Abstract
Background
The glial-lymphatic or glymphatic pathway is a fluid clearance pathway recently identified in the rodent brain. This pathway subserves the flow of cerebrospinal fluid (CSF) into the brain along arterial perivascular spaces and thence into the brain interstitium facilitated by aquaporin-4 (AQP4) water channels. The pathway then directs flows towards the venous perivascular and perineuronal spaces, ultimately clearing solutes from the neuropil into meningeal and cervical lymphatic drainage vessels. In rodents, the glymphatic pathway is primarily active during sleep, when the clearance of harmful metabolites such as amyloid β (Aβ) increases two-fold relative to the waking state. Glymphatic dysfunction has been demonstrated in animal models of traumatic brain injury (TBI), Alzheimer’s disease (AD) and micro-infarct disease, most likely in relation to perturbed expression of AQP4. The recent characterizations of the glymphatic and meningeal lymphatic systems calls for revaluation of the anatomical routes for CSF-ISF flow and the physiological role that these pathways play in CNS health.
Recent developments
Recent work has revealed that several features of the glymphatic and meningeal lymphatic systems are also present in humans. MRI imaging of intrathecally-administered contrast agent shows that CSF flows along pathways closely resembling the glymphatic system outlined in rodents. Furthermore, PET studies reveal that Aβ accumulates in the healthy brain after a single night of sleep deprivation, suggesting that the human glymphatic pathway might also be primarily active during sleep. Other PET studies have shown that CSF clearance of Aβ and tau tracers is reduced in patients with AD compared to healthy controls. The observed reduction in CSF clearance was associated with increasing grey matter Aβ levels in human brain, which is consistent with findings in mice showing that decreased glymphatic function leads Aβ accumulation. Altered AQP4 expression is also evident in brain tissue from AD or normal pressure hydrocephalus (NPH) patients; glymphatic MRI of NPH patients shows reduced CSF tracer entry and clearance.
Where next?
Future research is needed to confirm if specific factors driving glymphatic flow in rodents also apply to humans. Conducting longitudinal imaging studies to evaluate human CSF dynamics will determine if there is indeed a causal link between reduced brain solute clearance and the development of neurodegenerative diseases. Assessment of glymphatic function after stroke or TBI could identify if it correlates with neurological recovery. Gaining new insights into how behavior and genetics modify glymphatic function, and how this decompensates in disease should lead to the development of new preventive and diagnostic tools, as well as novel therapeutic targets.
Introduction
The mechanisms underlying solute clearance from the brain’s extracellular space have puzzled neurologists for centuries.1 Protein aggregates are a common feature in patients with Alzheimer’s disease, (AD) Parkinson’s disease, amyotrophic lateral sclerosis and other neurodegenerative diseases.2–4 This implies that reduced brain clearance could be a shared phenomenon in neurodegeneration. The cerebral parenchyma is devoid of lymphatic vessels, such that removal of protein waste has traditionally been attributed to extra- and intracellular degradation processes, including autophagy and ubiquitination,5–7 whereas a few proteins such as amyloid-β (Aβ) are also cleared by specific transport across the blood-brain barrier (BBB).8 Although transport of the interstitial fluid (ISF) within the brain parenchyma was traditionally attributed only to diffusion, several historical observations have revealed a lymphatic-like system that drains to the cervical lymphatics in the rodent brain, which might constitute an unappreciated, complementary aspect of brain clearance.9–11 Recent 2-photon microscopic studies of cerebrospinal fluid (CSF) flow in the live mouse brain indicated the existance of the glymphatic pathway, a novel brain clearance mechanism with substantial capacity.12,13
In the glymphatic pathway, convective influx of CSF is balanced by perivenous efflux of ISF, which rids the neuropil of toxic proteinaceous metabolites, including Aβ.12 Glymphatic function robustly increases during sleep, thereby eliminating various metabolic byproducts from the extracellular space that accumulate during wakefulness.14 Exciting advances in clinical studies have shown CSF flow patterns in human brain resembling that of the glymphatic pathway in rodents, and furthermore show that CSF clearance is reduced in patients with AD or idiopathic normal pressure hydrocephalus (iNPH).15–17 The elucidation of this newly-described pathway enhances our basic understanding of brain clearance and also presents a promising target for developing clinical tools for early risk assessment, diagnostics, prognostics and therapeutics for neurodegenerative diseases. With a more detailed understanding of CSF flow pathways within and outside the brain, including the dural lymphatic network, a whole new understanding of CSF dynamics is emerging.18–20 We intend in this Rapid Review to summarize the rapidly growing literature on CSF-mediated brain clearance in both humans and animal models, placing special emphasis on what is already known about clinical aspects of the human glymphatic pathway.
The Glymphatic Pathway
The glymphatic pathway is a highly-organized fluid transport system. In its initial segments, CSF from the subarachnoid space flows into brain through perivascular spaces of the large leptomeningeal arteries.12,13,18,20 With branching of the vascular tree, CSF is driven into the brain parenchyma through the perivascular spaces of penetrating arteries, also known as Virchow-Robins spaces (Fig. 1).12,21,22 From the perivascular space, CSF then flows across the glial basement membrane and astroglial endfeet bordering the brain parenchyma.12 Astrocytic endfeet densely express the water channel aquaporin-4 (AQP4), which facilitates the flow of CSF into the brain parenchyma, where it mixes with the ISF.12,23 In the interstitium, the fluid disperses via a polarized net fluid movement directed towards the venous perivascular and perineuronal spaces.12 Ultimately, CSF exits along the perineural sheaths of cranial and spinal nerves, meningeal lymphatic vessels and arachnoid granulations (Fig. 2).18–20,24,25 The relative importance of each of these drainage routes is still a matter of debate. However, a primary CSF egress site in both mice and humans is along the olfactory nerve, passing through the cribriform plate to the nasal mucosa reaching the cervical lymphatic vessels.20,24 Results of recent invasive animal studies as well as modeling studies have suggested that fluid movement occurs exclusively via diffusion in the extracellular space, with a component of convective flow present only in the perivascular spaces (Fig. 1).26,27 However, the technical challenges to studying fluid flow driven by the low-pressure gradients prevailing in the closed compartment of the brain calls for non-invasive imaging approaches. In humans, intrathecal contrast agent flows deep into the brain parenchyma achieving distances that exceed those expected by diffusion alone, suggesting that convective flow is also an important driver of fluid movement within the human brain.17 Furthermore, the notion that glymphatic function depends upon astrocytic AQP4 has been recently called into question due to a report of normal brain CSF clearance in AQP4 knockout animals.27 However, multiple independent groups have replicated these initial findings of AQP4-dependent CSF clearance through the glymphatic pathway28–33 and have further confirmed the key role that AQP4 plays in facilitating Aβ clearance.34 Readers seeking an in-depth discussion of fluid dynamics within the brain interstitium should consult these recent reviews.33,35–37
Figure 1. The glymphatic pathway.
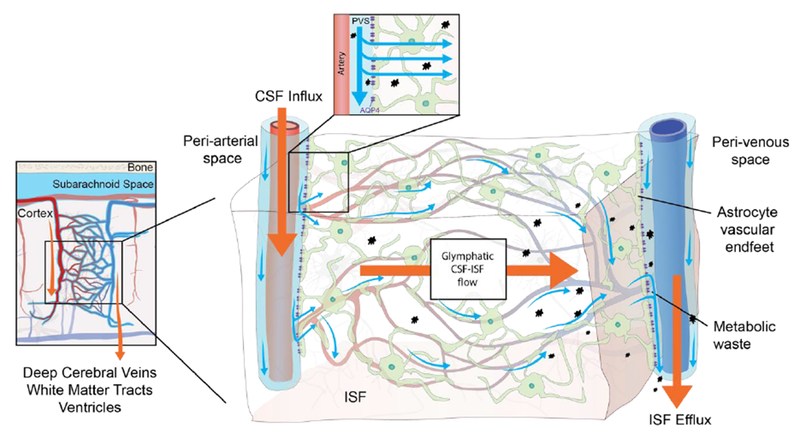
Rodent studies have shown that CSF from the subarachnoid space is driven into the perivascular space of major cerebral arteries on the brain surface from where it flows along the artery as it branches into penetrating arteries.12,13,22,39A similar pattern of CSF flow has been shown in patients undergoing MRI in combination with intrathecal contrast agent.15,46 In these patients it was observed that the CSF contrast agent flows along the large leptomeningeal cerebral arteries in an anterograde fashion, and that presence of contrast agent in the subarachnoid space precedes parenchymal uptake in adjacent brain regions.15 The microscopic details of CSF flow within the brain thus far all stem from animal research. These studies have shown that the perivascular space runs along the entire penetrating artery, known as the Virchow-Robin space, and continues to follow the vessel as it branches into arterioles and capillaries.12,13,21 In the murine brain, CSF influx into the extracellular space happens at every level of the perivascular space after entry to the brain parenchyma and is facilitated by a polarized expression of the AQP4 water channel towards the astrocytic end-feet that line the perivascular space.12 Whether a similar parenchymal CSF flow occurs in human brain has not yet been proven, but humans also harbor intracerebral perivascular spaces and polarized AQP4 expression towards astrocytic end-feet.55,58 The basis of fluid movement within the interstitium is still a matter of debate. Bulk flow clearance of ISF is a long-standing observation, which could be driven by multiple factors such as CSF inflow, arterial pulsatility, hydrostatic pressure gradients between the arterial and venous perivascular spaces, and osmotic gradients.8 Rodent studies show that ISF and its solutes move towards the venous perivascular space, where the fluid is taken up and drained by convection out of the brain parenchyma.12 This directional flow removes solutes from the brain parenchyma accumulated during neural activity.12
CSF, cerebrospinal fluid; ISF interstitial fluid, PVS: perivascular space; AQP4, aquaporin-4.
Figure 2. Cerebrospinal fluid efflux in humans.
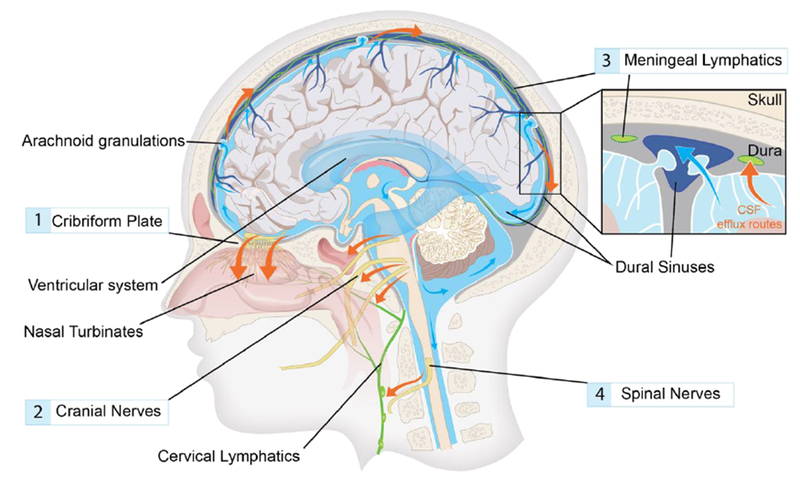
Cerebrospinal fluid (CSF) produced in the choroid plexus flows from the ventricles to the subarachnoid space of the brain and spinal cord. CSF contained in the subarachnoid space keeps the central nervous system buoyant and serves as a fluid source for glymphatic influx. Egress sites of cranial cerebrospinal fluid (red arrows) fall into three functionally distinct categories, namely the perineural sheaths surrounding cranial and spinal nerves,20,24 dural lymphatic vessels,18,19, and arachnoid granulations.1 The contribution and significance of each egress pathway is still a matter of debate. A main perineural egress site in both rodents and human is along the olfactory nerve through the cribriform plate (1) towards lymphatic vessels of the nasal mucosa.16,20 From here the CSF is drained to the cervical lymph nodes.43 Other significant perineural efflux pathways in rodents are the trigeminal, the glossopharyngeal, vagal, and spinal accessory nerves (2).20 Dural lymphatic vessels have also been shown to carry CSF towards the cervical lymphatic system (3). In rodents, these vessels exit the skull along the pterygopalatine artery, the veins that drain the sigmoid sinus and retroglenoid vein, and the foramina of the cranial nerves.18,19 In humans, meningeal lymphatic vessels have been visualized with MRI and were located around the dural sinuses, middle meningeal artery and cribiform plate.25 Arachnoid granulations are protrusions of the arachnoid membrane where CSF flows into the sagittal sinus, and constitute the only known egress site that drains directly to the blood stream.1 Traditionally, this site was thought to be the main cerebrospinal fluid egress site, but evidence suggests that under physiological intracranial pressure virtually no CSF leaves to the blood stream.1 The main egress site of CSF in the spinal cord is along the spinal nerves (4).
Factors driving flow in the glymphatic pathway
Bulk flow of CSF in the perivascular space in murine brain is partly driven by arterial pulsations arising from the cardiac systolic pressure wave.12,38,39 Ultrafast MR encephalography has revealed that pulsations related to the respiratory cycle and changes in vasomotor tone also propagate across the human brain, and could thus potentially contribute to glymphatic flow.40 In contrast, CSF flow within the ventricular compartments might be largely driven by respiration, which is also thought to be a significant driver of ISF efflux along perivenous spaces and white matter tracts.41 CSF production contributes to parenchymal influx, and inhibiting CSF production in rodents by treatment with acetazolamide significantly impairs CSF clearance.42,43 Body posture also modulates clearance; in sleeping mice, CSF clearance is more efficient with lateral head positioning than in the prone position, where CSF flow is more directed towards the spinal cord.44
Imaging the glymphatic pathway
The glymphatic pathway has been visualized in rodents using a variety of imaging approaches (Fig. 3). However, clinical imaging of the glymphatic pathway is an emerging field, and several innovative methods that visualize CSF flow in human brain have recently been developed. Noninvasive methods include T1-weighted MRI, which can quantify CSF flow within the ventricular system,41 and MRI diffusion tensor imaging, from which fractional anisotropy and diffusivity maps can be generated to calculate directional diffusivity along perivascular spaces.45 Ultrafast MR encephalography (MREG) scans the entire human brain volume in only 100 ms, which is 20-25 times faster than conventional fMRI, and thus provides sufficient temporal resolution to study the pulsations propagating through the brain parenchyma that might drive CSF movement.40 With the use of contrast agents, a detailed picture of glymphatic flow and clearance can be obtained. Thus, serial T1-weighted MRI scanning during 24 hours after intrathecal administration of the gadobutrol, delivered via lumbar puncture, can yield a detailed clinical assessment of glymphatic flow (Fig. 3).15,17 This MRI technique is capable of evaluating CSF flow pathways, perivascular influx, and parenchymal uptake and clearance rates.15 Since intrathecal contrast injection is minimally invasive, these studies have only been conducted in patients with a clinical indication for the procedure e.g. suspicion of CSF leakage or intracranial cysts.15,16 This imaging procedure revealed CSF transport in human brain along a glymphatic pathway resembling that in rodents, with an anterograde flow pattern of CSF from the subarachnoid space alongside the large cerebral arteries.15 There was significant signal enhancement across all brain regions, with contrast agent spreading centripetally from cortex to deeper brain regions.17 The largest contrast uptake in the CNS was seen in cerebral cortex, cerebral white matter, the limbic system and the cerebellar cortex.17 Brain-wide distribution of the contrast suggests that modulating glymphatic function might facilitate the delivery of intrathecal compounds. In patients with iNPH, contrast was also evident in periventricular white matter due to ventricular reflux secondary to retrograde CSF flow.15,17,46 PET-MRI presents a less invasive way to asses CSF clearance, whereby a BBB-permeable PET tracer can be administered intravenously. The tracer dynamics in the CSF provides detailed information on tracer uptake and clearance rates, while also potentially revealing the cranial outflow sites.44 Dual tracer PET-MRI studies with 11C-PiB or 18F-florbetaben allow correlation of brain Aβ deposition with altered CSF dynamics (Fig. 3).16,47
Figure 3. Glymphatic imaging modalities.
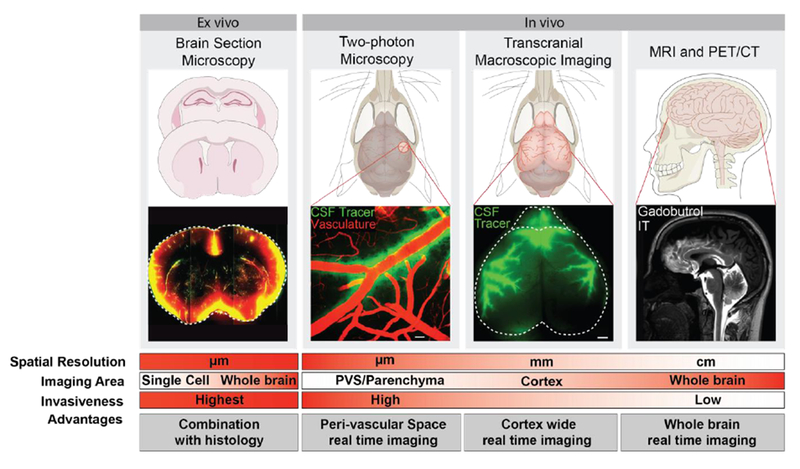
Analysis of the glymphatic pathway can be accomplished through multiple approaches. Ex vivo imaging is performed with micrometer-thick brain sections prepared from animals after injection of tracer into the CSF compartment.22,23 This method provides information on parenchymal distribution of CSF in the glymphatic pathway both on a brain-wide and regional scale, as well as at the cellular level, depending on the selected microscopic field of view. Combined with immunohistochemistry, the CSF tracer distribution can be compared to expression patterns of specific molecules e.g. AQP4 polarization towards the astrocytic end-feet12,23 In vivo imaging of the glymphatic pathway has been performed with 2-photon laser scanning microscopy (2PLSM) to map out flow mechanisms in the perivascular space in rodents.12 Optical access is acquired by surgically implanting an imaging window, i.e. a craniotomy covered with a glass coverslip. Affording high spatial resolution and the utilization of fluorescent tracers along with transgenic labeling of anatomical structures, 2PLSM is ideally suited for detailed study of perivascular space flow patterns and the factors impacting flow.12,27,65 For more global approaches, transcranial macroscopic imaging is used in combination with fluorescent CSF tracers. This process is less invasive since imaging is done through the intact skull bone of a mouse; it provides information on CSF flow patterns across the entire dorsal cortex. MRI and PET/CT is used in both basic as well as clinical neuroimaging studies of glymphatic flow. These are the least invasive methods, and the only approaches by which a 4-dimensional view of the CSF flow can be acquired in the entire brain of living animals and humans. 13,15,16,22 Ultrafast MR encephalography provides 3D imaging of the entire human brain volume in ~100 ms intervals, which provides sufficient temporal resolution to study pulsations propagating in brain parenchyma.40 The left panel and the two middle panels show representative images from mouse brain obtained by imaging methods only applicable in animal research. The right panel displays neuroimaging of glymphatic function in human brain, thus presenting techniques that are applicable in both clinical and basic science. [Left panel ex vivo section is reprinted with permission from Kress et al.,23 right panel MRI image is reprinted with permission from Ringstad et al.15]
Alzheimer’s disease and other dementias
Accumulation of Aβ plaques and neurofibrillary tangles of hyperphosphorylated tau are implicated in the cognitive decline seen in AD.48 Aβ, which plays a physiological role in synaptic regulation and neuronal survival,49,50 is degraded and cleared via multiple pathways.8 Findings from pre-clinical research suggest that the glymphatic pathway could indeed be a substantial factor in the net clearance of Aβ.12,34 Impaired glymphatic function in AQP4 knockout mice resulted in a 55% reduction in clearance of radiolabeled 125I-Aβ1-40 infused into the striatum compared to clearance in wild type mice.12 Furthermore, in a double hit mouse model of AD in which Aβ plaque formation is promoted by inserting chimeric mouse/human amyloid precursor protein mutations and human mutant presenilin-1 (APP/PS1) and AQP4 is deleted there was a 25-50 % increase in levels of soluble and insoluble Aβ compared to APP/PS1 mice expressing AQP4.34 The deletion of AQP4 did not alter the expression of proteins involved in synthesis or degradation of Aβ, suggesting that deletion of AQP4 results in reduced parenchymal clearance of Aβ.34 The reduction in Aβ clearance could be attributable to reduced efflux of ISF to the CSF, as well as reduced BBB transport, since impaired interstitial flow will lower the amount of Aβ available for BBB transport. However, AQP4 might also exert its protective function in mouse models of AD by modulating the inflammatory response to Aβ or by other yet unidentified mechanisms.34 In humans, a recent study found no association between candidate single nucleotide polymorphisms (SNPs) of AQP4 and Braak stage or neuritic plaque density post-mortem, but did find several gene variants that either slowed or increased the rate of cognitive decline.51,52 Authors suggested that the interaction between AQP4 and AD neuropathology could have been obscured by evaluating the end stages of the disease and therefore additional research using CSF AD biomarkers or amyloid PET in the early stages of the disease is needed. Genome-wide association studies in humans combined with longitudinal clinical imaging of CSF dynamics and brain Aβ levels are called for, to elucidate the role of AQP4 in AD neuropathology and to identify other genes with potential impact on the glymphatic pathway.
Other tracer studies in mice revealed a doubling in glymphatic clearance of 125I-Aβ1-40 during sleep compared to the awake state.14 Conversely, sleep deprivation in mice causes a marked reduction in the clearance of various CSF metabolites.14,42,43 Poor sleep quality is a known risk factor for cognitive decline and dementia in humans;53 and this risk might well be conferred via reduced glymphatic function. In humans, a single night of sleep deprivation leads to increased Aβ levels, in the hippocampal, parahippocampal and thalamic regions quantified by PET,47 and interruption of slow wave sleep leads to elevated CSF Aβ levels.54 While these findings support the hypothesis that reduced glymphatic function could account for the association between poor sleep and AD, further studies are needed to substantiate this relationship and rule out possible confounders (e.g. that sleep deprivation leads to increased neuronal activity and hence increased Aβ synthesis). A genetic study found that AQP4 SNPs moderated the relationship between decreased sleep quality and increased Aβ burden in humans,52 providing further weight to the link between sleep disturbance, glymphatic impairment and Aβ accumulation.
The prevalence of cognitive decline and dementia increases with age in humans. In aged mice, increased AQP4 expression, mislocalization of AQP4 expression away from the astrocytic end-feet, and reduced arterial wall pulsatility together, lead to a 40% reduction in 125I-Aβ1-40 clearance rate from the brain.23 Likewise, intraventricular and intracisternal tracers delivered to the CSF of mice show significant delay in reaching the blood stream and cervical lymph nodes in aged mice compared to young.20 This reduction in CSF outflow and impaired clearance of brain parenchyma, if also present in humans, could in part explain the accumulation of Aβ frequently seen with aging. Animal models of vascular disease, e.g. selective manipulation of arterial wall compliance, could shed further light on the vascular contribution to the reduced glymphatic function due to aging, whereas genetic manipulations that directly target AQP4 polarization, as opposed to global expression, could present new tools to address the role of AQP4 mislocalization. A similar pattern of perturbed AQP4 expression to that in aging mice has been found in a post-mortem immunohistochemical study of patients with AD.55 In this study, total AQP4 immunoreactivity increased with age but AQP4 polarity was preserved in cognitively intact older individuals while loss of perivascular AQP4 was a significant predictor of AD status and correlated with Aβ burden and Braak stage.55 These findings are consistent with the altered AQP4 expression observed in aged mice and suggests that loss of AQP4 polarization could favor the development of AD pathology in humans. However, it cannot be concluded from this study whether the process of AQP4 mislocalization is an effect of Aβ accumulation or if AQP4 mislocalization causes Aβ accumulation via reduced glymphatic function.
The relationship between Aβ plaque formation and glymphatic function has been investigated further in the APP/PS1 mouse model of AD.56 Influx and clearance rates of CSF tracers were reduced in the AD animals, and, interestingly, glymphatic dysfunction was found not only in aged transgenic animals with insoluble Aβ plaques, but also in young animals with no visible Aβ plaque formation.56 CSF injections of Aβ1-40 in normal mice caused a reduction of glymphatic influx, suggesting that soluble Aβ in the CSF can, by itself, perturb glymphatic flow.56 Hence, it seems that not only that reduced glymphatic function leads to decreased clearance of Aβ, but that Aβ can directly or indirectly disrupt flow in the glymphatic pathway, suggesting a feedforward mechanism. That Aβ can lead to reduced CSF turnover in humans, is supported by PET studies of patients with AD, who had a 23% reduction in ventricular CSF uptake of the tau tracer 18F-THK5117, along with 33% lower clearance of the tracer from the CSF.16 This reduced CSF turnover was associated with elevated brain Aβ levels visualized with dual tracer PET scanning.16 iNPH represents one of the most common, treatable, causes of dementia, and is also a risk factor for developing AD.15 In iNPH patients, the medial temporal lobe atrophy score –a structural MRI biomarker of AD– correlated with decreased clearance of intrathecally-delivered CSF contrast agent.57 Compared to reference subjects, iNPH patients showed delayed signal enhancement of gadobutrol delivered by lumbar puncture in cerebral CSF compartments such as the Sylvian fissure, brain vertex and in major cerebral artery perivascular spaces, as well as a significantly lower clearance rate of gadobutrol 24 h after contrast injection.15,17 In another study by the same group, immunohistochemical analysis of biopsy samples resected from frontal cortex of iNPH patients undergoing intracranial pressure monitoring, revealed reduced AQP4 expression in astrocytic endfeet compared to reference subjects.58 Though that finding does not directly link AQP4 mislocalization to reduced clearance in humans, it does support findings from animal studies and underscores the need for human trials combing histopathology with imaging of CSF flow.
Glymphatic dysfunction has also been linked to vascular dementia. In a rat model of multiple microinfarcts induced by injection of cholesterol crystals into the internal carotid artery, the enlargement of perivascular spaces, as well as AQP4 mislocalization, were both associated with the degree of cognitive impairment.59 These pathological changes occurred in conjunction with reduced CSF influx to the brain and impaired parenchymal clearance.59 Another research group found that glymphatic function was reduced at three days after infarction in mice, with severely impaired influx evident ipsilateral to multiple mini-strokes. After recovery for 14 days, glymphatic function was globally restored except in the microinfarct cores, where CSF solutes were retained at elevated concentrations compared to non-injured parenchyma (Fig. 4).60
Figure 4. Pathological changes to the glymphatic pathway.
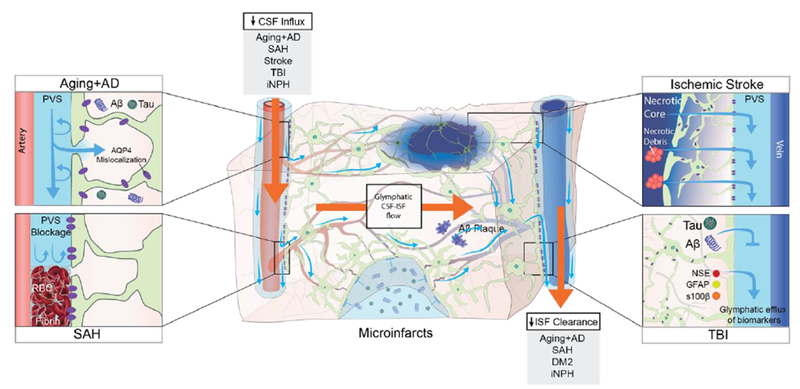
Aging and several diseases have been associated with a decrease in CSF influx to the glymphatic pathway and/or reduced clearance efficacy both in animals and in humans. In aging mice, the flow changes are likely caused by reduced vascular compliance, increased AQP4 expression and AQP4 mislocalization away from the astrocytic end-feet, which all cause reduced parenchymal influx of CSF.23 In a human postmortem study, AQP4 expression increased with age, albeit without AQP4 mislocalization.55 In murine models of AD, soluble and insoluble Aβ plaques provoke AQP4 mislocalization and impaired CSF influx.34,56 In AD patients, CSF clearance rate is reduced and exhibits an inverse relationship with Aβ levels.16 Post-mortem studies of AD patients identified AQP4 mislocalization and an increase in total AQP4 expression in AD patients compared to non-AD subjects.55 In hemorrhagic stroke in mice and gyrenchephalic non-human primates, blood components leaking into the PVS, especially fibrin/fibrinogen deposits, occlude the PVS, which leads to reduced CSF influx.61–63 In rodent models of ischemic stroke, necrotic cores are formed within the brain parenchyma, around which reactive astrocytes create a barrier (glial scar) to contain the injury and the toxic agents that form upon necrosis.64 Contents of the necrotic core leak through the permeable glial scar into the PVS.64 In mice, cerebral microinfarcts lead to a transient global reduction in glymphatic influx, and prolonged trapping of solutes within the infarct cores, probably due to reduced interstitial fluid turnover.60 TBI in mice leads to reduced glymphatic clearance, and biomarkers of the injured parenchyma are transported through the glymphatic pathway towards the cervical lymphatic system.42 In iNPH patients, glymphatic function is broadly impaired and characterized by both a delayed influx and a reduced clearance rate following intrathecal contrast injection.15 In rat models of diabetes mellitus type 2 (DM2), CSF tracers remain trapped within the brain parenchyma for prolonged periods, suggesting that perivenous efflux is decreased.68 This finding has not yet been replicated in humans, but we speculate that reduced brain clearance could contribute to the cognitive decline that is often seen in DM2 patients.
CSF, cerebrospinal fluid; ISF interstitial fluid, PVS: perivascular space; AQP4, aquaporin-4, AD, Alzheimer’s Disease; SAH, subarachnoid hemorrhage; TBI, traumatic brain injury; iNPH, idiopathic normalpressure hydrocephalus; DM2, diabetes mellitus type 2; GFAP, glial fibrillary acidic protein; S100B, S100 calcium binding protein; NSE, neuron-specific enolase.
Stroke
Hemorrhagic stroke
Mouse models as well as a gyrencephalic non-human primate model of subarachnoid hemorrhage (SAH) showed impaired CSF inflow along periarterial influx routes starting at 24 hours after the insult.61–63 This glymphatic inhibition was attributed to the occlusion of perivascular spaces by blood components, specifically fibrin/fibrinogen deposits; intraventricular delivery of the fibrinolytic, tissue-type plasminogen activator restored CSF flow in these rodents (Fig. 4).61,63 However, intracerebral hemorrhage induced by injecting collagenase into the right striatum of mice did not significantly affect glymphatic influx.61 Nonetheless, intraventricular fibrinolysis with atelplase will soon undergo clinical testing for treating delayed cognitive impairment after aneurysmal SAH (FIVHeMA trial: NCT03187405).63
Ischemic stroke
Rodent models of acute ischemic stroke impair CSF inflow in the ipsilateral cortex at three hours after middle cerebral artery occlusion (MCAO), as revealed by MRI and histological examination.61 Glymphatic influx had recovered 24 hours after spontaneous arterial recanalization.61 Interestingly, a murine study evaluating clearance of fluorescent tracers from the necrotic infarct core at seven weeks after a hypoxic MCAO showed that solutes trapped in the core leak through the glial scar and are transported from brain along perivascular spaces (Fig. 4).64 The ISF within the core contained elevated levels of pro-inflammatory cytokines and was toxic to cultured cortical and hippocampal neurons even at seven weeks after the MCAO, suggesting a beneficial role of glymphatic clearance in disease resolution.64 Cortical spreading depression, which is a mass depolarization of neurons initiating in the ischemic core potentially leading to neuronal death in surrounding hypoxic tissue after stroke, has also been implicated in impaired glymphatic flow.65 In mice, cortical spreading depression induced in the non-hypoxic brain leads to a transient complete closure of the perivascular spaces around both arteries and veins lasting up to ten minutes, followed by a gradual reopening that does not recover to the baseline caliber within the first 30 minutes, and leads to a reduction of interstitial flow.65 The glymphatic pathway’s involvement in stroke all stems from findings in animal research, and needs to be reproduced in human trials.
Traumatic brain injury
Traumatic brain injury (TBI) in mice leads to decreased glymphatic influx and impaired clearance of intracortically-injected radiotracers from the brain interstitium, persisting for more than 28 days after the injury.66 Impaired glymphatic function in AQP4 knockout mice exacerbated the resultant accumulation of phosphorylated tau and axonal degeneration after TBI – processes that lead to cognitive decline.66 Importantly, glymphatic efflux in murine brain may transport biomarkers of TBI to the general circulation.42 Thus, levels of GFAP, S100B and neuron-specific enolase (NSE) were elevated in blood serum at 18 hours after TBI in wild type mice, but inhibition of glymphatic efflux prevented the rise in these serum biomarkers (Fig. 4).42 In mice, the efflux of lactate from the brain parenchyma is also in part dependent on glymphatic efflux.43 If a similar glymphatic efflux of potential biomarkers occurs in humans after TBI, some of the interventions commonly applied in clinical practice, e.g. sleep deprivation due to frequent neurological examination and ventriculotomy after severe TBI, seem likely to inhibit glymphatic function, thus potentially interfering in biomarker efflux which could contribute to the difficulties in identifying a TBI blood test. 67
Conclusion and future directions
The characterization of the structural and biochemical components of the glymphatic pathway has brought new understanding of a highly organized fluid transport and clearance system in the CNS. The pathway entails a convective inflow of CSF within periarterial spaces and convective outflow of CSF-ISF towards perivenous spaces, which results in directionalized fluid flow through the brain parenchyma. Facilitated by polarized AQP4 expression on astroctytic end-feet, glymphatic flow rids the interstitium of potentially toxic metabolites.12 The pathway is especially active during sleep, when clearance rate of exogenous tracers more than doubles.14 While the pathway was delineated in rodents, an increasing number of human studies have supported the anatomical, physiological and pathological mechanisms underlying flow in the glymphatic pathway. In this Rapid Review, we present the involvement of the glymphatic pathway in dementia, stroke and TBI. However, glymphatic pathway dysfunction is also evident in animal models of diabetes mellitus, depression, migraine with aura, multiple sclerosis and chronic alcohol use (Fig. 4).31,65,68–70 Significant inter-individual differences in CSF inflow are seen in healthy humans,15 and we speculate that a trait of low glymphatic flow may contribute to the individual risk of developing sporadic neurodegenerative diseases. Conversely, glymphatic flow and clearance rates are increased in rodents after physical exercise, changes in body posture, intake of omega-3 polyunsaturated fatty acids, and low dose alcohol (0.5 g/kg), providing several, easily-implemented, interventions.29,44,70–72 Much work remains in charting out the details of the human glymphatic pathway, identifying factors that impair or enhance its function, as well as mapping the time-course of pathological changes in glymphatic function during human disease. If the glymphatic pathway proves as significant for human brain homeostasis as in rodents, these innovations could give rise to new prognostic and diagnostic tools as well as novel therapeutic targets.
Search strategy and selection criteria
A literature search was conducted on PubMed using the keywords “glymphatic”, “glial lymphatic”, “dural lymphatics”, “brain clearance”, and “perivascular spaces”. The search identified more than 2000 studies in rodents, non-human primates, and humans. Only studies published after January 2015 and in the English language were included in this review. Seminal papers relevant to the review that were published before January 2015 were also included. The final reference list was generated based on relevance to the topic covered in this Rapid Review.
Acknowledgements
The authors have received funding from the Cure Alzheimer Fund, Lundbeck Foundation, the Novo Nordisk Foundation, the National Institute of Neurological Disorders and Stroke, the National Institute of Aging, Foundation Leducq Transatlantic Networks of Excellence program, the European Union’s Horizon 2020 research and innovation programme under grant agreement No 666881 (SVDs@target), and the Joint Programming Neurodegenerative Disease (JPND), DACAPO-AD. We would like to thank Dan Xue for assistance with illustrations, and Prof. Paul Cumming for comments on the manuscript.
Glossary
- Glossary of Glymphatic Pathway Terminology
The glymphatic pathway constitutes three distinct anatomical regions, including the periarterial and perivenous spaces, as well as the interposed brain parenchyma (Fig. 1).12 Fluid flow within the pathway is dependent on cerebrospinal fluid from the subarachnoid space being driven into the periarterial space.12 Within each anatomical region of the pathway, distinct factors contribute to the fluid dynamics, some of which are not yet fully understood.14,38,39 Terminology developed during the study of the glymphatic pathway broadly covers overlapping anatomical regions and the associated fluid dynamics. For the benefit of readers new to this field we explain this terminology below.
- Glymphatic function
refers to the entire pathway and the flow efficiency within it. As the glymphatic pathway distributes constituents of the cerebrospinal fluid to the entire brain12 and clears exogenously injected or endogenous metabolites,12,34 the term glymphatic function broadly refers to the efficacy in which cerebrospinal fluid distributes its solvents to the brain parenchyma and the clearance rate of parenchymal substrates.
- Glymphatic influx
refers to the efficiency in which CSF distribute endogenous contents or injected tracers to the brain parenchyma in both experimental animals and in human brain. Changes can be caused by reduced inflow in the periarterial space, or by congestion in the downstream segments, including the interstitial space and perivenous efflux. Mechanisms are still far from understood but reduced arterial compliance in aging or extracellular space volume during wakefulness can reduce periarterial inflow.14,23
- Glymphatic efflux
refers to the efficiency by which endogenous metabolites or tracers injected into the cerebrospinal fluid or brain parenchyma are cleared from brain by fluid flow. Mechanisms underlying impaired glymphatic efflux are not fully understood but are likely related to reduced perivenous or perineuronal efflux as well as slow or reduced flow in the entirety of the glymphatic pathway. Impaired glymphatic efflux have been observed in human patients suffering from idiopathic normal pressure hydrocephalus.15
Footnotes
Declaration of interests
The authors declare no conflict of interest.
Bibliography
- 1.Pollay M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res 2010; 7: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown RH, Al-Chalabi A. Amyotrophic Lateral Sclerosis. N Engl J Med 2017; 377(2): 162–72. [DOI] [PubMed] [Google Scholar]
- 3.Sevigny J, Chiao P, Bussiere T, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature 2016; 537(7618): 50–6. [DOI] [PubMed] [Google Scholar]
- 4.Peng C, Gathagan RJ, Covell DJ, et al. Cellular milieu imparts distinct pathological alpha-synuclein strains in alpha-synucleinopathies. Nature 2018; 557(7706): 558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem 2015; 84: 435–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hjerpe R, Bett JS, Keuss MJ, et al. UBQLN2 Mediates Autophagy-Independent Protein Aggregate Clearance by the Proteasome. Cell 2016; 166(4): 935–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thibaudeau TA, Anderson RT, Smith DM. A common mechanism of proteasome impairment by neurodegenerative disease-associated oligomers. Nat Commun 2018; 9(1): 1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarasoff-Conway JM, Carare RO, Osorio RS, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 2015; 11(8): 457–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casley-Smith JR, Foldi-Borcsok E, Foldi M. The prelymphatic pathways of the brain as revealed by cervical lymphatic obstruction and the passage of particles. British Journal of Experimental Pathology 1976; 57: 179–88 [PMC free article] [PubMed] [Google Scholar]
- 10.Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a Paravascular Fluid Circulation in the Mammalian Central Nervous-System, Provided by the Rapid Distribution of Tracer Protein Throughout the Brain from the Subarachnoid Space. Brain Research 1985; 326(1): 47–63. [DOI] [PubMed] [Google Scholar]
- 11.Cserr HF, Cooper DN, Milhorat TH. Flow of cerebral interstitial fluid as indicated by the removal of extracellular markers from rat caudate nucleus. Experimental Eye Research 1977; 25: 461–73. [DOI] [PubMed] [Google Scholar]
- 12.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012; 4(147): 147ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iliff JJ, Lee H, Yu M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. Journal of Clinical Investigation 2013; 123(3): 1299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013; 342(6156): 373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ringstad G, Vatnehol SAS, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 2017; 140(10): 2691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Leon MJ, Li Y, Okamura N, et al. Cerebrospinal Fluid Clearance in Alzheimer Disease Measured with Dynamic PET. J Nucl Med 2017; 58(9): 1471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ringstad G, Valnes LM, Dale AM, et al. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight 2018; 3(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 2015; 212(7): 991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015; 523(7560): 337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Q, Ineichen BV, Detmar M, Proulx ST. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun 2017; 8(1): 1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannocks M-J, Pizzo ME, Huppert J, et al. Molecular characterization of perivascular drainage pathways in the murine brain. Journal of Cerebral Blood Flow & Metabolism 2017: 0271678X1774968-0271678X1774968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pizzo ME, Wolak DJ, Kumar NN, et al. Intrathecal antibody distribution in the rat brain: surface diffusion, perivascular transport and osmotic enhancement of delivery. J Physiol 2018; 596(3): 445–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kress BT, Iliff JJ, Xia M, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 2014; 76(6): 845–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res 2004; 1(1): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Absinta M, Ha SK, Nair G, et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holter KE, Kehlet B, Devor A, et al. Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc Natl Acad Sci U S A 2017; 114(37): 9894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith AJ, Yao X, Dix JA, Jin BJ, Verkman AS. Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife 2017; 6: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murlidharan G, Crowther A, Reardon RA, Song J, Asokan A. Glymphatic fluid transport controls paravascular clearance of AAV vectors from the brain. JCI Insight 2016; 1(14): e88034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren H, Luo C, Feng Y, et al. Omega-3 polyunsaturated fatty acids promote amyloid-beta clearance from the brain through mediating the function of the glymphatic system. FASEB J 2017; 31(1): 282–93. [DOI] [PubMed] [Google Scholar]
- 30.Luo C, Yao X, Li J, et al. Paravascular pathways contribute to vasculitis and neuroinflammation after subarachnoid hemorrhage independently of glymphatic control. Cell Death Dis 2016; 7: e2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia M, Yang L, Sun G, Qi S, Li B. Mechanism of depression as a risk factor in the development of Alzheimer’s disease: the function of AQP4 and the glymphatic system. Psychopharmacology (Berl) 2017; 234(3): 365–79. [DOI] [PubMed] [Google Scholar]
- 32.Achariyar TM, Li B, Peng W, et al. Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol Neurodegener 2016; 11(1): 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci (Lond) 2017; 131(17): 2257–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Z, Xiao N, Chen Y, et al. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Molecular Neurodegeneration 2015; 10(1): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG. The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’ system? Acta Neuropathol 2018; 135(3): 387–407. [DOI] [PubMed] [Google Scholar]
- 36.Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest 2017; 127(9): 3210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plog BA, Nedergaard M. The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu Rev Pathol 2018; 13: 379–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iliff JJ, Wang M, Zeppenfeld DM, et al. Cerebral Arterial Pulsation Drives Paravascular CSF-Interstitial Fluid Exchange in the Murine Brain. Journal of Neuroscience 2013; 33(46): 18190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bedussi B, Almasian M, de Vos J, VanBavel E, Bakker EN. Paravascular spaces at the brain surface: Low resistance pathways for cerebrospinal fluid flow. J Cereb Blood Flow Metab 2017: 271678X17737984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiviniemi V, Wang X, Korhonen V, et al. Ultra-fast magnetic resonance encephalography of physiological brain activity - Glymphatic pulsation mechanisms? J Cereb Blood Flow Metab 2016; 36(6): 1033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dreha-Kulaczewski S, Joseph AA, Merboldt KD, Ludwig HC, Gartner J, Frahm J. Inspiration Is the Major Regulator of Human CSF Flow. Journal of Neuroscience 2015; 35(6): 2485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plog BA, Dashnaw ML, Hitomi E, et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci 2015; 35(2): 518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lundgaard I, Lu ML, Yang E, et al. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J Cereb Blood Flow Metab 2017; 37(6): 2112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee H, Xie L, Yu M, et al. The Effect of Body Posture on Brain Glymphatic Transport. J Neurosci 2015; 35(31): 11034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Japanese Journal of Radiology 2017; 35(4): 172–8. [DOI] [PubMed] [Google Scholar]
- 46.Eide PK, Ringstad G. MRI with intrathecal MRI gadolinium contrast medium administration: a possible method to assess glymphatic function in human brain. Acta Radiol Open 2015; 4(11): 2058460115609635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shokri-Kojori E, Wang G-J, Wiers CE, et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proceedings of the National Academy of Sciences 2018: 201721694-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Gu BJ, Masters CL, Wang YJ. A systemic view of Alzheimer disease - insights from amyloid-beta metabolism beyond the brain. Nat Rev Neurol 2017; 13(10): 612–23. [DOI] [PubMed] [Google Scholar]
- 49.Brothers HM, Gosztyla ML, Robinson SR. The Physiological Roles of Amyloid-beta Peptide Hint at New Ways to Treat Alzheimer’s Disease. Front Aging Neurosci 2018; 10: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar DK, Choi SH, Washicosky KJ, et al. Amyloid-beta peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med 2016; 8(340): 340ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burfeind KG, Murchison CF, Westaway SK, et al. The effects of noncoding aquaporin-4 single-nucleotide polymorphisms on cognition and functional progression of Alzheimer’s disease. Alzheimers Dement (N Y) 2017; 3(3): 348–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rainey-Smith SR, Mazzucchelli GN, Villemagne VL, et al. Genetic variation in Aquaporin-4 moderates the relationship between sleep and brain Abeta-amyloid burden. Transl Psychiatry 2018; 8(1): 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ju YES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology - a bidirectional relationship. Nat Rev Neurol 2015; 10(2): 115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ju YS, Ooms SJ, Sutphen C, et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-beta levels. Brain 2017; 140(8): 2104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeppenfeld DM, Simon M, Haswell JD, et al. Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol 2017; 74(1): 91–9. [DOI] [PubMed] [Google Scholar]
- 56.Peng W, Achariyar TM, Li B, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis 2016; 93(2016): 215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eide PK, Ringstad G. Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: A glymphatic magnetic resonance imaging study. Journal of Cerebral Blood Flow & Metabolism 2018: 0271678X1876097-0271678X1876097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eide PK, Hansson HA. Astrogliosis and impaired aquaporin-4 and dystrophin systems in idiopathic normal pressure hydrocephalus. Neuropathol Appl Neurobiol 2017. [DOI] [PubMed] [Google Scholar]
- 59.Venkat P, Chopp M, Zacharek A, et al. White matter damage and glymphatic dysfunction in a model of vascular dementia in rats with no prior vascular pathologies. Neurobiol Aging 2017; 50: 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang M, Ding F, Deng S, et al. Focal Solute Trapping and Global Glymphatic Pathway Impairment in a Murine Model of Multiple Microinfarcts. The Journal of Neuroscience 2017; 37(11): 2870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaberel T, Gakuba C, Goulay R, et al. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: A new target for fibrinolysis? Stroke 2014; 45(10): 3092–6. [DOI] [PubMed] [Google Scholar]
- 62.Golanov EV, Bovshik EI, Wong KK, et al. Subarachnoid hemorrhage – Induced block of cerebrospinal fluid flow: Role of brain coagulation factor III (tissue factor). Journal of Cerebral Blood Flow & Metabolism 2017: 0271678X1770115-0271678X1770115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goulay R, Flament J, Gauberti M, et al. Subarachnoid Hemorrhage Severely Impairs Brain Parenchymal Cerebrospinal Fluid Circulation in Nonhuman Primate. Stroke 2017; 48(8): 2301–5. [DOI] [PubMed] [Google Scholar]
- 64.Zbesko JC, Nguyen T-VV, Yang T, et al. Glial scars are permeable to the neurotoxic environment of chronic stroke infarcts. Neurobiology of Disease 2018; 112(December 2017): 63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schain AJ, Melo-Carrillo A, Strassman AM, Burstein R. Cortical Spreading Depression Closes Paravascular Space and Impairs Glymphatic Flow: Implications for Migraine Headache. J Neurosci 2017; 37(11): 2904–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iliff JJ, Chen MJ, Plog BA, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 2014; 34(49): 16180–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Plog BA, Nedergaard M. Why have we not yet developed a simple blood test for TBI? Expert Review of Neurotherapeutics 2015; 15(5): 465–8. [DOI] [PubMed] [Google Scholar]
- 68.Jiang Q, Zhang L, Ding G, et al. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab 2017; 37(4): 1326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fournier AP, Gauberti M, Quenault A, Vivien D, Macrez R, Docagne F. Reduced spinal cord parenchymal cerebrospinal fluid circulation in experimental autoimmune encephalomyelitis. J Cereb Blood Flow Metab 2018: 271678X18754732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lundgaard I, Wang W, Eberhardt A, et al. Beneficial effects of low alcohol exposure, but adverse effects of high alcohol intake on glymphatic function. Sci Rep 2018; 8(1): 2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He XF, Liu DX, Zhang Q, et al. Voluntary Exercise Promotes Glymphatic Clearance of Amyloid Beta and Reduces the Activation of Astrocytes and Microglia in Aged Mice. Front Mol Neurosci 2017; 10(May): 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.von Holstein-Rathlou S, Petersen NC, Nedergaard M. Voluntary running enhances glymphatic influx in awake behaving, young mice. Neurosci Lett 2018; 662(September 2017): 253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


