Abstract
Tropical reefs are commonly transitioning from coral- to macroalgal-dominance, producing abrupt, and often lasting, shifts in community composition and ecosystem function. Although negative effects of macroalgae on corals are well documented, whether such effects vary with spatial scale or the density of macroalgae remains inadequately understood, as does the legacy of their impact on coral growth. Using closely adjacent coral- versus macroalgal-dominated areas, we tested effects of macroalgal competition on the Indo-Pacific corals Acropora millepora and Porites cylindrica. When corals were transplanted to areas of: i) macroalgal-dominance, ii) macroalgal-dominance but with nearby macroalgae removed, or iii) coral-dominance lacking macroalgae, coral growth was equivalently high in plots without macroalgae and low (62–90% less) in plots with macroalgae, regardless of location. In a separate experiment, we raised corals above the benthos in each area and exposed them to differing densities of the dominant macroalga Sargassum polycystum. Coral survivorship was high (≥ 93% after 3 months) and did not differ among treatments, whereas the growth of both coral species decreased as a function of Sargassum density. When Sargassum was removed after two months, there was no legacy effect of macroalgal density on coral growth over the next seven months; however, there was no compensation for previously depressed growth. In sum, macroalgal impacts were density dependent, occurred only if macroalgae were in close contact, and coral growth was resilient to prior macroalgal contact. The temporal and spatial constraints of these interactions suggest that corals may be surprisingly resilient to periodic macroalgal competition, which could have important implications for ecosystem trajectories that lead to reef decline or recovery.
Keywords: coral reef, macroalgae, coral-algal competition, Fiji
INTRODUCTION
Local and global disturbances are negatively impacting foundation species and altering ecological communities in ways that reduce ecosystem function and services (Scheffer et al. 2001, Folke et al. 2004). These ecosystem shifts represent a fundamental change in the structure and function of these systems and once established, many ecosystem shifts are difficult to reverse (Scheffer & Carpenter 2003, Folke et al. 2004). Conceptual models suggest that the stability of the different state arises from interactions among elements of the new state that form feedbacks, reinforcing and maintaining the state (Mumby & Steneck 2008; Hughes et al. 2010). Despite their potential importance, there is a critical gap in our knowledge of feedback mechanisms, how they build or erode the resilience of ecosystems, and the time courses over which they establish or weaken. This understanding is required to predict, avoid, and reverse undesirable ecosystem shifts.
On tropical reefs, corals provide topographically complex habitat for hundreds of thousands of species (Fisher et al. 2015) and economic goods and services for millions of people (Moberg & Folke 1999). However, recent natural and human-induced stressors (Harvell et al. 2007, Hoegh-Guldberg et al. 2007, Hughes et al. 2017) have decimated these foundation species, with many reefs transitioning to structurally simplified systems with low coral cover and increased cover of macroalgae that compete with corals (Mumby & Steneck 2008, Hughes et al. 2010). As competitive interactions between corals and macroalgae increase, macroalgae are expected to hasten coral decline, limit coral recovery (Burkepile & Hay 2008, Mumby & Steneck 2008, Hughes et al. 2010), and enhance macroalgal resilience via positive feedbacks (Hoey & Bellwood 2011, Dell et al. 2016, van de Leemput 2016). Macroalgae can harm corals via physical mechanisms such as shading, abrasion, and overgrowth (McCook et al. 2001), chemical mechanisms such as allelopathy (Rasher et al. 2011, Vieira et al. 2016), suppression of coral settlement (Kuffner et al. 2006, Paul et al. 2011, Dixson et al. 2014), or disruption of coral microbiomes that protect against coral pathogens (Nugues et al. 2004, Smith et al. 2006, Barott et al. 2012, Zaneveld et al. 2016). Macroalgae also alter coral interactions with corallivores (Wolf & Nugues 2013, Clements & Hay 2015, Brooker et al. 2016).
Despite evidence that macroalgal competition harms corals (McCook et al. 2001, Birrell et al. 2008), there are few field-based manipulative experiments investigating the long-term consequences of macroalgal competition for coral fitness (e.g., Box & Mumby 2007, Hughes et al. 2007, Ferrari et al. 2012a). Especially lacking are investigations of how the density of macroalgae, and the proximity to natural, multispecies assemblages of macroalgae common to degraded reefs affect corals. Studies to date have focused primarily on the impacts of an individual macroalga on an individual coral, rather than how impacts vary with macroalgal density or when contacting single species (experimentally) versus the multispecies assemblages that occur in the field.
We conducted manipulative field experiments to investigate the long-term effects of macroalgal competition on growth and survivorship of the corals Acropora millepora and Porites cylindrica, both common to Indo-Pacific reefs. We used a coral-dominated, no-take Marine Protected Area (MPA) and adjacent macroalgal-dominated fished area to investigate: 1) the long-term effects of differing macroalgal cover on coral growth and survivorship, 2) whether a history of macroalgal presence altered coral resistance or resilience to competition, 3) the effects of algal density on coral growth and survivorship, and 4) the resilience of coral growth following algal removal.
MATERIALS AND METHODS
Study site and organisms
This study was conducted within neighboring sections of shallow (1.5–2.5 m deep) lagoonal back reefs that were either coral-dominated (a no-take MPA) or macroalgal-dominated (a fished area) at Votua Village along the Coral Coast of Viti Levu, Fiji (18° 13.05’S, 177°42.97’E). Both areas are similar in depth and physical regimes, but differ in reef community assemblages, which diverged from a similar benthic state across the entire area when the MPA was established about ~11 years before our experiment (Simpson 2010). Within the MPA, corals are now abundant (~55% cover) and macroalgae rare (< 3%) on hard substrates, while the fished area supports few corals (~4% cover), few herbivorous fishes, and high cover of macroalgae (~91%; Rasher et al. 2013).
Our study consisted of two field-based manipulative experiments assessing the long-term (3–9 month duration) effects of macroalgae on coral growth and survivorship. In each case, we used the corals Acropora millepora and Porites cylindrica (hereafter Acropora and Porites), which are common on reefs throughout the Indo-Pacific and are representative of coral families differing in growth rates (Darling et al. 2012) and tolerances to various stressors (e.g., macroalgal allelopathy, Acanthaster spp. predation, bleaching; Pratchett 2007, Rasher et al. 2011, Bonaldo & Hay 2014).
Influence of proximity to natural macroalgal assemblages on coral growth and survival
To determine the effect of natural macroalgal assemblages and environmental legacy effects on coral growth and survivorship, we conducted a reciprocal transplant experiment using corals from the macroalgal- versus coral-dominated areas. Corals collected from each area were reciprocally transplanted to benthic plots (0.5 × 0.5 m) in each area where macroalgae were either (i) naturally present (macroalgal-dominated area), (ii) routinely removed at ~3-week intervals (macroalgal-dominated area) or (iii) naturally absent (coral-dominated area) (Fig. S1). In December 2013, five branches (6–8 cm in length) were collected from each of 20 colonies of Acropora and Porites within the coral-dominated MPA and macroalgal-dominated fished area at Votua Reef (100 branches species−1 area−1). Individual branches were affixed into the cut-off necks of inverted plastic bottles using epoxy (Emerkit) and the screw-off top of bottles was secured, inverted, to the substrate with a nail (see Video S1 in Supplementary Material for example of this experimental method). This procedure allowed us to easily transplant individuals to our benthic plots and to detach and reattach them for periodic weighing with minimal disturbance. Corals were initially interspersed on galvanized metal racks (~1.5 m water depth, and 0.75 m above the substratum) in their area of origin for ~1-month to allow acclimation and recovery from fragmentation. During this time, we established a series of twenty benthic plots for each of the three treatments (i.e., macroalgae present, macroalgae removed, macroalgae naturally absent), each of which were interspersed haphazardly within a ~100 m stretch of reef at ~1.5 m depth and marked with flagging tape. Adjacent plots were separated by a minimum of ~4 m. Following the recovery period, one branch from each colony of each species (Acropora and Porites) and each area (coral- and macroalgal-dominated) was haphazardly selected and allocated to a plot within each treatment (4 branches plot−1 treatment−1). Corals were screwed into one of four bottle caps haphazardly embedded within the benthos near the center of their designated plot. Bottle caps, and hence corals, within each plot were separated by ~15–20 cm. This reciprocal transplantation allowed us to compare whether corals responded differently when grown in plots without macroalgae in the coral- and macroalgal-dominated areas, and whether a coral’s environmental legacy (i.e., originating in the coral- or macroalgal-dominated area) influenced its performance in different plots and/or areas.
Coral growth and survivorship were monitored at five intervals over the 36 weeks between 22 January and 4 October 2014 (Fig. S3). Corals were ‘unscrewed’ from the substratum and weighed in the field using an electronic scale (OHAUS Scout Pro) enclosed within a plastic container that was mounted to a tripod holding it above the water surface. Twenty-four to 48 hours before weighing sessions, each coral’s bottle-top/epoxy base was lightly brushed to remove fouling organisms. During weighing sessions, each coral was gently shaken 30 times to remove excess water, weighed, and then immediately placed back into the water and reattached to the substrate. At the end of the experiment, corals were separated from their epoxy base and each coral and base weighed separately. This allowed the relative change in coral mass (as a percentage of initial mass) to be determined for each sampling period.
Differences in growth (% change in mass) among surviving conspecifics of different locations (macroalgal- versus coral-dominated area), plots (macroalgae present versus absent), and origins (macroalgal- versus coral-dominated area) were assessed using the “compareGrowthCurves” function in the R (version 3.3.2) package “statmod”. P-values were adjusted for multiple pairwise comparisons using Hommel’s method. Differences in total mortality among conspecifics of different locations, plots, and origins were compared using Fisher’s exact tests, with p-values adjusted for multiple contrasts using the Bonferroni method.
During each assessment of coral mass, we simultaneously surveyed the percent cover and canopy height of macroalgae immediately surrounding corals within plots where macroalgae were not removed to document changes in the benthic community that might affect coral growth (for methods, see Supplementary Material), such as seasonal changes in abundance of macroalgal species like Sargassum that dominate the fished area (Rasher et al. 2013, Dell et al. 2016).
Influence of Sargassum density on coral growth
To investigate the effect of macroalgal density on the growth and survivorship of corals, we exposed branches of Acropora and Porites to different densities of Sargassum polycystum for 3 months. Sargassum is a canopy-forming macroalga that dominated macroalgal assemblages (71–94%) in our benthic plots and is abundant on degraded reefs in Fiji and worldwide (e.g., Hughes 1994, Ledlie et al. 2007, Rasher et al. 2013, Chong-Seng et al. 2014). To create standardized units of Sargassum-coral contact, 6–8 cm length branches of Acropora and Porites corals were collected from colonies within both the macroalgal- and coral-dominated areas of Votua Reef (15 colonies species−1 area−1) and individually epoxied into the cut-off necks of inverted plastic bottles during November 2013 (as described above). Each coral and its epoxy/bottle-top base was then screwed into a bottle cap embedded within a cement cone and interspersed on one of four galvanized metal racks (Fig. S1), positioned so that rack tops were about 50 cm above the reef substratum and at ~1 m depth during low tide. Racks were located in the area where the coral was collected (i.e., transplants were not reciprocal), but were elevated above the reef substrata to isolate corals from confounding factors associated with the benthos (e.g., sand scour, benthic predators). Corals were allowed to acclimate for ~1 month, after which they were exposed to one of four algal treatments.
In December 2013, whole Sargassum thalli (length = 15–20 cm) were collected from the macroalgal-dominated area and either 0, 1, 3, or 6 thalli were inserted into a three-stranded rope (length = 18–20 cm) that was slipped over two 4-cm nails embedded 180° apart on the upper surface of the cement cone (following Rasher & Hay 2010, see Fig. S2). The base of each Sargassum thallus was held 2–4 cm from the coral, such that the thallus was lightly contacting the experimental corals. All racks were caged with 1 cm2-grid galvanized metal mesh to exclude large herbivorous fishes, and all cages were brushed weekly to remove fouling organisms. During weekly maintenance, any Sargassum displaced from the ropes (e.g., because of wave action) was replaced. Sargassum density treatments were applied to corals for three months (December 19–20, 2013 to March 15–16, 2014), and the mass of corals (including their epoxy/bottle-top base) were assessed after two (February 13–14) and three months of contact. At the end of this three-month period, all algae were removed (as was the mesh caging), and the corals were maintained for a further six months to evaluate any legacy effects of past macroalgal contact (Fig. S3). After six months of further growth with no macroalgal contact, each coral was separated from its base, and the bases and corals were weighed separately to allow relative growth rates to be calculated.
To compare the effects of Sargassum density and coral origin (coral- and macroalgal-dominated area) on coral growth, differences in relative growth (as percentage of initial weight) at three months (the algal density treatment) and nine months (six months following algal removal), as well as the total change in mass (g) for Acropora and Porites during the entire nine-month experiment, were analyzed using generalized least square (GLS) models in R (v. 3.3.2) (R Core Team 2016) with the package nlme (Pinheiro et al. 2017). In each case, we used model selection to sequentially test nested GLS models via likelihood ratio tests to obtain the optimal fixed structure for each model (following Zuur et al. 2009). When necessary, the varIdent argument was used to control for heteroscedasticity. Following model selection, the significance of remaining fixed terms was tested using likelihood ratio tests. Subsequent multiple comparisons of means were performed using the generalized linear hypothesis test (glht) and Tukey (HSD) test in the multcomp package (Hothorn et al. 2008).
RESULTS
Coral growth and survival in plots with versus without natural macroalgal assemblages
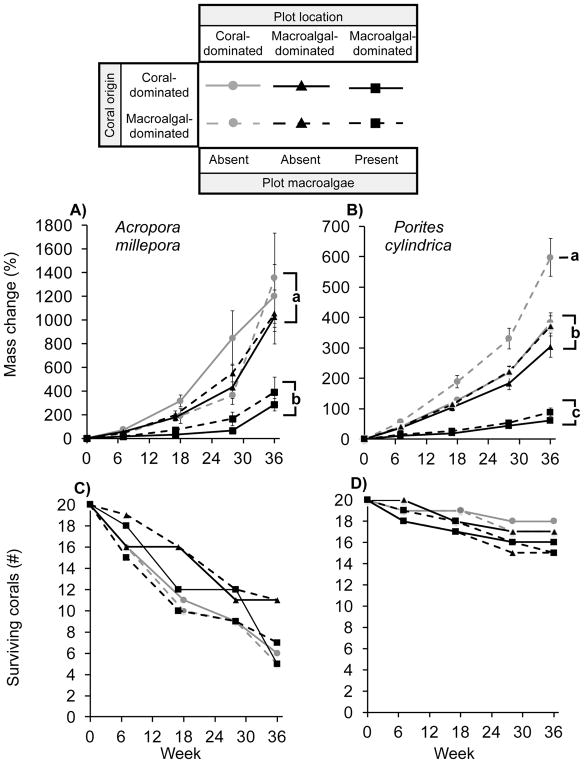
When transplanted to benthic plots, surviving Acropora increased in mass by ~11.3–14.5 × over the 36-week period if they were not surrounded (≥15 cm) by macroalgae (i.e., macroalgae removed and macroalgae absent plots). By contrast, Acropora surrounded by natural macroalgal assemblages increased in mass by only ~3.9–4.9 x (a 57–72% reduction in growth). These patterns were unaffected by coral origin or location to which they were transplanted, so long as macroalgae had been removed within ~15 cm of the transplants (Fig. 1A). Similarly, surviving Porites in plots without macroalgae increased in mass ~4.0–7.0 x, while those surrounded by macroalgae increased only ~1.6–1.9 x (a 52–77% reduction; Fig. 1B). Interestingly, Porites from the macroalgal-dominated area that were transplanted to the coral-dominated area exhibited 1.6–2 x greater growth than Porites in areas cleared of macroalgae or Porites collected from, and transplanted to, the coral-dominated area (Fig. 1B).
Fig. 1.
(Top) Percentage change in coral mass (mean ± SE) during a 36-week period (January–October 2014) for Acropora millepora (A) and Porites cylindrica (B) originally from the coral- or macroalgal-dominated area that were embedded within coral- or macroalgal-dominated area plots (with natural algal assemblages either left in place or physically removed within the fished area location). Growth differences among conspecifics were analyzed using the “compareGrowthCurves” function in the R package “statmod.” Letters to the right of lines indicate significant groupings via Hommel’s method (p < 0.05). (Bottom) The number of Acropora (C) and Porites (D) that survived throughout the duration of the experiment. Survival did not differ significantly as a function of treatment for either species.
After 36 weeks, the mortality of Acropora (45–75%, 9–15 of 20 individuals per treatment) was greater than that of Porites (10–25%, 2–5 of 20; p < 0.001; Fisher Exact test), but did not differ among treatments for either species (Acropora: p = 1.000–0.105, Porites: p = 1.000–0.408, Fisher Exact tests; Fig. 1C & D).
During our experiment, percent cover of macroalgae surrounding corals in the macroalgal-dominated area where we did not remove macroalgae ranged from 81–97%, with Sargassum accounting for ~71–94% of total cover (Fig. S4). Macroalgal cover and canopy height were greatest when sampled in the Austral summer (January and March) and lowest during the Austral winter (May and August) (Fig. S4). Macroalgal cover or height in the coral-dominated area or in our removal treatments was not measured because it was always minimal; we visually estimated cover and height in these areas as below 1% and 0.5 cm, respectively.
Influence of Sargassum density on coral growth
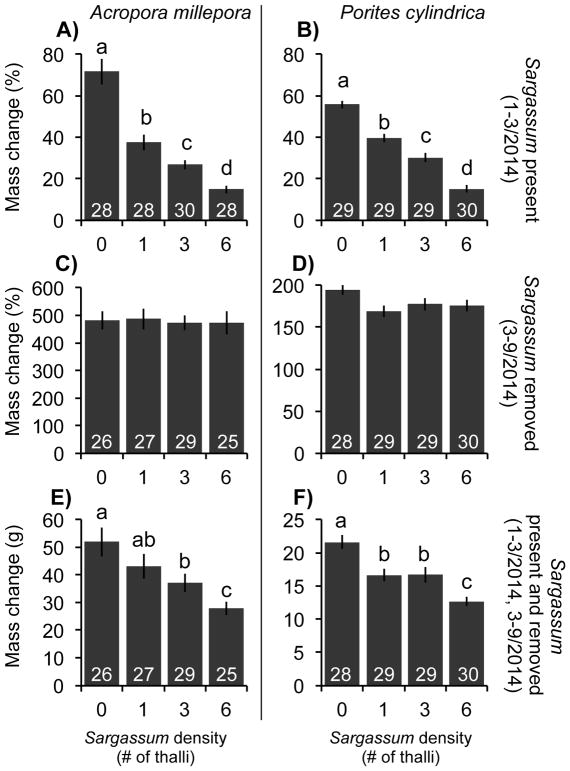
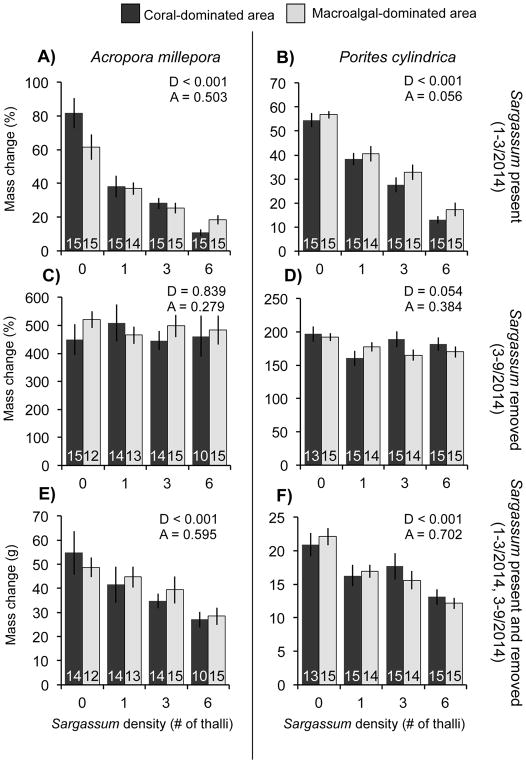
When surrounded by Sargassum on experimental racks, growth of Acropora and Porites strongly decreased with increasing Sargassum density (p < 0.001; Fig. 2A & B); effects did not vary by coral origin (L = 0.449, p = 0.503 for Acropora, L = 3.661, p = 0.056 for Porites; Fig. 3A). The presence of a single Sargassum thalli reduced Acropora growth by ~48% compared to Acropora without macroalgae (Fig. 2A). Increasing the density of Sargassum to three and six thalli reduced growth by a further ~15% in each case (Fig. 2A). Growth of Porites adjacent to one Sargassum thalli was reduced by ~29% compared to Porites without Sargassum, while 3- and 6-fold increases in the density of Sargassum reduced growth by ~16 and 27%, respectively (Fig. 2B). Survivorship was high for both species; only 5% of Acropora and 2% of Porites died during this three-month period (Fig. 2A & B).
Fig. 2.
(Top) Percentage change in mass (mean ± SE) for the corals Acropora millepora (A) and Porites cylindrica (B) over two months (January–March 2014) of contact with differing densities of Sargassum polycystum. (Middle) Percentage change in mass (mean ± SE) during March–September 2014 for Acropora (C) and Porites (D) previously exposed to different densities of surrounding Sargassum, but with no Sargassum present during this period of growth assessment. (Bottom) Total mass change (g) (mean ± SE) during January–September 2014 for Acropora (E) and Porites (F) initially exposed to different densities of Sargassum for three months (December-March 2014), but with Sargassum then removed and absent for the next 7 months (March–September 2014). For all graphs, data for each species were analyzed using generalized least-squares (GLS) models. Letters denote significant differences (p < 0.05) among algal density treatments via Tukey tests. Numbers within bars indicate sample size.
Fig. 3.
(Top) Percentage change in mass (mean ± SE) for the corals Acropora millepora (A) and Porites cylindrica (B) over two months (January–March 2014) of contact by differing densities of Sargassum polycystum. (Middle) Percentage change in mass (mean ± SE) during March–September 2014 for Acropora (C) and Porites (D) previously exposed to different densities of surrounding Sargassum, but with no Sargassum present during this period of growth assessment. (Bottom) Total mass change (g) (mean ± SE) during January–September 2014 for Acropora (E) and Porites (F) initially exposed to different densities of Sargassum for two months (January–March 2014), but with Sargassum then removed and absent for the next 7 months (March–September 2014). For all graphs, data for each species were analyzed by sequentially testing nested GLS models via likelihood ratio tests to obtain the optimal fixed structure for each model. P-values from these analyses are presented above each figure. D = inclusion of Sargassum density as a fixed term; A = inclusion of area (coral- or macroalgal-dominated area) as a fixed term. Numbers within bars indicate sample size.
Six months after the removal of the Sargassum treatments, the absolute growth (g increase) of each species was still depressed as a function of past Sargassum density (p < 0.001; Fig. 2E & F), but did not vary by coral origin (L = 0.282, p = 0.595 for Acropora, L = 0.146, p = 0.702 for Porites; Fig. 3E & F), thus resembling patterns established during the first three months when Sargassum was present. However, once the size of the corals at three months was taken into account, the relative growth rates (% growth) after Sargassum was removed did not differ as a function of previous Sargassum density (L = 0.844, p = 0.839 for Acropora, L = 7.650, p = 0.054 for Porites; Fig. 2C & D) or coral origin (L = 1.171, p = 0.279 for Acropora, L = 0.759, p = 0.384 for Porites; Fig. 3C & D). Eighty-nine percent of Acropora and 97% of Porites on the racks survived through the entire experimental period (Fig. 2E & F); this was considerably greater than the 25–55% survival of Acropora and the 75–90% survival of Porites on the natural substrate over this time period (Fig. 1C & D).
DISCUSSION
Resolving the temporal and spatial scales at which macroalgae can negatively impact corals is critical for predicting, avoiding, and reversing phase-shifts on reefs (Mumby & Steneck 2008, Hughes et al. 2010, Graham et al. 2013). We found (1) macroalgae had a dramatic effect on coral growth, irrespective of previous macroalgal exposure or whether corals were located within coral- or macroalgal-dominated reefs, (2) negative effects on coral growth increased with increasing macroalgal density, and (3) these effects were broadly consistent for the two taxonomically-disparate corals we investigated; however, (4) negative growth effects were eliminated if macroalgae within about 15 cm were removed, and (5) the rate at which macroalgal effects on corals commence or cease were immediate. Together, these findings have implications for understanding the spatial and temporal scales at which feedbacks form and are broken.
Reefs may shift from coral- to macroalgal-dominance and not return to their coral-dominated state due to alterations in the growth, mortality, and/or recruitment of corals, or a range of other processes (Mumby & Steneck 2008, Graham et al. 2015). Although we found coral growth to be suppressed by the presence and density of macroalgae, there were no legacy effects of prior macroalgal exposure on future coral growth. Our results show that the growth of corals within a degraded system can rapidly recover if close-proximity macroalgae are removed. Following three months of contact by differing densities of Sargassum, all corals on our experimental racks immediately recovered growth rates that equaled those of control corals once Sargassum was removed. Thus, macroalgae did not produce a persistent negative feedback on coral growth following removal. We also found no negative effects of growing within a macroalgal-dominated habitat, as might be expected if macroalgal release of dissolved organic carbon (DOC) was affecting the general area by suppressing coral health via alterations of coral microbiomes or other critical processes (Barott & Rohwer 2012, Morrow et al. 2013). Both previous investigations finding that macroalgal dominance did not enhance reef-scale DOC concentrations (Dinsdale et al. 2008, Nelson et al. 2011) and our data suggest that if water-soluble macroalgal exudates are affecting corals, then impacts will be very localized, operating at scales of centimeters or less near the coral-macroalgal interface (Smith et al. 2006, Morrow et al. 2013, Jorissen et al. 2016).
We did not investigate the specific mechanisms by which close-proximity macroalgae reduced coral growth, but these may include a variety of physical (e.g., shading, abrasion, increased sedimentation) or small-scale (mm-cm) chemical or microbially mediated effects (McCook et al. 2001, River & Edmunds 2003, Rasher et al. 2011, Vieira et al. 2016, Zaneveld et al. 2016). Interestingly, the relationship between coral growth and Sargassum density appeared curve-linear, with the greatest reductions in growth realized following the addition of a single Sargassum thallus. Further increases in the density of Sargassum led to smaller reductions in coral growth. Such relationships may provide some insights into the underlying mechanisms, however, the limited number of densities examined preclude generalizations, and one previous study demonstrated a more linear decrease in the growth of Montipora corals with increasing macroalgal density (Clements & Hay 2015). Further experiments will be necessary to determine whether our findings are broadly applicable to interactions between other species of coral and macroalgae, as well as whether algal effects vary with interaction duration and/or in combination with other stressors (Zaneveld et al. 2016).
While the presence or absence of macroalgae strongly influenced coral growth, survivorship was statistically indistinguishable for conspecific corals in our benthic plots whether macroalgae was present or absent. Corals elevated off of the benthos also exhibited comparable survivorship when surrounded by multiple densities of Sargassum, suggesting that competition with Sargassum may be costly for corals in terms of growth, but rarely results in whole colony mortality over the time periods we investigated. In contrast, other macroalgal species that are strongly allelopathic can cause mortality for some corals (including Acropora millepora) over periods of only days to two or three weeks (Rasher et al. 2011). Other benthic disturbances, such as sand scouring, damage from dislodged coral heads during storms, and/or crown-of-thorns sea star predation were observed in several instances (C. Clements, personal observation) and may have contributed to coral mortality on the natural benthos. Reef decline is commonly characterized by punctuated disturbance events (e.g., hurricanes, crown-of-thorns outbreaks, bleaching events) that reduce coral cover, followed by periods of relative stasis rather than coral recovery (Hughes 1994, Gardner et al. 2003, Graham et al. 2015). Our findings suggest that macroalgal competition may limit the re-growth of established corals and growth of new corals, and may also impose opportunity costs associated with delayed growth (e.g., increased mortality risk, and decreased competitive ability and fecundity; Hall & Hughes 1996, Zilberberg & Edmunds 2001, Edmunds & Gates 2004). Therefore, even low densities of macroalgae could inhibit recovery of corals between disturbance events; contributing to the “ratcheting down” of coral reef ecosystems. However, if natural processes (e.g., herbivory, seasonality; Ferrari et al. 2012b, Duran et al. 2016) keep macroalgae in check, it appears that remaining corals should be able to rapidly recover their growth potential.
Other studies have documented evidence that canopy-forming macroalgae like Sargassum experience enhanced growth (Dell et al. 2016) and reduced herbivory (Hoey & Bellwood 2011, Dell et al. 2016) when growing in dense stands – constituting positive feedbacks that reinforce Sargassum dominance. Our data demonstrate that the density of Sargassum also impacts coral growth, which may increase Sargassum’s ability to monopolize space and further reinforce Sargassum dominance. Conversely, reductions in the density of Sargassum may promote proportional increases in growth and recovery of existing corals; increasing reef structural complexity and recruitment of herbivorous fishes (Mumby & Steneck 2008, Graham & Nash 2013) that could undermine Sargassum dominance (Hoey & Bellwood 2011; Rasher et al. 2013). Targeted reductions of direct interactions between macroalgae and corals may also help promote coral growth, recovery, and reproductive potential of corals currently inhabiting macroalgal-dominated reefs (Graham et al. 2013).
Our study highlights the negative impacts of macroalgae that are common to degraded reefs. However, our data also demonstrate that some corals may be resilient to macroalgal competition depending on the temporal and spatial scales of these interactions and how they impact trajectories of benthic community structure on disturbed reefs. Our findings dovetail with evidence from previous studies, suggesting that preserving or restoring critical ecosystem processes such as herbivory can limit macroalgae and lead to enhanced coral persistence and recovery (Mumby & Harborne 2010, Gilmour et al. 2013). Understanding the context-dependencies inherent to common coral-algal interactions will be particularly important as global-scale disturbances continue to challenge management and conservation of vulnerable coral reef ecosystems.
Supplementary Material
Acknowledgments
We thank the Fijian government and the Korolevu-i-Wai district elders for collection and research permissions, and D. Dakuidreketi and D. Godard for assistance in the field. Financial support provided by NSF grant OCE- 0929119, NIH ICBG grant U19TW007401, and the Teasley Endowment to Georgia Tech.
LITERATURE CITED
- Barott KL, Rodriguez-Mueller B, Youle M, Marhaver KL, Vermeij MJ, Smith JE, Rohwer FL. Microbial to reef scale interactions between the reef-building coral Montastraea annularis and benthic algae. Proc R Soc B. 2012;279:1655–1664. doi: 10.1098/rspb.2011.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barott KL, Rohwer FL. Unseen players shape benthic competition on coral reefs. Trends Microbiol. 2012;20:621–628. doi: 10.1016/j.tim.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Birrell CL, McCook LJ, Willis BL, Diaz-Pulido GA. Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. In: Gibson RN, Atkinson RJA, Gordon JDM, editors. Oceanogr Mar Biol Annu Rev. Vol. 46. 2008. pp. 25–63. [Google Scholar]
- Bonaldo RM, Hay ME. Seaweed-coral interactions: variance in seaweed allelopathy, coral susceptibility, and potential effects on coral resilience. PLOS ONE. 2014;9:e85786. doi: 10.1371/journal.pone.0085786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box SJ, Mumby PJ. Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar Ecol Prog Ser. 2007;342:139–149. [Google Scholar]
- Brooker RM, Brandl SJ, Dixson DL. Cryptic effects of habitat declines: coral-associated fishes avoid coral-seaweed interactions due to visual and chemical cues. Sci Rep. 2016;6:18842–18842. doi: 10.1038/srep18842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkepile DE, Hay ME. Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc Nat Acad Sci USA. 2008;105:16201–16206. doi: 10.1073/pnas.0801946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong-Seng KM, Nash KL, Bellwood DR, Graham NAJ. Macroalgal herbivory on recovering versus degrading coral reefs. Coral Reefs. 2014;33:409–419. [Google Scholar]
- Clements CS, Hay ME. Competitors as accomplices: seaweed competitors hide corals from predatory sea stars. Proc R Soc B. 2015;282:221–229. doi: 10.1098/rspb.2015.0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling ES, Alvarez-Filip L, Oliver TA, McClanahan TR, Cote IM, Bellwood D. Evaluating life-history strategies of reef corals from species traits. Ecol Lett. 2012;15:1378–1386. doi: 10.1111/j.1461-0248.2012.01861.x. [DOI] [PubMed] [Google Scholar]
- Dell CL, Longo GO, Hay ME. Positive feedbacks enhance macroalgal resilience on degraded coral reefs. PLOS ONE. 2016;11:e0155049. doi: 10.1371/journal.pone.0155049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinsdale EA, Pantos O, Smriga S, Edwards RA, Angly F, Wegley L, Hatay M, Hall D, Brown E, Haynes M, Krause L, Sala E, Sandin SA, Thurber RV, Willis BL, Azam F, Knowlton N, Rohwer F. Microbial ecology of four coral atolls in the northern Line Islands. PLOS ONE. 2008;3:e1584. doi: 10.1371/journal.pone.0001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixson DL, Abrego D, Hay ME. Chemically mediated behavior of recruiting corals and fishes: a tipping point that may limit reef recovery. Science. 2014;345:892. doi: 10.1126/science.1255057. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Duran A, Collado-Vides L, Burkepile DE. Seasonal regulation of herbivory and nutrient effects on macroalgal recruitment and succession in a Florida coral reef. PeerJ. 2016;4:e2643. doi: 10.7717/peerj.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds PJ, Gates RD. Size-dependent differences in the photophysiology of the reef coral Porites astreoides. Biol Bull. 2004;206:61–64. doi: 10.2307/1543536. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Gonzalez-Rivero M, Mumby PJ. Size matters in competition between corals and macroalgae. Mar Ecol Prog Ser. 2012a;467:77–88. [Google Scholar]
- Ferrari R, Gonzalez-Rivero M, Ortiz JC, Mumby PJ. Interaction of herbivory and seasonality on the dynamics of Caribbean macroalgae. Coral Reefs. 2012b;31:683–692. [Google Scholar]
- Fisher R, O’Leary RA, Low-Choy S, Mengersen K, Knowlton N, Brainard RE, Caley MJ. Species richness on coral reefs and the pursuit of convergent global estimates. Curr Biol. 2015;25:500–505. doi: 10.1016/j.cub.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Folke C, Carpenter S, Walker B, Scheffer M, Elmqvist T, Gunderson L, Holling CS. Regime shifts, resilience, and biodiversity in ecosystem management. Annu Rev Ecol Evol Syst. 2004;35:557–581. [Google Scholar]
- Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR. Long-term region-wide declines in Caribbean corals. Science. 2003;301:958–960. doi: 10.1126/science.1086050. [DOI] [PubMed] [Google Scholar]
- Gilmour JP, Smith LD, Heyward AJ, Baird AH, Pratchett MS. Recovery of an isolated coral reef system following severe disturbance. Science. 2013;340:69. doi: 10.1126/science.1232310. [DOI] [PubMed] [Google Scholar]
- Graham NAJ, Bellwood DR, Cinner JE, Hughes TP, Norström AV, Nyström M. Managing resilience to reverse phase shifts in coral reefs. Front Ecol Environ. 2013;11:541–548. [Google Scholar]
- Graham NAJ, Jennings S, MacNeil MA, Mouillot D, Wilson SK. Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature. 2015;518:94–97. doi: 10.1038/nature14140. [DOI] [PubMed] [Google Scholar]
- Graham NAJ, Nash KL. The importance of structural complexity in coral reef ecosystems. Coral Reefs. 2013;32:315–326. [Google Scholar]
- Haas AF, Nelson CE, Wegley Kelly L, Carlson CA, Rohwer F, Leichter JJ, Wyatt A, Smith JE. Effects of coral reef benthic primary producers on dissolved organic carbon and microbial activity. PLOS ONE. 2011;6:e27973. doi: 10.1371/journal.pone.0027973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall VR, Hughes TP. Reproductive strategies of modular organisms: comparative studies of reef-building corals. Ecology. 1996;77:950–963. [Google Scholar]
- Harvell D, Jordan-Dahlgren E, Merkel S, Rosenberg E, Raymundo L, Smith G, Weil E, Willis B Global Envrionm Facility C. Coral disease, environmental drivers, and the balance between coral and microbial associates. Oceanography. 2007;20:172–195. [Google Scholar]
- Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- Hoey AS, Bellwood DR. Suppression of herbivory by macroalgal density: a critical feedback on coral reefs? Ecol Lett. 2011;14:267–273. doi: 10.1111/j.1461-0248.2010.01581.x. [DOI] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- Hughes TP. Catastrophes, phase-shifts, and large scale degradation of a Caribbean coral-reef. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L, Moltschaniwskyj N, Pratchett MS, Steneck RS, Willis B. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol. 2007;17:360–365. doi: 10.1016/j.cub.2006.12.049. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Graham NAJ, Jackson JBC, Mumby PJ, Steneck RS. Rising to the challenge of sustaining coral reef resilience. Trends Ecol Evol. 2010;25:633–642. doi: 10.1016/j.tree.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TC, Butler IR, Byrne M, Cantin NE, Comeau S, Connolly SR, Cumming GS, Dalton SJ, Diaz-Pulido G, Eakin CM, Figueira WF, Gilmour JP, Harrison HB, Heron SF, Hoey AS, Hobbs J-PA, Hoogenboom MO, Kennedy EV, Kuo C-y, Lough JM, Lowe RJ, Liu G, McCulloch MT, Malcolm HA, McWilliam MJ, Pandolfi JM, Pears RJ, Pratchett MS, Schoepf V, Simpson T, Skirving WJ, Sommer B, Torda G, Wachenfeld DR, Willis BL, Wilson SK. Global warming and recurrent mass bleaching of corals. Nature. 2017;543:373–377. doi: 10.1038/nature21707. [DOI] [PubMed] [Google Scholar]
- Jorissen H, Skinner C, Osinga R, de Beer D, Nugues MM. Evidence for water-mediated mechanisms in coral-algal interactions. Proc R Soc B. 2016:283. doi: 10.1098/rspb.2016.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffner IB, Walters LJ, Becerro MA, Paul VJ, Ritson-Williams R, Beach KS. Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol Prog Ser. 2006;323:107–117. [Google Scholar]
- Ledlie MH, Graham NAJ, Bythell JC, Wilson SK, Jennings S, Polunin NVC, Hardcastle J. Phase shifts and the role of herbivory in the resilience of coral reefs. Coral Reefs. 2007;26:641–653. [Google Scholar]
- McCook L, Jompa J, Diaz-Pulido G. Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs. 2001;19:400–417. [Google Scholar]
- Moberg F, Folke C. Ecological goods and services of coral reef ecosystems. Ecol Econ. 1999;29:215–233. [Google Scholar]
- Morrow KM, Liles MR, Paul VJ, Moss A, Chadwick NE. Bacterial shifts associated with coral–macroalgal competition in the Caribbean Sea. Mar Ecol Prog Ser. 2013;488:103–117. [Google Scholar]
- Mumby PJ, Harborne AR. Marine reserves enhance the recovery of corals on Caribbean reefs. PLOS ONE. 2010;5:e8657. doi: 10.1371/journal.pone.0008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby PJ, Steneck RS. Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol Evol. 2008;23:555–563. doi: 10.1016/j.tree.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Nelson CE, Alldredge AL, McCliment EA, Amaral-Zettler LA, Carlson CA. Depleted dissolved organic carbon and distinct bacterial communities in the water column of a rapid-flushing coral reef ecosystem. ISME J. 2011;5:1374–1387. doi: 10.1038/ismej.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugues MM, Smith GW, van Hooidonk RJ, Seabra MI, Bak RPM. Algal contact as a trigger for coral disease. Ecol Lett. 2004;7:919–923. [Google Scholar]
- Paul VJ, Kuffner IB, Walters LJ, Ritson-Williams R, Beach KS, Becerro MA. Chemically mediated interactions between macroalgae Dictyota spp. and multiple life-history stages of the coral Porites astreoides. Mar Ecol Prog Ser. 2011;426:161–170. [Google Scholar]
- Pratchett MS. Feeding preferences of Acanthaster planci (Echinodermata: Asteroidea) under controlled conditions of food availability. Pac Sci. 2007;61:113–120. [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. URL http://www.R-project.org/ [Google Scholar]
- Rasher DB, Hay ME. Chemically rich seaweeds poison corals when not controlled by herbivores. Proc Natl Acad Sci USA. 2010;107:9683–9688. doi: 10.1073/pnas.0912095107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasher DB, Hoey AS, Hay ME. Consumer diversity interacts with prey defenses to drive ecosystem function. Ecology. 2013;94:1347–1358. doi: 10.1890/12-0389.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasher DB, Stout EP, Engel S, Kubanek J, Hay ME. Macroalgal terpenes function as allelopathic agents against reef corals. Proc Natl Acad Sci USA. 2011;108:17726–17731. doi: 10.1073/pnas.1108628108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- River GF, Edmunds PJ. Mechanisms of interaction between macroalgae and scleractinians on a coral reef in Jamaica. J Exp Mar Biol Ecol. 2001;261:159–172. doi: 10.1016/s0022-0981(01)00266-0. [DOI] [PubMed] [Google Scholar]
- Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;413:591–596. doi: 10.1038/35098000. [DOI] [PubMed] [Google Scholar]
- Scheffer M, Carpenter SR. Catastrophic regime shifts in ecosystems: linking theory to observation. Trends Ecol Evol. 2003;18:648–656. [Google Scholar]
- Simpson R. MSc thesis. University of South Pacific; Suva, Fiji: 2010. Assessing MPA effectiveness through observing the relative abundances of community selected indicator populations over time. A case study of the Korolevu-i-wai qoliqoli on the Coral Coast, Fiji. [Google Scholar]
- Smith JE, Shaw M, Edwards RA, Obura D, Pantos O, Sala E, Sandin SA, Smriga S, Hatay M, Rohwer FL. Indirect effects of algae on coral: algae-mediated, microbe-induced coral mortality. Ecol Lett. 2006;9:835–845. doi: 10.1111/j.1461-0248.2006.00937.x. [DOI] [PubMed] [Google Scholar]
- van de Leemput IA, Hughes TP, van Nes EH, Scheffer M. Multiple feedbacks and the prevalence of alternate stable states on coral reefs. Coral Reefs. 2016;35:857–865. [Google Scholar]
- Vieira C, Thomas OP, Culioli G, Genta-Jouve G, Houlbreque F, Gaubert J, De Clerck O, Payri CE. Allelopathic interactions between the brown algal genus Lobophora (Dictyotales, Phaeophyceae) and scleractinian corals. Sci Rep. 2016;6:18637. doi: 10.1038/srep18637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AT, Nugues MM. Synergistic effects of algal overgrowth and corallivory on Caribbean reef-building corals. Ecology. 2013;94:1667–1674. doi: 10.1890/12-0680.1. [DOI] [PubMed] [Google Scholar]
- Zaneveld JR, Burkepile DE, Shantz AA, Pritchard CE, McMinds R, Payet JP, Welsh R, Correa AM, Lemoine NP, Rosales S, Fuchs C, Maynard JA, Thurber RV. Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales. Nat Commun. 2016;7:11833. doi: 10.1038/ncomms11833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberberg C, Edmunds PJ. Competition among small colonies of Agaricia: the importance of size asymmetry in determining competitive outcome. Mar Ecol Prog Ser. 2001;221:125–133. [Google Scholar]
- Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM. Mixed effects models and extensions in ecology with R. Springer; New York: 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.