Abstract
Osteocytes are master orchestrators of bone remodeling; they control osteoblast and osteoclast activities both directly via cell-to-cell communication and indirectly via secreted factors, and they are the main postnatal source of sclerostin and RANKL (receptor activator of NF-kB ligand), two regulators of osteoblast and osteoclast function. Despite progress in understanding osteocyte biology and function, much remains to be elucidated. Recently developed osteocytic cell lines—together with new genome editing tools—has allowed a closer look at the biology and molecular makeup of these cells. By using single-cell cloning, we identified genes that are associated with high Sost/sclerostin expression and analyzed their regulation and function. Unbiased transcriptome analysis of high- vs. low-Sost/sclerostin–expressing cells identified known and novel genes. Dmp1 (dentin matrix protein 1), Dkk1 (Dickkopf WNT signaling pathway inhibitor 1), and Phex were among the most up-regulated known genes, whereas Srpx2, Cd200, and carbonic anhydrase III (CAIII) were identified as novel markers of differentiated osteocytes. Aspn, Enpp2, Robo2, Nov, and Serpina3g were among the transcripts that were most significantly suppressed in high-Sost cells. Considering that CAII was recently identified as being regulated by Sost/sclerostin and capable of controlling mineral homeostasis, we focused our attention on CAIII. Here, we report that CAIII is highly expressed in osteocytes, is regulated by parathyroid hormone both in vitro and in vivo, and protects osteocytes from oxidative stress.—Shi, C., Uda, Y., Dedic, C., Azab, E., Sun, N., Hussein, A. I., Petty, C. A., Fulzele, K., Mitterberger-Vogt, M. C., Zwerschke, W., Pereira, R., Wang, K., Divieti Pajevic, P. Carbonic anhydrase III protects osteocytes from oxidative stress.
Keywords: bone homeostasis, sclerostin, PTH
Osteocytes—bone cells encased in mineralized matrix—have recently risen to become the principal orchestrators of bone modeling and remodeling. They are the main postnatal source of sclerostin—encoded by the Sost gene (1–5)—and receptor activator of NF-kB ligand (RANKL) (6, 7), two major regulators of osteoblast and osteoclast function, respectively. Sclerostin is a cysteine-rich protein with homology to gremlin and is a current target for osteoporosis treatment. It acts by binding to LDL receptor–related protein 5/6 and inhibiting the WNT signaling pathway (5, 8). Genetic inactivation of sclerostin, both in human and in animal models, is associated with skeletal abnormalities that are characterized by high bone mass (1–4, 9), whereas its overexpression in animals induces osteopenia. Its expression is regulated by mechanical forces and hormonal stimuli. Loading suppresses sclerostin, whereas disuse and unloading induces its expression (10–12). We have recently reported that Sost/sclerostin is suppressed by parathyroid hormone (PTH) via an Hdac5-Mef2c–mediated mechanism (13) and its suppression is required for the full anabolic effects of the hormone (14). Despite progress in understanding the function and regulation of this protein, much remains to be elucidated. Osteocytes are also the main source of RANKL, the ligand for RANK, a cytokine that is essential for osteoclast differentiation, function, and survival (15–18). Despite growing knowledge of the function of osteocytes, several aspects of their role in bone metabolism remain unknown. Moreover, few studies suggest that osteocytes might be differentially regulated depending on their in vivo localization (19).
Newly formed osteocytes—those recently embedded into the mineralized matrix—express E11/gp38 (or podoplanin) and Dmp1 (dentin matrix protein 1) and have low sclerostin expression. As the osteocyte matures and its distance from the endosteal or periosteal surface increases—or, in the case of larger primates, its distance from the Haversian canal—the expression of sclerostin seems to be more evident (20, 21).
Recently developed osteocytic cell lines (11, 22), together with new genome editing tools (23), have allowed additional exploration of the biology and molecular makeup of mature osteocytes. By using single-cell clonal populations of the osteocytic cell line, Ocy454, we have identified genes that are associated with high Sost/sclerostin expression and analyzed their regulation and functions. Clonal cells were classified according to their Sost/sclerostin expression, and microarray analysis of high- and low-Sost clones delineated the genetic makeup of these two populations. We identified more than 500 genes that were differentially up- or down-regulated in high-Sost–expressing cells compared with low-Sost–expressing cells, and carbonic anhydrase III (CAIII) was identified as a novel marker of differentiated osteocytes. Aspn and Enpp2 were significantly reduced in high-Sost cells, which further confirmed the results of previous studies (24).
CAIII is a member of a multigene family that is composed of several zinc metalloenzymes with various tissue distributions and intracellular locations. These enzymes catalyze the interconversion between carbon dioxide and the bicarbonate ion and, thus, are involved in critical physiologic processes that are connected with respiration and ion exchange. The family consists of 13 active isoenzymes that are expressed in mammals, of which 12 are expressed and function in humans (25–27). CAII was recently identified as being regulated by Sost/sclerostin and capable of controlling mineral homeostasis (28). By using a combination of in vitro and ex vivo models, we demonstrated that CAIII expression increases with osteoblast/osteocyte differentiation, is independent of Sost expression, and protects osteocytes from oxidative stress. The enzyme is regulated at both the transcriptional and protein levels by PTH, prostaglandin E2, and forskolin, which indicates a cAMP-mediated pathway. We also demonstrated that CAIII protects mature osteocytes from hypoxia and oxidative stress.
In summary, we report here—for the first time to our knowledge—that mature osteocytes express a high level of CAIII, which plays a functional role in protecting these cells from hypoxic and oxidative stresses.
MATERIALS AND METHODS
Peptides and compounds
Synthetic human PTH was synthesized by Dr. Askhok Khatri (Peptide/Protein Core Facility, Massachusetts General Hospital, Boston, MA, USA). Forskolin (F6886) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were from Thermo Fisher Scientific (Waltham, MA, USA) and Sigma-Aldrich.
Cells and culture media
Ocy454 cells, were isolated from the long bones of 4-wk-old SV40TAg and 8KbDmp1–green fluorescent protein (GFP) double-transgenic mice as previously described (11). Cells were routinely maintained on type I collagen-coated flasks (Corning Biocoat; Corning, Corning, NY, USA) in α-minimum essential medium that contained 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific) and 1% antibiotic antimycotic (Thermo Fisher Scientific). In the experimental setting, cells were plated at 0.5–1.0 × 105 cells/ml and allowed to reach confluence at a permissive temperature (33°C) for 3 or 4 d. Cells were then moved to a semipermissive temperature (37°C) and cultured for the indicated times to induce differentiation. To induce oxidative stress, cells were treated with H2O2 for 4 to 16 h at the concentrations indicated in each experiment. For hypoxia experiments, cells were cultured in hypoxic chambers (StemCell Technologies, Vancouver, BC, Canada) with 1% O2 in an incubator at 37°C for 24 h. Cell proliferation was assessed by PrestoBlue Cell Viability Reagent (Thermo Fisher Scientific) according to manufacturer recommendations. HEK293T cells were maintained in high-glucose DMEM (Thermo Fisher Scientific) with l-glutamine that was supplemented with 10% heat-inactivated FBS.
For in vitro sclerostin treatment, cells were plated at 1.0 × 105 cells/ml and allowed to reach confluence at the permissive temperature (33°C) for 3 or 4 d. Cells were then moved to a semipermissive temperature (37°C) and cultured for an additional 14 d. Cells were treated with recombinant sclerostin (R&D Systems, Minneapolis, MN, USA; 50 ng/ml) on d 9 and 12, and RNA was extracted on d 14. For acute treatments, Ocy454 cells were plated as described above and cultured at a semipermissive temperature (37°C) for 7 or 14 d before being treated for 4 h with 25, 50 and 100 ng/ml of recombinant sclerostin.
For single-cell cloning, cells were sorted for GFP expression and automatically plated on 96-well plates. At 24 h postsorting, plates were visually examined, and wells with individual cells were marked as positive. Cells were grown to confluence at the permissive temperature (33°C), then expanded for additional studies.
Primary calvaria osteoblasts (provided by the Bone Cell Core, Center for Skeletal Research, MGH) were isolated from 3- to 5-d-old C57BL/6 pups after standard sequential collagenase digestions. Calvaria cells (first passage) were expanded in 10-cm dishes before seeding at 1 × 105 cells/ml in 12-well plates, then cultured for the indicated time before RNA isolation as described below. Starting at d 0 (2 d after seeding), cells were grown in growth medium (α-minimum essential medium + 10% FBS + 1% antibiotic antimycotic) or mineralizing medium (growth medium that was supplemented with 50 μg/ml ascorbic acid and 10 mM β-glycerophosphate).
Real-time quantitative PCR
Total RNA was extracted by using the RNAeasy Plus Mini Kit (Qiagen, Germantown, MD, USA) according to manufacturer recommendations and were quantified by using NanoDrop 2000 (Thermo Fisher Scientific). cDNA was synthesized by using qScript cDNA SuperMix (Quantabio, Beverly, MA, USA) or Takara PrimeScript RT Reagent Kit with gDNA Eraser (Clontech, Mountain View, CA, USA) from 0.5 to 1.0 μg of total RNA, followed by SYBR Green (Power SYBR; Thermo Fisher Scientific) semiquantitative PCR (StepOnePlus; Thermo Fisher Scientific). Primer sequences are listed in Supplemental Table 1. Gene expression between samples was calculated by using the comparative Ct method (−∆∆Ct), β-actin, 18S, or RPL13A as reference gene and were normalized with control or vehicle-treated cells or by normalizing β-actin expression. Experiments were run in triplicate, unless otherwise indicated.
Sclerostin ELISA
Conditioned medium (48–72 h) was harvested from Ocy454 single-cell clones as indicated in the figure legends and stored at −80°C until further use. After harvesting the supernatant, cell proliferation per well was determined by using PrestoBlue Cell Viability Reagent (Thermo Fisher Scientific). High-binding 96-well plates (21-377-203; Thermo Fisher Scientific) were coated with sclerostin Ab VI capture Ab (3 μg/ml) in PBS for 1 h at room temperature. Plates were washed with wash buffer (PBS + 0.5% Tween 20) and blocked with blocking buffer (wash buffer that was supplemented with 1% bovine serum albumin and 1% normal goat serum) for 1 h at room temperature. Samples (60 μl/well) were then added along with sequentially diluted murine recombinant sclerostin protein (R&D Systems) to obtain a standard curve, and plates were incubated overnight at 4°C. After washing with wash buffer 3 times, plates were incubated with horseradish peroxidase (HRP)–coupled sclerostin Ab VII detection Ab (0.5 μg/ml) in blocking buffer for 1 h at room temperature. HRP activity was detected with Ultra TMB ELISA substrate (34028; Pierce, Rockford, IL, USA), stopped by 2N sulfuric acid, and read at an optical density value of 450 nm with TriStar LB 941 (Berthold Technologies, Oak Ridge, TN, USA).
Western blot
Whole-cell lysates from in vitro cell cultures were prepared by using lysis buffer that contained 50 mM Tris base, 1 mM EDTA, 1.5 mM MgCl2, 150 mM NaCl, 1% Triton X-100, and 10% glycerol that was supplemented with protease and phosphatase inhibitors (Sigma-Aldrich). Protein concentrations were quantified by using Bradford Protein Assay (Protein Assay Dye Reagent; Bio-Rad, Hercules, CA, USA) with sequentially diluted bovine serum albumin (New England Biolabs, Ipswich, Massachusetts, USA) to obtain a standard curve, and ∼15–20 μg of protein was separated on a 7 or 10% polyacrylamide gel (Thermo Fisher Scientific) and transferred to a PVDF membrane (Bio-Rad) or nitrocellulose membrane (Amersham Biosciences, Little Chalfont, United Kingdom; for phosphorylated protein) using the Wet/Tank Blotting System (Bio-Rad) according to manufacturer recommendations. The membrane was blocked with blocking buffer [Tris-buffered saline containing 0.1% Tween 20 supplemented with 5% bovine serum albumin (Sigma-Aldrich) or 5% nonfat dry milk (Santa Cruz Biotechnology, Santa Cruz, CA, USA)] for 1 h, then incubated overnight at 4°C with the appropriate primary Ab. The following primary Abs were used: anti-CAIII (1:500; Abcam, Cambridge, MA, USA), anti–HIF-1α (1:500; Cell Signaling Technology, Danvers, MA, USA), anti–phospho-HDAC4/5/7 (S246/S259/S155; 1:1000; Cell Signaling Technology), anti-HDAC4 (1:1000; Abcam) and anti–β-tubulin (1:2000; Cell Signaling Technology). After washing with Tris-buffered saline containing 0.1% Tween 20, the membrane was hybridized with the appropriate HRP-conjugated secondary Ab (anti-rabbit IgG, 1:2000; Cell Signaling Technology) in blocking buffer for 1 h at room temperature. The membrane was then developed by using ECL or SuperSignal West Femto (Thermo Fisher Scientific) and signal intensity was detected with a charge-coupled device camera on G:Box (Syngene, Cambridge, United Kingdom). Densitometric analysis of protein bands was performed by using GeneTools software (Syngene). For Western blotting of secreted proteins, 4 ml of conditioned medium was transferred to a centrifugal filter unit with a 10-kDa MW cutoff (Millipore, Billerica, MA, USA) and spun down at 850 rpm (136 g) for 10 min to reduce the volume to 400 µl per the manufacturer’s recommendations. Reversible Protein Stain Kit (Thermo Fisher Scientific) was used to detect the total number of proteins transblotted on the membrane. Detected total proteins were used as loading control for conditioned medium samples.
CRISPR/Cas9 synergistic activation mediators and gene deletion
Optimized single-guided RNA targeting CAIII were designed by using the online Synergistic Activation Mediator (SAM) Cas9 activator design tool (http://sam.genome-engineering.org/). Lenti-sgRNA(MS2) zeo backbone, lenti-dCAS-VP64_Blast, and lenti-MS2-P65-HSF1_Hygro were all obtained from Addgene (Cambridge, MA, USA) and were gifts from F. Zhang [Massachusetts Institute of Technology, Cambridge, MA, USA (29)]. Sg-RNAs were cloned into lenti-sgRNA(MS2) zeo backbone to generate CAIII overexpression plasmid according to Addgene protocols. Four independent sgRNAs were designed and tested together with 2 controls.
For PTH receptor (PPR) deletion, sgRNAs that target the receptor (Pth1r) were designed by using online designing tools [Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) Design from F. Zhang’s laboratory, and sgRNA Designer from Broad Institute, Cambridge, MA, USA]. Optimized gRNA sequences were subcloned into PX458 (a gift from F. Zhang; Addgene) (30), a plasmid that coexpresses sgRNA, Cas9, and eGFP. Ocy454 cells were transfected by using FuGene HD (Promega, Madison, WI, USA; 1 μg of plasmid/well of a 6-well plate). Forty-eight hours later, eGFPhi cells were recovered by fluorescence-activated cell sorting (FACS)–based sorting and plated in 96-well plates at 1 cell/well. Growth medium was changed once weekly, and 3 wk later, colonies were identified by visual inspection. Colonies were then expanded and analyzed for the loss of the targeted receptor’s function by measuring second messenger (cAMP) activity upon ligand (PTH) treatment.
Lentivirus production
HEK293T cells were cultured as previously described. One day before transfection, cells were plated at 7 × 105 cell/ml in 5 ml DMEM + 10% FBS without antibiotics in a 6-cm dish. Cells were transfected the next day when they reached ∼80% confluency. For each dish, 1 μg of plasmid that contained the vector of interest—750 ng psPAX2 (Addgene) and 250 ng pMD2.G (Addgene)—was transfected by using 6 μl FuGene HD transfection reagent (Promega). PsPAX2 and pMD2.G were gifts from D. Trono (Ecole Polytechnique Federale, Lausanne, France). Twelve to fifteen hours after transfection, medium was replaced with 5 ml DMEM + 20% FBS with antibiotic antimycotic. Condition medium was harvested 48 h later, spun down at 1000 rpm and 4°C for 10 min, and virus supernatant was collected, portioned into aliquots, and stored at −80°C.
Lentivirus infection
Ocy454 cells were plated at 1 × 105cells/ml on a 12-well plate 1 d before infection. Cells were infected with 100 μl of lentivirus in the presence of 10 μg/ml polybrene. Medium was changed 1 d after infection with selection agent (300 μg/ml zeocin, 5 μg/ml blasticidin, and 400 μg/ml hygromycin). Antibiotic treatment continued for 14 d.
Lentiviral short hairpin RNA
For short hairpin RNA (shRNA), lentiviruses were produced in HEK293T cells in a pLKO.1-puro backbone, a gift from B. Weinberg (Addgene) (31). Viral packaging was performed by using standard protocols (http://www.broadinstitute.org/rnai/public/resources/protocols). In brief, HEK293T cells were plated at 2.2 × 105 cells/ml and transfected the next day with shRNA-expressing plasmid along with psPAX2 and MD2.G by using FuGene HD. Medium was changed the next day and collected 48 h later.
Osteocyte-enriched bone explants
Osteocyte-enriched bone explants (OEBEs) were generated from femurs and tibiae of 6- to 8-wk-old male C57BL/6NCr animals (Charles River Laboratories, Wilmington, MA, USA). In brief, after removing the epiphyses and flushing out bone marrow, explants were sequentially digested by using collagenase and EDTA to completely remove endosteal and periosteal osteoblasts and bone marrow cells, as described previously (32, 33). OEBEs were cultured at 37°C in 5% CO2 for 2 d before treatment with 100 nM of human PTH(1–34) for 4 h. For osteocyte-specific gene expression, RNA was isolated from OEBEs that were homogenized in Trizol (Thermo Fisher Scientific) by using a tissue homogenizer (TissueLyser; Qiagen), then RNA was extracted with Trizol.
In vivo sclerostin injection
All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital (Boston, MA, USA) and Boston University. For sclerostin treatment, 8- to 10-wk-old male mice (C57BL/6NCrl; Charles River Laboratories) were injected with 100 µg/kg body weight recombinant sclerostin (R&D Systems) 5 d/wk for 3 wk. No specific procedure was used to randomly assign mice to the vehicle or treatment groups. Mice were maintained under standard laboratory conditions on a 12-h light/dark schedule. Water and food (standard chow Teklad global 15% protein rodent diet; Envigo, Huntington, United Kingdom) were provided ad libitum. Mice were housed in ventilated cages at 4–5 animals cage.
Reactive oxygen species
Reactive oxygen species (ROS) assay was performed by labeling cells with 2′,7′-dichlorofluorescein diacetate (DCF-DA). In brief, cells were plated at 5 × 104 cells/ml in 6- or 12-well plates and cultured for 3–4 d at 33°C before being moved to 37°C. On d 4 or 7 of culture at 37°C, cells were treated with 1 mM H2O2 for 4 h. Thirty minutes before the end of the 4-h H2O2 treatment, cells were labeled with 20 μM DCF-DA (Sigma-Aldrich) and trypsinized with TrypLE Express (Thermo Fisher Scientific). After washing with ice-cold PBS that contained 2% FBS, DCF-DA–positive cell populations were quantified by flow cytometry analysis (LSRII; BD Biosciences, San Jose, CA, USA).
Cleaved caspase-3
Cleaved caspase-3 (Asp175) Ab (Cell Signaling Technology) was used to detect cell apoptosis upon treatment with H2O2. Similar to the ROS assay described above, cells were plated at 5 × 104 cells/ml in 6- or 12-well plates and were cultured for 3–4 d at 33°C and for 3 d at 37°C. Cells were then treated with 1 mM H2O2 for 4 h, trypsinized with TrypLE, and fixed with 10% formalin for 15 min at 37°C with gentle agitation. Cells were subsequently permeabilized in 90% ice-cold methanol and incubated with cleaved caspase-3 Ab (1:200) for 1 h at room temperature or overnight at 4°C according to the manufacturer’s protocol. Anti-rabbit IgG Alexa Fluor 594 conjugate (Cell Signaling Technology) was used as secondary Ab. After washing with PBS, the cleaved caspase-3–positive cell population was analyzed by flow cytometry (LSRII; BD Biosciences).
Microarray analysis
Mouse Gene 2.0 ST CEL files were normalized to produce gene-level expression values by using the Robust Multiarray Average [PubMed, National Center for Biotechnology Information, Bethesda, MD, USA; https://www.ncbi.nlm.nih.gov/pubmed/ (ID: 12925520)] in the affy package (v.1.36.1; ID: 14960456) that is included in the Bioconductor software suite (v.2.12; ID: 15461798) and an Entrez gene-specific probe set mapping (17.0.0) from the Molecular and Behavioral Neuroscience Institute (Brainarray) at the University of Michigan (ID: 16284200). Differential expression was assessed by using the moderated (empirical Bayesian) t test in the limma package (v.3.14.4). Microarray analysis was performed using Affymetrix (Santa Clara, CA, USA).
Statistical analysis
Data are presented as means ± sd. Statistically significant differences between groups and treatments were determined by ANOVA using Prism (GraphPad Software, La Jolla, CA, USA). Values of P < 0.05 were accepted as significant. Each experiment was conducted in triplicate and repeated several times, unless otherwise specified in the figure legends.
RESULTS
Ocy454 single-cell cloning and characterization
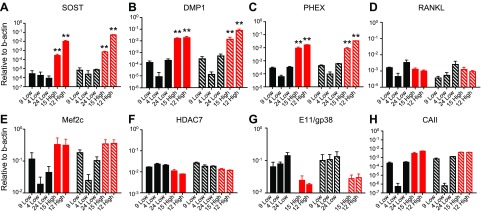
As previously reported, the osteocytic cell line, Ocy454, was originally isolated by FACS for Dmp1-GFP–positive cells (11). This method provided a heterogeneous population of Dmp1-GFP–positive cells with various degrees of Sost and Dmp1 expression, depending on their stage of maturation. In an effort to identify cells expressing high levels of Sost/sclerostin, we single-cell cloned (with FACS) high-GFP–expressing Ocy454 cells and screened them for sclerostin expression. Cells were maintained at 33°C until confluence, then were shifted to a nonpermissive temperature (37°C), and conditioned medium was collected after 7 d in culture. Among the 37 clones tested, we selected 2 high-Sost– (Ocy454-12H and Ocy454-15H) and 3 low-Sost–expressing clones (Ocy454-4L, Ocy454-9L, and Ocy454-24L) for additional characterization. These clones were chosen because they regulated Sost expression in response to PTH (data not shown). Gene analysis by semiquantitative real-time PCR confirmed high Sost mRNA expression in Ocy454-12H and Ocy454-15H (Fig. 1A) after both 7 and 14 d in culture at a nonpermissive temperature (37°C). High Sost expression was associated with increased levels of Dmp1 (Fig. 1B) and Phex (Fig. 1C), whereas RANKL was not significantly different among clones, although it tended to be lower in high Sost clones (Fig. 1D). We previously reported that Sost/sclerostin expression in Ocy454 cells is controlled by Mef2C via an Hdac4/5-dependent mechanism, whereas RANKL is controlled by the cAMP-regulated transcriptional coactivator 2 transcription factor (13, 34). As expected, Mef2C mRNA was significantly increased in Ocy454-12H and Ocy454-15H compared with low-Sost–expressing clones (Fig. 1E), whereas HDAC4/5 transcripts were unchanged (Supplemental Fig 1A, B). Of interest, HDAC7 expression was significantly reduced in high-Sost clones (Fig. 1F). In addition, E11/gp38 expression was significantly lower in high-Sost clones, which further confirmed that these cells are highly differentiated osteocytes (Fig. 1G). Taken together, these data demonstrate that Ocy454 cells are composed of populations of more differentiated cells (high Sost/sclerostin) and less mature osteocytes, in which the level of Sost, Dmp1, Phex are significantly reduced, but E11/gp38 is significantly increased. Of interest, increased cell differentiation was not associated with significant changes in RANKL or Fgf23 expression (Supplemental Fig. 1C), and their expression did not change with time in culture (14 vs. 7 d; Fig. 1D and Supplemental Fig. 1C).
Figure 1.
Ocy454 subclone characterization. Ocy454 clones: 3 low- (9-Low, 4-Low, and 24-Low) and 2 high-sclerostin–expressing clones (15-High and 12-High) were used for additional characterization. A–C, E, H) Semiquantitative PCR Sost (A), Dmp1 (B), Phex (C), Mef2c (E), and CAIII (H) are significantly increased in 2 high (15-High and 12-High; red bars) clones compared with 3 low (9-Low, 4-Low and 24-Low; black bars) clones after 7 (solid bars) and 14 (hatched bars) d in culture (regular medium, 37°C). D, F, G) Hdac7 (F) and E11/gp38 (G) expression is significantly lower in 15- and 12-High compared with 9-, 4-, and 24-Low, whereas RANKL (D) is unchanged across subclones. Gene expression is normalized to β-actin. Data are expressed as means ± sd of triplicates. Each experiment was repeated at 3–4 times. Two-way ANOVA with Tukey’s multiple comparison test was performed. *P < 0.05, **P < 0.01.
Transcriptome profile of high- and low-Sost–expressing clones
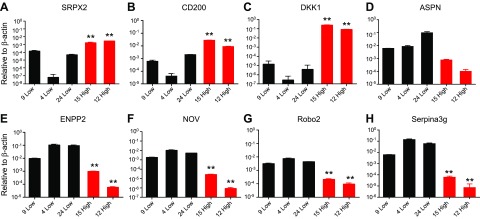
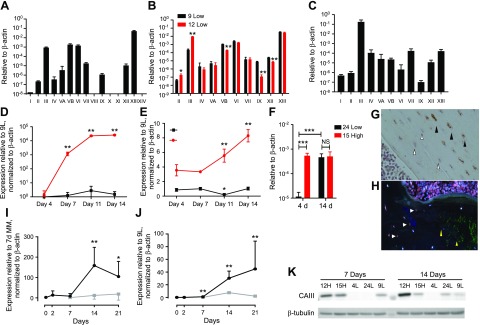
To identify novel (and known) genes that are differentially expressed in these two cell populations, we performed microarray analysis (Affymetrix). We found that 264 genes were significantly increased in high- vs. low-Sost–expressing cells (cutoff, 1.5-fold), and that 254 genes were significantly down-regulated. Table 1 lists the top 10 up- and down-regulated genes. Real-time quantitative PCR validation of the top 5 genes in each group confirmed the microarray results (Fig. 2). Among up-regulated genes, we identified sushi-repeat-containing protein, X-linked 2 (Srpx2) (Fig. 2A), Dmp1(Fig. 1B), CD200 antigen (Cd200) (Fig. 2B), Phex (Fig. 1C), and Dkk1 (Dickkopf WNT signaling pathway inhibitor 1; Fig. 2C), whereas the most down-regulated genes were Asporin (Aspn) (Fig. 2D), ectonucleotide pyrophosphatase/phosphodiesterase 2 (Enpp2; Fig. 2E), nephroblastoma overexpressed gene (Nov; Fig. 2F), roundabout guidance receptor 2 (Robo2; Fig. 2G), and serine (or cysteine) peptidase inhibitor, clade A, member 3G (Serpina3g; Fig. 2H). CAIII was also significantly increased (12.8-fold) in high- vs. low-Sost–expressing cells (Table 1 and Fig. 1H). It has been reported that sclerostin regulates the expression of CAII in human primary osteocyte-like cells and mouse MLO-Y4 cells (28); therefore, we investigated CAIII regulation and its functional role in Ocy454 cells. Expression of CA isoforms demonstrated that CAIII, CAVB, CAVI, CAVII, CAXII, and CAXIII were abundantly expressed in Ocy454 cells (Fig. 3A), and among these CAs, 6 subtypes were differentially expressed in high- vs. low-Sost–expressing cells, with CAIII being the most significant (Fig. 3B). CAIII was also the most abundantly expressed CA in mRNA from mouse long bones (Fig. 3C) and in OEBEs from femurs and tibiae of C57BL/6N (Supplemental Fig. 1D). Time course analysis demonstrated increased CAIII transcript with time in culture, reaching maximal expression by d 14 (Fig. 3E), whereas Sost was already significantly increased after 7 d in culture (Fig. 3D) in Ocy454-12H vs. Ocy454-9L. A significant increase in CAIII expression over time (4 vs. 14 d in culture) was also present in other clones, as shown in Fig. 3F. In Ocy454-24L, CAIII expression increased more than 100-fold (d 4 vs. 14), whereas in Ocy454-15H, the transcript was already maximally expressed after 4 d in culture (Fig. 3F). We next evaluated CAIII expression during osteoblast differentiation. Both CAIII and Sost expression significantly increased in primary calvaria osteoblasts that underwent differentiation (Fig. 3J, I), which further demonstrated that CAIII is abundant in young and mature osteocytes. Indeed, immunohistochemical analysis of mouse (Fig. 3G) and human bones (Fig. 3H) showed CAIII-positive osteocytes. Of interest, in human samples, it seemed that CAIII did not colocalized with sclerostin-positive osteocytes. Immunofluorescent staining revealed diffuse cytoplasmic staining of CAIII in Ocy454 cells (Supplemental Fig. 1E), which confirmed the expression of protein in these cells. Western blot analysis of whole-cell lysates confirmed CAIII expression in Ocy454-12H and Ocy454-15H subclones (Fig. 3K) after both 7 and 14 d in culture at a nonpermissive temperature (37°C).
TABLE 1.
Top 10 up- and down-regulated genes
| Gene | Fold change | P |
|---|---|---|
| Up-regulated transcripts | ||
| Srpx2 | 23.6 | 3.3E−03 |
| Dmp1 | 17.0 | 3.5E−03 |
| Dkk1 | 15.4 | 2.5E−03 |
| Phex | 15.0 | 1.8E−03 |
| Cd200 | 13.3 | 9.3E−03 |
| Car3 | 12.8 | 2.4E−02 |
| Megf10 | 12.6 | 1.1E−05 |
| Spns2 | 10.6 | 5.4E−06 |
| B4galnt3 | 10.6 | 5.7E−03 |
| Slc8a3 | 9.9 | 2.2E−03 |
| Down-regulated transcripts | ||
| Aspn | −83.8 | 1.1E−02 |
| Enpp2 | −52.2 | 4.9E−03 |
| Nov | −47.9 | 2.2E−03 |
| Robo2 | −29.9 | 6.9E−04 |
| Serpina3g | −17.6 | 3.5E−02 |
| Itm2a | −16.3 | 2.7E−02 |
| Igfbp2 | −14.2 | 1.4E−02 |
| Plk2 | −14.2 | 4.2E−03 |
| Sdpr | −13.6 | 4.4E−02 |
| 6330406I15Rik | −12.5 | 3.1E−02 |
Figure 2.
Transcripts that are differentially regulated in high- and low-expressing clones. A–C) Semiquantitative PCR for Srpx2 (A), CD200 (B), and DKK1 (C). These transcripts are significantly increased in and 2 high (15-High and 12-High; red bars) clones compared with 3 low (9-Low, 4-Low and 24-Low; black bars) clones after 7 d of differentiation at a nonpermissive temperature (37°C). D–H) Aspn (D), Enpp2 (E), Nov (F), Robo2 (G), and Serpina3g (H) are significantly lower in 15- and 12-High compared with 9-, 4-, and 24-Low. Gene expression is normalized to β-actin. Data are expressed as means ± sd of triplicates. Each experiment was repeated at 3–4 times. One-way ANOVA with Tukey’s multiple comparison test was performed. **P < 0.01.
Figure 3.
CA expression and regulation. Cells were plated at 10 × 105 cells/ml and cultured at a permissive temperature (33°C) for 3–4 d before being moved to a nonpermissive temperature (37°C). A) Semiquantitative PCR for all CA isoforms. CAIII, CAVB, CAVI, and CAXIII were highly expressed in Ocy454 cells. Data are normalized to β-actin. B) CA isoform expression in 12H (high) and 9L (low) Sost cells. CAII and CAIII were significantly increased in 12H, whereas CAVB, CAIX, CAXII, and CAXIII were significantly reduced. C) CA expression in mouse femurs (n = 5). Among the isoforms, CAIII is the most abundant in bone. D, E) Time course expression of Sost (D) and CAIII (E) in Ocy454-12H (red line) and Ocy454-9L (black line). F) CAIII expression in 24L (black bars) and 15H (red bars) after 7 and 14 d in culture. G) Immunohistochemistry analysis of mouse femur. CAIII-positive osteocytes (black arrowheads) are more abundant in the center of the cortex, whereas CAIII-negative cells (open arrowheads) are closer to the endosteal surface. Original magnification, ×40. H) Immunofluorescence for CAIII (red, white arrow) and sclerostin (green, yellow arrows) in human iliac bone. I, J) Expression of Sost (I) and CAIII (J) in primary osteoblasts that were isolated from mouse calvaria were cultured up to 3 wk in the absence (gray line) or presence of 50 μg/ml ascorbic acid and 10 mM β-glycerophospate (mineralization medium, black line). Data are expressed as means ± sd. One-way ANOVA with Tukey’s multiple comparison test was performed. K) Western blot analysis of whole-cell lysate after 7 and 14 d. CAIII is highly expressed in 12H and 15H clones already at 7 d. NS, not significant. *P < 0.05, **P < 0.01 (vs. d 0 mineralization medium); ***P < 0.001, ΨP < 0.01 (vs. d 7 growth medium only). All experiments were performed in triplicate.
Hormonal regulation of CAIII in Ocy454 cells
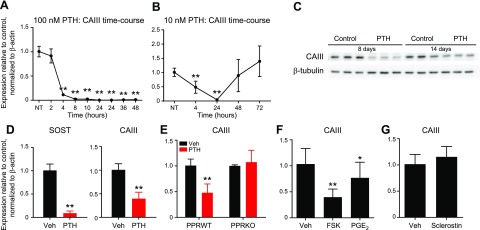
We next investigated whether CAIII was regulated by hormonal stimuli. Human PTH(1–34) (at 10 or 100nM) significantly suppressed mRNA and protein CAIII expression in Ocy454 cells by 4 h (Fig. 4A, C), and the suppression was still present after 48 h. When cells were treated with lower PTH doses (10 nM), the effect was reversed after 24 h (Fig. 4B). Similarly, this effect was also present in ex vivo organ culture. OEBEs from femurs and tibiae of C57BL/6N mice were treated with 100 nM human PTH(1–34) for 4 h before RNA isolation. PTH significantly suppressed CAIII expression (Fig. 4D), which demonstrated that osteocytes in ex vivo settings down-regulate CAIII upon PTH treatment. As expected, Sost was significantly suppressed upon PTH administration (Fig. 4D). Moreover, the hormonal regulation of CAIII was abolished when the receptor was ablated from Ocy454 cells (PPR-knockout; Fig. 4E), which demonstrated a PPR-mediated mechanism. CAIII was also significantly down-regulated by forskolin—an adenylato cyclase activator—and prostaglandin E2, which indicated a cAMP-dependent mechanism (Fig. 4F). Considering that sclerostin regulates skeletal CAII expression in osteocytes (28), we investigated whether this protein could also affect CAIII expression. To this end, Ocy454 cells were treated with sclerostin (50 ng/ml) for 6 d before RNA isolation. Under these conditions, no regulation of CAIII mRNA expression was observed (Fig. 4G). Similar results were obtained when Ocy454 cells were acutely (4 h) treated with increasing doses of recombinant protein (25, 50, and 100 ng/ml) after 7 or 14 d in culture (Supplemental Fig. 2C, D) or when animals (n = 5) were injected with sclerostin (100 μg/kg) for 5 d/wk for 3 wk before RNA isolation (Supplemental Fig. 2A, B).
Figure 4.
CAIII regulation by PTH and sclerostin. Cells were plated at 10 × 105 cells/ml and cultured at a permissive temperature (33°C) for 3–4 d before being moved to a nonpermissive temperature (37°C). A, B) Time course of CAIII expression after treatment with 100 nM (A) or 10 nM (B) PTH(1–34). CAIII transcripts were significantly suppressed by the hormone up to 48 h. When lower doses were used (B), the effect was reversed by 48–72 h. C) PTH (100 nM for 24 h) significantly suppressed CAIII protein levels. D) PTH significantly suppressed CAIII and Sost expression in OEBEs. Femurs and tibiae from C57BL/6N mice were deprived of epiphysis and bone marrow and cultured for a few days before PTH treatment (n = 3). E) Ocy454 cells that lacked PTH1R expression [PPR-knockout (KO)] were treated with PTH for 4 h before RNA extraction. In the absence of PPR expression, there was no regulation of CAIII upon PTH treatment (red bars). F) Treatment of Ocy454 with forskolin (FSK; 10 μM) and prostaglandin E2 (PGE2; 100 mM) for 4 h significantly suppressed CAIII expression. G) Treatment with sclerostin (50 ng/ml) for 5 d did not regulate CAIII gene expression in Ocy454 cells. Data in panels A, B and D–G are relative to β-actin and normalized to NT or vehicle. NT, no treatment; Veh, vehicle; WT, wild-type. All experiments were performed in triplicate and repeated several times. Data are expressed as means ± sd. One-way ANOVA Tukey’s multiple comparison test was performed. *P < 0.05, **P < 0.01 (vs. NT or vehicle).
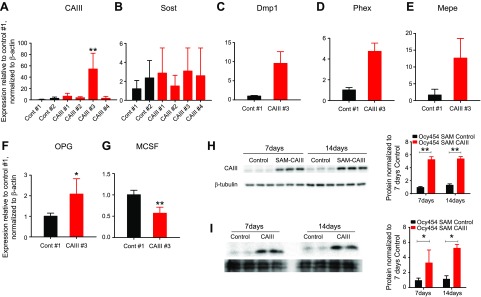
We next investigated whether the overexpression or deletion of CAIII in Ocy454 cells affected gene expression and cellular function. The CRISPR/Cas9 SAM technique was used to increase CAIII levels in Ocy454 (SAM-CAIII) cells by 40- to 80-fold compared with control cells (Fig. 5A). Dmp1, Phex, and Mepe were all significantly up-regulated in SAM-CAIII cells (Fig. 5C–E), whereas Sost was unaffected (Fig. 5B). Of interest, in SAM-CAIII cells, there was also a significant increase in osteoprotegerin expression and a reduction in M-CSF (Fig. 5F, G). Increased CAIII expression in SAM-CAIII was also confirmed at the protein level (Fig. 5H). Of interest, in Ocy454 cells, when overexpressed, CAIII was also secreted, as demonstrated by the detection of the protein in conditioned medium from controls and SAM-CAIII cells (Fig. 5I). Down-regulation of CAIII using shRNAs reduced CAIII expression (Fig. 6A), but did not affected Sost expression (Fig. 6B). Altogether, these data demonstrate that CAIII is highly expressed in mature osteocytes and is regulated by PTH and prostaglandin and that its overexpression induces changes in few osteocytic transcripts.
Figure 5.
CAIII overexpression in Ocy454 cells. CAIII was overexpressed in Ocy454 cells by using the CRISPR/Cas9 SAM technique. A) Two controls (Cont #1 and 2) and 4 SAM-CAIII guided-RNAs (CAIII #1–4) were used to stably transfect Ocy454 cells. Real-time quantitative PCR analysis demonstrated that one gRNA (CAIII #3) significantly increased CAIII expression compared with control. B–G) SAM-CAIII #3 significantly increased Sost (B), Dmp1 (C), Phex (D), Mepe (E), osteoprotegerin (OPG; F), and M-CSF (G) expression compared with control. SAM-CAIII #3 was used in panels D–G. Data are expressed as means ± sd. H) Western blot analysis of whole-cell lysates of SAM-CAIII #3 cells cultured for 7 and 14 d. CAIII protein is significantly increased in SAM cells (red bars) compared with control (black bar). Quantification is shown on the right. I) Western blot of conditioned medium from controls and SAM-CAIII cells. Quantification is shown on the right and was normalized by β-tubulin. Each experiment was repeated 3 times. *P < 0.05, **P < 0.01.
Figure 6.
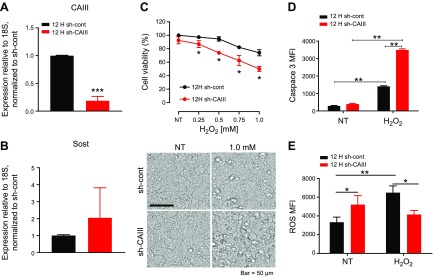
CAIII protects osteocytes from oxidative stress. Ocy454-12H cells were infected with lentiviral shCAIII to knock down CAIII expression. A, B) Quantitative real-time PCR analysis of CAIII (A) and Sost (B) expression in 12H shCAIII cells. C) 12H shCAIII cell viability under H2O2 treatment (6 h after 7 d in culture under 37°C). In the absence of CAIII, Ocy454 cells were more sensitive to H2O2 treatment compared with control, as demonstrated by the significantly reduced viability upon treatment with H2O2. Morphologic analysis of cells that were treated with H2O2 displayed signs of cell death (birifrangent nuclei), and these signs are more evident in shCAIII cells. D) Caspase-3 cleavage in shCAIII cells (red bars) that were exposed to 1 mM H2O2 for 4 h is increased compared with control cells (black bars). E) Basal increase in ROS was observed in shCAIII cells (red bars) compared with control cells (black bars). Upon H2O2 exposure, there was a significant increase in ROS in control cells but not in shCAIII cells. Each experiment was run in triplicates and performed at least 3 times. Data are expressed as means ± sd. Two-way ANOVA with Tukey’s multiple comparison test was performed. *P < 0.05, **P < 0.01 (vs. control or H2O2 treatment), ***P < 0.001.
CAIII protects osteocytes from H2O2 oxidative stress
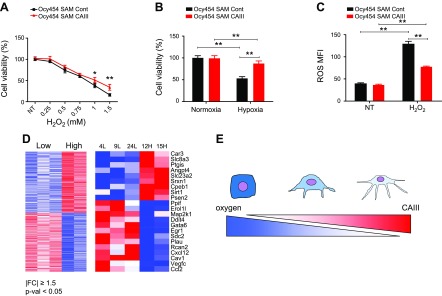
CAs enzymes are involved in a variety of physiologic processes, including pH and CO2 homeostasis, gluconeogenesis, bone resorption, calcification, and tumors. Among the various CAs, CAIII has been shown to protect kidney and rat fibroblasts from H2O2-induced oxidative stress (27, 35, 36). To investigate whether CAIII exerted a protective effect in osteocytes, we treated Ocy454 cells that overexpressed (SAM-CAIII) or lacked (shCAIII) CAIII with H2O2 and measured cell viability, apoptosis, ROS, and gene expression. In the absence of CAIII, Ocy454 cells were more sensitive to H2O2 treatment, as demonstrated by reduced cell viability and increased cell death (Fig. 6C) and a significant increase in cleaved caspase-3–positive cells (Fig. 6D). Of interest, Ocy454-12H shCAIII demonstrated a significant increase in basal ROS activity (Fig. 6E), and exposure to H2O2 was not associated with a significant ROS increase. At the molecular level, H2O2 treatment was associated with a significant suppression of CAIII and Hif1α expression in both cells. RANKL, Dmp1, and osteoprotegerin were also significantly down-regulated by treatment with H2O2 (Supplemental Fig. 3) in both cell lines. In cells that overexpressed CAIII, there was an increase in cell viability and a reduced sensitivity to H2O2 exposure (Fig. 7A). In cells that lacked CAIII, the protective effect of CAIII was present in postconfluent cells as early as 3 d (Supplemental Fig. 4) and persisted until 7 d (Fig. 6C). As expected, SAM-CAIII cells were more resistant to exposure to oxidative stress, as shown by a reduced increase in ROS activity upon H2O2 treatment (Fig. 7C). To investigate whether CAIII played a role in osteocyte response to hypoxic conditions, we exposed SAM-CAIII cells to low oxygen (1% O2) for 24 h. Hypoxic conditions reduced cell viability by 50% in control cells but only by 20% in SAM-CAIII, which confirmed a protective role of this enzyme in osteocytes (Fig. 7B). By using microarray analysis, we found that 518 genes—with a cutoff of 1.5-fold change and a moderated P-value threshold of 0.05—were differentially regulated in high- vs. low-Sost–expressing clones. The Database for Annotation, Visualization, and Integrated Discovery (DAVID; National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA; https://david.ncifcrf.gov/) revealed, among the 518 genes, that 22 genes are involved in response to oxidative stress and/or hypoxia. Nine of the 22 genes, including CAIII, were up-regulated in high- vs. low-Sost–expressing cells (Fig. 7D and Supplemental Table 1)
Figure 7.
CAIII overexpression protects osteocytes from cell death and ROS. A) CAIII-overexpressing Ocy454 cells (SAM-CAIII) displayed an increase in cell viability and reduced sensitivity to H2O2 exposure at various doses. B) SAM-CAIII cells were exposed to hypoxia (1% O2) for 24 h. C) H2O2-induced ROS activity is reduced in SAM-CAIII cells compared with SAM control. D) Unbiased global transcriptome analysis with microarray (Affymetrix) identified 518 genes (left) that were significantly up- or down-regulated in high-Sost–expressing clones (|FC| ≥1.5; P < 0.05; Supplemental Table 1). Expression values are shown as a colored representation (heatmap) in which each row corresponds to an Entrez Gene ID and each column corresponds to a clone (from left to right: 4L, 9L, 24L, 12H, and 15H). Red and blue indicate expression values ≥2 sd above and below, respectively, the row-wise mean (white) computed across all clones. Among those 518 genes, Functional Annotation Tools (DAVID) indicated that 22 genes (right), including CAIII, were involved in the response to hypoxic and/or oxidative stress (Supplemental Table 2). E) Schematic diagram of the relationship between CAIII expression and oxygen levels in osteocytic cell differentiation. As osteoblasts differentiate into osteocytes after being entrapped in the mineralized bone matrix in vivo, the oxygen level available for differentiating osteoblasts is decreased, whereas the expression of CAIII, which protects osteocytes from cell death and ROS, is increased.
In summary, we report that osteocytes can be classified according to their Sost/sclerostin expression. High-Sost–expressing cells (possibly more mature osteocytes) express genes that are involved in matrix mineralization, hypoxia, and oxidative stress (Fig. 7D and Table 1).
DISCUSSION
The primary objective of this study was to identify novel and known genes that are associated with terminally differentiated, high-Sost–expressing osteocytes by using an unbiased transcriptomic approach. Among genes that were expressed in mature osteocytes, we identified CAIII as a novel PTH-regulated transcript. Functionally, CAIII protects osteocytes from H2O2-induced oxidative stresses and cell death. Osteocyte apoptosis plays an essential role in both physiologic and pathologic bone remodeling. Metabolic stress, impaired metabolite transport, and hypoxic conditions have been proposed as major triggers of osteocyte apoptosis and subsequent bone remodeling. By using in vivo multiphoton microscopy, Frikha-Benayed et al. (37) demonstrated that osteocytes in the cortical bone exhibit a well-defined spatial heterogeneity, and they respond to hypoxic stress differently depending on their location. Osteocytes near the periosteum have higher mitochondrial content than do osteocytes that are deeper in the cortex, but most of these mitochondria are poorly functional (37). Here, we report that CAIII expression is elevated in high-Sost/sclerostin–expressing osteocytes and protects these cells from hypoxic stress. CAIII overexpression in the Ocy454-12H subclone was associated with reduced cell death upon H2O2 exposure and reduced ROS production. Conversely, when CAIII was knocked down using lentiviral shRNA, Ocy454-12H cells were more sensitive to oxidative stresses, which demonstrated that this enzyme exerts a protective effect on osteocytes.
CAIII is highly abundant in slow skeletal muscle (10%), adipocytes (24%), and liver (8%), and despite its notable abundance in these tissues, its physiologic role remains unknown. CAIII functions include hydrase activity to maintain intracellular pH, antioxidative activity to protect muscle from oxidative stress and oxidative phosphorylation, and energy metabolism. In adipose cells, this enzyme regulates peroxisome proliferation-activated receptor-γ2, and its down-regulation in preadipocyte enhances adipogenesis by regulating peroxisome proliferation-activated receptor-γ2 gene expression (38). Similarly, in C2C12 cells, a transactivating transcriptional activator–CAIII fused protein decreased the rate of cell apoptosis during hypoxia/reoxygenation, which indicated an antioxidative activity of this enzyme in muscle cells (26). CAIII has been shown to reduce protein oxidation and to defend cells from H2O2-induced apoptosis (36). Knockout mice—deficient in CAIII—have normal growth, development, and lifespan, which suggests that this enzyme does not have an essential role (perhaps as a result of the redundancy of this class of enzymes) (39). Despite the high expression of CAIII in muscle, adipose tissue, and liver, little is known about its transcriptional regulation. In liver, transcription of CAIII gene is inhibited by the aryl hydrocarbon receptor ligand, 3-methylchlathrene. In humans, training and hypoxic conditions induce CAIII expression (27), whereas its levels are reduced in the muscle of patients with myasthenia gravis, which suggests a possible pathologic implication. Compared with other CA isoenzymes, CAIII has lower specific activity for CO2 hydration-dehydration reaction and it has a unique phosphatase activity (40, 41). Previous studies have demonstrated that CAIII protects Rat1 rat fibroblastic cells from H2O2-induced ROS and therefore acts as an antioxidant (36). Here, we demonstrate, for the first time to our knowledge, that CAIII is highly expressed in bone tissues and that this enzyme exerts similar functions in osteocytes—and possibly mature osteoblasts. More recently, CAIII was shown to be up-regulated in proximal tubule cells of mice that lacked Clcn5, a well-defined model of Dent’s disease (35). CAIII is expressed in bone cells and, in particular, in osteocytes, and its expression increases when cells mature and differentiate, as demonstrated by high Sost expression. In bone, as in other tissues, this enzyme works to protect cells from ROS-induced cell death and oxidative stress. We can hypothesize that, as the osteocyte becomes embedded in the mineralized bone, it is exposed to increasing hypoxic condition that is counterbalanced by increased expression of CAIII. Initial analysis of human samples suggests that CAIII and sclerostin might not colocalize in osteocytes. Additional studies are needed to further elucidate the functions of CAIII in skeletal tissues.
In this study, we used a heterogeneous osteocytic cell population (i.e., Ocy454 cells) to identify cells with high and low Sost/sclerostin expression as a hallmark of more and less differentiated cells, respectively. As expected, high-Sost–expressing clones displayed high levels of Dmp1, Phex, Mepe, and Dkk1 transcripts, which further validated their mature phenotype. Among novel genes that were significantly increased in these cells, we identified Sprx2, Cd200, and CAIII. Sprx2 is an extracellular matrix and cell adhesion protein that is involved in cancer and cancer progression. It has also been previously reported to be expressed in cartilage (42). Cd200 is a transmembrane protein that belongs to the Ig family, and it binds to its receptor, Cd200R, abundantly expressed on myeloid-derived cells and T lymphocytes. In bone, Cd200 is expressed in osteoblast precursors, whereas its receptor is present on osteoclasts. The protein is present in hypertrophic chondrocytes and, in Ocy454 cells, is regulated by PTH (34). Among genes that are significantly suppressed in highly differentiating Ocy454, we identified Aspn, Enpp2, and Nov. Aspn is a member of the small leucine-rich repeat proteoglycan/protein family that is believed to be important for the mineralization of bone and teeth. In teeth, Asp is expressed in odontoblasts and ameloblasts, and its expression is regulated by fluoride. Whereas Enpp1 has been implicated in bone mineralization and insulin signaling (43, 44), Enpp2 has not been associated with bone function. Enpps are transmembrane glycoproteins, and Enpp2 has lysophospholipid hydrolyzing activity. They are conserved in vertebrates and hydrolyze pyrophosphate or phosphodiester bonds in various extracellular compounds. In mammals, there are 7 Enpps with distinct substrate specificities and tissue distributions. Enpp2 (or autotaxin) is a secreted lysophospholipase D that is capable of hydrolyzing lysophosphatidylcholine to produce lysophosphatidic acid, which activates GPCRs. Nov/CCN3 is a member of the CCN protein family and has been shown to inhibit osteoblast differentiation and bone regeneration. CCN3-KO animals do not have a skeletal phenotype, whereas its overexpression induces osteopenia. Robo2 has been previously identified, together with its receptor, Slit2, in bone cells. In particular, Robo1, Robo2, and Slit2 were observed to be expressed during osteoblast differentiation (45), but the precise roles of these proteins in skeletal homeostasis is less understood.
In summary, we have established and characterized clonal Ocy454 cells that express various levels of Sost/sclerostin and identified gene signatures that are associated with high-Sost–expressing cells. Among transcripts that are highly expressed in high-Sost cells, we identified CAIII as a novel marker of mature osteocytes. CAIII expression increases with cell differentiation, is regulated by PTH, both in vitro and ex vivo, and protects osteocytes from oxidative stress. Additional studies are needed to investigate in vivo the role of CAIII in PTH skeletal effects. Finally, gene profiling demonstrates that, as osteocytes become embedded into the mineralized matrix, they up-regulate genes that are involved in responses to oxidative stress.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was partially supported by U.S. National Institutes of Health (NIH) National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants AR060221, AR059655, and NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant DK079161 (to P.D.P.). The Center for Skeletal Research Core (NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant P30-AR066261) provided primary calvaria osteoblasts and hypoxic chambers and conducted radioimmunoassays for cAMP. This work was also supported by the Boston University Flow Cytometry Core Facility and the Boston University Microarray Core Facility (Clinical & Translational Science Institute Grant U54-TR001012). The authors declare no conflicts of interest.
Glossary
- CA
carbonic anhydrase
- DCF-DA
2′,7′-dichlorofluorescein diacetate
- Dkk1
Dickkopf WNT signaling pathway inhibitor 1
- Dmp1
dentin matrix protein 1
- FACS
fluorescence-activated cell sorting
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- HRP
horseradish peroxidase
- OEBE
osteocyte-enriched bone explant
- PPR
parathyroid hormone receptor
- PTH
parathyroid hormone
- RANKL
receptor-activator of NF-kB ligand
- ROS
reactive oxygen species
- SAM
synergistic activation mediator
- shRNA
short hairpin RNA
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
C. Shi, Y. Uda, and P. Divieti Pajevic designed the study; C. Shi, Y. Uda, C. Dedic, E. Azab, N. Sun, K. Fulzele, and R. Pereira conducted the study; C. Shi, Y. Uda, C. Dedic, E. Azab, N. Sun, A. I. Hussein, C. A. Petty, R. Pereira, and P. Divieti Pajevic analyzed the data; C. Shi, Y. Uda, R. Pereira, and P. Divieti Pajevic interpreted the data; C. Shi and P. Divieti Pajevic drafted the manuscript; M. C. Mitterberger-Vogt and W. Zwerschke contributed materials; and C. Shi, Y. Uda, C. Dedic, E. Azab, N. Sun, A. I. Hussein, C. A. Petty, M. C. Mitterberger-Vogt, W. Zwerschke, R. Pereira, K. Wang, and P. Divieti Pajevic revised the manuscript and provided critical approval of the final version.
REFERENCES
- 1.Balemans W., Ebeling M., Patel N., Van Hul E., Olson P., Dioszegi M., Lacza C., Wuyts W., Van Den Ende J., Willems P., Paes-Alves A. F., Hill S., Bueno M., Ramos F. J., Tacconi P., Dikkers F. G., Stratakis C., Lindpaintner K., Vickery B., Foernzler D., Van Hul W. (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 10, 537–543 [DOI] [PubMed] [Google Scholar]
- 2.Balemans W., Patel N., Ebeling M., Van Hul E., Wuyts W., Lacza C., Dioszegi M., Dikkers F. G., Hildering P., Willems P. J., Verheij J. B., Lindpaintner K., Vickery B., Foernzler D., Van Hul W. (2002) Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J. Med. Genet. 39, 91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunkow M. E., Gardner J. C., Van Ness J., Paeper B. W., Kovacevich B. R., Proll S., Skonier J. E., Zhao L., Sabo P. J., Fu Y., Alisch R. S., Gillett L., Colbert T., Tacconi P., Galas D., Hamersma H., Beighton P., Mulligan J. (2001) Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am. J. Hum. Genet. 68, 577–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim C. A., Honjo R., Bertola D., Albano L., Oliveira L., Jales S., Siqueira J., Castilho A., Balemans W., Piters E., Jennes K., Van Hul W. (2008) A known SOST gene mutation causes sclerosteosis in a familial and an isolated case from Brazilian origin. Genet. Test. 12, 475–479 [DOI] [PubMed] [Google Scholar]
- 5.van Bezooijen R. L., ten Dijke P., Papapoulos S. E., Löwik C. W. (2005) SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev. 16, 319–327 [DOI] [PubMed] [Google Scholar]
- 6.Nakashima T., Hayashi M., Fukunaga T., Kurata K., Oh-Hora M., Feng J. Q., Bonewald L. F., Kodama T., Wutz A., Wagner E. F., Penninger J. M., Takayanagi H. (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234 [DOI] [PubMed] [Google Scholar]
- 7.Xiong J., Onal M., Jilka R. L., Weinstein R. S., Manolagas S. C., O’Brien C. A. (2011) Matrix-embedded cells control osteoclast formation. Nat. Med. 17, 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X., Zhang Y., Kang H., Liu W., Liu P., Zhang J., Harris S. E., Wu D. (2005) Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 280, 19883–19887 [DOI] [PubMed] [Google Scholar]
- 9.Balemans W., Van Den Ende J., Freire Paes-Alves A., Dikkers F. G., Willems P. J., Vanhoenacker F., de Almeida-Melo N., Alves C. F., Stratakis C. A., Hill S. C., Van Hul W. (1999) Localization of the gene for sclerosteosis to the van Buchem disease-gene region on chromosome 17q12-q21. Am. J. Hum. Genet. 64, 1661–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spatz J. M., Fields E. E., Yu E. W., Divieti Pajevic P., Bouxsein M. L., Sibonga J. D., Zwart S. R., Smith S. M. (2012) Serum sclerostin increases in healthy adult men during bed rest. J. Clin. Endocrinol. Metab. 97, E1736–E1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spatz J. M., Wein M. N., Gooi J. H., Qu Y., Garr J. L., Liu S., Barry K. J., Uda Y., Lai F., Dedic C., Balcells-Camps M., Kronenberg H. M., Babij P., Pajevic P. D. (2015) The Wnt inhibitor sclerostin is up-regulated by mechanical unloading in osteocytes in vitro. J. Biol. Chem. 290, 16744–16758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robling A. G., Niziolek P. J., Baldridge L. A., Condon K. W., Allen M. R., Alam I., Mantila S. M., Gluhak-Heinrich J., Bellido T. M., Harris S. E., Turner C. H. (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 283, 5866–5875 [DOI] [PubMed] [Google Scholar]
- 13.Wein M. N., Spatz J., Nishimori S., Doench J., Root D., Babij P., Nagano K., Baron R., Brooks D., Bouxsein M., Pajevic P. D., Kronenberg H. M. (2015) HDAC5 controls MEF2C-driven sclerostin expression in osteocytes. J. Bone Miner. Res. 30, 400–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saini V., Marengi D. J., Barry K. J., Fulzele K. S., Heiden E., Liu X., Dedic C., Maeda A., Lotinun S., Baron R., Pajevic P. D.Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J. Biol. Chem. 288, 20122–20134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyle W. J., Simonet W. S., Lacey D. L. (2003) Osteoclast differentiation and activation. Nature 423, 337–342 [DOI] [PubMed] [Google Scholar]
- 16.Hsu H., Lacey D. L., Dunstan C. R., Solovyev I., Colombero A., Timms E., Tan H. L., Elliott G., Kelley M. J., Sarosi I., Wang L., Xia X. Z., Elliott R., Chiu L., Black T., Scully S., Capparelli C., Morony S., Shimamoto G., Bass M. B., Boyle W. J. (1999) Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc. Natl. Acad. Sci. USA 96, 3540–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong Y. Y., Yoshida H., Sarosi I., Tan H. L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A. J., Van G., Itie A., Khoo W., Wakeham A., Dunstan C. R., Lacey D. L., Mak T. W., Boyle W. J., Penninger J. M. (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323 [DOI] [PubMed] [Google Scholar]
- 18.Li J., Sarosi I., Yan X. Q., Morony S., Capparelli C., Tan H. L., McCabe S., Elliott R., Scully S., Van G., Kaufman S., Juan S. C., Sun Y., Tarpley J., Martin L., Christensen K., McCabe J., Kostenuik P., Hsu H., Fletcher F., Dunstan C. R., Lacey D. L., Boyle W. J. (2000) RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc. Natl. Acad. Sci. USA 97, 1566–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaman G., Pitsillides A. A., Rawlinson S. C. F., Suswillo R. F. L., Mosley J. R., Cheng M. Z., Platts L. A. M., Hukkanen M., Polak J. M., Lanyon L. E. (1999) Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J. Bone Miner. Res. 14, 1123–1131 [DOI] [PubMed] [Google Scholar]
- 20.Guo D., Bonewald L. F. (2009) Advancing our understanding of osteocyte cell biology. Ther. Adv. Musculoskelet. Dis. 1, 87–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang W., Harris M. A., Heinrich J. G., Guo D., Bonewald L. F., Harris S. E. (2009) Gene expression signatures of a fibroblastoid preosteoblast and cuboidal osteoblast cell model compared to the MLO-Y4 osteocyte cell model. Bone 44, 32–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woo S. M., Rosser J., Dusevich V., Kalajzic I., Bonewald L. F. (2011) Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J. Bone Miner. Res. 26, 2634–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang F., Wen Y., Guo X. (2014) CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum. Mol. Genet. 23(R1), R40–R46 [DOI] [PubMed] [Google Scholar]
- 24.Paic F., Igwe J. C., Nori R., Kronenberg M. S., Franceschetti T., Harrington P., Kuo L., Shin D. G., Rowe D. W., Harris S. E., Kalajzic I. (2009) Identification of differentially expressed genes between osteoblasts and osteocytes. Bone 45, 682–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang H., Ren H. M., Shang X. L., Liu X. Y. (2014) Detection of the phosphatase activity of carbonic anhydrase III on a nitrocellulose membrane following 2D gel electrophoresis. Mol. Med. Rep. 10, 1887–1892 [DOI] [PubMed] [Google Scholar]
- 26.Shang X., Bao Y., Chen S., Ren H., Huang H., Li Y. (2012) Expression and purification of TAT-fused carbonic anhydrase III and its effect on C2C12 cell apoptosis induced by hypoxia/reoxygenation. Arch. Med. Sci. 8, 711–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shang X., Chen S., Ren H., Li Y., Huang H. (2009) Carbonic anhydrase III: the new hope for the elimination of exercise-induced muscle fatigue. Med. Hypotheses 72, 427–429 [DOI] [PubMed] [Google Scholar]
- 28.Kogawa M., Wijenayaka A. R., Ormsby R. T., Thomas G. P., Anderson P. H., Bonewald L. F., Findlay D. M., Atkins G. J. (2013) Sclerostin regulates release of bone mineral by osteocytes by induction of carbonic anhydrase 2. J. Bone Miner. Res. 28, 2436–2448 [DOI] [PubMed] [Google Scholar]
- 29.Konermann S., Brigham M. D., Trevino A. E., Joung J., Abudayyeh O. O., Barcena C., Hsu P. D., Habib N., Gootenberg J. S., Nishimasu H., Nureki O., Zhang F. (2015) Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517, 583–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ran F. A., Hsu P. D., Wright J., Agarwala V., Scott D. A., Zhang F. (2013) Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart S. A., Dykxhoorn D. M., Palliser D., Mizuno H., Yu E. Y., An D. S., Sabatini D. M., Chen I. S., Hahn W. C., Sharp P. A., Weinberg R. A., Novina C. D. (2003) Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 9, 493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fulzele K., Krause D. S., Panaroni C., Saini V., Barry K. J., Liu X., Lotinun S., Baron R., Bonewald L., Feng J. Q., Chen M., Weinstein L. S., Wu J. Y., Kronenberg H. M., Scadden D. T., Divieti Pajevic P.Myelopoiesis is regulated by osteocytes through Gsalpha-dependent signaling. Blood 121, 930–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fulzele K., Lai F., Dedic C., Saini V., Uda Y., Shi C., Tuck P., Aronson J. L., Liu X., Spatz J. M., Wein M., Pajevic P. D. (2017) Osteocyte-secreted Wnt signaling inhibitor sclerostin contributes to beige adipogenesis in peripheral fat depots. J. Bone Miner. Res. 32, 373–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wein M. N., Liang Y., Goransson O., Sundberg T. B., Wang J., Williams E. A., O’Meara M. J., Govea N., Beqo B., Nishimori S., Nagano K., Brooks D. J., Martins J. S., Corbin B., Anselmo A., Sadreyev R., Wu J. Y., Sakamoto K., Foretz M., Xavier R. J., Baron R., Bouxsein M. L., Gardella T. J., Divieti-Pajevic P., Gray N. S., Kronenberg H. M. (2016) SIKs control osteocyte responses to parathyroid hormone. Nat. Commun. 7, 13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gailly P., Jouret F., Martin D., Debaix H., Parreira K. S., Nishita T., Blanchard A., Antignac C., Willnow T. E., Courtoy P. J., Scheinman S. J., Christensen E. I., Devuyst O. (2008) A novel renal carbonic anhydrase type III plays a role in proximal tubule dysfunction. Kidney Int. 74, 52–61 [DOI] [PubMed] [Google Scholar]
- 36.Roy P., Reavey E., Rayne M., Roy S., Abed El Baky M., Ishii Y., Bartholomew C. (2010) Enhanced sensitivity to hydrogen peroxide-induced apoptosis in Evi1 transformed Rat1 fibroblasts due to repression of carbonic anhydrase III. FEBS J. 277, 441–452 [DOI] [PubMed] [Google Scholar]
- 37.Frikha-Benayed D., Basta-Pljakic J., Majeska R. J., Schaffler M. B. (2016) Regional differences in oxidative metabolism and mitochondrial activity among cortical bone osteocytes. Bone 90, 15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitterberger M. C., Kim G., Rostek U., Levine R. L., Zwerschke W. (2012) Carbonic anhydrase III regulates peroxisome proliferator-activated receptor-γ2. Exp. Cell Res. 318, 877–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim G., Lee T. H., Wetzel P., Geers C., Robinson M. A., Myers T. G., Owens J. W., Wehr N. B., Eckhaus M. W., Gros G., Wynshaw-Boris A., Levine R. L. (2004) Carbonic anhydrase III is not required in the mouse for normal growth, development, and life span. Mol. Cell. Biol. 24, 9942–9947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cabiscol E., Levine R. L. (1995) Carbonic anhydrase III. Oxidative modification in vivo and loss of phosphatase activity during aging. J. Biol. Chem. 270, 14742–14747 [DOI] [PubMed] [Google Scholar]
- 41.Cabiscol E., Levine R. L. (1996) The phosphatase activity of carbonic anhydrase III is reversibly regulated by glutathiolation. Proc. Natl. Acad. Sci. USA 93, 4170–4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang H., Zhao J., Zhang L., Zhao J., Zhuang Y., Liang P. (2016) SRPX2 enhances the epithelial-mesenchymal transition and temozolomide resistance in glioblastoma Cells. Cell. Mol. Neurobiol. 36, 1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kato K., Nishimasu H., Mihara E., Ishitani R., Takagi J., Aoki J., Nureki O. (2012) Expression, purification, crystallization and preliminary X-ray crystallographic analysis of Enpp1. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 68, 778–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kato K., Nishimasu H., Okudaira S., Mihara E., Ishitani R., Takagi J., Aoki J., Nureki O. (2012) Crystal structure of Enpp1, an extracellular glycoprotein involved in bone mineralization and insulin signaling. Proc. Natl. Acad. Sci. USA 109, 16876–16881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun H., Dai K., Tang T., Zhang X. (2009) Regulation of osteoblast differentiation by slit2 in osteoblastic cells. Cells Tissues Organs 190, 69–80 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.