Abstract
A series of 2-(2-diethylamino)-ethoxychalcone and 6-prenyl(or its isomers)-flavanones 10a,b and 11a–g were synthesized and evaluated for their vasorelaxant activities against rat aorta rings pretreated with 1 μM phenylephrine (PE). Several compounds showed potent vasorelaxant activities. Compound 10a (EC50 = 7.6 μM, Emax = 93.1%), the most potent one, would be a promising structural template for development of novel and more efficient vasodilators. Further, 2D-QSAR analysis of compounds 10a,b and 11c-e as well as thirty previously synthesized flavonoids 1-3 and 12-38 using Enhanced Replacement Method-Multiple Linear Regression (ERM-MLR) was further performed based on an optimal set of molecular descriptors (H5m, SIC2, DISPe, Mor03u and L3m), leading to a reliable model with good predictive ability (Rtrain2 = 0.839, Qloo2 = 0.733 and Rtest2 = 0.804). The results provide good insights into the structure- activity relationships of the target compounds.
Keywords: flavonoids, molecular descriptors, vasorelaxant agents, qsar, erm-mlr
1. Introduction
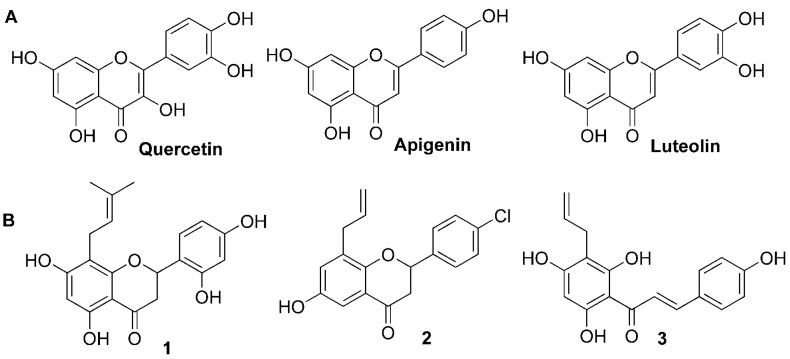
Flavonoids, a class of plant secondary metabolites, are polyphenols based around a phenylbenzopyrone structure [1,2]. According to their different skeletons, they are categorized into flavones, flavanones, chalcones, flavonols, isoflavones and aurones, etc. [3]. Associations of dietary flavonoid intake with lower incidence of cardiovascular disease have been reported in several epidemiological studies [4,5,6,7]. Since one of the main causes of cardiovascular diseases is the involvement of increasing tonicity or loss of relaxation capacity of vascular tissues, vasodilators are a beneficial treatment for cardiovascular diseases. Until now, a large number of flavonoids such as quercetin, luteolin and apegenin (Figure 1A) have been found to show vasorelaxant activities [8,9,10]. In addition, flavonoids can reduce the superoxide levels of vascular endothelium under oxidative stress conditions and improve endothelial cell disfunction, which is also crucial for treatment of cardiovascular diseases [11,12].
Figure 1.
The 2D structure of the flavonoids with potent vasorelaxant activities.
Previously, some quercetin analogues were synthesized by our group and evaluated for their vasorelaxant activities, the results of which indicated that the LogP values of the synthesized flavonoids were correlated with their vasorelaxant activities [13]. In order to further investigate the effect of lipophilic change on vasorelaxant activity, the prenyl (or allyl) group was introduced into various flavonoid scaffolds (e.g., chalcones, flavanones, flavones and aurones). Some of them exhibited potent vasorelaxant activity, such as 8-prenyl (or allyl)-flavanone derivatives 1, 2 and chalcone derivative 3 (Figure 1B; the EC50 values of 1, 2 and 3 are 9.3, 4.6 and 24.0 μM, respectively) [14,15,16]. These results prompted us to discover more potent lipophilic flavonoid derivatives and investigate the comprehensive structure-activity relationship of these compounds.
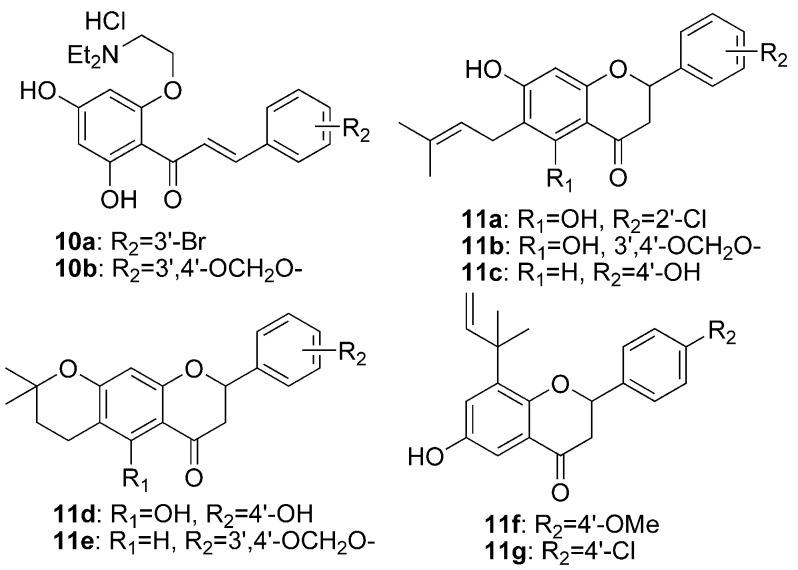
In this study, 6-prenyl(or its isomers)-flavanones and 2-(2-diethylamino)-ethoxychalcone derivatives 10a,b and 11a–g (Figure 2) were prepared, considering the effect of prenyl(or its isomers) in the C-6 position of flavanones as well as the introduction of 2-(diethylamino)ethyl groups in chalcones. The vasorelaxant activities of the synthesized flavonoids were assayed on rat-aorta rings pretreated with 1 μM phenylephrine (PE). Furthermore, Enhanced Replacement Method-Multiple Linear Regression (ERM-MLR) was applied to select the most optimal set of molecular descriptors and set up a linear model to probe the quantitative structure-activity relationships (QSAR) of the target compounds.
Figure 2.
The structures of the target flavonoids synthesized in this study.
2. Results and Discussion
2.1. Chemistry
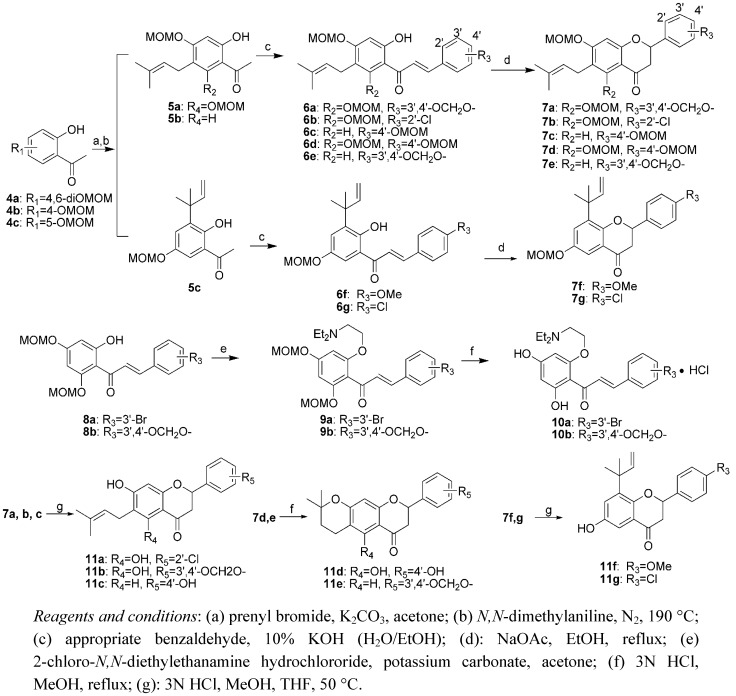
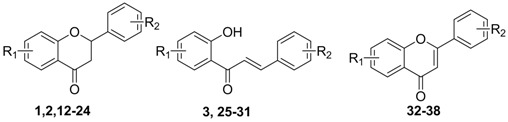
The synthetic pathway to the nine prenylflavonoids 10a,b and 11a–g is outlined in Scheme 1. Acetophenone 4 was allylated with prenyl bromide and successively heated at 220 °C to afford Claisen rearrangement products 5. Condensation of 5 with the corresponding benzaldehydes in aqueous alcoholic alkali at room temperature afforded chalcones 6. Cyclization of 6 in a solution of sodium acetate in ethanol under reflux conditions gave flavanone 7. Compound 9 was obtained by the treatment of chalcone 8 with 2-chloro-N,N-diethylethanamine hydrochloride in the presence of potassium carbonate in acetone. Deprotection of the methoxymethyl groups of 7a-c, 7f, 7g and 9a,b were carried out in 3N HCl/MeOH/THF (2:5:5, v/v) to give the expected products 11a-c, 11f, 11g and 10a,b, while demethoxymethylation of compounds 7d and 7e in 3N HCl/MeOH (1/1, v/v) was observed to afford dihydropyranoflavones 11d and 11e bearing cyclic prenyl groups. The structures of all the synthesized compounds were elucidated by 1H-NMR and ESI-MS.
Scheme 1.
Synthesis of prenylflavonoids 10a,b and 11a–g.
2.2. Vasorelaxant Activity and SAR
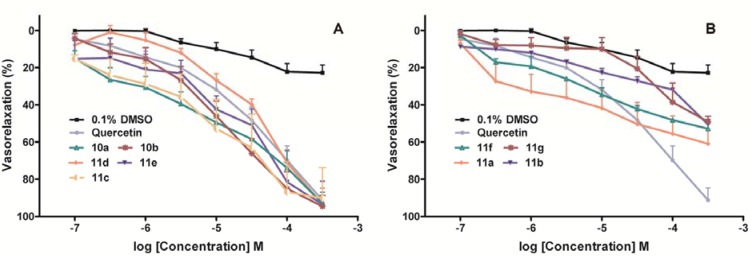
Vasorelaxant activities of compounds 10a,b and 11a-g were evaluated against aortic rings with endothelium pre-contracted with 1 μM phenylephrine. The promoted relaxation of all the compounds was dose-dependent manner, with the maximal effect observed at 300 μM. Several compounds showed more potent vasorelaxation than the positive control quercetin based on the result of either Emax or EC50. As shown in Table 1, flavonoids 10a,b and 11c-e inducing 50% relaxation at small concentration (EC50 < 100 μM) with good efficacy (Emax > 90%) were considered to be good relaxing agents, while the remaining compounds 11a,b,f and 11g were regarded as weak vasodilators (EC50 > 100 μM; Emax < 70%). Concentration–relaxation curves of the flavonoids in two categories are shown in Figure 3. The effects on vasorelaxant activities of prenyl (or its isomer) on C-6 of flavonoids were investigated, showing that the introduction of a cyclic prenyl group resulted in good vasorelaxant activity, as exemplified in dihydropyranoflavones 11d and 11e (11d: EC50 = 78.7 μM, Emax = 93.5%; 11e: EC50 = 53.5 μM, Emax = 93.6%). The introduction of a 6-prenyl or 8-(1,1-dimethyl)allyl group on A ring of flavanone (e.g., 11a,b,f and 11g) led to the weak to moderate activity, except for compound 11c. The 2-(2-diethylamino)ethoxychalcone derivatives 10a,b showed better vasorelaxant activities (EC50 of 10a and 10b were 7.6 and 13.7 μM, respectively), indicating that replacement of prenyl with a 2-(diethylamino)ethyl group on chalcone skeletons retained the potent activity.
Table 1.
The vasorelaxant activities of flavonoids 10a,b and 11a-g, in rat aorta rings pre-contracted with PE.
| Compd. | EC50(μM) | Emax(%) | Compd. | EC50(μM) | Emax(%) |
|---|---|---|---|---|---|
| Quercetin | 244 | 91.3 | 11c | 19.9 | 90.3 |
| 10a | 7.6 | 93.1 | 11d | 78.7 | 93.5 |
| 10b | 13.7 | 99.5 | 11e | 53.5 | 93.6 |
| 11a | N.D. | 66.2 | 11f | N.D. | 52.9 |
| 11b | N.D. | 50.6 | 11g | N.D. | 49.1 |
Figure 3.
Effects of flavonoids on relaxation in aortic rings with endothelium pre-contracted with 1 μM phenylephrine. Flavonoids were added cumulatively to achieve the appropriate concentrations. Results are expressed as means ± standard error of mean in terms of percentage relaxation of the contraction to PE (n = 3 ~ 4). (A) Flavonoids with highly vasorelaxation effect; (B) Flavonoids with weakly vasorelaxation effect.
2.3. Development of QSAR Model Using ERM-MLR
For a quantitative understanding of the structure-vasorelaxant activities relationship of the flavonoids, ERM-MLR was applied to set up a linear QSAR model to explore the EC50 of the newly synthesized five flavonoids 10a,b and 11c-e with efficacious EC50 and the previously reported thirty flavonoids 1-3 and 12-38 [14,15,16,19]. At first, the calculated molecular descriptors that stayed constant for all molecules were eliminated and one of each correlated pair was deleted according to the results of correlation analysis, resulting in a pool of 299 descriptors for further QSAR model development. Then, ERM-MLR was applied to select an optimal set of molecular descriptors and four QSAR models with different number of variables (n = 2~5) were obtained considering that the number of variables selected should be kept lower than 20 percent of the number of coupounds in training set in avoidance of overlay problem. Commonly, the higher values of correlation coefficient (R2) for both training and test set, leave-one-out (loo) cross correlation coefficient (Qloo2) and the lower standard deviation value (SD), the better the results. Thus, five molecular descriptors in ERM-MLR model has an optimal influence on improving correlation (Table 2). The physical-chemical meanings of the molecular descriptors in the best model are listed in Table 3, forming the equation as given in Equation (1):
| −Log(EC50) = 0.442*H5m − 0.465*SIC2 + 0.287*DISPe − 0.518*Mor03u − 0.574*L3m + 4.376 (1) |
Table 2.
The statistical parameters of obtained ERM-MLR models.
| No. | ERM-MLR | n a | |||
|---|---|---|---|---|---|
| Rtrain2 | Qloo2 | Rtest2 | |||
| 1 | 0.603 | 0.483 | 0.366 | n = 2 | |
| 2 | 0.734 | 0.648 | 0.336 | n = 3 | |
| 3 | 0.786 | 0.705 | 0.527 | n = 4 | |
| 4 | 0.839 | 0.733 | 0.804 | n = 5 | |
a The number of molecular descriptors selected in ERM-MLR QSAR models.
Table 3.
The molecular descriptors involved in the ERM-MLR models and their corresponding definition.
| Symbol | Class | Definition |
|---|---|---|
| H5m | GETAWAY descriptors | H autocorrelation of lag 5/weighted by atomic masses |
| SIC2 | information indices | structural information content (neighborhood symmetry of |
| 2-order) information indices | ||
| DISPe | geometrical descriptors | d COMMA2 value/weighted by atomic Sanderson |
| electronegativities | ||
| Mor03u | 3D-MoRSE descriptors | Mor03u 3D-MoRSE - signal 03/unweighted |
| L3m | WHIM descriptors | 3rd component size directional WHIM index/weighted by |
| atomic masses |
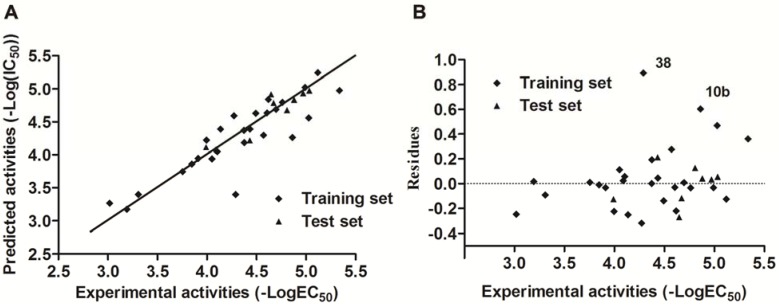
The quality of the fit for the resulting model [Equation (1)] can be judged by the determination coefficient (Rtrain2 = 0.839), the standard deviation (SD = 0.263) and the Fishers estimate of statistical significance value (F = 21.9). Its predictive ability is characterized by leave-one cross validation (Qloo2 = 0.733, SD = 0.303) and test set validation (Rtest2 = 0.804, SD = 0.145). The predictions provided by Equation (1) are shown in Table 4. In Figure 4A the predictions in function of the experimental coefficients are plotted, suggesting that the thirty-five data points (overall data set) follow a straight line trend. In addition, Figure 4B shows the graph between the residuals vs. experimental vasorelaxant activity values. The lower residuals obtained in both the training and test set of compounds in the model indicated that the developed model are valuable and has the capability to establish the relationship between the structure and vasorelaxant activity for flavonoids used in this study. For example, the vasorelaxant activities of the highly potent compound 10a and 11d were accurately predicted using the established QSAR model. The values of H5m, SIC2, DISPe, Mor03u and L3m are optimal for Equation(1), contributing to the fact that the prediction activity is as good as its actual activity (predictive –log(EC50) of 10a: 5.245; actual −log(EC50) of 10a: 5.119), while the unfavorable values of H5m, SIC2, DISPe, Mor03u and L3m led to the lower prediction activity compared with compound 10a but close to its actual activity (predictive –log(EC50) of 11d: 4.048; actual −log(EC50) of 11d: 4.104). However, inaccurate prediction of several compounds was also observed. As exemplified by compound 38, the weak prediction [predictive –log(EC50) of 38: 3.399] may due to the weak activities exhibited by flavone skeleton compounds [actual −log(EC50) of compounds 33~37: <4.088], but compound 38 actually exhibited moderate activity [actual −log(EC50) of 38: 4.290], resulting in the worst fit of 38 (residual: 0.891). In fact, this problem would be fixed in further study, accompanied that more vasorelaxant flavonoids were identified and applied in improving the model.
Table 4.
The experimental and prediction vasorelaxant activities of flavonoids 1-3, 10a,b, 11c-e and 12-38 used in the established QSAR model.
| Cmpd. | R1 | R2 | Exp. activities | Pred. | Res. | Ref. | |
|---|---|---|---|---|---|---|---|
| EC50(μM) | p(EC50) | pEC50(μM) | |||||
| 1 a | 5,7-diOH-8-prenyl | 2',4'-diOH | 9.3 | 5.032 | 4.977 | 0.055 | [16] |
| 2 | 6-OH-8-allyl | 4'-Cl | 4.6 | 5.337 | 4.976 | 0.361 | [14] |
| 3 | 3-allyl-4,6-diOH | 4'-OH | 24.0 | 4.620 | 4.841 | −0.221 | [15] |
| 10a | / | / | 7.6 | 5.119 | 5.245 | −0.126 | / |
| 10b | / | / | 13.7 | 4.863 | 4.260 | 0.603 | / |
| 11c | / | / | 19.9 | 4.762 | 4.796 | −0.034 | / |
| 11d | / | / | 78.7 | 4.104 | 4.048 | 0.056 | / |
| 11e | / | / | 53.5 | 4.272 | 4.592 | −0.32 | / |
| 12 | 5,7-diOH | 3'-Br | 42.4 | 4.373 | 4.372 | 0.001 | [14] |
| 13 | 5,7-diOH | 3',4'-OCH2O- | 20.1 | 4.697 | 4.688 | 0.009 | [14] |
| 14 | 6-OH-8-allyl | 4'-OH | 34.5 | 4.991 | 5.024 | −0.033 | [14] |
| 15a | 5,7-diOH-8-allyl | 3'-OH | 37.0 | 4.432 | 4.219 | 0.213 | [14] |
| 16 | 5,7-diOH-8-allyl | 4'-OH | 32.1 | 4.493 | 4.632 | −0.139 | [14] |
| 17 | 7-OH-8-allyl | 3',4'-OCH2O- | 9.4 | 5.027 | 4.558 | 0.469 | [14] |
| 18 | 7-OH-8-allyl | 4'-OH | 26.9 | 4.570 | 4.295 | 0.275 | [14] |
| 19 | 7-OH-8-allyl | 4'-Cl | 142 | 3.848 | 3.858 | −0.01 | [14] |
| 20a | 5,7-diOH-8-prenyl | 3',4'-OCH2O- | 13.2 | 4.879 | 4.837 | 0.042 | [19] |
| 21 | 5,7-diOH-8-prenyl | 3',4',5'-triMeO- | 24.8 | 4.606 | 4.636 | −0.03 | [19] |
| 22 | 5,7-diOH-8-prenyl | 3'-MeO-4'-OH | 101 | 3.996 | 4.221 | −0.225 | [19] |
| 23a | 5,7-diOH-8-prenyl | 3'-Br | 15.6 | 4.807 | 4.680 | 0.459 | [19] |
| 24 | 5,7-diOH-8-prenyl | 4'-OH | 72.7 | 4.138 | 4.389 | −0.251 | [16] |
| 25a | 4,6-diOH | 3'-Br | 21.3 | 4.672 | 4.789 | −0.117 | [14] |
| 26a | 4,6-diOH | 3',4'-OCH2O- | 22.5 | 4.648 | 4.919 | −0.271 | [14] |
| 27 | 3-allyl-4-OH | 4'-OH | 89.1 | 4.050 | 3.937 | 0.113 | [14] |
| 28 | 3-prenyl-4,6-diOH | 3',4'-OCH2O- | 42.1 | 4.376 | 4.184 | 0.192 | [19] |
| 29a | 3-prenyl-4-OH | 4'-OH | 102 | 3.991 | 4.116 | −0.125 | [15] |
| 30 | 4-OH-5-prenyl | 3'-OH | 123 | 3.910 | 3.943 | −0.033 | [15] |
| 31a | 4-OH-5-prenyl | 2', 4'-diOH | 10.7 | 4.971 | 4.937 | 0.034 | [15] |
| 32 | 5,7-diOH | 3'-Br | 36.8 | 4.434 | 4.389 | 0.045 | [14] |
| 33 | 7-OH | 3',4'-OCH2O- | 81.7 | 4.088 | 4.065 | 0.023 | [14] |
| 34 | 5,7-diOH-8-prenyl | 3'-Br | 959 | 3.018 | 3.266 | −0.248 | [19] |
| 35 | 5,7-diOH-8-prenyl | 3',4',5'-triMeO- | 643 | 3.192 | 3.175 | 0.017 | [19] |
| 36 | 5,7-diOH-8-prenyl | 3'-MeO-4'-OH | 493 | 3.307 | 3.399 | −0.092 | [19] |
| 37 | 5,7-diOH-8-prenyl | 2',4'-diOH | 176 | 3.754 | 3.744 | 0.01 | [16] |
| 38 | 6-OH-8-allyl | 4'-OH | 51.3 | 4.290 | 3.399 | 0.891 | [14] |
a The compounds were used as test set.
Figure 4.
Predicted vs. experimental vasorelaxant activities (−LogEC50) of the QSAR model developed by EMR-MLR.
3. Conclusions
In this study, some synthesized flavonoid derivatives were characterized as agents with remarkable vasorelaxant activities. The preliminary structure-activity relationships studies revealed that cyclic prenyl and 2-(diethylamino)ethyl groups are beneficial for vasorelaxant activity. 2D-QSAR analysis using ERM-MLR was performed to set up a statistically reliable model with good predictive ability (Rtrain2 = 0.839, Qloo2 = 0.733 and Rtest2 = 0.804), and five descriptors (H5m, SIC2, DISPe, Mor03u and L3m) were found to be closely correlated with the observed vasorelaxant activities of the target compounds.
4. Experimental
4.1. General
Melting points were measured on a Büchi B-540 apparatus and are uncorrected. All 1H-NMR spectra were recorded on a 400 MHz spectrometer (Brüker AM). Chemical shifts were expressed as δ values in parts in million (ppm) relative to tetramethylsilane (TMS). Mass spectral data were obtained on an Esquire-LC-00075 spectrometer. Reagents and solvents were purchased from common commercial suppliers and were used without further purification. Compounds 4a-c, 6c, 7c, 8a,b and 11c were prepared according to the procedures reported in previous references [14,17].
4.2. General Method for Synthesis of Prenylated Acetophenones 5
A solution of compound 4, prenyl bromide and potassium carbonate in DMF (100 mL) was heated at 100 °C under a N2 atmosphere for 6 h, and then the reaction mixture was poured into cold water and extracted with ethyl acetate. The organic phase was washed with brine and dried over anhydrous sodium sulfate. After removal of the solvent, the residue was dissolved in N,N-dimethylaniline (50 mL) and then heated at 190 °C for 3~5 h. The mixture was concentrated under vacuum, and the residue was purified by column chromatography on silica gel using (30/1, v/v) as eluent to give compounds 5.
2-Hydroxy-5-(3,3-dimethyl)allyl-4,6-dimethoxymethoxyacetophenone (5a): Reagents: compound 4a (10.0 g, 39.1 mmol), prenyl bromide (8.73 g, 58.6 mmol) and potassium carbonate (10.8 g, 78.2 mmol). Product: yellow oil (4.93 g, 39%); 1H-NMR (CDCl3, δ): 1.68 (s, 3H), 1.76 (s, 3H), 2.69 (s, 3H), 3.30 (d, 1H, J = 6.4 Hz), 3.45 (s, 3H), 3.51 (s, 3H), 4.95 (s, 2H), 5.14 (m, 1H), 5.21 (s, 2H), 6.46 (s, 1H), 12.93 (s, 1H, OH). ESI-MS: m/z [M-H]− 323.
2-Hydroxy-5-(3,3-dimethyl)allyl-4-dimethoxymethoxyacetophenone (5b): Reagents: compound 4b (10.0 g, 51.0 mmol), prenyl bromide (11.4 g, 76.5 mmol) and potassium carbonate (14.1 g, 102.0 mmol). Product: yellow oil (7.4 g, 55%). 1H-NMR (CDCl3, δ): 1.72 (s, 3H), 1.75 (s,3H), 2.55 (s, 3H), 3.25 (d, 2H, J = 7.2Hz), 3.47 (s, 3H), 5.23 (s, 2H), 5.26 (m, 1H), 6.60(s,1H), 7.43(s,1H), 12.54(s,1H). ESI-MS: m/z [M-H]− 263.
2-Hydroxy-3-(1,1-dimethyl)allyl-5-dimethoxymethoxyacetophenone (5c): Reagents: compound 4c (10.0 g, 51.0 mmol), prenyl bromide (11.4 g, 76.5 mmol) and potassium carbonate (14.1 g, 102.0 mmol). Product: yellow oil (6.19 g, 46%). 1H-NMR (CDCl3, δ): 1.44 (s, 3H), 1.47 (s, 3H), 2.55 (s, 3H), 3.48 (s, 3H), 4.85 (d, 1H, J = 17.2 Hz), 4.93 (d, 1H, J = 10.8 Hz), 5.24 (s, 2H), 6.05 (dd, 1H, J = 10.8, 17.2 Hz), 7.06 (d, 1H, J = 2.0 Hz), 7.33 (d, 1H, J = 2.0 Hz), 12.87 (s, 1H, OH). ESI-MS: m/z [M-H]− 263.
4.3. General Method for Synthesis of Prenylated Chalcones 6
To a cold solution of the acetophenone 5 and appropriate benzaldehyde in H2O-EtOH (1/4, v/v, 3 mL), 20% KOH in H2O-EtOH (1/4, v/v, 3 mL) was added with stirring. The resulting mixture was stirred under nitrogen at room temperature for 36 h, and then poured into ice-water (50 mL). The solution was acidified to pH ~ 2 with 1 N HCl, and extracted with ethyl acetate (20 mL × 3 times). The organic phase was washed with brine, dried over anhydrous sodium sulfate, and concentrated in vacuo. The residue was purified by column chromatography on silica gel using petroleum ether-ethyl acetate as eluant to give the desired compound 6.
2-Hydoxy-2'-chloro-4,6-dimethoxymethoxy-5-(3,3-dimethyl)allylchalcone (6a): Reagents: compound 5a (500.2 mg, 1.54 mmol), 2-chlorobenzaldehyde (227.8 mg, 1.62 mmol). Eluent: petroleum ether- ethyl acetate (20:1, v/v). Product: yellow oil (468.7 mg, 68%). 1H-NMR (CDCl3, δ): 1.68 (s, 3H,), 1.78 (s, 3H), 3.32 (d, 2H, J = 6.8 Hz), 3.43 (s, 3H), 3.49 (s, 3H), 5.21 (m, 1H), 5.25 (s, 2H), 5.26 (s, 2H), 6.39 (s, 1H,), 7.30 (m, 2H), 7.43 (m, 1H), 7.67 (m, 1H), 7.88 (d, 1H, J = 16.0 Hz), 8.12 (d, 1H, J = 16.0 Hz), 13.86 (s, 1H, OH). ESI-MS: m/z [M+H]+ 447.
2-Hydoxy-3',4'-dioxymethylene-4,6-dimethoxymethoxy-5-(3,3-dimethyl)allylchalcone (6b): Reagents: compound 5a (500.0 mg, 1.54 mmol), 3,4-dioxymethylenebenzaldehyde (243.1 mg, 1.62 mmol). Eluent: petroleum ether-ethyl acetate (18:1, v/v). Product: yellow oil (443.3 mg, 63%). 1H-NMR (CDCl3, δ): 1.70 (s, 3H,), 1.74 (s, 3H), 3.31 (d, 2H, J = 6.8 Hz), 3.50 (s, 3H), 3.54 (s, 3H), 5.21 (m, 1H), 5.24 (s, 2H), 5.28 (s, 2H), 6.01 (s, 2H), 6.43 (s, 1H), 6.83 (d, 1H, J = 7.6 Hz), 7.06 (dd, 1H, J = 7.6, 2.0 Hz), 7.08 (d, 1H, J = 2.0 Hz), 7.71 (d, 1H, J = 16.0 Hz), 7.78 (d, 1H, J = 16.0 Hz), 13.90 (s, 1H). ESI-MS: m/z [M+H]+ 457.
2-Hydoxy-4,4',6-trimethoxymethoxy-5-(3,3-dimethyl)allylchalcone (6d): Reagents: compound 5a (500.2 mg, 1.54 mmol), 3,4-dimethoxymethoxybenzaldehyde (269.1 mg, 1.62 mmol). Eluent: petroleum ether-ethyl acetate (18:1, v/v). Product: yellow oil (437.2 mg, 60%) . 1H-NMR (CDCl3, δ): 1.68 (s, 3H), 1.79 (s, 3H), 3.32 (d, 2H, J = 6.8 Hz), 3.47 (s, 3H), 3.52 (s, 3H), 3.53 (s, 3H), 5.21 (m, 1H), 5.22 (s, 2H), 5.23 (s, 2H), 5.25 (s, 2H), 6.42 (s, 1H,), 7.06 (d, 2H, J = 8.8 Hz), 7.55 (d, 2H, J = 8.8 Hz), 7.76 (d, 1H, J = 15.6 Hz), 7.85 (d, 1H, J = 15.6 Hz), 13.82 (s, 1H). ESI-MS: m/z [M+H]+ 473.
2-Hydoxy-3',4'-dioxymethylene-4-methoxymethoxy-5-(3,3-dimethyl)allylchalcone (6e): Reagents: compound 5b (500.3 mg, 1.90 mmol), 3,4-dimethoxymethoxybenzaldehyde (298.5 mg, 1.99 mmol). Eluent: petroleum ether-ethyl acetate (20:1, v/v). Product: yellow oil (540.3 mg, 72%). 1H-NMR (CDCl3, δ): 1.77 (s, 3H,), 1.78 (s, 3H), 3.31 (d, 2H, J = 6.8 Hz), 3.50 (s, 3H), 5.26 (s, 2H), 5.30 (m, 1H), 6.06 (s, 2H), 6.66 (s, 1H), 6.88 (d, 1H, J = 8.4 Hz), 7.15 (d, 1H, J = 8.4 Hz), 7.18 (d, 1H, J = 1.2 Hz), 7.40 (d, 1H, J = 16.0 Hz), 7.62 (s, 1H), 7.81 (d, 1H, J = 16.0 Hz), 13.30 (s, 1H, OH). ESI-MS: m/z [M+H]+ 397.
2-Hydoxy-3-(1,1-methyl)allyl-4'-methoxy-5-methoxymethoxychalcone (6f): Reagents: compound 5c (500.1 mg, 1.89 mmol), 4-methoxybenzaldehyde (270.5 mg, 1.99 mmol). Eluent: petroleum ether- ethyl acetate (25:1, v/v). Product: yellow oil (180.9 mg, 25%). 1H-NMR (CDCl3, δ): 1.52 (s, 3H), 1.57 (s, 3H), 3.52 (s, 3H), 3.86 (s, 3H), 5.01 (d, 1H, J = 18.0 Hz), 5.02 (d, 1H, J = 9.6 Hz), 5.15 (s, 2H), 6.26 (dd, J = 18.0, 9.6 Hz), 6.94 (d, 2H, J = 8.4 Hz), 7.25 (d, 1H, J = 2.0 Hz), 7.46 (d, 1H, J = 2.0 Hz), 7.47 (d, 1H, J = 15.6 Hz), 7.61 (d, 2H, J = 8.4 Hz), 7.88 (d, 1H, J = 15.6 Hz), 13.40 (s, 1H, OH). ESI-MS: m/z [M+H]+ 383.
2-Hydoxy-3-(1,1-methyl)allyl-4'-chloro-5-methoxymethoxychalcone (6g): Reagents: compound 5c (500 mg, 1.89 mmol), 4-chlorobenzaldehyde (279.4 mg, 1.99 mmol). Eluent: petroleum ether-ethyl acetate (25:1, v/v). Product: yellow oil (219.6 mg, 30%). 1H-NMR (CDCl3, δ): 1.52 (s, 3H), 1.55 (s, 3H), 3.51 (s, 3H), 5.01 (d, 1H, J = 17.2 Hz), 5.03 (d, 1H, J = 9.2 Hz), 5.15 (s, 2H), 6.26 (dd, J = 17.2, 9.2 Hz), 7.28 (d, 1H, J = 2.0 Hz), 7.40 (d, 2H, J = 8.4 Hz), 7.45 (d, 1H, J = 2.0 Hz), 7.55 (d, 1H, J = 15.6 Hz), 7.58 (d, 2H, J = 8.4 Hz), 7.83 (d, 1H, J = 15.6 Hz), 13.6 (s, 1H, OH). ESI-MS: m/z [M+H]+ 387.
4.4. General Method for Synthesis of Prenylated Flavanones 7
A solution of 6 and sodium acetate (500 mg, 6.10 mmol) in ethanol (5 mL) containing 3 drops of water was refluxed for 24 h. The mixture was poured into cold water (30 mL) and extracted with ethyl acetate (10 mL × 3 times). The organic phase was washed with brine, dried over sodium sulfate. After removing the solvent, the residue was purified by column chromatography on silica gel using petroleum ether-ethyl acetate as eluent to give 7.
2'-Chloro-5,7-dimethoxymethoxy-6-(3,3-dimethyl)allylflavanone (7a): Reagents: compound 6a (300 mg, 0.67 mmol); Eluent: petroleum ether-ethyl acetate (15:1, v/v). Product: pale yellow syrup (162 mg, 54%). 1H-NMR (CDCl3, δ): 1.66 (s, 3H), 1.69 (s, 3H), 2.75 (dd, 1H, J = 16.4 Hz, 2.8 Hz), 2.93 (dd, 1H, J = 13.4, 16.4 Hz), 3.36 (d, 2H, J = 6.8 Hz), 3.48 (s, 3H), 3.52 (s, 3H), 5.17 (m, 1H), 5.20 (s, 2H), 5.23 (s, 2H), 5.68 (dd, 1H, J = 2.8, 13.4 Hz), 6.65 (s, 1H), 7.30 (td, 1H, J = 2.0, 7.6 Hz), 7.36 (td, 1H, J = 2.0, 7.6 Hz), 7.39 (dd, 1H, J =2.0, 7.6 Hz), 7.20 ( dd, 1H, J =2.0, 7.6 Hz). ESI-MS: m/z [M+H]+ 447.
3',4'-Dioxymethylene-5,7-dimethoxymethoxy-6-(3,3-dimethyl)allylflavanone (7b): Reagents: compound 6b (300.2 mg, 0.66 mmol). Eluent: petroleum ether-ethyl acetate (15:1, v/v). Product: pale yellow syrup (204.1 mg, 68%). 1H-NMR (CDCl3, δ): 1.71 (s, 3H), 1.73 (s, 3H), 2.76 (dd, 1H, J = 16.8 Hz, 2.8 Hz), 2.98 (dd, 1H, J = 12.8, 16.4 Hz), 3.27 (d, 2H, J = 7.2 Hz), 3.42 (s, 3H), 3.47 (s, 3H), 5.22 (s, 2H), 5.26 (m, 1H), 5.27 (s, 2H), 5.34 (dd, J = 2.8, 12.8Hz), 5.99 (s, 2H), 6.69 (s, 1H), 6.83 (d, 1H, J = 8.0 Hz), 6.90 (dd, 1H, J = 2.0, 8.0), 6.98 (d, 1H, J = 2.0 Hz), 7.69 (s, 1H). ESI-MS: m/z [M+H]+ 457.
4',5,7-Trimethoxymethoxy-6-(3,3-dimethyl)allylflavanone (7d): Reagent: compound 6d (300.5 mg, 0.64 mmol). Eluent: petroleum ether-ethyl acetate (15:1, v/v). Product: pale yellow syrup (156.3 mg, 52%). 1H-NMR (CDCl3, δ): 1.68 (s, 3H), 1.70 (s, 3H), 2.82 (dd, 1H, J = 16.4 Hz, 2.8 Hz), 2.98 (dd, 1H, J =13.4, 16.4 Hz), 3.33 (d, 2H, J = 7.2 Hz), 3.48 (s, 3H), 3.50 (s, 3H), 3.54 (s, 3H), 5.20 (m, 1H), 5.22 (s, 2H), 5.23 (s, 2H), 5.26 (s, 2H), 5.37 (dd, 1H, J = 2.8 Hz, 13.4 Hz), 6.68 (s, 1H), 7.10 (d, 2H, J = 8.0 Hz), 7.39 (d, 2H, J = 8.0 Hz). ESI-MS: m/z [M+H]+ 473.
3',4'-Dioxymethylene-5-methoxymethoxy-6-(3,3-dimethyl)allylflavanone (7e): Reagents: compound 6e (300.2 mg, 0.76 mmol). Elent: petroleum ether-ethyl acetate (12:1, v/v). Product: pale yellow syrup (180.1 mg, 60%). 1H-NMR (CDCl3, δ): 1.71 (s, 3H), 1.73 (s, 3H), 2.76 (dd, 1H, J = 16.8 Hz, 2.8 Hz), 2.98 (dd, 1H, J =12.8, 16.4 Hz), 3.27 (d, 2H, J = 7.2 Hz), 3.47 (s, 3H), 5.22 (s, 2H), 5.26 (m, 1H), 5.34 (dd, J = 2.8, 12.8Hz), 5.99 (s, 2H), 6.69 (s, 1H), 6.83 (d, 1H, J = 8.0 Hz), 6.90 (dd, 1H, J = 2.0, 8.0), 6.98 (d, 1H, J = 2.0 Hz), 7.69 (s, 1H). ESI-MS: m/z [M+H]+ 397.
4'-Methoxy-6-methoxymethoxy-8-(1,1-dimethyl)allyl-flavanone (7f): Reagent: compound 6f (300.0 mg, 0.79 mmol). Elu-nt: petroleum ether-ethyl acetate (15:1, v/v). Product: pale yellow syrup (150 mg, 50%). 1H-NMR (CDCl3, δ): 1.41 (s, 3H), 1.45 (s, 3H), 2.85 (dd, J = 2.0, 16.8 Hz, 1H), 3.02 (dd, J = 13.2, 16.8 Hz, 1H), 3.48 (s, 3H), 3.92 (s, 3H), 4.94 (d, 1H, J = 17.2 Hz), 4.95 (d, 1H, J = 10.8 Hz), 5.21 (s, 2H), 5.24 (dd, J = 2.0, 13.2 Hz), 6.09 (dd, 1H, J = 10.8, 17.2 Hz), 7.01 (d, 2H, J = 8.4 Hz), 7.12 (d, 1H, J = 2.4 Hz), 7.38 (d, 1H, J = 2.4 Hz), 7.40 (d, 2H, J = 8.4 Hz). ESI-MS: m/z [M+H]+ 383.
4'-Chloro-6-methoxymethoxy-8-(1,1-dimethyl)allyl-flavanone (7g): Reagents: compound 6g (300.3 mg, 0.78 mmol). Eluent: petroleum ether-ethyl acetate (15:1, v/v). Product: pale yellow syrup (129.1 mg, 43%). 1H-NMR (CDCl3 δ): 1.42 (s, 3H), 1.48 (s, 3H), 2.83 (dd, 1H, J = 2.0, 16.8 Hz), 3.95 (dd, 1H, J = 13.2, 16.8 Hz), 3.44 (s, 3H), 4.90 (d, 1H, J = 17.2 Hz), 4.98 (d, 1H, J = 10.8 Hz), 5.25 (s, 2H), 5.32 (dd, 1H, J = 2.0, 13.2 Hz), 6.03 (dd, 1H, J = 10.8, 17.2 Hz), 7.13 (d, 1H, J = 2.4 Hz), 7.39 (d, 1H, J = 2.4 Hz), 7.44 (brd, 4H). ESI-MS: m/z [M+H]+ 387.
4.5. General Method for Synthesis of Compounds 10
A solution of compound 8, 2-chloro-diethylethanamine hydrochloride and potassium carbonate in acetone (5 mL) was refluxed for 10 h. After cooling to room temperature, the mixture was filtered and the solution was concentrated in vacuo. The residue was purified by column chromatography on silica gel using petroleum ether-ethyl acetate as eluant (3:1, v/v) to give 9, which was further dissolved in 5 mL 3N HCl/methanol (1/4, v/v) and refluxed for 1 h. Then, the reaction mixture was evaporated in vacuo to give yellow solid and washed with ether to afford 10.
2-(2-(Diethylamino)ethoxy-3'-bromo-4,6-dimethoxymethoxychalcone (10a): Reagents: compound 8a (200 mg, 0.47 mmol), 2-chloro-N,N-diethylethanamine hydrochlororide (122 mg, 0.71 mmol) and potassium carbonate (194.6 mg, 1.41 mmol). Product: yellow solid (167.3 mg, 72%), m.p. 180–182 °C. 1H-NMR (MeOH-d4, δ): 1.08 (d, 1H, J = 6.8 Hz), 3.10 (m, 4H), 3.48 (m, 2H), 4.38 (brs, 2H), 6.03 (s, 1H), 6.07 (s, 1H), 7.40 (d, 1H, J = 8.0 Hz), 7.51 (d, 1H, J = 16.0 Hz), 7.63 (d, 1H, J = 8.0 Hz ), 7.65 (d, 1H, J = 16.0 Hz), 7.78 (d, 1H, J = 8.0 Hz), 8.01 (s, 1H), 10.06 (s, 1H, OH), 10.37 (s, 1H, OH), 12.48 (s, 1H, OH). ESI-MS: m/z [M+H]+ 434.
2-(2-(Diethylamino)ethoxy-3',4'-dioxymethylene-4,6-dimethoxymethoxy-chalcone (10b): Reagents: compound 8b (201 mg, 0.52 mmol), 2-chloro-N,N-diethylethanamine hydrochlororide (133.7 mg, 0.78 mmol) and potassium carbonate (215.3 mg, 1.56 mmol). Product: yellow solid (161.8 mg, 78%), m.p. 227–230 °C. 1H-NMR (MeOH-d4, δ): 1.10 (d, 1H, J = 6.8 Hz), 3.10 (m, 4H), 3.49 (m, 2H), 4.39 (brs, 2H), 6.02 (s, 1H), 6.06 (s, 1H), 6.10 (s, 2H), 6.97 (d, 1H, J = 8.0 Hz), 7.25 (d, 1H, J = 8.0 Hz), 7.46 (s, 1H), 7.45 (d, 1H, J = 16.0 Hz), 7.51 (d, 1H, J = 16.0 Hz), 10.07 (s, 1H, OH), 10.55 (s, 1H, OH), 12.55 (s, 1H, OH). ESI-MS: m/z [M+H]+ 400.
4.6. General Method for Synthesis of Compounds 11
A solution of 7 in 3N HCl/methanol (1/4, v/v, 3 mL) was refluxed for 2 h, then poured into cold water (15 mL) and extracted with ethyl acetate (5 mL × 3 times). The organic phase was washed with brine and then dried over anhydrous sodium sulfate. After removal of the solvent, the residue was purified by column chromatography on silica gel using petroleum ether-ethyl acetate as eluant to give 11.
2'-Chloro-5,7-dihydroxy-6-(3,3-dimethyallyl)flavanone (11a): Reagents: compound 11a (150 mg, 0.34 mmol). Eluent: petroleum ether-ethyl acetate (6:1, v/v). Product: white solid (90.3 mg, 75%), m.p. 130–133 °C. 1H-NMR (acetone-d6, δ): 1.59 (s, 3H), 1.69 (s, 3H), 2.78 (dd, 1H, J = 2.8, 17.2 Hz), 3.07 (dd, 1H, J = 12.8, 17.2 Hz), 3.12 (d, 2H, J = 6.8 Hz), 5.17 (m, 1H), 5.79 (dd, 1H, J = 2.8, 12.8 Hz), 5.99 (s, 1H), 7.39–7.48 (m, 3H), 7.77 (dd, 1H, J =1.6, 7.6 Hz), 9.66 (s, 1H), 12.35 (s, 1H). ESI-MS: m/z [M+H]+ 359.
3',4'-Dioxymethylene-5,7-dihydroxy-6-(3,3-dimethyl)allylflavanone (11b): Reagents: compound 11b (150 mg, 0.33 mmol). Eluent: petroleum ether-ethyl acetate (6:1, v/v). Product: white solid (79.9 mg, 68%), m.p. 156–158 °C. 1H-NMR (acetone-d6, δ): 1.59 (s, 3H), 1.68 (s, 3H), 2.69 (dd, J =3.2, 16.8 Hz, 1H), 3.10 (dd, 1H, J = 12.8, 16.8 Hz), 3.20 (d, 2H, J = 7.2 Hz), 5.18 (m, 1H), 5.39 (dd, 1H, J = 12.8 Hz, 3.2 Hz), 5.98 (s, 2H), 6.00 (s, 1H), 6.83 (d, 1H, J = 8.0 Hz), 6.97 (d, J = 8.0 Hz), 7.04 (s, 1H), 9.53 (s, 1H), 12.39 (s, 1H). ESI-MS: m/z [M+H]+ 369.
2-(4'-Hydroxyphenyl)-5-hydroxy-6,7-dihydro-8,8-dimethyl-4H,8H-benzo[1,2-b;5,4-b']dipyran-4-one (11d): Reagents: compound 11d (150 mg, 0.32 mmol). Eluent: petroleum ether-ethyl acetate (6:1, v/v). Product: white solid (41.1 mg, 38%), m.p. 122–125 °C. 1H-NMR (DMSO-d6, δ): 1.33 (s, 6H), 1.82 (t, 2H, J = 7.2 Hz), 2.58 (t, 2H, J = 7.2 Hz), 2.73 (dd, 1H, J = 2.8, 17.2 Hz), 3.16 (dd, 1H, J = 12.8, 17.2 Hz), 5.42 (dd, 1H, J = 2.8, 12.8 Hz), 5.84 (s, 1H), 6.89 (d, 1H, J = 8.0 Hz), 7.38 (dd, 1H, J = 8.0 Hz), 8.51 (1H, OH), 12.57 (1H, OH). ESI-MS: m/z [M+H]+ 341.
2-(3,4-Dioxymethylenephenyl)-6,7-dihydro-8,8-dimethyl-4H,8H-benzo[1,2-b;5,4-b']dipyran-4-one (11e): Reagents: compound 11e (150 mg, 0.38 mmol). Eluent: petroleum ether: ethyl acetate (6:1, v/v). Product: white solid (60 mg, 45%), m.p. 95–98 °C .1H-NMR (DMSO-d6, δ): 1.34 (s, 3H), 1.35 (s, 3H), 1.81 (t, 1H, J = 6.8 Hz), 2.76 (t, 1H, J = 6.8 Hz), 2.77 (dd, 1H, J = 2.4, 17.2 Hz), 2.98 (dd, 1H, J = 12.8, 17.2 Hz), 5.32 (dd, 1H, J = 2.4, 12.8 Hz), 5.97 (s, 2H), 6.38 (s, 1H), 6.82 (d, 1H, J = 7.6 Hz), 6.90 (dd, 1H, J = 2.0, 7.6 Hz), 6.97 (d, 1H 1H, J = 2.0 Hz), 7.26 (s, 1H), 7.67 (s, 1H). ESI-MS: m/z [M+H]+ 353.
4'-Methoxy-6-hydroxy-8-(1,1-dimethyl)allyl-flavanone (11f): Reagents: compound 11f (150 mg, 0.39 mmol). Eluent: petroleum ether: ethyl acetate (10:1, v/v). Product: yellow syrup (55.7, 42%). 1H-NMR (CDCl3 δ): 1.43 (s, 3H), 1.44 (s, 3H), 2.82 (dd, J = 2.0, 16.8 Hz, 1H), 3.00 (dd, J = 13.2, 16.8 Hz, 1H), 3.84 (s, 3H), 4.89 (d, 1H, J = 17.2 Hz), 4.95 (d, 1H, J = 10.8 Hz), 4.97 (s, 1H, OH), 5.30 (dd, J = 2.0, 13.2 Hz), 6.11 (dd, 1H, J = 10.8, 17.2 Hz), 6.95 (d, 2H, J = 8.4 Hz), 7.11 (d, 1H, J = 2.4 Hz), 7.27 (d, 1H, J = 2.4 Hz), 7.40 (d, 2H, J = 8.4 Hz). ESI-MS: m/z [M+H]+ 339.
4'-Chloro-6-hydroxy-8-(1,1-dimethyl)allyl-flavanone (11g): Reagents: compound 11g (100 mg, 0.26 mmol). Eluent: petroleum ether-ethyl acetate (10:1, v/v). Product: yellow solid (29.2 mg, 33%), m.p. 90–92 °C. 1H-NMR (CDCl3 δ): 1.42 (s, 3H), 1.45 (s, 3H), 2.83 (dd, 1H, J = 2.0, 16.8 Hz), 3.92 (dd, 1H, J = 13.2, 16.8 Hz), 4.89 (d, 1H, J = 17.2 Hz), 4.95 (d, 1H, J = 10.8 Hz), 5.00 (s, 1H, OH), 5.30 (dd, 1H, J = 2.0, 13.2 Hz), 6.09 (dd, 1H, J = 10.8, 17.2 Hz), 7.13 (d, 1H, J = 2.4 Hz), 7.36 (d, 1H, J = 2.4 H), 7.41 (brd, 4H). ESI-MS: m/z [M+H]+ 343.
4.7. Vasorelaxant Activities Assay
Vascular rings were prepared from the aorta of male Male Sprague-Dawley rats (four to six months old and weighing on average 250 g), and contraction studies were performed following the general procedure detailed in the literature [18]. After an equilibration period of at least 1 h, isometric contractions induced by PE (1 μM) were obtained. When contraction of the tissue in response to this vasoconstrictor agent had stabilized (after about 20 min), cumulatively increasing concentrations of the tested compounds were added to the bath at 15~20 min intervals (the time needed to obtain steady-state relaxation). Control tissues were simultaneously subjected to the same procedures, but omitting the compounds and adding the vehicle. The flavonoids-induced maximal relaxation (Emax) in aortic rings was calculated as a percentage of the contraction in response to PE (1 μM). The half maximum effective concentration (EC50) was defined as the concentration of the flavonoids that induced 50% of maximum relaxation from the contraction elicited by PE (1 μM) and was calculated from the concentration response curve by nonlinear regression (curve fit) using GraphPad Prism (Version 4.0).
4.8. Computational Methods
4.8.1. Data Preparation
For the development of 2D-QSAR model, five newly synthesized compounds and thirty previous compounds [14,15,16,19] with available EC50 were selected. They were divided into training set (26 molecules) and test set (nine molecules) considering both structural diversity and wide coverage of biological activity ranges. The structures of all compounds were optimized in Discovery Studio 2.0 software (Accelrys, Inc. San Diego, CA, USA). The resulted geometry of molecules were inputted into Dragon software [20], which can calculate constitutional descriptors, topological descriptors, walk and path counts, information indices, 2D autocorrelations, edge adjacency indices, Burden eigenvalue descriptors, Burden eigenvalue descriptors, etc. After the calculation of the molecular descriptors, those that stayed constant for all molecules were eliminated, and pairs of variables with a correlation coefficient greater than 0.75 were classified as intercorrelated with one of each correlated pair was deleted.
4.8.2. Enhanced Replacement Method-Multiple Linear Regressions (ERM-MLR)
Enhanced Replacement Method (ERM) proposed by Mercader et al. [21] is a modified version of Replacement Method (RM) [22,23]. The purpose of both algorithms is to choose an optimal subset of d (d < D) descriptors from the pool of D descriptors with minimum standard deviation SD:
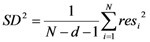 |
where N is the number of molecules in the training set, and resi the residual for molecule i (difference between the experimental and predicted property). Considering that SD(dn) is a distribution on a discrete space of D!/d!(D–d) disordered points dn, ERM produces linear models that are quite similar with the full search (FS) but with much less computational work. First, an initial set of descriptors dk was selected at random. And one of the descriptors, say Xki, with all the remaining descriptors (D-d) was replaced by other descriptor, one by one, and the set with the smallest value of SD was kept. Second, from the resulting set the descriptor with the greatest SD in its coefficient is chosen and all the remaining D-d descriptors, one by one, until the set remains unmodified. More detailed information about these algorithms can be found in reference [21].
4.8.3. Leave-One-Out Cross Validation
Validation of the models was required to test the predictive ability and generalization of the methods by cross validation as well as test set prediction. The leave-one-out cross validation, a special case of the cross-validation technique [24] was employed to find the promising QSAR model. Given n samples available in a data set and m candidate models, each model is trained with n − 1 samples and then is tested on the sample that was left out. This process is repeated n times until every sample in the data set have been used once as a cross-validation instance. Finally, cross validation correlation coefficient (Qloo2) of LOO-CV, a measure of the model generalization capability, for all candidate models can be obtained as below:
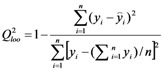 |
where yi is the desired output and 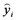 is the actual output of the model, and n is the number of compounds in the analyzed set.
is the actual output of the model, and n is the number of compounds in the analyzed set.
Acknowledgements
The authors are grateful for support from the Medical Scientific Research Foundation of Zhejiang Province (NO. 2010KYA061), and the Administration of Traditional Chinese Medicine of Zhejiang Province (NO. 2009CB033).
Footnotes
Sample Availability: Samples of the compounds 10a,b and 11a-g are available from the authors.
References
- 1.Grassi D., Desideri G., Croce G., Tiberti S., Aggio A., Ferri C. Flavonoids, vascular function and cardiovascular protection. Curr. Pharm. Design. 2009;10:1072–1084. doi: 10.2174/138161209787846982. [DOI] [PubMed] [Google Scholar]
- 2.Harborne J.B., Williams C.A. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/S0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 3.Bravo L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1986;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 4.García-Lafuente A., Guillamón E., Villares A., Rostagno M.A., Martínez J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009;58:537–552. doi: 10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- 5.Tripoli E., Guardia M.L., Giammanco S., Majo D.D., Giammanco M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007;104:466–479. doi: 10.1016/j.foodchem.2006.11.054. [DOI] [Google Scholar]
- 6.Stoclet J.C., Chataigneau T., Ndiaye M., Oak M.H., El Bedoui J., Chataigneau M., Schini-Kerth V.B. Vascular protection by dietary polyphenols. Eur. J. Pharmacol. 2004;500:299–313. doi: 10.1016/j.ejphar.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 7.Kris-Etherton P.M., Keen C.L. Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr. Opin. Lipidol. 2002;13:41–49. doi: 10.1097/00041433-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Lin Y.L., Dai Z.K., Lin R.J., Chu K.S., Chen I.J., Wu J.R., Wu B.N. Baicalin, a flavonoid from Scutellaria baicalensis Georgi, activates large-conductance Ca2+-activated K+ channels via cyclic nucleotide-dependent protein kinases in mesenteric artery. Phytomedicine. 2010;17:760–770. doi: 10.1016/j.phymed.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y.C., Leung S.W.S., Yeung D.K.Y., Hu L.H., Chen G.H., Che C.M., Man Y.K.R. Structure–activity relationships of flavonoids for vascular relaxation in porcine coronary artery. Phytochemistry. 2007;68:1179–1188. doi: 10.1016/j.phytochem.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Benavente-Garcia O., Castillo J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008;56:6185–6205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- 11.Ma X., Li Y., Gao Q., Ye Z., Lu X., Wang H., Jiang H., Bruce I.C., Xia Q. Inhibition of superoxide anion-mediated impairment of endothelium by treatment with luteolin and apigenin in rat mesenteric artery. Life Sci. 2008;83:110–117. doi: 10.1016/j.lfs.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Woodman O.L., Meeker W.F., Boujaoude M. Vasorelaxant and antioxidant activity of flavonols and flavones: Structure-activity relationships. J. Cardiovasc. Pharmacol. 2005;46:302–309. doi: 10.1097/01.fjc.0000175431.62626.07. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z., Hu Y., Wu H., Jiang H. Synthesis and biological evaluation of flavonoids as vasorelaxant agents. Bioorg. Med. Chem. Lett. 2004;14:3949–3952. doi: 10.1016/j.bmcl.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 14.Dong X. Liu, T., Yan J., Wu P., Chen J., Hu Y. Synthesis, biological evaluation and quantitative structure-activities relationship of flavonoids as vasorelaxant agents. Bioorg. Med. Chem. 2009;17:716–726. doi: 10.1016/j.bmc.2008.11.052. [DOI] [PubMed] [Google Scholar]
- 15.Dong X., Chen J., Jiang C., Liu T., Hu Y. Design, synthesis, and biological evaluation of prenylated chalcones as vasorelaxant agents. Arch. Pharm. 2009;342:428–432. doi: 10.1002/ardp.200800229. [DOI] [PubMed] [Google Scholar]
- 16.Dong X., Qi L., Jiang C., Chen J., Wei E., Hu Y. Synthesis, biological evaluation of prenylflavonoids as vasorelaxant and neuroprotective agents. Bioorg. Med. Chem. Lett. 2009;19:3196–3198. doi: 10.1016/j.bmcl.2009.04.120. [DOI] [PubMed] [Google Scholar]
- 17.Dong X., Fan Y., Yu L., Hu Y. Synthesis of four natural prenylflavonoids and their estrogen-like activities. Arch. Pharm. 2007;340:372–376. doi: 10.1002/ardp.200700057. [DOI] [PubMed] [Google Scholar]
- 18.Qian L., Wang H., Qiu W., Huang H., Bruce I.C., Xia Q. Interleukin-2 protects against endothelial dysfunction induced by high glucose levels in rats. Vascul. Pharmacol. 2006;45:374–382. doi: 10.1016/j.vph.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Dong X., Liu Y., Yan J., Jiang C., Chen J., Liu T., Hu Y. Identification of SVM-based classification model, synthesis and evaluation of prenylated flavonoids as vasorelaxant agents. Bioorg. Med. Chem. 2008;16:8151–8160. doi: 10.1016/j.bmc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 20.Talete srl. DRAGON for Windows software for molecular descriptor calculation. [(accessed on 14 September 2011)]. Available online: http://talete.mi.it.
- 21.Mercader A.G., Duchowicz P.R., Fernández F.M., Castro E.A. Modified and enhanced replacement method for the selection of molecular descriptors in QSAR and QSPR theories. Chemom. Intell. Lab. Syst. 2008;92:138–144. doi: 10.1016/j.chemolab.2008.02.005. [DOI] [Google Scholar]
- 22.Duchowicz P.R., Castro E.A., Fernández F.M. Alternative algorithm for the search of an optimal set of descriptors in QSAR - QSPR studies. MATCH Commun. Math. Comput. Chem. 2006;55:179–192. [Google Scholar]
- 23.Duchowicz P.R., Fernández M., Caballero J., Castro E.A., Fernández F.M. QSAR for non-nucleoside inhibitors of HIV-1 reverse transcriptase. Bioorg. Med. Chem. 2006;14:5876–5889. doi: 10.1016/j.bmc.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Gong G. Cross-validation, the jackknife and the bootstrap excess error estimation in forward regression logistic regression. Am. Stat. Assoc. 1986;81:108–113. doi: 10.1080/01621459.1986.10478245. [DOI] [Google Scholar]