Abstract
The 4-thiazolidinones 3a–d were used as a key intermediates for the synthesis of 2-arylimino-5-arylidene-4-thiazolidinones derivatives 7a–p via nucleophilic addition reactions with the arylidene malononitrile. Moreover the 4-thiazolidinones 3a and 3c condensed with the DMF-DMA to form the corresponding enamines 8 and 9 depending on the reaction conditions. Otherwise the 4-thiazolidinone 3b reacts regioselectively with DMF-DMA to afford the enaminones 10 and 11, respectively. The latter reacts with many heterocyclic amines affording polyfunctionally substituted fused pyrimidine derivatives 13–18. The enamine 8b was also reacted with the 3-amino-1,2,4-triazole to afford the acyclic product 19, which could not be further cyclized to the corresponding tricyclic system 20. Moreover the 4-thiazolidinone 3c reacted with the benzenediazonium chloride to afford the arylhydrazones 12. The X-ray single crystal technique was employed in this study for structure elucidation and Z/E potential isomerism configuration determination. The X-ray crystallographic analyses of eight products could be obtained, thus establishing with certainty the structures proposed in this work.
Keywords: 4-thiazolidinone, enaminones, arylidene malononitrile, dimethylformamide dimethylacetal, azolopyrimidine
1. Introduction
One of the main objectives of organic and medicinal chemistry is the design, synthesis and production of molecules having value as human therapeutic agents and the treatment of infectious diseases still remains an important and challenging problem because of a combination of factors, including emerging infectious diseases and the increasing number of multi-drug resistant microbial pathogens, of particular relevance for Gram positive bacteria [1,2]. On the other hand, a recent survey of novel small-molecule therapeutics revealed that the majority of them result from an analogue-based approach and that their market value represents two-thirds of all drug sales [3]. There are numerous biologically active molecules with five membered rings, containing two heteroatoms among which is the 4-thiazolidinone ring system which is a core structure in various synthetic compounds and an important scaffold known to be associated with several biological activities such as hypnotic activity [4,5], antitubercular [6], anticonvulsant [7,8], antibacterial [9,10], anticancer [11,12], antihistaminic [13,14], antifungal [15], anti-inflammatory [16], antiviral [17] and cardiovascular effects [18]. Consequently, the combination of the 4-thiazolidinone template with substituted pyran or fused azolopyrimidine moieties which are also known to having several biological activities [19,20,21,22,23] in one molecule can be considered as promising approach in drug-like molecules design which is the goal of our study.
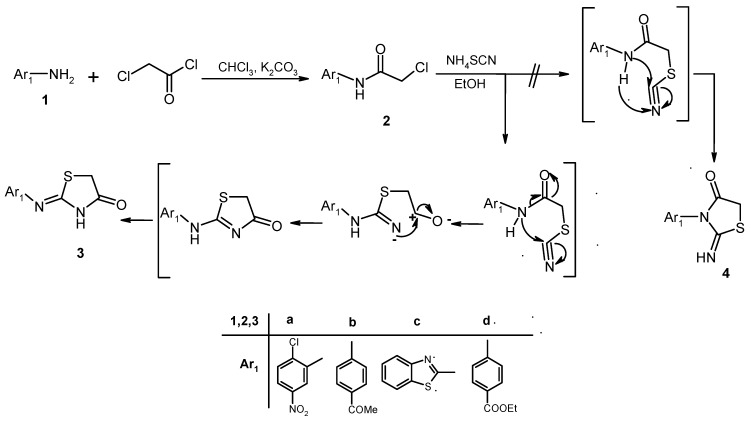
2. Results and Discussion
Several protocols for the synthesis of 4-thiazolidinones are available in the literature [24,25,26]. Here 2-chloro-N-(2-chloro-5-nitrophenyl)acetamide (2a), N-(4-acetylphenyl)-2-chloroacetamide (2b), 2-chloro-N-(benzothiazol-2-yl)acetamide (2c) and ethyl-4-(2-chloroacetamido)benzoate (2d) were synthesized using a procedure reported earlier [25] starting from 2-chloro-5-nitroaniline (1a), 4-amino acetophenone (1b), 2-aminobenzothiazole (1c) and benzocaine (1d), respectively, upon heterocyclization of 2a–d in the presence of ammonium thiocyanate in refluxing ethanol, efficiently produced 2-(2-chloro-5-nitrophenyl-2-ylimino)thiazolidin-4-one (3a), 2-(4-acetylphenylimino)thiazol- idin-4-one (3b), 2-(benzothiazol-2-ylimino)thiazolidin-4-one (3c) and ethyl-4-(4-oxothiazolidin-2-ylideneamino)benzoate (3d), through intramolecular cyclization and the Dimroth-like rearrangements [27] (cf. Scheme 1). The lactam structure for 3a–d was confirmed based on the 1H-NMR spectra which exhibit the NH proton at δ ≈ 12.35 ppm, accounting for a lactam proton but not for an imine proton which is expected around 9.0 ppm. The lactam structure was also confirmed through the X-ray single crystal structure determination for 3a, 3c and 3d (cf. Figure 1, Figure 2, Figure 3, Figure 4 and Table 1, Table 2). This was considered to be a strong evidence for this type of ring closure which gives 3 and not 4 [25] as mentioned by Vicini, et al. [26].
Scheme 1.
Synthesis of the 4-thiazolidinones 3a–d.
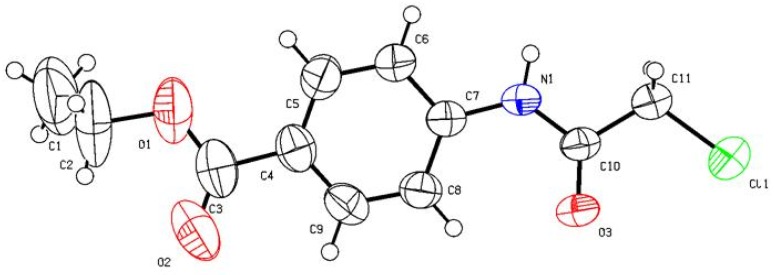
Figure 1.
ORTEP plot of the X-ray crystallographic data determined for 2d. Crystallo- graphic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 874278 [28].
Figure 2.
ORTEP plot of the X-ray crystallographic data determined for 3a. Crystallo- graphic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 874280 [29].
Figure 3.
ORTEP plot of the X-ray crystallographic data determined for 3c. Crystallo- graphic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 874281 [30].
Figure 4.
ORTEP plot of the X-ray crystallographic data determined for 3d. Crystallo- graphic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 874279 [31].
Table 1.
Selected bond lengths and bond angles for 3a.
| Bond | Bond length(Å) | Bond | Bond angle(°) |
|---|---|---|---|
| N2-C5 | 1.420 | C5-N2-C7 | 118.30 |
| N2-C7 | 1.281 | N2-C7-N3 | 121.20 |
| N3-C8 | 1.375 | S1-C7-N2 | 126.70 |
| N3-C7 | 1.362 | S1-C7-N3 | 112.10 |
| S1-C7 | 1.749 | S1-C9-C8 | 108.20 |
| S1-C9 | 1.803 | C7-N3-C8 | 117.40 |
| C8-C9 | 1.505 | N3-C8-C9 | 110.70 |
Table 2.
Selected bond lengths and bond angles for 3c.
| Bond | Bond length(Å) | Bond | Bond angle(°) |
|---|---|---|---|
| N2-C7 | 1.385 | C7-N2-C8 | 120.17 |
| N2-C8 | 1.282 | N2-C8-N3 | 118.36 |
| N3-C8 | 1.381 | S2-C8-N2 | 130.73 |
| N3-C10 | 1.358 | S2-C8-N3 | 110.91 |
| S2-C8 | 1.747 | S2-C9-C10 | 107.64 |
| S2-C9 | 1.815 | C8-N3-C10 | 118.21 |
| C9-C10 | 1.501 | N3-C10-C9 | 111.27 |
Inspection of the crystallographically determined lengths of the N2-C7 bond (1.28 Å) in 3a, N2-C8 bond (1.30 Å) in 3c and N1-C8 bond (1.28 Å) in 3d showed that they are typical C=N bond lengths.
It was of interest to explore the scope, limitations and generality of the 4-thiazolidinones 3 as a precursor for the synthesis of some polyfunctionally substituted fused pyran derivatives for which we might expect a wide spectrum of bioresponses. Thus the active methylene in the 4-thiazolidinones 3a-d underwent nucleophilic addition reaction to the double bond of a variety of arylidene malononitriles 5 via a Michael type addition [32] reaction, by refluxing in ethanol containing few drops of piperidine to give a substance whose structure should be either the pyranothiazole derivative 6 (route a) or the arylidene derivatives 7 (route b). The actual structure of the product was assigned as 5-arylidene-4-thiazolidinone derivatives 7 based on the molecular mass of the product which in agreement with this structure, moreover the 1H-NMR lacked the pyran H-4 signal which should appear at approximately δ = 4.0–5.0 ppm [33]. Also the 1H-NMR spectroscopic data showed a NH signal and was devoid of an amino group signal which should have appeared if the reaction product were 6. Compounds 7 were also obtained via refluxing of 4-thiazolidinones 3a–d with the appropriate aldehydes in glacial acetic acid containing sodium acetate. The Z configuration of the products was confirmed via the X-ray crystallographic analysis (cf. Scheme 2 and Figure 5). Attempts to isolate the targeted pyranothiazole derivative 6 failed. The main advantage of method a over method b is that it gives a higher yield, as illustrated in the Experimental section.
Scheme 2.
Synthesis of the 5-benzylidene-4-thiazolidinone derivatives 7.
Figure 5.
ORTEP plot of the X-ray crystallographic data determined for 7c containing a DMSO molecule. Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 875772 [34].
In order to synthesis a new class of enamine derivatives the active methylene in the 4-thiazol- idinones 3a and 3c was condensed with dimethylformamide dimethylacetal (DMF-DMA) in dioxane to yield the corresponding enamines 8a,b (cf. Scheme 3).
Scheme 3.
Reaction of 3a-c with DMF-DMA and bezenediazonium chloride.
The fact that only the Z-isomer could be formed was confirmed by X-ray single crystal determination (cf. Figure 6 and Table 3). The methylated enamines 9a,b were also formed as products when the reaction was carried out in toluene in the presence of excess DMF-DMA. On the other hand the 4-thiazolidinones 3b condensed with DMF-DMA regioselectively in toluene to afford the enaminones 10 and 11, respectively, depending on the reaction conditions. The structure and configuration of the enaminone 11 was also determined via the X-ray single crystal technique (cf. Figure 7). Moreover the active methylene in 3c was coupled with the benzenediazonium chloride in EtOH/NaOH to form the corresponding arylhydrazone 12 (cf. Scheme 3).
Figure 6.
ORTEP plot of the X-ray crystallographic data determined for 8b. Crystallo- graphic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 874404 [35].
Table 3.
Selected bond lengths and bond angles for 8b.
| Bond | Bond length(Å) | Bond | Bond angle(°) |
|---|---|---|---|
| N1-C4 | 1.369 | C5-N1-C4 | 117.48 |
| N1-C5 | 1.296 | N1-C5-N3 | 120.98 |
| N3-C5 | 1.363 | N1-C5-S2 | 128.88 |
| N3-C10 | 1.380 | N3-C5-S2 | 110.11 |
| S2-C5 | 1.753 | C10-C6-S2 | 110.70 |
| S2-C6 | 1.773 | C5-N3-C10 | 117.96 |
| C6-C7 | 1.367 | N3-C10-C6 | 110.06 |
| N2-C7 | 1.326 | C5-S2-C6 | 91.11 |
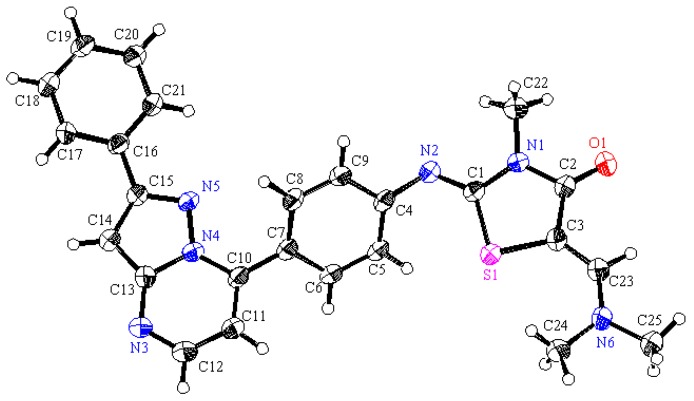
Figure 7.
ORTEP plot of the X-ray crystallographic data determined for 11. Crystallo- graphic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 880545 [36].
The foregoing results prompted us to investigate the behavior of the enaminone 11 towards some N-nucleophiles such as heterocyclic amines as potential precursors for polyfunctionally substituted fused pyrimidine derivatives which display a broad spectrum of biological activity [19,20,21,22,23].
Thus, reaction of the enaminone 11 with equimolar amounts of various heterocyclic amines, namely 3-amino-1,2,4-triazole, 3-phenyl-1H-pyrazole-5-amine, 5-amino-N-(2-chloro-5-nitrophenyl)-1H-pyrazole-4-carboxamide, 5-amino-N-(4,6-dimethylpyrimidin-2-yl)-1H-pyrazole-4-carboxamide upon reflux in pyridine, afforded the corresponding 1,2,4-triazolo[1,5-a]pyrimidine (13) and pyrazolo[1,5-a] pyrimidine derivatives 14–16 (cf. Scheme 4). The identity of the products was established on the basis of elemental analyses and spectral background in each case also, the X-ray single crystal technique was employed for this purpose and for determination of the configuration of the products (cf. Figure 8). In the same manner, the enaminone 11 reacts regioselectively with 2-aminobenzimidazole under the same experimental condition to give the benzimidazo[1,2-a]pyrimidine derivative 17. The structure of compound 17 was established on the basis of elemental analysis and spectral data of the isolated reaction product. Formation of the fused pyrimidine derivatives 13–17 from the enaminone 11 and N,N-binucleophiles proceeds by initial substitution of the dimethylamino group followed by cyclization. Similarly, the reaction of the enaminone 11 with 2-amino-4-phenylthiophene-3-carbonitrile in refluxing pyridine furnished only one isolable product, which was identified as 18. The spectral data of the isolated product were in complete agreement with the assigned structure. For example, the IR spectrum of the reaction product 18 revealed bands due to nitrile function, NH and two carbonyl at 2206, 3116, 1699, 1654 cm−1, respectively (cf. Scheme 4).
Scheme 4.
Reactions of the enaminone 11 with heterocyclic amines.
Figure 8.
ORTEP plot of the X-ray crystallographic data determined for 14. Crystallo- graphic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 880544 [37].
In attempts to synthesis a novel class of fused azoloazinethiazolidinone derivatives 20 we reacted the enamine 8b with 3-amino-1,2,4-triazole as an example of a heterocyclic amine but the formed product was found to be the acyclic one 19 and not the fused one 20. Attempts to effect cyclisation of 19 into 20 under a variety of conditions failed (cf. Scheme 5).
Scheme 5.
Reaction of 8b with 3-amino-1,2,4-triazole.
3. Experimental
3.1. General
Melting points were recorded on a Griffin melting point apparatus and are reported uncorrected. IR spectra were recorded using KBr disks using a Perkin-Elmer System 2000 FT-IR spectrophotometer. 1H-NMR (400 MHz) or (600 MHz) and 13C-NMR (100 MHz) or (150 MHz) spectra were recorded at 25 °C or as reported in CDCl3 or DMSO-d6 as solvent with TMS as internal standard on a Bruker DPX 400 or 600 super-conducting NMR spectrometer. Chemical shifts are reported in ppm. Mass spectra were measured using a high resolution GC-MS (DFS) Thermo spectrometer with EI (70 EV). Microanalyses were performed on a LECO CHNS-932 Elemental Analyzer. The crystal structures were determined by a Rigaku R-AXIS RAPID diffractometer and Bruker X8 Prospector at Kuwait University. The compounds 5-amino-N-(2-chloro-5-nitrophenyl)-1H-pyrazole-4-carboxamide and 5-amino-N-(4,6-dimethylpyrimidin-2-yl)-1H-pyrazole-4-carboxamide were prepared according to the literature procedure [38].
3.2. General Procedure for the Preparation of 2-Chloro-N-(heteroaryl)acetamides 2a–d
A solution of the appropriate amines 1a–d (10 mmol) and chloroacetyl chloride (1.12 g, 10 mmol) in chloroform (50 mL) was refluxed in the presence of K2CO3 (15 mmol) for about 10 h. Then the solvent was removed in vacuo and the residue was stirred with water (100 mL) and filtered. The solid product is then washed with 5% NaHCO3 solution and subsequently with water. The crude product is dried and crystallized from appropriate solvent to furnish pure solid product.
2-Chloro-N-(2-chloro-5-nitrophenyl)acetamide (2a). Recrystallized from isopropyl alcohol as pale yellow crystals, yield: 93%, m.p. 120–121 °C; IR (KBr): 𝑣/cm−1 3354 (NH), 1686 (CO); 1H-NMR (DMSO-d6): δ = 4.47 (s, 2H, CH2), 7.84 (d, J = 8.0 Hz, 1H, Ar-H), 8.06 (d, J = 8.0 Hz, 1H, Ar-H), 8.73 (s, 1H, Ar-H), and 10.23 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 43.03 (CH2), 115.96, 119.91, 129.44, 129.77, 134.58, 147.17 and 164.18 ppm (Ar-C and CO) MS (EI): m/z (%) 248 (M+, 11.55), 249 (M++1, 3.75). Anal. calcd. for C8H6Cl2N2O3 (249.05): C, 38.58; H, 2.43; N, 11.25. Found: C, 38.66; H, 2.50; N, 11.18.
N-(4-Acetylphenyl)-2-chloroacetamide (2b). Recrystallized from EtOH as yellow crystals, yield: 89%, m.p. 145–146 °C; IR (KBr): 𝑣/cm−1 3286 (NH), 1707, 1658 (2CO); 1H-NMR (DMSO-d6): δ = 2.53 (s, 3H, CH3), 4.31 (s, 2H, CH2), 7.73 (d, J = 8.4 Hz, 2H, Ar-H), 7.94 (d, J = 8.4 Hz, 2H, Ar-H) and 10.63 ppm (s, 1H, NH); m/z (%) 211 (M+, 39.20), 212 (M++1, 7.65). Anal. calcd. for C10H10ClNO2 (211.65): C, 56.75; H, 4.76; N, 6.62. Found: C, 56.84; H, 4.72; N, 6.58.
N-Benzothiazol-2-yl-2-chloroacetamide (2c) [39]. Recrystallized from EtOH as white crystals, yield: 96%, m.p. 145–146 °C; IR (KBr): 𝑣/cm−1 3348 (NH), 1665 (CO); 1H-NMR (DMSO-d6): δ = 4.47 (s, 2H, CH2), 7.32 (t, J = 7.6 Hz, 1H, Ar-H), 7.45 (t, J = 7.6 Hz, 1H, Ar-H), 7.77 (d, J = 7.6 Hz, 1H, Ar-H), 7.99 (d, J = 7.6 Hz, 1H, Ar-H) and 12.74 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 43.02 (CH2), 121.16, 122.26, 124.26, 126.70, 131.94, 148.87, 158.06 and 166.43 ppm (Ar-C and CO); MS (EI): m/z (%) 226 (M+, 26.4), 227 (M++1, 5.6). Anal. calcd. for C9H7ClN2OS (226.69): C, 47.69; H, 3.11; N, 12.36; S, 14.14. Found: C, 47.77; H, 3.09; N, 12.44; S, 14.23.
Ethyl-4-(2-chloroacetamido)benzoate (2d). Recrystallized from EtOH as white crystals, yield: 91%, m.p. 106–107 °C; IR (KBr): 𝑣/cm−1 3275 (NH), 1722, 1678 (2CO); 1H-NMR (CDCl3): δ = 1.36 (t, J = 7.2 Hz, 3H, CH3), 4.17 (s, 2H, CH2), 4.34 (q, J = 7.2 Hz, 2H, CH2), 7.63 (d, J = 8.0 Hz, 2H, Ar-H), 8.01 (d, J = 8.0 Hz, 2H, Ar-H) and 8.56 ppm (s, 1H, NH); 13C-NMR (CDCl3): δ = 14.22 (CH3), 42.84 (CH2), 60.93 (CH2), 119.12, 126.74, 130.68, 140.75, 164.15 and 165.92 ppm (Ar-C and CO); MS (EI): m/z (%) 241 (M+, 68.84), 242 (M++1, 21.72). Anal. calcd. for C11H12ClNO3 (241.68): C, 54.67; H, 5.00; N, 5.80. Found: 54.75; H, 4.93; N, 5.71. Crystallographic Analysis for 2d: The crystals were mounted on a glass fiber. All measurements were performed on Bruker X8 Prospector. The data were collected at a temperature of 20 ± 1 °C to a maximum θ value of 66.61° using the ω scanning technique. The structure was solved by direct method using SHELXS-97 (Sheldrick, 2008) and refined by Full-matrix least-squares on F2. The non-hydrogen atoms were refined anisotropically. Data were corrected for absorption effects using the multi-scan method (SADABS). Crystal Data: C11H12ClNO3, M = 241.68, monoclinic, a = 4.7349(4) Å, b = 28.541(2) Å, c = 9.1098(6) Å, V = 1203.41(16) Å3, α = γ = 90.00°, β = 102.173(4)°, space group: P 1 21/n 1, Z = 4, Dcalc = 1.334 Mg cm−3, No. of reflections measured 4589, θmax = 66.34°, R1 = 0.06. Figure 1 illustrates the structure as determined. Full data can be obtained on request from the CCDC [28].
3.3. General Procedure for the Synthesis of 2-(Arylimino)thiazolidin-4-ones 3a–d
A solution of 2-chloro-N-(heteroaryl or aryl)acetamides 2a–d (10 mmol) and ammonium thiocyanate (15 mmol) in absolute ethanol (30 mL) was refluxed for 4 h and allowed to stand overnight. The formed precipitate was filtered off, washed with water and then recrystallised from the appropriate solvent.
(Z)-2-(2-Chloro-5-nitrophenylimino)thiazolidin-4-one (3a). Recrystallized from EtOH as yellow crystals, yield: 76%, m.p. 198–199 °C; IR (KBr): 𝑣/cm−1 3136 (NH), 1738 (CO); 1H-NMR (DMSO-d6): δ = 4.09 (s, 2H, CH2), 7.81 (d, J = 8.4 Hz, 1H, Ar-H), 7.89 (s, 1H, Ar-H), 7.98 (d, J = 8.4 Hz, 1H, Ar-H) and 12.23 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 34.57 (CH2), 116.96, 119.99, 131.12, 133.19, 146.50, 146.78, 160.11 and 173.73 (Ar-C and CO); MS (EI): m/z (%) 271 (M+, 100), 272 (M++1, 32.25). Anal. calcd. for C9H6ClN3O3S (271.68): C, 39.79; H, 2.23; N, 15.47; S, 11.80; Found: C, 39.86; H, 2.14; N, 15.36; S, 11.88. Crystallographic Analysis for 3a: The crystals were mounted on a glass fiber. All measurements were performed on a Rigaku R-AXIS RAPID diffractometer using filtered Mo-Kα radiation. The data were collected at a temperature of 20 ± 1 °C to a maximum 2θ value of 55.0° using the ω scanning technique. The structure was solved by charge flipping method and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically. Hydrogen atoms were refined using the riding model. Crystal Data: C9H6ClN3O3S, M = 271.68, orthorhombic, a = 20.1723(7) Å, b = 11.3976(5) Å, c = 20.145(2) Å, V = 4631.6(5) Å3, α = β = γ = 90.00°, space group: Pbca (#61), Z = 16, Dcalc = 1.558 g cm−3, No. of reflections measured 5236, 2θmax = 54.9°, R1 = 0.0683. Figure 2 shows the structure as determined. Full data can be obtained on request from the CCDC [29].
(Z)-2-(4-Acetylphenylimino)thiazolidin-4-one (3b). Recrystallized from EtOH/dioxane (1:1) as buff crystals, yield: 81%, m.p. 260–261 °C; IR (KBr): 𝑣/cm−1 3271 (NH), 1717, 1666 (2CO); 1H-NMR (DMSO-d6): δ = 2.55 (s, 3H, CH3), 4.03 (s, 2H, CH2), 7.06 (d, J = 8.0 Hz, 1H, Ar-H), 7.86 (d, J = 8.0 Hz, 1H, Ar-H), 7.96 (d, J = 8.0 Hz, 2H, Ar-H) and 11.92 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 26.49 (CH3), 37.39 (CH2), 118.56, 129.57, 132.26, 142.57, 164.90, 174.01 and 196.49 (Ar-C and CO); MS (EI): m/z (%) 234 (M+, 78.0), 235 (M++1, 15.80). Anal. calcd. for C11H10N2O2S (234.28): C, 56.40; H, 4.30; N, 11.96; S,13.69. Found: C, 56.33; H, 4.26; N, 11.85; S,13.55.
(Z)-2-(Benzothiazol-2-ylimino)thiazolidin-4-one (3c) [36]. Recrystallized from an EtOH/dioxane (1:1) mixture pale yellow crystals, yield: 73%, m.p. 201–202 °C; IR (KBr): 𝑣/cm−1 3145 (NH), 1734 (CO); 1H-NMR (DMSO-d6): δ = 4.12 (s, 2H, CH2), 7.39 (t, J = 7.6 Hz, 1H, Ar-H), 7.51 (t, J = 7.6 Hz, 1H, Ar-H), 7.85 (d, J = 7.6 Hz, 1H, Ar-H), 8.01 (d, J = 7.6 Hz, 1H, Ar-H) and 12.35 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 35.68 (CH2), 121.82, 122.40, 124.65, 126.82, 133.49, 151.30, 166.56, 169.29 and 174.78 (Ar-C and CO); MS (EI): m/z (%) 249 (M+, 100), 250 (M++1, 14.6). Anal. calcd. for C10H7N3OS2 (249.31): C, 48.18; H, 2.83; N, 16.85; S, 25.72. Found: C, 48.09; H, 2.94; N, 16.94; S, 25.65. Crystallographic Analysis for 3c: The crystals were mounted on a glass fiber. All measurements were performed on a Rigaku R-AXIS RAPID diffractometer using filtered Mo-Kα radiation. The data were collected at a temperature of 20 ± 1 °C to a maximum 2θ value of 55.0° using the ω scanning technique. The structure was solved by charge flipping method and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically. Hydrogen atoms were refined using the riding model. Crystal Data: C10H7N3OS2, M = 249.31, monoclinic, a = 18.289(2) Å, b = 5.0485(1) Å, c = 11.5483(4) Å, V = 1066.30(9) Å3, α = γ = 90.00°, β = 90.077(6)°, space group: P21/c, Z = 4, Dcalc = 1.553 g cm−3. No. of reflections measured 2444, 2θmax = 54.9°, R1 = 0.0305. Figure 3 illustrates the structure as determined. Full data can be obtained on request from the CCDC [30].
(Z)-Ethyl-4-(4-oxothiazolidin-2-ylideneamino)benzoate (3d). Recrystallized from EtOH as pale yellow crystals, yield: 80%, m.p. 186–187 °C; IR (KBr): 𝑣/cm−1 3200 (NH), 1719, 1672 (2CO); 1H-NMR (DMSO-d6): δ = 1.31 (t, J = 7.2 Hz, 3H, CH3), 4.03 (s, 2H, CH2), 4.29 (q, J = 7.2 Hz, 2H, CH2), 7.06 (d, J = 7.8 Hz, 1H, Ar-H), 7.85 (d, J = 7.8 Hz, 1H, Ar-H), 7.96 (d, J = 7.8 Hz, 2H, Ar-H) and 11.54 ppm (br, 1H, NH); 13C-NMR (DMSO-d6): δ = 14.20 (CH3), 34.42 (CH2), 60.58 (CH2), 119.71, 121.31, 125.51, 130.48, 142.80, 157.92, 165.72 and 174.33 (Ar-C and CO); MS (EI): m/z (%) 264 (M+, 100), 265 (M++1, 17.80). Anal. calcd. For C12H12N2O3S (264.31): C, 54.53; H, 4.58; N, 10.60; S, 12.13; Found: 54.64; H, 4.49; N, 10.67; S, 12.06. Crystallographic Analysis for 3d. The crystals were mounted on a glass fiber. All measurements were performed on Bruker X8 Prospector. The data were collected at a temperature of 20 ± 1 °C to a maximum θ value of 66.61° using the ω scanning technique. The structure was solved by direct method using SHELXS-97 (Sheldrick, 2008) and refined by Full-matrix least-squares on F2. The non-hydrogen atoms were refined anisotropically. Data were corrected for absorption effects using the multi-scan method (SADABS). Crystal Data. C12H12N2O3S, M = 264.31, triclinic, a = 4.0829(3) Å, b = 5.5436(4) Å, c = 26.974(2) Å, V = 605.71(8) Å3, α = 84.625(6)°, β = 89.051(6)°, γ = 85.220(6)°, space group: P-1, Z = 2, Dcalc = 1.449 Mg cm−3, No. of reflections measured 5850, θmax = 66.0°, R1 = 0.0623. Figure 4 shows the structure as determined. Full data can be obtained on request from the CCDC [31].
3.4. General Procedure for the Synthesis of 2-Arylimino-5-arylidene-4-thiazolidinones 7a–p
Method A: Independent mixtures of 2-(heteroaryl or arylimino)thiazolidin-4-ones 3a–d (5 mmol) and the appropriate arylidene malononitrile 5 (0.77 g, 5 mmol) in ethanol (30 mL) containing few drops of piperidine (5 drops) were stirred at reflux for 3 h. Then, the reaction mixtures were cooled to room temperature. The solid which formed was collected by filtration, washed with hot ethanol, and recrystallized from the appropriate solvent to afford 7a–p respectively, as pure substances.
Method B: A mixture of 2-(heteroaryl or arylimino)thiazolidin-4-ones 3a–d (5 mmol) and the appropriate arylaldehyde (5 mmol) in acetic acid (25 mL) containing sodium acetate (10 mmol) was refluxed for 5 h. The reaction mixture was then cooled to room temperature and poured into ice-cold water. The precipitate was filtered off and washed with water and the resulting crude product was purified by recrystallization from the appropriate solvent.
(2Z,5Z)-5-Benzylidene-2-(2-chloro-5-nitrophenylimino)thiazolidin-4-one (7a). Recrystallized from dioxane/DMF (1:2) mixture as creamy crystals; yield: (A: 84%, B: 71%), m.p. 240–241 °C; IR (KBr): 𝑣/cm−1 3121 (NH), 1729 (CO); 1H-NMR (DMSO-d6): δ = 7.42–7.49 (m, 5H, Ar-H), 7.71 (s, 1H, olefinic CH), 7.82 (d, J = 7.6 Hz, 1H, Ar-H), 8.02–8.05 (m, 2H, Ar-H) and 12.86 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 117.28, 120.51, 122.10, 129.25, 129.80, 130.15, 130.62, 131.18, 133.01, 146.07, 146.93, 154.28, 160.98 and 167.22 ppm (Ar-C and CO); MS (EI): m/z (%) 359 (M+, 82.3), 360 (M++1, 20.22). Anal. calcd. for C16H10ClN3O3S (359.79): C, 53.41; H, 2.80; N, 11.68; S, 8.91. Found: C, 53.34; H, 2.68; N, 11.75; S, 8.84.
(2Z,5Z)-2-(2-Chloro-5-nitrophenylimino)-5-(4-methylbenzylidene)thiazolidin-4-one (7b). Obtained from dioxane/DMF (1:1) mixture as yellowish white crystals; yield: (A: 83%, B: 68%), m.p. 250–251 °C; IR (KBr): 𝑣/cm−1 3132 (NH), 1731 (CO); 1H-NMR (DMSO-d6): δ = 3.32 (s, 3H, CH3), 7.27 (d, J = 8.0 Hz, 2H, Ar-H), 7.39 (d, J = 8.0 Hz, 2H, Ar-H), 7.68 (s, 1H, olefinic CH), 7.84 (d, J = 7.6 Hz, 1H, Ar-H), 8.02–8.04 (m, 2H, Ar-H) and 12.82 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 21.03(CH3), 117.25, 120.43, 120.81, 129.84, 130.20, 130.69, 131.15, 133.01,140.36, 146.10, 146.89, 154.28, 160.84 and 167.25 ppm (Ar-C and CO); MS (EI): m/z (%) 373 (M+, 71.75), 374 (M++1, 18.60). Anal. calcd. for C17H12ClN3O3S (373.82): C, 54.62; H, 3.24; N, 11.24; S, 8.58. Found: C, 54.70; H, 3.17; N, 11.31; S, 8.66.
(2Z,5Z)-2-(2-Chloro-5-nitrophenylimino)-5-(4-methoxybenzylidene)thiazolidin-4-one (7c). Obtained from DMSO as yellow crystals; yield: (A: 77%, B: 65%), m.p. 247–248 °C; IR (KBr): 𝑣/cm−1 3136 (NH), 1726 (CO); 1H-NMR (DMSO-d6): δ = 3.78 (s, 3H, OCH3), 7.03 (d, J = 8.4 Hz, 2H, Ar-H), 7.49 (d, J = 8.4 Hz, 2H, Ar-H), 7.69 (s, 1H, olefinic CH), 7.87 (d, J = 8.0 Hz, 1H, Ar-H), 8.04–8.10 (m, 2H, Ar-H) and 12.77 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 55.39 (CH3), 114.83, 117.32, 118.88, 120.42, 125.47, 130.65, 131.18, 131.84, 133.06, 146.24, 146.93, 154.46, 160.75 and 167.38 ppm (Ar-C and CO); MS (EI): m/z (%) 389 (M+, 66.25), 390 (M++1, 13.94). Anal. calcd. for C17H12ClN3O4S (389.82): C, 52.38; H, 3.10; N, 10.78; S, 8.23. Found: C, 52.43; H, 3.03; N, 10.84; S, 8.17. Crystallographic Analysis for 7c: The crystals were mounted on a glass fiber. All measurements were performed on a Rigaku R-AXIS RAPID diffractometer using filtered Mo-Kα radiation. The data were collected at a temperature of 20 ± 1 °C to a maximum 2θ value of 55.0° using the ω scanning technique. The structure was solved by charge flipping method and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically. Hydrogen atoms were refined using the riding model. Crystal Data: C17H12ClN3O4S, M = 389.82, monoclinic, a = 21.540(3) Å, b = 8.1718(9) Å, c = 25.489(3) Å, V = 4170.4(8) Å3, α = γ = 90.00°, β = 111.639(8)°, space group: P21/c, Z = 8, Dcalc = 1.490 g cm−3, No. of reflections measured 6925, 2θmax = 50.0°, R1 = 0.0470. Figure 5 illustrates the structure as determined. Full data can be obtained on request from the CCDC [34].
(2Z,5Z)-2-(2-Chloro-5-nitrophenylimino)-5-(4-nitrobenzylidene)thiazolidin-4-one (7d). Recrystallized from dioxane/DMF (1:2) mixture as yellow crystals; yield: (A: 73%, B: 59%), m.p. 263–264 °C; IR (KBr): 𝑣/cm−1 3292 (NH), 1733 (CO); 1H-NMR (DMSO-d6): δ = 7.78 (d, J = 8.4 Hz, 2H, Ar-H), 7.83 (s, 1H, olefinic CH), 7.88 (d, J = 8.0 Hz, 1H, Ar-H), 8.06–8.08 (m, 2H, Ar-H), 8.27 (d, J = 8.4 Hz, 2H, Ar-H) and 13.07 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 117.27, 120.69, 124.22, 126.62, 128.02, 130.76, 131.26, 132.99, 139.32; 145.88, 146.93, 147.24, 153.67 and 166.87 ppm (Ar-C and CO); MS (EI): m/z (%) 404 (M+, 55.30), 405 (M++1, 14.62). Anal. calcd. For C16H9ClN4O5S (404.79): C, 47.48; H, 2.24; N, 13.84; S, 7.92. Found: C, 47.56; H, 2.27; N, 13.91; S, 7.86.
(2Z,5Z)-5-(4-Chlorobenzylidene)-2-(2-chloro-5-nitrophenylimino)thiazolidin-4-one (7e). Recrystallized from DMF as yellowish white crystals; yield: (A: 75%, B: 66%), m.p. 253–254 °C; IR (KBr): 𝑣/cm−1 3128 (NH), 1724 (CO); 1H-NMR (DMSO-d6): δ = 7.51–7.52 (m, 4H, Ar-H), 7.71 (s, 1H, olefinic CH), 7.84 (d, J = 8.0 Hz, 1H, Ar-H), 8.03–8.05 (m, 2H, Ar-H) and 12.89 ppm (s, 1H, NH); MS (EI): m/z (%) 393 (M+, 45.30), 394 (M++1, 12.95). Anal. calcd. for C16H9Cl2N3O3S (394.24): C, 48.75; H, 2.30; N, 10.66; S, 8.13. Found: C, 48.82; H, 2.23; N, 10.58; S, 8.19.
(2Z,5Z)-2-(2-Chloro-5-nitrophenylimino)-5-(thiophen-2-ylmethylidene)thiazolidin-4-one (7f). Obtained from dioxane as pale brown crystals; yield: (A: 81%, B: 70%), m.p. 266–267 °C; IR (KBr): 𝑣/cm−1 3112 (NH), 1727 (CO); 1H-NMR (DMSO-d6): δ = 7.24 (t, J = 4.8 Hz, 1H, Ar-H), 7.62 (d, J = 4.8 Hz, 1H, Ar-H), 7.86 (s, 1H, olefinic CH), 7.90 (d, J = 4.8 Hz, 1H, Ar-H), 8.00 (s, 1H, Ar-H), 8.05–8.07 (m, 2H, Ar-H) and 12.83 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 117.26, 119.64, 120.51, 123.98, 128.85, 131.18, 132.45, 133.10, 133.94, 137.16, 146.10, 146.88, 153.71 and 166.94 ppm (Ar-C and CO); MS (EI): m/z (%) 365 (M+, 86.77), 366 (M++1, 23.48). Anal. calcd. For C14H8ClN3O3S2 (365.82): C, 45.97; H, 2.20; N, 11.49; S, 17.53. Found: C, 46.05; H, 2.26; N, 11.43; S, 17.44.
(2Z,5Z)-Ethyl-4-(5-benzylidene-4-oxothiazolidin-2-ylideneamino)benzoate (7g). Recrystallized from DMF as yellow crystals; yield: (A: 74%, B: 69%), m.p. 291–292 °C; IR (KBr): 𝑣/cm−1 3199 (NH), 1709, 1678 (2CO); 1H-NMR (DMSO-d6): δ = 1.33 (t, J = 6.8 Hz, 3H, CH3), 4.32 (q, J = 6.8 Hz, 2H, CH2), 7.15 (d, J = 7.2 Hz, 2H, Ar-H), 7.47–8.00 (m, 8H, Ar-H and olefinic CH) and 12.34 ppm (br, 1H, NH); MS (EI): m/z (%) 352 (M+, 19.50), 353 (M++1, 5.20). Anal. calcd. for C19H16N2O3S (352.41): C, 64.76; H, 4.58; N, 7.95; S, 9.10; Found: C, 64.84; H, 4.65; N, 8.01; S, 9.18.
(2Z,5Z)-2-(4-Acetylphenylimino)-5-benzylidenethiazolidin-4-one (7h). Recrystallized from DMF as pale brown crystals; yield: (A: 90%, B: 79%), m.p. above 300 °C; IR (KBr): 𝑣/cm−1 3199 (NH), 1711, 1676 (CO); 1H-NMR (DMSO-d6 at 110 °C): δ = 2.58 (s, 3H, CH3), 7.41–7.43 (m, 3H, Ar-H), 7.47–7.56 (m, 4H, Ar-H), 7.69 (s, 1H, olefinic CH), 8.00 (d, J = 8.0 Hz, 2H, Ar-H) and 12.34 ppm (s, 1H, NH); MS (EI): m/z (%) 322 (M+, 15.45), 323 (M++1, 4.15). Anal. calcd. for C18H14N2O2S (322.39): C, 67.06; H, 4.38; N, 8.69; S, 9.95. Found: C, 66.98; H, 4.51; N, 8.63; S, 10.03.
(2Z,5Z)-2-(4-Acetylphenylimino)-5-(4-methylbenzylidene)thiazolidin-4-one (7i). Recrystallized from DMF as pale brown crystals; yield: (A: 93%, B: 81%), m.p. above 300 °C; IR (KBr): 𝑣/cm−1 3196 (NH), 1710, 1674 (2CO); 1H-NMR (DMSO-d6 at 110 °C): δ = 2.35 (s, 3H, CH3), 2.56 (s, 3H, CH3), 7.30 (d, J = 7.6 Hz, 2H, Ar-H), 7.43–7.45 (m, 4H, Ar-H), 7.65 (s, 1H, olefinic CH), 7.98 (d, J = 8.0 Hz, 2H, Ar-H) and 11.84 ppm (s, 1H, NH); MS (EI): m/z (%) 336 (M+, 55.0), 337 (M++1, 13.50). Anal. calcd. for C19H16N2O2S (336.42): C, 67.84; H, 4.79; N, 8.33; S, 9.53. Found: C, 67.91; H, 4.75; N, 8.39; S, 9.45.
(2Z,5Z)-2-(4-Acetylphenylimino)-5-(4-methoxybenzylidene)thiazolidin-4-one (7j). Recrystallized from DMF as yellow crystals; yield: (A: 89%, B: 75%), m.p. above 300 °C; IR (KBr): 𝑣/cm−1 3192 (NH), 1713, 1672 (2CO); 1H-NMR (DMSO-d6 at 110 °C): δ = 2.56 (s, 3H, CH3), 3.82 (s, 3H, OCH3), 7.04 (d, J = 8.4 Hz, 2H, Ar-H), 7.39–7.49 (m, 4H, Ar-H), 7.64 (s, 1H, olefinic CH), 7.98 (d, J = 8.0 Hz, 2H, Ar-H) and 11.81 ppm (s, 1H, NH); 13C-NMR (DMSO-d6 at 110 °C): δ = 26.68 (CH3), 55.96 (CH3), 115.09, 115.43, 1121.44, 126.75, 130.03, 130.26, 130.76, 131.91, 134.11, 143.95, 161.31, 166.74 and 196.84 ppm (Ar-C and CO); MS (EI): m/z (%) 352 (M+, 46.20), 353 (M++1, 12.15). Anal. calcd. for C19H16N2O3S (352.41): C, 64.76; H, 4.58; N, 7.95; S, 9.10. Found: C, 64.79; H, 4.65; N, 7.86; S, 8.99.
(2Z,5Z)-2-(4-Acetylphenylimino)-5-(4-nitrobenzylidene)thiazolidin-4-one (7k). Recrystallized from DMF as yellow crystals; yield: (A: 92%, B: 84%), m.p. above 300 °C; IR (KBr): 𝑣/cm−1 3198 (NH), 1718, 1674 (2CO); 1H-NMR (DMSO-d6 at 110 °C): δ = 2.57 (s, 3H, CH3), 7.48–7.78 (m, 4H, Ar-H), 7.98 (s, 1H, olefinic CH), 8.10–8.27 (m, 4H, Ar-H) and 12.09 ppm (s, 1H, NH); MS (EI): m/z (%) 367 (M+, 100), 368 (M++1, 23.80). Anal. calcd. for C18H13N3O4S (367.39): C, 58.85; H, 3.57; N, 11.44; S, 8.73. Found: C, 58.77; H, 3.66; N, 11.48; S, 8.79.
(2Z,5Z)-2-(4-Acetylphenylimino)-5-(4-chlorobenzylidene)thiazolidin-4-one (7l). Recrystallized from DMF as pale brown crystals; yield: Yield: (A: 95%, B: 86%), m.p. above 300 °C; IR (KBr): 𝑣/cm−1 3196 (NH), 1715, 1678 (2CO); 1H-NMR (DMSO-d6 at 110 °C): δ = 2.56 (s, 3H, CH3), 7.40–7.54 (m, 6H, Ar-H), 7.67 (s, 1H, olefinic CH), 7.98 (d, J = 8.4 Hz, 2H, Ar-H) and 11.94 ppm (s, 1H, NH); MS (EI): m/z (%) 356 (M+, 78.50), 357 (M++1, 19.95). Anal. calcd. for C18H13ClN2O2S (356.83): C, 60.59; H, 3.67; N, 7.85; S, 8.99. Found: C, 60.67; H, 3.64; N, 7.88; S, 8.93.
(2Z,5Z)-2-(4-Acetylphenylimino)-5-(thiophen-2-ylmethylidene)thiazolidin-4-one (7m). Recrystallized from DMF as yellow crystals; yield: (A: 87%, B: 78%), m.p. above 300 °C; IR (KBr): 𝑣/cm−1 3195 (NH), 1716, 1672 (2CO); 1H-NMR (DMSO-d6 at 110 °C): δ = 2.58 (s, 3H, CH3), 7.20–8.00 (m, 8H, Ar-H and olefinic CH) and 11.95 ppm (s, 1H, NH); MS (EI): m/z (%) 328 (M+, 49.55), 329 (M++1, 10.25). Anal. calcd. for C16H12N2O2S2 (328.41): C, 58.52; H, 3.68; N, 8.53; S, 19.53. Found: C, 58.59; H, 3.76; N, 8.56; S, 19.44.
(2Z,5Z)-2-(Benzothiazol-2-ylimino)-5-(4-methylbenzylidene)thiazolidin-4-one (7n). Recrystallized from dioxane as yellow crystals; yield: (A: 86%, B: 73%), m.p. 247–248 °C; IR (KBr): 𝑣/cm−1 3125 (NH), 1696 (CO); 1H-NMR (DMSO-d6): δ = 2.37 (s, 3H, CH3), 7.35–7.39 (m, 3H, Ar-H), 7.50 (t, J = 7.6 Hz, 1H, Ar-H), 7.58 (d, J = 8.0 Hz, 2H, Ar-H), 7.74 (s, 1H, olefinic CH), 7.93 (d, J = 7.6 Hz, 1H, Ar-H), 7.99 (d, J = 7.6 Hz, 1H, Ar-H) and 12.88 ppm (s, 1H, NH); MS (EI): m/z (%) 351 (M+, 52.6), 352 (M++1,12.2). Anal. calcd. for C18H13N3OS2 (351.45): C, 61.52; H, 3.73; N, 11.96; S, 18.25 Found: C, 61.45; H, 3.81; N, 12.02; S, 18.32.
(2Z,5Z)-2-(Benzothiazol-2-ylimino)-5-(thiophen-2-ylmethylidene)thiazolidin-4-one (7o). Obtained from dioxane as yellow crystals; yield: (A: 86%, B: 79%), m.p. 276–277 °C; IR (KBr): 𝑣/cm−1 3136 (NH), 1692 (CO); 1H-NMR (DMSO-d6): δ = 7.33 (t, J = 5.4 Hz, 1H, Ar-H), 7.39 (t, J = 7.6 Hz, 1H, Ar-H), 7.52 (t, J = 7.6 Hz, 1H, Ar-H), 7.73 (s, 1H, olefinic CH), 7.91 (d, J = 7.6 Hz, 1H, Ar-H), 8.01 (d, J = 7.6 Hz, 1H, Ar-H), 8.06–8.10 (m, 2H, Ar-H) and 12.89 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 121.67, 121.94, 122.12, 124.48, 125.81, 126.56, 129.00, 133.16, 133.43, 134.92, 137.38, 150.75, 158.06, 166.83 and 168.22, ppm (Ar-C and CO); MS (EI): m/z (%) 343 (M+, 80.5), 344 (M++1, 15.7). Anal. calcd. for C15H9N3OS3 (343.45): C, 52.46; H, 2.64; N, 12.23; S, 28.01. Found: C, 52.38; H, 2.73; N, 12.31; S, 27.94.
(2Z,5Z)-2-(Benzothiazol-2-ylimino)-5-[4-(dimethylamino)benzylidene]thiazolidin-4-one (7p). Obtained from dioxane as orange crystals; yield: (A: 90%, B: 77%), m.p. 299–300 °C; IR (KBr): 𝑣/cm−1 3129 (NH), 1711 (CO); 1H-NMR (DMSO-d6): δ = 3.04 (s, 6H, 2CH3), 6.87 (d, J = 8.4 Hz, 2H, Ar-H), 7.36 (t, J = 8.0 Hz, 1H, Ar-H), 7.50 (t, J = 8.0 Hz, 1H, Ar-H), 7.55 (d, J = 8.4 Hz, 2H, Ar-H), 7.66 (s, 1H, olefinic CH), 7.93 (d, J = 8.0 Hz, 1H, Ar-H), 8.00 (d, J = 8.0 Hz, 1H, Ar-H) and 12.68 ppm (s, 1H, NH); MS (EI): m/z (%) 380 (M+, 26.75), 381 (M++1, 7.65). Anal. calcd. for C19H16N4OS2 (380.49): C, 59.98; H, 4.24; N, 14.72; S, 16.85. Found: C,60.07; H, 4.16; N, 14.65; S, 16.89.
3.5. General Procedure for the Synthesis of the Enamines 8
Mixture of 3a or 3c (5 mmol), N,N-dimethylformamide dimethylacetal (DMF-DMA) (0.6 g, 5 mmol) in dioxane (20 mL) were stirred at reflux for 12 h. The separated solid product obtained on standing at room temperature was collected by filtration, washed by EtOH and recrystallized from dioxane to afford the corresponding enamines 8 as orange crystal.
(2Z,5Z)-2-(2-Chloro-5-nitrophenylimino)-5-[(dimethylamino)methylidene]thiazolidin-4-one (8a). Yield: 75%, m.p. 260–261 °C; IR (KBr): 𝑣/cm−1 2383 (NH), 1708 (CO); 1H-NMR (DMSO-d6): δ = 3.01 (s, 6H, 2CH3), 7.49 (s, 1H, olefinic CH), 7.79 (d, J = 8.4 Hz, 1H, Ar-H), 7.89–795 (m, 2H, Ar-H), and 11.77 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 42.22 (2CH3), 84.91, 117.90, 119.92, 131.56, 133.97, 144.76, 147.41, 147.81, 156.48 and 168.40 ppm (Ar-C and CO); MS (EI): m/z (%) 326 (M+, 57.25), 327 (M++1, 11.50). Anal. calcd. for C12H11ClN4O3S (326.76): C, 44.11; H, 3.39; N, 17.15; S, 9.81. Found: C, 44.18; H, 3.32; N, 17.24; S, 9.90.
(2Z,5Z)-2-(Benzothiazol-2-ylimino)-5-[(dimethylamino)methylidene]thiazolidin-4-one (8b). Yield: 79%, m.p. 205–206 °C; IR (KBr): 𝑣/cm−1 3133 (NH), 1670 (CO); 1H-NMR (DMSO-d6): δ = 3.18 (s, 6H, 2CH3), 7.25 (t, J = 7.6 Hz, 1H, Ar-H), 7.39 (t, J = 7.6 Hz, 1H, Ar-H), 7.63 (s, 1H, olefinic CH), 7.75 (d, J = 7.6 Hz, 1H, Ar-H), 7.88 (d, J = 7.6 Hz, 1H, Ar-H) and 12.07 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 41.71 (2CH3), 87.52, 120.76, 121.69, 123.48, 126.03, 132.53, 146.55, 151.19, 160.35, 167.19 and 168.97 ppm (Ar-C and CO); MS (EI): m/z (%) 304 (M+, 76.40), 305 (M++1, 15.30). Anal. calcd. for C13H12N4OS2 (304.39): C, 51.30; H, 3.97; N, 18.41; S, 21.07. Found: C, 51.38; H, 4.05; N, 18.48; S, 21.13. Crystallographic Analysis for 8b: The crystals were mounted on a glass fiber. All measurements were performed on Bruker X8 Prospector. The data were collected at a temperature of 20 ± 1 °C to a maximum θ value of 66.61° using the ω scanning technique. The structure was solved by direct method using SHELXS-97 (Sheldrick, 2008) and refined by Full-matrix least-squares on F2. The non-hydrogen atoms were refined anisotropically. Data were corrected for absorption effects using the multi-scan method (SADABS). Crystal Data: C13H12N4OS2, M = 304.39, monoclinic, a = 7.9497(5) Å, b = 7.2661(5) Å, c = 24.1772(17) Å, V = 1384.30(16) Å3, α = γ = 90.00°, β = 97.597(3)°, space group: P 1 21/c 1, Z = 4, Dcalc = 1.461 Mg cm−3, No. of reflection measured 7648, θmax = 66.69°, R1 = 0.0317. Figure 6 illustrates the structure as determined. Full data can be obtained on request from the CCDC [35].
3.6. General Procedure for the Synthesis of the Enamines 9
Mixtureof 3a or 3c (5 mmol), N,N-dimethylformamide dimethylacetal (DMF-DMA) (1.2 g, 10 mmol) in toluene (30 mL) were stirred at reflux for 6 h. The separated solid product obtained on standing at room temperature was collected by filtration, washed by EtOH and recrystallized from EtOH/dioxane (1:2) mixture to afford the corresponding enamines 9 as orange crystal.
(2Z,5Z)-2-(2-Chloro-5-nitrophenylimino)-5-[(dimethylamino)methylidene]-3-methylthiazolidin-4-one (9a). Yield: 72%, m.p. 199–200 °C; IR (KBr): 𝑣/cm−1 1703 (CO); 1H-NMR (DMSO-d6): δ = 3.04 (s, 6H, 2CH3), 3.25 (s, 3H, CH3), 7.63 (s, 1H, olefinic CH), 7.81 (d, J = 8.4 Hz, 1H, Ar-H), 7.90 (s, 1H, Ar-H) and 7.95 ppm (d, J = 8.4 Hz, 1H, Ar-H); 13C-NMR (DMSO-d6): δ = 28.85 (CH3), 41.93 (2CH3), 82.92, 117.29, 119.58, 131.16, 133.46, 144.92, 146.94, 147.09, 155.87 and 166.40 ppm (Ar-C and CO); MS (EI): m/z (%) 340 (M+, 41.55), 341 (M++1, 9.70). Anal. calcd. for C13H13ClN4O3S (340.79): C, 45.82; H, 3.85; N, 16.44; S, 9.41. Found: C, 45.91; H, 3.79; N, 16.37; S, 9.36.
(2Z,5Z)-2-(Benzothiazol-2-ylimino)-5-[(dimethylamino)methylidene]-3-methylthiazolidin-4-one (9b). Yield: 79%, m.p. 211–212 °C; IR (KBr): 𝑣/cm−1 1684 (CO); 1H-NMR (DMSO-d6): δ = 3.20 (s, 6H, 2CH3), 3.23 (s, 3H, CH3), 7.27 (t, J = 7.6 Hz, 1H, Ar-H), 7.40 (t, J = 7.6 Hz, 1H, Ar-H), 7.64 (s, 1H, olefinic CH), 7.76 (d, J = 7.6 Hz, 1H, Ar-H) and 7.90 ppm (d, J = 7.6 Hz, 1H, Ar-H); 13C-NMR (DMSO-d6): δ = 29.44 (CH3), 42.61 (2CH3), 85.93, 120.96, 121.72, 123.51, 126.06, 132.76, 146.58, 151.13, 159.38, 165.92 and 168.71 ppm (Ar-C and CO); MS (EI): m/z (%) 318 (M+, 33.9), 319 (M++1, 7.45). Anal. calcd. for C14H14N4OS2 (318.42): C, 52.81; H, 4.43; N, 17.60; S, 20.14. Found: C, 52.74; H, 4.49; N, 17.52; S, 20.25.
3.7. (2Z,5Z)-2-(4-Acetylphenylimino)-5-[(dimethylamino)methylidene]thiazolidin-4-one (10)
A mixture of 3b (10 mmol), N,N-dimethylformamide dimethylacetal (DMF-DMA) (1.2 g, 10 mmol) in toluene (25 mL) was stirred at reflux for 5 h. The separated solid product obtained on standing at room temperature was collected by filtration, washed by EtOH and recrystallized from dioxane/DMF (1:1) mixture as reddish brown crystals. Yield: 86%, m.p. 299–300 °C; IR (KBr): 𝑣/cm−1 3194, 1678, 1657 (2CO); 1H-NMR (DMSO-d6): δ = 2.55 (s, 3H, COCH3), 3.07 (s, 6H, 2CH3), 7.23 (d, J = 8.4 Hz, 2H, Ar-H), 7.57 (s, 1H, olefinic CH), 7.95 (d, J = 8.4 Hz, 2H, Ar-H) and 11.13 ppm (s, 1H, NH); MS (EI): m/z (%) 289 (M+, 55.50), 290 (M++1, 10.80). Anal. calcd. for C14H15N3O2S (289.36): C, 58.11; H, 5.23; N, 14.52; S, 11.08. Found: C, 58.24; H, 5.17; N, 14.43; S, 10.95.
3.8. (2Z,5Z)-5-[(Dimethylamino)methylidene]-3-methyl-2-{4-[(E)-4-methlypent-2-enoyl]phenylimino} thiazolidin-4-one (11)
A mixture of 3b (10 mmol), N,N-dimethylformamide dimethylacetal (DMF-DMA) (3.6 g, 30 mmol) in toluene (75 mL) was stirred at reflux for 72 h. The separated solid product obtained on standing at room temperature was collected by filtration, washed with EtOH and recrystallized from EtOH/dioxane (2:1) mixture to afford the corresponding enaminone 11 as deep orange crystals. Yield: 81%, m.p. 236–238 °C; IR (KBr): 𝑣/cm−1 1686, 1677 (2CO); 1H-NMR (DMSO-d6): δ = 2.91 (s, 3H, CH3), 3.02 (s, 6H, 2CH3), 3.14 (s, 3H, CH3), 3.21 (s, 3H, CH3), 5.85 (d, J = 12 Hz, 1H, olefinic CH=CH), 7.00 (d, J = 8.4 Hz, 2H, Ar-H), 7.55 (s, 1H, olefinic CH), 7.71 (d, J = 12 Hz, 1H, olefinic CH=CH), 7.90 (d, J = 8.4 Hz, 2H, Ar-H); 13C-NMR (DMSO-d6): δ = 28.87 (CH3), 37.14 (CH3), 41.80 (2CH3), 24.53 (CH3) 83.57, 90.71, 121.03, 128.72, 135.72, 144.14, 151.63, 152.80, 153.93, 166.65 and 184.84 ppm (Ar-C and CO); MS (EI): m/z (%) 358 (M+, 100), 359 (M++1, 22.55). Anal. calcd. for C18H22N4O2S (358.47): C, 60.31; H, 6.19; N, 15.63; S, 8.94. Found: C, 60.25; H, 6.23; N, 15.57; S, 8.96. Crystallographic Analysis for 11: The crystals were mounted on a glass fiber. All measurements were performed on a Rigaku R-AXIS RAPID diffractometer using filtered Mo-Kα radiation. The data were collected at a temperature of 20 ± 1 °C to a maximum 2θ value of 55.0° using the ω scanning technique. The structure was solved by charge flipping method and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically. Hydrogen atoms were refined using the riding model. Crystal Data: C18H22N4O2S, M = 358.47, triclinic, a = 7.275(2) Å, b = 10.923(2) Å, c = 24.1772(17) Å, V = 887.0(3) Å3, α = 105.825(8)°, β = 99.679(7)°, γ = 95.624(7)°, space group: P-1 (#2), Z = 2, Dcalc = 1.342 g cm−3, No. of reflections measured 3236, θmax = 50.7°, R1 = 0.0558. Figure 7 illustrates the structure as determined. Full data can be obtained on request from the CCDC [36].
3.9. (2Z)-2-(Benzothiazol-2-ylimino)-5-(phenylhydrazono)thiazolidin-4-one (12)
A cold solution of benzenediazonium chloride (10 mmol) was prepared by adding a solution of sodium nitrite (1.4 g dissolved in 10 mL water) to cold solution of aniline hydrochloride (0.93 g of aniline in 10 mL, 6M HCl) with stirring. The resulting solution of benzenediazonium chloride was then added to a cold solution of 4-thiazolidinone 3c (2.49 g, 10 mmol) in ethanol (100 mL) in the presence of sodium hydroxide (6.0 g, 15 mmol). The reaction mixture was stirred at room temperature for 1h and poured into ice cold water, the formed solid product was collected by filtration and washed with water then recrystallized from an EtOH to afford 12 as pale brown crystals, yield: 68%, m.p. 154–155 °C; IR (KBr): 𝑣/cm−1 3250, 3125 (2NH), 1723 (CO); 1H-NMR (DMSO-d6): δ = 6.98 (t, J = 8.0 Hz, 1H, Ar-H), 7.33–7.41 (m, 4H, Ar-H), 7.46–7.53 (m, 2H, Ar-H), 7.98 (d, J = 8.0 Hz, 1H, Ar-H), 8.01 (d, J = 8.0 Hz, 1H, Ar-H), 11.01 (s, 1H, NH) and 12.67 ppm (s, 1H, NH); MS (EI): m/z (%) 353 (M+, 39.70), 354 (M++1, 7.33). Anal. calcd. for C16H11N5OS2 (353.43): C, 54.38; H, 3.14; N, 19.82; S, 18.14. Found: C, 54.50; H, 3.25; N, 19.75; S, 18.20.
3.10. General Procedure for the Synthesis of Azolopyrimidines 13–18
Independent mixtures of 11 (1.075 g, 3 mmol) and the appropriate heteroaromatic amine (3 mmol) in pyridine (20 mL) were stirred at reflux for 24 h. The reaction mixtures were cooled to room temperature and poured into ice cold water then acidified with hydrochloric acid (2 N), forming solids that were collected by filtration and washed with water then MeOH and recrystallized from the indicated solvent.
(2Z,5Z)-5-[1-(Dimethylamino)methylidene]-3-methyl-2-[4-(1,2,4-triazolo[1,5-a]pyrimidin-7-yl)phenyl-imino]thiazolidin-4-one (13). Recrystallized from EtOH as yellow crystals, yield: 95%, m.p. 212–213 °C; IR (KBr): 𝑣/cm−1 1701 (CO); 1H-NMR (DMSO-d6): δ = 3.05 (s, 6H, 2CH3), 3.24 (s, 3H, CH3), 7.22 (d, J = 8.4 Hz, 2H, Ar-H), 7.58 (s, 1H, olefinic CH), 7.66 (d, J = 4.8 Hz, 1H, pyrimidine H), 8.30 (d, J = 8.4 Hz, 2H, Ar-H), 8.75 (s, 1H, triazole H) and 8.92 ppm (d, J = 4.8 Hz, 1H, pyrimidine H); 13C-NMR (DMSO-d6): δ = 28.87 (CH3), 41.65 (2CH3), 83.26, 108.91, 121.66, 124.54, 131.03, 144.34, 146.92, 152.37, 153.27, 154.83, 155.42, 155.86 and 166.50 (Ar-C and CO); MS (EI): m/z (%) 379 (M+, 100), 380 (M++1, 23.50). Anal. calcd. for C18H17N7OS (379.45): C, 56.98; H, 4.52; N, 25.84; S, 8.45. Found: C, 56.92; H, 4.44; N, 25.73; S, 8.54.
(2Z,5Z)-5-[1-(Dimethylamino)methylidene]-3-methyl-2-[4-(2-phenylpyrazolo[1,5-a]pyrimidin-7-yl)- phenylimino]thiazolidin-4-one (14). Recrystallized from dioxane as yellow crystals, yield: 92%, m.p. 251–252 °C; IR (KBr): 𝑣/cm−1 1690 (CO); 1H-NMR (DMSO-d6): δ = 3.06 (s, 6H, 2CH3), 3.25 (s, 3H, CH3), 7.23 (d, J = 8.4 Hz, 2H, Ar-H), 7.30–7.33 (m, 2H, pyrimidine H and pyrazole H), 7.45 (t, J = 7.6 Hz, 1H, Ar-H), 7.51 (t, J = 7.6 Hz, 2H, Ar-H), 7.59 (s, 1H, olefinic CH), 8.07 (d, J = 7.6 Hz, 2H, Ar-H), 8.34 (d, J = 8.4 Hz, 2H, Ar-H) and 8.58 ppm (d, J = 4.8 Hz, 1H, pyrimidine H); 13C-NMR (DMSO-d6 at 110 °C): δ = 29.23 (CH3), 42.28 (2CH3), 84.92, 93.65, 107.45, 121.93, 126.24, 126.85, 129.17, 129.29, 131.11, 133.34, 144.57, 145.56, 149.82, 151.57, 152.16, 153.31, 155.35 and 167.13 (Ar-C and CO); MS (EI): m/z (%) 454 (M+, 100), 455 (M++1, 21.95). Anal. calcd. for C25H22N6OS (454.56): C, 66.06; H, 4.88; N, 18.49; S, 7.05. Found: C, 66.11; H, 4.91; N, 18.55; S, 7.12. Crystallographic Analysis for 14: The crystals were mounted on a glass fiber. All measurements were performed on a Rigaku R-AXIS RAPID diffractometer using filtered Mo-Kα radiation. The data were collected at a temperature of 20 ± 1 °C to a maximum 2θ value of 55.0° using the ω scanning technique. The structure was solved by charge flipping method and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically. Hydrogen atoms were refined using the riding model. Crystal Data: C25H22N6OS, M = 454.56, monoclinic, a = 7.6658(8) Å, b = 10.612(2) Å, c =29.279(4) Å, V = 2378.2(5) Å3, α = γ = 90.00°, β = 93.200(7)°, space group: P21/c (#14), Z = 4, Dcalc = 1.392 g cm−3, No. of reflection measured 4333, θmax = 50.6°, R1 = 0.066. Figure 8 illustrates the structure as determined. Full data can be obtained on request from the CCDC [37].
7-{4-[(Z)5-(1-Dimethylaminomethylidene)-3-methyl-4-oxo-thiazolidin-(2Z)-ylideneamino]phenyl}-N-(2-chloro-5-nitrophenyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (15). Recrystallized from dioxane/ DMF (2:1) mixture as orange crystals, yield: 87%, m.p. 294–295 °C; IR (KBr): 𝑣/cm−1 3246 (NH), 1697, 1680 (2CO); 1H-NMR (DMSO-d6 at 110 °C): δ = 3.07 (s, 6H, 2CH3), 3.27 (s, 3H, CH3), 7.24 (d, J = 8.4 Hz, 2H, Ar-H), 7.55 (s, 1H, olefinic CH), 7.59 (d, J = 4.8 Hz, 1H, pyrimidine H), 7.86 (d, J = 7.8 Hz, 1H, Ar-H), 7.97 (d, J = 7.8 Hz, 1H, Ar-H), 8.29 (d, J = 8.4 Hz, 2H, Ar-H), 8.81 (s, 1H, pyrazole H), 8.94 (d, J = 4.8 Hz, 1H, pyrimidine H), 9.48 (s, 1H, Ar-H), and 10.86 ppm (s, 1H, NH); MS (EI): m/z (%) 576 (M+, 100), 577 (M++1, 44.85). Anal. calcd. for C26H21ClN8O4S (577.03): C, 54.12; H, 3.67; N, 19.42; S, 5.56. Found: C, 54.19; H, 3.65; N, 19.38; S, 5.64.
7-{4-[(Z)5-(1-Dimethylaminomethylidene)-3-methyl-4-oxo-thiazolidin-(2Z)-ylideneamino]phenyl}-N-(4,6-dimethylpyrimidin-2-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide (16). Recrystallized from EtOH/ dioxane (1:1) mixture as orange crystals, yield: 93%, m.p. above 300 °C; IR (KBr): 𝑣/cm−1 3277 (NH), 1688, 1676 (2CO); 1H-NMR (DMSO-d6): δ = 2.42 (s, 6H, 2CH3), 3.06 (s, 6H, 2CH3), 3.24 (s, 3H, CH3), 7.01 (s, 1H, 4,6-dimethylpyrimidin H), 7.23 (d, J = 8.4 Hz, 2H, Ar-H), 7.60 (s, 1H, olefinic CH), 7.62 (d, J = 4.8 Hz, 1H, pyrimidine H), 8.26 (d, J = 8.4 Hz, 2H, Ar-H), 8.79 (s, 1H, pyrazole H), 8.98 (d, J = 4.8 Hz, 1H, pyrimidine H) and 10.60 ppm (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 23.49 (2CH3), 28.91 (CH3), 42.18 (2CH3), 83.31, 104.76, 109.01, 115.59, 121.59, 124.62, 131.40, 144.46, 146.17, 146.72, 147.36, 152.36, 152.43, 153.25, 157.07, 157.97, 166.53 and 167.84 (Ar-C and CO); MS (EI): m/z (%) 527 (M+, 94.80), 528 (M++1, 30.15). Anal. calcd. for C26H25N9O2S (527.61): C, 59.19; H, 4.78; N, 23.89; S, 6.08. Found: C, 59.25; H, 4.71; N, 23.98; S, 6.15.
(2Z,5Z)-2-[4-(Benzimidazo[1,2-a]pyrimidine-4-yl)phenylimino]-5-[1-(dimethylamino)methylidene]-3-methylthiazolidin-4-one (17). Recrystallized from EtOH/ dioxane (1:1) mixture as yellow crystals, yield: 96%, m.p. 281–282 °C; IR (KBr): 𝑣/cm−1 1692 (CO); 1H-NMR (DMSO-d6): δ = 3.09 (s, 6H, 2CH3), 3.27 (s, 3H, CH3), 6.69 (d, J = 8.0 Hz, 1H, Ar-H), 7.06–7.10 (m, 2H, 1Ar-H and pyrimidine H), 7.27 (d, J = 8.4 Hz, 2H, Ar-H), 7.48 (t, J = 8.0 Hz, 1H, Ar-H), 7.61 (s, 1H, olefinic CH), 7.71 (d, J = 8.4 Hz, 2H, Ar-H), 7.88 (d, J = 8.0 Hz, 1H, Ar-H) and 8.86 ppm (d, J = 4.8 Hz, 1H, pyrimidine H); 13C-NMR (DMSO-d6): δ = 28.93 (CH3), 42.25 (2CH3), 83.47, 108.24, 114.75, 119.58, 120.76, 122.31, 125.77, 127.06, 127.20, 129.75, 144.25, 144.35, 149.54, 151.36, 151.64, 153.51, 155.86 and 166.65 (Ar-C and CO); MS (EI): m/z (%) 428 (M+, 100), 429 (M++1, 24.10). Anal. calcd. for C23H20N6OS (428.52): C, 64.47; H, 4.70; N, 19.61; S, 7.48. Found: C, 64.54; H, 4.69; N, 19.64; S, 7.52.
2((E)-3-{4-[(Z)5-(1-Dimethylaminomethylidene)-3-methyl-4-oxo-thiazolidin-(2Z)-ylideneamino]phen- yl}-3-oxoprop-1-enylamino)-4-phenylthiophene-3-carbonitrile (18). Recrystallized from dioxane as orange crystals, yield: 81%, m.p. 270–271 °C; IR (KBr): 𝑣/cm−1 3116 (NH), 2206 (CN), 1699, 1654 (2CO); 1H-NMR (DMSO-d6): δ = 3.04 (s, 6H, 2CH3), 3.22 (s, 3H, CH3), 6.43 (d, J = 8.8 Hz, 1H, olefinic CH=CH), 7.10 (t, J = 7.6 Hz, 2H, Ar-H), 7.31 (s, 1H, thiophene H), 7.43–7.53 (m, 3H, Ar-H), 7.58 (s, 1H, olefinic CH), 7.60–7.65 (m, 2H, Ar-H), 7.83 (d, J = 8.8 Hz, 1H, olefinic CH=CH), 7.90 (d, J = 7.6 Hz, 1H, Ar-H), 8.04 (d, J = 7.6 Hz, 1H, Ar-H) and 12.97 ppm (brd, 1H, NH); MS (EI): m/z (%) 513 (M+, 100), 514 (M++1, 29.80). Anal. calcd. for C27H23N5O2S2 (513.64): C, 63.14; H, 4.51; N, 13.63; S, 12.48. Found: C, 63.21; H, 4.44; N, 13.57; S, 12.39.
3.11. (2Z,5Z)-2-(Benzothiazol-2-ylimino)-5-[(1H-1,2,4-triazol-3-ylamino)methylidene]thiazolidin-4-one (19)
A mixture of the enamine 8b (1.25 g, 5 mmol) and 3-amino-1,2,4-triazol (0.42 g, 5 mmol) in acetic acid (20 mL) containing anhydrous sodium acetate (10 mmol) was refluxed for 24 h. The reaction mixture was then cooled to room temperature and then poured into ice-cold water. The precipitate was filtered off and washed with water and the resulting crude product was purified by recrystallization from dioxane as brown crystals, yield: 74%, m.p. above 300 °C; IR (KBr): 𝑣/cm−1 3195, 3142, 3115 (3NH), 1683 (CO); 1H-NMR (DMSO-d6): δ = 7.32 (t, J = 7.6 Hz, 1H, Ar-H), 7.46 (t, J = 7.6 Hz, 1H, Ar-H), 7.75 (d, J = 7.6 Hz, 1H, Ar-H), 7.95 (d, J = 7.6 Hz, 1H, Ar-H), 7.63 (d, J = 10.8 Hz, 1H, CHNH), 8.44 (br d, 1H, triazole H), 11.03 (s, 1H, NH), 12.24 (s, 1H, NH) and 13.76 ppm (br, 1H, NH); 13C-NMR (DMSO-d6): δ = 96.37, 120.91, 121.98, 123.94, 126.38, 132.88, 135.05, 143.78, 151.19, 158.97, 160.10, 167.66 and 168.94 ppm (Ar-C and CO); MS (EI): m/z (%) 343 (M+, 7.90), 344 (M++1, 2.08). Anal. calcd. for C13H9N7OS2 (343.39): C, 45.47; H, 2.64; N, 28.55; S, 18.67. Found: C, 45.53; H, 2.73; N, 28.62; S, 18.54.
4. Conclusions
The results of the study described above have led to the development of a simple approach for the synthesis of a novel class of fused azolopyrimidines and 2-arylimino-5-arylidene-4-thiazolidinones, substances with potentially interesting biological and medicinal properties. Furthermore, the observations made during this work showed that the reaction of 4-thiazolidinones with arylidene malononitrile afforded only the 2-arylimino-5-arylidene-4-thiazolidinones and not corresponding pyranothiazole. In addition a new class of enaminone derivatives was synthesized, which can be used as precursors for the synthesis of a variety of interesting heterocyclic compounds.
Acknowledgments
Support of this work was provided by the University of Kuwait through a research grant (SC03/11). The facilities of Analab/SAF supported by research grants GS01/01, GS01/05, GS01/03 and GS03/08 are gratefully acknowledged.
Footnotes
Sample Availability: Samples of the compounds 2, 3, 7, 9, 11 and 13–18 are available from the authors.
References and Notes
- 1.Tenover F.C., McDonald L.C. Vancomycin-resistant staphylococci and enterococci: epidemiology and control. Curr. Opin. Infect. Dis. 2005;18:300–305. doi: 10.1097/01.qco.0000171923.62699.0c. [DOI] [PubMed] [Google Scholar]
- 2.Muroi H., Nihei K., Tsujimoto K., Kubo I. Synergistic effects of anacardic acids and methicillin against methicillin resistant Staphylococcus aureus. Bioorg. Med. Chem. 2004;12:583–587. doi: 10.1016/j.bmc.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 3.Wermuth C.G. Similarity in drugs: reflections on analogue design. Drug Discov. Today. 2006;11:348–354. doi: 10.1016/j.drudis.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhari S.K., Verma M., Chaturvedi A.K., Parmar S.S. Substituted thiazolidones: Selective inhibition of nicotinamide adenine dinucleotide-dependent oxidations and evaluation of their cns activity. J. Pharm. Sci. 1975;64:614–617. doi: 10.1002/jps.2600640408. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhary M., Parmar S.S., Chaudhary S.K., Chaturvedi A.K., Rama Sastry B.V. CNS depressant activity of pyrimidylthiazolidones and their selective inhibition of NAD-dependent pyruvate oxidation. J. Pharm. Sci. 1976;65:443–446. doi: 10.1002/jps.2600650336. [DOI] [PubMed] [Google Scholar]
- 6.Babaoglu K., Page M.A., Jones V.C., McNeil M.R., Dong C., Naismith J.H., Lee R.E. Novel inhibitors of an emerging target in mycobacterium tuberculosis; substituted thiazolidinones as inhibitors of dTDP-rhamnose synthesis. Bioorg. Med. Chem. Lett. 2003;13:3227–3230. doi: 10.1016/S0960-894X(03)00673-5. [DOI] [PubMed] [Google Scholar]
- 7.Dwivedi C., Gupta T.K., Parmar S.S. Substituted thiazolidones as anticonvulsants. J. Med. Chem. 1972;15:553–554. doi: 10.1021/jm00275a031. [DOI] [PubMed] [Google Scholar]
- 8.Parmar S.S., Dwivedi C., Chaudhari A., Gupta T.K. Substituted thiazolidones and their selective inhibition of nicotinamide-adenine dinucleotide dependent oxidations. J. Med. Chem. 1972;15:99–101. doi: 10.1021/jm00271a030. [DOI] [PubMed] [Google Scholar]
- 9.Bondock S., Khalifa W., Fadda A.A. Synthesis and antimicrobial evaluation of some new thiazole, thiazolidinone and thiazoline derivatives starting from 1-chloro-3,4-dihydronaphthalene-2-carboxaldehyde. Eur. J. Med. Chem. 2007;42:948–954. doi: 10.1016/j.ejmech.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 10.Vicini P., Geronikaki A., Incerti M., Zani F., Dearden J., Hewitt M. 2-Heteroarylimino-5-benzylidene-4-thiazolidinones analogues of 2-thiazolylimino-5-benzylidene-4-thiazolidinones with antimicrobial activity: Synthesis and structure–activity relationship. Bioorg. Med. Chem. 2008;16:3714–3724. doi: 10.1016/j.bmc.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Gududuru V., Hurh E., Dalton J.T., Miller D.D. Synthesis and antiproliferative activity of 2-aryl-4-oxo-thiazolidin-3-yl-amides for prostate cancer. Bioorg. Med. Chem. Lett. 2004;14:5289–5293. doi: 10.1016/j.bmcl.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 12.Ottanà R., Carotti S., Maccari R., Landini I., Chiricosta G., Caciagli B., Vigorita M.G., Mini E. In vitro antiproliferative activity against human colon cancer cell lines of representative 4-thiazol- idinones. Part I. Bioorg. Med. Chem. Lett. 2005;15:3930–3933. doi: 10.1016/j.bmcl.2005.05.093. [DOI] [PubMed] [Google Scholar]
- 13.Agrawal V.K., Sachan S., Khadikar P.V. QSAR studies on antihistaminic activity of some thiazolidine-4-ones. Acta Pharm. 2000;50:281–290. [Google Scholar]
- 14.Diurno M.V., Mazzoni O., Correale G., Monterrey I.G., Calignano A., La Rana G., Bolognese A. Synthesis and structure–activity relationships of 2-(substituted phenyl)-3-[3-(N,N-dimethylamino)- propyl]-1,3-thiazolidin-4-ones acting as H1-histamine antagonists. II Farmaco. 1999;54:579–583. doi: 10.1016/S0014-827X(99)00064-6. [DOI] [PubMed] [Google Scholar]
- 15.Omar K., Geronikaki A., Zoumpoulakis P., Camoutsis C., Sokovic M., Ciric A., Glamoclija J. Novel 4-thiazolidinone derivatives as potential antifungal and antibacterial drugs. Bioorg. Med. Chem. 2010;18:426–432. doi: 10.1016/j.bmc.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 16.Vigorita M.G., Ottanà R., Monforte F., Maccari R., Monforte M.T., Trovato A., Taviano M.F., Miceli N., De Luca G., Alcaro S., Ortuso F. Chiral 3,3′-(1,2-ethanediyl)-bis[2-(3,4-dimethoxyphenyl)-4-thiazolidinones] with anti-inflammatory activity. Part 11: Evaluation of COX-2 selectivity and modeling. Bioorg. Med. Chem. 2003;11:999–1006. doi: 10.1016/S0968-0896(02)00518-7. [DOI] [PubMed] [Google Scholar]
- 17.Rawal R.K., Prabhakar Y.S., Katti S.B., De Clercq E. 2-(Aryl)-3-furan-2-ylmethyl-thiazolidin-4-ones as selective HIV-RT Inhibitors. Bioorg. Med. Chem. 2005;13:6771–6776. doi: 10.1016/j.bmc.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki Y., Akima M., Tamura K. Effects of CP-060S, a novel cardioprotective drug, on cardiac function and myocardial oxygen consumption. Gen. Pharmacol. 1999;32:57–63. doi: 10.1016/S0306-3623(98)00062-7. [DOI] [PubMed] [Google Scholar]
- 19.Svetlik J., Veizerová L., Mayer T.U., Catarinella M. Monastrol analogs: A synthesis of pyrazolo-pyridine, benzopyranopyrazolopyridine, and oxygen-bridged azolopyrimidine derivatives and their biological screening. Bioorg. Med. Chem. Lett. 2010;20:4073–4076. doi: 10.1016/j.bmcl.2010.05.085. [DOI] [PubMed] [Google Scholar]
- 20.Petroski R.E., Pomeroy J.E., Das R., Bowman H., Yang W., Chen A.P., Foster A.C. Indiplon is a high-affinity positive allosteric modulator with selectivity for alpha1 subunit-containing GABAA receptors. J. Pharmacol. Exp. Ther. 2006;317:369–377. doi: 10.1124/jpet.105.096701. [DOI] [PubMed] [Google Scholar]
- 21.Mirza N.R., Rodgers R.J., Mathiasen L.S. Comparative cue generalization profiles of L-838, 417, SL651498, zolpidem, CL218,872, ocinaplon, bretazenil, zopiclone, and various benzodiazepines in chlordiazepoxide and zolpidem drug discrimination. J. Pharmacol. Exp. Ther. 2006;316:1291–1299. doi: 10.1124/jpet.105.094003. [DOI] [PubMed] [Google Scholar]
- 22.Weitzel K.W., Wickman J.M., Augustin S.G., Strom J.G. Zaleplon: A pyrazolopyrimidine sedative-hypnotic agent for the treatment of insomnia. Clin. Ther. 2000;22:1254–1267. doi: 10.1016/S0149-2918(00)83024-6. [DOI] [PubMed] [Google Scholar]
- 23.Burchat A.F., Calderwood D.J., Friedman M.M., Hirst G.C., Li B., Rafferty P., Ritter K., Skinner B.S. Pyrazolo[3,4-d]pyrimidines containing an extended 3-substituent as potent inhibitors of Lck—A selectivity insight. Bioorg. Med. Chem. Lett. 2002;12:1687–1690. doi: 10.1016/S0960-894X(02)00196-8. [DOI] [PubMed] [Google Scholar]
- 24.Siavosh M., Andreas S., Heymo H., Emerich E., Thomas B., Mathias S., Thomas M., Josef S.F., Thomas B.L. [4-(Imidazol-1-yl)thiazol-2-yl]phenylamines. a novel Class of highly potent colchicine site binding tubulin inhibitors: Synthesis and cytotoxic activity on selected human cancer cell lines. J. Med. Chem. 2006;49:5769–5776. doi: 10.1021/jm060545p. [DOI] [PubMed] [Google Scholar]
- 25.Chavan A.A., Pai N.R. Synthesis and antimicrobial screening of 5-arylidene-2-imino-4-thiazolidinones. ARKIVOC. 2007;16:148–155. [Google Scholar]
- 26.Vicini P., Geronikaki A., Anastasia K., Incerti M., Zani F. Synthesis and antimicrobial activity of novel 2-thiazolylimino-5-arylidene-4-thiazolidinones. Bioorg. Med. Chem. 2006;14:3859–3864. doi: 10.1016/j.bmc.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 27.Subbotina J.O., Fabian W.M.F., Tarasov E.V., Volkova N.N., Bakulev V.A. Synthetic and Theoretical Aspects of New Dimroth Rearrangement of 6-Aminopyran-2-ones to 6-Hydroxy- pyridin-2-ones via Carbamoyl Ketenes. Eur. J. Org. Chem. 2005:2914–2923. doi: 10.1002/ejoc.200400875. [DOI] [Google Scholar]
- 28.Crystallographic data for 2d (ref. CCDC 874278) can be obtained on request from the director, Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EW, UK.
- 29.Crystallographic data for 3a (ref. CCDC 874280) can be obtained on request from the director, Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EW, UK.
- 30.Crystallographic data for 3c (ref. CCDC 874281) can be obtained on request from the director, Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EW, UK.
- 31.Crystallographic data for 3d (ref. CCDC 874279) can be obtained on request from the director, Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EW, UK.
- 32.Behbehani H., Ibrahim H.M., Makhseed S., Elnagdi M.H., Mahmoud H. 2-Aminothiophenes as building blocks in heterocyclic synthesis: Synthesis and antimicrobial evaluation of a new class of pyrido[1,2-a]thieno[3,2-e]pyrimidine, quinoline and pyridin-2-one derivatives. Eur. J. Med. Chem. 2012;52:61–65. doi: 10.1016/j.ejmech.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Behbehani H., Ibrahim H.M., Makhseed S. Studies with 3-oxoalkanonitriles: Reactivity of 3-oxo-(1-methylindoloyl)propanenitrile. Heterocycles. 2009;78:3081–3090. doi: 10.3987/COM-09-11818. [DOI] [Google Scholar]
- 34.Crystallographic data for 7c (ref. CCDC 875772) can be obtained on request from the director, Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EW, UK.
- 35.Crystallographic data for 8b (ref. CCDC 874404) can be obtained on request from the director, Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EW, UK.
- 36.Crystallographic data for 11 (ref. CCDC 880545) can be obtained on request from the director, Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EW, UK.
- 37.Crystallographic data for 14 (ref. CCDC 880544) can be obtained on request from the director, Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EW, UK.
- 38.Behbehani H., Ibrahim H.M., Makhseed S. Synthesis of 7-substituted pyrazolo[1,5-a]-pyrimidine-3-carboxamides as potential non benzodiazepine hypnotics. ARKIVOC. 2010;2:267–282. [Google Scholar]
- 39.Patil V., Tilekar K., Mehendale-Munj S., Mohan R., Ramaa C.S. Synthesis and primary cytotoxicity evaluation of new 5-benzylidene-2,4-thiazolidinedione derivatives. Eur. J. Med. Chem. 2010;45:4539–4544. doi: 10.1016/j.ejmech.2010.07.014. [DOI] [PubMed] [Google Scholar]