Abstract
A new dienamide, (2E,4E)-7-(3',4'-dimethoxyphenyl)-N-ethyl-6-(R)-hydroxyhepta-2,4-dienamide, named (-)-kunstleramide (1), were isolated from the bark of Beilschmiedia kunstleri Gamble together with one neolignan: (+)-kunstlerone (2) and seven known alkaloids: (+)-nornuciferine (3), (-)-isocaryachine (4), (+)-cassythicine (5), (+)-laurotetanine (6), (+)-boldine (7), noratherosperminine (8), (+)-N-demethylphyllocaryptine (9). Their structures were established from spectroscopic techniques, most notably 1D- and 2D-NMR, UV, IR, OR, circular dichroism (CD) spectra and LCMS-IT-TOF. (-)-Kunstleramide (1) exhibited very poor dose-dependent inhibition of DPPH activity, with an IC50 value of 179.5 ± 4.4 μg/mL, but showed a moderate cytotoxic effect on MTT assays of A375, A549, HT-29, PC-3 and WRL-68 with EC50 values of 64.65, 44.74, 55.94, 73.87 and 70.95 µg/mL, respectively.
Keywords: Beilschmiedia kunstleri, lauraceae, alkaloid, dienamide, antioxidant, cytotoxicity
1. Introduction
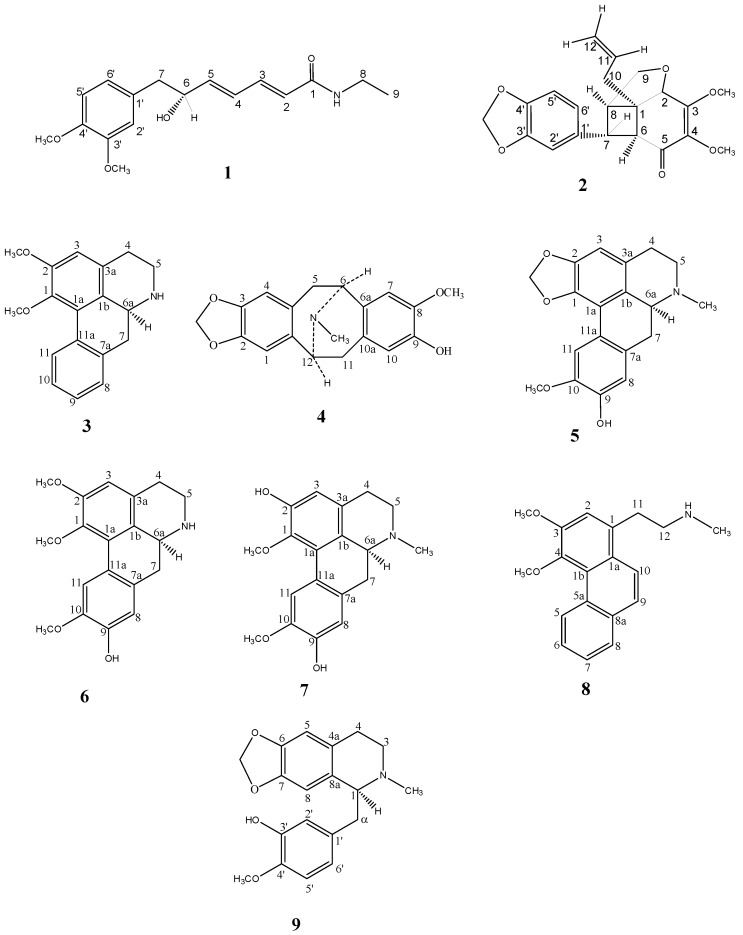
In continuation of our research on the medicinal plants from Malaysian flora [1,2,3], we have performed a phytochemical investigation on the bark of a Malaysian Lauraceae, Beilschmiedia kunstleri Gamble, which has led to the isolation of a new dienamide, (2E,4E)-7-(3',4'-dimethoxyphenyl)-N-ethyl-6-(R)-hydroxyhepta-2,4-dienamide, named (−)-kunstleramide (1). In addition, seven known alkaloids: (+)-nornuciferine (3) [4], (−)-isocaryachine (4) [5], (+)-cassythicine (5) [6], (+)-laurotetanine (6) [7], (+)-boldine (7) [8], noratherosperminine (8) [9], (+)-N-demethylphyllocaryptine (9) [8] and one neolignan, (+)-kunstlerone (2) [10], were also isolated (Figure 1). This paper describes the structural elucidation, the DPPH activity with an IC50 value and cytotoxic effect of (−)-kunstleramide (1).
Figure 1.
Chemical structures of compounds 1–9.
There are no previous reports on dienamides isolated from the Lauraceae species, although they are reported to occur in another plant species such as Zanthoxylum ailanthoides Sieb. and Zucc. (Rutaceae), Piper maingayi (Piperaceae) and Fagara zanthoxyloides Lam. (Rutaceae) [11,12,13,14]. Recently, we have reported one new antioxidant neolignan from the leaves of Beilschmiedia species [10].
2. Results and Discussion
(−)-Kunstleramide (1) was isolated as a yellowish amorphous solid. The LC-MS-IT-TOF revealed a pseudomolecular ion peak at m/z 328.1531 [M+Na+], thus suggesting a molecular formula of C17H23NO4 (calc. 305.1525). The IR spectrum showed the presence of the bands at 1,612 and 1,659 cm−1, due to C=C and C=O, and 3,351 cm−1 due to NH and OH stretching vibrations, respectively [15,16]. The 1H-NMR spectrum (Table 1) established the presence of three aromatic protons at δ 6.66 (1H, d, J = 1.8 Hz, H-2'), 6.67 (1H, dd, J = 8.2, 1.8 Hz, H-6') and 6.76 (1H, d, J = 8.2 Hz, H-5') and two methoxyl groups at δ 3.79 (3H, s, 4'-OCH3) and 3.81 (3H, s, 3'-OCH3).
Table 1.
1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectral data of (−)-kunstleramide (1) in CDCl3.
| Position | 1H (δH, Hz) | 13C |
|---|---|---|
| C=O | - | 165.8 |
| C-2 | 5.74, d (15.1) | 124.2 |
| C-3 | 7.15, dd (15.1, 8.9) | 140.0 |
| C-4 | 6.26, dd (15.1, 11.2) | 127.7 |
| C-5 | 6.06, dd (15.1, 5.1) | 142.3 |
| C-6 | 4.37, m | 72.6 |
| C-7 | 2.66, dd (Jβ = 13.1, 5.1),2.80, dd (Jα = 13.1, 5.1) | 43.4 |
| C-8C-9 | 3.30, q (7.4)1.10, t (7.4) | 34.614.9 |
| C-1' | - | 129.5 |
| C-2' | 6.66, d (1.8) | 112.6 |
| C-3' | - | 147.9 |
| 3’-OMeC-4'4'-OMe | 3.81, s-3.79, s | 55.9149.955.9 |
| C-5' | 6.76, d (8.2) | 111.3 |
| C-6' | 6.67, dd (8.2, 1.8) | 121.6 |
| N-HO-H | 5.43, br s 1.67, br s | -- |
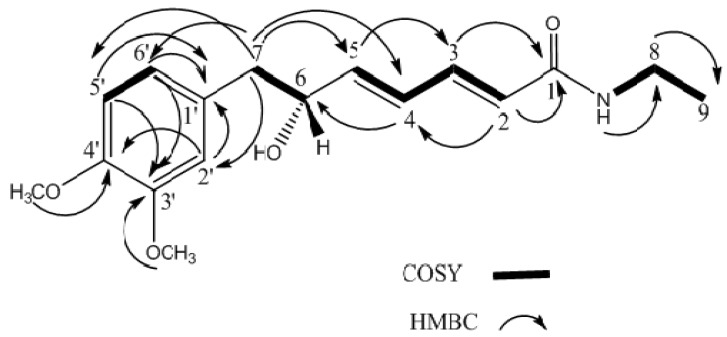
Signals representing an N-ethyl attached to C=O appeared as a quartet at δ 3.30 (methylene) and a triplet at δ 1.10 for the methyl group. The H-6 methine appeared as a multiplet at δ 4.37 and the H-7 methylene showed two doublet of doublets at δ 2.66 and 2.80 (J = 13.1 and 5.1 Hz), respectively. The olefinic proton was observed at δ 5.74 (J = 15.1 Hz) as a doublet for H-2. A doublet of doublets at δ 7.15 (J = 15.1 and 8.9 Hz) was assigned to H-3. Another two olefenic protons, H-4 and H-5 appeared at δ 6.26 (J = 15.1 and 11.2 Hz) as doublet of doublets and at δ 6.06 (J = 15.1 and 5.1 Hz) as a doublet of doublets, respectively. Therefore, the structure of compound 1 was unambiguously determined as (2E,4E), which was also confirmed by H-H COSY, H-H NOESY, and DEPT spectra [16]. 13C-NMR and DEPT spectra (Table 1) showed three methyls, two methylenes, eight methines, three quaternary carbons and one carbonyl. The sp2 carbon of C-2 bearing an ethyl amide group gave a signal at δ124.2, while the carbonyl group was observed at δ 165.8 [16]. The correlations of H-2 and H-8 with C-1, and H-N with C-8 in the HMBC spectrum further confirmed the position of ethyl amide fragment at C-1. In addition, the cross-peaks in the COSY spectrum were observed between H-2/H-3, H-3/H-4, H-4/H-5, H-5/H-6, H-6/H-7 and H-5'/H-6'. The chemical shifts of proton and carbon were shown in Table 1 and the H-H correlations and H-C connectivity are shown in Figure 2.
Figure 2.
Selected 2D-NMR (HMBC and COSY) correlations of (-)-kunstleramide(1).
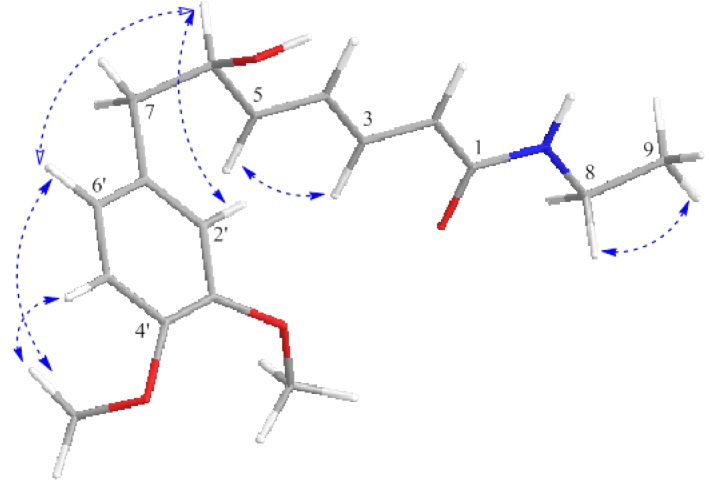
The configuration of proton at C-6 was confirmed based on the analysis of NOESY spectrum. NOESY correlations were observed between H-9 (δ 1.10)/H-8 (δ 3.30), H-3 (δ 7.15)/H-5 (δ 6.06), H-7α (δ 2.80)/H-7β (δ 2.66), H-6 (δ 4.37)/6' (δ 6.67), H-2' (δ 6.66)/H-6 (δ 4.37), H-6' (δ 6.67)/4'-OCH3 (δ 3.79) and H-5' (δ 6.76)/4'-OCH3 (δ 3.79) respectively (Figure 3).
Figure 3.
Selected NOESY correlation of (−)-kunstleramide (1).
Consequently, the relative configuration of C-6 proton was R and the optical rotation is −15.48°. Since compound (1) is a chiral, there is a Cotton effect at the position of the chromophore band. The CD (circular dichroism) showed two Cotton effect curves with opposite sign around λmax 262 nm separated by a Davydov splitting. Therefore, the chirality of compound 1 is due to the chiralities between the electric dipole transition moments of both chromophore (the benzene and the unsaturated amide) [17,18]. Based on the above spectroscopic data, it was confirmed that compound 1 was (2E,4E)-7-(3',4'-dimethoxyphenyl)-N-ethyl-6-(R)-hydroxyhepta-2,4-dienamide, and it was named (−)-kunstleramide.
2.1. Antioxidant Activity
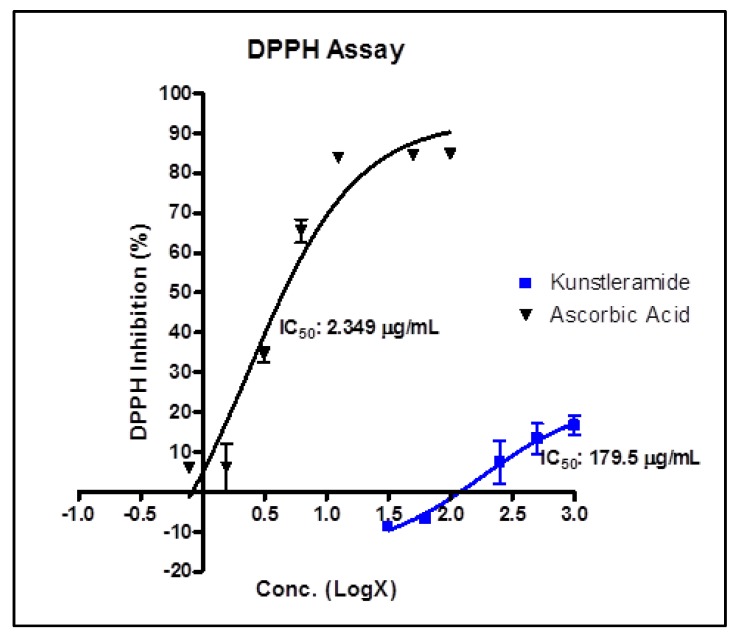
The antioxidant activity of (−)-kunstleramide (1) was tested using a DPPH assay. Antioxidants are substances that may protect cells from the damage caused by unstable molecules known as free radicals [19]. Free radicals from oxidative stress are involved in many disorders like neurodegenerative diseases and cancer [20]. The new dienamide, (−)-kunstleramide (1) exhibited very poor DPPH activity, with an IC50 value of 179.5 ± 4.4 μg/mL compared to ascorbic acid (Figure 4). Factors such as growth conditions, stability of the specific antioxidant components, including variations in the process of extraction can influence the variations in the antioxidant activity [21].
Figure 4.
IC50 values of (−)-kunstleramide (1). AA: ascorbic acid as control. Results are means ± SD of two replicates.
2.2. Cytotoxic Activity
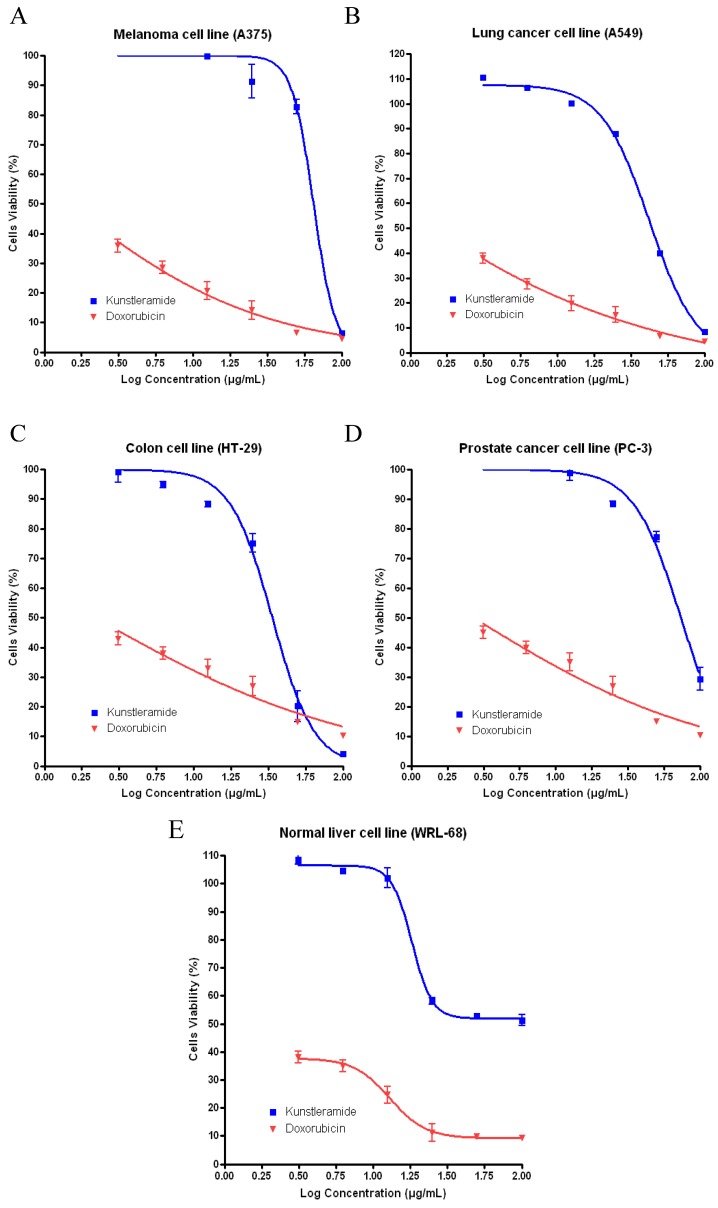
To evaluate the cytotoxic activity, the new compound (−)-kunstleramide (1) was tested with a series of different doses on A549, PC-3, A375, HT-29 and WRL-68, respectively (Figure 5). After 24 h, cell viability was determined by the MTT assay. Test agents induced cell cytotoxicity in a concentration dependent manner. These dose titration curves allowed determining EC50 for the test agents towards different cell lines (Table 2).
Figure 5.
Dose-response curves (using GraphPad Prism) tested with (−)-kunstleramide (1) and doxorubicin (positive control) in the MTT assays towards (A) A375, (B) A549, (C) HT-29, (D) PC-3 and (E) WRL-68.
Table 2.
Effect of compounds (−)-kunstleramide (1) and doxorubicin (positive control) on different cells type expressed as EC50 values in 24 h MTT assay. [EC50 ± S.D (µg/mL)].
| Cell line | New Dienamide | Doxorubicin |
|---|---|---|
| A375 | 64.65 | 1.364 |
| A549 | 44.74 | 1.550 |
| HT-29 | 55.94 | 1.957 |
| PC-3 | 73.87 | 2.125 |
| WRL-68 | 70.95 | 1.731 |
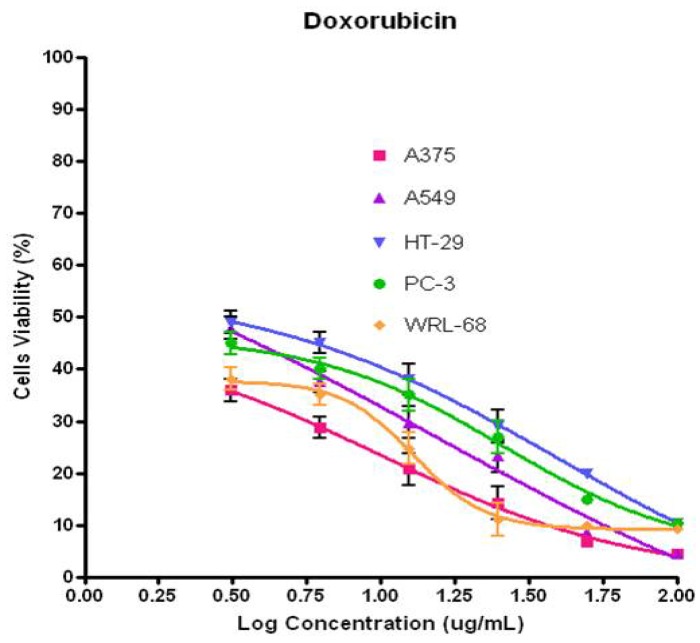
From Figure 5, (−)-kunstleramide (1) showed cytotoxic effect on several of the cancer cell lines with different EC50 values as compared to the standard, Doxorubicin (Figure 6). This compound showed moderately cytotoxic effect. (−)-Kunstleramide (1) demonstrated dose-depended cytotoxic effects EC50 values of 64.65, 44.74, 55.94, 73.87 and 70.95 µg/mL; in A375, A549, HT-29, PC-3 and WRL-68, respectively. These results indicate that cell lines differ in their sensitivity to the same test agent, which may be determined by multiple cell type-specific signalling cascades and transcription factor activities.
To our knowledge, the cytotoxic potentials of (−)-kunstleramide (1) have not been examined and the underlying molecular mechanisms remain to be discovered.
Figure 6.
Dose-response curves (using GraphPad Prism) tested with doxorubicin (positive control) in the MTT assays towards A375, A549, HT-29, PC-3 and WRL-68.
3. Experimental
3.1. General
The Fourier Transform Infrared (FT-IR) spectra were obtained with CHCl3 (NaCl window technique) on a Perkin Elmer 2000 instrument. The ultraviolet spectra (UV) were obtained in MeOH on a Shimadzu UV-310 ultraviolet-visible spectrophotometer. The OR (optical rotation) was recorded on a JASCO (Japan) P1020 Polarimeter equipped with a tungsten lamp (MeOH as solvent) and CD (circular dichroism) data was recorded on a JASCO (Japan) J-815 spectrometer equipped with a tungsten lamp (MeOH as solvent). Mass spectra were obtained using LCMS-IT-TOF, Shimadzu spectrometer Series Mass Selective Detector, Agilent Technologies 6530 Accurate-Mass Q-TOF LC/MS, with ZORBAX Eclipse XDB-C18 Rapid Resolution HT 4.6 mm i.d. × 50 mm × 1.8 μm column. Solvent used was methanol (CH3OH). The Auto mass Multi Thermofinnigan was used for HR ESI analysis and EIMS spectra were obtained on Shimadzu LCMS-IT-TOF Mass Spectrometer, QP2000A spectrometer 70 eV. NMR spectra were recorded in deuterated chloroform (CDCl3) and deuterated methanol CD3OD) on JEOL LA400 FT-NMR and JEOL ECA 400 FT-NMR as a JEOL JNM-FX400 (400 MHz for 1H and 100 MHz for 13C; unless stated otherwise) and signal of spectra calibrated using TMS. Chemical shifts were reported in ppm on δ scale, and the coupling constants were measured in Hertz (Hz). Silica gel 60, 70-230 mesh ASTM (Merck 7734) was used for various column chromatography methods such as CLC and FLC.TLC aluminum sheets and PTLC (20 × 20 cm Silica gel 60 F254) were used in the thin layer chromatography analysis. The TLC and PTLC spots were visualized under UV light (254 and 366 nm) followed by spraying with Dragendorff’s reagent for detection of alkaloids. Dragendorff’s reagent and Mayer’s reagent were used for alkaloid screening. All solvents used were of AR grade except those used for bulk extraction.
3.2. Plant Materials
The bark of Beilschmiedia kunstleri Gamble (Lauraceae) was collected at Hutan Simpan Sungai Tekam, Jerantut, Pahang, Malaysia by the phytochemical group of the Department of Chemistry, University of Malaya. The voucher specimen (KL5627) of this plant has been deposited at the Herbarium of the Department of Chemistry, University of Malaya, Kuala Lumpur, Malaysia.
3.3. Extraction and Isolation of Chemical Constituents
Dried, ground stem bark of Beilschmiedia kunstleri Gamble (1.50 kg) was first defatted with hexane (5.0 L) for 72 h and then the hexane extract was filtered. The residual bark material was air-dried and moistened with 15% NH4OH. It was then extracted with CH2Cl2 (10.0 L) for 5 days. After filtration, the supernatant was concentrated using rotary evaporator under reduced pressure to a volume of 500 mL, followed by acidic extraction with 5% HCl until a negative Mayer’s test result was obtained. The hydrochloric acid portion was washed with CH2Cl2, basified with NH4OH to pH 11 and re-extracted with CH2Cl2. The CH2Cl2 extract was washed with distilled H2O, dried over anhydrous sodium sulphate, filtered and evaporated to give crude alkaloid (2.90 g). The extraction of alkaloids was repeated by using MeOH as the solvent and crude alkaloid (10.00 g) was obtained. The crude alkaloid (2.90 g) was subjected to exhaustive column chromatography over silica gel using CH2Cl2 and gradually increasing the polarity with methanol to give 12 fractions. Fraction 9 gave a new compound 1 (38.05 mg, 1.30%, PTLC Merck KGaA silica gel 60 F254; CH2Cl2-MeOH; 90:10), fraction 10 gave alkaloid 3 (23.55 mg, 0.80%, CH2Cl2-MeOH; 85:15), fraction 7 afforded alkaloid 4 (19.52 mg, 0.67%, CH2Cl2-MeOH; 95:5), fraction 3 gave alkaloid 5 (19.00 mg, 0.65%, CH2Cl2-MeOH; 98:2), fraction 1 gave alkaloid 6 (32.20 mg, 1.13%, CH2Cl2-MeOH; 98:2), fraction 6 afforded alkaloid 8 (24.80 mg, 0.85%, CH2Cl2-MeOH; 96:4) and fraction 11 gave alkaloid 9 (27.21 mg, 0.93%, CH2Cl2-MeOH; 80:20).
The crude methanol extract (10.00 g) was subjected to column chromatography over silica gel to yield five fractions. Fraction 3 gave compound 2 (24.45 mg, 0.83%, CH2Cl2-MeOH; 50:50), fraction 2 afforded alkaloid 5 (29.50 mg, 1%, CH2Cl2-MeOH; 60:40) and fraction 1 gave alkaloid 7 (32.20 mg, 1.13%, CH2Cl2-MeOH; 98:2).
(−)-Kunstleramide (1) with [α25D]: = −15.48° (C = 4.2 × 10−2 M, MeOH), was obtained as a yellowish amorphous solid; UV (MeOH) λmax: 210 and 262 nm [14,15]; CD (MeOH) λmax 260 (Δε + 4.0), 265 (Δε −3.5); (IR bands (KBr): 3,351.5, 1,659.4, 1,612.2, 351.5 cm −1[15,16]; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3): (Table 1); LC-MS, MHz: 328.1531 [M+Na]+ (calc. 328.1525 for C17H23NO4).
3.4. Antioxidant Assay
The DPPH assay was performed according to the method reported by Orhan et al. [22] and Brem et al. [23], with modifications. Briefly, 0.02% stable DPPH free radical (50 µL) in methanol (100 mL) was added to standard/sample/control (20 µL) and methanol (130 µL, total assay volume 200 µL) in a 96-well plate. Ascorbic Acid (vitamin C) was used as the standard and blank solvent methanol as the negative control. The absorbance was read at 517 nm using SUNRISE Microplate Absorbance Reader after 30 min of incubation at room temperature. The percentage of DPPH free radical inhibition activity was determined according to the formula:
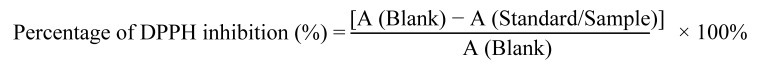
where A (Blank) refers to the absorbance of the blank solvent and DPPH at 517 nm while A(Standard/Sample) refers to the absorbance of Ascorbic Acid and the samples at 517 nm. This formula was also used to determine the concentration of each sample required to quench 50% of the DPPH free radical activity (IC50 value) [24].
3.5. Statistical Analyses
Each experiment was performed at least twice. Results are expressed as the means value ± standard deviation (SD). Log IC50 calculations were performed using the built-in algorithms for dose-response curves with variable slope using Graphpad Prism software (version 4.0; GraphPad Software Inc., San Diego, CA, USA). A fixed maximum value of the dose-response curve was set to the maximum obtained value for each drug.
3.5. Cytotoxic Activity Studies
3.5.1. Cell Culture
All the cells used in this study were obtained from American Type Cell Collection (ATCC) and maintained in a 37 °C incubator with 5% CO2 saturation. A375 human melanoma, HT-29 human colon adenocarcinoma cells and WRL-68 normal hepatic cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM), whereas A549 non-small cell lung cancer cells and PC-3 prostate adenocarcinoma cells were maintained in RPMI medium. Both medium were supplemented with 10% fetus calf serum (FCS), 100 units/mL penicillin, and 0.1 mg/mL streptomycin.
3.5.2. Cellular Viability
Different cell types mentioned above were used to evaluate the inhibitory effect of kunstleramide (1) on cell growth using the MTT assay. The MTT assay was modified as described by Cheah et al. and Mosmann [25,26]. Briefly, cells were seeded at a density of 1 × 105 cells/mL in a 96-well plate and incubated for 24 h at 37 °C, 5% CO2. The next day, cells were treated with the compounds respectively and incubated for another 24 h. After 24 h, MTT solution at 2 mg/mL was added and incubatefor 1 h. Absorbance at 570 nm was measured and recorded using a Plate Chameleon V microplate reader (Hidex, Turku, Finland). Results were expressed as a percentage of control giving percentage cell viability after 24 h exposure to test agent. The potency of cell growth inhibition for each test agent was expressed as an EC50 value, defined as the concentration that caused a 50% loss of cell growth. Viability was defined as the ratio (expressed as a percentage) of absorbance of treated cells to untreated cells [27].
4. Conclusions
To the knowledge of the authors, (−)-kunstleramide (1) is the first ethyl dienamide reported in the family of Lauraceae which bears a hydroxyl group at C-6 and two methoxyl groups attached to C-3' and C-4'. This is the first communication on a dienamide from Beilschmiedia kunstleri. The neolognan (+)-kunstlerone (2) and alkaloids 3-9, belonging to the aporphine, benzylisoquinoline, morphinandienone and pavine type of alkaloids were also isolated from this plant. (−)-Kunstleramide (1) exhibited very poor DPPH, activity with an IC50 value of 179.5 ± 4.4 μg/mL compared with the DPPH inhibitor ascorbic acid. Kunstleramide (1) showed moderate cytotoxic effect with EC50 values. This study revealed that this plant showed promising cytotoxic activity but poor DPPH activity. Further investigation should be carried out to evaluate the cytotoxicity of compound 1 at lower concentrations and its mechanism.
Acknowledgements
The authors acknowledge the financial support provided by University of Malaya Research Grant (UMRG 045/11BIO), Centre of Natural Products and Drugs Development (CENAR), Postgraduate Research Grant of University of Malaya (PS366/2010B) and High Impact Research (HIR) Grant of University of Malaya (F000009-21001). We also acknowledge the support provided by Din Mat Nor and Rafly Syamsir for the plant samples, and Hairin Taha for editorial assistance.
Supplementary Materials
Supplementary materials can be found at: http://www.mdpi.com/1420-3049/17/4/4197/s1.
Footnotes
Sample Availability: Not available.
References and Notes
- 1.Kochummen K.M. In: Tree Flora of Malaya: A Manual For Foresters. Ng F.S.P., editor. Vol. 4. Longman; Selangor, Malaysia: 1989. p. 98. [Google Scholar]
- 2.Pudjiastuti P., Mukhtar M.R., Hadi A.H.A., Saidi N., Morita H., Litaudon M., Awang K. (6,7-Dimethoxy-4-methylisoquinolinyl)-(4'-methoxyphenyl)-methanone, a new benzylisoquinoline alkaloid from Beilschmiedia brevipes. Molecules. 2010;15:2339–2346. doi: 10.3390/molecules15042339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Funasaki M., Lordello A.L., Viana A.M., Santa-Catarina C., Floh E.I.S., Yoshida M., Kato M.J. Neolignans and sesquiterpenes from leaves and embryogenic cultures of Ocotea catharinensis (Lauraceae) J. Braz. Chem. Soc. 2009;20:853–859. doi: 10.1590/S0103-50532009000500008. [DOI] [Google Scholar]
- 4.Cava M.P., Rao K.V., Douglas B., Weisbach J.A. Alkaloids of Cassytha Americana. J. Org. Chem. 1968;33:2443–2446. [Google Scholar]
- 5.Chung-hsiung C., Shoei-shen L., Chung-fang L., Jinn W., Jack L.B. A caryachnine N-methosalt from Cryptocarya chinensis and PMR spectral characteristics of some quaternary pavine alkaloids. J. Nat. Prod. 1979;42:163–167. doi: 10.1021/np50002a005. [DOI] [PubMed] [Google Scholar]
- 6.Pinheiro M.L.B., Xavier C.M., Souza A.D.L.D., Rabelo D.D.M., Batista C.L., Batista R.L., Costa E.V., Campos F.R., Barison A., Valdez R.H., et al. Acanthoic acid and other constituents from the stem of Annona amazonica (Annonaceae) J. Braz. Chem. Soc. 2009;20:1095–1102. [Google Scholar]
- 7.Babkock P.A., Segelman A.B. Alkaloids of Lindera benzoin (L.) Blume (lauraceae), I: Isolation and identification of laurotetanine. J. Pharm. Sci. 1974;63:1495–1496. doi: 10.1002/jps.2600630944. [DOI] [PubMed] [Google Scholar]
- 8.Cave A., Leboeuf M., Moskowitz H., Ranaivo A., Bick I., Sinchai W., Nieto M., Sevenet T., Cabalion P. Alkaloids of Cryptocarya phyllostemon. Aust. J. Chem. 1989;42:2243–2263. doi: 10.1071/CH9892243. [DOI] [Google Scholar]
- 9.Ramana M.M.V., Prashant V.P. Tandem acylation-cycloalkylation with cyclohexene-1-acetic acid: A new entry to phenanthrene alkaloids. Nat. Prod. Res. 1996;8:317–324. [Google Scholar]
- 10.Mollataghi A., Hamid A.H.A., Awang K., Jamaludin M., Litaudon M., Mukhtar M.R. (+)-Kunstlerone, a new antioxidant neolignan from the leaves of Beilschmiedia kunstleri Gamble. Molecules. 2011;16:6582–6590. doi: 10.3390/molecules16086582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad F., Jamil S., Ibrahim A.Z., Read R.W. Alkenylalamides from Piper maingayi Hk. F. Pertanika. J. Sci. Technol. 1996;4:167–171. [Google Scholar]
- 12.Marques J.V., Oliveira A.D., Raggi L., Young M.C.M., Kato M.J. Antifungal activity of natural and synthetic amides from piper species. J. Braz. Chem. Soc. 2010;21:1807–1813. doi: 10.1590/S0103-50532010001000003. [DOI] [Google Scholar]
- 13.Cheng M.J., Tsai I.L., Chen I.S. Chemical constituents from the root bark of Formosan Zanthoxylum ailanthoides. J. Chin. Chem. Soc. 2003;50:1241–1246. [Google Scholar]
- 14.Chaaib F., Queiroz E.F., Ndjoko K., Diallo D., Hostettmann K. Antifungal and antioxidant compounds from the root bark of Fagara zanthoxyloides. Planta Med. 2003;69:316–320. doi: 10.1055/s-2003-38877. [DOI] [PubMed] [Google Scholar]
- 15.Zhao J., Muhammad I., Dunbar D.C., Mustafa J., Khan I.A. New alkamides from maca (Lepidium meyenii) J. Agric. Food Chem. 2005;53:690–693. doi: 10.1021/jf048529t. [DOI] [PubMed] [Google Scholar]
- 16.Caturla F., Najera C. Lithiated (E)-N-isopropyl-5-tosyl-4-pentenamide: Synthetic applications as new δ-acyldienyl anion equivalent. Tetrahedron. 1998;54:11255–11270. doi: 10.1016/S0040-4020(98)00658-9. [DOI] [Google Scholar]
- 17.Harada N., Nakanishi K. Exciton chirality method and its application to configurational and conformational studies of natural products. Acc. Chem. Res. 1972;5:257–263. [Google Scholar]
- 18.Smith H.E. Chiroptical properties of the benzene chromophore. A method for the determination of the absolute configurations of benzene compounds by application of the benzene sector and benzene chirality rules. Chem. Rev. 1998;98:1709–1740. doi: 10.1021/cr9410124. [DOI] [PubMed] [Google Scholar]
- 19.Blot W.J., Li J.Y., Taylor P.R., Guo W., Dawsey S., Wang G.Q., Yang C.S., Zheng S.F., Gail M., Li G.Y., et al. Nutrition intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J. Natl. Cancer Inst. 1993;85:1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 20.Uddin N., Rahman A., Uddin N.A., Rana S., Akter R., Chowdhury M.A. Antioxidant, cytotoxic and antimicrobial properties of Eclipta alba ethanol extract. Int. J. Biol. Med. Res. 2010;1:341–346. [Google Scholar]
- 21.Price J.A., Sanny C.G., Sherlin D. Application of manual assessment of oxygen radical absorbent capacity (ORAC) for use in high throughput assay of “total” antioxidant activity of drugs and natural products. J. Pharmacol. Toxicol. Methods. 2006;54:56–61. doi: 10.1016/j.vascn.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Orhan M.K., Naz Q., Ejaz A., Yilmaz G., Kan Y., Konuklugil B., Sener B., Choudhary M.I. Antioxidant and anticholinesterase evaluation of selected Turkish Salvia species. Food Chem. 2007;103:1247–1254. doi: 10.1016/j.foodchem.2006.10.030. [DOI] [Google Scholar]
- 23.Brem B., Seger C., Pacher T., Harti M., Hadacek F., Hofer O., Vajrodaya S., Greger H. Antioxidant dehydrotocopherols as a new chemical character of Stemona species. Phytochemistry. 2004;65:2719–2729. doi: 10.1016/j.phytochem.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 24.Enayat S., Banerjee S. Comparative antioxidant activities of extracts from leaves, bark and catkins of Salixa egyptiaca species. Food Chem. 2009;116:23–28. doi: 10.1016/j.foodchem.2009.01.092. [DOI] [Google Scholar]
- 25.Cheah S.C., Appleton D.R., Lee S.T., Lam M.L., Hadi A.H.A., Mustafa M.R. Panduratin a inhibits the growth of A549 cells through induction of apoptosis and inhibition of NF-Kappa B translocation. Molecules. 2011;16:2583–2598. doi: 10.3390/molecules16032583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosmann T. Rapid colorimetric assay for the cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 27.Braga P.A., Dos Santos D.A., Da Silva M.F., Vieira P.C., Fernandes J.B., Houghton P.J., Fang R. In vitro cytotoxicity activity on several cancer cell lines of acridone alkaloids and N-phenylethyl-dienamide derivatives from Swinglea glutinosa (Bl.) Merr. Nat. Prod. Res. 2007;21:47–55. doi: 10.1080/14786410600907002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.