Abstract
Simian immunodeficiency virus (SIV) infection in rhesus macaques is often characterized by high viremia and CD4 T cell depletion. By contrast, SIV infection in African nonhuman primate natural hosts is typically nonpathogenic despite active viral replication. Baboons are abundant in Africa and have a geographical distribution that overlaps with natural hosts, but they do not harbor SIVs. Previous work has demonstrated baboons are resistant to chronic SIV infection and/or disease in vivo but the underlying mechanisms remain unknown. Using in vitro SIVmac infections, we sought to identify SIV restriction factors in baboons by comparing observations to the pathogenic rhesus macaque model. SIVmac replicated in baboon PBMC but had delayed kinetics compared to rhesus PBMC. However, SIVmac replication in baboon and rhesus isolated CD4 cells were similar to the kinetics seen for rhesus PBMC, demonstrating intracellular restriction factors do not play a strong role in baboon inhibition of SIVmac replication. Here, we show CD8 T cells contribute to the innate SIV-suppressive activity seen in naïve baboon PBMC. As one mechanism of restriction, we identified higher production of MIP-1α, MIP-1β, and RANTES by baboon PBMC. Contact between CD4 and CD8 T cells resulted in maximum production of these chemokines and suppression of viral replication, whereas neutralization of CCR5-binding chemokines in baboon PBMC increased viral loads. Our studies indicate baboon natural restriction of SIVmac replication is largely dependent on CD4-extrinsinc mechanisms mediated, in part, by CD8 T cells.
Keywords: baboon, AIDS, cytokine, chemokine, CCR5
1. Introduction
Human immunodeficiency virus (HIV) resulted from multiple cross-species transmission events of simian immunodeficiency viruses (SIV). Accidental or experimental SIV infection of a nonnatural nonhuman primate (NHP) host, like SIVmac infection of rhesus macaques, causes a disease resembling human Acquired Immunodeficiency Syndrome (AIDS), characterized by high viremia, CD4 T cell depletion, and opportunistic infections [1]. In contrast to macaques, SIV infection of natural NHP hosts, including SIVagm infection of African green monkeys and SIVsmm infection of sooty mangabey monkeys, typically does not cause disease despite active viral replication [2, 3]. Understanding the mechanisms that underlie differences in disease outcomes for natural and nonnatural NHP hosts have proven valuable for the study of AIDS progression. Nevertheless, neither animal model is able to resist or clear SIV infection.
Baboons (Papio hamadryas sp.) are abundant in Africa, along with natural NHP hosts, but are not endemically infected with SIV. This is unexpected when considering the geographical distribution of baboon subspecies vastly overlaps with that of natural NHP hosts, primarily African green monkeys. Furthermore, African green monkeys comprise a significant portion of a baboon’s diet [4]. Despite exposure to SIV through co-habitation and predation, baboons still remain largely SIV-negative. Field studies in Tanzania and Ethiopia have documented some baboons to be positive for antibodies to SIVagm, but the prevalence was less than 1% [5]. Interestingly, one of the seropositive baboons harbored fragments of an integrated SIVagm variant, suggesting the animal had been infected although was not producing virus [6].
Previous studies have interrogated SIV tropism for baboons in vivo and in vitro. Benveniste et al. challenged baboons with an SIV strain from pig-tailed macaques (SIVMne) and reported no clinical signs of disease, undetectable virus in circulation and tissues, and lack of seroconversion up to 1 year post-inoculation [7]. Earlier in vitro studies demonstrated baboon lymphocytes are susceptible to infection with SIVmac, but virus growth was less efficient than in rhesus macaque lymphocytes [8, 9]. In support of this finding, Cranage et al. showed baboons can support persistent SIVmac infection in vivo; however, baboons did not progress to disease or develop any tissue pathology at the microscopic level [10]. The processes by which baboons resist infection and/or disease in vivo and restrict virus growth in vitro remain unclear. Previous studies have investigated immune correlates of viral suppression in a baboon model of HIV-2 infection, a virus genetically close to SIVsmm and SIVmac [11, 12]. However, these animals were challenged with dual-tropic HIV-2 strains, which do not model the CCR5-tropic SIVs baboons would encounter in the wild. Further investigation in an appropriate SIV-baboon system could uncover mechanisms of natural SIV resistance in baboons that can be applied towards the development of novel antiviral strategies against HIV.
In this study, we used in vitro infections to identify the key baboon cell types involved in SIV suppression and to elucidate a mechanism of viral restriction. Here we report that SIVmac has an equal capacity to bind, enter, and replicate in baboon and rhesus macaque isolated CD4 cells. However, virus growth is dampened in baboon PBMC, where other immune cell types are present. Restriction in baboon PBMC is mediated, in part, by contact of CD4 cells with CD8 T as well as by high production of MIP-1α/CCL3, MIP-1β/CCL4, and RANTES/CCL5, chemokines that compete with the virus for access to the entry co-receptor, CCR5.
2. Materials and Methods
2.1. Animals and cell separation
Whole blood in EDTA was obtained from SIV seronegative baboons (Papio hamadryas sp., n = 74) and Indian rhesus macaques (Macaca mulatta, n = 57) from the Southwest National Primate Research Center (SNPRC) at the Texas Biomedical Research Institute (TBRI). Distribution of age and gender of the animals is shown in Supplementary Table 1. Animal care and treatments were all in accordance with protocols approved by the TBRI Institutional Animal Care and Use Committee (IACUC). Animals were serologically screened for simian T-lymphotropic virus (STLV) and SIV antibodies by Luminex assay. Peripheral blood mononuclear cells (PBMC) were isolated by gradient centrifugation using Lymphocyte Separation Medium (Cellgro, Corning). Cells were washed twice with PBS before phenotyping by flow cytometry. CD4 cells were sorted from freshly isolated PBMC by positive selection using magnetic beads coated with anti-CD4 (clone L200) antibodies, as per the manufacturer’s instructions (IMag™ Human CD4 T Lymphocyte Enrichment Set-DM, BD Biosciences). Purity of the positive fraction was assessed by flow cytometry using a clone of anti-CD4 antibody that differed from that used for sorting (CD4-APC, clone 13B8.2, Beckman-Coulter).
2.2. Flow cytometry
PBMC were stained with various combinations of the following monoclonal antibodies: CD3-V500 (clone SP34.2, BD-Biosciences), CD4-PerCp-Cy5.5 (clone L200, BD-Biosciences) or CD4-APC, clone 13B8.2, Beckman-Coulter), CD8-FITC (clone 3B5, Invitrogen, ThermoFisher), CCR5-PE (clone 3A9, BD-Biosciences). After 30 min of incubation at 4°C, cells were washed with cold PBS then fixed in PBS containing 1.6% methanol-free formaldehyde (Polysciences). Data was collected on a three-laser CyAn ADP (Beckman-Coulter) and analyzed on FlowJo version 10 software.
2.3. PBMC and CD4 cell infections
Prior to infection, freshly isolated PBMC or CD4 cells were cultured for 48 hr in Roswell Park Memorial Institute (RPMI) 1640 medium with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, 25 mM HEPES, 1% non-essential amino acids (NEAA), and 1% L-glutamine. For some experiments, cells were stimulated for 48 hr with 5 μg/mL Phytohemagglutinin-L (PHA-L) (Sigma-Aldrich). Cells were resuspended in fresh medium and infected with SIVmac251 at a M.O.I. of 0.01 TCID50/cell by Magnetofection™ (OZ Biosciences) according to the manufacturer’s protocol. Briefly, cells were pelleted in 5 ml polypropylene tubes. Virus was incubated with cationic magnetic nanoparticles (ViroMag R/L, OZ Biosciences) at 4°C for 15 min before addition to pelleted cells. The final volume in the tube after addition of virus to cells did not exceed 500 μL. Tubes were placed at 37°C for 2 hr over a magnet to concentrate the nanoparticle-associated virus onto the cell pellet. Cells were washed twice to remove unbound virus. Cultures were maintained at 1-2 x 106 cells/mL in complete RPMI-10 with 50 U/mL IL-2 (NIH AIDS Reagent Program). For some infections, cells were pre-treated and cultured in 1 μM of the CCR5 antagonist maraviroc (NIH AIDS Reagent Program). Chemokines were inhibited using polyclonal goat IgG neutralizing antibodies against MIP-1α, MIP-1β, and RANTES (R&D Systems). Infected cultures received 2 μg/mL of each antibody; an equal volume of normal goat serum was added as a control. Twice weekly, half the media was changed and treatments replenished appropriately. Supernatant was saved for quantification of viral loads and cytokines.
For single-cycle infections, CD4 cells were infected at a M.O.I. of 1 TCID50/cell. Cells were incubated over a magnet with nanoparticle-associated virus at 4°C for 1 hr to allow binding but restrict entry. Cells were washed twice with ice cold PBS before harvesting an aliquot of cells for analysis. Remaining cells were resuspended in media and placed at 37°C for 1 hr to promote entry and progression through replication stages. Cells were then cultured in 200 nM of the protease inhibitor darunavir (NIH AIDS Reagent Program) to prevent subsequent rounds of infection. Cells were harvested at 12, 24, and 48 h.p.i. Cellular RNA was extracted with the RNeasy Mini Kit (Qiagen) then treated with DNase (TURBO DNA-free™ kit, Ambion, ThermoFisher). The Gentra Puregene Cell Kit (Qiagen) was used to extract and RNase-treat cellular DNA. Viral RNA was isolated from culture supernatants using the QIAamp Viral RNA Mini Kit (Qiagen). Before isolation, supernatant samples were spiked with a known amount of Qβ bacteriophage (Attostar) to control for efficient extraction and amplification of recovered RNA.
2.4. CD4, CD8 T, and NK cell co-cultures
CD8 T and NK cells were positively sorted from CD4-depleted PBMC by flow cytometry. CD4 depleted-PBMC were stained with antibodies to CD3-APC (clone Sp34.2, BD-Biosciences), CD8-Pacific Blue (clone 3B5, Invitrogen, ThermoFisher), and NKG2A-PE (clone Z199, Beckman-Coulter). CD8 T cells were sorted as CD3+CD8+CD4− and NK cells as CD3−NKG2A+. Sorting was performed on a four-laser FACS Aria III (BD-Biosciences). Data from the sorting was collected on FACSDiva version 8 software and analyzed on FlowJo version 10 software. Cells were cultured overnight at 37°C before downstream application. CD4 cells were infected in isolation at a M.O.I. of 0.01 as described in section 2.3. After washes to remove unbound virus, sorted CD8 T and NK cells were added to uninfected or infected CD4 cell cultures in a 48-well plate in various combinations. The ratio of CD4 to CD8 T cells was 1:1 and CD4 to NK cells was 1:0.3. Ratios were selected to reflect the relative abundance of these cell types in baboon PBMC based on previous flow cytometry data from our laboratory [13]. For contact-dependency studies, CD8 T and NK cells were added to the surface of a 0.4 μm permeable membrane (Transwell®, Corning) in a 24-well plate. CD4 cells were cultured in the lower compartment. Segregation between the two compartments was confirmed by flow cytometry. The input and concentration of each cell type was equal across all conditions.
2.5. Luminex assays
Viral loads were monitored by measurement of SIV p27 in culture supernatant. Briefly, samples were left undiluted or diluted with RPMI-10 at 1:5, 1:10, or 1:100. Samples were lysed in buffer containing 0.2% Tween 20 for 1 hr at room temperature then incubated with capture beads coupled to an anti-SIV Gag p27 antibody (clone 55-2F12, NIH AIDS Reagent Program) at 4°C overnight. The following day, samples were washed then incubated for 1 hr at room temperature with plasma from an SIV+ rhesus macaque (containing anti-SIV antibodies). Lastly, samples were exposed to goat anti-human IgG-PE (Santa Cruz Biotechnology) antibody for 1 hr at room temperature before reading. The concentration of p27 in samples was determined by generating a standard curve from a SIVmac239 viral stock of known concentration [14].
Culture supernatants were assayed for nonhuman primate cytokines and chemokines as previously described [15]. The following analytes were included in the multiplex panel: G-CSF, GM-CSF, Gro-α, IFN- α, IFN-γ, IL-1β, IL-1Ra, IL-4, IL-6, IL-8, IL-10, IL-13, IL-17, IP-10, MCP-1, MIG, MIP-1α, MIP-1β, Perforin, RANTES, sCD40L, TNF-α, TNF-β. Samples were diluted with RPMI-10 at 1:3 or 1:5. Concentrations were determined using human cytokines and chemokines as standards. All plates were read on the Luminex® 100/200 xMAP system (Luminex Corp.) and data was analyzed using MasterPlex™ QT version 1.0 (MiraiBio Corp.)
2.6. Real-time PCR
The sequences for all primers and probes are listed in Table 1. Real-time RT-PCR reactions were prepared using the RNA Ultra Sense™ One-Step Quantitative RT-PCR system (Applied Biosystems, ThermoFisher). Viral loads in supernatant were determined by quantification of SIV gag using published primer and probe sequences [16]. Qβ bacteriophage (internal control) was detected using primers and probe purchased from the manufacturer (Attostar). The final concentration of SIV gag primers and probe were 200 nM and 100 nM, respectively. 5 μL of template RNA was added to a final reaction volume of 25 μL. Real-time RT-PCR reactions were carried out as follows: 50°C for 20 min, 95°C for 2 min, 40 cycles of 95°C for 15 sec and 60°C for 1 min. The concentration of SIVmac was determined using a standard curve generated from SIVmac genomic RNA ranging from 10 to 106 copies/μL.
Table 1.
Sequence of primers used for RT-PCR and PCR.
| Name+ | Sequence (5′f to 3′f) |
|---|---|
| SIV gag [16] | |
| SIVgag510F | GCC AGG ATT TCA GGC ACT GT |
| SIVgag592R | GCT TGA TGG TCT CCC ACA CAA |
| SIVgag535P | (FAM)-AAG GTT GCA CCC CCT ATG ACAT TAA TCA GAT GTT A-(TAMRA) |
| SIV 2-LTR [18] | |
| 2LTRF | TAA GCT AGT GTG TGT TCC CAT |
| 2LTRR | CTC CTG TGC CTC ATC TGA TAC A |
| 2LTRP | (FAM)-AGC CGC CGC CTG GTC AAC TCG-(TAMRA) |
| MIP1A/CCL3 | |
| MIP1A17F | CCG GCA GAT TCC ACA GAA TT |
| MIP1A247R | TCT GGA CCC ACT CCT TAC TG |
| MIP1A192P | (FAM)-ACC TGC CGG CCT CTC TTG GTT AGG-(TAMRA) |
| MIP1B/CCL4 | |
| MIP1B13F | GTG ACT GTC CTG TCT CTC CT |
| MIP1B143R | ACA AAG TTG CGA GGA AGC TT |
| MIP1B86P | (FAM)-ACC CTC CCA CCT CCT GCT GCT-(TAMRA) |
| GAPDH | |
| G590F | CAA CAG CCT CAA GAT CGT CA |
| G697R | GTG GTC ATG AGT CCT TCC AC |
| G633P | (FAM)-TGC TTA GCA CCC CTG GCC AAG GT-(TAMRA) |
| OSM [17] | |
| OSMF | CCT CGG GCT CAG GAA CAA C |
| OSMR | GGC CTT CGT GGG CTC AG |
| OSMP | (VIC)- TAC TGC ATG GCC CAG CTG CTG GAC AA-(MGBNFQ) |
F = forward primer, R = reverse primer, P = probe
For measurement of cell-associated viral RNA and host gene expression, 30–50 ng of template RNA, as determined by spectrophotometry (NanoDrop™-2000), was added to each real-time RT-PCR reaction. Reaction volumes and cycle conditions were as described above. The sequences for Oncostatin-M (OSM) primers and probe were obtained from Bruce et al. (2005) [17]. Sequences of primers and probes for MIP1A, MIP1B, and GAPDH are listed in the Table 1. A final concentration of 200 nM was used for all primers and 100 nM for all probes. The amount of cell-associated SIVmac and chemokine expression was relatively quantified as 2 Ct using endogenous control genes GAPDH and OSM, respectively.
Real-time PCR reactions for quantification of SIV DNA replication stages were set up per the manufacturer’s instructions (PlatinumR Quantitative PCR SuperMix-UDG, Invitrogen, ThermoFisher). Reactions were carried out in a 25 μL volume containing 30–50 ng of template DNA. PCR conditions were 50°C for 2 min, 95°C for 2 min, 40 cycles of 95°C for 15 sec and 60°C for 1 min. Primers and probe targeting SIV 2-LTR were designed and used in the assay as described elsewhere [18]. The levels of SIV gag and 2-LTR DNA were represented as 2 Ct, using OSM as an endogenous control.
Primers and probes were designed on Geneious R7 (Biomatters). All real-time PCR assays were run on an ABI 7500 system. Data was collected and analyzed on the accompanying SDS System Software version 1.5.1.
2.7. Statistics
Prism 6 (GraphPad Software) was used to create figures and perform statistical analysis. The statistical test used for each experiment is noted in the figure legends. Significance was defined as a P value of <0.05.
3. Results
3.1. SIVmac251 infection is restricted in baboon PBMC
Previous studies reported baboon PBMC are permissive to SIVmac and SIVMne in vitro [8, 9]. We sought to confirm these findings as well as compare the kinetics of SIVmac251 growth between unstimulated and PHA-stimulated PBMC. In accordance with the literature, we observed that although baboon PBMC are susceptible to SIVmac251 infection, virus growth is stunted relative to the kinetics seen in rhesus macaque PBMC (Fig. 1A). The levels of p27 in the supernatant of baboon cultures did not reach the same levels measured in rhesus macaque cultures until about 17 days post-infection (d.p.i.). PBMC from some baboon donors remained largely refractory to SIVmac251. Stimulation with PHA allowed cells from almost all baboon donors to replicate virus, but kinetics were still delayed compared to rhesus macaques (Fig. 1B). All rhesus macaque donors were permissive even without PBMC stimulation.
FIG 1.
SIVmac251 growth is restricted in baboon PBMC. PBMC were isolated from whole blood of rhesus macaques (open circles) or baboons (solid circles) then cultured for 48 hr in (A) RPMI-10 or (B) RPMI-10 containing PHA. PBMC were then infected with SIVmac251 at an M.O.I. of 0.01. Viral loads were quantified by measuring p27 in the supernatant by Luminex. In both panels, each curve represents one animal (n = 10). Repeated measures two-way ANOVA was used for statistical analysis (*P ≤ 0.05, ***P ≤ 0.001, ****P ≤ 0.0001).
Restriction of SIVmac251 in baboon PBMC could be explained by a lower abundance of SIV target cells and entry receptors, specifically CCR5+CD4+ cells which are the major target of SIV infection. However, flow cytometry analysis confirmed that baboons and rhesus macaques had comparable levels of CD4+ and CD4+CCR5+ T cells (Fig. 2A). Furthermore, there were no significant differences in surface expression levels of CD4 or CCR5 on unstimulated CD4+ T cells, although PHA stimulation did reduce surface CCR5 on rhesus macaque cells (Fig. 2B). Differences in viral loads between species in the RPMI condition was not due to initial activation status either, as unstimulated baboon and rhesus macaque CD4 cells in PBMC had similar expression of activation markers CD69, HLA-DR, and CD25 (Fig. S1).
FIG 2.
Baboon and rhesus macaque PBMC have similar levels of SIV target cells and entry receptors. PBMC were isolated from whole blood of rhesus macaques (open circles, n = 9) and baboons (solid circles, n = 8). PBMC were phenotyped by flow cytometry following isolation (Fresh) and after 48 hr in culture with RPMI-10 (RPMI) or RPMI-10 containing PHA. A) Percentage of CD4+ T cells within lymphocytes and percentage of CCR5+ cells within CD4 + T cells. B) Median fluorescence intensity (MFI) of CD4 and CCR5 expression on CD4 T cells. In all panels, lines indicate the median and each point represents one animal. Mann-Whitney tests were used for statistical analysis (***P ≤ 0.001). n.s.: not significant.
It is known that SIV entry can be achieved through alternative co-receptors, such as CXCR6 (Bonzo) and GPR15 (Bob) [19–21]. Therefore, we sought to confirm CCR5 as the principal co-receptor used by SIVmac251 in baboon CD4 cells. Treatment of baboon sorted CD4 cells (Fig. S2A) with maraviroc, a CCR5 antagonist [22], inhibited SIVmac251 growth to nearly undetectable levels, strongly implicating CCR5 as the major entry co-receptor (Fig. S2B). Thus, despite having similar levels of target cells and entry receptors as rhesus macaques, SIVmac251 replication is less efficient in baboon PBMC.
3.2. SIVmac251 replication in a single-cycle infection is similar in baboon and rhesus macaque isolated CD4 cells
Our flow cytometry analysis revealed baboon and rhesus macaque CD4 T cells have similar levels of CD4 and CCR5 surface expression; however, this does not exclude the possibility that SIVmac251 has a weak capacity to engage baboon receptors. Therefore, we determined if SIVmac251 equally bound baboon and rhesus macaque CD4 cells in isolation. Quantification of cell-bound viral RNA, while internalization was inhibited by low temperatures, showed no significant difference in the amount of surface-bound SIVmac251 between CD4 cells from both species (Fig. 3A).
FIG 3.
The levels of SIVmac251 binding, intracellular replication, and egress are similar between baboon and rhesus macaque isolated CD4 cells. CD4 cells were sorted from baboon (dark bars) or rhesus macaque (light bars) PBMC and cultured for 48 hr before infection. Cells were infected with SIVmac251 at an M.O.I. of 1; viral binding was synchronized by keeping cells at 4°C for 1 hr. (A) Cells were washed twice before RNA extraction; SIV RNA was measured by real-time RT-PCR, using GAPDH mRNA to normalize RNA input and for relative quantification (2 Ct) (n = 5). (B-C) After incubation at 4°C, cells were placed at 37°C for 1 hr to allow entry. A protease inhibitor, darunavir, was added to cultures to restrict the virus to a single replication cycle. Cells were lysed at indicated time points and SIV DNA was measured by real-time PCR using Oncostatin-M gene to normalize DNA input (n = 3). (D) Supernatant was harvested at 48 h.p.i. and SIV RNA was quantified by real-time RT-PCR (n = 5). In all panels, bars represent the mean ± SD. n.s.: not significant.
We next asked if baboon intracellular factors were responsible for SIVmac251 restriction. Sorted CD4 cells from rhesus macaque and baboon donors were infected in isolation with SIVmac251 and cultured in the presence of the protease inhibitor, darunavir, to confine the virus to a single replication cycle. A real-time PCR assay modeled after Butler et al. [23] was used to quantify SIV replication stages over the course of 48 hr. Total SIV DNA was amplified to quantify stages after reverse transcription. The 2-long terminal repeat (2-LTR) circle, a circular form of the HIV/SIV genome, serves as a marker of nuclear transport, as it is formed exclusively in the nucleus and its levels are proportional to the amount of imported viral DNA [24]. Baboon and rhesus macaque CD4 cells had comparable levels of total SIVmac251 DNA (Fig. 3B) and 2-LTR DNA (Fig. 3C) at 12, 24, and 48 h.p.i. Quantification of extracellular SIVmac251 genomic RNA revealed no significant differences in viral release between species (Fig. 3D). Total SIV DNA represents the contribution of nascent linear cDNA, circular DNA, and integrated DNA. As there was no asynchrony in progression from nuclear import to extracellular RNA and total SIV DNA levels were similar between species, it is likely that integrated DNA levels are also similar. Thus, the kinetics of SIVmac251 reverse transcription, nuclear import, integration, and egress are similar between baboon and rhesus macaque CD4 cells in isolation.
3.3. SIVmac251 growth in isolated CD4 cells is comparable between baboons and rhesus macaques
In our previous experiments we limited SIV infection of CD4 cells to a single round of replication. However, the antiviral host defense includes intracellular restriction factors, such as APOBEC3 and SERINC3/5, which act on subsequent rounds of replication [25–27]. We evaluated the potential contribution of such processes in restricting SIVmac251 in baboons by performing a multi-round infection in isolated CD4 cells. We observed that, in the absence of other cell types, SIVmac251 grows to the same efficiency in baboon and rhesus macaque CD4 cells (Fig. 4A). Noticeable, and in contrast to the previous observations in PBMC, the majority of baboon CD4 cell donors were permissive to infection without PHA stimulation.
FIG 4.
SIVmac251 growth is similar in baboon and rhesus macaque isolated CD4 cells. (A) CD4 cells were sorted from baboon (closed triangles, n = 10) or rhesus macaque (open triangles, n = 8) PBMC, cultured for 48 hr in RPMI-10, then infected with SIVmac251 at a M.O.I of 0.01. (B-C) Species-matched compilation of viral loads data from infections in PBMC (shown in Fig. 1A) and sorted CD4 cells (shown in Fig. 4A). (D) PBMC and sorted CD4 cells from the same baboon donor (n = 5) were infected with SIVmac251 at a MOI of 0.01. (E) PBMC were isolated from STLV seronegative (n = 5) and seropositive (n = 5) baboon donors of the same age (4 y/o). PBMC were infected with SIVmac251 at a MOI of 0.01. Viral loads were quantified by measuring p27 in the supernatant by Luminex. In all panels, each curve represents one animal. Repeated measures two-way ANOVA was used for statistical analysis (*P ≤ 0.05, ****P ≤ 0.0001).
When evaluating SIVmac251 replication kinetics in rhesus macaque cultures, it was clear that PBMC and CD4 cells from all donors were largely permissive to infection. There was no evidence of strong contributions from other immune cell types in restricting viral replication in rhesus macaque PBMC, as SIV growth kinetics in PBMC were comparable to those in isolated CD4 cells (Fig. 4B). Similar to rhesus macaques, there was little variation in viral growth kinetics among most baboon CD4 cell donors (Fig. 4C); however, and differently from what was seen for rhesus macaque cells, roughly 50% of baboon PBMC donors were refractory to SIVmac251 infection when compared to CD4 cells. To address if restriction of SIVmac251 in baboon PBMC but not in isolated CD4 cells was consistent between donors, we contemporaneously infected PBMC and CD4 cells isolated from the same donor. In accordance with our previous data, PBMC from all baboon donors were susceptible to infection but grew the virus to varying titers by 17 d.p.i. (4.50 x 104 ± 5.09 x 104 pg/mL of p27). In contrast, CD4 cell viral loads were more uniform across all donors and were roughly 2 logs higher than in PBMC at peak infection (5.53 x 106 « 2.37 x 106 pg/mL p27) (Fig. 4D).
Because a considerable number of wild and captive baboons harbor simian T-lymphotropic virus (STLV) [28–30], we evaluated if underlying STLV infection was associated with the observed variability in SIVmac251 growth kinetics between baboon donors. No significant difference was observed in SIVmac251 replication between PBMC from age-matched STLV- and STLV+ baboon donors (Fig. 4E), indicating STLV status does not impact the outcome of SIVmac251 infection in baboon PBMC.
Our findings from isolated baboon CD4 cell infections demonstrate a very permissive environment and suggest that intracellular restriction factors are likely not responsible for the inhibition of SIVmac251 replication observed in baboon PBMC.
3.4. CD8 T and NK cells restrict SIVmac251 infection in baboon CD4 cells
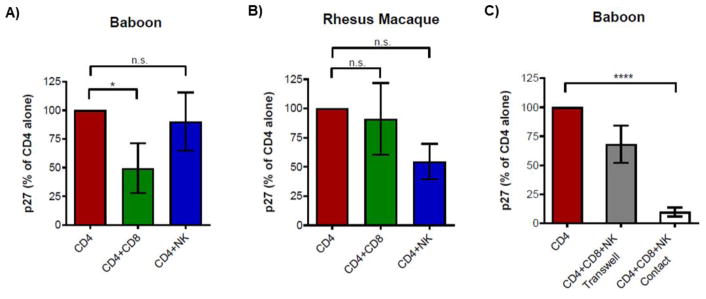
We hypothesized that inhibition of SIVmac in baboon PBMC could be mediated by other immune cell types present in PBMC but absent in sorted CD4 cultures. To mimic the baboon PBMC environment in a more controlled fashion as well as identify key SIV-suppressive cell types, we performed add-back studies in CD4 cultures. CD8 T and NK cells were of primary interest because of their canonical roles in antiviral defense. CD4, CD8 T, and NK cells were sorted from PBMC isolated from baboons and rhesus macaques (Fig. S3). CD4 cells were infected in isolation then co-cultured with autologous CD8 T and NK cells in various combinations, at ratios reflective of their relative abundance to CD4 cells in baboon [13] and rhesus PBMC. The input and concentration of CD4 cells was normalized across all conditions. As expected, CD4 cells cultured alone had the highest levels of p27 at peak infection. The presence of NK cells had varying degrees of impact on SIVmac251 replication between baboon and rhesus macaque donors (Fig. 5A and 5B). Interestingly, the addition of CD8 T cells to baboon CD4 cultures could reduce viral loads by an average of 50% whereas CD8 T cells in rhesus macaque CD4 cultures had no significant effect on reducing viral loads. It is important to note that all donors were SIV-naïve, and so restriction of SIVmac by baboon CD8 T cells was not due to a cellular memory response as has previously been reported for other viral strains [12].
FIG 5.
CD8 T cells suppress SIVmac251 infection in baboon CD4 cells. Sorted CD4 cells were infected with SIVmac251 and cultured in isolation or with the addition of autologous CD8 T and NK cells. (A-B) The same number of SIV-infected CD4 cells from baboons (n = 6) or rhesus macaques ( n = 3) were cultured alone, with CD8 T cells, or with NK cells. (C) CD8 T and NK cells were cultured with infected CD4 cells in contact or separated by a 0.4 μm membrane (Transwell). In all panels, viral loads were quantified by measuring p27 in the supernatant by Luminex. Bar graphs display SIV p27 values normalized to the CD4 alone condition at peak infection (10–14 d.p.i.) (mean ± SEM). One-way ANOVA was used for statistical analysis (*P ≤ 0.05, ****P ≤ 0.0001). n.s.: not significant.
We asked if the SIV-suppressive effects of CD8 T and NK cells in baboons were contact-dependent. Compared to cultures that had CD8 T or NK cells in contact, restriction of SIVmac251 was diminished when CD8 T and NK cells were segregated from infected CD4 cells by a semi-permeable membrane (Fig. 5C). Here, we also observed CD4 cultures containing both CD8 T and NK cells had peak p27 levels that were, on average, 75% lower than in cultures containing CD4 cells alone. This finding was consistent across all baboon donors. Contact-dependent inhibition of SIVmac251 replication in baboon CD4 cells was not due to cytotoxic responses, as there was no significant loss of CD4 cells during co-culture with CD8 T or NK cells (data not shown). Altogether, these studies show baboon CD8 T cells require contact with CD4 cells to mediate control of SIVmac251 through a mechanism of innate immunity.
3.5. Baboon PBMC produce higher levels of CCR5-binding chemokines
Lastly, we analyzed the cytokine milieu in baboon cultures to aid in the identification of mechanisms responsible for SIVmac251 restriction. Our Luminex multiplex platform included 22 analytes that varied in classification and function (cytokines, chemokines, growth factors, cytolytic proteins, etc.). Early after infection, there were no significant differences in levels of most analytes between these species including key antiviral response regulators, TNF-α and IFN-γ (Fig. S4A). However, three chemokines that showed significantly different levels between rhesus macaque and baboon cells were MIP-1α (macrophage inflammatory protein-1 alpha, CCL3), MIP-1β (CCL4), and RANTES (regulated on activation, T cell expressed and secreted, CCL5), which were of early interest because they are the natural ligands for CCR5, the primary co-receptor used by HIV and SIV for entry into CD4 cells [31]. The levels of MIP-1α, MIP-1β, and RANTES were roughly 10-fold higher in baboon PBMC than in rhesus macaque PBMC at 3 d.p.i. (Fig. 6A). High production of these chemokines by baboon PBMC was independent of infection, as levels were similar in uninfected cultures. This trend was also evident before infection, particularly for MIP-1α (Fig. S4B). Differences between baboon and rhesus macaque MIP-1α and MIP-1β production were confirmed at the mRNA level, indicating the high chemokine concentration measured in baboon cultures was not due to differences in binding to baboon or rhesus macaque molecules by the anti-human chemokine antibodies used in our Luminex assays (Fig. S5).
FIG 6.
Elevated production of CCR5-binding chemokines contributes to SIVmac251 restriction in baboon PBMC. (A) Identification of MIP-1α, MIP-1β, and RANTES in PBMC supernatants at 3 d.p.i. PBMC were isolated from baboons (dark bars) and rhesus macaques (light bars) and cultured for 48 hr in medium alone (RPMI) or medium containing PHA then infected with SIVmac251at an M.O.I. of 0.01 (n = 7). Mann-Whitney tests were used for statistical analysis. Top and bottom of boxes represent first and third quartile, band is median, whiskers show minimum and maximum values. (B-C) PBMC were infected with SIVmac251 at an M.O.I. of 0.01. Cultures were treated with medium, normal goat serum, or goat polyclonal anti-chemokine neutralizing antibodies. Viral loads were assessed by measuring p27 in the supernatant by Luminex. Bar graphs display p27 MFI values normalized to the untreated condition at peak infection (10 d.p.i.) (mean ± SEM) (RPMI: n = 6, PHA: n = 5). Paired t-test was used for statistical analysis. (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001). (D-E) Correlation analysis for total CCR5-binding chemokine concentration at 3 d.p.i. versus log of SIV p27 concentrations at 10 d.p.i. in baboon (closed 20 circles, n = 14) or rhesus macaque (open circles, n = 13) PBMC. Line represents linear regression and each point represents a different donor.
We then evaluated the contribution of MIP-1α, MIP-1β, and RANTES in suppressing SIVmac251 infection in baboon PBMC by inhibiting their activity with neutralizing antibodies. Our preliminary studies showed that neutralization of a single CCR5-binding chemokine had little to no effect on p27 levels (data not shown). However, simultaneous neutralization of all three chemokines in baboon cultures increased viral loads at 10 d.p.i. by roughly 2-fold in unstimulated PBMC (Fig. 6B) and 4 fold in PHA-stimulated PBMC (Fig. 6C); this effect could be seen at an earlier time in infection (6 d.p.i.) (Fig. S6). Furthermore, we observed a statistically significant correlation between increased CCR5-binding chemokine production and reduced viral loads in baboon PBMC (Fig. 6D). None of these findings were observed in rhesus macaque PBMC cultures, which also lacked an inverse correlation between chemokine production and viral loads (Fig. 6E).
Thus, the combined effects of MIP-1α, MIP-1β, and RANTES contribute to early restriction of SIVmac in baboon PBMC.
3.6. CD8 T and NK cells increase CCR5-binding chemokine levels in baboon CD4 cell cultures
Our add-back studies in isolated baboon CD4 cell cultures demonstrated CD8 T cells contribute to restriction of SIVmac251. CD4 T, CD8 T, and NK cells have all been reported to produce antiviral concentrations of MIP-1α, MIP-1β, and RANTES [31–36]. Therefore, we asked if CD8 T cells reduced viral loads by boosting production of CCR5-binding chemokines. Cytokine analysis showed baboon CD4 cells in isolation produced MIP-1α, MIP-1β, and RANTES (Fig. 7A). By contrast, the majority of CD8 T cells cultured alone produced undetectable levels of these chemokines. In some donors, the addition of just NK cells to CD4 cultures moderately elevated production of CCR5-binding chemokines (Fig. 7A). However, the concentrations of all chemokines increased in CD4 cultures by approximately 2- to 4-fold in the presence of CD8 T cells. The addition of CD8 T or NK cells to rhesus macaque CD4 cultures did not have a significant impact on elevating CCR5-binding chemokine production, and the levels of CCR5 binding chemokines were much lower than in baboons, as observed before for PBMC cultures (Fig 7B).
FIG 7.
CD8 T and NK cells contribute to high levels of CCR5-binding chemokines in baboon cultures. Luminex cytokine assays performed on supernatants obtained from cultures at 3.d.p.i. (A) CD8 T and NK cells were cultured alone, with uninfected or infected CD4 cells in the indicated combinations (n = 4 - 6). (B) Infected CD4 T cells from baboons (n = 6) and rhesus macaques (n = 3) were cultured with autologous CD8 T and NK cells. Graph shows total CCR5-binding chemokine levels. (C) CD8 T and NK cells were cultured with infected CD4 cells separated by a 0.4 μm membrane (Transwell) or in contact (n = 6). For box and whiskers plots, top and bottom of box represent first and third quartile, band is median, whiskers show minimum and maximum values. One-way ANOVA was used for statistical analysis (*P ≤ 0.05).
Lastly, we extended our analysis to transwell co-cultures where we observed contact between CD4, CD8 T, and NK cells provided maximum suppression of SIVmac251 (Fig. 5C). Interestingly, this condition also had the highest levels of MIP-1α, MIP-1β, and RANTES (Fig 7C). Segregation of CD8 T and NK cells from infected CD4 cells abrogated higher production of CCR5-binding chemokines, which was mirrored by a lack of SIVmac251 suppression. These findings support a model whereby interactions between baboon CD8 T and CD4 cells allow elevated production of CCR5-binding chemokines that suppress SIVmac growth by interfering with co-receptor binding.
4. Discussion
Although noninvasive studies in the wild have suggested SIVcpz infection is pathogenic for chimpanzees [37], studies of wild or captive African green monkeys, sooty mangabeys, and mandrills show a general lack of disease during SIV infection [2, 3, 38–40]. Nonpathogenic infection has long been a key area of HIV research to aid in the development of AIDS therapies. Studies in these animals could provide some insight into treating or preventing HIV-associated pathologies. Nevertheless, natural NHP hosts fail to control viral replication and thus are not an ideal model to uncover mechanisms for preventing or clearing infection. Although more than 90% of the African NHP species tested have been shown to be infected with an SIV [41], wild baboons do not harbor SIV, even though a significant portion of their diet comprises African green monkeys, a species where SIV prevalence is roughly 50% [42]. Baboon resistance to SIV challenge in vivo was first reported in 1988 [7]. Nearly 30 years later, the mechanisms underlying resistance still remain unclear. Using in vitro SIVmac infections, we sought to uncover mechanisms of SIV restriction in baboons that could explain two previously reported findings: (1) Baboon PBMC support SIVmac growth in vitro but replication kinetics are dampened (2) Baboons are susceptible to SIVmac in vivo but do not develop AIDS. A recent study identified immunogenetic factors in natural hosts that were highly divergent from rhesus macaques and so may have a strong role in preventing AIDS [43]. It would be tempting to speculate baboons resist disease during SIVmac infection by these mechanisms identified in natural hosts. However, this study revealed baboons have more homology to macaques than to African green monkeys or sooty mangabeys in these immunogenetic factors heavily implicated in AIDS resistance. An earlier study by Apetrei et al. also showed baboon T cells share more immunophenotypic features with rhesus macaques than natural hosts [44]. Thus, baboons likely resist SIVmac-induced AIDS and infection with other SIV strains through mechanisms distinct from natural hosts, which may include direct control of viral replication.
In this study, we used in vitro infections to identify key cell types and mechanisms responsible for SIVmac251 restriction in baboon PBMC. Although this in vitro system does not represent what may occur in vivo, PBMC infection has been shown to be an accurate predictor of the outcome of infection in rhesus macaques exposed to SIV [45, 46]. In accordance with our findings, other groups have reported dampened SIVmac growth kinetics in baboon PBMC relative to rhesus macaque PBMC [8, 9]. As PHA stimulation is often used to enhance in vitro retroviral infection by activating T cells and triggering cell division, we also compared SIVmac251 growth kinetics in unstimulated and stimulated PBMC from both species. PHA-stimulated baboon and rhesus macaque PBMC did show increased viral loads and growth kinetics relative to donor-matched unstimulated PBMC; however, SIVmac251 was still detectable in PBMC from both species even without stimulation. This allowed us to maintain cells in a natural state while interrogating innate antiviral responses that could otherwise be clouded by initial activation with PHA. Indeed, the difference in SIVmac251-suppressive activity between baboons and rhesus macaques was more apparent in unstimulated PBMC.
We evaluated if differences in the abundance of SIV target cells and entry receptors were responsible for dampened SIVmac251 infection in baboon PBMC. Pandrea et al. previously showed natural NHP hosts for SIV have a lower frequency of CD4+CCR5+ T cells and reduced CCR5 expression on CD4+ T cells compared to nonnatural NHP hosts. In this same study, the authors reported that baboons and rhesus macaques had comparable levels in both parameters [47]. We confirmed the latter findings in a larger baboon cohort as well as established similar levels of CD4+ T cells and surface CD4 expression on CD4+ T cells in baboon and rhesus macaque PBMC. Furthermore, we demonstrated CCR5 to be the main co-receptor used by SIVmac251 on baboon cells, since treatment with maraviroc prevented infection.
It is known that host intracellular restriction factors, such as APOBEC3G and TRIM-5α, can block infection by interfering with different stages of viral replication; viruses, in turn, have adapted strategies to overcome mechanisms of host restriction [25]. As a consequence of this co-evolution, restriction factors accumulate species-specific amino acid differences which can impose barriers to cross-species transmission [48, 49]. Thus, we explored if baboon intracellular restriction factors were more effective against SIVmac251 than those from rhesus macaques. Here, in synchronized single-cycle infections, there were no significant differences in SIVmac251 replication kinetics between baboon and rhesus macaque CD4 cells, as measured by the levels of 2-LTR circles (indicating efficient nuclear transport) and viral loads in the supernatant (indicating a lack of interference with viral budding). In contrast to the delay in SIVmac251 replication seen in baboon PBMC, virus growth in a multi-round infection was unrestricted in baboon isolated CD4 cells. Taken together, these observations indicate intracellular restriction factors, typically associated with species adaptation, do not play a significant antiviral role against SIVmac251 in baboons. Future work will be aimed at evaluating if these observations are applicable across other strains of SIV, such as SIVagm.
Unrestricted growth of SIVmac251 in baboon isolated CD4 cells raised the possibility that suppression of SIVmac251 in baboon PBMC is mediated by cell types removed during enrichment for CD4 cells. Our add-back studies established contributions from baboon CD8 T in restricting SIVmac251 growth in a contact-dependent manner. While CD8 T or NK cells alone could modestly reduce CD4 cell viral loads, the strongest inhibition of viral growth was seen in CD4 cultures containing both CD8 T and NK cells. While this dramatic response could be an additive effect, evidence of synergy between cell types was observed. For some baboon donors, the addition of NK cells alone had no impact on viral loads, but when NK cells were added to CD4 cultures containing CD8 T cells, SIVmac251 suppression was boosted beyond that observed by adding only CD8 T cells. These observations point to a potentially complex interplay between all cell types, whereby the antiviral or immunomodulatory activity of one cell type is activated or amplified in the presence of a different cell type.
Suppression of HIV and SIV replication by CD8 T and NK cells is well-known [50, 51]. CD8 T cells, in particular, have been reported to have a strong role in controlling SIV and HIV-2 infection in rhesus macaques and baboons, respectively [12, 52–54]. However, in these previous studies, significant suppression of viral replication in vitro was seen by CD8 T cells from infected but not uninfected animals, indicative of major contributions from an antigen-specific memory response; in addition, suppression was largely attributed to cytolytic activity [12, 52]. Our in vitro studies did not show any significant inhibition of SIVmac growth by CD8 T cells from SIV-naïve rhesus macaques. On the contrary, the strong viral inhibition by naïve baboon CD8 T cells demonstrates major contributions from components of innate immunity. Furthermore, we observed little to no CD4 cell loss in SIV-infected baboon cultures containing CD8 T cells, suggesting direct lysis of infected cells does not play a major role in the contact-dependent suppression of SIVmac251 growth by these baboon cell types. In support of our findings, several studies have reported contributions from non-cytolytic CD8 T cell responses in controlling HIV and SIV production [55, 56]. Currently, we are elucidating the role of these and other innate effector functions of baboon CD4 and CD8 T cells.
While interrogating the cytokine milieu, we identified elevated CCR5-binding chemokines as one mechanism of SIVmac251 restriction in baboon cells. When in culture, whether stimulated or not, baboon PBMC produced higher levels than rhesus macaques of CCR5 ligands MIP-1α, MIP-1β, and RANTES, which can all interfere with HIV and SIV entry by hindering access to docking sites or causing receptor internalization [31, 57, 58]. Neutralization of these chemokines increased viral loads in baboon PBMC but not in rhesus macaque PBMC, demonstrating their importance in limiting virus spread in baboon PBMC. Previous studies have shown CD4 T and CD8 T cells can serve as sources of MIP-1α, MIP-1β, and RANTES during HIV infection [31–36]. Analysis of baboon co-cultures revealed CD4 cells in isolation could produce these chemokines, but levels were highest in CD4 cultures containing CD8 T cells, with some contribution from NK cells. Although baboon CD4 cells alone produce higher levels of CCR5-binding chemokines than any of the rhesus macaque conditions, additional production from CD8 T cells may be necessary to reach antiviral concentrations of these chemokines in baboon cultures, which could partially explain why SIVmac growth was unrestricted in isolated baboon CD4 cells when compared to rhesus macaques. The impact of soluble factors in restricting viral growth is commonly addressed using transwells. In this regard, we saw no restriction of SIVmac251 when CD8 T and NK cells were segregated from CD4 cells. However, we uncovered that contact between these cell types was required for elevated production of CCR5-binding chemokines, which was mirrored by a reduction in viral loads. Although CD4 cells can be a source of MIP-1α, MIP-1β, and RANTES in baboon PBMC, CD8 T cells may elevate levels to antiviral concentrations by also producing these chemokines or by promoting their production by CD4 cells. Identification of the major cell source(s) of CCR5-binding chemokines as well as the signals that boost their production in baboon PBMC will require future investigation.
The ability of baboon PBMC to produce higher levels of MIP-1α, MIP-1β, and RANTES than rhesus macaque PBMC in vitro is, to our knowledge, a novel finding that may represent a mechanism for both limiting SIV replication and, by extension, preventing infection-associated pathology in vivo. Indeed, previous reports of pathogenic infections of baboons with HIV-2 employed a dual-tropic virus, which could circumvent CCR5 restriction [11, 59]. The relevance of CCR5 ligands in controlling HIV infection and disease in humans has been previously explored. Numerous studies have reported better clinical profiles in HIV-infected individuals whose cells produce higher levels of MIP-1α and/or MIP-1β in vitro [60–62]. A polymorphism in the RANTES promoter results in increased transcription activity and is associated with slower progression to AIDS [63, 64]. Naturally, chemokine-mediated inhibition of HIV and SIV entry has served as a springboard for the development of HIV therapeutics. Maraviroc, a CCR5-antagonist, was approved in 2007 for treatment of CCR5-tropic HIV infection; when used as a component of combined antiretroviral therapy, maraviroc could reduce viral loads by over 1 log [65].
We can only speculate on the conditions that favored the emergence of high CCR5 ligand production in baboons. As one possibility, specific underlying microbial infections could activate an immune response that includes elevated production of CCR5-binding chemokines. Studies have shown HIV patients infected with Orientia tsutsugamushi (bacterium that causes scrub typhus) [66, 67] or Hepatitis G/GB virus C [68, 69] have reduced viral loads and/or higher CD4 T cell counts. In vitro stimulation of PBMC with either pathogen increased secretion of RANTES [70, 71]. Although we found STLV-1 status had no impact on the outcome of SIVmac infection in baboon PBMC, protection from SIV may be elicited by other infections currently endemic in wild and captive baboon colonies. Alternatively, baboons may have evolved a higher capacity for chemokine production to overcome now extinct microbes.
One limitation of using PBMC to identify SIV-suppressive cell types is the exclusion of other immune cell types, including granulocytes. Investigation into the potential impact of these cell types in baboon SIV resistance could uncover other antiviral mechanisms. In addition, in vitro systems do not model the complexities of in vivo HIV and SIV infection. Our findings have provided insight into the key cell types responsible for baboon restriction of SIVmac251 in vitro and as such, will be the focus of future investigation in vivo.
To summarize, we have shown SIVmac251 growth is inhibited in the mixed-cell environment of baboon PBMC but not in isolated CD4 cells. By contrast, rhesus macaque PBMC and CD4 cells were equally permissive for viral growth. CD8 T cells provided suppression of SIVmac251 infection in baboon CD4 cells. We identified one mechanism of restriction that involves a high capacity for production of MIP-1α, MIP-1β, and RANTES, chemokines that limit SIV infection by interfering with viral binding to CCR5. Baboon resistance to SIV is likely mediated by multiple independent mechanisms but these studies have provided the foundation of our search for other factors.
Supplementary Material
HIGHLIGHTS.
PBMC and CD4 cells from naïve rhesus macaques are equally permissive to SIVmac.
SIVmac is restricted in PBMC but not in isolated CD4 cells from naïve baboons.
Baboon, but not rhesus macaque, CD8 T cells suppress of SIVmac replication.
Suppression by baboon CD8 T cells is due to components of innate immunity.
CD8 T cells elevate levels of CCR5 ligands that interfere with SIV entry.
Acknowledgments
We would like to acknowledge veterinarians, veterinary technicians, and personnel from the Southwest National Primate Research Center for their continued support.
FUNDING
This investigation used resources that were supported by the Southwest National Primate Research Center grant P51 OD011133 from the Office of Research Infrastructure Programs, National Institutes of Health. Additional funding was provided by Texas Biomed Forum and SNPRC Pilot Project Programs.
Footnotes
DECLARATION OF INTERESTS
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Letvin NL, Daniel MD, Sehgal PK, Desrosiers RC, Hunt RD, Waldron LM, MacKey JJ, Schmidt DK, Chalifoux LV, King NW. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985;230(4721):71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- 2.Hahn BH, Shaw GM, De Cock KM, Sharp PM. AIDS as a zoonosis: scientific and public health implications. Science. 2000;287(5453):607–614. doi: 10.1126/science.287.5453.607. [DOI] [PubMed] [Google Scholar]
- 3.Silvestri G, Paiardini M, Pandrea I, Lederman MM, Sodora DL. Understanding the benign nature of SIV infection in natural hosts. J Clin Invest. 2007;117(11):3148–3154. doi: 10.1172/JCI33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hausfater G. Predatory Behavior of Yellow Baboons. Behaviour. 1976;56(1/2):44–68. [Google Scholar]
- 5.Kodama T, Silva DP, Daniel MD, Phillips-Conroy JE, Jolly CJ, Rogers J, Desrosiers RC. Prevalence of Antibodies to SIV in Baboons in Their Native Habitat. AIDS Res Hum Retroviruses. 1989;5(3):337–343. doi: 10.1089/aid.1989.5.337. [DOI] [PubMed] [Google Scholar]
- 6.van Rensburg EJ, Engelbrecht S, Mwenda J, Laten JD, Robson BA, Stander T, Chege GK. Simian immunodeficiency viruses (SIVs) from eastern and southern Africa: Detection of a SIVagm variant from a chacma baboon. J Gen Virol. 1998;79:1809–1814. doi: 10.1099/0022-1317-79-7-1809. [DOI] [PubMed] [Google Scholar]
- 7.Benveniste RE, Morton WR, Clark EA, Tsai CC, Ochs HD, Ward JM, Kuller L, Knott WB, Hill RW, Gale MJ. Inoculation of Baboons and Macaques with Simian Immunodeficiency Virus Mne, a Primate Lentivirus Closely Related to Human Immunodeficiency Virus Type 2. J Virol. 1988;62(6):2091–2101. doi: 10.1128/jvi.62.6.2091-2101.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kannagi M, Yetz JM, Letvin NL. In vitro growth characteristics of simian T-lymphotropic virus type III. Proc Natl Acad Sci U S A. 1985;82(20):7053–7057. doi: 10.1073/pnas.82.20.7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benveniste RE, Arthur LO, Tsai CC, Sowder R, Copeland TD, Henderson LE, Oroszlan S. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV-III/LAV and other lentiviruses. J Virol. 1986;60(2):483–490. doi: 10.1128/jvi.60.2.483-490.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cranage MP, Cook N, Stott EJ, Cook R, Baskerville A, Greenaway PJ. Transmission studies with simian immunodeficiency virus of macaques; persistent infection of baboons. Intervirology. 1992;34(2):53–61. doi: 10.1159/000150263. [DOI] [PubMed] [Google Scholar]
- 11.Barnett SW, Murthy KK, Herndier BG, Levy JA. An AIDS-like condition induced in baboons by HIV-2. Science. 1994;266(5185):642–646. doi: 10.1126/science.7939718. [DOI] [PubMed] [Google Scholar]
- 12.Blackbourn DJ, Locher CP, Ramachandran B, Barnett SW, Murthy KK, Carey KD, Brasky KM, Levy JA. CD8+ cells from HIV-2-infected baboons control HIV replication. AIDS. 1997;11(6):737–46. doi: 10.1097/00002030-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Giavedoni LD, Schlabritz-Loutsevitch N, Hodara VL, Parodi LM, Hubbard GB, Dudley DJ, McDonald TJ, Nathanielsz PW. Phenotypic changes associated with advancing gestation in maternal and fetal baboon lymphocytes. J Reprod Immunol. 2004;64(1–2):121–132. doi: 10.1016/j.jri.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Del Prete GQ, Scarlotta M, Newman L, Reid C, Parodi LM, Roser JD, Oswald K, Marx PA, Miller CJ, Desrosiers RC, Barouch DH, Pal R, Piatak M, Jr, Chertova E, Giavedoni LD, O’Connor DH, Lifson JD, Keele BF. Comparative characterization of transfection- and infection-derived simian immunodeficiency virus challenge stocks for in vivo nonhuman primate studies. J Virol. 2013;87(8):4584–4595. doi: 10.1128/JVI.03507-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giavedoni LD. Simultaneous detection of multiple cytokines and chemokines from nonhuman primates using luminex technology. J Immunol Methods. 2005;301(1–2):89–101. doi: 10.1016/j.jim.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Leutenegger CM, Higgins J, Matthews TB, Tarantal AF, Luciw PA, Pedersen NC, North TW. Real-time TaqMan PCR as a specific and more sensitive alternative to the branched-chain DNA assay for quantitation of simian immunodeficiency virus RNA. AIDS Res Hum Retroviruses. 2001;17(3):243–251. doi: 10.1089/088922201750063160. [DOI] [PubMed] [Google Scholar]
- 17.Bruce AG, Bakke AM, Thouless ME, Rose TM. Development of a real-time QPCR assay for the detection of RV2 lineage-specific rhadinoviruses in macaques and baboons. Virol J. 2005;2:2. doi: 10.1186/1743-422X-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannioui A, Bourry O, Sellier P, Delache B, Brochard P, Andrieu T, Vaslin B, Karlsson I, Roques P, Le Grand R. Dynamics of viral replication in blood and lymphoid tissues during SIVmac251 infection of macaques. Retrovirology. 2009;6:106. doi: 10.1186/1742-4690-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riddick NE, Hermann EA, Loftin LM, Elliott ST, Wey WC, Cervasi B, Taaffe J, Engram JC, Li B, Else JG, Li Y, Hahn BH, Derdeyn CA, Sodora DL, Apetrei C, Paiardini M, Silvestri G, Collman RG. A novel CCR5 mutation common in sooty mangabeys reveals SIVsmm infection of CCR5-null natural hosts and efficient alternative coreceptor use in vivo. PLoS Pathog. 2010;6(8):e1001064. doi: 10.1371/journal.ppat.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng H, Unutmaz D, KewalRamani VN, Littman DR. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388(6639):296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 21.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two Orphan Seven-Transmembrane Segment Receptors Which Are Expressed in CD4-positive Cells Support Simian Immunodeficiency Virus Infection. J Exp Med. 1997;186(3):405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, Mori J, Rickett G, Smith-Burchnell C, Napier C, Webster R, Armour D, Price D, Stammen B, Wood A, Perros M. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49(11):4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler SL, Hansen MS, Bushman FD. A quantitative assay for HIV DNA integration in vivo. Nat Med. 2001;7:631–634. doi: 10.1038/87979. [DOI] [PubMed] [Google Scholar]
- 24.Bukrinsky MI, Sharova N, Dempsey MP, Stanwick TL, Bukrinskaya AG, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci U S A. 1992;89(14):6580–4. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malim MH, Bieniasz PD. HIV restriction factors and mechanisms of evasion. Cold Spring Harb Perspect Med. 2012;2:1–16. doi: 10.1101/cshperspect.a006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosa A, Chande A, Ziglio S, De Sanctis V, Bertorelli R, Goh SL, McCauley SM, Nowosielska A, Antonarakis SE, Luban J, Santoni FA, Pizzato M. HIV-1 Nef promotes infection by excluding SERINC5 from virion incorporation. Nature. 2015;526(7572):212–217. doi: 10.1038/nature15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Usami Y, Wu Y, Gottlinger HG. SERINC3 and SERINC5 restrict HIV-1 infectivity and are counteracted by Nef. Nature. 2015;526(7572):218–223. doi: 10.1038/nature15400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.d’Offay JM, Eberle R, Sucol Y, Schoelkopf L, White MA, Valentine BD, White GL, Lerche NW. Transmission Dynamics of Simian T-lymphotropic Virus Type 1 (STLV1) in a Baboon Breeding Colony: Predominance of Female-to-female Transmission. Comp Med. 2007;57(1):105–114. [PubMed] [Google Scholar]
- 29.Takemura T, Yamashita M, Shimada MK, Ohkura S, Shotake T, Ikeda M, Miura T, Hayami M. High Prevalence of Simian T-Lymphotropic Virus Type L in Wild Ethiopian Baboons. J Virol. 2002;76(4):1642–1648. doi: 10.1128/JVI.76.4.1642-1648.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voevodin A, Samilchuk E, Allan J, Rogers J, Broussard S. Simian T-Lymphotropic Virus Type 1 (STLV-1) Infection in Wild Yellow Baboons (Papio hamadryas cynocephalus) from Mikumi National Park, Tanzania. Virology. 1997;228(2):350–359. doi: 10.1006/viro.1996.8408. [DOI] [PubMed] [Google Scholar]
- 31.Cocchi F, Devico AL, Garzino-demo A, Arya SK, Gallo RC, Lussot P. Identification of RANTES, MIP-1a, and MIP-1b as the major HIV-suppressive factors produced by CD8 T cells. Science. 1995;270(5243):1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 32.Levine BL, Mosca JD, Riley JL, Carroll RG, Vahey MT, Jagodzinski LL, Wagner KF, Mayers DL, Burke DS, Weislow OS, St Louis DC, June CH. Antiviral effect and ex vivo CD4+ T cell proliferation in HIV-positive patients as a result of CD28 costimulation. Science. 1996;272(5270):1939–1943. doi: 10.1126/science.272.5270.1939. [DOI] [PubMed] [Google Scholar]
- 33.Riley JL, Carroll RG, Levine BL, Bernstein W, St Louis DC, Weislow OS, June CH. Intrinsic resistance to T cell infection with HIV type 1 induced by CD28 costimulation. J Immunol. 1997;158(11):5545–5553. [PubMed] [Google Scholar]
- 34.Mengozzi M, Malipatlolla M, De Rosa SC, Herzenberg LA, Herzenberg LA, Roederer M. Naïve CD4 T cells inhibit CD28-costimulated R5 HIV replication in memory CD4 T cells. Proc Natl Acad Sci U S A. 2001;98(20):11644–11649. doi: 10.1073/pnas.211205098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fehniger TA, Herbein G, Yu H, Para MI, Bernstein ZP, O’Brien WA, Caligiuri MA. Natural killer cells from HIV-1+ patients produce C-C chemokines and inhibit HIV-1 infection. J Immunol. 1998;161(11):6433–6438. [PubMed] [Google Scholar]
- 36.Oliva A, Kinter AL, Vaccarezza M, Rubbert A, Catanzaro A, Moir S, Monaco J, Ehler L, Mizell S, Jackson R, Li Y, Romano JW, Fauci AS. Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J Clin Invest. 1998;102(1):223–231. doi: 10.1172/JCI2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keele BF, Jones JH, Terio KA, Estes JD, Rudicell RS, Wilson ML, Li Y, Learn GH, Beasley TM, Schumacher-Stankey J, Wroblewski E, Mosser A, Raphael J, Kamenya S, Lonsdorf EV, Travis DA, Mlengeya T, Kinsel MJ, Else JG, Silvestri G, Goodall J, Sharp PM, Shaw GM, Pusey AE, Hahn BH. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature. 2009;460(7254):515–519. doi: 10.1038/nature08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.VandeWoude S, Apetrei C. Going wild: lessons from naturally occurring T-lymphotropic lentiviruses. Clin Microbiol Rev. 2006;19(4):728–762. doi: 10.1128/CMR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pandrea I, Sodora DL, Silvestri G, Apetrei C. Into the wild: simian immunodeficiency virus (SIV) infection in natural hosts. Trends Immunol. 2008;29(9):419–428. doi: 10.1016/j.it.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandrea I, Apetrei C. Where the Wild Things Are: Pathogenesis of SIV Infection in African Nonhuman Primate Hosts. Curr HIV/AIDS Rep. 2010;7(1):28–36. doi: 10.1007/s11904-009-0034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peeters M, Ma D, Liegeois F, Apetrei C. Chapter 3 - Simian Immunodeficiency Virus Infections in the Wild. In: Silvestri G, editor. Natural Hosts of SIV. Elsevier; Amsterdam: 2014. [Google Scholar]
- 42.Ma D, Jasinska A, Kristoff J, Grobler JP, Turner T, Jung Y, Schmitt C, Raehtz K, Feyertag F, Martinez Sosa N, Wijewardana V, Burke DS, Robertson DL, Tracy R, Pandrea I, Freimer N, Apetrei C. SIVagm Infection in Wild African Green Monkeys from South Africa: Epidemiology, Natural History, and Evolutionary Considerations. PLoS Pathog. 2013;9(1):e1003011. doi: 10.1371/journal.ppat.1003011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palesch D, Bosinger SE, Tharp GK, Vanderford TH, Paiardini M, Chahroudi A, Johnson ZP, Kirchhoff F, Hahn BH, Norgren RB, Patel NB, Sodora DL, Dawoud RA, Stewart C-B, Seepo SM, Harris RA, Liu Y, Raveendran M, Han Y, English A, Thomas GWC, Hahn MW, Pipes L, Mason CE, Muzny DM, Gibbs RA, Sauter D, Worley K, Rogers J, Silvestri G. Sooty mangabey genome sequence provides insight into AIDS resistance in a natural SIV host. Nature. 2018;553:77. doi: 10.1038/nature25140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Apetrei C, Gaufin T, Gautam R, Vinton C, Hirsch V, Lewis M, Brenchley J, Pandrea I. Pattern of SIVagm Infection in Patas Monkeys Suggests that Host Adaptation to SIV Infection May Result in Resistance to Infection and Virus Extinction. J Infect Dis. 2010;202(S3):S371–S376. doi: 10.1086/655970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seman AL, Pewen WF, Fresh LF, Martin LN, Murphey-Corb M. The replicative capacity of rhesus macaque peripheral blood mononuclear cells for simian immunodeficiency virus in vitro is predictive of the rate of progression to AIDS in vivo. J Gen Virol. 2000;81(10):2441–2449. doi: 10.1099/0022-1317-81-10-2441. [DOI] [PubMed] [Google Scholar]
- 46.Margolis L, Glushakova S, Chougnet C, Shearer G, Markham P, Robert-Guroff M, Benveniste R, Miller CJ, Cranage M, Hirsch V, Franchini G. Replication of simian immunodeficiency virus (SIV) in ex vivo lymph nodes as a means to assess susceptibility of macaques in vivo. Virology. 2000;275(2):391–7. doi: 10.1006/viro.2000.0528. [DOI] [PubMed] [Google Scholar]
- 47.Pandrea I, Apetrei C, Gordon S, Barbercheck J, Dufour J, Bohm R, Sumpter B, Roques P, Marx Pa, Hirsch VM, Kaur A, Lackner Aa, Veazey RS, Silvestri G. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2007;109(3):1069–1076. doi: 10.1182/blood-2006-05-024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krupp A, McCarthy KR, Ooms M, Letko M, Morgan JS, Simon V, Johnson WE. APOBEC3G Polymorphism as a Selective Barrier to Cross-Species Transmission and Emergence of Pathogenic SIV and AIDS in a Primate Host. PLoS Pathog. 2013;9(10):e1003641. doi: 10.1371/journal.ppat.1003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirmaier A, Wu F, Newman RM, Hall LR, Morgan JS, O’Connor S, Marx PA, Meythaler M, Goldstein S, Buckler-White A, Kaur A, Hirsch VM, Johnson WE. TRIM5 Suppresses Cross-Species Transmission of a Primate Immunodeficiency Virus and Selects for Emergence of Resistant Variants in the New Species. PLoS Biol. 2010;8(8):e1000462. doi: 10.1371/journal.pbio.1000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giavedoni LD, Velasquillo MC, Parodi LM, Hubbard GB, Hodara VL. Cytokine expression, natural killer cell activation, and phenotypic changes in lymphoid cells from rhesus macaques during acute infection with pathogenic simian immunodeficiency virus. J Virol. 2000;74(4):1648–57. doi: 10.1128/jvi.74.4.1648-1657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shieh TM, Carter DL, Blosser RL, Mankowski JL, Zink MC, Clements JE. Functional analyses of natural killer cells in macaques infected with neurovirulent simian immunodeficiency virus. J Neurovirol. 2001;7(1):11–24. doi: 10.1080/135502801300069593. [DOI] [PubMed] [Google Scholar]
- 52.Kannagi M, Chalifoux LV, Lord CI, Letvin NL. Suppression of simian immunodeficiency virus replication in vitro by CD8+ lymphocytes. J Immunol. 1988;140(7):2237–42. [PubMed] [Google Scholar]
- 53.Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, Irwin CE, Safrit JT, Mittler J, Weinberger L, Kostrikis LG, Zhang L, Perelson AS, Ho DD. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189(6):991–8. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283(5403):857–60. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 55.Levy JA, Mackewicz CE, Barker E. Controlling HIV pathogenesis: the role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol Today. 1996;17(5):217–224. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- 56.Lu W, Chen S, Lai C, Lai M, Fang H, Dao H, Kang J, Fan J, Guo W, Fu L, Andrieu J-M. Suppression of HIV Replication by CD8(+) Regulatory T-Cells in Elite Controllers. Front Immunol. 2016;7:134. doi: 10.3389/fimmu.2016.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alkhatib G, Locati M, Kennedy PE, Murphy PM, Berger EA. HIV-1 Coreceptor Activity of CCR5 and Its Inhibition by Chemokines: Independence from G Protein Signaling and Importance of Coreceptor Downmodulation. Virology. 1997;234(2):340–348. doi: 10.1006/viro.1997.8673. [DOI] [PubMed] [Google Scholar]
- 58.Amara A, Gall SL, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier J-L, Arenzana-Seisdedos F. HIV Coreceptor Downregulation as Antiviral Principle: SDF-1α–dependent Internalization of the Chemokine Receptor CXCR4 Contributes to Inhibition of HIV Replication. J Exp Med. 1997;186(1):139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Locher CP, Witt SA, Herndier BG, Abbey NW, Tenner-Racz K, Racz P, Kiviat NB, Murthy KK, Brasky K, Leland M, Levy JA. Increased virus replication and virulence after serial passage of human immunodeficiency virus type 2 in baboons. J Virol. 2003;77(1):77–83. doi: 10.1128/JVI.77.1.77-83.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garzino-Demo A, Moss RB, Margolick JB, Cleghorn F, Sill A, Blattner WA, Cocchi F, Carlo DJ, DeVico AL, Gallo RC. Spontaneous and antigen-induced production of HIV-inhibitory β-chemokines are associated with AIDS-free status. Proc Natl Acad Sci U S A. 1999;96(21):11986–11991. doi: 10.1073/pnas.96.21.11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cocchi F, DeVico AL, Yarchoan R, Redfield R, Cleghorn F, Blattner WA, Garzino-Demo A, Colombini-Hatch S, Margolis D, Gallo RC. Higher macrophage inflammatory protein (MIP)-1α and MIP-1β levels from CD8(+) T cells are associated with asymptomatic HIV-1 infection. Proc Natl Acad Sci U S A. 2000;97(25):13812–13817. doi: 10.1073/pnas.240469997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garzino-Demo A, DeVico AL, Conant KE, Gallo RC. The role of chemokines in human immunodeficiency virus infection. Immunol Rev. 2000;177(1):79–87. doi: 10.1034/j.1600-065x.2000.17711.x. [DOI] [PubMed] [Google Scholar]
- 63.Liu H, Chao D, Nakayama EE, Taguchi H, Goto M, Xin X, Takamatsu J-k, Saito H, Ishikawa Y, Akaza T, Juji T, Takebe Y, Ohishi T, Fukutake K, Maruyama Y, Yashiki S, Sonoda S, Nakamura T, Nagai Y, Iwamoto A, Shioda T. Polymorphism in RANTES chemokine promoter affects HIV-1 disease progression. Proc Natl Acad Sci U S A. 1999;96(8):4581–4585. doi: 10.1073/pnas.96.8.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McDermott DH, Beecroft MJ, Kleeberger CA, Al-Sharif FM, Ollier WE, Zimmerman PA, Boatin BA, Leitman SF, Detels R, Hajeer AH, Murphy PM. Chemokine RANTES promoter polymorphism affects risk of both HIV infection and disease progression in the Multicenter AIDS Cohort Study. AIDS. 2000;14(17):2671–2678. doi: 10.1097/00002030-200012010-00006. [DOI] [PubMed] [Google Scholar]
- 65.Gulick RM, Lalezari J, Goodrich J, Clumeck N, DeJesus E, Horban A, Nadler J, Clotet B, Karlsson A, Wohlfeiler M, Montana JB, McHale M, Sullivan J, Ridgway C, Felstead S, Dunne MW, van der Ryst E, Mayer H. Maraviroc for Previously Treated Patients with R5 HIV-1 Infection. N Engl J Med. 2008;359(14):1429–1441. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watt G, Kantipong P, de Souza M, Chanbancherd P, Jongsakul K, Ruangweerayud R, Loomis-Price LD, Polonis V, Myint KS, Birx DL, Brown AE, Krishna S. HIV-1 suppression during acute scrub-typhus infection. Lancet. 2000;356(9228):475–479. doi: 10.1016/S0140-6736(00)02557-5. [DOI] [PubMed] [Google Scholar]
- 67.Watt G, Kantipong P, Jongsakul K, de Souza M, Burnouf T. Passive transfer of scrub typhus plasma to patients with AIDS: a descriptive clinical study. Qjm. 2001;94(11):599–607. doi: 10.1093/qjmed/94.11.599. [DOI] [PubMed] [Google Scholar]
- 68.Yeo AE, Matsumoto A, Hisada M, Shih JW, Alter HJ, Goedert JJ. Effect of hepatitis G virus infection on progression of HIV infection in patients with hemophilia. Multicenter Hemophilia Cohort Study. Ann Intern Med. 2000;132(12):959–963. doi: 10.7326/0003-4819-132-12-200006200-00006. [DOI] [PubMed] [Google Scholar]
- 69.Tillmann HL, Heiken H, Knapik-Botor A, Heringlake S, Ockenga J, Wilber JC, Goergen B, Detmer J, McMorrow M, Stoll M, Schmidt RE, Manns MP. Infection with GB Virus C and Reduced Mortality among HIV-Infected Patients. N Engl J Med. 2001;345(10):715–724. doi: 10.1056/NEJMoa010398. [DOI] [PubMed] [Google Scholar]
- 70.Moriuchi M, Tamura A, Moriuchi H. In vitro reactivation of human immunodeficiency virus-1 upon stimulation with scrub typhus rickettsial infection. Am J Trop Med Hyg. 2003;68(5):557–561. doi: 10.4269/ajtmh.2003.68.557. [DOI] [PubMed] [Google Scholar]
- 71.Nattermann J, Nischalke HD, Kupfer B, Rockstroh J, Hess L, Sauerbruch T, Spengler U. Regulation of CC chemokine receptor 5 in hepatitis G virus infection. AIDS. 2003;17(10):1457–1462. doi: 10.1097/00002030-200307040-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.