Despite the large number of elderly patients with cancer, they are often disproportionally underrepresented in clinical research trials. This article reports outcomes of elderly patients treated with first‐line chemotherapy within EORTC‐STBSG clinical trials.
Keywords: Elderly, Soft tissue, Sarcoma, Chemotherapy, Outcomes, European Organization for Research and Treatment of Cancer
Abstract
Background.
Almost half of patients diagnosed with soft tissue sarcoma (STS) are older than 65 years; however, the outcomes of elderly patients with metastatic disease are not well described.
Patients and Methods.
An elderly cohort of patients aged ≥65 years was extracted from the European Organization for Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group database of patients treated with first‐line chemotherapy for advanced STS within 12 EORTC clinical trials. Endpoints were overall survival (OS), progression‐free survival (PFS), and response rate (RR).
Results.
Of 2,810 participants in EORTC trials, there were 348 elderly patients (12.4%, median 68 years; interquartile range [IQR], 67–70; maximum 84 years) and 2,462 patients aged <65 years (median 49 years; IQR, 39–57). Most elderly patients had a performance status of 0 (n = 134; 39%) or 1 (n = 177; 51%). Leiomyosarcoma (n = 130; 37%) was the most common histological subtype. Lung metastases were present in 181 patients (52%) and liver metastases in 63 patients (18%). Overall, 126 patients (36%) received doxorubicin, 114 patients (33%) doxorubicin + ifosfamide, 43 patients (12%) epirubicin, 39 patients (11%) trabectedin, and 26 patients (7%) ifosfamide. Overall RR was 14.9% (n = 52), median PFS was 3.5 months (95% confidence interval [CI], 2.7–4.3), and median OS was 10.8 months (95% CI, 9.43–11.83). In patients aged <65 years, overall RR was 20.3% (n = 501), median OS was 12.3 months (95% CI, 11.9–12.9), and median PFS was 4.3 months (95% CI, 3.9–4.6).
Conclusion.
Elderly patients with metastatic STS treated with first‐line chemotherapy were largely underrepresented in these EORTC STS trials. Their outcomes were only slightly worse than those of younger patients. Novel trials with broader eligibility criteria are needed for elderly patients. These trials should incorporate geriatric assessments and measurements of age‐adjusted health‐related quality of life.
Implications for Practice.
This analysis demonstrates that elderly patients with advanced soft tissue sarcoma are underrepresented in clinical trials of first‐line chemotherapy by the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Furthermore, the elderly participants were generally of excellent performance status, which is not representative of an unselected elderly population. These data provide rationale for development of novel trials for elderly patients that are not only for “elite” patients but include comprehensive geriatric assessments for risk stratification. Because chemotherapy for advanced soft tissue sarcomas is largely given with palliative intent, incorporation of health‐related quality of life measures with traditional endpoints will provide a more holistic approach to future clinical trials.
Introduction
Global life expectancy is increasing annually. In 2015, the World Health Organization (WHO) estimated that this figure reached 71.4 years, exceeding 82 years in 12 countries [1]. Cancer is predominantly a disease of the elderly because of the cumulative acquisition of genetic abnormalities and lifetime exposure to carcinogens [2]. Currently more than 60% of all cancer diagnoses and 70% of cancer‐related deaths occur in patients aged >65 years [3]. In view of the aging population, it is widely acknowledged that the incidence of cancer will continue to rise significantly in the years to come [3]. The challenges of treating elderly patients with cancer are multifactorial. Physiological changes associated with aging, comorbid medical conditions, psychosocial factors, functional and nutritional status, and polypharmacy are several key issues that require careful consideration in elderly patients with cancer [4]. The interaction of these factors is complex, and their influence on cancer biology, treatment tolerance, compliance, efficacy, and outcomes remains uncertain [3]. Currently a multidisciplinary approach is recommended; however, it is undeniable that new guidelines specifically for elderly patients with cancer are urgently needed [2].
Despite the large number of elderly patients with cancer, they are often disproportionally underrepresented in clinical research trials [5]. Strict exclusion criteria, attrition (mortality, relocation), patient heterogeneity, costs, and longer recruitment processes contribute to this finding [6]. Data from studies in younger patients are often extrapolated to aid clinical decision making in elderly patients. Outcomes for elderly patients in clinical trials are not routinely distinguished from all data, thus limiting evidence‐based decision making in clinical practice. Furthermore, dose reductions are frequently implemented in elderly “frail” patients without clear evidence of treatment efficacy at lower dose levels [3].
Soft tissue sarcomas (STSs) are rare, heterogeneous tumors that account for approximately 1% of all adult solid malignancies [7]. Approximately half of patients with localized, intermediate, or high‐grade tumors will eventually develop metastatic disease [8]. Cytotoxic chemotherapy, usually an anthracycline‐based regimen, has been the mainstay of treatment since the 1970s [9]. Median overall survival for patients with advanced soft tissue sarcoma is around 12–19 months [10], [11], [12], [13]. STSs are common in elderly patients aged ≥65 years, with an age‐adjusted incidence of 11.3 cases per 100,000 population compared with 2.3 cases per 100,000 in those aged <65 years [14]. Although approximately 40%–50% of all patients diagnosed with STS are aged >65 years, the median age of patients in prospective first‐line chemotherapy trials for advanced STS ranges from 48 to 60 years [14], [15], [16], [17]. The objective of this study is to examine outcomes of elderly patients treated with first‐line chemotherapy within European Organization for Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group (STBSG) clinical trials.
Materials and Methods
Patient Sample
The EORTC‐STBSG database comprises 3,711 patients treated with first‐line chemotherapy in 15 EORTC advanced soft tissue sarcoma trials. In this analysis, patients with gastrointestinal stromal tumors (GISTs), those who had received prior (adjuvant or palliative) chemotherapy, and patients for whom age or time to treatment failure (discontinuation of treatment for any reason, including disease progression, toxicity, or death) was missing were excluded. Furthermore, we wished to focus on outcomes with currently used chemotherapy schedules, and consequently patients treated with cyclophosphamide, vincristine, adriamycin, dacarbazine (CYVADIC), ifosfamide 12 mg/m2, or brostallicin were not included in this analysis. Therefore, 2,810 patients from 13 trials were used for the descriptive part of this report. Elderly patients were defined as those aged at least 65 years. The randomized trial of doxorubicin versus doxorubicin plus ifosfamide (EORTC 62012) had an upper age range of 60 years (oldest patient, 63 years), and therefore patients in this trial did not contribute to the elderly subgroup. From the remaining 12 studies, 348 elderly patients were identified (supplemental online Appendix: Summary of EORTC‐STBSG clinical trials in this analysis and elderly patients per protocol). Ethical approval was not required for this analysis.
Endpoints
Endpoints for this analysis were overall survival (OS), progression‐free survival (PFS), and response to chemotherapy. PFS was defined as the time interval between the date of randomization, or the date of prospective registration in the nonrandomized trials, and the date of first report of progression or death, whichever came first. Patients who were alive and without progressive disease at the last follow‐up date were censored. OS was computed from the date of randomisation (in randomized trials) or the date of prospective registration (in nonrandomized trials) to the date of death. Patients who were alive at the last follow‐up date were censored. Response to chemotherapy was evaluated in all trials using WHO response criteria or RECIST and categorized as complete reponse, partial reseponse, stable disease, or progressive disease.
Covariates
The variables included in the study were demographic data, histological subytpe of sarcoma, and the extent of the disease at the time of inclusion in the trials and the assigned treatment. The demographic variables include age and performance status (PS) before the start of chemotherapy. PS was measured on the WHO scale (except for two trials in which it was retrospectively converted from the Karnosky scale to the WHO scale). As few patients had PS 3, PS 2 and 3 were combined in the same category, named PS 2+. Variables related to the history of sarcoma were prior radiotherapy and prior surgery, which had three categories: no surgery, partial surgery (including palliative surgery and other), and total surgery. For study 62012, data about primary surgery were not collected, therefore the variable was missing for all patients in that trial. Histopathological grade estimated by a panel of reference pathologists was preferred over the use of local diagnosis to ensure consistency and homogeneity of the database. Similarly, the reviewed histopathological cell type was preferred over the local diagnosis.
The treatment was aggregated in five categories: (liposomal) doxorubicin alone (doxorubicin 75 mg/m2, caelyx 35 mg/m2), epirubicin (epirubicin 75 mg/m2, epirubicin 50 mg/m2 [days 1–3], epirubicin 150 mg/m2), ifosfamide alone (ifosfamide 5 mg/m2, ifosfamide 3 mg/m2 [days 1–3], ifosfamide 9 mg/m2 [continuous infusion over 72 hours]), the combination of doxorubicin and ifosfamide (doxorubicin 50 mg/m2 and ifosfamide 5 mg/m2, doxorubicin 75 mg/m2 and ifosfamide 5 mg/m2, doxorubicin 75 mg/m2 and ifosfamide 10 mg/m2) and trabectedin (1.3 mg/m2 [3 hours’ infusion] or 1.5 mg/m2 [24 hours’ infusion]).
Statistical Analysis
All baseline variables are described. The categorical data are summarized by frequencies and percentages, and the continuous covariates are summarized by median, interquartile range, and overall range. The overall and progression‐free survival were estimated by the Kaplan‐Meier method. Medians are provided with corresponding 95% confidence intervals (CIs). Response to chemotherapy is summarized as a percentage with corresponding 95% CI.
Results
A total of 348 elderly patients with advanced soft tissue sarcoma who entered EORTC first‐line chemotherapy clinical trials between 1980 and 2012 were identified for this analysis.
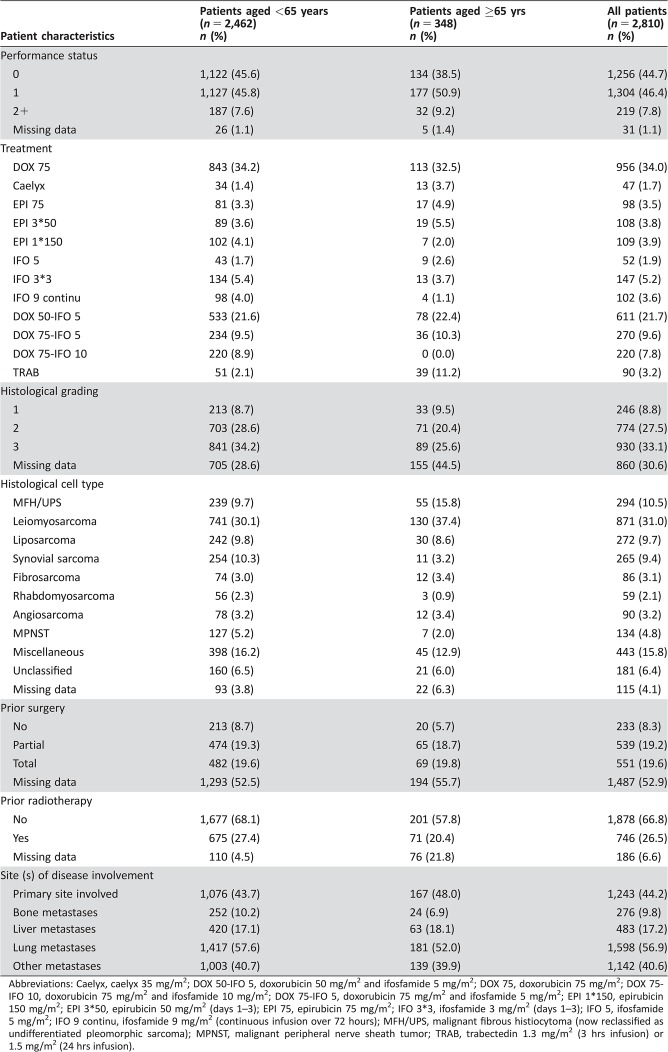
Patient Characteristics (Table 1)
Table 1. Patient characteristics.
Abbreviations: Caelyx, caelyx 35 mg/m2; DOX 50‐IFO 5, doxorubicin 50 mg/m2 and ifosfamide 5 mg/m2; DOX 75, doxorubicin 75 mg/m2; DOX 75‐IFO 10, doxorubicin 75 mg/m2 and ifosfamide 10 mg/m2; DOX 75‐IFO 5, doxorubicin 75 mg/m2 and ifosfamide 5 mg/m2; EPI 1*150, epirubicin 150 mg/m2; EPI 3*50, epirubicin 50 mg/m2 (days 1–3); EPI 75, epirubicin 75 mg/m2; IFO 3*3, ifosfamide 3 mg/m2 (days 1–3); IFO 5, ifosfamide 5 mg/m2; IFO 9 continu, ifosfamide 9 mg/m2 (continuous infusion over 72 hours); MFH/UPS, malignant fibrous histiocytoma (now reclassified as undifferentiated pleomorphic sarcoma); MPNST, malignant peripheral nerve sheath tumor; TRAB, trabectedin 1.3 mg/m2 (3 hrs infusion) or 1.5 mg/m2 (24 hrs infusion).
The median age of elderly patients was 68 years (IQR, 67–71), with a maximum of 84 years. Most patients had a PS of 0 (n = 134, 38.5%) or 1 (n = 177, 50.9%). A small number of patients had a PS of 2+ (n = 32, 9.2%). Histopathological grade was most commonly grade 3 (n = 89, 25.6%) or grade 2 (n = 71, 20.4%); however, data regarding tumor grade were missing for almost half of the patients (n = 155, 44.5%). The most frequent histological subtypes were leiomyosarcoma (n = 130, 37.4%), malignant fibrous histiocytoma (MFH) or undifferentiated pleomorphic sarcoma (UPS; n = 55, 15.8%), and liposarcoma (n = 30, 8.6%). Of note, MFH is no longer part of the currently used nomenclature and has been reclassified as UPS. There were 43 patients (12.3%) with unclassified or missing histological subtype. Overall, 167 patients (48%) had involvement of the primary site of disease, more than half of patients had pulmonary metastases (n = 181, 52.0%), 63 patients (18.1%) had liver metastases, 24 patients (6.9%) had bone metastases, and 139 patients (39.9%) had metastases at other sites.
Prior surgical details were missing for more than half of patients (n = 194, 55.7%); however, of the remaining 154 patients with available data, 65 patients had received previous partial surgery, and 69 patients had previous total surgery. Prior radiotherapy details were missing for 76 patients (21.8%), but of the remaining 272 patients, 71 patients (26%) had received prior radiotherapy.
Overall, 126 (36%) patients were treated with first‐line single‐agent doxorubicin, 114 patients (33%) with combination doxorubicin and ifosfamide, 43 patients (12%) with epirubicin, 39 patients (11%) with trabectedin, and 26 patients (7%) with single‐agent ifosfamide.
Outcomes
The median follow‐up time for elderly patients who were still alive at the time of their analyses was 9.5 months (IQR, 6–25). Of note, the median follow‐up time for patients treated with trabectedin who were still alive at the time of the clinical cutoff date was considerably shorter than for the other treatment groups (trabectedin, 6.7 months [IQR, 5.8–9.2] vs. doxorubicin + ifosfamide, 24 months [IQR, 7–38], doxorubicin alone, 14 months [IQR, 5.5–29], epirubicin, 24 months [IQR, 8–43], and ifosfamide alone, 4 months [IQR, 3.5–16]). At the time of their respective analyses, 84 patients (24.1%) were alive, and 264 patients (75.9%) were deceased.
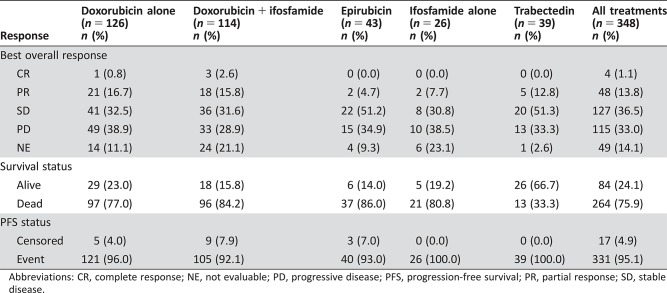
Response Rates (Table 2)
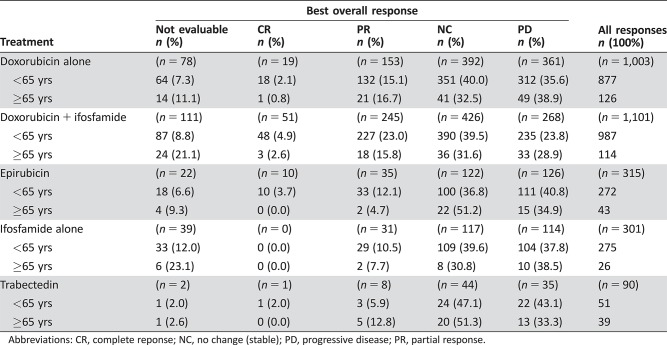
Table 2. Best overall response by elderly patients (all treatments).
Abbreviations: CR, complete response; NE, not evaluable; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease.
In total, 48 patients (13.8%) had a partial response and 4 patients (1.1%) had complete response. There were 127 patients (36.5%) with stable disease and 115 patients (33.0%) with progressive disease as best response. Response was not evaluable for 49 patients (14.1%). Radiological responses were primarily seen in patients treated with single‐agent doxorubicin (n = 22) or combination doxorubicin plus ifosfamide (n = 21).
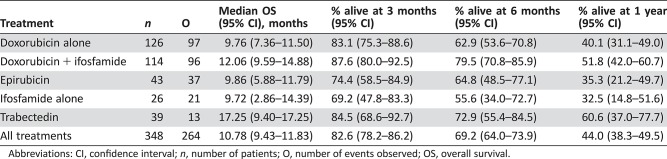
Overall Survival (Table 3)
Table 3. Overall survival of elderly patients (all treatments).
Abbreviations: CI, confidence interval; n, number of patients; O, number of events observed; OS, overall survival.
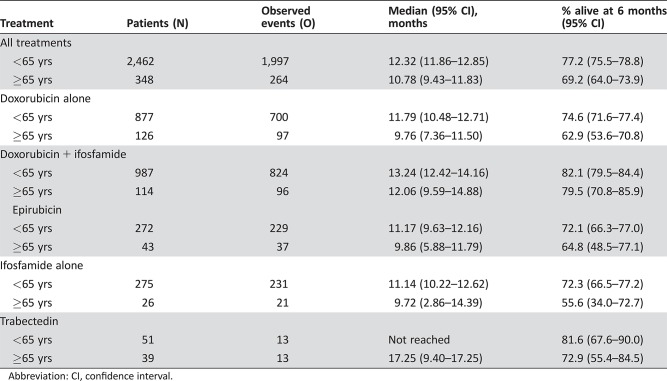
The median OS was 10.8 months (95% CI, 9.4–11.8); for single agent doxorubicin, 9.8 months (95% CI, 7.4–11.5); for doxorubicin plus ifosfamide, 12.1 months (95% CI, 9.6–14.9); for epirubicin, 9.9 months (95% CI, 5.9–11.8); for single‐agent ifosfamide, 9.7 months (95% CI, 2.9–14.4); and for trabectedin, 17.3 months (95% CI, 9.4–17.3). Because of the shorter follow‐up in the trabectedin group, there was some overestimation of overall survival in that group compared with the other treatment groups. The median OS across treatment groups at 3, 6, and 12 months, respectively, was 82.6%, 69.2%, and 44.0%. Kaplan‐Meier OS curves for all patients and for treatments received are available in the supplemental online material.
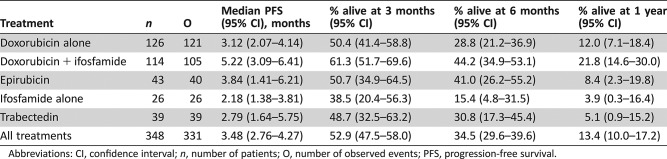
Progression‐Free Survival (Table 4)
Table 4. Progression‐free survival elderly patients (all treatments).
Abbreviations: CI, confidence interval; n, number of patients; O, number of observed events; PFS, progression‐free survival.
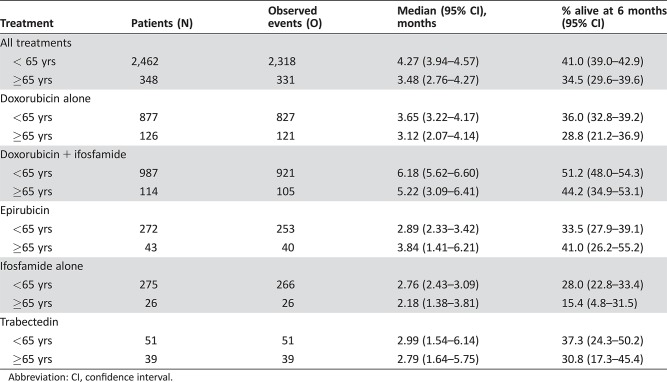
The median PFS was 3.48 months (95% CI, 2.76–4.27); for doxorubicin, 3.1 months (95% CI, 2.1–4.1); for doxorubicin plus ifosfamide, 5.2 months (95% CI, 3.1–6.4); for epirubicin, 3.8 months (95% CI, 1.4–6.2); for ifosfamide alone, 2.2 months (95% CI, 1.4–3.8); and for trabectedin, 2.8 months (95% CI, 1.6–5.8).
Older Elderly Patients (Aged ≥75 Years)
There were 31 patients aged ≥75 years. Their median survival was 10.1 months (95% CI, 4.8–13.0), and their median PFS was 3.4 months (95% CI, 1.4–5.5).
Comparison of Elderly (≥65 Years) and Younger Patients (<65 Years; Tables 5, 6, 7)
Table 5. Best overall response in elderly patients (≥65 years) versus younger patients (<65 years).
Abbreviations: CR, complete reponse; NC, no change (stable); PD, progressive disease; PR, partial response.
Table 6. Overall survival: Comparison of elderly (≥65 years) and younger (<65) patients.
Abbreviation: CI, confidence interval.
Table 7. Progression‐free survival: Comparison of elderly (≥65 years) and younger (<65) patients.
Abbreviation: CI, confidence interval.
There were 2,462 patients aged <65 years who were treated in 13 EORTC‐STBSG trials of the same chemotherapy regimens described above. The median age of these patients was 49 years (IQR, 39–57). Their median follow‐up was 46 months (IQR, 29–72).
Patient characteristics are summarized in Table 1. In patients aged <65 years, a higher proportion were PS 0 compared with elderly patients (45.6% vs. 38.5%); however, in both age groups, the majority of patients were either PS 0 or PS 1 (91.4% younger vs. 89.4% elderly). In patients aged <65 years, tumors were most commonly histopathological grade 3 (34.2%) or grade 2 (28.6%); however, tumor grading was missing for 705 patients (28.6%). As observed in elderly patients, the most common histological cell type in patients aged <65 years was leiomyosarcoma (n = 741, 30.1%). The second most frequent histological subtype in younger patients was synovial sarcoma (n = 254, 10.3%), which was only reported in 11 elderly patients (3.2%). UPS (MFH) was proportionally less common in patients aged <65 years compared with the elderly patient group (9.7% vs. 15.8%). The frequency of liposarcoma was similar in both age groups (9.8% vs. 8.5%).
The lung was the most common site of metastases in patients aged <65 years (57.6%). Liver metastases were present with similar frequency in younger and elderly patients (17.1% vs. 18.1%). A slightly higher proportion of younger patients had bone metastases compared with elderly patients (10.2% vs. 6.9%). Rates of prior partial surgery (19.3%) and total surgery (19.6%) in younger patients were almost identical to those in elderly patients. As seen in the elderly patient group, very few patients had received prior radiotherapy; 20.4% younger patients versus 26.5% elderly patients.
Treatments received by patients aged <65 years were compared with treatments received by elderly patients: doxorubicin (36% younger vs. 36% elderly), doxorubicin plus ifosfamide (40% younger vs. 33% elderly), epirubicin (11% younger vs. 12% elderly), ifosfamide alone (11% younger vs. 7% elderly), and trabectedin (2% younger vs. 11% elderly).
In patients aged <65 years, 424 patients (17.2%) achieved a partial response, and 77 patients (3.1%) achieved a complete response. There were 974 patients (39.6%) with stable disease, 784 patients (31.8%) with progressive disease, and 203 patients (8%) for whom disease was not evaluable. Therefore, compared with elderly patients, younger patients (<65 years) had a slightly higher radiological response rate (20.3% vs. 14.9%). Radiological responses in younger patients were primarily seen in those treated with combination doxorubicin plus ifosfamide (n = 227, 23.0%). Table 5 summarizes radiological responses in younger versus older patients according to treatment received.
Median OS was better in younger patients (<65 years) compared with elderly patients (≥65 years): the median OS was 12.3 months (95% CI, 11.9–12.9) in younger patients versus 10.8 months (95% CI, 9.4–11.8) in elderly patients (Table 6). Younger patients had a slightly better median PFS compared with elderly patients: the median PFS was 4.3 months (95% CI, 3.9–4.3) in younger patients compared with 3.5 months (95% CI, 2.8–4.3) in elderly patients (Table 7).
Discussion
Although almost half of patients diagnosed with STS are aged >65 years, elderly patients only represented 12% of participants in EORTC clinical trials of first‐line chemotherapy for advanced STS [15]. Furthermore, the median age of elderly patients in these studies was 68 years, which is relatively low. This was concordant with previous studies showing that elderly patients with cancer account for less than one quarter of all participants in clinical trials [18].
As expected, most elderly patients had a performance status of 0 or 1 as a result of strict eligibility criteria required for clinical trials. Good performance status (0 or 1) is an independent predictive factor for overall survival [19]. Lung and liver metastases were present with similar frequency in elderly and younger patients. Liver metastases are associated with poor response rates and worse overall survival in advanced STS treated with anthracyclines [19].
Although tumors were most commonly histopathological grade 3, a significant proportion of tumors in elderly and younger patients were grade 2. Given that histopathological grade predicts development of metastases in adult STS and that all patients had advanced disease, a higher proportion of grade 3 tumors was anticipated [8]. It is conceivable that patients with rapidly progressive grade 3 tumors were excluded because of frailty. The high number of grade 2 tumors is also noteworthy given that low histological grade is associated with lower response rates in patients with advanced STS treated with anthracycline chemotherapy and improved overall survival [19], [20]. Histological subtypes differed according to age group; synovial sarcomas were more common in young patients, and UPS (formerly known as MFH) was more frequent among elderly patients. Synovial sarcoma is associated with improved survival in patients with advanced STS treated with anthracyclines and with longer PFS in those treated with ifosfamide‐containing regimens [19], [20]. Conversely, UPS (MFH) is associated with reduced overall survival in patients treated with anthracyclines [19].
A minority of patients in this analysis had received prior radiotherapy. This was unexpected given that pre‐ or postoperative radiotherapy is now recommended for the majority of patients with intermediate or high‐grade tumors [21]. These results may reflect inaccurate data recording, lack of adherence with guidelines, and changes in practice in the last few decades [22].
Overall, elderly patients had shorter overall survival than younger patients. There was also a trend toward shorter progression‐free survival and lower response rates. Despite favorable baseline characteristics, these results demonstrate that older age (≥65 years) is an adverse prognostic factor for patients with advanced STS treated with first‐line chemotherapy. In reality, the heterogeneity of an unselected elderly population with STS encountered in clinical practice is not directly comparable with these patients. A previous single‐institution, retrospective review of 120 elderly patients with advanced STS (excluding GIST) treated with first‐line chemotherapy, most commonly single‐agent doxorubicin (60%), described a slightly better RR of 20%; however, the OS was 6.5 months (95% CI, 4.7–8.3) [23]. Another monoinstitutional study of 134 elderly patients with advanced soft tissue sarcomas (primary scalp, trunk, girdles, and extremities only) reported a median OS of 7.3 months [24]. In this study, one quarter of patients had PS 2 (n = 33, 25%). In addition, 40% of cases were high‐grade UPS (formerly MFH) or angiosarcomas, and these tumors were associated with worse prognosis <6 months [24]. An additional retrospective study evaluated 197 patients aged ≥75 years with advanced STS and reported a median PFS of 4 months (95% CI, 2.9–5.1) and OS of 10.9 months (95% CI, 8.3–13.5); however, baseline characteristics were indistinguishable from patients receiving best supportive care [25]. In this study, age ≥80 years, PS ≥2, and number of metastatic sites were independent prognostic variables for OS [25]. The majority of patients received an anthracycline‐based regime (63%), and patients were usually treated with single‐agent chemotherapy (83%) [25].
Doxorubicin has been standard first‐line chemotherapy for advanced STS since the 1970s; however, its side effects include myelosuppression, mucositis, and cardiotoxicity. Empirical dose reductions are often used in patients who are frail or have preexisting comorbidities, such as renal dysfunction, because of the risk of severe toxicity and hospitalization. Previous studies have demonstrated a dose‐response relationship with optimal antitumor activity at doses of ≥60 mg/m2 (administered every 3 weeks) and reduced efficacy at lower levels [26], [27]. Older patients may therefore be disadvantaged as a result of suboptimal treatment.
The combination of doxorubicin and ifosfamide has been shown to provide higher response rates but no improvement in overall survival and at the cost of increased toxicity [21]. In EORTC STBSG clinical trials 62842, 62851, 62883, and 62903, 114 elderly patients were treated with combination therapy (doxorubicin + ifosfamide). These patients had a median OS of 12 months (95% CI, 9.59–14.88) and PFS 5 months (95% CI, 3.09–6.41). There is often a misconception that elderly patients do not tolerate chemotherapy; however, these results demonstrate that even doublet chemotherapy can be used effectively in carefully selected patients.
We observed that radiological responses were mainly seen in elderly patients treated with doxorubicin or doxorubicin plus ifosfamide. It is probable that these patients were potentially the more “fit” elderly patients who were considered to be suitable for clinical trials of these more toxic chemotherapy drugs. In addition, two thirds of all elderly patients were treated with these chemotherapy regimens, and therefore more responses may be anticipated in a larger group. The higher overall response rate in younger patients may be at least partly explained by greater proportion of patients aged <65 years who received combination ifosfamide plus doxorubicin, which has a significantly higher response rate compared with single‐agent doxorubicin [16].
Determining which patients will tolerate chemotherapy is challenging. The ability of physicians to predict chemotherapy‐induced toxicity has been evaluated in patients with lung cancer [28]. This group found that severe toxicity and successful completion of treatment were equally likely in patients who were deemed eligible for treatment [28]. They suggested that more detailed geriatric assessments are needed to predict those patients who are at risk of toxicity [28]. The effectiveness of such tools has been tested in other tumor groups. The mini‐mental state examination and instrumental activities of daily living (IADL) were predictive of toxicity in elderly patients with metastatic colorectal cancer [29]. Comprehensive geriatric assessment has been used in patients with metastatic breast cancer (aged ≥65 years) to predict grade 3–4 toxicity [30]. The International Society of Geriatric Oncology consensus has determined the G8 assessment to be the most “robust, predictive/prognostic” tool for outcome measures in elderly patients [31]. The EORTC‐STBSG is now routinely incorporating the G8 assessment tool in its clinical trials.
Participants in research studies must be representative of the population of interest [5]. With an aging population, there is an increasing need for clinical trials that are designed to assess the optimal pharmacotherapeutic strategies for elderly patients. The feasibility of metronomic oral cyclophosphamide with prednisolone was evaluated in a group of 26 elderly patients (aged 66–88 years); the toxicity profile was favorable, the response rate was 26.9%, and the median PFS was 6.8 months [32]. Other trials include the EPAZ phase II noninferiority trial of pazopanib (800 mg once daily) versus doxorubicin (75 mg/m2) as first‐line therapy for patients aged ≥60 years with advanced STS (n = 120) and the E‐TRAB study in elderly patients (aged >60 years) treated with first‐line trabectedin and considered unsuitable for anthracycline‐based chemotherapy (n = 110) [33], [34]. The E‐TRAB study will analyze quality of life and patient‐reported outcome data in addition to overall survival [34].
The challenge is to design trials that are not only for “elite” older patients but consider the heterogeneity of older patients. Although the median overall survival was inferior for elderly patients, response rates were similar, suggesting that elderly patients can benefit from standard chemotherapy regimens that are routinely prescribed for younger patients. Although the patients described in this report were of good performance status, our data can provide a benchmark for designing future trials. Study designs should also consider tools that can accurately assess potential toxicity of treatments and enable stratification of patients. These trials should be done within a multidisciplinary team, including geriatricians and trained nursing staff to provide adequate support for patients. Given the marginal survival benefit of chemotherapy in advanced STS, clinical trials should also incorporate health‐related quality of life assessments as study endpoints. These data will enhance clinical decision making and enable clinicians to provide a holistic, evidence‐based approach to the care of elderly patients.
Although there were few patients in these EORTC‐STBSG clinical trials aged ≥75 years, their outcomes were similar to those aged ≥65 years. It is probable that these patients were highly selected older elderly individuals in order to meet eligibility criteria for these clinical trials. In a real‐life setting, the METASARC observational study (n = 2,165) reported that patients aged ≥75 years (n = 279) were significantly less likely to receive any systemic treatment for metastatic disease and more likely to be offered best supportive care than those aged <75 years [35]. This French group attributed this finding to the general reluctance of oncologists to prescribe anthracyclines to elderly patients in view of potential hematological and cardiotoxic effects of these drugs in older patients with functional decline or comorbid medical conditions [35]. The complex association of cellular senescence, aging, and cancer is beyond the scope of this article.
Limitations
The clincial trials in this EORTC‐STBSG database were not designed to evaluate age‐related differences in chemotherapy outcomes; therefore, p values have not been presented to avoid overinterpretation of the data. Selection bias because of the favorable baseline characteristics of clinical trial patients means that these results may not reflect outcomes in clinical practice. No details were available on treatments received upon progression of disease. However, because of the limited availability of effective second‐ or third‐line treatment options, it is likely that all patients received similar treatments. Additionally, this database contains historical data from patients recruited in clinical trials from the 1980s, and therefore results may be influenced by differences in concomitant standards of care.
Conclusion
Elderly patients with advanced STS have slightly worse outcomes than younger patients when treated with first‐line chemotherapy within clinical trials. In light of the aging population, there is an increasing need to design studies that specifically evaluate treatments in elderly patients, not only those with favorable characteristics. The results of this analysis can help in the design of future trials, which should incorporate geriatric tools to stratify patients and assess risk and include health‐related quality of life assessments as endpoints.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This research was supported by the EORTC Cancer Research Fund and the Biomedical Research Centre of the Royal Marsden Hospital and the Institute of Cancer Research.
Author Contributions
Conception/design: Eugenie Younger, Saskia Litière, Axel Le Cesne, Olivier Mir, Hans Gelderblom, Antoine Italiano, Sandrine Marreaud, Robin Lewis Jones, Alessandro Gronchi, Winette T.A. van der Graaf
Provision of study material or patients: Saskia Litière, Axel Le Cesne, Olivier Mir, Hans Gelderblom, Antoine Italiano, Sandrine Marreaud, Robin Lewis Jones, Alessandro Gronchi, Winette T.A. van der Graaf
Data analysis and interpretation: Saskia Litière, Eugenie Younger, Winette T.A. van der Graaf
Manuscript writing: Eugenie Younger
Final approval of manuscript: Eugenie Younger, Saskia Litière, Axel Le Cesne, Olivier Mir, Hans Gelderblom, Antoine Italiano, Sandrine Marreaud, Robin Lewis Jones, Alessandro Gronchi, Winette T.A. van der Graaf
Disclosures
Robin Lewis Jones: Adaptimmune, Blueprint, Clinigen, Eisai, Epizyme, Daichii, Deciphera, Immunedesign, Lilly, Merck, Pharmamar (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.World Health Organization. World Health Statistics 2016: Monitoring Health for the Sustainable Health Goals. Geneva, Switzerland: World Health Organization; 2016. http://www.who.int/gho/publications/world_health_statistics/2016/en/. Accessed March 1, 2018.
- 2. Terret C, Zulian GB, Naiem A et al. Multidisciplinary approach to the geriatric oncology patient. J Clin Oncol 2007;25:1876–1881. [DOI] [PubMed] [Google Scholar]
- 3. Berger NA, Savvides P, Koroukian SM et al. Cancer in the elderly. Trans Am Clin Climatol Assoc 2006;117:147–155; discussion 155–156. [PMC free article] [PubMed] [Google Scholar]
- 4. Balducci L. Studying cancer treatment in the elderly patient population. Cancer Control 2014;21:215–220. [DOI] [PubMed] [Google Scholar]
- 5. Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol 2005;23:3112–3124. [DOI] [PubMed] [Google Scholar]
- 6. Knechel NA. The challenges of enrolling older adults into intervention studies. Yale J Biol Med 2013;86:41–47. [PMC free article] [PubMed] [Google Scholar]
- 7. Burningham Z, Hashibe M, Spector L et al. The epidemiology of sarcoma. Clin Sarcoma Res 2012;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coindre JM, Terrier P, Guillou L et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: A study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 2001;91:1914–1926. [DOI] [PubMed] [Google Scholar]
- 9. Benjamin RS, Wiernik PH, Bachur NR. Adriamycin: A new effective agent in the therapy of disseminated sarcomas. Med Pediatr Oncol 1975;1:63–76. [DOI] [PubMed] [Google Scholar]
- 10. Blay JY, van Glabbeke M, Verweij J et al. Advanced soft‐tissue sarcoma: A disease that is potentially curable for a subset of patients treated with chemotherapy. Eur J Cancer 2003;39:64–69. [DOI] [PubMed] [Google Scholar]
- 11. Schroyen S, Adam S, Jerusalem G et al. Ageism and its clinical impact in oncogeriatry: State of knowledge and therapeutic leads. Clin Interv Aging 2014;10:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Italiano A, Mathoulin‐Pelissier S, Cesne AL et al. Trends in survival for patients with metastatic soft‐tissue sarcoma. Cancer 2011;117:1049–1054. [DOI] [PubMed] [Google Scholar]
- 13. Tap WD, Jones RL, Van Tine BA et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft‐tissue sarcoma: An open‐label phase 1b and randomised phase 2 trial. Lancet 2016;388:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Compare statistics by age. In: Howlader N, Noone AM, Krapcho M et al. SEER Cancer Statistics Review, 1975–2014. Bethesda, MD: National Cancer Institute; April 2017. Available from https://seer.cancer.gov/faststats/selections.php?series=age. Accessed March 1, 2018.
- 15. Nijhuis PH, Schaapveld M, Otter R et al. Epidemiological aspects of soft tissue sarcomas (STS)–consequences for the design of clinical STS trials. Eur J Cancer 1999;35:1705–1710. [DOI] [PubMed] [Google Scholar]
- 16. Judson I, Verweij J, Gelderblom H et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first‐line treatment of advanced or metastatic soft‐tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol 2014;15:415–423. [DOI] [PubMed] [Google Scholar]
- 17. Tap WD, Papai Z, Van Tine BA et al. Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft‐tissue sarcoma (TH CR‐406/SARC021): An international, multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2017;18:1089–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aapro MS, Köhne CH, Cohen HJ et al. Never too old? Age should not be a barrier to enrollment in cancer clinical trials. The Oncologist 2005;10:198–204. [DOI] [PubMed] [Google Scholar]
- 19. Van Glabbeke M, van Oosterom AT, Oosterhuis JW et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: An analysis of 2,185 patients treated with anthracycline‐containing first‐line regimens–a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 1999;17:150–157. [DOI] [PubMed] [Google Scholar]
- 20. Sleijfer S, Ouali M, van Glabbeke M et al. Prognostic and predictive factors for outcome to first‐line ifosfamide‐containing chemotherapy for adult patients with advanced soft tissue sarcomas: An exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer‐Soft Tissue and Bone Sarcoma Group (EORTC‐STBSG). Eur J Cancer 2010;46:72–83. [DOI] [PubMed] [Google Scholar]
- 21. Dangoor A, Seddon B, Gerrand C et al. UK guidelines for the management of soft tissue sarcomas. Clin Sarcoma Res 2016;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bagaria SP, Ashman JB, Daugherty LC et al. Compliance with National Comprehensive Cancer Network guidelines in the use of radiation therapy for extremity and superficial trunk soft tissue sarcoma in the United States. J Surg Oncol 2014;109:633–638. [DOI] [PubMed] [Google Scholar]
- 23. Yousaf N, Harris S, Martin‐Liberal J et al. First line palliative chemotherapy in elderly patients with advanced soft tissue sarcoma. Clin Sarcoma Res 2015;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Comandone A, Boglione A, Guibellino E et al. Metastatic soft tissue sarcomas in elderly patients: 18 years of monoinstitutional experience. Clin Oncol. 2017;2:1231. [Google Scholar]
- 25. Garbay D, Maki RG, Blay JY et al. Advanced soft‐tissue sarcoma in elderly patients: Patterns of care and survival. Ann Oncol 2013;24:1924–1930. [DOI] [PubMed] [Google Scholar]
- 26. O'Bryan RM, Baker LH, Gottlieb JE et al. Dose response evaluation of adriamycin in human neoplasia. Cancer 1977;39:1940–1948. [DOI] [PubMed] [Google Scholar]
- 27. Toma S, Palumbo R, Sogno G et al. Doxorubicin (or epidoxorubicin) combined with ifosfamide in the treatment of adult advanced soft tissue sarcomas. Ann Oncol 1992;3(suppl 2):S119–S123. [DOI] [PubMed] [Google Scholar]
- 28. Zauderer MG, Sima CS, Korc‐Grodzicki B et al. Toxicity of initial chemotherapy in older patients with lung cancers. J Geriatr Oncol 2013;4:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aparicio T, Jouve JL, Teillet L et al. Geriatric factors predict chemotherapy feasibility: Ancillary results of FFCD 2001‐02 phase III study in first‐line chemotherapy for metastatic colorectal cancer in elderly patients. J Clin Oncol 2013;31:1464–1470. [DOI] [PubMed] [Google Scholar]
- 30. Hamaker ME, Seynaeve C, Wymenga AN et al. Baseline comprehensive geriatric assessment is associated with toxicity and survival in elderly metastatic breast cancer patients receiving single‐agent chemotherapy: Results from the OMEGA study of the Dutch breast cancer trialists’ group. Breast 2014;23:81–87. [DOI] [PubMed] [Google Scholar]
- 31. Decoster L, Van Puyvelde K, Mohile S et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: An update on SIOG recommendations. Ann Oncol 2015;26:288–300. [DOI] [PubMed] [Google Scholar]
- 32. Mir O, Domont J, Cioffi A et al. Feasibility of metronomic oral cyclophosphamide plus prednisolone in elderly patients with inoperable or metastatic soft tissue sarcoma. Eur J Cancer 2011;47:515–519. [DOI] [PubMed] [Google Scholar]
- 33. Karch A, Koch A, Grünwald V. A phase II trial comparing pazopanib with doxorubicin as first‐line treatment in elderly patients with metastatic or advanced soft tissue sarcoma (EPAZ): Study protocol for a randomized controlled trial. Trials 2016;17:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kasper B, Reichardt P, Schuler M et al. Geriatric assessment of elderly chemotherapy‐naive patients treated with trabectedin for advanced soft tissue sarcomas (STS): The E‐TRAB study of the German Interdisciplinary Sarcoma Group (GISG‐13). Ann Oncol 2017;28(suppl 5):mdx387.050A. [Google Scholar]
- 35. Savina M, Le Cesne A, Blay JY et al. Patterns of care and outcomes of patients with metastatic soft tissue sarcoma in a real‐life setting: The METASARC observational study. BMC Med 2017;15:78. [DOI] [PMC free article] [PubMed] [Google Scholar]