Abstract
Models of differential susceptibility hypothesize that neural function may be a marker of differential susceptibility to context, but no studies have tested this hypothesis. Using a sample of 310 young men from low-income urban neighborhoods, this study investigated amygdala reactivity to facial expressions as a moderator of the relations between socioeconomic resources and later antisocial behavior and income. For individuals with high amygdala reactivity, greater socioeconomic resources at age 20 predicted less AB and greater income at age 22. For young men with low amygdala reactivity, however, socioeconomic resources at age 20 did not predict later outcomes. Amygdala reactivity to fearful facial expressions, key to the etiology of antisocial behavior, moderated links between resources and antisocial behavior. In contrast, amygdala reactivity more generally to multiple facial expressions moderated the effects of resources on later income attainment. Both interactions met rigorous quantitative criteria for patterns of differential susceptibility rather than diathesis-stress or vantage sensitivity. Moreover, these associations remained significant after inclusion of socioeconomic resources during earlier developmental periods. These results suggest that greater amygdala reactivity to facial expressions is a marker of greater susceptibility to context, for better or for worse, during the transition to adulthood.
Keywords: differential susceptibility, amygdala reactivity, socioeconomic resources, emerging adulthood, antisocial behavior
The U.S. Census Bureau estimates that 21.1% or 15.5 million children under age 18 live in poverty (DeNavas-Walt & Proctor, 2015). A wealth of literature indicates that child poverty and poverty-related adversities predict worse physical and mental health outcomes, worse well-being, and persistent poverty status across the lifespan (Duncan, Ziol-Guest, & Kalil, 2010; Bradley & Corwyn, 2002). Poverty is associated with a cluster of concurrent and later disadvantages, including poor employment opportunities, neighborhood dangerousness, and housing crowding and instability (Bradley & Corwyn, 2002). One of the most insidious effects of poverty and its related sequela is its intergenerational stability: poverty is often perpetuated across childhood into adulthood and across generations (Kendig, Mattingly, & Bianchi, 2007).
However, there is substantial variability in how individuals respond to socioeconomic adversity, such that some individuals exhibit poor socioemotional and vocational outcomes, while others function well at home and work (Alexander, Entwise, & Olson, 2014; Masten, 2001). One potential developmental window during which trajectories of socioeconomic risk may be malleable is during the transition to adulthood (i.e., late teens through early twenties), when youth experience changes in romantic and social relationships, increased privileges and responsibilities associated with reaching legal age, and potential independence from caregivers (Arnett, 2000). During this key period, youth may secure new resources or, conversely, engage in behaviors which may undermine their future economic prospects. For many youth, the transition to adulthood is when their family of origin’s socioeconomic resources can translate into their adult socioeconomic resources, for better or worse. Moreover, even for youth growing up in poverty, there is substantial variability in terms of the amount of socioeconomic resources they, or their family, may possess, leading to divergent outcomes even within relatively disadvantaged contexts. As evidence for this period being critical in socioeconomic trajectories, Obradović and colleagues (2006) measured indicators of socioemotional health from childhood to adulthood and found the greatest change in outcomes, such as interpersonal and work/educational competence, during emerging adulthood (i.e., ages 17–23 years), highlighting the notion that this developmental transition affords both risks and opportunities for psychosocial outcomes (Schulenberg, Sameroff, & Cicchetti 2004). Emerging adulthood may be even more critical for youth growing up in low-income, urban environments, where there are fewer opportunities for prosocial economic growth (Alexander et al., 2014).
As youth transition to adulthood in high-risk contexts, two salient indicators of how youth are functioning include engaging in (or desisting from) antisocial behavior (AB) and emerging income attainment. Without economic security, low-income young adults aged 18–24 are more likely to endorse substance use and mental health problems, be unemployed (and/or unenrolled in school), and to report crowded living arrangements than their more financially-solvent peers (Federal Interagency Forum on Child and Family Statistics, 2014). For youth living in low-income contexts, AB including aggression, violence, and rule-breaking (Loeber & Hay, 1997) indicates a poor transition. Antisocial behaviors, particularly more serious criminality, peak in early adulthood and predict a range of poor outcomes including substance use and depression (Odgers et al., 2008). Risk factors associated with poverty, including neighborhood violence, crowded and unstable housing, and unemployment have each been shown to predict the emergence and continuity of AB into adulthood, making AB an unfortunately common outcome for low-income, urban youth, particularly males (Pratt & Cullen, 2005). By contrast, finding steady income may be a marker of a successful transition to adulthood (Arnett, 2000; Alexander et al., 2014) and an important step towards preventing the perpetuation of intergenerational poverty (Kendig et al., 2007). Thus, while continued AB indicates difficulty with the transition to adulthood and the possibility of ensnaring young adults via incarceration (Moffitt, 1993), income attainment is a marker of a successful transition to adulthood in low-income contexts.
Differential Susceptibility to Context
Though socioeconomic adversity puts youth at risk for poor outcomes, there is tremendous individual variability in these outcomes (Alexander et al. 2014; Masten, 2001), due to attributes of both the context (e.g., presence of some protective factors within a risky context) and/or the individual (e.g., sensitivity to protective factors). One useful model for studying individual variation in response to adversity is differential susceptibility (Belsky & Pluess, 2009) or biological sensitivity to context (Ellis & Boyce, 2008). These models posit that individuals vary in the extent to which they respond to environmental influence, for better or for worse. For more “plastic” individuals, negative environments yield maladaptive outcomes (i.e., diathesis-stress; Monroe & Simmons, 1991) and positive environments predict adaptive outcomes (i.e., vantage sensitivity; Pluess & Belsky, 2013). Conversely, more “fixed” individuals exhibit less variation in behavior across all environments (Belsky & Pluess, 2009; Ellis & Boyce, 2008).
Emerging literature in this area has focused on identifying individual and potentially biologically-based markers of differential susceptibility to context, including temperament, physiological measures of arousal, and genetic variation (Belsky & Pluess, 2009; Boyce, 2016). For example, studies have identified that young children with high negative reactivity and emotional distress (i.e., difficult temperaments) are more susceptible to behavior problems when exposed to harsh parenting (Bradley & Corwyn, 2008), but also show greater attachment security in response to increases in maternal sensitivity (Klein Velderman, Bakermans-Kranenberg, Juffer, & van IJzendoorn, 2006). Similarly, children described as more stress reactive (e.g., lower respiratory sinus arrhythmia suppression, greater cortisol reactivity during stress challenge) have also been shown to be more sensitive to the effects of family adversity and family income on behavioral problems, school engagement, and executive function skills (Obradović, Bush, Stamperdahl, Adler, & Boyce, 2010; Obradović, Portilla, & Ballard, 2016). Additionally, short allele carriers of the serotonin transporter gene (SLC6A4) are at heightened risk for psychopathology in adverse contexts (Karg, Burmeister, Shedden, & Sen, 2011), but have also been shown to exert the greatest positive affect in supportive contexts (Hankin et al., 2011).
Amygdala Reactivity as a Marker of Differential Susceptibility
Findings linking early temperament, stress reactivity, and genetic variability in serotonin signaling to differential susceptibility have led researchers to suggest that neural reactivity, particularly in the amygdala, may be an important marker of differential susceptibility to context (Belsky & Pluess, 2009; Schriber & Guyer, 2016). The amygdala is a neural region critical for emotion processing, salience detection, and fear learning (LeDoux, 2000). Amygdala reactivity has been studied extensively in response to emotional faces (LeDoux, 2000; Whalen et al., 2001), and amygdala reactivity to these ecologically valid affective stimuli in adulthood has been found to be stable across a year (Manuck, Brown, Forbes, & Hariri, 2007). In adult samples, greater amygdala reactivity to emotional facial expressions (particularly fearful facial expressions) has been linked to several phenotypes associated with differential susceptibility, including negative emotionality (Etkin et al., 2004), stress reactivity (Henckens, Klumpers, Everaed, Kooijman, van Wingen, & Fernández, 2016), and genetic variation within the serotonin transporter (Munafó, Brown, & Hariri, 2008). The amygdala also plays a crucial role in the stress response (LeDoux, 2000) and underlying central nervous system sensitivity, systems hypothesized to form core endophenotypes for biological markers of differential susceptibility to context (Boyce, 2016; Belsky & Pluess, 2009). Moreover, greater amygdala reactivity to affective faces (Canli et al., 2001), particularly to fearful faces, predicts both maladaptive (e.g., externalizing problems; Hyde, Shaw, & Hariri, 2013) and adaptive (e.g., altruism; Marsh et al., 2008) outcomes. It may be that these differential associations between relatively high amygdala reactivity to fearful facial expressions and both positive and negative outcomes emerge as a function of exposure to different environment contexts. That is, greater neural sensitivity to others’ distress may be associated with positive outcomes (e.g., social competence) in promotive environments, but lead to hostile attributions and poorer outcomes (e.g., AB) in less supportive environments (Dodge, 2006).
The evidence noted above suggests that amygdala reactivity to fearful facial expressions is a likely marker of differential susceptibility to context, particularly when examining AB as the outcome (Blair, 2013; Hyde, Shaw, & Hariri, 2013). However, the amygdala is sensitive to multiple types of emotional faces (Fitzgerald, Angstadt, Kelsone, Nathan, & Phan, 2006) and is thought to play a broad role in salience detection (Davis & Whalen, 2001). Santos, Mier, Kirsch, and Meyer-Lindenberg (2011) showed that participants detected target faces faster than non-targets independent of affective valence, and that the degree of amygdala activation was similar when participants viewed emotional-threatening, emotional-non-threatening, and non-emotional target faces (see also Fitzgerald et al., 2006). As securing employment and steady income requires attention to multiple interpersonal cues across contexts (Liu, Peng, & Wong, 2014), it may be that a measure of general amygdala reactivity (i.e., to all faces) is a marker of differential susceptibility to context when examining broader outcomes such as income attainment.
Quantitative Concerns in Testing Models of Differential Susceptibility
Although research has identified multiple potential markers of differential susceptibility to context, many of these studies have two major limitations. The first is that, until recently, there were few guidelines and statistical approaches to confirm whether the pattern of findings fit a model of diathesis-stress (i.e., that some youth evince worse outcomes in poor environments), vantage sensitivity (i.e., that some youth show positive outcomes in promotive contexts), or differential susceptibility (i.e., that some youth do well in good environments, but poorly in bad environments). Widaman et al. (2012) and Roisman et al. (2012) have each offered conceptual and statistical requirements for determining the type of interaction found in studies testing differential susceptibility to context. Within these models, studies can examine several attributes of the interaction to assess the presence of differential susceptibility versus vantage sensitivity or diathesis-stress, such as calculating the crossover point of an interaction and its confidence interval with respect to the observed data to determine if the interaction is disordinal (i.e., indicative of differential susceptibility) or ordinal (i.e., indicative of diathesis-stress or vantage sensitivity) (see Figure 1). Thus, these rigorous approaches are critical in testing models of differential susceptibility to context.
Figure 1.
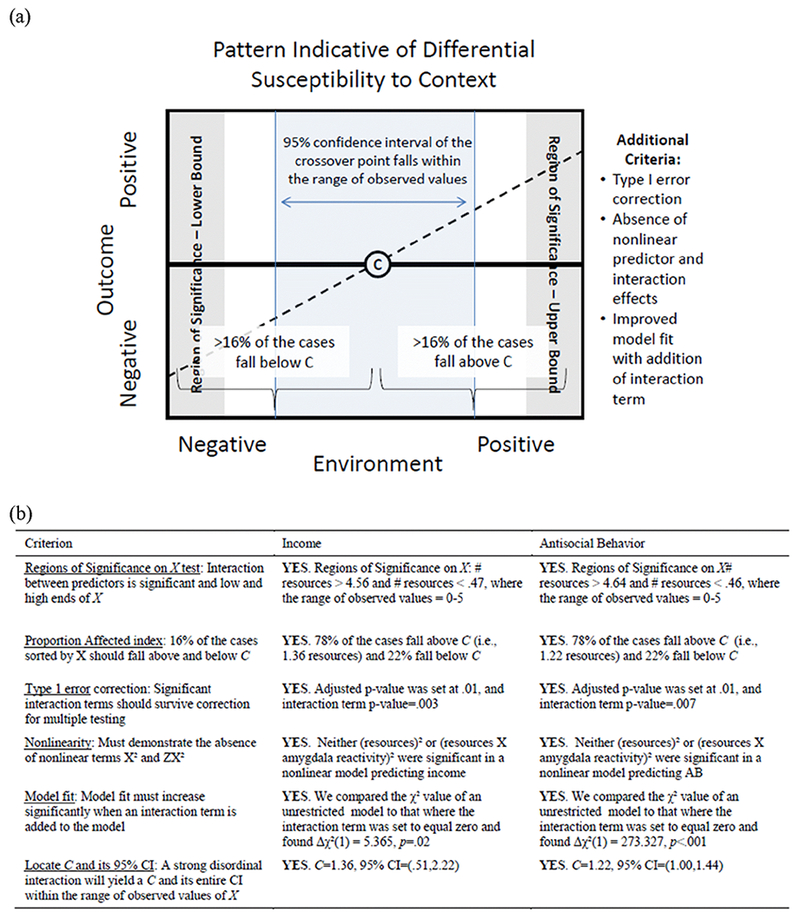
(a) Graphical depiction of the criteria indicative of differential susceptibility to context. (b) Criteria for differential susceptibility to context applied to predicted income (with amygdala reactivity all facial expressions as the moderator) and antisocial behavior (with amygdala reactivity to fearful facial expressions as the moderator) at age 22 in the current sample.
Note. C=crossover point
A second quantitative concern for evaluating these models is the reliance on measures of the environment and outcomes with restricted ranges. For example, a study of differential susceptibility that uses family adversity as the environmental indicator and behavioral problems as the outcome (i.e., a common scenario in existing studies of differential susceptibility to context) (e.g., Obradovic et al., 2010) would need to assume that the absence of adversity confers a “positive” environment and the absence of behavioral problems indicates “adaptive” or “resilient” functioning. One way to address this problem is to create a dimensional index of context such that one end of the scale reflects relatively supportive environments and the other risky or adverse contexts. Another is to focus on multiple outcomes that are truly dimensional, reflecting positive and negative functioning (e.g., income).
The Current Study
To address these gaps in the literature, we explored whether amygdala reactivity to facial expressions moderated the relations between a dimensional index of socioeconomic resources in young adulthood (age 20) and indicators of successful or unsuccessful transition to adulthood (i.e., AB and income attainment) two years later. We focused on the transition to adulthood as a developmental period during which change might be possible, even within a relatively impoverished sample. Moreover, to confirm that these effects were specific to this developmental window and not the result of stable trajectories of early risk exposure (Duncan et al., 2010), we examined whether amygdala reactivity to facial expressions moderated the relation between socioeconomic resources at other key developmental periods (i.e., early childhood and early adolescence, in separate models) and successful transition to adulthood. We were particularly interested in evaluating whether the pattern of findings for each model was consistent with a model of differential susceptibility, diathesis-stress, or vantage sensitivity, using quantitative recommendations by Widaman et al. (2012) and Roisman et al. (2012).
To evaluate these hypotheses in youth facing adversity, we used a well-characterized sample of young men followed since infancy who were at elevated risk for poor outcomes, including AB, based on their gender, their low family income, and living in an urban community (Shaw, Gilliom, Ingoldsby, & Nagin, 2003). Although the young men in this sample grew up in disadvantaged contexts, variation in socioeconomic resources increased for a minority of the sample during childhood and adolescence, allowing us to examine the interaction of promotive factors and individual markers of differential susceptibility to context. As prior research suggests that greater amygdala reactivity is related to other markers of differential susceptibility (e.g., high neuroticism, high stress reactivity), we hypothesized that youth with relatively high amygdala reactivity to facial expressions would be more “plastic” or “susceptible” to the behavioral and economic consequences of individual differences in socioeconomic resources. Conversely, we hypothesized that youth with relatively low amygdala reactivity would not demonstrate similar sensitivity to socioeconomic resources. However, because low amygdala reactivity, particularly to fearful facial expressions, is associated with more AB in this sample (Hyde, Shaw, Murray, Gard, Hariri, & Forbes, 2015) and elsewhere (for a review, see Hyde et al., 2013), we hypothesized that young men with low amygdala reactivity to fearful facial expressions (albeit less sensitive to contextual adversity) would have the worst outcomes overall (i.e., higher AB, lower income). Though exploratory, we hypothesized that amygdala reactivity to fearful facial expressions would be most important as a marker of differential susceptibility to context when examining AB as an outcome, and that amygdala reactivity to all faces might be most important when examining income attainment as an outcome.
Method
Participants
Participants were part of the Pitt Mother & Child Project (PMCP), an ongoing longitudinal study of child risk and resilience in low-income families (Shaw et al., 2003; Hyde et al., 2015). In 1991 and 1992, 310 low-income boys and their families were recruited from Allegheny County Women, Infant and Children (WIC) Nutritional Supplement Clinics when boys were between 6 and 17 months old. At the time of recruitment, 53% of the target children in the sample were European-American, 36% were African-American, 5% were biracial, and 6% were of other races (e.g., Hispanic-American or Asian-American). Two-thirds of mothers in the sample had 12 years of education or less. The mean per capita income was $241 per month ($2,892/year), and the mean Hollingshead SES score was 24.5, indicative of a low socioeconomic status (SES) sample. Thus, many boys in this study were considered at elevated risk for antisocial outcomes because of their male gender (Hyde et al., 2013), childhood SES, and urbanicity (Pratt & Cullen, 2005).
Target children and mothers were seen almost yearly from age 1.5 – 22 in the laboratory and/or home with assessments that included questionnaires, a psychiatric interview, and at age 20, an fMRI scanning session. Retention rates were generally high at each of the assessment time points, with data for these analyses available on 306 of the initial 310 participants (89%) at 24 months, 272 participants (88%) at ages 10, 11, or 12; and 258 (83%) and 255 (82%) of the original participants with some data at ages 20 and 22, respectively. Of the 186 men who consented and were able to participate in the MRI at age 20, valid data were available for 167 men (see Supplemental Table 1 for sources of data loss related to fMRI). Participants with usable imaging data did not differ from participants who dropped out at earlier ages on the Child Behavior Checklist externalizing scores at ages 2, or 3.5, maternal age, income, or educational attainment (ps > .1). Participants were reimbursed for their time at each assessment. All procedures were approved by the Institutional Review Board of the University of Pittsburgh IRB (most recent IRB#MOD09020252–06/PRO09020252; “Substance Use in Young Men: Genes, Brain Function, and Early Social Development”).
Measures
To create a dimensional index of promotive socioeconomic resources in young adulthood (20 years), we created an index of cumulative resources using methods commonly used in cumulative risk research. Literature in developmental psychopathology shows that the accumulation of multiple risk factors exerts larger effects on an outcome than any one risk factor alone (Sameroff, 2010). Thus, we sought to capture variability in the amount of resources (rather than the unique effects of each risk factor) by creating a cumulative index of socioeconomic resources (e.g., income, education, neighborhood safety) during young adulthood. Similar measures were created during key developmental periods: early (18 – 24 months) and middle childhood (10 – 12 years). Amygdala reactivity to facial expressions was captured at age 20, and adult outcomes including AB and income attainment were collected at age 22.
Socioeconomic Resource Indices
All resource indicators were created by dichotomizing socioeconomic resources, similar to previous cumulative risk research, where individuals received a score of “1” if present and a score of “0” if absent. For continuous measures that did not have clear cut-offs, criteria were established so that approximately 25% of the sample would meet criteria for each resource indicator, an approach that is consistent with prior research on cumulative risk (Sameroff, Seifer, Baldwin, & Baldwin, 1993; Ackerman, Izard, Schoff, Youngstrom, & Kogos, 1999; Sameroff, 2010; Trentacosta et al., 2013). Supplemental Table 2 includes additional descriptive information about the quartile cut-offs for the continuous socioeconomic resource indicators.
Table 1 presents the indicators that comprise the young adulthood resource index. We included indicators that have previously been shown to predict socioemotional and economic outcomes (i.e., antisocial behavior, income attainment) including (1) housing stability (Cutts et al., 2011; Fowler, Henry, & Marcal, 2015), (2) higher income (i.e., >200% of the poverty line, for a single person) (Kendig, Mattingly, & Bianchi, 2014; Sampson & Laub, 1994), (3) adequate living space (Evans & Kantrowitz, 2002), (4) neighborhood safety (Fauth, Leventhal, & Brooks-Gunn, 2004; Loeber & Hay, 1997), and (5) employment opportunity (Skogstad, Torsheim, Einarsen, & Hauge, 2011). Employment opportunity was defined as being employed, part-time or full-time, and scoring in the top quartile on the Work Characteristics Questionnaire (Conger, 1988) which assesses quality of the work environment (e.g., “This job provides good security”). Similar indices were created for early and middle childhood (see Table 1) to include six developmentally appropriate indicators. The young adulthood resources index ranged from 0 to 5 (M = 2.13, SD = .98, N = 238), and both the early (M = 2.67, SD = 1.33, N = 283) and middle childhood (M = 2.34, SD = 1.46, N = 233) indices ranged from 0 to 6. All three resource indices were normally distributed. If individuals were missing data on any of the individual indicators, they were identified as missing on the resource index at that age.
Table 1.
Economic resource indicators across childhood, sources, and percentage meeting criteria
| Indicator | Criteria | Source | % | |
|---|---|---|---|---|
| Young Adulthood (N=238) | Housing stability | One move or less in the last two years | Demographic interview | 74.8 |
| Income | > 200% of the poverty line, based on a single person | Demographic interview | 18 | |
| Adequate living space | More rooms than people in the housing structure | Demographic interview | 81.0 | |
| Neighborhood safety | Bottom quartile on neighborhood danger subscale | Neighborhood Questionnaire | 25.8 | |
| Employment opportunity | Employed (part- or full-time) AND top quartile on a measure of workplace opportunity | Revised Work Characteristics | 14.3 | |
| Middle Childhood (N=233) | Maternal marital stability | Mother married or living with partner at all time points 10 – 12 years | Demographic interview | 47.4 |
| Housing stability | No moves between 10 – 12 years | Demographic interview | 64.7 | |
| Family Income | Greater than 200% the poverty line based on family size at any time point 18 – 42 months | Demographic interview | 43.5 | |
| Neighborhood cohesion | Top quartile on neighborhood cohesion subscale | Me and My Neighborhood | 27.8 | |
| Neighborhood safety | Bottom quartile on neighborhood danger subscale | Me and My Neighborhood | 25.1 | |
| Maternal education | Greater than a high school degree | Demographics interview | 58.0 | |
| Early Childhood (N=283) | Maternal marital stability | Mother married or living with partner at all time points 18 – 42 months | Demographic interview | 48.8 |
| Maternal education | Greater than a high school education – mean across 18 – 42 months | Demographic interview | 51.1 | |
| Maternal age at birth of target child | Older than 21 years old | Demographic interview | 45.5 | |
| Family income | Greater than 200% the poverty line based on family size at any time point 18 – 42 months | Demographic interview | 9.1 | |
| Adequate living space | More rooms than people | Demographic interview | 79 | |
| Neighborhood safety | Bottom quartile of neighborhood danger 24 months | Neighborhood questionnaire | 28.4 | |
Antisocial Behavior
We created a composite measure of AB at age 22 that ranged from relatively common and normative behaviors to severe AB across multiple contexts and domains (i.e., workplace AB, trait-like impulsivity and AB, general delinquency/crime) by adding standardized total scores from the following three measures (1) the Self-Report of Delinquency Questionnaire (Elliot, Huizinga, & Ageton, 1985), (2) the Workplace Deviance Questionnaire (Bennett & Robinson, 2000), and (3) the Antisocial facet/subscale of the Self-Report of Psychopathy – Short Form (Neuman & Pardini, 2014). The SRD contains 53 items that assess the frequency with which an individual has engaged in aggressive and delinquent behavior, alcohol and drug use, and related offenses during the prior year, using a 3-point scale (0 = never, 1 = once/twice, 2 = more often; range = 0 – 43; α =.85; M = 9.83, SD = 7.09) (Elliot et al., 1985). The WDQ is a 25-item scale that measures common AB in the workplace (e.g., “Taken property from work without permission”, “Cursed someone at work”). Participants rate the frequency of each behavior on a 7-point likert scale (1 = never and 7 = daily; range = 0 – 80, α = .89; M = 11.97, SD = 15.34) (Bennett & Robinson, 2000). The SRP-SF is a 28-item scale that measures psychopathy along four dimensions including Interpersonal, Affective, Lifestyle, and Antisocial. To measure severe AB, we used the seven items that comprise the Antisocial facet (e.g., “I was convicted of a serious crime”, “I have assaulted a law enforcement official or social worker”). Participants rate agreement with each of the statements along a five-point likert scale (1 = disagree strongly and 5 = agree strongly; range = 0 – 21; α = .77; M = 4.15, SD = 4.15) (Neuman & Pardini, 2014). Inter-scale correlations ranged from r = .15-.47 (p < .01 to p < .001), and 250 participants had valid data on all three measures of AB. To control for the stability of AB, we added the total score of the SRD at age 20 to models in which age 22 AB was the outcome (the other two measures of AB were not collected at age 20). There was one participant with a value of AB +3 SD below the mean, but results did not change when this potential outlier was excluded from the models. Supplemental Figure 1 depicts the psychometrics of the AB composite.
Income Attainment
Income attainment was measured at age 22 using participant-reported monthly income in dollars, and did not include income from other household members (e.g., parents). Data was available for 230 participants (M = 1,027.82, SD = 984.94, range = 0 to 6,666). While age 22 monthly income varied across participants, the youth in our sample were, on average, living just above the poverty threshold (i.e., $11,170/year for a single person) (U.S. Department of Health and Human Services, 2012). Three participants reported monthly income +3 SD from the mean (> $3,982/month), but results were the same when these potential outliers were excluded from analyses. As both housing status (i.e., living with family or independently) and school enrollment (i.e., in school or not) could explain variation in income at age 22, we controlled for these variables in models predicting age 22 income. See Supplement (pp. 1) for additional information. Moreover, our results for models predicting income did not change in direction or statistical significance when we excluded the 70 young men who were enrolled in school at age 22 (results available upon request).
Amygdala Reactivity to Facial Expressions
Amygdala reactivity paradigm.
The experimental fMRI paradigm consisted of four blocks of a perceptual face processing task interleaved with five blocks of a sensorimotor control (see Supplemental Figure 2). During the face processing task, subjects viewed a trio of faces and selected one of two faces (bottom) identical to a target face (top). Each face processing block consisted of six images, balanced for sex, all derived from a standard set of pictures of facial affect (Ekman & Friesen, 1976). Each of the four face processing blocks consisted of a different emotional facial expression (i.e., anger, fear, surprise, neutral), and participants were randomly assigned to one of four different orders of block presentation. During the sensorimotor control blocks, participants viewed a trio of simple geometric shapes (circles, vertical and horizontal ellipses) and selected one of two shapes (bottom) identical to a target shape (top). All blocks were preceded by brief instructions (“Match Faces” or “Match Shapes”) lasting 2 s. In the face processing blocks, each of the six face trios was presented for 4 s with a variable interstimulus interval (ISI) of 2 to 6 s (mean = 4 s) for a total block length of 48 s. A variable ISI was used to minimize expectancy effects and resulting habituation, as well as to maximize amygdala reactivity throughout the paradigm. In the sensorimotor control blocks, each of the six shape trios was presented for 4 s with a fixed ISI of 2 s (total block length = 36 s; total task time = 390 s).
Bold fMRI acquisition parameters.
Each participant was scanned with a research-dedicated Siemens 3-T Tim Trio. Blood oxygenation level–dependent (BOLD) functional images were acquired with a gradient-echo echoplanar imaging (EPI) sequence (TR/TE = 2000/29 ms, FOV = 200×200), which covered 34 interleaved axial slices (3-mm slice thickness) aligned with the AC-PC plane and encompassing the entire cerebrum and most of the cerebellum to maximum coverage of limbic structures. All scanning parameters were selected to optimize the quality of the BOLD signal while maintaining a sufficient number of slices to acquire whole-brain data. Before collecting fMRI data for each participant, a reference echoplanar imaging scan was acquired and visually inspected for artifacts (e.g., ghosting) and good signal across the entire volume of acquisition. Additionally, an autoshimming procedure was conducted before the acquisition of BOLD data in each participant to minimize field inhomogeneities
Image processing and analysis.
Whole-brain image analysis was completed using the general linear model of SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). Images for each participant were grey matter segmented, realigned to the mean volume in the time series, unwarped to correct for head motion, co-registered to high resolution structural scans (MPRAGE) (TE/TR = 3.29/2200; Flip Angle = 9°; FOV = 256×192 mm2; Slice-Thickness = 1 mm; Matrix: 256×256; 192 continuous slices), spatially normalized into a standard stereotactic space (MNI template) using a 12-parameter affine model, and smoothed to minimize noise and residual differences in gyral anatomy with a Gaussian filter set at 6 mm FWHM. Functional images had a voxel size of 2mm. Voxelwise signal intensities were ratio-normalized to the whole-brain global mean. After preprocessing, the Artifact detection Tools (ART) software package (http://www.nitrc.org/projects/artifact_detect/) was used to detect global mean intensity and translation or rotational motion outliers (> 4.5 SD from the mean global brain activation, > 2 mm movement or 2o translation in any direction) within each participant’s data and to create a regressor accounting for the possible confounding effects of volumes as outliers. Additionally, because of the relatively extensive signal loss typically observed in the amygdala, single-subject BOLD fMRI data were only included in subsequent analyses if there was a minimum of 90% signal coverage in the amygdala region of interest (ROI) (defined as the bilateral amygdala using the Automated Anatomical Labeling [AAL] Atlas in the WFU PickAtlas Tool, version 1.04; Wake Forest University School of Medicine, Winston-Salem, NC) (see also Hyde et al., 2015).
BOLD fMRI data analysis.
The general linear model in SPM8 was used to estimate condition-specific (e.g., fearful faces > shapes) BOLD activation for each individual scan. Individual contrast images were then used in second-level random effects models to determine mean expression-specific reactivity using one-sample t-tests (i.e., main effects of the task). As our goal was to examine amygdala reactivity to specific contrasts within an anatomically defined ROI, the following contrasts were estimated and extracted from SPM8 to be used in regression models: fearful facial expressions > shapes, to measure neural reactivity to interpersonal distress (Whalen et al., 2001), and all faces > shapes to capture general amygdala reactivity during socioemotional processing. Several studies (e.g., Davis, Neta, Kim, Moran, & Whalen, 2016; Marusak, Zundel, Brown, Rabinak, & Thomason, 2016; Somerville, Kim, Johnstone, Alexander, & Whalen, 2004) indicate that purely “neutral” faces may be interpreted as hostile by participants. Thus, many studies have begun to use “calm” faces as the baseline condition, which combine neutral faces morphed with happy expressions (Sebastian et al., 2014; Viding et al., 2012). However, as our task did not have calm faces, we used shapes as the baseline condition in our contrasts, which helps to link our work to the numerous studies using this contrast with this task (see Hyde et al., 2013; Munafò, Brown, & Hariri, 2008). Contrast-specific BOLD parameter estimates were extracted from clusters in the left and right amygdala regions (defined using the AAL bilateral amygdala mask used to check for coverage) that showed activation to the contrast and survived correction for multiple comparisons across the entire brain using the Family-Wise Error correction in SPM8 (p < .05) (see Supplemental Table 3). As we did not have a priori hypothesis about laterality, to decrease multiple comparisons, we created a measure of mean amygdala activation across left and right clusters for each condition using the extracted main effect estimates (i.e., fearful faces > shapes and all faces > shapes) as has been done in previous work (Swartz, Knodt, Radtke, & Hariri, 2015). Using main effect estimates of left and right amygdala reactivity separately showed no differences in laterality, supporting our approach of creating a measure of mean amygdala reactivity across both hemispheres. There were two participants with values of amygdala reactivity +/− 3 SD around the mean, but results did not change when these potential outliers were excluded from the models.
Analytic Plan
Four regression models were computed to examine whether amygdala reactivity to all emotional facial expressions or fearful facial expressions moderated the relations between socioeconomic resources at age 20 and income attainment or AB at age 22. As both main effect predictors (i.e., amygdala reactivity and socioeconomic resource) were on different scales, we standardized both before creating the interaction terms (Aiken & West, 1991). Although we report the point estimates from the standardized regression in the text, graphical presentations of significant interactions use unstandardized point estimates to facilitate interpretation (i.e., to present the resource index on the observed scale from zero to five). Next, to confirm that our results were specific to early adulthood, we added resource indices in early and middle childhood, and their interaction terms with amygdala reactivity at age 20, to each of the above regression models. As the resources indices during early and middle childhood were highly correlated (r = .57, p < .001), we added each resource index to the original regression models separately to reduce multicollinearity of predictors. Finally, to stringently assess whether each of our four regression models yielded patterns of diathesis-stress, vantage sensitivity, or differential susceptibility to context, we followed recommendations by both Widaman et al., (2012) and Roisman et al. (2012) (see below). All models included participant race as a covariate.
All analyses were performed in Mplus (version 7.2) (Muthén & Muthén, 1998-2011) using FIML estimation. Participants with usable imaging data (n = 167) did not differ from other participants on the socioeconomic resources indices in early childhood, middle childhood, or young adulthood (ps = .12-.48), or on any of the measures of AB (ps = .43-.81). Participants with usable imaging data (versus without) reported higher income at age 22, t (228) = −2.23, p < .05. We used FIML estimation to include all participants with data at age 22 (n = 258) because this estimation provides unbiased estimates, even in the context of substantial missing data (McCartney, Burchinal & Burb, 2006). Our results were indistinguishable when income at age 22 was included as an auxiliary variable that contributed to the covariance matrix of available data (Graham, 2009). The results (i.e., main effects and interactions) were also parallel using listwise deletion. Significant interaction terms were graphed using the online utility by Preacher, Curran, & Bauer (2006) to determine regions of significance and simple slopes. Further probing of significant interaction terms was completed using SPSS (version 23).
Both Widaman et al., (2012) and Roisman et al. (2012) independently set forth a series of quantitative recommendations to evaluate patterns of differential susceptibility from diathesis-stress (see Figure 1 for a graphical depiction). We followed both methods of evaluating interaction patterns as a rigorous test of our hypothesis that amygdala reactivity is a marker of differential susceptibility to context. For each significant interaction, we (1) calculated the crossover point (C) and the 95% confidence interval of C to determine whether the interaction was ordinal (i.e., indicative of diathesis-stress or vantage sensitivity) or disordinal (i.e., indicative of differential susceptibility) (Widaman et al., 2012). (2) We calculated the regions of significance (RoS) on X test to assure that the moderator (i.e., amygdala reactivity) predicted the outcome variable (i.e., income or AB) at both the high and low ends of the observed distribution of the predictor (i.e., socioeconomic resources). (3) We calculated a proportion affected index by sorting the dataset with respect to the environmental predictor (e.g., socioeconomic resources) and identifying the proportion of cases that fell above and below C; if 16% of the cases fall above or below C, the model suggests differential susceptibility (Roisman et al., 2012). (4) We applied a Type I Bonferroni error correction to account for the number of statistical tests calculated (i.e., p < .05/4 tests = p < .01 adjusted), and (5) estimated an additional model that included X² (i.e., resources²) and ZX² (i.e., amygdala reactivity X resources²) to account for possible nonlinearity of the predictors. (6) We calculated the change in model fit when an interaction term was introduced into the model, which we addressed by comparing the χ2-value of an unrestricted model to a nested model where the interaction term was fixed to zero.
Results
Zero-order correlations (Table 2) indicated that, consistent with past research and our hypotheses, greater socioeconomic resources at age 20 were associated with less AB and greater income at age 22. Surprisingly, greater socioeconomic resources in early childhood were associated with greater AB at age 22 and less amygdala reactivity to fearful facial expressions at age 20, although these associations would not meet statistical significance after correcting for multiple comparisons. Amygdala reactivity to all faces was positively correlated with amygdala reactivity to fearful facial expressions and negatively correlated with income at age 22. Surprisingly, amygdala reactivity at age 20 was not significantly related to AB at age 22, and AB and income at age 22 were uncorrelated.
Table 2.
Zero-order correlations and Descriptive Statistics
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. Early Childhood Resources | - | ||||||
| 2. Middle Childhood Resources | .57***§ N = 241 | - | |||||
| 3. Young Adulthood Resources | .10 N = 256 | .18† N = 250 | - | ||||
| 4. Amygdala Reactivity Faces > Shapes | −.11 N = 251 | −.19† N = 210 | −.10 N = 251 | - | |||
| 5. Amygdala Reactivity Fear Faces > Shapes | −.14† N = 251 | −.21* N = 210 | −.02 N = 251 | .44***§ N = 167 | - | ||
| 6. Income Age 22 | .10 N = 255 | .20 N = 240 | .18**§ N = 255 | −.15* N = 242 | −.02 N = 242 | - | |
| 7. Antisocial Behavior Age 22 | .16* N = 255 | .11 N = 241 | −.15* N = 255 | −.05 N = 242 | −.02 N = 242 | .01 N = 236 | - |
| M (SD) | 2.67(1.33) | 2.63(1.34) | 2.13(.98) | .00(.24) | .00(.51) | $1,027.82 (984.95) | .00(1.93) |
| Min – Max | 0 – 6 | 0 – 5 | 0 – 5 | −.76 – .54 | −1.68 – 1.30 | $0 – $6,666 | −3.22 – 9.84 |
p < .10,
p < .05,
p < .01,
p < .001,
survives Bonferonni correction for multiple comparisons across all 21 correlations (p < .002)
Does amygdala reactivity moderate the link between low socioeconomic resources and subsequent antisocial behavior?
Based on previous research linking low amygdala reactivity to fearful facial expressions to concurrent AB in this sample (Hyde et al., 2015), we first examined whether amygdala reactivity to fearful facial expressions moderated the relation between socioeconomic resources at age 20 and AB at age 22. Greater socioeconomic resources and lower amygdala reactivity to fearful facial expressions at age 20 each had main effects and predicted lower AB two years later (Table 3). Moreover, amygdala reactivity to fearful facial expressions moderated the relation between socioeconomic resources and later AB. As shown in Figure 2, for individuals with relatively low amygdala reactivity to fear, socioeconomic resources at age 20 did not predict self-reported AB two years later, although these young men generally had the highest levels of AB. For young men with relatively high amygdala reactivity, however, there was a significant negative relation between socioeconomic resources at age 20 and AB at age 22. This interaction term continued to be significant even after accounting for early and middle childhood resource indices and their interactions with amygdala reactivity at age 20 (Supplemental Table 4). Following recommendations by Widaman et al. (2012) and Roisman et al. (2012), we found evidence for differential susceptibility to context (Figure 1). Compared to individuals with relatively low amygdala reactivity to interpersonal fear, individuals with high amygdala reactivity to fearful faces were more susceptible to the effects of socioeconomic resources on later AB. Finally, we found that general amygdala reactivity (i.e., to all faces) did not moderate the relation between socioeconomic resources and later AB (Table 3). Moreover, though amygdala reactivity to angry faces has also been linked to AB in some studies (see Hyde et al., 2013), post-hoc analyses further reiterated the importance of fear processing for AB in our sample. Amygdala reactivity to angry faces (versus shapes) did not show a significant main effect or interaction with socioeconomic resources when predicting AB (Supplemental Table 5).
Table 3.
Multiple Regression Analyses Predicting Income and Antisocial Behavior at Age 22
| Main Effect of Socioeconomic Resources | Main Effect of Amygdala Reactivity | Interaction | R2 | |||||
|---|---|---|---|---|---|---|---|---|
| Age 22 Outcome | Neural Contrast | B(SE) | β | B(SE) | β | B(SE) | β | |
| Income | All faces |
1.70 (.67) | .17* | −4.52 (1.33) | −.45**§ | 6.62 (2.41) | .33**§ | .13***§ |
| Fearful faces | 1.85 (.65) | .19**§ | −.91 (1.43) | −.09 | .69 (1.23) | .09 | .08* | |
| Antisocial Behavior | All faces |
−.18 (.13) | −.08 | −.12 (.27) | −.05 | .38 (.64) | .09 | .36***§ |
| Fearful faces | −.25 (.13) | −.12† | .68 (.21) | .32**§ | −.45 (.17) | −.26**§ | .38***§ | |
Note: N=258. Socioeconomic resources and amygdala reactivity measured at age 20. Amygdala reactivity represents a mean of activity across right and left amygdala regions of interest. All models include child race as a covariate. Models with antisocial behavior as the outcome also include antisocial behavior at age 20 as a covariate and models predicting income additionally include schooling status (i.e., in school or not) and housing status (i.e., living with family or independently) as covariates. Note that including income at age 20 as a covariate rather than as an indicator in the socioeconomic resource index, did not change that the results of our models predicting income at age 22.
p < .10.
p < .05.
p < .01.
p < .001.
survives Bonferonni correction for multiple comparisons across all 4 regressions (p < .012)
Figure 2.
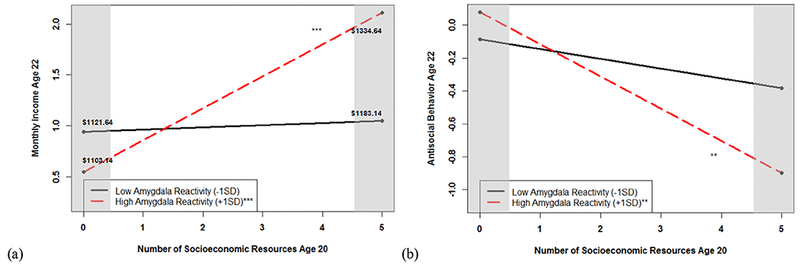
Shaded regions indicate the values of socioeconomic resources where the moderator (i.e., amygdala reactivity) significantly predicts the outcome (i.e., self-reported income or antisocial behavior). (a) Amygdala reactivity to all facial expressions moderates the relation between socioeconomic resources at age 20 and self-reported income at age 22. Monthly income was divided by 100 to reduce the variance of this variable for analytic purposes. While high amygdala reactivity is operationalized as > 1 SD above the mean for graphical purposes, the regions of significance for the moderator indicated that socioeconomic resources at age 20 predicted monthly income at age 22 only for individuals with high amygdala reactivity to all faces (calculated as values greater than −.10 where the mean was 0) (b) Amygdala reactivity to fearful facial expressions moderates the relation between socioeconomic resources at age 20 and antisocial behavior at age 22. While high amygdala reactivity is operationalized as > 1 SD above the mean for graphical purposes, the regions of significance for the moderator indicated that socioeconomic resources at age 20 antisocial behavior at age 22 only for individuals with high amygdala reactivity to fearful facial expressions (calculated as values greater than 0, or the mean).
N=258
** indicates the simple slope was significant at p < .01
*** indicates that the simple slope was significant at p < .001
Does amygdala reactivity moderate the link between socioeconomic resources and later income?
We next examined whether amygdala reactivity to fearful facial expressions or all faces moderated the relations between socioeconomic resources at age 20 and income at age 22. Unlike in models predicting AB, amygdala reactivity to fearful facial expressions did not moderate the link between socioeconomic resources at age 20 and income at age 22 (see Table 3). By contrast, in a second model, lower amygdala reactivity to all faces and greater socioeconomic resources each had main effects and predicted greater income two years later (see Table 3). We found that the relation between socioeconomic resources at age 20 and income at age 22 was moderated by amygdala reactivity to all faces. As shown in Figure 2, for individuals with relatively low amygdala reactivity to all faces, socioeconomic resources at age 20 did not predict later income (i.e., the simple slope was not significant). For individuals with relatively high amygdala reactivity, however, socioeconomic resources at age 20 predicted income at age 22 (i.e., the simple slope was significant), such that individuals with no resources at age 20 reported less monthly income (i.e., $754.50) at age 22 than individuals with five resources at age 20 (i.e., $872.90). Adding measures of socioeconomic resources from early or middle childhood and their interaction terms with amygdala reactivity at age 20, did not change the pattern or the statistical significance of these results, nor did these variables predict income at age 22 (Supplemental Table 4). Consistent with recommendations by Widaman et al. (2012) and Roisman et al. (2012), we probed this interaction term to determine if the pattern of results was consistent with a model of diathesis-stress, vantage sensitivity, or differential susceptibility to context. Across all six criteria (e.g., correcting for multiple comparisons, assessing alternative models, calculating the crossover point), these results indicated a pattern of differential susceptibility to context (see Figure 1). Compared to individuals with low amygdala reactivity to all faces, individuals with relatively high amygdala reactivity were more susceptible to the effects of socioeconomic resources on income attainment, for better or for worse. To understand if amygdala reactivity to any single face-type was most important in moderating the relation between socioeconomic resources at age 20 and income at age 22, we examined amygdala reactivity to each face-type in exploratory analyses. These post-hoc analyses revealed that amygdala reactivity to facial expressions of surprise (but not to angry or neutral faces versus shapes) moderated the relation between socioeconomic resources at age 20 and income at age 22 in a vantage-sensitivity pattern (Supplemental Table 5; Supplemental Figure 3), although this finding did not survive correction for multiple comparisons.
Discussion
In a sample of urban men from impoverished families, we found that amygdala reactivity during socioemotional processing was a marker of differential susceptibility to socioeconomic resources during the transition to adulthood. In line with our hypotheses, amygdala reactivity to fearful facial expressions moderated the relation between socioeconomic resources and later AB, while general interpersonal amygdala reactivity (i.e., to all faces) moderated the relation between socioeconomic resources in young adulthood and income attainment two years later. Controlling for multiple comparisons and socioeconomic resources earlier in development, both models revealed that the extent to which socioeconomic resources predicted AB and income attainment two years later were dependent on amygdala reactivity. In both cases, young men with relatively high amygdala reactivity were more sensitive, for better or for worse, to the effects of socioeconomic resources at age 20 on later income attainment and AB at age 22 (Figure 2). In contrast, young men with relatively low amygdala reactivity were less sensitive to these resources. Using recommendations by both Widaman et al. (2012) and Roisman et al. (2012), we found that both models met quantitative criteria for strong patterns of differential susceptibility rather than diathesis-stress or vantage sensitivity. That is, amygdala reactivity to facial expressions set some individuals at greater risk for poor outcomes in poor environments (i.e., diathesis-stress) and greater benefit for good outcomes in good environments (i.e., vantage sensitivity). Finally, we found evidence for developmental specificity in the transition to adulthood, such that these relations remained when controlling for resources during early and middle childhood and their interactions with amygdala reactivity at age 20.
Amygdala reactivity as a marker of differential susceptibility to context
Across both models predicting AB and income attainment, we found that relatively high amygdala reactivity to facial expressions identified young men who were more sensitive to the predictive effects of socioeconomic resources, for better or for worse. That high amygdala reactivity was a marker of sensitivity or “plasticity” to the environment is consistent with previous work and theory on differential susceptibility to context. Greater amygdala reactivity to emotional faces, particularly to facial expressions of fear, is associated with greater negative emotionality (Etkin et al., 2004), stress reactivity (Henckens et al., 2016), and genetic variants associated with serotonin genes (Munafo et al., 2008), which previously have been linked to differential susceptibility to context. Thus, it may be that amygdala reactivity to interpersonal emotion is an endophenotype or mechanism that might mediate existing differential susceptibility findings.
The role of amygdala reactivity in AB emergence and persistence during early adulthood
Based on robust links between fear processing and AB (Hyde et al., 2013), the specification of a fear probe in our AB models was not surprising. Whereas socioeconomic resources at age 20 predicted later AB, consistent with a range of studies demonstrating the role of resources in preventing AB (Shaw et al., 2012; Lober & Hay, 1997), this link was strongest for young men with high amygdala reactivity. By contrast, young men with relatively low amygdala reactivity to fearful facial expressions reported high AB across all levels of socioeconomic resources, consistent with a large body of work linking more serious and persistent AB (e.g., psychopathy, callous-unemotional traits) with low amygdala reactivity to fear (Hyde et al., 2013). Importantly, these results suggested differential susceptibility to context rather than diathesis-stress or vantage sensitivity (Roisman et al., 2012; Widaman et al., 2012) (see Figure 1), and were robust to inclusion of socioeconomic resources in early and middle childhood. Neural sensitivity to distress in others may be linked to less AB in positive environments because it may promote social skills and prosocial behavior (e.g., see studies linking higher amygdala reactivity to altruism; Marsh et al., 2014). In contrast, high neural sensitivity to distress in others, when paired with an inconsistent and dangerous, lower-resourced environment, may promote emotion dysregulation and reactive forms of AB via hostile attribution biases (Dodge, 2006; Hyde et al., 2013). Our results suggest that youth with relatively greater amygdala reactivity to fearful facial expressions may be more sensitive to socioeconomic resources during the transition to adulthood, and may be well-suited for resource-based prevention or intervention programs to reduce AB (e.g., cash transfers; Ozer, Fernald, Manley, & Gertler, 2009). However, our findings are several steps away from influencing prevention efforts, clinical practice, or social policy and thus, currently should be viewed as additions to a basic science “proof of concept”. The measure of general amygdala reactivity includes neural reactivity to all of the faces in our task (i.e., neutral, angry, fear, and surprise), and youth with AB show typical recognition and reactivity to angry and surprise facial expressions (Marsh & Blair, 2008). These non-significant results with the measure of general amygdala reactivity (and amygdala reactivity to angry faces versus shapes; Supplemental Table 5) converge with the extant literature to highlight the relevance of fear processing in AB.
The role of amygdala reactivity in emerging income attainment
Consistent with extant research reporting the stability of socioeconomic standing across development (Duncan et al., 2010; Kendig et al., 2014), we found, not surprisingly, that socioeconomic resources at age 20 predicted income attainment at age 22. Interestingly, however, given the expected strong correlation between these two factors, this relation was specific to young men with relatively high amygdala reactivity to all faces (contrasted with shapes). That this interaction predicted later income over and above current socioeconomic resources, resources in earlier developmental periods, and education enrollment and housing status make these findings all the more significant. As amygdala reactivity during broad socioemotional processing is linked to emotionality (Etkin et al., 2004) and prosociality (Marsh et al., 2014), it could be that youth with greater amygdala reactivity to multiple interpersonal emotions are higher on traits like neuroticism and extraversion (Canli et al., 2001) and more sensitive to interpersonal cues. In impoverished contexts, but with relatively more socioeconomic resources at the transition to adulthood, these youth may do well in interpersonal settings and secure consistent and better-paying employment. In contrast, in environments characterized by few socioeconomic resources, youth with greater sensitivity to interpersonal cues may exhibit emotional dysregulation in affectively-laden or ambiguous interpersonal contexts that undermines gainful employment and supportive interpersonal relationships (Liu et al., 2014). However, these potential explanations are speculative, as our initial research cannot address mechanisms underlying this pattern of differential susceptibility. Whereas our post-hoc analyses suggest that this effect may be strongest in relation to ambiguous facial expressions of surprise, based on the weak statistical findings and exploratory nature of the analyses, future replication and exploration is needed to examine the general versus specific effects of amygdala reactivity to faces as a marker of differential susceptibility.
These findings also highlight the need to quantitatively assess whether a pattern of results fits a model of diathesis-stress, vantage sensitivity, or differential susceptibility to context. While “visual inspection” of an interaction is a useful first step in this process (Roisman et al., 2012), a visual inspection of our results in Figure 2 would have suggested a pattern of vantage sensitivity. However, using guidelines from two independent research groups (Roisman et al., 2012; Widaman et al., 2012) (e.g., proportion affected index: at least 16% of the cases fell above and below the crossover point; evidence for a disordinal interaction), we found a pattern of differential susceptibility to context.
Implications for models of differential susceptibility to context
More broadly, one of our main goals was to apply best practices in testing of models of differential susceptibility to context. A limitation of past research is the reliance on environmental variables and outcomes with restricted ranges (Belsky & Pluess, 2009). Thus, in addition to quantitatively evaluating our results to reflect recent recommendations (Roisman et al., 2012; Widaman et al., 2012), we created an index of socioeconomic resources that was dimensional. That is, at either end of this scale, this index represented relatively poor resources in an already disadvantaged context or protective factors that could promote resilience. Similarly, although much work in this sample has focused on the development of AB (e.g., Shaw et al., 2003, 2012; Hyde et al., 2015), the lack of psychopathology itself has been debated as a marker of “resilience” (Masten, 2001); that low AB may be a positive outcome for young men in this sample may not be generalizable to other samples. Therefore, we examined both low levels of AB and a potential dimensional outcome that has major implications for future health, wealth, and happiness (i.e., income). This approach is consistent with suggestions in the resilience literature to measure competence across multiple domains to ensure a more thorough understanding of an individual’s level of functioning (Masten, 2001). However, future work should consider other markers of successful or unsuccessful transition to adulthood (e.g., parenting, romantic relationships, educational attainment), as well as other promotive contexts (e.g., schooling quality, social support) (Hyde, Gorka, Manuck, & Hariri, 2011). Using AB and income attainment as two measures of youth functioning at the transition to adulthood, we show that even among the most disadvantaged youth, both differential susceptibility factors and socioeconomic resources play a role in whether youth attain developmental competencies.
It is yet unclear whether differential susceptibility factors operate in a domain-general or a domain-specific manner (Belsky & Pluess, 2009; Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van Ijzendoorn, 2011). Whereas original conceptualizations suggested that heightened stress reactivity would function as a neurobiological marker of susceptibility to broadly construed dimensional environments and outcomes (Ellis & Boyce, 2008), empirical data suggests that there are domain-specific properties of some susceptibility factors. For example, Obradovic, Bush, and Boyce (2011) found that high respiratory sinus arrhythmia moderated the association between marital conflict and youth externalizing, but not internalizing, behaviors (see also Essex, Armstrong, Burk, Goldsmith, & Boyce, 2011, for another example of domain-specificity). Therefore, it may be that some markers of susceptibility are domain-general (e.g., temperament) while others are domain specific (e.g., reward sensitivity). Our current results suggest quite a bit of specificity (and in hypothesized directions) in that amygdala reactivity was a marker of differential susceptibility but in specific ways. Amygdala reactivity to fearful faces moderated paths to AB, whereas more general amygdala reactivity to all faces moderated links to income attainment.
Limitations
Although the well-characterized sample, longitudinal design, and sample size with neuroimaging data were strengths of this study, some notable limitations warrant consideration in interpreting the results. First, like many other studies examining amygdala reactivity (e.g., Carré, Fisher, Manuck, & Hariri, 2012; Fisher et al., 2009; Hariri, 2002; Swartz, Knodt, Radtke, & Hariri, 2015), our baseline condition was shapes. Thus, amygdala reactivity to fearful facial expressions may tap face processing or visual complexity more broadly than reactivity to fear stimuli specifically. Note that we did not use “neutral” faces as the baseline condition because studies suggest that true neutral (versus calm) faces robustly activate the amygdala and may be perceived as threatening (Marusak et al., 2016; Somerville et al., 2004). Additionally, our task did not contain “calm” faces that may be the most ideal baseline condition (Sebastian et al., 2014; Viding et al., 2012).
Second, while our measure of income attainment at age 22 was specific to the youth’s income (and not that of other household members), we were unable to distinguish legal from illegal income. We did test a model that included AB at age 22 as a covariate to attempt to parse any potential illegal income via AB; the findings did not change. Third, we tried to confirm that experiences during early adulthood were the most important predictors of age 22 outcomes by including similar indices of socioeconomic resources in early and middle childhood as covariates. However, these earlier indices were not identical to our resource index in early adulthood because some of the indicators at age 20 were not developmentally appropriate at earlier ages. Thus, we may have underestimated effects of experience at earlier developmental periods. Fourth, a further caveat to our findings was the surprising positive zero-order correlation between socioeconomic resources in early childhood and AB at age 22, as well as the non-significant association between income and AB age 22. These findings highlight the need for replication of our results in other samples. Moreover, we found no prospective zero-order relations between amygdala reactivity at age 20 and AB at age 22 despite previous work in this sample that reported cross-sectional associations (Hyde et al., 2015). In regression models, however, greater amygdala reactivity to fearful facial expressions predicted greater AB at age 22 after controlling for self-reported delinquency at age 20 (Table 3), suggesting that our findings are specific to changes in AB over time. However, we are cautious to interpret main effects in the presence of an interaction, particularly given that our measures of AB at ages 20 and 22 were not identical. It is important for future research to examine whether amygdala reactivity prospectively predicts AB after controlling for the stability of AB using repeated measures, and if this prediction is in the same direction as cross-sectional associations.
Finally, our results are based on a sample of young men in impoverished contexts during a brief period of time (i.e., their income at age 22 is only a snapshot of their socioeconomic trajectory), which may be appropriate for an investigation of vulnerability and resilience. However, to conclude that amygdala reactivity to interpersonal emotion is indeed a marker of differential sensitivity to context broadly, these results need to be replicated in other samples (i.e., of mixed gender, varied socioeconomic status, rural versus urban samples) and across the lifespan. It is also important to note that the families in our study were recruited from WIC Nutritional Supplement Clinics, which requires initiation on the part of the parent to receive aid, and thus our results may not translate to other boys in low income families.
Despite these caveats, the current findings suggest that amygdala reactivity during socioemotional processing is a marker of differential susceptibility to socioeconomic resources. For youth with relatively high, but not low, amygdala reactivity to facial expressions, socioeconomic context was a robust predictor of income and AB, consistent with theories of differential susceptibility. These findings can inform our understanding of differential susceptibility and why young adults demonstrate divergent outcomes even when exposed to similar contexts.
Supplementary Material
Acknowledgements
This work was support by the National Institutes of Health Grant Nos. R01 MH50907 (to DSS), R01 MH01666 (to DSS), and R01 DA026222 (to DSS and EEF). AMG was supported by T32 HD00710936 and LWH was supported by the National Science Foundation (award 1519686 to R. Crosnoe and E. Gershoff). We are grateful to the work of the staff of the Pitt Mother & Child Project for their many years of service, and to our study families for sharing their lives with us and making this research possible. The authors report no biomedical financial interests or potential conflicts of interest.
References
- Ackerman BP, Izard CE, Schoff K, Youngstrom EA, Kogos J (1999). Contextual Risk, Caregiver Emotionality, and the Problem Behaviors of Six-and Seven-Year-Old Children from Economically Disadvantaged Families. Child Development, 70(6), 1415–1427. https://doi.org/10.1111/1467-8624.00103 [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG (1991). Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage. [Google Scholar]
- Alexander K, Entwisle D, Olson L (2014). The long shadow: Family background, disadvantaged urban youth, and the transition to adulthood. New York, NY: Russell Sage Foundation. [Google Scholar]
- Arnett JJ (2000). Emerging adulthood: A theory of development from the late teens through the twenties. American Psychologist, 55(5), 469–480. http://doi.org/10.1037//0003-066X.55.5.469 [PubMed] [Google Scholar]
- Belsky J, Pluess M (2009). Beyond diathesis-stress: Differential susceptibility to the environmental influences. Psychological Bulletin, 135(6), 885–908. http://dx.doi.org.proxy.lib.umich.edu/10.1037/a0017376 [DOI] [PubMed] [Google Scholar]
- Bennett RJ, Robinson SL (2000). Development of a measure of workplace deviance. Journal of Applied Psychology, 85(3), 349 http://doi.org/10.1037/0021-9010.85.3.349 [DOI] [PubMed] [Google Scholar]
- Boyce WT (2016). Differential susceptibility of the developing brain to contextual adversity and stress. Neuropsychopharmacology, 41, 142–162. https://doi.org/10.1038/npp.2015.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF (2002). Socioeconomic status and child development. Annual Review of Psychology, 53(1), 371–399. http://doi.org/10.1146/annurev.psych.53.100901.135233 [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF (2008). Infant temperament, parenting, and externalizing behavior in first grade: a test of the differential susceptibility hypothesis. Journal of Child Psychology and Psychiatry, 49(2), 124–131. http://doi.org/10.1111/j.1469-7610.2007.01829.x [DOI] [PubMed] [Google Scholar]
- Bureau of Labor Statistics (2013, August). The Employment Situation – 2013 (BLS news release USDL-13-1527). Retrieved from https://www.bls.gov/opub/ted/2013/ted_20130806.htm. [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JDE (2001). An fMRI study of personality influences on brain reactivity to emotional stimuli. Behavioral Neuroscience, 115(1), 33–42. http://doi.org/10.1037//0735-7044.115.1.33 [DOI] [PubMed] [Google Scholar]
- Conger RD (1988). Work Characteristics Questionnaires. Adapted from diverse sources for the Iowa Youth & Family Project. Ames, IA: Iowa State University. [Google Scholar]
- Cutts DB, Meyers AF, Black MM, Casey PH, Chilton M, Cook JT, … Frank DA. (2011). US Housing Insecurity and the Health of Very Young Children. American Journal of Public Health, 101(8), 1508–1514. https://doi.org/10.2105/AJPH.2011.300139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNavas-Walt C and Proctor BD (2015). Income and poverty in the United States: 2014. U.S. Census Bureau, Current Population Reports, P60–252. Washington, DC: U.S. Government Printing Office. [Google Scholar]
- Duncan GJ, Ziol-Guest KM, Kalil A (2010). Early-Childhood Poverty and Adult Attainment, Behavior, and Health. Child Development, 81(1), 306–325. http://doi.org/10.1111/j.1467-8624.2009.01396.x [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV (1976). Measuring facial movement. Environmental Psychology and Nonverbal Behavior, 1(1), 56–75. [Google Scholar]
- Ellis BJ, Boyce WT (2008). Biological sensitivity to context. Current Directions in Psychological Science, 17(3), 183–187. http://doi.org/10.1111/j.1467-8721.2008.00571.x [Google Scholar]
- Elliott DS, Huizinga D, Ageton SS (1985). Explaining delinquency and drug use. Sage Publications. [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J (2004). Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron, 44(6), 1043–1055. https://doi.org/10.1016/j.neuron.2004.12.006 [DOI] [PubMed] [Google Scholar]
- Evans GW, Wells NM, Moch A (2003). Housing and Mental Health: A Review of the Evidence and a Methodological and Conceptual Critique. Journal of Social Issues, 59(3), 475–500. https://doi.org/10.1111/1540-4560.00074 [Google Scholar]
- Fauth RC, Leventhal T, Brooks-Gunn J (2004). Short-term effects of moving from public housing in poor to middle-class neighborhoods on low-income, minority adults’ outcomes. Social Science & Medicine, 59(11), 2271–2284. https://doi.org/10.1016/j.socscimed.2004.03.020 [DOI] [PubMed] [Google Scholar]
- Federal Interagency Forum on Child and Family Statistics. (2014). America’s Young Adults: Special Issue, 2014. Washington, DC: U.S. Government Printing Office. [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL (2006). Beyond threat: Amygdala reactivity across multiple expressions of facial affect. NeuroImage, 30(4), 1441–1448. https://doi.org/10.1016/j.neuroimage.2005.11.003 [DOI] [PubMed] [Google Scholar]
- Fowler PJ, Henry DB, & Marcal KE (2015). Family and housing instability: Longitudinal impact on adolescent emotional and behavioral well-being. Social Science Research, 53(Supplement C), 364–374. https://doi.org/10.1016/j.ssresearch.2015.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JW (2009). Missing data analysis: Making it work in the real world. Annual Review of Psychology, 60, 549–576. https://doi.org/10.1146/annurev.psych.58.110405.085530 [DOI] [PubMed] [Google Scholar]
- Hankin BL, Nederhof E, Oppenheimer CW, Jenness J, Young JF, Abela JRZ, … Oldehinkel AJ (2011). Differential susceptibility in youth: evidence that 5-HTTLPR x positive parenting is associated with positive affect “for better and worse.” Translational Psychiatry, 1(10), e44 https://doi.org/10.1038/tp.2011.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJAG, Klumpers F, Everaerd D, Kooijman SC, van Wingen GA, Fernández G (2016). Interindividual differences in stress sensitivity: basal and stress-induced cortisol levels differentially predict neural vigilance processing under stress. Social Cognitive and Affective Neuroscience, 11(4), 663–673. http://doi.org/10.1093/scan/nsv149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Shaw DS, Hariri AR (2013). Understanding youth antisocial behavior using neuroscience through a developmental psychopathology lens: Review, integration, and directions for research. Developmental Review, 33(3), 168–223. http://doi.org/10.1016/j.dr.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Shaw DS, Murray L, Gard A, Hariri AR, Forbes EE (2015). Dissecting the Role of Amygdala Reactivity in Antisocial Behavior in a Sample of Young, Low-Income, Urban Men. Clinical Psychological Science, 1–18. http://doi.org/10.1177/2167702615614511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S (2011). The Serotonin Transporter Promoter Variant (5-HTTLPR), Stress, and Depression Meta-analysis Revisited: Evidence of Genetic Moderation. Archives of General Psychiatry, 68(5), 444 https://doi.org/10.1001/archgenpsychiatry.2010.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendig SM, Mattingly MJ, Bianchi SM (2014). Childhood Poverty and the Transition to Adulthood: Childhood Poverty and Transition to Adulthood. Family Relations, 63(2), 271–286. https://doi.org/10.1111/fare.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein Velderman M, Bakermans-Kranenburg MJ, Juffer F, Van Ijzendoorn MH (2006). Effects of attachment-based interventions on maternal sensitivity and infant attachment: differential susceptibility of highly reactive infants. Journal of Family Psychology, 20(2), 266 http://dx.doi.org/10.1037/0893-3200.20.2.266 [DOI] [PubMed] [Google Scholar]
- LeDoux J (2000). Emotion circuits in the brain. Annual Reviews of Neuroscience, 23, 155–184. https://doi.org/10.1146/annurev.neuro.23.1.155 [DOI] [PubMed] [Google Scholar]
- Liu Y, Peng K, Wong C-S (2014). Career maturity and job attainment: the moderating roles of emotional intelligence and social vocational interest. International Journal for Educational and Vocational Guidance, 14(3), 293–307. http://doi.org/10.1007/s10775-014-9271-5 [Google Scholar]
- Loeber R, Hay D (1997). Key issues in the development of aggression and violence from childhood to early adulthood. Annual Review of Psychology, 48(1), 371–410. http://doi.org/10.1146/annurev.psych.48.1.371 [DOI] [PubMed] [Google Scholar]
- Manuck SB, Brown SM, Forbes EE, Hariri AR (2007). Temporal stability of individual differences in amygdala reactivity. The American Journal of Psychiatry. http://doi.org/10.1176/appi.ajp.2007.07040609 [DOI] [PubMed] [Google Scholar]
- Marsh AA, Blair RJR (2008). Deficits in facial affect recognition among antisocial populations: A meta-analysis. Neuroscience & Biobehavioral Reviews, 32(3), 454–465. https://doi.org/10.1016/j.neubiorev.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Stoycos SA, Brethel-Haurwitz KM, Robinson P, VanMeter JW, & Cardinale EM (2014). Neural and cognitive characteristics of extraordinary altruists. Proceedings of the National Academy of Sciences, 111(42), 15036–15041. http://doi.org/10.1073/pnas.1408440111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS (2001). Ordinary magic: Resilience processes in development. American Psychologist, 56(3), 227 http://doi.org/10.1037/0003-066X.56.3.227 [DOI] [PubMed] [Google Scholar]
- McCartney K, Burchinal MR, Bub KL (2006). Best practices in quantitative methods for developmentalists. Monographs of the Society for Research in Child Development, i–145. https://doi.org/10.1111/j.1540-5834.2006.07103001.x [DOI] [PubMed] [Google Scholar]
- Monroe SM, Simons AD (1991). Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychological Bulletin, 110(3), 406 http://dx.doi.org.proxy.lib.umich.edu/10.1037 [DOI] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR (2008). Serotonin Transporter (5-HTTLPR) Genotype and Amygdala Activation: A Meta-Analysis. Biological Psychiatry, 63(9), 852–857. https://doi.org/10.1016/j.biopsych.2007.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO (1998-2011). Mplus User’s Guide (Seventh Edition). Los Angeles, CA: Muthen & Muthen. [Google Scholar]
- Neumann CS, Pardini D (2014). Factor structure and construct validity of the Self-Report Psychopathy (SRP) Scale and the Youth Psychopathic Traits Inventory (YPI) in young men. Journal of Personality Disorders, 28(3), 419 http://doi.org/10.1521/pedi_2012_26_063 [DOI] [PubMed] [Google Scholar]
- Odgers CL, Moffitt TE, Broadbent JM, Dickson N, Hancox RJ, Harrington H, … Caspi A (2008). Female and male antisocial trajectories: From childhood origins to adult outcomes. Development and Psychopathology, 20(02), 673–716. https://doi.org/10.1017/S0954579408000333 [DOI] [PubMed] [Google Scholar]
- Obradovic J, Burt KB, Masten AS (2006). Pathways of Adaptation from Adolescence to Young Adulthood: Antecedents and Correlates. Annals of the New York Academy of Sciences, 1094(1), 340–344. http://doi.org/10.1196/annals.1376.046 [DOI] [PubMed] [Google Scholar]
- Obradović J, Bush NR, Stamperdahl J, Adler NE, & Boyce WT (2010). Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development, 81(1), 270–289. https://doi.org/10.1111/j.1467-8624.2009.01394.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradović J, Portilla XA, Ballard PJ (2015). Biological sensitivity to family income: differential effects on early executive functioning. Child Development. Retrieved from http://onlinelibrary.wiley.com/doi/10.1111/cdev.12475/pdf [DOI] [PubMed] [Google Scholar]
- Ozer EJ, Fernald LC, Manley JG, & Gertler PJ (2009). Effects of a conditional cash transfer program on children’s behavior problems. Pediatrics, 123(4), 630–637. http://doi.org/10.1542/peds.2008-2882 [DOI] [PubMed] [Google Scholar]
- Pratt TC, & Cullen FT (2005). Assessing macro-level predictors and theories of crime: A meta-analysis. Crime and Justice, 32, 373–450. https://doi.org/10.1086/655357 [Google Scholar]
- Pluess M, Belsky J (2013). Vantage sensitivity: Individual differences in response to positive experiences. Psychological Bulletin, 139(4), 901–916. http://doi.org/10.1037/a0030196 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ (2006). Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics, 31(4), 437–448. http://doi.org/10.3102/10769986031004437 [Google Scholar]
- Raykov T (1997). Estimation of composite reliability for congeneric measures. Applied Psychological Measurement, 21(2), 173–184. [Google Scholar]
- Roisman GI, Newman DA, Fraley RC, Haltigan JD, Groh AM, Haydon KC (2012). Distinguishing differential susceptibility from diathesis–stress: Recommendations for evaluating interaction effects. Development and Psychopathology, 24(02), 389–409. http://doi.org/10.1017/S0954579412000065 [DOI] [PubMed] [Google Scholar]
- Sameroff AJ (2010). A unified theory of development: A dialectic integration of nature and nurture. Child Development, 81(1), 6–22. http://doi.org/10.1111/j.1467-8624.2009.01378.x [DOI] [PubMed] [Google Scholar]
- Sameroff AJ, Seifer R, Baldwin A, Baldwin C (1993). Stability of intelligence from preschool to adolescence: The influence of social and family risk factors. Child development, 64(1), 80–97. https://doi.org/10.1111/j.1467-8624.1993.tb02896.x [DOI] [PubMed] [Google Scholar]
- Sampson RJ, Laub JH (1994). Urban poverty and the family context of delinquency: A new look at structure and process in a classic study. Child Development, 65(2), 523–540. https://doi.org/10.1111/j.1467-8624.1994.tb00767.x [PubMed] [Google Scholar]
- Santos A, Mier D, Kirsch P, Meyer-Lindenberg A (2011). Evidence for a general face salience signal in human amygdala. NeuroImage, 54(4), 3111–3116. https://doi.org/10.1016/j.neuroimage.2010.11.024 [DOI] [PubMed] [Google Scholar]
- Schriber RA, Guyer AE (2016). Adolescent neurobiological susceptibility to social context. Developmental Cognitive Neuroscience, 19, 1–18. https://doi.org/10.1016/j.dcn.2015.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenberg JE, Sameroff AJ, Cicchetti D (2004). The transition to adulthood as a critical juncture in the course of psychopathology and mental health. Development and Psychopathology, 16(04), 799–806. http://doi.org/10.1017/S0954579404040015 [DOI] [PubMed] [Google Scholar]
- Shaw DS, Gilliom M, Ingoldsby EM, Nagin DS (2003). Trajectories leading to school-age conduct problems. Developmental Psychology, 39(2), 189–200. http://doi.org/10.1037/0012-1649.39.2.189 [DOI] [PubMed] [Google Scholar]
- Shaw DS, Hyde LW, Brennan LM (2012). Early predictors of boys’ antisocial trajectories. Development and Psychopathology, 24(03), 871–888. https://doi.org/10.1017/S0954579412000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogstad A, Torsheim T, Einarsen S, Hauge LJ (2011). Testing the Work Environment Hypothesis of Bullying on a Group Level of Analysis: Psychosocial Factors as Precursors of Observed Workplace Bullying. Applied Psychology, 60(3), 475–495. https://doi.org/10.1111/j.1464-0597.2011.00444.x [Google Scholar]
- Swartz JR, Knodt AR, Radtke SR, Hariri AR (2015). A neural biomarker of psychological vulnerability to future life stress. Neuron, 85(3), 505–511. https://doi.org/10.1016/j.neuron.2014.12.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentacosta CJ, Hyde LW, Shaw DS, Dishion TJ, Gardner F, & Wilson M (2008). The relations among cumulative risk, parenting, and behavior problems during early childhood. Journal of Child Psychology and Psychiatry, 1211–1219. https://doi.org/10.1111/j.1469-7610.2008.01941.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2012). Annual Update of the HHS Poverty Guidelines (FR Doc No: 2012–1603). Washington, DC: U.S. Government Printing Office. [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL (2001). A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion, 1(1), 70 http://dx.doi.org/10.1037/1528-3542.1.1.70 [DOI] [PubMed] [Google Scholar]
- Widaman KF, Helm JL, Castro-Schilo L, Pluess M, Stallings MC, Belsky J (2012). Distinguishing ordinal and disordinal interactions. Psychological Methods, 17(4), 615 http://dx.doi.org/10.1037/a0030003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


