Abstract
We report here on the synthesis and in vitro anti-tumor effects of a series of novel 1,2,4-triazole (compounds 3–6), 4,5-dicyanoimidazole (compound 7), and purine (compounds 8–13) coumarin derivatives and their acyclic nucleoside analogues 14–18. Structures of novel compounds 3–18 were deduced from their 1H- and 13C-NMR and corresponding mass spectra. Results of anti-proliferative assays performed on a panel of selected human tumor cell lines revealed that compound 6 had moderate cytostatic activity against the HeLa cell line (IC50 = 35 µM), whereas compound 10 showed moderate activity against the HeLa (IC50 = 33 µM), HepG2 (IC50 = 25 µM) and SW620 (IC50 = 35 µM) cell lines. These compounds showed no cytotoxic effects on normal (diploid) human fibroblasts.
Keywords: : 1,2,4-triazole; 4,5-dicyanoimidazole and purine coumarin derivatives; acyclic nucleoside analogues; antitumor activity evaluation
1. Introduction
Coumarin (1,2-benzopyrone or 2H-1-benzopyran-2-one) and its derivatives are ubiquitously distributed in Nature and many of them exhibit diverse and useful biological activities [1,2]. These compounds have numerous medical applications including antitumor and anti-HIV therapy [3,4], central nervous system (CNS) stimulation [5], antibacterial [6,7], anti-inflammatory [8,9,10] and anti-coagulant properties [11]. In addition, hydroxycoumarins are known to be powerful chain-breaking anti-oxidants which can prevent free radical injury by scavenging reactive oxygen species [12,13]. Some coumarin derivatives display cytostatic properties, while others have cytotoxic activities [14]. For example, coumarin and its active metabolite, 7-hydroxycoumarin, have demonstrated growth-inhibitory activity in human cancer cell lines, such as A549 (lung), ACHN (renal), H727 (lung), MCF-7 (breast) and HL-60 (leukemia), and have also been reported to have anti-proliferative activity in prostate cancer, malignant melanoma and metastatic renal cell carcinoma in clinical trials [15,16,17,18]. The recent discovery of coumarins having weak estrogenic activity resulted in the use of such derivatives as therapeutic agents in preventing the emergence of menopause-related diseases, such as osteoporosis, increased risk of cardiovascular disease and cognitive deficiencies [19]. Furthermore, the substituted benzopyranobenzothiazinones exhibited estrogenic activity in MCF-7 breast carcinoma cells [20]. Of particular interest in breast cancer chemotherapy is the finding that some coumarin analogs and their active 7-hydroxycoumarin metabolites have sulfatase and aromatase inhibitory activities. Coumarin-based selective estrogen receptor modulators (SERMs) and coumarin estrogen conjugates have also been described as potential anti-breast cancer agents. Since breast cancer is the second leading cause of death in American women after lung cancer, there is a strong impetus to identify potential new drug treatments for breast cancer [21].
The anti-tumor activities of coumarin and its known metabolite 7-hydroxycoumarin were tested in several human tumor cell lines by Steffen et al. [22]. Both compounds inhibited cell proliferation of gastric carcinoma cell line (HSC-39), colon carcinoma cell line (Caco-2), hepatoma-derived cell line (Hep-G2) and lymphoblastic cell line (CCRF). Egan et al. [23] have synthesized, characterized and determined cytostatic and cytotoxic nature of 8-nitro-7-hydroxycoumarin using both human (including K-562 and HL-60) and animal cell lines grown in vitro. The effect of warfarin on tumor cell growth was studied [24]. Warfarin inhibits metastasis of Mtln3 rat mammary carcinoma without affecting primary tumor growth. Seven known coumarins showing significant cytotoxic activities on P388 cell lines were isolated from the roots of Angelica gigas (Umbelliferae) [25]. The cytotoxicity of 22 natural and semi-synthetic simple coumarins was evaluated in human small cell lungcarcinoma cell line GLC4 and human colorectal cancer cell line COLO 320 using the MTT assay [26]. Furthermore, a number of 4-hydroxycoumarin derivatives have been studied for their HIV integrase inhibitory potency [27]. The main purpose was to simplify the large structure of the compounds while maintaining their potency. It was found that the minimum active pharmacophore consisted of coumarin dimer containing aryl substituent on the central linker, methylene. Additionally, 1,2,4-triazole represents a unique template that is associated with anti-viral, anti-bacterial, anti-fungal, anti-inflammatory and CNS activity. Compounds incorporating 1,2,4-triazole rings have also been shown to be anti-tumor agents [28]. Pyrimidine, 1,2,4-triazole and purine derivatives are constituents of a number of useful drugs and are associated with many biological, pharmaceutical and therapeutic activities. Condensed pyrimidine, 1,2,4-triazole and purine derivatives have been reported as anti-microbial, analgesic, anti-viral, anti-inflammatory, anti-HIV, anti-tubercular, anti-tumor, anti-malarial, diuretic and cardiovascular [29,30,31,32] agents.
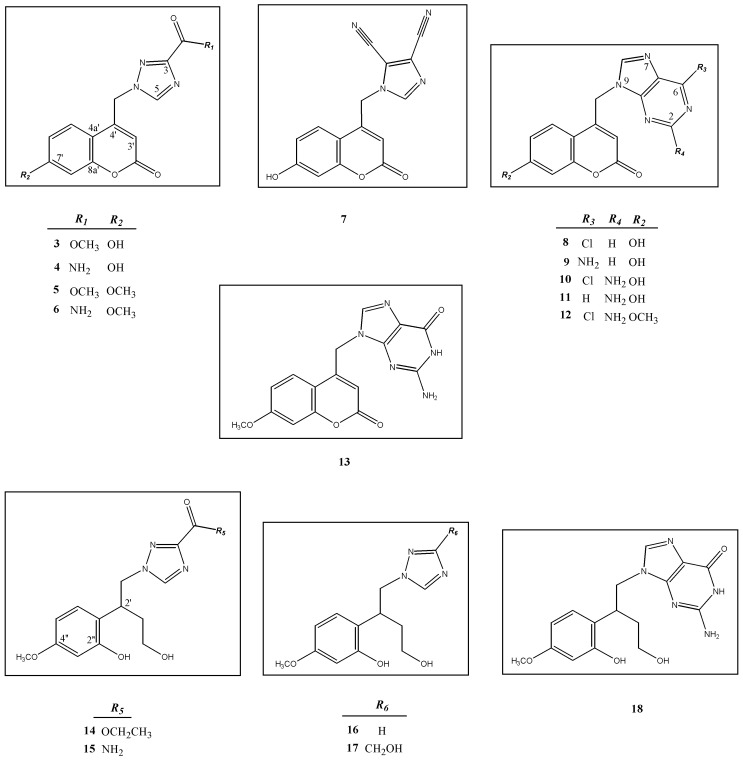
In light of these findings and based on our previous study [33], we efficiently synthesized a series of new 7-methoxy- or 7-hydroxycoumarin derivatives containing 1,2,4-triazole-3-carboxylic methyl ester (3 and5) or 3-carboxyamide moieties as heterocyclic constituents of ribavirin (compounds 4 and 6), 4,5-dicyanoimidazole (compound 7) or substituted purine derivatives (compounds 8–13), their open ring analogues (compounds 14–17) and acyclic nucleoside analogue (compound 18) (Figure 1).
Figure 1.
New coumarin derivatives containing 1,2,4-triazole (3–6), 4,5-dicyanoimidazole (7) and purine (8–13) moiety, their open ring analogues (14–17) as well as acyclic nucleoside analogue (18).
2. Results and Discussion
2.1. Chemistry
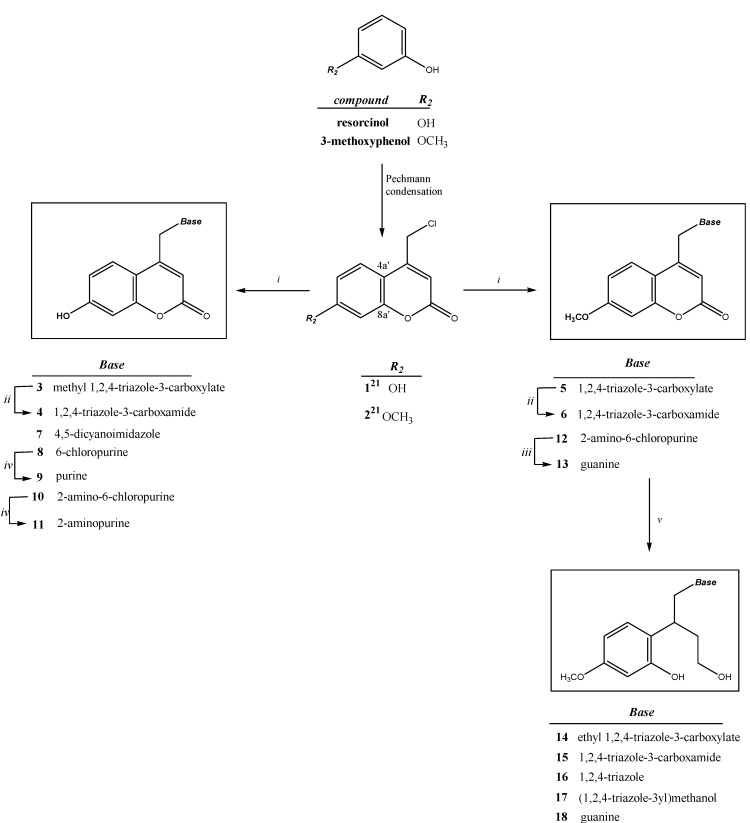
The syntheses of new structurally diverse 7'-methoxy- or 7'-hydroxycoumarin derivatives containing 1,2,4-triazole (compounds 3–6), 4,5-dicyanoimidazole (compound 7) and purine (compounds 8–13) moieties, their open ring analogues (compounds 14–17) and acyclic nucleoside analogue (compound 18) were carried out by the sequence of reactions shown in Scheme 1. These syntheses were performed by coupling of the synthetic precursors 4-(chloromethyl)-7-hydroxy-2H-chromen-2-one (1) or 4-(chloromethyl)-7-methoxy-2H-chromen-2-one (2) with appropriate heterocyclic bases. The synthesis of 4-chloromethylcoumarins 1 and 2 involving the Pechmann condensation was performed starting from resorcinol and 3-methoxyphenol, respectively, according to the pathway shown in Scheme 1 and as described previously [23].
Scheme 1.
Synthesis of new coumarin derivatives containing 1,2,4-triazole (3–6), 4,5-dicyanoimidazole (7) and purine (8–13) moiety, their open ring analogues (14–17) as well as acyclic nucleoside analogue (18).
Reagents and conditions: (i) DMF, NaH, 80 °C, nucleoside bases; (ii) gaseous NH3, MeOH, room temperature; (iii) 80% HCO2H, 100 °C; (iv) 80% HCO2H, 100 °C, then 29% aq. NH3, rt; (v) NaBH4, EtOH (dry), 70 °C.
Subsequent reduction of 5 and 6 with NaBH4 gave the corresponding open ring analogues 14–17, whereas reduction of 13 with NaBH4 gave the acyclic nucleoside analogue 18. During the reduction of the starting methyl ester 5 in ethanol solution with NaBH4 three products have been isolated, namely compounds 14, 16 and 17. Trans-esterification occured producing ethyl ester derivative 14. The methyl ester group of compound 5 was removed giving the open ring analogue 16. Reduction of methyl ester group of compound 5 occurred also giving open ring analogue 17 containing 1,2,4-triazole-yl-3-methanol moiety.
2.2. NMR Assignments
The structures of the newly synthesized compounds were deduced from the analysis of their 1H- and 13C-NMR and mass spectra. The assignment of 1H-NMR spectra was performed on the basis of the chemical shifts, substituent induced chemical shifts, signal intensities, magnitude and multiplicity of H-H coupling constants. The chemical shifts in 1H and 13C-NMR spectra (Table 1, Table 2 and Experimental) are in concordance with the proposed structures of the novel compounds and related coumarine derivatives [23].
2.3. Antiproliferative Effects
Compounds 3–18 were evaluated for their inhibitory activities against human tumor cell lines: HeLa (cervical carcinoma), MCF-7 (breast epithelial adenocarcinoma, metastatic), HepG2 (hepatocellular carcinoma), SW620 (colorectal adenocarcinoma, metastatic), as well as on normal (diploid) human fibroblasts (control cell line BJ) (Table 3). Table 2,Table 1. Of all evaluated compounds, only compound 6 containing a 1,2,4-triazole-3-carboxyamide moiety, the heterocyclic constituent of the ribavirin, showed moderate cytostatic activity against HeLa cells (IC50 = 35 µM), whereas compound 10 involving a modified guanine base showed moderate activity against HeLa (IC50 = 33 µM), HepG2 (IC50 = 25 µM) and SW620 (IC50 = 35 µM) cells.
Table 1.
1H-NMR (DMSO-d6) chemical shifts (δ/ppm) and H-H coupling constants (J/Hz) in 1H-NMR spectra for compounds 3–13 (for enumeration of atoms c.f. Figure 1).
| OH-7' | H-5 | H-8 | H-5' | H-6' | H-8' | NH2-2 | CH2-N | H-3' | OMe-7' | |
|---|---|---|---|---|---|---|---|---|---|---|
| 3 a | 10.72 (s, 1H) | 8.34 (s, 1H) | / | 7.72 (d, 1H, J = 8.8) | 6.87 (AB, dd, 1H, J = 2.3, 8.7) | 6.78 (d, 1H, J = 2.2) | / | 6.01 (s, 2H) | 5.30 (s, 1H) | / |
| 4 b | 10.72 (s, 1H) | 8.25 (s, 1H) | / | 7.74 (d, 1H, J = 8.7) | 6.85 (AB, dd, 1H, J = 2.0, 8.6) | 6.77 (d, 1H, J = 2.0) | / | 6.06 (s, 2H) | 5.26 (s, 1H) | / |
| 5 c | / | 8.09 (s, 1H) | / | 7.77 (d, 1H, J = 8.9) | 7.05 (AB, dd, 1H, J = 2.5, 8.6) | 7.01 (AB, dd, 1H, J = 2.6, 8.8) | / | 5.84 (s, 2H) | 5.76 (s, 1H) | 3.85 (s, 3H) |
| 6 d | / | 8.83 (s, 1H) | / | 7.73 (d, 1H, J = 8.9) | 7.07 (AB, dd, 1H, J = 2.0, 8.6) | 7.02 (AB, dd, 1H, J = 2.0, 8.8) | / | 5.81 (s, 2H) | 5.79 (s, 1H) | 3.87 (s, 3H) |
| 7 | 10.76 (s, 1H) | 8.45 (s, 1H) | / | 7.66 (d, 1H, J = 8.7) | 6.87 (AB, dd, 1H, J = 2.2, 8.7) | 6.80 (d, 1H, J = 2.1) | / | 5.82 (s, 2H) | 5.76 (s, 1H) | / |
| 8 d | 10.72 (s, 1H) | / | 8.82 (s, 1H) | 7.80 (d, 1H, J = 8.7) | 6.87 (AB, dd, 1H, J = 2.0, 8.6) | 6.78 (d, 1H, J = 2.1) | / | 5.81 (s, 2H) | 5.61 (s, 1H) | / |
| 9 f | 10.72 (s, 1H) | / | 8.10 (s, 1H) | 7.77 (d, 1H, J = 8.7) | 6.89 (AB, dd, 1H, J = 2.3, 8.7) | 6.81 (d, 1H, J = 2.2) | / | 5.65 (s, 2H) | 5.36 (s, 1H) | / |
| 10 | 10.72 (s, 1H) | / | 8.22 (s, 1H) | 7.79 (d, 1H, J = 8.7) | 6.87 (AB, dd, 1H, J = 2.2, 8.7) | 6.78 (d, 1H, J = 2.2) | 6.76 (s, 2H) | 5.55 (s, 2H) | 5.44 (s, 1H) | / |
| 11 g | 10.01 (s, 1H) | / | 7.78 (s, 1H) | 7.74 (d, 1H, J = 8.8) | 6.84 (AB, dd, 1H, J = 1.9, 8.7) | 6.76 (d, 1H, J = 2.2) | 6.75 (s, 2H) | 5.42 (s, 2H) | 5.23 (s, 1H) | / |
| 12 | / | / | 8.20 (s, 1H) | 7.85 (d, 1H, J = 8.8) | 7.01 (AB, dd, 1H, J = 2.0, 8.7) | 7.05 (d, 1H, J = 1.9) | 6.97 (s, 2H) | 5.56 (s, 2H) | 5.54 (s, 1H) | 3.87 (s, 3H) |
| 13 h | / | / | 7.78 (s, 1H) | 7.86 (d, 1H, J = 8.8) | 7.02 (AB, dd, 1H, J = 1.9, 8.7) | 7.06 (d, 1H, J = 1.9) | 6.53 (s, 2H) | 5.46 (s, 2H) | 5.41 (s, 1H) | 3.88 (s, 3H) |
a Compound 3: signal for COOCH3-triazole: 3.88 ppm (s, 3H); b Compound 4: signal for CONH2-triazole: 8.38 and 8.07 ppm (2 × s, 2 × 1H); c Compound 5: signal for COOCH3-triazole: 3.87 ppm (s, 3H); d Compound 6: signal for CONH2-triazole: 7.84 and 7.64 ppm (2 × s, 2 × 1H); e Compound 8: signal for H-2-purine: 8.80 ppm (s, 1H); f Compound 9: signal for NH2-6-purine: 7.97 ppm (s, 2H); H-2-purine: 8.10 ppm (s, 1H); g Compound 11: signal for H-6-purine: 8.45 ppm (s, 1H); h Compound 13: signal for NH-purine: 10.67 ppm (s, 1H).
Table 2.
1H-NMR (DMSO-d6) chemical shifts (δ/ppm) and H-H coupling constants (J/Hz) in 1H-NMR spectra for compounds 14–18 (for enumeration of atoms c.f. Figure 1).
| OH-2" | H-8 | H-5 | H-6" | H-5" | H-3" | OH-4' | OMe-4" | H-1' | H-2' | H-3' | H-4' | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14 a | 9.64 (s, 1H) | / | 8.11 (s, 1H) | 6.88 (AB, dd, 1H, J= 3.27, 8.43) | 6.36 (AB, dd, 1H, J = 2.34, 8.36) | 6.36 (d, 1H, J = 8.30) | 5.19 (t, 1H, J = 6.03) | 3.65 (s, 3H) | 3.17–3.24 (m, 2H) | 2.52–2.56 (m, 1H) | 1.80–1.61 (m, 2H) | 4.15–4.18 (m, 2H) |
| 15 b | 9.49 (s, 1H) | / | 8.25 (s, 1H) | 6.91 (d, 1H, J = 8.37) | 6.35 (d, 1H, J = 2.31) | 6.30 (AB, dd, 1H, J= 2.28, 8.34) | 5.11 (t, 1H, J = 6.01) | 3.65 (s, 3H) | 3.16–3.18 (m, 2H) | 2.32–2.35 (m, 1H) | 1.79–1.61 (m, 2H) | 4.37–4.40 (m, 2H) |
| 16 c | 9.47 (s, 1H) | / | 8.17 (s, 1H) | 6.87 (d, 1H, J = 8.40) | 6.34 (d, 1H, J = 2.28) | 6.28 (AB, dd, 1H, J = 2.28, 8.40) | 5.14 (t, 1H, J = 5.96) | 3.65 (s, 3H) | 3.25–3.17 (m, 2H) | 2.84–2.87 (m, 1H) | 1.79–1.61 (m, 2H) | 4.27–4.29 (m, 2H) |
| 17 d | 9.42 (s, 1H) | / | 8.07 (s, 1H) | 6.91 (d, 1H, J = 8.40) | 6.36 (d, 1H, J = 2.46) | 6.31 (AB, dd, 1H, J = 2.40, 8.34) | 5.11 (t, 1H, J = 6.06) | 3.66 (s, 3H) | 3.21–3.13 (m, 2H) | 2.33–2.37 (m, 1H) | 1.79–1.61 (m, 2H) | 4.34–4.37 (m, 2H) |
| 18 e | 9.46 (s, 1H) | 7.2 (s, 1H) | / | 6.92 (d, 1H, J = 8.34) | 6.32 (d, 1H, J = 1.95) | 6.29 (AB, dd, 1H, J = 2.35, 8.19) | 4.97 (t, 1H, J = 6.04) | 3.66 (s, 3H) | 3.26–3.17 (m, 2H) | 3.04–3.07 (m, 1H) | 1.82–1.62 (m, 2H) | 4.13– 4.16 (m, 2H) |
a Compound 14: signal for COOCH2CH3-triazole: 2.70 ppm (m, 2H); COOCH2CH3-triazole: 1.05 ppm (t, 3H,J = 7.1 Hz); b Compound 15: signal for CONH2-triazole: 7.69 and 7.50 ppm (2 × s, 2 × 1H); c Compound 16: signal for H-3: 7.88 ppm (s, 1H); d Compound 17: signal for OH-3-triazole: 4.37 ppm (d, 1H,J = 6.0 Hz); CH2-3-triazole: 4.30 (d, 2H, J = 7.4 Hz); e Compound 18: signal for NH-purine: 10.70 ppm (s, 1H); NH2-purine: 6.56 ppm (s, 2H).
These compounds showed no cytotoxic effects in normal fibroblasts. It appears that compounds 6 and 10 exert their effects in a different way that is probably attributable to a different genetic background of tumour cell lines. Compound 6 having a 1,2,4-triazole-3-carboxamide ligand is highly selective towards human cervix cancer (HeLa) cells. These cells harbor integrated Human Papillomavirus (HPV) genomes and express two viral oncogenes, E6 and E7, which inactivate the p53and pRB tumor suppressors. Compound 10 with a 2-amino-6-chloropurine ligand exerted noticeable cytostatic effect on other tumour cell lines as well, more precisely on hepatic carcinoma (HepG2) and colon cancer (SW620) cells both bearing mutations in the p53 gene probably through impairment od DNA synthesis. Similarly, literature data on 1,2,4-triazolylcoumarins indicate potential antitumor and anti-HIV activities of this class of compounds [34]. Similarly to compound 10 with chloropurine moiety, literature data report on chloropurine derivatives with cytostatic activity towards many cancer cell lines including colon cancer [35].
Table 3.
Inhibitory effects of compounds 3–18 on the growth of malignant tumor cell lines in comparison with their effects on the growth of normal diploid fibroblasts (BJ). The results are presented as IC50 values (μM).
| IC50a (μM) | |||||
|---|---|---|---|---|---|
| Substance No. | Cell lines | ||||
| HeLa | MCF-7 | HepG2 | SW620 | BJ | |
| 3 | >100 | >100 | >100 | >100 | >100 |
| 4 | >100 | >100 | >100 | >100 | >100 |
| 5 | >100 | >100 | >100 | >100 | >100 |
| 6 | 35.5 ± 13.5 | >100 | >100 | >100 | >100 |
| 7 | >100 | >100 | >100 | >100 | >100 |
| 8 | >100 | >100 | >100 | >100 | >100 |
| 9 | >100 | >100 | >100 | >100 | >100 |
| 10 | 34 ± 8.4 | >100 | 25.6 ± 12.6 | 35.4 ± 3.7 | >100 |
| 11 | >100 | >100 | >100 | >100 | >100 |
| 12 | >100 | >100 | >100 | >100 | >100 |
| 13 | >100 | >100 | >100 | >100 | >100 |
| 14 | >100 | >100 | >100 | >100 | >100 |
| 15 | >100 | >100 | >100 | >100 | >100 |
| 16 | >100 | >100 | >100 | >100 | >100 |
| 17 | >100 | >100 | >100 | >100 | >100 |
| 18 | >100 | >100 | >100 | >100 | >100 |
a IC50 represents the concentration of a drug that is required for 50% growth inhibition in vitro.
3. Experimental
3.1. General
The melting points (uncorrected) were determined with a Kofler micro hot-stage (Reichert, Vienna, Austria). Pre-coated Merck silica gel 60F-254 plates were used for thin layer chromatography (TLC) and the spots were detected under UV light (254 nm). Column chromatography (CLC) was performed using silica gel (0.063–0.2 mm, Sigma-Aldrich, Co., 3050 Spruce Street, St. Luis, MO 63103 USA); glass column was slurry-packed under gravity. Mass spectra were recorded on an Agilent 6410 instrument (Agilent Technoligies, Wilmington, NC, USA) equipped with electrospray interface and triple quadrupole analyzer (LC/MS/MS). High-performance liquid chromatography was performed on Agilent 1100 series system with UV detection (photodiode array detector) using Zorbax C18 reverse-phase analytical column (2.1 × 30 mm, 3.5 µm, Agilent). Structures of newly synthesized compounds were deduced on the basis of analysis of their 1H and 13C-NMR as well as their mass spectra and confirmed by elemental analysis. 1H and 13C-NMR spectra were acquired on a Bruker 300 MHz NMR spectrometer (Bruker Spectrospin, Rheinstetten, Germany). All data were recorded in solvent DMSO-d6 at 298 K and chemical shifts are referred to TMS. Individual resonances were assigned on the basis of their chemical shifts, signal intensities, multiplicity of resonances and H-H coupling constants. Elemental analyses were performed in the Central Analytic Service, Rudjer Bošković Institute Zagreb, Croatia, using a Perkin Elmer 2400 Elemental Analyser.
3.2. Procedures for the Preparation of Compounds
3.2.1. Compounds 3–13
Compounds 3–13 were prepared by the following general procedure: to a stirred solution containing methyl 1H-1,2,4-triazole-3-carboxylate, 1H-1,2,4-triazole, 1H-4,5-dicyanoimidazole, 2-amino-6-chloro-1H-purine or 6-chloro-1H-purine and NaH (1.5 equiv.) in DMF (30 mL), either 4-chloromethyl-7-hydroxycoumarin (1, 500 mg, 2.37 mmol) (for the preparation of compounds 3 and 4 and 7–11) or 4-chloromethyl-7-methoxycoumarin (2, 500 mg, 2.23 mmol) (for the preparation of compounds 4 and 5, 12 and 13) was added after 2h at room temperature under moisture-free conditions. The reaction mixture was stirred at 80 °C overnight, evaporated and the residual crude oil purified by column chromatography (CH2Cl2–MeOH = 50:1) to give pure compounds 3–13 as white solids.
3.2.2. Compound Data
Methyl 1-((7-hydroxy-2-oxo-2H-chromen-4-yl)methyl)-1,2,4-triazole-3-carboxylate (3).This compound was synthesized following the general procedure (Section 3.2.1) using methyl 1H-1,2,4-triazole-3-carboxylate (302 mg, 2.37 mmol) to give 3 (310 mg, 44%, m.p. = 216–218 °C). 13C-NMR (DMSO) δ/ppm: 162.1 (C-2a'), 160.3 (C=O-triazole), 158.2 (C-7a'), 155.4 (C-3-triazole), 151.3 (C-9a'), 145.4 (C-4a'), 140.5 (C-5-triazole), 126.4 (C-5a'), 113.7 (C-3a'), 109.9 (C-10a'), 108.4 (C-6a'), 103.0 (C-8a'), 53.5 (COOCH3-triazole), 50.8 (CH2-N); MS m/z 302.1 [M+1]. Elemental analysis. Calc. for C14H11N3O5: C 55.82, H 3.68, N 13.95, Found: C 55.96, H 3.74, N 13.97.
1-((7-Hydroxy-2-oxo-2H-chromen-4-yl)methyl)-1,2,4-triazole-3-carboxamide (4). Methyl 1-((7-hydroxy-2-oxo-2H-chromen-4-yl)methyl)-1,2,4-triazole-3-carboxylate (3, 200 mg, 0.66 mmol) was treated with NH3 (g) in MeOH (15 mL) solution at 0 °C in ice bath for 30 min. The resulting mixture was stirred at room temperature overnight. It was evaporated and the residual white solid was separated by column chromatography (CH2Cl2–MeOH = 40:1) to give white solids of 4 (151 mg, 79%, m.p. = 246–248 °C). 13C-NMR (DMSO) δ/ppm: 162.2 (C-2a'), 160.4 (C=O-triazole), 159.0 (C-7a'), 155.4 (C-3-triazole), 151.9 (C-9a'), 147.6 (C-4a'), 141.3 (C-5-triazole), 126.4 (C-5a'), 113.7 (C-3a'), 109.9 (C-10a'), 108.5 (C-6a'), 103.0 (C-8a'), 50.4 (CH2-N); MS m/z 287.1 [M+1]. Elemental analysis. Calc. for C13H10N4O4: C 54.55, H 3.52, N 19.57, Found: C 54.59, H 3.56, N 19.53.
Methyl 1-((7-Methoxy-2-oxo-2H-chromen-4-yl)methyl)-1,2,4-triazole-3-carboxylate (5). The compound was synthesized following the general procedure (Section 3.2.1) using methyl 1H-1,2,4-triazole-3-carboxylate (283 mg, 2.23 mmol) to give 5 (528 mg, 75%, m.p. = 234–237 °C). 13C-NMR (DMSO) δ/ppm: 162.8 (C-2a'), 159.7 (C=O-triazole), 160.8 (C-7a'), 155.1 (C-3-triazole), 150.6 (C-9a'), 149.6 (C-4a'), 141.7 (C-5-triazole), 125.8 (C-5a'), 112.4 (C-3a'), 110.6 (C-10a'), 110.3 (C-6a'), 101.2 (C-8a'), 56.0 (O-CH3), 52.2 (COOCH3-triazole), 49.2 (CH2-N); MS m/z 316.1 [M+1]. Elemental analysis. Calc. for C16H15N3O5: C 58.36, H 4.59, N 12.76, Found: C 58.42, H 4.61, N 12.81.
1-((7-Methoxy-2-oxo-2H-chromen-4-yl)methyl)-1,2,4-triazole-3-carboxamide (6). Methyl 1-((7-methoxy-2-oxo-2H-chromen-4-yl)methyl)-1,2,4-triazole-3-carboxylate (5, 500 mg, 1.59 mmol) was treated with NH3 (g) in MeOH (20 mL) solution at 0 °C in ice bath for 30 min. The resulting mixture was stirred at room temperature overnight. It was evaporated and the residual white solid was separated by column chromatography (CH2Cl2–MeOH = 40:1) to give white solids of 6 (402 mg, 84%, m.p. = 261–264 °C). 13C-NMR (DMSO) δ/ppm: 163.2 (C-2a'), 160.8 (C=O-triazole), 160.2 (C-7a'), 155.5 (C-3-triazole), 150.4 (C-9a'), 149.3 (C-4a'), 141.3 (C-5-triazole), 126.3 (C-5a'), 112.9 (C-3a'), 111.2 (C-10a'), 111.0 (C-6a'), 101.6 (C-8a'), 56.4 (O-CH3), 56.4 (CH2-N); MS m/z 301.1 [M+1]. Elemental analysis. Calc. for C14H12N4O4: C 56.00, H 4.03, N 18.66, Found: C 56.06, H 4.07, N 18.59.
1-((7-Hydroxy-2-oxo-2H-chromen-4-yl)methyl)-4,5-dicyanoimidazole (7). The compound was synthesized following the general procedure (Section 3.2.1) using 1H-4,5-dicyanoimidazole (280 mg, 2.37 mmol) to give 7 (489 mg, 71%, m.p. = 238–240 °C). 13C-NMR (DMSO) δ/ppm: 162.3 (C-2a'), 160.2 (C-7a'), 149.4 (C-9a'), 144.5 (C-4a'), 132.6 (C-5-imidazole), 126.3 (C-5a'), 122.7 and 122.9 (2 × C≡N-imidazole), 113.8 (C-3a'), 112.8 and 112.9 [(C-2+C-3)-imidazole)], 109.4 (C-10a'), 108.9 (C-6a'), 103.1 (C-8a'), 47.3 (CH2-N); MS m/z 293.1 [M+1]. Elemental analysis. Calc. for C15H8N4O3: C 61.65, H 2.76, N 19.17, Found: C 61.61, H 2.79, N 19.09.
1-((7-Hydroxy-2-oxo-2H-chromen-4-yl)methyl)-6-chloropurine (8). The compound was synthesized following the general procedure (Section 3.2.1) using 1H-6-chloropurine (367 mg, 2.37 mmol) to give 8 (512 mg, 66%, m.p. > 300 °C). 13C-NMR (DMSO) δ/ppm: 162.1 (C-2a'), 160.3 (C-7a'), 155.5 (C-9a'), 152.5 (C-4-purine), 152.4 (C-2-purine), 150.6 (C-6-purine), 149.9 (C-4a'), 143.6 (C-8-purine), 132.3 (C-5-purine), 126.4 (C-5a'), 113.7 (C-3a'), 109.8 (C-10a'), 109.2 (C-6a'), 103.1 (C-8a'), 44.0 (CH2-N); MS m/z 329.1 [M+1]. Elemental analysis. Calc. for C15H9ClN4O3: C 54.81, H 2.76, N 17.04, Found: C 54.76, H 2.72, N 17.08.
1-((7-Hydroxy-2-oxo-2H-chromen-4-yl)methyl)purine (9). Compound 8 (300 mg, 0.87 mmol) was heated in 85% aq formic acid (20 mL) at 100 °C for 3 h. Next, the mixture was evaporated and without further purification suspended in 90% aq EtOH (15 mL) and treated with 29% ammonia for 1h at room temperature, evaporated again and the residual solid was separated by column chromatography (CH2Cl2–MeOH = 30:1) to give 9 as white solid (197 mg, 73%, m.p. = 275–277 °C). 13C-NMR (DMSO) δ/ppm: 162.6 (C-2a'), 160.4 (C-7a'), 157.1 (C-9a'), 155.9 (C-4-purine), 151.8 (C-2-purine), 149.0 (C-4a'), 148.2 (C-6-purine), 145.1 (C-8-purine), 135.2 (C-5-purine), 126.2 (C-5a'), 113.9 (C-3a'), 109.6 (C-10a'), 108.3 (C-6a'), 103.1 (C-8a'), 43.7 (CH2-N); MS m/z 295.1 [M+1]. Elemental analysis. Calc. for C15H10N4O3: C 61.22, H 3.43, N 19.04, Found: C 61.13, H 3.39, N 19.07.
1-((7-Hydroxy-2-oxo-2H-chromen-4-yl)methyl)-2-amino-6-chloropurine (10). The compound was synthesized following the general procedure (Section 3.2.1) using 1H-2-amino-6-chloropurine (402 mg, 2.73 mmol) to give 10 (512 mg, 55%, m.p. > 300 °C). 13C-NMR (DMSO) δ/ppm: 162.1 (C-2a'), 160.5 (C-7a'), 160.3 (C-9a'), 154.6 (C-4-purine), 151.3 (C-2-purine), 150.9 (C-6-purine), 150.3 (C-4a'), 142.9 (C-8-purine), 126.3 (C-5a'), 125.9 (C-5-purine), 113.8 (C-3a'), 109.8 (C-10a'), 108.3 (C-6a'), 103.1 (C-8a'), 43.4 (CH2-N); MS m/z 343,72 [M+1]. Elemental analysis. Calc. for C15H10ClN5O3: C 52.41, H 2.93, N 20.37, Found: C 52.47, H 2.97, N 20.31.
1-((7-Hydroxy-2-oxo-2H-chromen-4-yl)methyl)-2-aminopurine (11). Compound 10 (400 mg, 1.16 mmol) was heated in 85% aq. formic acid (20 mL) at 100 °C for 3 h. Next, the mixture was evaporated and without further purification suspended in 90% aq. EtOH (15 mL) and treated with 29% ammonia for 1h at room temperature. The solution was evaporated and the residual solid was separated by column chromatography (CH2Cl2–MeOH = 40:1) to give 11 as white solid (197 mg, 52%, m.p. > 300 °C). 13C-NMR (DMSO) δ/ppm: 163.5 (C-2a'), 160.6 (C-7a'), 157.4 (C-9a'), 155.6 (C-4-purine), 150.9 (C-2-purine), 149.3 (C-6-purine), 152.4 (C-4a'), 153.4 (C-8-purine), 126.1 (C-5a'), 126.0 (C-5-purine), 114.2 (C-3a'), 109.1 (C-10a'), 107.2 (C-6a'), 103.1 (C-8a'), 43.1 (CH2-N); MS m/z 309,28 [M+1]. Elemental analysis. Calc. for C15H11N5O3: C 58.25, H 3.58, N 22.64, Found: C 58.18, H 3.54, N 22.69.
1-((7-Methoxy-2-oxo-2H-chromen-4-yl)methyl)-2-amino-6-chloropurine (12). The compound was synthesized following the general procedure (Section 3.2.1) using 1H-2amino-6-chloropurine (378 mg, 2.23 mmol) to give 12 (328 mg, 41%, m.p. > 300 °C). 13C-NMR (DMSO) δ/ppm: 162.8 (C-2a'), 160.0 (C-7a'), 159.7 (C-9a'), 155.0 (C-4-purine), 150.6 (C-2-purine), 150.1 (C-6-purine), 149.8 (C-4a'), 151.9 (C-8-purine), 125.6 (C-5a'), 125.4 (C-5-purine), 112.5 (C-3a'), 109.4 (C-10a'), 109.0 (C-6a'), 101.1 (C-8a'), 56.0 (O-CH3), 42.9 (CH2-N); MS m/z 357,75 [M+1]. Elemental analysis. Calc. for C16H12ClN5O3: C 53.72, H 3.38 N 19.58, Found: C 53.76, H 3.42, N 19.61.
1-((7-Methoxy-2-oxo-2H-chromen-4-yl)methyl)guanine (13).Compound 12 (500 mg, 1.40 mmol) was heated in 85% aq formic acid (20 mL) at 100 °C for 3 h. Next, the mixture was evaporated and the residual solid was separated by column chromatography (CH2Cl2–MeOH = 30:1) to give 13 as white solid (197 mg, 52%, m.p. > 300 °C). 13C-NMR (DMSO) δ/ppm: 162.8 (C-2a'), 160.9 (C-7a'), 159.8 (C-9a'), 156.9 (C-6-purine), 156.7 (C-4-purine), 154.0 (C-2-purine), 151.5 (C-4a'), 151.3 (C-8-purine), 125.6 (C-5a'), 121.9 (C-5-purine), 112.5 (C-3a'), 110.5 (C-10a'), 108.6 (C-6a'), 101.1 (C-8a'), 56.0 (O-CH3), 42.7 (CH2-N); MS m/z 340.1 [M+1]. Elemental analysis. Calc. for C16H13N5O4: C 56.64, H 3.86, N 20.64, Found: C 56.59, H 3.82, N 20.67.
3.2.3. Compounds 14–18
Compounds 14–18 were prepared according to the following general procedure: compounds 5, 6 and 12, 13 were treated with NaBH4 (3 equiv.) in EtOH (20 mL) at 70 °C for 5 h. The reaction mixture were evaporated and the residual oils were separated by column chromatography (CH2Cl2–MeOH = 10:1) to give colorless oils of the corresponding 1,2,4-triazole (compound 14–17) and guanine (compound 18) derivatives.
3.2.4. Compound Data
Ethyl 1-(4-hydroxy-2-(2-hydroxy-4-methoxyphenyl)butyl)-1,2,4-triazole-3-carboxylate (14). 1-(4-hydroxy-2-(2-hydroxy-4-methoxyphenyl)butyl)-1,2,4-triazole (16) and ethyl 1-(4-hydroxy-2-(2-hydroxy-4-methoxyphenyl)butyl)-1,2,4-triazole-3-hydroxymethyl (17). The compounds were synthesized following the general procedure (Section 3.2.3) using 1-((7-methoxy-2-oxo-2H-chromen-4-yl)methyl)-1,2,4-triazole-3-carboxylate (5, 1,000 mg, 3.17 mmol) to give 14 (289 mg, 31%), 16 (256 mg, 28%) and 17 (153 mg, 17%).
Compound 14: 13C-NMR (DMSO) δ/ppm: 171.7 (C=O-triazole), 163.9 (C-3-triazole), 159.5 (C-4"), 156.5 (C-2"), 145.2 (C-5-triazole), 129.7 (C-6"), 119.6 (C-1"), 104.7 (C-5"), 101.8 (C-3"), 66.3 (CH2-triazole), 59.1 (C-4'), 57.3 (C-1'), 55.2 (O-CH3), 53.0 (C-3'), 35.0 (C-2'), 16.7 (CH3-triazole); MS m/z 336.2 [M+1]. Elemental analysis. Calc. for C16H21N3O5: C 57.30, H 6.31, N 12.53, Found: C 57.21, H 6.27, N 12.45.
Compound 16: 13C-NMR (DMSO) δ/ppm: 158.6 (C-4"), 156.1 (C-2"), 151.6 (C-3-triazole), 144.1 (C-5-triazole), 129.2 (C-6"), 119.1 (C-1"), 104.2 (C-5"), 101.3 (C-3"), 59.9 (C-4'), 58.7 (C-1'), 54.7 (O–CH3), 52.5 (C-3'), 34.5 (C-2'); MS m/z 264.1 [M+1]. Elemental analysis. Calc. for C13H17N3O3: C 59.30, H 6.51, N 15.96, Found: C 59.36, H 6.46, N 15.91.
Compound 17: 13C-NMR (DMSO) δ/ppm: 149.2 (C-3-triazole), 156.6 (C-4"), 156.0 (C-2"), 144.7 (C-5-triazole), 129.7 (C-6"), 119.3 (C-1"), 104.8 (C-5"), 101.8 (C-3"), 59.3 (C-4'), 59.2 (C-1'), 57.3 (CH2-triazole), 55.2 (O-CH3), 53.7 (C-3'), 35.0 (C-2'); MS m/z 294.1 [M+1]. Elemental analysis. Calc. for C14H19N3O: C 57.33, H 6.53, N 14.33, Found: 57.27, H 6.49, N 14.29.
1-(4-Hydroxy-2-(2-hydroxy-4-methoxyphenyl)butyl)-1,2,4-triazole-3-carboxamide (15). This compound was synthesized following the general procedure (Section 3.2.3) using 1-((7-methoxy-2-oxo-2H-chromen-4-yl)methyl)-1,2,4-triazole-3-carboxamide 6 (1500 mg, 5.0 mmol) to give 15 (118 mg, 5.5%). 13C-NMR (DMSO) δ/ppm: 163.9 (C=O-triazole), 161.1 (C-3-triazole), 159.5 (C-4"), 156.1 (C-2"), 145.1 (C-5-triazole), 129.1 (C-6"), 119.3 (C-1"), 104.2 (C-5"), 101.4 (C-3"), 59.1 (C-4'), 61.4 (C-1'), 60.2 (O-CH3), 53.0 (C-3'), 35.0 (C-2'); MS m/z 307.1 [M+1]. Elemental analysis. Calc. for C14H18N4O4: C 54.89, H 5.92, N 18.29, Found: C 54.82, H 5.87, N 18.22.
1-(4-Hydroxy-2-(2-hydroxy-4-methoxyphenyl)butyl)guanine (18). This compound was synthesized following the general procedure (Section 3.2.3) using 1-((7-methoxy-2-oxo-2H-chromen-4-yl)methyl)-guanine (13, 483 mg, 1.42 mmol) to give 18 (11 mg, 2.2%). 13C-NMR (DMSO) δ/ppm: 169.2 (C-5-purine), 160.3 (C-6-purine), 159.1 (C-2-purine), 157.2 (C-4"), 156.8 (C-2"), 153.9 (C-4-purine), 142.7 (C-8-purine), 129.8 (C-6"), 119.7 (C-1"), 104.7 (C-5"), 101.8 (C-3"), 60.2 (C-4'), 59.3 (C-1'), 55.2 (O-CH3), 47.2 (C-3'), 35.3 (C-2'); MS m/z 346.1 [M+1]. Elemental analysis. Calc. for C16H19N5O4: C 55.64, H 5.55, N 20.28, Found: C 55.58, H 5.53, N 20.31.
3.2.5. Cytostatic Activity Assay
This study was set out to examine the anti-proliferative effects of coumarin derivates (3–18) on human tumor cell lines and normal (diploid) human fibroblasts.
Cell culturing: Human cell lines HeLa (cervical carcinoma), SW620 (colorectal adenocarcinoma, metastatic), MCF-7 (breast epithelial adenocarcinoma, metastatic), HepG2 (hepatocellular carcinoma) and BJ (normal diploid human fibroblasts) were cultured as monolayers and maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin in a humidified atmosphere with 5% CO2 at 37 °C.
Proliferation assay: The cell lines were inoculated into a series of standard 96-well microtiter plates on day 0 at seeding density of 3,000–6,000 cells per well depending upon their specific doubling times. Freshly prepared dilutions of test compounds in culture medium in the concentration range 1 × 10−8–1 × 10−4 M were added to the microtiter plates, and the cells were grown for further 72 h. The solvent (DMSO) was also tested for its potential inhibitory activity by adjusting its concentration to the values used for the preparation of the working concentrations (DMSO concentration never exceeded 0.1%). After 72 h of incubation, the cell growth rate was evaluated by performing the MTT assay [36]: experimentally determined absorbance values were transformed into the cell percentage growth (PG) using the formulas proposed by NIH and described previously [37]. This method directly relies on control cells behaving normally at the day of assay commencement because it compares the growth of treated cells with that of untreated cells in control wells on the same plate. The results are therefore a percentile difference from the calculated expected value. The IC50 value for each compound was calculated from dose-response curves using linear regression analysis by fitting the mean test concentrations that give PG values above and below the reference value. If, however, all of the tested concentrations produce PGs exceeding the respective reference level of effect (e.g., PG value of 50) for a given cell line, the highest tested concentration is assigned as the default value (in the screening data report that default value is preceded by a “>” sign). Each test point was performed in quadruplicate in three individual experiments. The results were statistically analyzed (ANOVA, Tukey post-hoc test at p < 0.05).
4. Conclusions
A series of novel coumarin derivatives containing heterocyclic bases 1,2,4-triazole (compounds 3–6), 4,5-dicyanoimidazole (compound 7) and purine (compounds 8–13) linked via methylenic spacer to 7'-methoxy- or 7'-hydroxycoumarin moieties were synthesized and evaluated for their potential cytostatic activity. The biological evaluation of these coumarin derivatives generally showed very weak antiproliferative effects for all tested compounds and no cytotoxicity on normal human fibroblasts. Only two compounds (compounds 6 and 10) exerted stronger effects at higher tested concentrations. This effect was non-specific and completely absent in the micromolar range. It appears that compounds 6 and 10 exert their effects in a different way that is probably attributable to a different genetic background of the tumour cell lines. Compound 6 having a 1,2,4-triazole-3-carboxamide ligand is highly selective towards human cervix cancer (HeLa) cells. These cells harbour integrated Human Papillomavirus (HPV) genomes and express two viral oncogenes, E6 and E7, which inactivate the p53 and pRB tumour suppressors. Compound 10 with a 2-amino-6-chloropurine ligand exerted noticeable cytostatic effect on other tumour cell lines as well, more precisely on hepatic carcinoma (HepG2) and colon cancer (SW620) cells both bearing mutations in the p53 gene probably through impairment of DNA synthesis. Similarly, literature data on 1,2,4-triazolylcoumarins indicate potential antitumor and anti-HIV activities of this class of compounds [34]. Like compound 10 with a chloropurine moiety, the literature data report on chloropurine derivatives with cytostatic activity towards many cancer cell lines including colon cancer [35].
Acknowledgements
Support for this study was provided by the Ministry of Science, Education and Sports of the Republic of Croatia (Projects Nos. 125-0982464-2922, 335-0982464-239, 335-0000000-3532).
Footnotes
Sample Availability: Samples of all compounds are available from the authors.
References
- 1.Egan D., O’kennedy R., Moran E., Cox D., Prosser E., Thornes R.D. The Pharmacology, Metabolism, Analysis, and Applications of Coumarin and Coumarin-Related Compounds. Drug Metab. Rev. 1990;22:503–529. doi: 10.3109/03602539008991449. [DOI] [PubMed] [Google Scholar]
- 2.Borges F., Roleira F., Milhazes N., Santana L., Uriarte E. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Curr. Med. Chem. 2005;12:887–916. doi: 10.2174/0929867053507315. [DOI] [PubMed] [Google Scholar]
- 3.Harvey R.G., Cortez C., Ananthanarayan T.P., Schmolka S. A new coumarin synthesis and its utilization for the synthesis of polycyclic coumarin compounds with anticarcinogenic properties. J. Org. Chem. 1988;53:3936–3943. [Google Scholar]
- 4.Kostova I., Raleva S., Genova P., Argirova R. Structure-Activity Relationships of Synthetic Coumarins as HIV-1 Inhibitors. Bioinorg. Chem. Appl. . 2006 doi: 10.1155/BCA/2006/68274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moffet R.S. Central Nervous System Depressants. VII.1 Pyridyl Coumarins. J. Med. Chem. 1964;7:446–449. doi: 10.1021/jm00334a010. [DOI] [PubMed] [Google Scholar]
- 6.Al-Haiza M.A., Mostafa M.S., El-Kady M.Y. Synthesis and Biological Evaluation of Some New Coumarin Derivatives. Molecules. 2003;8:275–286. doi: 10.3390/80200275. [DOI] [Google Scholar]
- 7.Musicki B., Periers A.-M., Laurin P., Ferroud D., Benedetti Y., Lachaud S., Chatreaux F., Haesslein J.L., Iltis A., Pierre C., et al. Improved antibacterial activities of coumarin antibiotics bearing 5',5'-dialkylnoviose: Biological activity of RU79115. Bioorg. Med. Chem. Lett. 2000;10:1695–1699. doi: 10.1016/s0960-894x(00)00304-8. [DOI] [PubMed] [Google Scholar]
- 8.Fylaktakidou K.C., Hadjipavlou-Litina D.J., Litinas K.E., Nicolaides D.N. Natural and Synthetic Coumarin Derivatives with Anti-Inflammatory/Antioxidant Activities. Curr. Pharm. Des. 2004;10:3813–3833. doi: 10.2174/1381612043382710. [DOI] [PubMed] [Google Scholar]
- 9.Bucolo C., Cuzzocrea S., Mazzon E., Caputi A.P. Effects of Cloricromene, a Coumarin Derivative, on Endotoxin-Induced Uveitis in Lewis Rats. Invest. Ophthalmol. Vis. Sci. 2003;44:1178–1184. doi: 10.1167/iovs.02-0559. [DOI] [PubMed] [Google Scholar]
- 10.Bucolo C., Ward K.W., Mazzon E., Cuzzocrea S., Drago F. Protective Effects of a Coumarin Derivative in Diabetic Rats. Invest. Ophthalmol. Vis. Sci. 2009;50:3846–3852. doi: 10.1167/iovs.08-3328. [DOI] [PubMed] [Google Scholar]
- 11.Payá M., Halliwell B., Hoult J.R. Interactions of a series of coumarins with reactive oxygen species: Scavenging of superoxide, hypochlorous acid and hydroxyl radicals. Biochem. Pharmacol. 1992;44:205–214. doi: 10.1016/0006-2952(92)90002-z. [DOI] [PubMed] [Google Scholar]
- 12.Marshall M.E., Ryles M., Butler K., Weiss L. Treatment of advanced renal cell carcinoma (RCC) with coumarin and cimetidine: long-term follow-up of patients on a phase I trial. J. Cancer Res. Clin. Oncol. 1994;120:535–538. [Google Scholar]
- 13.Marshall M.E., Mohler J.L., Edmonds K., Williams B., Butler K., Ryles M., Weiss L., Urban D., Bueschen A., Markiewicz M. An updated review of the clinical development of coumarin (1,2-benzopyrone) and 7-hydroxycoumarin. J. Cancer Res. Clin. Oncol. 1994;120:S39–S42. doi: 10.1007/BF01377124. [DOI] [PubMed] [Google Scholar]
- 14.Stanchev S., Momekov G., Jensen F., Manolov I. Synthesis, computational study and cytotoxic activity of new 4-hydroxycoumarin derivatives. Eur. J. Med. Chem. 2008;43:694–706. doi: 10.1016/j.ejmech.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Thornes R.D., Daly L., Lynch G., Breslin B., Browne H., Browne H.Y., Corrigan T., Daly P., Edwards G., Gaffney E., et al. Treatment with coumarin to prevent or delay recurrence of malignant melanoma. J. Cancer Res. Clin. Oncol. 1994;120:S32–S34. doi: 10.1007/BF01377122. [DOI] [PubMed] [Google Scholar]
- 16.Marshall M.E., Butler K., Fried A. Phase I evaluation of coumarin (1,2-benzopyrone) and cimetidine in patients with advanced malignancies. Mol. Biother. 1991;3:170–178. [PubMed] [Google Scholar]
- 17.Mohler J.L., Gomella L.G., Crawford E.D., Glode L.M., Zippe C.D., Fair W.R., Marshall M.E. Phase II evaluation of coumarin (1,2-benzopyrone) in metastatic prostatic carcinoma. Prostate. 1992;20:123–131. doi: 10.1002/pros.2990200208. [DOI] [PubMed] [Google Scholar]
- 18.Jung J.-C., Kim J.-C., Park O.-S. Simple and cost-effective syntheses of 4-hydroxycoumarins. Synth. Commun. 1999;29:3587–3595. doi: 10.1080/00397919908085993. [DOI] [Google Scholar]
- 19.Jacquot Y., Bermont L., Giorgi H., Refouvelet B., Adessi G., Daubrosse E., Xicluna A. Substituted benzopyranobenzothiazinones. Synthesis and estrogenic activity on MCF-7 breast carcinoma cells. Eur. J. Med. Chem. 2001;36:127–136. doi: 10.1016/S0223-5234(00)01207-1. [DOI] [PubMed] [Google Scholar]
- 20.Budzisz E., Brzezinska E., Krajewska U., Rozalski M. Cytotoxic effects, alkylating properties and molecular modelling of coumarin derivatives and their phosphonic analogues. Eur. J. Med. Chem. 2003;38:597–603. doi: 10.1016/S0223-5234(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 21.Musa M.A., Cooperwood J.S., Khan M.O.F. A Review of Coumarin Derivatives in Pharmacotherapy of Breast Cancer. Curr. Med. Chem. 2008;15:2664–2679. doi: 10.2174/092986708786242877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steffen U.S., Weber B., Siegers C. Antitumor-activities of coumarin, 7-hydroxy-coumarin and its glucuronide in several human tumor cell lines. Res. Commun. Mol. Pathol. Pharmacol. 1998;99:193–206. [PubMed] [Google Scholar]
- 23.Zagotto G., Gia O., Baccichetti F., Uriarte E., Palumbo M. Synthesis and Photobiological Properties of 4-Hydroxymethyl-4'-methylpsoralen Derivatives. Photochem. Photobiol. 1993;58:486–491. doi: 10.1111/j.1751-1097.1993.tb04919.x. [DOI] [PubMed] [Google Scholar]
- 24.McCulloch P., George W.D. Warfarin inhibits metastasis of Mtln3 rat mammary carcinoma without affecting primary tumour growth. Br. J.Cancer. 1989;59:179–183. doi: 10.1038/bjc.1989.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itokawa H., Yun J.S., Morita H., Takeya K. Cytotoxic coumarins from roots of Angelica gigas Nakai. Nat. Med. 1994;48:334–335. [Google Scholar]
- 26.Kolodziej H., Kayser O., Woerdenbag H.J., Van Uden W., Pras N. Examination for anti-Human Immunodeficienvy Virus—Type 1(HIV-1) effect of three 4-hydroxycoumarin (4-hc) derivatives. Z.Naturforsch. C. 1997;52:240–244. doi: 10.1515/znc-1997-3-416. [DOI] [PubMed] [Google Scholar]
- 27.Zhao H., Neamati N., Hong H., Mazumder A., Wang S., Sunder S., Milne G.W.A., Pommier Y., Burke T.R. Coumarin-Based Inhibitors of HIV Integrase. J. Med. Chem. 1997;40:242–248. doi: 10.1021/jm960450v. [DOI] [PubMed] [Google Scholar]
- 28.Singhal N., Sharma P.K., Dudhe R., Kumar N. Recent advancement of triazole derivatives and their biological significance. J. Chem. Pharm. Res. 2011;3:126–133. [Google Scholar]
- 29.Amr A.E., Nermien M.S., Abdulla M.M. Synthesis, reactions, and anti-inflammatory activity of heterocyclic systems fused to a thiophene moiety using citrazinic acid as synthon. Monatsh. Chem. 2007;138:699–707. [Google Scholar]
- 30.Fujiwara N., Nakajima T., Ueda Y., Fujita H., Kawakami H. Novel piperidinylpyrimidine derivatives as inhibitors of HIV-1 LTR activation. Bioorg. Med. Chem. 2008;16:9804–9816. doi: 10.1016/j.bmc.2008.09.059. [DOI] [PubMed] [Google Scholar]
- 31.Ballell L., Field R.A., Chung G.A.C., Young R.J. New thiopyrazolo[3,4-d]pyrimidine derivatives as anti-mycobacterial agents. Bioorg. Med. Chem. Lett. 2007;17:1736–1740. doi: 10.1016/j.bmcl.2006.12.066. [DOI] [PubMed] [Google Scholar]
- 32.Wagner E., Al-Kadasi K., Zimecki M., Sawka-Dobrowolska W. Synthesis and pharmacological screening of derivatives of isoxazolo[4,5-d]pyrimidine. Eur. J. Med. Chem. 2008;43:2498–2504. doi: 10.1016/j.ejmech.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 33.Završnik D., Muratović S., Makuc D., Plavec J., Cetina M., Nagl A., DeClercq E., Balzarini J., Mintas M. Benzylidene-bis-(4-hydroxycoumarin) and benzopyranocoumarin derivatives: synthesis, 1H/13C-NMR conformational and X-ray crystal structure studies and in vitro antiviral activity evaluations. Molecules. 2011;16:6023–6040. doi: 10.3390/molecules16076023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Soud Y.A., Al-Masoudi I.A., Saeed B., Beifuß U., Al-Masoudi N.A. Synthesis of new 1H-1,2,4-triazolylcoumarins and theirantitumor and anti-HIVactivities. Chem. Heterocycl. Comp. 2006;42:583–590. [Google Scholar]
- 35.Moon H.R., Kim H.O., Lee S.K., Choi W.J., Chun M.W., Jeong L.S. Synthesis and biological evaluation of novel thiapio dideoxynucleosides. Bioorg. Med. Chem. 2002;10:1499–1507. doi: 10.1016/s0968-0896(01)00417-5. [DOI] [PubMed] [Google Scholar]
- 36.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 37.Gazivoda T., Raić-Malić S., Krištafor V., Makuc D., Plavec J., Bratulic S., Kraljević Pavelić S., Pavelić K., Naesens L., Andrei G., et al. Synthesis, Cytostatic and Anti-HIV Evaluations of the New Unsaturated Acyclic C-5 Pyrimidine Nucleoside Analogues. Bioorg. Med. Chem. 2008;16:5624–5634. doi: 10.1016/j.bmc.2008.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]