Abstract
The screening of several Chinese medicinal herbs for nematocidal properties showed that the ethanol extract of Liriope muscari fibrous roots possessed significant nematocidal activity against the pine wood nematode (Bursaphelenchus xylophilus). From the ethanol extract, a new constituent (1,4-epoxy-cis-eudesm-6-O-β-D-glucopyranoside) and three known glycosides [1β,6α-dihydroxy-cis-eudesm-3-ene-6-O-β-D-glucopyranoside (liriopeoside A), 1β,6β-dihydroxy-cis-eudesm-3-ene-6-O-β-D-glucopyranoside, and 1α,6β-dihydroxy-5,10-bis-epi-eudesm-4(15)-ene-6-O-β-D-glucopyranoside] were isolated by bioassay-guided fractionation. The structures were elucidated by 1D and 2D NMR and MS techniques. 1,4-Epoxy-cis-eudesm-6-O-β-D-glucopyranoside possessed moderate nemato-cidal activity against B. xylophilus with a LC50 value of 339.76 μg/mL, while liriopeoside A (LC50 = 82.84 μg/mL) and 1β,6β-dihydroxy-cis-eudesm-3-ene-6-O-β-D-glucopyranoside (LC50 = 153.39 μg/mL) also exhibited nematocidal activity against B. xylophilus. The crude extract of L. muscari fibrous roots exhibited nematocidal activity against the pine wood nematode with a LC50 value of 182.56 μg/mL.
Keywords: Liriope muscari; Nematocidal activity; Bursaphelenchus xylophilus; 1,4-epoxy-cis-eudesm-6-O-β-D-glucopyranoside
1. Introduction
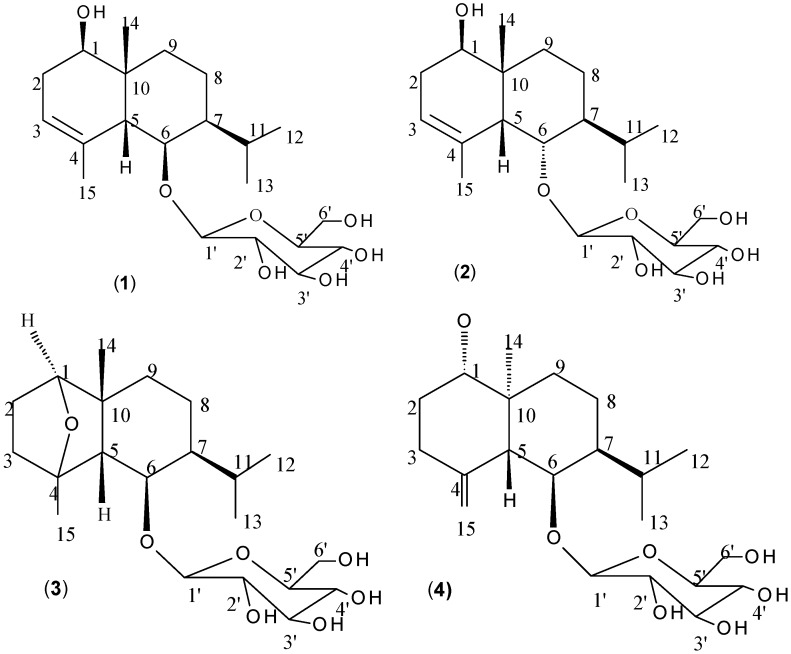
Liriope muscari (Decaisne) L.H. Bailey (Family: Liliaceae), is a species of low, herbaceous flowering plant from East Asia (China, Japan and Korea) occurring in shady forests at elevations of 330 to 4,600 feet. It is a perennial with grass-like evergreen foliage and lilac-purple flowers which produce single seeded berries on a spike in the fall [1]. The roots of this species are used as a substitute for Radix Ophiopogonis (Ophiopogon japonicus) in some areas of China [2,3]. Its roots have been employed in Traditional Chinese Medicine as an expectorant, antitussive, tonic agent and also used to treat various inflammation-related diseases such as pharyngitis, bronchitis, pneumonia, cough in addition to showing pharmacological effects on the cardiovascular system [3,4]. Previous phytochemical investigations revealed that L. muscari mainly contained amides, steroids, steroidal glycosides (saponins), triterpenoids, flavonoids and essential oil [5,6,7,8,9,10,11]. The essential oil of L. muscari had fumigant toxicity against the rice weevil (Sitophilus oryzae) but no strong toxicity was found [12]. However, during our screening program for new agrochemicals from local wild plants and Chinese medicinal herbs, the ethanol extract of L. muscari fibrous roots was found to possess nematocidal activity against the pine wood nematode [Bursaphelenchus xylophilus (Steiner and Buherer) Nickle]. We report here the isolation and characterization of a new eudesmane sesquiterpene glucoside, 1,4-epoxy-cis-eudesm-6-O-β-D-glucopyranoside (3) and three known glycosides (Figure 1) by bioassay-guided fractionation and their nematocidal assessment against B. xylophilus. Liriopeoside A (1, 1β,6β-dihydroxy-cis-eudesm-3-ene-6-O-β-D-glucopyranoside) was first isolated from L. muscari [9] and 1α,6β-dihydroxy-cis-eudesm-3-ene-6-O-β-D-glucopyranoside (2) and 1α,6β-dihydroxy-5,10-bis-epi-eudesm-4(15)-ene-6-O-β-D-glucopyranoside (4) were isolated from Aster scaber [15] and Parepigynum funingense [16], respectively.
Figure 1.
Structures of compounds isolated from Liriope muscari fibrous roots.
2. Results and Discussion
2.1. Chemistry
1,4-Epoxy-cis-eudesm-6-O-β-D-glucopyranoside (3, Figure 1) was obtained as a colorless powder from the ethanol extract of L. muscari fibrous roots. The 1H-NMR spectrum of compound 3 led to the identification of the following representative signals: two methyl singlet protons at d 1.40 and 1.06, two methyl doublet signal protons at δ 0.89 (d, J = 6.0 Hz) and 0.87 (d, J = 6.0 Hz), and an anomeric proton at δ 4.19 (d, J = 7.7 Hz). In the 13C-NMR (including DEPT) spectrum, 21 carbon signals appeared, which included four methyl carbons at δC = 31.2, 22.4, 22.3, and 20.7; five methylene carbons at δC = 61.7, 30.1, 29.4, 25.9 and 20.0; three oxygenated methine carbons at δC = 87.7, 85.5, and 76.2; three methine carbons at δC = 56.7, 43.1, and 26.6 and six signals assignable to the glucose moiety (δC = 102.8, 77.4, 77.2, 74.5, 70.7 and 61.7).
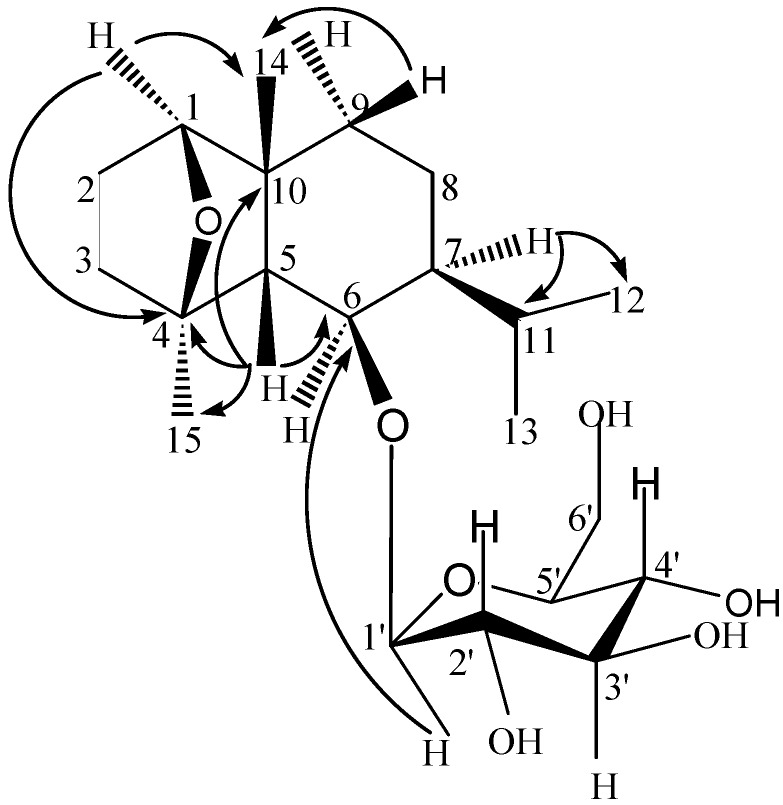
The NMR data of compound 3 were similar to those of 1β,4β,6β-trihydroxy-cis-eudesmane-6-O-β-D-glucopyranoside isolated from O. japonicus [9]. The major difference in compound 3 was the downfield chemical shifts for the carbons at C-l (δC = 85.5) and C-4 (δC = 87.7) in contrast to C-l (δC = 74.8) and C-4 (δC = 71.5) in compound 1β,4β,6β-trihydroxy-cis-eudesmane-6-O-β-D-glucopyranoside, uggesting that there was an epoxy bridge between C-1 and C-4, a fact confirmed by a key HMBC correlation between H-1 (δH = 3.74, t, J = 3.1 Hz) and C-4 (δC = 87.7) (Figure 2). Furthermore, the molecular formula of 1β,4β,6β-trihydroxy-cis-eudesmane-6-O-β-D-glucopyranoside was determined to be C21H38O, whereas the molecular formula of compound 3 was determined to be C21H36O7, suggesting a loss of a water molecule (18 amu).
Figure 2.
Key HMBC correlations of compound 3.
The HSQC and HMBC spectrum indicated that δ 3.74 (H-1) has connectivity with δ 31.2 (C-14), 87.7 (C-4) and 56.7 (C-5); δ 1.66 (H-5) has connectivity with δ 31.2 (C-14), 42.7 (C-10), 20.7 (C-15), 87.7 (C-4) and 76.2 (C-6); δ 1.40 (H-7) has connectivity with δ 26.6 (C-11) and 22.4 (C-12).
The coupling constant (J = 7.7 Hz) of the anomeric proton at δH = 4.19 indicated the D-glucose moiety was in the β-form. The glycosidic position was established by HMBC, with a long-range correlation observed between H-1′ (δH = 4.19, d, J = 7.7 Hz) and C-6 (δC = 76.2).
The relative configuration of compound 3 was deduced by comparing its 13C-NMR spectral data and coupling constants with those of 1β,4β,6β-trihydroxy-cis-eudesmane-6-O-β-D-glucopyranoside. The chemical shifts of C-5, C-6, C-7, C-10 and C-15 in compound 3 and those in 1β,4β,6β-trihydroxy-cis-eudesmane-6-O-β-D-glucopyranoside were similar, indicating that the H-6 and H-7 were α-oriented, the methyl group at C-4 was α-substituted. The coupling constant of H-1 (δH = 4.24, t, J = 3.2 Hz) for compound 3 was quite similar to that of 1β,4β,6β-trihydroxy-cis-eudesmane-6-O-β-D-glucopyranoside (δH = 3.68, t, J = 3.1 Hz), thus the configuration of H-1 was α-oriented. The proposed structure of compound 3 was in accordance with 1H-1H COSY, HSQC, and HMBC spectra. Therefore, the structure of compound 3 was established as 1,4-epoxy-cis-eudesm-6-O-β-D-glucopyranoside.
2.2. Biological Activity
The ethanol extract of L. muscari fibrous roots exhibited toxicity against the pine wood nematode (B. xylophilus) with an LC50 value of 182.56 μg/mL (Table 1).
Table 1.
Nematocidal activity of ethanol extract of Liriope muscari fibrous roots and isolated constituents against B. xylophilus.
| Treatments | Concentrations (μg/mL) | 72 h LC50 (μg/mL) | 95% Fiducial limits | Chi-Square Tests (χ2) |
|---|---|---|---|---|
| Ethanol extract | 50.00–800.00 | 182.56 | 19.79–24.46 | 9.45 |
| 1 | 50.00–800.00 | 82.84 | 76.80–93.49 | 1.95 |
| 2 | 50.00–800.00 | 153.39 | 140.44–169.46 | 5.83 |
| 3 | 50.00–800.00 | 339.76 | 302.99–366.65 | 3.97 |
| 4 | 50.00–800.00 | 465.68 | 435.88–508.33 | 2.28 |
| Carbofuran | 1.50–50.00 | 3.76 | 2.62–4.99 | 3.78 |
1β,6β-Dihydroxy-cis-eudesm-3-ene-6-O-β-D-glucopyranoside showed weak toxicity against B. xylophilus, with an LC50 value of 339.76 μg/mL. However, the other two isolated compounds, 1β,6α-dihydroxy-cis-eudesm-3-ene-6-O-β-D-glucopyranoside and 1,4-epoxy-cis-eudesm-6-O-β-D-gluco-pyranoside exhibited higher nematocidal activity against B. xylophilus, with LC50 values of 82.84 μg/mL and 153.39 μg/mL, respectively. Compared to the positive control, carbofuran (LC50 = 3.76 μg/mL), the most toxic compound was 22 times less activite against the pine wood nematode.
3. Experimental
3.1. General
Melting points were measured on a Buchi 535 apparatus and were uncorrected. High-resolution mass spectra were determined on a Bruker micrOTOF-Q spectrometer, equipped with Apollo II electrospray ionization source with ion funnel, operated in the positive ion mode. 1H- and 13C-NMR spectra were recorded on Bruker Avance DRX 500 instrument using DMSO-d6 as a solvent with TMS as internal standard.
3.2. Plant Material
Fresh fibrous roots of L. muscari were collected from Quanzhou City (24.54° N latitude and 118.37° E longitude), Fujian Province, China in May 2010. The species was identified by Dr. Liu. Q.R. (College of Life Sciences, Beijing Normal University), and the voucher specimens (BNU-CMH-Dushuahan-2010-05-24-012 were deposited at the Herbarium (BNU) of College of Life Sciences, Beijing Normal University. The roots were air-dried and ground to a powder using a grinding mill (Retsch Muhle, Germany).
3.3. Extraction and Isolation of Active Ingredients
The powder (5 kg) was extracted with 80% ethanol (10 L) at room temperature over a period of 2 weeks. The extracts were concentrated to afford a syrup (1.8 Kg), which was dissolved in water (1,800 mL) and chromatographed on a AB-8 resin (Nankai University, Tianjin, China) column (120 mm in diameter and 1.0 m in height) eluting with a gradient of EtOH-H2O (0:100, 10: 90, 50: 50, 90: 10) to give four fractions. The 90% ethanol eluent was concentrated under vacuum to obtain the crude glycosides (51 g) which were dissolved in water (500 mL), and then fractionated with n-BuOH (500 mL × 5) to yield the n-BuOH-soluble extract (45 g) after evaporation of the solvent. The n-BuOH-soluble extract was subjected to silica gel column chromatography (900 g, 160-200 mesh, Qingdao Marine Chemical Plant, Shandong Province, China), using a CHCl3-MeOH solvent system of increasing polarity (from 100% CHCl3, CHCl3:MeOH = 100:2, CHCl3:MeOH = 100:5, … 100% MeOH) to afford 60 fractions. Fractions 17–20 (2.0 g, elution with CHCl3-MeOH = 100:10) were combined according to their TLC similarity (precoated silica gel GF254 plates, Yantai Jiangyou Silica Gel Development Co. Ltd., Shandong, China) and separated by ODS (40 g, Agela Technologies, Tianjin, China) column chromatography to give five subfractions. The subfractions were purified by semi-preparative HPLC [Waters 2695-2996 liquid chromatograph, Agilent ZORBAX Eclipse XDB-C18 columns (250 mm × 4.6 mm and 250 mm × 9.4 mm i.d.)] to yield compound 1 (55 mg) using MeOH-H2O (35:65) as mobile phase at a flow rate of 3 mL/min, compound 2 (60 mg) using MeCN-H2O (22:78) as mobile phase at a flow rate of 3 mL/min, compound 3 (50 mg) using MeOH-H2O (25:75) as mobile phase at a flow rate of 3 mL/min and compound 4 (42 mg) using MeOH-H2O (40:60) as mobile phase at a flow rate of 3 mL/min.
3.5. Compound Characterization
1β,6β-Dihydroxy-cis-eudesm-3-ene-6-O-β-D-glucopyranoside (1). White powder, HR-ESIMS m/z: 423.2356 [M + Na]+. C21H36O7. 1H-NMR (DMSO-d6, 500 MHz) δ ppm: 4.24 (1H, t, J = 3.2 Hz, H-1), 2.26 (1H, d, J = 15 Hz, H-2), 1.87 (1H, m, H-2), 5.19 (1H, br, s, H-3), 2.54 (1H, s, H-5), 4.21 (1H, t, J = 2.6 Hz, H-6), 0.70 (1H, m, H-7), 1.49 (1H, m, H-8), 1.41 (1H, m, H-8), 1.24 (1H, m, H-9), 1.03 (1H, m, H-9), 1.79 (1H, m, H-11), 0.85 (3H, d, J = 6.8 Hz, H-12), 0.91 (3H, d, J = 6.5 Hz, H-13), 1.10 (3H, s, H-14), 1.64 (3H, s, H-15), 4.23 (1H, d, J = 7.7 Hz, H-1′). 13C-NMR (DMSO-d6, 125 MHz) δ ppm: 133.6 (C-4), 120.1 (C-3), 105.3 (C-1’), 77.5 (C-5’), 77.3 (C-6), 77.1 (C-1), 74.9 (C-3’), 73.8 (C-2’), 71.1 (C-4’), 61.9 (C-6’), 46.2 (C-5), 45.4 (C-7), 36.1 (C-10), 32.2 (C-9), 31.7 (C-2), 27.5 (C-11), 25.0 (C-14), 22.4 (C-15), 22.1 (C-13), 21.4 (C-12), 20.2 (C-8). The 1H- and 13C-NMR (DMSO-d6) spectral data are consistent with published data [9].
1α,6β-Dihydroxy-cis-eudesm-3-ene-6-O-β-D-glucopyranoside (2). White powder, HR-ESIMS m/z: 423.1462 [M + Na]+. C21H36O7. 1H-NMR (DMSO-d6, 500 MHz) δ ppm: 3.90 (1H, t, J = 3.2 Hz, H-1), 5.21 (1H, br.s, H-3), 2.01 (1H, m, H-5), 4.38 (1H, br.s, H-6), 1.50 (1H, m, H-7), 1.78 (1H, m, H-8), 1.64 (1H, m, H-8), 1.97 (1H, m, H-9), 1.59 (1H, m, H-9), 1.90 (1H, m, H-11), 4.25 (1H, d, J = 7.0 Hz, H-1’), 1.00 (3H, d, J = 6.0 Hz, H-12), 0.95 (3H, d, J = 6.5 Hz, H-13), 0.86 (3H, s, H-14), 1.79 (3H, s, H-15). 13C-NMR (DMSO-d6, 125 MHz) δ ppm: 135.9 (C-4), 120.4 (C-3), 102.5 (C-1’), 77.6 (C-5’), 78.2 (C-3’), 78.8 (C-1), 71.7 (C-6), 75.4 (C-2’), 71.8 (C-4’), 61.9 (C-6’), 45.1 (C-7), 46.7 (C-5), 37.6 (C-10), 34.8 (C-9), 31.7 (C-2), 30.0 (C-11), 22.2 (C-13), 22.1 (C-12), 12.7 (C-14), 21.2 (C-8), 22.1 (C-15). The 1H- and 13C-NMR (DMSO-d6) spectral data are consistent with published data [15].
1,4-Epoxy-cis-eudesm-6-O-β-D-glucopyranoside (3). White powder, mp 215.0–217.6 °C, HR-ESIMS m/z: 423.3416 [M + Na]+. C21H36O7. 1H-NMR (DMSO-d6, 500MHz) δ ppm: 3.74 (1H, t, J = 3.1 Hz, H-1), 1.51 (1H, m, H-2), 1.36 (1H, m, H-2), 1.85 (1H, m, H-3), 1.09 (1H, m, H-3), 1.66 (1H, d, J = 5.0 Hz, H-5), 3.78 (1H, d, J = 2.0 Hz, H-6), 1.40 (1H, m, H-7), 1.86 (1H, m, H-8), 1.48 (1H, m, H-8), 1.49 (1H, m, H-9), 1.19 (1H, m, H-9), 2.10 (1H, m, H-11), 0.89 (3H, d, J = 6.0 Hz, H-12), 0.87 (3H, d, J = 6.0 Hz, H-13), 1.06 (3H, s, H-14), 1.40 (3H, s, H-15), 4.19 (1H, d, J = 7.7 Hz, H-1’), 3.12 (1H, t, J = 7.7 Hz, H-2’), 3.07 (1H, m, H-3’), 2.92 (1H, t, J = 8.0 Hz, H-4’), 3.45 (1H, t, J = 7.8 Hz, H-5’), 3.68 (1H, dd, J = 12.0, 7.7 Hz, H-6’), 3.05 (1H, m, H-6’). 13C-NMR (DMSO-d6, 125MHz) δ ppm: 102.8 (C-1’), 87.7 (C-4), 85.5 (C-1), 77.4 (C-5’), 77.2 (C-3’), 76.2 (C-6), 74.5 (C-2’), 70.7 (C-4’), 61.7 (C-6’), 56.7 (C-5), 43.1 (C-7), 42.7 (C-10), 31.2 (C-14), 30.1 (C-3), 29.4 (C-9), 26.6 (C-11), 25.9 (C-8), 22.4 (C-12), 22.3 (C-13), 20.7 (C-15), 20.0 (C-2).
1α,6β-Dihydroxy-5,10-bis-epi-eudesm-4(15)-ene-6-O-β-D-glucopyranoside (4). White powder, HR-ESIMS m/z: 423.2210 [M + Na]+. C21H36O7. 1H-NMR (DMSO-d6, 500MHz) δ ppm: 2.25 (1H, br.s, H-11), 1.07 (3H, d, J = 6.4 Hz, H-12), 0.98 (3H, d, J = 6.4 Hz, H-13), 0.99 (3H, s, H-14), 5.64 (1H, br.s, H-15), 4.82 (1H, br.s, H-15), 3.89 (1H, m, H-1), 4.61 (1H, d, J = 7.7 Hz, H-1’). 13C-NMR (DMSO-d6, 125MHz) δ ppm: 146.9 (C-4), 108.3 (C-15), 100.4 (C-1’), 77.3 (C-1), 76.5 (C-5’), 75.6 (C-3’), 74.9 (C-2’), 71.1 (C-6), 70.7 (C-4’), 61.8 (C-6’), 53.8 (C-5), 46.8 (C-7), 40.5 (C-3), 34.4 (C-10), 31.4 (C-9), 29.4 (C-2), 29.2 (C-11), 21.5 (C-14), 21.0 (C-13), 20.2 (C-8),20.0 (C-12).The 1H- and 13C-NMR (DMSO-d6) spectral data are consistent with published data [16].
3.2. Nematocidal Assay
The pine wood nematode (B. xylophilus) was isolated from chips of infested pine wood collected in Taizhou city (28.41° N latitude and 121.27° E longitude), Zhejiang Province, China and extracted by Baermann funnel techniques [13]. The pine wood nematode isolate was rinsed three times with sterile distilled water and reared on a lawn of Botrytis cinerea cultured on potato dextrose agar medium in the dark at 25 °C. Petri-dishes (9 cm in diameter) with fully grown fungus were inoculated with B. xylophilus and left until fungal mycelia were completely consumed (3–5 days). The cultured nematodes (mixed stage) were separated from fungal cultures by centrifuging at 8,500 rpm at 20 °C, rinsed with sterile distilled water and collected. An aqueous suspension of the nematodes (ca. 5,000 nematodes per mL) was prepared by appropriate dilution for use as a working stock.
Range-finding studies were run to determine the appropriate testing concentrations. A serial dilution of the ethanol extract (pure compounds, five concentrations) was prepared. The crude extract/compounds were first dissolved in ethanol and the final concentration of the ethanol solution was 2%. The in vitro tests were performed in wells of 12-well plates. Solution containing test compounds/extract (20 μL) and dilution with about 100 nematodes (20 μL) were added to each well and the final volume of each vial was 1 mL. The plates were incubated in an incubator at 25 °C. Dead and active nematodes were recorded in an interval of 24 h for 72 h using a microscope (×20). The nematodes were considered dead if they gave no response to physical stimuli such as mechanical stirring and pricking with the point of a needle. Six replicates were performed for each treatment. A 2% alcohol in H2O solution was used as a negative control and carbofuran as a positive control. Results from all replicates for the pure compounds and ethanol extract were subjected to probit analysis using the PriProbit Program V1.6.3 to determine LC50 values and their 95% confidence intervals [14].
4. Conclusions
Based on mass screening of medicinal herbs, the ethanol extract of L. muscari fibrous roots was found to possess toxicity against the pine wood nematode (B. xylophilus). A novel and three known eudesmane sesquiterpene glycosides were isolated and identified from the ethanol extract of L. muscari by bioactivity-guided fractionation. The four isolated constituents and the crude extract exhibited nematocidal activity against the pine-wood nematode (B. xylophilus).
Acknowledgments
This project was supported by State Key Laboratory of Earth Surface Processes and Resource Ecology and the Hi-Tech Research and Development of China 2011AA10A202. We thank Liu Q.R. from the College of Life Sciences, Beijing Normal University, Beijing 100875, for the identification of the investigated medicinal herb.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/16/11/9017/s1.
Footnotes
Sample Availability: Samples of the crude extracts and pure compounds are available from the authors.
References and Notes
- 1.Committee of Flora of China. Flora of China. Vol. 15. Science Press; Beijing, China: 1978. pp. 128–130. Number 2. [Google Scholar]
- 2.Jiangsu New Medical College. Encyclopedia of Chinese Medicinal Substances. Shanghai People’s Publisher; Shanghai, China: 1986. p. 2082. [Google Scholar]
- 3.Yu B.Y., Xu G.J., Jin R.L., Xu L.S. Drug resources and identification of commercial drugs on Radix Ophiopogonis. Chung-kuo Yao ko ta Hsueh Hsueh Pao. 1991;22:150–153. (in Chinese with English abstract) [Google Scholar]
- 4.Tian Y.Q., Kou J.P., Li L.Z., Yu B.Y. Anti-inflammatory effects of aqueous extract from Radix Liriope muscari and its major active fraction and component. Chin. J. Nat. Med. 2011;9:222–226. [Google Scholar]
- 5.Yu B.Y., Hirai Y., Shoji J.Z., Xu G.J. Comparative studies on the constituents of ophiopogonis tuber and its congeners. VI. Studies on the constituents of the subterranean part of Liriope spicata var. prolifera and L. muscari. Chem. Pharm. Bull. 1990;38:1931–1935. doi: 10.1248/cpb.38.1931. [DOI] [Google Scholar]
- 6.Yu B.Y., Yin X., Rong Z.Y., Yang T.M., Zhang C.H., Xu G.J. Biological activities of ruscogenin 1-O-[β-D-glucopyranosyl (1→2)] [β-D-xylopyranosyl (1→3)]-β-D-fucopyranoside from tuberous roots of Liriope muscari (Deene.) Bailey. Chung-kuo Yao ko ta Hsueh Hsueh Pao. 1994;25:286–288. [Google Scholar]
- 7.Wu F.H., Cao J.S., Jiang J.Y., Yu B.Y., Xu Q. Ruscogenin glycoside (Lm-3) isolated from Liriope muscari improves liver injury by dysfunctioning liver-infiltrating lymphocytes. J. Pharm. Pharmacol. 2001;53:681–688. doi: 10.1211/0022357011775802. [DOI] [PubMed] [Google Scholar]
- 8.Liu J.L., Chen T., Yu B.Y., Xu Q. Ruscogenin glycoside (Lm-3) isolated from Liriope muscari inhibits lymphocyte adhesion to extracellular matrix. J. Pharm. Pharmacol. 2002;54:959–966. doi: 10.1211/002235702760089081. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Z.H., Wu T., Bligh S.W.A., Bashall A., Yu B.Y. cis-Eudesmane sesquiterpene glycosides from Liriope muscari and Ophiopogon japonicus. J. Nat. Prod. 2004;67:1761–1763. doi: 10.1021/np049864e. [DOI] [PubMed] [Google Scholar]
- 10.Cheng Z.H., Wu T., Yu B.Y., Xu L.S. Studies on Chemical constituents of Liriope muscari. Zhong Cao Yao. 2005;36:823–826. (in Chinese with English abstract) [Google Scholar]
- 11.Cheng Z.H., Wu T., Guo Y.L., Yu B.Y., Xu L.S. Two new steroidal glycosides from Liriope muscari. Chin. Chem. Lett. 2006;17:31–34. [Google Scholar]
- 12.Lee S.E., Lee B.H., Choi W.S., Park B.S., Kim J.G., Campbell B.C. Fumigant toxicity of volatile natural products from Korean spices and medicinal plants towards the rice weevil, Sitophilus oryzae (L.) Pest Manag. Sci. 2001;57:548–553. doi: 10.1002/ps.322. [DOI] [PubMed] [Google Scholar]
- 13.Viglierchio D.R., Schmit R.V. On the methodology of nematode extraction from field samples: Baermann funnel modifications. J. Nematol. 1983;15:438–444. [PMC free article] [PubMed] [Google Scholar]
- 14.Sakuma M. Probit analysis of preference data. Appl. Entomol. Zool. 1998;33:339–347. [Google Scholar]
- 15.Hak C.K., Ock R.C., Kang C.L., Kang R.L. Cerebrosides and terpene glycosides from the root of Aster scaber. Arch. Pharm. Res. 2003;26:132–137. doi: 10.1007/BF02976658. [DOI] [PubMed] [Google Scholar]
- 16.Hua Y., Zhou J., Chen C.X. A new eudesmane sesquiterpene glycoside from Parepigynum funingense. Chin. Chem. Lett. 2007;18:73–75. doi: 10.1016/j.cclet.2006.11.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.