Abstract
Background
Cerebral malaria (CM) is often fatal, and severe brain swelling is a predictor of CM-related mortality. CM is characterized by elevated circulating pro-inflammatory cytokines TNF and IFN-γ and anti-inflammatory cytokine IL-10, however whether cytokine levels correlate with brain swelling severity is unknown. This study therefore was conducted to investigate the relationship between cytokine levels and brain swelling severity in children presenting with CM.
Methods
A total of 195 Malawian children presenting with CM were recruited and had the concentrations of plasma cytokines determined and compared to brain swelling severity, determined by MRI examination, and graded as severe, moderate, mild or none.
Results
Levels of IL-1β, IL-6, IL-8 and IL-10 did not differ between CM patients with and without severe brain swelling. Compared to children without brain swelling, IL-12 levels were higher in children with severe swelling (p < 0.01, no swelling 1 pg/mL, IQR [1] vs. severe swelling 18.7 pg/mL, IQR [1–27]), whereas TNF concentrations were higher in children with moderate brain swelling compared to children with no swelling (p < 0.01, no swelling 3 pg/mL, IQR [1–20] vs. moderate swelling 24 pg/mL, IQR [8–58]. Multivariate analysis showed that no single cytokine independently predicted brain swelling.
Conclusion
Severe brain swelling in paediatric CM was independent of tested blood pro-inflammatory and anti-inflammatory cytokines which are markers of systemic inflammation.
Electronic supplementary material
The online version of this article (10.1186/s12936-018-2590-0) contains supplementary material, which is available to authorized users.
Keywords: Cerebral malaria, Brain swelling, Cytokines, Plasmodium falciparum, Africa
Background
Despite substantial progress in reducing the burden of malaria globally [1], Plasmodium falciparum malaria still accounts for hundreds of thousands of paediatric deaths annually in sub-Saharan Africa [2]. The exact mechanisms that contribute to death are not fully known. Clinically, P. falciparum malaria infections present with a spectrum of severity ranging from asymptomatic infections to uncomplicated malaria illness (UM), and more severe forms of disease including cerebral malaria (CM), severe malarial anaemia and/or respiratory distress [3]. CM is the major contributor to mortality [3] and often results in debilitating neurological impairments in survivors [4]. Although some investigators have reported an association between overproduction of some pro-inflammatory cytokines, such as tumour necrosis factor (TNF), interferon gamma (IFN-γ), interleukin 6 (IL-6) and interleukin 1 beta (IL-1β) and CM pathogenesis [5], disease severity and death [6] others reported lack of association between TNF concentration and malaria severity in Ugandan children [7].
Studies have shown that inflammatory cytokines can affect the integrity and functions of the blood brain barrier (BBB) leading to vasogenic oedema and protein extravasation [8–10]. Previous work has identified increased intracranial pressure and brain swelling in paediatric patients with CM [4, 11], and recent work conducted in Malawi identified brain swelling, as shown by magnetic resonance imaging (MRI), as a key risk factor for a fatal outcome [12]. The mechanisms and processes leading to brain swelling in CM remain uncharacterized. Here, the association was investigated between severity of brain swelling on MRI and peripheral blood cytokine levels in children with clinical CM to expand on the current understanding of the role of inflammatory processes in CM pathogenesis. Malaria retinopathy was included in the definition of CM to improve specificity. In addition, the relationships between cytokine concentrations and two clinical parameters: duration of coma and parasite density were investigated. The two clinical parameters were investigated because total parasite load measured as plasma HRP-2 concentration has previously been shown to predict progression to CM [13], and pro-inflammatory cytokines TNF and IFN-γ and anti-inflammatory cytokine interleukin 10 (IL-10) have been associated with CM [5, 14, 15].
Methods
Study area and study population
Children aged between 6 months and 12 years old presenting with CM at Queen Elizabeth Central Hospital (QECH) in Blantyre, Malawi were recruited from January 2009 to June 2016. CM was defined based on the WHO definition of fever (temperature > 37.5 °C) with asexual stage P. falciparum parasites on blood film microscopy combined with a Blantyre coma score of 2 or less at admission and 4 h later, after eliminating other potential causes of seizures, such as hypoglycaemia. Coma duration prior to presentation was determined by asking the guardian the time the child became comatose.
All study participants underwent a direct and indirect dilated ophthalmological funduscopic examination. Children with retinal findings characteristic of malaria, including retinal whitening, haemorrhages and vessel discoloration [16, 17], were classified as retinopathy positive (Ret+ CM) and children with normal ocular fundi as retinopathy negative (Ret− CM). The WHO criteria for CM are highly sensitive for true CM, but the inclusion of retinal examination has been shown to improve CM specificity. Parasitaemic children with Ret− CM are commonly found to have a non-malaria aetiology of coma [12, 17]. Therefore, only Ret+ CM cases were included in the analysis. Data from HIV-infected participants were excluded from the final analysis since studies done in this population have shown that that there is synergy between the two infections [9], and that HIV infection on its own also has an independent effect on the cytokine profiles of the infected individuals [18]. Informed consent was obtained from parents or guardians for all the children enrolled in the study. A 5 mL venous blood sample was collected in sodium heparin tubes from each participant at recruitment. After centrifugation, plasma was stored at − 80 °C until the day of analysis. Participants from 2009 to 2013 were treated with IV quinine, whereas patients from 2014 to 2016 were treated with IV artesunate as recommended by the National Malaria Control Programme of Malawi.
Brain swelling analysis
Brain scans were performed using a 0.35-T Signa Ovation Excite MRI scanner (General Electric, Milwaukee, USA). Two radiologists, unaware of each other’s readings and each patient’s retinopathy status and clinical outcome, interpreted each MRI scan. Brain swelling was graded for severity on the basis of pre-specified criteria [12]. Overall brain swelling was scored on the basis of the appearance of the cerebral hemispheres on a scale from 1 to 8, with a score of 1 indicating marked atrophy, 2 mild atrophy, 3 normal brain size, 4 slight swelling, 5 mild swelling, 6 moderate swelling, 7 substantial swelling with diffuse sulcal and cisternal effacement but no evidence of herniation, and 8 sulcal and cisternal effacement with evidence of herniation [12]. Scores of 7 and 8 were pre-specified as severe brain swelling because the radiologists considered these scores to indicate a life-threatening condition [12]. In this study, brain swelling was defined as follows: score of ≤ 3: normal brain size; scores of 4–5: mild swelling; score of 6: moderate swelling; scores of 7–8: severe swelling.
Cytokine analysis
Plasma cytokine concentrations were measured using a human inflammatory cytokines cytometric bead array (CBA) (Becton–Dickinson), a multiplex assay that allows for the simultaneous quantification of IL-1β, IL-6, interleukin 8 (IL-8), IL-10, interleukin 12 (IL-12) and TNF. This combination of cytokines was chosen based on their previously reported roles in malaria and specifically in CM [15, 19]. A 50 μL aliquot of each sample was mixed with 50 μL of the capture beads mixture. Samples were diluted 1:10 using assay diluent. Subsequent steps were performed according to manufacturer’s instructions (BD CBA Instruction Manuals, 2016). Samples were acquired on CyAn ADP flow cytometer (Beckman Coulter) and analysed using BD FCAP software version 3.0 (San Jose, CA, USA).
Statistical analysis
Data were analysed using Stata version 14.0 (Stata Corp, TX, USA) and GraphPad Prism 5 (GraphPad, CA, USA). Medians and inter quartile range (IQR) were computed for continuous variables after log transformation. Kruskal–Wallis test was used to compare medians across more than two groups and between-group comparisons of cytokine concentrations for the different groups were assessed with Dunn’s multiple comparison test. Mann–Whitney U test was used to assess bivariate association with brain swelling for continuous variables that were not normally distributed. Fisher’s exact test was used to assess bivariate association with brain swelling for categorical variables because the sample size was small. Univariate and multivariate logistic regression models were fitted to investigate independent factors associated with brain swelling. The models were used to estimate the crude, adjusted odds ratios (ORs) and associated 95% confidence intervals (CI). Variables with p-value ≤ 0.2 in univariate analysis were included in the multivariate model shown in Tables 1, 2. Correlations between different variables were determined by Microsoft Excel and cytokine network analysis and a correlation matrix heatmap was done using R package version 3.2.0 presented in Fig. 4. Graphs in Figs. 1, 2, 3 were plotted using GraphPad Prism 5. Differences were considered to be statistically significant if the p values were less than or equal to 0.05.
Table 1.
Demographic characteristics of the study participants: univariate and multivariable logistic regression model showing the association between brain swelling and demographic characteristics of the study participants
| Variable | Characteristic | No severe swelling | Severe swelling | p-value* | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value** | OR | 95% CI | p-value*** | |||||
| Gender | Female (%) | 33 (37.5) | 55 (62.5) | 0.24 | 0.68 | 0.38; 1.22 | 0.195 | 1.04 | 0.28; 3.87 | 0.954 |
| Male (%) | 50 (46.73) | 57 (53.27) | ||||||||
| Age (months) | Median (IQR) | 48 (31; 68) | 42 (28.0; 65.5) | 0.29 | 1 | 0.99; 1.00 | 0.523 | ND | ||
| Hours in coma prior to admission | Median (IQR) | 2.08 (1.79; 2.48) | 2.20 (1.79; 3.04) | 0.22 | 1.25 | 0.89; 1.75 | 0.191 | 1.31 | 0.64; 2.66 | 0.462 |
| Parasitaemia (parasites log10/μL) | Median (IQR) | 10.83 (8.16; 11.78) | 10.64 (7.46; 12.41) | 0.66 | 1.03 | 0.93; 1.14 | 0.597 | ND | ND | |
In this analysis brain swelling scores of 3 to 6 were considered to not have severe swelling and scores of 7 and 8 were considered to have severe brain swelling. Variables with p-value ≤ 0.2 in univariate analysis were included in the multivariate model. Group comparisons were done using Mann–Whitney U test except for age, where Fisher’s Exact test was used. Results for parasitaemia were log transformed (log10)
ND not done
* Univariate association
** Simple logistic regression
*** Multivariable logistic regression
Table 2.
Univariate and multivariate associations with brain swelling: univariate association and multivariable logistic regression model showing the association between brain swelling and a number of cytokines
| Variable | Characteristic | No severe swelling | Severe swelling | p-value* | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | OR | 95% CI | p-value** | OR | 95% CI | p-value*** | |||
| IL-10 (pg/mL) | Median (IQR) | 5.28 (4.44; 6.32) | 5.42 (4.40; 6.37) | 0.61 | 1.04 | 0.87; 1.26 | 0.644 | ND | ||
| IL-1β (pg/mL) | Median (IQR) | 2.07 (1.35; 2.77) | 2.02 (1.31; 2.58) | 0.69 | 1 | 0.79; 1.27 | 0.996 | ND | ||
| IL-6 (pg/mL) | Median (IQR) | 5.11 (4.23; 5.95) | 5.27 (4.29; 6.38) | 0.26 | 1.11 | 0.91; 1.35 | 0.297 | ND | ||
| IL-12 (pg/mL) | Median (IQR) | 2.73 (1.42; 3.20) | 3.11 (2.87; 3.29) | 0.05 | 1.37 | 0.94; 1.99 | 0.103 | 1.24 | 0.74; 2.08 | 0.415 |
| IL-8 (pg/mL) | Median (IQR) | 4.27 (3.58; 5.04) | 4.46 (3.65; 5.50) | 0.16 | 1.19 | 0.93; 1.52 | 0.159 | 1.91 | 0.98; 3.71 | 0.056 |
| TNF (pg/mL) | Median (IQR) | 2.82 (2.26; 3.32) | 3.03 (2.50; 3.82) | 0.07 | 1.24 | 0.95; 1.63 | 0.119 | 1.41 | 0.76; 2.76 | 0.275 |
| Ratio (IL10/TNF) | Median (IQR) | 2.24 (1.32; 3.45) | 2.23 (0.85; 3.58) | 0.77 | 0.96 | 0.82; 1.13 | 0.662 | ND | ||
| Ratio (IL-10/IL-6) | Median (IQR) | 0.20 (0.51; 0.85) | 0.14 (0.73; 0.77) | 0.44 | 0.9 | 0.69; 1.18 | 0.448 | ND | ||
| Ratio (IL-10/IL-1β) | Median (IQR) | 3.43 (2.26; 4.51) | 3.62 (2.37; 4.46) | 0.96 | 1 | 0.83; 1.21 | 0.962 | ND | ||
| Ratio (IL-10/IL-12) | Median (IQR) | 2.57 (1.07; 3.92) | 2.64 (0.68; 3.55) | 0.69 | 0.94 | 0.75; 1.19 | 0.632 | ND | ||
| Ratio (IL-10/IL-8) | Median (IQR) | 0.96 (0.12; 1.72) | 0.92 (0.33; 1.72) | 0.67 | 0.93 | 0.76; 1.14 | 0.496 | ND | ||
In this analysis brain swelling scores of 3 to 6 were considered to not have severe swelling and scores of 7 and 8 were considered to have severe swelling. Variables with p-value ≤ 0.2 in univariate analysis were included in the multivariate model. Group comparisons were done using Mann–Whitney U test. Results for cytokine concentrations and parasitaemia were log transformed (log10). Multivariable logistic regression model analysis was based on only those variables with p-value ≤ 0.2 in univariate analysis
ND not done
* Univariate association
** Simple logistic regression
*** Multivariable logistic regression
Fig. 4.
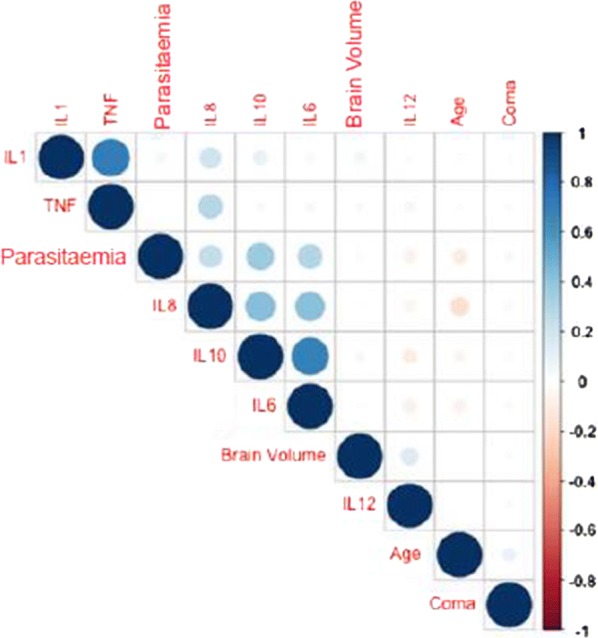
Correlation between plasma concentration of cytokines with disease parameters in children with CM. A heatmap representing the correlation between different cytokines with other variables associated with acute CM (n = 195) such as parasitaemia levels, age, length of coma and brain swelling in Malawian children. Circle size and darkness signify increased positive correlation (shade of colour is proportional to the probability of dependence between variables from + 1 to − 1)
Fig. 1.
Plasma cytokine levels in CM children stratified by severity of brain swelling. Median plasma cytokine concentrations (pg/mL) at hospital presentation in CM children with varying severity of brain swelling on MRI, normal (score < 3, n = 23), mild (score 4,5, n = 58), moderate (score 6, n = 53), severe (score 7,8, n = 61); a IL-1β, b IL-10, c IL-6, d IL-12, e IL-8, f TNF. Kruskal–Wallis test was used for group analysis, p value of ≤ 0.05 was considered statistically significant. The bar represents a median value
Fig. 2.
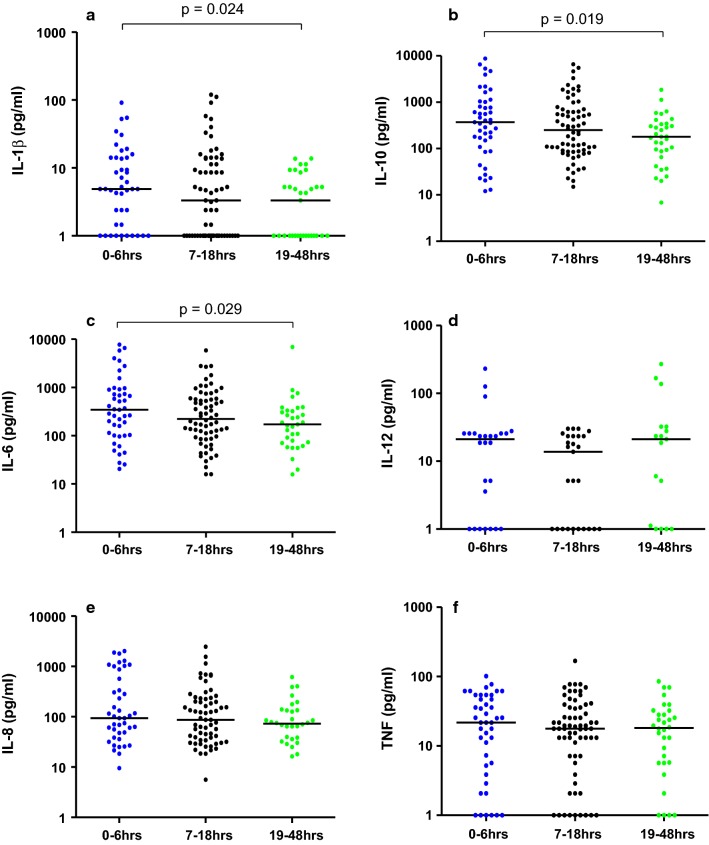
Plasma cytokine levels in CM children stratified by duration of coma prior to admission. Median plasma cytokine levels (pg/mL) at hospital presentation in CM children categorized into three groups based on the length of coma prior to admission [0–6 h (n = 45); 7–18 h, (n = 68); 19–48 h, (n = 33)]; a IL-1β, b IL-10, c IL-6, d IL-12, e IL-8, f TNF. Kruskal–Wallis test was used for paired group analysis, p value of ≤ 0.05 was considered statistically significant. The bar represents a median value. All the medians were similar for all the cytokines
Fig. 3.
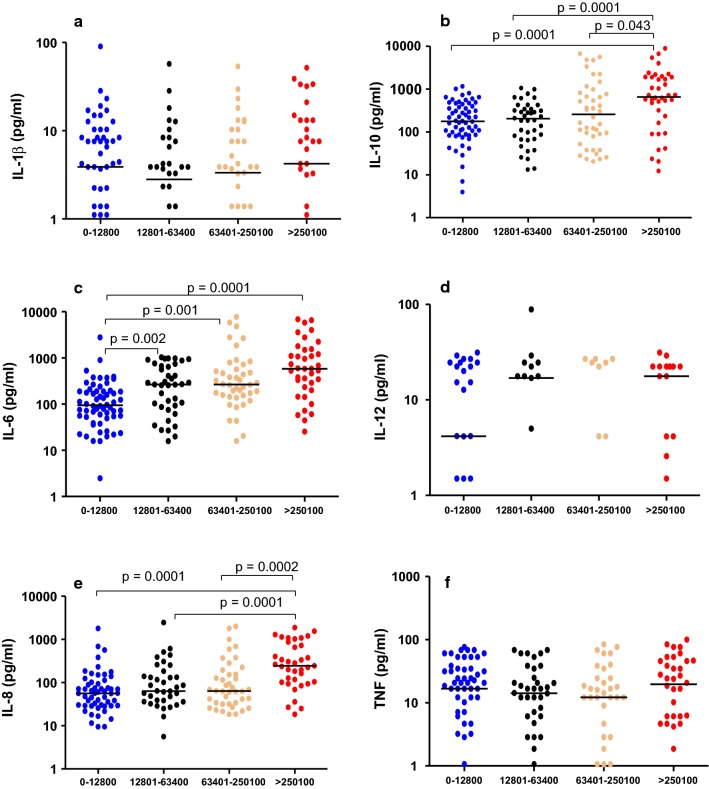
Plasma cytokine levels in CM children stratified by peripheral parasitaemia. Median plasma cytokine levels (pg/mL) at hospital presentation in children with CM, categorized into four groups based on peripheral blood parasite load per microliter of blood [1–12,800, (n = 60); 12,801–63,400, (n = 39); 63,401–250,100, (n = 42) and > 250,100, (n = 38)] at presentation; a IL-1β, b IL-10, c IL-6, d IL-12, e IL-8, f TNF. Kruskal–Wallis test was used for group analysis, p value of ≤ 0.05 was considered statistically significant. The bar represents a median value
Results
Demographic characteristics of study patients
A total of 195 HIV-uninfected children with CM and malarial retinopathy aged between 6 months and 12 years were studied. Convenience sampling was used based on the availability of plasma samples and MRI data. Sample collection dates span from 2009 to 2015. Of the 195 children, 107 (55%) were male. Twenty-eight (14%) study participants died. Based on the MRI interpretation [20], 22 children had no brain swelling, 58 had mild swelling, 53 had moderate swelling and 62 had severe brain swelling. Previous published work demonstrated a correlation between severe swelling and death [12]; hence during analysis the children with no severe swelling were compared with those that had severe swelling (Tables 1, 2).
Univariate and multivariate associations of cytokines with brain swelling
In univariate analyses there were no differences in cytokine levels between children with no severe swelling and those with severe swelling (Table 2). Variables with p ≤ 0.2 in univariate analysis (IL-8, IL-12 and ratio TNF) were included in the multivariate model. Just as in univariate analysis, there were no independent predictors of brain swelling with variables included in the model (Table 2). There were also no differences in cytokine levels as well as demographic characteristics between children with no swelling and those with swelling (Additional file 1: Tables S1, S2, respectively).
Plasma cytokine levels in children with CM and different degrees of brain swelling
Plasma levels of the following cytokines did not significantly differ between children with severe brain swelling and those who had no swelling, mild or moderate swelling (Fig. 1a–f): IL-1β, TNF, IL-10, IL-6, and IL-8. Children with severe brain swelling had significantly higher levels of IL-12 than those with no swelling: 18.7 pg/mL [1–27] vs. 1 pg/mL [1] (p = 0.01) (Fig. 1d). Children with moderate brain swelling had significantly higher TNF levels compared to those who had no swelling: 24 pg/mL [8–58] vs. 3 [1–20] (p < 0.01), and those who had mild swelling: 13 pg/mL [3–25] (p < 0.05) (Fig. 1f).
IL-1β, IL-6, IL-10 plasma levels were lower in those with longer coma duration prior to admission
Because many cytokines have short half-lives in plasma spanning 4 to 12 h [18], the relationship between plasma cytokine levels and the estimated time the children were in coma before presenting at the hospital for admission was investigated. CM pathogenesis is likely a progressive process. It was therefore hypothesized that the time the disease remains untreated (i.e., the time from initial onset of coma to presentation at the Paediatric Research Ward) is an important determinant of disease severity. Although time in coma prior to presentation to the hospital is only an approximation of the onset of the disease process, it is used as an indicator of progression along this disease pathway. Given the transient kinetics of peripheral cytokines, consideration of the time from initial disease onset might be important.
Based on the distribution of the coma duration prior to admission, children were stratified into three groups: 0–6 h coma, 7–18 h, 19–48 h. Compared to children who presented with ≥ 19 h after coma onset, children who presented with ≤ 6 h of coma prior to admission had significantly higher levels of the cytokines IL-1β: 5 pg/mL [1.46–14] vs. 3 [1–7], (p = 0.024) (Fig. 2a), IL-10: 369 pg/mL [129–1030] vs. 180 [77–340], (p = 0.019), (Fig. 2b), and IL-6: 345 pg/mL [102–907] vs. 172 [72–327], (p = 0.029) (Fig. 2c).
Plasma cytokine levels of IL-6, IL-8 and IL-10 were associated with high parasitaemia
The relationship between peripheral cytokine levels and parasite density on admission was investigated. Study participants were stratified into four groups based on quintile ranges of parasite density per microlitre of blood (1–12,800, 12,801–63,400, 63,401–250,100, and > 250,100). Plasma cytokine levels of IL-10: (657 [212–1868] vs. 177 [85–423] pg/mL) (Fig. 3b); IL-6: (584 [225–1512] vs. 94 [53–190] pg/ml) (Fig. 3c), and IL-8: (245 [104–802] vs. 57 [29–95] pg/mL) (Fig. 3e) were significantly higher in children in the highest parasitaemia quintile (> 250,100) compared to those with the lowest parasite density (1–12,800) (p < 0.0001). Concentrations of the cytokines IL-10, IL-8 and IL-6 correlated with parasite density in the correlation analysis (Fig. 4).
Cytokine network and correlation with brain swelling in cerebral malaria
To investigate the relationships between peripheral blood concentrations of the cytokines IL-10, IL-1β, IL-6, IL-8, IL-12, and TNF and age, parasitaemia, length of coma, and brain swelling, correlation analyses were performed. There were correlations between levels of some of the cytokines: IL-8 correlated with IL-1β, IL-6, and TNF, whereas IL-10 correlated with IL-8 and IL-6. This result demonstrates that cytokines work in concert and provide a plasma cytokine profile in CM in children. This cytokine network is critical in driving the pro-inflammatory response as well as immune response counter regulation. As shown in Fig. 4, there was no association between cytokine concentrations and brain swelling or duration of coma.
Discussion
Cerebral malaria has a high mortality rate but an understanding of the mechanisms leading to death remains unclear. Factors including inflammatory cytokines [5, 15], markers of endothelial activation [21, 22], coagulation dysfunction [23], and total parasite load [24] have all been implicated, and combinations of these biomarkers can improve predictive value [25]. More recently, a strong association was described between severity of brain swelling on MRI and mortality from CM in Malawian children, with 84% of children who died having severe brain swelling [12]. Given the strong associations between elevations of certain systemic cytokines, disease severity [5, 15] and outcome [26] it was hypothesized that an elevated systemic inflammatory response, as indicated by elevated plasma cytokine concentrations of inflammatory cytokines IL-1β, TNF, IL-6, and IL-8, may be directly or indirectly associated with brain swelling. In a unique series of 195 children with CM who underwent MRI scanning, concentrations of a selection of pro- and anti-inflammatory cytokines were measured in blood plasma collected at hospital presentation, and the relationships were investigated between plasma cytokines, brain swelling, coma duration, and parasite density in these children.
Overall, there were weak associations between pro- and anti-inflammatory cytokine concentrations and brain swelling, possibly reflecting the different temporal dynamics of cytokines and brain swelling, or the difficulties of assessing tissue-specific inflammation in peripheral blood. Higher concentrations of IL-12 in patients with severe and moderate brain swelling compared to patients without swelling, and of TNF in moderate (but not severe swelling) compared to children without swelling were found, but concentrations of other cytokines did not differ with the severity of the brain swelling. Data on the relationship between IL-12 and CM are limited [27]. In contrast, studies aimed at investigating the association between high TNF levels and malaria severity as well as death have yielded conflicting results with some studies showing an association [5, 28, 29] and another study showing no association [7]. The weak relationship between circulating TNF and brain swelling observed may be because local production of TNF at sites of sequestration is critical, and is not accurately reflected in circulating levels. Additionally, the lack of association between severe brain swelling and TNF concentrations may reflect differences in the kinetics of the different processes, with cytokine concentrations potentially peaking earlier in the disease process than the brain swelling.
To investigate how differences in duration of coma might affect cytokine concentrations, children were categorized according to duration of coma prior to admission. Children who presented within 6 h of coma onset had higher concentrations of IL-1β, IL-6, and IL-10 than those who presented more than 18 h after coma onset. While the precise time of onset of the malaria episode was not known, loss of consciousness implies significant neurological dysfunction. Coma duration was not associated with CM brain swelling. It is possible that the onset of coma is associated with some kind of a cytokine storm that gradually diminishes as cytokine levels decline with time [30, 31]. This suggests different kinetics to the two processes.
Parasite biomass, measured as concentration of plasma HRP2 (a biomarker of sequestered and circulating parasites) has previously been correlated with malaria severity and progression to CM [13, 32], and it is this sequestration that is thought to directly or indirectly contribute to CM pathogenesis through activation of the inflammasome pathway in endothelial cells [33] and possibly by inducing endothelial barrier disruption [34]. Some researchers have demonstrated a strong association between coma, congestion and microvascular obstruction in human CM leading to the hypothesis that CM brain swelling could, in part, be caused by congestion and increased hydrostatic pressure which leads to oedema [24]. Although there were strong associations between peripheral blood parasite density and concentrations of cytokines IL-6, IL-8 and IL-10, there was no significant association between peripheral blood parasite density and severe brain swelling (Fig. 4). The observed association between peripheral parasite density and cytokine levels may indicate compartmentalized immune response to circulating parasitaemia [35].
Cytokines, such as TNF, IFN-γ, IL-6 and IL-1β, are likely to be beneficial in the early stages of the infection in parasite clearance, but might actually contribute to the pathogenesis associated with CM [36], and to disease severity and death [37] if produced in an unregulated manner. In contrast, anti-inflammatory cytokines, including IL-10, are also elevated in children with CM, but are thought to be involved in counteracting the effect of the pro-inflammatory cytokines and down-regulating their production [38]. The findings of this study are instead more consistent with inflammatory and counter-regulatory cytokine responses that are appropriately increased as parasite burden increased. In a heat map analysis, there were no significant correlations between cytokine concentrations and brain swelling (Fig. 4), suggesting that peripheral blood cytokine concentrations were not major drivers of brain swelling in this population. Overall, the results of this and previous studies concerning cytokines in malaria suggest that peripheral cytokine levels are elevated in CM but that within the CM cases circulating cytokines appear not to correlate with either brain swelling or death [5]. If cytokines do not directly contribute to brain swelling, there must be other signals that initiate and/or perpetuate swelling (such as an increased responsiveness of the endothelial cells to cytokines or cytokine-independent activation of the endothelial cells).
The strengths of this study include the large number of children undergoing an MRI scan, and the use of retinal examination, resulting in stringently defined CM [17]. By analysing plasma cytokine levels in 195 children with CM, the study had sufficient statistical power (> 80%) to observe significant differences in the various parameters tested. Limitations include only a single time point for blood sampling, which varied between children relative to onset of coma and probably to time of infection. Blood samples collected at different time points from onset of coma for each child would have permitted exploration of the relationship between cytokine levels and duration in coma in more detail. Second, like all human cohort studies of pre-fatal CM, samples from this study were collected from the peripheral blood, and not from the sites of parasite sequestration and disease pathology. Peripheral blood cytokine concentrations may not accurately reflect those at the site of disease in the cerebral vasculature. There is evidence that inflammatory cytokines are produced within the central nervous system [39], and a recent study reported higher cytokine concentrations in cerebral spinal fluid (CSF) than in plasma from CM patients [39], although a second study did not find any correlation between CSF and serum cytokine levels [40]. Whether measurement of cytokines in CSF, or in brain tissue, would provide stronger correlates of brain swelling, remains to be studied.
Conclusions
Although inflammatory cytokine concentrations in peripheral blood at time of presentation are associated with parasite load and time of coma onset, they do not appear to directly correlate with brain swelling severity on MRI in strictly defined CM. These findings might reflect the different temporal dynamics of cytokines and brain swelling, or the difficulties of assessing tissue-specific inflammation in peripheral blood. Further investigation should study the relationship between markers of endothelial cell activation and brain swelling.
Additional file
Additional file 1: Table S1. Demographic characteristics of the study participants. Table S2. Univariate and multivariate associations with brain swelling.
Authors’ contributions
VH, SR, KS and WM conceived the study. VH, MN, SK and MJP performed the investigations. VH, BK, AC and WM analysed the data. VH, WM, TT and KS wrote the report. SR, KS, TT, WM and SK oversaw the research. All authors contributed to the study design and reviewed the report. All authors read and approved the final manuscript.
Acknowledgements
The authors are thankful to the enrolled children and their guardians for participation in the study, the staff of the Blantyre Malaria Project, Queen Elizabeth Central Hospital, and Ndirande Health Centre in Blantyre, Malawi for their willing cooperation with this study, especially Patricia Phula and our project research nurses. This work was supported by a grant to SJR from the National Medical Health Research Council (Australia).
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable. The study was approved by the College of Medicine Research and Ethics Committee (COMREC), University of Malawi and the Michigan State University Institutional Review Board (MSU-IRB) and written consent was obtained from the parents or guardians of each participating child.
Funding
This work was partly supported by the National Health and Medical Research Council of Australia and by the US National Institutes of Health (5R01AI034969-14), and the Michigan State University College of Osteopathic Medicine.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- BBB
blood brain barrier
- CBA
cytometric bead array
- CI
confidence interval
- CM
cerebral malaria
- COMREC
College of Medicine Research and Ethics Committee
- HIV
human immunodeficiency virus
- HRP2
histidine-rich protein 2
- IFN-γ
interferon gamma
- IL
interleukin
- iRBCs
infected red blood cells
- IV
intravenous
- MRI
magnetic resonance imaging
- MSU-IRB
Michigan State University Institutional Review Board
- OR
odds ratio
- QECH
Queen Elizabeth Central Hospital
- Ret−
retinopathy negative
- Ret+
retinopathy positive
- TNF
tumour necrosis factor
- UM
uncomplicated malaria
- WHO
World Health Organization
Contributor Information
Visopo Harawa, Phone: +265 884 199 758, Email: vharawa@gmail.com.
Wilson Mandala, Phone: +265 888 858 454, Email: wmandala2002@gmail.com.
References
- 1.Murray CJL, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–1070. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Global Malaria Programme . World malaria report 2015. Geneva: World Health Organization; 2015. [Google Scholar]
- 3.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 4.Newton CR, Peshu N, Kendall B, Kirkham FJ, Sowunmi A, Waruiru C, et al. Brain swelling and ischaemia in Kenyans with cerebral malaria. Arch Dis Child. 1994;70:281–287. doi: 10.1136/adc.70.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grau GE, Taylor TE, Molyneux ME, Wirima JJ, Vassalli P, Hommel M, et al. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 6.Mshana RN, Boulandi J, Mshana NM, Mayombo J, Mendome G. Cytokines in the pathogenesis of malaria: levels of IL-I beta, IL-4, IL-6, TNF-alpha and IFN-gamma in plasma of healthy individuals and malaria patients in a holoendemic area. J Clin Lab Immunol. 1991;34:131–139. [PubMed] [Google Scholar]
- 7.Lovegrove FE, Tangpukdee N, Opoka RO, Lafferty EI, Rajwans N, Hawkes M, et al. Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS ONE. 2009;4:e4912. doi: 10.1371/journal.pone.0004912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dieye Y, Mbengue B, Dagamajalu S, Fall MM, Loke MF, Nguer CM, et al. Cytokine response during non-cerebral and cerebral malaria: evidence of a failure to control inflammation as a cause of death in African adults. PeerJ. 2016;4:1965. doi: 10.7717/peerj.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hochman SE, Madaline TF, Wassmer SC, Mbale E, Choi N, Seydel KB, et al. Fatal pediatric cerebral malaria is associated with intravascular monocytes and platelets that are increased with HIV coinfection. MBio. 2015;6:e01390-15. doi: 10.1128/mBio.01390-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2012;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newton CR, Crawley J, Sowumni A, Waruiru C, Mwangi I, English M, et al. Intracranial hypertension in Africans with cerebral malaria. Arch Dis Child. 1997;76:219–226. doi: 10.1136/adc.76.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seydel KB, Kampondeni SD, Valim C, Potchen MJ, Milner DA, Muwalo FW, et al. Brain swelling and death in children with cerebral malaria. N Engl J Med. 2015;372:1126–1137. doi: 10.1056/NEJMoa1400116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox LL, Taylor TE, Pensulo P, Liomba A, Mpakiza A, Varela A, et al. Histidine-rich protein 2 plasma levels predict progression to cerebral malaria in malawian children with Plasmodium falciparum infection. J Infect Dis. 2013;208:500–503. doi: 10.1093/infdis/jit176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwiatkowski D, Hill AV, Sambou I, Twumasi P, Castracane J, Manogue KR, et al. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336:1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 15.Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, et al. Serum levels of the proinflammatory IL-8, IL-10, tumor necrosis factor alpha, and IL-12 (p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun. 2004;72:5630–5637. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molyneux ME, Taylor TE, Wirma JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Int J Med. 1988;2:441–459. [PubMed] [Google Scholar]
- 17.Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–145. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 18.Mora DJ, Ferreira-Paim K, Andrade-Silva LE, Bragine T, Rocha IH, De Melo Ribeiro B, et al. Cytokine patterns in a prospective cohort of HIV-infected patients with cryptococcal meningitis following initiation of antifungal and antiretroviral therapy. PLoS ONE. 2017;12:1–17. doi: 10.1371/journal.pone.0176304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Othoro C, Lal AA, Nahlen B, Koech D, Orago ASS, Udhayakumar V. A low interleukin-10 tumor necrosis factor-α ratio is associated with malaria anemia in children residing in a holoendemic malaria region in Western Kenya. J Infect Dis. 1999;179:279–282. doi: 10.1086/314548. [DOI] [PubMed] [Google Scholar]
- 20.Potchen MJ, Kampondeni SD, Ibrahim K, Bonner J, Seydel KB, Taylor TE, et al. Neurointerp: a method for facilitating neuroimaging research on cerebral malaria. Neurology. 2013;81:585–588. doi: 10.1212/WNL.0b013e31829e6ed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown H, Hien TT, Day N, Mai NT, Chuong LV, Chau TT, et al. Evidence of blood–brain barrier dysfunction in human cerebral malaria. Neuropathol Appl Neurobiol. 1999;25:331–340. doi: 10.1046/j.1365-2990.1999.00188.x. [DOI] [PubMed] [Google Scholar]
- 22.Hanson J, Lee SJ, Hossain MA, Anstey NM, Charunwatthana P, Maude RJ, et al. Microvascular obstruction and endothelial activation are independently associated with the clinical manifestations of severe falciparum malaria in adults: an observational study. BMC Med. 2015;13:122. doi: 10.1186/s12916-015-0365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moxon CA, Wassmer SC, Milner DA, Chisala NV, Taylor TE, Seydel KB, et al. Loss of endothelial protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria in African children. Blood. 2013;122:842–851. doi: 10.1182/blood-2013-03-490219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponsford MJ, Medana IM, Prapansilp P, Hien TT, Lee SJ, Dondorp AM, et al. Sequestration and microvascular congestion are associated with coma in human cerebral malaria. J Infect Dis. 2012;205:663–671. doi: 10.1093/infdis/jir812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehrhardt S, Wichmann D, Hemmer CJ, Burchard GD, Brattig NW. Circulating concentrations of cardiac proteins in complicated and uncomplicated Plasmodium falciparum malaria. Trop Med Int Health. 2004;9:1099–1103. doi: 10.1111/j.1365-3156.2004.01303.x. [DOI] [PubMed] [Google Scholar]
- 26.Jallow M, Casals-Pascual C, Ackerman H, Walther B, Walther M, Pinder M, et al. Clinical features of severe malaria associated with death: a 13-year observational study in The Gambia. PLoS ONE. 2012;7:1–8. doi: 10.1371/journal.pone.0045645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marquet S, Doumbo O, Cabantous S, Poudiougou B, Argiro L, Safeukui I, et al. A functional promoter variant in IL12B predisposes to cerebral malaria. Hum Mol Genet. 2008;17:2190–2195. doi: 10.1093/hmg/ddn118. [DOI] [PubMed] [Google Scholar]
- 28.Mandala WL, Msefula CL, Gondwe EN, Gilchrist JJ, Graham SM, Pensulo P, et al. Lymphocyte perturbations in Malawian children with severe and uncomplicated malaria. Clin Vaccine Immunol. 2016;23:95–103. doi: 10.1128/CVI.00564-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Storm J, Craig AG. Pathogenesis of cerebral malaria-inflammation and cytoadherence. Front Cell Infect Microbiol. 2014;4:100. doi: 10.3389/fcimb.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark IA, Budd AC, Alleva LM, Cowden WB. Human malarial disease: a consequence of inflammatory cytokine release. Malar J. 2006;5:85. doi: 10.1186/1475-2875-5-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark IA, Vissel B. The meteorology of cytokine storms, and the clinical usefulness of this knowledge. Semin Immunopathol. 2017;39:505–516. doi: 10.1007/s00281-017-0628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dondorp AM, Desakorn V, Pongtavornpinyo W, Sahassananda D, Silamut K, Chotivanich K, et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2005;2:e204. doi: 10.1371/journal.pmed.0020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 34.Kessler A, Dankwa S, Bernabeu M, Seydel K, Smith J, Kim K, et al. Linking EPCR-binding PfEMP1 to brain swelling in pediatric cerebral malaria. Host Microbe. 2017;22:1–14. doi: 10.1016/j.chom.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Ombrain MC, Robinson LJ, Stanisic DI, Taraika J, Bernard N, Michon P, et al. Association of early interferon-gamma production with immunity to clinical malaria: a longitudinal study among Papua New Guinean children. Clin Infect Dis. 2008;47:1380–1387. doi: 10.1086/592971. [DOI] [PubMed] [Google Scholar]
- 36.Day NPJ, Hien TT, Schollaardt T, Loc PP, Chuong LV, Chau TTH, et al. The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J Infect Dis. 1999;180:1288–1297. doi: 10.1086/315016. [DOI] [PubMed] [Google Scholar]
- 37.Kinra P, Dutta V. Serum TNF alpha levels: a prognostic marker for assessment of severity of malaria. Trop Biomed. 2013;30:645–653. [PubMed] [Google Scholar]
- 38.Boeuf PS, Loizon S, Awandare GA, Tetteh JK, Addae MM, Adjei GO, et al. Insights into deregulated TNF and IL-10 production in malaria: implications for understanding severe malarial anaemia. Malar J. 2012;11:253. doi: 10.1186/1475-2875-11-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shabani E, Ouma BJ, Idro R, Bangirana P, Opoka RO, Park GS, et al. Elevated cerebrospinal fluid tumour necrosis factor is associated with acute and long-term neurocognitive impairment in cerebral malaria. Parasite Immunol. 2017;39:e12438. doi: 10.1111/pim.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.John CC, Panoskaltsis-Mortari A, Opoka RO, Park GS, Orchard PJ, Jurek AM, et al. Cerebrospinal fluid cytokine levels and cognitive impairment in cerebral malaria. Am J Trop Med Hyg. 2008;78:198–205. doi: 10.4269/ajtmh.2008.78.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Demographic characteristics of the study participants. Table S2. Univariate and multivariate associations with brain swelling.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.