Abstract
Background
Plants of Allium spp., including garlic (A. sativum) and onions (A. cepa), are known to be oxidatively toxic to canine erythrocytes resulting in Heinz body hemolytic anemia in dogs. In humans, these plants have been used as medicinal agents for multiple diseases since ancient times. Especially, fresh garlic extracted over a prolonged period produces less irritative and odorless aged garlic extract (AGE), containing unique and beneficial organosulfur compounds that can help prevent many kinds of diseases. In this study, the safety and efficacy of long-term oral administration of AGE is evaluated in dogs. The objectives are to confirm the safe dosage for long-term use and beneficial functions of AGE for dogs and to plan and design a canine health supplement or a preventive agent for multiple diseases based on the data of this study.
Results
Beagles were orally administered AGE (45 or 90 mg/kg body weight once a day) or an equivalent amount of water as control for 12 weeks. In AGE-treated groups, at 12 weeks post-administration at a dose of 90 mg/kg, there were no observable changes in the clinical signs, complete blood count, and serum biochemical parameters. Heinz bodies and eccentrocytes, the markers of oxidative damage in erythrocytes, did not appear in blood smear examination. In order to further evaluate the beneficial effects of AGE on health of dogs, the expressions of nuclear factor erythroid 2-related factor 2 (Nrf2) gene (NFE2L2) and Nrf2-regulated phase II antioxidant enzyme genes (NQO1, GCLM, HMOX1, and SOD2) were determined in whole blood between pre- and post-AGE administration. The expression of NFE2L2 gene was significantly upregulated in the AGE-treated groups [45 (p < 0.05) and 90 mg/kg (p < 0.01), 8 weeks] as compared to in the control group. Among the Nrf2-regulated enzymes examined, the expressions of NQO1 [45 (p < 0.05) and 90 mg/kg (p < 0.01), 8 weeks] and GCLM [45 (p < 0.05) and 90 mg/kg (p < 0.01), 12 weeks] genes were significantly upregulated.
Conclusion
The long-term oral administration of AGE at a dose of 90 mg/kg/day for 12 weeks did not show any adverse effects in dogs. Furthermore, the administration of AGE upregulated the gene expressions of canine Nrf2 and Nrf2-regulated phase II antioxidant enzymes. These results suggest that AGE might safely contribute to the health of dogs provided that the appropriate dosage is used.
Electronic supplementary material
The online version of this article (10.1186/s12917-018-1699-2) contains supplementary material, which is available to authorized users.
Keywords: Aged garlic extract (AGE), Dog, Nuclear factor erythroid 2-related factor 2 (Nrf2), Phase II antioxidant enzyme, NAD(P)H quinone dehydrogenase 1 (NQO1), Glutamate-cysteine ligase modifier subunit (GCLM)
Background
Garlic (Allium sativum) has been used by humans since ancient times not only as food but also as a therapeutic agent for treatment of cardiovascular diseases, gastrointestinal disorders, bronchial asthma, and other disorders [1, 2]. Favorable biological and pharmacological properties of garlic that contribute to the treatment of such diseases and disorders have been documented in humans and rodents [3–7]. However, intake of either garlic or onion (A. cepa) causes hemolytic anemia, a potentially life-threatening event, in dogs due to the oxidation of erythrocytes that result in the formation of Heinz bodies and eccentrocytes [8, 9]. Therefore, the medicinal properties of these Allium plants have never been utilized for improvement in the health of dogs. It would be ideal if the medicinal properties could be utilized as a canine health supplement or a preventive agent for multiple diseases to maintain the health of dogs.
Fresh garlic extracted over a prolonged period produces a less irritative and odorless aged garlic extract (AGE) [6], which contains stable and water-soluble sulfur-containing amino acids, such as S-allylcysteine (SAC) and S-1-propenylcysteine (S1PC) [10–12]. Clinical studies on human subjects have demonstrated favorable pharmacological effects of AGE in atherosclerosis [13, 14], metabolic syndromes [15], and hypertension [16]. Numerous experimental studies have also revealed positive effects of AGE and its components, SAC and S1PC, in anti-oxidation [17], anti-aging [18, 19], immunomodulation [20, 21], anti-hypertensive [22, 23], anti-fatigue [24, 25], hepato-protective [26], anti-inflammatory [27, 28], and cardio-protective activities [29].
To examine the safety of AGE in animals, acute and 6-month-long administration studies were conducted in mice and rats, whose results suggested its safety nature; for example, AGE administration resulted in less gastric mucosal irritation as compared to when raw garlic was administered [30, 31]. However, to our knowledge, no studies have yet been conducted on the safety and efficacy of AGE in dogs. Therefore, this study was performed to evaluate the toxicity and safety of 12-week-long oral administration of AGE in dogs. Furthermore, amongst a variety of potential biological effects of AGE, nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway and Nrf2-regulated phase II antioxidant enzymes were selected for evaluation of AGE efficacy on health of dogs, because the Nrf2 signaling pathway is expected to be a key to the multiple functions of AGE.
The objectives of this study are to confirm the safe dosage for long-term use and beneficial functions of AGE for dogs and to plan and design a canine health supplement or a preventive agent for multiple diseases based on the data of this study.
Results
Clinical outcome, food consumption, and body weight
All the dogs were kept active without any severe clinical deterioration during the study period. Most of the dogs were willing to drink AGE when it was fed. Coat condition and urine color were normal in all dogs during the experimental period.
Soft feces were observed in three dogs fed AGE (45 mg/kg), but not in any dogs that were fed AGE (90 mg/kg) and the control group (Additional file 1). The occurrence of soft feces was once to seven times in the three dogs over 84 days of the experimental period. Vomiting was observed four times in one dog fed AGE (45 mg/kg) and once in one dog fed AGE (90 mg/kg), but not in the control dogs (Additional file 1).
All the dogs consumed 230 g of dry food fed every day during the experimental period. There was no statistically significant difference in body weights among the three groups during the experimental period (Additional file 2)
Hematology
There was almost no observable change in the data obtained from complete blood count (CBC) and blood smear examinations, except for a slightly significant change (p < 0.05) of the reticulocyte count between the group administered AGE at a dose of 45 mg/kg group and the control group a week after the administration (Table 1). Heinz bodies, eccentrocytes, and other types of poikilocytosis were not found even by an elaborate microscopic observation in any dog during the experimental period.
Table 1.
Changes in the complete blood count and oxidative damage markers in erythrocytes in dogs fed aged garlic extract (45 mg/kg or 90 mg/kg body weight once a day) for 12 weeks
| Parameters | Dose (mg/kg) | Week 0 | Week 1 | Week 4 | Week 8 | Week 12 |
|---|---|---|---|---|---|---|
| RBC | 0 (Control) | 7.68 ± 0.49 | 7.59 ± 0.68 | 7.48 ± 0.59 | 7.18 ± 0.62 | 7.40 ± 0.86 |
| (106/μl) | 45 | 7.14 ± 0.71 | 7.40 ± 1.14 | 7.32 ± 0.78 | 6.97 ± 0.72 | 7.36 ± 0.59 |
| 90 | 7.38 ± 0.42 | 7.42 ± 0.64 | 6.93 ± 0.87 | 6.80 ± 0.80 | 7.34 ± 0.67 | |
| Hb | 0 (Control) | 17.0 ± 1.5 | 17.0 ± 2.3 | 16.5 ± 2.0 | 15.6 ± 1.9 | 16.4 ± 2.4 |
| (g/dl) | 45 | 16.3 ± 1.9 | 16.9 ± 2.8 | 16.4 ± 1.9 | 15.5 ± 1.7 | 16.6 ± 1.6 |
| 90 | 16.3 ± 0.6 | 16.6 ± 0.7 | 15.4 ± 1.5 | 15.0 ± 1.2 | 16.3 ± 0.7 | |
| Ht | 0 (Control) | 50.6 ± 4.0 | 50.0 ± 5.9 | 49.0 ± 4.9 | 46.6 ± 5.1 | 47.8 ± 6.1 |
| (%) | 45 | 47.4 ± 5.6 | 48.9 ± 7.8 | 48.1 ± 5.3 | 45.8 ± 5.0 | 47.7 ± 5.5 |
| 90 | 46.9 ± 2.2 | 47.2 ± 1.6 | 44.8 ± 3.3 | 43.7 ± 2.2 | 46.5 ± 1.8 | |
| MCV | 0 (Control) | 65.9 ± 4.3 | 65.9 ± 3.8 | 65.5 ± 3.8 | 64.9 ± 3.0 | 64.5 ± 2.7 |
| (fl) | 45 | 66.3 ± 1.9 | 66.1 ± 1.8 | 65.8 ± 2.5 | 65.7 ± 2.3 | 64.6 ± 2.6 |
| 90 | 63.5 ± 2.7 | 63.9 ± 3.4 | 64.9 ± 3.5 | 64.6 ± 4.2 | 63.6 ± 3.9 | |
| MCH | 0 (Control) | 22.2 ± 1.1 | 22.3 ± 1.3 | 22.0 ± 1.2 | 21.8 ± 0.9 | 22.2 ± 0.8 |
| (pg) | 45 | 22.8 ± 0.8 | 22.9 ± 0.9 | 22.4 ± 0.8 | 22.3 ± 0.8 | 22.5 ± 0.5 |
| 90 | 22.1 ± 0.8 | 22.5 ± 1.0 | 22.2 ± 0.8 | 22.1 ± 0.9 | 22.3 ± 1.1 | |
| MCHC | 0 (Control) | 33.7 ± 1.0 | 33.9 ± 1.1 | 33.6 ± 1.2 | 33.5 ± 1.1 | 34.4 ± 1.1 |
| (g/dl) | 45 | 34.4 ± 0.5 | 34.6 ± 0.5 | 34.1 ± 0.1 | 33.9 ± 0.1 | 34.8 ± 0.7 |
| 90 | 34.7 ± 0.6 | 35.2 ± 0.5 | 34.4 ± 0.9 | 34.2 ± 1.1 | 35.2 ± 0.5 | |
| WBC | 0 (Control) | 10.9 ± 0.9 | 9.3 ± 0.5 | 9.8 ± 1.5 | 12.1 ± 1.7 | 11.2 ± 2.6 |
| (103/μl) | 45 | 10.3 ± 0.8 | 9.8 ± 1.3 | 8.7 ± 1.2 | 10.4 ± 0.9 | 10.2 ± 0.7 |
| 90 | 12.3 ± 1.2 | 9.9 ± 1.1 | 10.6 ± 1.5 | 11.8 ± 3.2 | 10.3 ± 1.9 | |
| PLT | 0 (Control) | 341 ± 62 | 331 ± 51 | 349 ± 55 | 355 ± 33 | 379 ± 47 |
| (103/μl) | 45 | 346 ± 48 | 339 ± 41 | 348 ± 101 | 342 ± 82 | 355 ± 157 |
| 90 | 325 ± 40 | 343 ± 125 | 357 ± 93 | 324 ± 45 | 336 ± 64 | |
| Reticulocyte | 0 (Control) | 8 ± 5 | 2 ± 1 | 7 ± 6 | 3 ± 1 | 3 ± 2 |
| (‰) | 45 | 9 ± 5 | 8 ± 2* | 9 ± 6 | 7 ± 5 | 6 ± 3 |
| 90 | 5 ± 1 | 4 ± 1 | 5 ± 3 | 3 ± 1 | 4 ± 1 | |
| Heinz body | 0 (Control) | 0 | 0 | 0 | 0 | 0 |
| (‰) | 45 | 0 | 0 | 0 | 0 | 0 |
| 90 | 0 | 0 | 0 | 0 | 0 | |
| Eccentrocyte | 0 (Control) | 0 | 0 | 0 | 0 | 0 |
| (‰) | 45 | 0 | 0 | 0 | 0 | 0 |
| 90 | 0 | 0 | 0 | 0 | 0 |
Data are presented as the mean ± standard deviation (n = 3). RBC erythrocyte count, Hb hemoglobin concentration, Ht hematocrit value, MCV mean corpuscular volume, MCH mean corpuscular hemoglobin, MCHC mean corpuscular hemoglobin concentration, WBC leukocyte count, PLT platelet count. *p < 0.05, compared with the water-treated control group
Serum biochemistry
Among all the serum biochemical parameters examined, there was no tendency of increase or decrease on administration of AGE (Table 2). All the parameters were kept within the canine normal ranges. A slightly significant difference (p < 0.05) was observed in lactate dehydrogenase (LDH) activity between groups administered AGE (45 and 90 mg/kg) and the control group at weeks 4 and 8, in alkaline phosphatase (ALP) activity between the AGE (45 mg/kg)-administered and the control groups at week 12; and in total bilirubin (T-Bil) concentration between the AGE (90 mg/kg)-administered and the control groups before the administration. These significant changes did not result from the changes caused by AGE, but occurred due to the original difference for each individual within the normal ranges.
Table 2.
Changes in serum biochemical parameters in dogs fed aged garlic extract (45 mg/kg or 90 mg/kg body weight once a day) for 12 weeks
| Parameters | Dose (mg/kg) | Week 0 | Week 1 | Week 4 | Week 8 | Week 12 |
|---|---|---|---|---|---|---|
| LDH | 0 (Control) | 49.2 ± 6.7 | 41.2 ± 0.5 | 73.8 ± 14.2 | 102.0 ± 39.0 | 72.0 ± 24.9 |
| (U/l) | 45 | 44.8 ± 5.7 | 44.2 ± 14.8 | 45.4 ± 11.4* | 42.7 ± 8.5* | 54.0 ± 14.4 |
| 90 | 45.2 ± 9.3 | 32.2 ± 4.9 | 38.0 ± 2.4* | 36.0 ± 8.7* | 40.0 ± 10.4 | |
| ALT | 0 (Control) | 37.9 ± 5.7 | 39.2 ± 7.8 | 35.2 ± 4.9 | 35.0 ± 2.0 | 32.7 ± 4.7 |
| (U/l) | 45 | 47.8 ± 6.4 | 52.5 ± 20.6 | 43.9 ± 11.5 | 41.3 ± 10.2 | 34.7 ± 14.0 |
| 90 | 35.6 ± 2.8 | 39.1 ± 8.9 | 32.3 ± 4.1 | 29.7 ± 5.1 | 35.3 ± 6.0 | |
| AST | 0 (Control) | 34.6 ± 9.3 | 31.1 ± 8.9 | 36.0 ± 13.1 | 38.0 ± 12.5 | 33.7 ± 13.2 |
| (U/l) | 45 | 33.4 ± 3.8 | 29.8 ± 4.4 | 33.7 ± 5.0 | 30.7 ± 5.5 | 30.3 ± 3.8 |
| 90 | 33.2 ± 2.6 | 28.6 ± 1.2 | 29.6 ± 2.7 | 29.3 ± 0.6 | 28.3 ± 2.1 | |
| ALP | 0 (Control) | 108 ± 32 | 104 ± 34 | 97 ± 28 | 90 ± 21 | 84 ± 20 |
| (U/l) | 45 | 200 ± 56 | 198 ± 54 | 182 ± 45 | 164 ± 39 | 179 ± 43* |
| 90 | 141 ± 61 | 126 ± 53 | 150 ± 75 | 142 ± 78 | 121 ± 42 | |
| CK | 0 (Control) | 124 ± 17 | 110 ± 26 | 136 ± 25 | 162 ± 39 | 127 ± 28 |
| (U/l) | 45 | 113 ± 19 | 100 ± 14 | 112 ± 20 | 95 ± 26 | 111 ± 24 |
| 90 | 125 ± 30 | 104 ± 36 | 104 ± 26 | 104 ± 20 | 100 ± 16 | |
| TP | 0 (Control) | 5.89 ± 0.43 | 6.13 ± 0.31 | 6.53 ± 0.19 | 6.04 ± 0.47 | 6.20 ± 0.58 |
| (g/dl) | 45 | 5.85 ± 0.14 | 5.94 ± 0.16 | 6.30 ± 0.40 | 5.86 ± 0.22 | 6.19 ± 0.18 |
| 90 | 6.06 ± 0.60 | 6.31 ± 0.61 | 6.85 ± 0.50 | 6.29 ± 0.29 | 6.41 ± 0.40 | |
| Alb | 0 (Control) | 2.68 ± 0.27 | 2.67 ± 0.30 | 2.61 ± 0.24 | 2.45 ± 0.17 | 2.50 ± 0.18 |
| (g/dl) | 45 | 2.60 ± 0.22 | 2.52 ± 0.24 | 2.55 ± 0.20 | 2.41 ± 0.19 | 2.42 ± 0.28 |
| 90 | 2.69 ± 0.17 | 2.61 ± 0.17 | 2.57 ± 0.18 | 2.34 ± 0.01 | 2.39 ± 0.06 | |
| Glb | 0 (Control) | 3.21 ± 0.27 | 3.47 ± 0.19 | 3.92 ± 0.07 | 3.59 ± 0.32 | 3.70 ± 0.42 |
| (g/dl) | 45 | 3.24 ± 0.09 | 3.42 ± 0.15 | 3.75 ± 0.55 | 3.45 ± 0.35 | 3.77 ± 0.46 |
| 90 | 3.37 ± 0.44 | 3.69 ± 0.47 | 4.28 ± 0.35 | 3.95 ± 0.30 | 4.02 ± 0.45 | |
| Glu | 0 (Control) | 98.0 ± 2.3 | 100.2 ± 1.8 | 94.5 ± 2.4 | 91.0 ± 10.8 | 87.7 ± 6.8 |
| (mg/dl) | 45 | 110.2 ± 2.4 | 106.2 ± 7.2 | 103.7 ± 5.8 | 97.0 ± 6.2 | 96.3 ± 2.5 |
| 90 | 103.9 ± 11.2 | 97.5 ± 7.1 | 93.1 ± 3.3 | 90.7 ± 2.5 | 91.3 ± 6.7 | |
| TG | 0 (Control) | 30.6 ± 8.5 | 35.8 ± 9.8 | 26.7 ± 1.6 | 29.3 ± 2.5 | 32.7 ± 7.0 |
| (mg/dl) | 45 | 29.0 ± 5.0 | 29.0 ± 5.0 | 30.2 ± 1.9 | 30.7 ± 6.5 | 30.7 ± 8.6 |
| 90 | 33.0 ± 12.4 | 36.2 ± 8.3 | 30.2 ± 7.4 | 31.3 ± 5.7 | 30.0 ± 9.2 | |
| T-Cho | 0 (Control) | 136 ± 34 | 135 ± 26 | 124 ± 23 | 116 ± 24 | 141 ± 51 |
| (mg/dl) | 45 | 128 ± 14 | 126 ± 17 | 121 ± 19 | 117 ± 11 | 119 ± 9 |
| 90 | 144 ± 43 | 147 ± 41 | 149 ± 41 | 135 ± 49 | 129 ± 45 | |
| BUN | 0 (Control) | 12.8 ± 2.4 | 14.0 ± 1.9 | 12.1 ± 0.7 | 11.9 ± 2.6 | 11.7 ± 3.4 |
| (mg/dl) | 45 | 13.2 ± 1.5 | 14.6 ± 1.9 | 13.2 ± 1.4 | 11.8 ± 0.6 | 13.0 ± 0.9 |
| 90 | 12.1 ± 0.6 | 12.1 ± 0.4 | 12.5 ± 1.0 | 12.0 ± 0.6 | 11.4 ± 1.8 | |
| Cre | 0 (Control) | 0.62 ± 0.10 | 0.66 ± 0.10 | 0.66 ± 0.09 | 0.62 ± 0.11 | 0.61 ± 0.11 |
| (mg/dl) | 45 | 0.70 ± 0.09 | 0.70 ± 0.11 | 0.67 ± 0.05 | 0.62 ± 0.03 | 0.64 ± 0.04 |
| 90 | 0.66 ± 0.12 | 0.64 ± 0.11 | 0.63 ± 0.08 | 0.59 ± 0.06 | 0.64 ± 0.06 | |
| T-Bil | 0 (Control) | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.01 |
| (mg/dl) | 45 | 0.10 ± 0.02 | 0.10 ± 0.03 | 0.10 ± 0.03 | 0.08 ± 0.02 | 0.10 ± 0.01 |
| 90 | 0.12 ± 0.01* | 0.09 ± 0.01 | 0.10 ± 0.02 | 0.07 ± 0.02 | 0.09 ± 0.02 | |
| CRP | 0 (Control) | 1.28 ± 0.90 | 1.63 ± 1.22 | 1.04 ± 0.87 | 0.78 ± 0.43 | 0.75 ± 0.43 |
| (mg/dl) | 45 | 1.44 ± 0.63 | 1.56 ± 0.70 | 0.85 ± 0.96 | 0.61 ± 0.56 | 0.74 ± 0.89 |
| 90 | 0.92 ± 0.19 | 1.05 ± 0.41 | 1.02 ± 0.19 | 0.62 ± 0.15 | 0.44 ± 0.30 | |
| Ca | 0 (Control) | 9.65 ± 0.44 | 9.94 ± 0.24 | 9.83 ± 0.26 | 9.27 ± 0.15 | 9.50 ± 0.10 |
| (mg/dl) | 45 | 9.87 ± 0.31 | 9.89 ± 0.19 | 9.71 ± 0.51 | 9.30 ± 0.26 | 9.50 ± 0.36 |
| 90 | 9.71 ± 0.17 | 9.70 ± 0.35 | 9.94 ± 0.43 | 9.23 ± 0.15 | 9.63 ± 0.15 | |
| iP | 0 | 3.31 ± 0.31 | 3.47 ± 0.23 | 3.31 ± 0.27 | 3.03 ± 0.25 | 3.40 ± 0.20 |
| (mg/dl) | 45 | 3.40 ± 0.26 | 3.51 ± 0.44 | 3.61 ± 0.41 | 3.30 ± 0.44 | 3.63 ± 0.25 |
| 90 | 3.40 ± 0.47 | 3.63 ± 0.39 | 3.56 ± 0.29 | 3.30 ± 0.26 | 3.33 ± 0.65 | |
| Na | 0 (Control) | 150.3 ± 3.0 | 152.3 ± 1.7 | 151.3 ± 1.9 | 145.7 ± 0.6 | 145.3 ± 0.6 |
| (mEq/l) | 45 | 154.4 ± 1.0 | 152.4 ± 1.6 | 151.3 ± 0.4 | 147.7 ± 0.6 | 145.3 ± 1.2 |
| 90 | 151.0 ± 0.9 | 149.3 ± 0.6 | 150.8 ± 2.5 | 144.7 ± 1.5 | 145.0 ± 1.0 | |
| K | 0 (Control) | 4.81 ± 0.26 | 4.91 ± 0.24 | 5.07 ± 0.17 | 4.60 ± 0.14 | 5.05 ± 0.15 |
| (mEq/l) | 45 | 4.71 ± 0.33 | 4.92 ± 0.26 | 5.01 ± 0.09 | 4.55 ± 0.27 | 4.96 ± 0.17 |
| 90 | 4.56 ± 0.35 | 4.82 ± 0.20 | 4.66 ± 0.34 | 4.44 ± 0.35 | 4.65 ± 0.35 | |
| Cl | 0 (Control) | 118.0 ± 2.4 | 118.3 ± 1.4 | 117.5 ± 0.6 | 113.7 ± 1.5 | 112.7 ± 1.5 |
| (mEq/l) | 45 | 118.9 ± 1.8 | 118.4 ± 2.0 | 118.2 ± 2.2 | 112.7 ± 1.5 | 112.0 ± 1.0 |
| 90 | 119.3 ± 0.5 | 118.5 ± 0.6 | 119.4 ± 2.4 | 113.7 ± 1.5 | 113.7 ± 0.6 |
Data are presented as the mean ± standard deviation (n = 3). LDH lactate dehydrogenase, ALT alanine aminotransferase, AST aspartate aminotransferase, ALP alkaline phosphatase, CK creatine kinase, TP total protein, Alb albumin, Glb globulin, Glu glucose, TG triglyceride, T-Chol total cholesterol, BUN blood urea nitrogen, Cre creatinine, T-Bil total bilirubin, CRP C-reactive protein, Ca calcium, iP inorganic phosphorus, Na sodium, K potassium, Cl chloride. *p < 0.05, compared with the water-treated control group
Gene expression of Nrf2 and Nrf2-regulated phase II antioxidant enzymes
The expression of Nrf2 gene (NFE2L2) increased significantly in the AGE [45 (p < 0.05) and 90 mg/kg (p < 0.01)]-administered groups compared to the control group after 8 weeks (Fig. 1). There was no statistical difference between the two AGE groups and the control group at week 12, although the gene expressions were slightly higher in the AGE groups than in the control group. Among the four Nrf2-regulated phase II antioxidant enzyme genes, the expressions of NAD(P)H quinone dehydrogenase 1 (NQO1), glutamate-cysteine ligase modifier subunit (GCLM), and heme oxygenase 1 (HMOX1) genes were higher in both the AGE groups than in the control group (Fig. 2). The expression of NQO1 gene increased significantly in the AGE [45 (p < 0.05) and 90 mg/kg (p < 0.01)]-administered groups as compared to the control group at week 8. The expression of GCLM gene increased significantly only in the AGE [45 (p < 0.05) and 90 mg/kg (p < 0.01)]-administered groups as compared to the control group at week 12. The expression of superoxide dismutase 2 (SOD2) gene did not change on administration of AGE during the experimental period.
Fig. 1.
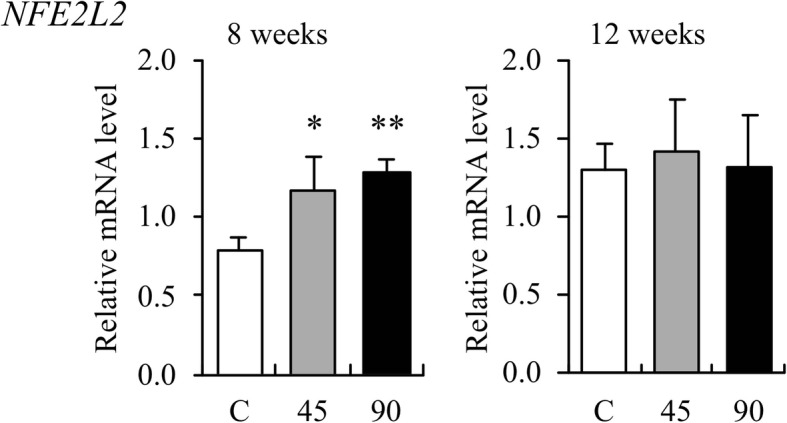
The effect of aged garlic extract (AGE) on the expression of canine NFE2L2 gene in the whole blood. Relative mRNA expression levels in whole blood samples from dogs administered AGE at doses of 45 and 90 mg/kg/day at weeks 8 and 12 are shown as fold induction compared with those in water-treated control group (C). The expression level is calculated as the ratio of expression after administration to that before administration of AGE. Data are presented as the mean ± standard deviation of the results from three independent experiments. *p < 0.05 and **p < 0.01 vs control with a statistical significance
Fig. 2.
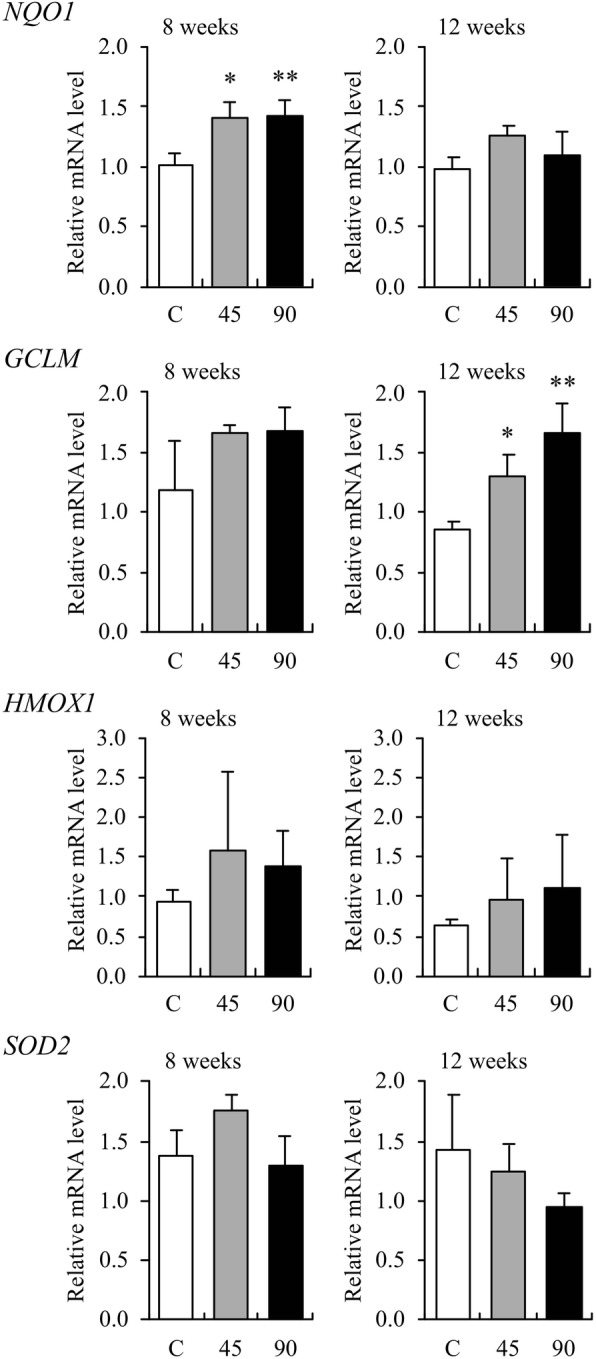
The effect of aged garlic extract (AGE) on the expression of canine NQO1, GCLM, HMOX1, and SOD2 genes in the whole blood.
Relative mRNA expression levels in whole blood samples from dogs administered AGE at doses of 45 and 90 mg/kg/day at weeks 8 and 12 are shown as fold induction compared with those in water-treated control group (C). The expression level is calculated as the ratio of expression after administration to that before administration of AGE. Data are presented as the mean ± standard deviation of the results from three independent experiments. *p < 0.05 and **p < 0.01 vs control with a statistical significance
Discussion
It has experimentally been demonstrated that the constituents of garlic have the potential to oxidize canine erythrocyte membranes and hemoglobin, inducing hemolysis associated with the appearance of eccentrocytes and Heinz bodies [9]. In addition, there have been a few cases of naturally occurring garlic-induced hemolytic anemia in canines [8, 32, 33]. Therefore, this has led to the opinion that foods containing garlic should not be fed to dogs [9]. Sodium 2-propenyl thiosulfate (2PTS) was identified to be present in boiled garlic as one of the causative agents of garlic-induced hemolytic anemia in dogs [34]. It can oxidize canine erythrocytes [34] and has the potential to induce Heinz body hemolytic anemia like one of the causative agents of onion-induced hemolytic anemia: sodium n-propyl thiosulfate (NPTS) [35–37].
In the present study, AGE was orally administered to dogs at a maximum dose of 90 mg/kg body weight once daily for long-term (12 weeks). The data on hematology (Table 1) and serum biochemistry (Table 2) showed no abnormal or toxic changes in CBC, hemolysis (reticulocytes, LDH, and T-Bil), erythrocyte oxidation (Heinz bodies and eccentrocytes), proteins [total protein (TP), albumin (Alb), and globulin (Glb)], hepatocyte damage [alanine aminotransferase (ALT) and aspartate aminotransferase (AST)], cholestasis [ALP, T-Bil, total cholesterol (T-Cho)], renal function [blood urea nitrogen (BUN) and creatinine (Cre)], muscular damage [creatine kinase (CK) and AST], glucose metabolism [glucose (Glu)], lipid metabolism [triglyceride (TG) and T-Cho)], acute phase protein [C-reactive protein (CRP)], minerals [calcium (Ca) and inorganic phosphorus (iP)], and electrolytes [sodium (Na), potassium (K), and chloride (Cl)], except for a few significant differences between each AGE-treated and the control groups due to each dog’s original value for a given variable within the normal ranges. The survival time of canine erythrocytes is estimated to be 100 days [38]. The administration period of AGE in the present study was approximately 84% of this survival time, when no Heinz bodies, eccentrocytes, or any other poikilocytes were detected in the blood smears from all the dogs. This suggests that continuous oral administration of AGE at a dose of 90 mg/kg does not cause anemia in normal dogs.
It is known that compounds formed from various precursors in fresh garlic, causes strong irritation to the gastric mucosa [10, 39], which can induce vomiting and diarrhea. It has been reported that AGE, compared with other processed garlic, causes less gastric irritation in dogs and rats [23, 39]. In the present study, soft feces or vomiting was temporarily observed in all dogs in the AGE (45 mg/kg)-administered group, but was observed just once in one dog at the 90 mg/kg dose. Therefore, these temporal soft feces and vomiting were presumably accidental occurrences in healthy dogs, and not associated with the administration of AGE. However, since the occurrence of these symptoms was slightly more prevalent in one dog (no. 6, Additional file 1) in the AGE (45 mg/kg)-administered group compared with that in other dogs, AGE may be associated with slight irritation of the gastrointestinal system in certain dogs. One of the most sensitive inflammation markers, CRP [40], did not increase in any of the dogs, suggesting that AGE does not stimulate inflammation of the canine gastrointestinal mucosa. Based on these clinical observations, AGE-based supplements or drugs for dogs should be designed together with certain mucoprotective agents. Furthermore, neither appetite reduction nor weight loss were observed in any of the dogs used in the present study.
These results from the evaluation of toxicity and safety of AGE demonstrate that the long-term oral administration of AGE at a dose of 90 mg/kg/day is completely safe in normal Beagles unlike intact garlic and other garlic preparations. Based on the safety of AGE in dogs, it is expected to not contain sodium alk(en)yl thiosulfates, including 2PTS and NPTS, which are oxidatively toxic to canine erythrocytes and have the potential to induce hemolytic anemia. However, Japanese and Korean dogs like Shiba Inus, Akita Inus, and Jindo dogs, include a special phenotype that usually shows no clinical manifestation, but these dogs are more susceptible to onion-induced hemolytic anemia and NPTS than normal dogs when the same doses of onions and NPTS are administered [35, 36, 41, 42]. Therefore, a lower-dose of AGE than 90 mg/kg/day should be considered safe in such dogs. Furthermore, cats are generally recognized as the species most susceptible to erythrocyte oxidation; this is partially because feline hemoglobin (Hb) molecule appears to be more susceptible to oxidative denaturation than does the Hb of other species [43], and thus, AGE should not be fed to cats without demonstration of the safe dose like in the present study.
Transcription factor, Nrf2, is a major regulator of cellular responses against environmental stresses [44, 45]. This factor induces the expressions of phase II antioxidant and detoxification enzymes after the exposure of environmental stresses. Kelch-like ECH-associated protein 1 (Keap1) is an adaptor subunit of Cullin 3-based E3 ubiquitin ligase, which acts as a sensor for oxidative and electrophilic stresses and subsequently regulates the activity of Nrf2. Without any environmental stress, Nrf2 is effectively ubiquitinated by the ligase activity of Keap1 and degraded rapidly in the proteasome system, resulting in the continuous suppression of the cellular Nrf2 activity. When cells are exposed to oxidative or electrophilic stresses, Keap1 loses its ability to ubiquitinate Nrf2, enabling Nrf2 to translocate to and accumulate in the nucleus. Accumulated intranuclear Nrf2 dimerizes with Maf proteins to form an Nrf2–Maf heterodimer that can bind the antioxidant response element or electrophile response element located in the regulatory regions of many cytoprotective enzymes on DNA, and finally activates its target genes. Well-known active compounds extracted from raw garlic, i.e., allicin, ajoene, and diallyl trisulfide, are demonstrated to produce their functions via Keap1–Nrf2 signaling pathway because these compounds have sulfur molecules which can deactivate Keap1 by reacting with its sulfhydryl groups [46–48]. However, these active compounds extracted from raw garlic should not be used for canine health because they have the potential to be oxidatively toxic to canine erythrocytes.
The AGE is highly bioavailable and exerts biological activity in both humans and experimental animals [10]. The process of aging gently modifies harsh and irritating compounds from raw garlic and naturally generates unique and beneficial compounds, such as SAC and S1PC through both enzymatic and natural chemical reactions. Both AGE and SAC perform their functions via Keap1–Nrf2 signaling pathway [10, 49], and S1PC is suspected to be an Nrf2 inducer. In the present study, the gene expressions of canine Nrf2 and Nrf2-regulated phase II antioxidant enzymes were analyzed to evaluate the potential multiple functions of AGE contributing to the health of dogs. As a result, among the four Nrf2-regulated phase II antioxidant enzyme genes, the expressions of NQO1 and GCLM were observed to increase significantly by the long-term oral administration of AGE (Fig. 2). This suggests that AGE acts as an Nrf2 inducer and upregulates multiple genes related to antioxidation as well as detoxication via the Keap1–Nrf2 signaling pathway in dogs. The Nrf2 inducers in AGE might be SAC and S1PC, or other active compounds that have not been yet identified. Furthermore, the expression of NFE2L2 gene increased significantly by the administration of AGE (Fig. 1). This may be due to a compensatory reaction to the decreased amounts of Nrf2 in the cytoplasm following its transport to the nucleus. Otherwise, AGE may contain certain compound(s) that can directly upregulate canine NFE2L2 gene and result in an increase of Nrf2. In this case, AGE might upregulate the Keap1–Nrf2 signaling pathway in a synergetic manner.
Conclusions
The long-term oral administration of AGE at a dose of 90 mg/kg/day for 12 weeks did not show any adverse effects in dogs. Furthermore, AGE administration upregulated the gene expressions of canine Nrf2 and Nrf2-regulated phase II antioxidant enzymes. These results suggest that AGE might safely contribute to the health of dogs provided that the appropriate dosage is used.
Methods
Preparation of AGE
The AGE was prepared by Wakunaga Pharmaceutical Co., Ltd. and was manufactured under license issued by the Ministry of Health and Welfare of Japan using the following steps: sliced raw garlic cloves were dipped into aqueous ethanol and extracted over 10 months at room temperature. The extract was dried at 65 °C for 25 h using a circulation dryer (HOH-A3, Takabayashi Rika Co., Ltd., Tokyo, Japan) with crystalline cellulose (CEOLUS UF-F702, Asahi Kasei Chemicals Corporation, Tokyo, Japan) to obtain a final AGE concentration of 33% (w/w). This product was pulverized into powder and stored at 4 °C until further use.
Experimental animals and treatments
This experiment was conducted at the Research Institute for Animal Science in Biochemistry and Toxicology (Sagamihara, Kanagawa, Japan). Nine Beagles, aged 3 years with mean body weight of 11.6 kg (10.1–12.9 kg), were obtained from the Institute for Animal Reproduction (Kasumigaura, Ibaraki, Japan) and Kitayama Labes Co., Ltd. (Ina, Nagano, Japan) and returned to the original companies after this study. Before the experimentation, all the dogs were confirmed to be healthy based on physical examination, CBC, and serum biochemical analysis. The experimental facility was maintained at a temperature of 23 °C under a 12-h light–dark cycle (light 7 am–7 pm). Before and during the study, each dog was fed 230 g of dry food (TC-1, Oriental Yeast Co., Ltd., Tokyo, Japan) once daily. Water was provided ad libitum. The nine dogs were randomly divided into three groups: a water-treated control group and two AGE-treated groups (45 and 90 mg AGE/kg body weight once a day), with each group consisting of three dogs (two males and one female). At the time of administration, stored AGE powder was dissolved in deionized water to prepare net concentrations of 22.5 and 45 mg/ml. Forced oral doses of AGE solution in water in amounts of 2 ml/kg body weight (0, 45, and 90 mg/kg, respectively) were administered once daily approximately one hour before feeding for 12 weeks using plastic syringes.
Clinical observation and measurement of body weight
The animals were inspected daily for their appetite, coat condition, feces characters, urine color, and behaviors before administration of AGE and an hour after it. The dogs were given 230 g of the above-mentioned dry food once daily, and the remaining amount of food was measured at 24 h after the last feeding. The dogs were weighed on the initial day of the administration and then in weeks 4, 8, and 12 after it.
Hematology and serum biochemistry
Approximately 5.5 ml of blood was collected from the cephalic vein just before administration of AGE and then 1, 4, 8, and 12 weeks after. One ml of the blood was put into a tube with ethylenediaminetetraacetic acid dipotassium salt for hematological examinations, including CBC and blood smear examinations. Serum was obtained from approximately 4 ml of the collected blood for biochemical analysis.
The CBC, including counts of red blood cell (RBC), white blood cell (WBC), platelets (PLT), Hb concentration, hematocrit (Ht) value, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC), was determined using a hematology analyzer (XT-2000iV, Sysmex, Tokyo, Japan). Blood smears were stained with neutral red and brilliant green for the detection of reticulocytes and Heinz bodies in the erythrocytes, whereas May-Grünwald-Giemsa stain was used for the detection of eccentrocytes and any other types of poikilocytosis. Counts of reticulocytes, erythrocytes with Heinz bodies, and eccentrocytes were determined by microscopic observation. Activities of LDH, ALT, AST, ALP, and CK, as well as concentrations of TP, Alb, Glb, Glu, TG, T-Cho, BUN, Cre, T-Bil, CRP, Ca, iP, Na, K, and Cl were determined using an automated biochemical analyzer (CA-BM6050, JEOL, Tokyo, Japan).
RNA extraction and quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) analysis
For qRT-PCR analysis, 0.5 ml of the collected blood samples was mixed with 1.3 ml of RNAlater RNA stabilization reagent (Invitrogen, Waltham, MA, USA) and kept at − 20 °C until further use. Whole blood cell precipitate was prepared by melting and centrifugation of the stored samples. Total RNA was extracted using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) and complementary DNA was synthesized using PrimeScript II (TaKaRa Bio, Shiga, Japan) according to the manufacture’s protocol. Moving on, qRT-PCR was performed using SYBR green as the detection reagent along with pairs of primers for each targeted gene (Table 3). Expressions of the NFE2L2 and Nrf2-regulated phase II antioxidant enzyme genes: NQO1, GCLM, HMOX1, and SOD2, were measured on the basis of mRNA levels of house-keeping actin-beta (ACTB) gene using the comparative 2−ΔΔCT method.
Table 3.
Primers used for quantitative real-time reverse transcription polymerase chain reaction
| Genes | Primers | Sequence (5′ to 3′) | NCBI Reference |
|---|---|---|---|
| Nuclear factor erythroid 2-related factor 2 (NFE2L2) | |||
| cNrf2-P1 | CCCATCGGAAACCAGTGCAT | XM_014110726.1 | |
| cNrf2-M1 | CATCTACGAACGGGAATGTCTCTG | ||
| NAD(P)H quinone dehydrogenase 1 (NQO1) | |||
| cNqo1-P1 | GAAGCCGCAGACCTGGTGAT | XM_848524.4 | |
| cNqo1-M1 | GCACTCGCTCGAACCAGCCT | ||
| Glutamate-cysteine ligase modifier subunit (GCLM) | |||
| cGCLM-P2 | GCCTCCAGACTTGACTGCAT | XM_005621897.2 | |
| cGCLM-M2 | GGATGCTTTCCTGAAGGGCT | ||
| Heme oxygenase 1 (HMOX1) | |||
| cHmox-P1 | CTTTCAGAAGGGCCAGGTGAC | NM_001194969.1 | |
| cHmox-M1 | TGCTCGATCTCCTCCTCCAG | ||
| Superoxide dismutase 2 (SOD2) | |||
| cSod2-P1 | ATCGAGGAGAAGTATCTGGAGG | XM_533463.5 | |
| cSod2-M1 | CCTGAGCCTTGGACACCGAC | ||
| Actin beta (ACTB) | |||
| cActB-P1 | CGTGAGAAGATGACCCAGAT | NM_001195845.2 | |
| cActB-M1 | CGTGAGGATCTTCATGAGGTAGT | ||
Statistical analyses
Statistical analyses were performed using the SPSS software (ver. 11.5.1 J, ADACS, Tokyo, Japan). Data were shown as mean ± standard deviation. Statistical significance was determined by one-way analysis of variance (ANOVA) using Dunnett’s multi-comparison procedures. Differences with p < 0.05 were considered statistically significant.
Additional files
The number of days when soft feces or vomiting was observed before and 1 h after the administration of AGE during the experimental period (84 days). (DOCX 19 kb)
Change in body weight before and 4, 8, and 12 weeks after the administration of AGE during the experimental period. (DOCX 13 kb)
Acknowledgements
The authors gratefully acknowledge the financial support and encouragement of Mr. Kanji Wakunaga, Managing Director of Wakunaga Pharmaceutical Co., Ltd.
Funding
Funding was provided by Wakunaga Pharmaceutical Co., Ltd., which prepared AGE used in the present study, in order to confirm the safe dosage and beneficial functions of AGE for dogs and design a canine health supplement based on the data of this study. The funder did not have any role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article. All the datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 2PTS
Sodium 2-propenyl thiosulfate
- AGE
Aged garlic extract
- Alb
Albumin
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- ANOVA
Analysis of variance
- AST
Aspartate aminotransferase
- BUN
Blood urea nitrogen
- Ca
Calcium
- CBC
Complete blood count
- CK
Creatine kinase
- Cl
Chloride
- Cre
Creatinine
- CRP
C-reactive protein
- Glb
Globulin
- Glu
Glucose
- Hb
Hemoglobin
- Ht
Hematocrit
- iP
Inorganic phosphorus
- K
Potassium
- Keap1
Kelch-like ECH-associated protein 1
- LDH
Lactate dehydrogenase
- MCH
Mean corpuscular hemoglobin
- MCHC
Mean corpuscular hemoglobin concentration
- MCV
Mean corpuscular volume
- Na
Sodium
- NPTS
Sodium n-propyl thiosulfate
- Nrf2
Nuclear factor erythroid 2-related factor 2
- PLT
Platelet
- qRT-PCR
Quantitative real-time reverse transcription-polymerase chain reaction
- RBC
Red blood cell (erythrocyte)
- S1PC
S-1-propenylcysteine
- SAC
S-allylcysteine
- T-Bil
Total bilirubin
- T-Cho
Total cholesterol
- TG
Triglyceride
- TP
Total protein
- WBC
White blood cell (leukocyte)
Authors’ contributions
OY supervised the project, designed the study, reviewed the results, and drafted the final version of the manuscript. TT carried out the experiments on qRT-PCR analysis, analyzed the results, and drafted the manuscript. MU analyzed the results and drafted the manuscript. HJ participated in the design of the study. AY participated in the design of the study and analyzed the results. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The experiments in the present study were approved by the institutional committee for animal experimentation and ethics at the Research Institute for Animal Science in Biochemistry and Toxicology (Permit Number: 14–154). Not applicable for the consent of participate.
Consent for publication
Not applicable.
Competing interests
Some of the authors (TT, MU, and HJ) are employed by the company which provided AGE and the funding for this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Osamu Yamato, Email: osam@vet.kagoshima-u.ac.jp.
Tadamitsu Tsuneyoshi, Email: tsuneyoshi_td@wakunaga.co.jp.
Mitsuyasu Ushijima, Email: ushijima_m@wakunaga.co.jp.
Hiroshi Jikihara, Email: jikihara_h@wakunaga.co.jp.
Akira Yabuki, Email: yabu@vet.kagoshima-u.ac.jp.
References
- 1.Hahn G. History, folk medicine, and legendary uses of garlic. In: Koch HP, Lawson LD, editors. Garlic: the science and therapeutic application of Allium sativum L. and related species. 2nd ed. Williams & Wilkins. 1996. pp. 1–24. [Google Scholar]
- 2.Bayan L, Koulivand PH, Gorji A. Garlic: a review of potential therapeutic effects. Avicenna J Phytomed. 2014;4:1–13. doi: 10.4103/2231-0770.127413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HK. Garlic supplementation ameliorates UV-induced photoaging in hairless mice by regulating antioxidative activity and MMPs expression. Molecules. 2016;21:70. doi: 10.3390/molecules21010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qamar A, Siddiqui A, Kumar H. Fresh garlic amelioration of high-fat-diet induced fatty liver in albino rats. J Pak Med Assoc. 2015;65:1102–1107. [PubMed] [Google Scholar]
- 5.Wang HP, Yang J, Qin LQ, Yang XJ. Effect of garlic on blood pressure: a meta-analysis. J Clin Hypertens. 2015;17:223–231. doi: 10.1111/jch.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001;131:955S–962S. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 7.Gardner CD, Chatterjee LM, Carlson JJ. The effect of a garlic preparation on plasma lipid levels in moderately hypercholesterolemic adults. Atherosclerosis. 2001;154:213–220. doi: 10.1016/S0021-9150(00)00466-4. [DOI] [PubMed] [Google Scholar]
- 8.Yamato O, Kasai E, Katsura T, Takahashi S, Shiota T, Tajima M, Yamasaki M, Maede Y. Heinz body hemolytic anemia with eccentrocytosis due to ingestion of Chinese chive (Allium tuberosum) and garlic (Allium sativum) in a dog. J Am Anim Hosp Assoc. 2005;41:68–73. doi: 10.5326/0410068. [DOI] [PubMed] [Google Scholar]
- 9.Lee KW, Yamato O, Tajima M, Kuraoka M, Omae S, Maede Y. Hematologic changes associated with the appearance of eccentrocytes after intragastric administration of garlic extract to dogs. Am J Vet Res. 2000;61:1446–1450. doi: 10.2460/ajvr.2000.61.1446. [DOI] [PubMed] [Google Scholar]
- 10.Colín-González AL, Santana RA, Silva-Islas CA, Chánez-Cárdenas ME, Santamaría A, Maldonado PD. The antioxidant mechanisms underlying the aged garlic extract- and S-allylcysteine-induced protection. Oxidative Med Cell Longev. 2012;2012:907162. doi: 10.1155/2012/907162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kodera Y, Ushijima M, Amano H, Suzuki J, Matsutomo T. Chemical and biological properties of S-1-propenyl-L-cysteine in aged garlic extract. Molecules. 2017;22:E570. doi: 10.3390/molecules22040570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsutomo T, Kodera Y. Development of an analytic method for sulfur compounds in aged garlic extract with the use of a postcolumn high performance liquid chromatography method with sulfur-specific detection. J Nutr. 2016;146:450S–455S. doi: 10.3945/jn.114.208520. [DOI] [PubMed] [Google Scholar]
- 13.Budoff MJ, Ahmadi N, Gul KM, Liu ST, Flores FR, Tiano J, Takasu J, Miller E, Tsimikas S. Aged garlic extract supplemented with B vitamins, folic acid and L-arginine retards the progression of subclinical atherosclerosis: a randomized clinical trial. Prev Med. 2009;49:101–107. doi: 10.1016/j.ypmed.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto S, Nakanishi R, Li D, Alani A, Rezaeian P, Prabhu S, Abraham J, Fahmy MA, Dailing C, Flores F, Hamai S, Broersen A, Kitslaar P, Budoff MJ. Aged garlic extract reduces low attenuation plaque in coronary arteries of patients with metabolic syndrome in a prospective randomized double-blind study. J Nutr. 2016;146:427S–432S. doi: 10.3945/jn.114.202424. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Arbeláez Diego, Lahera Vicente, Oubiña Pilar, Valero-Muñoz Maria, de las Heras Natalia, Rodríguez Yudy, García Ronald Gerardo, Camacho Paul Anthony, López-Jaramillo Patricio. Aged Garlic Extract Improves Adiponectin Levels in Subjects with Metabolic Syndrome: A Double-Blind, Placebo-Controlled, Randomized, Crossover Study. Mediators of Inflammation. 2013;2013:1–6. doi: 10.1155/2013/285795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ried K, Frank OR, Stocks NP. Aged garlic extract reduces blood pressure in hypertensives: a dose-response trial. Eur J Clin Nutr. 2013;67:64–70. doi: 10.1038/ejcn.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borek C. Antioxidant health effects of aged garlic extract. J Nutr. 2001;131:1010S–1015S. doi: 10.1093/jn/131.3.1010S. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa T, Kodera Y, Hirata D, Blackwell K, Mizunuma M. Natural thioallyl compounds increase oxidative stress resistance and lifespan in Caenorhabditis elegans by modulating SKN-1/Nrf. Sci Rep. 2016;6:21611. doi: 10.1038/srep21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moriguchi T, Saito H, Nishiyama N. Aged garlic extract prolongs longevity and improves spatial memory deficit in senescence-accelerated mouse. Biol Pharm Bull. 1996;19:305–307. doi: 10.1248/bpb.19.305. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki J, Yamaguchi T, Matsutomo T, Amano H, Morihara N, Kodera Y. S-1-Propenylcysteine promotes the differentiation of B cells into IgA-producing cells by the induction of Erk1/2-dependent Xbp1 expression in Peyer's patches. Nutrition. 2016;32:884–889. doi: 10.1016/j.nut.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Kyo E, Uda N, Kasuga S, Itakura Y. Immunomodulatory effects of aged garlic extract. J Nutr. 2001;131:1075S–1079S. doi: 10.1093/jn/131.3.1075S. [DOI] [PubMed] [Google Scholar]
- 22.Matsutomo T, Ushijima M, Kodera Y, Nakamoto M, Takashima M, Morihara N, Tamura K. Metabolomic study on the antihypertensive effect of S-1-propenylcysteine in spontaneously hypertensive rats using liquid chromatography coupled with quadrupole-Orbitrap mass spectrometry. J Chromatogr B. 2017;1046:147–155. doi: 10.1016/j.jchromb.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 23.Harauma A, Moriguchi T. Aged garlic extract improves blood pressure in spontaneously hypertensive rats more safely than raw garlic. J Nutr. 2006;136:769S–773S. doi: 10.1093/jn/136.3.769S. [DOI] [PubMed] [Google Scholar]
- 24.Morihara N, Ushijima M, Kashimoto N, Sumioka I, Nishihama T, Hayama M, Takeda H. Aged garlic extract ameliorates physical fatigue. Biol Pharm Bull. 2006;29:962–966. doi: 10.1248/bpb.29.962. [DOI] [PubMed] [Google Scholar]
- 25.Ushijima M, Sumioka I, Kakimoto M, Yokoyama K, Uda N, Matsuura H, kyo E, Suzuki A, Kasuga S, Itakura Y, Brenda L, Amagase H. Effect of garlic and garlic preparations on physiological and psychological stress in mice. Phytother Res. 1997;11:226–230. doi: 10.1002/(SICI)1099-1573(199705)11:3<226::AID-PTR85>3.0.CO;2-E. [DOI] [Google Scholar]
- 26.Nakagawa S, Kasuga S. Matsuura. Prevention of liver damage by aged garlic extract and its components in mice Phytother Res. 1989;3:50–53. [Google Scholar]
- 27.Miki S, Inokuma K, Takashima M, Nishida M, Sasaki Y, Ushijima M, Suzuki J, Morihara N. Aged garlic extract suppresses the increase of plasma glycated albumin level and enhances the AMP-activated protein kinase in adipose tissue in TSOD mice. Mol Nutr Food Res. 2017;61:1600797. doi: 10.1002/mnfr.201600797. [DOI] [PubMed] [Google Scholar]
- 28.Morihara N, Hino A, Miki S, Takashima M, Suzuki J. Aged garlic extract suppresses inflammation in apolipoprotein E-knockout mice. Mol Nutr Food Res. 2017;61:1700308. doi: 10.1002/mnfr.201700308. [DOI] [PubMed] [Google Scholar]
- 29.Chuah SC, Moore PK, Zhu YZ. S-allylcysteine mediates cardioprotection in an acute myocardial infarction rat model via a hydrogen sulfide-mediated pathway. Am J Physiol Heart Circ Physiol. 2007;293:H2693–H2701. doi: 10.1152/ajpheart.00853.2007. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa S, Masamoto K, Sumiyoshi H, Harada H. Acute toxicity test of garlic extract. J Toxicol Sci. 1984;9:57–60. doi: 10.2131/jts.9.57. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa S, Masamoto K, Sumiyoshi H, Kunihiro K, Fuwa T. Effect of raw and extracted-aged garlic juice on growth of young rats and their organs after peroral administration. J Toxicol Sci. 1980;5:91–112. doi: 10.2131/jts.5.91. [DOI] [PubMed] [Google Scholar]
- 32.Kang MH, Park HM. Hypertension after ingestion baked garlic (Allium sativum) in a dog. J Vet Med Sci. 2010;72:515–518. doi: 10.1292/jvms.09-0434. [DOI] [PubMed] [Google Scholar]
- 33.Caldin M, Carli E, Furlanello T, Solano-Gallego L, Tasca S, Patron C, Lubas G. A retrospective study of 60 cases of eccentrocytosis in the dog. Vet Clin Pathol. 2005;34:224–231. doi: 10.1111/j.1939-165X.2005.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 34.Yamato O, Sugiyama Y, Matsuura H, Lee KW, Goto K, Hossain MA, Maede Y, Yoshihara T. Isolation and identification of sodium 2-propenyl thiosulfate from boiled garlic (Allium sativum) that oxidizes canine erythrocytes. Biosci Biotechnol Biochem. 2003;67:1594–1596. doi: 10.1271/bbb.67.1594. [DOI] [PubMed] [Google Scholar]
- 35.Yamato O, Hayashi M, Kasai E, Tajima M, Yamasaki M, Maede Y. Reduced glutathione accelerates the oxidative damage produced by sodium n-propylthiosulfate, one of the causative agents of onion-induced hemolytic anemia in dogs. Biochim Biophys Acta. 1999;1427:175–182. doi: 10.1016/S0304-4165(99)00023-9. [DOI] [PubMed] [Google Scholar]
- 36.Yamato O, Hayashi M, Yamasaki M, Maede Y. Induction of onion-induced haemolytic anaemia in dogs with sodium n-propylthiosulphate. Vet Rec. 1998;142:216–219. doi: 10.1136/vr.142.9.216. [DOI] [PubMed] [Google Scholar]
- 37.Yamato O, Yoshihara T, Ichihara A, Maede Y. Novel Heinz body hemolysis factors in onion (Allium cepa) Biosci Biotechnol Biochem. 1994;58:221–222. doi: 10.1271/bbb.58.221. [DOI] [PubMed] [Google Scholar]
- 38.Harvey JW. The erythrocyte: physiology, metabolism, and biochemical disorders. In: Kaneko JJ, Harvey JW, Bruss ML, editors. Clinical biochemistry of domestic animals. 6th ed. Burlington academic. 2008. pp. 173–240. [Google Scholar]
- 39.Hoshino T, Kashimoto N, Kasuga S. Effects of garlic preparations on the gastrointestinal mucosa. J Nutr. 2001;131:1109S–1113S. doi: 10.1093/jn/131.3.1109S. [DOI] [PubMed] [Google Scholar]
- 40.Bayramli G, Ulutas B. Acute phase protein response in dogs with experimentally induced gastric mucosal injury. Vet Clin Pathol. 2008;37:312–316. doi: 10.1111/j.1939-165X.2008.00060.x. [DOI] [PubMed] [Google Scholar]
- 41.Yamato O, Lee KW, Chang HS, Tajima M, Maede Y. Relation between erythrocyte reduced glutathione and glutamate concentrations in Korean Jindo dogs with erythrocytes possessing hereditary high activity of Na-K-ATPase and a high concentration of potassium. J Vet Med Sci. 1999;61:1179–1182. doi: 10.1292/jvms.61.1179. [DOI] [PubMed] [Google Scholar]
- 42.Yamoto O, Maede Y. Susceptibility to onion-induced hemolysis in dogs with hereditary high erythrocyte reduced glutathione and potassium concentrations. Am J Vet Res. 1992;53:134–137. [PubMed] [Google Scholar]
- 43.Harvey JW, Kaneko JJ. Glucose metabolism of mammalian erythrocytes. J Cell Physiol. 1976;89:219–224. doi: 10.1002/jcp.1040890205. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki T, Yamamoto M. Stress sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. J Biol Chem. 2017;292:16817–16824. doi: 10.1074/jbc.R117.800169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki T, Yamamoto M. Molecular basis of the Keap1-Nrf2 system. Free Radic Biol Med. 2015;88:93–100. doi: 10.1016/j.freeradbiomed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Kim S, Lee HG, Park SA, Kundu JK, Keum YS, Cha YN, Na HK, Surh YJ. Keap1 cysteine 288 as a potential target for diallyl trisulfide-induced Nrf2 activation. PLoS One. 2014;9:e85984. doi: 10.1371/journal.pone.0085984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bat-Chen W, Golan T, Peri I, Ludmer Z, Schwartz B. Allicin purified from fresh garlic cloves induces apoptosis in colon cancer cells via Nrf2. Nutr Cancer. 2010;62:947–957. doi: 10.1080/01635581.2010.509837. [DOI] [PubMed] [Google Scholar]
- 48.Kay HY, Won Yang J, Kim TH, Lee DY, Kang B, Ryu JH, Jeon R, Kim SG. Ajoene, a stable garlic by-product, has an antioxidant effect through Nrf2-mediated glutamate-cysteine ligase induction in HepG2 cells and primary hepatocytes. J Nutr. 2010;140:1211–1219. doi: 10.3945/jn.110.121277. [DOI] [PubMed] [Google Scholar]
- 49.Hiramitsu K, Tsuneyoshi T, Ogawa T, Morihara N. Aged garlic extract enhances heme oxygenase-1 and glutamate-cysteine ligase modifier subunit expression via the nuclear factor erythroid 2-related factor 2-antioxidant response element signaling pathway in human endothelial cells. Nutr Res. 2016;36:143–149. doi: 10.1016/j.nutres.2015.09.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The number of days when soft feces or vomiting was observed before and 1 h after the administration of AGE during the experimental period (84 days). (DOCX 19 kb)
Change in body weight before and 4, 8, and 12 weeks after the administration of AGE during the experimental period. (DOCX 13 kb)
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article. All the datasets used and analysed during the current study are available from the corresponding author on reasonable request.


