Abstract
Background:
The arterial switch operation (ASO) is the gold standard operative correction of neonates with transposition of the great arteries and intact ventricular septum, with excellent operative survival. The associations between patient and surgeon characteristics and outcomes are well understood, but the associations between variation in preoperative care and outcomes are less well studied.
Methods:
A multicenter retrospective cohort study of infants undergoing neonatal ASO between 1/2010 and 9/2015 at hospitals contributing data to the Pediatric Health Information Systems Database was performed. The association between preoperative care (timing of ASO, preoperative use of balloon atrial septostomy, prostaglandin infusion, mechanical ventilation, and vasoactive agents) and operative outcomes (mortality, length of stay, and cost) was studied using multivariable mixed effects models.
Results:
Over the study period, 2,159 neonates at 40 hospitals were evaluated. Perioperative mortality was 2.8%. Between hospitals, the use of adjuvant therapies and timing of arterial switch operation varied broadly. At the subject-level, older age at ASO was associated with higher mortality risk (age >6 days OR: 1.90, 95% CI: 1.11–3.26, p=0.02), cost, and length of stay. Receipt of a balloon atrial septostomy was associated with lower mortality risk (OR: 0.32, 95% CI: 0.17–0.59, p<0.001), cost, and length of stay. Later hospital median age at ASO was associated with higher odds of mortality (OR: 1.15 per day, 95% CI: 1.02 to 1.29, p=0.03), longer length of stay (p<0.004), and higher cost (p<0.001). Other hospital factors were not independently associated with the outcomes of interest.
Conclusion:
There was significant variation in preoperative care between hospitals. Some potentially modifiable aspects of perioperative care (timing of ASO and septostomy) were significantly associated with mortality, length of stay, and cost. Further research on the perioperative care of neonates is necessary to determine if modifying practice based on the observed associations translates into improved outcomes.
Keywords: Congenital heart disease, pediatrics, mortality, volume, economics, congenital heart surgery
Background:
Transposition of the great arteries (TGA) is the second most common form of cyanotic congenital heart disease. The arterial switch operation (ASO) first described by Jatene and colleagues in 19751 has replaced atrial switch operations as the operation of choice in North American and European countries. Average operative mortality is ~5% in the United States and Europe2,3. Previous studies have identified patient-level factors (low birth weight4–7and variations in coronary anatomy8–13) and both hospital- and surgeon-experience14 as factors that are associated with operative mortality. The associations between preoperative care (specifically timing of ASO and use of other adjuvant therapies) and perioperative outcomes have not received the same attention in the current era.
Neonates with TGA inevitably experience hypoxemia. In some patients, the degree of hypoxemia is tolerable, but, in others, insufficient atrial-level mixing, pulmonary parenchymal disease, or other issues can lead to shock and end organ dysfunction. Adjuvant therapies, specifically Rashkind balloon atrial septostomy (BAS), prostaglandin infusion (PGE), mechanical ventilation, and inotropic agents, have the potential to improve systemic arterial oxygen saturation and tissue oxygen delivery, but operative correction remains the ultimate treatment for hypoxia. Adjuvant therapies improve oxygen saturation in many cases. Availability of these therapies has created a more flexible window in which ASO can be performed. At the same time, each of these adjuvant therapies incurs not only economic cost but also risk of complications. To our knowledge, the associations between inter-hospital variation in preoperative care and perioperative outcome have not been thoroughly evaluated.
The use of adjuvant therapies and timing of surgery are determined by both individual patient condition and hospital habitual practice. Disentangling these factors requires data from both a large number of patients and a number of hospitals with a range of practice patterns. To address this, we performed a multicenter retrospective cohort study using data from the Pediatric Health Information Systems (PHIS) Database, evaluating the association between preoperative care (timing of ASO and preoperative BAS, PGE, and mechanical ventilation) and perioperative outcomes (mortality, cost, and length of stay (LOS)).
Methods:
Data source:
The PHIS database is an administrative database that contains data from inpatient, emergency department, ambulatory surgery, and observation encounters from 47 not-for-profit, tertiary care pediatric hospitals in the United States that are affiliated with the Children’s Hospital Association (Overland Park, KS). Data quality and reliability are assured through a joint effort between the Children’s Hospital Association and participating hospitals. The data warehouse function for the PHIS database is managed by Truven Health Analytics (Ann Arbor, MI). Participating hospitals provide discharge/encounter data including demographics, diagnoses, and procedures. Data are de-identified at the time of data submission and are subjected to a number of reliability and validity checks. A data-use agreement was signed between study investigators and Children’s Hospital Association. The institutional review board (IRB) of The Children’s Hospital of Philadelphia has previously determined that all studies using data from PHIS do not represent human subjects research in accordance with the Common Rule (45 CFR 46.102(f)) and are exempt from IRB review. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure, as this would be a violation of our data use agreement.
Study Population:
Patients were identified using International Classification of Disease, ninth revision codes (ICD-9). We included neonates (<30 days at admission) with a diagnosis of transposition of the great arteries who underwent an arterial switch operation (ICD-9: 35.84) during the incident hospitalization with date of birth between 1/1/2010 and 9/13/2015. These dates were chosen to create a birth cohort with consistent data prior to the adoption of ICD-10 codes. Patients who underwent Rastelli-type operations or atrial switch operations were explicitly excluded. ICD-9 codes for procedures were used for cohort identification because these were more sensitive than diagnosis codes as described previously15–18. Cases from hospitals that did not contribute data to PHIS in at least two-thirds of the study years were excluded as described previously15–18. Preoperative ECMO was exceedingly rare. Cases in which it was utilized were excluded, because they were not representative either typical pre-operative practice. Patients in whom ASO was performed at age >30 days represented <1% of cases. They were excluded since the focus of the study was on factors associated with outcomes in neonatal ASO.
Study Measures:
Data were extracted from the PHIS database by direct query using ICD-9 codes for diagnoses and procedures and Clinical Transaction Codes (CTC) for pharmaceutical products.
The primary exposure for this study was age at ASO. Age at ASO was calculated directly. Secondary exposures were 1) BAS, 2) PGE infusion, 3) mechanical ventilation, and 4) inotrope infusion(s). Exposure to the latter three was defined as receipt of the service >1/2 days of service immediately prior to operation, >3 total days of the preoperative period, or the two days immediately prior to the operation. This definition was chosen to exclude cases in which therapies were used transiently during initial stabilization and evaluation and those in which changes were made on the day of the operation. Information about these practices was collected for each patient. Patterns of resource utilization for each hospital were also studied as exposures, specifically the median age at ASO and the proportion of cases receiving BAS, PGE, mechanical ventilation, and inotrope infusions.
Three primary outcomes were identified prior to analysis: 1) death prior to hospital discharge, 2) LOS, and 3) total cost. The sensitivity of identifying other post-operative complications was potentially limited, so they were not measured. Patient-level covariates included subject age, sex, race (white, black, Asian, other, or missing), insurance payer (private, Medicaid, other governmental insurance, or other), presence of genetic syndrome, presence of non-cardiac congenital anomalies, and history of prematurity. Gestational age of subjects was subdivided into 26–30 weeks, 31–34 weeks, 35–37 weeks, and >=37 weeks for descriptive purposes but dichotomized (either > or <=37 weeks) for subsequent analyses. Data about non-cardiac medical conditions (divided by system as has been previously described19) were collected as was the incidence of NEC and sepsis. However, it is not possible in this database to determine which of these codes were present preoperatively and which occurred post-operatively. Including them as covariates in subsequent analyses would introduce risk of obscuring pre-operative risk, and, thus, they were not included in our primary analyses. Sensitivity analyses were performed to evaluate the maximal degree to which excluding these covariates influenced the study results.
Measurable hospital-level covariates included hospital annual cardiac (CT) surgical volume and ASO-specific operative volume. Hospital annual CT surgical volumes were calculated for the study period as described previously15. There was strong correlation between annual CT surgical volume and number of ASO performed (p<0.001, r2 0.85), but because mean annual total CT surgical volume is more stable and reflective of programmatic size than ASO-specific volume, it was used for all subsequent analyses.
As described previously16,17,20,21, several steps were taken to obtain cost data comparable between hospitals and across the entire study period. PHIS receives billing data directly from hospitals and converts these charges to costs using hospital- and department-specific ratios of costs to charges. Costs are also adjusted for regional wage-price indices to provide costs comparable between hospitals across the country. Total costs for an entire hospitalization/encounter can be retrieved within the confines of our data-use agreement, but more detailed cost-reports (i.e. department-level or itemized costs) are not released. We further adjusted cost data to account for inflation using the Consumer Price Index for medical care, as compiled by the United States Bureau of Labor Statistics (http://data.bls.gov/cgi-bin/dsrv). All costs are expressed as year 2015 United States dollars (2015US$).
Statistical Analysis:
The characteristics of the patients in the study population were described by calculating standard descriptive statistics. Continuous variables were expressed as mean + standard deviation or median (inter-quartile range (IQR) and range) as appropriate. The proportion of cases with adverse outcomes are reported along with a 95% confidence interval (95% CI).
A priority in the design of an observational study is to minimize bias and confounding. As described above, we restricted the study population to neonates undergoing ASO, further restricting cases that deviated from current conventional practice (late ASO or pre-operative ECMO). We then sought to adjust for measurable confounders (e.g. genetic syndrome or prematurity). There are also potential confounding variables that were poorly characterized in the database (NEC and sepsis). Because of the uncertainty in their definition these were excluded from our initial analysis. Finally, there are aspects of patient anatomy and physiology (size of atrial communication or oxygen saturation) that are not measured in the database and cannot be included in our analysis. However, with relatively large number of patients per hospital, it is unlikely that these characteristics would be distributed unevenly between centers.
To adjust for measurable confounders (patient and hospital characteristics), multivariable mixed effects models using generalized linear models were used. A binomial frequency distribution (with logit link) was used for models of mortality, and a gamma distribution (with log link) was used for the models of cost and LOS. Cost and length of stay are uniformly positive continuous outcomes with significant skew. There is not a single standard frequency distribution for these outcomes, but data from simulation studies supports the use of the gamma distribution22, and it has been used previously with good model fit to study congenital heart disease 16,17,21. For each model, fixed effects were covariates as described below. A random intercept was added to account for covariance within individual hospitals. To avoid bias, no bi-variable screening or model refinement was performed23. Conditional standardization was used to estimate outcomes for a standard-risk patient as well as 95% confidence intervals.
Analyses of the outcomes were performed in two steps. First, the association between patient-level factors and use of adjuvant therapies for individual infants was performed. Outcomes were as described above. Covariates included the primary exposures described, prematurity, and identified genetic syndrome.
Second, we sought to study the association between hospital characteristics independent of patient characteristics and other adjuvant therapies by adding pre-specified hospital characteristics to the models based on patient-level factors and adjuvant therapies calculated in the previous step. The proportion of ASO patients receiving BAS, ventilator support, or prostaglandin infusion, the mean age at ASO for each hospital, and the annual hospital volume of CT surgical cases were included in these models. We hypothesized that at the hospital level, delayed ASO would be associated with higher utilization of adjuvant therapies and also would be associated with worse outcomes (higher mortality, higher cost, and longer LOS).
Based on reported in-hospital mortality after ASO (~5%)2,3, we expected that the number of in-hospital deaths would be insufficient for a multi-variable analysis including all relevant covariates24. Instead, a set of exploratory analyses was performed, separately adding each of the hospital characteristics to the previously calculated patient-characteristics-adjusted model for in-hospital mortality. Also, prior to analysis it was not clear that the association between age at ASO and death would be linear and monotonic. Therefore, a second set of analyses were performed evaluating the association between the risk of death and one of three age criteria: 1) age at ASO <25th percentile, 2) age at ASO >50th percentile, and 3) age at ASO divided into three strata (<25th percentile, 25th-50th percentile, and >50th percentile). Because of the low event rate, analysis using splines or other methodologies was not feasible.
Several pre-specified secondary analyses were performed. First, a model was developed using post-operative LOS (rather than total LOS) as an outcome to determine if associations seen in models of total LOS were the result of preoperative and/or postoperative care. Analysis was performed for total LOS and cost models were repeated restricted to patients who survived to hospital discharge to measure the degree to which expired patients affected our estimates. Analyses of multivariable models for LOS and cost were calculated including patient characteristics with each of the hospital characteristics individually to evaluate for covariance between hospital characteristics resulting in bias (data not shown). Post-hoc sensitivity analyses were performed to determine whether correlation between use of BAS and timing of ASO affected observed associations. Isolating either factor did not affect the observed associations (data not shown).
Two post-hoc analyses were performed. Because of clinical interest, the effect of including subjects with preoperative ECMO on risk of mortality, LOS, and cost was performed. Because they reflect a distinct deviation from standard pre-operative management, models for hospital characteristics were not recalculated with the addition of cases with preoperative ECMO. Secondly, to explore the degree to which the degree of prematurity was associated with outcome, additional post-hoc models stratifying the population into term neonate and those with 35–37 week, 31–37 week, and <=30 week gestations were calculated. Further exploration was not possible because of small numbers of subjects born very prematurely. Sensitivity analyses adding necrotizing enterocolitis (NEC) and sepsis to models of patient characteristics were performed to evaluate the maximum degree that omitting them had on associations demonstrated in the primary model.
Missing data were generally infrequent (<1% for most variables). However, data for race was missing for a significant number of patients. To mitigate potential bias, a separate categorical variable for “missing race” was generated. Otherwise cases with missing data were excluded by case restriction, and no imputation was applied, since the benefit for addressing these rare instances was minimal. The primary analyses were pre-specified, and other analyses should be considered exploratory. No formal adjustment for multiple comparisons was made.
All data analysis was performed using Stata MP 13 (Statacorp, College Station, TX). The threshold for statistical significance was p<0.05.
RESULTS:
Patient characteristics:
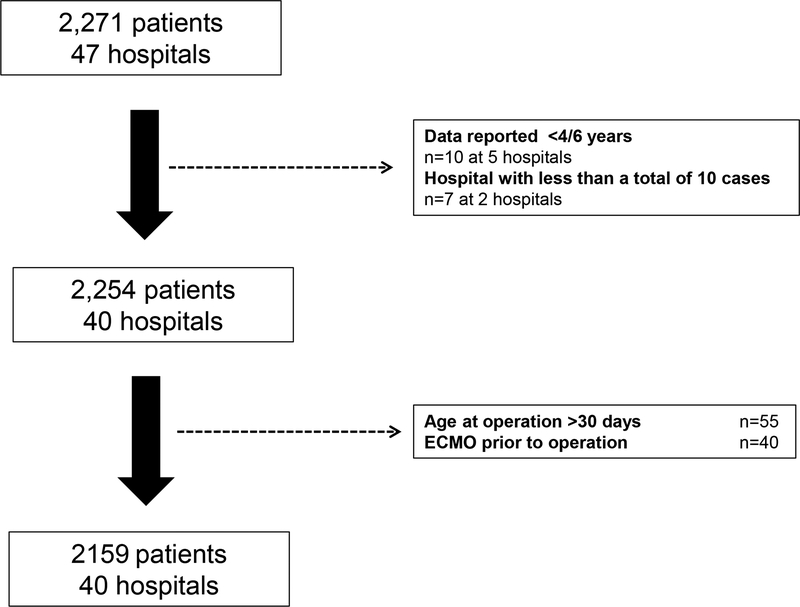
Over the study period, 2,159 patients at 40 hospitals meeting met inclusion criteria (Figure 1). They were 63% white and 68% male, with 8% born prematurely (Table 1). In terms of pre-operative care, 49% underwent BAS, 60% received PGE infusion, 37% underwent mechanical ventilation, and 1% received inotropes. Median age at ASO was 6 days (IQR: 4–9).
Figure 1: Study Population.
Abbreviations: ECMO extracorporeal membrane oxygenation
Table 1.
Study Population
| N | 2159 |
|---|---|
| Hospitals | 40 |
| Age at admission (days) | 0 (IQR: 0–1) |
| Female sex | 32% (685) |
| Race | |
| White | 63% (1,368) |
| Black | 7% (146) |
| Asian | 3% (59) |
| Other/missing | 27% (586) |
| Payer | |
| Private | 47% (1022) |
| Medicaid | 43% (917) |
| Other government | 5% (108) |
| Other | 5% (105) |
| Prematurity | |
| 26–30 weeks gestation | 0.2% (5) |
| 31–34 weeks gestation | 2% (53) |
| 35–39 weeks gestation | 15% (314) |
| Term | 83% (1787) |
| Non-cardiac congenital anomaly | 5% (104) |
| Balloon atrial septostomy | 49% (1131) |
| Prostaglandin infusion | 60% (1294) |
| Mechanical ventilation/respiratory support | 37% (802) |
| Inotropes | 1% (20) |
| Age at arterial switch operation | 6 (IQR: 4–9) |
Abbreviations: IQR interquartile range
In-hospital mortality was 2.8% (95% CI: 2.1–3.6%, Table 2). Median hospital LOS was 18 days (IQR: 14–26) with median post-operative LOS of 12 days (IQR: 9–19). Median total cost of hospitalization was 2015US$116,504 (IQR: 87,414–167,415).
Table 2.
Outcomes
| Death prior to discharge % (95% CI, n) | 2.8% (2.1–3.6%, n=60) |
| Initiation of ECMO % (95% CI, n) | 2.1% (1.5–2.8%, n=45) |
| New dialysis % (95% CI, n) | 4.7% (3.9–5.7%, n=102) |
| Total length of stay | 18 days (IQR: 14–26) |
| Post-operative length of stay | 12 days (IQR: 9–19) |
| Total cost of hospitalization (US2015$) | 116,504 (IQR: 87,414 to 167,415) |
Abbreviations: CI: confidence interval, ECMO extracorporeal membrane oxygenation
Hospital characteristics:
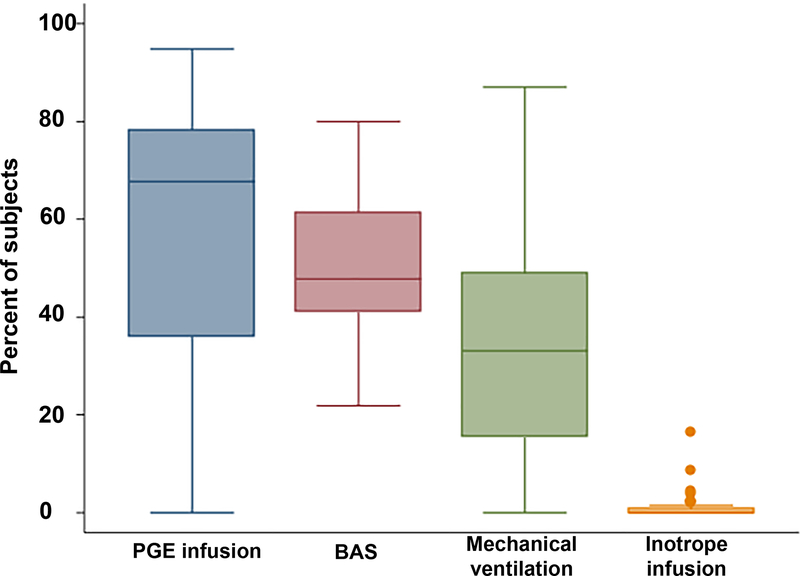
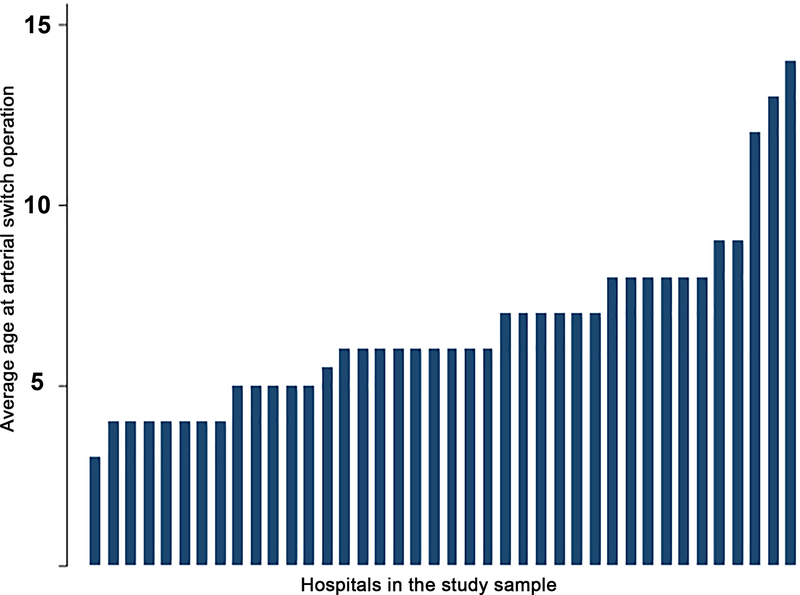
The median annual number of ASO performed at the hospitals in the study sample was 8 (IQR: 6–11). The median hospital annual CT surgical volume was 224 cases (IQR: 135–302). The propensity to use adjuvant therapies varied significantly between hospitals in the study sample (Figure 2). The median hospital percentage of cases receiving PGE was 67% (IQR 36–78%), with a range that spanned 18% to 95%. Similar broad ranges were also seen in the utilization of BAS (Figure 3 median, 48%, IQR: 41–61%, range: 22–80%), mechanical ventilation 33% (IQR: 15%−49%, range: 0–87%), and age at ASO (median: 6 days, IQR: 5–8, range; 3–14, Figure 4). Inotropes were used infrequently and in a more consistent fashion between hospitals (median: 0, IQR: 0–6%, range: 0–16%).
Figure 2: Hospital variation in preoperative care:
This box-and-whiskers plot depicts the distribution of the propensity to utilize prostaglandin (PGE) infusion, balloon atrial septostomy (BAS), mechanical ventilation, and inotrope infusions at the hospitals in the study sample. The horizontal line marks the percentage of cases treated with the adjuvant therapy at the median hospital. Upper and lower limits of the box depict the utilization rate at the 25th and 75th percentile hospitals. Whiskers are drawn to the adjacent value under the limit of 1.5 times the inter-quartile range. Values outside this limit are marked with filled circles.
Figure 3: Hospital variation in utilization of balloon atrial septostomy:
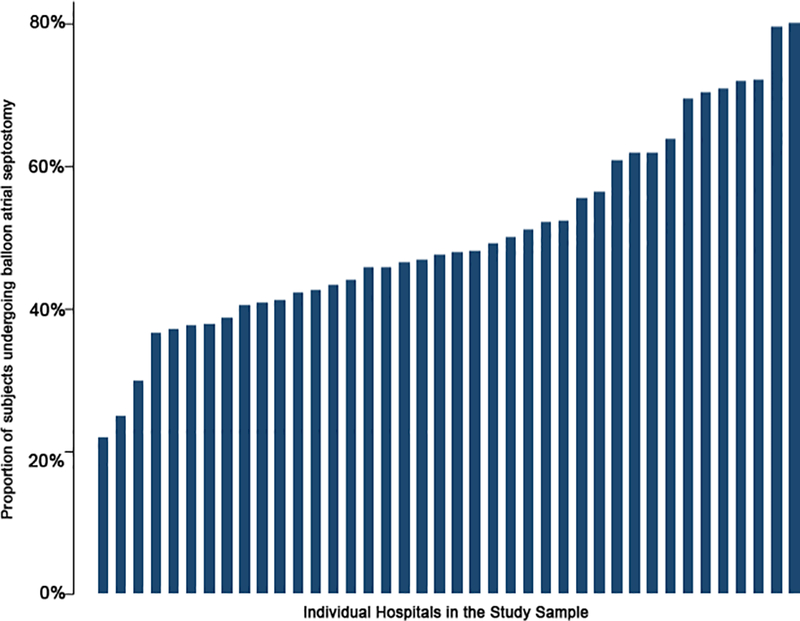
This bar graph depicts the proportion of cases at which a balloon atrial septostomy was performed at each hospital (y-axis) in the study sample (x-axis, sorted by ascending propensity for septostomy).
Figure 4: Hospital variation in age at arterial switch operation:
This bar graph depicts the median age of arterial switch operation (y-axis) at each hospital in the study sample (x-axis, sorted by ascending age at operation).
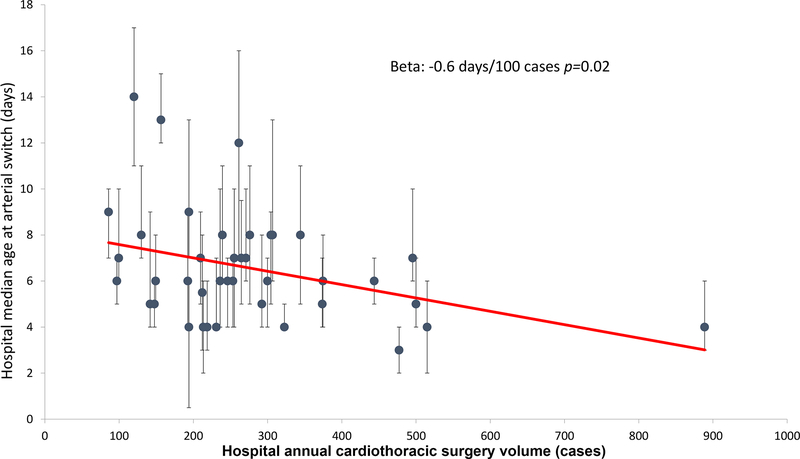
Correlations between hospital characteristics were measured (Supplementary Table 1). Larger annual hospital surgical volume was associated with earlier age at ASO (r2=0.12, p=0.03 Figure 5). Increasing hospital propensity for BAS was associated with later average age of ASO (r2=0.20, p=0.004). Though not statistically significant, there was a positive correlation between hospital propensity for PGE and hospital propensity for mechanical ventilation use (r2=0.09, p=0.06). Other hospital characteristics were not significantly associated with each other.
Figure 5: Association between hospital median age at arterial switch and annual cardiothoracic operative volume:
This scatter plot depicts the association between hospital surgical volume and the median age of arterial switch at each hospital in the study sample (blue circles) with inter-quartile ranges depicted (brackets). Though there is a significant correlation between increasing size and decreasing age at ASO (solid red line). There is still broad variation in the distribution (r2=0.15).
Associations between patient characteristics and outcomes:
A multivariable model was developed to evaluate patient factors associated with in-hospital mortality (Table 3). The standardized risk of mortality for a patient with no risk factors operated on at day of life 6 was 3.7% (95% CI: 2.3–5.8%). Preoperative exposure to inotropes was associated with higher odds of death (OR 4.79, 95% CI: 1.00 to 23.01, p=0.05), while BAS was associated with lower odds of mortality (OR: 0.32, 95% CI: 0.17–0.58, p<0.001). Genetic condition (OR: 6.00, 95% CI: 2.30 to 15.64, p<0.001) and prematurity (OR: 2.84, 95% CI: 1.44 to 5.58, p=0.002) were both associated with higher risk of in-hospital mortality. The use of other adjuvant therapies was not associated with hospital mortality, nor was age at ASO (p=0.38) when age was treated as a continuous variable. However, in pre-specified analyses where age of ASO was not treated as a continuous variable, significant associations between age at ASO and mortality were seen. Specifically, ASO performed older than median age (6 days) was associated with higher risk of mortality (OR: 1.90, 95% CI: 1.11–3.26, p=0.02). Performing the arterial switch early (<25th percentile at 4 days) was not associated with a significant difference in risk (p=0.62). A model evaluating a non-linear association between age at ASO and death did not demonstrate a significant difference for age <4 days versus age 4–6 days (p=0.25), but ASO at >6 days was associated with higher risk than ASO at 4–6 days (OR: 1,88, 95% CI: 1.00–3.50, p=0.048).
Table 3:
Multivariable model of preoperative factors associated with in-hospital mortality, length of stay, and cost
| Mortality | Length of stay | Cost | ||||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) |
p | Ratio (95% CI) |
p | Ratio (95% CI) |
p | |
| Age at arterial switch operation (per day) | 1.02 (0.98 to 1.07) | 0.31 | 1.03 (1.03 to 1.04) | <0.001 | 1.02 (1.02 to 1.03) | <0.001 |
| Genetic condition | 6.00 (2.30 to 15.64) | 0.001 | 1.99 (1.68 to 2.36) | <0.001 | 1.95 (1.65 to 2.31) | <0.001 |
| Prematurity | 2.84 (1.44to 5.58) | 0.002 | 1.32 (1.21 to 1.44) | <0.001 | 1.26 (1.16 to 1.38) | <0.001 |
| Preoperative prostaglandin infusion | 0.74 (0.43 to 1.26) | 0.26 | 1.01 (0.95 to 1.06) | 0.77 | 1.00 (0.95 to 1.06) | 0.92 |
| Preoperative inotrope infusion | 4.79 (1.12 to 24.9) | 0.04 | 1.05 (0.83 to 1.33) | 0.67 | 1.38 (1.09 to 1.75) | 0.007 |
| Preoperative mechanical ventilation | 1.14 (0.65 to 2.00) | 0.64 | 1.12 (1.06 to 1.18) | <0.001 | 1.17 (1.11 to 1.23) | <0.001 |
| Balloon atrial septostomy | 0.32 (0.17–0.59) | <0.001 | 0.84 (0.80 to 0.88) | <0.001 | 0.85 (0.81 to 0.88) | <0.001 |
CI confidence interval
Analogous models for total hospital LOS and hospital cost were calculated (Table 3). Standardized LOS was 23 days (95% CI: 21 to 25 days), and standardized cost of hospitalization was 2015US$147,127 (95% CI: 133,530 to 162,108). Prematurity and presence of a genetic syndrome were both associated with higher total LOS and hospital cost as was increasing age at ASO. In terms of other preoperative factors, BAS was associated with a lower total LOS and cost, while preoperative mechanical ventilation was associated with higher total LOS and cost. Exposure to inotropes preoperatively was associated with significantly higher cost, but not LOS. In a secondary analysis, a model for post-operative LOS was also calculated (Supplementary Table 2) for which point estimates and confidence intervals were similar to the model for total LOS, with a standardized post-operative length of stay of 18 days (95% CI: 17 to 20). Sensitivity analyses including NEC and sepsis in primary models for mortality, LOS, and cost were performed with no effect on the associations described (data not shown).
Association between hospital practice patterns and outcome:
As expected, the number of in-hospital deaths was prohibitively low to include all hospital-characteristics of interest in a single model. Therefore, a series of models were calculated adding individual center characteristics to the previously calculated model for mortality (Supplementary Tables 3–7). Increasing hospital median age of ASO was associated with higher odds of mortality (OR: 1.15 per day, 95% CI: 1.02 to 1.29, p=0.03). Increasing annual CT surgical volume (OR: 0.83, 95% CI: 0.70 to 0.99, p=0.04) was associated with lower odds of in-hospital mortality. No other hospital characteristics were associated with odds of mortality.
A multivariable model was calculated to study the association between hospital practices and total LOS adjusted for patient characteristics (Table 4). Increasing hospital average age at ASO was associated with longer LOS (p=0.004). The point estimate for increasing CT surgical volume suggested a shorter LOS (ratio: 0.96, 95% CI: 0.92 to 1.00) but this was not statistically significant (p=0.05). In the analogous model for hospital cost (Table 4), increasing median center age at ASO was associated with increased cost (p<0.001) independent of hospital annual CT surgical volume. The point estimate for the associations between each hospital’s propensity to use PGE (ratio: 1.32, 95% CI: 0.97 to 1.81, p=0.08) and cost was suggestive of a significant association between increasing use of PGE and higher cost, but the association was not statistically significant. Similarly, the point estimate for the association between hospital CT surgical volume (ratio: 0.96, 95% CI: 0.91–1.01, p=0.10) and cost suggested that an association between higher surgical volume and lower cost, but the association was not statistically significant. Other pre-identified center-level covariates were not associated with higher cost.
Table 4:
Multivariable model of preoperative and hospital factors associated with total hospital length of stay and cost
| Length of stay | Cost | |||
|---|---|---|---|---|
| Ratio (95% CI) | p | Ratio (95% CI) | p | |
| Age at ASO (per day) | 1.03 (1.03 to 1.04) | <0.001 | 1.02 (1.02 to 1.03) | <0.001 |
| Genetic condition | 1.98 (1.67 to 2.35) | <0.001 | 1.95 (1.64 to 2.30) | <0.001 |
| Prematurity | 1.32(1.22 to 1.44) | <0.001 | 1.27 (1.17 to 1.38) | <0.001 |
| Preoperative prostaglandin infusion | 1.01 (0.96 to 1.07) | 0.65 | 1.00 (0.95 to 1.05) | 1.00 |
| Preoperative inotrope infusion | 1.06 (0.83 to 1.34) | 0.65 | 1.38 (1.09 to 1.74) | 0.008 |
| Preoperative mechanical ventilation | 1.12 (1.07 to 1.19) | <0.001 | 1.17 (1.11 to 1.24) | <0.001 |
| Balloon atrial septostomy | 0.84 (0.80 to 0.88) | <0.001 | 0.84 (0.81 to 0.88) | <0.001 |
| Hospital average age at ASO (per day) | 1.04 (1.01 to 1.08) | 0.004 | 1.07 (1.03 to 1.11) | <0.001 |
| Hospital propensity to pursue BAS | 0.89 (0.55 to 1.42) | 0.41 | 0.74 (0.41 to 1.32) | 0.31 |
| Hospital propensity for mechanical ventilation | 1.05 (0.81 to 1.37) | 0.70 | 0.97 (0.70 to 1.35) | 0.87 |
| Hospital propensity for prostaglandin | 0.90 (0.70 to 1.16) | 0.41 | 1.32 (0.97 to 1.81) | 0.08 |
| Hospital CT surgical volume (per 100 cases) | 0.96 (0.92 to 1.00) | 0.05 | 0.96 (0.91 to 1.01) | 0.10 |
Abbreviations: ASO arterial switch operation, BAS balloon atrial septostomy, CI confidence interval, CT cardiothoracic
A pre-specified secondary analysis of post-operative LOS demonstrated similar associations as the model for total LOS (Supplementary Table 8). Increasing hospital average age at ASO was associated with longer post-operative LOS (p=0.001). The point estimate of the association between hospital CT surgical volume and post-operative length of stay still suggested that increasing volume was associated with shorter post-operative length of stay (ratio: 0.95) but the association was no longer statistically significant (p=0.08).
Sensitivity analyses restricting analyses of cost and total length of stay to patients who survived to hospital discharge were performed. There were no changes in the estimates or significance of associations observed in the previous two models (data not shown).
Post-hoc analyses:
Two post-hoc analyses were performed. First, patients with pre-procedural ECMO were included and the associations between ECMO and outcomes were evaluated. Pre-operative ECMO was associated with higher risk of in-hospital mortality (OR: 6.03, 95% CI: 1.87 to 19.41, p=0.03) and higher cost (ratio: 1.58, 95% CI: 1.33 to 1.89, p<0.001). No significant association was seen with LOS (p=0.35). Associations between other individual-level covariates and mortality, LOS, and cost were unchanged (data not shown).
We also evaluated whether the observed associations between prematurity on outcome were proportional to the degree of prematurity. In the study population, 91% of patients were born at term, 5% between 35–37 weeks gestation, 3% between 31–35 weeks, and 0.6% <=30 weeks. Relative to term gestation, gestational age <= 30 weeks was associated with higher odds of mortality (OR: 21.08, 95% CI: 3.21 to 138.25, p=0.001). The point estimates for the odds of mortality in gestational age 31–35 (OR: 2.86) and 35–37 weeks (OR: 2.26,) were consistent with higher risk but they were not statistically significant (p=0.06) (Supplementary Table 9).
DISCUSSION:
This retrospective multicenter cohort study sought to study the association between perioperative care and outcomes following ASO. The ASO has become the dominant operation to provide anatomic and physiologic correction of TGA. Contemporary series demonstrate excellent operative success and operative mortality2,3,25,26. Previously, studies have demonstrated an association between patient characteristics (birth weight <2.5 kg5–7,27 and variations in coronary artery origins8–11,13) and operative mortality. Increasing center and surgeon experience also have been shown to be associated with lower risk of operative mortality14. However, there has been less attention paid to the associations between (potentially modifiable) preoperative care and outcomes. We sought to evaluate these in a large multicenter administrative database.
In the current study, there was significant variation in practice with large differences in the utilization of BAS, PGE, and mechanical ventilation prior to ASO as well as timing of eventual ASO. This was in sharp contrast to the small and relatively consistent proportion of patients who received inotropes, suggesting more consensus regarding the use of this therapy. The presence of variation in the utilization of the other practices is consistent with 1) uncertainty as to the optimal preoperative care for neonates awaiting ASO and 2) the potential to improve outcomes by identifying potentially influential factors. Across medicine, efforts to reduce practice variation have improved outcomes and increased value in care28–38. The observed magnitude of this variation could not be explained by each hospital’s case-mix. Importantly, there is sufficient variation across hospitals in the sample to allow for the evaluation the associations between hospital practice and outcomes.
Timing of the arterial switch operation has been of interest since the introduction of the operation. Seminal research from the Congenital Heart Surgeons Society provided evidence that earlier ASO was associated with lower risk of early mortality39. However, in an earlier era, patients undergoing ASO included older patients with potentially different risk factors for adverse outcome. Contemporary series, in comparison, demonstrate ASO is uniformly performed relatively early in neonatal life26,27,40. Conventional wisdom continues to support the notion that delaying ASO allows for increased stability, allowing for transition from fetal to neonatal circulation, maturation of the kidneys and liver, and evaluation/identification of other congenital anomalies. However, the optimal duration of this delay has not been clearly defined, and uncertainty regarding this is manifest in the tremendous variation in timing of ASO between hospitals seen in the current study. Single-center studies have also demonstrated that within their centers, ASO in the first few days of life was not associated with higher mortality, and that delaying ASO incurred greater hospital cost40,41. In addition, provocative case series of ASO performed in the first hours of life have demonstrated excellent technical success and mortality comparable to series with conventional timing42–44. In the current study, early ASO was not associated with higher mortality, while ASO at a later age was associated with higher mortality, LOS, and cost at both the level of the individual patient and in terms of hospital practice.
Care must be taken in interpreting the observed associations. First, because PHIS is an administrative database, it does not contain information all of the potentially relevant clinical factors (e.g. anatomic variants or degree of cyanosis). At this time, to our knowledge, there are no evidence-based practice guidelines for thresholds of cyanosis or hypoxemia to guide whether BAS should be performed and/or timing of ASO. The absence of these guidelines was part of the motivation to pursue this study and likely a contributor to the observed variability in practice. It is also unlikely that over a relatively large number of patients per hospital that degree of cyanosis is distributed unevenly. Individual patients might also have a non-cardiac clinical condition (sepsis, NEC, or respiratory issues) that simultaneously would delay ASO and increase the likelihood of mortality, morbidity, and prolonged hospitalization. Some of the variation in timing of ASO might be due to these factors. However, it is less plausible that these factors occur in sufficiently different frequency between hospitals to explain the tremendous variation in the median age at ASO at different centers (which is as low as 3 days at some centers and over 10 days at other centers), and the observed associations between the hospital median age of ASO and outcome. This is evidence of systematic differences in timing of ASO between centers and an association between those differences and outcomes. There is a statistically significant correlation between smaller hospital surgical volume and later ASO. However, there is significant variability across the span of hospital sizes in terms of age of ASO, allowing for separate evaluation of these factors. Second, the associations identified in an observational study do not imply causation. Further studies using experimental or quasi-experimental designs (such as a clinical trial) are necessary to accomplish this. A clinical trial would address this most directly. It also important to note that it is not possible to determine the mechanisms that underlie these associations. Despite these limitations, the observed associations point to potentially modifiable aspects of care that could improve outcomes and value delivered with patients with TGA. Further research is necessary to determine if the benefit of early ASO is directly related to timing (due to reduced exposure to cyanosis and risk of iatrogenic events) or whether earlier ASO at individual hospitals is an indicator of hospital surgical program quality. Differentiating between these factors is an important step in determining how to translate these observations into practice.
The observed association between BAS and lower mortality, cost, and LOS was unexpected. There has been controversy regarding the use of BAS, originating from a report that BAS was associated with higher risk of stroke in this population45. Subsequent studies demonstrated that risk of brain injury in this population was more strongly correlated with severity and duration of post-natal hypoxia than BAS46, but how to determine which patients receive BAS remains controversial. In the current study some centers utilized BAS in <20% of cases, while in some hospitals it was applied to >80% of cases (a greater than four-fold difference). As with timing of ASO, further research is necessary to determine the mechanism underlying the observed association (e.g. an intrinsic benefit to improving mixing at atrial level and effective pulmonary blood flow prior to undergoing cardiopulmonary bypass or improved saturations allowing for reduced exposure to other adjuvants and associated risk of complications). It is also important to identify whether the superior outcomes associated with BAS are universal or whether they are restricted to a subset of patients.
At the hospital level, the use of PGE and mechanical ventilation were not significantly associated with perioperative outcomes. Preoperative use of mechanical ventilation was associated with shorter LOS and lower cost. Previous studies evaluating the association between these therapies and outcome have had equivocal results47. There remains widespread practice variation in the use of both PGE and mechanical ventilation. Along with the variation seen in ASO timing and BAS (which appear to have significant associations with outcome), there is value in identifying which patients would benefit from each of these therapies.
There are additional limitations to this study. First, it is not possible to determine if patients were prenatally diagnosed, the location of their delivery, and the proximity of the delivery hospital to the center at which ASO was performed. Though data are equivocal in several studies5,48–50, the interplay between these factors with hemodynamic status of the patient and timing of ASO are important targets for future studies. We also did not include surgeon-specific data as this was beyond the scope of this study. Second, retrospective observational studies are limited to reporting the range of practices that exist. However, in this case, there is tremendous variability in the range of practices observed. Thirdly, the study was restricted to short-term outcomes. The study period does not allow for evaluation of the association between identified factors and the risk of re-intervention and longer-term mortality. Additionally, the study population was fixed, limiting analysis of mortality because of its rarity and placing the study at risk of type II error. Lastly, PHIS contains data from US primary children’s hospitals. This limits the generalizability of the study since the sample contains many large academic centers. However, the fact that there is significant variation in practice in this relatively homogenous sample reaffirms that there is no consensus in the care of neonates prior to ASO.
CONCLUSION:
Preoperative care of neonates awaiting ASO is highly variable between hospitals, beyond what can be explained by differences in case-mix. There is evidence that aspects of this preoperative care (timing of ASO and use of BAS) may influence perioperative outcome. Despite the inherent limitations of an observational study, these findings suggest that further research into the optimal preoperative care of neonates with TGA-IVS has the potential to improve outcomes in this population.
Supplementary Material
Clinical Perspective:
What is new (word count: 97) :
There is significant variation in the care of neonates with transposition of the great arteries (TGA) before arterial switch operation (ASO) beyond what could be explained by variability in case-mix.
Some of these differences in practice were associated with differences in clinical outcome.
At the individual subject level, receipt of balloon atrial septostomy was associated with lower mortality, length of stay, and cost.
ASO in older neonates was associated with increased mortality, length of stay, and cost.
Receipt of ASO at hospitals that habitually performed ASO later in life was also associated with increased risk of mortality.
What are the clinical implications (word count: 79):
Perioperative care should continue to be individualized based on patient anatomy and physiology though careful consideration of the potential risks of delaying ASO should be considered, as should the potential benefit of septostomy.
At the hospital-level, these findings should be considered in organizing pre-operative care of infants with transposition of the great arteries, prioritizing efficient evaluation and treatment of patients prior to ASO.
Specifically, obstacles to timely arterial switch operation should be identified, and efforts made to overcome them.
ACKNOWLEDGEMENTS:
This work was supported in part by the Cardiac Center Clinical Research Core at The Children’s Hospital of Philadelphia.
FUNDING SOURCES: Dr. O’Byrne receives research support from the National Institute of Health/National Heart Lung and Blood Institute (K23 HL130420–01). The funding agencies had no role in the planning or execution of the study, nor did they edit the manuscript as presented. The manuscript represents the opinions of the authors alone.
Footnotes
DISCLOSURES: None.
REFERENCE:
- 1.Jatene AD, Fontes VF, Paulista PP, Souza LC, Neger F, Galantier M, Sousa JE. Anatomic correction of transposition of the great vessels. J Thorac Cardiovasc Surg. 1976;72:364–370. [PubMed] [Google Scholar]
- 2.O’Brien SM, Clarke DR, Jacobs JP, Jacobs ML, Lacour-Gayet FG, Pizarro C, Welke KF, Maruszewski B, Tobota Z, Miller WJ, Hamilton L, Peterson ED, Mavroudis C, Edwards FH. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138:1139–1153. [DOI] [PubMed] [Google Scholar]
- 3.Sarris GE, Chatzis AC, Giannopoulos NM, Kirvassilis G, Berggren H, Hazekamp M, Carrel T, Comas JV, Di Carlo D, Daenen W, Ebels T, Fragata J, Hraska V, Ilyin V, Lindberg HL, Métras D, Pozzi M, Rubay J, Sairanen H, Stellin G, Urban A, van Doorn C, Ziemer G, European Congenital Heart Surgeons Association. The arterial switch operation in Europe for transposition of the great arteries: a multi-institutional study from the European Congenital Heart Surgeons Association. J Thorac Cardiovasc Surg. 2006;132:633–639. [DOI] [PubMed] [Google Scholar]
- 4.Fricke TA, Bulstra AE, Naimo PS, Bullock A, Robertson T, d’Udekem Y, Brizard CP, Konstantinov IE. Excellent Long-Term Outcomes of the Arterial Switch Operation in Patients With Intramural Coronary Arteries. Ann Thoracic Surg. 2016;101:725–729. [DOI] [PubMed] [Google Scholar]
- 5.Lara DA, Fixler DE, Ethen MK, Canfield MA, Nembhard WN, Morris SA. Prenatal diagnosis, hospital characteristics, and mortality in transposition of the great arteries. Birth Def Res A Clin Mol Teratol. 2016;106:739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prandstetter C, Hofer A, Lechner E, Mair R, Sames-Dolzer E, Tulzer G. Early and mid-term outcome of the arterial switch operation in 114 consecutive patients. Clin Res Cardiol. 2007;96:723–729. [DOI] [PubMed] [Google Scholar]
- 7.Fricke TA, Bulstra AE, Loyer BR, Weintraub RG, d’Udekem Y, Brizard CP, Konstantinov IE. Outcomes of the Arterial Switch Operation in Children Less Than 2.5 Kilograms. Ann Thoracic Surg. 2017;103:840–844. [DOI] [PubMed] [Google Scholar]
- 8.Mayer JE, Sanders SP, Jonas RA, Castañeda AR, Wernovsky G. Coronary artery pattern and outcome of arterial switch operation for transposition of the great arteries. Circulation. 1990;82:IV139–145. [PubMed] [Google Scholar]
- 9.Wernovsky G, Mayer JE, Jonas RA, Hanley FL, Blackstone EH, Kirklin JW, Castañeda AR. Factors influencing early and late outcome of the arterial switch operation for transposition of the great arteries. J Thorac Cardiovasc Surg. 1995;109:289–301– discussion 301–302. [DOI] [PubMed] [Google Scholar]
- 10.Pasquali SK, Hasselblad V, Li JS, Kong DF, Sanders SP. Coronary Artery Pattern and Outcome of Arterial Switch Operation for Transposition of the Great Arteries: A Meta-Analysis. Circulation. 2002;106:2575–2580. [DOI] [PubMed] [Google Scholar]
- 11.Metton O, Calvaruso D, Gaudin R, Mussa S, Raisky O, Bonnet D, Sidi D, Vouhé PR. Intramural coronary arteries and outcome of neonatal arterial switch operation. Euro J Card Thorac Surg. 2010;37:1246–1253. [DOI] [PubMed] [Google Scholar]
- 12.Brown JW, Park HJ, Turrentine MW. Arterial switch operation: factors impacting survival in the current era. Ann Thorac Surg. 2001;71:1978–1984. [DOI] [PubMed] [Google Scholar]
- 13.Moll M, Michalak KW, Sobczak-Budlewska K, Moll JA, Kopala M, Szymczyk K, Dryżek P, Moll JJ. Coronary Artery Anomalies in Patients With Transposition of the Great Arteries and Their Impact on Postoperative Outcomes. Ann Thoracic Surg. 2017;104:1620–1628. [DOI] [PubMed] [Google Scholar]
- 14.Karamlou T, Jacobs ML, Pasquali S, He X, Hill K, O’Brien S, McMullan DM, Jacobs JP. Surgeon and Center Volume Influence on Outcomes After Arterial Switch Operation: Analysis of the STS Congenital Heart Surgery Database. Ann Thoracic Surg. 2014;98:904–911. [DOI] [PubMed] [Google Scholar]
- 15.O’Byrne ML, Glatz AC, Shinohara RT, Jayaram N, Gillespie MJ, Dori Y, Rome JJ, Kawut S. Effect of center catheterization volume on risk of catastrophic adverse event after cardiac catheterization in children. Am Heart J. 2015;169:823–832.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Byrne ML, Glatz AC, Mercer-Rosa L, Gillespie MJ, Dori Y, Goldmuntz E, Kawut S, Rome JJ. Trends in pulmonary valve replacement in children and adults with tetralogy of Fallot. Am J Cardiol. 2015;115:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Byrne ML, Gillespie MJ, Shinohara RT, Dori Y, Rome JJ, Glatz AC. Cost comparison of Transcatheter and Operative Pulmonary Valve Replacement (from the Pediatric Health Information Systems Database). Am J Cardiol. 2016;117:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Byrne ML, Glatz AC, Hanna BD, Shinohara RT, Gillespie MJ, Dori Y, Rome JJ, Kawut SM. Predictors of Catastrophic Adverse Outcomes in Children With Pulmonary Hypertension Undergoing Cardiac Catheterization: A Multi-Institutional Analysis From the Pediatric Health Information Systems Database. J Am Coll Cardiol. 2015;66:1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatr. 2001;107:1–5. [DOI] [PubMed] [Google Scholar]
- 20.O’Byrne ML, Gillespie MJ, Shinohara RT, Dori Y, Rome JJ, Glatz AC. Cost comparison of transcatheter and operative closures of ostium secundum atrial septal defects. Am Heart J. 2015;169:727–735.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Byrne ML, Shinohara RT, Grant EK, Kanter JP, Gillespie MJ, Dori Y, Rome JJ, Glatz AC. Increasing propensity to pursue operative closure of atrial septal defects following changes in the instructions for use of the Amplatzer Septal Occluder device: An observational study using data from the Pediatric Health Information Systems database. Am Heart J. 2017;192:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ. 2005;24:465–488. [DOI] [PubMed] [Google Scholar]
- 23.Sun G-W, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. Journal of Clinical Epidemiology. 1996;49:907–916. [DOI] [PubMed] [Google Scholar]
- 24.Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. AmJEpidemiol. 2003;158:280–287. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs ML, O’Brien SM, Jacobs JP, Mavroudis C, Lacour-Gayet F, Pasquali SK, Welke K, Pizarro C, Tsai F, Clarke DR. An empirically based tool for analyzing morbidity associated with operations for congenital heart disease. J Thorac Cardiovasc Surg. 2013;145:1046–1057.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schidlow DN, Jenkins KJ, Gauvreau K, Croti UA, Giang DTC, Konda RK, Novick WM, Sandoval NF, Castañeda A. Transposition of the Great Arteries in the Developing World Surgery and Outcomes. J Am Coll Cardiol. 2017;69:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fricke TA, d’Udekem Y, Richardson M, Thuys C, Dronavalli M, Ramsay JM, Wheaton G, Grigg LE, Brizard CP, Konstantinov IE. Outcomes of the Arterial Switch Operation for Transposition of the Great Arteries: 25 Years of Experience. Ann Thoracic Surg. 2012;94:139–145. [DOI] [PubMed] [Google Scholar]
- 28.Chan PS, Oetgen WJ, Buchanan D, Mitchell K, Fiocchi FF, Tang F, Jones PG, Breeding T, Thrutchley D, Rumsfeld JS, Spertus JA. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry’s PINNACLE (Practice Innovation And Clinical Excellence) program. J Am Coll Cardiol. 2010;56:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan PS, Maddox TM, Tang F, Spinler S, Spertus JA. Practice-level variation in warfarin use among outpatients with atrial fibrillation (from the NCDR PINNACLE program). Am J Cardiol. 2011;108:1136–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hira RS, Kennedy K, Jneid H, Alam M, Basra SS, Petersen LA, Ballantyne CM, Nambi V, Chan PS, Virani SS. Frequency and practice-level variation in inappropriate and nonrecommended prasugrel prescribing: insights from the NCDR PINNACLE registry. J Am Coll Cardiol. 2014;63:2876–2877. [DOI] [PubMed] [Google Scholar]
- 31.Maddox TM, Chan PS, Spertus JA, Tang F, Jones P, Ho PM, Bradley SM, Tsai TT, Bhatt DL, Peterson PN. Variations in coronary artery disease secondary prevention prescriptions among outpatient cardiology practices: insights from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2014;63:539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hira RS, Kennedy K, Nambi V, Jneid H, Alam M, Basra SS, Ho PM, Deswal A, Ballantyne CM, Petersen LA, Virani SS. Frequency and practice-level variation in inappropriate aspirin use for the primary prevention of cardiovascular disease: insights from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence registry. J Am Coll Cardiol. 2015;65:111–121. [DOI] [PubMed] [Google Scholar]
- 33.Harahsheh AS, O’Byrne ML, Pastor B, Graham DA, Fulton DR. Pediatric Chest Pain—Low-Probability Referral. Clin Pediatr. 2017;56:1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedman KG, Rathod RH, Farias M, Graham D, Powell AJ, Fulton DR, Newburger JW, Colan SD, Jenkins KJ, Lock JE. Resource utilization after introduction of a standardized clinical assessment and management plan. Cong Heart Dis. 2010;5:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fonarow GC, Yancy CW, Heywood JT, ADHERE Scientific Advisory Committee, Study Group, and Investigators. Adherence to heart failure quality-of-care indicators in US hospitals: analysis of the ADHERE Registry. Arch Intern Med. 2005;165:1469–1477. [DOI] [PubMed] [Google Scholar]
- 36.Arnold SV, Spertus JA, Masoudi FA, Daugherty SL, Maddox TM, Li Y, Dodson JA, Chan PS. Beyond medication prescription as performance measures: optimal secondary prevention medication dosing after acute myocardial infarction. J Am Coll Cardiol. 2013;62:1791–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterson PN, Chan PS, Spertus JA, Tang F, Jones PG, Ezekowitz JA, Allen LA, Masoudi FA, Maddox TM. Practice-level variation in use of recommended medications among outpatients with heart failure: Insights from the NCDR PINNACLE program. Circ Heart Fail. 2013;6:1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Komajda M, Lapuerta P, Hermans N, Gonzalez-Juanatey JR, van Veldhuisen DJ, Erdmann E, Tavazzi L, Poole-Wilson P, Le Pen C. Adherence to guidelines is a predictor of outcome in chronic heart failure: the MAHLER survey. Eur Heart J. 2005;26:1653–1659. [DOI] [PubMed] [Google Scholar]
- 39.Kirklin JW, Blackstone EH, Tchervenkov CI, Castañeda AR. Clinical outcomes after the arterial switch operation for transposition. Patient, support, procedural, and institutional risk factors. Congenital Heart Surgeons Society. Circulation. 1992;86:1501–1515. [DOI] [PubMed] [Google Scholar]
- 40.Anderson BR, Ciarleglio AJ, Hayes DA, Quaegebeur JM, Vincent JA, Bacha EA. Earlier arterial switch operation improves outcomes and reduces costs for neonates with transposition of the great arteries. J Am Coll Cardiol. 2014;63:481–487. [DOI] [PubMed] [Google Scholar]
- 41.Cain MT, Cao Y, Ghanayem NS, Simpson PM, Trapp K, Mitchell ME, Tweddell JS, Woods RK. Transposition of the great arteries--outcomes and time interval of early neonatal repair. World J Pediatr Cong Heart Surg. 2014;5:241–247. [DOI] [PubMed] [Google Scholar]
- 42.Nevvazhay T, Chernogrivov A, Biryukov E, Biktasheva L, Karchevskaya K, Sulejmanov S, Kalinicheva J, Artemiev N. Arterial switch in the first hours of life: no need for Rashkind septostomy? Eur J Cardiothorac Surg. 2012;42:520–523. [DOI] [PubMed] [Google Scholar]
- 43.Chasovskyi K, Fedevych O, Vorobiova G, Zhovnir V, Maksimenko A, Boychenko O, Lysak Y, Cohen G, Yemets I. Arterial switch operation in the first hours of life using autologous umbilical cord blood. Ann Thoracic Surg. 2012;93:1571–1576. [DOI] [PubMed] [Google Scholar]
- 44.Chasovskyi K, Mykychak Y, Rudenko N, Vorobyova H, Yemets I. Five-year Experience With Arterial Switch Operation in the First Hours of Life. Sem Thorac Cardiovasc Surg. 2017;29:70–76. [DOI] [PubMed] [Google Scholar]
- 45.McQuillen PS, Hamrick SEG, Perez MJ, Barkovich AJ, Glidden DV, Karl TR, Teitel D, Miller SP. Balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries. Circulation. 2006;113:280–285. [DOI] [PubMed] [Google Scholar]
- 46.Petit CJ, Rome JJ, Wernovsky G, Mason SE, Shera DM, Nicolson SC, Montenegro LM, Tabbutt S, Zimmerman RA, Licht DJ. Preoperative brain injury in transposition of the great arteries is associated with oxygenation and time to surgery, not balloon atrial septostomy. Circulation. 2009;119:709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Butts RJ, Ellis AR, Bradley SM, Hulsey TC, Atz AM. Effect of prostaglandin duration on outcomes in transposition of the great arteries with intact ventricular septum. Congenit Heart Dis. 2012;7:387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartlett JM, Wypij D, Bellinger DC, Rappaport LA, Heffner LJ, Jonas RA, Newburger JW. Effect of prenatal diagnosis on outcomes in D-transposition of the great arteries. Pediatr. 2004;113:e335–e340. [DOI] [PubMed] [Google Scholar]
- 49.Blyth M, Howe D, Gnanapragasam J, Wellesley D. The hidden mortality of transposition of the great arteries and survival advantage provided by prenatal diagnosis. Brit J Obstet Gynecol. 2008;115:1096–1100. [DOI] [PubMed] [Google Scholar]
- 50.Bonnet D, Coltri A, Butera G, Fermont L, Le Bidois J, Kachaner J, Sidi D. Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality. Circulation. 1999;99:916–918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.