Abstract
Inappropriate antibiotic use is a key factor in the emergence of antibiotic resistance. The majority of antibiotics are prescribed in primary care, where upper respiratory tract infection (URTI) is a common presentation. Inappropriate antibiotic prescribing in URTI is common globally and has increased markedly in developing and transitional countries. Antibiotic stewardship is crucial to prevent the emergence and spread of resistant microbes. This project aimed to reduce inappropriate antibiotic prescribing in URTI in a non-governmental organisation’s primary care outreach clinics in Kolkata, India, from 62.6% to 30% over 4 months. A multifaceted intervention to reduce inappropriate antibiotic use in non-specific URTI was implemented. This consisted of a repeated process of audit and feedback, interactive training sessions, one-to-one case-based discussion, antibiotic guideline development and coding updates. The primary outcome measure was antibiotic prescribing rates. A baseline audit of all patients presenting with non-specific URTI over 8 weeks in November and December 2016 (n=222) found that 62.6% were prescribed antibiotics. Postintervention audit over 4 weeks in April 2017 (n=69) showed a marked reduction in antibiotic prescribing to 7.2%. An increase in documentation of examination findings was also observed, from 52.7% to 95.6%. This multifaceted intervention was successful at reducing inappropriate antibiotic prescribing, with sustained reductions demonstrated over the 4 months of the project. This suggests that approaches previously used in Europe can successfully be applied to different settings.
Keywords: quality improvement, antibiotic management, PDSA, audit and feedback, general practice
Problem
Upper respiratory tract infection (URTI) is a common presentation in primary care, accounting for up to 10% of presentations.1 The use of antibiotics in nasopharyngitis, common cold and non-specific URTI does not improve outcomes; in fact, patients report increased adverse events compared with placebo.2 Thus, the majority of patients with URTI do not require antibiotics. Overuse of antibiotics contributes to the emergence of antibiotic resistance.3 4 Despite the risk to individual and population health, inappropriate antibiotic use is widespread in URTI.5–7
The outreach clinics of a non-governmental organisation provide free healthcare to disadvantaged people living in Kolkata and West Bengal, serving homeless and slum communities. Anyone living below the poverty line is entitled to free medical care and may be entitled to benefits packages, for example, food supplies. The organisation employs 10 full-time doctors. Patients self-refer to clinics or are referred by government hospitals for extra support. Patients often present with complex medical and social needs, creating a pressured working environment. The outreach clinics are routinely staffed by a doctor, a pharmacist and support healthcare workers, with an average attendance of 30 patients per half-day session. URTI is a common presentation in the outreach clinics; on average, 100 patients present with URTI each month.
A baseline audit of 222 patients presenting with non-specific URTI over 8 weeks found antibiotics were prescribed in 62.6% of cases. This project aimed to reduce antibiotic prescribing in non-specific URTI from 62.6% to 30% in 4 months.
Background
Antibiotic resistance is a matter of global concern; increasing antibiotic use drives the emergence of resistance. Hence, as well as risking unwarranted side effects to the individual patient (such as drug side effects), inappropriate use of antibiotics also poses risks to community and public health.8 Patients treated with antibiotics for respiratory infections in primary care develop bacterial resistance to that antibiotic. This can persist for up to 12 months, increasing the prevalence of resistant organisms in the community.9 In developed countries, 80%–90% of antibiotics are prescribed in primary care settings; the majority of these are prescribed for respiratory tract infections.10 11 The rates of inappropriate antibiotic prescription are worryingly high; a major study of US ambulatory care visits estimated a third of all antibiotics prescribed were inappropriate.7
Antibiotic prescribing in primary care varies markedly within European countries. In 2015, antibiotic prescribing ranged from 10.7 defined daily dose per 1000 inhabitants per day in the Netherlands to 36.1 in Greece.12 In Dutch primary care, 19% of patients presenting with URTI in 2010 were prescribed antibiotics.13 In the UK, the median general practice in a large study prescribed antibiotics at 38% of consultations for URTI.14 Antibiotic prescribing for URTI in primary care in developing and transitional countries has increased from rates of approximately 44% in 1982 to 71% in 2006.15 In China and Thailand, over 80% were prescribed antibiotics.16 17 Pooled data for India shows antibiotic prescribing in URTI of over 70%.18
Why do doctors prescribe antibiotics inappropriately? Doctors’ knowledge of antibiotic prescribing guidelines and the risks of antimicrobial resistance may be inadequate.19–23 Older GPs are less likely to prescribe according to antibiotic guidelines, suggesting difficulty changing established practice in light of new evidence.24 Doctors may disagree with evidence-based guidelines, instead relying on their own clinical experience to guide them25; for example, doctors in India report using antibiotics as prevention in patients from poorer communities due to concerns about higher prevalence of bacterial infection.26 Barriers towards putting evidence into practice include time pressure, financial considerations and doctors’ perceptions of patients’ expectations.26–30
A number of randomised controlled trials in primary care in Europe have shown significant reductions in antibiotic prescribing (both all cause and specifically in URTI) through programmes comprising educational events and feedback on practice prescribing habits.31–33 For example, the Stemming the Tide of Antibiotic Resistance (STAR) programme, consisting of prescribing feedback, seminars and online education, led to a 4.2% reduction in all-cause antibiotic prescribing without increased hospital admissions or reconsultations in a Welsh primary care setting.33
Interventions that use a number of different approaches to improve antibiotic prescribing tend to be most successful. Audit and feedback alone generally lead to small improvements in professional practice.34 Interactive educational sessions are more effective than lectures.35
Measurement
Initial data collection reviewed all cases coded as upper respiratory tract infection in the outreach primary care clinics over 8 weeks in November and December 2016. The coding for URTI was a generic code with no subdivisions. Ear infections were coded differently and were not included. All notes are paper based and were reviewed manually.
In case notes audited, the predominant symptoms were cough, cold and runny nose. There were no positive examination findings apart from cervical lymphadenopathy and coryza. Some notes did not record symptoms but simply documented ‘URTI’; these were included. Case notes were excluded from analysis when the coding was judged to be incorrect (eg, patients with cough and fever with focal chest findings on examination). Inclusion criteria were developed to select a patient population in which antibiotics were not indicated. Therefore, cases where likely diagnosis might warrant antibiotic use (eg, tonsillitis, sinusitis) were excluded; these cases comprised 9 out of 231 patients coded as URTI. An Excel spreadsheet was used to store and analyse the data collected.
Subsequent audits used the same criteria but were carried out over 4-week periods. The coding system was updated in the second Plan, Do, Study, Act (PDSA) cycle. Following this, audit included patients coded as common cold or non-specific URTI.
In November and December 2016, 222 patients meeting inclusion criteria were seen. Antibiotics were prescribed in 62.6%. Two antibiotics were prescribed in 1.8%. Ten different antibiotics were prescribed; the most common being amoxicillin (31.7%), cefadroxil (23.7%) and co-trimoxazole (12.9%). Examination findings were documented in 47.3% of patients. Supportive treatment (such as paracetamol, cough syrup and antihistamines) was prescribed in 87% of patients.
Design
A multifaceted intervention to reduce inappropriate antibiotic prescribing was planned. This comprised audit prescribing feedback, educational seminars, one-on-one case-based discussion, guideline development and coding alterations.
Pooled audit feedback on URTI prescribing was presented at pre-established monthly doctors’ meetings, attended by the organisation’s doctors and chaired by the deputy CEO. Giving individual as well as pooled prescribing feedback was considered, but it was felt that this could alienate staff and reduce engagement. Furthermore, pooled feedback might motivate doctors who were prescribing at higher rates to modify their practice in line with colleagues. Doctors and medical management gathered around a table; PowerPoint was used for presentations.
Three seminars (1 hour) combining PowerPoint and round table discussion were planned for the doctors’ meetings to provide the evidence for antibiotic use in URTI and to encourage interactive discussion around the rationale for antibiotic prescribing. The hypothesis was that eliciting doctors’ views in a supportive environment would help to generate open debate. Allowing staff to share their prescribing motivations without censure made it possible to understand why inappropriate antibiotic prescribing was taking place. Meetings were not taped or transcribed; however, issues raised by staff was recorded during the meetings. Staff feedback was used to tailor training sessions around their specific issues and concerns. Staff engagement was critical to the long-term sustainability of this project.
One-on-one case-based discussions in the clinics were planned, as it was anticipated that these would yield more specific information on the reasons behind inappropriate antibiotic use and give an opportunity to suggest alternative management strategies. These were delivered in all outreach clinics on an informal basis (weekly–fortnightly) prompted by doctors’ queries or quality improvement team concerns around inappropriate prescribing identified in consultations.
The development of antibiotic guidelines for the organisation was planned; these were approved by management and frontline staff. This aimed to increase the sustainability of the process by providing agreed references for best practice. An update to the coding system was used to differentiate within URTI and encourage more focused diagnosis and thus decision making and management. This was done in collaboration with staff and management and subdivided URTI from a generic category into specific subsections.
Strategy
The PDSA model was used to trial interventions and assess their impact on reducing inappropriate antibiotic prescribing in URTI.
PDSA cycle 1
In this cycle, the aim was to raise awareness of the problem of inappropriate antibiotic prescribing in URTI through audit feedback and to explore the reasons behind this.
Training sessions took place in the monthly doctors’ meetings. The initial session comprised an interactive group discussion on the baseline audit findings. Staff recognised that antibiotic prescribing levels were high. They identified potential problems, including side effects to the individual patient, the financial cost to the organisation and the emergence of antibiotic resistance. However, doctors felt that, given the ready availability of antibiotics from pharmacies without prescription, changing their individual antibiotic prescribing habits would make little difference to antibiotic resistance. Reasons behind the high levels of antibiotic prescribing were explored: doctors described having a lower threshold for antibiotic prescribing in the mobile clinics because of difficulties with follow-up. Doctors were concerned that, due to limited health knowledge, patients might not seek help appropriately if their symptoms deteriorated. Doctors felt that patients expected to be prescribed antibiotics. They felt that patients might go on to buy inappropriate antibiotics over the counter from pharmacies if they withheld them. The evidence around antibiotic use in URTI was presented and discussed. After discussion and consensus building, all doctors agreed that antibiotics should not routinely be prescribed in common cold and non-specific URTI.
Case-based discussions took place on an ad hoc basis when potentially inappropriate antibiotic prescribing in URTI was identified in clinic. This provided useful insights, including the fact that purulent nasal discharge was seen as an indication for antibiotic use. Relevant evidence was shared by the quality improvement team and alternative management strategies were discussed.
The following audit demonstrated a reduction in antibiotic prescribing in URTI from 62.6% to 52.2%.
PDSA cycle 2
The aim of this cycle was to further reduce antibiotic prescribing by discussing alternative management strategies, by giving clear guidelines on when antibiotics were indicated and by encouraging a greater focus on diagnosis through updating the coding system.
The interventions outlined in the first PDSA cycle continued. Training focused on strategies to limit antibiotic prescribing built around the motivations elicited previously. For example: the use of safety netting to ensure patients are reviewed if they deteriorate; the use of examination to exclude serious bacterial infection such as pneumonia; testing for dengue and malaria in febrile patients with URTI symptoms; patient education on self-care and the risks of inappropriate antibiotic use in self-limiting conditions; and identifying and addressing the patient’s expectations.
The organisation did not have antimicrobial guidelines. Guidelines were developed based on national and international recommendations and evidence, adapted to local availability of drugs. Unfortunately, local resistance data were unavailable, and therefore, national resistance data were used to inform the guidelines. Guidelines were circulated to the medical management team and frontline staff for their input before finalisation.
The coding system previously had a single code for URTI. The hypothesis was that changing the coding to an umbrella code of URTI with specific subsections (common cold, pharyngitis, tonsillitis, sinusitis and other/non-specific URTI) would encourage more targeted prescribing and thus reduce indiscriminate use of antibiotics. The implementation of the coding change took longer than anticipated, as the entire coding system was updated in the process, and changes needed to be agreed by all key parties. The new coding system was implemented on April 2017.
In the audit following this PDSA cycle, antibiotic use dropped dramatically to 7.8%.
Results
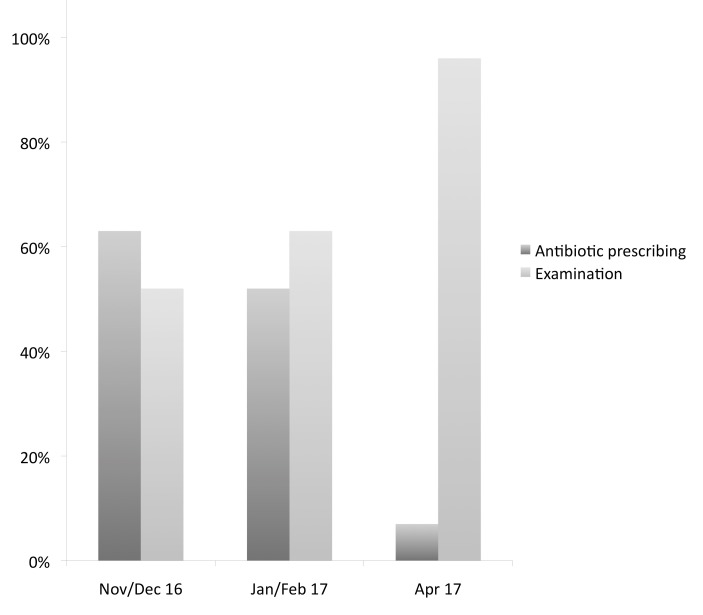
The main outcome measure was antibiotic prescribing rates. Selection criteria for the audit meant that only patients without a documented indication for antibiotics were included in analysis. Pre–post intervention analysis revealed a dramatic drop in antibiotic prescribing, from 62.6% to 7.2%, following 4 months of the multifaceted intervention. It was also noted that the rate of documented examinations increased during the project (see figure 1).
Figure 1.
Antibiotic prescribing (%) and documented examination findings (%) over time.
Baseline audit, carried out over 8 weeks in November and December 2016, showed 62.6% of patients received antibiotics and 52.7% had documented examinations (n=222).
Repeat audit after the first PDSA cycle showed a reduction in antibiotic prescribing to 52.2% and an increase in documented examinations to 63%. Audit was carried out over 4 weeks using the same selection criteria as the baseline audit (n=92). Nine different antibiotics were prescribed, most commonly amoxicillin (37.5%), cefadroxil (14.6%) and co-trimoxazole (14.6%). AMedications for symptomatic relief (paracetamol, antihistamines and cough syrup) were prescribed in 89.2%.
Repeat audit after the second PDSA cycle showed a marked reduction in antibiotics prescribing to 7.2% and an increase in documented examinations to 95.6% (n=69). Audit was carried out over 4 weeks; selection criteria were updated due to the new coding system. Patients coded as ‘common cold’ and ‘non-specific URTI’ were selected. Amoxicillin, cefadroxil and co-amoxiclav were used. Medications for symptomatic relief were prescribed in 89.8% (see table 1).
Table 1.
Summary of data
| Cycle | Time (weeks) |
Patients (N) | Examinations | Examinations (%) | Antibiotics | Antibiotics (%) | Symptom relief medications | Symptom relief medications (%) |
| Baseline | 8 | 222 | 117 | 52.7 | 139 | 62.6 | 194 | 87.3 |
| PDSA 1 | 4 | 92 | 58 | 63 | 48 | 52.2 | 82 | 89.1 |
| PDSA 2 | 4 | 69 | 66 | 95.6 | 5 | 7.2 | 62 | 89.8 |
PDSA, Plan, Do, Study, Act.
An important benefit of the project was the development of a new disease coding system for the outreach clinics. The disease coding system in use was outdated, with many common diagnoses not listed (eg, hypertension). It did not allow for more than one disease code and did not differentiate new and follow-up cases. Informal feedback from doctors, healthcare workers and management following its introduction was positive.
Lessons and limitations
This project aim was to reduce inappropriate antibiotic prescribing in URTI by approximately half, from 62.6% to 30%, through the use of a multifaceted intervention. The reduction in antibiotic prescribing was dramatic and better than anticipated, with rates of 7.2% in the postintervention audit.
A key strength of the project was the use of staff engagement to drive change. Following baseline audit feedback and discussion, the deputy CEO in charge of the medical projects became a strong advocate for reducing unnecessary antibiotic prescribing in URTI. Her support in conjunction with her authority within the organisation gave increased credibility to the project. The use of interactive sessions allowed staff to discuss their concerns and motivations for antibiotic prescribing in an open environment. This in turn allowed the development of strategies to address specific staff concerns. For example, some doctors said that they prescribed antibiotics as they were worried about missing an underlying bacterial infection. Examining the patient was suggested to help differentiate URTI and pneumonia. During the project, there was a marked rise in documentation of examinations, suggesting that this was a strategy that doctors found helpful.
The increase in examinations documented may have improved diagnostic accuracy (assuming the change is due to a real increase in examination rather than an increase in documentation). For example, a patient presenting with cough who had focal chest signs but was not examined in the baseline audit may have been diagnosed as URTI, whereas in the postintervention audit, the patient may have been diagnosed with pneumonia. This would be an unexpected advantage of the project; it is important that serious infections such as pneumonia receive appropriate management and follow-up.
The focus on common cold and non-specific URTI was both a strength and a limitation of the project. Following initial training sessions and audit feedback, it was possible to obtain group consensus that antibiotics were not appropriate in these patients. However, the majority of doctors felt that the use of antibiotics in sore throat and sinusitis was almost always indicated; they felt that the evidence put forward did not apply to their patient populations. The narrower focus in this intervention made it easier to deliver and reinforce clear messages. However, further work on improving antibiotic prescribing in tonsillitis and sinusitis would be beneficial.
Multiple interventions were trialled concurrently. Previous studies have shown multifaceted interventions to be effective. It is likely that the interventions had a synergistic impact and that the concurrent use of interventions led to the more rapid change seen in the second PDSA cycle. However, this design means it is not possible to analyse the impact of any one intervention.
A major potential limitation of the study is the impact of the Hawthorne effect. The very fact of observing prescribing behaviour can increase physician compliance with guidelines.36 To avoid this effect, a control group would also be needed. Since paper notes were analysed retrospectively, the audit process itself would not have affected the baseline data. After this, staff were aware of the audit process. Given that this was the first project looking at antibiotic prescribing in the organisation, this alone may have had a dramatic impact on the effect size of the interventions.
A new doctor was recruited between baseline data collection and the initial PDSA cycle. Their prescribing practice may have affected changes seen between the December–November baseline audit and the January–February audit. As prescribing data were pooled, it is not possible to determine the extent and direction of this effect.
The project relied on changing staff prescribing patterns, largely through training and audit feedback; this is a potential limitation. This process was not routine in the organisation before the project. Its importance has been recognised by medical management, who are driving future audit projects to improve service quality. An ongoing process of audit and feedback is likely to be crucial to making these changes sustainable long term.
In future, it would be helpful to think about introducing more system changes to support these changes, such as enabling the pharmacy team to directly query inappropriate antibiotic requests.
Seasonal variation may impact the results: the number of patients presenting with non-specific URTI decreased between the initial 8 weeks audit and the final audit. The time of year may influence doctors’ perceptions of risks of bacterial infection and thus impact prescribing practice.
It was not possible to collect all relevant data to assess the broader impact of the intervention. Although the organisation provides a primary care service, there is no notification by hospitals or other healthcare providers of patient attendances. Therefore, it was not possible to collect data on hospital presentations or admissions during this study. The ultimate goal of reducing inappropriate antibiotic use is to reduce the risk of antimicrobial resistance and to prevent unnecessary side effects. It was beyond the scope of this study to examine the impact on antimicrobial resistance in bacterial isolates.
Conclusion
The multifaceted intervention implemented in this project (comprising audit feedback, group training sessions, one-to-one case-based discussion, guideline development and coding updates) resulted in a marked reduction in inappropriate antibiotic prescribing in URTI. This supports randomised controlled trials of multifaceted interventions in this field, which have shown significant impact on inappropriate antibiotic prescribing in URTI. Previous studies have largely been in European countries; this project shows similar interventions can be successful in lower income countries and can be successful in settings outside a government healthcare system. The outcomes of this project, alongside the worrying increase in antibiotic prescribing in URTI in developing and transitional countries,15 suggests that further work in this context is urgent and could bring substantial benefits.
This intervention would be most suitable for adaptation to a similar setting with minimal resources, as it is low cost and relies on basic materials only. Some facets of the intervention could be adapted to other settings, including the use of audit feedback, group interactive discussion and one-to-one case-based discussions. In clinical settings using computer-based systems, the use of prompts associated with antibiotic prescribing would be a helpful addition. The development of antimicrobial guidelines and changes to the coding system were specific to the organisation’s needs; however, similar changes could be made as required in different situations.
Ongoing audit and monitoring is planned to assess the project’s sustainability; senior management support and staff engagement should ensure that the benefits demonstrated over the project continue. The next planned intervention is to engage patients, through the delivery of patient and community education sessions on self care in URTI.
Acknowledgments
Special thanks to the doctors involved in the project for their efforts in changing practice: the success is theirs. I would like to particularly thank Dr Ghosh, deputy CEO. Dr Ghosh’s support and mentorship have been critical to the success of this project. I would also like to thank my mentor, Dr Fox, for his valuable input during my time in Kolkata. I would like to thank the Calcutta Rescue Fund UK and the Clare Wand Fund for their invaluable support of my role.
Footnotes
Contributors: ADL designed the data collection process, collected the data, implemented the multifaceted intervention, analysed the data and drafted the paper.
Funding: The author has not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Barton E, Spencer R. URTIs: recommended diagnosis and treatment in general practice. Prescriber 2011;22:23–36. 10.1002/psb.743 [DOI] [Google Scholar]
- 2. Kenealy T, Arroll B. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst Rev 2013;6:CD000247 10.1002/14651858.CD000247.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Toner E, Adalja A, Gronvall GK, et al. Antimicrobial resistance is a global health emergency. Health Secur 2015;13:153–5. 10.1089/hs.2014.0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ventola CL. The antibiotic resistance crisis. Pharm Ther 2015;40:277–83. [PMC free article] [PubMed] [Google Scholar]
- 5. Akkerman AE, Kuyvenhoven MM, van der Wouden JC, et al. Prescribing antibiotics for respiratory tract infections by GPs: management and prescriber characteristics. Br J Gen Pract 2005;55:114–8. [PMC free article] [PubMed] [Google Scholar]
- 6. Ab Rahman N, Teng CL, Sivasampu S. Antibiotic prescribing in public and private practice: a cross-sectional study in primary care clinics in Malaysia. BMC Infect Dis 2016;16:208 10.1186/s12879-016-1530-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA 2016;315:1864–73. 10.1001/jama.2016.4151 [DOI] [PubMed] [Google Scholar]
- 8. Levy SB. Antibiotic availability and use: consequences to man and his envronment. J Clin Epidemiol 1991;45(Suppl 2):83–7. 10.1016/0895-4356(91)90117-R [DOI] [PubMed] [Google Scholar]
- 9. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010;340:c2096 10.1136/bmj.c2096 [DOI] [PubMed] [Google Scholar]
- 10. Tyrstrup M, Beckman A, Mölstad S, et al. Reduction in antibiotic prescribing for respiratory tract infections in Swedish primary care- a retrospective study of electronic patient records. BMC Infect Dis 2016;16:709 10.1186/s12879-016-2018-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Disease Surveillance Centre, Scientific Advisory Committee. SARI: a Strategy for the control of Antimicrobial Resistance in Ireland. Dublin: NDSC, 2001. [Google Scholar]
- 12. European Centre for Disease Prevention and Control, 2016. Summary of the latest data on antibiotic consumption in the European Union ESAC-Net surveillance data November 2016. https://ecdc.europa.eu/sites/portal/files/documents/antibiotics-ESAC-Net%20Summary%202016_0.pdf
- 13. van den Broek d’Obrenan J, Verheij TJ, Numans ME, et al. Antibiotic use in Dutch primary care: relation between diagnosis, consultation and treatment. J Antimicrob Chemother 2014;69:1701–7. 10.1093/jac/dku005 [DOI] [PubMed] [Google Scholar]
- 14. Gulliford MC, Dregan A, Moore MV, et al. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open 2014;4:e006245 10.1136/bmjopen-2014-006245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization. Medicines use in primary care in developing and transitional countries: fact book summarizing results from studies reported between 1990 and 2006: WHO press, 2009. (cited 4 Jul 2017). [Google Scholar]
- 16. Jiang Q, Yu BN, Ying G, et al. Outpatient prescription practices in rural township health centers in Sichuan Province, China. BMC Health Serv Res 2012;12:324 10.1186/1472-6963-12-324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Issarachaikul R, Suankratay C. Antibiotic prescription for adults with upper respiratory tract infection and acute bronchitis at King Chulalongkorn Memorial Hospital, Thailand. Asian Biomed 2013;7:15–20. [Google Scholar]
- 18. Holloway KA, Rosella L, Henry D. The Impact of WHO Essential Medicines Policies on Inappropriate Use of Antibiotics. PLoS One 2016;11:e0152020 10.1371/journal.pone.0152020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bai Y, Wang S, Yin X, et al. Factors associated with doctors' knowledge on antibiotic use in China. Sci Rep 2016;6:23429 10.1038/srep23429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lucet JC, Nicolas-Chanoine MH, Roy C, et al. Antibiotic use: knowledge and perceptions in two university hospitals. J Antimicrob Chemother 2011;66:936–40. 10.1093/jac/dkq541 [DOI] [PubMed] [Google Scholar]
- 21. Thriemer K, Katuala Y, Batoko B, et al. Antibiotic prescribing in DR Congo: a knowledge, attitude and practice survey among medical doctors and students. PLoS One 2013;8:e55495 10.1371/journal.pone.0055495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanchez GV, Roberts RM, Albert AP, et al. Effects of Knowledge, Attitudes, and Practices of Primary Care Providers on Antibiotic Selection, United States. Emerg Infect Dis 2014;20:2041–7. 10.3201/eid2012.140331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pulcini C, Williams F, Molinari N, et al. Junior doctors' knowledge and perceptions of antibiotic resistance and prescribing: a survey in France and Scotland. Clin Microbiol Infect 2011;17:80–7. 10.1111/j.1469-0691.2010.03179.x [DOI] [PubMed] [Google Scholar]
- 24. Tell D, Engström S, Mölstad S. Adherence to guidelines on antibiotic treatment for respiratory tract infections in various categories of physicians: a retrospective cross-sectional study of data from electronic patient records. BMJ Open 2015;5:e008096 10.1136/bmjopen-2015-008096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hisham R, Liew SM, Ng CJ, Cj N, et al. Rural Doctors' Views on and Experiences with Evidence-Based Medicine: The FrEEDoM Qualitative Study. PLoS One 2016;11:e0152649 10.1371/journal.pone.0152649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kotwani A, Wattal C, Katewa S, et al. Factors influencing primary care physicians to prescribe antibiotics in Delhi India. Fam Pract 2010;27:684–90. 10.1093/fampra/cmq059 [DOI] [PubMed] [Google Scholar]
- 27. Schwartz RK, Soumerai SB, Avorn J. Physician motivations for nonscientific drug prescribing. Soc Sci Med 1989;28:577–82. 10.1016/0277-9536(89)90252-9 [DOI] [PubMed] [Google Scholar]
- 28. Lopez-Vazquez P, Vazquez-Lago JM, Figueiras A. Misprescription of antibiotics in primary care: a critical systematic review of its determinants. J Eval Clin Pract 2012;18:473–84. 10.1111/j.1365-2753.2010.01610.x [DOI] [PubMed] [Google Scholar]
- 29. Teixeira Rodrigues A, Roque F, Falcão A, et al. Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents 2013;41:203–12. 10.1016/j.ijantimicag.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 30. Hassali MA, Kamil TK, Md Yusof FA, et al. General practitioners' knowledge, attitude and prescribing of antibiotics for upper respiratory tract infections in Selangor, Malaysia: findings and implications. Expert Rev Anti Infect Ther 2015;13:1–10. 10.1586/14787210.2015.1012497 [DOI] [PubMed] [Google Scholar]
- 31. Vervloet M, Meulepas MA, Cals JW, et al. Reducing antibiotic prescriptions for respiratory tract infections in family practice: results of a cluster randomized controlled trial evaluating a multifaceted peer-group-based intervention. NPJ Prim Care Respir Med 2016;26:15083 10.1038/npjpcrm.2015.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gjelstad S, Høye S, Straand J, et al. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing (Rx-PAD) study). BMJ 2013;347:f4403 10.1136/bmj.f4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Butler CC, Simpson SA, Dunstan F, et al. Effectiveness of multifaceted educational programme to reduce antibiotic dispensing in primary care: practice based randomised controlled trial. BMJ 2012;344:d8173 10.1136/bmj.d8173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jamtvedt G, Young JM, Kristoffersen DT, et al. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2006;2:CD000259. [DOI] [PubMed] [Google Scholar]
- 35. Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev 2005;4:CD003539 10.1002/14651858.CD003539.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peleg AY, Paterson DL. Modifying antibiotic prescribing in primary care. Clin Infect Dis 2006;42:1231–3. 10.1086/503042 [DOI] [PubMed] [Google Scholar]