Abstract
In the behavioral variant of frontotemporal dementia (bvFTD), left-lateralized salience network dysfunction reduces basal activity in the parasympathetic nervous system, a branch of the autonomic nervous system that reduces arousal and fosters empathy and prosociality. Here we examined whether resting parasympathetic deficits in bvFTD related to diminished prosocial behavior. Eighty participants (30 with bvFTD, 25 with Alzheimer’s disease [AD], and 25 healthy controls) completed a “helping task” in which we quantified participants’ spontaneous reactions to an experimenter who struggled to find a lost key. Participants also underwent an assessment of baseline autonomic nervous system activity and structural magnetic resonance imaging. An exploratory factor analysis of participants’ behaviors during the helping task revealed four factors: empathic concern, consolation, disengagement, and impatience. Patients with bvFTD had lower empathic concern and greater disengagement and impatience than the AD and healthy control groups. Patients with bvFTD had lower resting respiratory sinus arrhythmia and faster respiration and heart rates than patients with AD and healthy controls, a pattern consistent with parasympathetic dysfunction. Skin conductance level was also lower in bvFTD than in the other groups. Lower baseline respiratory sinus arrhythmia and faster baseline respiration rates, but not skin conductance level, predicted lower prosocial helping behaviors. Voxel-based morphometry analyses revealed that atrophy in the bilateral medial pulvinar nucleus of the thalamus, midcingulate cortex, and caudate was associated with lower empathic concern and consolation, and atrophy in the bilateral medial pulvinar nucleus of the thalamus, left frontoinsula, and left ventral striatum was associated with greater disengagement and impatience. Left-lateralized frontoinsula atrophy was associated with not only lower respiratory sinus arrhythmia but also with lower consolation and greater disengagement. This study offers evidence for prosocial behavior deficits in bvFTD and suggests that left-lateralized salience network atrophy reduces patients’ resting parasympathetic activity and motivation to help others in need.
Keywords: neurodegenerative disease, vagus nerve, salience network, compassion
1. Introduction
The behavioral variant of frontotemporal dementia (bvFTD) is a neurodegenerative disorder characterized by dysfunction in emotion systems that promote interpersonal sensitivity. Hallmark bvFTD clinical features include loss of empathy, apathy, and disinhibition (Rascovsky et al., 2011), symptoms that make it difficult for patients to navigate the social world and to maintain lasting relationships. On standardized tests, patients with bvFTD exhibit impaired comprehension of complex social cues such as humor, prosody, and sarcasm; perspective-taking; and moral decision-making (Baez et al., 2016; Clark et al., 2015; Dara et al., 2012; Gleichgerrcht, Torralva, Roca, Pose, & Manes, 2011; Shany-Ur et al., 2012).
Deterioration of socioemotional behavior in bvFTD often reflects disconnection and degeneration in the salience network (Zhou et al., 2010), a distributed brain system that supports visceromotor emotion generation and homeostatic regulation (Seeley et al., 2007), and in appraisal networks that support semantic processing and social cognition (Ranasinghe et al., 2016). With hubs in the frontoinsula and anterior cingulate cortex, the salience network also includes the thalamus, amygdala, hypothalamus, ventral striatum, and periaqueductal gray (Benarroch, 1993; Critchley, 2005; Seeley et al., 2007). In bvFTD, atrophy and dysfunction in the salience network are associated with reduced social interest and interpersonal engagement (Bickart et al., 2014; Seeley, 2010; Shany-Ur & Rankin, 2011; Toller et al., 2018). Loss of empathy is a core feature of the bvFTD syndrome (Baez et al., 2014; Rascovsky et al., 2007), and patients have significant difficulty identifying emotions in other people (Goodkind et al., 2015; Kumfor & Piguet, 2012; Snowden et al., 2008). Laboratory-based assessments that measure the affective responsiveness of patients themselves (Levenson et al., 2008) have identified specific areas of deficient emotional reactivity in bvFTD. Patients with bvFTD have blunted autonomic and behavioral reactions to disgusting stimuli and embarrassing situations (Eckart, Sturm, Miller, & Levenson, 2012; Sturm, Allison, Rosen, Miller, & Levenson, 2006; Sturm, Ascher, Miller, & Levenson, 2008), deficits that reflect atrophy of salience network structures such as the anterior cingulate cortex and insula (Sturm, Sollberger, et al., 2013; Verstaen et al., 2016; Woolley et al., 2015). Deficits in empathy and emotion generation may hamper patients’ sensitivity to social cues and responsivity to others’ needs (de Waal, 2012; Decety, Bartal, Uzefovsky, & Knafo-Noam, 2016).
Recent studies have determined that salience network disruption in bvFTD not only impacts emotion generation but also alters resting sympathetic and parasympathetic autonomic nervous system activity (Guo et al., 2016; Joshi et al., 2014). Although mobilization of the sympathetic system occurs primarily during phasic moments of heightened arousal, tonic engagement of the parasympathetic system at rest promotes homeostatic equilibrium and a quiet internal milieu (Craig, 2005; Levenson, 2003; Porges, 2001). After negative emotional events, parasympathetic activity decreases physiological arousal to restore baseline functioning (Yuan, McCarthy, Holley, & Levenson, 2010). The salience network interacts with brainstem nuclei that activate the vagus nerve and attenuate heart and respiration rates to paces that are lower than those set by pacemaker cells and create variability in their patterned outflow (Dick, Baekey, Paton, Lindsey, & Morris, 2009; Dick et al., 2008). Baseline respiratory sinus arrhythmia (RSA), a parasympathetic measure of vagally-mediated heart rate variability (Berntson et al., 1997; Oppenheimer, Kedem, & Martin, 1996), is reduced in bvFTD and reflects diminished connectivity between left-lateralized cortical salience network nodes and damage to left hemisphere structures (Guo et al., 2016). Asymmetric atrophy and dysfunction in the left frontoinsula, therefore, may be deleterious for parasympathetic activity in bvFTD.
Parasympathetic dysfunction may underlie some of the socioemotional symptoms that emerge in bvFTD. In mammals, the parasympathetic nervous system promotes interpersonal attunement by orienting attention to the needs and feelings of others (Porges, 2001). In humans, higher resting parasympathetic activity is associated with better emotion regulation, cognitive control, and emotional expressivity, abilities that are essential for social behavior and communication (Beauchaine, 2001; Thayer & Lane, 2000). Individuals with higher baseline parasympathetic activity have higher affiliative traits such as optimism and agreeableness, and they report higher levels of positive emotional experience in their everyday lives (Oveis et al., 2009). Compassion is a positive emotion that motivates people to help others who are struggling (Decety et al., 2016; Goetz, Keltner, & Simon-Thomas, 2010), and in healthy adults, higher resting RSA is associated with greater prosocial tendencies (Stellar, Cohen, Oveis, & Keltner, 2015) such as helping and consolation, actions that reduce suffering and foster well-being. Taken together, these studies suggest that the parasympathetic system is essential for fostering positive emotions and other-oriented empathic actions.
Decline in empathy is a core feature of bvFTD, but relatively little is known about the disease’s impact on prosocial behavior. Although previous decision-making studies have found that patients with bvFTD prioritize their own needs over those of others (Melloni et al., 2016; O’Callaghan et al., 2016; Sturm et al., 2017), patients’ responsivity to others’ emotional needs during a standardized yet ecologically valid situation has not been assessed. We developed a novel laboratory-based task to examine prosocial behavior in a realistic situation in which patients encountered an experimenter who needed help. We compared patients with bvFTD to healthy controls and to patients with Alzheimer’s disease-type dementia (henceforth, AD), a neurodegenerative disorder that targets the default mode network, a network dedicated to episodic memory and mental time travel (Buckner, Andrews-Hanna, & Schacter, 2008; Buckner et al., 2005; Greicius, Srivastava, Reiss, & Menon, 2004). Default mode network decline in AD is accompanied by salience network hyperconnectivity (Fredericks et al.; Zhou et al., 2010) and enhanced emotional sensitivity in some contexts. Patients with AD may, for example, exhibit anxiety and heightened responsivity to others’ affective states (Sturm, Yokoyama, et al., 2013), making it possible that some forms of empathy and prosociality are preserved, or even possibly enhanced, in this disease. We also measured resting autonomic nervous system physiology and examined whether baseline parasympathetic and sympathetic activity were associated with prosocial actions. We hypothesized that prosocial behavior would be impaired in bvFTD, but not AD, compared to healthy controls and that prosocial helping deficits would relate to baseline parasympathetic dysfunction. Neuroanatomically, we anticipated that salience network atrophy would be associated with prosocial behavior deficits in bvFTD. Given the critical role of the left frontoinsular cortex in parasympathetic activity, we also investigated whether left-lateralized frontoinsula atrophy was a common neural substrate underlying impairment in both RSA and prosociality in bvFTD.
2. MaterialsandMethods
2.1. Participants
Eighty participants, including 30 patients with bvFTD (Rascovsky et al., 2011), 25 with AD (McKhann et al., 1984), and 25 healthy controls completed the study. Participants underwent a multidisciplinary team evaluation at the University of California, San Francisco (UCSF) Memory and Aging Center that included a clinical interview, neurological exam, functional assessment, and neuropsychological testing (assessment of verbal and visual episodic memory, executive function, language, and visuospatial functioning). Functional assessments of dementia severity were obtained using the Clinical Dementia Rating Scale (CDR; Morris, 1993). The CDR Total (scores range from 0 to 3) and Sum of the Boxes (CDR-SB) scores (scores range from 0 to 18, with higher scores on both CDR measures indicating greater functional impairment) were computed for each participant, providing indices of disease severity. The healthy controls were recruited from advertisements; underwent an identical neurological, cognitive, and imaging work-up as the patients; and were free of current or previous neurological or psychiatric disorders. Height and weight were collected at the time of testing to quantify body mass index (BMI). The study was approved by the Committee on Human Research at the University of California, San Francisco and all participants, or their surrogates, gave their informed consent before participating in the study. Table 1 presents the demographic, cognitive, and functional data for each group.
Table 1.
Demographic and clinical information for the diagnostic groups.
| bvFTD | AD | Healthy Controls |
|
|---|---|---|---|
| N | 30 | 25 | 25 |
| Age (Mean ± SD) | 63.5 ± 8.4 | 62.0 ± 9.4 | 67.4 ± 5.9 |
| Sex (male / female) | 17 / 13 | 11 / 14 | 11 / 14 |
| Body mass index† | 27.9 ± 5.4 | 24.7 ± 3.6 | 26.5 ± 4.2 |
| Education (Mean ± SD) | 16.4 ± 3.3 | 17.6 ± 4.3 | 17.7 ± 1.6 |
| CDR Total (Mean ± SD)*†‡ | 1.4 ± 0.6 | 0.7 ± 0.3 | 0.0± 0.0 |
| CDR-SB (Mean ± SD)*†‡ | 7.7 ± 3.2 | 4.4 ± 1.7 | 0.0± 0.0 |
| MMSE (Mean ± SD)*‡ | 23.7 ± 4.4 | 22.2 ± 3.3 | 29.4 ± 0.9 |
| California Verbal Learning Test-Short Form 10-minute Recall (/9) |
2.3 ± 3.0 | 2.1 ± 2.2 | n/a |
| California Verbal Learning Test-2 Long Form 10-minute Recall (/16) |
n/a | n/a | 13.3 ± 2.2 |
| Benson Figure Copy 10-minute Recall (/17)*†‡ |
7.8 ± 5.1 | 4.1 ± 2.7 | 13.1 ± 2.1 |
| Modified Trails (correct lines per minute)*†‡ |
15.6 ± 13.2 | 4.8 ± 5.5 | 45.2 ± 15.3 |
| Modified Trails Errors*† | 2.8 ± 3.0 | 1.2 ± 1.2 | 0.2 ± 0.4 |
| Phonemic Fluency (# correct in 60 seconds)*†‡ |
6.8 ± 5.1 | 10.1 ± 4.6 | 18.6 ± 3.5 |
| Semantic Fluency (# correct in 60 seconds)*‡ |
9.6 ± 6.5 | 12.3 ± 5.1 | 25.0 ± 6.3 |
| Design Fluency Correct (# correct in 60 seconds)*‡ |
5.1 ± 4.4 | 3.9 ± 2.8 | 13.2 ± 3.1 |
| Design Fluency Repetitions*† | 4.1 ± 4.7 | 1.3 ± 1.5 | 1.4 ± 1.7 |
| Digits Backward*‡ | 3.3 ± 1.4 | 3.1 ± 1.1 | 5.8 ± 1.7 |
| Benson Figure Copy (/17)†‡ | 14.0 ± 3.0 | 8.4 ± 5.2 | 15.4 ± 1.0 |
| Calculations (/5)*†‡ | 3.2 ± 1.4 | 2.3 ± 1.2 | 4.7 ± 0.7 |
| Boston Naming Test Spontaneous Correct (/15)*‡ |
12.1 ± 3.4 | 12.2 ± 2.4 | 14.7 ± 0.6 |
| Peabody Picture Vocabulary Test (/16)* |
13.4 ± 3.4 | 14.6 ± 1.4 | 15.9 ± 0.3 |
| Stroop Color Naming (# correct in 60 seconds)*†‡ |
56.0 ± 23.4 | 38.8 ± 17.0 | 92.3 ± 15.6 |
| Stroop Inhibition (# correct in 60 seconds)*‡ |
25.5 ± 19.0 | 16.1 ± 13.7 | 54.3 ± 10.9 |
indicates a Tukey-corrected pairwise difference between bvFTD and healthy controls at p< .05
indicates a Tukey-corrected pairwise difference between AD and bvFTD
indicates a Tukey-corrected pairwise difference between AD and healthy controls.
2.2. Laboratory Assessment of Emotion
2.2.1. Procedure
Participants’ emotional functioning was assessed at the UCSF Center for Psychophysiology and Behavior. Participants were seated in a comfortable chair in a well-lit experiment room. Sensors were applied to participants to obtain continuous measures of physiological activity, and participants were videotaped with a remotely controlled video camera throughout the testing session. Participants were notified that they were being filmed during the informed consent process at the beginning of the testing session.
2.2.2. Tasks
2.2.2.1. Resting Baseline.
At the beginning of the testing session, participants sat 4.25 feet away from a 21.5-inch computer monitor. To measure baseline physiology, participantsProsocial behavior deficits in bvFTD were asked to clear their minds and to watch a black “X” on a white background for two minutes.
2.2.2.2. Helping Task.
We developed a laboratory-based task designed to elicit helping and consolation behaviors in a realistic yet controlled environment. Participants sat quietly during another 90-second resting baseline during which they again viewed an “X” on the monitor. At the start of this baseline period, they only were told that the experimenter would remove the physiological sensors after this baseline. At the end of the baseline, the experimenter, who was seated next to the participant, stated that she needed to check the physiological signals for a final time. The experimenter then walked across the room to inspect the signals on another monitor that was located on a table against the wall. As she moved the mouse on the screen, she “accidentally” knocked a key off the table so that it landed on the floor behind a file cabinet and out of the sight of the participant.
The task was semi-scripted, and at the end of the baseline period the experimenter stated the following comments intermittently over the course of the 90-second task:
“Thanks [walks over to the monitor across the room] —just sit tight before we remove the sensors. I just need to check something on this computer really quickly [knocks the key off the table with the mouse]. Oh shoot… [crouches down to look for the key]. I just dropped the key to the filing cabinet, and I need to use it today! Oh this is bad. This is so bad. I don’t see it. Oh no…I can’t find it at all. I’m so stupid. This is the third time I’ve dropped something today. I’m an idiot! I can’t be t his clumsy anymore. I can’t believe I dropped it again. This is not good—hey, I can’t see it from here. Sorry, I just really need to find this key [crouches under the table near participant]. I’m so dumb for losing this key. Ow—I can’t see anything under here. It’s too d ark.”
After approximately 90 seconds, the experimenter found the key and then conducted a validity check by asking participants whether they had believed (yes/no) that she had needed help finding the key. She then asked participants to rate their subjective experience of various positive and negative emotions (i.e., compassion, tenderness or love, surprised, awe, sad, amused, afraid, and disgusted) during the task on a scale from 0 (not at all) to 4 (extremely). Finally, the experimenter debriefed the participants about the purpose of the task and disclosed that she dropped the key on purpose to assess their reaction to someone who needed help. She then thanked them for the help they had offered. The task was inspired by other tasks designed to elicit helping behaviors (Piff, Dietze, Feinberg, Stancato, & Keltner, 2015; Vohs, Mead, & Goode, 2006) but was designed to minimize cognitive demands for patients with dementia.
2.2.3. Measures
2.2.3.1. Behavior.
A team of trained coders later coded participants’ behavior during the helping task. Twenty behaviors were coded, including verbal (e.g., offering to help) and non-verbal (e.g., looking around the room for an object that could be used to help) behaviors (see Table 2). During each 15-second bin of the task, a code was given that best captured the participant’s behavior on an intensity scale of 0 (not present), 1 (present), and 2 (frequent). The coding system was developed to capture a wide range of behaviors that occurred during the task. The coders were naïve to the types of neurodegenerative syndromes that were being investigated and were blind to the participants’ diagnoses and the study hypotheses. The coders did not make judgments about whether the behaviors they observed were prosocial or not but only categorized participants’ actions. Coders were trained on the coding system until they were reliable. Twenty percent of videotapes were double-coded, and interrater reliability was high (Krippendorf’s alpha= .81). For videos coded by more than one coder, the codes were averaged before being summed across the trial.
Table 2.
Varimax Rotated Factor Loadings From an Exploratory Factor Analysis of 17 Verbal and Non-Verbal Behaviors Observed in the Helping Task. The behavioral examples are specific actions that illustrate each behavioral code. For each factor, the three behavioral codes with the strongest loadings on each factor are shown in bold.
| Behavioral Code |
Behavioral Example |
Factor 1: Empathic Concern |
Factor 2: Consolation |
Factor 3: Disengagement |
Factor 4: Impatience |
|---|---|---|---|---|---|
| Verbal | |||||
| Asking questions |
“What did you drop?” |
0.48 | 0.15 | −0.1 | 0.22 |
| Making suggestions |
“You could use a ruler to get the key out.” |
0.62 | 0.06 | −0.02 | −0.07 |
| Offering to help |
“Let me give you a hand.” |
−0.13 | −0.18 | −0.06 | −0.21 |
| Offering reassurance |
“You’ll find it eventually.” |
−0.15 | 0.3 | −0.05 | 0.1 |
| Validating | “Oh gosh, I don’t see it.” |
0.34 | 0.46 | −0.14 | −0.16 |
| Making self- deprecatory comments |
“I drop things all the time too!” |
0.18 | 0.23 | −0.04 | −0.09 |
| Making consoling noises |
“Aww.” | 0 | 0.58 | −0.02 | −0.06 |
| Asking unrelated questions |
“I’m going to drink my Gatorade.” |
0.08 | 0.19 | 0.21 | 0.28 |
| Non-Verbal | |||||
| Looking impatient |
Tapped foot | −0.07 | −0.21 | 0.03 | 0.46 |
| Looking annoyed |
Rolled eyes | −0.21 | −0.19 | 0.34 | 0.50 |
| Laughing | Laughed or giggled |
0.24 | 0.73 | 0.05 | 0.04 |
| Face- touching |
Touched face, neck, or head |
0 | 0.22 | 0.23 | 0.05 |
| Leaning toward experimenter |
Leaned in chair toward experimenter |
0.41 | 0.15 | −0.18 | −0.27 |
| Gazing toward experimenter |
Looked at experimenter |
0.01 | 0.13 | −0.72 | −0.05 |
| Gazing around room |
Looked around the room (in apparent effort to help) |
0.87 | −0.08 | −0.04 | −0.18 |
| Staring off into space |
Passively looked into space and not at the experimenter |
−0.37 | −0.03 | 0.82 | −0.04 |
| Looking away from experimenter |
Actively looked at another object in the room and not at experimenter |
−0.18 | 0.16 | −0.17 | 0.54 |
2.2.3.2. Physiological Recordings.
Continuous recordings of autonomic nervous system physiology were obtained using Biopac MP150 bioamplifiers and a computer equipped with data acquisition software: (1) heart rate: Electrodes were placed in a bipolar configuration on opposite sides of the participant’s chest; the heart rate was calculated as the number of R waves from the electrocardiogram per minute, (2) respiration rate: A pneumatic bellows or respiration transducer was stretched around the thoracic region, and the respiration rate was measured as the number of inspirations per minute, (3) skin conductance level (SCL): A constant-voltage device was used to pass a small voltage between Ag/AgCl Silver 8mm electrodes (using an electrolyte of sodium chloride) attached to the palmar surface of the middle phalanges of the ring and index fingers of the non-dominant hand, and (4) respiratory sinus arrhythmia (RSA): RSA was calculated as the difference in milliseconds between the shortest inter-beat interval during inspiration and the longest inter-beat interval during expiration, the peak-valley approach to assessing RSA. We used the cardiac and respiratory data to compute RSA, a measure of vagally-mediated parasympathetic activity (Berntson et al., 1997; Grossman & Kollai, 1993). We used SCL, a measure of sympathetic activity (Critchley, 2002), as an autonomic control measure because we expected it would be unrelated to prosocial behavior.
Physiological data were processed using a custom pipeline scripted in AcqKnowledge software (v4.4, www.biopac.com). Briefly, algorithms identified and marked the signature components of each waveform, and these marks were then visually inspected for errors and noise. Outliers in the raw data were considered to be +/− 3 standard deviations from the mean level during the trial; these periods were interpolated if their duration was three seconds or less and deleted if their duration was greater than three seconds. Mean resting heart rate, respiration rate, SCL, and RSA levels were computed during the 120-second resting baseline period. The natural log of RSA (lnRSA) was used in all analyses to improve proximity to a normal distribution. Outliers in the averaged physiological data were also examined and excluded if they were +/− 3 standard deviations from the mean during the baseline. This resulted in one patient with AD being excluded for RSA and respiration rate and one patient with bvFTD being Prosocial behavior deficits in bvFTD excluded for SCL. An additional patient with AD who had a pacemaker was excluded from the heart rate and RSA analyses.
2.3. Structural Neuroimaging
Fifty participants (29 bvFTD and 21 healthy controls) underwent research-quality 3T structural magnetic resonance imaging (MRI). Patients were scanned within four months of the behavioral assessment, and healthy controls within 12 months. Images were obtained on a 3.0 Tesla Siemens (Siemens, Iselin, NJ) TIM Trio scanner equipped with a 12-channel head coil located at the UCSF Neuroscience Imaging Center. Whole brain images were acquired using volumetric MPRAGE (160 sagittal slices; slice thickness= 1.0 mm; FOV= 256 × 230 mm2; matrix 256 × 230; voxel size 1.0 × 1.0 × 1.0 mm3; TR= 2300 ms; TE= 2.98 ms; flip angle= 9°). Four p atients with bvFTD were excluded for significant motion or white matter disease.
2.3.1. Voxel-Based Morphometry
Structural T1 images were visually inspected for movement artifact, corrected for bias field, segmented into gray matter, white matter, and cerebrospinal fluid, and spatially normalized to Montreal Neurological Institute space using Statistical Parametric Mapping 12 (Friston, Ashburner, Kiebel, Nichols, & Penny, 2007). In all preprocessing steps, default parameters were utilized with the exception of using the light clean-up procedure in the morphological filtering step. Default tissue probability priors (voxel size: 2.0 × 2.0 × 2.0 mm 3) of the International Consortium for Brain Mapping were used. Segmented images were visually inspected for adequate gray-white segmentation. Each participant’s image was warped to a template to optimize inter-subject registration. Gray matter maps were then smoothed with an 8 mm full-width at half-maximum Gaussian kernel.
2.4. Analyses
All statistical analyses were carried out in R Project (R Core Team, 2015).
2.4.1. Validity Checks
Belief question. Participants who reported that they believed that the experimenter needed help finding the key were given a score of 1. Participants who reported that they did not believe, as well as those who expressed any doubt at all, were given a score of 0.
2.4.1.1. Self-reported experience.
We examined whether the task elicited the target emotions of compassion and tenderness/love and used analyses of covariance (ANCOVA), controlling for age and sex, to determine whether there were differences in these positive emotions among the diagnostic groups.
2.4.2. Helping Behavior
The helping task was semi-scripted and, therefore, had a somewhat variable duration; therefore, we analyzed the first 75 seconds of the task for each participant. The task was terminated early for one patient with bvFTD after she stated that she did not want to help and that she wanted to leave after 45 seconds. In this case, we prorated her scores by dividing each behavioral code by the task duration (i.e., 45 seconds) and then multiplying these values by 75 (to make her scores comparable to the rest of the sample who completed the task for 75 seconds).
We conducted an exploratory factor analysis of the behavioral codes during the helping task, a data-driven approach that allowed us to identify the latent components in the data. For each participant, we summed the intensities of each code in the helping task to compute a total score for each code (scores ranged from 0–10). We excluded three variables (encouragement, endearment, and self-deprecation) that occurred in less than five percent of the participants; 17 variables remained and entered the factor analysis. We used the components method to create the factor scores: first, we z-scored each code; second, we multiplied each z-score by its loading on the factor of interest; third, we summed those values to create the factor score. We then used a multivariate analysis of covariance (MANCOVA), controlling for age and sex, to examine whether there were group differences in the factor scores. Follow-up ANCOVAs with Tukey-corrected pairwise comparisons then were used to isolate significant pairwise differences for each factor. In a set of exploratory analyses, we also used ANCOVAs, followed by Tukey-corrected pairwise comparisons, to compare the individual behaviors among the groups.
2.4.3. Physiological Analyses
We first used ANCOVAs (same covariates as above) to examine whether there were baseline physiological differences among the groups. We also examined whether there were any main effects of diagnosis on BMI that may account for any differences in physiological activity. Next, we conducted multiple regressions across the entire sample (controlling for age, sex, and diagnosis [dummy coded with two variables to account for the three diagnostic groups]) to examine whether baseline physiology predicted behavior during the helping task.
2.4.4. Voxel-Based Morphometry Analyses
We conducted structural neuroimaging analyses to identify the neural correlates of RSA and each behavioral factor score. In general, we expected that atrophy in bvFTD would be associated with lower RSA and lower prosocial behavior (i.e., lower empathic concern, lower consolation, greater impatience, and greater disengagement). Previous studies of AD have shown that heightened emotional sensitivity in AD reflects enhanced functional connectivity (Fredericks et al.; Zhou et al., 2010) despite atrophy (Sturm, Yokoyama, et al., 2013) in certain emotion-relevant structures. Thus, we omitted the patients with AD from the neuroimaging analyses because we expected that atrophy in the bvFTD and AD groups would have opposite relationships with prosocial behavior (i.e., a positive correlation in bvFTD but a negative correlation in AD), and our primary hypotheses focused on the neuroanatomical underpinnings of helping deficits in bvFTD.
2.4.4.1. Gray matter correlations.
We conducted separate voxel-based morphometry analyses to correlate RSA and each of the factor scores with gray matter structural maps. We included age, sex, total intracranial volume (a sum of the total gray matter, white matter, and cerebrospinal fluid, to account for individual differences in head size), and diagnosis as nuisance covariates in our linear models. A priori significance was established at uncorrected praw<.005.To offset the loss of power incurred by correction for multiple comparisons, we masked our analyses to structures within the salience network: anterior cingulate cortex, midcingulate cortex, insula, amygdala, caudate, putamen, pallidum, and thalamus. One thousand permutation analyses using combined peak and extent thresholds were run to derive a study-specific error distribution to determine the one-tailed T-threshold for multiple comparisons correction at pFWE<.05 (Nichols & Holmes, 2002). Permutation analysis is a resampling approach to significance testing by which a test statistic is compared to the null distribution derived from the present study’s dataset and thus is an accurate representation of Type 1 error at pFWE< .05 across the entire brain (Kimberg, Coslett, & Schwartz, 2007). Images were overlaid using MRIcron (http://mccauslandcenter.sc.edu/CRNL) on the Montreal Neurological Institute template brain.
2.4.4.2. Structural lateralization correlations.
Previous research has shown that RSA impairment in bvFTD reflects left-lateralized frontoinsula atrophy (Guo et al., 2016). We utilized this same approach and computed left-over-right lateralization indices, which quantify the differential atrophy between the left and right sides of the brain, from the preprocessed structural images. The average gray matter intensity in each voxel on the left side (VL) and on the right side (VR) of the brain were entered into the following equation: (VL − VR)/(VL + VR). Thus, lower scores indicated more severe atrophy on the left than right, and higher scores indicated more severe atrophy on the right than left. We performed voxel-wise regression analyses, also masked to the salience network, to examine whether left-lateralized atrophy related to diminished RSA. Next, we explored whether similar patterns of left-lateralized salience network atrophy also related to prosocial helping deficits.
3. Results
3.1. Validity Checks
3.1.1. Belief
The majority of participants (90%) believed the experimental manipulation: 97% of patients with bvFTD, 96% of patients with AD, and 76% of healthy controls reported that they believed that the experimenter needed help finding the key. One patient with bvFTD did not respond to the validity check question, three healthy controls reported they did not believe the experimenter needed help, and three healthy controls reported that they were uncertain about the validity of the task. We binarized these responses (1= believed the task, 0= did not believe or expressed uncertainty) and included this variable as an additional covariate in follow-up analyses to determine whether any participant doubt impacted our results.
3.1.2. Self-Reported Experience
As expected, compassion and tenderness/love were the emotions that participants in all groups reported feeling most strongly during the helping task (Table 3). There were no group differences in mean levels of self-reported compassion, F(2,40)= 0.51, p= .605, ηp2= .03, or tenderness/love, F(2,40)= 0.63, p= .539, ηp2= .03.
Table 3.
Physiological Activity, Behavior, and Self-Reported Experience. Baseline physiological activity, behavior, and subjective experience were measured during the helping task.
| bvFTD M(SD) |
AD M(SD) |
Healthy Controls M(SD) |
|
|---|---|---|---|
| Self-reported experience | |||
| Compassion | 1.80 (1.37) | 2.38 (1.20) | 2.08 (1.26) |
| Tenderness or love | 1.00 (1.41) | 1.38 (1.26) | 0.77 (0.73) |
| Surprised | 1.07 (1.49) | 0.94 (1.24) | 0.85 (0.80) |
| Awe | 0.60 (1.10) | 0.31 (1.01) | 0.08 (0.28) |
| Sad | 0.53 (1.06) | 0.69 (0.95) | 0.39 (0.51) |
| Amused | 1.20 (1.52) | 0.50 (0.73) | 0.77 (0.93) |
| Afraid | 0.20 (0.78) | 0.06 (0.25) | 0.08 (0.28) |
| Disgusted | 0.27 (0.80) | 0.00 (0.00) | 0.00 (0.00) |
| Behavior: Factor Scores | |||
| Empathic concern*† | −1.39 (2.00) | 1.18 (2.19) | 0.50 (2 .23) |
| Consolation | −0.42 (1.75) | 0.59 (2.01) | −0.09 (1.79) |
| Disengagement*† | 0.78 (2.24) | −0.44 (1.18) | −0.50 (1.4 5) |
| Impatience*† | 0.61 (1.95) | −0.61 (0.94) | −0.13 (0.94) |
| Behavior: Individual Behavioral Codes | |||
| Asking questions | 0.96 (1.09) | 1.3 (1.43) | 1.88 (1.59) |
| Making suggestions* | 0.62 (1.32) | 1.12 (1.06) | 1.84 (1.49) |
| Offering to help | 0.37 (1.16) | 0.30 (0.46) | 0.56 (0.92) |
| Offering reassurance | 1.35 (2.11) | 0.7 (0.96) | 0.68 (0.85) |
| Validating† | 0.45 (0.75) | 1.66 (1.66) | 1.30 (1.32) |
| Making self-deprecatory comments | 0.07 (0.25) | 0.08 (0.28) | 0.52 (2.02) |
| Making consoling noises | 0.33 (0.61) | 0.32 (0.85) | 0.40 (0.65) |
| Asking unrelated questions | 0.10 (0.31) | 0.18 (0.48) | 0 (0) |
| Looking impatient | 0.28 (0.98) | 0 (0) | 0.20 (0.58) |
| Looking annoyed | 0.97 (1.77) | 0.16 (0.80) | 0.31 (0.75) |
| Laughing | 0.60 (1.07) | 1.20 (1.32) | 0.54 (1.02) |
| Face-touching | 1.15 (1.84) | 1.14 (2.14) | 1.21 (1.89) |
| Leaning toward experimenter†‡ | 1.02 (1.68) | 2.94 (2.53) | 1.06 (1.51) |
| Gazing toward experimenter | 5.92 (2.89) | 6.56 (2.59) | 7.21 (2.65) |
| Gazing around room† | 1.02 (1.48) | 2.7 (2.16) | 1.88 (1. 73) |
| Staring off into space*† | 2.27 (3.06) | 0.40 (0.87) | 0. 67 (1.79) |
| Looking away from experimenter | 1.11 (1.56) | 0.50 (0.76) | 0.65 (0.98) |
| Baseline Physiological Activity | |||
| Heart rate (beats per minute)*† | 73.47 (9.47) | 61.82 (7.85) | 65.29 (14.42) |
| Respiration rate (breaths per minute)* | 17.14 (5.53) | 14.88 (3.01) | 12.71 (3.07) |
| Respiratory sinus arrhythmia (ms)* | 17.30 (9.09) | 23.83 (10.42) | 36.25 (34.05) |
| Skin conductance level (microsiemens)* |
1.12(0.71) | 1.57 (0.87) | 1.70 (0.86) |
indicates a difference between bvFTD and healthy controls at p< .05
indicates a Tukey-corrected pairwise difference between AD and bvFTD
indicates a Tukey-corrected pairwise difference between AD and healthy controls.
3.2. Helping Behavior
3.2.1. Exploratory Factor Analysis
The Kaiser-Meyer-Olkin (KMO) test (KMO= 0.55) and Bartlett’s test of sphericity ( p< .0001) confirmed the appropriateness of exploratory factor analysis on the behavioral data (Hutcheson & Sofroniou, 1999). We examined a scree plot of eigenvalues for each factor to determine the number of factors to retain. There were distinct points of inflexion after two and four factors, which suggested that the amount of variance explained was much less for subsequent factors. We selected a four-factor solution. The first factor (“empathic concern”) was defined by behaviors that demonstrated active helping behaviors (e.g., making suggestions). The second factor (“consolation”) was defined by behaviors that were less active but supported and encouraged the experimenter as she struggled (e.g., making validating comments). The third factor (“disengagement”) was defined by behaviors that suggested the participant was not interested in engaging with the experimenter during the task (e.g., staring off into space). The fourth factor (“impatience”) was defined by behaviors that indicated that the participant was eager for the session to end and for the experimenter to find the key on her own (e.g., looking annoyed by pressing lips). See Table 2 for a complete list of all of the behavioral codes and their rotated factor loadings. The four factors were only moderately correlated, which suggests that they were not overlapping constructs or bipolar opposites of the same construct (John & Benet-Martinez, 2000): empathic concern and consolation, r(78)= .50, p<.001; empathic concern and disengagement, r(78)= −.53, p<.001; empathic concern and impatience, r(78)= −.51, p<.001; consolation and disengagement, r(78)= −.31, p= .005; consolation and impatience, r(78)= −.19, p= .098; and disengagement and impatience, r(78)= .40, p= <.001.
3.2.2. Behavioral Analyses
The MANCOVA with the four factors as dependent variables revealed a significant main effect of diagnosis, F(8,146)= 3.03, p= .004, ηp2= .14. Individual ANOVAs revealed a main effect of diagnosis for empathic concern, F(2,75)= 11.17, p < .001, ηp2= .23; disengagement, F(2,75)= 5.39, p= .007, ηp2= .13; and impatience, F(2,75)= 6.14, p= .003, ηp2= .14. Follow-up pairwise comparisons showed that patients with bvFTD displayed lower empathic concern than the AD (p< .001) and healthy control (p= .002) groups; greater disengagement than the AD (p=.026) and healthy control (p= .015) groups; and greater impatience than the AD (p= .004) and healthy control (p= .043) groups (Figure 1). The groups did not differ in their consolation behavior, F(2,75)= 1.64, p= .200, ηp2= .04. See Table 3. Although patients with AD did not differ significantly from healthy controls in any of the behavioral factor scores, on average they had the highest empathic concern and consolation and the lowest impatience. When we controlled for responses to our validity check question regarding participants’ belief in the task, all main effects of diagnosis remained significant.
Figure 1. Behaviors During the Helping Task.
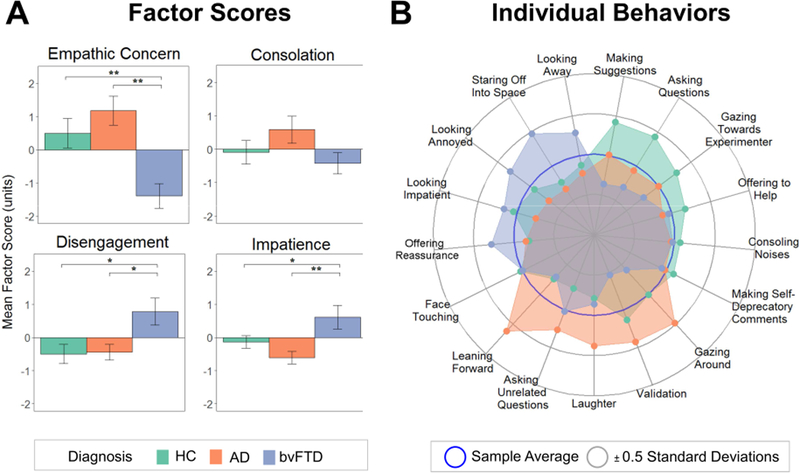
(A) Bar graphs depict each diagnostic group’s mean factor scores, which were derived from the verbal and non-verbal behaviors the participants exhibited during the helping task. Error bars are standard error of the mean. (B) The radial plot shows the individual behaviors that were included in the factor scores and their mean levels in each diagnostic group. AD= Alzheimer’s disease, bvFTD= behavioral variant frontotemporal dementia, HC= healthy controls.
An exploratory examination of the individual behaviors revealed main effects of diagnosis on making suggestions (bvFTD < healthy controls, p= .011), validating (bvFTD < AD, p= .004), leaning forward (bvFTD < AD, p= .001; AD > healthy controls, p= .021), gazing around the room (bvFTD <AD, p= .002), looking annoyed (bvFTD >AD, p= .043), and staring off into space (bvFTD > AD, p= .006; bvFTD > healthy controls, p= .027). See Table 3 and Figure 1.
3.3. Physiological Activity
We found main effects of diagnosis on baseline RSA, F(2,67)= 10.00, p< .001, ηp2 =.23; respiration rate, F(2,74)= 9.15, p< .001, ηp2= .20; and SCL, F(2,65)= 3.9, p= .025, ηp2= .11 (see Figure 2). Compared to healthy controls, patients with bvFTD had lower resting RSA (p< .001), faster respiration rates (p< .001), and lower SCL (p= .024). We also detected a main effect of diagnosis on baseline heart rate, F(2,68)= 7.50, p= .001, ηp2= .18, with post hoc Tukey tests showing that patients with bvFTD had faster heart rates than the AD (p= .001) and healthy control (p= .045) groups (see Table 3). There was a main effect of diagnosis on BMI, F(2,75)= 3.40, p= .039, ηp2= .08, with post hoc tests showing that BMI was lower in AD than in bvFTD (p= .029). When we added BMI as an additional covariate to our analyses, the main effects of diagnosis remained for RSA, F(2,64)= 8.01, p< .001, ηp2= .20; respiration rate, F(2,71)= 8.51, p< .001, ηp2= .19; SCL, F(2,62)= 4.97, p= .010, ηp2= .14; and heart rate, F(2,65)= 5.52, p= .006, ηp2= .15. All of the Tukey-corrected post hoc pairwise comparisons also remained significant with the exception of heart rate, which continued to be significantly faster in bvFTD compared to AD (p= .008) but heart rate in bvFTD was no longer different from the healthy controls (p=.118).
Figure 2. Baseline Physiology and Associated Behaviors.
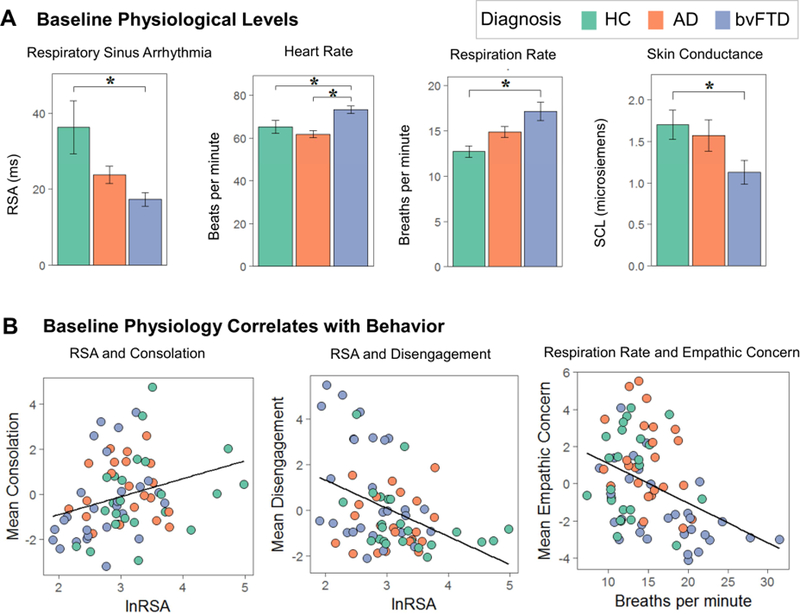
(A) Mean levels of resting baseline physiology are shown for each of the diagnostic groups. (B) Multiple regression analyses (controlling for age, sex, and diagnosis) found that lower baseline RSA was associated lower consolation and greater disengagement and that faster baseline respiration rates were associated with greater empathic concern as well as greater disengagement and impatience (scatters not shown). AD= Alzheimer’s disease, bvFTD= behavioral variant frontotemporal dementia, HC= healthy controls, lnRSA= natural log of respiratory sinus arrhythmia, RSA= respiratory sinus arrhythmia, SCL= skin conductance level.
The multiple regression analyses showed that lower baseline RSA was associated with lower consolation, F(1,66)= 5.65, p= .020, ηp2= .08, and greater disengagement, F(1,66)= 5.93,p= .018, ηp2= .08, during the helping task. Faster respiration rates were associated with lower empathic concern, F(1,73)= 7.52, p= .008, ηp2= .09; greater disengagement, F(1,73)= 8.00, p=.006, ηp2= .10; and greater impatience, F(1,73)= 19.15, p< .001, ηp2= .21 (see Figure 2). In contrast, neither baseline SCL nor heart rate predicted any behavioral factor score.
3.4. Structural Correlates of Parasympathetic Dysfunction and Prosocial Behavior Deficits
The voxel-based morphometry analyses of the structural gray matter maps did not find any regions that were significantly associated with lower RSA, but they did reveal an association between salience network atrophy and prosocial behavior deficits. At the most stringent statistical threshold (pFWE<.05), lower empathic concern was associated with smaller gray matter volume in bilateral midcingulate cortex, and greater impatience was associated with smaller gray matter volume in the bilateral medial pulvinar nucleus of the thalamus (pFWE<.05), among other thalamic nuclei (see Table 4 and Figure 3). At uncorrected levels (praw<.005), lower prosocial behaviors (i.e., lower empathic concern and consolation) were associated with smaller gray matter volume in the bilateral midcingulate cortex and bilateral thalamus, and lower consolation was associated with smaller volume in the right caudate. Greater disengagement was associated with smaller gray matter volume in the left ventral striatum and left frontoinsula.
Table 4.
Neural Correlates of Prosocial Behavior During the Helping Task. Smaller gray matter volume was associated with lower empathic concern and consolation but greater disengagement and impatience in bvFTD and healthy controls (controlling for age, sex, diagnosis, and total intracranial volume). Montreal Neurological Institute (MNI) coordinates given for maximum T- score for the cluster (cluster size > 70 mm3, p<.005, uncorrected).
| Anatomical region | Cluster volume (mm3) |
MNI | Maximum T-score |
|||
|---|---|---|---|---|---|---|
| coordinates | ||||||
| x | y | z | ||||
| Empathic Concern | ||||||
| Left Midcingulate Cortex* | 408 | 0 | −5 | 51 | 4.48 | |
| Right Midcingulate Cortex | † | |||||
| Right Thalamus | 1215 | 3 | −24 | 8 | 3.80 | |
| Right Midcingulate Cortex | 128 | 2 | −26 | 53 | 3.35 | |
| Right Midcingulate Cortex | 95 | 14 | −33 | 44 | 3.20 | |
| Left Thalamus | 81 | −14 | −33 | 2 | 2.96 | |
| Consolation | ||||||
| Right Caudate | 284 | 20 | −20 | 20 | 3.47 | |
| Right Thalamus | † | |||||
| Left Caudate | 78 | −20 | −20 | 20 | 3.39 | |
| Left Thalamus | 716 | −5 | −21 | 5 | 3.30 | |
| Left Midcingulate Cortex | 192 | −6 | −45 | 50 | 3.20 | |
| Disengagement | ||||||
| Left Ventral Striatum | 807 | −20 | 12 | −8 | 3.41 | |
| Left Frontoinsula | 128 | −32 | 17 | −8 | 2.97 | |
| Impatience | ||||||
| Right Thalamus* | 2336 | 15 | −27 | 3 | 5.76 | |
| Left Thalamus* | 1877 | −9 | −24 | 2 | 4.74 | |
denotes clusters in which voxels remain significant at pFWE<.05.
signifies these regions were included in th e cluster above.
Color bars indicate the T-scores.
Figure 3. Neural Correlates of Behaviors During the Helping Task.
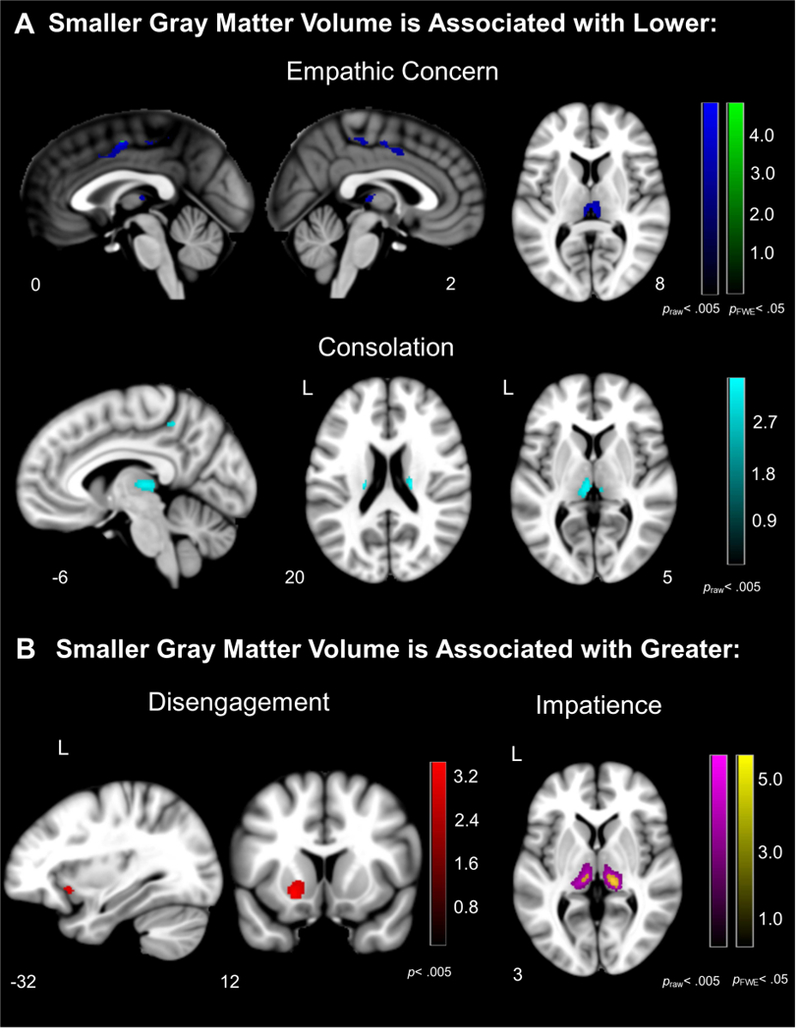
Voxel-based morphometry analyses revealed that (A) atrophy in the bilateral midcingulate cortex, thalamus, and caudate were associated with lower empathic concern and consolation, and (B) atrophy in the bilateral thalamus, left frontoinsula, and left ventral striatum were associated with greater disengagement and impatience in bvFTD and healthy controls. Color bars represent T-scores at p< .005, uncorrected (T> 2.70) and pFWE<.05 (T> 4.21) are presented. Statistical maps are superimposed on the Montreal Neurological Institute template brain.
The voxel-wise analyses of the lateralization indices showed that lower baseline RSA was associated with left-worse-than-right atrophy in the frontoinsula (−35, 14, −17; peak T = 2.97;cluster volume= 37 mm3), midcingulate cortex (−11, 2, 35; peak T = 3.30; cluster volume 61 mm3), and putamen/pallidum (−12, 24, −6; peak T = 2.85; cluster volume= 10 mm3), praw<.005,(Figure 4). We next examined whether lower consolation and greater disengagement, the two behavioral factor scores that RSA predicted in the multiple regression analyses, were also associated with left-lateralized atrophy. Lower consolation (−35, 21, 0; peak T = 3.36; cluster volume= 88 mm3; and −33, 17, −12; peak T = 3.11; cluster volume= 84 mm3) and higher disengagement (−35, 17, −6; peak T = 3.51; cluster volume= 135 mm3) were associated with left-worse-than-right frontoinsula atrophy, praw<.005, (Figure 4). Lower consolation was also associated with left-worse-than-right ventral striatum atrophy (−12, 18, −9; peak T = 3.47; cluster volume= 658 mm3).
Figure 4. Left-Lateralized Frontoinsula Atrophy Predicts Diminished Parasympathetic Activity and Prosocial Behavior Deficits.
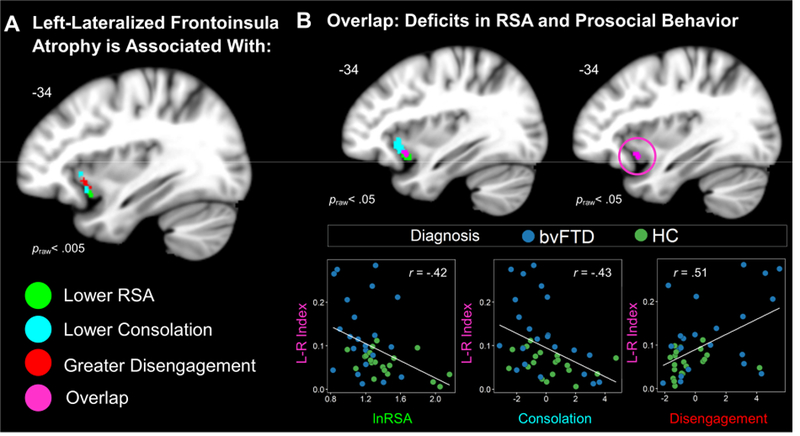
(A) Left-greater-than-right FI atrophy correlated with lower RSA, lower consolation behavior, and greater disengagement (praw< .005). (B) Lowering the threshold (praw< .05) revealed an overlapping cluster (violet) where voxel intensity significantly correlated with RSA, consolation, and disengagement. We extracted the gray matter volume from this cluster in each participant. Scatterplots are shown to illustrate the independent associations that left-lateralized frontoinsula atrophy had with all three measures. Statistical maps of the lateralization results are superimposed on the left hemisphere of the Montreal Neurological Institute template brain. Maps represent T-scores at praw< .005, uncorrected (T> 2.70) and praw< .05, uncorrected (T> 1.68). Frontoinsula (FI), left (L), respiratory sinus arrhythmia (RSA), right (R).
Discussion
The results of the present study indicate that parasympathetic nervous system dysfunction relates to diminished prosocial behavior in bvFTD. During a laboratory-based task designed to evoke spontaneous, other-oriented helping behaviors, patients with bvFTD showed lower levels of empathic concern than patients with AD and healthy controls. Consolation behaviors were also low in bvFTD though this difference did not reach statistical significance. The bvFTD group, however, demonstrated high levels of disengagement and impatience, behaviors that were uncommon in the other groups and reflected disinterest and annoyance as the experimenter struggled to find a lost key. At the level of the factor scores, most prosocial behaviors were significantly higher in AD than in bvFTD, and certain other-oriented actions (e.g., leaning forward) were significantly higher in AD than in both the bvFTD and healthy control groups. On average, patients with AD displayed the highest empathic concern and consolation and the lowest impatience of all of the groups. There were no group differences in self-reported positive emotional experience during the task, however. Assessment of resting autonomic nervous system physiology revealed that patients with bvFTD had lower baseline RSA, faster heart and respiration rates, and lower SCL than the healthy controls (in the pairwise comparisons, AD did not differ from either the bvFTD or the control group on any measure except for heart rate, which was slower in AD than in bvFTD). Across the sample, baseline deficits that likely reflect parasympathetic dysfunction predicted lower prosociality during the helping task: lower resting RSA predicted lower consolation and greater disengagement whereas faster resting respiration rate predicted lower empathic concern, greater disengagement, and greater impatience. Baseline alterations in SCL, a sympathetic measure, and heart rate, which reflects both parasympathetic and sympathetic influences, were not related to prosocial behavior. Neuroanatomically, smaller gray matter volume in salience network structures was associated with prosocial behavior deficits (i.e., lower empathic concern and consolation and greater disengagement and impatience). Within the salience network, left-lateralized frontoinsula atrophy was associated with lower RSA, lower consolation, and greater disengagement, which suggested that tissue loss in the left frontoinsula disrupted both parasympathetic activity and prosocial actions in bvFTD.
When we encounter others who are struggling, shared feeling states motivate us to take actions that alleviate their distress (Batson et al., 1997; Decety et al., 2016; Decety & Sommerville, 2003; Nummenmaa, Hirvonen, Parkkola, & Hietanen, 2008; Zaki & Ochsner, 2012). Rather than expressing empathic concern or consolation, prosocial behaviors that prioritize the needs of others (Decety et al., 2016; Decety & Jackson, 2004), patients with bvFTD exhibited high levels of disengagement and impatience in response to the struggling experimenter. Basal RSA was impaired in bvFTD and predicted diminished prosocial behaviors, a pattern that is consistent with previous studies that have found a privileged relationship between the parasympathetic system and socioemotional functioning (Beauchaine, 2001; Porges, 2001; Thayer & Lane, 2000). Although previous studies have found mixed evidence for baseline SCL deficits in bvFTD (Guo et al., 2016; Joshi et al., 2014), our results suggest resting parasympathetic and sympathetic activity are impaired in bvFTD but that only parasympathetic deficits underlie patients’ prosocial behavior defi cits. Patients with bvFTD did not significantly differ from the other groups in their self-reported experience of compassion or tenderness/love during the task, however, which may reflect their tendency to endorse numerous types of subjective emotional experience, both appropriate and inappropriate, due to atrophy in interoceptive pathways (Chen et al., 2017).
Our neuroimaging results showed that the midcingulate cortex and thalamocortical systems were critical for prosocial behavior. Low empathic concern and consolation were associated with atrophy in the bilateral midcingulate cortex, a hub in pain pathways that has strong connections with skeletomotor systems (Vogt, 2016); atrophy in this region may have reduced patients’ sensitivity to the experimenter’s state and motivation to act in prosocial ways. Atrophy in the thalamus was also associated with diminished empathic concern and consolation as well as greater impatience. The thalamic clusters that emerged across these neuroimaging analyses were bilateral and included the medial pulvinar nucleus and extended into the vicinity of the parvocellular part of the mediodorsal nucleus, structures that receive direct input from the nucleus of the solitary tract, the first central relay for vagal afferents and the sensory axons of several other cranial nerves (Beckstead & Norgren, 1979; Krauth et al., 2010; Mufson & Mesulam, 1984). Focal thalamic dysfunction may alter thalamocortical loops that are critical for parasympathetic activity, emotions, empathy, and socioemotional behavior (Benarroch, 2015; Bruneau, Pluta, & Saxe, 2012; Craig, 2002; Guillery & Sherman, 2002; Mufson & Mesulam, 1984; Nummenmaa et al., 2008). In bvFTD, medial pulvinar atrophy is related to reduced salience network connectivity (Lee et al., 2014), a disruption that may diminish parasympathetic activity (Guo et al., 2016) and impede the transmission of afferent signals (Schmahmann & Pandya, 2008) that typically promote prosocial actions.
Within the salience network, left-lateralized frontoinsular connections appear to be critical for both prosocial motivation and parasympathetic regulation. Our analysis of the gray matter structural maps showed that greater disengagement was associated with atrophy in both the left ventral striatum, a region implicated in processing rewarding cues including enjoyable social interactions (Haber & Knutson, 2010; Morelli, Sacchet, & Zaki, 2015), and the left frontoinsula. Consistent with previous studies (Guo et al., 2016), our analysis of the gray matter lateralization indices also pointed to a critical role for the left frontoinsula in parasympathetic control. Left-greater-than-right frontoinsula atrophy was associated with lower RSA as well as lower consolation and greater disengagement (the two behavioral factors that RSA predicted in the regression analyses). Taken together, these findings suggest that this region may play a critical role in a common pathway that both produces RSA and encourages prosocial behavior. We speculate that in bvFTD, asymmetric left frontoinsula atrophy leads to failure of the vagal brake (i.e., lower resting RSA) and shifts patients toward a physiological ceiling (i.e., faster heart and respiration rates), creating a restricted physiological range for adaptive arousal increases that facilitate vicarious emotion simulation and prosocial motivation. Thus, left-lateralized parasympathetic deficits may reduce patients’ ability to share others’ emotions and render them unmoved, unhelpful, disengaged, and impatient.
There are several limitations to the present study that should be considered. First, because many participants talked and moved in their chair during the helping task, we quantified verbal and non-verbal behavior as our primary dependent measures of interest but did not examine physiological reactivity during the helping task itself. Emotions such as compassion are accompanied by parasympathetic activity (Stellar et al., 2015) and, therefore, we may have failed to detect important associations between physiological reactivity and prosociality. Second, although the patients with AD had the highest levels of empathic concern and consolation, their levels of prosocial behavior were significantly higher than the bvFTD but not the healthy control group. Our previous studies have shown that certain forms of emotional empathy are heightened in AD (Sturm, Yokoyama, et al., 2013), and enhanced sensitivity to the experimenter’s struggle may account for this group’s elevated prosocial tendencies. It is possible that with a larger sample the prosocial elevations in AD, as measured by the factor scores, would have reached statistical significance. We speculate that higher emotional empathy in AD may motivate prosocial feelings but leave patients less equipped to actively help because they are flooded by distress. Indeed, the patients with AD tended to engage in more passive positive behaviors (e.g., leaning forward) than active problem-solving strategies (e.g., making suggestions). Our focus on behavior and not physiological reactivity, therefore, may have underestimated the extent to which patients with AD were emotionally impacted by the task. Third, the thalamus, and medial pulvinar nucleus in particular, is an early and predominant site of disruption in genetic cases of bvFTD caused by mutations in the C9ORF72 gene (Lee et al., 2014; Sha et al., 2012; Whitwell et al., 2012). Although we did not examine how genetic status may have influenced our results, future studies that examine empathy and prosocial behavior in patients with the C9ORF72 mutation may help to elucidate the role of the thalamus in socioemotional behavior.
Emotions motivate action and are essential for maintaining social relationships (Frijda, Kuipers, & ter Schure, 1989). We demonstrated that in bvFTD, a disease that targets emotion systems, patients are less responsive to, and more disinterested in, a person in need of aid. Our results indicated that dysfunction in the parasympathetic nervous system, which is essential for promoting social engagement and emotions such as compassion (Oveis et al., 2009; Stellar et al., 2015), was associated with diminished prosocial responses to an individual who was struggling. We propose that in bvFTD, disruption of sensorimotor affect-sharing systems as well as parasympathetic pathways may interfere with patients’ ability to downregulate physiological arousal and access internal cues that motivate helpful acts.
Acknowledgements
We would like to thank Lily Gordon, Samantha Wong, and Megan Frank for their assistance with the behavioral coding. We are grateful for the patients, healthy controls, and families that participated in our study.
Funding
This project was supported by grants from the NIH National Institute on Aging (P50AG023501, P01AG019724, R01AG052496, R01AG032306, R01AG057204, 1K23AG040127, and 1K23AG045289), The Larry L. Hillblom Foundation (2013-A-029-SUP and 2005/2T), the John Douglas French Foundation; the Consortium for Frontotemporal Dementia Research, and the Tau Consortium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baez S, Manes F, Huepe D, Torralva T, Fiorentino N, Richter F, … Ibanez A (2014). Primary empathy deficits in frontotemporal dementia. Front Aging Neurosci, 6, 262. doi:10.3389/fnagi.2014.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez S, Morales JP, Slachevsky A, Torralva T, Matus C, Manes F, & Ibanez A (2016). Orbitofrontal and limbic signatures of empathic concern and intentional harm in the behavioral variant frontotemporal dementia. Cortex, 75, 20–32. doi:10.1016/j.cortex.2015.11.007 [DOI] [PubMed] [Google Scholar]
- Batson CD, Sager K, Garst E, Kang M, Rubchinsky K, & Dawson K (1997). Is empathy-induced helping due to self–other merging? Journal of Personality and Social Psychology, 73(3), 495–509. [Google Scholar]
- Beauchaine T (2001). Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol, 13(2), 183–214. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, & Norgren R (1979). An autoradiographic examination of the central distribution of the trigeminal, facial, glossopharyngeal, and vagal nerves in the monkey. J Comp Neurol, 184(3), 455–472. doi:10.1002/cne.901840303 [DOI] [PubMed] [Google Scholar]
- Benarroch EE (1993). The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc, 68(10), 988–1001. doi:10.1016/S0025-6196(12)62272-1 [DOI] [PubMed] [Google Scholar]
- Benarroch EE (2015). Pulvinar: associative role in cortical function and clinical correlations. Neurology, 84(7), 738–747. doi:10.1212/wnl.0000000000001276 [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger T Jr., Eckberg DL, Grossman P, Kaufman PG, Malik M, …vand der Molen MW (1997). Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology, 34, 623–648. doi:10.1111/j.1469-8986.1997.tb02140.x [DOI] [PubMed] [Google Scholar]
- Bickart KC, Brickhouse M, Negreira A, Sapolsky D, Barrett LF, & Dickerson BC (2014). Atrophy in distinct corticolimbic networks in frontotemporal dementia relates to social impairments measured using the Social Impairment Rating Scale. Journal of Neurology, Neurosurgery and Psychiatry, 85(4), 438–448. doi:10.1136/jnnp-2012-304656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau EG, Pluta A, & Saxe R (2012). Distinct roles of the ‘shared pain’ and ‘theory of mind’ networks in processing others’ emotional suffering. Neuropsychologia, 50(2), 219–231. doi:10.1016/j.neuropsychologia.2011.11.008 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. doi:10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, … Mintun MA (2005). Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. Journal of Neuroscience, 25(34), 7709–7717. doi:10.1523/jneurosci.2177-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KH, Lwi SJ, Hua AY, Haase CM, Miller BL, & Levenson RW (2017). Increased subjective experience of non-target emotions in patients with frontotemporal dementia and Alzheimer’s disease. Curr Opin Behav Sci, 15, 77–84. doi:10.1016/j.cobeha.2017.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CN, Nicholas JM, Henley SM, Downey LE, Woollacott IO, Golden HL, … Warren JD (2015). Humour processing in frontotemporal lobar degeneration: A behavioural and neuroanatomical analysis. Cortex, 69, 47–59. doi:10.1016/j.cortex.2015.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience, 3, 655–666. doi:10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Craig AD (2005). Forebrain emotional asymmetry: A neuroanatomical basis? Trends in Cognitve Science, 9(12), 566–571. doi:10.1016/j.tics.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Critchley HD (2002). Electrodermal responses: what happens in the brain. Neuroscientist, 8(2), 132–142. [DOI] [PubMed] [Google Scholar]
- Critchley HD (2005). Neural mechanisms of autonomic, affective, and cognitive integration. The Journal of Comparative Neurology, 493, 154–166. doi:10.1002/cne.20749 [DOI] [PubMed] [Google Scholar]
- Dara C, Kirsch-Darrow L, Ochfeld E, Slenz J, Agranovich A, Vasconcellos-Faria A, …Kortte KB (2012). Impaired emotion processing from vocal and facial cues in frontotemporal dementia compared to right hemisphere stroke. Neurocase doi:10.1080/13554794.2012.701641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal FB (2012). The antiquity of empathy. Science, 336(6083), 874–876. doi:10.1126/science.1220999 [DOI] [PubMed] [Google Scholar]
- Decety J, Bartal IB, Uzefovsky F, & Knafo-Noam A (2016). Empathy as a driver of prosocial behaviour: highly conserved neurobehavioural mechanisms across species. Philos Trans R Soc Lond B Biol Sci, 371(1686), 20150077. doi:10.1098/rstb.2015.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, & Jackson PL (2004). The functional architecture of human empathy. Behavioral and Cognitive Neuroscience Reviews, 3(2), 71–100. doi:10.1177/1534582304267187 [DOI] [PubMed] [Google Scholar]
- Decety J, & Sommerville JA (2003). Shared representations between self and other: A social cognitive neuroscience view. Trends in Cognitive Science, 7(12), 527–533. [DOI] [PubMed] [Google Scholar]
- Dick TE, Baekey DM, Paton JF, Lindsey BG, & Morris KF (2009). Cardio-respiratory coupling depends on the pons. Respir Physiol Neurobiol, 168(1–2), 76–85. doi:10.1016/j.resp.2009.07.009 [DOI] [PubMed] [Google Scholar]
- Dick TE, Shannon R, Lindsey BG, Nuding SC, Segers LS, Baekey DM, & Morris KF (2008). Pontine respiratory-modulated activity before and after vagotomy in decerebrate cats. J Physiol, 586(17), 4265–4282. doi:10.1113/jphysiol.2008.152108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckart JA, Sturm VE, Miller BL, & Levenson RW (2012). Diminished disgust reactivity in behavioral variant frontotemporal dementia. Neuropsychologia, 50(5), 786–790. doi:10.1016/j.neuropsychologia.2012.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericks CA, Sturm VE, Brown JA, Hua AY, Bilgel M, Wong DF, … Seeley WW Early affective changes and increased connectivity in preclinical Alzheimer’s disease. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring doi:10.1016/j.dadm.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijda NH, Kuipers P, & ter Schure E (1989). Relations among emotion, appraisal, and emotional action readiness. Journal of Personality and Social Psychology, 57(2), 212–228. [Google Scholar]
- Friston KJ, Ashburner J, Kiebel S, Nichols T, & Penny WD (Eds.). (2007). Statistical Parametric Mapping: The Analysis of Functional Brain Images London: Academic Press. [Google Scholar]
- Gleichgerrcht E, Torralva T, Roca M, Pose M, & Manes F (2011). The role of social cognition in moral judgment in frontotemporal dementia. Soc Neurosci, 6(2), 113–122. doi:10.1080/17470919.2010.506751 [DOI] [PubMed] [Google Scholar]
- Goetz JL, Keltner D, & Simon-Thomas E (2010). Compassion: an evolutionary analysis and empirical review. Psychol Bull, 136(3), 351–374. doi:10.1037/a0018807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind MS, Sturm VE, Ascher EA, Shdo SM, Miller BL, Rankin KP, & Levenson RW (2015). Emotion recognition in frontotemporal dementia and Alzheimer’s disease: A new film-based assessment. Emotion, 15(4), 416–427. doi:10.1037/a0039261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, & Menon V (2004). Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America, 101(13), 4637–4642. doi:10.1073/pnas.0308627101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, & Kollai M (1993). Respiratory sinus arrhythmia, cardiac vagal tone, and respiration: within- and between-individual relations. Psychophysiology, 30(5), 486–495. [DOI] [PubMed] [Google Scholar]
- Guillery RW, & Sherman SM (2002). The thalamus as a monitor of motor outputs. Philos Trans R Soc Lond B Biol Sci, 357(1428), 1809–1821. doi:10.1098/rstb.2002.1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CC, Sturm VE, Zhou J, Gennatas ED, Trujillo AJ, Hua AY, … Seeley WW (2016). Dominant hemisphere lateralization of cortical parasympathetic control as revealed by frontotemporal dementia. Proc Natl Acad Sci U S A, 113(17), E2430–2439. doi:10.1073/pnas.1509184113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, & Knutson B (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26. doi:10.1038/npp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson GD, & Sofroniou N (1999). The multivariate social scientist: Introductory statistics using generalized linear models London: Sage Publications. [Google Scholar]
- John OP, & Benet-Martinez V (2000). Measurement: Reliability, construct validation, and scale construction. In Reis HT & Judd CM (Eds.), Handbook of research methods in social and personality psychology (pp. 339–369). New York: Cambridge University Press. [Google Scholar]
- Joshi A, Mendez MF, Kaiser N, Jimenez E, Mather M, & Shapira JS (2014). Skin conductance levels may reflect emotional blunting in behavioral variant frontotemporal dementia. J Neuropsychiatry Clin Neurosci, 26(3), 227–232. doi:10.1176/appi.neuropsych.12110332 [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Coslett HB, & Schwartz MF (2007). Power in Voxel-based lesion-symptom mapping. Journal of Cognitive Neuroscience, 19(7), 1067–1080. doi:10.1162/jocn.2007.19.7.1067 [DOI] [PubMed] [Google Scholar]
- Krauth A, Blanc R, Poveda A, Jeanmonod D, Morel A, & Szekely G (2010). A mean three-dimensional atlas of the human thalamus: generation from multiple histological data. Neuroimage, 49(3), 2053–2062. doi:10.1016/j.neuroimage.2009.10.042 [DOI] [PubMed] [Google Scholar]
- Kumfor F, & Piguet O (2012). Disturbance of emotion processing in frontotemporal dementia: a synthesis of cognitive and neuroimaging findings. Neuropsychol Rev, 22(3), 280–297. doi:10.1007/s11065-012-9201-6 [DOI] [PubMed] [Google Scholar]
- Lee SE, Khazenzon AM, Trujillo AJ, Guo CC, Yokoyama JS, Sha SJ, … Seeley WW (2014). Altered network connectivity in frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. Brain, 137(Pt 11), 3047–3060. doi:10.1093/brain/awu248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson RW (2003). Blood, sweat, and fears: The autonomic architecture of emotion. Annals of the New York Academy of Sciences, 1000, 348–366. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Ascher E, Goodkind M, McCarthy M, Sturm V, & Werner K (2008). Chapter 25 Laboratory testing of emotion and frontal cortex. Handb Clin Neurol, 88, 489–498. doi:10.1016/s0072-9752(07)88025-0 [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, & Stadlan EM (1984). Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology, 34(7), 939–944. [DOI] [PubMed] [Google Scholar]
- Melloni M, Billeke P, Baez S, Hesse E, de la Fuente L, Forno G, … Ibanez A (2016). Your perspective and my benefit: multiple lesion models of self-other integration strategies during social bargaining. Brain, aww231 doi:10.1093/brain/aww231 [DOI] [PubMed] [Google Scholar]
- Morelli SA, Sacchet MD, & Zaki J (2015). Common and distinct neural correlates of personal and vicarious reward: A quantitative meta-analysis. Neuroimage, 112, 244–253. doi:10.1016/j.neuroimage.2014.12.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, & Mesulam MM (1984). Thalamic connections of the insula in the rhesus monkey and comments on the paralimbic connectivity of the medial pulvinar nucleus. J Comp Neurol, 227(1), 109–120. doi:10.1002/cne.902270112 [DOI] [PubMed] [Google Scholar]
- Nichols TE, & Holmes AP (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping, 15(1), 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Hirvonen J, Parkkola R, & Hietanen JK (2008). Is emotional contagion special? An fMRI study on neural systems for affective and cognitive empathy. Neuroimage, 43(3), 571–580. doi:10.1016/j.neuroimage.2008.08.014 [DOI] [PubMed] [Google Scholar]
- O’Callaghan C, Bertoux M, Irish M, Shine JM, Wong S, Spiliopoulos L, …Hornberger M (2016). Fair play: social norm compliance failures in behavioural variant frontotemporal dementia. Brain, 139(Pt 1), 204–216. doi:10.1093/brain/awv315 [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Kedem G, & Martin WM (1996). Left-insular cortex lesions perturb cardiac autonomic tone in humans. Clinical Autonomic Research, 6(3), 131–140. [DOI] [PubMed] [Google Scholar]
- Oveis C, Cohen AB, Gruber J, Shiota MN, Haidt J, & Keltner D (2009). Resting respiratory sinus arrhythmia is associated with tonic positive emotionality. Emotion, 9(2), 265–270. doi:10.1037/a0015383 [DOI] [PubMed] [Google Scholar]
- Piff PK, Dietze P, Feinberg M, Stancato DM, & Keltner D (2015). Awe, the small self, and prosocial behavior. J Pers Soc Psychol, 108(6), 883–899. doi:10.1037/pspi0000018 [DOI] [PubMed] [Google Scholar]
- Porges SW (2001). The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol, 42(2), 123–146. doi:10.1016/S0167-8760(01)00162-3 [DOI] [PubMed] [Google Scholar]
- Ranasinghe KG, Rankin KP, Pressman PS, Perry DC, Lobach IV, Seeley WW, … Miller BL (2016). Distinct Subtypes of Behavioral Variant Frontotemporal Dementia Based on Patterns of Network Degeneration. JAMA Neurol, 73(9), 1078–1088. doi:10.1001/jamaneurol.2016.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Kipps CM, Johnson JK, Seeley WW, Mendez MF, … Miller BL (2007). Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): Current limitations and future directions. Alzheimer Disease and Associated Disorders, 21(4), S14–S18. doi:10.1097/WAD.0b013e31815c3445 [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, … Miller BL (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134(Pt 9), 2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, & Pandya DN (2008). Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex, 44(8), 1037–1066. doi:10.1016/j.cortex.2008.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW (2010). Anterior insula degeneration in frontotemporal dementia. Brain Struct Funct, 214(5–6), 465–475. doi:10.1007/s00429-010-0263-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, … Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. doi:10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SJ, Takada LT, Rankin KP, Yokoyama JS, Rutherford NJ, Fong JC, …Boxer AL (2012). Frontotemporal dementia due to C9ORF72 mutations: clinical and imaging features. Neurology, 79(10), 1002–1011. doi:10.1212/WNL.0b013e318268452e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shany-Ur T, Poorzand P, Grossman SN, Growdon ME, Jang JY, Ketelle RS, …Rankin KP (2012). Comprehension of insincere communication in neurodegenerative disease: lies, sarcasm, and theory of mind. Cortex, 48(10), 1329–1341. doi:10.1016/j.cortex.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shany-Ur T, & Rankin KP (2011). Personality and social cognition in neurodegenerative disease. Curr Opin Neurol, 24(6), 550–555. doi:10.1097/WCO.0b013e32834cd42a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Austin NA, Sembi S, Thompson JC, Craufurd D, & Neary D (2008). Emotion recognition in Huntington’s disease and frontotemporal dementia. Neuropsychologia, 46(11), 2638–2649. [DOI] [PubMed] [Google Scholar]
- Stellar JE, Cohen A, Oveis C, & Keltner D (2015). Affective and physiological responses to the suffering of others: Compassion and vagal activity. Journal of Personality and Social Psychology, 108(4), 572–585. doi:10.1037/pspi0000010 [DOI] [PubMed] [Google Scholar]
- Sturm VE, Allison SC, Rosen HJ, Miller BL, & Levenson RW (2006). Self-conscious emotion deficits in frontotemporal lobar degeneration. Brain, 129(9), 2508–2516. doi:10.1093/brain/awl145 [DOI] [PubMed] [Google Scholar]
- Sturm VE, Ascher EA, Miller BL, & Levenson RW (2008). Diminished self-conscious emotional responding in frontotemporal lobar degeneration patients. Emotion, 8(6), 861–869. doi:10.1037/a0013765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Perry DC, Wood K, Hua AY, Alcantar O, Datta S, … Kramer JH (2017). Prosocial deficits in behavioral variant frontotemporal dementia relate to reward network atrophy. Brain and Behaviour, e00807. [DOI] [PMC free article] [PubMed]
- Sturm VE, Sollberger M, Seeley WW, Rankin KP, Ascher EA, Rosen HJ, … Levenson RW (2013). Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity. Soc Cogn Affect Neurosci, 8(4), 468–474. doi:10.1093/scan/nss023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Yokoyama JS, Seeley WW, Kramer JH, Miller BL, & Rankin KP (2013). Heightened emotional contagion in mild cognitive impairment and Alzheimer’s disease is associated with temporal lobe degeneration. Proceedings of the National Academy of Sciences of the United States of America, 110(24), 9944–9949. doi:10.1073/pnas.1301119110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2000). A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord, 61(3), 201–216. [DOI] [PubMed] [Google Scholar]
- Toller G, Brown J, Sollberger M, Shdo SM, Bouvet L, Sukhanov P, … Rankin KP (2018). Individual differences in socioemotional sensitivity are an index of salience network function. Cortex, 103, 211–223. doi:10.1016/j.cortex.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstaen A, Lwi S, Haase CM, Sturm VE, Miller BL, & Levenson RW (2016). Insular atrophy and diminished disgust reactivity. Emotion, 16(6), 903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA (2016). Midcingulate cortex: Structure, connections, homologies, functions and diseases. J Chem Neuroanat, 74, 28–46. doi:10.1016/j.jchemneu.2016.01.010 [DOI] [PubMed] [Google Scholar]
- Vohs KD, Mead NL, & Goode MR (2006). The psychological consequences of money. Science, 314(5802), 1154–1156. doi:10.1126/science.1132491 [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Boeve BF, Senjem ML, Gunter JL, DeJesus-Hernandez M, … Josephs KA (2012). Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain, 135(Pt 3), 794–806. doi:10.1093/brain/aws001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Strobl EV, Sturm VE, Shany-Ur T, Poorzand P, Grossman S, … Rankin KP (2015). Impaired Recognition and Regulation of Disgust Is Associated with Distinct but Partially Overlapping Patterns of Decreased Gray Matter Volume in the Ventroanterior Insula. Biol Psychiatry doi:10.1016/j.biopsych.2014.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JW, McCarthy M, Holley SR, & Levenson RW (2010). Physiological down-regulation and positive emotion in marital interaction. Emotion, 10(4), 467–474. doi:10.1037/a0018699 [DOI] [PubMed] [Google Scholar]
- Zaki J, & Ochsner KN (2012). The neuroscience of empathy: progress, pitfalls and promise. Nat Neurosci, 15(5), 675–680. doi:10.1038/nn.3085 [DOI] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, …Seeley WW (2010). Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain, 133(Pt 5), 1352–1367. doi:10.1093/brain/awq075 [DOI] [PMC free article] [PubMed] [Google Scholar]


