Abstract
The bidirectional communication between the central nervous system (CNS) and the gut microbiota plays a pivotal role in human health. Increasing numbers of studies suggest that the gut microbiota can influence the brain and behavior of patients. Various metabolites secreted by the gut microbiota can affect the cognitive ability of patients diagnosed with neurodegenerative diseases. Nearly one in every ten Korean senior citizens suffers from Alzheimer’s disease (AD), the most common form of dementia. This review highlights the impact of metabolites from the gut microbiota on communication pathways between the brain and gut, as well as the neuroinflammatory roles they may have in AD patients. The objectives of this review are as follows: (1) to examine the role of the intestinal microbiota in homeostatic communication between the gut microbiota and the brain, termed the microbiota–gut–brain (MGB) axis; (2) to determine the underlying mechanisms of signal dysfunction; and (3) to assess the impact of signal dysfunction induced by the microbiota on AD. This review will aid in understanding the microbiota of elderly people and the neuroinflammatory roles they may have in AD.
Keywords: gut microbiota, MGB axis, germ-free animal, probiotic, neurodegenerative diseases, Alzheimer’s disease
1. Introduction
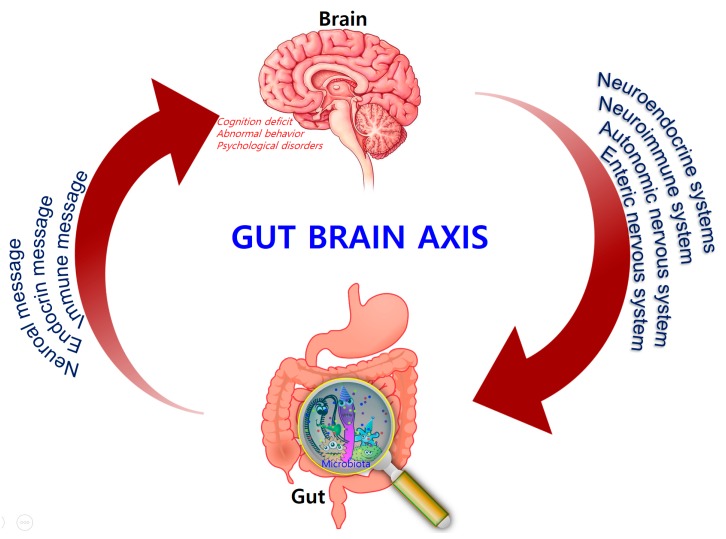
Comprising trillions of symbiotic microorganisms, the gut microbiota is an essential element for the maintenance of the host’s health [1,2,3]. This microbial ecosystem consists mainly of bacteria, of which most are strict anaerobes, and also fungi and viruses. The four main phyla in adults consist of Bacteroidetes (~48%) and Firmicutes (~51%), which make up the highest proportion, as well as Proteobacteria and Actinobacteria, which are found in relatively low amounts (1%) [4]. Alterations in the composition of the gut microbiota, caused by dietary changes, antibiotic exposure, and infection, lead to the loss of homeostasis, which is implicated in the development of several diseases in humans, such as colorectal cancer, metabolic syndrome, obesity, allergies, inflammatory bowel disease (IBD), type 2 diabetes, heart failure, and neurodegenerative disorders [5,6,7,8]. Recent evidence points to a causative link between pathogens and changes in the intestinal microbiota composition, along with inflammatory changes in various tissues and organs including brain tissue [7]. Hence, gut microbes may alter levels of neurotransmitter-related metabolites, affecting gut-to-brain communication and/or altering brain function (Figure 1).
Figure 1.
Bidirectional signaling between the gastrointestinal tract and the brain is regulated at the neural, hormonal, and immunological levels.
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by a progressive loss of memory, language, and cognitive ability. According to the classical “amyloid cascade” model, the disease results from the over production of amyloid-beta peptide (Aβ), following the disruption of homeostatic mechanisms which regulate the proteolytic cleavage of the amyloid precursor protein (APP). Amyloids associated with AD consist largely of perivascular amyloid enriched in the 42-amino-acid Aβ42 peptide. Aβ42 is thought to initiate the neuroinflammatory process characteristic of AD pathology [8]. Plaque formation is also a natural response to infection by trapping invading microorganisms, further contributing to the collateral damage of healthy tissue that results from neuroinflammation. However, recent work suggests that the cascade model does not fully explain AD pathogenesis [9] and that alterations in the gut microbiome may play a significant role in the progression of the disease [10].
Over the last decade, several publications demonstrated the regulatory influence of the gut microbiota on the innate and adaptive immune response, as well as the importance of the interactions between the endogenous microbiota and the host’s central nervous system (CNS) [11,12]. Given the unsuccessful AD treatments employed so far, gut modification or recondition strategies started attracting the attention of the scientific community regarding the pathogenesis of CNS diseases, with emphasis on Alzheimer’s and Parkinson’s diseases [13]. In this review, we examine the gut microbiota and its interaction with the CNS, as well as its modulatory role in the neuroinflammatory process in Alzheimer’s disease.
2. The Intestinal Microbiota and Homeostasis
Early colonization of certain enterotypes can have a long-lasting influence on the health status of the host [14,15,16]. Human microbial colonization begins at birth. Infants born vaginally are initially colonized with microbial colonies that have a maternal signature (enriched in Lactobacillus and Prevotella spp.), while those delivered by caesarean section harbor colonies that more closely resemble the skin microbiota (enriched in Staphylococcus and Propionibacterium spp.). The microbiota then diversifies over the first few weeks of life to form a complex, anaerobe-dominated microbial community [17]. At the same time, the hypothalamic–pituitary–adrenal (HPA) axis becomes activated, which has an impact on the enteric nervous system (ENS) that innervates the gastrointestinal tract (GIT). Finally, the human gut microbiota rapidly expands and reaches an adult-like stage by three years of age. Shifts from Bifidobacterium to Clostridia and Bacteriodetes occur as the host develops from an infant into an adult [18]. Reductions in the level of Faecalibacterium prauznitzii and its anti-inflammatory relatives occur as young adults mature [6]. The composition of the microbiota is altered throughout the lifespan and is dependent on dietary and environmental factors, disease state, and other factors. In addition, several strategies suggest that Porphyromonas gingivalis causes an inflammatory response in the liver through the increased expression of pro-inflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6), as well as fat storage-inducing transmembrane protein 2 (Fitm2) and perilipin 2 (Plin2), associated with lipid droplet formation, which subsequently increases neuroinflammation and causes neurodegenerative changes and AD [12,19,20].
The microbiota has long been known to play a relevant role in the health of the host. It can help break down certain nutrients, which can then be metabolized by host cells, and some of these products are involved in neural function. As such, gut bacteria produce amino acids (i.e., gamma-amino butyric acid (GABA) and tryptophan) [21,22], and monoamines (i.e., serotonin, histamine, and dopamine), which play a significant role in the brain as neurotransmitters, or as neurotransmitter precursors [23,24]. These neuroactive products can target the CNS via the blood stream and can also influence neurons in the ENS.
In the homeostatic state, a healthy GIT has a normal and stable commensal intestinal microbiota. This provides the host with nutrition and energy through the production of vitamins [25,26,27], aids in the maintenance of intestinal epithelial barrier integrity, aids in resistance to pathogens, and plays a role in the metabolic and immune systems [28,29,30]. Furthermore, the idea that the interplay between the gut and brain can modulate behavior is emerging as an exciting concept in health and disease. The interaction between them forms an essential relationship for maintaining GI homeostasis. These essential communication pathways between the immune system and the CNS depend on neurotransmitters, neuropeptides, cytokines, hormones, and growth factors, amongst other things. Experimental evidence suggests that the gut microbiota can alter levels of circulating cytokines, which in turn can have a significant effect on several brain functions [31]. Moreover, both the afferent branch of the vagus nerve and modulation of systemic tryptophan, the precursor of the neurotransmitter serotonin, are highly implicated in relaying messages from the gut microbiota to the brain [32]. In addition, an imbalance in the gut microbiota can result in diseases such as inflammatory bowel disease (IBD), irritable bowel syndrome (IBS) [33,34], gastroenteric infections [35], allergic diseases, diabetes, and metabolic syndromes [36,37,38].
3. The Microbiota–Gut–Brain (MGB) Axis
In the 1880s, William James and Carl Lange first introduced the concept that bidirectional communication between the CNS and intestinal organs plays a role in emotional regulation [38,39]. Forty years later, the idea that the brain plays an important role in regulating GI function was developed by Walter Cannon [40]. A number of rodent studies also showed that the gut–brain axis is a focus of research in different fields, ranging from basic microbiology to translational applications. Consequently, the potential involvement of the gut microbiota in brain function emerged. This involvement pertains to the core microbiome, distinct enterotypes, and age-related shifts in composition, which are harmful to health [41]. The concept of the MGB axis is well established. The neuroendocrine and neuroimmune systems, in addition to the sympathetic and parasympathetic arms of the autonomic nervous system (ANS) and the ENS, are key pathways in gut–brain communication. Although the exact mechanisms mediating gut–brain interactions are not fully understood, they were suggested to involve endocrine, immune, and neural pathways (vagus nerve and enteric nervous system), leading to possible alteration in AD patients or aggravating inflammation.
The concept has now expanded and has become a quickly evolving area of research that led to convergence of research efforts in the fields of neuroscience, psychiatry, gastroenterology, and microbiology—disciplines that were previously considered to have distinct and separate research objectives and focuses.
Studies investigating the effects of intestinal microbiota composition on brain function predominantly involve animal models of behavioral disorders such as anxiety, depression, and cognitive dysfunction. Accumulating evidence suggests that the composition of the gut microbiota may also have a role in several other metabolic conditions involving the CNS [42,43]. For instance, when confronted with stress, the brain may alter the composition of the gut microbiota through the HPA axis, which can regulate cortisol secretion and effect immune cell activity, both locally in the gut and systemically. Other experiments indicate that the gut microbiota and probiotic agents can alter levels of circulating cytokines, which in turn can have a significant effect on some brain functions [31,43]. In addition, both the afferent branch of the vagus nerve and modulation of systemic tryptophan are strongly implicated in relaying signals from the gut to the brain [44,45].
The MGB axis is vital for maintaining gut homeostasis. Dysregulation of the MGB axis was implicated in various disease states, the most common being chronic functional GI disorders such as IBS, which can induce depression and can also result in decreased cognitive function [46,47]. The work done by Bravo and Tillisch et al. implicated microbiota–brain signaling in alterations of resting brain activity in key circuits involved in pain, emotion, and cognition [48]. Researchers in this area are increasingly giving recognition to the microbiota itself as being an active and highly influential contributing factor in this bidirectional communication network.
4. Disrupting Microbiota Effects on Brain and Behavior
The gut microbiota has become a focus of studies on the brain and behavior. Alterations in the gut microbiota can modulate the peripheral and central nervous systems, resulting in altered brain function, which gives further evidence for the existence of the MGB axis. Early studies in humans demonstrate that altering the microbiota with beneficial bacteria or probiotics can lead to changes in brain function, as well as subjective reports of mood. Experimental approaches in MGB-axis research include the use of germ-free animals, animals with pathogenic bacterial infections, and animals exposed to probiotic agents or antibiotics [49].
Germ-free animal studies are conducted in animals born and reared in sterile conditions, eliminating the opportunity for postnatal colonization of the GIT. Thus, research conducted in germ-free animals highlighted the important role the gut microbiota plays in the development of both physiologic and metabolic abnormalities [49]. When compared with conventional animals, germ-free animals exhibit abnormal gastrointestinal motility, increased expression of genes encoding transporters throughout the gut, and an altered response to inflammatory pain [50,51,52]. In addition, germ-free animals have an immature and dysregulated immune system, with abnormal immunoglobulin A (IgA) production [52,53,54,55,56] and decreased numbers of intestinal mast cells [57].
The absence of gut bacteria during development affects the HPA axis [58], which has a significant role in the stress response. Studies in germ-free animals clearly demonstrate a relationship between the gut microbiota and stress- and anxiety-related behaviors. Further studies should concentrate on the influence of time, sex, strain, and other species factors on this relationship.
Infection studies are used to assess the effects of pathogenic bacteria on brain and behavior which are mediated largely through activation of the immune system [59]. Lyte et al. showed that infection-induced c-Fos expression, a marker for cellular activation, and abnormal anxiety-like behaviors can be triggered by stress [58]. Bercik et al. sought to determine the effects of chronic gut inflammation on behavior. In this study, mice were infected with Trichuris muris, which is very closely related to the human parasite T. trichiura, and alterations in anxiety-like behavior and brain-derived neurotrophic factor (BDNF) expression were examined [60]. Mice were either treated with anti-inflammatory agents, the probiotic B. longum, or vagotomy. All treatments normalized the infection-induced behavioral changes; however, the microbiota may communicate with the brain via several routes.
Probiotics are considered to be a novel and safe way of maintaining a healthy intestinal microbiota by producing antimicrobial agents or metabolic compounds that suppress the growth of other microorganisms [61,62] or by competing with other intestinal microbes for receptors and binding sites on the intestinal mucosa [63]. The use of probiotics was shown to improve behaviors associated with stress-related psychiatric conditions and induce neuronal plasticity. Consequently, they appear to protect the brain from stress-induced insults to the brain circuitry. For instance, probiotic-induced promotion of neurogenesis, such as increases in BDNF, in the hippocampus normalizes the abnormal response of the HPA axis. Increases in BDNF can downregulate the expression of inflammatory cytokines, decrease oxidative stress, and also improve the nutritional state [64]. A number of studies also showed that most probiotics from the Lactobacilli family have the potential to reduce corticosterone levels and colonic permeability, and can also affect the excitability of enteric neurons and colonic motility in the maternal separation rat model of early life stress [65]. Certain probiotic strains might be useful for improving cognitive and emotional aspects of mental health disorders. Furthermore, combining probiotic treatment with psychopharmacology may allow for the administration of lower doses of pharmacologically active compounds, thus improving their safety and reducing their toxic side effects. However, probiotic therapies have limitations, including a poor ability to establish a stable population within the recipient. Furthermore, in many instances, pathogenesis may be contributed to by broad functions conserved across many different species, such as the ability to produce metabolites that are immunomodulatory, or that directly influence brain activity. Here, it may be the absence of suitable drivers of beneficial behavior that is limiting, rather than the absence of microbes capable of exhibiting them. In such instances, the broad-scale alteration of the microbiome using selective dietary microbial growth substrates or prebiotics may be more appropriate and result in longer-lasting change. For example, consumption of fructooligosaccharides or a nondigestible galactooligosaccharide formulation (BGOS) elevates BDNF levels and N-methyl-d-aspartate receptor (NMDAR) subunit expression in rats [60].
Antibiotics are normally used to remove or prevent bacterial colonization in the human body, without targeting specific types of bacteria. As a result, broad-spectrum antibiotics can greatly affect the composition of the gut microbiota, reduce the bio-diversity of the fecal microbiota, and delay colonization for a long period after administration. A number of studies showed that different antibiotic treatments result in short- and/or long-term changes in the intestinal microbiota in both humans and animals. The actions of several antibiotics on gut microbial diversity are outlined in Table 1 [66,67,68,69,70,71,72,73].
Table 1.
Antibiotic treatment effects on the microbiota (both human and animals).
| Antibiotics | Dosage | Subjects | Changes in Microbial Composition | Type and Duration of Study | Reference |
|---|---|---|---|---|---|
| Ciprofloxacin | 500 mg, 3× per day for 5 days | Healthy adults | ↓Clostridiales | Diversity 8 months | [14] |
| Amoxicillin | 375 mg, 3× per day for 5 days | Healthy adults | ↓Bifidobacterium ↑Enterobacteriaceae ↑Metabolic dysfunction |
Parallel intervention 26 days | [66] |
| Amoxicillin | 60 mg/mL 8–11 days | Rats | ↑Proteobacteria ↑Haptoglobin levels ↓Diversity Index |
Intestinal permeability 8 days | [67] |
| Vancomycin | 500 mg, 3× per day for 7 days | Male adults. Metabolic syndrome | ↓Gram-positive bacteria (Firmicutes) ↑Gram-negative bacteria (Proteobacteria) ↓Peripheral insulin sensitivity |
Single blinded randomized controlled 1 week | [68] |
| Vancomycin | 0.2 mg/mL for 8 weeks | NOD mice | ↑Escherichia, ↑Lactobacillus ↑Sutterella |
Disease (type 1 diabetes) 40 weeks | [69] |
| Metronidazole | 500 mg/L for 4 weeks | Mice | ↓Alpha diversity ↓Bacteroidetes ↑Akkermansia muciniphila |
Glucose metabolism 4 weeks | [70] |
| Ampicillin and Gentamicin | Parenteral treatment (within 48 h of birth) | Newborn babies | ↑Proteobacteria ↓Actinobacteria and Lactobacillus |
Developmental 2 months | [71] |
| Cefalexin | 50 mg/kg, 4× per day for 4 days | Newborn babies | ↑Enterococcus spp. and ↑Enterobacteriaceae |
Developmental 7 days | [72] |
| Clindamycin | 150 mg, 4× per day for 7 days | Healthy adults | ↑Frequencies of highly antibiotic-resistant clones ↓Bacteroides diversity |
Diversity 24 months | [73] |
| F-quinolones and β-lactams Combination | Variable dose 7 days | Admitted patients | ↑Bacteroidetes ↓25% microbial taxa |
Infection 1 week | [74] |
| Clarithromycin Metronidazole Combination | 400 + 250 mg, 2× per day for 7 days | Helicobacter pylori-infected adults | ↓Diversity, particularly Actinobacteria in faeces ↑ermB gene levels |
Diversity 6 months | [75] |
Note: ↓, decrease; ↑, Increase; NOD, non-obese diabetic.
5. Microbiota and Neurodegenerative Diseases
Amounting evidence suggests that gut microbiota plays an important role in the development of brain, and that there is a bidirectional relationship between the brain, gut, and the bacteria within the gut which is referred as the brain–gut–microbiome axis [74]. The microbiota can affect regulation of the MGB axis via immunological, neuroendocrine, and direct neural mechanisms. The gut microbiota is known to increase local and systemic inflammation due to lipopolysaccharide (LPS) from pathogenic bacteria and the synthesis of pro-inflammatory cytokines [75,76]. These microorganisms are able to produce neurotransmitters and neuromodulators, such as short-chain fatty acids (SCFAs), biogenic amines (e.g., histamine), and other amino-acid-derived metabolites such as serotonin [77] or GABA [78,79]. In addition, bacterial enzymes may also synthesize neurotoxic metabolites such as d-lactic acid and ammonia [80]. Signaling molecules secreted by the gut microbiota are transferred via the lymphatic and systemic circulation throughout the CNS where they then affect behavior and modulate brain plasticity and cognitive function [81]. This implicates the importance of the gut microbiota in the development and function of the CNS, and in the pathophysiology of chronic brain diseases [80]. Microbiome species and their secretory products are extremely powerful pro-inflammatory and innate-immune activators in the host [23,82,83,84,85,86,87,88,89,90].
A recent study provided additional evidence of the role of molecules secreted by gut microbiota in the brain inflammatory cascade. Two kinds of immune cells in the CNS were reported—the microglia and astrocytes. They communicate through the use of vascular endothelial growth factor B (VEGF-B) and transforming growth factor alpha (TGF-α) to regulate neuroinflammation [91]. This communication is made possible through a ligand-activated transcription factor called aryl hydrocarbon receptor (AHR), which was previously thought to have an exclusive role in processing environmental toxins. In this study, experimental autoimmune encephalitis (EAE) was induced in genetically engineered mice with AHR, which can be easily deleted in microglia (except brain cells and other immune cells) through drug treatment. Elimination of microglial AHR significantly exacerbated EAE, but did not affect immune responses outside the CNS. This suggested that AHR activation in microglia inhibits inflammation in the CNS. The study also demonstrated the direct involvement of AHR in the expression of TGF-α and VEGF-B. Additional in vivo and in vitro analyses of these cytokines showed their role in regulating pro-inflammatory reactivity of astrocytes. TGF-α reduces astrocyte inflammatory responses in EAE, and its expression in microglia is impeded by AHR deletion. In contrast, VEGF-B increases astrocyte inflammatory responses in EAE, and its expression is enhanced by AHR deletion [91]. In addition, CNS inflammation was reduced in antibiotic-treated mice by supplementation with the tryptophan metabolites indole, indoxyl-3-sulfate (I3S), indole-3-propionic acid (IPA), and indole-3-aldehyde (IAld), or the bacterial enzyme tryptophanase [92]. It is worth noting that several of the aforementioned metabolites are proven quorum antagonists [93,94]. In addition to metabolites bacterial peptides readily cross the blood-brain barrier as well [95], which begs the question do these quorum molecules induce significant aberrations in communication between specific neural structures of the brain (dopaminergic (DA) neurons) [96].
The connection between the kind of gut microbiota and AD pathology was shown in a study that used transgenic mouse models. Harach et al. observed a significant shift in the diversity of gut microbiota of APP transgenic mice with that of non-transgenic wild-type mice through sequencing of 16S ribosomal RNA (rRNA) from their fecal samples [97]. Germ-free APP transgenic mice were generated, in which a significant decrease in cerebral Aβ pathology was observed when compared to control mice with intestinal microbiota. Interestingly, an increase in cerebral Aβ pathology was observed in germ-free APP transgenic mice when these were colonized with gut microbiota acquired from conventionally raised APP transgenic mice [98].
The specific role of gut microbiota in modulating neuro-immune functions well beyond the gastrointestinal tract may constitute an important influence on the process of neurodegeneration, especially that associated with AD [41,99]. Bacteria and fungi, as a component of human gut microbiota, secrete amyloid protein in CNS, resulting in Aβ accumulation and increased risk of AD [100,101,102,103,104,105,106,107,108]. Limiting amyloid accumulation via periodic fasting-mimicking diet (FMD) was proposed as an alternative approach to increase the protection of multiple systems in mice and possibly humans [109]. Preliminary results of the randomized, parallel-group, three-arm pilot trial to test the feasibility of prolonged fasting and ketogenic diet in relapsing-remitting multiple sclerosis (IGEL) (clinicaltrials.gov identifier: NCT01538355) showed a reduction in the number of autoimmune lymphocytes, an increase in the β-hydroxybutyrate concentrations in plasma, and a reduction in relapses and expanded disability status scale in the FMD and ketogenic diet groups. FMD ameliorates CNS damage and behavioral outcomes in EAE mice by increasing the corticosterone levels and regulatory T (Treg) cell numbers, and reducing the levels of pro-inflammatory cytokines, T-helper 1 (Th1) and Th17 cells, and antigen-presenting cells (APCs) [110]. These effects are possibly related to an arrest of B- and T-cell development and concomitant selective localization of mature B- and T-cell numbers in the bone marrow seen in fasting mice [111].
Recent studies suggest that gut microbiota is altered in AD patients and may be involved in the pathogenesis of AD. Several bacteria taxa in AD patients were different from those in controls at taxonomic levels, such as Bacteroides Actinobacteria, Ruminococcus, Lachnospiraceae, and Selenomonadales, from 43 AD patients using 16S ribosomal RNA sequencing [112]. In addition, many bacteria are capable of synthesizing and releasing many neurotransmitters and neuromodulators themselves, as well as neuropeptides from enteroendocrine cells, indicating a possible involvement of gut microbiota in the development of AD pathology (Table 2) [23,86,87,88,89,90,97,99,112,113,114].
Table 2.
Potential links between gut microbiota and the development of Alzheimer’s disease (AD).
| Gut Microbiota | Metabolite Product | Effects on Nervous System Function | References |
|---|---|---|---|
| Escherichia, Bacillus, Lactococcus, Lactobacillus, Streptococcus | Dopamine | System activity, Parkinson’s disease, AD, and depression-related | [86,87,88] |
| Lactobacillus, Bacillus | Acetylcholine | Acting on neurotransmitters in the central and peripheral nervous systems, and cognitive function, particularly closely related to learning and memory | [89] |
| Lactobacillus, Lactococcus, Streptococcus, Enterococcus | Histamine | Regulate neurotransmitters; sleep and cognitive function related | [23,90] |
| Gram-negative bacteria | Endotoxin | Induce inflammation, release large amounts of inflammatory cytokines (TNF-α, IL-6, and IL-8, etc.), obesity, IR, and diabetes, and are closely related to the occurrence of AD | [97,113] |
| Actinobacteria, Bacteroidales, Ruminococcaceae, Selenomonadales, and Lachnoclostridium | Neural, endocrine, and immune pathways | Impairment and brain amyloidosis, neuroinflammation | [112] |
| Chlamydiophila pneumoniae, Helicobacter pylori, Toxoplasma gondii | Pro-inflammatory cytokines and induction of oxidative | The presence of the bacteria in astrocytes, microglia, neurons, and in infected cells close to senile plaques and intracellular neurofibrillary tangles | [101,102,103,104] |
| stress, immune regulation, and apoptosis | |||
| Viruses (HSV-1, HIV, human cytomegalo-virus, and hepatitis C) | Microbiome-derived amyloids | Microbial amyloids may play a role in the homeostasis and pathology of the CNS with particular reference to AD | [105,106,107] |
| Porphyromonas gingivalis | Pro-inflammatory cytokines TNF-α, IL-6, and IL-1β | Subsequently increase neuroinflammation and cause neurodegenerative changes and AD | [19,20] |
| Acetobacter and Lactobacillus | Regulating acetate (short-chain fatty acids—SCFA) in Drosophila model | Participate in AD pathogenesis by influencing SCFA level | [99,108] |
TNF-α: tumor necrosis factor alpha; IL-6: interleukin 6; IL-8: interleukin 8; CNS: central nervous system; SCFA: short-chain fatty acids; IR: Insulin resistance; HSV-1, Herpes Simplex Virus 1; HIV, Human Immunodeficiency Virus; CNS: central nervous system.
6. The Role of Inflammation in Alzheimer’s Disease
Inflammatory reactions could be both beneficial and detrimental to the brain, depending on strengths of their activation in various stages of neurodegeneration [112]. Regulation of immuno-inflammatory control is one of the relevant processes involved in the pathogenesis of neurodegenerative disorders. AD shares several common properties with other neurodegenerative disorders, such as accumulation of misfolded proteins (Aβ) and hyperphosphorylated tau, evidence for a prion-like spread of pathology with misfolded proteins and neuroinflammation [9]. The pro-inflammatory gut microbiota dysbiosis in AD patients could trigger inflammation-induced formation and aggregation of cerebral amyloid-β, proving to be an effective strategy for preventing or reducing the risk of AD [115]. Bacterial strains known to produce functional extracellular amyloid fibers are Escherichia coli, Salmonella enterica, Salmonella typhimurium, Bacillus subtilis, Mycobacterium tuberculosis, and Staphylococcus aureus [115]. For instance, E. coli endotoxin was shown to induce the formation of Aβ fibrils in vitro, implying their involvement in AD pathogenesis [115]. Numerous studies established a role for neuroinflammation in AD pathology [116]. The amyloid cascade hypothesis is the prevailing theory that the presence of Aβ plaques primarily results in synaptic dysfunction and dementia in AD. However, this was challenged by Honig et al. when they showed that treatment of AD patients every week for four weeks with 400 mg of solanezumab, which clears soluble Aβ from the brain, did not significantly affect cognitive decline [117,118]. Moreover, the drug efficacy can be affected by some factors such as the presence of the blood–brain barrier. Additional research presented evidence of other key players of neuroinflammation in the CNS, such as microglia, astrocytes, the complement system, and cytokines. More specifically, cytokines play a central role in neuroinflammation [119,120]. Several studies demonstrated abnormally elevated levels of inflammatory cytokines, such as interleukin IL-1β and TNF, in AD patients. Activated microglia were shown to be involved in the secretion of pro-inflammatory cytokines such as IL-1, IL-6, TNF-α, and TGF-β, thereby contributing to the progress of neurological disorders [82,83,91,121]. In AD particularly, proper cessation of inflammation is compromised due to the constant deposition of Aβ plaques, as well as the positive feedback loop between inflammation and amyloid precursor protein (APP) processing [118]. In addition, studies on AD pathogenesis showed that microglia produce Aβ, which by itself is pro-inflammatory and causes activation of several inflammatory mediators [12,122,123,124,125].
Activated astrocytes are supportive cells that provide trophic and metabolic maintenance for neurons and are also influential in neuroinflammation in AD. Changes in astrocyte morphology, gene expression, protein composition, and activity can be observed in AD, which in turn can compromise astrocyte function [126]. Several transgenic mouse models showed that activated astrocytes accumulate in the brain before any plaque or tangle pathology can be observed [119], suggesting that astrocyte activation may be involved in AD pathogenesis. Astrocytes outnumber microglia in the brain and have a greater influence on long-term neuroinflammation. They also secrete pro-inflammatory cytokines and chemokines to process and clear away accumulated Aβ. The additional deposition of Aβ then results in a positive feedback loop that furthers astrocyte activation resulting in the release of more pro-inflammatory factors [118]. Thus, it is essential to further explore the key inflammatory components in AD pathogenesis in order to develop better treatments for these disorders (Figure 2).
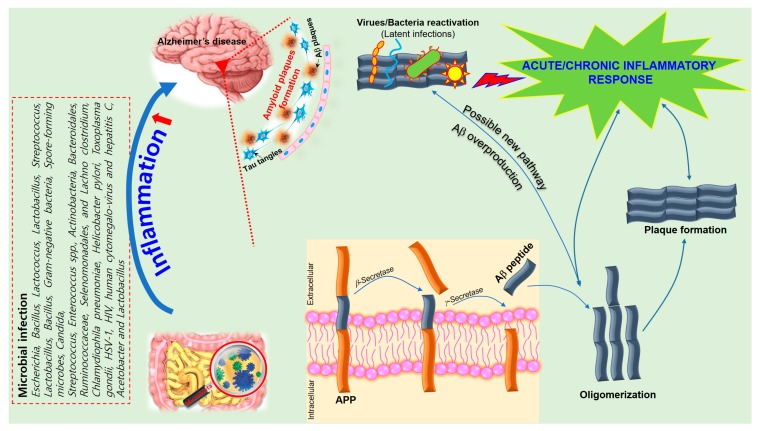
Figure 2.
A schematic of the hypothetical chain of events via which brain infection may lead to pathological amyloid-β peptide (Aβ) plaque formation in the brain. The amyloid precursor protein (APP) is processed by secretases into different peptides, including Aβ. The gut microbiota plays a significant role in the development of Alzheimer’s disease (AD) since Aβ functions as an antimicrobial peptide via oligomerization and plaque formation, trapping invading microorganisms, including bacteria, fungi, viruses, and protist parasites (detailed in Table 2). Aβ plaque formation in response to infection could result in a neuroinflammatory effect of microbiota on AD and neurodegeneration due to collateral damage in plaque-surrounding tissue.
7. Neuroinflammatory Effects of Microbiota on AD
In AD, the cerebellum is replete of microglia in areas of amyloid deposition, and cerebellar volume is reduced [127]. Molecular layer gliosis and atrophy in the vermis is also severe. Loss of Purkinje neurons occurs in the vermis, cerebellar hemispheres, and the inferior olivary nucleus [128,129]. Atrophy of the molecular layer by 24% and the granular layer by 22% correlates with a decrease in Purkinje cell numbers [130]. This might be related to BDNF, which is essential for the maintenance and survival of neurons, and which has pleiotropic effects on neuronal development, differentiation, synaptogenesis, and the synaptic plasticity that underlies neuronal circuit formation and cognition. Decreased amounts of BDNF were found in AD brains [131,132]. Interestingly, mice deficient in BDNF have altered development of GI-tract innervations [133,134]. BDNF expression was found to be reduced in the hippocampus and cortex of germ-free mice, and reduced expression of BDNF was found to be specifically associated with increased anxiety and progressive cognitive dysfunction [91,135].
Pro-inflammatory cytokines are already known to enhance APP expression, upregulate β-secretase messenger RNA (mRNA), and increase Aβ formation in the hippocampus. Similarly, bacterial amyloid is recognized as a pathogen-associated molecular pattern that can cause activation of Toll-like receptor-2 (TLR2), which was also reported to induce Notch1 upregulation and activation of microglia, and may enhance processes leading to the development of AD or Parkinson’s disease (PD) [135]. Functional bacterial amyloid may be the source of (1) misfolding of neuronal proteins through cross-seeding and (2) activation of the innate immune system and priming of neuroinflammation. The unique structure of this foreign amyloid might induce specific misfolding patterns which could be responsible for the variety of phenotypes in neurodegenerative disorders. APP expression may also influence the kind of microbiota that thrive in the gut. Reduction in populations of Firmicutes, Verrucomicrobia, Proteobacteria, and Actinobacteria were observed in the gut of conventionally raised amyloid precursor protein (APP) gene with Swedish mutation and presenilin 1 gene (PS1) with deletion of exon 9 (APPPS1) transgenic mice when compared to normal wild-type controls, both aged eight months [98]. In contrast, an increase in Bacteroidetes and Tenericutes phyla were observed [98]. Subsequently, germ-free generated APPPS1 mice showed decreased levels of cerebral Aβ42 when compared to conventionally raised APPPS1 mice, further supporting the possible influence of gut microbiota on APP expression [98].
Chromogranin A (CHGA) is a neuroinflammatory factor which is frequently present in AD senile plaques associated with microglial activation [136]. Wu and Nakanishi found that CHGA activated both nuclear factor kappa B (NF-κB) and pro-caspase-1, while Aβ only activated pro-caspase-1. For activation of pro-caspase-1, both CHGA and Aβ require the enzymatic activity of cathepsin B (CatB). In the AD brain, highly activated microglia showed intense immunoreactivity for CatB and IL-1β, and they surround CHGA-positive plaques more frequently than Aβ-positive plaques. This suggests different pathways for CHGA- and Aβ-induced microglial production of IL-1β, which may help us gain a better understanding of the pathological significance of neuroinflammation in AD [137].
In addition, chronic gut inflammation may increase the breakdown of blood–brain barrier, LPS permeability, and the generation of pro-inflammatory cytokines. Elevated levels of LPS were found in the plasma of AD patients compared to healthy controls [82]. In an animal study, peripheral administration of LPS led to neuroinflammation with an increase in levels of IL-6. Moreover, overexpression of IL-1β resulted in a robust increase in tau phosphorylation in the triple transgenic mouse model of AD [121,135]. Furthermore, recent studies suggested that increased concentrations of circulating LPS, promoted by changes in intestinal permeability, may play a pivotal role in insulin resistance. Neuronal insulin resistance can increase the risk of developing AD, and insulin treatment may enhance memory function. Carvalho et al. found that antibiotic treatment greatly modifies the gut microbiota by reducing levels of Bacteroidetes and Firmicutes and circulating LPS levels. This modulation consequently improved glucose and insulin tolerance and activity in metabolically active tissues [138].
Furthermore, Villaran and co-workers reported that peripheral inflammation in the form of dextran sodium sulfate (DSS)-induced colitis can aggravate LPS-induced neuroinflammation and neurodegeneration as shown by increased mRNA transcripts of TNF-α, inducible nitric oxide synthase (iNOS), and IL-6 in the midbrain [4]. Interestingly, even rats with colitis alone, which received no midbrain injection of LPS, showed increased levels of TNF-α, iNOS, and IL-6 mRNA in the midbrain. Other studies also demonstrated that recurring systemic infections can increase the probability of developing multiple sclerosis (MS), AD or PD [139]. Table 2 listed the effects of some particular gut microbiota and their corresponding metabolites on nervous system function. Also included are endotoxins produced by Gram-negative bacteria, which further impact neuroinflammation through inflammatory cytokines in conditions like obesity, diabetes, and possibly AD. LPS is also a structural component of Gram-negative bacterial membranes in the gut. When the bacteria die, LPS is shed from their membranes and floats freely in the lumen as a bacterial endotoxin. LPS is harmless and is isolated from the bloodstream if the intestinal lining is intact. However, damage to the lining can cause LPS to penetrate and cause low-grade inflammation. LPS from the intestinal tract was found to be abundant in the neocortex and hippocampus of AD-affected brains [140].
Hence, the microbiota is closely related to neurological dysfunction and plays a significant role in neuroinflammation through the secretion of pro-inflammatory cytokines. Changes in the homeostatic state of the microbiota leads to increased intestinal permeability, which may promote the translocation of bacteria and endotoxins across the epithelial barrier, inducing an immunological response associated with the production of pro-inflammatory cytokines. The activation of both enteric neurons and glial cells may result in various neurological disorders [139].
8. Conclusions
This review described the importance of the MGB axis and the bidirectional communication between the gut and brain in regulating the health of the host. A healthy GIT in a homeostatic state has a normal and stable commensal intestinal microbiota and provides the host with nutrition and energy by producing vitamins. Braak and colleagues hypothesized that disease begins in the gut and spreads from the gut to the brain via the MGB axis. Gut-related inflammation is essential in provoking endotoxemia, systemic inflammation, and neuroinflammation, which can contribute to neurodevelopmental disorders and the onset of psychiatric illness in later life.
Previous studies provided considerable evidence of the effect of gut microbiota on the CNS, as well as its neuroinflammatory role in neurodegenerative diseases like AD. The production of amino acids by gut microbiota, which serve as components for neurotransmitters, can influence neurons both in the CNS and ENS. The kind of gut microbiota also plays a role in AD pathology as shown by cross-colonization experiments using germ-free and control APP transgenic mice. Pro-inflammatory cytokines secreted by microglia and astrocytes, and continuous Aβ deposition create a positive feedback loop that furthers neurodegeneration. This is especially made more significant by a dysregulated MGB axis, as well as chronic gut inflammation, which increases intestinal permeability, leading to the release of more pro-inflammatory factors in the CNS. Thus, it is essential to direct future studies on cell signaling and cytokine regulation to suppress neuroinflammation in AD.
Author Contributions
Writing—Original Draft Preparation, V.V.G. and S.Y.W.; Literature Search, A.J.; Writing—Review & Editing, S.S.A.A., S.Y.K., and J.H.; Supervision, J.H.
Funding
This research was funded by a National Research Foundation of Korea (NRF) awarded by the Korean government (NRF, No 2017R1A2B4012636).
Conflicts of Interest
There are no conflicts of interest to declare.
References
- 1.Fung T.C., Olson C.A., Hsiao E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017;20:145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sender R., Fuchs S., Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villaran R.F., Espinosa-Oliva A.M., Sarmiento M., De Pablos R.M., Arguelles S., Delgado-Cortes M.J., Sobrino V., Van Rooijen N., Venero J.L., Herrera A.J., et al. Ulcerative colitis exacerbates lipopolysaccharide-induced damage to the nigral dopaminergic system: Potential risk factor in Parkinson’s disease. J. Neurochem. 2010;114:1687–1700. doi: 10.1111/j.1471-4159.2010.06879.x. [DOI] [PubMed] [Google Scholar]
- 5.O’Hara A.M., Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morais G.C.P., Arruda M.M., Bonadia J.C.A., Pozzan G. Cardiac amyloidosis: A challenging diagnosis. Autops. Case Rep. 2014;4:9–17. doi: 10.4322/acr.2014.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sperry B.W., Tang W.H.W. Amyloid heart disease: Genetics translated into disease-modifying therapy. Heart. 2017;103:812–817. doi: 10.1136/heartjnl-2016-309914. [DOI] [PubMed] [Google Scholar]
- 8.Rogers G.B., Keating D.J., Young R.L., Wong M.L., Licinio J., Wesselingh S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry. 2016;21:738–748. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bostanciklioğlu M. Intestinal Bacterial Flora and Alzheimer’s Disease. Neurophysiology. 2018;50:140–148. doi: 10.1007/s11062-018-9728-0. [DOI] [Google Scholar]
- 10.Morris G., Berk M., Maes M., Puri B.K. Could Alzheimer’s Disease Originate in the Periphery and If So How So? Mol. Neurobiol. 2018 doi: 10.1007/s12035-018-1092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukiw W.J., Bazan N.G. Survival signalling in Alzheimer’s disease. Pt 6Biochem. Soc. Trans. 2006;34:1277–1282. doi: 10.1042/BST0341277. [DOI] [PubMed] [Google Scholar]
- 12.Bagyinszky E., Giau V.V., Shim K., Suk K., An S.S.A., Kim S. Role of inflammatory molecules in the Alzheimer’s disease progression and diagnosis. J. Neurol. Sci. 2017;376:242–254. doi: 10.1016/j.jns.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 13.Cammarota G., Ianiro G., Bibbo S., Gasbarrini A. Gut microbiota modulation: Probiotics, antibiotics or fecal microbiota transplantation? Intern. Emerg. Med. 2014;9:365–373. doi: 10.1007/s11739-014-1069-4. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt T.S.B., Raes J., Bork P. The Human Gut Microbiome: From Association to Modulation. Cell. 2018;172:1198–1215. doi: 10.1016/j.cell.2018.02.044. [DOI] [PubMed] [Google Scholar]
- 15.Cox L.M., Weiner H.L. Microbiota Signaling Pathways that Influence Neurologic Disease. Neurotherapeutics. 2018;15:135–145. doi: 10.1007/s13311-017-0598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Toole P.W., Jeffery I.B. Microbiome-health interactions in older people. Cell. Mol. Life Sci. CMLS. 2018;75:119–128. doi: 10.1007/s00018-017-2673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson J.K., Holmes E., Kinross J., Burcelin R., Gibson G., Jia W., Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 18.Yatsunenko T., Rey F.E., Manary M.J., Trehan I., Dominguez-Bello M.G., Contreras M., Magris M., Hidalgo G., Baldassano R.N., Anokhin A.P., et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arimatsu K., Yamada H., Miyazawa H., Minagawa T., Nakajima M., Ryder M.I., Gotoh K., Motooka D., Nakamura S., Iida T., et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 2014;4:4828. doi: 10.1038/srep04828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding Y., Ren J., Yu H., Yu W., Zhou Y. Porphyromonas gingivalis, a periodontitis causing bacterium, induces memory impairment and age-dependent neuroinflammation in mice. Immun. Ageing. 2018;15:6. doi: 10.1186/s12979-017-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briguglio M., Dell’Osso B., Panzica G., Malgaroli A., Banfi G., Zanaboni Dina C., Galentino R., Porta M. Dietary Neurotransmitters: A Narrative Review on Current Knowledge. Nutrients. 2018;10:591. doi: 10.3390/nu10050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyte M., Villageliú D.N., Crooker B.A., Brown D.R. Symposium review: Microbial endocrinology—Why the integration of microbes, epithelial cells, and neurochemical signals in the digestive tract matters to ruminant health1. J. Dairy Sci. 2018;101:5619–5628. doi: 10.3168/jds.2017-13589. [DOI] [PubMed] [Google Scholar]
- 23.Thomas C.M., Hong T., van Pijkeren J.P., Hemarajata P., Trinh D.V., Hu W., Britton R.A., Kalkum M., Versalovic J. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS ONE. 2012;7:e31951. doi: 10.1371/journal.pone.0031951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wall R., Cryan J.F., Ross R.P., Fitzgerald G.F., Dinan T.G., Stanton C. Bacterial neuroactive compounds produced by psychobiotics. Adv. Exp. Med. Biol. 2014;817:221–239. doi: 10.1007/978-1-4939-0897-4_10. [DOI] [PubMed] [Google Scholar]
- 25.Kau A.L., Ahern P.P., Griffin N.W., Goodman A.L., Gordon J.I. Human nutrition, the gut microbiome, and immune system: Envisioning the future. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukudo S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J. Gastroenterol. 2007;42(Suppl. 17):48–51. doi: 10.1007/s00535-006-1942-7. [DOI] [PubMed] [Google Scholar]
- 27.Nishino R., Mikami K., Takahashi H., Tomonaga S., Furuse M., Hiramoto T., Aiba Y., Koga Y., Sudo N. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol. Motil. 2013;25:521–528. doi: 10.1111/nmo.12110. [DOI] [PubMed] [Google Scholar]
- 28.Hooper L.V., Littman D.R., Macpherson A.J. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmes E., Kinross J., Gibson G.R., Burcelin R., Jia W., Pettersson S., Nicholson J.K. Therapeutic modulation of microbiota-host metabolic interactions. Sci. Transl. Med. 2012;4:137rv6. doi: 10.1126/scitranslmed.3004244. [DOI] [PubMed] [Google Scholar]
- 30.Santilli A.D., Dawson E.M., Whitehead K.J., Whitehead D.C. Nonmicrobicidal Small Molecule Inhibition of Polysaccharide Metabolism in Human Gut Microbes: A Potential Therapeutic Avenue. ACS Chem. Biol. 2018;13:1165–1172. doi: 10.1021/acschembio.8b00309. [DOI] [PubMed] [Google Scholar]
- 31.Forsythe P., Bienenstock J. Immunomodulation by commensal and probiotic bacteria. Immunol. Investig. 2010;39:429–448. doi: 10.3109/08820131003667978. [DOI] [PubMed] [Google Scholar]
- 32.Bercik P., Park A.J., Sinclair D., Khoshdel A., Lu J., Huang X., Deng Y., Blennerhassett P.A., Fahnestock M., Moine D., et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halfvarson J., Brislawn C.J., Lamendella R., Vazquez-Baeza Y., Walters W.A., Bramer L.M., D’Amato M., Bonfiglio F., McDonald D., Gonzalez A., et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2017;2:17004. doi: 10.1038/nmicrobiol.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moser G., Fournier C., Peter J. Intestinal microbiome-gut-brain axis and irritable bowel syndrome. Wiener Medizinische Wochenschrift. 2018;168:62–66. doi: 10.1007/s10354-017-0592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atamna A., Elis A., Gilady E., Gitter-Azulay L., Bishara J. How obesity impacts outcomes of infectious diseases. Eur. J. Clin. Microbiol. Infect. Dis. 2017;36:585–591. doi: 10.1007/s10096-016-2835-1. [DOI] [PubMed] [Google Scholar]
- 36.Le Roy C.I., Beaumont M., Jackson M.A., Steves C.J., Spector T.D., Bell J.T. Heritable components of the human fecal microbiome are associated with visceral fat. Gut microbes. 2018;9:61–67. doi: 10.1080/19490976.2017.1356556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mennini M., Dahdah L., Artesani M.C., Fiocchi A., Martelli A. Probiotics in Asthma and Allergy Prevention. Front. Pediatr. 2017;5:165. doi: 10.3389/fped.2017.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulders R.J., de Git K.C.G., Schele E., Dickson S.L., Sanz Y., Adan R.A.H. Microbiota in obesity: Interactions with enteroendocrine, immune and central nervous systems. Obes. Rev. 2018;19:435–451. doi: 10.1111/obr.12661. [DOI] [PubMed] [Google Scholar]
- 39.Stilling R.M., Dinan T.G., Cryan J.F. Microbial genes, brain & behavior—Epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014;13:69–86. doi: 10.1111/gbb.12109. [DOI] [PubMed] [Google Scholar]
- 40.Dinan T.G., Cryan J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017;595:489–503. doi: 10.1113/JP273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohler C.A., Maes M., Slyepchenko A., Berk M., Solmi M., Lanctot K.L., Carvalho A.F. The Gut-Brain Axis, Including the Microbiome, Leaky Gut and Bacterial Translocation: Mechanisms and Pathophysiological Role in Alzheimer’s Disease. Curr. Pharm. Des. 2016;22:6152–6166. doi: 10.2174/1381612822666160907093807. [DOI] [PubMed] [Google Scholar]
- 42.Cussotto S., Sandhu K.V., Dinan T.G., Cryan J.F. The Neuroendocrinology of the Microbiota-Gut-Brain Axis: A Behavioural Perspective. Front. Neuroendocrinol. 2018;51:80–101. doi: 10.1016/j.yfrne.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Münger E., Montiel-Castro A.J., Langhans W., Pacheco-López G. Reciprocal Interactions Between Gut Microbiota and Host Social Behavior. Front. Integr. Neurosci. 2018;12:21. doi: 10.3389/fnint.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowan C.S.M., Hoban A.E., Ventura-Silva A.P., Dinan T.G., Clarke G., Cryan J.F. Gutsy Moves: The Amygdala as a Critical Node in Microbiota to Brain Signaling. BioEssays. 2018;40 doi: 10.1002/bies.201700172. [DOI] [PubMed] [Google Scholar]
- 45.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Distrutti E., Monaldi L., Ricci P., Fiorucci S. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World J. Gastroenterol. 2016;22:2219–2241. doi: 10.3748/wjg.v22.i7.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hestad K.A., Engedal K., Whist J.E., Farup P.G. The Relationships among Tryptophan, Kynurenine, Indoleamine 2,3-Dioxygenase, Depression, and Neuropsychological Performance. Front. Psychol. 2017;8:1561. doi: 10.3389/fpsyg.2017.01561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tillisch K., Labus J., Kilpatrick L., Jiang Z., Stains J., Ebrat B., Guyonnet D., Legrain-Raspaud S., Trotin B., Naliboff B., et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–1401. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Palma G., Collins S.M., Bercik P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut Microbes. 2014;5:419–429. doi: 10.4161/gmic.29417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Backhed F. Programming of host metabolism by the gut microbiota. Ann. Nutr. Metab. 2011;58(Suppl. 2):44–52. doi: 10.1159/000328042. [DOI] [PubMed] [Google Scholar]
- 51.Luczynski P., McVey Neufeld K.A., Oriach C.S., Clarke G., Dinan T.G., Cryan J.F. Growing up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior. Int. J. Neuropsychopharmacol. 2016:19. doi: 10.1093/ijnp/pyw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hansen C.H., Nielsen D.S., Kverka M., Zakostelska Z., Klimesova K., Hudcovic T., Tlaskalova-Hogenova H., Hansen A.K. Patterns of early gut colonization shape future immune responses of the host. PLoS ONE. 2012;7:e34043. doi: 10.1371/journal.pone.0034043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olszak T., An D., Zeissig S., Vera M.P., Richter J., Franke A., Glickman J.N., Siebert R., Baron R.M., Kasper D.L., et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pabst O., Cerovic V., Hornef M. Secretory IgA in the Coordination of Establishment and Maintenance of the Microbiota. Trends Immunol. 2016;37:287–296. doi: 10.1016/j.it.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 55.McCoy K.D., Ronchi F., Geuking M.B. Host-microbiota interactions and adaptive immunity. Immunol. Rev. 2017;279:63–69. doi: 10.1111/imr.12575. [DOI] [PubMed] [Google Scholar]
- 56.Macpherson A.J., Geuking M.B., McCoy K.D. Homeland security: IgA immunity at the frontiers of the body. Trends Immunol. 2012;33:160–167. doi: 10.1016/j.it.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 57.Girolamo F., Coppola C., Ribatti D. Immunoregulatory effect of mast cells influenced by microbes in neurodegenerative diseases. Brain Behav. Immun. 2017;65:68–89. doi: 10.1016/j.bbi.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 58.Lyte M., Li W., Opitz N., Gaykema R.P., Goehler L.E. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol. Behav. 2006;89:350–357. doi: 10.1016/j.physbeh.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 59.Hoban A.E., Stilling R.M., Moloney G., Shanahan F., Dinan T.G., Clarke G., Cryan J.F. The microbiome regulates amygdala-dependent fear recall. Mol. Psychiatry. 2018;23:1134–1144. doi: 10.1038/mp.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bercik P., Verdu E.F., Foster J.A., Macri J., Potter M., Huang X., Malinowski P., Jackson W., Blennerhassett P., Neufeld K.A., et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- 61.He B., Hoang T.K., Wang T., Ferris M., Taylor C.M., Tian X., Luo M., Tran D.Q., Zhou J., Tatevian N., et al. Resetting microbiota by Lactobacillus reuteri inhibits T reg deficiency-induced autoimmunity via adenosine A2A receptors. J. Exp. Med. 2017;214:107–123. doi: 10.1084/jem.20160961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Shea E.F., Cotter P.D., Stanton C., Ross R.P., Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: Bacteriocins and conjugated linoleic acid. Int. J. Food Microbiol. 2012;152:189–205. doi: 10.1016/j.ijfoodmicro.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 63.Grant M.C., Baker J.S. An overview of the effect of probiotics and exercise on mood and associated health conditions. Crit. Rev. Food Sci. Nutr. 2017;57:3887–3893. doi: 10.1080/10408398.2016.1189872. [DOI] [PubMed] [Google Scholar]
- 64.Ventura M., O’Flaherty S., Claesson M.J., Turroni F., Klaenhammer T.R., van Sinderen D., O’Toole P.W. Genome-scale analyses of health-promoting bacteria: Probiogenomics. Nat. Rev. Microbiol. 2009;7:61–71. doi: 10.1038/nrmicro2047. [DOI] [PubMed] [Google Scholar]
- 65.Vanhaecke T., Aubert P., Grohard P.A., Durand T., Hulin P., Paul-Gilloteaux P., Fournier A., Docagne F., Ligneul A., Fressange-Mazda C., et al. L. fermentum CECT 5716 prevents stress-induced intestinal barrier dysfunction in newborn rats. Neurogastroenterol. Motil. 2017;29:e13069. doi: 10.1111/nmo.13069. [DOI] [PubMed] [Google Scholar]
- 66.Ladirat S.E., Schoterman M.H., Rahaoui H., Mars M., Schuren F.H., Gruppen H., Nauta A., Schols H.A. Exploring the effects of galacto-oligosaccharides on the gut microbiota of healthy adults receiving amoxicillin treatment. Br. J. Nutr. 2014;112:536–546. doi: 10.1017/S0007114514001135. [DOI] [PubMed] [Google Scholar]
- 67.Tulstrup M.V.-L., Christensen E.G., Carvalho V., Linninge C., Ahrné S., Højberg O., Licht T.R., Bahl M.I. Antibiotic Treatment Affects Intestinal Permeability and Gut Microbial Composition in Wistar Rats Dependent on Antibiotic Class. PLoS ONE. 2015;10:e0144854. doi: 10.1371/journal.pone.0144854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vrieze A., Out C., Fuentes S., Jonker L., Reuling I., Kootte R.S., van Nood E., Holleman F., Knaapen M., Romijn J.A., et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol. 2014;60:824–831. doi: 10.1016/j.jhep.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 69.Candon S., Perez-Arroyo A., Marquet C., Valette F., Foray A.P., Pelletier B., Milani C., Ventura M., Bach J.F., Chatenoud L. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes. PLoS ONE. 2015;10:e0125448. doi: 10.1371/journal.pone.0125448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodrigues R.R., Greer R.L., Dong X., KN D.S., Gurung M., Wu J.Y., Morgun A., Shulzhenko N. Antibiotic-Induced Alterations in Gut Microbiota Are Associated with Changes in Glucose Metabolism in Healthy Mice. Front. Microbiol. 2017;8:2306. doi: 10.3389/fmicb.2017.02306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fouhy F., Guinane C.M., Hussey S., Wall R., Ryan C.A., Dempsey E.M., Murphy B., Ross R.P., Fitzgerald G.F., Stanton C., et al. High-throughput sequencing reveals the incomplete, short-term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob. Agents Chemother. 2012;56:5811–5820. doi: 10.1128/AAC.00789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka S., Kobayashi T., Songjinda P., Tateyama A., Tsubouchi M., Kiyohara C., Shirakawa T., Sonomoto K., Nakayama J. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol. Med. Microbiol. 2009;56:80–87. doi: 10.1111/j.1574-695X.2009.00553.x. [DOI] [PubMed] [Google Scholar]
- 73.Jernberg C., Lofmark S., Edlund C., Jansson J.K. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007;1:56–66. doi: 10.1038/ismej.2007.3. [DOI] [PubMed] [Google Scholar]
- 74.Panda S., El khader I., Casellas F., Lopez Vivancos J., Garcia Cors M., Santiago A., Cuenca S., Guarner F., Manichanh C. Short-term effect of antibiotics on human gut microbiota. PLoS ONE. 2014;9:e95476. doi: 10.1371/journal.pone.0095476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jakobsson H.E., Jernberg C., Andersson A.F., Sjolund-Karlsson M., Jansson J.K., Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS ONE. 2010;5:e9836. doi: 10.1371/journal.pone.0009836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rieder R., Wisniewski P.J., Alderman B.L., Campbell S.C. Microbes and mental health: A review. Brain Behave. Immunity. 2017;66:9–17. doi: 10.1016/j.bbi.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 77.Albenberg L.G., Wu G.D. Diet and the intestinal microbiome: Associations, functions, and implications for health and disease. Gastroenterology. 2014;146:1564–1572. doi: 10.1053/j.gastro.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Asti A., Gioglio L. Can a bacterial endotoxin be a key factor in the kinetics of amyloid fibril formation? J. Alzheimer’s Dis. JAD. 2014;39:169–179. doi: 10.3233/JAD-131394. [DOI] [PubMed] [Google Scholar]
- 79.Collins S.M., Surette M., Bercik P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 80.Hanstock T.L., Mallet P.E., Clayton E.H. Increased plasma d-lactic acid associated with impaired memory in rats. Physiol. Behav. 2010;101:653–659. doi: 10.1016/j.physbeh.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 81.De Haas E.N., van der Eijk J.A.J. Where in the serotonergic system does it go wrong? Unravelling the route by which the serotonergic system affects feather pecking in chickens. Neurosci. Biobehav. Rev. 2018;95:170–188. doi: 10.1016/j.neubiorev.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 82.Hernandez-Rapp J., Martin-Lannerée S., Hirsch T.Z., Launay J.-M., Mouillet-Richard S. Hijacking PrP(c)-dependent signal transduction: When prions impair Aβ clearance. Front. Aging Neurosci. 2014;6:25. doi: 10.3389/fnagi.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Daulatzai M.A. Chronic functional bowel syndrome enhances gut-brain axis dysfunction, neuroinflammation, cognitive impairment, and vulnerability to dementia. Neurochem. Res. 2014;39:624–644. doi: 10.1007/s11064-014-1266-6. [DOI] [PubMed] [Google Scholar]
- 84.Johnson K.V., Foster K.R. Why does the microbiome affect behaviour? Nat. Rev. Microbiol. 2018;16:647–655. doi: 10.1038/s41579-018-0014-3. [DOI] [PubMed] [Google Scholar]
- 85.Rhee S.H., Pothoulakis C., Mayer E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsavkelova E.A., Botvinko I.V., Kudrin V.S., Oleskin A.V. Detection of neurotransmitter amines in microorganisms with the use of high-performance liquid chromatography. Dokl. Biochem. 2000;372:115–117. [PubMed] [Google Scholar]
- 87.Shishov V.A., Kirovskaia T.A., Kudrin V.S., Oleskin A.V. (Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12) Prikladnaia Biokhimiia i Mikrobiologiia. 2009;45:550–554. doi: 10.1134/S0003683809050068. [DOI] [PubMed] [Google Scholar]
- 88.Özogul F. Effects of specific lactic acid bacteria species on biogenic amine production by foodborne pathogen. Int. J. Food Sci. Technol. 2011;46:478–484. doi: 10.1111/j.1365-2621.2010.02511.x. [DOI] [Google Scholar]
- 89.Kawashima K., Misawa H., Moriwaki Y., Fujii Y.X., Fujii T., Horiuchi Y., Yamada T., Imanaka T., Kamekura M. Ubiquitous expression of acetylcholine and its biological functions in life forms without nervous systems. Life Sci. 2007;80:2206–2209. doi: 10.1016/j.lfs.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 90.Landete J.M., De las Rivas B., Marcobal A., Munoz R. Updated molecular knowledge about histamine biosynthesis by bacteria. Crit. Rev. Food Sci. Nutr. 2008;48:697–714. doi: 10.1080/10408390701639041. [DOI] [PubMed] [Google Scholar]
- 91.Rothhammer V., Borucki D.M., Tjon E.C., Takenaka M.C., Chao C.-C., Ardura-Fabregat A., de Lima K.A., Gutiérrez-Vázquez C., Hewson P., Staszewski O., et al. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018;557:724–728. doi: 10.1038/s41586-018-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wekerle H. Brain inflammatory cascade controlled by gut-derived molecules. Nature. 2018;557:642–643. doi: 10.1038/d41586-018-05113-0. [DOI] [PubMed] [Google Scholar]
- 93.Cho H.S., Lee J.H., Ryu S.Y., Joo S.W., Cho M.H., Lee J. Inhibition of Pseudomonas aeruginosa and Escherichia coli O157:H7 biofilm formation by plant metabolite epsilon-viniferin. J. Agric. Food Chem. 2013;61:7120–7126. doi: 10.1021/jf4009313. [DOI] [PubMed] [Google Scholar]
- 94.Gao J., Xu K., Liu H., Liu G., Bai M., Peng C., Li T., Yin Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018;8:13. doi: 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wynendaele E., Verbeke F., Stalmans S., Gevaert B., Janssens Y., Van De Wiele C., Peremans K., Burvenich C., De Spiegeleer B. Quorum Sensing Peptides Selectively Penetrate the Blood-Brain Barrier. PLoS ONE. 2015;10:e0142071. doi: 10.1371/journal.pone.0142071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schluter J., Schoech A.P., Foster K.R., Mitri S. The Evolution of Quorum Sensing as a Mechanism to Infer Kinship. PLoS Comput. Biol. 2016;12:e1004848. doi: 10.1371/journal.pcbi.1004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Levi M., Keller T.T., van Gorp E., ten Cate H. Infection and inflammation and the coagulation system. Cardiovasc. Res. 2003;60:26–39. doi: 10.1016/S0008-6363(02)00857-X. [DOI] [PubMed] [Google Scholar]
- 98.Harach T., Marungruang N., Duthilleul N., Cheatham V., Mc Coy K.D., Frisoni G., Neher J.J., Fåk F., Jucker M., Lasser T., et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017;7:41802. doi: 10.1038/srep41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feng Q., Chen W.-D., Wang Y.-D. Gut Microbiota: An Integral Moderator in Health and Disease. Front. Microbiol. 2018;9:151. doi: 10.3389/fmicb.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hill J.M., Lukiw W.J. Microbial-generated amyloids and Alzheimer’s disease (AD) Front. Aging Neurosci. 2015;7:9. doi: 10.3389/fnagi.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roubaud-Baudron C., Krolak-Salmon P., Quadrio I., Megraud F., Salles N. Impact of chronic Helicobacter pylori infection on Alzheimer’s disease: Preliminary results. Neurobiol. Aging. 2012;33:1009.e11–1019.e19. doi: 10.1016/j.neurobiolaging.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 102.Gerard H.C., Dreses-Werringloer U., Wildt K.S., Deka S., Oszust C., Balin B.J., Frey W.H., 2nd, Bordayo E.Z., Whittum-Hudson J.A., Hudson A.P. Chlamydophila (Chlamydia) pneumoniae in the Alzheimer’s brain. FEMS Immunol. Med. Microbiol. 2006;48:355–366. doi: 10.1111/j.1574-695X.2006.00154.x. [DOI] [PubMed] [Google Scholar]
- 103.Kountouras J., Gavalas E., Zavos C., Stergiopoulos C., Chatzopoulos D., Kapetanakis N., Gisakis D. Alzheimer’s disease and Helicobacter pylori infection: Defective immune regulation and apoptosis as proposed common links. Med. Hypotheses. 2007;68:378–388. doi: 10.1016/j.mehy.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 104.Prandota J. Possible link between Toxoplasma gondii and the anosmia associated with neurodegenerative diseases. Am. J. Alzheimer’s Dis. Dement. 2014;29:205–214. doi: 10.1177/1533317513517049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ball M.J. Limbic predilection in Alzheimer dementia: Is reactivated herpesvirus involved? Can. J. Neurol. Sci. Le J. Can. Sci. Neurol. 1982;9:303–306. doi: 10.1017/S0317167100044115. [DOI] [PubMed] [Google Scholar]
- 106.Lurain N.S., Hanson B.A., Martinson J., Leurgans S.E., Landay A.L., Bennett D.A., Schneider J.A. Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J. Infect. Dis. 2013;208:564–572. doi: 10.1093/infdis/jit210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chiu W.C., Tsan Y.T., Tsai S.L., Chang C.J., Wang J.D., Chen P.C. Hepatitis C viral infection and the risk of dementia. Eur. J. Neurol. 2014;21:1068-59. doi: 10.1111/ene.12317. [DOI] [PubMed] [Google Scholar]
- 108.Kong Y., Jiang B., Luo X. Gut microbiota influences Alzheimer’s disease pathogenesis by regulating acetate in Drosophila model. Future Microbiol. 2018;13:1117–1128. doi: 10.2217/fmb-2018-0185. [DOI] [PubMed] [Google Scholar]
- 109.Jiang N.M., Cowan M., Moonah S.N., Petri W.A., Jr. The Impact of Systemic Inflammation on Neurodevelopment. Trends Mol. Med. 2018;24:794–804. doi: 10.1016/j.molmed.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peruzzotti-Jametti L., Pluchino S. Targeting Mitochondrial Metabolism in Neuroinflammation: Towards a Therapy for Progressive Multiple Sclerosis. Trends Mol. Med. 2018;24:838–855. doi: 10.1016/j.molmed.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 111.Deussing J.M., Arzt E. P2X7 Receptor: A Potential Therapeutic Target for Depression? Trends Mol. Med. 2018;24:736–747. doi: 10.1016/j.molmed.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 112.Zhuang Z.Q., Shen L.L., Li W.W., Fu X., Zeng F., Gui L., Lu Y., Cai M., Zhu C., Tan Y.L., et al. Gut Microbiota is Altered in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. JAD. 2018;63:1337–1346. doi: 10.3233/JAD-180176. [DOI] [PubMed] [Google Scholar]
- 113.Wang X., Quinn P.J. Endotoxins: Lipopolysaccharides of gram-negative bacteria. Sub-Cell. Biochem. 2010;53:3–25. doi: 10.1007/978-90-481-9078-2_1. [DOI] [PubMed] [Google Scholar]
- 114.Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., Nagler C.R., Ismagilov R.F., Mazmanian S.K., Hsiao E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pistollato F., Sumalla Cano S., Elio I., Masias Vergara M., Giampieri F., Battino M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016;74:624–634. doi: 10.1093/nutrit/nuw023. [DOI] [PubMed] [Google Scholar]
- 116.Calvo-Ochoa E., Arias C. Cellular and metabolic alterations in the hippocampus caused by insulin signalling dysfunction and its association with cognitive impairment during aging and Alzheimer’s disease: Studies in animal models. Diabetes/Metab. Res. Rev. 2015;31:1–13. doi: 10.1002/dmrr.2531. [DOI] [PubMed] [Google Scholar]
- 117.Honig L.S., Vellas B., Woodward M., Boada M., Bullock R., Borrie M., Hager K., Andreasen N., Scarpini E., Liu-Seifert H., et al. Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. N. Engl. J. Med. 2018;378:321–330. doi: 10.1056/NEJMoa1705971. [DOI] [PubMed] [Google Scholar]
- 118.Lin L., Zheng L.J., Zhang L.J. Neuroinflammation, Gut Microbiome, and Alzheimer’s Disease. Mol. Neurobiol. 2018;55:8243–8250. doi: 10.1007/s12035-018-0983-2. [DOI] [PubMed] [Google Scholar]
- 119.Schwab C., Klegeris A., McGeer P.L. Inflammation in transgenic mouse models of neurodegenerative disorders. Biochim. Biophys. Acta. 2010;1802:889–902. doi: 10.1016/j.bbadis.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 120.Burns J.M., Honea R.A., Vidoni E.D., Hutfles L.J., Brooks W.M., Swerdlow R.H. Insulin is differentially related to cognitive decline and atrophy in Alzheimer’s disease and aging. Biochim. Biophys Acta. 2012;1822:333–339. doi: 10.1016/j.bbadis.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moore A.H., Wu M., Shaftel S.S., Graham K.A., O’Banion M.K. Sustained expression of interleukin-1beta in mouse hippocampus impairs spatial memory. Neuroscience. 2009;164:1484–1495. doi: 10.1016/j.neuroscience.2009.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang W.-Y., Tan M.-S., Yu J.-T., Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015;3:136. doi: 10.3978/j.issn.2305-5839.2015.03.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hansen D.V., Hanson J.E., Sheng M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018;217:459–472. doi: 10.1083/jcb.201709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen P.L., Wang W.J., Rao Y.Q., Li J., Cheng M.J. Serum containing Gengnianchun formula suppresses amyloid β-induced inflammatory cytokines in BV-2 microglial cells by inhibiting the NF-κB and JNK signaling pathways. Mol. Med. Rep. 2018;17:5043–5048. doi: 10.3892/mmr.2018.8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Trovato Salinaro A., Pennisi M., Di Paola R., Scuto M., Crupi R., Cambria M.T., Ontario M.L., Tomasello M., Uva M., Maiolino L., et al. Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer’s disease and Alzheimer-linked pathologies: Modulation by nutritional mushrooms. Immun. Ageing. 2018;15:8. doi: 10.1186/s12979-017-0108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schwartz K., Boles B.R. Microbial amyloids—Functions and interactions within the host. Curr. Opin. Microbiol. 2013;16:93–99. doi: 10.1016/j.mib.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Andersen K., Andersen B.B., Pakkenberg B. Stereological quantification of the cerebellum in patients with Alzheimer’s disease. Neurobiol. Aging. 2012;33:197.e11–197.e20. doi: 10.1016/j.neurobiolaging.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 128.Sjobeck M., Englund E. Alzheimer’s disease and the cerebellum: A morphologic study on neuronal and glial changes. Dement. Geriatr. Cognit. Disord. 2001;12:211–218. doi: 10.1159/000051260. [DOI] [PubMed] [Google Scholar]
- 129.Mavroudis I.A., Fotiou D.F., Adipepe L.F., Manani M.G., Njau S.D., Psaroulis D., Costa V.G., Baloyannis S.J. Morphological changes of the human purkinje cells and deposition of neuritic plaques and neurofibrillary tangles on the cerebellar cortex of Alzheimer’s disease. Am. J. Alzheimer’s Dis. Dement. 2010;25:585–591. doi: 10.1177/1533317510382892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wegiel J., Wisniewski H.M., Dziewiatkowski J., Badmajew E., Tarnawski M., Reisberg B., Mlodzik B., De Leon M.J., Miller D.C. Cerebellar atrophy in Alzheimer’s disease-clinicopathological correlations. Brain Res. 1999;818:41–50. doi: 10.1016/S0006-8993(98)01279-7. [DOI] [PubMed] [Google Scholar]
- 131.Reddy Y.M., Singh D., Nagarajan D., Pillarisetti J., Biria M., Boolani H., Emert M., Chikkam V., Ryschon K., Vacek J., et al. Atrial fibrillation ablation in patients with gastroesophageal reflux disease or irritable bowel syndrome-the heart to gut connection! J. Interv. Card. Electrophysiol. 2013;37:259–265. doi: 10.1007/s10840-013-9807-5. [DOI] [PubMed] [Google Scholar]
- 132.Churchill L., Taishi P., Wang M., Brandt J., Cearley C., Rehman A., Krueger J.M. Brain distribution of cytokine mRNA induced by systemic administration of interleukin-1beta or tumor necrosis factor alpha. Brain Res. 2006;1120:64–73. doi: 10.1016/j.brainres.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 133.Foster J.A., Rinaman L., Cryan J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang R., Miller R.G., Gascon R., Champion S., Katz J., Lancero M., Narvaez A., Honrada R., Ruvalcaba D., McGrath M.S. Circulating endotoxin and systemic immune activation in sporadic Amyotrophic Lateral Sclerosis (sALS) J. Neuroimmunol. 2009;206:121–124. doi: 10.1016/j.jneuroim.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ghosh S., Wu M.D., Shaftel S.S., Kyrkanides S., LaFerla F.M., Olschowka J.A., O’Banion M.K. Sustained Interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer’s mouse model. J. Neurosci. 2013;33:5053–5064. doi: 10.1523/JNEUROSCI.4361-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Terada K., Yamada J., Hayashi Y., Wu Z., Uchiyama Y., Peters C., Nakanishi H. Involvement of cathepsin B in the processing and secretion of interleukin-1beta in chromogranin A-stimulated microglia. Glia. 2010;58:114–124. doi: 10.1002/glia.20906. [DOI] [PubMed] [Google Scholar]
- 137.Wu Z., Sun L., Hashioka S., Yu S., Schwab C., Okada R., Hayashi Y., McGeer P.L., Nakanishi H. Differential pathways for interleukin-1beta production activated by chromogranin A and amyloid beta in microglia. Neurobiol. Aging. 2013;34:2715–2725. doi: 10.1016/j.neurobiolaging.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 138.Carvalho B.M., Guadagnini D., Tsukumo D.M.L., Schenka A.A., Latuf-Filho P., Vassallo J., Dias J.C., Kubota L.T., Carvalheira J.B.C., Saad M.J.A. Modulation of gut microbiota by antibiotics improves insulin signalling in high-fat fed mice. Diabetologia. 2012;55:2823–2834. doi: 10.1007/s00125-012-2648-4. [DOI] [PubMed] [Google Scholar]
- 139.Tilvis R.S., Kahonen-Vare M.H., Jolkkonen J., Valvanne J., Pitkala K.H., Strandberg T.E. Predictors of cognitive decline and mortality of aged people over a 10-year period. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004;59:268–274. doi: 10.1093/gerona/59.3.M268. [DOI] [PubMed] [Google Scholar]
- 140.Cattaneo A., Cattane N., Galluzzi S., Provasi S., Lopizzo N., Festari C., Ferrari C., Guerra U.P., Paghera B., Muscio C., et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]