Abstract
Engineering of functional vascularized liver tissues hold great promise in addressing donor organ shortage for transplantation. Whole organ decellularization is a cell removal method that retains the native vascular structures of the organ such that it can be anastomosed with the recipient circulation after recellularization with healthy cells. However, a main hurdle to successful implantation of bioengineered organ is the inability to efficiently re-endothelialize the vasculature with a functional endothelium, resulting in blood clotting which is the primary cause of failure in early transplant studies. Here, we present an efficient approach for enhancing re-endothelialization of decellularized rat liver scaffolds by conjugating the REDV cell-binding domain to improve attachment of endothelial cells (EC) on vascular wall surfaces. In order to facilitate expression and purification of the peptide, REDV was fused with elastin-like peptide (ELP) that confers thermally triggered aggregation behavior to the fusion protein. After validating the adhesive properties of the REDV-ELP peptide, we covalently coupled REDV-ELP to the blood vasculature of decellularized rat livers and seeded EC using perfusion of the portal vein. We showed that REDV-ELP increased cell attachment, spreading and proliferation of EC within the construct resulting in uniform endothelial lining of the scaffold vasculature. We further observed that REDV-ELP conjugation dramatically reduced platelet adhesion and activation. Altogether, our results demonstrate that this method allowed functional re-endothelialization of liver scaffold and show great potential toward the generation of functional bioengineered liver for long-term transplantation.
Keywords: Bioengineered liver, Endothelialization, Elastin-like polypeptide, REDV peptide, transplantation
Graphical Abstract
1. Introduction
Liver transplantation is a lifesaving intervention and remains the only viable and definitive treatment for patients suffering from decompensated cirrhosis, certain malignancies and genetic diseases associated with disordered liver metabolism. While the number of liver transplants in the United States is large with 7,841 transplants performed in 2016, 1,410 patients died while on the liver transplantation waiting list as a result of severe shortage of organs. In 2016, there were 14,087 individuals listed for liver transplantation in the United States, a number that far exceeds the number of transplants performed each year [1]. Strategies that offer new treatment options to this unmet clinical need for functional, replacement liver are continuously being investigated.
Tissue engineering may offer alternative solutions to cover the donor organ gap by creating artificial tissues. Whole liver engineering is a new approach that has shown promising success in recent years. The first step consists of whole-organ decellularization through detergent perfusion, via the vascular system, that allows removal of cellular components while preserving structural, mechanical and chemical properties of the native tissue [2]. Subsequently, the resulting preserved extracellular matrix (ECM) is reseeded with the organ-specific cells to regenerate a functional organ [3].
The decellularization process has been shown to be successful for a number of bioengineered tissues and organs such as heart [4], lung [5] [6], kidney [7], urinary bladder [8] and liver [9] [10]. This technique has been under development for many years leading to optimized protocols that allow the evaluation of the scaffold quality (collagen, laminin, fibronectin and glycosaminoglycan contents), while monitoring cell removal [11] [12] [13]. Importantly, decellularized matrices retain the microvascular structure, which favors connection of the tissue directly with patient vasculature upon transplantation, permitting immediate circulation and delivery of nutrients and oxygen [14] [15]. Nevertheless, the recellularization process remains challenging and several obstacles need to be addressed for clinical translation of recellularized liver transplantation. One major hurdle to successful organ bioengineering is vascular patency [16] [17]. Collagen has the ability to activate the extrinsic coagulation cascade when in contact with blood. Thus, a sufficient coverage of the vascular network by endothelial cells (EC) has to be achieved to prevent thrombosis and provide proper vascular function after in vivo transplantation. While studies have reported the re-endothelialization of scaffolds by perfusion, demonstrating the ability of EC to adhere to the internal surface of blood vessels [18] [9] [19] [20] [21], the anti-coagulant effect of EC remained insufficient to prevent thrombosis beyond 3 days post-implantation due to limited coverage of vessel and microvessel walls of the decellularized scaffold. Therefore, enhancing re-endothelialization efficiency would be a major breakthrough for the development of a transplantable, recellularized organ.
The goal of this study was to develop an inexpensive and scalable technique using artificial extracellular matrix proteins to increase attachment of EC to the decellularized liver matrix, and so enhance re-endothelialization efficiency. The biopolymer used in this study consists of domains derived from elastin and fibronectin named the repetitive elastin-like polypeptide (ELP) and the CS5 domain, respectively. ELP is an engineered biopolymer composed of tandemly repeated blocks of (VPGVG), a sequence motif derived from the hydrophobic domain of tropoelastin, the soluble precursor form of elastin. ELPs have been proven to be non-immunogenic, biologically compatible and to retain elasticity and mechanical properties of native elastin [22]. This peptide has been used for several applications in regenerative medicine including cartilage and intervertebral disc regeneration [23] [24] [25], wound healing [26], vascular graft [27] [28] [29] and ocular [30] tissue engineering. An interesting property of ELPs is their ability to undergo thermally triggered phase separation. At temperatures below their inverse transition temperature, they are soluble in aqueous solutions. However as the temperature is raised above their transition temperature, they undergo a temperature induced contraction and self-assembly, rendering them insoluble. This property enables recombinant ELPs to be expressed in bacteria and rapidly purified to high homogeneity using inverse temperature cycling (ITC). In order to facilitate endothelial cell binding to vessel walls, we have genetically fused ELP to five internal peptide sequence (REDV) of the CS5 segment of fibronectin. This tetrapeptide has been reported to be the minimal active sequence within the CS5 site of the alternatively spliced type III connecting segment (IIICS) region of the fibronectin [31] and the integrin heterodimer alpha 4 beta 1 has been identified as its receptor [32]. Importantly, substrates containing covalently immobilized REDV-containing peptides selectively support the attachment and spreading of human umbilical vein endothelial cells over that of fibroblasts, vascular smooth muscle cells and blood platelets [33]. Thus, the ELP sequences of REDV-ELP give the biopolymer mechanical properties similar to that of native elastin and facilitate its expression [22] while the peptide sequence REDV support attachment and spreading of endothelial cells.
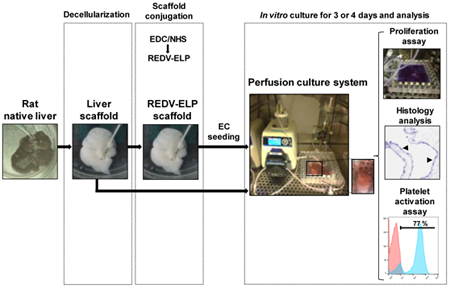
In this study, we hypothesized that the immobilization of the REDV-ELP peptide on the decellularized vasculature would increase the ability of vessel surfaces to induce cell attachment supporting the growth of an endothelial monolayer on vascular walls. We validated the adhesive properties of REDV-ELP in vitro by testing endothelial cell adhesion on gelatin and decellularized liver matrix conjugated or not with the biopolymer. Then, we covalently coupled REDV-ELP to the blood vasculature of decellularized rat livers and seeded EC using static and ramping perfusion to bioengineer a fully endothelialized organs. We evaluated the efficiency of REDV-ELP coating on liver scaffolds by monitoring cell proliferation overtime using a perfusion culture system. We further characterized the re-endothelialization efficacy by performing histological and molecular analysis of the repopulated scaffolds and tested its functionality ex vivo using platelet-rich plasma perfusion.
2. Material and methods
2.1. Cloning, expression, purification and characterization of REDV-ELP construct
As previously described by Dooley et al. [34] and Devalliere et al. [26] an empty pET24a(+) plasmid was modified to incorporate BseRI and AcuI endonuclease restriction sites as well as short leader and trailer sequences. A short cassette encoding five pentapeptide ELP repeats, (VPGVG)5, was generated by annealing two overlapping oligonucleotides with appropriate overhangs for ligation into the modified pET24a(+) vector linearized with BseRI. ELP cassettes were concatenated by recursive directional ligation by plasmid reconstruction (PRe-RDL) until the desired lengths of 40 pentapeptide repeats were achieved and a sequence encoding five (REDV)5 was appended to the N-terminus of the ELP cassette. Sequencing of the final cloned product was performed to ensure accuracy of the construct. The resulting plasmid was transformed into BL21(DE3) E. coli for expression.
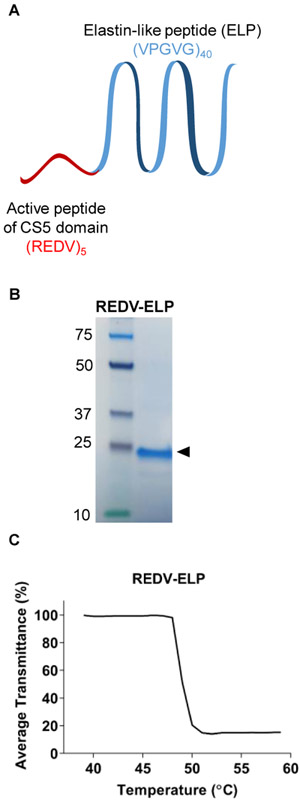
REDV-ELP fusion protein was expressed in terrific broth (TB) media supplemented with Kanamycin (100 μg/mL) and purified by inverse transition cycling (ITC), as previously described [34]. Briefly, after carrying out expression at 37 °C and 220 RPM, cells were harvested by centrifugation and lysed via microtip sonication. Cell debris was separated from the soluble protein by centrifugation and polyethyleneimine (0.7 % w/v) was used to precipitate and eliminate the nucleic acid contaminants. The ELP fusion protein was further purified by performing three rounds of ITC. ELP samples were heated to 45 °C to induce their precipitation. The precipitated proteins were separated from host cell contaminants by centrifugation at 10,000 xg for 15 minutes in a warmed centrifuge. The soluble fraction was discarded and the precipitated pellet was resuspended in ice cold PBS. The samples were then centrifuged at 4 °C at 15,000 xg for 15 minutes to remove any insoluble contamination, thus completing one round of ITC. Purity was verified by SDS-PAGE after completion of 3 cycles (Fig. 1B). ELP fusion transition temperature (Tt) was calculated using a temperature controlled Bio Rad Benchmark Plus microplate spectrophotometer. REDV-ELP solution (25 μM; 0.5g/L) was prepared in PBS in a 96 well plate, warmed from 40 to 60 °C over 20 min and optical density readings were taken at 350 nm each minute. All readings were made in triplicate. The resulting transmittance profile is given in Fig. 1C. The data were fitted to a four parameter logistic (4 PL) curve and the Tt was defined as the inflection point temperature at half the minimal transmittance.
Fig. 1.
Characterization of REDV-ELP peptide. (A) Schematic of REDV-ELP fusion protein. (B) Representative protein analysis showing purified REDV-ELP after 3 rounds of inverse transition cycling. (C) Representative percentage of average transmittance measurements at 350 nm of REDV-ELP (25 lM).
REDV-ELP was labeled with FITC to visualize the fusion protein attachment to the extracellular matrix. FITC labeling was performed by adding a 5 molar excess of FITC solution prepared in dimethylformamide to 1 ml of REDV-ELP dissolved in sodium carbonate buffer (100 mM, pH 8.6). After 2 hours incubation, unbound FITC was separated from FITC-labeled REDV-ELP using G-50 Sephadex® gel filtration columns (GE Healthcare, Chicago, IL, USA), followed by overnight dialysis using 7K MWCO cassette (ThermoFisher Scientific, Waltham, MA, USA).
2.2. Cell culture
Human EA.hy926 endothelial cells (EC) from American Type Culture Collection (ATCC, Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2% L-glutamine, 1% penicillin and streptomycin in a humidified incubator at 37 °C and 5% CO2. Suspension of EA.hy926 cells were obtained from confluent culture using trypsin-EDTA (0.05%) solution (Gibco, Waltham, MA, USA) and cell concentration was subsequently determined using a hemocytometer. Primary rat hepatocytes were isolated using the two step perfusion protocol described previously [35]. Tissue culture dishes were coated with a collagen solution that was nine parts type I rat tail collagen (1.25 mg/ml) and 1 part 10× DMEM and incubated for 1 h at 37 °C to form a collagen gel. After gelation, cells were seeded in 6-well plate at 1.5 ×106 cells per well in hepatocyte culture medium consisted of Williams' E (Sigma-Aldrich, Natick, MA, USA), 5% FBS (Hyclone, Logan, Ut, USA), 0.5 U/ml insulin (Eli Lilly, Indianapolis, IN, USA), 20 ng/ml epidermal growth factor (ThermoFisher Scientific), 14 ng/ml glucagon (Bedford Laboratories, Bedford, OH, USA), 7.5 μg/ml hydrocortisone (GE Healthcare, Chicago, IL, USA), 100 U/ml penicillin and 100 μg/ml streptomycin (ThermoFisher Scientific), and incubated in 90% air and 10% CO2 at 37 °C. Culture medium was collected daily and analyzed for urea content measured with diacetylmonoxime using a commercially available kit (StanBio Laboratory, Boerny, TX, USA) and albumin content using Enzyme Linked Immunosorbent Assay kit (Bethyl laboratories, Montgomery, TX, USA).
2.3. In vitro cell attachment assay
To evaluate the ability of REDV-ELP coating to stimulate adhesion and spreading of EC, a solution of 10 μM of FITC-labeled REDV-ELP was directly applied on gelatin and incubated for 2h (absorption method) or conjugated to gelatin by covalent immobilization. For immobilization, 1% gelatin coating was treated with a solution of 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) (Sigma-Aldrich) (0.05 M) and N-hydroxysuccinimide esters (NHS) (Sigma-Aldrich) (0.06 M) in 2-(N-morpholino)ethanesulfonic acid (MES) buffer (pH 5.6; 0.05 M) for 30 min at room temperature. Subsequently, 5, 10 or 20 μM of REDV-ELP fusion protein in phosphate buffered solution (PBS) was applied on gelatin and incubated for 2 h at room temperature. After washing with PBS to remove unreacted REDV-ELP, 2×105 cells were seeded in 24-well plates coated with either 1% gelatin conjugated with REDV-ELP or 1% gelatin only. Medium was removed 1 or 2 h after seeding in order to remove non-adherent cells. Cultures were washed with PBS, fixed for 10 min in 1% paraformaldehyde and stained with phalloidin alexa 546 (1:40 dilution; Thermo Fisher Scientific, Waltham, MA, USA) for F-actin labeling and 4′,6-Diamidino-2-phenylindole (DAPI; 1:30,000 dilution; ThermoFisher Scientific) for nuclear staining. The Cells were examined using an EVOS FL microscope; cell number and spreading analyzed using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA).
To evaluate the ability of the decellularized liver matrix (DLM) to support the attachment of EA.hy926 EC, 100 μm slices of native liver were cut and placed on glass microscope slides. O-rings of 2 cm diameter and 2 mm of height were glued on top of the liver slice to delimit the disc area of tissue that will be decellularized by immersion using increasing concentration of SDS: 0.01% SDS for 8 h, 0.1% SDS for 8 h, 0.2% SDS for 1 h, 0.5% SDS for 1 h. The liver slices were then washed with distilled water, followed by 1% Triton X-100 (Sigma-Aldrich, St. Louis, MO) for 30 min. Finally, the DLM slices were washed with PBS for 1 h and sterilized in a PBS solution containing 0.1% peracetic acid (Sigma) and 4% ethanol (Sigma) for 3 h. The discs of DLM were conjugated with FITC-ELP or REDV-ELP using EDC/NHS coupling chemistry, unconjugated DLM were used as negative control. After treatment, discs were washed with PBS and 500 μL of cell suspension containing 5×104 EA.hy926 EC was applied to the disc surfaces. The number of adherent cells was assessed after 2 h incubation by immunofluorescence staining. DLM slices were washed with PBS, fixed for 10 min in 1% paraformaldehyde and stained with phalloidin alexa 546 and DAPI as previously described. DLM was observed without staining due to extracellular matrix autofluorescence in green channel. For proliferation assay, the number of viable EC was evaluated every day using the fluorometric assay PrestoBlue® cell viability reagent (Thermo Fisher Scientific) over a period of 7 days. PrestoBlue™ is a resazurin-based reagent that is metabolized by live cells and gives rise to a high fluorescent product that diffuses into surrounding medium without the need of cell lysis. PrestoBlue™ solution can be used to indicate long-term viability and growth of a cell population due to its minimal cytotoxicity during the assay period and the possibility to efficiently wash it out after a measurement, enabling repetitive measurements during the entire culture period [36]. Briefly, PrestoBlue® was added to fresh media at a concentration of 1:10 and applied on DLM discs area at 37 °C in a 5% CO2 humidified air incubator for 1 h. One hundred microliters of solution from each DLM slice was then transferred to a 96-well black flat bottom plate (Thermo Fisher Scientific) and fluorescence (Excitation 535 nm, Emission 615 nm) was measured on a Synergy 2 microplate reader (Biotek, Winooski, Vermont). Cell number is proportional to fluorescence intensity expressed as relative fluorescence unit (RFU).
2.4. Liver harvest
All animal procedures were conducted in accordance to the Institutional Animal Care and Use Committee (IACUC) at Massachusetts General Hospital. Rat livers were collected from female Lewis rats (Charles River Laboratories, Wilmington, MA, USA) weighing 150–200 g. After anesthetization, a transverse incision across the abdomen was made. Ligaments connecting the liver lobes to the body cavity and to other lobes were carefully cut. The stomach and intestinal tract were removed to the side to expose the portal vein. An 18-gauge catheter was inserted into the portal vein and connected to a syringe filled with PBS (Sigma-Aldrich, St. Louis, MO, USA). After cutting the inferior vena cava, 20 mL of PBS was slowly injected to clear the organ of blood. The catheter was secured with 5–0 silk sutures and the liver was carefully excised from the body cavity by freeing more ligaments and ligating the superior vena cava, bile duct, and portal vein inferior to the catheter. After excision, the liver was placed in a petri dish filled with PBS and the livers were kept at −80°C until decellularization.
2.5. Liver decellularization
Rat livers were thawed and decellularization was performed according to an established protocol described in our previous work [9] [11]. The perfusion setup consisted of a Masterflex peristaltic pump and a bubble trap. Briefly, livers were thawed, weighed, and cleared with PBS perfusion overnight. Next, livers were perfused with 0.01% SDS (Sigma-Aldrich, St. Louis, MO, USA) for 5 min and with PBS for 1 h. These two steps were repeated thrice, with each repetition increasing the length of 0.01% SDS perfusion by an increment of 5 min. After the last hour of PBS perfusion, livers were perfused with 0.01% SDS overnight followed by 0.1% SDS for 4h, 0.2% SDS for 1 h, and 0.5% SDS for 1 h. The livers were washed with distilled water for 15 min to remove SDS followed by 1% Triton X-100 (Sigma-Aldrich) for 30 min to remove any residual cellular components in the scaffolds. Finally, the decellularized livers were washed with PBS for 1 h. The perfusion rate was kept at 1.2 mL/min throughout.
2.6. Re-endothelialization of decellularized liver scaffold
To improve re-endothelialization of vasculatures within the liver scaffold, REDV-ELP was conjugated to the acellular liver scaffold. 30 mL of a solution of 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) (Sigma-Aldrich, Natick, MA, USA) (0.05 M) and N-hydroxysuccinimide esters (NHS) (Sigma-Aldrich) (0.06 M) in 2-(N-morpholino)ethanesulfonic acid (MES) buffer (pH 5.6; 0.06 M) were injected within rat liver scaffold through the portal vein catheter and incubated for 30 min at room temperature. Then, 10 mL of phosphate buffered solution (PBS) containing 10 μM of REDV-ELP were injected, followed by 2 h incubation at room temperature. Scaffolds were washed with 30 mL of PBS to remove unconjugated REDV-ELP peptides.
For re-endothelialization of the REDV-ELP conjugated liver matrix and untreated liver matrix, a first injection of 4.5 mL of 10×106/mL of EA.hy926 endothelial cells suspended in complete DMEM was performed through the portal vein. The seeded cells were then allowed to attach for 1h and liver placed into a specially designed perfusion chamber for in vitro culture. The perfusion chamber features two hermetically sealed silicon sheets, forming a pouch filled with culture medium. This design avoids rigid surfaces, preventing development of pressure spots, while enabling sterile culture of the recellularized grafts. A second injection of 3 mL of 10×106/mL EA.hy926 endothelial cells was then made through the perfusion chamber inlet to the scaffold’s portal vein, followed by a 180-degree rotation of the perfusion chamber from the original configuration. Rotation of the perfusion chamber allows a more even distribution of cells within the matrix. After 1 h incubation, continuous perfusion of the recellularized liver scaffolds was started at 2 mL/min using a perfusion setup consisted of the perfusion chamber, a Masterflex peristaltic pump, an oxygenator and a bubble trap. The perfusion setup contained 50 mL of complete DMEM and was maintained at 37 °C in a 5% CO2 humidified air incubator. The flow rate was gradually increased to 5 mL/min after 24 h and maintained at 8 mL/min after day 2.
2.7. Histology
Samples of recellularized liver were fixed in 10% formalin for preparation of histological analysis. Samples were embedded in paraffin for tissue slicing, sliced at 10 μm, and stained with hematoxylin and eosin.
2.8. In vitro functional testing of the recellularized scaffold
4 REDV-ELP livers and 4 control livers were cultured for 4 days and cell viability was assessed using PrestoBlue every day. Evaluation of ECs by reverse transcription quantitative PCR and histology was performed on the same cultured livers on day 4. As no proliferation was observed between day 3 and 4 of culture, platelet activation and adhesion assays were achieved on day 3 using 4 independent REDV-ELP livers compared to 4 control livers. In total, 8 REDV-ELP livers and 8 control livers were recellularized and analyzed in this study.
To assess cell viability and proliferation in the liver constructs, perfusion media was replaced by 40 mL of PrestoBlue® reagent diluted in fresh media at a concentration of 1:10. Re-endothelialized livers were perfused with PrestoBlue® solution at 2 mL/min for 1 h at 37 °C every day. Fluorescence was read using Synergy 2 microplate reader at 535 nm (ex)/615 nm (em). After incubation, PrestoBlue mixture was replaced by 50 mL of fresh media.
To evaluate the function of ECs grown on decellularized scaffolds conjugated or not with REDV-ELP, total RNA was extracted from re-endothelialized liver scaffolds using Nucleospin RNA from Macherey-Nagel (Bethlehem, PA, USA), according to manufacturer’s instructions. Reverse transcription was performed using ImProm-II reverse transcription system from Promega (Madison, WI, USA) and quantitative PCRs were performed using ViiA 7 Real-Time PCR system (Thermo Fisher Scientific). For quantification, duplicates were normalized by the concomitant quantification of GAPDH. Relative expression was calculated according to the −2−ΔΔCt method. Custom primers were obtained from Massachusetts General Hospital DNA core; their sequences are listed in Table 1.
Table 1.
Oligonucleotide primer pairs for qPCR. VE-Cadherin = vascular endothelial cadherin; iNOS = inducible nitric oxide synthase; eNOS = endothelial nitric oxide synthase; VEGF = vascular endothelial growth factor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase transcript variant 1.
| Human VE-CAD | F 5’-GGCAAGATCAAGTCAAGCGTG-3’ R 5'-ACGTCTCCTGTCTCTGCATCG-3' |
| Human iNOS | F 5’-TCCCGAAGTTCTCAAGGCAC-3’ R 5’-CATAGCGGATGAGCTGAGCA-3’ |
| Human eNOS | F 5’-CAGTGTCCAACATGCTGCTGGAAATTG-3’ R 5'-TAAAGGTCTTCTTCCTGGTGATGCC-3' |
| Human VEGF | F 5’-GGCAGAAGGAGGAGGGACAGAATC-3’ R 5'-CATTTACACGTCTGCGGATCTTGT-3' |
| Human GAPDH | F 5’-ACAGTCAGCCGCATCTTCTT-3’ R 5'-GACAAGCTTCCCGTTCTCAG-3' |
In order to assess the efficiency of endothelialization, four REDV-ELP conjugated liver and four unconjugated liver scaffolds were retrieved after 3 days in the perfusion chamber, and perfused with freshly prepared platelet-rich plasma (PRP). Blood was drawn from female Lewis rats (Charles River Laboratories) in tubes containing sodium citrate (0.105 M, 3.2%), followed by centrifugation at 160G for 15 min at room temperature and collection of PRP layer. Liver scaffolds were perfused with PRP (3×108 cells/ml) and incubated 1 h at 37 °C. Scaffolds were then rinsed with PBS and platelets collected into a polypropylene tube containing paraformaldehyde 4% for fixation. Platelets were centrifuged at 1200g for 5 min and incubated with 10 μL of CD62-P (P-selectin marker) (Biolegend, San Diego, CA, USA) in 400 μL of cold PBS with 1% BSA. Tubes were placed on ice for 25 min, then washed twice with cold PBS with 1% BSA. The samples were analyzed on a flow cytometer (BD Facscalibur™, BD Biosciences, San Jose, CA, USA).
For immunofluorescence staining following PRP perfusion, deparaffinization and rehydration was achieved by immersing slides three times in xylene at room temperature for 5 min, and then transferred sequentially into two washes of 10 min each of 100% ethanol, 95% ethanol, 70% ethanol and 50% ethanol. Sections were rinsed in deionized water. For antigen retrieval, slides were boiled in 10 mM sodium citrate buffer (pH 6.0) and then maintained at a sub-boiling temperature for 10 min. Slides were washed by immersion in deionized water for 5 min. Tissue was permeabilized by incubating the sections twice for 10 min with 1% normal goat serum (NGS) in PBS with 0.4% Triton X-100 and slides were blocked by incubating the tissue sections with 5% NGS in PBS for 30 min. Sections were stained using Integrin αIIb (1:50 dilution; Santa Cruz Biotechnology, Dallas, TX, USA) antibody diluted in 1% NGS in PBS with 0.4% Triton X-100 and incubated at 4 °C overnight. After two PBS washes, goat anti-mouse Alexa 555 (1:1,000 dilution; Thermo Fisher Scientific) was diluted in 1% NGS in PBS with 0.4% Triton X-100 and incubated for 2 h at room temperature. Slides were rinsed in PBS, and incubated 5 min with DAPI (1:30,000 dilution; ThermoFisher Scientific) for nuclear staining.
2.9. Statistics
Statistical analysis was performed using GraphPad Prism 7 software (Graphpad Software Inc., San Diego, CA, USA). The p value was calculated for the indicated groups by using Student t-test analysis, a value of p < 0.05 was considered statistically significant. Results are expressed as mean ± SEM.
3. Results
3.1. Production and characterization of REDV-ELP
We have previously reported the use of the elastin-like peptide cassette containing repeats of the pentapeptide (VPGVG) and demonstrated its stability and efficacy in delivering biologically active protein [26] [34]. As schematically shown in Fig. 1A, we fused five REDV peptides to the ELP cassette generating a polypeptide of 20.3 kDa. A high yield of 40 g of fusion protein per liter of bacterial culture was obtained. Following production and purification processes, purity was confirmed by loading 5 μL per well of REDV-ELP fusion protein solution on SDS-PAGE gel. Analysis revealed a single band with the appropriated molecular weight of ~20 kDa, confirming the efficiency of the ITC process to purify the polypeptides from the bacterial lysate (Fig. 1B). It was previously shown that the transition temperature (Tt) of the ELP biopolymer is concentration-dependent where Tt was found to increase with decreasing concentration of peptides [37]. In order to demonstrate that REDV-ELP peptides remained as a homogeneous population of monomers during immobilization process, we further investigated the transition temperature (Tt) of the ELP fusion protein. The phase transition in response to increasing temperature was evaluated by turbidimetry and showed a Tt of approximatively 50 °C for a concentration of 0.5 g/L of protein (25 μM). This result confirmed that REDV-ELP peptides do not aggregate at conditions used for immobilization (5, 10 and 20 μM at 37 °C).
3.2. In vitro assessment of cell-binding properties of REDV-ELP
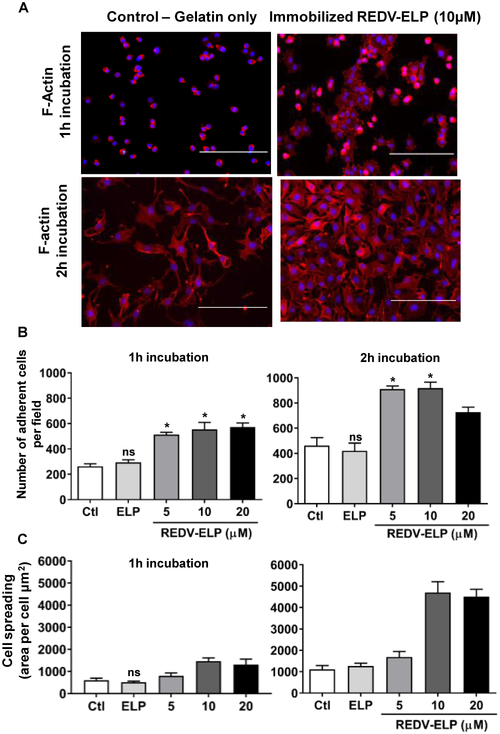
We tested the ability of REDV-ELP immobilization to enhance attachment and spreading of EA.hy926 endothelial cells on gelatin. Different concentrations of REDV-ELP (5, 10 and 20 μM) were conjugated onto gelatin-coated coverslips by covalent immobilization. EC were then added to the gelatin-coated surface conjugated or not with ELP (20 μM) or REDV-ELP, and cells were allowed to adhere to the matrix for 1 or 2 h. As illustrated in Figure 2A and B, the number of cells attached to the gelatin was significantly increased in the presence of 10 μM of REDV-ELP by 2.1 and 1.9 fold compared to cells attached to untreated gelatin or conjugated with ELP after 1 and 2 h incubation, respectively. The cell area was quantified and showed that conjugation of 10 μM of REDV-ELP promoted cell spreading with an increase of ~75% of the cell area in contact with the gelatin matrix 2h after cell attachment (4710 ± 501.7 μm2 for REDV-ELP cell area vs 1107 ± 180.7 μm2 for control cell area) (Fig 2C). These results showed that REDV-ELP immobilization enhanced EC attachment and ability to fully spread on gelatin, leading to a reduced time for complete spreading and coverage of adhesion surface. ELP conjugation induced no modification of cell attachment and spreading as compared to untreated gelatin, indicating that ELP itself has no effect on the cell-adhesion properties of the matrix and confirming that REDV active peptides effectively accelerated EC recruitment and adhesion. As there was no significant difference between 10 and 20 μM, we chose to pursue this study with 10 μM of REDV-ELP as the highest protein coupling concentration.
Fig. 2.
Effect of REDV-ELP conjugation on EC attachment and spreading on gelatin coated coverslips. Human EA.hy926 EC were allowed to attach for 1 or 2 h on gelatin coated coverslips conjugated or not with 20 lM of ELP or 5, 10 or 20 lM of REDV-ELP fusion protein. (A) Immunofluorescence evaluation of cell-binding properties of REDV-ELP conjugated gelatin (10 lM). After PBS washing, cells were fixed and incubated with phalloidin (red) and DAPI(blue) for F-actin and nucleus staining, respectively. The number of cells per field was determined by quantifying the number of nucleus (B), and F-actin staining was used to determine cell surface area (C) using ImageJ software in 5 different fields per experiment (means ± SEM). Histogram is representative of 3 independent experiments. *P< 0.05, **P < 0.01, ***P < 0.001 vs. control. Scale bars = 400 lm.***(For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
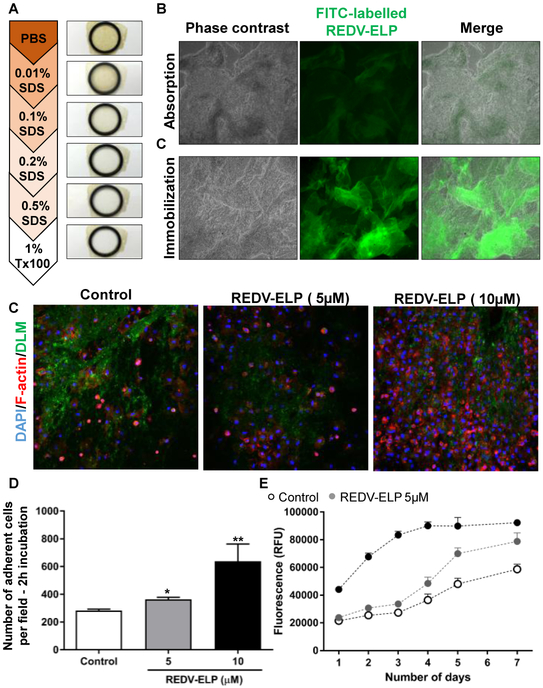
To determine whether REDV-ELP conjugation could improve EC retention on decellularized liver matrix (DLM), REDV-ELP peptides were covalently coupled on slices of native liver previously decellularized using increasing concentration of SDS (Fig. 3A), following our protocol established for whole rat liver [11] [38]. Conjugation onto DLM was confirmed using fluorescently labeled REDV-ELP and showed greater ELP attachment compared to absorption method (incubation of FITC-labeled REDV-ELP with DLM), underlining the advantage of using crosslinking agent (Fig 3B). As FITC labeling can affect the binding abilities of the protein, unlabeled REDV-ELP was used for the following experiments. Immunofluorescence staining confirmed that pre-coating of DLM using 10 μM of REDV-ELP resulted in a significant increase in cell attachment (2.25 fold) while 5 μM coating only increased the cell adhesion by 1.28 fold, compared to uncoated DLM (Fig. 3C and D). PrestoBlue™ solution was used as a fluorometric assay to repetitively quantify the viable cell number cultured on DLM over a period of 7 days. As shown in Figure 3E, the number of cells attached to the DLM after one day of culture is significantly increased by 2.1 fold using 10 μM of REDV-ELP compared to untreated DLM, confirming immunofluorescent staining results (44,157 ± 2,112 RFU on REDV-ELP DLM vs 21,489 ± 995 RFU on untreated DLM). To investigate the proliferative capacity of the EC on DLM, cell viability assay was performed over 7 days, and the results demonstrated that the cell growth was accelerated by REDV-ELP with cell population doubling after 3 day versus 5 days for untreated DLM (Fig 3E). Fluorescence reached a plateau after 5 days of cultivation in REDV-ELP condition due to complete coverage of the DLM surface by cells (data not shown). Altogether, these results indicate that immobilization of REDV-ELP peptides provides improved conditions for the survival and proliferation of EC on DLM.
Fig. 3.
Effect of REDV-ELP conjugation on EC attachment and proliferation on DLM. (A) Macroscopic view of DLM disc showing decoloration of the tissue over decellularization process. Disc of native liver were decellularized using increasing concentration of SDS: 0.01%, 0.1%, 0.2% and 0.5% SDS. The liver slices were then washed with distilled water, followed by 1% Triton X-100. (B) Micrographs of DLM discs conjugated with FITC-labeled REDV-ELP. Immobilization of FITC-labeled ELP on discs was performed by absorption or by covalent binding using EDC/NHS coupling chemistry and fluorescence visualized under microscope. Scale bar = 500 lm. (C) Microscopic analysis of cellbinding properties of REDV-ELP conjugated DLM. Human EA.hy926 EC were allowed to attach for 2 h on DLM conjugated or not with 5 or 10 lM of REDV-ELP fusion protein. After PBS washing, cells were immunostained for F-actin, and visualized under confocal microscope. Scale bar = 400 lm. (D) Quantification of adherent cells assessed by counting the number of nucleus in 10 different fields using ImageJ software (means ± SEM), *P < 0.05, **P < 0.01 vs. control. (E) Fluorescence intensity of PrestoBlue™ reagent after incubation on DLM conjugated or not with 5 or 10 lM of REDV-ELP over a period of 7 days. Results are expressed as relative fluorescent unit (means ± SEM).
3.3. Structural and functional characterization of re-endothelialized liver scaffolds
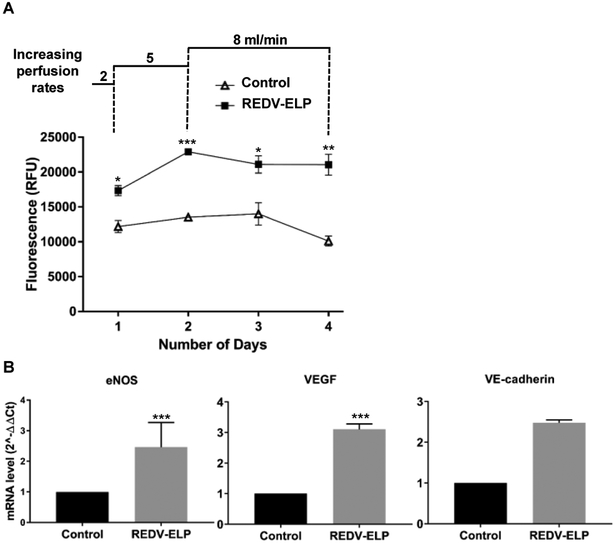
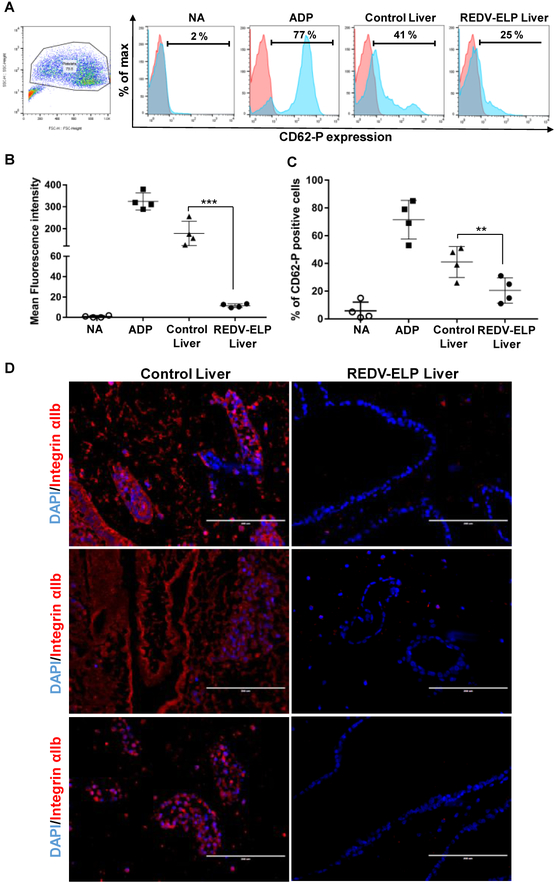
After decellularization, REDV-ELP peptides were conjugated or not (control liver scaffolds) onto the vascular surfaces of rat liver scaffolds. Two injections of cell suspension were performed through the liver vasculature using the portal vein and cells allowed to attach for one hour after each injection. Based on our previous cell seeding experiments, we have selected a flow rate of 2 ml/min to start perfusion of the scaffold and progressively increased the flow rate up to 8 ml/min to prevent cell clogging within small capillaries and stimulate cell alignment in the vasculature. To assess viable cell numbers and cell proliferation ability of the reseeded EC in the scaffolds, liver constructs were perfused for one hour every day with a PrestoBlue™ solution. As shown in Fig. 4A, REDV-ELP conjugation increased significantly the fluorescence intensity (17,330 ± 735 RFU for REDV-ELP conjugated liver vs. 12,183 ± 865 RFU for untreated liver) indicating a higher number of cells attached in REDV-ELP scaffold as compared to untreated liver scaffold after one day of culture. A significant increase of cell population was observed after 2 days of perfusion in re-endothelialized liver scaffolds conjugated with REDV-ELP, demonstrating a rapid cell proliferation, while cell population did not significantly grow within the control scaffolds (22,900 ± 257 RFU for REDV-ELP conjugated liver vs. 13,531 ± 125 RFU for untreated liver, P < 0.001). Then, the number of cells remained stable between day 2 and 4 and no significant growth was observed in REDV-ELP liver scaffold as shown by stable fluorescence intensity whereas untreated scaffolds exhibited a slight decrease of cell viability on day 4. To further characterize reseeded scaffolds, histological analysis was performed at day 4. H&E staining of re-endothelialized liver scaffolds showed greater cell spreading and surface coverage on REDV-ELP coated vasculature compared to untreated livers. As illustrated in Fig. 5, vessel walls conjugated with REDV-ELP were covered by a monolayer of well-spread EC whereas uneven coverage was observed on vessel surfaces of control liver, with the presence of round shaped cells that suggests the failure of appropriate attachment and spreading of EC (Fig 5, panel control 1 and 4). Moreover, control scaffold showed vessel blockage with the presence of EC obstructing the vessel lumen (Fig 5, panel control 3 and 5). Lower magnification photos of re-endothelialized scaffolds are presented in Fig. S1 for overview pictures. To ensure that EC did not present an altered phenotype, that could lead to EC activation, unexpected responses to hemodynamic forces, thrombosis and ultimately graft failure, the expression of endothelial nitric oxide synthase (eNOS), inducible nitric oxide synthase (iNOS), vascular endothelial growth factor (VEGF) and vascular endothelial-cadherin (VE-cadherin) were tested by quantitative PCR after 4 days of perfusion. The expression level of eNOS, VEGF and VE-cadherin was found to be increased in EC grown within the REDV-ELP conjugated scaffolds by 2.5 ± 0.5, 3.1 ± 0.1, 2.5 ± 0.04, respectively, when compared to EC grown within control untreated scaffolds (Fig. 4B). The expression level of iNOS was undetectable in both conditions. The raw data of the PCR experiments are presented in Fig. S2 and show the expression level of the different genes. To determine whether endothelialized liver matrices were functional in inhibiting platelet activation, we perfused both re-endothelialized scaffolds conjugated or not with REDV-ELP with platelet-rich plasma (PRP) through the portal vein. We hypothesized that improved EC lining of the vessel lumen would render the surface blood compatible, thus significantly reducing platelet adhesion in REDV-ELP liver scaffolds. After PRP injection, the effect of re-endothelialization was determined by quantifying platelet activation marker CD62-P by flow cytometry (Fig. 6A). Adenosine diphosphate (ADP) stimulation was used as a control of platelets activation and showed a mean fluorescent intensity (MFI) of 324 ± 20 with 71 ± 7 % of CD62-P positive platelets, confirming the platelets functionality (Fig. 6B). The platelets injected in the REDV-ELP conjugated liver scaffolds showed a significant decrease of activation compared to platelets incubated within control scaffolds, with a level of CD62-P marker expression of 13 ± 1 MFI and 178 ± 28 MFI, respectively (P < 0.01). The percentage of CD62-P positive platelets was also found lower after injection into the REDV-ELP conjugated liver matrices (18 ± 4 %) compared to untreated liver matrices (41 ± 6 %) as shown in Fig. 6C. Consistent with these results, immunostaining with integrin αIIb antibodies showed that a minimal number of platelets were found on vasculature of the REDV-ELP re-endothelialized scaffolds, whereas high levels of platelet accumulation were observed within untreated control liver scaffold. Altogether, these results indicate that conjugation of REDV-ELP facilitates EC seeding resulting in formation of uniform and functional EC monolayer, dramatically reducing activation and adhesion of platelets to the vessel walls.
Fig. 4.
Re-endothelialization of acellular rat liver scaffolds. After decellularization, liver scaffolds were covalently conjugated or not (control) with 10 lM of REDV-ELP. Matrices were re-endothelialized using static seeding followed by a gradually increase perfusion rates from 2 to 5 and 8 mL/min. (A) Fluorescence intensity of PrestoBlueTM reagent after perfusion of recellularized matrices over a period of 4 days (n = 4 for each condition). *P < 0.05, **P < 0.01, ***P < 0.001 vs. control. (B) Level of expression of eNOS, VEGF and VE-cadherin of EC grown within control or REDV-ELP scaffolds after 4 days of perfusion determined by RT-qPCR. Results were normalized to GAPDH and shown as the means ± SEM from three independent experiments, ***P < 0.001 vs. control.
Fig. 5.
H&E staining of re-endothelialized liver scaffolds. After decellularization, scaffolds were covalently conjugated or not (control) with 10 lM of REDV-ELP. Matrices were re-endothelialized and perfused for 4 days. The left lateral lobe (1), caudate lobe (2), inferior right lateral lobe (3), superior right lateral lobe (4) and median lobe (5) were collected, fixed in 10% formalin, embedded in paraffin for tissue slicing and stained with hematoxylin and eosin. Scale bar = 100 lm. * shows endothelial cells partially detached form the vessel wall or vessel clogged by endothelial cells accumulation in lumen. shows vessel walls covered by a monolayer of well-spread endothelial cells.
Fig. 6.
Functional analysis of re-endothelialized liver scaffolds. After 3 days of perfusion, liver scaffolds were injected with platelet-rich plasma, incubated for 1 h at 37 °C. (A) Flow cytometry analysis of platelet activation. Platelets were collected from liver scaffolds, and immunostained with CD62-P antibodies. Representative dot plot of platelets population and histograms showing CD62-P expression level of non-activated platelets (NA), platelets stimulated with ADP or collected from unconjugated liver (control) or REDV-ELP conjugated liver. (B) Mean fluorescence intensity of 4 independent experiments showing CD62-P expression level in each sample: NA, ADP, control liver and REDVELP liver (means ± SEM) **P < 0.01 vs. control liver. (C) Percentage of CD62-P positive platelets in each sample: NA, ADP, control liver and REDV-ELP liver (means ± SEM) ***P < 0.001 vs. control liver. (D) Representative immunostaining of re-endohelialized scaffolds using anti-integrin αIIb antibodies (red) and DAPI (blue). Scale bar = 200 lm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Reconstruction of functional liver graft requires the addition of hepatic parenchymal cells. As a preliminary test to evaluate potential side effects of REDV-ELP conjugation on hepatocytes function and seeding, we investigated the metabolic activity and morphology of hepatocytes cultured on REDV-ELP coated collagen. We quantified hepatocyte albumin production and urea synthesis and monitored cell morphology during 4 days (Fig. S3). No statistical differences were observed in the cumulative urea amounts synthesized by hepatocytes cultivated on collagen or REDV-ELP collagen (Fig. S3C). Similarly, the difference in albumin cumulative production by hepatocytes cultivated on collagen or REDV-ELP collagen was not statistically different (Fig. S3B), showing no change in hepatocytes functionality or toxicity of REDV-ELP to hepatocytes.
4. Discussion
The generation of a transplantable bioengineered liver graft represents a potential treatment for liver disease and a strategy to solve organ shortage. While several studies have demonstrated that decellularized liver provides an adequate scaffold for hepatocytes grafting, enabling cell-specific functions in perfusion culture, Uygun et al. have observed that the post-transplant survival remains limited to only 8 h due to widespread thrombosis in the transplanted graft [9]. It was shown that exposed extracellular matrix in vascular network was responsible for platelets activation and formation of thrombus. To address early graft failure due to thrombosis related effect, the coverage of the vascular network by EC need to be improved to prevent platelet adherence to vessel walls. The aim of this study was to improve blood compatibility in bioengineered rat livers by conjugating REDV-ELP peptides to the vessel surface in order to stimulate EC integrin binding and facilitate cell attachment, spreading and stabilize seeded cells on the vessel surfaces.
The fusion of REDV to ELP offers several advantages. First, it provides an inexpensive way to purify the protein by exploiting the ELP's thermal responsiveness using a non-chromatographic separation method. A recent study has described that conjugation of anti-endothelial cell antibodies facilitates cell coverage of the vessel walls allowing to withstand physiological blood flow within pig liver for 24 h following transplantation [19]. While these results represent a major advance towards the generation of an engineered liver, the use of antibodies remains extremely expensive which makes this strategy unlikely to be translated into clinical applications. The use of REDV-ELP peptides would be an affordable and safe alternative since ELPs have been shown to be easily purified for a low cost as well as biocompatible. Furthermore, we have previously shown that the use of ELP as a carrier increases peptide stability, promoting peptide activity and potency [34] [26]. As an artificial ECM component, ELPs conjugation does not alter matrix properties and can be engineered to mimic natural elastin. The approach of combining biomaterials with cells and growth factors has been proven to be insufficient to recapitulate the complexity of tissue regeneration. Decellularized matrices, when properly prepared, provide a microenvironment naturally dense of molecular cues able to maintain organ-specific cells functions. As opposed to surface coating techniques that mask extracellular molecules, REDV-ELP peptide conjugation provides an additional cell adhesion signal to the ECM that promotes endothelial cell attachment and growth, and maintains elasticity as well as structural and mechanical integrity of ECM.
Another approach to efficiently enhance hemocompatibility, and consequently prevent thrombosis in graft, is the surface modification of matrices using polyethylene glycol (PEG) [39], zwitterionic polymers [40], heparin [41] [42] or thrombomodulin [43], just to name a few. However, these modified surfaces may be excessively hydrophilic, limiting the initial EC adhesion and formation of confluent endothelial lining on vessel walls. In order to improve both biocompatibility and EC adhesion, many peptide sequences have been discovered and grafted onto artificial vascular grafts, such as RGD [44], REDV[45], GRGDSP from fibronectin, IKVAV a laminin-derived recognition sequence [46] or DGEA, a collagen type I sequence [47]. While these peptides have been used for vascular tissue engineering for a decade, to our knowledge we are the first to have developed this technique for whole-organ bioengineering. Our results showed that chemical modification of the liver vasculature with REDV active peptide effectively accelerated EC recruitment and functional endothelialization. Unlike the general adhesive property of RGD peptide, REDV has been shown to initiate cell-specific binding of EC by targeting the adhesion receptor α4β1 integrin while inhibiting platelets adhesion and decreasing adhesion, proliferation and migration of smooth muscle cells (SMC) [33]. Its specific affinity with ECs over platelets and SMC makes REDV peptide an excellent candidate for re-endothelialization of bioengineered organs.
Our data further showed that in addition to increase cell attachment, cell proliferation was accelerated during the first days of the culture when cells were seeded on REDV-ELP conjugated ECM compared to unconjugated ECM. This result was supported by our experiments using DLM and whole organ perfusion that demonstrated higher cell growth in presence of REDV-ELP on the matrices as well as a higher level of VEGF, a well-known angiogenic factor and crucial growth factor for vascular EC, expressed by cells seeded on REDV-ELP conjugated scaffold compared to unconjugated liver. These observations may be explained by a latent cellular growth period in the unconjugated condition due to a low cell seeding density in absence of REDV. It has been previously reported in the literature that a lower cell seeding density can lead to a longer lag phase and a lower observed cell growth, which are presumed to be due to a decrease in cell-cell interactions [48]. As shown in overview pictures (Fig. S1), EC attached to the vascular surfaces and also penetrated into the parenchyma space of the scaffolds, particularly in REDV-ELP conjugated liver. In future work, after treating the scaffold with REDV-ELP, we intend to infuse hepatocytes into the scaffold to allow them to settle within the parenchyma regions before injecting the EC. This 2 step recellularization process would limit spreading of EC into parenchymal region.
While proliferative capacity was increased by REDV-ELP conjugation in re-endothelialized liver, a decrease of cell viability was observed in the control liver on day 4. Robust EC adhesion to its underlying matrix is necessary to prevent EC detachment under flow shear stress. We hypothesize that poor attachment of EC on unconjugated liver scaffold lead to anoikis, where anchorage-dependent cells detach from surrounding ECM due to inadequate cell-matrix interactions and undergo apoptosis. This assumption was supported by histology analysis of reseeded liver that showed unspread and detached cells in the control liver, as well as cell accumulation in vessel lumen. Furthermore, cells seeded on REDV-ELP conjugated scaffold showed a higher expression level of VE-cadherin suggesting a better cell-cell contact and lining of blood vessels by EC. Thus, the conjugation of REDV-ELP reinforced the binding of α4β1 integrin expressing EC resulting to an increase of cell retention to the ECM under flow conditions.
We observed that cell number reached a plateau after 2 days in REDV-ELP scaffolds. This decrease in cell proliferation may be due to nutrient limitation as the cell number to medium volume ratio is higher for the perfusion culture compared to plate cultures. The use of a richer (20% FBS) or more complex medium with epidermal growth factor or VEGF could improve cell proliferative capacity. Another hypothesis is the development of shunts that hinder the perfusion of the scaffold, limiting the coverage of the vasculature and cell proliferation. Our future work will focus on improving the perfusion of the liver by delivering cells and medium through portal vein, hepatic artery and vena cava.
The phenotypic expression of eNOS was investigated as a measure of endothelial cell function. Unconjugated scaffold exhibited cells with a low expression of eNOS while the REDV-ELP conjugated scaffold showed higher expression of this endothelial cell phenotypic marker. These results are in agreement with previous studies reporting that synthetic elastin peptides induced endothelium vasorelaxation and increase endothelial Ca2+ level [49] [50]. The endothelial dysfunction marker iNOS was not expressed under either conditions, confirming that REDV-ELP conjugation did not alter endothelial phenotype.
Our study further demonstrated the feasibility to increase functional endothelialization of decellularized rat liver as shown by the dramatic decrease of platelets adhesion and activation induced by REDV-ELP conjugation. Although these data present a promising strategy to bioengineer organ, several questions need to be addressed to achieve long-term implantation. Our future work will be conducted in pig livers, which represent a clinically relevant scale. In order to pursue our effort to generate a functional human-sized bioengineered liver, we will test vascular patency under normal physiological vascular conditions after transplantation in pig. To achieve a fully functional organ, studies are planned to include both parenchymal and endothelial cells using different seeding strategies to evaluate endothelialization effect on hepatocytes function in vivo. Our work so far is promising and shows that REDV-ELP conjugation does not adversely affect hepatocyte viability and function.
EC source and species used to repopulate an organ-specific ECM scaffold are important considerations for ultimate functionality and clinical translation. Liver sinusoidal endothelial cells are known to be difficult to culture in vitro and traditional methods of isolation from the liver initiate a process of endothelial cell dedifferentiation [51] rendering them unfit for organ engineering given the current state of knowledge. Primary human umbilical vein endothelial cells (HUVECs) are considered to be the best source of allogeneic cells for clinical therapy. However, poor availability, high variability, loss of morphology, proliferative ability and function in culture over prolonged periods of time, limit their use for whole organ engineering that requires large numbers of well-defined, controllable cells. For initial studies, EA.hy926 are used as an alternative to primary cells because they have extended life span, are well characterized, showed stable expression of endothelial markers and significant similarity regarding their functions when compared to HUVECs [52]. An ideal autologous cell source would be one that can proliferate or self-renew as needed and give rise to the heterogeneous types of cells necessary to form a functional organ. Induced pluripotent stem (iPS) cells are considered as a sustainable cell source for cell therapies such as tissue engineering and are of particular interest as they could be patient-specific and would reduce or remove the need for immunosuppression upon transplantation. However, stem cells derived from current methods retain epigenetic memory, which could impair their potential to differentiate into cells of all lineages by predisposing iPS cells to differentiate more readily into their parental cells than others [53] [54]. Nevertheless, the use of iPS for liver engineering represent an important direction for research and further studies are in need to obtain scalable and efficient methods to differentiate pluripotent cells to liver cells equivalent [55] [56] [57].
Source of livers for clinical trials of decellularization techniques remain to be defined. Cadaveric human livers not used for transplantation could constitute an appropriate liver source for preparing scaffolds for whole liver grafts. Livers rejected for transplant because of excessive steatosis or level of ischemia that are beyond acceptable limits, could be accessible for decellularization. Importantly, some of the pathologies that lead to rejection for transplant could have effects on the quality of ECM and future studies will have to determine if this damage will impair recellularization process and functionality. Another limitation to consider is the high level of heterogeneity between individuals described by Mattei et al. [58]. Authors have shown that human liver ECM from different donors cannot be reproducibly decellularized using a universal cell-removal protocol. For ease of standardization and quality assurance, pig livers represent another potential source of organs. Porcine livers are large enough to hold sufficient number of cells to support a failing human liver and possess mechanostructural and physiological features that have been shown to be donor-independent [59]. However, use of xenogeneic tissues could lead to hyperacute transplant rejection because of recipient antibodies against galactose-α-1,3-galactose (the α-gal epitope), which is expressed by pig tissue [60]. Nevertheless, after decellularization, xenogeneic tissue does not seem to generate an adverse immune response, but a constructive remodeling response was shown in an African Green monkey abdominal wall resection model [61]. Therefore, porcine livers could also constitute a viable source of organs.
5. Conclusion
In conclusion, this study has demonstrated that conjugation of REDV-ELP efficiently promoted endothelial cell attachment, survival, proliferation and retention onto rat liver scaffold which consequently reduced platelet adhesion and activation. These findings demonstrate the feasibility of re-endothelializing a whole liver vasculature using this strategy and show great potential toward the generation of functional bioengineered liver for long-term transplantation.
Supplementary Material
Statement of significance.
There is a critical need for novel organ replacement therapies as the grafts for transplantation fall short of demand. Recent advances in tissue engineering, through the use of decellularized scaffolds, have opened the possibility that engineered grafts could be used as substitutes for donor livers. However, successful implantation has been challenged by the inability to create a functional vasculature. Our research study reports a new strategy to increase efficiency of endothelialization by increasing the affinity of the vascular matrix for endothelial cells. We functionalized decellularized liver scaffold using elastin-like peptides grafted with REDV cell binding domain. We showed that REDV-ELP conjugation improve endothelial cell attachment and proliferation within the scaffold, demonstrating the feasibility of re-endothelializing a whole liver vasculature using our technique.
Acknowledgements
We would like to thank MGH Pathology Services for assistance with histological analysis. This work was accomplished using funding from the National Institutes of Health (R01DK084053 M.L.Y. and B.E.U., R00DK088962 B.E.U.), Shriners Hospitals for Children (J.D.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
B.E.U. has a financial interest in Organ Solutions, LLC, that is reviewed and arranged by MGH and Partners HealthCare in accordance with their conflict of interest policies. The rest of the authors of this manuscript have no conflicts of interest to disclose.
References
- [1].Waiting list candidates by organ type ∣ UNOS, (n.d.). https://unos.org/data/transplant-trends/waiting-list-candidates-by-organ-type/ (accessed December 5, 2017).
- [2].Barakat O, Abbasi S, Rodriguez G, Rios J, Wood RP, Ozaki C, Holley LS, Gauthier PK, Use of decellularized porcine liver for engineering humanized liver organ, J. Surg. Res 173 (2012) e11–25. doi:10.1016/j.jss.2011.09.033. [DOI] [PubMed] [Google Scholar]
- [3].Pla-Palacín I, Sainz-Arnal P, Morini S, Almeida M, Baptista PM, Liver Bioengineering Using Decellularized Whole-Liver Scaffolds, Methods Mol. Biol Clifton NJ: (2017). doi:10.1007/7651_2017_98. [DOI] [PubMed] [Google Scholar]
- [4].Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, Taylor DA, Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart, Nat. Med 14 (2008) 213–221. doi:10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- [5].Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, Herzog E, Niklason LE, Tissue-engineered lungs for in vivo implantation, Science. 329 (2010) 538–541. doi:10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP, Regeneration and orthotopic transplantation of a bioartificial lung, Nat. Med 16 (2010) 927–933. doi:10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- [7].Sullivan DC, Mirmalek-Sani S-H, Deegan DB, Baptista PM, Aboushwareb T, Atala A, Yoo JJ, Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system, Biomaterials. 33 (2012) 7756–7764. doi:10.1016/j.biomaterials.2012.07.023. [DOI] [PubMed] [Google Scholar]
- [8].Bolland F, Korossis S, Wilshaw S-P, Ingham E, Fisher J, Kearney JN, Southgate J, Development and characterisation of a full-thickness acellular porcine bladder matrix for tissue engineering, Biomaterials. 28 (2007) 1061–1070. doi:10.1016/j.biomaterials.2006.10.005. [DOI] [PubMed] [Google Scholar]
- [9].Uygun BE, Soto-Gutierrez A, Yagi H, Izamis M-L, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, Hertl M, Nahmias Y, Yarmush ML, Uygun K, Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix, Nat. Med 16 (2010) 814–820. doi:10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lorvellec M, Scottoni F, Crowley C, Fiadeiro R, Maghsoudlou P, Pellegata AF, Mazzacuva F, Gjinovci A, Lyne A-M, Zulini J, Little D, Mosaku O, Kelly D, De Coppi P, Gissen P, Mouse decellularised liver scaffold improves human embryonic and induced pluripotent stem cells differentiation into hepatocyte-like cells, PloS One. 12 (2017) e0189586. doi:10.1371/journal.pone.0189586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Geerts S, Ozer S, Jaramillo M, Yarmush ML, Uygun BE, Nondestructive Methods for Monitoring Cell Removal During Rat Liver Decellularization, Tissue Eng. Part C Methods. 22 (2016) 671–678. doi:10.1089/ten.TEC.2015.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang Y, Nicolas CT, Chen HS, Ross JJ, De Lorenzo SB, Nyberg SL, Recent Advances in Decellularization and Recellularization for Tissue-Engineered Liver Grafts, Cells Tissues Organs. 204 (2017) 125–136. doi:10.1159/000479597. [DOI] [PubMed] [Google Scholar]
- [13].Peloso A, Katari R, Tamburrini R, Duisit J, Orlando G, Glycosaminoglycans as a measure of outcome of cell-on-scaffold seeding (decellularization) technology, Expert Rev. Med. Devices 13 (2016) 1067–1068. doi:10.1080/17434440.2016.1249849. [DOI] [PubMed] [Google Scholar]
- [14].Maghsoudlou P, Georgiades F, Smith H, Milan A, Shangaris P, Urbani L, Loukogeorgakis SP, Lombardi B, Mazza G, Hagen C, Sebire NJ, Turmaine M, Eaton S, Olivo A, Godovac-Zimmermann J, Pinzani M, Gissen P, De Coppi P, Optimization of Liver Decellularization Maintains Extracellular Matrix Micro-Architecture and Composition Predisposing to Effective Cell Seeding, PloS One. 11 (2016) e0155324. doi:10.1371/journal.pone.0155324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Soto-Gutierrez A, Zhang L, Medberry C, Fukumitsu K, Faulk D, Jiang H, Reing J, Gramignoli R, Komori J, Ross M, Nagaya M, Lagasse E, Stolz D, Strom SC, Fox IJ, Badylak SF, A whole-organ regenerative medicine approach for liver replacement, Tissue Eng. Part C Methods 17 (2011) 677–686. doi:10.1089/ten.TEC.2010.0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zheng C-X, Sui B-D, Hu C-H, Qiu X-Y, Zhao P, Jin Y, Reconstruction of structure and function in tissue engineering of solid organs: Toward simulation of natural development based on decellularization, J. Tissue Eng. Regen. Med (2018). doi:10.1002/term.2676. [DOI] [PubMed] [Google Scholar]
- [17].Pellegata AF, Tedeschi AM, De Coppi P, Whole Organ Tissue Vascularization: Engineering the Tree to Develop the Fruits, Front. Bioeng. Biotechnol 6 (2018) 56. doi:10.3389/fbioe.2018.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bao J, Wu Q, Sun J, Zhou Y, Wang Y, Jiang X, Li L, Shi Y, Bu H, Hemocompatibility improvement of perfusion-decellularized clinical-scale liver scaffold through heparin immobilization, Sci. Rep 5 (2015) 10756. doi:10.1038/srep10756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ko IK, Peng L, Peloso A, Smith CJ, Dhal A, Deegan DB, Zimmerman C, Clouse C, Zhao W, Shupe TD, Soker S, Yoo JJ, Atala A, Bioengineered transplantable porcine livers with re-endothelialized vasculature, Biomaterials. 40 (2015) 72–79. doi:10.1016/j.biomaterials.2014.11.027. [DOI] [PubMed] [Google Scholar]
- [20].Kojima H, Yasuchika K, Fukumitsu K, Ishii T, Ogiso S, Miyauchi Y, Yamaoka R, Kawai T, Katayama H, Yoshitoshi-Uebayashi EY, Kita S, Yasuda K, Sasaki N, Komori J, Uemoto S, Establishment of practical recellularized liver graft for blood perfusion using primary rat hepatocytes and liver sinusoidal endothelial cells, Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg 18 (2018) 1351–1359. doi:10.1111/ajt.14666. [DOI] [PubMed] [Google Scholar]
- [21].Yang W, Chen Q, Xia R, Zhang Y, Shuai L, Lai J, You X, Jiang Y, Bie P, Zhang L, Zhang H, Bai L, A novel bioscaffold with naturally-occurring extracellular matrix promotes hepatocyte survival and vessel patency in mouse models of heterologous transplantation, Biomaterials. 177 (2018) 52–66. doi:10.1016/j.biomaterials.2018.05.026. [DOI] [PubMed] [Google Scholar]
- [22].Welsh ER, Tirrell DA, Engineering the extracellular matrix: a novel approach to polymeric biomaterials. I. Control of the physical properties of artificial protein matrices designed to support adhesion of vascular endothelial cells, Biomacromolecules. 1 (2000) 23–30. [DOI] [PubMed] [Google Scholar]
- [23].Betre H, Setton LA, Meyer DE, Chilkoti A, Characterization of a genetically engineered elastin-like polypeptide for cartilaginous tissue repair, Biomacromolecules. 3 (2002) 910–916. [DOI] [PubMed] [Google Scholar]
- [24].Betre H, Ong SR, Guilak F, Chilkoti A, Fermor B, Setton LA, Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide, Biomaterials. 27 (2006) 91–99. doi:10.1016/j.biomaterials.2005.05.071. [DOI] [PubMed] [Google Scholar]
- [25].Betre H, Liu W, Zalutsky MR, Chilkoti A, Kraus VB, Setton LA, A thermally responsive biopolymer for intra-articular drug delivery, J. Control. Release Off. J. Control. Release Soc 115 (2006) 175–182. doi:10.1016/j.jconrel.2006.07.022. [DOI] [PubMed] [Google Scholar]
- [26].Devalliere J, Dooley K, Hu Y, Kelangi SS, Uygun BE, Yarmush ML, Co-delivery of a growth factor and a tissue-protective molecule using elastin biopolymers accelerates wound healing in diabetic mice, Biomaterials. 141 (2017) 149–160. doi:10.1016/j.biomaterials.2017.06.043. [DOI] [PubMed] [Google Scholar]
- [27].Liu JC, Tirrell DA, Cell response to RGD density in cross-linked artificial extracellular matrix protein films, Biomacromolecules. 9 (2008) 2984–2988. doi:10.1021/bm800469j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nicol A, Gowda DC, Urry DW, Cell adhesion and growth on synthetic elastomeric matrices containing Arg-Gly-Asp-Ser-3, J. Biomed. Mater. Res 26 (1992) 393–413. doi:10.1002/jbm.820260309. [DOI] [PubMed] [Google Scholar]
- [29].Heilshorn SC, DiZio KA, Welsh ER, Tirrell DA, Endothelial cell adhesion to the fibronectin CS5 domain in artificial extracellular matrix proteins, Biomaterials. 24 (2003) 4245–4252. [DOI] [PubMed] [Google Scholar]
- [30].Martínez-Osorio H, Juárez-Campo M, Diebold Y, Girotti A, Alonso M, Arias FJ, Rodríguez-Cabello JC, García-Vázquez C, Calonge M, Genetically engineered elastin-like polymer as a substratum to culture cells from the ocular surface, Curr. Eye Res 34 (2009) 48–56. doi:10.1080/02713680802542053. [DOI] [PubMed] [Google Scholar]
- [31].Humphries MJ, Akiyama SK, Komoriya A, Olden K, Yamada KM, Identification of an alternatively spliced site in human plasma fibronectin that mediates cell type-specific adhesion, J. Cell Biol 103 (1986) 2637–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mould AP, Komoriya A, Yamada KM, Humphries MJ, The CS5 peptide is a second site in the IIICS region of fibronectin recognized by the integrin alpha 4 beta 1. Inhibition of alpha 4 beta 1 function by RGD peptide homologues, J. Biol. Chem 266 (1991) 3579–3585. [PubMed] [Google Scholar]
- [33].Massia SP, Hubbell JA, Vascular endothelial cell adhesion and spreading promoted by the peptide REDV of the IIICS region of plasma fibronectin is mediated by integrin alpha 4 beta 1, J. Biol. Chem 267 (1992) 14019–14026. [PubMed] [Google Scholar]
- [34].Dooley K, Devalliere J, Uygun BE, Yarmush ML, Functionalized Biopolymer Particles Enhance Performance of a Tissue-Protective Peptide under Proteolytic and Thermal Stress, Biomacromolecules. 17 (2016) 2073–2079. doi:10.1021/acs.biomac.6b00280. [DOI] [PubMed] [Google Scholar]
- [35].Balis UJ, Behnia K, Dwarakanath B, Bhatia SN, Sullivan SJ, Yarmush ML, Toner M, Oxygen consumption characteristics of porcine hepatocytes, Metab. Eng 1 (1999) 49–62. doi:10.1006/mben.1998.0105. [DOI] [PubMed] [Google Scholar]
- [36].Ren X, Tapias LF, Jank BJ, Mathisen DJ, Lanuti M, Ott HC, Ex vivo non-invasive assessment of cell viability and proliferation in bio-engineered whole organ constructs, Biomaterials. 52 (2015) 103–112. doi:10.1016/j.biomaterials.2015.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Meyer DE, Chilkoti A, Purification of recombinant proteins by fusion with thermally-responsive polypeptides, Nat. Biotechnol 17 (1999) 1112–1115. doi:10.1038/15100. [DOI] [PubMed] [Google Scholar]
- [38].Chen Y, Geerts S, Jaramillo M, Uygun BE, Preparation of Decellularized Liver Scaffolds and Recellularized Liver Grafts, Methods Mol. Biol. Clifton NJ (2017). doi:10.1007/7651_2017_56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nakao A, Nagaoka S, Mori Y, Hemocompatibility of hydrogel with polyethyleneoxide chains, J. Biomater. Appl 2 (1987) 219–234. doi:10.1177/088532828700200203. [DOI] [PubMed] [Google Scholar]
- [40].Chen S-H, Chang Y, Ishihara K, Reduced Blood Cell Adhesion on Polypropylene Substrates through a Simple Surface Zwitterionization, Langmuir ACS J. Surf. Colloids 33 (2017) 611–621. doi:10.1021/acs.langmuir.6b03295. [DOI] [PubMed] [Google Scholar]
- [41].Bruinsma BG, Kim Y, Berendsen TA, Ozer S, Yarmush ML, Uygun BE, Layer-by-layer heparinization of decellularized liver matrices to reduce thrombogenicity of tissue engineered grafts, J. Clin. Transl. Res 1 (2015). doi:10.18053/jctres.201501.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hussein KH, Park K-M, Kang K-S, Woo H-M, Heparin-gelatin mixture improves vascular reconstruction efficiency and hepatic function in bioengineered livers, Acta Biomater. 38 (2016) 82–93. doi:10.1016/j.actbio.2016.04.042. [DOI] [PubMed] [Google Scholar]
- [43].Sperling C, Salchert K, Streller U, Werner C, Covalently immobilized thrombomodulin inhibits coagulation and complement activation of artificial surfaces in vitro, Biomaterials. 25 (2004) 5101–5113. doi:10.1016/j.biomaterials.2003.12.014. [DOI] [PubMed] [Google Scholar]
- [44].Zheng W, Wang Z, Song L, Zhao Q, Zhang J, Li D, Wang S, Han J, Zheng X-L, Yang Z, Kong D, Endothelialization and patency of RGD-functionalized vascular grafts in a rabbit carotid artery model, Biomaterials. 33 (2012) 2880–2891. doi:10.1016/j.biomaterials.2011.12.047. [DOI] [PubMed] [Google Scholar]
- [45].Wei Y, Ji Y, Xiao L, Lin Q, Ji J, Different complex surfaces of polyethyleneglycol (PEG) and REDV ligand to enhance the endothelial cells selectivity over smooth muscle cells, Colloids Surf. B Biointerfaces 84 (2011) 369–378. doi:10.1016/j.colsurfb.2011.01.028. [DOI] [PubMed] [Google Scholar]
- [46].Tashiro K, Sephel GC, Weeks B, Sasaki M, Martin GR, Kleinman HK, Yamada Y, A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth, J. Biol. Chem 264 (1989) 16174–16182. [PubMed] [Google Scholar]
- [47].Weber LM, Hayda KN, Haskins K, Anseth KS, The effects of cell-matrix interactions on encapsulated beta-cell function within hydrogels functionalized with matrix-derived adhesive peptides, Biomaterials. 28 (2007) 3004–3011. doi:10.1016/j.biomaterials.2007.03.005. [DOI] [PubMed] [Google Scholar]
- [48].Giannotta M, Trani M, Dejana E, VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity, Dev. Cell 26 (2013) 441–454. doi:10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- [49].Faury G, Ristori MT, Verdetti J, Jacob MP, Robert L, Effect of elastin peptides on vascular tone, J. Vasc. Res 32 (1995) 112–119. [DOI] [PubMed] [Google Scholar]
- [50].Faury G, Garnier S, Weiss AS, Wallach J, Fülöp T, Jacob MP, Mecham RP, Robert L, Verdetti J, Action of tropoelastin and synthetic elastin sequences on vascular tone and on free Ca2+ level in human vascular endothelial cells, Circ. Res 82 (1998) 328–336. [DOI] [PubMed] [Google Scholar]
- [51].Maintenance of hepatic sinusoidal endothelial cell phenotype in vitro using organ-specific extracellular matrix scaffolds. - PubMed - NCBI, (n.d.). https://www-ncbi-nlm-nih-gov.ezp-prod1.hul.harvard.edu/pubmed/?term=Maintenance+of+Hepatic+Sinusoidal+Endothelial+Cell+Phenotype+In+Vitro+Using+Organ-Specific+Extracellular+Matrix+Scaffolds (accessed June 13, 2018). [DOI] [PubMed]
- [52].Bouïs D, Hospers GAP, Meijer C, Molema G, Mulder NH, Endothelium in vitro: A review of human vascular endothelial cell lines for blood vessel-related research, Angiogenesis. 4 (2001) 91–102. doi:10.1023/A:1012259529167. [DOI] [PubMed] [Google Scholar]
- [53].Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. - PubMed - NCBI, (n.d.). https://www-ncbi-nlm-nih-gov.ezp-prod1.hul.harvard.edu/pubmed/?term=Incomplete+DNA+methylation+underlies+a+transcriptional+memory+of+somatic+cells+in+human+iPS+cells (accessed June 14, 2018). [DOI] [PMC free article] [PubMed]
- [54].Reprogramming of cell fate: epigenetic memory and the erasure of memories past. - PubMed - NCBI, (n.d.). https://www-ncbi-nlm-nih-gov.ezp-prod1.hul.harvard.edu/pubmed/25820261 (accessed June 14, 2018). [DOI] [PMC free article] [PubMed]
- [55].Zaret KS, Grompe M, Generation and regeneration of cells of the liver and pancreas, Science. 322 (2008) 1490–1494. doi:10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lanzoni G, Cardinale V, Carpino G, The hepatic, biliary, and pancreatic network of stem/progenitor cell niches in humans: A new reference frame for disease and regeneration, Hepatol. Baltim. Md 64 (2016) 277–286. doi:10.1002/hep.28326. [DOI] [PubMed] [Google Scholar]
- [57].Starokozhko V, Hemmingsen M, Larsen L, Mohanty S, Merema M, Pimentel RC, Wolff A, Emnéus J, Aspegren A, Groothuis G, Dufva M, Differentiation of human-induced pluripotent stem cell under flow conditions to mature hepatocytes for liver tissue engineering, J. Tissue Eng. Regen. Med 12 (2018) 1273–1284. doi:10.1002/term.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mattei G, Magliaro C, Pirone A, Ahluwalia A, Decellularized Human Liver Is Too Heterogeneous for Designing a Generic Extracellular Matrix Mimic Hepatic Scaffold, Artif. Organs 41 (2017) E347–E355. doi:10.1111/aor.12925. [DOI] [PubMed] [Google Scholar]
- [59].Mattei G, Di Patria V, Tirella A, Alaimo A, Elia G, Corti A, Paolicchi A, Ahluwalia A, Mechanostructure and composition of highly reproducible decellularized liver matrices, Acta Biomater. 10 (2014) 875–882. doi:10.1016/j.actbio.2013.10.023. [DOI] [PubMed] [Google Scholar]
- [60].Galili U, Induced anti-non gal antibodies in human xenograft recipients, Transplantation. 93 (2012) 11–16. doi:10.1097/TP.0b013e31823be870. [DOI] [PubMed] [Google Scholar]
- [61].Daly KA, Stewart-Akers AM, Hara H, Ezzelarab M, Long C, Cordero K, Johnson SA, Ayares D, Cooper DKC, Badylak SF, Effect of the alphaGal epitope on the response to small intestinal submucosa extracellular matrix in a nonhuman primate model, Tissue Eng. Part A 15 (2009) 3877–3888. doi:10.1089/ten.TEA.2009.0089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.