Abstract
Aims
The present study was conducted to explore the effect of interleukin-33 (IL-33) on glycogen synthase kinase-3β (GSK-3β) activation involving Tau phosphorylation, a critical causative factor for Alzheimer's disease (AD).
Main methods
Experiments were performed using PC-12 cells. Target proteins were knocked-down by transfecting with the siRNA for each protein. The kinase activities were assessed by monitoring phosphorylation of Thr308 and Ser473 for Akt and phosphorylation of Ser9 and Tyr216 for GSK-3β in the Western blotting.
Key findings
Exogenously applied IL-33 activated Akt and inactivated GSK-3β. IL-33-induced Akt activation and GSK-3β inactivation were significantly inhibited by knocking-down myeloid differentiation factor 88 (MyD88), tumor necrosis factor receptor associated factor 6 (TRAF6), receptor-interacting protein (RIP), or phosphatidylinositol 3 kinase (PI3K). IL-33 neutralized amyloid β1-42 (Aβ1-42)-induced Akt inactivation and GSK-3β activation.
Significance
The results of the present study show that IL-33 inactivates GSK-3β through an ST2-independent MyD88/TRAF6/RIP/PI3K/Akt pathway and inhibits Aβ1-42-induced GSK-3β activation. This suggests that IL-33 could restrain GSK-3β-mediated Tau phosphorylation in AD.
Keyword: Biochemistry
1. Introduction
Interleukin-33 (IL-33), that belongs to the IL-1 family [1], is well-recognized to serve as a proinflammatory cytokine and a nuclear factor in a variety of cells [2, 3, 4]. Extracellular IL-33 transmits the signals through a heterodimeric receptor complex, comprising ST2 and interleukin-1 receptor accessory protein (IL-1RAcP) on the plasma membrane [5]. The receptor complex, activated by IL-33, recruits and activate myeloid differentiation factor 88 (MyD88), followed by activation of interleukin-1 receptor-associated kinase 4 (IRAK4) [1]. Then, IRAK4 phosphorylates IRAK1, to bind to and activate tumor necrosis factor (TNF) receptor associated factor 6 (TRAF6). TRAF6 activates transforming growth factor-β (TGF-β)-activated kinase 1 (TAK1), followed by activation of mitogen-activated protein kinase (MAPK) cascades. The death receptor kinase receptor-interacting protein (RIP), alternatively, associates with TRAF6 and activates phosphatidylinositol 3 kinase (PI3K) [6, 7, 8].
Accumulating evidence has pointed to the role of IL-33 in cognitive functions. Exogenously applied IL-33 potentiates responses of α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA) receptor, a major excitatory neurotransmitter receptor, expressed in Xenopus oocytes [9]. IL-33 deficiency suppresses Schaffer collateral/CA1 long-term potentiation (LTP), a cellular model of learning and memory, in the mouse hippocampus, which was neutralized by adding IL-33 [10]. These findings suggest that IL-33 is required for expression of LTP. In the water maze test, the acquisition latency in IL-33-deficient mice was significantly prolonged as compared with the latency in wild-type control mice [10], suggesting the role of IL-33 in spatial learning and memory.
IL-33 exerts protective and toxic bidirectional actions in many organs, depending on its doses. IL-33 induces microglia-mediated neuroinflammation also [11], while it exhibits neuroprotection effects [12, 13]. Moreover, IL-33 promotes microglial synapse engulfment and neural circuit development [14].
Alzheimer's disease (AD) is characterized by extensive deposition of Aβ called amyloid plaque and formation of neurofibrillary tangles (NFTs). When hyperphosphorylated, Tau detaches from the microtubules and forms insoluble fibrils, referred to as paired helical filaments (PHFs), and NFTs comprising aggregation of PHFs [15, 16]. Aβ activates glycogen synthase kinase-3β (GSK-3β), to phosphorylate Tau, thereby initiating formation of PHFs and NFTs [17]. We have found that amyloid β1-42 (Aβ1-42) significantly reduces phosphorylation of GSK-3β at Ser9 and enhances phosphorylation of Tau at Ser202/Thr205/Ser396 in hippocampal slices from wild-type mice [18]. This indicates that Aβ1–42 induces GSK-3β activation and promotes Tau phosphorylation. We have also found that the levels of GSK-3β phosphorylation at Ser9 and Tau phosphorylation at Ser396 in the hippocampus from 5xFAD AD model mice at 6 months of age are significantly lower and higher, respectively, as compared with each of the levels in the hippocampus from wild-type mice [18]. It is shown that the levels of Aβ1–42 in the 5xFAD mouse brain increase in an age-dependent manner [19]. Moreover, we have found that Tau hyperphosphorylation in the hippocampus of 5xFAD mice precedes high GSK-3β activation [20]. Collectively, these results further support the notion that Aβ1–42 triggers GSK-3β activation, thereby causing Tau hyperphosphorylation in the AD brain.
Higher levels of IL-33 are found in the AD brain cells and Aβ1-42 upregulates IL-33 in cultured mouse astrocytes [21]. IL-33 reverses synaptic plasticity impairment and memory deficits in APP/PS1 mice, an animal model of AD [22]. IL-33 reduces soluble Aβ levels and amyloid plaque deposition by promoting microglia-engaged phagocytosis of Aβ [22]. Moreover, IL-33 may prevent conversion from mild cognitive impairment to AD [23]. Thus, IL-33 appears to play a role in AD.
The present study was conducted to understand the effects of IL-33 on the activity of GSK-3β involving Tau phosphorylation, a critical causative factor for AD. The results show that IL-33 inactivates GSK-3β through an ST2-independent MyD88/TRAF6/RIP/PI3K/Akt pathway and suppresses Aβ1-42-induced GSK-3β activation.
2. Materials and methods
2.1. Cell culture
PC-12 cells, obtained from RIKEN Cell Bank (Tsukuba, Japan), were cultured in DMEM with 10% (v/v) heat-inactivated FBS and 10% (v/v) heat-inactivated horse serum supplemented with penicillin (100 U/ml), and streptomycin (0.1 mg/mL), in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. PC-12 cells were differentiated by treatment with nerve growth factor (100 ng/mL) for 5 days.
2.2. Protein knockdown
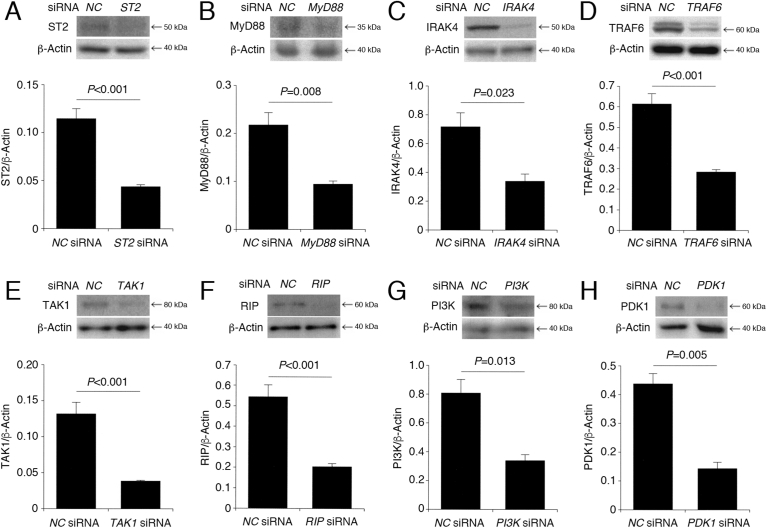
Protein knockdown was carried out by the method previously described [24]. Briefly, the siRNAs to silence the ST2-, MyD88-, IRAK4-, TRAF6-, TAK1-, RIP-, PI3K-, and 3-phosphoinositide-dependent protein kinase-1 (PDK1)-targeted genes and the negative control siRNA (NC siRNA) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Ambion (Carlsbad, CA, USA). siRNAs were transfected into PC-12 cells using a Lipofectamine reagent (Invitrogen, Carlsbad, CA, USA), and cells were used for experiments 48 h after transfection. Knock-down of the relevant proteins was confirmed in the Western blot analysis using antibodies against ST2 (Thermo Fisher Scientific, Tokyo, Japan), MyD88 (R&D Systems Inc., MN, USA), IRAK4 (Bioss Antibodies Inc., MA, USA), TRAF6 (Boster Biological Technology, CA, USA), TAK1 (Bioss Antibodies Inc.), RIP (Santa Cruz Biotechnology, Inc., TX, USA), PI3K (R&D Systems Inc.), and PDK1 (Thermo Fisher Scientific) anti-β-actin antibody (Sigma, St. Louis, MO, USA) (Fig. 1A–H). The host species of all the primary antibodies used here were mouse. Antibodies were used in 1000–2000 times dilution.
Fig. 1.
PC-12 cells were transfected with siRNAs for negative control (NC), ST2 (A), MyD88 (B), IRAK4 (C), TRAF6 (D), TAK1 (E), RIP (F), PI3K (G), and PDK1 (H), and 48 h later Western blotting was carried out. The signal intensity for each protein was normalized by the signal intensity for β-actin. In the graphs, each column represents the mean (±SEM) normalized signal intensity (n = 8 independent Western blot results). P values, unpaired t-test.
Full Western blot images were not stored and therefore cannot be provided for any of the blots shown in the figures.
2.3. Assay of activities for Akt and GSK-3β
PC-12 cells, transfected without and with the siRNA, were treated with full-length IL-33 (1 ng/mL), which was kindly provided by Prof. Yoshimoto (Hyogo College of Medicine), for 10–30 min. In a different set of experiments, cells were incubated with Aβ1-42 protein fragment (1 μM) (Abcam, Cambridge, UK) for 3h, and IL-33 (1 ng/mL) was added 30 min prior to the end of the incubation. After treatment, cells were homogenized with a sonicator in an ice-cold PBS containing 1% (v/v) protease inhibitor cocktail (Nacalai, Kyoto, Japan), and then centrifuged at 3,000 rpm for 5 min at 4 °C. The supernatants (20 μg of protein) were loaded on 10% (w/v) acrylamide gel and electrophoresed. Separated proteins were transferred onto polyvinylidene difluoride membrane. Blotting membranes were blocked with TBS-T (150 mM NaCl, 0.1% Tween20 and 20 mM Tris, pH 7.5) containing 5% (w/v) bovine serum albumin (Wako, Osaka, Japan) and reacted with antibodies against Akt (Cell Signaling, Beverly, MA, USA), phospho-Thr308-Akt (pT308-Akt) (Cell Signaling), phospho-Ser473-Akt (pS473-Akt) (Cell Signaling), GSK-3β (Cell Signaling), phospho-Ser9-GSK-3β (pS9-GSK-3β) (Cell Signaling), and phospho-Tyr216-GSK-3β (pY216-GSK-3β) (BD Biosciences, San Jose, CA, USA), followed by a horseradish peroxidase-conjugated goat anti-mouse IgG antibody. The host species of all the primary antibodies used was mouse. Antibodies were used in 500–1000 times dilution. Immunoreactivity was detected with an ECL kit (GE Healthcare, Piscataway, NJ, USA) and visualized using a chemiluminescence detection system (GE Healthcare).
Activities of Akt and GSK-3β were analyzed by calculating the immunoreactive signal intensity for pT308-Akt and pS473-Akt relative to the signal intensity for Akt or the immunoreactive signal intensity for pS9-GSK-3β and pY216-GSK-3β relative to the signal intensity for GSK-3β.
Full Western blot images were not stored and therefore cannot be provided for any of the blots shown in the figures.
2.4. Statistical analysis
The statistical software STATA was purchased from Light Stone (Tokyo, Japan), and statistical analysis was carried out using unpaired t-test and analysis of variance (ANOVA) followed by a Bonferroni correction.
3. Results
3.1. IL-33 activates Akt and inactivates GSK-3β
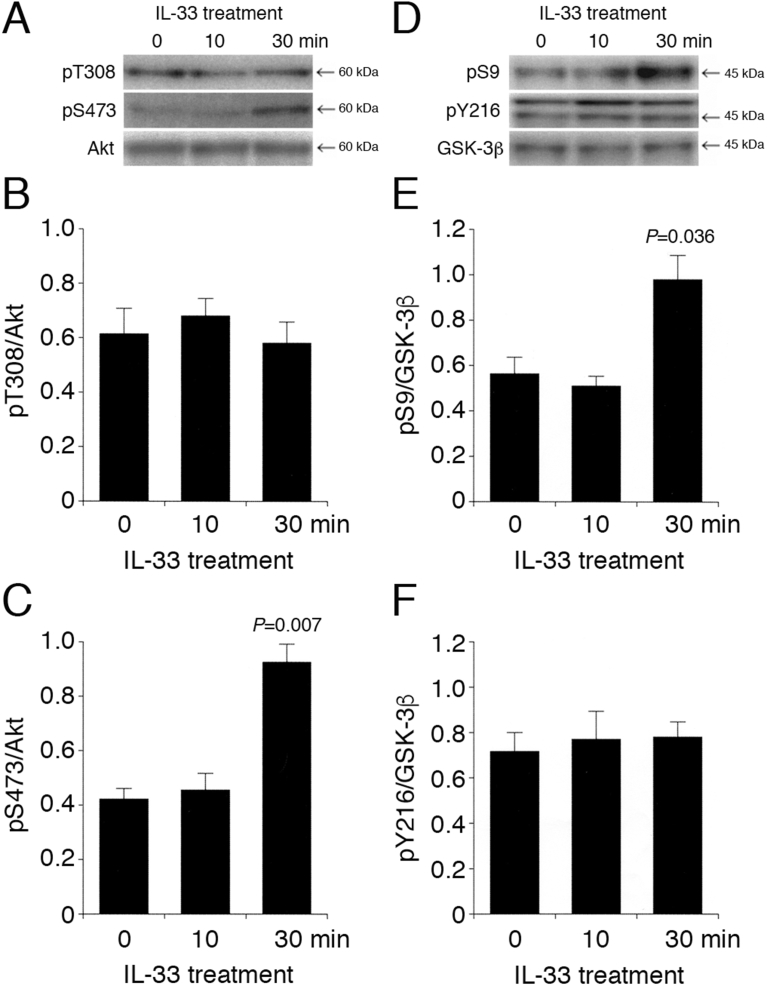
My initial attempt was to assess the effect of IL-33 on the Akt and GSK-3β activities in differentiated PC-12 cells. Akt is activated by phosphorylation at Thr308 and/or Ser473. GSK-3β is inactivated by phosphorylation at Ser9 and activated by phosphorylation at Tyr216 [25].
In my earlier study, IL-33 at a concentration of 1 ng/mL restored the suppression of Schaffer collateral/CA1 LTP in IL-33-deficient mice [10]. The same concentration of IL-33 (1 ng/mL), therefore, was used in the present study. IL-33 significantly enhanced pS473-Akt 30 min after treatment, although it had no effect on pT308-Akt (Fig. 2A–C). Otherwise, IL-33 significantly enhanced pS9-GSK-3β 30 min after treatment, while pY216-GSK-3β was not affected (Fig. 2D–F). Collectively, these results indicate that IL-33 promotes Akt activation and GSK-3β inactivation.
Fig. 2.
IL-33 activates Akt and inactivates GSK-3β. PC-12 cells were treated with IL-33 (1 ng/mL) for the periods of time as indicated, followed by Western blotting using antibodies against Akt, pT308-Akt, pS473-Akt, GSK-3β, pS9-GSK-3β, and pY216-GSK-3β. (A) (D) Western blot images. The arrow shown in (D) indicates the signal band at 46 kDa for pY216-GSK-3β. (B) (C) In the graphs, each column represents the mean (±SEM) signal intensity for pT308-Akt and pS473-Akt relative to the signal intensity for Akt (n = 8 Western blot results from independent experiments). (E) (F) In the graphs, each column represents the mean (±SEM) signal intensity for pS9-GSK-3β and pY216-GSK-3β relative to the signal intensity for GSK-3β (n = 8 Western blot results from independent experiments). P values shown in the graphs, ANOVA followed by a Bonferroni correction.
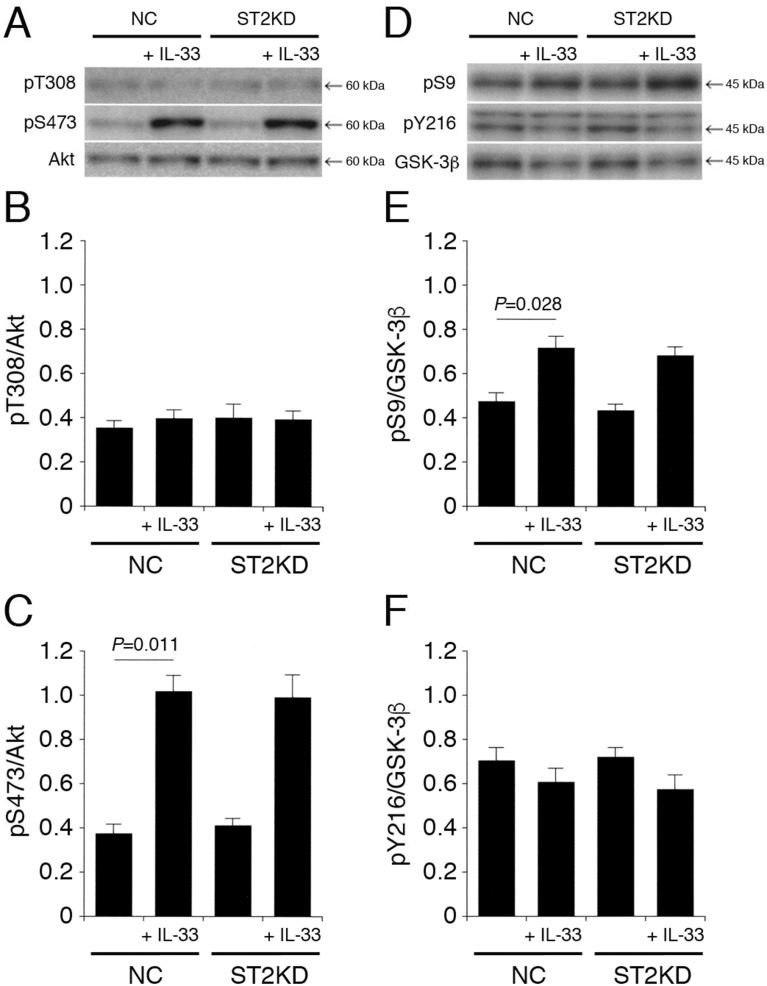
3.2. ST2 is not implicated in IL-33-induced Akt activation and GSK-3β inactivation
Subsequently, it was examined whether the effects of IL-33 on the Akt and GSK-3β activities are mediated by the IL-33 receptor ST2. IL-33 had no effect on pT308-Akt and pY216-GSK-3β in cells transfected with the NC siRNA or the ST2 siRNA (Fig. 3A,B,D,F). IL-33 significantly enhanced pS473-Akt and pS9-GSK-3β in cells transfected with the NC siRNA, but the effects were not affected by knocking-down ST2 (Fig. 3A,C,D,E). This indicates that IL-33 promotes Akt activation and GSK-3β inactivation in an ST2-independent manner.
Fig. 3.
ST2 is not implicated in IL-33-induced Akt activation and GSK-3β inactivation. PC-12 cells, transfected with the NC siRNA or the ST2 siRNA, were treated with IL-33 (1 ng/mL) for 30 min, followed by Western blotting using antibodies against Akt, pT308-Akt, pS473-Akt, GSK-3β, pS9-GSK-3β, and pY216-GSK-3β. (A) (D) Western blot images. The arrow shown in (D) indicates the signal band at 46 kDa for pY216-GSK-3β. (B) (C) In the graphs, each column represents the mean (±SEM) signal intensity for pT308-Akt and pS473-Akt relative to the signal intensity for Akt (n = 8 Western blot results from independent experiments). (E) (F) In the graphs, each column represents the mean (±SEM) signal intensity for pS9-GSK-3β and pY216-GSK-3β relative to the signal intensity for GSK-3β (n = 8 Western blot results from independent experiments). P values shown in the graphs, ANOVA followed by a Bonferroni correction.
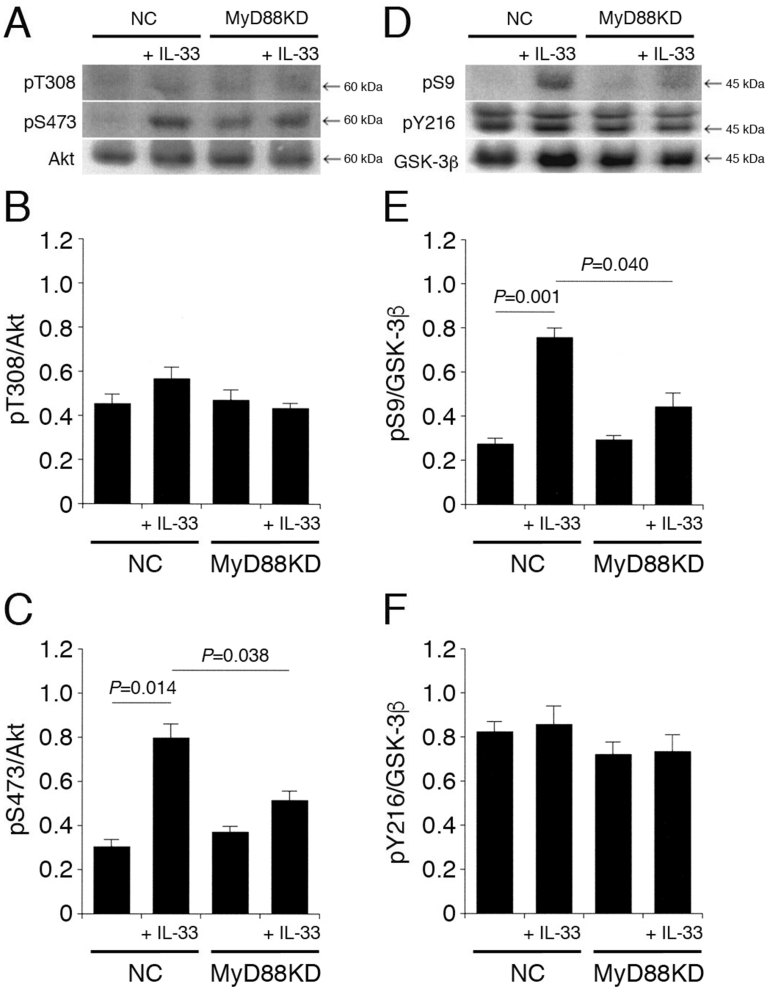
3.3. MyD88 is implicated in IL-33-induced Akt activation and GSK-3β inactivation
As mentioned before, MyD88 functions downstream the IL-33 receptor complex ST2/IL-1RAcP. IL-33 had no effect on pT308-Akt and pY216-GSK-3β in cells transfected with the NC siRNA or the MyD88 siRNA (Fig. 4A,B,D,F). However, IL-33 significantly enhanced pS473-Akt and pS9-GSK-3β in cells transfected with the NC siRNA, and the effects were clearly inhibited by knocking-down MyD88 (Fig. 4A,C,D,E). These results indicate that IL-33 promotes Akt activation and GSK-3β inactivation in an MyD88-dependent manner.
Fig. 4.
MyD88 is implicated in IL-33-induced Akt activation and GSK-3β inactivation. PC-12 cells, transfected with the NC siRNA or the MyD88 siRNA, were treated with IL-33 (1 ng/mL) for 30 min, followed by Western blotting using antibodies against Akt, pT308-Akt, pS473-Akt, GSK-3β, pS9-GSK-3β, and pY216-GSK-3β. (A) (D) Western blot images. The arrow shown in (D) indicates the signal band at 46 kDa for pY216-GSK-3β. (B) (C) In the graphs, each column represents the mean (±SEM) signal intensity for pT308-Akt and pS473-Akt relative to the signal intensity for Akt (n = 8 Western blot results from independent experiments). (E) (F) In the graphs, each column represents the mean (±SEM) signal intensity for pS9-GSK-3β and pY216-GSK-3β relative to the signal intensity for GSK-3β (n = 8 Western blot results from independent experiments). P values shown in the graphs, ANOVA followed by a Bonferroni correction.
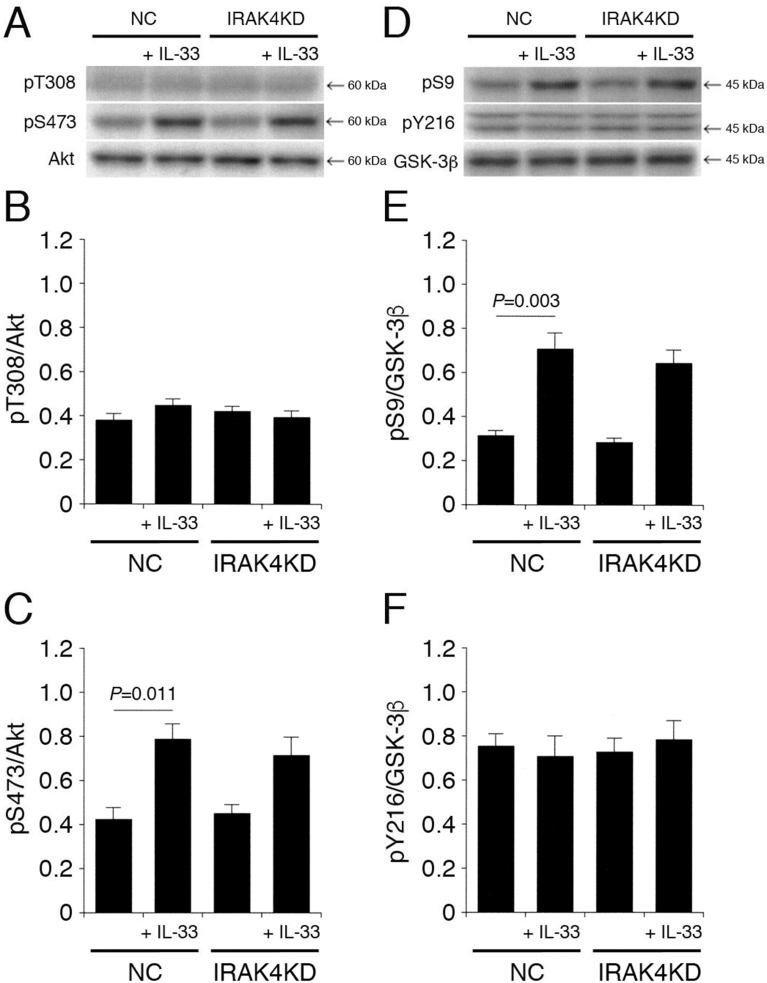
3.4. IRAK4 is not implicated in IL-33-induced Akt activation and GSK-3β inactivation
Since MyD88 activates IRAK4, to phosphorylate IRAK1, it was also evaluated whether IL-33 effects implicate IRAK4 activity. IL-33 had no effect on pT308-Akt and pY216-GSK-3β in cells transfected with the NC siRNA or the IRAK4 siRNA (Fig. 5A,B,D,F). Furthermore, IL-33 significantly enhanced pS473-Akt and pS9-GSK-3β in cells transfected with the NC siRNA, but the effects were not affected by knocking-down IRAK4 (Fig. 5A,C,D,E), indicating that IL-33 promotes Akt activation and GSK-3β inactivation in an IRAK4-independent manner.
Fig. 5.
IRAK4 is not implicated in IL-33-induced Akt activation and GSK-3β inactivation. PC-12 cells, transfected with the NC siRNA or the IRAK4 siRNA, were treated with IL-33 (1 ng/mL) for 30 min, followed by Western blotting using antibodies against Akt, pT308-Akt, pS473-Akt, GSK-3β, pS9-GSK-3β, and pY216-GSK-3β. (A) (D) Western blot images. The arrow shown in (D) indicates the signal band at 46 kDa for pY216-GSK-3β. (B) (C) In the graphs, each column represents the mean (±SEM) signal intensity for pT308-Akt and pS473-Akt relative to the signal intensity for Akt (n = 8 Western blot results from independent experiments). (E) (F) In the graphs, each column represents the mean (±SEM) signal intensity for pS9-GSK-3β and pY216-GSK-3β relative to the signal intensity for GSK-3β (n = 8 Western blot results from independent experiments). P values shown in the graphs, ANOVA followed by a Bonferroni correction.
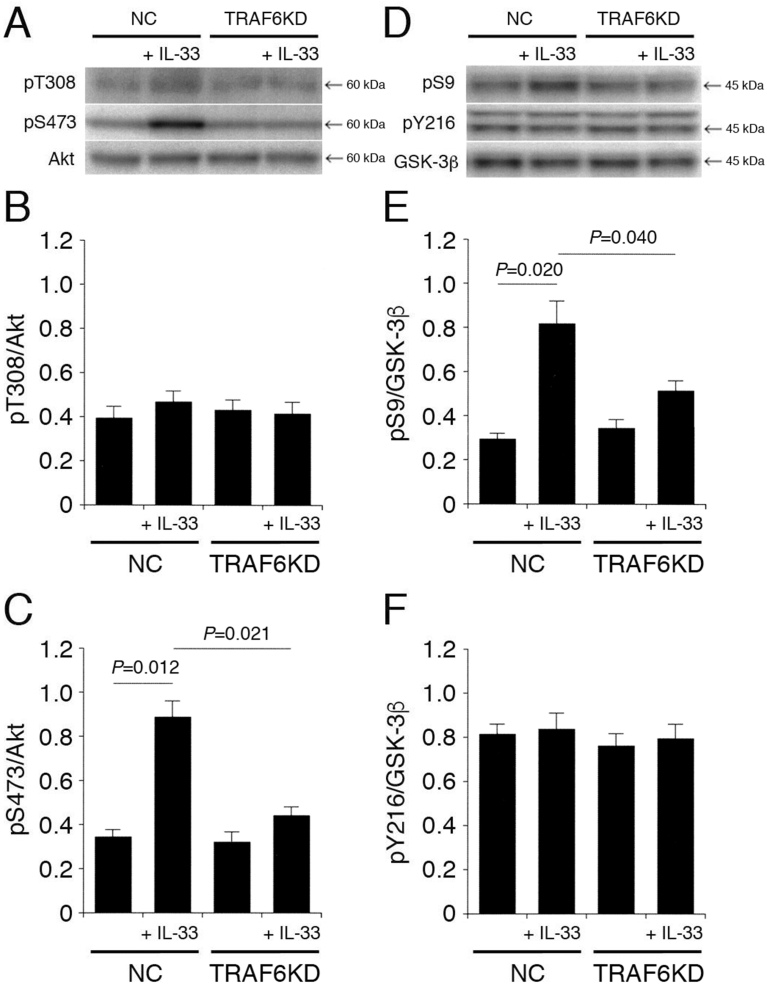
3.5. TRAF6 is implicated in IL-33-induced Akt activation and GSK-3β inactivation
Phosphorylated IRAK1 binds to and activates TRAF6. Again, IL-33 had no effect on pT308-Akt and pY216-GSK-3β in cells transfected with the NC siRNA or the TRAF6 siRNA (Fig. 6A,B,D,F). Moreover, IL-33 significantly enhanced pS473-Akt and pS9-GSK-3β in cells transfected with the NC siRNA. However, the effects were apparently inhibited by knocking-down TRAF6 (Fig. 6A,C,D,E). This indicates that IL-33 promotes Akt activation and GSK-3β inactivation in a TRAF6-dependent manner.
Fig. 6.
TRAF6 is implicated in IL-33-induced Akt activation and GSK-3β inactivation. PC-12 cells, transfected with the NC siRNA or the TRAF6 siRNA, were treated with IL-33 (1 ng/mL) for 30 min, followed by Western blotting using antibodies against Akt, pT308-Akt, pS473-Akt, GSK-3β, pS9-GSK-3β, and pY216-GSK-3β. (A) (D) Western blot images. The arrow shown in (D) indicates the signal band at 46 kDa for pY216-GSK-3β. (B) (C) In the graphs, each column represents the mean (±SEM) signal intensity for pT308-Akt and pS473-Akt relative to the signal intensity for Akt (n = 8 Western blot results from independent experiments). (E) (F) In the graphs, each column represents the mean (±SEM) signal intensity for pS9-GSK-3β and pY216-GSK-3β relative to the signal intensity for GSK-3β (n = 8 Western blot results from independent experiments). P values shown in the graphs, ANOVA followed by a Bonferroni correction.
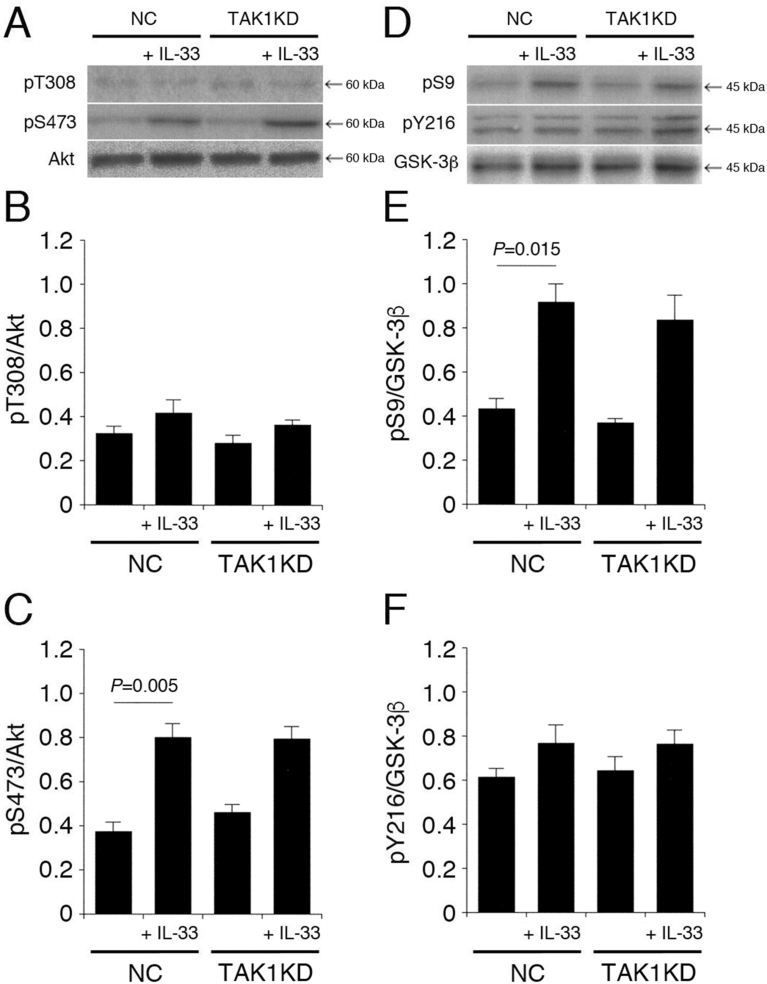
3.6. TAK1 is not implicated in IL-33-induced Akt activation and GSK-3β inactivation
As previously described, TRAF6 activates TAK1 by forming an IRAK1/TRAF6/TAK1/TAK binding protein 1 (TAB1)/TAB2/Ubc13/UeV1A complex, followed by activation of MAPK cascades [26]. Next, it was examined if IL-33 signaling involves TAK1. Once again, IL-33 had no effect on pT308-Akt and pY216-GSK-3β in cells transfected with the NC siRNA or the TAK1 siRNA (Fig. 7A,B,D,F). IL-33 significantly enhanced pS473-Akt and pS9-GSK-3β in cells transfected with the NC siRNA, but the effects were not affected by knocking-down TAK1 (Fig. 7A,C,D,E). This indicates that IL-33 promotes Akt activation and GSK-3β inactivation in a TAK1-independent manner.
Fig. 7.
TAK1 is not implicated in IL-33-induced Akt activation and GSK-3β inactivation. PC-12 cells, transfected with the NC siRNA or the TAK1 siRNA, were treated with IL-33 (1 ng/mL) for 30 min, followed by Western blotting using antibodies against Akt, pT308-Akt, pS473-Akt, GSK-3β, pS9-GSK-3β, and pY216-GSK-3β. (A) (D) Western blot images. The arrow shown in (D) indicates the signal band at 46 kDa for pY216-GSK-3β. (B) (C) In the graphs, each column represents the mean (±SEM) signal intensity for pT308-Akt and pS473-Akt relative to the signal intensity for Akt (n = 8 Western blot results from independent experiments). (E) (F) In the graphs, each column represents the mean (±SEM) signal intensity for pS9-GSK-3β and pY216-GSK-3β relative to the signal intensity for GSK-3β (n = 8 Western blot results from independent experiments. P values shown in the graphs, ANOVA followed by a Bonferroni correction.
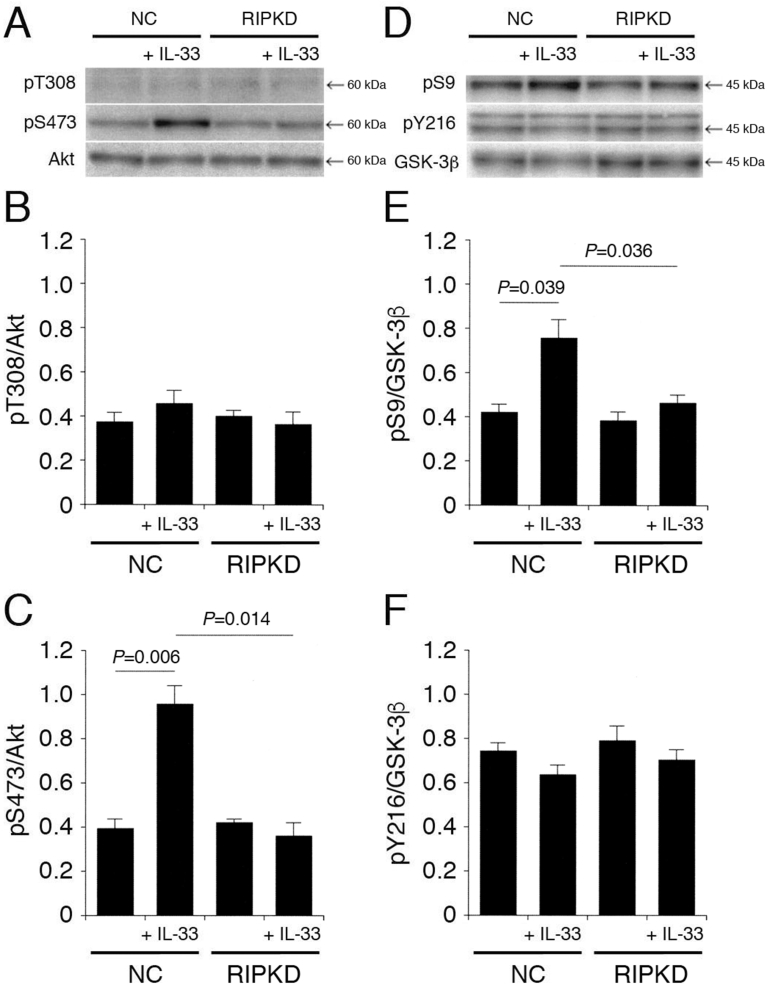
3.7. RIP is implicated in IL-33-induced Akt activation and GSK-3β inactivation
It is possible that PI3K is activated through a RIP/TRAF6 pathway [6, 7, 8]. IL-33 had no effect on pT308-Akt and pY216-GSK-3β in cells transfected with the NC siRNA or the RIP siRNA (Fig. 8A,B,D,F). Furthermore, IL-33 significantly enhanced pS473-Akt and pS9-GSK-3β in cells transfected with the NC siRNA. However, these effects were abolished by knocking-down RIP (Fig. 8A,C,D,E), indicating that IL-33 promotes Akt activation and GSK-3β inactivation in a RIP-dependent manner. This also indicates that IL-33 could activate PI3K through a RIP/TRAF6 pathway.
Fig. 8.
RIP is implicated in IL-33-induced Akt activation and GSK-3β inactivation. PC-12 cells, transfected with the NC siRNA or the RIP siRNA, were treated with IL-33 (1 ng/mL) for 30 min, followed by Western blotting using antibodies against Akt, pT308-Akt, pS473-Akt, GSK-3β, pS9-GSK-3β, and pY216-GSK-3β. (A) (D) Western blot images. The arrow shown in (D) indicates the signal band at 46 kDa for pY216-GSK-3β. (B) (C) In the graphs, each column represents the mean (±SEM) signal intensity for pT308-Akt and pS473-Akt relative to the signal intensity for Akt (n = 8 Western blot results from independent experiments). (E) (F) In the graphs, each column represents the mean (±SEM) signal intensity for pS9-GSK-3β and pY216-GSK-3β relative to the signal intensity for GSK-3β (n = 8 Western blot results from independent experiments). P values shown in the graphs, ANOVA followed by a Bonferroni correction.
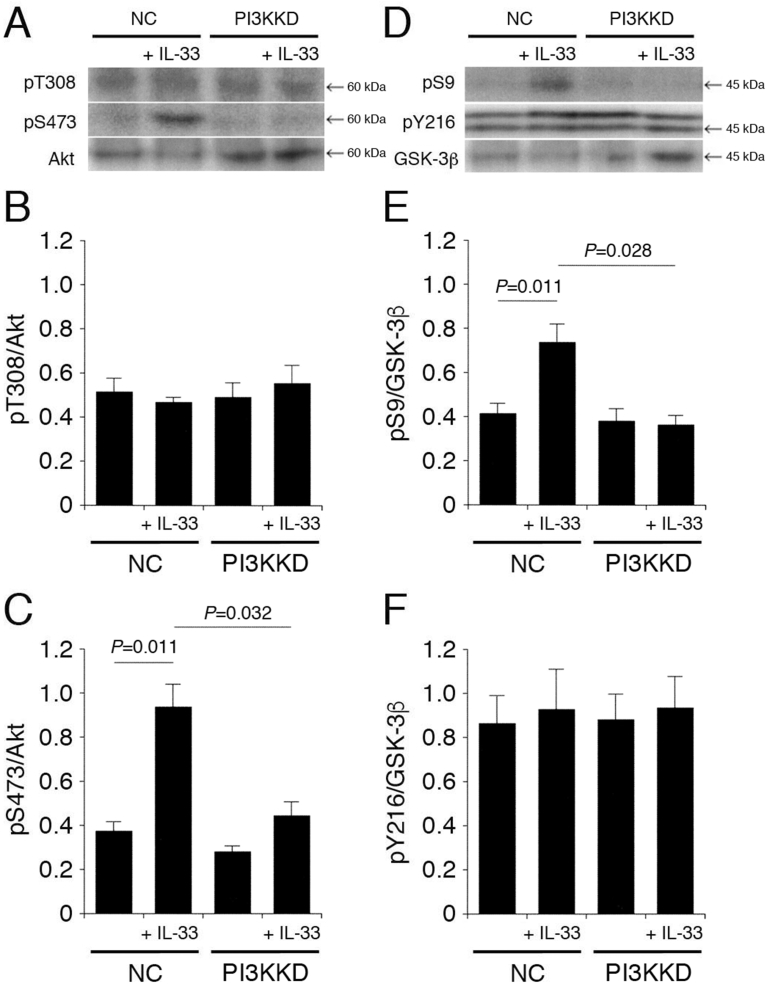
3.8. PI3K is implicated in IL-33-induced Akt activation and GSK-3β inactivation
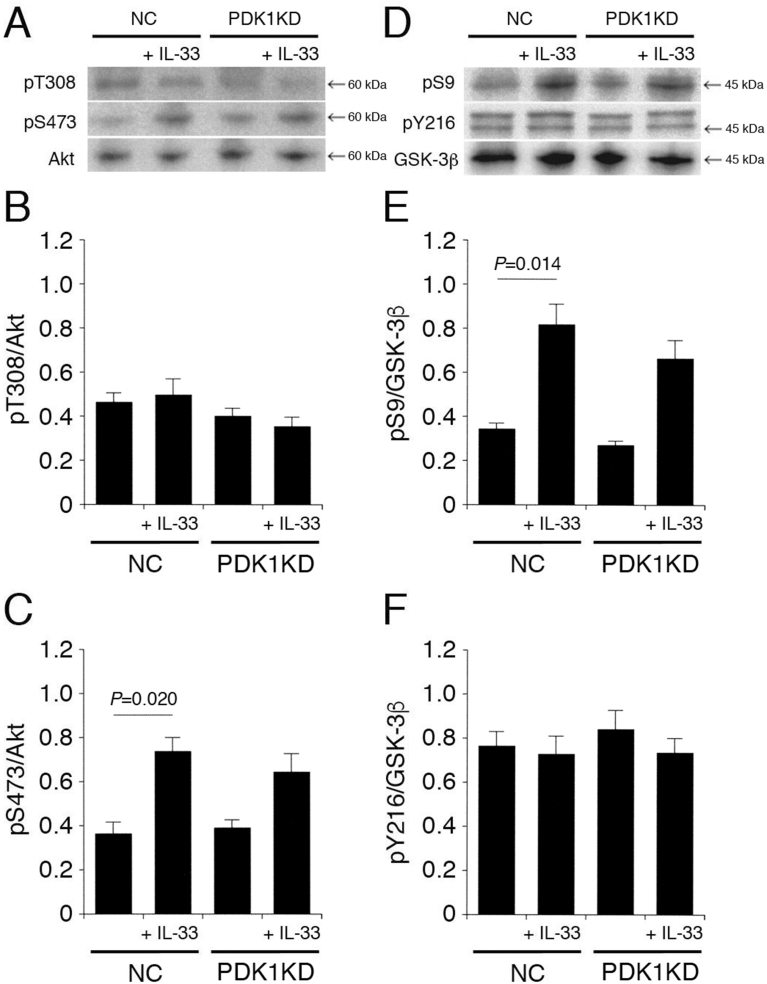
Akt is activated through a PI3K/PDK1 pathway. IL-33 had no effect on pT308-Akt and pY216-GSK-3β in cells transfected with the NC siRNA, the PI3K siRNA (Fig. 9A,B,D,F), or the PDK1 siRNA (Fig. 10A,B,D,F). IL-33 significantly enhanced pS473-Akt and pS9-GSK-3β in cells transfected with the NC siRNA, and the effects were abolished by knocking-down PI3K (Fig. 9A,C,D,E), but not PDK1 (Fig. 10A,C,D,E). This indicates that IL-33 promotes Akt activation and GSK-3β inactivation in a PI3K-dependent and PDK-1-independent manner.
Fig. 9.
PI3K is implicated in IL-33-induced Akt activation and GSK-3β inactivation. PC-12 cells, transfected with the NC siRNA or the PI3K siRNA, were treated with IL-33 (1 ng/mL) for 30 min, followed by Western blotting using antibodies against Akt, pT308-Akt, pS473-Akt, GSK-3β, pS9-GSK-3β, and pY216-GSK-3β. (A) (D) Western blot images. The arrow shown in (D) indicates the signal band at 46 kDa for pY216-GSK-3β. (B) (C) In the graphs, each column represents the mean (±SEM) signal intensity for pT308-Akt and pS473-Akt relative to the signal intensity for Akt (n = 8 Western blot results from independent experiments). (E) (F) In the graphs, each column represents the mean (±SEM) signal intensity for pS9-GSK-3β and pY216-GSK-3β relative to the signal intensity for GSK-3β (n = 8 Western blot results from independent experiments). P values shown in the graphs, ANOVA followed by a Bonferroni correction.
Fig. 10.
PDK1 is not implicated in IL-33-induced Akt activation and GSK-3β inactivation. PC-12 cells, transfected with the NC siRNA or the PDK1 siRNA, were treated with IL-33 (1 ng/mL) for 30 min, followed by Western blotting using antibodies against Akt, pT308-Akt, pS473-Akt, GSK-3β, pS9-GSK-3β, and pY216-GSK-3β. (A) (D) Western blot images. The arrow shown in (D) indicates the signal band at 46 kDa for pY216-GSK-3β. (B) (C) In the graphs, each column represents the mean (±SEM) signal intensity for pT308-Akt and pS473-Akt relative to the signal intensity for Akt (n = 8 Western blot results from independent experiments). (E) (F) In the graphs, each column represents the mean (±SEM) signal intensity for pS9-GSK-3β and pY216-GSK-3β relative to the signal intensity for GSK-3β (n = 8 Western blot results from independent experiments). P values shown in the graphs, ANOVA followed by a Bonferroni correction.
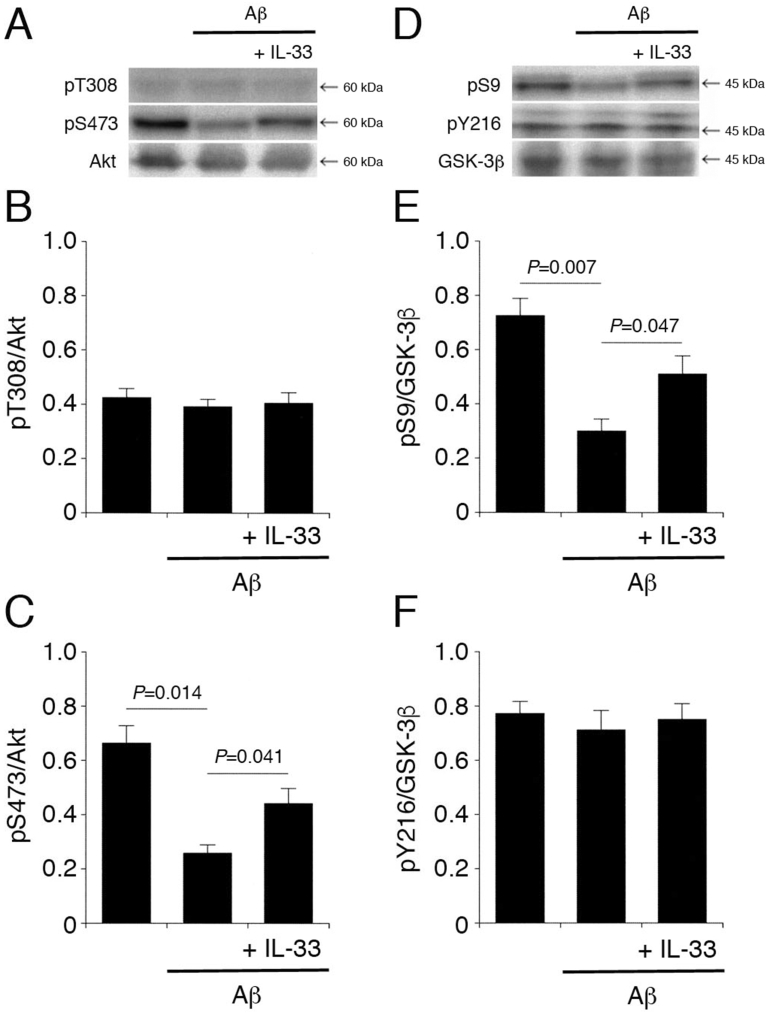
3.9. IL-33 neutralizes Aβ1-42-induced Akt-inactivation and GSK-3β activation
My final attempt was to see the effect of IL-33 on Aβ-induced GSK-3β activation. It is confirmed in the cell-free kinase assay that Akt induces GSK-3β phosphorylation at Ser9, i.e., Akt inactivates GSK-3β; conversely, inhibition of the Akt activity causes GSK-3β activation [18]. Aβ1-42 markedly reduced pS473-Akt and pS9-GSK-3β, although pT308-Akt and pY216-GSK-3β were not affected (Fig. 11A–F). This implies that Aβ1-42 attenuates the Akt activity and enhances the GSK-3β activity. IL-33 significantly reversed Aβ1-42-induced reduction of pS473-Akt and pS9-GSK-3β (Fig. 11A,C,D,E). This indicates that IL-33 neutralizes Aβ1-42-induced GSK-3β activation by enhancing the Akt activity.
Fig. 11.
IL-33 neutralizes Aβ1-42-mediated Akt inactivation and GSK-3β activation. PC-12 cells were incubated with Aβ1-42 (1 μM) for 3h, and IL-33 (1 ng/mL) was added 30 min prior to the end of the incubation, followed by Western blotting using antibodies against Akt pT308-Akt, pS473-Akt, GSK-3β, pS9-GSK-3β, and pY216-GSK-3β. (A) (D) Western blot images. The arrow shown in (D) indicates the signal band at 46 kDa for pY216-GSK-3β. (B) (C) In the graphs, each column represents the mean (±SEM) signal intensity for pT308-Akt and pS473-Akt relative to the signal intensity for Akt (n = 8 Western blot results from independent experiments). (E) (F) In the graphs, each column represents the mean (±SEM) signal intensity for pS9-GSK-3β and pY216-GSK-3β relative to the signal intensity for GSK-3β (n = 8 Western blot results from independent experiments). P values shown in the graphs, ANOVA followed by a Bonferroni correction.
4. Discussion
Akt is activated by phosphorylation at Thr308 and/or Ser473, and Akt inactivates GSK-3β by phosphorylating at Ser9. In the present study, it was observed that IL-33 enhanced pS473-Akt and pS9-GSK-3β in PC-12 cells. This indicates that IL-33 activates Akt, thereby phosphorylating and inactivating GSK-3β.
Extracellular IL-33 binds to ST2 and activates the heterodimeric receptor complex ST2/IL-1RAcP on the plasma membrane, followed by the sequential activation of MyD88, IRAK4, TRAF6, TAK1, and MAPK [5]. Unexpectedly, the effects of IL-33 on the Akt and GSK-3β activities were not affected by knocking-down ST2, while the effects were clearly inhibited by knocking-down MyD88, a downstream target of ST2/IL-1RAcP. This indicates that IL-33 activates Akt and inactivates GSK-3β in an ST2-independent and MyD88-dependent manner.
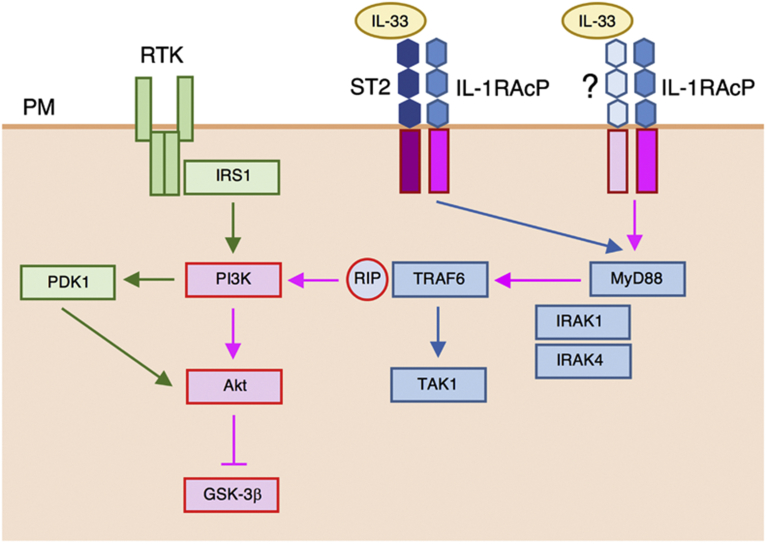
Then, the question to address is how IL-33 activates MyD88 in an ST2-independent manner. In my earlier study, it was demonstrated that Schaffer collateral/CA1 LTP was suppressed in IL-33- or MyD88-deficient mice, but the LTP was not affected in ST2-deficient mice [10]. This, taken together with the present findings, suggests that IL-33 might activate MyD88 through a yet unidentified IL-33 receptor associating with IL-1RAcP, instead of ST2 (Fig. 12).
Fig. 12.
A putative pathway underlying IL-33-induced Akt activation and GSK-3β inactivation. IL-33 activates MyD88, regardless of ST2, followed by activation of the downstream effector TRAF6. Then, TRAF6 recruits RIP, to activate PI3K and in turn, Akt. Akt inactivates GSK-3β by phosphorylating at Ser9. PM, plasma membrane.
MyD88 triggers IRAK4-mediated IRAK1 phosphorylation, to activate TRAF6. IL-33-induced Akt activation and GSK-3β inactivation were significantly inhibited by knocking-down TRAF6, while the effects were not affected by knocking-down IRAK4. This indicates that IL-33 activates Akt and inactivates GSK-3β by targeting TRAF6, regardless of IRAK4. IL-33-induced Akt activation and GSK-3β inactivation were not inhibited by knocking-down TAK1, a downstream target of TRAF6. This argues no implication of TAK1 in the effects of IL-33 here.
Akt is activated through a major pathway along a receptor tyrosine kinase (RTK)/insulin receptor substrate 1 (IRS-1)/PI3K/PDK1/Akt axis, to inactivate GSK-3β by phosphorylating at Ser9. IL-33-induced Akt activation and GSK-3β inactivation were cancelled by knocking-down PI3K, but not PDK1. This indicates that IL-33 activates PI3K prior to activation of Akt. Several avenues of evidence have pointed to a pathway linked to IL-33-induced PI3K activation or Akt activation [26, 27, 28, 29]. How IL-33 activates PI3K or Akt, however, remained to be elucidated. IL-33 is shown to promote inflammation-induced lymphangiogenesis through an ST2-dependent TRAF6-mediated Akt activation pathway [30]. This pathway does not account for IL-33-induced Akt activation showed here, since the IL-33 effect was independent of ST2. On the other hand, TRAF6 is recognized to recruit RIP, to activate PI3K [6, 7, 8]. This raises the possibility that IL-33 could activate PI3K through a RIP/TRAF6 pathway. Expectedly, IL-33-induced Akt activation and GSK-3β inactivation were abolished by knocking-down RIP. Overall, the results of the present study lead to a conclusion that IL-33 activates Akt through an ST2-independent MyD88/TRAF6/RIP/PI3K pathway, to phosphorylate and inactivate GSK-3β (Fig. 12).
Aβ has been long thought to be a causative factor in AD. Recent interest highlights Tau protein as another critical factor in AD. Phosphorylated Tau forms PHFs and NFTs, causing tauopathies, a class of neurodegenerative diseases, that include not only AD but frontotemporal dementia and parkinsonism linked to chromosome 17, progressive supranuclear palsy, Pick's disease, and corticobasal degeneration [31]. Tau is phosphorylated by serine/threonine protein kinases such as GSK-3β, cyclin-dependent kinase 5 (Cdk5)/p25, extracellular signal-regulated kinase 2 (ERK2), p70 ribosomal protein S6 kinase (p70-S6K), microtubule affinity-regulating kinase (MARK), SAD kinase (SADK), protein kinase A (PKA), calcium/calmodulin-dependent protein kinase II (CaMKII) or non-RTKs such as Fyn and c-Abl [32, 33, 34, 35, 36]. Of the protein kinases GSK-3β plays a central role in Tau phosphorylation relevant to tauopathies. Inhibition/inactivation of GSK-3β, therefore, could restrain Tau phosphorylation.
Aβ activates Fyn, which activates GSK-3β by phosphorylating at Tyr216 [37, 38]. Aβ, alternatively, inhibits PI3K/Akt, leading to activation of GSK-3β, to induce Tau phosphorylation [39, 40]. Soluble Aβ oligomers inhibit insulin signaling relevant to Akt activation, thereby activating GSK-3β and increasing Tau phosphorylation [41]. Akt, PKCε, PKA, integrin-linked kinase (ILK), CaMKII, p90 ribosomal protein S6 kinase (p90RSK), and cGMP-dependent protein kinase 1 (PrkG1), on the other hand, inactivate GSK-3β by phosphorylating at Ser9 [18, 42, 43, 44, 45, 46]. In our earlier study, Aβ1–42 significantly reduced phosphorylation of GSK-3β at Ser9 in mouse hippocampal slices [18], indicating that Aβ1–42 activates GSK-3β. It is recognized that β-catenin is a substrate of GSK-3β [47]. Aβ1–42 significantly enhanced phosphorylation of β-catenin at Ser33/Ser37/Thr41 in mouse hippocampal slices, and the effect was restrained by DCP-LA, a selective PKCε activator that acts as a potential inhibitor of GSK-3β [18], providing convincing evidence for Aβ1–42-induced GSK-3β activation. Moreover, Aβ1–42 significantly enhanced phosphorylation of Tau at Ser202/Thr205/Ser396 in mouse hippocampal slices, and the effect was also clearly inhibited by DCP-LA [18], indicating that Aβ1–42 promotes Tau phosphorylation by activating GSK-3β. Notably, DCP-LA suppressed GSK-3β activation and Tau phosphorylation in the 5xFAD mouse hippocampus and ameliorated spatial learning and memory impairment in 5xFAD mice. Like DCP-LA, IL-33 neutralized Aβ1-42-induced GSK-3β activation. This raises the possibility that IL-33 could prevent GSK-3β-mediated Tau phosphorylation in AD. IL-33, thus, could become a promising target in the development of therapeutic drugs of tauopathy including AD.
In conclusion, the results of the present study show that IL-33 suppresses GSK-3β activation through an ST2-independent MyD88/TRAF6/RIP/PI3K/Akt pathway and neutralizes Aβ1-42-induced Akt inactivation and GSK-3β activation. This may represent fresh insight into the IL-33 signal transduction pathways relevant to prevention of Tau phosphorylation.
Declarations
Author contribution statement
Tomoyuki Nishizaki: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The author declares no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T.K., Zurawski G., Moshrefi M., Qin J., Li X., Gorman D.M., Bazan J.F., Kastelein R.A. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Liew F.Y., Pitman N.I., McInnes I.B. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat. Rev. Immunol. 2009;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 3.Han P., Mi W.L., Wang Y.Q. Research progress on interleukin-33 and its roles in the central nervous system. Neurosci. Bull. 2011;27:351–357. doi: 10.1007/s12264-011-1025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oboki K., Ohno T., Kajiwara N., Saito H., Nakae S. IL-33 and IL-33 receptors in host defense and diseases. Allergol. Int. 2010;59:143–160. doi: 10.2332/allergolint.10-RAI-0186. [DOI] [PubMed] [Google Scholar]
- 5.Roussel L., Erard M., Cayrol C., Girard J.P. Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Rep. 2008;9:1006–1012. doi: 10.1038/embor.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanz L., Diaz-Meco M.T., Nakano H., Moscat J. The atypical PKC-interacting protein p62 channels NF-κB activation by the IL-1-TRAF6 pathway. EMBO J. 2000;19:1576–1586. doi: 10.1093/emboj/19.7.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivarelli M.S., McDonald D., Miller M., Cusson N., Kelliher M., Geha R.S. RIP links TLR4 to Akt and is essential for cell survival in response to LPS stimulation. J. Exp. Med. 2004;200:399–404. doi: 10.1084/jem.20040446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park S., Zhao D., Hatanpaa K.J., Mickey B.E., Saha D., Boothman D.A., Story M.D., Wong E.T., Burma S., Georgescu M.M., Rangnekar V.M., Chauncey S.S., Habib A.A. RIP1 activates PI3K-Akt via a dual mechanism involving NF-κB-mediated inhibition of the mTOR-S6K-IRS1 negative feedback loop and down-regulation of PTEN. Cancer Res. 2009;69:4107–4111. doi: 10.1158/0008-5472.CAN-09-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishizaki T. Does IL-33 potentiate or depress AMPA receptor response? Ind. J. Med. Res. Pharmaceut. Sci. 2017;4:42–47. [Google Scholar]
- 10.Nishizaki T. IL-33 acts to express Schaffer collateral/CA1 LTP and regulate learning and memory by targeting MyD88. Neural Plast. 2017;2017:2531453. doi: 10.1155/2017/2531453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao K., Liao X., Lu J., Yao S., Wu F., Zhu X., Shi D., Wen S., Liu L., Zhou H. IL-33/ST2 plays a critical role in endothelial cell activation and microglia-mediated neuroinflammation modulation. J. Neuroinflammation. 2018;15:136. doi: 10.1186/s12974-018-1169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao Y., Luo C.L., Li L.L., Ye G.H., Gao C., Wang H.C., Huang W.W., Wang T., Wang Z.F., Ni H., Chen X.P., Tao L.Y. IL-33 provides neuroprotection through suppressing apoptotic, autophagic and NF-κB-mediated inflammatory pathways in a rat model of recurrent neonatal seizure. Front. Mol. Neurosci. 2017;10:423. doi: 10.3389/fnmol.2017.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Y., Ma L., Luo C.L., Wang T., Zhang M.Y., Shen X., Meng H.H., Ji M.M., Wang Z.F., Chen X.P., Tao L.Y. IL-33 exerts neuroprotective effect in mice intracerebral hemorrhage model through suppressing inflammation/apoptotic/autophagic pathway. Mol. Neurobiol. 2017;54:3879–3892. doi: 10.1007/s12035-016-9947-6. [DOI] [PubMed] [Google Scholar]
- 14.Vainchtein I.D., Chin G., Cho F.S., Kelley K.W., Miller J.G., Chien E.C., Liddelow S.A., Nguyen P.T., Nakao-Inoue H., Dorman L.C., Akil O., Joshita S., Barres B.A., Paz J.T., Molofsky A.B., Molofsky A.V. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science. 2018;359:1269–1273. doi: 10.1126/science.aal3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundke-Iqbal I., Iqbal K., Tung Y.C., Quinlan M., Wisniewski H.M., Binder L.I. Abnormal phosphorylation of the microtubule-associated protein ?? (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. U.S.A. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brion J.P., Couck A.M., Passareiro E., Flament-Durand J. Neurofibrillary tangles of Alzheimer's disease: an immunohistochemical study. J. Submicr. Cytol. 1985;17:89–96. [PubMed] [Google Scholar]
- 17.Hernandez F., Lucas J.J., Avila J. GSK3 and tau: two convergence points in Alzheimers disease. J. Alzheimers Dis. 2013;33:S141–S144. doi: 10.3233/JAD-2012-129025. [DOI] [PubMed] [Google Scholar]
- 18.Kanno T., Tsuchiya A., Tanaka A., Nishizaki T. Combination of PKCε activation and PTP1B inhibition effectively suppresses Aβ-induced GSK-3β activation and Tau phosphorylation. Mol. Neurobiol. 2016;53:4787–4797. doi: 10.1007/s12035-015-9405-x. [DOI] [PubMed] [Google Scholar]
- 19.Oakley H., Cole S.L., Logan S., Maus E., Shao P., Craft J., Guillozet-Bongaarts A., Ohno M., Disterhoft J., Van Eldik L., Berry R., Vassar R. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanno T., Tsuchiya A., Nishizaki T. Hyperphosphorylation of Tau at Ser396 occurs in the much earlier stage than appearance of learning and memory disorders in 5XFAD mice. Behav. Brain Res. 2014;274:302–306. doi: 10.1016/j.bbr.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 21.Xiong Z., Thangavel R., Kempuraj D., Yang E., Zaheer S., Zaheer A. Alzheimer’s disease: evidence for the expression of interleukin-33 and its receptor ST2 in the brain. J. Alzheimers Dis. 2014;40:297–308. doi: 10.3233/JAD-132081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu A.K., Hung K.W., Yuen M.Y., Zhou X., Mak D.S., Chan I.C., Cheung T.H., Zhang B., Fu W.Y., Liew F.Y., Ip N.Y. IL-33 ameliorates Alzheimer's disease-like pathology and cognitive decline. Proc. Natl. Acad. Sci. U.S.A. 2016;113:E2705–E2713. doi: 10.1073/pnas.1604032113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.La Rosa F., Saresella M., Baglio F., Piancone F., Marventano I., Calabrese E., Nemni R., Ripamonti E., Cabinio M., Clerici M. Immune and imaging correlates of mild cognitive impairment conversion to Alzheimer's disease. Sci. Rep. 2017;7:16760. doi: 10.1038/s41598-017-16754-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 25.Forde J.E., Dale T.C. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell. Mol. Life Sci. 2007;64:1930–1944. doi: 10.1007/s00018-007-7045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh M.C., Lee J., Choi Y Y. Tumor necrosis factor receptor- associated factor 6 (TRAF6) regulation of development, function, and homeostasis of the immune system. Immunol. Rev. 2015;266:72–92. doi: 10.1111/imr.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroeger K.M., Sullivan B.M., Locksley R.M. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38α-dependent pathway. J. Leukoc. Biol. 2009;86:769–778. doi: 10.1189/jlb.0708452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salmond R.J., Mirchandani A.S., Besnard A.G., Bain C.C., Thomson N.C., Liew F.Y. IL-33 induces innate lymphoid cell-mediated airway inflammation by activating mammalian target of rapamycin. J. Allergy Clin. Immunol. 2012;130:1159–1166. doi: 10.1016/j.jaci.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinto S.M., Subbannayya Y., Rex D.A.B., Raju R., Chatterjee O., Advani J., Radhakrishnan A., Keshava Prasad T.S., Wani M.R., Pandey A. A network map of IL-33 signaling pathway. J. Cell Commun. Signal. 2018;12:615–624. doi: 10.1007/s12079-018-0464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han L., Zhang M., Liang X., Jia X., Jia J., Zhao M., Fan Y. Interleukin-33 promotes inflammation-induced lymphangiogenesis via ST2/TRAF6-mediated Akt/eNOS/NO signalling pathway. Sci. Rep. 2017;7:10602. doi: 10.1038/s41598-017-10894-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kneynsberg A., Combs B., Christensen K., Morfini G., Kanaan N.M. Axonal degeneration in tauopathies: disease relevance and underlying mechanisms. Front. Neurosci. 2017;11:572. doi: 10.3389/fnins.2017.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patrick G.N., Zukerberg L., Nikolic M., de la Monte S., Dikkes P., Tsai L.H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 33.Jeganathan S., Hascher A., Chinnathambi S., Biernat J., Mandelkow E.M., Mandelkow E. Proline-directed pseudo-phosphorylation at AT8 and PHF1 epitopes induces a compaction of the paperclip folding of Tau and generates a pathological (MC-1) conformation. J. Biol. Chem. 2008;283:32066–32076. doi: 10.1074/jbc.M805300200. [DOI] [PubMed] [Google Scholar]
- 34.Pei J.J., Björkdahl C., Zhang H., Zhou X., Winblad B. p70 S6 kinase and tau in Alzheimer's disease. J. Alzheimers Dis. 2008;14:385–392. doi: 10.3233/jad-2008-14405. [DOI] [PubMed] [Google Scholar]
- 35.Ren Q.G., Wang Y.J., Gong W.G., Xu L., Zhang Z.J. Escitalopram ameliorates Tau hyperphosphorylation and spatial memory deficits induced by protein kinase A activation in Sprague Dawley rats. J. Alzheimers Dis. 2015;47:61–71. doi: 10.3233/JAD-143012. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto H., Yamauchi E., Taniguchi H., Ono T., Miyamoto E. Phosphorylation of microtubule-associated protein tau by Ca2+/calmodulin-dependent protein kinase II in its tubulin binding sites. Arch. Biochem. Biophys. 2002;408:255–262. doi: 10.1016/s0003-9861(02)00556-8. [DOI] [PubMed] [Google Scholar]
- 37.Hu M., Waring J.F., Gopalakrishnan M., Li J. Role of GSK-3β activation and α7 nAChRs in Aβ1-42-induced tau phosphorylation in PC12 cells. J. Neurochem. 2008;106:1371–1377. doi: 10.1111/j.1471-4159.2008.05483.x. [DOI] [PubMed] [Google Scholar]
- 38.Li C., Götz J. Somatodendritic accumulation of Tau in Alzheimer's disease is promoted by Fyn-mediated local protein translation. EMBO J. 2017;36:3120–3138. doi: 10.15252/embj.201797724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbott J.J., Howlett D.R., Francis P.T., Williams R.J. Aβ1-42 modulation of Akt phosphorylation via α7 nAChR and NMDA receptors. Neurobiol. Aging. 2008;29:992–1001. doi: 10.1016/j.neurobiolaging.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Takashima A. GSK-3β and memory formation. Front. Mol. Neurosci. 2012;5:47. doi: 10.3389/fnmol.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tokutake T., Kasuga K., Yajima R., Sekine Y., Tezuka T., Nishizawa M., Ikeuchi T. Hyperphosphorylation of Tau induced by naturally secreted amyloid-β at nanomolar concentrations is modulated by insulin-dependent Akt-GSK3β signaling pathway. J. Biol. Chem. 2012;287:35222–35233. doi: 10.1074/jbc.M112.348300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shelly M., Lim B.K., Cancedda L., Heilshorn S.C., Gao H., Poo M.M. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science. 2010;327:547–552. doi: 10.1126/science.1179735. [DOI] [PubMed] [Google Scholar]
- 43.Naska S., Park K.J., Hannigan G.E., Dedhar S., Miller F.D., Kaplan D.R. An essential role for the integrin-linked kinase-glycogen synthase kinase-3β pathway during dendrite initiation and growth. J. Neurosci. 2006;26:13344–13356. doi: 10.1523/JNEUROSCI.4462-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song B., Lai B., Zheng Z., Zhang Y., Luo J., Wang C., Chen Y., Woodgett J.R., Li M. Inhibitory phosphorylation of GSK-3 by CaMKII couples depolarization to neuronal survival. J. Biol. Chem. 2010;285:41122–41134. doi: 10.1074/jbc.M110.130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valerio A., Ghisi V., Dossena M., Tonello C., Giordano A., Frontini A., Ferrario M., Pizzi M., Spano P., Carruba M.O., Nisoli E. Leptin increases axonal growth cone size in developing mouse cortical neurons by convergent signals inactivating glycogen synthase kinase-3β. J. Biol. Chem. 2006;281:12950–12958. doi: 10.1074/jbc.M508691200. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Z., Wang Z., Gu Y., Feil R., Hofmann F., Ma L. Regulate axon branching by the cyclic GMP pathway via inhibition of glycogen synthase kinase 3 in dorsal root ganglion sensory neurons. J. Neurosci. 2009;29:1350–1360. doi: 10.1523/JNEUROSCI.3770-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wada A. Lithium and neuropsychiatric therapeutics: neuroplasticity via glycogen synthase kinase-3β, β-catenin, and neurotrophin cascades. J. Pharmacol. Sci. 2009;110:14–28. doi: 10.1254/jphs.09r02cr. [DOI] [PubMed] [Google Scholar]