Abstract
Many studies on intracellular calcium ([Ca2+]i) and intracellular pH (pHi) have been carried out due to their importance in regulation of different cellular functions. However, most of the previous studies are focused on human or mammalian cells. The purpose of the present study was to characterize the effect of Rhodojaponin-III (R-III) on [Ca2+]i and pHi and the proliferation of Sf9 cells. R-III strongly inhibited Sf9 cells proliferation with a time- and dose-dependent manner. Flow cytometry established that R-III interfered with Sf9 cells division and arrested them in G2/M. By using confocal scanning technique, effects of R-III on intracellular free calcium ([Ca2+]i) and intracellular pH (pHi) in Sf9 cells were determined. R-III induced a significant dose-dependent (1, 10, 100, 200 μg/mL) increase in [Ca2+]i and pHi of Sf9 cells in presence of Ca2+-containing solution (Hanks) and an irreversible decrease in the absence of extra cellular Ca2+. We also found that both extra cellular Ca2+ and intracellular Ca2+ stores contributed to the increase of [Ca2+]i, because completely treating Sf9 cells with CdCl2 (5 mM), a Ca2+ channels blocker, R-III (100 μg/mL) induced a transient elevation of [Ca2+]i in case of cells either in presence of Ca2+ containing or Ca2+ free solution. In these conditions, pHi showed similar changes with that of [Ca2+]i on the whole. Accordingly, we supposed that there was a certain linkage for change of [Ca2+]i, cell cycle arrest, proliferation inhibition in Sf9 cells induced by R-III.
Keywords: rhodojaponin-III, intracellular free calcium, intracellular pH, Sf9 cells arrest
1. Introduction
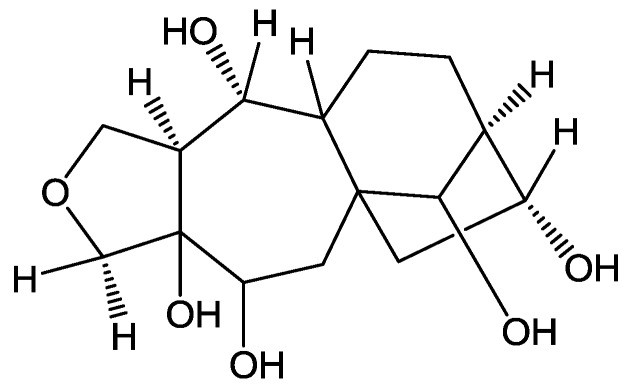
Rhodojaponin-III (R-III, Figure 1) show structure as Figure 1 is a grayanoid diterpene isolated from Rhododendron molle and determined as the main insecticidal ingredient in the plant [1]. It is an effective natural insecticide against more than 40 species of agricultural pests [2]. Previous studies indicate that R-III shows many anti-insect properties including potent antifeedant, oviposition, ovicides, antimolting, growth inhibitor, contact and/or stomach toxicity [3], which is related to the nervous, digestive, endocrine and reproductive systems of insects. There have been some studies on the mode of action of R-III on insects, although the precise molecular mechanism is not well understood. Some researchers demonstrated that R-III remarkably decreases the contents of acetylcholine (ACh) and has reversible activated effects on Na+-K+-ATPase and Ca2+-Mg2+-ATPase activities [4], indicating its interference with insect nervous system through blocked the transition of nervous impulse [5], in which Ca2+ as an intracellular second messenger plays a key role.
Figure 1.
Structure of Rhodojaponin-III.
Intracellular free calcium ([Ca2+]i) is a highly versatile intracellular second messenger and signal transducer in both excitable and non-excitable cells. It is involved in many functions in proliferative cells, including gene expression, protein synthesis, cell secretion, motility, metabolism, cell-cycle progression and apoptosis cell death [6,7]. Under normal conditions, [Ca2+]i concentration is maintained at 10–100 nM, but sustained Ca2+ release from intracellular Ca2+ stores, Ca2+ influx through receptor- or voltage-dependent Ca2+ channels or blockage of re-uptake can perturb [Ca2+]i homeostasis [7]. A variety of physical, chemical, or biological stimuli can modify [Ca2+]i which may lead to cellular physiological changes such as cell arrest or cell death [8,9].
Intracellular pH (pHi) is becoming evident to many aspects of cell physiology, and protons may also function as a second messenger in a manner similar to that of Ca2+ [10]. Relatively small changes in pHi could have a profound effect on a variety of cellular functions. For example, changes in pHi take place in response to growth, tumor promotion, DNA synthesis [11], protein synthesis, activation of the ion channel [12], apoptosis, proliferation and transformation [13]. High pHi can sensibilize cellular proteins such as enzymes, ion channels and ion transporters [14] and pHi shifts may have significant effects on calcium regulation in cells. It has been established in previous research that pHi and [Ca2+]i are closely linked. In effect, pHi has been described as being able to affect intracellular Ca2+ homeostasis and contribute to the length, magnitude, and frequency of the Ca2+ signal through the modulation of voltage-dependent or -independent plasma membrane Ca2+ channels and/or through regulation of the mobilization of Ca2+ from internal stores [10]. On the other hand, Ca2+ has been described as inducing pHi variation, particularly in neurons [15]. In several cellular models cytosolic alkalinization is a sufficient signal to release calcium from intracellular pools [16].
Although many studies on [Ca2+]i and pHi have been carried out due to their importance in regulation of different cellular functions, most of the previous studies are focused on human or mammalian cells and similar studies in insect cells are lacking. Studying the mode of action of botanical pesticide against insects has been greatly simplified by the finding that its effects can be seen in cultured insect cells [17,18]. Therefore, the purpose of this study is to principally characterize the effect of R-III on intracellular Ca2+ and pHi in Sf9 cells (isolated from Spodoptera frugiperda pupal ovarian tissue). Otherwise, we primarily discuss the possible interactions among changes of [Ca2+]i level, cell cycle and cell proliferation, and the possible linkage between changes of intracellular Ca2+ and that of pHi in Sf9 cells induced by R-III, all of which are helpful to explore some new clues for the further study on insecticidal mechanism of R-III.
2. Results and Discussion
2.1. Effect of R-III on the Proliferation of Sf9 Cells
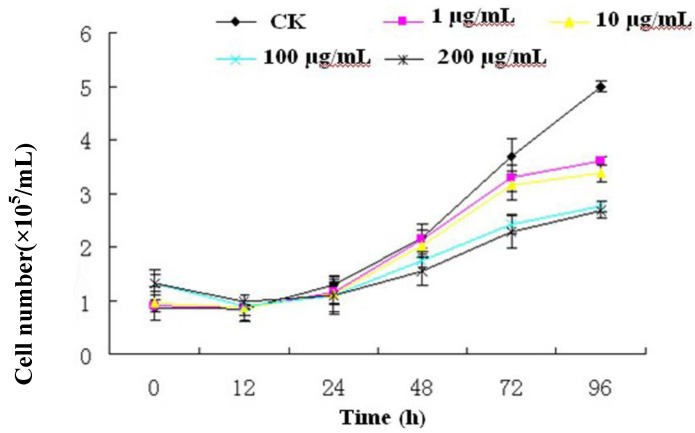
To investigate the effect of R-III on the proliferation of Sf9 cells, cell viability was measured by Trypan blue exclusion assay. As shown in Figure 2, the inhibition effect of R-III on Sf9 cells was not significant and survival cell after 24 h of treatment with 1, 10, 100 and 200 μg/mL of R-III was about 1.0 × 105 cells/mL, similar to that of control. After 24 h of treatments with different concentrations of R-III, cell viability decreased in a time- and dose-dependent manner.
Figure 2.
Effects of R-III on the proliferation of Sf9 cells. The cells were grown in presence of 1, 10, 100 and 200 μg/mL of R-III for the times shown in the figure. Survival cell number was counted by means of Trypan blue exclusion with a standard haemocytometer. Each result derived from the mean of three repetitions.
Survival cell number was 3.2 × 105, 3.0 × 105, 2.2×105, 2.1 × 105 cells/mL, which was far lower than that of the control (3.8 × 105 cells/mL) after 72 h of treatment with R-III at the concentrations of 1, 10, 100 and 200 μg/mL respectively. By 96 h of treatments with R-III, survival cell number in control (5.0 × 105 cells/mL) had greatly outstripped the R-III treatment. In addition, the inhibition effect on Sf9 cells of R-III at the concentrations of 100 μg/mL and 200 μg/mL was significantly higher than that of 1 μg/mL and 10 μg/mL. However, the inhibition effect between 100 μg/mL and 200 μg/mL or between 1 μg/mL and 10 μg/mL showed no significant difference. The results in this assay indicated that R-III strongly inhibited the proliferation of Sf9 cells in a time- and dose-dependent manner.
2.2. Effect of R-III on Cell Cycle
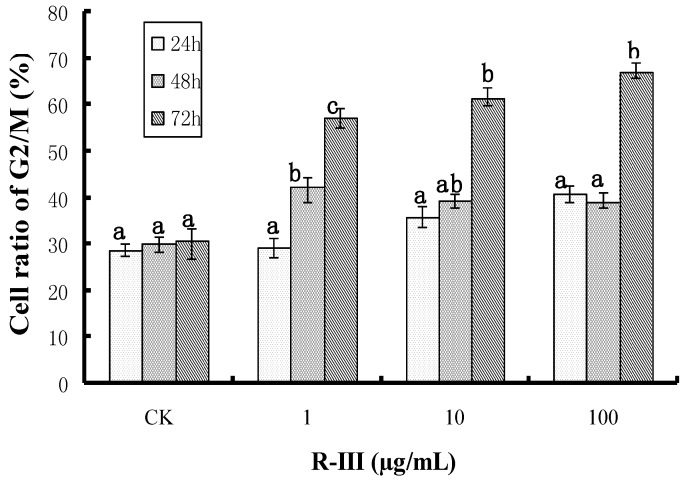
In order to further clarify the effects of R-III on the proliferation of Sf9 cells, we checked the effect of R-III on cell cycle by flow cytometry. As shown in Figure 3, an arrest in G2/M cell cycle phase became evident in Sf9 cells treated with R-III, showing a time- and dose-dependent manner. After 24 h of treatment with 1, 10 and 100 μg/mL of R-III, the percentages of cells in G2/M phase increased to 29.2%,35.8%,and 40.7% respectively. While after 48 h of the same treatment, the percent of cells in G2/M phase increased to 42.2%, 39.4% and 39.1% and became 57.1%, 61.3% and 67% respectively, after 72 h of treatment. Comparing to the treated groups, the percent of G2/M phase cells in control was lower (28.6%, 29.9% and 30.5% for 24, 48 and 72 h, respectively) and kept in a steady state within the treated time. Results suggested that R-III like the antimitotic agents such as colchicine and azadirachtin [19] interfered with Sf9 cells division and arrests them in G2/M, showing strong inhibitory activity to the cell growth and proliferation.
Figure 3.
Effects of R-III on cell cycle.The cells were grown in presence of 1, 10, and 100 μg/mL of R-III for the times shown in the figure. Cell cycle was arrested in G2/M in Sf9 cells and showed a time- and dose-dependent manner. Cells that treated with 0.1% DMSO were used as control. The error bars represent mean ± SEM for data derived from three repetitions. Treatment means sharing the same letter were not significantly different from each other (P < 0.05).
2.3. Effects of R-III on [Ca2+]i and pHi in Sf9 Cells
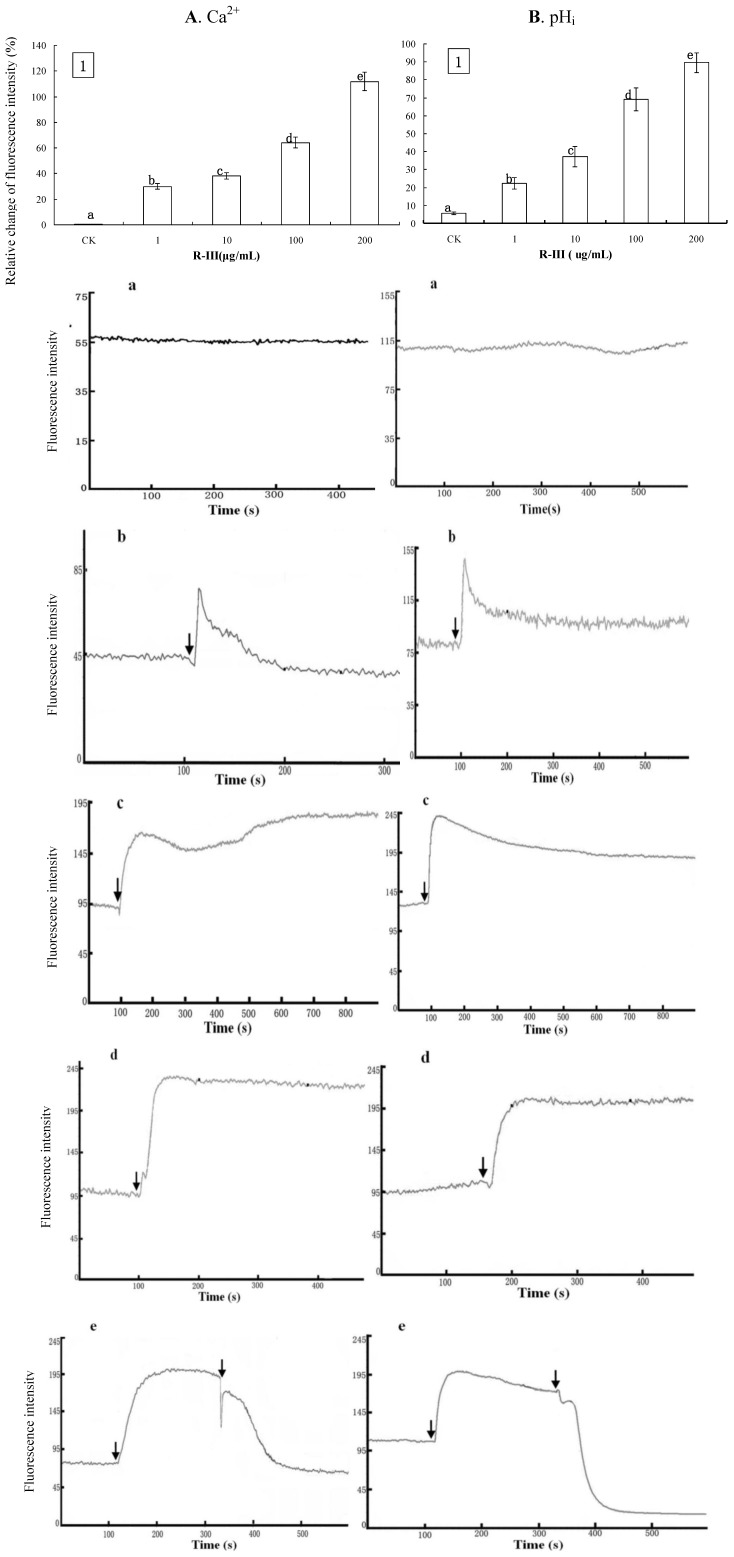
In order to know the effects of R-III on [Ca2+]i, Sf9 cells were exposed to Hanks, a buffer solution containing Ca2+. Ca2+ influx took place in Sf9 cells stimulated by R-III, which elicited a significant increase of [Ca2+]i. As shown in Figure 4A1, comparing to control, Sf9 cells showed gradual increase of [Ca2+]i by 29.9%, 38.28%, 64.21% and 111.78%, after the stimulation with 1, 10, 100 and 200 μg/mL of R-III respectively, showing a dose-dependent fashion. Under these conditions, we observed a similar change of pHi with that of [Ca2+]i. As shown in Figure 4B1, Sf9 cells presented a gradual increase of pHi by 22.27%, 37.13%, 69.17% and 89.58% after stimulation with 1, 10, 100 and 200 μg/mL of R-III respectively, also in a dose-dependent fashion. The increase of pHi was only 5.7% in control. In order to further investigate the effects of R-III on [Ca2+]i in Sf9 cells, we checked the time-dependent changes of Ca2+ fluorescence signals in Sf9 cells induced by R-III. As shown in Figure 4A-a, a flat trace in control indicated no change of [Ca2+]i in cells. A transient elevation of [Ca2+]i characterized by a fluorescence intensity increase followed by a recovery to basal level was observed in Sf9 cells stimulated with low concentration of R-III (1 μg/mL) (Figure 4A-b), suggesting that cells can regulate [Ca2+ ]i to keep intracellular Ca2+ homeostasis in case of slight external stimulation.
Figure 4.
Effect of R-Ⅲ on [Ca2+]i and pHi in Sf9 cells in presence of Hanks. (A1). changes of [Ca2+]i in Sf9 cells stimulated by R-III at various concentrations as indicated by relative change of Fluo-3AM fluorescence intensity; (B1). Changes of pHi in Sf9 cells stimulated by R-III at various concentrations as indicated by relative change of Snarf1M fluorescence intensity; (A a–d). Dynamic changes of [Ca2+]i indicated by a dynamic trace of Fluo-3AM fluorescence intensity in case of Sf9 cells treated with 0, 1, 100, 200 µg/mL of R-Ⅲ respectively; (B a–d). pHi profile in cells subject to the protocol in (A a–d); (A-e). Dynamic variation of [Ca2+]i in Sf9 cells treated with 100 µg/mL of R-III for two times; (B-e). pHi profile in cells subject to the protocol in (A-e). Arrows indicated the addition of R-Ⅲ.Results of representative experiment derived from three repetitions and 4–6 cells were measured in each repetition. The error bars represent mean ± SEM for data derived from value of relative fluorescence intensity in each time interval. Treatment means sharing the same letter were not significantly different from each other (P < 0.05).
When exposed to 100 and 200 μg/mL of R-III, Sf9 cells showed a rapid rise of [Ca2+]i, which reached to a high steady state [Figure 4A-(c,d)]. Previous studies have established that elevation of [Ca2+]i may derive from extra cellular Ca2+influx through calcium channels or transporters [20] or the Ca2+release from intracellular Ca2+ stores induced by intracellular inositol 1,4,5-trisphosphate (IP3), synthesized in response to external stimulation [21]. Under this condition, we applied the second stimulation of R-III, which caused [Ca2+]i declining sharply in a index fashion to a steady state lower than the basal level (Figure 4A-e). In this assay, we also found that the changes of pHi were in line with that of [Ca2+]i, as shown in the traces in Figure 4B-(a,d) and the second stimulation of R-III also produced a sharply decrease of pHi to a steady state lower than the basal level (Figure 4B-e). Although the mechanisms of the sharp decrease of [Ca2+]i and pHi are not clear yet, an interpretation from groups of Li et al. [22] who investigated the modulation effect of glutamate on [Ca2+]i of inner hair cell of the guinea pig cochlea and found similar phenomenon may enlighten us on this study: Excessive stimulation of glutamate may cause toxicity on cells and increase the penetration of plasma membrane (PM) which gives rise to [Ca2+]i efflux. Since R-III was a botanic pesticide showing significant toxicity to many kinds of insect, the second stimulation of R-III possibly produced toxic effect on Sf9 cells causing [Ca2+]i efflux, in agreement with viewpoint of [22], and [Ca2+]i efflux exchanged for H+ influx through Ca2+-ATPase in PM [23], eliciting decrease of pHi.
2.4. Effects of R-III on [Ca2+]i and pHi in Sf9 Cells in Presence of Dhanks
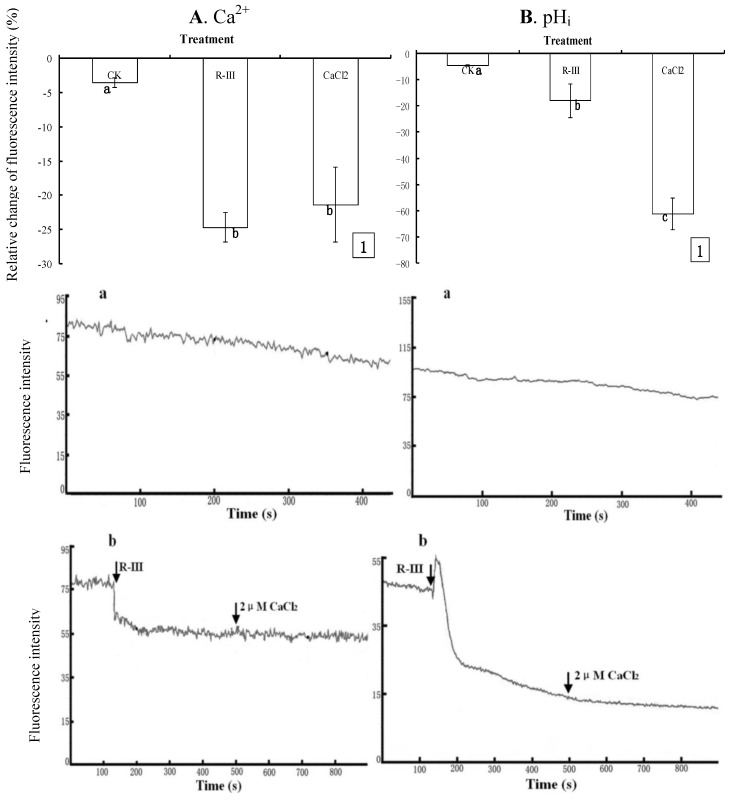
To further clarify the effect of R-III on [Ca2+]i and pHi, Sf9 cells were exposed to Dhanks, a Ca2+-free buffer solution, and recoded the change of fluorescence intensity in Sf9 cells stimulated with R-III (100 μg/mL) at 130 s. Comparing to the control with only a slightly decrease of [Ca2+]i (3.52%) ( 5A1, A-a), [Ca2+]i sharply decreased by 24.64% after the stimulation of R-III (Figure 5A1), and kept in a steady state (Figure 5A-b), indicating [Ca2+]i efflux in Sf9 cells induced by R-III, and no increase of [Ca2+]i was observed in case of re-addition of CaCl2 (2 μM) to the Ca2+-free buffer solution at 500 s (Figure 5A-b), suggesting that Ca2+ efflux in cells was irreversible. Previous study finds that although glucose oxidase induce a rapid decrease in rat endothelial cells exposed in Ca2+ free buffer, re-addition of Ca2+ to the extracellular buffer may activate store operated Ca2+ entry to cause large [Ca2+]i increases [24]. However, store operated Ca2+ entry in Sf9 cells was not activated by the re-addition of Ca2+ in this assay. The results further proved that it was the Ca2+ influx that elicited the substantial increase of [Ca2+]i in Sf9 cells stimulated by R-III in case of cell exposure to Hanks in the experiment above [Figure 4A-(b,e)]. Under these conditions, pHi also showed significant decrease (17.85%), and sustained decrease (61.02%) was observed even if addition of CaCl2 at 130 s after stimulation (Figure 5B1). Knowing from dynamic change of fluorescence signals in Sf9 cells induced by R-III (Figure 5B-b), R-III induced a transient rise of pHi, followed by a decline to a steady level much lower than basal level in Sf9 cells, and no recovery of pHi was observed even if addition of CaCl2 at 130 s after stimulation. In contrast, the control showed only a slightly decrease of pHi (Figure 5B-a). Interestingly, [Ca2+]i showed no transient increase in the same conditions ( 5A- b). The results in this assay indicated that R-III not only induced [Ca2+]i in Sf9 cells decline through Ca2+ efflux but also elicited the intracellular acidification, possibly through H+ entry in exchange for Ca2+ extrusion by the Ca2+-ATPase in cell PM [23].
Figure 5.
Effects of R-III on [Ca2+]i and pHi in Sf9 cells in presence of Dhanks. (A1). Changes of [Ca2+]i when cells were stimulated by 100 μg/mL in 130 s and subsequent addition of 2 μM CaCl2 in 500 s, as indicated by relative change of Fluo-3AM fluorescence intensity; (A-a). Control; (A-b). Dynamic changes of [Ca2+]i in the same conditions with (A1); (B). pHi profile in cells subjected to the protocol in (A). Results of representative experiment derived from three repetitions and 4–6 cells were measured in each repetition. The error bars represent mean ± SEM for data derived from value of relative fluorescence intensity (vs. control) in each time interval. Treatment means sharing the same letter were not significantly different from each other (P < 0.05). The negative value meant decrease of relative fluorescence intensity in cells.
Ca2+ signaling plays a crucial role in the function of almost all cell types as an intracellular second messenger [25]. For example, many researches prove that changes in [Ca2+]i homeostasis are associated with induction of apoptotic [26] or cell death [27]. An experimental report coming from group of Wang et al. [28] provides a convincing interpretation for the role of Ca2+ in participation in apoptotic cell death. In their study, the authors found that H2O2-induced apoptosis of tobacco protoplasts primarily involves in the increase of [Ca2+]i resulting from the entry of extra cellular Ca2+. In recent years, some reports show that calcium signal is a key component of the molecular switch mechanism in cell division cycle [29].Through the interplay with several proteins, [Ca2+]i participates in regulating key steps in the cell cycle such as reentry of quiescent cells into proliferation and the transition through the G1/S, G2/M, and the metaphase/anaphase boundaries [30,31,32]. Moreover, mitosis can be initiated by IP3R-induced calcium transients [33]. Disturbance of [Ca2+]i homeostasis such as increase of [Ca2+]i level in response to external stimulation may interfere with cells division cycle, resulting in cell cycle arrest [34]. In present study, Sf9 cells showed significant changes of [Ca2+]i induced by R-III [Figure 4A-(b,e) and Figure 5A-b]. Otherwise, R-III also produced cell cycle arrest in G2/M (Figure 3) and strongly inhibited Sf9 cells proliferation (Figure 2), although apoptosis was not observed. Our results suggested that there was a certain linkage for change of [Ca2+]i, cell cycle arrest, cell proliferation inhibition in Sf9 cells induced by R-III. Moreover, we tentatively hypothesize that disturbance of [Ca2+]i homeostasis in Sf9 cells induced by R-III may result in cell cycle arrest, which finally causes inhibition of insect cells proliferation or even cell death (including apoptopic cell death). This dual negative effect would significantly decrease the absolute number of cells, and finally induce remarkable decrease of survival cell number in R-III treatment.
2.5. The Contribution of Intracellular Ca2+ Stores to the Changes of Intracellular Ca2+ and pHi
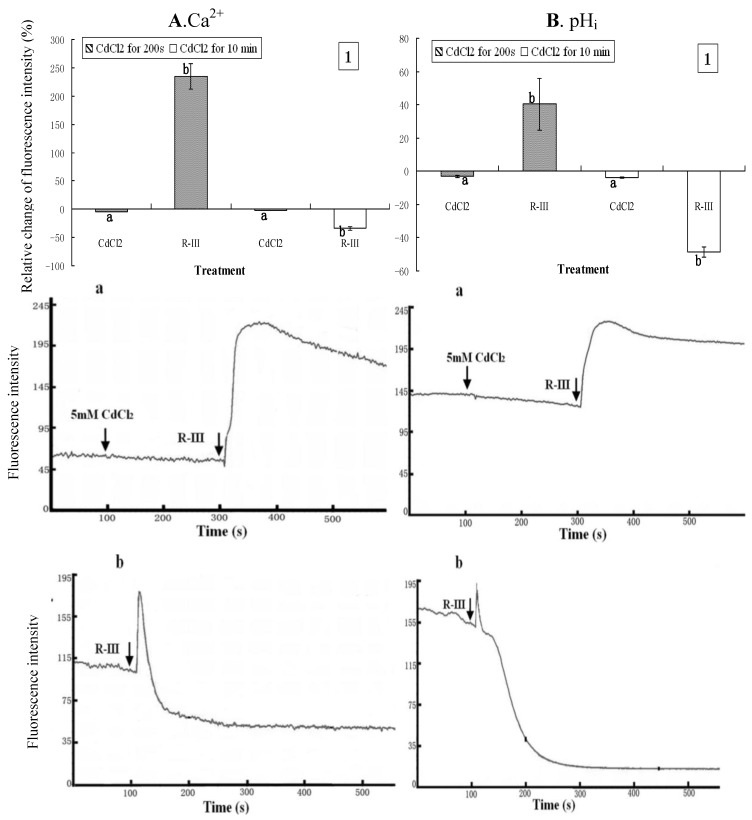
Intracellular Ca2+ stores such as mitochondria or endoplasmic reticulum may be the other principal source of Ca2+ [35]. In present study, to examine the contribution of intracellular Ca2+ stores to the changes of intracellular Ca2+, CdCl2, a blocker of Ca2+ channels was used to treat the cells that were then exposed to Hanks in the following experiments. CdCl2 (5 mM) was applied to treat the cells for 200 s prior to the stimulation of R-III (100 μg/mL). As shown in Figure 6A1 and Figure 6B1, [Ca2+]i and pHi in Sf9 cells incubated with CdCl2 showed a slight decrease (4.52% and 3.03% respectively). After treatment with R-III, [Ca2+]i and pHi rose by 235% and 40.32% respectively. As indicated in dynamic change of trace (Figure 6A-a and B-a), [Ca2+]i increased immediately, but followed by a gradual decrease when cells were stimulated by R-III, suggesting that Cd2+ gradually blocked the Ca2+ channels to inhibit the Ca2+ influx.
Figure 6.
Effect of Ca2+ channels blockon [Ca2+]i and pHi in R-III-induced Sf9 cells in presence of Hanks. (A1). changes of [Ca2+]i in Sf9 cells treated with 0.5 mM CdCl2 for 200 s and10 min prior to stimulate with R-III (100 μg/mL), as indicated by relative change of Fluo-3AM fluorescence intensity; (A-a). Dynamic changes of [Ca2+]i in Sf9 cells treated with 0.5 mM CdCl2 for 200 s prior to stimulate with R-III (100 μg/mL); (A-b). Dynamic changes of [Ca2+]i in Sf9 cells incubated with CdCl2 (5 mM) for 10 min prior to stimulated with R-III (100 μg/mL); (B). pHi profile in cells subjected to the protocol in (A). Results of representative experiment derived from three repetitions and 4–6 cells were measured in each repetition. The error bars represent mean ± SEM for data derived from value of relative fluorescence intensity in each time interval. Treatment means sharing the same letter were not significantly different from each other (P < 0.05). The negative value meant decrease of relative fluorescence intensity in cells.
Under these conditions, pHi changed in similar fashion with [Ca2+]i. When we used CdCl2 (5 mM) to incubate with Sf9 cells for 10 min to block the Ca2+ channels completely, and then stimulated with R-III (100 g/mL), both [Ca2+]i and pHi decreased sharply by rate of 33.85% and 48.74% respectively (Figure 6A1 and 6B1). However, in this condition, we found in dynamic change of trace of Figure 6A-b and Figure 6B-b that both [Ca2+]i and pHi showed a transient elevation before decreasing sharply. Since Cd2+ had blocked Ca2+ channels completely and inhibited Ca2+ influx, the transient increase of [Ca2+]i mainly derived from Ca2+ released from intracellular Ca2+ stores. It is well established that inositol 1,4,5-trisphosphate (IP3), synthesized in response to external stimulation, induces the release of Ca2+ from intracellular stores [21]. In this assay, stimulation of R-III may also induce the synthesis and increase of IP3 to promote release of Ca2+ from intracellular stores through the Ca2+-ATPase. Otherwise, the Ca2+ sustained release from intracellular Ca2+ stores may likely give rise to its depletion, which could activate store-operated Ca2+ channels to promote the Ca2+ influx in mammalian non-excitable cells [36], whereas Ca2+ channels had been blocked completely by CdCl2, and no Ca2+ entry but efflux characterized by sharp decline of [Ca2+]i to a level far lower than basal level occurred in this study (Figure 6A-b). Under this condition, we observed a proportional change of pHi with that of [Ca2+]i. We hypothesized that Ca2+ released from intracellular stores through the Ca2+-ATPase in exchange for H+ entry intracellular stores resulted in the transient increase of pHi, and the Ca2+-ATPase of PM was activated by the transient increase of [Ca2+]i and the sustained stimulation of R-III. [Ca2+]i effused through Ca2+-ATPase in exchange for H+ entry intracellular cytosol, which caused the decrease of pHi. The results in this assay demonstrated that both extra calcium influx and Ca2+ release from intracellular Ca2+ stores contributed to the elevation of [Ca2+]i in Sf9 cells stimulated by R-III, and pHi showed proportional change with [Ca2+]i+ in response to the stimulation of R-III.
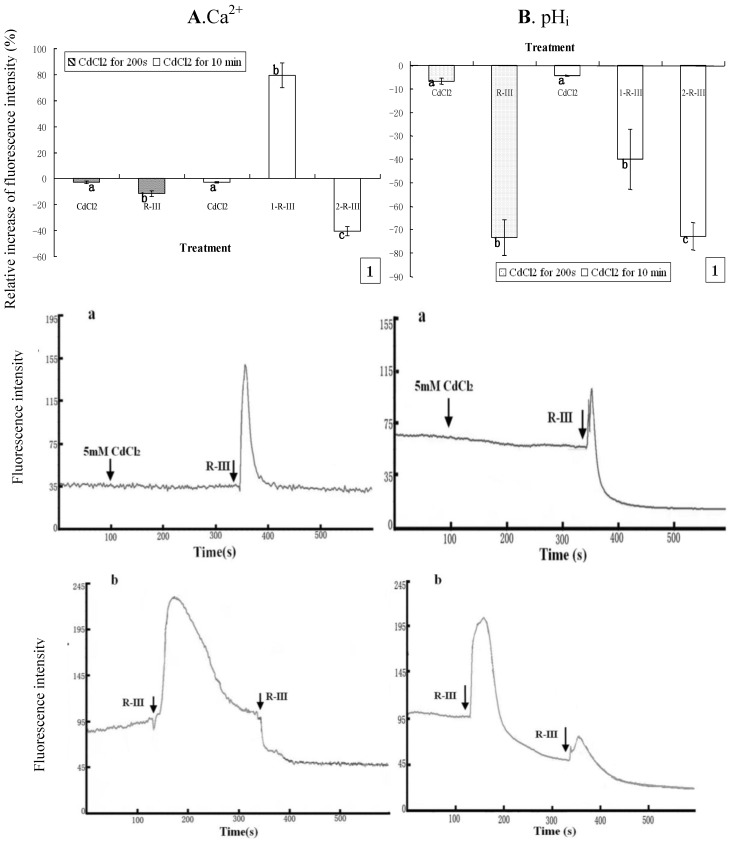
To further clarify the sources of Ca2+ and the response of intracellular Ca2+ stores in Sf9 cells stimulated by R-III, we repeated the above experiment with the only different condition of cells being exposed to Dhanks. As shown in Figure 7Aand Figure 7B, [Ca2+]i and pHi of Sf9 cells indicated only a slight decline in case of incubation with CdCl2 for 200s or 10 min. However, the subsequent addition of stimulation by R-III to Sf9 cells after incubation with CdCl2 for 200 s gave rise to a dramatic decrease of pHi by rate of 73.36% and only a slight decrease of [Ca2+]i (11.55%) ( 7A1 and Figure 7B1). Under this condition, we got the information from Figure 7A-a that [Ca2+]i showed only a transient increase followed by a rapid recovery to the basal level, which explained well the only slight change of [Ca2+]i in this time interval. Since cells were in presence of Ca2+ free solution, and Ca2+ channels in PM were at least partially blocked, the transient increase of [Ca2+]i should mainly derive from intracellular Ca2+ stores, which further prove the contribution of intracellular Ca2+ stores to the changes of intracellular Ca2+.
Figure 7.
Effect of Ca2+ channels block on [Ca2+]i and pHi in R-III-induced Sf9 cells in presence of Dhanks. (A1). changes of [Ca2+]i in Sf9 cells treated with 0.5 mM CdCl2 for 200 s and 10 min prior to stimulate with R-III (100 μg/mL), as indicated by relative change of Fluo-3AM fluorescence intensity; (Aa). Dynamic changes of [Ca2+]i in Sf9 cells incubated with CdCl2 (5 mM) for 200 s prior to stimulate with R-III (100 μg/mL); (A-b). Dynamic changes of [Ca2+]i in Sf9 cells incubated with CdCl2 (5 mM) for 10 min prior to stimulate with R-III (100 μg/mL) for two times; (B). pHi profile in cells subjected to the protocol in (A). Results of representative experiment derived from three repetitions and 4–6 cells were measured in each repetition. The error bars represent mean ± SEM for data derived from value of relative fluorescence intensity in each time interval. Treatment means sharing the same letter were not significantly different from each other (P < 0.05). The negative value meant decrease of relative fluorescence intensity in cells.
Meanwhile, pHi under these conditions also showed a transient increase similar with that of [Ca2+]i, but decline finally to a state much lower than the basal level (Figure 7B-a), which induced a high rate of pHidecrease (Figure 7A1) differing from the change of [Ca2+]i. Nevertheless, cells pretreated for 10 min with CdCl2 (5 mM) presented an markedly increase of [Ca2+]i by rate of 79.43% in the first stimulation of R-III for 200 s, and the second stimulation of R-III induced [Ca2+]i decrease by 40.35% (Figure 7A1). Knowing from the dynamic trace in Figure 7A-b, [Ca2+]i in Sf9 cells showed a transient elevation followed by a gradual decline to basal level in the first stimulation of R-III, which made the peak of dynamic trace much wider than that of Figure 6A-b, suggesting that [Ca2+]i was much higher in this time interval. In contrast, R-III induced a decrease of pHi by rate of 39.85% in first stimulation and 72.78% in the second stimulation (Figure 7B1). We found from Figure 7B-b that pHi in Sf9 cell also showed a transient elevation, but followed by a rapid decline in the first stimulation of R-III. The peak of dynamic trace also became wider comparing to that of Figure 6A-b. Although the reason for these phenomenon was not clear, the results in this assay further indicated that pHi showed proportional change with [Ca2+]i+ in response to the stimulation of R-III on the whole.
It is known that the functional relationships and crosstalk between calcium and pH receive more and more attention, specially, on human cells, but little on insect cells. Although many studies show that changes of pHiare associated with that of [Ca2+]i in a number of cell types, a clear relationships between the steady state level of pHi and [Ca2+]i is not observed in present because interrelationships between pHi and [Ca2+]i are rather complex and depend on the cell type [37]. A few studies show that cytosolic alkalinization shift is associated with the increase of [Ca2+]i [38] and that acidification shift is associated with the decrease of [Ca2+]i [39]. More specifically, an experimental report on crayfish muscle fibre from Kaila and Voipio [40] shows that resting cytosolic calcium is decreased by intracellular alkalosis. In present study, the changes of pHi in Sf9 cells induced by R-III show distinct proportion with that of [Ca2+]i, which also suggests that cytosolic alkalinization or acidification are associated with changes of [Ca2+]i, but the specific interaction mechanism of pHi and [Ca2+]i in these conditions remains unclear, and need further researches to clarify.
3. Experimental
3.1. Reagents
The Rhodojaponin-III (98%) was isolated by using HPLC from the flowers of R. molle in the Laboratory of Insect Toxicology, South China Agricultural University. Fura-3/AM, Snarf1/AM were purchased from Sigma and stock solutions of all molecules were initially dissolved in dimethyl sulfoxide (DMSO), diluted to their final concentration in phosphate buffer solution (135 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, and 8 mM K2HPO4, pH 7.2) and stored at −20 °C until used. All other chemicals were from standard commercial sources and reagent grade or the highest purity.
3.2. Cell Culture
Sf9 cells were obtained from State Key Laboratory of Biocontrol, Sun Yat-sen University, and were cultured in Grace’s medium (GIBCO-BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (heat-inactivated) and 1% penicillin/streptomycin. Cells were cultured at 27 °C and subcultured every 3 days. Confluent cells with >95% viability (tested with Trypan blue exclusion) were used in all experiments.
3.3. Cell Growth Curve
Sf9 cells were seeded onto 24-well plates (2 × 104 cells per well). When cells were adherent, at concentrations of 1, 10,100 and 200 μg/mL was added to treated cells for serial times (0, 12, 24, 48, 72 and 96 h). Cells that cultured with 0.1% DMSO at the same times were used as control. After harvested, survival cell number for each time point was counted using Trypan blue exclusion test with a standard haemocytometer. Growth curve was made after analyzing the data.
3.4. Flow Cytometry
Sf9 cells were seeded onto a 25 cm2 plastic tissue culture flask (2 × 105 cells per flask). When the density was 1 × 106 cells/mL, cells were treated with R-III at concentrations of 1, 10, 100 and 200 μg/mL for serial times (24, 48 and 72 h). After harvested, cells were re-suspended and washed twice with phosphate buffer solution (PBS) (137 mM NaCl, 2.7 mM KCl, 100 mM Na2HPO4, 2 mM KH2PO4, pH 7.2). After that, cells were fixed in cold 70% ethanol, and stored below −20 °C over night. Prior to analysis, ethanol was removed and fixed cells were washed twice with PBS (pH 7.2). Cells were re-suspended in PI solution (50 μg/mL PI, 0.1% Triton X-100, 0.1 mM EDTA, 50 μg/mL RNase A) and incubated for 30 min at room temperature. After that cells were processed in a FACSCalibur (Becton Dickinson, USA). At least 2.0 × 104 cells were counted in each assay. The fraction of the total cell population presented in G2/M phase was obtained from DNA histograms using Cell Quest and Modfit Software (Becton Dickinson, USA). All cytometry experiments were performed on cells in log phase of growth. Cells that cultured with 0.1% DMSO at the same times were used as control.
3.5. Fluorescence Measurements
Cell treatment: [Ca2+]i measurements from Sf9 cells were performed with the fluorescent Ca2+ indicator fluo-3/AM. Cells were cultured in 35-mm polystyrenetissue culture dishes (Nunc, Denmark) and washed twice with PBS, than loaded with 1 uM membrane-permeant acetoxymethyl ester of the dye (fluo-3/AM) for 45min at 37 °C. After dye loading, cells were washed twice with Dhanks solution (137.93 mM NaCl, 5.33 mM KCl, 4.17 mM NaHCO3, 0.441 mM KH2PO4, 0.338 mM Na2HPO4, 5.56 mM D-Glucose, pH 7.2). The pre-treatment measurement of pHi was carried out same as [Ca2+]i measurements, except that the final concentration of dye solution were 10 μM (Snarf1/AM).
Fluorescence measurement: Fluo-3/AM and Snarf1/AM is one of the most suitable Ca2+and pH indicators for CLSM, and widely used to monitor [Ca2+]i and pHi in various cells. It can be excited by an argon ion laser at 488 nm, and its emitted fluorescence (at wavelengths 520 nm) increases with increasing [Ca2+]i [41] or pHi. To measure the Fluo-3/AM and Snarf1/AM fluorescence, laser scanning confocal microscopy (Leica TCS SP2AOBS, Germany) was used to scan the cells with good silhouette and recorded fluorescence at intervals of 6 s for more than 400 s, and room temperature was kept in 20–23 °C during the experiments. According to the experimental design, drug was added from concentrated solutions with a pipette directly into the culture dishes through a small hole on top of the cuvette lid, and in control assays the same volume of DMSO was added. Results were analyzed using the Leica confocal software, and got time-dependent curves of calcium fluorescence signal. Although fluorescence recordings could not be calibrated to count the absolute value of [Ca2+]i [22], [Ca2+]i change could be shown by relative change of fluorescence intensity.
3.6. Statistical Analysis
Data analysis was carried out using SAS software (SAS Institute Inc.) and Microsoft Excel software. Differences between the treatments were determined by Tukey’s multiple range tests (P < 0.05 being considered significant).
4. Conclusions
R-III displayed strong inhibitory activity on the proliferation of Sf9 cells, and interfered with the Sf9 cell division cycle and arrested them in G2/M. In addition, R-III perturbed [Ca2+]i homeostasis by inducing [Ca2+]i influx or efflux in Sf9 cells in the presence of Ca2+-containing or Ca2+-free buffer solution. In these conditions, pHi showed proportional changes with that of [Ca2+]i on the whole. According to the results and discussion in this paper, we supposed that there was a certain linkage for change of intracellular calcium, cell cycle arrest, cell proliferation inhibition in Sf9 cells induced R-III and that cytosolic alkalinization or acidification shifts are associated with changes of [Ca2+]i level in Sf9 cells induced R-III.
Acknowledgements
We would like to thank National Nature Science Foundation (Number 31071713) and Research Fund for the Doctoral Program of Higher Education of China (20094404110019) for providing funds, Xiang Jing Qin (School of Life Sciences, Sun Yat-sen University, China) for her kind assistance in writing.
Footnotes
Sample Availability: Rhodojaponin-III is available from the authors.
References
- 1.Klocke J.A., Hu M.Y., Chiu S.F., Kubo I. Grayanoid diterpenes insect antifeedants and insecticides from Rhododendron molle. Phytochemistry. 1991;30:1797–1800. doi: 10.1016/0031-9422(91)85015-R. [DOI] [Google Scholar]
- 2.Hu M.Y., Zhong G.H., Wu Q.S., Chiu S.F. The biological activities of yellow azalea, Rhododendron molle G. Don against the vegetable leafminer, Liriomyza sativae (Diptera: Agromyzidae) Entomologia Sinica. 2000;7:65–70. [Google Scholar]
- 3.Zhong G.H., Hu M.Y., Wei X.Y., Weng Q.F., Xie J.J., Liu J.X., Wang W.X. Grayanane diterpenoids from the flowers of Rhododendron molle with cytotoxic activity against a Spodoptera frugiperda cell line. J. Nat. Prod. 2005;68:924–926. doi: 10.1021/np049645t. [DOI] [PubMed] [Google Scholar]
- 4.Zhong G.H., Hu M.Y., Chiu S.F., Cheng D.M. Effects of Rhodojaponin-III on nervous System of larvae of imported cabbage worm, Pieris rapae L. Chin. J. Pestic. Sci. 2000;2:13–18. [Google Scholar]
- 5.Feng X., Chiu S.F. Preliminary studies on the biological activity of extracts from Rhododendron molle against insect pests and their mode of action. J. South China Agric. Univ. 1990;11:135–142. [Google Scholar]
- 6.Berridge M.J., Lipp P., Bootman M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell. Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez J.C., Wilkins J.R. Changes in intracellular calcium concentration in response to hypertonicity in bovine articular chondrocytes. Comp. Biochem. Physiol. 2004;A137:173–182. doi: 10.1016/j.cbpb.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Pretorius E., Bornman M.S. Calcium-mediated aponecrosis plays a central role in the pathogenesis of estrogenic chemical-induced neurotoxicity. Med. Hypotheses. 2005;65:893–904. doi: 10.1016/j.mehy.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 9.Alfonso A., Cabado A.G., Vieytes M.R., Botana L.M. Calcium-pH crosstalks in rat mast cells: Cytosolic alkalinization, but not intracellular calcium release, is a sufficient signal for degranulation. Br. J. Pharmacol. 2000;130:1809–1816. doi: 10.1038/sj.bjp.0703490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee C.H., Cragoe E.J.J., Edwards A.M. Control of hepatocyte DNA synthesis by intracellular pH and its role in the action of tumor promoters. J. Cell. Physiol. 2003;195:61–69. doi: 10.1002/jcp.10225. [DOI] [PubMed] [Google Scholar]
- 11.Kim H.S., Lee Y.S., Kim D.K. Doxorubicin exerts cytotoxic effects through cell cycle arrest and Fas-mediated cell death. Pharmacology. 2009;84:300–309. doi: 10.1159/000245937. [DOI] [PubMed] [Google Scholar]
- 12.Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levin J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 13.Shrode L.D., Tapper H., Grinstein S. Role of intracellular pH in proliferation, transformation, and apoptosis. J. Bioenerg. Biomembr. 1997;29:393–399. doi: 10.1023/A:1022407116339. [DOI] [PubMed] [Google Scholar]
- 14.Deitmer J.W., Rose C.R. pH regulation and protonsignaling by glial-cells. Progr. Neurobiol. 1996;48:73–103. doi: 10.1016/0301-0082(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 15.Schwiening C.J., Willoughby D. Depolarization-induced pH microdomams and their relationship to calcium transients in isolated snail neurones. J. Physiol. 2002;538:371–382. doi: 10.1113/jphysiol.2001.013055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nitschke R., Riedel A., Ricken S., Leipziger J., Benning N., Fischer K.G., Greger R. The effect of intracellular pH on cytosolic Ca2+ in HT29 cells. Pflügers. Arch. Eur. J. Physiol. 1996;433:98–108. doi: 10.1007/s004240050254. [DOI] [PubMed] [Google Scholar]
- 17.Salehzadeh A., Akhkha A., Cushley W., Adams R.L.P., Kusel J.R., Strang R.H.C. The antimitotic effect of the neem terpenoid azadirachtin on cultured insect cells. Insect Biochem. Mol. Biol. 2003;33:681–689. doi: 10.1016/S0965-1748(03)00057-2. [DOI] [PubMed] [Google Scholar]
- 18.Huang X.Y., Li O.W., Xu H.H. Induction of programmed death and cytoskeletal damage on Trichoplusiani BTI-Tn-5B1-4 cells by azadirachtin. Pestic. Biochem. Phys. 2010;98:289–295. doi: 10.1016/j.pestbp.2010.06.020. [DOI] [Google Scholar]
- 19.Salehzadeh A., Akhkha A., Cushley W., Adams R.L.P., Kusel J.R., Strang R.H.C. The antimitotic effect of the neem terpenoid azadirachtin on cultured insect cells. Insect Biochem. Mol. Biol. 2003;33:681–689. doi: 10.1016/S0965-1748(03)00057-2. [DOI] [PubMed] [Google Scholar]
- 20.Berridge M.J. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/S0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 21.Dupont G., Goldbeter A. Properties of intracellular Ca2+ waves generated by a model based on Ca(2+)-induced Ca2+ release. Biophys. J. 1994;67:2191–2204. doi: 10.1016/S0006-3495(94)80705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X.Q., Sun J.H., Yu N., Sun Y.R., Tan Z.L., Jiang S.Q., Li N., Zhou C.X. Glutamate induced modulation of free Cain isolated inner hair cells of the guinea pig cochlea. Chin. J. Otorhinolaryngol. 2001;36:101–104. [PubMed] [Google Scholar]
- 23.Schwiening C.J., Kennedy H.J., Thomas R.C. Calcium-hydrogen exchange by the plasma membrane Ca2+-ATPase of voltage-clamped snail neurons. Proc. Roy. Soc. B-Biol. Sci. 1993;253:285–289. doi: 10.1098/rspb.1993.0115. [DOI] [PubMed] [Google Scholar]
- 24.Volk T., Hensel M., Kox W.J. Transient Ca2+ changes in endothelial cells induced by low doses of reactive oxygen species: Role of hydrogen peroxide. Mol. Cell. Biochem. 1997;171:11–21. doi: 10.1023/A:1006886215193. [DOI] [PubMed] [Google Scholar]
- 25.Berridge M. J. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Rizzuto R., Marchi S., Bonora M., Aguiari P., Bononi A., De Stefani D., Giorgi C., Leo S., Rimessi A., Siviero R., Zecchini E., Pinton P. Ca2+ transfer from the ER to mitochondria: when, how and why. Biochim. Biophys. Acta. 2009;1787:1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao W.C., Huang C.C., Lu Y.C., Chi C.C., Chu S.T., Su H.H., Kuo C.C., Cheng J.S., Tseng L.L., Ho C.M., et al. Maprotiline-induced Ca2+ fluxes and apoptosis in human osteosarcoma cells. Drug Develop. Res. 2010;71:268–274. doi: 10.1002/ddr.20371. [DOI] [Google Scholar]
- 28.Wang Y., Lin J.S., Wang F.X. Calcium-mediated mitochondrial permeability transition involved in hydrogen peroxide-induced apoptosis in tobacco protoplasts. J. Integr. Plant Biol. 2006;48:433–439. doi: 10.1111/j.1744-7909.2006.00219.x. [DOI] [Google Scholar]
- 29.Michael W. Calcium microdomains and cell cycle control. Cell Calcium. 2006;40:585–592. doi: 10.1016/j.ceca.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takuwa N., Zhou W., Kumada M., Takuwa Y. Ca2+ dependent stimulation of retino blastoma gene product phosphorylation and p34cdc2 kinase activation in serum stimulated human fibroblasts. J. Biol. Chem. 1993;268:138–145. [PubMed] [Google Scholar]
- 31.Parry H.D., McDougall A.D., Whitaker M. Microdomains bounded by endoplasmic reticulum segregate cell cycle calcium transients in syncytial Drosophila embryos. J. Cell. Biol. 2005;171:47–59. doi: 10.1083/jcb.200503139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parry H.D., McDougall A.D., Whitaker M. Endoplasmic reticulum generates calcium signalling microdomains around the nucleus and spindle in syncytial Drosophila embryos. Biochem. Soc. Transact. 2006;34:385–388. doi: 10.1042/BST0340385. [DOI] [PubMed] [Google Scholar]
- 33.FitzHarris G., Larman M., Richards C., Carroll J. An increase in [Ca2+]i is sufficient but not necessary for driving mitosis in early mouse embryos. J. Cell Sci. 2005;118:4563–4575. doi: 10.1242/jcs.02586. [DOI] [PubMed] [Google Scholar]
- 34.Jana S., Jana O., Monika L., Lucie R., Zdeněk K., Miroslav S. Brassinosteroids cause cell cycle arrest and apoptosis of human breast cancer cells. Chem.-Biol Interact. 2010;188:487–496. doi: 10.1016/j.cbi.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Lohr C., Rose C.R., Deitmer J.W. Extracellular Ca2+ changes during transmitter application in the leech central nervous system. Neurosci. Lett. 1996;205:57–60. doi: 10.1016/0304-3940(96)12371-5. [DOI] [PubMed] [Google Scholar]
- 36.Glitsch M.G., Bakowski D., Parekh A.B. Store-operated Ca2+entry depends on mitochondrial Ca2+ uptake. EMBO J. 2002;21:6744–6754. doi: 10.1093/emboj/cdf675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brustovetsky N., Dubinsky J.M. Dual responses of CNS mitochondria to elevated calcium. J. Neurosci. 2000;20:103–113. doi: 10.1523/JNEUROSCI.20-01-00103.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snowdowne K.W., Way B., Thomas G., Chen H.Y., Cashman J.R. pHi controls cytoplasmic calcium in rat parotid cells. Biochim. Biophys. Acta. 1992;1108:145–152. doi: 10.1016/0005-2736(92)90019-I. [DOI] [PubMed] [Google Scholar]
- 39.Kaila K., Voipio J. Dependence of intracellular free calcium and tension on membrane potential and intracellular pH in single crayfish muscle fibres. Pflügers. Arch. Eur. J. Physiol. 1990;416:501–511. doi: 10.1007/BF00382682. [DOI] [PubMed] [Google Scholar]
- 40.Sedova M., Blatter L.A. Intracellular sodium sodulates sitochondrial calcium signaling in vascular endothelial cells. J. Biol. Chem. 2000;275:35402–35407. doi: 10.1074/jbc.M006058200. [DOI] [PubMed] [Google Scholar]
- 41.Kao J.P.Y. Practical aspects of measuring [Ca2+] with fluorescent indicators. Methods Cell Biol. 1994;40:155–181. doi: 10.1016/S0091-679X(08)61114-0. [DOI] [PubMed] [Google Scholar]