocrine disrupting chemicals found in food packaging and personal care/household products (Mervish et al., 2014; Schettler, 2006). Although life-course exposures to these chemicals can adversely impact health, pregnancy is an especially sensitive window, and epidemiological studies demonstrate that pregnant women have widespread exposure to these chemicals (Fisher et al., 2015; Yan et al., 2009). In placental mammals, the establishment and maintenance of a successful pregnancy, and the offspring’s life-long health, rely on finely tuned developmental trajectories of placental and fetal tissues (reviewed in Godfrey, 2002 and Jansson and Powell, 2007). As we have reviewed elsewhere (Strakovsky and Schantz, 2018), recent human studies suggest associations between BPA or phthalate exposures and numerous placental molecular endpoints. These include hormone-related mRNA expression (Adibi et al., 2010, 2017), micro-RNA expression (LaRocca et al., 2016; Zhong et al., 2018), long noncoding RNA expression (Machtinger et al., 2018), and DNA methylation (Grindler et al., 2018; LaRocca et al., 2014; Nahar et al., 2015; Zhao et al., 2015, 2016). Furthermore, there is evidence that phthalate exposure in humans is also associated with changes to placental anthropometry (length, breadth, surface area, and thickness) (Zhu et al., 2018). However, the current literature in humans is still limited in its ability to pinpoint specific mechanisms by which these chemicals target the human placenta to cause these effects and to ultimately impact placental function.
These knowledge gaps are due, in part, to the challenges in assessing phenotypic and molecular endpoints within the large and heterogeneous human placenta, with studies showing that even the most careful sampling schemes to assess gene expression can result in great within-placental variability (Adibi et al., 2009). Other challenges for making mechanistic evaluations within the human placenta thus far have included the following: (1) the fact that chemical exposures have been assessed at various points in pregnancy, whereas placental tissues are typically only available at birth, and (2) lack of reliable information about the role of fetal sex in modifying associations of exposures with placental molecular endpoints. As will be discussed throughout this review, there are undoubtedly challenges for modeling placental outcomes in experimental animal and cell models. Nevertheless, given the difficulties that exist in human placental sampling, such models are invaluable for unraveling the mechanisms of action of environmental chemicals within the placenta. The primary goal of this review is to address these challenges and opportunities for modeling placental structural, functional, and molecular outcomes in response to BPA and phthalates.
ANIMAL MODELS
Given the challenges in both human exposure assessment and placental sampling, animal models are important for assessing the underlying mechanisms behind the associations observed in humans. First, unlike in humans, the small size of the rodent placenta allows for histological evaluation of whole placentas as well as for site- and structure-specific molecular analysis in response to chemical exposures. Second, rodent models provide excellent opportunities for sex-specific evaluations of the placental-fetal unit, given that each rodent pup develops with its own placenta. As will be described below, important factors to consider when designing such models include the structural, functional, and epigenetic differences between species, exposure window, chemical dose, and placental sex, among others (Tables 1 and 2).
Table 1.
Effects of Bisphenol A (BPA) Exposure on Placental Endpoints in Rodent Models
| Model or Strain | BPA Dose | Dosing Mode | Dosing Window (GD) | Sex | Summary of Outcomes | Ref. |
|---|---|---|---|---|---|---|
| ICR mice | 2.5 μg/kg | Oral | 6.5–17.5 | Male | Effects on nuclear receptor mRNA expression, including hormone receptors: YES | Imanishi et al. (2003) |
| Female | Effects on nuclear receptor mRNA expression, including hormone receptors: YES | |||||
| (some outcomes different compared with males.) | ||||||
| ICR mice | 10 mg/kg | SC | 0–7 | NR | Effects on placental weight, anatomy, and structure: YES | Tachibana et al. (2007) |
| ER-luc mice | 50 mg/kg | Oral | 8–15 | Combined | Effect on estrogen receptor gene activity: YES | Ter Veld et al. (2009) |
| 6.66 μl/g bw | IP | 14 | ||||
| JF1 mice | 0.2 mg/kg | Oral | 8.5–12.5 | NR | Effects on placental imprinting: NO | Kang et al. (2011) |
| Mating between C57BL/6(B6) and B6 or B7 mice | 10 μg/kg | In diet (estimated exposure from 50 μg/kg or 50 mg/kg in diet) | 2 weeks premating—GD9.5 | NR | Effects on loss-of-imprinting and expression of imprinted genes: YES | Susiarjo et al. (2013) |
| Effects on global methylation: NO | ||||||
| 10 mg/kg | Effects on loss-of-imprinting and expression of imprinted genes: YES | |||||
| Effects on global and CpG-specific methylation: YES | ||||||
| 2 weeks premating—GD12.5 | Effects on placental anatomy and structure: SOME | |||||
| 10 mg/kg | 5.5–12.5 | Effects on loss-of-imprinting: NO | ||||
| ICR mice | 2 mg/kg | Oral | 13–16 | NR | Effects on hormonal mRNA expression: NO | Tan et al. (2013) |
| Effects on protein expression of downstream pathways: SOME | ||||||
| 20 mg/kg | Effects on hormonal mRNA expression: NO | |||||
| Effects on protein expression of downstream pathways: SOME | ||||||
| 200 mg/kg | Effects on hormonal mRNA expression: YES | |||||
| Effects on protein expression of downstream pathways: YES | ||||||
| CD-1 mice | 0.5 mg/kg | Oral | 1–11 | Combined | Effects on placental anatomy and structure: SOME | Tait et al. (2015) |
| Nuclear protein content of growth-related protein: YES | ||||||
| 50 mg/kg | Effects on placental anatomy and structure: YES | |||||
| Nuclear protein content of growth-related protein: NO | ||||||
| (unique gene signature compared with low dose) | ||||||
| ICR mice | 50 mg/kg | SC | 11.5–16.5 | Combined | Effects on mRNA expression of some metal cation transporters: SOME | Lee et al. (2016) |
| Kunming mice | 5 | SC | 0.5–5.5 | NR | Effects on proteins related to trophoblast migration: YES | Lan et al. (2017)a |
| 40 | Effects on proteins related to trophoblast migration: YES | |||||
| Polypay×Dorset sheep | 0.5 mg/kg | SC | 30–100 | Combined | Effects on placental weight: (NO), number of placentomes: NO, tissue composition: NO | Gingrich et al. (2018) |
| Effects on syncytialization-related protein and mRNA: NO, binucleate cells: NO | ||||||
| BPS: 0.5 mg/kg | Effects on placental weight: (NO), number of placentomes: NO, tissue composition: NO | |||||
| Effects on syncytialization-related protein and mRNA: YES, binucleate cells: YES |
BPA, bisphenol A; BPS, bisphenol S; GD, gestational day; IP, intraperitoneal; LOI, loss of imprinting; ND, not detected; NR, not reported; prot, protein; SC, subcutaneous.
Authors also showed histological data and reported (at 5, 10, and 40 mg/kg BPA) increased ITGB1 and ITGA5, a shift in the sizes of the layers, and increased presence of glycogenosomes and vacuoles by BPA, but without quantification or statistical comparisons.
Table 2.
Effects of Phthalate Exposure on Placental Endpoints in Rodent Models
| Model or Strain | Phthalate Dose (mg/kg) | Phthalate | Dosing Mode | Dosing Window (GD) | Sex | Summary of Outcomes | Ref. |
|---|---|---|---|---|---|---|---|
| Sprague Dawley rats | 750 | DEHP | Oral | 0–19 | Combined | Effects on lipid-related mRNA or protein expression in junctional zone: SOME | Xu et al. (2008) |
| Effects on prostaglandin-related mRNA or protein expression in junctional zone: SOME | |||||||
| Effects on lipid-related mRNA or protein expression in the labyrinth: SOME | |||||||
| Effects on overall prostaglandin-related mRNA expression in junctional zone: NO | |||||||
| Effects on overall prostaglandin production: YES | |||||||
| 1500 | Effects on lipid-related mRNA or protein expression in junctional zone: SOME | ||||||
| Effects on prostaglandin-related mRNA or protein expression in junctional zone: SOME | |||||||
| Effects on lipid-related mRNA or protein expression in the labyrinth: YES | |||||||
| Effects on prostaglandin-related mRNA expression in junctional zone: NO | |||||||
| Effects on overall prostaglandin production: YES | |||||||
| Effects on overall fatty acid distribution: SOME | |||||||
| ER-luc mice | 100 | DEHP | Oral | 8–15 | Combined | Effect on estrogen receptor gene activity: NO | Ter Veld et al. (2009) |
| DIHP | Effect on estrogen receptor gene activity: YES | ||||||
| 6.66 μl/g bw | DEHP | IP | 14 | Effect on estrogen receptor gene activity: NO | |||
| DIHP | Effect on estrogen receptor gene activity: YES | ||||||
| JF1 mice | 750 | DEHP | Oral | 8.5–12.5 | NR | Loss-of-imprint: SOME | Kang et al. (2011) |
| CD-1 mice | 125 | DEHP | Oral | 1–9 | NR | Effects on placental anatomy and structure: YES | Zong et al. (2015) |
| Effects on development-, apoptosis-, and vascularization-related genes: SOME | |||||||
| 1–13 | Effects on placental weight, anatomy, and structure: SOME | ||||||
| Effects on vascularization: NO | |||||||
| Effects on development-, apoptosis-, and vascularization-related genes: YES | |||||||
| 250 | 1–9 | Effects on placental anatomy and structure: YES | |||||
| Effects on development-, apoptosis-, and vascularization-related genes: SOME | |||||||
| 1–13 | Effects on placental weight, anatomy, and structure: YES | ||||||
| Effects on vascularization: YES | |||||||
| Effects on development-, apoptosis-, and vascularization-related genes: YES | |||||||
| 500 | 1–9 | Effects on placental anatomy and structure: YES | |||||
| Effects on development-, apoptosis-, and vascularization-related genes: SOME | |||||||
| 1–13 | Effects on placental weight, anatomy, and structure: YES | ||||||
| Effects on vascularization: YES | |||||||
| Effects on development-, apoptosis-, and vascularization-related genes: YES | |||||||
| ICR mice | 200 | DEHP | Oral | 0–6 or 7–12 or13–17 | Combined and by sex | Effects on placental weight and anatomy: YES—in males and if exposed on GD7-12 | Shen et al. (2017) |
| Effects on placental structure and proliferation: YES—on GD7-12 | |||||||
| Wistar rats | 500 | DBP | Oral | 6–18 to F0 | Combined | Effects on placental weight: YES—in undosed F1, F2, F3 generations. | Mahaboob Basha and Radha (2017) |
| Wistar rats | 20 | DHP | Oral | 6–19 | NR | Effects on placental shape: SOME | Ahbab et al. (2017) |
| Effects on markers of placental damage: NO | |||||||
| Effects on protein content of estrogen receptors: YES | |||||||
| Effects on marker of proliferation: YES | |||||||
| 100 | Effects on placental shape: SOME | ||||||
| Effects on markers of placental damage: SOME | |||||||
| Effects on protein content of estrogen receptors: YES | |||||||
| Effects on marker of proliferation: YES | |||||||
| 500 | Effects on placental shape: YES | ||||||
| Effects on markers of placental damage: SOME | |||||||
| Effects on protein content of estrogen receptors: YES | |||||||
| Effects on marker of proliferation: YES | |||||||
| 20 | DCHP | Effects on placental shape: YES | |||||
| Effects on markers of placental damage: SOME | |||||||
| Effects on protein content of estrogen receptors: YES | |||||||
| Effects on marker of proliferation: YES | |||||||
| 100 | Effects on placental shape: SOME | ||||||
| Effects on markers of placental damage: SOME | |||||||
| Effects on protein content of estrogen receptors: YES | |||||||
| Effects on marker of proliferation: YES | |||||||
| 500 | Effects on placental shape: YES | ||||||
| Effects on markers of placental damage: YES | |||||||
| Effects on protein content of estrogen receptors: YES | |||||||
| Effects on marker of proliferation: YES |
--, no effect observed; DEHP, di(2-ethylhexyl)phthalate; DHP, di-n-hexyl phthalate; DCHP, dicyclohexyl phthalate; GD, gestational day; IHC, immunohistochemistry; IP, intraperitoneal; NR, not reported; prot, protein.
Animal Model Selection
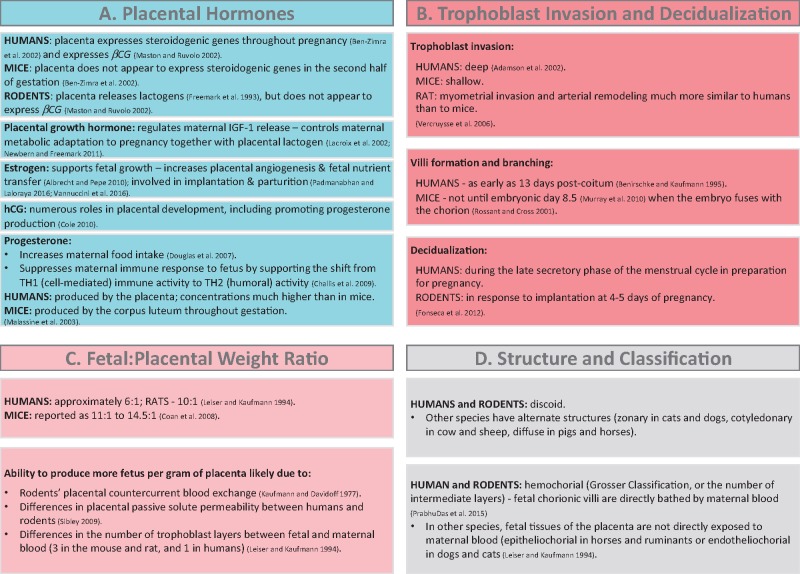
Although the rodent is the most-widely utilized animal model in studies assessing the effects of BPA and phthalates on the placenta, there are important developmental and structural differences between human and rodent placentas (reviewed in Carter, 2007) (cited and reviewed in Figure 1). These differences include placental hormone synthesis and function (Figure 1A), trophoblast invasion, remodeling of spiral arteries that carry blood and nutrients to the fetus, the timing and process of decidualization and placental development, the formation and branching of villi (Figure 1B), and the fetal: placental weight ratio, which is driven by fundamental species differences in maternal-fetal blood flow (Figure 1C).
Figure 1.
Species differences (specifically focusing on humans and rodents) in A, placental hormones, B, trophoblast invasion and decidualization, C, the fetal: placental weight ratio, and D, placental structure and classification (Adamson et al., 2002; Albrecht and Pepe, 2010; Benirschke and Kaufmann, 1995; Ben-Zimra et al., 2002; Challis et al., 2009; Coan et al., 2008; Cole, 2010; Douglas et al., 2007; Fonseca et al., 2012; Freemark et al., 1993; Kaufmann and Davidoff, 1977; Lacroix et al., 2002; Leiser and Kaufmann, 1994; Malassine et al., 2003; Maston and Ruvolo, 2002; Murray et al., 2010; Newbern and Freemark, 2011; Padmanabhan and Laloraya, 2016; PrabhuDas et al., 2015; Rossant and Cross, 2001; Sibley, 2009; Vannuccini et al., 2016; Vercruysse et al., 2006).
Despite these differences, there are numerous reasons for using rodent models to study the effects of environmental chemicals on the placenta. First, like humans, rodents develop a single discoid placenta. Humans and rodents also both have a hemochorial placenta, in which fetal chorionic villi are directly bathed by maternal blood (Figure 1D). In regard to epigenetic regulation, aspects of placental imprinting differ between rodents and humans (Monk et al., 2006). However, given that genomic imprinting is important for both human and rodent placental development (Frost and Moore, 2010), rodent studies have been useful for assessing the capacity of environmental chemicals to disrupt placental imprinting. Other useful models of human placental development also exist, and include nonhuman primates and sheep. However, few studies using these models have assessed the effects of endocrine disruptors on placental endpoints (except 1 study in sheep discussed later), therefore here, we have focused primarily on currently available studies in rodents.
Exposure Timing
Developmental toxicity studies targeting specific windows of fetal organ development are valuable for gaining insight into fetal organogenesis. However, the role of the placenta in mediating these outcomes is difficult to assess if dosing begins after the earliest stages of implantation and placental development/trophoblast invasion (as early as gestational day [GD] 3.5 in mice) (Yamanaka et al., 2006). Furthermore, these studies do not accurately model exposures in humans, as women are exposed to BPA and phthalates on a daily basis, prior to and throughout pregnancy.
Of the studies described here, only one began dosing prior to mating, whereas others started on GD 0 or 1, or much later (Tables 1 and 2). However, there is evidence for window-specific effects of phthalates on placental pathology. A recent study in ICR mice dosed with high-dose (500 mg/kg) di-(2-ethylhexyl) phthalate (DEHP) found decreased placental weight and diameter (males only) and decreased blood sinusoid area and cell proliferation (males and females analyzed together) only in dams dosed at GD7-12, but not GD0-6 or GD13-17 (Shen et al., 2017). Timing of exposure is especially significant when assessing placental epigenetic endpoints, as the earliest rapid stages of placental cell differentiation occur under strict epigenetic control (Maltepe et al., 2010). In JF1 mice, neither BPA (0.2 mg/kg/day) nor DEHP (750 mg/kg/day) gavaged from GD8.5 to GD12.5 had major effects on loss-of-imprinting or the expression of imprinted genes (Kang et al., 2011). BPA also had no effect on either placental or embryonic loss-of-imprinting in pregnant C57BL/6 dosed from GD5.5 to GD12.5 (Susiarjo et al., 2013). However, when the C57BL/6 mice were dosed with BPA beginning two weeks prior to mating until GD9.5 or GD12.5, BPA disturbed loss-of-imprinting, altered the mRNA expression of several imprinted genes, reduced placental CpG and average global DNA methylation, and decreased DNA methylation of 1 imprinted gene (Susiarjo et al., 2013). Although germline imprints (those passed to future generations) resist the first wave of active demethylation immediately following fertilization (Sanz et al., 2010), the earliest regulation of placental imprinting occurs, in part, independently of DNA methyltransferase 1 (DNMT1) mechanisms that regulate extra-placental imprinting (Court et al., 2014). These early dynamic processes may be especially sensitive to disruption by environmental chemicals. Therefore, given the role of epigenetics in regulating transcription, downstream translation, and ultimately placental development, it is critical that studies assessing the effects of chemicals on the placenta begin dosing prior to mating or immediately following fertilization.
Bisphenol dose and compound selection
Animal studies assessing nonplacental endpoints suggest a nonmonotonic dose-response for BPA, likely reflecting effects on both endocrine and non-endocrine pathways. Placental studies, however, have thus far utilized relatively high doses and limited dose-response curves. Despite this, several studies do suggest a dose-response relationship for BPA. For example, in ICR mice, most effects on placental mRNA and protein expression were observed only at the higher BPA doses (200 and 20 mg/kg vs 2 mg/kg) (Tan et al., 2013). In a study using CD-1 mice, 0.5 versus 50 mg/kg/day BPA also had dose-dependent effects on placental size, size of the spongiotrophoblast layer, total areas of maternal blood spaces, and embryonic labyrinthine capillaries. In this study, of all genes affected by 0.5 or 50 mg/kg BPA (compared with control), only 77 were shared between the 2 treatment groups, with 0.5 mg/kg BPA generally having a larger effect than 50 mg/kg. The 2 BPA doses also led to different significantly enriched KEGG pathways and different affected protein hubs (Tait et al., 2015). BPA has also been shown to dose-dependently affect epigenetic endpoints in other tissues (Ho et al., 2015), and while the Susiarjo et al. study demonstrated dose-dependent effects on placental imprinting and DNA methylation, and included a lower dose of BPA (estimated 10 µg/kg bw/day), additional studies are warranted using wider ranges of doses, including those more in line with human exposures (estimated to be well <1–2 µg/kg bw/day in the general population; Teeguarden and Hanson-Drury, 2013).
Only one animal study has assessed the effects of bisphenols other than BPA. Interestingly, a study in sheep found that bisphenol S (BPS), which is currently being used as a replacement for BPA in many products, altered the expression of syncytialization-related proteins/mRNA and decreased the number of trophoblast-derived binucleate cells, whereas the same dose of BPA had no effect on these outcomes (Gingrich et al., 2018). Pregnant women are exposed to various bisphenols (Kolatorova et al., 2018; Wan et al., 2018), so additional studies in animals may be warranted to investigate the effects of these compounds on the placenta.
Phthalate dose and compound selection
Animal studies assessing effects of phthalates on placental endpoints are also limited, have utilized relatively high doses, and have primarily focused on 1 phthalate, DEHP. In pregnant CD-1 mice, DEHP (125, 250, or 500 mg/kg/day) had dose-dependent effects on total placental and labyrinthine area, whereas all DEHP doses altered placental weight, spongiotrophoblast area, small-branched fetal vessels, proliferation in several placental zones, and the mRNA expression of numerous genes (Zong et al., 2015). In Sprague Dawley rats, DEHP (750 or 1500 mg/kg/day) dose- and zone-specifically induced placental mRNA and protein expression of Ppara and Pparg, the expression of several fatty acid transport-related genes/proteins, and placental content of several long-chain polyunsaturated fatty acids. However, both doses induced cytochrome p4504A1 (CYP4A1) mRNA and protein, and reduced labyrinthine cyclooxygenase (COX)-2 protein and prostaglandin formation from placental homogenates (Xu et al., 2008). It is likely that the lack of a dose-response in these studies was due to the high phthalate doses used in these experiments, which were chosen because previous studies had used similar doses, and not because they were relevant to human exposures. Authors discussed that approximate plasma concentrations of mono-(2-ethylhexyl) phthalate (MEHP) (the major metabolite of DEHP) in healthy women at term are 2.05 ± 1.47 μg/ml (referenced from Latini et al., 2003). However, authors of this animal study discussed unpublished results showing that 750 and 1500 mg/kg DEHP treatment led to maternal MEHP plasma concentrations of 65 or 136 μg/ml (respectively). Given that these internal doses are greatly out of the range of human exposures, additional studies using lower concentrations of DEHP are needed to corroborate these findings.
Two more-recent studies reported placental outcomes in response to phthalates other than DEHP, but also at relatively high doses. In Wistar rats, 500 mg/kg DBP dosed to the F0 generation decreased placental weight in F1, F2, and F3 generations, accompanied by numerous reproductive and developmental disturbances in offspring (Mahaboob Basha and Radha, 2017). Another study in Wistar rats found that all doses (20, 100, 500 mg/kg) of di-n-hexyl phthalate (DHP) or dicyclohexyl phthalate (DCHP) decreased placental protein expression of PCNA, PPARγ, ERα, ERβ, and AR. However, effects on placental weight, diameter, and length differed by chemical and dose, as did most pathological findings within the trophoblasts, spongiotrophoblast, and basal zone (Ahbab et al., 2017). Although this study included a dose-response curve, even the lowest concentration (20 mg/kg) may not be relevant to human exposures, as urinary MCHP (the major metabolite of DCHP) was below the level of detection for most people in the recent NHANES (CDC, 2017), and little is known about human DHP exposure.
Based on these findings, studies with more extensive dose-response curves that are more in line with human exposures will be essential to begin unraveling the mechanisms responsible for associations of phthalates and BPA exposures with placental outcomes in humans. This task is complicated by species differences in toxicokinetics, exposure, metabolism, and elimination rates of both BPA and phthalates (Doerge et al., 2011; Rusyn et al., 2006; Silva et al., 2007; Thayer et al., 2015). In humans, exposure is estimated based on internal doses of parent compounds or excretion of their metabolites. In animals, however, doses of parent compounds are known, but not the circulating or excreted concentrations of chemicals and their metabolites. Establishing exposure “dose” in humans is difficult given the multiple exposure pathways for BPA and phthalates and the paucity of data on human pharmacokinetics of these compounds. Therefore, in future animal studies, assessment of circulating and excreted concentrations of chemicals and their metabolites will be critical to allow exposure/dose comparisons between humans and model animals.
Sex-specific placental outcomes
The placenta develops from both maternal and fetal tissues, and consequently has sexually dimorphic responses to environmental and dietary cues (Rosenfeld, 2015). Studies in humans do suggest sex-specific associations between exposure to BPA or phthalates and placental outcomes (Adibi et al., 2017; LaRocca et al., 2014). However, of the animal studies described here, only 2 specifically discussed “placental sex.” In ICR mice, 200 mg/kg DEHP decreased placental weight and diameter in male but not female placentas (Shen et al., 2017), whereas 2.5 μg/kg/day BPA differentially affected placental mRNA expression depending on placental sex, either in magnitude or direction (Imanishi et al., 2003). As previously discussed, rodent models are especially useful for assessing sex-specific associations between placental and fetal outcomes, as each pup develops with its own placenta. Given the reported sexually dimorphic associations of BPA/phthalates with placental outcomes in humans and some previous animal studies, future animal models should leverage the rodent fetal-placental unit to assess sex-specific placental effects of BPA and phthalates.
IN VITRO SYSTEMS
Cell culture experiments are indispensable for establishing mechanisms of action of BPA and phthalates on placental function. In placental in vitro models, these functional/phenotypic endpoints include standard measures of cell health (viability, proliferation, necrosis), and placenta-specific measures of function and development (apoptosis, migration, invasion, and differentiation). Similar to cancer, appropriate placental development is characterized by increased migration, invasion, differentiation (Knofler and Pollheimer, 2013), and apoptosis (which is exaggerated in instances of placental disease; Sharp et al., 2010), thereby providing phenotypic readouts for the effects of BPA and phthalates. As will be discussed in following sections, similar to animal models, chemical concentration and length of exposure are critical for assessing effects of these chemicals, as is model selection. Models utilized in currently available studies include classic immortalized cell lines, as well as more physiologically relevant placental primary cultures to more-accurately model placental exposures (Tables 3 and 4).
Table 3.
Effects of Bisphenol A (BPA) in Placental In Vitro Models
| Cell Model | µM BPAa | Treatment Length (h) | Summary of Outcomes | Ref. |
|---|---|---|---|---|
| JEG-3 | 25 | 10 min, 30 min, 1, 2 | Effects on aromatase activity: YES | Nativelle-Serpentini et al. (2003) |
| 18 | Effects on aromatase activity: YES | |||
| 50 | 10 min | Effects on aromatase activity/mRNA: NO | ||
| 30 min, 1 | Effects on aromatase activity: NO | |||
| 2 | Effects on aromatase activity: YES | |||
| 18 | Effects on aromatase activity: YES | |||
| 100 | 10 min, 2 | Effects on aromatase activity: YES | ||
| 30 min, 1 | Effects on aromatase activity: NO | |||
| 18 | Effects on aromatase activity: YES | |||
| BeWo | 0.1, 10, 50 | approximately 1 | Effects on measure of drug efflux: YES | Jin and Audus (2005) |
| Microsomes @term | 500 | 15 min | Effects on aromatase activity: YES | Benachour et al. (2007) |
| 1° Cytotrophoblasts | 8.8×10−4, 8.8×10−3 | 24 | Effects on measure of apoptosis: NO, necrosis: YES, TNF-α mRNA: YES, TNF-α prot: NO | Benachour and Aris (2009) |
| 0.0876, 0.438 | Effects on measure of apoptosis: YES, necrosis: YES, TNF-α mRNA: YES, TNF-α prot: YES | |||
| 0.876 | Effects on measure of apoptosis: YES, necrosis: NO, TNF-α mRNA: NO, TNF-α prot: NO | |||
| 8.76, 87.6 | Effects on measure of apoptosis: YES, necrosis NO, TNF-α mRNA: NO, TNF-α prot: YES | |||
| 438, 876 | Effects on measure of apoptosis: YES, necrosis YES, TNF-α mRNA: NO, TNF-α prot: YES | |||
| JEG-3 | 1 | 24 | Effects on aromatase mRNA/protein expression or promoter activity, aromatase activity: NO | Huang and Leung (2009) |
| PL30 | Effect on aromatase mRNA: NO | |||
| JEG-3 | 5 | Effects on aromatase mRNA/protein expression or promoter activity, aromatase activity: YES | ||
| PL30 | Effect on aromatase mRNA: NO | |||
| JEG-3 | 25 | Effects on aromatase mRNA/protein expression or promoter activity, aromatase activity: YES | ||
| PL30 | Effect on aromatase mRNA: YES | |||
| JEG-3 | 50 | Effects on aromatase mRNA/protein expression or promoter activity, aromatase activity: YES | ||
| PL30 | Effect on aromatase mRNA: YES | |||
| JEG-3, PL30 | 100 | Effects on aromatase mRNA/protein expression or promoter activity, aromatase activity: YES | ||
| 3A, HTR-8/Svneo | 1.095 | 6 days | Effects on miR-146a expression: NO | Avissar-Whiting et al. (2010) |
| 3A | 10.95 | Effects on miR-146a expression/survival after miR-146a overexpression: NO | ||
| HTR-8/Svneo | Effects on miR-146a expression: NO | |||
| 3A | 109.51 | Effects on miR-146a and other miR expression: YES; altered survival after miR-146a overexpression: NO | ||
| TCL-1 | Effects on miR expression: NO | |||
| HTR-8/Svneo | Effects on miR-146a and other miR expression: YES | |||
| JEG-3 | 1×10−3 | 24 | Effects on proliferation: NO (also in BeWo cells) | Morice et al. (2011) |
| 48 | Effects on viability or proliferation: NO (also in BeWo cells) | |||
| 0.01 | 24 | Effects on proliferation: NO (also in BeWo cells) | ||
| 48 | Effects on viability or proliferation: NO (also in BeWo cells) | |||
| 0.1 | 1 or 6? | Effects on mRNA of cell cycle-related genes: SOME | ||
| 8 | Effects on mRNA of an apoptosis-related gene: YES | |||
| 24 | Effects on proliferation: YES (NO in BeWo) | |||
| 48 | Effect on viability: NO, apoptosis: YES, proliferation: YES (NO in BeWo) | |||
| 1 | 1 or 6? | Effects on mRNA of cell cycle-related genes: SOME | ||
| 8 | Effects on mRNA of an apoptosis-related gene: YES | |||
| 24 | Effects on proliferation: YES (NO in BeWo)—potentially through Errγ | |||
| 48 | Effects on proliferation: YES (NO in BeWo), viability: NO, apoptosis: YES | |||
| 10 | 24 | Effects on proliferation: YES (NO in BeWo); | ||
| 48 | Effects on proliferation: YES (NO in BeWo), viability: NO | |||
| 100 | 48 | Effects on viability: YES | ||
| JEG-3 | 1, 5 | 24 | Effects on CRH mRNA/protein expression and promoter activity: NO, upstream proteins: SOME | Huang and Leung (2012)b |
| 25 | Effects on CRH mRNA/protein expression and promoter activity: YES, upstream proteins: SOME | |||
| 50 | Effects on CRH mRNA/protein expression and promoter activity: YES, upstream proteins: YES | |||
| 1° Villi | 5×10−4 | 24 | Effect on β-hCG in media with/without BPA-conditioned decidualized stromal cells (DSCs): NO;Effect on proinflammatory cytokine MIF: YES—not if co-cultured with BPA-conditioned DSCs | Mannelli et al. (2014) |
| 48 | Effect on β-hCG in media with/without BPA-conditioned decidualized stromal cells (DSCs): NO | |||
| BeWo | 0.01 | 48 | Effect on proliferation: NO, invasion into endometrial cells YES; invasion-related mRNA/prot: YES, DNA methylation-related mRNA/prot: SOME | Wang et al. (2015) |
| 0.1 | Effect on proliferation: NO | |||
| 1 | Effect on proliferation: YES, invasion into endometrial cells: YES; invasion-related mRNA/prot: YES, DNA methylation-related mRNA/prot: SOME | |||
| 10 | Effect on proliferation: YES, invasion-related prot: YES, DNA methylation-related mRNA/prot: SOME | |||
| 100 | Effect on proliferation: NO, invasion into endometrial cells: YES, invasion-related mRNA/prot: YES, DNA methylation-related mRNA/prot: YES | |||
| 1000 | Effect on proliferation: YES | |||
| 1° Trophoblasts | 0.438 | 24 | Effects on 11B-HSD2 activity, mRNA, prot: NO | Rajakumar et al. (2015) |
| 1.095, 2.19, 4.38 | 24 | Effects on 11B-HSD2 activity, mRNA, prot: YES | ||
| 8.76 | 3 | Effects on 11B-HSD2 activity, mRNA, prot: NO | ||
| 6, 12 | Effects on 11B-HSD2 activity, mRNA, prot: YES | |||
| 24 | Effects on 11B-HSD2 activity, mRNA, prot: YES Effects on nutrient transport- and hormone-related mRNA: YES |
|||
| HTR-8/Svneo | 1×10−9 | 24, 48 | Effect on viability: NO, proliferation: NO, migration: NO, invasion: NO, DNA content: NO | Spagnoletti et al. (2015) |
| 72 | Effect on viability: NO, proliferation: NO, migration: NO, invasion: NO, DNA content: YES | |||
| 1×10−7 | 24, 48 | Effect on viability: NO, proliferation: NO, migration: NO, invasion: NO, DNA content: YES | ||
| 72 | Effect on viability: NO, proliferation: NO, migration: NO, invasion: YES, DNA content: YES | |||
| 1×10−5 | 24 | Effect on viability: NO, proliferation: NO, migration: NO, invasion: NO, DNA content: NO, interaction w/ HUVECs: NO | ||
| 48 | Effect on viability: NO, proliferation: NO, migration: YES, invasion: YES, DNA content: YES (not reversed by ICI), interaction w/ HUVECs: NO | |||
| 72 | Effect on viability: NO, proliferation: NO, migration: YES, invasion: YES, DNA content: YES; differentiation: NO, cell cycle-related protein: NO | |||
| 1×10−3 | 24 | Effect on viability: NO, proliferation: NO, migration: NO, invasion: NO, DNA content: NO | ||
| 48 | Effect on viability: NO, proliferation: NO, migration: YES, invasion: YES, DNA content: YES | |||
| 72 | Effect on viability: NO, proliferation: NO, migration: YES, invasion: YES, DNA content: YES; differentiation: YES, cell cycle-related protein: YES | |||
| 0.1 | 24 | Effect on viability: NO, proliferation: NO, migration: NO, invasion: NO, DNA content: NO | ||
| 48 | Effect on viability: NO, proliferation: NO, migration: YES, invasion: YES, DNA content: YES | |||
| 72 | Effect on viability: NO, proliferation: NO, migration: YES, invasion: YES, DNA content: YES; differentiation: YES, cell cycle-related protein: NO | |||
| 1° in first trimester | 1×10−3 | 24, 48 | Effects on chemical efflux-related protein: NO | Sieppi et al. (2016) |
| 1° at term | 24 | Effects on chemical efflux-related protein/mRNA: NO | ||
| 48 | Effects on chemical efflux-related protein: YES—partly reversed by ICI mRNA: NO | |||
| 1° in first trimester | 0.1 | 24, 48 | Effects on chemical efflux-related protein: NO | |
| 1° at term | 24 | Effects on chemical efflux-related protein: NO; mRNA: YES | ||
| 48 | Effects on chemical efflux-related protein/mRNA: NO | |||
| JEG-3 | 0.01–500 | 24 | Effects on cytotoxicity at 138–218 μM: YES; ROS generation: YES; aromatase activity at IC50=71 μM: YES | Perez-Albaladejo et al. (2017) |
| HTR-8/Svneo | 0.1 | 24 | Effects on migration: NO | Lan et al. (2017) |
| 1, 10, 50 | Effects on migration: YES | |||
| 0.1 | 48 | Effects on proliferation: NO, migration: YES; invasion-related proteins: NO; adhesion-related protein: YES (NO mRNA), mesenchymal proteins/mRNA: NO | ||
| 1 | Effects on proliferation: NO, migration: YES; invasion-related proteins: SOME; adhesion-related protein: YES (NO mRNA), mesenchymal proteins/mRNA: NO. Effects potentially through ERK signaling | |||
| 10, 50 | Effects on proliferation: NO, migration: YES; invasion-related proteins: YES; adhesion-related protein and mRNA: YES, mesenchymal proteins/mRNA: NO | |||
| 1° villi in first trimester | 50 | 48, 72 | Effects on migration: YES; adhesion-related protein: NO |
Doses from studies were converted to μM to facilitate comparison across studies.
Authors also reported increased binding of CRE at the CRH promoter and CREB phosphorylation by BPA, but quantified data were not presented to include in this dose-response table.
Table 4.
Effects of Phthalates in Placental In Vitro Models
| Cell Model | μM Phthalates | Phthalate | Treatment Length (h) | Outcomes | Ref. |
|---|---|---|---|---|---|
| HRP-1 | 25, 50, 100, 200 | DEHP, MEHP, EHA | mRNA:4 Prot: 12 | Effects on lipid-related mRNA/prot expression: SOME (also some differences by phthalate metabolite). | Xu et al. (2005) |
| 50 | DEHP, MEHP, EHA | 2, 4, 8, 12, 24 | Effects on lipid-related mRNA/prot: SOME (also some differences by phthalate metabolite; effects on mRNA tended to precede protein response, without consistent time-specific patterns) | ||
| 24 | Effects on fatty acid distribution: SOMEEffects on transport of arachidonic acid: YES (MEHP, EHA), and docosahexaenoic acid: YES (DEHP) | ||||
| HRP-1 | 50 | DEHP | 24 | Effects on free fatty acid: YES, cholesterol esters: NO, diacylglycerol: NO, triacylglycerol: YES, phosphatidylcholine: NO, phosphatidylethanolamine: YES, phosphatidylserine: YES, lysophosphatidylcholine: YES, sphingomyelin: NO, saturated fatty acids: YES, monounsaturated fatty acids: NO, polyunsaturated fatty acids: YES, ω3 fatty acids: SOME, ω6 fatty acids: SOME, ω7 fatty acids: NO, ω9 fatty acids: NO | Xu et al. (2006) |
| MEHP | Effects on free fatty acids: YES, cholesterol esters: YES, diacylglycerol: YES, triacylglycerol: YES, phosphatidylcholine: NO, phosphatidylethanolamine: YES, phosphatidylserine: NO, lysophosphatidylcholine: YES, sphingomyelin: YES saturated fatty acids: YES, monounsaturated fatty acids: YES, polyunsaturated fatty acids: YES, ω3 fatty acids: SOME, ω6 fatty acids: SOME, ω7 fatty acids: YES, ω9 fatty acids: YES | ||||
| EHA | Effects on free fatty acids: YES, cholesterol esters: YES, diacylglycerol: YES, triacylglycerol: YES, phosphatidylcholine: YES, phosphatidylethanolamine: YES, phosphatidylserine: NO, lysophosphatidylcholine: NO, sphingomyelin: NO, saturated fatty acids: YES, monounsaturated fatty acids: YES, polyunsaturated fatty acids: YES, ω3 fatty acids: YES, ω6 fatty acids: YES, ω7 fatty acids: YES, ω9 fatty acids: YES↑, ↑, -- | ||||
| HTR-8/ Svneo | 11.25 | MEHP | 1 | Effect on ROS production: NO | Tetz et al. (2013) |
| 22.5 | 1 | Effect on ROS production: NO | |||
| 24 | Effects on marker of apoptosis: NO, viability: NO | ||||
| 48 | Effect on viability: NO | ||||
| 45 | 1 | Effect on ROS production: YES | |||
| 24 | Effects on marker of apoptosis: NO, viability: NO | ||||
| 48 | Effect on viability: NO | ||||
| 90 | 1 | Effect on ROS production: YES | |||
| 4, 8 | Effect on PTGS2 (COX2) mRNA: YES | ||||
| 24 | Effects on marker of apoptosis: NO, viability: NO; PTGS2 (COX2) mRNA: NO, other oxidative stress-related mRNA: NO | ||||
| 48 | Effect on viability: NO | ||||
| 180 | 1 | Effect on ROS production: YES | |||
| 4, 8 | Effect on PTGS2 (COX2) mRNA: YES | ||||
| 24 | Effects on marker of apoptosis: YES, viability: NO, PTGS2 (COX2) mRNA: YES, other oxidative stress-related mRNA: SOME | ||||
| 48 | Effect on viability: YES | ||||
| HTR-8/Svneo | 10 | MEHP | 72 | Effect on viability: NO | Meruvu et al. (2015) |
| 25 | 2, 4, 8 | Effect on ROS production: YES | |||
| 72 | Effects on ROS production: NO, cytotoxicity: NO | ||||
| 50 | 2 | Effect on ROS production: YES | |||
| 4, 8 | Effects on ROS production: YES, miR-16: NO | ||||
| 24 | Effect on miR-16: NO | ||||
| 48 | Effects on apoptosis: NO, miR-16: NO | ||||
| 72 | Effects on viability: NO, cytotoxicity: NO, miR-16: NO, ROS production: NO | ||||
| 100 | 2 | Effect on ROS production: YES | |||
| 4 | Effects on ROS production: YES, miR-16: YES; BCL-2 mRNA: YES | ||||
| 8 | Effects on ROS production: YES, miR-16: NO | ||||
| 24 | Effects on miR-16: NO, BCL-2 mRNA: NO | ||||
| 48 | Effects on apoptosis: YES, miR-16: YES, BCL-2 mRNA: NO | ||||
| 72 | Effects on ROS production: NO, viability: NO, cytotoxicity: NO; miR-16 inhibition prevents MEHP-induced ↓BCL-2/BAX | ||||
| 180 | 2 | Effect on ROS production: YES | |||
| 4 | Effects on ROS production: YES, miR-16: YES; BCL-2 mRNA: YES | ||||
| 8 | Effects on ROS production: YES, miR-16: YES | ||||
| 24 | Effects on miR-16: YES; BCL-2 mRNA: YES | ||||
| 48 | Effectss on apoptosis: NO, miR-16: YES; BCL-2 mRNA: YES | ||||
| 72 | Effects on ROS production: YES, cytotoxicity: YES (180 and 360 uM); Effect on viability: YES; miR-16 inhibition prevents MEHP-induced ↓BCL-2/BAX | ||||
| 360 | 72 | Effects on ROS production: YES, cytotoxicity: YES | |||
| THP-1, 1° placental macropha-ges (PM) | 10, 45 | MEHP | 24 | Effects on PGE2 release (PM): NO, PGF2 release (PM): NO | Tetz et al. (2015) |
| 90 | 24 | Effects on PGE2 release (PM): NO, PGF2 release (PM): YES | |||
| 180 | 2, 4 | Effect on PGE2 release (PM): NO | |||
| 8 | Effects on PGE2 release (PM): NO, COX1 prot (THP-1 and PM): NO, COX2 prot (THP-1 and PM): YES | ||||
| 24 | Effects on PGE2 and PGF2 release (PM): YES; COX and COX-2 inhibition decreased MEHP-stimulated PGE2 release (THP-1 and PM) | ||||
| HTR-8/Svneo | 25 | MEHP | 24 | Effects on viability: NO, ROS production: YES | Meruvu et al. (2016) |
| 48 | Effect on viability: NO | ||||
| 50 | 4 | Effects on miR expression: NO | |||
| 24 | Effects on viability: NO, ROS production: YES | ||||
| 48 | Effects on viability: NO, miR expression: NO | ||||
| 100 | 4 | Effects on miR expression: SOME, cell cycle- and oxidative stress-related mRNA: SOME | |||
| 24 | Effects on viability: NO, ROS production: YES, cell cycle- and oxidative stress-related mRNA: YES | ||||
| 48 | Effects on viability: NO, miR expression: NO, cell cycle- and oxidative stress-related mRNA: YES | ||||
| 180 | 4 | Effects on miR expression: YES, cell cycle- and oxidative stress-related mRNA: YES | |||
| 24 | Effects on viability: YES, ROS production: YES, cell cycle- and oxidative stress-related mRNA: YES | ||||
| 48 | Effects on viability: YES, miR expression: YES, cell cycle- and oxidative stress-related mRNA: YES | ||||
| 1° cytotropho-blasts | 1, 10, 50, 150, 300, 500 | MEHP | 24 | Effect on cell survival: YES (300 and 500 μM) | Wang et al. (2016) |
| 1, 10, 50 | MEHP | ? | Effects on CRH and COX-2 expression: NO | ||
| 100, 150 | MEHP | 24 | Effects on CRH and COX-2 mRNA/prot: YES, related nuclear factor protein and localization at CRH and COX-2 promoters: YES, potentially through NIK nuclear factor | ||
| JEG-3 microsomes | 100 | Di-phthalates | 12 | Effects on aromatase activity: by DCHP and BBOP: YES, by DMP, DEP, DPrP, DBP, DPP, DHP, DEHP, DNOP DINP: NO | Xu, et al. (2016) |
| Effects on progesterone production: by DPrP, DBP, DPP, DCHP, BBOP, DHP (DEHP, DNOP, DINP with 8Br-cAMP stimulation): YES, by DMP, DEP, DEHP, DNOP, DINP: NO | |||||
| Effects on estradiol production: by DCHP, BBOP: YES, by DPrP, DBP, DPP, DHP, DMP, DEP, DEHP, DNOP, DINP: NO | |||||
| Mono-phthalates | Effects on aromatase activity: by MMP, MBP, MEHP: NO | ||||
| Effects on progesterone production: by MBP, MEHP: YES, by MMP: NO | |||||
| Effects on estradiol production: by MMP, MBP, MEHP: NO | |||||
| HTR-8/Svneo | 1 | MEHP | 24 | Effects on viability or necrosis: NO, invasion: NO, invasion-related protein/mRNA expression/activity: NO/NO/NO | Gao et al. (2017) |
| 10 | Effects on viability or necrosis: NO, invasion: YES, invasion-related protein/mRNA expression/activity: YES/NO/NO | ||||
| 100 | Effects on viability or necrosis: NO, invasion: YES, invasion-related protein/mRNA expression/activity: YES/NO/YES | ||||
| PPAR-γ inhibitors reversed: MEHP-induced decrease in invasion and invasion-related protein activity. | |||||
| 200 | Effects on viability or necrosis: NO, invasion: YES, invasion-related protein/mRNA expression/activity: YES/NO/YES | ||||
| JEG-3 | 0.01–500 | DMP | 24 | Effects on cytotoxicity: NO, ROS production: NO, aromatase activity: YES (at >50 μM); treatment concentrations were above those in media after 24 h | Perez-Albaladejo et al. (2017) |
| DBP | Effects on cytotoxicity: YES (at 466 μM), ROS production: NO, aromatase activity (YES: 104 μM); treatment concentrations were above those in media after 24 h | ||||
| BBP | Effects on cytotoxicity NO, ROS production: NO, aromatase activity (YES: 167 μM); treatment concentrations were above those in media after 24 h | ||||
| DEHP | Effects on cytotoxicity NO, ROS production: NO, aromatase activity: NO; treatment concentrations were above those in media after 24 h |
Prot, protein; DEHP, di(2-ethylhexyl)phthalate; EHA, 2-ethylhexanoic acid; MEHP, mono-(2-ethylhexyl) phthalate.
Cell Models
Models assessing the impacts of environmental chemicals on placental molecular signaling have included immortalized cell lines, primary trophoblasts, cytotrophoblasts, microsomes, chorionic villi explants, and placental macrophages. For both immortalized and primary cells, the differences between their signaling and physiological responses to treatments represent fundamental differences in physiological functions of cells from which they are derived (Bilban et al., 2010). For example, in human HTR-8/SVneo placental cells, BPA had no effect on cell viability or proliferation, whereas a range of BPA concentrations decreased cell migration and invasion, reflecting the invasive nature of these first-trimester-derived trophoblasts (Spagnoletti et al., 2015). The invasive nature of HTR-8/SVneo cells was confirmed in another study, showing that BPA increased MMP-9 (but not MMP-2) protein, involved in cell invasion and migration (Lan et al., 2017), and both proteins were increased by BPA in BeWo cells, a first-trimester-derived trophoblast choriocarcinoma cell line (Wang et al., 2015).
Differences in gene transcription underlie the functional differences between placental cell lines and cell types, and may likely regulate their response to environmental chemicals. For example, in both JEG-3 choriocarcinoma immortalized cells and PL30 cells derived from third-trimester placentas, BPA concentration-dependently inhibited CYP19 gene expression, but the baseline expression of CYP19 appeared to be much greater in JEG-3 than in PL30 cells, reflecting the innate differences of an important hormonal regulator between these cells (Huang and Leung, 2009). A microarray study in several SV40-transformed placental cell lines showed that BPA altered 25 miRs in 3 A cells and 60 miRs in HTR-8 cells, without significant changes in TCL-1 cells (Avissar-Whiting et al., 2010). Authors suggested that these contrasting responses to BPA are likely due to stage-specific differences between cell lines, as HTR-8 cells are derived from first trimester extravillous cells from the termination of a normal pregnancy and 3 A cells from first trimester villous cells, whereas TCL-1 cells come from third trimester villous cells. Although this is a possible explanation, the maintenance and growth of these cells often varies depending on the supplier and even from lab-to-lab, so additional systematic studies may be needed to confirm these findings in primary cell lines.
The mechanisms by which BPA targets the placenta are still largely unknown, but there is some evidence that different cells may uniquely interact with specific toxicants. The estrogen-related receptor gamma (ERRγ1), a receptor that binds BPA with high affinity, has been suggested to drive the preferential accumulation of BPA within the placenta (Takeda et al., 2009). In one study, the baseline mRNA expression of ERRγ1 was higher in JEG-3 and human isolated extravillous cytotrophoblasts than in BeWo cells. Importantly, the expression of ERRγ1 was 20–25 and 50-fold higher in first and third trimester placentas, respectively, when compared with JEG-3 cells. Other ERRγ subtypes (ERRγ2 and ERRγ3) were not expressed at all in JEG-3 or BeWo cells, but were expressed in first-trimester placentas. Furthermore, in JEG-3, but not BeWo cells, BPA concentration-dependently decreased a marker of DNA synthesis rate partly through ERRγ (Morice et al., 2011). These studies in a variety of cell models suggest that, as with well-known cell models of cancer initiation, progression, and metastasis, future placental studies must select cell models based on their genetic and developmental characteristics. For example, immortalized cells from first trimester placentas (eg, HTR-8, BeWo, JEG-3) may be better suited for studies related to invasion and vascularization, whereas third trimester immortalized cells (eg, TCL-1) or primary cells from term placentas may be best when assessing effects on placental nutrient transfer or hormonal signaling.
BPA Concentration and Treatment Length
BPA’s nonmonotonicity and mechanism-of-action require careful selection of both treatment concentrations and timing. For example, in human placental chorionic villi explants, BPA increased a proinflammatory cytokine associated with immune adaptations in pregnancy after 24 h of treatment, but the effect was absent at 48 h (Mannelli et al., 2014). In primary cytotrophoblasts from term placentas of uncomplicated pregnancies treated with 0.0002 to 0.2 μg/ml BPA, apoptosis was induced by 0.02–200 μg/ml BPA, but not by the 2 lower concentrations, whereas necrosis and tumor necrosis factor α (TNF-α) mRNA and protein were induced by the lower BPA concentrations. Conversely, several higher concentrations of BPA had no effect on TNF-α mRNA, decreased TNF-α protein, and decreased necrosis (Benachour and Aris, 2009). In primary cultures of human placental trophoblasts, 11-β-dehydrogenase isozyme 2 (11β-HSD2) activity, protein content, and gene expression were also concentration-dependently increased by BPA (increased by 0.25–2.0 μg/ml but not 0.1 μg/ml BPA) (Rajakumar et al., 2015). Another study in placental explants showed that in term but not first trimester placental villus cultures, 48-h treatment with 1 nM but not 100 nM BPA decreased the ABCG2 protein, which transports a variety of compounds in human placental syncytiotrophoblasts (Sieppi et al., 2016), highlighting the importance of both chemical concentration and developmental timing.
Similarly, in immortalized cells, the mRNA and protein expression of CRH in JEG-3 cells was increased by 25 and 50 μM BPA, but not by 1 or 5 μM BPA (Huang et al., 2012). In addition, in JEG-3 cells, BPA had both time- and concentration-dependent effects on aromatase activity, increasing activity at some concentrations and treatment windows and decreasing activity at others (Nativelle-Serpentini et al., 2003). In BeWo cells, BPA increased cell proliferation at 1 and 10 μM, whereas other concentrations (0.01, 0.1, and 100 μM) had no impact on proliferation, and the highest concentration (1000 μM) decreased proliferation. These outcomes were accompanied by dose-specific effects on E-cadherin mRNA and protein, but relatively consistent effects across doses on invasion and markers of invasion (MMP-9, MMP-2, TIMP-1, and TIMP-2 protein) (Wang et al., 2015). Similarly, in HTR-8/SVneo cells, BPA had both concentration- and time-specific effects on cell migration and concentration-specific effects on markers of invasion (MMP-9 and TIMP-3 protein) (Lan et al., 2017). Taken together, these studies confirm the concentration- and time-dependent nature of BPA’s effects on downstream targets and highlight the importance of selecting physiologically relevant concentrations and treatment lengths for investigating potential mechanisms of action of BPA using in vitro models. Specifically, many of these studies support the nonmonotonicity of BPA—where effects on some pathways were only observed at lower or higher concentrations, whereas other pathways were targeted by the lowest and highest, but not middle BPA concentrations. This suggests that while both low and high concentrations of BPA impact placental pathways, BPA’s mechanisms of action may differ at the 2 ends of the dose-response curve. These data also reinforce the need for extensive dose-response curves that first: include the expected range of exposure in humans and second: allow for the interpretation of BPA concentration-specific mechanisms-of-action within the placenta.
Phthalate Concentration and Treatment Length
Numerous in vitro studies have assessed the effects of MEHP, the major DEHP metabolite, on placental cellular endpoints. For example, CRH and COX-2 regulate parturition in humans and have been linked with preterm birth, and in human placental cytotrophoblasts, only higher concentrations of MEHP (100 μM and 150 μM) increased the expression of these proteins (Wang et al., 2016). In HTR-8/SVneo cells treated with a range of MEHP concentrations, only the highest concentration (180 μM) increased ROS production, oxidative DNA damage, and apoptosis, whereas both 90 and 180 μM concentrations induced the mRNA expression of prostaglandin-endoperoxide synthase 2 (PTGS2/COX-2) (Tetz et al., 2013). In THP-1 cells (human monocyte cells derived from an infant with acute monocytic leukemia), this group later showed that only the highest concentration (180 μM, after 24 h of treatment, not 2–8 h) induced prostaglandin E2 release (Tetz et al., 2015). A more recent study in HTR-8/SVneo cells showed that invasion was decreased and TIMP-1 protein increased by 10, 100, and 200 μM MEHP, but not by the lowest concentration (1 μM), whereas MMP-9 activity was only decreased at 100 and 200 μM MEHP (Gao et al., 2017). In addition, in HTR-8/SVneo cells, 50, 100, or 180 μM MEHP induced ROS production, but the most pronounced effects were at the highest concentration and primarily after 72 h. In another study, 50 μM MEHP had no effect on miR-16 (which authors discussed is altered in pregnancy pathologies), whereas 100 μM MEHP increased miR-16 at 4 and 48 h, and 180 μM increased miR-16 at all timepoints (4, 8, 24, 48 h) (Meruvu et al., 2015). The same group later showed that intracellular ROS production in HTR-8/SVneo cells was increased by a range of MEHP concentrations, but the expression of several miRs was only increased at higher concentrations (100 or 180 μM), with the exception of miR-17-5p (Meruvu et al., 2016). These concentrations correspond to 30 and 54 μg/ml MEHP (respectively), whereas pregnant women have been shown to have much lower median plasma concentrations of 0.68 ± 0.85 μg/ml (Latini et al., 2003), and ranging from 0.17 to 6.74 μg/ml (Li et al., 2013). These studies suggest that most MEHP concentrations affect transcription and translation, but that cellular phenotypic changes are typically only observed at higher MEHP concentrations. It is possible that the expected chronic low-dose MEHP exposure in humans can be modeled in cells with short-term treatment at higher concentrations. However, it is more likely that as with BPA, MEHP’s mechanisms-of-action differ at the extremes of the concentration curve. Therefore, in order to understand the mechanisms by which MEHP affects the placenta, more studies are warranted assessing a variety of endpoints using broader and more human-relevant concentration curves.
As previously described, pregnant women are exposed daily to an array of phthalates. However, few cell studies have assessed outcomes in response to DEHP metabolites other than MEHP. However, 2 in vitro studies treated rat HRP-1 placental cells with DEHP or 2 of its metabolites, MEHP and 2-ethylhexanoic acid (EHA). DEHP and both metabolites had concentration- and time-specific effects on the expression of placental fatty acid uptake and metabolism-related genes. All 3 also increased the uptake rates of several essential fatty acids, whereas only MEHP and EHA increased arachidonic acid transport, and only DEHP increased DHA transport (Xu et al., 2005). In a follow-up study in HRP-1 cells, DEHP, MEHP, and EHA also had unique effects on the overall contents of several lipid classes (Xu et al., 2006).
Another current limitation in the field is that only 2 in vitro studies thus far have investigated phthalates other than DEHP and its metabolites. One assessed the structure-activity relationship of 100 μM of 11 diphthalates and 3 monophthalates on progesterone and estradiol production in JEG-3 cells. Estradiol production was only decreased by DCHP and BBOP, whereas 5 diphthalates decreased progesterone production, with no effects of monophthalates on either hormone (Table 4) (Xu et al., 2016), suggesting that the ability of phthalates to inhibit aromatase and 3β-hydroxysteroid dehydrogenase 1 (3β-HSD1), and to decrease estradiol and progesterone production is highly dependent on their chemical structure. Another study treated JEG-3 cells with 0.01–500 μM DMP, DBP, BBP, and DEHP. None of the phthalates affected ROS production, but DMP increased aromatase activity at concentrations >50 μM, DBP and BBP decreased aromatase activity at IC50 = 104 and 167 μM (respectively), whereas DEHP had no effect on aromatase activity (Perez-Albaladejo et al., 2017). Phthalate mixtures used in other experimental models have shown their distinct phenotypic effects compared with DEHP alone (Zhou et al., 2017a,b). Such approaches in placental studies will be valuable for establishing these chemicals’ mechanisms of action when present as complex mixtures, as they are in humans.
CONCLUSIONS AND FUTURE DIRECTIONS
Placental Actions of BPA and Phthalates
As summarized in Tables 1–4, studies assessing effects of BPA or phthalates on placental outcomes in experimental animal and cell models have focused on hormones, epigenetics, inflammation/oxidative stress, cellular damage, and nutrient transfer.
BPA and phthalates are known endocrine disruptors, and there is substantial evidence that BPA can disrupt placental hormones, their receptors, or regulatory enzymes, whereas studies related to the effects of phthalates on placental hormones are more limited. In addition to hormones, placental nutrient transfer capacity is the best-characterized measure of placental efficiency (Burton and Fowden, 2012). Therefore, more studies are warranted in all models to investigate the effects of BPA and phthalates on placental energy metabolism, and fetal nutrient supply.
Although there is great interest in the role of epigenetics in placental development, additional data are needed related to the ability of BPA and phthalates to disrupt placental epigenetic signaling in experimental models. Currently, evidence for the ability of BPA or phthalates to induce placental inflammation or oxidative stress comes primarily from cell models; given that oxidative stress and inflammation are proposed to mediate associations of BPA or phthalate exposure with pregnancy outcomes (Ferguson et al., 2017; Veiga-Lopez et al., 2015; Watkins et al., 2015), more studies in animal and cell models are warranted to investigate these mechanisms. Finally, there is substantial evidence from experimental models that BPA and phthalates cause frank damage to placental vasculature, structure, and function. However, these findings should be substantiated in animal models using concentrations of both chemicals that are in line with human exposures.
Summary of Animal and Cell Models
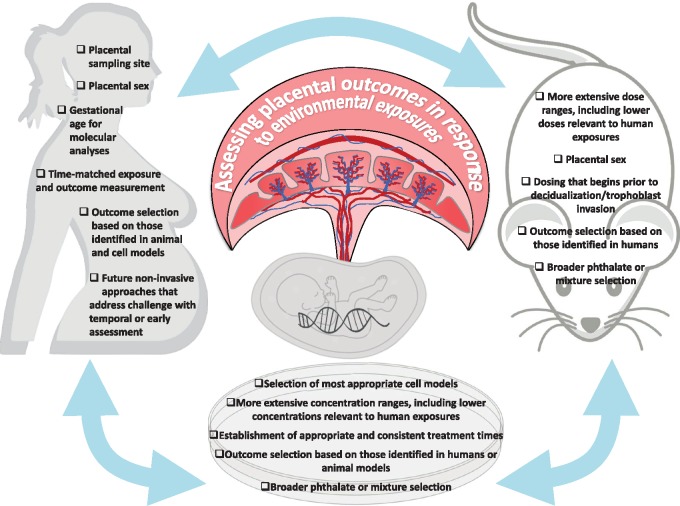
Given the challenges in human placental sampling (Figure 2), various experimental models have been employed to address mechanisms of placental disruption by BPA and phthalates. However, these studies vary in their design and measured outcomes, and few accurately reflect human exposures to these chemical. Therefore, both animal and cell models would benefit from use of more extensive BPA/phthalate doses and concentrations (Figure 2). Furthermore, exposures in animal models should ideally begin prior to decidualization, and treatment in cell models should be timed appropriately, with more extensive time ranges. Assessing sex-specific outcomes in animal models is also critical, as is the selection of precisely applicable cell models. In the case of phthalates, future animal and cell studies should assess exposure to a wider variety of phthalates (beyond DEHP and its metabolites), and at more relevant doses, with the ultimate goal of modeling more complex exposures to the mixtures of phthalates that occur in humans. Finally, given broad outcome selection in animal and cell models, a useful approach moving forward would be to design mechanistic animal and cell studies that parallel placental endpoints altered in humans in response to BPA and phthalates. Such approaches will be indispensable in helping to unravel the mechanisms involved in placental toxicity and the placenta-mediated effects of EDCs on fetal development.
Figure 2.
An integrated approach between human, animal, and cell studies will be needed to determine the precise effects of environmental exposures on the placenta. To accomplish this, as illustrated in the figure, numerous factors should be carefully considered when establishing animal and in vitro models, and using findings from animal or in vitro studies to inform questions in humans. Furthermore, these factors should be taken into account when drawing conclusions regarding mechanisms of action of BPA and phthalates from the currently available literature.
FUNDING
This publication was supported by the National Institute of Environmental Health Sciences grant K99ES024795A and R00ES024795 (to R.S.), and ES022848 (to S.S.), and U.S. Environmental Protection Agency grant RD83543401 (to S.S.). National Institute of Health Office of the Director grant OD023272 (to SS). Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication. The authors declare no financial conflict of interest.
REFERENCES
- Adamson S. L., Lu Y., Whiteley K. J., Holmyard D., Hemberger M., Pfarrer C., Cross J. C. (2002). Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev. Biol. 250, 358–373. [DOI] [PubMed] [Google Scholar]
- Adibi J. J., Buckley J. P., Lee M. K., Williams P. L., Just A. C., Zhao Y., Bhat H. K., Whyatt R. M. (2017). Maternal urinary phthalates and sex-specific placental mRNA levels in an urban birth cohort. Environ. Health 16, 35.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi J. J., Hauser R., Williams P. L., Whyatt R. M., Thaker H. M., Nelson H., Herrick R., Bhat H. K. (2009). Placental biomarkers of phthalate effects on mRNA transcription: Application in epidemiologic research. Environ. Health 8, 20.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi J. J., Whyatt R. M., Hauser R., Bhat H. K., Davis B. J., Calafat A. M., Hoepner L. A., Perera F. P., Tang D., Williams P. L. (2010). Transcriptional biomarkers of steroidogenesis and trophoblast differentiation in the placenta in relation to prenatal phthalate exposure. Environ. Health Perspect. 118, 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahbab M. A., Guven C., Kockaya E. A., Barlas N. (2017). Comparative developmental toxicity evaluation of di-n-hexyl phthalate and dicyclohexyl phthalate in rats. Toxicol. Ind. Health 33, 696–716. [DOI] [PubMed] [Google Scholar]
- Albrecht E. D., Pepe G. J. (2010). Estrogen regulation of placental angiogenesis and fetal ovarian development during primate pregnancy. Int. J. Dev. Biol. 54, 397408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar-Whiting M., Veiga K. R., Uhl K. M., Maccani M. A., Gagne L. A., Moen E. L., Marsit C. J. (2010). Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod. Toxicol. 29, 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benachour N., Aris A. (2009). Toxic effects of low doses of Bisphenol-A on human placental cells. Toxicol. Appl. Pharmacol. 241, 322–328. [DOI] [PubMed] [Google Scholar]
- Benachour N., Moslemi S., Sipahutar H., Seralini G. E. (2007). Cytotoxic effects and aromatase inhibition by xenobiotic endocrine disrupters alone and in combination. Toxicol. Appl. Pharmacol. 222, 129–140. [DOI] [PubMed] [Google Scholar]
- Benirschke K., Kaufmann P. (1995). Pathology of the Human Placenta, 3rd ed Springer-Verlag, New York. [Google Scholar]
- Ben-Zimra M., Koler M., Melamed-Book N., Arensburg J., Payne A. H., Orly J. (2002). Uterine and placental expression of steroidogenic genes during rodent pregnancy. Mol. Cell Endocrinol. 187, 223–231. [DOI] [PubMed] [Google Scholar]
- Bilban M., Tauber S., Haslinger P., Pollheimer J., Saleh L., Pehamberger H., Wagner O., Knofler M. (2010). Trophoblast invasion: Assessment of cellular models using gene expression signatures. Placenta 31, 989–996. [DOI] [PubMed] [Google Scholar]
- Burton G. J., Fowden A. L. (2012). Review: The placenta and developmental programming: Balancing fetal nutrient demands with maternal resource allocation. Placenta 33, S23–S27. [DOI] [PubMed] [Google Scholar]
- Carter A. M. (2007). Animal models of human placentation – a review. Placenta 28(Suppl. A), S41–S47. [DOI] [PubMed] [Google Scholar]
- CDC. (2017). Centers for Disease Control and Prevention (CDC) Fourth national report on human exposure to environmental chemicals. https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Mar2018.pdf, last accessed September 19, 2018.
- Challis J. R., Lockwood C. J., Myatt L., Norman J. E., Strauss J. F., Petraglia F. (2009). Inflammation and pregnancy. Reprod. Sci. 16, 206–215. [DOI] [PubMed] [Google Scholar]
- Coan P. M., Angiolini E., Sandovici I., Burton G. J., Constancia M., Fowden A. L. (2008). Adaptations in placental nutrient transfer capacity to meet fetal growth demands depend on placental size in mice. J. Physiol. 586, 4567–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole L. A. (2010). Biological functions of hCG and hCG-related molecules. Reprod. Biol. Endocrinol. 8, 102.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court F., Tayama C., Romanelli V., Martin-Trujillo A., Iglesias-Platas I., Okamura K., Sugahara N., Simon C., Moore H., Harness J. V., et al. (2014). Genome-wide parent-of-origin DNA methylation analysis reveals the intricacies of human imprinting and suggests a germline methylation-independent mechanism of establishment. Genome Res. 24, 554–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerge D. R., Twaddle N. C., Vanlandingham M., Fisher J. W. (2011). Pharmacokinetics of bisphenol A in neonatal and adult CD-1 mice: Inter-species comparisons with Sprague-Dawley rats and rhesus monkeys. Toxicol. Lett. 207, 298–305. [DOI] [PubMed] [Google Scholar]
- Douglas A. J., Johnstone L. E., Leng G. (2007). Neuroendocrine mechanisms of change in food intake during pregnancy: A potential role for brain oxytocin. Physiol. Behav. 91, 352–365. [DOI] [PubMed] [Google Scholar]
- Ferguson K. K., Chen Y. H., VanderWeele T. J., McElrath T. F., Meeker J. D., Mukherjee B. (2017). Mediation of the Relationship between Maternal Phthalate Exposure and Preterm Birth by Oxidative Stress with Repeated Measurements across Pregnancy. Environ. Health Perspect. 125, 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M., Arbuckle T. E., Mallick R., LeBlanc A., Hauser R., Feeley M., Koniecki D., Ramsay T., Provencher G., Bérubé R., et al. (2015). Bisphenol A and phthalate metabolite urinary concentrations: Daily and across pregnancy variability. J. Expo. Sci. Environ. Epidemiol. 25, 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca B. M., Correia-da-Silva G., Teixeira N. A. (2012). The rat as an animal model for fetoplacental development: A reappraisal of the post-implantation period. Reprod. Biol. 12, 97–118. [DOI] [PubMed] [Google Scholar]
- Freemark M., Kirk K., Pihoker C., Robertson M. C., Shiu R. P., Driscoll P. (1993). Pregnancy lactogens in the rat conceptus and fetus: Circulating levels, distribution of binding, and expression of receptor messenger ribonucleic acid. Endocrinology 133, 1830–1842. [DOI] [PubMed] [Google Scholar]
- Frost J. M., Moore G. E. (2010). The importance of imprinting in the human placenta. PLoS Genet. 6, e1001015.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F., Hu W., Li Y., Shen H., Hu J. (2017). Mono-2-ethylhexyl phthalate inhibits human extravillous trophoblast invasion via the PPARgamma pathway. Toxicol. Appl. Pharmacol. 327, 23–29. [DOI] [PubMed] [Google Scholar]
- Gingrich J., Pu Y., Roberts J., Karthikraj R., Kannan K., Ehrhardt R., Veiga-Lopez A. (2018). Gestational bisphenol S impairs placental endocrine function and the fusogenic trophoblast signaling pathway. Arch. Toxicol. 92, 1861–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey K. M. (2002). The role of the placenta in fetal programming-a review. Placenta 23(Suppl. A), S20–S27. [DOI] [PubMed] [Google Scholar]
- Grindler N. M., Vanderlinden L., Karthikraj R., Kannan K., Teal S., Polotsky A. J., Powell T. L., Yang I. V., Jansson T. (2018). Exposure to Phthalate, an Endocrine Disrupting Chemical, Alters the First Trimester Placental Methylome and Transcriptome in Women. Sci. Rep. 8, 6086.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. M., Cheong A., Lam H. M., Hu W. Y., Shi G. B., Zhu X., Chen J., Zhang X., Medvedovic M., Leung Y. K., et al. (2015). Exposure of Human Prostaspheres to Bisphenol A Epigenetically Regulates SNORD Family Noncoding RNAs via Histone Modification. Endocrinology 156, 3984–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Leung L. K. (2009). Bisphenol A downregulates CYP19 transcription in JEG-3 cells. Toxicol. Lett. 189, 248–252. [DOI] [PubMed] [Google Scholar]
- Huang H., Tan W., Wang C. C., Leung L. K. (2012). Bisphenol A induces corticotropin-releasing hormone expression in the placental cells JEG-3. Reprod. Toxicol. 34, 317–322. [DOI] [PubMed] [Google Scholar]
- Imanishi S., Manabe N., Nishizawa H., Morita M., Sugimoto M., Iwahori M., Miyamoto H. (2003). Effects of oral exposure of bisphenol A on mRNA expression of nuclear receptors in murine placentae assessed by DNA microarray. J. Reprod. Dev. 49, 329–336. [DOI] [PubMed] [Google Scholar]
- Jansson T., Powell T. L. (2007). Role of the placenta in fetal programming: Underlying mechanisms and potential interventional approaches. Clin. Sci. (Lond.) 113, 1–13. [DOI] [PubMed] [Google Scholar]
- Jin H., Audus K. L. (2005). Effect of bisphenol A on drug efflux in BeWo, a human trophoblast-like cell line. Placenta 26(Suppl. A), S96–S103. [DOI] [PubMed] [Google Scholar]
- Kang E. R., Iqbal K., Tran D. A., Rivas G. E., Singh P., Pfeifer G. P., Szabo P. E. (2011). Effects of endocrine disruptors on imprinted gene expression in the mouse embryo. Epigenetics 6, 937–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann P., Davidoff M. (1977). The guinea-pig placenta. Adv. Anat. Embryol. Cell Biol. 53, 5–91. [DOI] [PubMed] [Google Scholar]
- Knofler M., Pollheimer J. (2013). Human placental trophoblast invasion and differentiation: A particular focus on Wnt signaling. Front. Genet. 4, 190.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolatorova L., Vitku J., Hampl R., Adamcova K., Skodova T., Simkova M., Parizek A., Starka L., Duskova M. (2018). Exposure to bisphenols and parabens during pregnancy and relations to steroid changes. Environ. Res. 163, 115–122. [DOI] [PubMed] [Google Scholar]
- Lacroix M. C., Guibourdenche J., Frendo J. L., Muller F., Evain-Brion D. (2002). Human placental growth hormone – a review. Placenta 23(Suppl. A), S87–S94. [DOI] [PubMed] [Google Scholar]
- Lan X., Fu L.-J., Zhang J., Liu X.-Q., Zhang H.-J., Zhang X., Ma M.-F., Chen X.-M., He J.-L., Li L.-B., et al. (2017). Bisphenol A exposure promotes HTR-8/SVneo cell migration and impairs mouse placentation involving upregulation of integrin-beta1 and MMP-9 and stimulation of MAPK and PI3K signaling pathways. Oncotarget 8, 51507–51521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca J., Binder A. M., McElrath T. F., Michels K. B. (2014). The impact of first trimester phthalate and phenol exposure on IGF2/H19 genomic imprinting and birth outcomes. Environ. Res. 133, 396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca J., Binder A. M., McElrath T. F., Michels K. B. (2016). First-trimester urine concentrations of phthalate metabolites and phenols and placenta miRNA expression in a cohort of U.S. women. Environ. Health Perspect. 124, 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G., De Felice C., Presta G., Del Vecchio A., Paris I., Ruggieri F., Mazzeo P. (2003). Exposure to Di(2-ethylhexyl)phthalate in humans during pregnancy. A preliminary report. Biol. Neonate 83, 22–24. [DOI] [PubMed] [Google Scholar]
- Lee J. H., Ahn C., Kang H. Y., Hong E. J., Hyun S. H., Choi K. C., Jeung E. B. (2016). Effects of Octylphenol and Bisphenol A on the Metal Cation Transporter Channels of Mouse Placentas. Int. J. Environ. Res. Public Health 13, 965.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser R., Kaufmann P. (1994). Placental structure: In a comparative aspect. Exp. Clin. Endocrinol. 102, 122–134. [DOI] [PubMed] [Google Scholar]
- Li L. X., Chen L., Meng X. Z., Chen B. H., Chen S. Q., Zhao Y., Zhao L. F., Liang Y., Zhang Y. H. (2013). Exposure levels of environmental endocrine disruptors in mother-newborn pairs in China and their placental transfer characteristics. PLoS One 8, e62526.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtinger R., Zhong J., Mansur A., Adir M., Racowsky C., Hauser R., Brennan K., Karlsson O., Baccarelli A. A. (2018). Placental lncRNA Expression Is Associated With Prenatal Phthalate Exposure. Toxicol. Sci. 163, 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaboob Basha P., Radha M. J. (2017). Gestational di-n-butyl phthalate exposure induced developmental and teratogenic anomalies in rats: A multigenerational assessment. Environ. Sci. Pollut. Res. Int. 24, 4537–4551. [DOI] [PubMed] [Google Scholar]
- Malassine A., Frendo J. L., Evain-Brion D. (2003). A comparison of placental development and endocrine functions between the human and mouse model. Hum. Reprod. Update 9, 531–539. [DOI] [PubMed] [Google Scholar]
- Maltepe E., Bakardjiev A. I., Fisher S. J. (2010). The placenta: Transcriptional, epigenetic, and physiological integration during development. J. Clin. Invest. 120, 1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannelli C., Ietta F., Carotenuto C., Romagnoli R., Szostek A. Z., Wasniewski T., Skarzynski D. J., Paulesu L. (2014). Bisphenol A alters beta-hCG and MIF release by human placenta: An in vitro study to understand the role of endometrial cells. Mediators Inflamm. 2014, 1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maston G. A., Ruvolo M. (2002). Chorionic gonadotropin has a recent origin within primates and an evolutionary history of selection. Mol. Biol. Evol. 19, 320–335. [DOI] [PubMed] [Google Scholar]
- Meruvu S., Zhang J., Bedi Y. S., Choudhury M. (2015). Mono-(2-ethylhexyl) phthalate induces apoptosis through miR-16 in human first trimester placental cell line HTR-8/SVneo. Toxicol. In Vitro. 31, 35–42. [DOI] [PubMed] [Google Scholar]
- Meruvu S., Zhang J., Choudhury M. (2016). Mono-(2-ethylhexyl) phthalate increases oxidative stress responsive miRNAs in first trimester placental cell line HTR8/SVneo. Chem. Res. Toxicol. 29, 430–435. [DOI] [PubMed] [Google Scholar]
- Mervish N., McGovern K. J., Teitelbaum S. L., Pinney S. M., Windham G. C., Biro F. M., Kushi L. H., Silva M. J., Ye X., Calafat A. M., et al. (2014). Dietary predictors of urinary environmental biomarkers in young girls, BCERP, 2004-7. Environ. Res. 133, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk D., Arnaud P., Apostolidou S., Hills F. A., Kelsey G., Stanier P., Feil R., Moore G. E. (2006). Limited evolutionary conservation of imprinting in the human placenta. Proc. Natl. Acad. Sci. U.S.A. 103, 6623–6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morice L., Benaitreau D., Dieudonne M. N., Morvan C., Serazin V., de Mazancourt P., Pecquery R., Dos Santos E. (2011). Antiproliferative and proapoptotic effects of bisphenol A on human trophoblastic JEG-3 cells. Reprod. Toxicol. 32, 69–76. [DOI] [PubMed] [Google Scholar]
- Murray S. A., Morgan J. L., Kane C., Sharma Y., Heffner C. S., Lake J., Donahue L. R. (2010). Mouse gestation length is genetically determined. PLoS One 5, e12418.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar M. S., Liao C., Kannan K., Harris C., Dolinoy D. C. (2015). In utero bisphenol A concentration, metabolism, and global DNA methylation across matched placenta, kidney, and liver in the human fetus. Chemosphere 124, 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nativelle-Serpentini C., Richard S., Seralini G. E., Sourdaine P. (2003). Aromatase activity modulation by lindane and bisphenol-A in human placental JEG-3 and transfected kidney E293 cells. Toxicol. In Vitro 17, 413–422. [DOI] [PubMed] [Google Scholar]
- Newbern D., Freemark M. (2011). Placental hormones and the control of maternal metabolism and fetal growth. Curr. Opin. Endocrinol. Diabetes Obes. 18, 409–416. [DOI] [PubMed] [Google Scholar]
- Padmanabhan R. A., Laloraya M. (2016). Estrogen-Initiated Protein Interactomes During Embryo Implantation. Am. J. Reprod. Immunol. 75, 256–262. [DOI] [PubMed] [Google Scholar]
- Perez-Albaladejo E., Fernandes D., Lacorte S., Porte C. (2017). Comparative toxicity, oxidative stress and endocrine disruption potential of plasticizers in JEG-3 human placental cells. Toxicol. In Vitro 38, 41–48. [DOI] [PubMed] [Google Scholar]
- PrabhuDas M., Bonney E., Caron K., Dey S., Erlebacher A., Fazleabas A., Fisher S., Golos T., Matzuk M., McCune J. M., et al. (2015). Immune mechanisms at the maternal-fetal interface: Perspectives and challenges. Nat. Immunol. 16, 328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumar C., Guan H., Langlois D., Cernea M., Yang K. (2015). Bisphenol A disrupts gene expression in human placental trophoblast cells. Reprod. Toxicol. 53, 39–44. [DOI] [PubMed] [Google Scholar]
- Rosenfeld C. S. (2015). Sex-Specific Placental Responses in Fetal Development. Endocrinology 156, 3422–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J., Cross J. C. (2001). Placental development: Lessons from mouse mutants. Nat. Rev. Genet. 2, 538–548. [DOI] [PubMed] [Google Scholar]
- Rusyn I., Peters J. M., Cunningham M. L. (2006). Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit. Rev. Toxicol. 36, 459–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz L. A., Kota S. K., Feil R. (2010). Genome-wide DNA demethylation in mammals. Genome Biol. 11, 110.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettler T. (2006). Human exposure to phthalates via consumer products. Int. J. Androl. 29, 134–139. discussion 181-5. [DOI] [PubMed] [Google Scholar]
- Sharp A. N., Heazell A. E., Crocker I. P., Mor G. (2010). Placental apoptosis in health and disease. Am. J. Reprod. Immunol. 64, 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R., Zhao L. L., Yu Z., Zhang C., Chen Y. H., Wang H., Zhang Z. H., Xu D. X. (2017). Maternal di-(2-ethylhexyl) phthalate exposure during pregnancy causes fetal growth restriction in a stage-specific but gender-independent manner. Reprod. Toxicol. 67, 117–124. [DOI] [PubMed] [Google Scholar]
- Sibley C. P. (2009). Understanding placental nutrient transfer – why bother? New biomarkers of fetal growth. J. Physiol. 587, 3431–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieppi E., Vahakangas K., Rautio A., Ietta F., Paulesu L., Myllynen P. (2016). The xenoestrogens, bisphenol A and para-nonylphenol, decrease the expression of the ABCG2 transporter protein in human term placental explant cultures. Mol. Cell Endocrinol. 429, 41–9. [DOI] [PubMed] [Google Scholar]
- Silva M. J., Samandar E., Reidy J. A., Hauser R., Needham L. L., Calafat A. M. (2007). Metabolite profiles of di-n-butyl phthalate in humans and rats. Environ. Sci. Technol. 41, 7576–7580. [DOI] [PubMed] [Google Scholar]
- Spagnoletti A., Paulesu L., Mannelli C., Ermini L., Romagnoli R., Cintorino M., Ietta F. (2015). Low concentrations of Bisphenol A and para-Nonylphenol affect extravillous pathway of human trophoblast cells. Mol. Cell Endocrinol. 412, 56–64. [DOI] [PubMed] [Google Scholar]
- Strakovsky R, S., and Schantz, S. L (2018). Impacts of bisphenol A (BPA) and phthalate exposures on epigenetic outcomes in the human placenta. Environmental Epigenetics 4(3), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susiarjo M., Sasson I., Mesaros C., Bartolomei M. S. (2013). Bisphenol a exposure disrupts genomic imprinting in the mouse. PLoS Genet. 9, e1003401.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana T., Wakimoto Y., Nakamuta N., Phichitraslip T., Wakitani S., Kusakabe K., Hondo E., Kiso Y. (2007). Effects of bisphenol A (BPA) on placentation and survival of the neonates in mice. J. Reprod. Dev. 53, 509–514. [DOI] [PubMed] [Google Scholar]
- Tait S., Tassinari R., Maranghi F., Mantovani A. (2015). Bisphenol A affects placental layers morphology and angiogenesis during early pregnancy phase in mice. J. Appl. Toxicol. 35, 1278–1291. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Liu X., Sumiyoshi M., Matsushima A., Shimohigashi M., Shimohigashi Y. (2009). Placenta expressing the greatest quantity of bisphenol A receptor ERR{gamma} among the human reproductive tissues: Predominant expression of type-1 ERRgamma isoform. J. Biochem. 146, 113–122. [DOI] [PubMed] [Google Scholar]
- Tan W., Huang H., Wang Y., Wong T. Y., Wang C. C., Leung L. K. (2013). Bisphenol A differentially activates protein kinase C isoforms in murine placental tissue. Toxicol. Appl. Pharmacol. 269, 163–168. [DOI] [PubMed] [Google Scholar]
- Teeguarden J. G., Hanson-Drury S. (2013). A systematic review of Bisphenol A “low dose” studies in the context of human exposure: A case for establishing standards for reporting “low-dose” effects of chemicals. Food Chem. Toxicol. 62, 935–948. [DOI] [PubMed] [Google Scholar]
- Ter Veld M. G., Zawadzka E., Rietjens I. M., Murk A. J. (2009). Estrogenicity of food-associated estrogenic compounds in the fetuses of female transgenic mice upon oral and IP maternal exposure. Reprod. Toxicol. 27, 133–139. [DOI] [PubMed] [Google Scholar]
- Tetz L. M., Aronoff D. M., Loch-Caruso R. (2015). Mono-ethylhexyl phthalate stimulates prostaglandin secretion in human placental macrophages and THP-1 cells. Reprod. Biol. Endocrinol. 13, 56.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetz L. M., Cheng A. A., Korte C. S., Giese R. W., Wang P., Harris C., Meeker J. D., Loch-Caruso R. (2013). Mono-2-ethylhexyl phthalate induces oxidative stress responses in human placental cells in vitro. Toxicol. Appl. Pharmacol. 268, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer K. A., Doerge D. R., Hunt D., Schurman S. H., Twaddle N. C., Churchwell M. I., Garantziotis S., Kissling G. E., Easterling M. R., Bucher J. R., et al. (2015). Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ. Int. 83, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannuccini S., Bocchi C., Severi F. M., Challis J. R., Petraglia F. (2016). Endocrinology of human parturition. Ann. Endocrinol. (Paris) 77, 105–113. [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A., Pennathur S., Kannan K., Patisaul H. B., Dolinoy D. C., Zeng L., Padmanabhan V. (2015). Impact of gestational bisphenol A on oxidative stress and free fatty acids: Human association and interspecies animal testing studies. Endocrinology 156, 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercruysse L., Caluwaerts S., Luyten C., Pijnenborg R. (2006). Interstitial trophoblast invasion in the decidua and mesometrial triangle during the last third of pregnancy in the rat. Placenta 27, 22–33. [DOI] [PubMed] [Google Scholar]
- Wan Y., Huo W., Xu S., Zheng T., Zhang B., Li Y., Zhou A., Zhang Y., Hu J., Zhu Y., et al. (2018). Relationship between maternal exposure to bisphenol S and pregnancy duration. Environ. Pollut. 238, 717–724. [DOI] [PubMed] [Google Scholar]
- Wang X. K., Agarwal M., Parobchak N., Rosen A., Vetrano A. M., Srinivasan A., Wang B., Rosen T. (2016). Mono-(2-Ethylhexyl) Phthalate Promotes Pro-Labor Gene Expression in the Human Placenta. PLoS One 11, e0147013.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Y., Lu J., Zhang Y. Z., Zhang M., Liu T., Qu X. L. (2015). Effect of Bisphenol A on invasion ability of human trophoblastic cell line BeWo. Int. J. Clin. Exp. Pathol. 8, 14355–14364. [PMC free article] [PubMed] [Google Scholar]
- Watkins D. J., Ferguson K. K., Anzalota Del Toro L. V., Alshawabkeh A. N., Cordero J. F., Meeker J. D. (2015). Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico. Int. J. Hyg. Environ. Health 218, 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R. A., Mao B., Li S., Liu J., Li X., Li H., Su Y., Hu G., Lian Q. Q., Ge R. S. (2016). Structure-activity relationships of phthalates in inhibition of human placental 3beta-hydroxysteroid dehydrogenase 1 and aromatase. Reprod. Toxicol. 61, 151–161. [DOI] [PubMed] [Google Scholar]
- Xu Y., Agrawal S., Cook T. J., Knipp G. T. (2008). Maternal di-(2-ethylhexyl)-phthalate exposure influences essential fatty acid homeostasis in rat placenta. Placenta 29, 962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Cook T. J., Knipp G. T. (2005). Effects of di-(2-ethylhexyl)-phthalate (DEHP) and its metabolites on fatty acid homeostasis regulating proteins in rat placental HRP-1 trophoblast cells. Toxicol. Sci. 84, 287–300. [DOI] [PubMed] [Google Scholar]
- Xu Y., Knipp G. T., Cook T. J. (2006). Effects of di-(2-ethylhexyl)-phthalate and its metabolites on the lipid profiling in rat HRP-1 trophoblast cells. Arch. Toxicol. 80, 293–298. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y., Ralston A., Stephenson R. O., Rossant J. (2006). Cell and molecular regulation of the mouse blastocyst. Dev. Dyn. 235, 2301–2314. [DOI] [PubMed] [Google Scholar]
- Yan X., Calafat A., Lashley S., Smulian J., Ananth C., Barr D., Silva M., Ledoux T., Hore P., Robson M. G. (2009). Phthalates Biomarker Identification and Exposure Estimates in a Population of Pregnant Women. Hum. Ecol. Risk Assess 15, 565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Chen J., Wang X., Song Q., Xu H. H., Zhang Y. H. (2016). Third trimester phthalate exposure is associated with DNA methylation of growth-related genes in human placenta. Sci. Rep. 6, 33449.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Shi H. J., Xie C. M., Chen J., Laue H., Zhang Y. H. (2015). Prenatal phthalate exposure, infant growth, and global DNA methylation of human placenta. Environ. Mol. Mutagen. 56, 286–292. [DOI] [PubMed] [Google Scholar]
- Zhong J., Baccarelli A. A., Mansur A., Adir M., Nahum R., Hauser R., Bollati V., Racowsky C., Machtinger R. (2018). Maternal Phthalate and Personal Care Products Exposure Alters Extracellular Placental miRNA Profile in Twin Pregnancies. Reprod Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Gao L., Flaws J. A. (2017a). Exposure to an environmentally relevant phthalate mixture causes transgenerational effects on female reproduction in mice. Endocrinology. 158(6), 1739–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Gao L., Flaws J. A. (2017b). Prenatal exposure to an environmentally relevant phthalate mixture disrupts reproduction in F1 female mice. Toxicol. Appl. Pharmacol. 318, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. D., Gao H., Huang K., Zhang Y. W., Cai X. X., Yao H. Y., Mao L. J., Ge X., Zhou S. S., Xu Y. Y., et al. (2018). Prenatal phthalate exposure and placental size and shape at birth: A birth cohort study. Environ. Res. 160, 239–246. [DOI] [PubMed] [Google Scholar]
- Zong T., Lai L., Hu J., Guo M., Li M., Zhang L., Zhong C., Yang B., Wu L., Zhang D., et al. (2015). Maternal exposure to di-(2-ethylhexyl) phthalate disrupts placental growth and development in pregnant mice. J. Hazard. Mater. 297, 25–33. [DOI] [PubMed] [Google Scholar]