Abstract
Modern nutrition is often characterized by the excessive intake of different types of carbohydrates ranging from digestible polysaccharides to refined sugars that collectively mediate noxious effects on human health, a phenomenon that we refer to as ‘‘carbotoxicity.’’ Epidemiological and experimental evidence combined with clinical intervention trials underscore the negative impact of excessive carbohydrate uptake, as well as the beneficial effects of reducing carbs in the diet. We discuss the molecular, cellular, and neuroendocrine mechanisms that link exaggerated carbohydrate intake to disease and accelerated aging as we outline dietary and pharmacologic strategies to combat carbotoxicity.
Qualitative nutritional cues play a major role in determining physical shape and fitness. This applies to micronutrients that may have positive effects on human health (such as the minimally required doses of vitamins and other micronutrients including oligoelements and polyamines) (Madeo et al., 2018) or toxic effects, as demonstrated for trans-unsaturated fatty acids (Hadj Ahmed et al., 2018) and excessive doses of salt (Lanaspa et al., 2018). In addition, the proportion of macronutrients, i.e., carbohydrates, fat, and protein may impact whole-body metabolism, driven by the fact they are not fully interconvertible at the metabolic level. Thus, any kind of caloric intake can yield fatty acids and sterols (that are synthesized from the central metabolic intermediate acetyl-CoA), contrasting with the fact that fatty acids cannot be converted into glucose or other sugars (Pietrocola et al., 2015). The human body is capable of synthesizing most amino acids from carbohydrates or lipids, yet is not able to generate essential amino acids. As a result, variations in the supply of the three macronutrients, carbs, fat, and protein have short-term effects on systemic metabolism as well as longterm effects on body composition.
An excess of lipids (or particular classes of lipids) has been known for long to have toxic effects, coining the expression ‘‘lipotoxicity.’’ Curiously enough, however, the word ‘‘carbotoxicity’’ has not been introduced into the biochemical and medical vocabulary. This may appear surprising in view of the overwhelming evidence that excessive ingestion of different classes of carbohydrates, whether they are monosaccharides (like glucose and fructose), disaccharides (like sucrose), or polysaccharides (such as starch and glycogen), undermines human health and ultimately favors the development of metabolic syndrome, diabetes, obesity, and their multiple co-morbidities. This perspective will be dedicated to the mechanistic exploration of carbotoxicity.
Carbohydrates in Human Nutrition: History and Epidemiology
The (pre)history of humanity is marked by three steps that have led to a steady rise in the daily uptake of carbohydrates in all industrialized and most developing countries of the world.
The first major change in food composition is linked to the switch from the primitive nutrition of hunter-gatherers to agriculture focusing on a range of cereals (in Europe), rice (in Asia), corn (in Mesoamerica), and potatoes (in South America), tied to a rise in the prevalence of caries (Forshaw, 2014). Pre-agricultural nutrition consisting in the consumption of animals (meat, fish, insects, etc.) and a range of plant products (fruits, seeds, tubers, nuts, roots, bulbs, etc.) markedly varied in the average daily carbohydrate consumption depending on the ecoenvironment and hence geographical latitude. Thus, ethnographic analyses led to the estimation that carbs amounted to one third of the total energy over a wide range of latitude intervals (11° –40° north or south of the equator). However, with increasing latitude, carbohydrate intake decreased markedly undercutting 15% for hunter-gatherers living in the tundra and northern coniferous forests (Ströhle and Hahn, 2011). Thus, the range of energy intake from carbohydrates in the diets of most hunter-gatherer societies was much lower than the quantities currently recommended for human nutrition (45%–65% according to the Food and Nutrition Board, Institute of Medicine, National Academies, US) (https://health.gov/dietaryguidelines/2015/guidelines/).
The second major change in carbohydrate uptake is marked by the mass production and consumption of refined sugars. The history of sugar is intimately linked to the labor-intensive cultivation and extraction of sugarcane, first in tropical Southeast Asia, later in the medieval Islamic world and, finally, in the West Indies and tropical parts of the Americas, tied to the history of modern slavery. It is only in the 19th and 20th centuries that sucrose was massively produced in Europe from beets using less labor-intensive methods. Before sugar became a low-cost commodity, overweight and obesity was a privilege of the aristocracy. However, England became the first European country in which obesity became endemic through larger segments of the population, correlating with the massive importation of ever cheaper cane sugar from the 18th century (Johnson et al., 2017). Indeed, the availability of sugar transformed the eating habits of Europeans as they started consuming jams, candy, sweetened tea or coffee, cocoa, processed foods, and other sweet victuals.
The third and most important surge in carbohydrate intake is linked to the rising ingestion of ultra-processed food items manufactured by the food industry after World War II as well as that of sodas, first in the United States and then spreading over the rest of the world. High-fructose corn syrup (HFSC), a North American invention, emerged after 1975 and now is consumed at a rate of 27.5 kg per capita in the United States (Johnson et al., 2007). On a per-weight and per-calorie basis, sugar in its various forms, as well as carbohydrates from cereals or corn, are far less expensive than protein or fats, favoring their incorporation into mass products that maximize profits (Fiolet et al., 2018).
Large analyses of dietary patterns have led to the conclusion that high carbohydrate intake is associated with higher risk of total mortality, whereas total fat and individual types of fat (saturated, monounsaturated, or polyunsaturated) were related to lower overall mortality, as shown for instance in a prospective cohort study enrolling 135,335 individuals all over the world (Dehghan et al., 2017). Thus, at odds with what has been speculated for decades, it appears that digestible carbs are more toxic than lipids. In contrast, the overall ingestion of fiber, which is mostly composed by indigestible carbohydrates, is associated with cardiovascular health as well as with reduced overall mortality (Veronese et al., 2018).
While the total carbohydrate consumption had reached its peak around the year 2000 in Europe and the United States, the amount of added sugar, mostly sucrose and HFSC has been increasing in most countries in the world. The intake of free sugars per person per year amounts to 49 kg in the United States and 35 kg in Europe, an amount that is estimated to multiply cardiovascular mortality by a factor of 2–3 with respect to people who consume <9.1 kg sugar per year, as recommended by the World Health Organization (Yang et al., 2014). The increased intake of added sugar, in particular through sweetened beverages, is associated with higher weight gain with visceral fat depots (Maersk et al., 2012), type 2 diabetes (Siegel et al., 2012), dyslipidemia, non-alcoholic fatty liver disease (NAFLD) (Zelber-Sagi et al., 2007), blood pressure, cardiovascular mortality (Te Morenga et al., 2014), and rheumatoid arthritis (Hu et al., 2014), as well as the progression of age-related macular degeneration (Chiu et al., 2007).
Mechanisms of Carbotoxicity
Several putative toxic metabolites of glucose have been identified. These include dihydroxyacetone phosphate (DHAP) and methylglyoxal that contribute to the generation of advanced glycation end products (AGEs) (Weimer et al., 2014). DHAP formed during glycolysis is one of the two products of break-down of fructose 1,6-bisphosphate, along with glyceraldehyde 3-phosphate. Glycerol-3-phosphate dehydrogenase (GPDH) catalyzes the conversion of glycerol-3-phosphate to DHAP. DHAP is rapidly and reversibly isomerized by triosephosphate isomerase (TPI) to innocuous glyceraldehyde 3-phosphate. Familial TPI deficiency leads to an intractable progressive disease that eventuates in neuromuscular failure, hemolytic anemia, susceptibility to infection, and premature death of patients. Importantly, a Drosophila model of TPI loss-of-function mutation revealed that the disease phenotype might not be linked to a bioenergetic deficit but rather to the accumulation of DHAP (Celotto et al., 2006) or perhaps to its aberrant conversion into methylglyoxal (MG) (Gnerer et al., 2006). Dihydroxyacetone and methylglyoxal react with free amino groups of lysine and arginine and with thiol groups of cysteine contained in proteins, leading to the production of AGEs. Such AGEs have been implicated in the pathogenesis of multiple chronic degenerative processes triggered by aging, obesity, and diabetes (Frimat et al., 2017).
Fructose is naturally contained in fruit, although at comparatively low doses compared to industrial food items, and fruit consumption is epidemiologically associated with reduced obesity (Sharma et al., 2016). Low-dose fructose is cleared by the small intestine to generate glucose and hence does not reach the portal circulation (Jang et al., 2018), while high-dose fructose present in added sugars such as sucrose and corn syrup can trigger the manifestation of all signs of metabolic syndrome in rodents and non-human primates coupled to increased adiposity, and these effects occur independently of excessive energy intake, likely due to reduced physical activity (Johnson et al., 2009; Rendeiro et al., 2015). In combination with HFD, fructose (but not glucose) increases hepatic SREBP1c and fatty acid synthesis genes, resulting in enhanced lipogenesis and reduced liver insulin sensitivity (Softic et al., 2017). Similarly, administration of isocaloric diets containing either glucose or fructose to humans reveals a higher potential for fructose to induce visceral obesity and insulin resistance (Stanhope et al., 2009), increased de novo lipogenesis (resulting in the production of palmitic acid, a major driver of arteriosclerosis), decreased fat oxidation, increased liver fat, postprandial triglycerides, cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL)-C, and C-reactive protein (Jameel et al., 2014). Measurements of hypothalamic regional cerebral blood flow after glucose versus fructose ingestion led to the identification of different central nervous effects of both monosaccharides on the brain of healthy adult volunteers (Page et al., 2013), in line with the observation that specific regions of the mouse brain are capable of fructose metabolism (Oppelt et al., 2017). Functional magnetic resonance imaging of the brain of healthy volunteers revealed that ingestion of fructose increases brain reactivity to food cues in the visual cortex more than glucose, paralleling the observation that fructose stimulated greater hunger and desire for food than glucose (Luo et al., 2015).
How is it possible to explain the relatively high toxicity of fructose compared to glucose (Figure 1)? High-dose fructose enters the portal circulation and then hepatocytes though the trans-porters GLUT2 and GLUT5. Fructose is phosphorylated by fructokinase to fructose-1-phosphate (a reaction that consumes ATP), followed by the metabolism by aldolase B to generate D-glyceraldehyde and DHAP (Lyssiotis and Cantley, 2013), hence generating the same metabolites as does glucose. However, in contrast to glycolysis, the first steps of which are under feedback regulation inhibiting excessive glucose utilization, fructose catabolism is unrestrained allowing for limitless utilization of fructose carbons for gluconeogenesis, lactate production, acetyl-CoA synthesis, and consequent lipogenesis (Lim et al., 2010). Hence, fructose is highly lipogenic, especially if is combined with the uptake of saturated fatty acids (Chiu et al., 2018). In the liver, the activation of fructokinase C results in the generation of fructose-1-phosphate coupled to a transient drop in ATP and phosphate. Fructose-1-phosphate can allosterically activate glucokinase-regulatory protein, thus causing the shuttling of glucokinase from the nucleus to the cytoplasm, ultimately enhancing glucose uptake and glycolysis (Choi et al., 2013). The depletion of phosphate indirectly causes the formation of the proinflammatory metabolite uric acid secondary to the activation of adenosine monophosphate (AMP) deaminase, which converts AMP to inosine monophosphate (IMP), resulting in purine nucleotide turnover. Uric acid can stimulate hepatic lipogenesis via inducing mitochondrial oxidative stress that results in the inhibition of aconitase-2 in the Krebs cycle, causing the accumulation of citrate and the stimulation of ATP citrate lyase and fatty-acid synthase (Lanaspa et al., 2012a). This pathway appears to be clinically important because allopurinol, a xanthine oxidase inhibitor that blocks uric acid generation, reduces fatty liver triggered by fructose (Lanaspa et al., 2012b). The fructokinase C-mediated depletion of ATP and consequent AMPK activation results in a transient block in protein synthesis, induction of oxidative stress, and mitochondrial dysfunction in hepatocytes (Jensen et al., 2018). Fructose may stimulate the activation of sterol regulatory element binding transcription factor 1 (SREBP-1c) and carbohydrate responsive-element binding protein (ChREBP), resulting in lipogenesis and gluconeogenesis, respectively. Fructose also causes an increase in small intestinal permeability due to disruption of the tight junctions, and this phenotype is not found in fructokinase C-deficient mice (Jensen et al., 2018). Of note, antibiotics can prevent hepatosteatosis induced by high-fructose diet, supporting a role for microbial products such as lipopolysaccharide in the portal circulation in this disease process as well (Bergheim et al., 2008). In conclusion, there are multiple mechanisms explaining how excessive fructose may induce metabolic syndrome.
Figure 1. Fructose Metabolism in the Liver and Its Comparison with Glucose Metabolism.
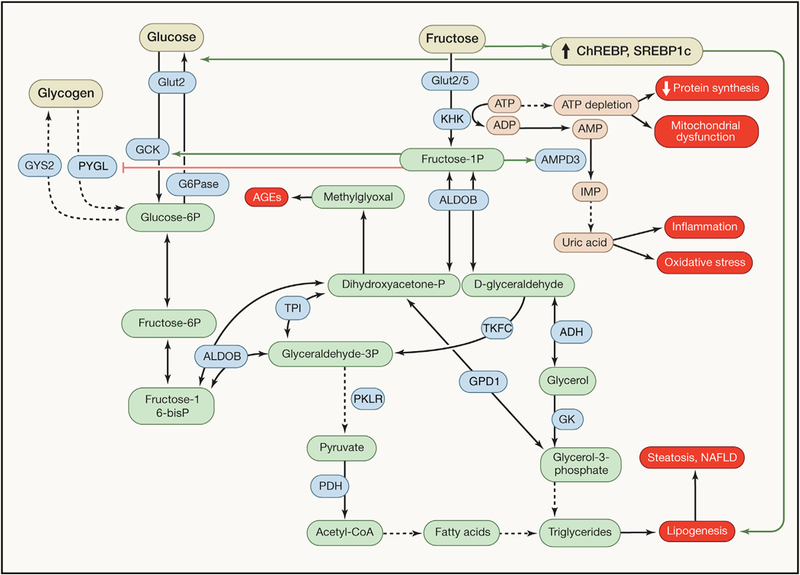
Fructose enters hepatocytes mainly through the transporter GLUT2, although other transporters may also be involved. Then, it is phosphorylated to fructose-1-phosphate by fructokinase in a reaction coupled to ATP and phosphate depletion, which indirectly causes the formation of uric acid. This metabolite contributes to the generation of a transient block in protein synthesis, mitochondrial dysfunction, inflammation, and oxidative stress. Fructose-1-phosphate activates (green lines) or inhibits (red lines) metabolic enzymes and is cleaved to generate D-glyceraldehyde and dihydroxyacetone phosphate (DHAP), which are then used for gluconeogenesis, lactate production, acetyl-CoA synthesis, and lipogenesis. Excessive lipogenesis causes hepatic steatosis and non-alcoholic fatty liver disease (NAFLD). DHAP is also converted to methylglyoxal that contributes to the formation of advanced glycation end products (AGEs). Chronic fructose consumption also induces key transcription factors such as carbohydrate-responsive element-binding protein (ChREBP) and sterol-regulatory element-binding protein 1c (SREBP1c), further resulting in gluconeogenesis and lipogenesis, respectively. Glucose also enters hepatocytes via GLUT2 transport, but—in contrast to the unrestrained fructose catabolism—the first steps in glycolysis are under feedback regulation, inhibiting excessive glucose uptake and utilization, and limiting carbotoxicity (Hannou et al., 2018).
Fructose may also function as an endogenous metabolite that mediates the toxicity of glucose. Aldose reductase converts glucose into sorbitol, which then can be metabolized by sorbitol dehydrogenase to fructose. Importantly, mice that are unable to generate fructose due to the knockout of aldose reductase, or to metabolize fructose due to the knockout of fructokinase (ketohexokinase [KHK]) are resistant to the negative impact of supplementing their drinking water with glucose (10%) with respect to increased energy uptake, body weight, visceral fat, hepatosteatosis, hyperinsulinemia, and hyperleptinemia (Lanaspa et al., 2013). Fructokinase-deficient mice are also protected against diabetic nephropathy (Lanaspa et al., 2014), ischemic acute kidney injury (Andres-Hernando et al., 2017), and high-salt induced renal aging (Roncal-Jimenez et al., 2016). Fructose may favor the development of cardiomyopathy in the context of pressure overload, when activation of hypoxia-inducible factor (HIF) induces the splicing factor SF3B1, thereby causing a switch from fructokinase isoform A to isoform C, the latter being endowed with much higher efficiency for driving fructose uptake and the hypertrophy of cardiomyocytes (Mirtschink et al., 2015). Of note, it appears that high-salt diet activates the aldose reductase/sorbitol dehydrogenase pathway in the hypothalamus and the liver, thus increasing endogenous fructose levels (Lanaspa et al., 2018). Fructokinase deficiency protects the mice against high salt-induced leptin resistance and hyperphagia that cause metabolic syndrome including fatty liver, transaminitis, insulin resistance, elevated blood pressure, and obesity (Lanaspa et al., 2018).
Another potentially toxic monosaccharide is mannose, which is either absorbed following the digestion of mannose-containing polysaccharides and glycoproteins or can be generated as an endogenous metabolite from glucose. Mannose is phosphorylated by liver hexokinase to mannose-6-phosphate, converted by phosphomannose isomerase into fructose-6-phosphate, and then enters the glycolytic pathway or is converted to glucose-6-phosphate for gluconeogenesis. Circulating mannose levels do not undergo major fluctuations due to fasting or post-prandial fluctuations, yet they correlate well with BMI, insulin resistance, and the ability to predict the risk of several chronic diseases including type 2 diabetes, cardiovascular disease, and albuminuria (Lee et al., 2016; Mardinoglu et al., 2017). This increase in mannose levels may be linked to a reduction in the liver expression of the mannose-consuming hexokinase 1 and 2 (Lee et al., 2016). Whether increases in mannose level then perturb protein glycosylation patterns or exert other yet-to-be-discovered toxic effects remains to be explored.
Administration of glucose-containing carbohydrates leads to an increase in postprandial glycemia, thus triggering the release of insulin by pancreatic islet β-cells, favoring glucose uptake by numerous cell types. When this reflex is stimulated by rapidly absorbable sugar (glucose, sucrose, corn syrup), it may ‘‘overshoot,’’ causing a dip in glycemia that stimulates appetite. Beyond these aspects, it is possible that carbohydrate-rich food leads to an additive response, as this has been suggested by neural imaging revealing the downregulation of dopamine 2 receptors in the midbrain of obese individuals (Carter et al., 2016; Volkow et al., 2008). It appears plausible, although remains to be confirmed, that artificial sweeteners have a similar effect either through a Pavlovian conditioning effect or through an action on sweet-taste receptors in the digestive system, thus ultimately causing hyperphagia and weight gain (Pepino, 2015). These findings may contribute to the explanation that ingestion of artificial sweeteners is epidemiologically linked to abdominal obesity (Chia et al., 2016).
Combating Carbotoxicity by Diets
There are several types of diet that curb carbs, for instance, the so-called low-carb diet, typically <20% of caloric intake (i.e., well below official recommendations) and the no-carb diet, which is mostly composed of animal source foods. One particular variant of diets designed to reduce carbotoxicity is based on the avoidance of food items with a high ‘‘glycemic index,’’ which measures the speed at which glycemia ramps up post-ingestion, creating a divide between fast-digesting simple carbohydrates causing a rapid increase in blood glucose and slower-digesting complex carbohydrates, such as whole grains, causing a slower raise in glucose levels (Jenkins et al., 1981). This latter concept is still the basis of nutritional recommendations for the management of diabetes, although low-carb regimens might eventually take over.
Below a certain threshold, a scarcity in carbohydrates, together with limited protein supply, causes ketogenesis, converting dietary fat and body fat into ketone bodies that are used to fuel organs that do not oxidize fatty acids for energy, especially the brain (Figure 2). Ketogenesis is coupled to decreases in plasma insulin and insulin growth factor-1 (IGF1), increased glucagon, hepatic glycogenesis and gluconeogenesis, lipolysis of adipose tissue, a rise in free fatty acids, enhanced β-oxidation, generation of acetyl-CoA, and a consequent increase in circulating ketone bodies (Brown-Borg, 2017). Some organs and parts of the brain still require glucose, which can be produced from protein, namely, from glucogenic amino acids (all natural amino acids except leucine and lysine), and from glycerol obtained by the breakdown of triglycerides. A ketogenic diet is composed of few carbohydrates (<30–40 g/day corresponding to ~5% of energy), high fat (>60% of energy), and adequate protein. Ketone bodies (in particular β-hydroxybutyrate) have broad effects explained by their capacity to suppress class I histone deacetylases, thus affecting epigenetic regulation (Tognini et al., 2017), to inhibit the NLRP3 inflammasome, thus aborting the production of interleukin-1β (Youm et al., 2015), to activate G protein-coupled receptors (Offermanns, 2017), and to induce covalent histone modifications (lysine β-hydroxybutyrylation of H3K9) associated with starvation response genes (Xie et al., 2016). Ketone bodies may also alter the metabolism of neurotransmitters such as glutamate and gamma-amino butyric acid (GABA), improve mitochondrial function, decrease oxidative stress, and activate the peroxisome proliferator activated receptor (PPAR) and AMPK pathways (Newman et al., 2017; Roberts et al., 2017).
Figure 2. Ketogenesis and Effects of Ketogenic Diets.
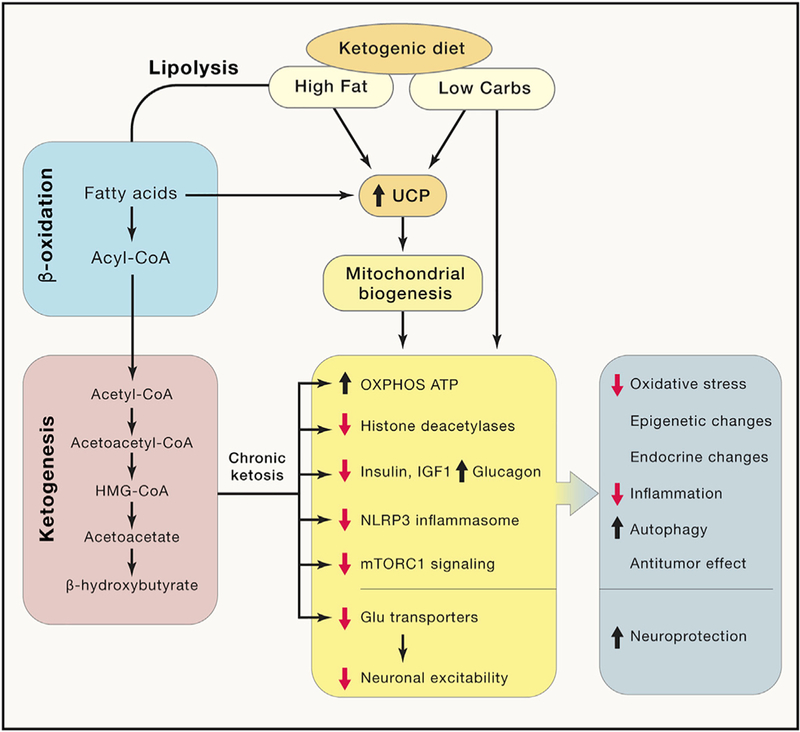
High-fat and low-carbohydrate diets cause ketogenesis and convert dietary fat and body fat into ketone bodies, such as acetoacetate and β-hydroxybutyrate. Chronic ketosis is coupled to an increase in uncoupling proteins (UCPs) and mitochondrial activity, a decrease in histone deacetylases, reduction of insulin, IGF-1, and mTORC1 signaling, inhibition of NLRP3 inflammasome, and impairment of neuronal excitability. As a consequence, ketogenic diets are associated with a number of positive effects on human health including protection against oxidative stress, beneficial epigenetic and endocrine changes, reduced inflammation, increased autophagy, antitumor activities, and neuroprotection.
Oral administration of β-hydroxybutyrate is sufficient to reduce visceral fat mass and to reduce the LDL/HDL ratio in rats (Caminhotto et al., 2017), suggesting that this ketone body has beneficial effects on its own. Intraperitoneal injection of β-hydroxybutyrate also has anticonvulsant effects on kainate-induced epilepsy in rats (Si et al., 2017). Indeed, a ketogenic diet is used for the treatment of children with intractable epilepsy, as well as for the treatment of glucose transporter 1 deficiency syndrome (GLUT1-DS, IMIM 606777) without any major side effects (apart from a reduction in growth) reported (Heussinger et al., 2017).
These findings support the contention that low-carb diets including ketogenic diets are safe and compatible with longterm health. Indeed, in mice, a constant ketogenic diet or periodic cycles thereof on alternate weeks increase the median lifespan and improves the health span, while avoiding age-associated memory decline (Newman et al., 2017; Roberts et al., 2017). The molecular mechanism through which these positive effects are obtained are still largely obscure. Interestingly, feeding mice a ketogenic diet inhibits MTORC1 activity in the liver (Roberts et al., 2017) and in the small bowel mucosa (Wang et al., 2017), which might lead to autophagy induction in the intestine, knowing that this is important for longevity in model organisms (Gelino et al., 2016). Ketogenic diet also induces autophagy in the hippocampus (Wang et al., 2018), increases energy expenditure and respiratory exchange rates, and improves blood lipid profiles in several distinct mouse strains (Barrington et al., 2018).
Clinical tests suggest that overweight adults with type 2 diabetes respond better to low-carbohydrate ketogenic diets than to moderate carbohydrate, caloric restricted diet with respect to HbA1c, weight loss, and reduced medication (Sainsbury et al., 2018; Saslow et al., 2017). Patients with NAFLD respond well to a carbohydrate-restricted, protein-rich diet that reportedly decreases hepatic de novo lipogenesis, increases mitochondrial β-oxidation leading to ketogenesis, and provokes a rise in folate-producing Streptococci within the gut, correlating with higher serum folate concentrations (Mardinoglu et al., 2018).
Altogether, the aforementioned examples underscore that carb-low diets, in particular ketogenic diets, are safe and can be used to prevent or reverse a variety of pathological states. Importantly, there is anecdotic evidence indicating that abandoning the ketogenic diet may have immediate negative consequences such as relapse from epileptic seizures (Elia et al., 2017) or migraine (Di Lorenzo et al., 2018), illustrating specific cases of acute, disease-related carbotoxicity.
As discussed above, carbotoxicity may be linked to increased production of AGEs. One of the sources of AGE is the ingestion of food containing AGEs generated by exposure to high temperature. In randomized trials, restriction of AGEs (by low-temperature cooking protocols) reduced cholesterol, HDL, and CRP levels in prediabetic patients (Di Pino et al., 2016) and ameliorated insulin resistance in obese people with metabolic syndrome (Vlassara et al., 2016). It is also possible to reduce the absorption of AGEs by means of agents such as sevelamer, which was originally designed and Food and Drug Administration (FDA)-approved for absorbing phosphate in the gut (Yubero-Serrano et al., 2015).
Pharmacological Strategies for Reducing Carbotoxicity
There are several strategies to reduce carbotoxicity that are based on the administration of pharmacological agents rather than on the simple avoidance of excessive carbohydrate ingestion (Figure 3).
Figure 3. Pharmacological Targets for Counteracting Carbotoxicity.
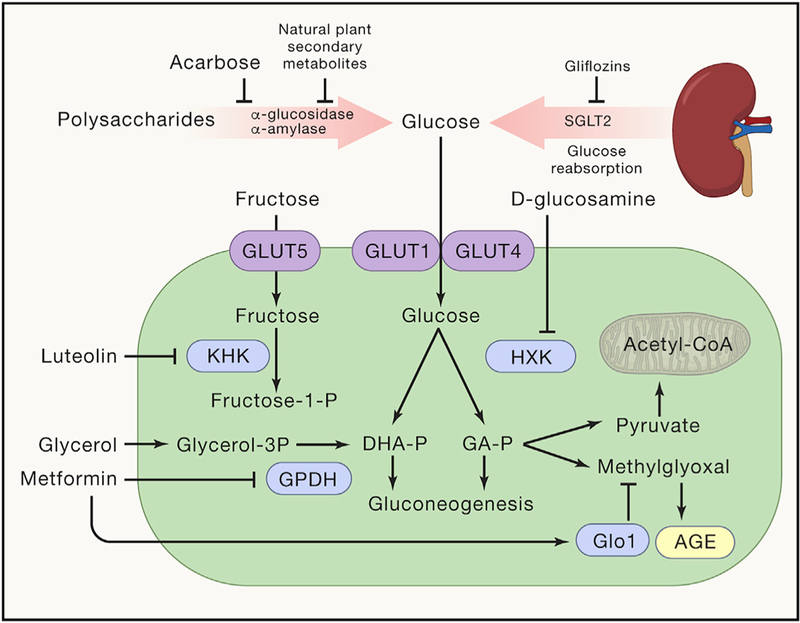
Shown are agents that inhibit sugar (re)absorption in the gut and kidney, agents that affect the conversion of glucose into fructose, as well as agents that affect glucose metabolism. A detailed discussion of these pathways is provided in the text.
Acarbose intake reduces intestinal absorption of glucose by inhibiting the release of α-glucosidase, an enzyme responsible of the breakdown of glucose from complex carbohydrates. Chronic acarbose administration prolongs the lifespan of mice (Harrison et al., 2014). Importantly, the clinical efficiency of acarbose has been related to shifts in the microbiome induced by the agent (Gu et al., 2017). Of note, leguminous plants contain natural inhibitors of α-glucosidase and α-amylase (Brewer et al., 2016), potentially paving the way for the isolation and development of other inhibitors of carbohydrate absorption.
Gliflozins, which are inhibitors of sodium/glucose cotransporter 2 (SGLT2), prevent the recovery of glucose from the glomerular filtrate, thus causing urinary glucose excretion. SGLT2 inhibitors, such as dapagliflozin, also have antihypertensive effects and reduce cardiovascular morbidity and mortality in type 2 diabetes patients compared with use of other glucose-lowering drugs (Birkeland et al., 2017; Zinman et al., 2015).
D-glucosamine, an inhibitor of glycolysis, is marketed as an over-the-counter food supplement for the treatment of osteoarthritis, with little if any evidence supporting its efficacy. Supplementation of aging C57BL/6 mice with D-glucosamine mediated lifespan extension correlating with an induction of mitochondrial biogenesis as well as lowered blood glucose levels (Weimer et al., 2014). Moreover, D-glucosamine use is associated with reduced mortality in an observational population study (Bell et al., 2012) and reduced the levels of CRP in a randomized clinical study (Navarro et al., 2015).
The mitochondrial isoform of GPDH is competitively inhibited by metformin, an antidiabetic agent with prominent antiaging effects. GPDH catalyzes the conversion of glycerol 3-phosphate into DHAP, meaning that inhibition of GPDH blocks hepatic gluconeogenesis. Indeed, knockout of GPDH in mice or knockdown of hepatic GPDH in rats phenocopies the salutary effects of metformin on glucose metabolism in vivo (Madiraju et al., 2014). It is tempting to speculate that metformin inhibits the accumulation of AGEs due to its capacity to inhibit GPDH and hence to deplete DHAP as well as methylglyoxal, an effect that has been observed in diabetic patients (Beisswenger et al., 1999). However, metformin has also been shown to increase the expression and activity of the enzyme that detoxifies methylglyoxal, which is glyoxalase 1 (Glo1) (Kender et al., 2014). Moreover, the mode of action of metformin is still a matter of debate, and there are alternative hypotheses on its pharmacological targets including the gut microbiota (Gupta et al., 2016).
Fructokinase inhibition might constitute yet another strategy to reduce the toxicity of exogenous or endogenous, glucose-derived fructose (Ishimoto et al., 2013). Thus, luteolin, 3′,4′,5,7tetrahydroxyflavone, a common flavonoid that exists in many types of plants, mediates nephroprotective effects in a model of ischemic acute kidney injury, phenocopying the effect of the fructokinase knockout (Andres-Hernando et al., 2017). However, it is unknown whether this mode of action explains the capacity of luteolin to retard age-associated neuroinflammation and memory loss in mice (Jang et al., 2010).
Concluding Remarks and Outlook
The findings summarized here indicate that carbotoxicity is a major driver of obesity and its comorbidities, calling for policies that reduce the excessive ingestion of refined carbohydrates and added sugar. There are a number of questions that must be addressed at the experimental and clinical levels before a general recommendation to reduce the ingestion of digestible sugars and polysaccharides beyond a precise threshold can be accurately proposed.
Which proportion of macronutrients is needed to optimize the health effects in different age groups? For example, there is evidence that caloric restriction extends health and survival, yet it loses such beneficial effects if imposed late in life (Longo and Panda, 2016). An extra supply of dietary glucose may even postpone the premature death of telomerase-deficient mice with an accelerated aging phenotype (Missios et al., 2014). Thus, different proportions of carbohydrate, fat, and protein might be indicated for young, median-aged, and elderly adults.
Importantly, variations in the overall quantity and composition of the diet with respect to the relative proportion of macronutrients reportedly can affect the longevity of distinct inbred mouse strains in a differential fashion, meaning that a particular diet may reduce the lifespan of one mouse strain but extend that of another strain (Solon-Biet et al., 2015). This points to the possibility that dietary recommendations would have be ‘‘personalized’’ for each individual.
Although genetic differences may affect the response to dietary interventions, there is also evidence that variations in the intestinal microbiota play a major role in the individual response to dietary challenge with carbohydrates (Zeevi et al., 2015). There is an urgent need to identify host-specific or microbiota-associated biomarkers that guide nutritional interventions in a personalized fashion.
Specific disease states may influence the urgent need for carbohydrates. For example, viral and bacterial infections have been shown to be differentially influenced in their innate immune responses by external glucose supply (Wang et al., 2016). Protein (but not carbohydrate) restriction stimulates anticancer immune responses in mice in the context of chemotherapy (Rubio-Patino et al., 2018). Hence, the question has to be addressed how dietary cues may affect immunometabolism in a favorable fashion, knowing that each immune cell subtype differs in its metabolic needs (Bantug et al., 2018).
The requirement for a specific design of diets has not been resolved for healthy adults facing particular challenges. While short-term needs of muscular activity may be stimulated by carbohydrate ingestion, it is still a matter of debate whether the long-term preparation of athletes practicing endurance sports might profit from ketogenic diets to increase oxidative phosphorylation (McSwiney et al., 2018). Similarly, it is known which proportion of carbohydrates optimizes intellectual output in the short-term (i.e., during an exam) or over long periods (i.e., scientific creativity). And what is the best nutritional preparation for surgical interventions, just to give another example?
All these questions need to be addressed by modern molecularly oriented and standardized scientific methods (rather than observational studies reporting correlations) before firm recommendations can be provided for the nutritional management of healthy individuals and patients facing the challenges of avoiding and reversing disease, respectively.
ACKNOWLEDGMENTS
G.K. is supported by the Ligue contre le Cancer, Agence National de la Recherche (ANR), Cancéropôle Ile-de-France, Chancelerie des universités de Paris (Legs Poix), a donation by Elior, the European Commission (ArtForce), European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR), Fondation Carrefour, Institut National du Cancer (INCa), Inserm (HTE), LeDucq Foundation, the LabEx Immuno-Oncology, the RHU Torino Lumière, the Seerave Foundation, the SIRICs SOCRATE, and CARPEM. C.L.-O. is supported by grants from European Research Council (DeAge), Ministerio de Economía y Competitividad, Instituto de Salud Carlos III (Ciberonc), and Progeria Research Foundation. F.M. is grateful to the Austrian Science Fund FWF (Austria; P23490-B20, P29262, P24381, P29203, and P27893) and DKplus Metabolic and Cardiovascular Diseases (W1226), as well as to Bundesministerium für Wissenschaft, Forschung und Wirtschaft, and the Karl-Franzens University for grants ‘‘Unkonventionelle Forschung’’ and ‘‘flysleep.’’ We acknowledge support from NAWI Graz and the BioTechMed-Graz flagship project ‘‘EPIAge.’’ R.d.C. is funded by the Intramural Research Program of the National Institute on Aging, NIH.
REFERENCES
- Andres-Hernando A, Li N, Cicerchi C, Inaba S, Chen W, Roncal-Jimenez C, Le MT, Wempe MF, Milagres T, Ishimoto T, et al. (2017). Protective role of fructokinase blockade in the pathogenesis of acute kidney injury in mice. Nat. Commun 8, 14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantug GR, Galluzzi L, Kroemer G, and Hess C (2018). The spectrum of T cell metabolism in health and disease. Nat. Rev. Immunol 18, 19–34. [DOI] [PubMed] [Google Scholar]
- Barrington WT, Wulfridge P, Wells AE, Rojas CM, Howe SYF, Perry A, Hua K, Pellizzon MA, Hansen KD, Voy BH, et al. (2018). Improving metabolic health through precision dietetics in mice. Genetics 208, 399–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisswenger PJ, Howell SK, Touchette AD, Lal S, and Szwergold BS (1999). Metformin reduces systemic methylglyoxal levels in type 2 diabetes. Diabetes 48, 198–202. [DOI] [PubMed] [Google Scholar]
- Bell GA, Kantor ED, Lampe JW, Shen DD, and White E (2012). Use of glucosamine and chondroitin in relation to mortality. Eur. J. Epidemiol 27, 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergheim I, Weber S, Vos M, Krämer S, Volynets V, Kaserouni S, McClain CJ, and Bischoff SC (2008). Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J. Hepatol 48, 983–992. [DOI] [PubMed] [Google Scholar]
- Birkeland KI, Jørgensen ME, Carstensen B, Persson F, Gulseth HL, Thuresson M, Fenici P, Nathanson D, Nyström T, Eriksson JW, et al. (2017). Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol 5, 709–717. [DOI] [PubMed] [Google Scholar]
- Brewer RA, Gibbs VK, and Smith DL Jr. (2016). Targeting glucose metabolism for healthy aging. Nutr. Healthy Aging 4, 31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM (2017). Disentangling high fat, low carb, and healthy aging. Cell Metab 26, 458–459. [DOI] [PubMed] [Google Scholar]
- Caminhotto RO, Komino ACM, de Fatima Silva F, Andreotti S, Sertié RAL, Boltes Reis G, and Lima FB (2017). Oral β-hydroxybutyrate increases ketonemia, decreases visceral adipocyte volume and improves serum lipid profile in Wistar rats. Nutr. Metab. (Lond.) 14, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A, Hendrikse J, Lee N, Yücel M, Verdejo-Garcia A, Andrews ZB, and Hall W (2016). The Neurobiology of ‘‘food addiction’’ and its implications for obesity treatment and policy. Annu. Rev. Nutr 36, 105–128. [DOI] [PubMed] [Google Scholar]
- Celotto AM, Frank AC, Seigle JL, and Palladino MJ (2006). Drosophila model of human inherited triosephosphate isomerase deficiency glycolytic enzymopathy. Genetics 174, 1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia CW, Shardell M, Tanaka T, Liu DD, Gravenstein KS, Simonsick EM, Egan JM, and Ferrucci L (2016). Chronic low-calorie sweetener use and risk of abdominal obesity among older adults: a cohort study. PLoS ONE 11, e0167241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CJ, Milton RC, Klein R, Gensler G, and Taylor A (2007). Dietary carbohydrate and the progression of age-related macular degeneration: a prospective study from the Age-Related Eye Disease Study. Am. J. Clin. Nutr 86, 1210–1218. [DOI] [PubMed] [Google Scholar]
- Chiu S, Mulligan K, and Schwarz JM (2018). Dietary carbohydrates and fatty liver disease: de novo lipogenesis. Curr. Opin. Clin. Nutr. Metab. Care 21, 277–282. [DOI] [PubMed] [Google Scholar]
- Choi JM, Seo MH, Kyeong HH, Kim E, and Kim HS (2013). Molecular basis for the role of glucokinase regulatory protein as the allosteric switch for glucokinase. Proc. Natl. Acad. Sci. USA 110, 10171–10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan M, Mente A, Zhang X, Swaminathan S, Li W, Mohan V, Iqbal R, Kumar R, Wentzel-Viljoen E, Rosengren A, et al. ; Prospective Urban Rural Epidemiology (PURE) study investigators (2017). Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 390, 2050–2062. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo C, Coppola G, Di Lenola D, Evangelista M, Sirianni G, Rossi P, Di Lorenzo G, Serrao M, and Pierelli F (2018). Efficacy of modified Atkins ketogenic diet in chronic cluster headache: an open-label, single-arm, clinical trial. Front. Neurol 9, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pino A, Currenti W, Urbano F, Mantegna C, Purrazzo G, Piro S, Purrello F, and Rabuazzo AM (2016). Low advanced glycation end product diet improves the lipid and inflammatory profiles of prediabetic subjects. J. Clin. Lipidol 10, 1098–1108. [DOI] [PubMed] [Google Scholar]
- Elia M, Klepper J, Leiendecker B, and Hartmann H (2017). Ketogenic diets in the treatment of epilepsy. Curr. Pharm. Des 23, 5691–5701. [DOI] [PubMed] [Google Scholar]
- Fiolet T, Srour B, Sellem L, Kesse-Guyot E, Allès B, Méjean C, Deschasaux M, Fassier P, Latino-Martel P, Beslay M, et al. (2018). Consumption of ultra-processed foods and cancer risk: results from NutriNet-Santé prospective cohort. BMJ 360, k322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forshaw R (2014). Dental indicators of ancient dietary patterns: dental analysis in archaeology. Br. Dent. J 216, 529–535. [DOI] [PubMed] [Google Scholar]
- Frimat M, Daroux M, Litke R, Nevière R, Tessier FJ, and Boulanger E (2017). Kidney, heart and brain: three organs targeted by ageing and glycation. Clin. Sci. (Lond.) 131, 1069–1092. [DOI] [PubMed] [Google Scholar]
- Gelino S, Chang JT, Kumsta C, She X, Davis A, Nguyen C, Panowski S, and Hansen M (2016). Intestinal autophagy improves healthspan and longevity in C. elegans during dietary restriction. PLoS Genet 12, e1006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnerer JP, Kreber RA, and Ganetzky B (2006). wasted away, a Drosophila mutation in triosephosphate isomerase, causes paralysis, neurodegeneration, and early death. Proc. Natl. Acad. Sci. USA 103, 14987–14993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Wang X, Li J, Zhang Y, Zhong H, Liu R, Zhang D, Feng Q, Xie X, Hong J, et al. (2017). Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat. Commun 8, 1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Bala M, Gupta S, Dua A, Dabur R, Injeti E, and Mittal A (2016). Efficacy and risk profile of anti-diabetic therapies: Conventional vs traditional drugs-A mechanistic revisit to understand their mode of action. Pharmacol. Res 113 (Pt A), 636–674. [DOI] [PubMed] [Google Scholar]
- Hadj Ahmed S, Kharroubi W, Kaoubaa N, Zarrouk A, Batbout F, Gamra H, Najjar MF, Lizard G, Hininger-Favier I, and Hammami M (2018). Correlation of trans fatty acids with the severity of coronary artery disease lesions. Lipids Health Dis 17, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, Fernandez E, Flurkey K, Javors MA, Nadon NL, et al. (2014). Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan prefer-entially in males. Aging Cell 13, 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannou SA, Haslam DE, McKeown NM, and Herman MA (2018). Fruc-tose metabolism and metabolic disease. J. Clin. Invest 128, 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heussinger N, Della Marina A, Beyerlein A, Leiendecker B, Hermann-Alves S, Dalla Pozza R, and Klepper J (2017). 10 patients, 10 years - long term follow-up of cardiovascular risk factors in Glut1 deficiency treated with ketogenic diet therapies: a prospective, multicenter case series. Clin. Nutr Published online November 11, 2017. https://doi.org/10.1016/j.clnu.2017.11.001. [DOI] [PubMed] [Google Scholar]
- Hu Y, Costenbader KH, Gao X, Al-Daabil M, Sparks JA, Solomon DH, Hu FB, Karlson EW, and Lu B (2014). Sugar-sweetened soda consumption and risk of developing rheumatoid arthritis in women. Am. J. Clin. Nutr 100, 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto T, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, Orlicky DJ, Cicerchi C, McMahan RH, Abdelmalek MF, Rosen HR, Jackman MR, et al. (2013). High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology 58, 1632–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameel F, Phang M, Wood LG, and Garg ML (2014). Acute effects of feeding fructose, glucose and sucrose on blood lipid levels and systemic inflammation. Lipids Health Dis 13, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Dilger RN, and Johnson RW (2010). Luteolin inhibits microglia and alters hippocampal-dependent spatial working memory in aged mice. J. Nutr 140, 1892–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang C, Hui S, Lu W, Cowan AJ, Morscher RJ, Lee G, Liu W, Tesz GJ, Birnbaum MJ, and Rabinowitz JD (2018). The small intestine converts dietary fructose into glucose and organic acids. Cell Metab 27, 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, and Goff DV (1981). Glycemic index of foods: a physiological basis for carbohydrate exchange. Am. J. Clin. Nutr 34, 362–366. [DOI] [PubMed] [Google Scholar]
- Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, Nakagawa T, Kuwabara M, Sato Y, Kang DH, et al. (2018). Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol 68, 1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, and Sánchez-Lozada LG (2007). Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr 86, 899–906. [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Perez-Pozo SE, Sautin YY, Manitius J, Sanchez-Lozada LG, Feig DI, Shafiu M, Segal M, Glassock RJ, Shimada M, et al. (2009). Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr. Rev 30, 96–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RJ, Sánchez-Lozada LG, Andrews P, and Lanaspa MA (2017). Perspective: a historical and scientific perspective of sugar and its relation with obesity and diabetes. Adv. Nutr 8, 412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kender Z, Fleming T, Kopf S, Torzsa P, Grolmusz V, Herzig S, Schleicher E, Rácz K, Reismann P, and Nawroth PP (2014). Effect of metformin on methylglyoxal metabolism in patients with type 2 diabetes. Exp. Clin. Endocrinol. Diabetes 122, 316–319. [DOI] [PubMed] [Google Scholar]
- Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, Roncal-Jimenez CA, Ishimoto T, Li N, Marek G, Duranay M, et al. (2012a). Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem 287, 40732–40744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanaspa MA, Sanchez-Lozada LG, Cicerchi C, Li N, Roncal-Jimenez CA, Ishimoto T, Le M, Garcia GE, Thomas JB, Rivard CJ, et al. (2012b). Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS ONE 7, e47948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanaspa MA, Ishimoto T, Li N, Cicerchi C, Orlicky DJ, Ruzycki P, Rivard C, Inaba S, Roncal-Jimenez CA, Bales ES, et al. (2013). Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat. Commun 4, 2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanaspa MA, Ishimoto T, Cicerchi C, Tamura Y, Roncal-Jimenez CA, Chen W, Tanabe K, Andres-Hernando A, Orlicky DJ, Finol E, et al. (2014). Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J. Am. Soc. Nephrol 25, 2526–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanaspa MA, Kuwabara M, Andres-Hernando A, Li N, Cicerchi C, Jensen T, Orlicky DJ, Roncal-Jimenez CA, Ishimoto T, Nakagawa T, et al. (2018). High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc. Natl. Acad. Sci. USA 115, 3138–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Zhang C, Kilicarslan M, Piening BD, Bjornson E, Hallström BM, Groen AK, Ferrannini E, Laakso M, Snyder M, et al. (2016). Integrated network analysis reveals an association between plasma mannose levels and insulin resistance. Cell Metab 24, 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, and Lustig RH (2010). The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol 7, 251–264. [DOI] [PubMed] [Google Scholar]
- Longo VD, and Panda S (2016). Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab 23, 1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Monterosso JR, Sarpelleh K, and Page KA (2015). Differential effects of fructose versus glucose on brain and appetitive responses to food cues and decisions for food rewards. Proc. Natl. Acad. Sci. USA 112, 6509–6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssiotis CA, and Cantley LC (2013). Metabolic syndrome: F stands for fructose and fat. Nature 502, 181–182. [DOI] [PubMed] [Google Scholar]
- Madeo F, Eisenberg T, Pietrocola F, and Kroemer G (2018). Spermidine in health and disease. Science 359, eaan2788. [DOI] [PubMed] [Google Scholar]
- Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, et al. (2014). Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510, 542–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maersk M, Belza A, Stødkilde-Jørgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen SB, Astrup A, and Richelsen B (2012). Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am. J. Clin. Nutr 95, 283–289. [DOI] [PubMed] [Google Scholar]
- Mardinoglu A, Stancáková A, Lotta LA, Kuusisto J, Boren J, Blüher M, Wareham NJ, Ferrannini E, Groop PH, Laakso M, et al. (2017). Plasma mannose levels are associated with incident type 2 diabetes and car-diovascular disease. Cell Metab 26, 281–283. [DOI] [PubMed] [Google Scholar]
- Mardinoglu A, Wu H, Bjornson E, Zhang C, Hakkarainen A, Rasanen SM, Lee S, Mancina RM, Bergentall M, Pietilainen KH, et al. (2018). An integrated understanding of the rapid metabolic benefits of a carbohy-drate-restricted diet on hepatic steatosis in humans. Cell Metab 27, 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSwiney FT, Wardrop B, Hyde PN, Lafountain RA, Volek JS, and Doyle L (2018). Keto-adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metabolism 81, 25–34. [DOI] [PubMed] [Google Scholar]
- Mirtschink P, Krishnan J, Grimm F, Sarre A, Hörl M, Kayikci M, Fank-hauser N, Christinat Y, Cortijo C, Feehan O, et al. (2015). HIF-driven SF3B1 induces KHK-C to enforce fructolysis and heart disease. Nature 522, 444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missios P, Zhou Y, Guachalla LM, von Figura G, Wegner A, Chakkarap-pan SR, Binz T, Gompf A, Hartleben G, Burkhalter MD, et al. (2014). Glucose substitution prolongs maintenance of energy homeostasis and life-span of telomere dysfunctional mice. Nat. Commun 5, 4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro SL, White E, Kantor ED, Zhang Y, Rho J, Song X, Milne GL, Lampe PD, and Lampe JW (2015). Randomized trial of glucosamine and chondroitin supplementation on inflammation and oxidative stress biomarkers and plasma proteomics profiles in healthy humans. PLoS ONE 10, e0117534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, Huang Y, Haldar S, and Verdin E (2017). Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab 26, 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns S (2017). Hydroxy-carboxylic acid receptor actions in metabolism. Trends Endocrinol. Metab 28, 227–236. [DOI] [PubMed] [Google Scholar]
- Oppelt SA, Zhang W, and Tolan DR (2017). Specific regions of the brain are capable of fructose metabolism. Brain Res 1657, 312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page KA, Chan O, Arora J, Belfort-Deaguiar R, Dzuira J, Roehmholdt B, Cline GW, Naik S, Sinha R, Constable RT, and Sherwin RS (2013). Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA 309, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino MY (2015). Metabolic effects of non-nutritive sweeteners. Physiol. Behav 152 (Pt B), 450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrocola F, Galluzzi L, Bravo-San Pedro JM, Madeo F, and Kroemer G (2015). Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab 21, 805–821. [DOI] [PubMed] [Google Scholar]
- Rendeiro C, Masnik AM, Mun JG, Du K, Clark D, Dilger RN, Dilger AC, and Rhodes JS (2015). Fructose decreases physical activity and in-creases body fat without affecting hippocampal neurogenesis and learning relative to an isocaloric glucose diet. Sci. Rep 5, 9589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, Perez G, Gutierrez-Casado E, Koike S, Knotts TA, et al. (2017). A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab 26, 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncal-Jimenez CA, Ishimoto T, Lanaspa MA, Milagres T, Hernando AA, Jensen T, Miyazaki M, Doke T, Hayasaki T, Nakagawa T, et al. (2016). Aging-associated renal disease in mice is fructokinase dependent. Am. J. Physiol. Renal Physiol 311, F722–F730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Patino C, Bossowski JP, De Donatis GM, Mondragon L, Villa E, Aira LE, Chiche J, Mhaidly R, Lebeaupin C, Marchetti S, et al. (2018). Low-protein diet induces IRE1alpha-dependent anticancer immunosurveil-lance. Cell Metab 27, 828–842. [DOI] [PubMed] [Google Scholar]
- Sainsbury E, Kizirian NV, Partridge SR, Gill T, Colagiuri S, and Gibson AA (2018). Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: a systematic review and meta-analysis. Diabetes Res. Clin. Pract 139, 239–252. [DOI] [PubMed] [Google Scholar]
- Saslow LR, Daubenmier JJ, Moskowitz JT, Kim S, Murphy EJ, Phinney SD, Ploutz-Snyder R, Goldman V, Cox RM, Mason AE, et al. (2017). Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes. Nutr. Diabetes 7, 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SP, Chung HJ, Kim HJ, and Hong ST (2016). Paradoxical effects of fruit on obesity. Nutrients 8, E633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si J, Wang S, Liu N, Yang X, Wang Y, Li L, Wang J, and Lv X (2017). Anticonvulsant effect of exogenous β-hydroxybutyrate on kainic acid-induced epilepsy. Exp. Ther. Med 14, 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel KR, Echouffo-Tcheugui JB, Ali MK, Mehta NK, Narayan KM, and Chetty V (2012). Societal correlates of diabetes prevalence: an analysis across 94 countries. Diabetes Res. Clin. Pract 96, 76–83. [DOI] [PubMed] [Google Scholar]
- Softic S, Gupta MK, Wang GX, Fujisaka S, O’Neill BT, Rao TN, Willoughby J, Harbison C, Fitzgerald K, Ilkayeva O, et al. (2017). Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J. Clin. Invest 127, 4059–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, Walters KA, Simanainen UK, McMahon AC, Ruohonen K, Ballard JW, Raubenheimer D, Handelsman DJ, Le Couteur DG, and Simpson SJ (2015). Macronutrient balance, reproductive function, and lifespan in aging mice. Proc. Natl. Acad. Sci. USA 112, 3481–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. (2009). Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in over-weight/obese humans. J. Clin. Invest 119, 1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ströhle A, and Hahn A (2011). Diets of modern hunter-gatherers vary substantially in their carbohydrate content depending on ecoenvironments: results from an ethnographic analysis. Nutr. Res 31, 429–435. [DOI] [PubMed] [Google Scholar]
- Te Morenga LA, Howatson AJ, Jones RM, and Mann J (2014). Dietary sugars and cardiometabolic risk: systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am. J. Clin. Nutr 100, 65–79. [DOI] [PubMed] [Google Scholar]
- Tognini P, Murakami M, Liu Y, Eckel-Mahan KL, Newman JC, Verdin E, Baldi P, and Sassone-Corsi P (2017). Distinct circadian signatures in liver and gut clocks revealed by ketogenic diet. Cell Metab 26, 523–538. [DOI] [PubMed] [Google Scholar]
- Veronese N, Solmi M, Caruso MG, Giannelli G, Osella AR, Evangelou E, Maggi S, Fontana L, Stubbs B, and Tzoulaki I (2018). Dietary fiber and health outcomes: an umbrella review of systematic reviews and meta-analyses. Am. J. Clin. Nutr 107, 436–444. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Cai W, Tripp E, Pyzik R, Yee K, Goldberg L, Tansman L, Chen X, Mani V, Fayad ZA, et al. (2016). Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: a randomised controlled trial. Diabetologia 59, 2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding YS, Wong C, Ma Y, and Pradhan K (2008). Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 42, 1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Huen SC, Luan HH, Yu S, Zhang C, Gallezot JD, Booth CJ, and Medzhitov R (2016). Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell 166, 1512–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhou Y, Rychahou P, Fan TW, Lane AN, Weiss HL, and Evers BM (2017). Ketogenesis contributes to intestinal cell differentiation. Cell Death Differ 24, 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BH, Hou Q, Lu YQ, Jia MM, Qiu T, Wang XH, Zhang ZX, and Jiang Y (2018). Ketogenic diet attenuates neuronal injury via autophagy and mitochondrial pathways in pentylenetetrazol-kindled seizures. Brain Res 1678, 106–115. [DOI] [PubMed] [Google Scholar]
- Weimer S, Priebs J, Kuhlow D, Groth M, Priebe S, Mansfeld J, Merry TL, Dubuis S, Laube B, Pfeiffer AF, et al. (2014). D-Glucosamine supplementation extends life span of nematodes and of ageing mice. Nat. Commun 5, 3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Zhang D, Chung D, Tang Z, Huang H, Dai L, Qi S, Li J, Colak G, Chen Y, et al. (2016). Metabolic regulation of gene expression by histone lysine β-hydroxybutyrylation. Mol. Cell 62, 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, and Hu FB (2014). Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern. Med 174, 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, et al. (2015). The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med 21, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yubero-Serrano EM, Woodward M, Poretsky L, Vlassara H, and Striker GE; AGE-less Study Group (2015). Effects of sevelamer carbonate on advanced glycation end products and antioxidant/pro-oxidant status in patients with diabetic kidney disease. Clin. J. Am. Soc. Nephrol 10, 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, et al. (2015). Personalized nutrition by prediction of glycemic responses. Cell 163, 1079–1094. [DOI] [PubMed] [Google Scholar]
- Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Blendis L, Halpern Z, and Oren R (2007). Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. J. Hepatol 47, 711–717. [DOI] [PubMed] [Google Scholar]
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. ; EMPA-REG OUTCOME Investigators (2015). Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med 373, 2117–2128. [DOI] [PubMed] [Google Scholar]


