Abstract
We examined whether attained SES moderated genetic and environmental sources of individual differences in cognitive performance using pooled data from nine adult twin studies. Prior work concerning SES moderation of cognitive performance has focused on rearing SES. The current adult sample of 12,196 individuals (aged 27-98 years) allowed for the examination of common sources of individual differences between attained SES and cognitive performance (signaling potential gene-environment correlation mechanisms, rGE), as well as sources of individual differences unique to cognitive performance (signaling potential gene-environment interaction mechanisms, GxE). Attained SES moderated sources of individual differences in 4 cognitive domains, assessed via performance on 5 cognitive tests ranging 2,149 to 8,722 participants. Attained SES moderated common sources of influences for 3 domains and influences unique to cognition in all 4 domains. The net effect was that genetic influences on the Common pathway tended to be relatively more important at the upper end of attained SES indicating possible active rGE, whereas, genetic influences for the Unique pathway were proportionally stable or less important at the upper end of attained SES. As a noted exception, at the upper end of attained SES, genetic influences unique to perceptual speed were amplified and genetic influences on the Common pathway were dampened. Accounting for rearing SES did not alter attained SES moderation effects on cognitive performance, suggesting mechanisms germane to adulthood. Our findings suggest the importance of gene-environment mechanisms through which attained SES moderates sources of individual differences in cognitive performance.
Keywords: GxE interaction, gene-environment correlation, socioeconomic status, cognitive performance, older adults
An individual’s socioeconomic status (SES) is among the most pervasive contexts associated with overall health and cognitive performance, with positive correlations between SES and individual outcomes throughout the lifespan (Adler et al., 1994; Hackman, Farah, & Meaney, 2010; Mortensen et al., 2014). According to Oakes and Rossi (2003) SES can be primarily conceptualized as individuals’ differential access to resources (e.g. goods, services, and knowledge), and can be extended to include the hierarchically-defined contexts in which individuals utilize their own social and economic capital in attempts to gain these resources and improve their SES. As individuals enter adulthood, their attained SES emerges through their own education and occupational endeavors, contributing to their position in the surrounding social hierarchy. However, an individual’s attained SES may also be facilitated or impeded by availability of early-life resources via rearing environment (i.e. rearing SES), genetic transmission of psychological traits (Plomin & Bergeman, 1991), reinforcement by one’s environment (Beam, Turkheimer, Dickens, & Davis, 2015; Dickens & Flynn, 2001; Turkheimer, Beam, Sundet, & Tambs, 2017), and available spousal support in adulthood (Bernasco, de Graaf, & Ultee, 1998; Verbakel & de Graaf, 2007). Given its individual-level emergence, attained SES has unique salience, warranting examination of underlying genetic and environmental mechanisms likely at work in its association with adult cognitive functioning.
Most of the literature examining differences in heritability of cognition as a function of rearing SES level has focused on child and adolescent twin samples (for recent reviews see Tucker-Drob & Bates, 2015; Turkheimer & Horn, 2014). The Scarr-Rowe hypothesis states that heritability for IQ will be higher in privileged environments, so that any differences in IQ will be mainly attributable to genetic differences between individuals (Rowe, Jacobson, & Van den Oord, 1999; Scarr-Salapatek, 1971). Such results would be indicative of gene-environment interaction, often characterized as genetic sensitivity to the environment (Kendler & Eaves, 1986). Evidence from several U.S. samples has suggested that rearing SES moderates individual differences in IQ and IQ subtests during childhood and adolescence, with additive genetic variance in IQ greater at higher rearing SES levels (Tucker-Drob & Bates, 2015; Turkheimer & Horn, 2014). These results provide support for the Scarr-Rowe hypothesis (Turkheimer, Harden, D’Onofrio, & Gottesman, 2009). However, there are inconsistent findings with respect to rearing SES moderation across studies and countries, with weaker or null effects in non-U.S. (i.e., Central and Northern European) samples (see Gottschling, Riemann, Spinath, & Diewald, 2016; Tucker-Drob & Bates, 2015). The few studies examining these effects in adults have suggested that rearing SES does not moderate additive genetic variance in adult IQ or reading ability (Grant et al., 2010; Kremen et al., 2005; van der Sluis, Willemsen, de Geus, Boomsma, & Posthuma, 2008). Given the dearth of studies examining rearing SES on cognition in adulthood, there are no indications of moderation patterns differing by country or region. Importantly, because twins share their rearing SES, all studies of rearing SES are limited to considering only variance in cognitive abilities independent of SES, but whatever drives the association between higher SES and better cognition may lie in the covariance between them.
Considering attained SES as a potential moderator of individual differences in adult cognitive performance presents the opportunity to explore the full covariance of SES with cognitive performance (Johnson, 2007). Experience Producing Drive (EPD) theory posits that individuals seek out experiences compatible with genetically-influenced drives (i.e. motivations and preferences) within the environmental offerings that complement those drives (Bouchard, 1997; Johnson, 2010). Attained SES emerges actively via contributions to constructing one’s own environments (sometimes called niche-picking) and passively through genetic and environmental resources, resulting in gene-environment correlations (Plomin, DeFries, & Loehlin, 1977). Gene-environment correlations (rge) are the associations between genetic influences and exposures to particular environments/contexts (Kendler & Eaves, 1986). In early life, rge largely stems from biological parents providing rearing environments correlated with their children’s genotype (passive rge). Other mechanisms include an individual’s own behaviors (active rge) and others’ response to an individual’s genetically influenced phenotypic characteristics (evocative rge), leading to different environmental and contextual exposures (Kendler & Eaves, 1986; Scarr & McCartney, 1983). Active and evocative rge accumulate in an increasingly individual way throughout the lifespan (Reynolds, Finkel, & Zavala, 2014; Scarr & McCartney, 1983) with recent empirical work supportive of increased rge over time (Beam et al., 2015). Attained SES is thus likely to be a contextual marker that signals the types and frequencies of environmental experiences an individual has both created and encountered throughout adulthood – experiences that may affect maintenance of cognitive performance in adulthood.
An example of a contextual experience that an individual both encounters and (to varying degrees) helps create is in their occupation, and may provide insight to mechanisms via which SES moderates cognitive performance. Cognitively complex occupations are often sought and obtained by individuals with high cognitive ability and high SES. In turn, cognitive complexity of work has been observed to predict later higher intellectual flexibility and function (Marquié et al., 2010; Schooler, Mulatu, & Oates, 1999). Schooler and Caplan (2008) argued that reciprocal processes between work complexity and intellectual function likely magnify psychological differences between individuals of high and low SES. Nevertheless, individuals who retire from cognitively complex occupations may be at greater risk for accelerated cognitive declines (Finkel, Andel, Gatz, & Pedersen, 2009), underscoring the importance of maintaining cognitive stimulating contexts. Additionally, a potentially reciprocal relationship between attained SES and cognitive performance necessitates testing the direction of any moderating effect.
The household is another important environmental context maintained by adults, one that is often an active collaboration between an individual and their spouse. Spouses tend to be similar on characteristics such as educational attainment and intelligence (Buss, 1985; Watkins & Meredith, 1981; Watson et al., 2004), and examinations of mechanisms for mate selection indicate that individuals seek these similarities in potential spouses (Reynolds, Baker, & Pedersen, 2000; Zietsch, Verweij, Hrath, & Martin, 2012). Selection on social background, or social homogamy, may also play a role (Reynolds et al., 2000; Zietsch et al., 2012), but principally the consequence is the same, i.e., that spouses tend to be similar for educational attainment and intelligence. Spouse selection is worth considering for older aging cohorts in which fewer occupational opportunities were afforded to women. Studies indicate that spousal support and spousal educational attainment is beneficial to both men and women in their own occupational pursuits (Airsman & Sharda, 1993; Bernasco et al., 1998; Verbakel & de Graaf, 2007). In older cohorts, there is evidence that men’s occupational attainment was protected from downward mobility by their wife’s level of educational attainment (Verbakel & de Graaf, 2007), indicating that women in older cohorts may have used their available social capital gained through educational achievement (and associated social standing) to support their husband’s occupational aspirations. As such, though gender-specific roles within households may adapt to the socio-historical context over time, it is evident that attained SES within a household is reflective of the characteristics and contributions of both partners, including leveraging of each individual’s social and cognitive resources.
We examined the moderating roles of attained SES in genetic and environmental influences on cognitive performances in a cross-sectional sample of adult twins aged 27-98 years in pooled data from nine studies in the consortium on Interplay of Genes and Environment across Multiple Studies (IGEMS). We used household-level attained SES in the current study to better capture the contributions of both men and women to spouses’ shared access to economic and social resources across socio-historical contexts. We examined four cognitive domains: verbal ability, perceptual speed, short-term/working memory, and spatial reasoning. We included age as a moderator of cognitive performance and attained SES, as age has been observed to moderate genetic and environmental influences on cognitive performance (Johnson, McGue, & Deary, 2014; Pahlen et al., 2018; Tucker-Drob & Bates, 2015), and in light of possible birth-cohort moderation of factors indexing SES (e.g. educational attainment, see Colodro-Conde, Rijsdijk, Tornero-Gomez, Sanchez-Romera, & Ordonana, 2015; Heath et al., 1985). In this first examination of attained SES as a moderator of cognitive performance in adults, we addressed the following questions:
Does attained SES moderate genetic influences, environmental influences, or both on cognitive performance in each cognitive domain? If so, is moderation occurring via gene-environment correlation and/or gene x environment interaction mechanisms?
Does cognitive functioning have moderating influences on genetic and environmental influences underlying attained SES?
How does adjusting for moderation effects of rearing SES on cognitive performance affect any moderation patterns observed for attained SES?
Will national/regional differences in moderation patterns be observed?
Method
Participants
Participants were drawn from nine twin studies representing three countries (Sweden, Denmark, and the United States) taking part in the IGEMS consortium (Pedersen et al., 2013). Participants were included if they or their co-twins had at least one score among five cognitive tests at the first cognitive assessment. Individuals were also required to have Mini-Mental State Examination scores of 24 or above, as scores below this point are considered indicative of possible cognitive impairment (Tombaugh & McIntyre, 1992); screening excluded 7.8% of those with cognitive data (excluded N=1,136). For the moderation analyses, both members of the twin pair had to have information regarding adult household SES, excluding 16.1% or 2,338 individuals with cognitive data. The final sample of participants for the current study was 12,196 (50.3% female). Participants’ mean age was 60.0 years (sd=12.7), ranging from 27.0 to 97.9 years of age (see Supplemental Figure 8 for age distribution). Complete twin pairs included 2,458 monozygotic (MZ) pairs, 2,642 same-sex dizygotic (SS-DZ) pairs, and 998 opposite-sex dizygotic (OS-DZ) pairs. Demographic characteristics of participants and test availability by study are shown in Table 1. The IGEMS consortium received approval for the project titled Gene-Environment Interplay from the University of Southern California University Park Institutional Review Board, which assigned the Study ID: UP-16-00315. All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees.
Table 1.
Demographic Characteristics of Participants by Study
| Individuals | # of Complete Twin Pairs | Mean Age | Mean Attained SES | Cognitive Tests | ||||
|---|---|---|---|---|---|---|---|---|
| Study | N | % Female | MZ | SSDZ | OSDZ | (SD) | (SD) | |
| Swedish Studies: | ||||||||
| SATSA | 726 | 54.3% | 138 | 225 | 0 | 63.2 (8.2) | 45.5 (9.9) | SYN, SD, DF, DB, BD |
| GENDER | 484 | 50.0% | 0 | 0 | 242 | 74.6 (2.6) | 46.9 (9.5) | SYN, SD, BD |
| OCTO-Twin | 604 | 64.2% | 130 | 172 | 0 | 83.3 (2.9) | 43.3 (9.7) | SYN, SD, DF, DB, BD |
| TOSS | 1644 | 62.5% | 362 | 460 | 0 | 44.9 (4.9) | 51.5 (9.5) | SYN |
| Danish Studies: | ||||||||
| LSADT | 2034 | 61.1% | 403 | 600 | 14 | 75.4 (4.5) | 46.7 (8.9) | SD, DF, DB |
| MADT | 3748 | 49.2% | 662 | 599 | 613 | 56.4 (6.3) | 50.0 (9.9) | SD, DF, DB |
| US Studies: | ||||||||
| VETSA | 1218 | 0.0% | 346 | 263 | 0 | 55.4 (2.5) | 49.1 (9.0) | SYN, DF, DB |
| MTSADA | 750 | 61.3% | 217 | 158 | 0 | 55.8 (12.6) | 52.8 (9.3) | SD, BD |
| MIDUS twins | 988 | 54.4% | 193 | 158 | 127 | 52.1 (10.6) | 51.6 (12.4) | DB |
| Total | 12196 | 50.3% | 2458 | 2642 | 998 | 60.0 (12.7) | 49.12 (10.1) | SYN, SD, DF, DB, BD |
Note. SYN= Synonyms, SD= Symbol Digit, DB= Digits Backward, DF= Digits Forward, BD= Block Design; MZ= Monozygotic, SSDZ=Same-Sex Dizygotic, OSDZ=Opposite-Sex Dizygotic. The 9 studies include the Swedish Adoption/Twin Study of Aging (SATSA), Aging in Men and Women (Gender), Origins of Variance in the Oldest-Old (OCTO-Twin), Twin-Offspring Study in Sweden (TOSS), Longitudinal Study of Aging Danish Twins (LSADT), the Middle-Aged Danish Twins Study (MADT), The Vietnam Era Twin Study of Aging (VETSA), the Minnesota Twin Study of Adult Development and Aging (MTSADA), and the twin sample from Midlife Development in the United States (MIDUS).
Measures
Cognitive Measures.
We examined five tests where data were available from more than one country (see Table 1). Scores for each cognitive test were first pooled by test-study combination. After being adjusted for main effects of sex, each of these samples was divided into 10-year age groups and placed on a T-score scale with means of 50 and standard deviations of 10 in the 50-59.99 year age group. Cognitive test scores were then winsorized within ±3 SD by age group. See the Supplemental Materials and Pahlen et al. (2018) for further details on harmonization and scaling. We examined the following cognitive tests:
Synonyms (verbal ability) –
These were multiple-choice tests in which participants were asked to select the best synonym for each target word. Studies differed in numbers of items, specific words used, and number of response options. This basic format was available in SATSA, GENDER, OCTO-Twin, TOSS, and VETSA (N=4,307).
Digits Forward (short-term memory span) –
In this test, participants are orally presented with increasing strings of digits and asked to recall the digits in the order they are presented. The number of trials and length of digit strings varied across studies. Due to variations in scoring methods by study, we scored maximum correct span length. This format was available in five studies (SATSA, OCTO-Twin, LSADT, MADT, and VETSA; N=7,860).
Digits Backward (short-term/working memory span) –
This test is the same as Digits Forward, but participants are asked to report the digits in reverse order. We scored maximum correct span length. This format was available in six studies (SATSA, OCTO-Twin, LSADT, MADT, VETSA and MIDUS; N=8,722).
Block Design (spatial reasoning) –
Participants were asked to reproduce two-dimensionally-presented geometric shapes using sets of colored blocks. Four studies administered this test (SATSA, GENDER, OCTO-Twin, and MTSADA). Swedish studies (SATSA, GENDER, OCTO-Twin) administered the Kohs Block Design test (Stone, 1985) whereas a U.S. study (MTSADA) used the WAIS-R (Wechsler, 1981) Block Design subtest. The total N was 2,148.
Symbol Digit/Digit Symbol (perceptual speed) –
Studies in Sweden and Denmark (SATSA, Gender, OCTO-Twin, LSADT, and MADT) administered Symbol Digit, in which participants are asked to orally report numbers that correspond to presented target symbols. One U.S. study (MTSADA) administered WAIS-R (Wechsler, 1981) Digit Symbol in which participants are asked to write symbols that correspond to target numbers. The total N was 7,019.
Attained SES.
Participants reported their occupations and their spouses’ occupations. Occupational coding for participants and spouses was rationally harmonized within IGEMS by matching available occupational titles and descriptions among studies. A socioeconomic-status index based on the 9-point occupational component of the Hollingshead Index (Hollingshead, 1975) was used to score the harmonized IGEMS occupational variable as follows: 1=service worker; 2=unskilled worker; 3=semi-skilled worker; 4=skilled-worker; 5=clerical & sales; 6=semi-professionals; 7=minor professionals; 8=lesser professionals; 9=major professionals. Though only the occupational component of the Hollingshead Index was used for the present study, it is worth noting that this occupational component is typically used as interval in nature (Anokhin, Golosheykin, Grant, & Heath, 2011; Cirino et al., 2002; Szabó, Kelemen, & Kéri, 2014). Similarly, the occupational component was used as a continuous measure of attained SES in the present study.
To obtain household-level attained SES, the higher of participant and spouse SES scores was taken. Participants categorized as housewives/husbands were excluded if no spousal occupation information was available (N=76) or if participants did not report their occupation but indicated their spouse was a housewife/husband (N=6). All SES values were linearly transformed to a T-score scale (age groups relative to the 50-59.99 base groups). See Supplemental Materials for additional details on how attained SES was created.
Rearing SES.
Parental occupation was available for 11,755 individuals (from all 9 studies) based on participant report. Parental occupation coding was harmonized similarly to participant occupation, though coding of household-level rearing SES was done independently of attained SES as many studies comprising IGEMS used different occupation coding schemes for parental and participant SES. The same 9-point SES scale was used in the final coding of parental occupations. Twins reared together (representing 94% of the IGEMS sample and 97% of the current study sample with available information on rearing SES) received the same rearing SES score. Rearing SES was standardized and T-scored in the same manner as attained SES. Please refer to the Supplemental Materials for additional details on how rearing SES was created.
Statistical Analysis
To test whether environmental and genetic influences on cognitive performance differed by level of attained SES, we adapted the bivariate moderation model developed by Purcell (2002) by including continuous age (in years, centered on age 60) as an additional moderator of cognitive performance. This particular model is an extension of the classic twin model, in which the degree of similarities and differences between identical (monozygotic) twins and fraternal (dizygotic) twins are utilized to decompose the phenotypic variance within a trait into the estimated genetic and environmental sources of influence. In the classical twin model, influences due to additive genetic (A) effects are correlated 1.0 between identical twins and correlated .50 for fraternal twins. Shared environmental (C) variance, environmental influences that serve to increase familial resemblance, is correlated 1.0 for both identical and fraternal twins. Nonshared environmental (E) variance, any environmental sources of influence, including measurement error, that serve to reduce familial resemblance, is uncorrelated between twins (for a more in-depth review of classic twin models, see Boomsma, Busjahn, & Peltonen, 2002).
We chose the bivariate moderation model proposed by Purcell (2002) to capture and decompose all phenotypic variance in cognitive performance, particularly the covariance between cognitive performance and attained SES. Model estimates were used to calculate cognitive performance variance trends by level of the moderators (e.g. attained SES and continuous age) using the coefficients referred to in the path diagram in Figure 1. Although sex was separately regressed from cognitive scores, and use of household-level SES as the indicator of attained SES largely equated levels of SES in males and females, all models were tested with sex as a covariate on the means model to control any residual inflation of within-pair similarity. Model fitting was initially conducted using Mx 1.70a (Neale, Boker, Xie, & Maes, 2003). Full and best-fitting results were confirmed in Mplus 8.0 (Muthen & Muthen, 2012) and 95% confidence intervals for model estimates and plot estimates were obtained by adapting a script from Dinescu, Horn, Duncan, and Turkheimer (2016) for use with the moderation models in the current study.
Figure 1.
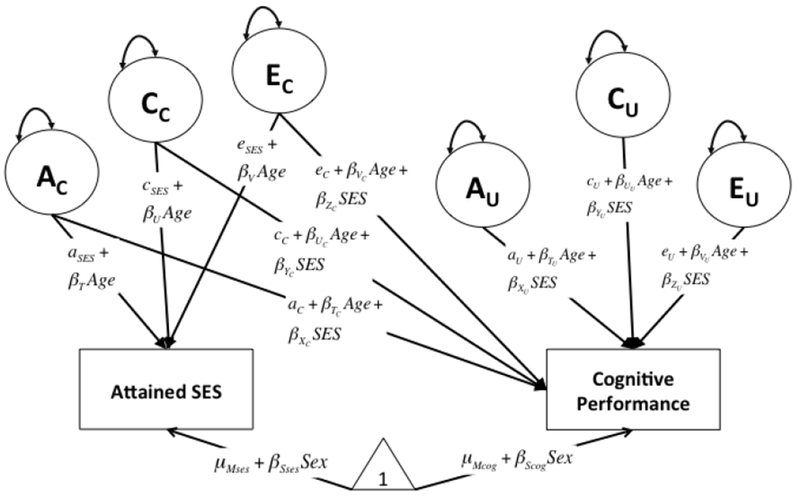
Full Moderation model showing genetic and environmental influences on cognitive performance as moderated by Attained SES and age. As in the traditional bivariate twin model, total phenotypic variance is partitioned into latent variables A, C, and E (nonshared environmental variance). Please note, diagram is simplified showing one twin for clarity (not shown are the twin pathways, e.g., additive genetic (A) effects correlated 1.0 for identical twins and .50 for fraternal twins). AC, CC, and EC refer to the sources of genetic and environmental influences common to attained SES and cognitive performance. AU, CU, and EU refer to the sources of genetic and environmental influences unique to cognitive performance. Model estimates can be used to calculate cognitive performance variance trends by level of Attained SES. For example, total raw genetic variance by level of attained SES, controlling for age, is calculated as: .
In the bivariate moderation model, there are six pathways on which attained SES could moderate influences on cognitive performance: (1) three common variance paths, denoted AC, CC, and EC, from attained SES to cognitive performance reflecting the sources of covariance (i.e. common influences) between SES and cognition with moderation indicated by the linear slope terms along those paths: βXC, βYC, βZC; and (2) genetic and environmental influences unique to cognitive performance, analogous to those considered in prior studies utilizing rearing SES, denoted AU, CU, and EU, and moderation is again indicated by the linear slope terms along those paths: βXU, βYU, βZU. Explicitly including the variance components in common between attained SES and cognition in the adapted bivariate moderation model allowed examination of G x E interaction in the presence of gene-environment correlation (rGE), essentially enabling interpretation of whether attained SES moderates adult cognitive performance adjusting for correlated or reciprocal processes with cognitive ability.
For each cognitive test, moderation on the common paths (i.e. AC, CC, and EC) and the unique paths (i.e. AU, CU, and EU) were systematically tested via omnibus model-fitting. In addition to chi-square difference tests from nested models using an alpha level of p<.01, best-fitting models were selected via comparison of Akaike’s Information Criterion (AIC; Akaike, 1983) and the sample size adjusted Bayesian Information Criterion (BIC; Raftery, 1995), with smaller values indicating better-fitting models. In cases of equivalent or competing AIC and BIC indications for best-fitting model, we favored models with fewer estimated parameters as more parsimonious.
We then used the estimates from the Full Bivariate Moderation Model and the best-fitting model to examine how attained SES moderated individual differences in cognitive performance by calculating the genetic and environmental variances by level of attained SES. For example, raw genetic variance in cognitive performance at attained SES (controlling age moderation) is calculated as and can be plotted for the range of attained SES values (see A variance paths in Figure 1). A, C, and E variances were calculated analogously and plotted to allow visual examination of the variances across levels of attained SES. To allow for clear interpretation of the attained SES effect on cognitive performance for each of the A, C, and E components, moderation on common variance paths signaling rGE and moderation on the unique variance paths signaling GxE were plotted in side-by-side plots for each cognitive test (Figure 3). Parallel proportional variance plots were constructed to illustrate the overall patterns in the relative contributions of genetic and environmental sources of variance on cognitive performance (Figure 4).
Figure 3.
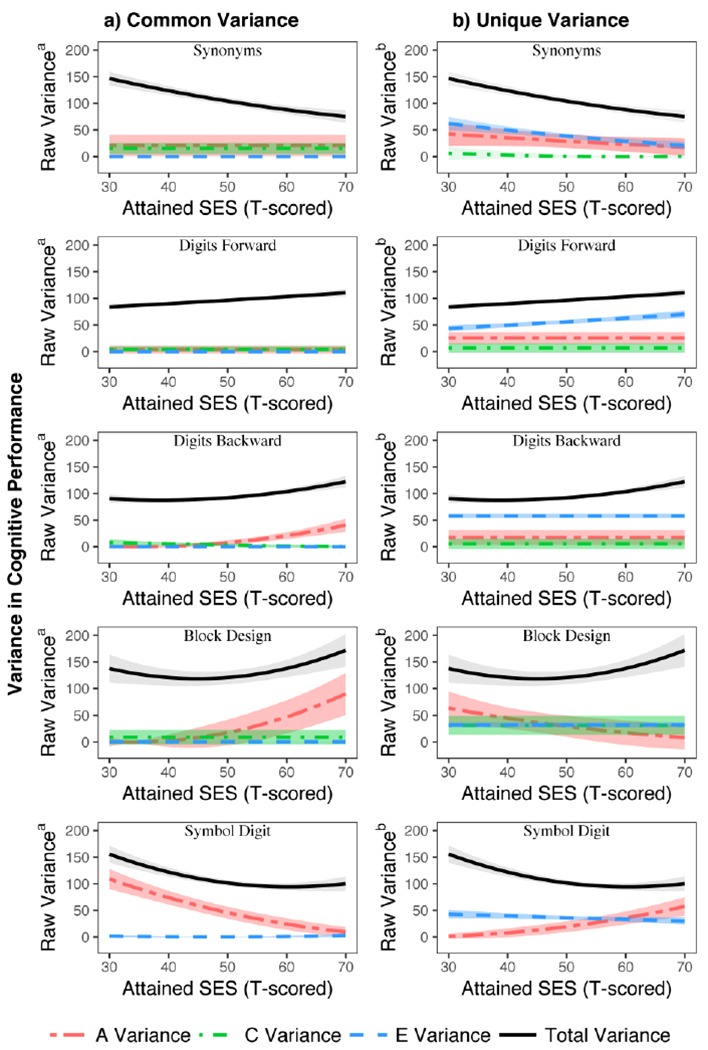
The raw variance estimates for the common and unique paths are plotted side-by-side for each cognitive test, with 95% confidence intervals indicated by the accompanying shaded areas. Total raw variance, summing common and unique variance estimates, is drawn in each plot. Plotted estimates adjusted for age moderation of the ACE variance components and gender effects for mean level performance.
Figure 4.
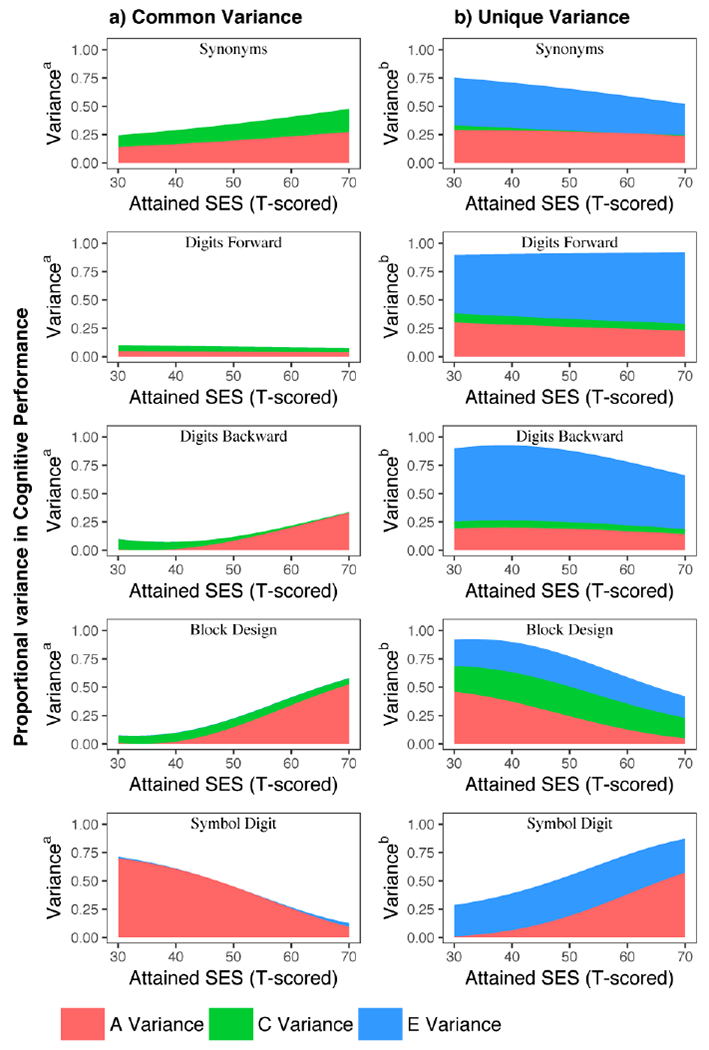
The proportional variance estimates for the common and unique paths are plotted side-by-side for each cognitive test. Total raw variance, summing common and unique variance estimates, was to calculate the proportions for both pathways, as such, the common and unique plots for each cognitive test effectively sum to 1. Plotted estimates adjusted for age moderation of the ACE variance components and gender effects for mean level performance.
Under the Purcell model, uniformly nonlinear main effects on mean level can be misinterpreted as moderation of covariance (Rathouz, Van Hulle, Rodgers, Waldman, & Lahey, 2008; Van Hulle, Lahey, & Rathouz, 2013). We addressed this problem by fitting models of uniformly nonlinear main effects prior to testing for significant moderating effects on variance common to SES and cognitive function. Where such models fit better, they indicate that relatively uniform nonlinear main effects are more likely than common moderation. Where they do not, the actual situation remains unclear – as other unmodeled alternatives could be present. Additionally, we used mixed effects regression analyses accounting for pair dependencies to directly address whether attained SES has a linear or nonlinear relationship with cognitive performance.
Sensitivity analyses.
We performed additional analyses to test the direction of moderation between attained SES and cognitive performance. In addition to performing tests with the raw data using Mplus 8.0 (Muthen & Muthen, 2012), imputed data sets were estimated using the mice package in R 3.3.3 (R Core Team, 2014) and analyzed for the reason that complete cognitive data for twin pairs was sparser than attained SES data (van Buuren & Groothuis-Oudshoorn, 2011). We also tested models controlling for rearing SES by including it as a covariate in the Full Moderation Bivariate model, on mean level ability and as a moderator of the ACE variance pathways for cognitive performance to evaluate whether rearing SES would alter observed attained SES moderation patterns (Tucker-Drob & Bates, 2015). Further, we evaluated whether effects might differ by region, comparing U.S. to Scandinavian samples (Tucker-Drob & Bates, 2015). More details regarding these analyses and additional sensitivity analyses using participants’ individually-attained SES can be found in the Supplemental Materials.
Results
Phenotypic patterns and twin pair descriptive statistics for attained SES and cognitive performance were considered prior to proceeding with biometric model fitting. Consistent with much prior research and as evident in the Loess plot in Figure 2 displaying general age trends for attained SES and cognitive performance, younger individuals tended to have higher mean attained SES. Mixed effects regression analyses (Proc Mixed, SAS 9.4; SAS Inc, Cary, NC), accounting for pair dependencies, confirmed this statistically (p<.01; see Supplemental Table S1). Younger age groups also had higher mean cognitive performance than older age groups, with a loss of 0.05 to 0.54 T-score points per year above age 60. As shown in Figure 2, this trend was particularly evident for Symbol Digit, Block Design, and to a lesser degree, Synonyms. Individuals with higher attained SES had higher mean performances on all cognitive tests than individuals with lower attained SES (all p<0.01; see Supplemental Table S1), with 0.163 to 0.253 point average gains in cognitive performance for each unit increase over mean attained SES (controlling for age effects). There were small but significant interactions between attained SES and age for Synonyms (p<0.01), Symbol Digit (p=0.01), and Digits Backward (p<0.01), in which higher SES mitigated the age effect. Neither Digits Forward nor Block Design evidenced significant attained SES-by-age differences (p=0.43 and p=0.25, respectively).
Figure 2.
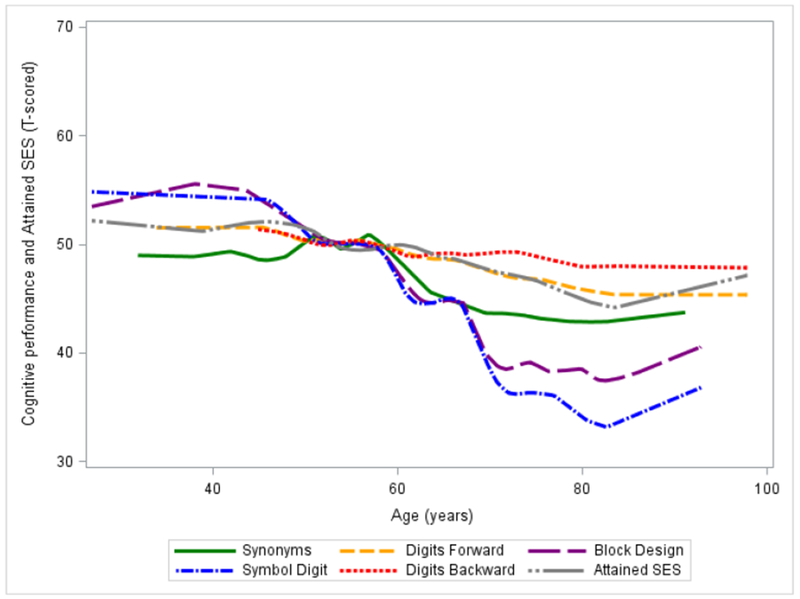
Loess Curve indicating trends in T-scored Cognitive Test Performance and T-scored Attained SES by Participant Age (in years)
The correlation between attained SES and rearing SES was r=.27. Table 2 shows the phenotypic correlations for both attained SES and rearing SES with cognitive performance. The phenotypic correlations ranged from small to moderate in magnitude, with the strongest relationships evident for Synonyms and Symbol Digit with both attained SES and rearing SES. Correlations within pairs (by zygosity) for attained SES were modestly higher in MZ twins than DZ twins. Within-pair correlations (by zygosity) for cognitive tests were higher in MZ twins than DZ twins, and the magnitude of twin correlations by cognitive test are roughly consistent with previous work in older adult studies (McClearn et al., 1997; McGue & Christensen, 2001; Pedersen, Plomin, Nesselroade, & McClearn, 1992). Cross-trait correlations between cognitive tests and co-twin´s attained SES were small to moderate and positive, and they too were higher in MZ than DZ pairs (see Table 3). Twin correlations by birth cohorts before and after 1940 are presented in Table S3 of the Supplemental Materials.
Table 2.
Phenotypic correlations of cognitive performance with attained and rearing SES.
| Cognitive Trait | NCOG | Attained SES* | Rearing SES* | Attained SES** (partial) |
|---|---|---|---|---|
| Synonyms (SYN) | 3866 | .33 | .23 | .29 |
| Digits Forward (DF) | 7860 | .19 | .13 | .16 |
| Digits Backward (DB) | 8722 | .19 | .13 | .16 |
| Block Design (BD) | 2148 | .24 | .17 | .21 |
| Symbol Digit (SD) | 7019 | .29 | .27 | .26 |
Notes.
Correlations estimated in Mx, restricting sample to pairs with complete Attained SES data.
Correlations adjusted for sex as well as age and nonlinear age effects.
Partial correlations further control for rearing SES.
Table 3.
Twin Correlations and cross-trait correlations for attained SES and cognitive test scores pooled across studies.
| N pairs Complete pairs (incomplete pairs) |
Within-Trait Twin Correlations | Cross-Trait Correlations between SES & Cognitive Tests | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Trait | MZ | SSDZ | OSDZ | MZ | SSDZ | OSDZ | MZ | SSDZ | OSDZ |
| Attained SES | 2458 | 2642 | 998 | .38 | .29 | .27 | -- | -- | -- |
| Synonyms (SYN) | 875 (68) | 963 (122) |
201 (39) |
.66 | .38 | .39 | .31 | .22 | .24 |
| Digits Forward (DF) | 1522 (146) | 1580 (273) | 610 (17) | .41 | .28 | .20 | .17 | .13 | .17 |
| Digits Backward (DB) | 1670 (197) | 1701 (317) | 710 (46) | .39 | .21 | .26 | .18 | .12 | .15 |
| Block Design (BD) | 374 (72) | 378 (127) | 203 (39) | .64 | .40 | −.10 | .24 | .11 | .09 |
| Symbol Digit (SD) | 1227 (210) | 1238 (363) | 707 (102) | .59 | .32 | .34 | .24 | .18 | .19 |
Note. Correlations estimated in Mx, restricting sample to pairs with complete data on Attained SES scores.
MZ= Monozygotic, SSDZ=Same-Sex Dizygotic, OSDZ=Opposite-Sex Dizygotic. Included incomplete twin pairs e.g. one twin without cognitive assessment in parentheses
SES moderation of cognitive performance
Evidence for moderation of cognitive performance by attained SES was observed for all cognitive tests (see Supplement Table S2 for model-fit statistics). Biometrical moderation model-fitting suggested mechanisms by which attained SES moderates each cognitive test differ, but general patterns emerged. Moderation via common (correlated) paths between attained SES and cognitive performance tended to indicate relatively greater genetic influences at high levels of attained SES. In contrast, attained SES moderation on variance unique to cognitive performance tended to indicate relatively smaller genetic influences at high levels of attained SES. A noted exception to this pattern, genetic influences unique to perceptual speed were amplified and common genetic influences were dampened at high levels of attained SES. Model parameter estimates, including 95% confidence intervals, are included in Table S4 of the Supplemental materials. Model estimates of the ACE raw variances as moderated by attained SES are plotted for the best-fitting model for each cognitive test in Figure 3 (see Supplemental Figure S1 for full moderation model results). Age moderation of cognitive performance and of attained SES was also tested to control any age effects and retained in all SES-moderation analyses (all ps < 0.01; see Supplemental Materials for age moderation results, including Supplemental Figure S2).
For Synonyms, model fit comparison Δχ2 (3)=106.8, p=5.36E-23 indicated the best-fitting model was SES moderation via the unique A, C, & E pathways. Overall phenotypic (total) variance for Synonyms was lower at higher levels of attained SES (Figure 3), driven mainly by smaller nonshared environmental influences unique to cognitive performance. The genetic variance unique to cognitive performance was also smaller at higher levels of attained SES. Still, proportionally, the genetic component was the main source of variance in cognitive performance at higher levels of attained SES (Figure 4). Shared environmental influences tended to be quite small overall, though at higher attained SES, common C variance was slightly greater and unique C variance was slightly smaller. Proportional variance plots (Figure 4) illustrate the overall patterns in the relative contributions of genetic and environmental sources of variance on cognitive performance. The patterns suggests that common genetic influences are proportionally amplified at higher levels of attained SES for Synonyms, while there is a modest relative attenuation by level of SES for unique genetic influences and more notable attenuation of nonshared environmental influences (right panel).
For Digits Forward, model fit comparison Δχ2 (1)=24.3, p=8.24E-07 indicated moderation was via unique moderation of nonshared environmental paths. Here — in contrast to Synonyms— overall phenotypic variance tended to be greater at higher levels of attained SES (Figure 3). Contributions from nonshared environmental influences were modestly larger at higher levels of attained SES. Genetic influences and shared environmental influences were constant across levels of attained SES. Additive genetic influences unique to cognitive performance were small. Genetic and environmental sources of variance common between attained SES and cognitive performance were near zero. Proportional variance plots (Figure 4) suggest unique genetic influences are proportionally attenuated at higher levels of attained SES while nonshared environmental effects are higher.
For Digits Backward, model fit comparison Δχ2 (3)= 53.8, p=1.24E-11 indicated moderation was on the common paths between attained SES and cognition, including both genetic and environmental influences. As with Digits Forward, overall phenotypic variance tended to be slightly greater at higher levels of attained SES (Figure 3). The main source of difference in variance was slightly greater common genetic influences at higher levels of attained SES. Shared environmental influences and nonshared environmental influences on the common pathways were close to zero. The main sources of individual differences on test performance were nonshared environmental and genetic influences unique to cognition, which were not moderated by attained SES. Proportional variance plots (Figure 4) suggests common genetic influences are amplified while unique genetic influences are modestly attenuated at higher levels of attained SES, with greater attenuation of nonshared environmental effects at higher levels of attained SES.
For Block Design, model fit comparison Δχ2 (3)= 19.4, p=6.13E-05 specified the best-fitting model showing genetic moderation, via both common and unique paths. Overall phenotypic variance was greater at higher levels of attained SES, mainly due to greater additive genetic influences in common sources of variance (see Figure 3). In contrast, genetic sources of variance unique to cognitive performance were smaller at higher levels of attained SES. Shared and nonshared environmental influences on cognitive performance were stable across levels of attained SES in the best-fitting model. Proportional variance plots (Figure 4) suggests common genetic influences are amplified while unique genetic influences are attenuated at higher levels of attained SES.
Finally, for Symbol Digit, there was evidence for non-additive genetic influences based on related work with these data (Pahlen et al., 2018) as well as quadratic age moderation, so we used an AE model for performance, estimating C only for attained SES and a quadratic term when adjusting for age. The full AE-moderation model fit best , Δχ2 (4)= 79.7, p=2.02E-16 specified, indicating SES moderated performance via both common and unique paths. Overall phenotypic variance for Symbol Digit performance tended to be lower at higher levels of attained SES (Figure 3). This was due mainly to smaller additive genetic influences on common variance at higher levels of attained SES. In contrast, attained SES moderated genetic variance unique to cognitive performance, such that genetic influences were larger at higher levels of attained SES. In proportional variance plots (Figure 4), estimates for Symbol Digit indicate large common genetic influences at low attained SES that become increasingly attenuated at high levels of attained SES whereas the unique variance estimates are amplified at high levels of attained SES. See Figure S7 in the Supplemental Materials for comparison plots showing the full moderation model for Symbol Digit as both an ACE and an AE model. Please note, variance plots presented for the ACE model should be interpreted with caution, given analyses in a previous study with the same sample indicated possible dominance genetic effects (Pahlen et al., 2018).
Along with model comparisons, tests of uniformly nonlinear main effects were conducted prior to further analyses for Symbol Digit, Digits Backward, and Block Design as initial model fitting suggested common variance moderation. The significance of the test for Digits Backward [χ2(1) = 5.08, p < .025] suggested that one cannot rule out uniformly nonlinear main effects as an alternative to common moderation of variance. The test was not significant for either Symbol Digit [χ2(1) = 0.49, p = 0.486] or Block Design [χ2(1) = 0.19, p = 0.664]. Additional multi-level regression analyses controlling for sex, age, and rearing SES compared linear and quadratic models for the relationship between attained SES and cognitive performance. Results indicated that for all cognitive tests, adding a quadratic did not improve model fit, and quadratic terms in the models were not significant (see Supplemental Table S2).
Rearing SES adjustments.
Full moderation models controlling for mean level effects and moderation by rearing SES indicated that attained SES moderation patterns of estimated ACE variance components for cognitive performance were very similar to the unadjusted models (see Supplemental Figure S3). Given the divergent trends for attained SES moderation of genetic influences for Synonyms and Symbol Digit (Supplemental Figure S3), yet similar patterns of moderation by levels of rearing SES in the bivariate twin models (Supplemental Figure S4), we examined univariate models of rearing SES moderating the two cognitive tests. Results indicated that patterns of estimated ACE variance components across levels of rearing SES were similar to those observed in the full bivariate moderation models (see Supplemental Figure S5).
Differences by Region.
We tested for differences in SES moderation between the United States and Scandinavia for additive genetic, common environment (where specified), and nonshared environment. Results suggested non-significant differences in moderation by region for four of five cognitive tests (i.e., testing if βXCusa=βXCscan, βXUusa=βXUscan) for moderation of A (p = .053 - .913), C (p = .260 - .960), or E (p = .158 - .920). For Symbol Digit performance, A-moderation was significantly different between the two regions (p = 0.001; Supplemental Table S5). But as the type of Symbol Digit test administered is confounded with country/region, this confounding may be the source of observed regional differences.
Does cognition moderate genetic and environmental influences on SES?
For each of the five cognitive tests, model-fit statistics were worse when cognitive ability moderated common and unique attained SES variance components compared to when attained SES moderated common and unique cognitive performance variance components (see Supplemental Table S6). Compared to the “No Moderation” models, AIC and BIC values were larger when cognitive ability moderated attained SES than vice versa. Further, imputed data sets were analyzed as an additional sensitivity analysis and demonstrated a similar pattern of increased AIC and BIC values, such that attained SES was considered to moderate cognitive ability more so than vice versa.
Individually-attained SES as a moderator
Full model results for individually-attained SES as the moderator of cognitive performance are shown in Supplemental Figure S6. The moderation patterns of estimated ACE variance components by level of individually- attained SES were very similar to our primary models with household-level attained SES with the exception of Symbol Digit (with moderation patterns similar to the other tests), indicating the effects of attained SES on cognition are fairly robust.
Discussion
In a body of research examining genetic-by-rearing SES interactions on cognitive performance, genetic variance in cognition was generally smaller in low SES conditions, particularly in the United States (Tucker-Drob & Bates, 2015; Turkheimer & Horn, 2014). When we examined this question with respect to attained SES in adults (controlling age moderation), we generally observed the opposite pattern. The overall pooled sample included 12,196 (50.3% female), though sample size for each cognitive test depended on data collected in each study within the IGEMS consortium. For verbal ability, memory, and spatial reasoning, moderation of genetic influences unique to cognitive performance were relatively constant along the range of attained SES levels. However, for perceptional speed, high attained SES appeared to amplify genetic influences unique to the cognitive test, signaling potential GxE mechanisms. Controlling rearing SES did not alter these findings. Our results indicated reciprocal associations between attained SES and cognitive ability. Furthermore, the direction-of-moderation results provide evidence that attained SES is likely a strong moderator of genetic and environmental influences on cognitive abilities, remaining an influential source of these associations in adulthood.
Pathways of Moderation
Using attained SES as a moderator allowed us to test whether attained SES moderated variance in cognitive performance via common sources of genetic and environmental influences between attained SES and cognitive performance as well as unique influences on cognitive performance (Purcell, 2002). Moderation of cognitive performance via unique pathways likely indicates a contextual effect of attained SES on adult cognitive performance akin to the effect of rearing SES on childhood and adolescent cognitive performance, though the direction of effect was generally in the opposite direction. Strictly speaking, in the twin model approach, attained SES is a trait of the individual as much as cognitive performance. Even so, we can conceptualize that an individual’s access to social and economic resources is not solely determined by cognitive ability. Both rearing SES and attained SES may provide varying degrees of opportunities for cognitive enrichment. There was also some evidence that attained SES moderated common variance in verbal ability, perceptual speed, and short-term/working memory (Digits Backward), implicating part of the moderation arose due to genetic and environmental influences affecting both attained SES and cognitive performance (rGE).
In some ways, multivariate modeling results pose more questions than answers. While there is evidence that rGE processes account for a portion of the relation between attained SES and cognitive performance, it is difficult to pin down the underlying mechanisms. Genetic selection is a likely candidate and appears to operate more strongly at one end of the SES continuum than the other depending on cognitive domain, operating at low SES for perceptual speed and high SES for spatial reasoning. For example, one explanation is that twins genetically predisposed to a trait (e.g., conscientiousness) might keep selecting environments, and are reinforced by those environments, making them more adept on certain abilities and wealthier compared to their less conscientious co-twins. Yet, the results suggest there is no clear selection pattern that benefits high SES versus low SES for all cognitive abilities
A second possibility – one we consider highly speculative – is that given higher mean cognitive performance with higher attained SES, greater genetic influences on common (correlated) variance at higher attained SES signals some combination of population-level genetic stratification contributing to both genetically-influenced niche-picking and/or greater opportunities for lifestyles that support cognitive performance (e.g., spatial reasoning and short-term/working memory (Digits Backward)). Uniformly nonlinear main effects may account for some of the common moderation effects we observed (see Supplemental Results and Rathouz et al., 2008) Indeed, follow-up analyses indicated that this alternative explanation may be likely for short-term/working memory, although multi-level analyses including covariates did not support non-linear SES moderation for any of the cognitive tests. Irrespective of the underlying process, the main conclusion that can be gleaned from these results is that attained SES and cognitive performance in adulthood are reciprocally related.
Though the observed patterns for attained SES moderation of cognitive performance are nuanced, general patterns in the present findings do show consistency with previous work on SES and cognitive ability. Rearing SES and SES in adulthood have shown evidence of additive, positive impact on level of mid- and late-life cognitive functioning (Fors, Lennartsson, & Lundberg, 2009; Hurst et al., 2013; Lyu & Burr, 2016). Providing evidence that these associations operate beyond mean-level associations in both childhood and adulthood, sensitivity analyses with both rearing SES and attained SES as moderators of performance on cognitive tests suggested that each component of SES differentially affects genetic and environmental influences on cognitive ability. These findings provide further support for the importance of assessing both rearing and attained SES in investigating determinants of adult cognitive performance.
Attained SES: Amplifier or Suppressor of Etiological Influences Unique to Cognition?
By modeling the common (correlated) variance between attained SES and cognition, moderation effects observed on variance unique to cognitive performance provide evidence for a causal role of attained SES (Purcell, 2002). Moderation of genetic influences on cognitive performance differ by cognitive test, consistent with differing heritability estimates for cognitive traits in older adults (Finkel & Reynolds, 2009; Lee, Henry, Trollor, & Sachdev, 2010). Notably, genetic influences were relatively stable across attained SES for the three cognitive tests encompassed by the Wechsler verbal IQ domain but varied across attained SES for the two tests encompassed by the performance IQ domain.
We highlight a couple of interpretations that constitute rich areas of further research on the relation between attained SES and cognitive ability. First, genetic etiology of nonverbal abilities may be sensitive to the level of goods, services, and knowledge people acquire throughout adulthood, with greater genetic variability indicating both better and worse ability levels given attained SES. The high end of SES attainment, thus, may confer greater variability in genetic influence for some abilities (processing speed) yet lower genetic influence for other abilities (perceptual organization). Low end points of SES attainment, on the other hand, may confer opposite patterns. We provide one example – the nature of professional demands at different levels of attained SES – purely for illustrative purposes. Genetic variability for processing speed abilities may be greater at higher attained SES than lower attained SES because of professional specialization. Those who practice law and medicine will be in the same SES strata but will be better or worse at processing information depending on the demands of the their specific practice (trial attorney versus contract law). Conversely, the processing speed ability needed to perform menial labor jobs in those in lower SES strata will vary less across profession (cashier versus construction).
Second, differentiation and specialization of cognitive abilities may also explain differences in moderation of cognitive abilities by attained SES (Tucker-Drob, 2009). At higher attained SES levels, the patterns of genetic and environmental influences were less organized, e.g., smaller genetic influences and smaller shared environmental influences on verbal ability and spatial reasoning abilities with greater genetic influences on perceptual speed ability, than at lower attained SES levels. Nonshared environmental influences predominate (mostly) at the lower levels of attained SES. As part of this interpretation, low SES contexts may be governed by less predictable nonshared environmental experiences overall, resulting in experiences that lead to increased variability in cognitive performance (Turkheimer & Gottesman, 1991). Conversely, higher attained SES may enable protective processes that dampen genetic influences on cognitive functioning in favor of relatively greater environmental influences, consistent with a ‘social context as compensation’ GxE mechanism (Shanahan & Hofer, 2005).
Greater genetic influences unique to perceptual speed as observed at higher attained SES levels is consistent with some of the rearing-SES-cognition literature (Tucker-Drob & Bates, 2015; Turkheimer & Horn, 2014) and with theories that high SES contexts may lead to greater heritability via accumulation of experiences in enriched environments that support expression of full genetic potential (Bronfenbrenner & Ceci, 1994; Bronfenbrenner & Morris, 2006; Shanahan & Hofer, 2005). Given the fairly stable nature of other cognitive abilities in middle adulthood, perceptual speed may uniquely benefit from enriching experiences at higher levels of attained SES as decline can begin in early adulthood (Christensen, 2001; Deary et al., 2009). This decline is often attributed to changes in the structural integrity of the brain, such that perceptual speed may act as a marker of neurological health particularly important for adults prior to apparent cognitive declines (Deary et al., 2009; Johnson et al., 2014). Notably, perceptual speed was the only cognitive test in this sample with nonlinear age moderation (Pahlen et al., 2018). Our findings, controlling for age moderation, suggest that genes that influence cognition may remain sensitive to SES contexts. These GxE mechanisms appear to operate primarily in adulthood, as removing covariance with rearing SES resulted in very little difference in the moderating effects of attained SES.
Given the combinations of cognitive tests currently available in the IGEMS twin samples, the creation of a general intelligence ‘g’ measure similar to the intelligence measures utilized in rearing SES studies was not feasible. Though not substitutable as a g measure, perceptual speed shares common genetic influences and is a marker of general intelligence in adulthood (Lee et al., 2012; Tucker-Drob, Reynolds, Finkel, & Pedersen, 2014; Verhaeghen & Salthouse, 1997). Results for perceptual speed potentially afford us a glimpse of how attained SES may moderate those mutual sources of genetic influences (e.g. genes) on g in adults. Namely, attained SES may moderate at least some of the genetic influences on g in patterns similar to that observed for rearing SES, albeit through contextual experiences particularly beneficial to maximizing genetic potential for cognitive functioning in adulthood. Moreover, given the potential GxE mechanisms noted here and evidence regarding social, cognitive, and health lifestyle choices in midlife as risk factors for cognitive decline (Baumgart et al., 2015), further research is warranted regarding specific genetic pathways by which attained SES contexts may be influencing adult cognitive abilities.
Direction of Moderation Between Cognitive Performance and Attained SES
Though there was some evidence that cognitive abilities moderated sources of individual differences in attained SES for all except spatial reasoning, attained SES moderating cognitive performance provided the clearer story for our data (based on model fit comparisons). Taken together, these results are consistent with the interactionist perspective, which proposes that attained SES and individual characteristics, including cognition, emerge via reciprocal relations that make disentangling causal forces difficult (Conger & Donnellan, 2006). Analyses reversing direction of moderation supported our focus on attained SES as the primary moderating variable in the associations. This finding is in keeping with the notion that effects of rearing SES are “handed off” to attained SES as people age. Although attained SES is influenced by cognitive ability more so than rearing SES, the current results suggest that attained SES may become a consistent contextual factor influencing cognitive performance in adulthood. Further modeling of attained SES and cognitive ability measures in longitudinal twin studies of aging are in a better position to clarify how genetic and environmental selection processes unfold over time, particularly to account for differences in verbal and nonverbal measures observed here.
Implications and conclusions
SES is a complex variable, conceptualized in numerous ways throughout research and academic discourse (Oakes & Rossi, 2003). Within epidemiological and psychological literature, how SES is measured and operationalized is important for understanding what contexts are being captured when assessing and interpreting SES effects on individual-level processes (Bruna Galobardes, Shaw, Lawlor, & Lynch, 2006; B. Galobardes, Shaw, Lawlor, Lynch, & Davey Smith, 2006). The current study used household-level attained SES as representing individuals’ access to resources and related hierarchical social and economic contexts. Moreover, attained SES likely reflects the joint efforts or agency of spouses and is hence appropriate given the changing demographics of women in the workforce over the historical birth cohorts represented in the IGEMS studies. Evaluating individually-attained SES as a moderator did not reveal marked differences in the overall findings, supporting our use of household-level attained SES that allowed greater inclusion of women.
There were some limitations to the current study that should be mentioned. Cross-sectional studies can only offer a snapshot of rather dynamic genetic and environmental processes. Due in part to persisting differential mortality by SES into old age (Bassuk, Berkman, & Amick, 2002; Huisman et al., 2004), it is likely that the older adult participants from the IGEMS studies, particularly individuals with low attained SES, represent selective members of the population. For this reason, attained SES moderation of adult cognitive performance in the general population may not be fully captured here. The multiple birth cohorts in varying environments likely differed in SES structure both by country and by birth cohort, which may produce spurious results. We were able to test and verify no evidence for U.S. versus Scandinavia regional differences in moderation patterns for verbal ability, short-term/working memory, and spatial reasoning; however, we were unable to confirm results for perceptual speed due to confounding of cognitive test form within region. Given these limitations, other twin cohorts may be able to provide further insight regarding the role of attained SES as a moderator of cognitive performance in adults.
In this study, we extended examination of moderation of genetic and environmental influences on cognitive performance by considering whether attained SES moderated individual differences in adult cognition across four cognitive abilities (via five cognitive tests). Perhaps most importantly, use of adult twin samples with attained SES and adult cognitive assessments allowed examination of the full variance between SES and cognitive performance. Our findings indicate that attained SES is moderating cognitive performance, in part, through active gene-environment correlation processes. This was evident in the relatively larger common genetic influences at higher levels of attained SES for verbal ability, working memory, and spatial reasoning. This is in addition to the mechanisms through which rearing SES moderates cognitive performance. At high levels of SES, genetic influences unique to perceptual speed were amplified whereas unique genetic influences for verbal performance and spatial reasoning were attenuated. These findings signal gene x attained SES mechanisms that may be particularly relevant to adult cognitive performance. The fact that we also observed moderating effects of cognitive abilities on attained SES suggests the presence of reciprocal effects. Our findings indicate multiple genetic and environmental pathways via which SES may be supporting maintenance of different cognitive domains in older adults.
Supplementary Material
Acknowledgement:
Financial Support: IGEMS is supported by the National Institutes of Health grant no. R01 AG037985 and R56 AG037985. SATSA was supported by grants R01 AG04563, R01 AG10175, the MacArthur Foundation Research Network on Successful Aging, the Swedish Council For Working Life and Social Research (FAS) (97:0147:1B, 2009-0795) and Swedish Research Council (825-2007-7460, 825-2009-6141). OCTO-Twin was supported by grant R01 AG08861. Gender was supported by the MacArthur Foundation Research Network on Successful Aging, The Axel and Margaret Ax:son Johnson’s Foundation, The Swedish Council for Social Research, and the Swedish Foundation for Health Care Sciences and Allergy Research. TOSS was supported by grant R01 MH54610 from the National Institutes of Health. The Danish Twin Registry is supported by grants from The National Program for Research Infrastructure 2007 from the Danish Agency for Science and Innovation, the Velux Foundation and the US National Institute of Health (P01 AG08761). The Minnesota Twin Study of Adult Development and Aging was supported by NIA grant R01 AG06886. VETSA was supported by National Institute of Health grants NIA R01 AG018384, R01 AG018386, R01 AG022381, and R01 AG022982, and, in part, with resources of the VA San Diego Center of Excellence for Stress and Mental Health. The Cooperative Studies Program of the Office of Research & Development of the United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. This MIDUS study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development and by National Institute on Aging Grant AG20166. Christopher R. Beam was supported by the National Institute on Aging (T32 AG000037-37) and an Alzheimer’s Association Research Fellowship (AARF-17-505302). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA/NIH, or the VA.
Footnotes
*References cited only in the Supplemental Materials.
Contributor Information
Catalina Zavala, Center for Economic and Social Research, University of Southern California, Los Angeles, CA 90089.
Christopher R. Beam, Department of Psychology, University of Southern California, Los Angeles, CA 90089
Brian K. Finch, Center for Economic and Social Research, University of Southern California, Los Angeles, CA 90089
Margaret Gatz, Center for Economic and Social Research, University of Southern California, Los Angeles, CA 90089; Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, SE 171 77 Stockholm, Sweden.
Wendy Johnson, Centre for Cognitive Ageing and Cognitive Epidemiology and Department of Psychology, University of Edinburgh, EH-89JZ Edinburgh, UK.
William S. Kremen, Department of Psychiatry and Center for Behavior Genetics of Aging, University of California, La Jolla, CA 92093 VA Center of Excellence for Stress and Mental Health, La Jolla, CA 92161.
Jenae M. Neiderhiser, Department of Psychology, Penn State University, University Park, PA 16802
Nancy L. Pedersen, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, SE 171 77 Stockholm, Sweden Department of Psychology, University of Southern California, Los Angeles, CA 90089.
Chandra A. Reynolds, Department of Psychology, University of California Riverside, Riverside, CA 92521
References
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, & Syme SL (1994). Socioconomic Status and Health: The Challenge of the Gradient. American Psychologist, 49(1), 15–24. doi:http://dx.doi.org/10.1037/0003-066X.49.1.15 [DOI] [PubMed] [Google Scholar]
- Airsman LA, & Sharda BD (1993). A Comparative Study of The Occupational Attainment Processes of White Men and Women in The United States: The Effects of Having Ever Married, Spousal Education, Children and Having Ever Divorced. [http://www.jstor.org/stable/41602274]. Journal of Comparative Family Studies, 24(2), 171–187. [Google Scholar]
- Akaike H (1983). Information measures and model selection. Bulletin of the International Statistical Institute, 50(1), 277–291. [Google Scholar]
- Anokhin AP, Golosheykin S, Grant JD, & Heath AC (2011). Heritability of Delay Discounting in Adolescence: A Longitudinal Twin Study. Behavior Genetics, 41(2), 175–183. doi:10.1007/s10519-010-9384-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Antonides G. (2011). The Division of Household Tasks and Household Financial Management. Zeitschrift Fur Psychologie-Journal of Psychology, 219(4), 198–208. doi:10.1027/2151-2604/a000073 [Google Scholar]
- Bassuk SS, Berkman LF, & Amick IIIBC (2002). Socioeconomic Status and Mortality among the Elderly: Findings from Four US Communities. American Journal of Epidemiology, 155(6), 520–533. doi:10.1093/aje/155.6.520 [DOI] [PubMed] [Google Scholar]
- Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, & Johns H (2015). Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer’s & Dementia, 11(6), 718–726. doi:https://doi.org/10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- Beam CR, Turkheimer E, Dickens WT, & Davis DW (2015). Twin Differentiation of Cognitive Ability Through Phenotype to Environment Transmission: The Louisville Twin Study. Behavior Genetics, 45(6), 622–634. doi:10.1007/s10519-015-9756-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasco W, de Graaf PM, & Ultee WC (1998). Coupled Careers: Effects of Spouse’s Resources on Occupational Attainment in the Netherlands. European Sociological Review, 14(1), 15–31. doi:10.1093/oxfordjournals.esr.a018225 [Google Scholar]
- Boomsma D, Busjahn A, & Peltonen L (2002). Classical twin studies and beyond. Nature Reviews Genetics, 3(11), 872–882. doi:10.1038/nrg932 [DOI] [PubMed] [Google Scholar]
- Bouchard TJ (1997). Experience Producing Drive Theory: how genes drive experience and shape personality. Acta Paediatrica, 86(S422), 60–64. doi:10.1111/j.1651-2227.1997.tb18347.x [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U, & Ceci SJ (1994). Nature-nuture reconceptualized in developmental perspective: A bioecological model. Psychological Review, 101(4), 568–586. doi:http://dx.doi.org/10.1037/0033-295X.101.4.568 [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U, & Morris PA (2006). The Bioecological Model of Human Development Handbook of Child Psychology (1 ed., Vol. 1, pp. 793–828): Wiley. [Google Scholar]
- Buss DM (1985). Human Mate Selection: Opposites are sometimes said to attract, but in fact we are likely to marry someone who is similar to us in almost every variable. American Scientist, 73(1), 47–51. [Google Scholar]
- Christensen H (2001). What cognitive changes can be expected with normal ageing? The Australian and New Zealand journal of psychiatry, 35(6), 768–775. [DOI] [PubMed] [Google Scholar]
- Cirino PT, Chin CE, Sevcik R. a., Wolf M, Lovett M, & Morris RD (2002). Measuring Socioeconomic Status: Reliability and Preliminary Validity for Different Approaches. Assessment, 9(2), 145–155. doi:10.1177/10791102009002005 [DOI] [PubMed] [Google Scholar]
- Colodro-Conde L, Rijsdijk F, Tornero-Gomez MJ, Sanchez-Romera JF, & Ordonana JR (2015). Equality in Educational Policy and the Heritability of Educational Attainment. PLoS ONE, 10(11), e0143796. doi:10.1371/journal.pone.0143796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger RD, & Donnellan MB (2006). An Interactionist Perspective on the Socioeconomic Context of Human Development. Annual Review of Psychology, 58(1), 175–199. doi:10.1146/annurev.psych.58.110405.085551 [DOI] [PubMed] [Google Scholar]
- Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, … Starr JM (2009). Age-associated cognitive decline. British Medical Bulletin, 92(1), 135–152. doi:10.1093/bmb/ldp033 [DOI] [PubMed] [Google Scholar]
- Dickens WT, & Flynn JR (2001). Heritability Estimates Versus Large Environmental Effects : The IQ Paradox Resolved. Psychological Review, 108(2), 346–369. [DOI] [PubMed] [Google Scholar]
- Dinescu D, Horn EE, Duncan G, & Turkheimer E (2016). Socioeconomic modifiers of genetic and environmental influences on body mass index in adult twins. Health Psychology, 35(2), 157–166. doi:http://dx.doi.org/10.1037/hea0000255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, Andel R, Gatz M, & Pedersen NL (2009). The role of occupational complexity in trajectories of cognitive aging before and after retirement. Psychology and Aging, 24(3), 563–573. doi:10.1037/a0015511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, & Reynolds CA (2009). Behavioral Genetic Investigations of Cognitive Aging In Kim Y-K (Ed.), Handbook of Behavior Genetics (pp. 101–112): Springer; New York. [Google Scholar]
- Fors S, Lennartsson C, & Lundberg O (2009). Childhood Living Conditions, Socioeconomic Position in Adulthood, and Cognition in Later Life : Exploring the Associations. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 64B(6), 750–757. doi:10.1093/geronb/gbp029. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Shaw M, Lawlor DA, & Lynch JW (2006). Indicators of socioeconomic position (part 1). Journal of Epidemiology and Community Health, 60(1), 7–12. doi:10.1136/jech.2004.023531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galobardes B, Shaw M, Lawlor DA, Lynch JW, & Davey Smith G (2006). Indicators of socioeconomic position (part 2). J Epidemiol Community Health, 60(2), 95–101. doi:10.1136/jech.2004.028092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschling J, Riemann R, Spinath F, & Diewald M (2016). Gene x Socioeconomic Status Interaction on Cognitive Ability: Insights from the German TwinLife Study. Paper presented at the 18th European Conference on Personality, Timisoara, Romania Conference paper retrieved from http://www.twin-life.de/en/2016 [Google Scholar]
- Grant MD, Kremen WS, Jacobson KC, Franz C, Xian H, Eisen S. a., … Lyons MJ (2010). Does parental education have a moderating effect on the genetic and environmental influences of general cognitive ability in early adulthood? Behavior Genetics, 40(4), 438–446. doi:10.1007/s10519-010-9351-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, & Meaney MJ (2010). Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews Neuroscience, 11(9), 651–659. doi:10.1038/nrn2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Berg K, Eaves LJ, Solaas MH, Corey LA, Sundet J, … Nance WE (1985). Education policy and the heritability of educational attainment. Nature, 314(6013), 734–736. doi:doi:10.1038/314734a0 [DOI] [PubMed] [Google Scholar]
- Hollingshead AB (1975). Four Factor Index of Social Status. Department of Sociology. Yale University; New Heaven, CT. [Google Scholar]
- Huisman M, Kunst AE, Andersen O, Bopp M, Borgan JK, Borrell C, … Mackenbach JP (2004). Socioeconomic inequalities in mortality among elderly people in 11 European populations. Journal of Epidemiology and Community Health, 58(6), 468. doi:10.1136/jech.2003.010496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst L, Stafford M, Cooper R, Hardy R, Richards M, & Kuh D (2013). Lifetime Socioeconomic Inequalities in Physical and Cognitive Aging. American Journal of Public Health, 103(9), 1641–1648. doi:10.2105/AJPH.2013.301240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W (2007). Genetic and environmental influences on behavior: Capturing all the interplay. Psychological Review, 114(2), 423–440. doi:10.1037/0033-295X.114.2.423 [DOI] [PubMed] [Google Scholar]
- Johnson W (2010). Extending and testing Tom Bouchard’s Experience Producing Drive Theory. Personality and Individual Differences, 49(4), 296–301. doi:http://dx.doi.org/10.1016/j.paid.2009.11.022 [Google Scholar]
- *Johnson W, Deary I, McGue M, & Christensen K (2009). Genetic and environmental transactions linking cognitive ability, physical fitness, and education in late life. Psychology and Aging, 24(1), 48–62. doi:10.1037/a0013929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Johnson W, & Krueger R (2005). Genetic Effects on Physical Health: Lower at Higher Income Levels. Behavior Genetics, 35(5), 579–590. doi:10.1007/s10519-005-3598-0 [DOI] [PubMed] [Google Scholar]
- Johnson W, McGue M, & Deary IJ (2014). Normative Cognitive Aging In Finkel D & Reynolds CA (Eds.), Behavior Genetics of Cognition Across the Lifespan (Vol. 1, pp. 135–167). New York, NY: Springer. [Google Scholar]
- Kendler KS, & Eaves LJ (1986). Models for the joint effect of genotype and environment on liability to psychiatric illness. American Journal of Psychiatry, 143(3), 279–289. doi:10.1176/ajp.143.3.279 [DOI] [PubMed] [Google Scholar]
- Kremen WS, Jacobson KC, Xian H, Eisen S. a., Waterman B, Toomey R, … Lyons MJ (2005). Heritability of word recognition in middle-aged men varies as a function of parental education. Behavior Genetics, 35(4), 417–433. doi:10.1007/s10519-004-3876-2 [DOI] [PubMed] [Google Scholar]
- Lee T, Henry JD, Trollor JN, & Sachdev PS (2010). Genetic influences on cognitive functions in the elderly: A selective review of twin studies. Brain Research Reviews, 64(1), 1–13. doi:http://dx.doi.org/10.1016/j.brainresrev.2010.02.001 [DOI] [PubMed] [Google Scholar]
- Lee T, Mosing MA, Henry JD, Trollor JN, Lammel A, Ames D, … Sachdev PS (2012). Genetic Influences on Five Measures of Processing Speed and Their Covariation with General Cognitive Ability in the Elderly: The Older Australian Twins Study. Behavior Genetics, 42(1), 96–106. doi:10.1007/s10519-011-9474-1 [DOI] [PubMed] [Google Scholar]
- Lyu J, & Burr JA (2016). Socioeconomic Status Across the Life Course and Cognitive Function Among Older Adults:An Examination of the Latency, Pathways, and Accumulation Hypotheses. Journal of Aging and Health, 28(1), 40–67. doi:10.1177/0898264315585504 [DOI] [PubMed] [Google Scholar]
- Marquié JC, Duarte LR, Bessières P, Dalm C, Gentil C, & Ruidavets JB (2010). Higher mental stimulation at work is associated with improved cognitive functioning in both young and older workers. Ergonomics, 53(11), 1287–1301. doi:10.1080/00140139.2010.519125 [DOI] [PubMed] [Google Scholar]
- McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, & Plomin R (1997). Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science, 276, 1560–1563. doi:10.1126/science.276.5318.1560 [DOI] [PubMed] [Google Scholar]
- McGue M, & Christensen K (2001). The heritability of cognitive functioning in very old adults: Evidence from Danish twins aged 75 years and older. Psychology and Aging, 16(2), 272–280. doi:10.1037/0882-7974.16.2.272 [DOI] [PubMed] [Google Scholar]
- Mortensen EL, Flensborg-Madsen T, Molbo D, Fagerlund B, Christensen U, Lund R, … Avlund K (2014). The Relationship Between Cognitive Ability and Demographic Factors in Late Midlife. Journal of Aging and Health, 26(1), 37–53. doi:10.1177/0898264313508780 [DOI] [PubMed] [Google Scholar]
- Muthén LK and Muthén BO (1998-2017). Mplus User’s Guide. Eighth Edition Los Angeles, CA: Muthén & Muthén [Google Scholar]
- Neale MC, Boker SM, Xie G, & Maes HH (2003). Mx: Statistical modeling . Richmond, VA: Department of Psychiatry; Virginia Institute for Psychiatric and Behavior Genetics, Virginia Commonwealth University. [Google Scholar]
- Oakes JM, & Rossi PH (2003). The measurement of SES in health research: current practice and steps toward a new approach. Social Science & Medicine, 56(4), 769–784. doi:http://doi.org/10.1016/S0277-9536(02)00073-4 [DOI] [PubMed] [Google Scholar]
- Pahlen S, Hamdi NR, Dahl Aslan AK, Horwitz BN, Panizzon MS, Petersen I, … McGue M (2018). Age-moderation of genetic and environmental contributions to cognitive functioning in mid- and late-life for specific cognitive abilities. Intelligence, 68, 70–81. doi:https://doi.org/10.1016/j.intell.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen NL, Christensen K, Dahl AK, Finkel D, Franz CE, Gatz M, … Reynolds CA (2013). IGEMS: The Consortium on Interplay of Genes and Environment Across Multiple Studies. Twin Research and Human Genetics, 16(Special Issue 01), 481–489. doi:10.1017/thg.2012.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen NL, Plomin R, Nesselroade JR, & McClearn GE (1992). A quantitative genetic analysis of cognitive abilities during the second half of the life span. Psychological Science, 3(6), 346–353. [Google Scholar]
- Plomin R, & Bergeman CS (1991). The nature of nurture: Genetic influence on “environmental” measures. Behavioral and Brain Sciences, 14, 373–427. doi:10.1017/S0140525×00070278 [Google Scholar]
- Plomin R, DeFries JC, & Loehlin JC (1977). Genotype-environment interaction and correlation in the analysis of human behavior. Psychological bulletin, 84(2), 309–322. doi:10.1037/0033-2909.84.2.309 [PubMed] [Google Scholar]
- Purcell S (2002). Variance Components Models for Gene–Environment Interaction in Twin Analysis. Twin Research and Human Genetics, 5(06), 554–571. doi:10.1375/136905202762342026 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2014). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- Raftery AE (1995). Bayesian model selection in social research. Sociological methodology, 25, 111–164. doi:10.2307/271063 [Google Scholar]
- Rathouz P, Van Hulle C, Rodgers J, Waldman I, & Lahey B (2008). Specification, Testing, and Interpretation of Gene-by-Measured-Environment Interaction Models in the Presence of Gene–Environment Correlation. Behavior Genetics, 38(3), 301–315. doi:10.1007/s10519-008-9193-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CA, Baker LA, & Pedersen NL (2000). Multivariate models of mixed assortment: Phenotypic assortment and social homogamy for education and fluid ability. Behavior Genetics, 30(6), 455–476. doi:10.1023/A:1010250818089 [DOI] [PubMed] [Google Scholar]
- *Reynolds CA., Finkel D, McArdle JJ, Gatz M, Berg S, & Pedersen NL (2005). Quantitative genetic analysis of latent growth curve models of cognitive abilities in adulthood. Developmental Psychology, 41(1), 3–3. doi:10.1037/0012-1649.41.1.3 [DOI] [PubMed] [Google Scholar]
- Reynolds CA, Finkel D, & Zavala C (2014). Gene by Environment Interplay in Cognitive Aging. In Finkel D & Reynolds CA (Eds.), (Vol. 1, pp. 169–199): Springer; New York. [Google Scholar]
- Rowe DC, Jacobson KC, & Van den Oord EJCG (1999). Genetic and Environmental Influences on Vocabulary IQ: Parental Education Level as Moderator. Child Development, 70(5), 1151–1162. doi:10.1111/1467-8624.00084 [DOI] [PubMed] [Google Scholar]
- Scarr S, & McCartney K (1983). How people make their own environments: A theory of genotype→ environment effects. Child Development, 424–435. doi:https://doi.org/10.1093/geronj/45.4.P156 [DOI] [PubMed] [Google Scholar]
- Scarr-Salapatek S (1971). Race, social class, and IQ. Science, 174(4016), 1285–1295. doi:10.1126/science.174.4016.1285 [DOI] [PubMed] [Google Scholar]
- Schooler C, & Caplan LJ (2008). Those who have, get: Social structure, environmental complexity, intellectual functioning, and self-directed orientations in the elderly. Social structures and aging individuals: Continuing challenges, 20, 131–153. [Google Scholar]
- Schooler C, Mulatu MS, & Oates G (1999). The continuing effects of substantively complex work on the intellectual functioning of older workers. Psychology and Aging, 14(3), 483–506. doi:10.1037/0882-7974.14.3.483 [DOI] [PubMed] [Google Scholar]
- Shanahan MJ, & Hofer SM (2005). Social Context in Gene–Environment Interactions: Retrospect and Prospect. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 60(Special Issue 1), 65–76. doi:10.1093/geronb/60.Special_Issue_1.65 [DOI] [PubMed] [Google Scholar]
- Stone M (1985). Kohs block design test In Keyser DJ & Sweetland RC (Eds.), Test Critiques II. Kansas City: Test Corporation of America. [Google Scholar]
- Szabó C, Kelemen O, & Kéri S (2014). Low-grade inflammation disrupts structural plasticity in the human brain. Neuroscience, 275, 81–88. doi:http://doi.org/10.1016/j.neuroscience.2014.06.002 [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, & McIntyre NJ (1992). The Mini-Mental State Examination: A Comprehensive Review. Journal of the American Geriatrics Society, 40(9), 922–935. doi:10.1111/j.1532-5415.1992.tb01992.x [DOI] [PubMed] [Google Scholar]
- Tucker-Drob EM (2009). Differentiation of cognitive abilities across the life span. Developmental Psychology, 45(4), 1097–1118. doi:10.1037/a0015864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, & Bates TC (2015). Large Cross-National Differences in Gene × Socioeconomic Status Interaction on Intelligence. Psychological Science. doi:10.1177/0956797615612727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Reynolds CA, Finkel D, & Pedersen NL (2014). Shared and unique genetic and environmental influences on aging-related changes in multiple cognitive abilities. Developmental Psychology, 50(1), 152– 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E, Beam CR, Sundet JM, & Tambs K (2017). Interaction between Parental Education and Twin Correlations for Cognitive Ability in a Norwegian Conscript Sample. Behavior Genetics, 47(5), 507–515. doi:10.1007/s10519-017-9857-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E, & Gottesman II (1991). Individual differences and the canalization of human behavior. Developmental Psychology, 27(1), 18–22. doi:10.1037/0012-1649.27.1.18 [Google Scholar]
- Turkheimer E, Harden KP, D’Onofrio B, & Gottesman II (2009). The Scarr–Rowe interaction between measured socioeconomic stautus and the heritability of cognitive ability In McCartney K & Weinberg RA (Eds.), Experience and development: A festschrift in honor of Sandra Wood Scarr (1 ed., pp. 81–97): Psychology Press. [Google Scholar]
- Turkheimer E, & Horn EE (2014). Interactions Between Socioeconomic Status and Components of Variation in Cognitive Ability In Finkel D & Reynolds CA (Eds.), Behavior Genetics of Cognition Across the Lifespan (Vol. 1, pp. 41–68). New York, NY: Springer. [Google Scholar]
- van Buuren S, & Groothuis-Oudshoorn K (2011). mice: Multivariate Imputation by Chained Equations in R. 2011, 45(3), 67. doi:10.18637/jss.v045.i03 [Google Scholar]
- *van der Sluis S, Posthuma D, & Dolan CV (2012). A Note on False Positives and Power in G × E Modelling of Twin Data. Behavior Genetics, 42(1), 170–186. doi:10.1007/s10519-011-9480-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluis S, Willemsen G, de Geus EJC, Boomsma DI, & Posthuma D (2008). Gene-environment interaction in adults’ IQ scores: measures of past and present environment. Behavior Genetics, 38(4), 348–360. doi:10.1007/s10519-008-9212-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hulle C, Lahey B, & Rathouz P (2013). Operating Characteristics of Alternative Statistical Methods for Detecting Gene-by-Measured Environment Interaction in the Presence of Gene–Environment Correlation in Twin and Sibling Studies. Behavior Genetics, 43(1), 71–84. doi:10.1007/s10519-012-9568-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbakel E, & de Graaf PM (2007). Resources of the Partner: Support or Restriction in the Occupational Career? Developments in the Netherlands Between 1940 and 2003. European Sociological Review, 24(1), 81–95. [Google Scholar]
- Verhaeghen P, & Salthouse TA (1997). Meta-analyses of age–cognition relations in adulthood: Estimates of linear and nonlinear age effects and structural models. Psychological bulletin, 122(3), 231–249. doi:http://dx.doi.org/10.1037/0033-2909.122.3.231 [DOI] [PubMed] [Google Scholar]
- Watkins MP, & Meredith W (1981). Spouse similarity in newlyweds with respect to specific cognitive abilities, socioeconomic status, and education. Behavior Genetics, 11(1), 1–21. doi:10.1007/BF01065824 [DOI] [PubMed] [Google Scholar]
- Watson D, Klohnen EC, Casillas A, Nus Simms E, Haig J, & Berry DS (2004). Match Makers and Deal Breakers: Analyses of Assortative Mating in Newlywed Couples. Journal of Personality, 72(5), 1029–1068. doi:10.1111/j.0022-3506.2004.00289.x [DOI] [PubMed] [Google Scholar]
- Wechsler D (1981). WAIS-R manual: Wechsler adult intelligence scale-revised: Psychological Corporation. [Google Scholar]
- Zietsch BP, Verweij KJH, Hrath AC, & Martin NG (2012). Variation in human mate choice: Simultaneously investigating heritability, parental influence, sexual iprinting, and assortative mating. American Naturalist, 177(5), 605–616. doi:10.1086/659629.Variation [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


