Abstract
Adult male and female GFAP-TK transgenic rats experienced six weeks of chronic intermittent ethanol vapor inhalation (CIE). During the last week of CIE, a subset of male and female TK rats were fed with Valcyte to ablate neural progenitor cells (NPCs). Seventy-two hours after CIE cessation, all CIE and age matched ethanol naïve controls experienced auditory trace fear conditioning (TFC). Twenty-four hours later all animals were tested for cue-mediated retrieval in the fear context. Adult male CIE rats showed a significant burst in NPCs paralleled by reduction in fear retrieval compared to naïve controls and Valcyte treated CIE rats. Adult female CIE rats did not show a burst in NPCs and showed similar fear retrieval compared to naïve controls and Valcyte treated CIE rats, indicating that CIE-mediated impairment in fear memory and its regulation by NPCs was sex dependent. Valcyte significantly reduced Ki-67 and NeuroD labeled cells in the dentate gyrus (DG) in both sexes, demonstrating a role for NPCs in reduced fear retrieval in males. Valcyte prevented adaptations in GluN2A receptor expression and synaptoporin density in the DG in males, indicating that NPCs contributed to alterations in plasticity-related proteins and mossy fiber projections that were associated with reduced fear retrieval. These data suggest that DG NPCs born during withdrawal and early abstinence from CIE are aberrant, and could play a role in weakening long-term memory consolidation dependent on the hippocampus.
Keywords: NeuroD, Ki-67, CIE, GluN, Synaptoporin, Trace Fear Conditioning
Introduction
Moderate to severe alcohol use disorder has been reported to result in cognitive deficits in humans (Brandt et al., 1983; Glenn & Parsons, 1991; Sullivan et al., 2000). Clinical studies have identified the deleterious effects of chronic alcohol exposure in the hippocampus, which has been shown to be particularly sensitive to alcohol-induced damage (Bengochea & Gonzalo, 1990; Sullivan et al., 1995; Durazzo et al., 2011). However, what is less well known is how neuroadaptations in the hippocampus produced by alcohol contribute to alcohol-induced behavioral deficits dependent on the hippocampus.
Pavlovian conditioning is a form of associative learning where a neutral conditional stimulus (CS; e.g. tone) becomes associated with an aversive unconditional stimulus (US; e.g. footshock). Through pairings of the two stimuli, the CS comes to elicit a conditional response (CR). Trace fear conditioning (TFC) is a procedure where termination of the CS precedes onset of the US. That is, a "trace interval" is imposed between the CS and US; thus, the two stimuli are temporally discontiguous. It has been established that TFC with a trace interval greater than 10s depends on the hippocampus (Huerta et al., 2000; McEchron et al., 2000; Quinn et al., 2002; Chowdhury et al., 2005). Notably, when adult rats that experienced ethanol neonatally are tested on a TFC paradigm, they demonstrate hippocampus-specific memory impairments (Goodfellow et al., 2016). These impairments are paralleled by neuroadaptations in hippocampal glutamate receptor function and signaling (Goodfellow et al., 2016), suggesting that ethanol-induced hippocampal glutamatergic neuroadaptations, a form of hippocampal plasticity, could contribute to ethanol-induced impairments in TFC (Bast et al., 2003; Szapiro et al., 2003).
The adult hippocampus also harbors neural stem cells and these cells generate neural progenitor cells (NPCs; Ki-67+ cells) that give rise to transiently amplifying neuroblasts and immature neurons (NeuroD+ cells) that mature into granule cell neurons in the dentate gyrus (DG) through a process called neurogenesis (Enikolopov et al., 2015; Goncalves et al., 2016). Adult neurogenesis in the hippocampus contributes to hippocampal plasticity and certain hippocampal functions (Toni et al., 2008; Ming & Song, 2011; Sahay et al., 2011; Spalding et al., 2013; Frankland & Josselyn, 2016). For example, types of learning that depend on the hippocampus, including TFC, increase the number of immature neurons (doublecortin+ or NeuroD+ cells) without enhancing proliferation of NPCs (Ki-67+ cells or 2-hour-old bromodeoxyuridine+ cells), suggesting that NeuroD+ young neurons are involved in the formation of trace memories (Gould et al., 1999). Furthermore, mechanistic studies show that young neurons in the hippocampus play a role in TFC, with studies in adult rats suggesting neurogenesis is needed for formation of trace memories involving fearful stimuli (Shors et al., 2001; Shors et al., 2002; Achanta et al., 2009).
In the context of ethanol experience, several widely accepted rodent models of moderate to severe alcohol use disorder demonstrate reduction of neurogenesis during ethanol experience (Nixon, 2006; Mandyam & Koob, 2012; Staples & Mandyam, 2016). One such model, namely the chronic intermittent ethanol vapor exposure (CIE) model, implements daily cycles of intoxication via ethanol vapors and withdrawal to induce clinical signs of alcoholism, such as somatic withdrawal symptoms and escalated ethanol drinking in rats (Valdez et al., 2002; O'Dell et al., 2004). Proliferation (Ki-67+cells), differentiation (doublecortin+ cells) and neurogenesis of newly born neurons is also hindered in adult rats that experience CIE (Richardson et al., 2009; Hansson et al., 2010); however, this suppression is transient, and forced abstinence from CIE produces a rebound or burst effect observed as increases in NPCs (Hansson et al., 2010; Somkuwar et al., 2016). Few studies have investigated the capacity of the NPCs born during the rebound effect to survive and express neuronal and glial markers, and findings indicate reduced stability and neurogenesis of NPCs born during excessive proliferation (Somkuwar et al., 2016). We therefore hypothesize that NPCs born during forced abstinence from CIE are aberrant and contribute to memory impairments dependent on the hippocampus. We used the pharmacogenetic rat model (Snyder et al., 2016) in which NPCs can be selectively and inducibly ablated. Our data demonstrates that NPCs born during the proliferative burst in abstinent CIE male rats contribute to reduced fear retrieval when tested in a TFC paradigm, and inhibiting this process assisted with maintaining intact fear responses. Our data also demonstrates sex specific effects of this phenomena as females did not demonstrate burst of NPCs during withdrawal and abstinence from CIE and had intact fear responses.
Methods
Animals:
Transgenic rats expressing HSV-TK under the human GFAP promoter (GFAP-TK) were generated on a Long–Evans background (Snyder et al., 2016). These rats were bred at the Scripps Research Institute. Rats were weaned at 21–24 d of age, pair-housed, and genotyped by PCR (TransnetYX). The rats were housed two-three/cage in a temperature-controlled (22°C) vivarium on a 12 h/12 h light/dark cycle (lights on 8:00 P.M- 8:00 A.M.). All procedures were performed during the dark phase of the light/dark cycle. Food and water access was available ad libitum. All rats weighed approximately 180-250 g and were 8 weeks old at the beginning of the study. All experimental procedures were carried out in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication number 85–23, revised 1996) and approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Chronic Intermittent Ethanol vapor exposure (CIE):
During CIE, male (n = 39, housed 2 per cage) and female (n = 39, housed 3 per cage) rat cages were housed in specialized chambers and exposed to a 14-h on / 10-h off schedule for the alcohol vapors. Using a peristaltic pump (model QG-6, FMI Laboratory, Fluid Metering), 95% ethanol from a large reservoir was delivered to a heated flask at a regulated flow rate (95% ethanol vaporized at a drip rate of 2.5 - 4 mls per min for 14h a day followed by 10h of withdrawal). The vaporized ethanol was carried to the vapor chambers containing the rat cages by controlled air flow (regulated by a pressure gauge). The air and ethanol flow rates were optimized to result in blood alcohol levels (BALs) between 125 and 250 mg/dl of or 27.2 and 54.4 mM (Gilpin et al., 2008a); these BALs are 2-3 times the BAL observed in binge drinking, but not high enough to abolish righting reflex (Ernst et al., 1976; Courtney & Polich, 2009). CIE males and females either experienced TFC (n = 25) or not (n =14) and were euthanized 96h after cessation of CIE.
Tail bleeding for BAL measures:
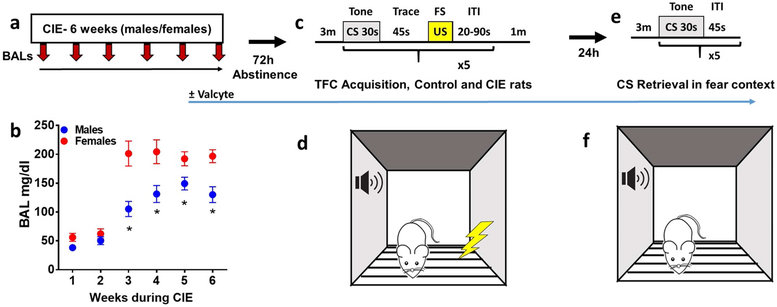
For measuring BALs, tail bleeding was performed on male and female rats, once a week on the same day every week, during hours 13-14 of CIE (total 14h exposure/day) according to (Gilpin et al., 2008b). Rats were gently restrained while the tip of the tail (1 mm) was punched with a 22g needle. Tail blood (0.2 ml) was collected and centrifuged. Plasma (5 μL) was used for measurement of blood alcohol levels (BALs) using an Analox AM1 analyzer (Analox Instruments USA Inc., MA). Single-point calibrations were performed for each set of samples with reagents provided by Analox Instruments (25–400 mg/dl or 5.4–87.0 mM). When blood samples were outside the target range (125–250 mg/dl), vapor levels were adjusted accordingly. Mean BALs during the 6 week CIE exposure is reported in Figure 1.
Figure 1:
Schematic of CIE weeks and TFC procedure in TK rats. (a) Timeline of CIE, blood collection for BALs during CIE and Valcyte administration. (b) BALs during each week of CIE in male and female TK rats. *p<0.05 vs. females. n = 38 males, n = 34 females. 72h after cessation of CIE, CIE naïve and CIE male and female TK rats were trained on TFC (c-d) and 24h after training all animals were tested for CS retrieval (e-f). Freezing was monitored during the 3 minute baseline session and during CS and trace intervals.
Estrous Cycle Tracking:
All female rats were vaginally swabbed with a sterile cotton swab soaked in 0.9% saline. Samples were applied to Superfrost® Plus slides and dried overnight and stained with Cresyl Echt Violet Solution (Abcam) to determine stage of estrous based on cell morphology (Cora et al., 2015). Vaginal swabs were performed after the last CIE session and after fear retrieval session.
Suppression of Neurogenesis:
Neurogenesis was suppressed by feeding the animals the orally available prodrug, valganciclovir (Valcyte, Roche), which is enzymatically converted to ganciclovir. Valcyte (7.5 mg) was given in a 0.5 g pellet of a 1:1 mixture of ground chow and peanut butter. To minimize neophobia, rats were exposed to the chow–peanut butter mixture in their home cage for 2-4 days prior to drug treatment. On drug treatment days, each rat was separated into an empty cage without bedding and individual Valcyte pellets were placed on the wall of the cage and monitored for feeding activity to ensure consistent dosing. Once the animal consumed the drug pellet the animal was moved back to the housing chamber. The entire feeding procedure lasted between 4-7 minutes and care was taken to reduce any stressful experience. Valcyte treatment (1x/d) was initiated during week 6 of CIE and was continued until the day of euthanasia. All CIE animals consumed the vehicle or Valcyte chow/peanut butter pellet (+/− Valcyte; males: n = 11 Valcyte−, n = 14 Valcyte+; females, n = 15 Valcyte−, n = 10 Valcyte+).
Trace Fear Conditioning:
Apparatus:
Fear conditioning was conducted in a set of four identical chambers housed within sound-attenuating boxes (Med Associates chambers connected to AnyMaze interface and Video tracking system). The floor was composed of stainless steel rods through which 0.5mA shocks were delivered. Each chamber was illuminated by an overhead 7.5-W bulb and was connected to its own shock generator-scrambler. Ventilation fans provided constant background noise (~60 dB). Chambers were cleaned with a solution of quatricide disinfectant between animals. All training and testing sessions were conducted in the same chamber for each rat.
Training:
72h after CIE cessation, CIE males (n = 25) and females (n = 25) and CIE naïve males (no CIE, n = 16) and females (no CIE, n = 13) were trained with TFC. We used a conditioning protocol based on previous reports to produce approximately equivalent freezing levels in both sexes. For training sessions of trace conditioning, the animals received 5 series of CS-US presentations that occurred with varied inter-trial intervals. The CS was a 30-sec tone cue (80 dB) and the US was a 1-sec foot shock (0.5 mA). The CS and US were separated by an empty 45-sec trace interval. The first CS presentation occurred following a 3-min baseline period and the final shock was followed by a 1-min post-shock period.
CS retrieval in fear context:
Twenty-four hours after training, animals were placed back in their original chambers for 3-min baseline period after which they were presented with 5 CS only presentations with each CS (30-sec tone cue) separated by 45s inter-interval. Immediately after the retrieval test, animals were returned to their home cages.
Assess freezing behavior:
Freezing behavior has been reported in a variety of ways: e.g. compute freezing during acquisition and retrieval without considering baseline freezing (Gewirtz & Davis, 1997; Marsicano et al., 2002; Schafe et al., 2005; Han et al., 2007; Gogolla et al., 2009; Monfils et al., 2009), or by subtracting baseline freezing (Reijmers et al., 2007). We report freezing behavior during acquisition and retrieval using the latter method, where baseline freezing was subtracted from each rat to compute freezing during acquisition and retrieval (Jacobs et al., 2010).
Brain tissue processing:
The groups of animals used for behavior and brain tissue processing is indicated in supplementary Figure 1. One hour after the fear retrieval session, TFC rats (no CIE and CIE), age matched experimentally naïve controls (n = 6 males, n = 6 females) and CIE only (TFC naïve rats euthanized 72h or 96 h after CIE) males (n = 14) and females (n = 14) were killed by rapid decapitation and the brains were isolated, and dissected along the midsagittal plane. The experimentally naïve group (control group) did not experience CIE or TFC. They were housed in cages similar to the CIE cages and were handled similarly. They were habituated to the TFC chamber, and did not experience any CS or US. They were not given any vehicle or Valcyte diet and were not tail bled for blood sampling. The left hemisphere was snap frozen and the right hemisphere was postfixed in 4% paraformaldehyde for immunohistochemistry. For tissue fixation, the hemispheres were incubated at room temperature for 36 hours and subsequently at 4°C for 48 hours with fresh paraformaldehyde replacing the old solution every 24 hours. Finally, the hemispheres were transferred to sucrose solution (30% sucrose with 0.1% sodium azide) for cryoprotection and stored until tissue sectioning was conducted (Cohen et al., 2015). Subsequently, the tissue was sliced in 40μm sections along the coronal plane on a freezing microtome. Sections were stored in a 1xPBS solution with 0.1% sodium azide at 4°C for histochemical analysis.
Immunohistochemistry (IHC):
Every eighteenth section through the hippocampus (anterio-posterior −2.5 to −6.3 mm from bregma) was mounted on Superfrost® Plus slides and dried overnight (Somkuwar et al., 2016). Six to eight sections per rat were stained for Ki-67 (1:700, Rabbit polyclonal, Thermo Scientific) and NeuroD (1:500, Goat polyclonal, Santacruz Biotechnology) followed by biotin-tagged secondary antibodies and visualized with DAB. For Ki-67 and NeuroD analyses, all immunoreactive cells in the subgranular zone and granule cell layer were counted per animal. In addition to cell counting, area measures of the granule cell layers were also determined for each section for each animal using StereoInvestigator software (MBF), and the raw cell counts per section per animal were divided by the area of the granule cell layer and are indicated as cells per mm2 of the granule cell layer per animal.
For morphometric analysis of the density of mossy fiber projections, dorsal hippocampal sections (representing −2.56 and −4.8mm from bregma, 4 sections per rat) were separately stained for synaptoporin (1:50, Rabbit polyclonal, SynapticSystems) followed by biotin-tagged secondary antibodies and visualized with DAB (Staples et al., 2017). For mossy fiber density, images were captured at 10x and synaptoporin in the hilus of the dorsal DG was evaluated by quantifying DAB stain (% area stained; (Galinato et al., 2018)) using ImageJ software (NIH). Briefly, the mossy fiber tracts were contoured using the polygonal selection feature. A circular area above the CA3 was used to quantify non-specific/ background staining.
Western Blotting:
Procedures optimized for measuring both phosphoproteins and total proteins were employed (Kim et al., 2015). Tissue punches from dorsal hippocampal formation enriched in the dentate gyrus from 500 μm thick sections were homogenized on ice by sonication in buffer (320 mM sucrose, 5 mM HEPES, 1 mM EGTA, 1mMEDTA, 1% SDS, with Protease Inhibitor Cocktail and Phosphatase Inhibitor Cocktails II and III diluted 1:100; Sigma), heated at 100 °C for five minutes, and stored at−80 °C until determination of protein concentration by a detergent-compatible Lowry method (Bio-Rad, Hercules, CA). Samples were mixed (1:1) with a Laemmli sample buffer containing β-mercaptoethanol. Each sample containing protein from one animal was run (20 μg per lane) on 8–12% SDS-PAGE gels (Bio-Rad) and transferred to polyvinylidene fluoride membranes (PVDF pore size 0.2 μm). Blots were blocked with 2.5% bovine serum albumin (for phosphoproteins) or 5% milk (w/v) in TBST (25 mM Tris–HCl (pH 7.4), 150 mM NaCl and 0.1% Tween 20 (v/v)) for 16–20 h at 4 °C and were incubated with the primary antibody for 16–20 h at 4 °C: antibody to tGluN2A (1:200, Santa Cruz Biotechnology cat. no. sc-9056, predicted molecular weight 177 kDa, observed band ~170 kDa), antibody to tCamKII (1:200, Abcam cat. no. ab52476, predicted molecular weight 47 and 60 kDa, observed band ~47 and 60 kDa), antibody to tumor necrosis factor alpha (TNFα; Abcam, ab9635; 1:500, predicted molecular weight 20 kDa, observed band ~17 kDa (Kinsella et al., 2016)). Blots were then washed three times for 15min in TBST, and then incubated for 1 h at room temperature (24 °C), appropriately with horseradish peroxide-conjugated goat antibody to rabbit or horseradish peroxide–conjugated goat antibody to mouse (1:10,000, BioRad) in TBST. After another three washes for 15 min with TBST, immunoreactivity was detected using SuperSignal West Dura chemiluminescence detection reagent (Thermo Scientific) and collected using HyBlot CL Autoradiography film (Denville Scientific) and a Kodak film processor. For normalization purposes, membranes were incubated with 0.125% coomassie stain for 5 minutes and washed three times for 5-10 minutes in destain solution. Densitometry was performed using ImageStudio software (Li-Cor Biosciences). X-ray films were digitally scanned at 600 dpi resolution, then bands of interest were selected in identically sized selection boxes within the imaging program which included a 3 pixel extended rectangle for assessment of the background signal. The average signal of the pixels in the ‘background’ region (between the exterior border of the region of interest selection box and the additional 3 pixel border) was then subtracted from the signal value calculated for the band of interest. This was repeated for coomassie, and the signal value of the band of interest following subtraction of the background calculation was then expressed as a ratio of the corresponding coomassie signal (following background subtraction). This ratio of expression for each band was then expressed as a percent of the drug naïve control animals included on the same blot.
Statistical analyses:
Parametric statistical analysis were used to analyze our datasets based on the assumption that our data fit a normal distribution and satisfy the sample size for adequate statistical power. Changes in BALs was assessed as a repeated measures two-way ANOVA (sex × week). For TFC analysis, the main dependent variable was the amount of time rats spent engaged in freezing behavior. Freezing was defined as the absence of all movement except for that required for respiration. The average percent time spent freezing was calculated using the AnyMaze software (StoeltingCo.com). The AMI scoring parameters were chosen and freezing was analyzed as a percentage of each minute during the baseline, training and testing sessions. None of the behaviors were hand scored. Baseline freezing was evaluated as percent freezing, and freezing during training and testing sessions are indicated as change in percent freezing from baseline freezing. Changes in freezing behavior during TFC acquisition was assessed as repeated measures two-way ANOVA (TFC session × treatment) in each sex. Changes in freezing behavior during CS retrieval was analyzed by one-way ANOVA in each sex. Cell counts for each marker (expressed as positive cells per mm2) and raw values of protein expression from Western blotting were analyzed by three-way ANOVA (sex × CIE × TFC). Significant interaction or ANOVA was followed by post-hoc analysis using Newman-Keuls multiple comparisons test. All graphs and statistical analysis were generated using Graph Pad version 7 for PC and p<0.05 was considered statistically significant.
Results
CIE produces higher BALs in females compared to male rats
Adult male and female rats experienced CIE for 6 weeks (Figure 1a-b). 72h after the last CIE session, CIE rats and age matched controls were trained on TFC (Figure 1c-d) and 24h later were tested for CS retrieval (Figure 1e-f). BALs were monitored during CIE in males and females. BALs increased as a function of the alcohol flow rates and remained within an acceptable range over the six week period in male and female rats (Figure 1b). The amount of alcohol experienced by each rat reached the desired range by the second week of CIE exposure as indicated by the increases in BALs. There were sex differences in BALs while vapors were delivered, with females demonstrating greater BALs than males during weeks 3 to 6. Repeated measures two-way ANOVA demonstrated a significant sex × week interaction (F[5,269]=3.1, p = 0.008), main effect of weeks of CIE (F[5,269]=39.1, p < 0.001) and main effect of sex (F[1,269]=44.1, p < 0.001) over the six weeks of CIE exposure. Post hoc analysis demonstrated higher BALs in females during weeks 3 to 6 compared with males (ps < 0.05; Figure 1b).
CIE and TFC does not alter estrus phase in females
We determined the estrus phase of females in CIE naïve and CIE groups before TFC and after TFC. The number of females in each phase are as follows: (CIE naïve: diestrus (D)- 1, proestrus (P)- 5, estrus (E)- 13; CIE: D-4, P-9, E-12; CIE naïve + TFC: D-3, P-2, E-14; CIE + TFC: D-5, P-4, E-16). Two-way ANOVA did not reveal significant effects of CIE and TFC on estrus cycle stages (effect of treatment F (3, 6) = 0.7619, p = 0.5), however, revealed changes in estrus phase before and after TFC in both groups (estrus phase F (2, 6) = 24.11, p = 0.001). These findings indicate that CIE and TFC did not alter estrus cycle in females, supporting previous findings (Lebron-Milad et al., 2013; Priddy et al., 2017).
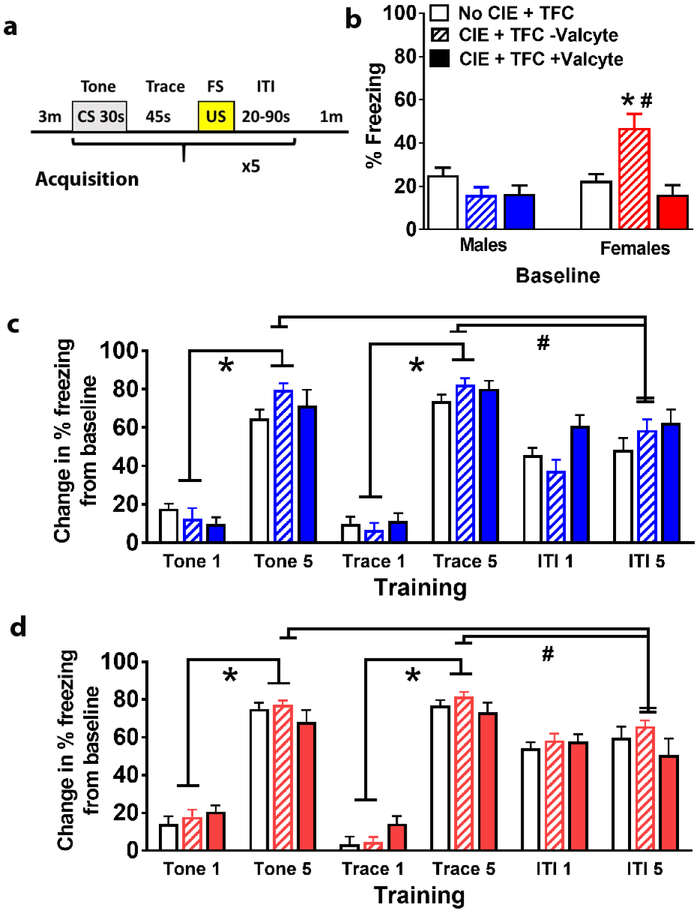
Abstinent CIE females show enhanced freezing during acclimation and this is abolished by Valcyte treatment
General locomotor activity was assessed in both sexes in all treatment groups before training period. Two-way ANOVA did not detect a significant treatment × sex interaction F[2,72] = 0.2, p = 0.8, or main effect of treatment F[2,72] = 0.8, p = 0.4, however, detected a main effect of sex F[1,72] = 9.5, p = 0.002; supplementary Figure 2). Baseline freezing behavior was obtained during the 3 minute acclimation period before training (Figure 2a). All the groups within each sex were evaluated separately. Freezing behavior did not differ between control males and females during acclimation (Figure 2b). Baseline freezing was higher in CIE females compared with control females and this effect was abolished with Valcyte treatment (Figure 2b). Valcyte treated males and females did show differences in freezing behavior. Two-way ANOVA demonstrated a significant treatment × sex interaction F[2,72] = 8.888, p = 0.004, main effect of treatment F[2,72] = 5.245, p = 0.007, and main effect of sex F[1,72] = 6.392, p = 0.01. Post hoc analysis indicated higher freezing in CIE− Valcyte females compared to controls and CIE+ Valcyte females (ps < 0.05; Figure 2b). Therefore, freezing behavior during training and CS retrieval is presented as change in percent freezing during training or retrieval period from percent freezing during baseline to eliminate the confounding effect of baseline freezing (Jacobs et al., 2010).
Figure 2:
Male and female TK rats after CIE have distinct responses during TFC. (a) Schematic of TFC protocol. (b) Mean (±SE) freezing did not differ between treatment groups in males during the 3 min baseline. Mean freezing was significantly higher in Valcyte− CIE females compared to other treatment groups during the 3 minute baseline. *p<0.05 vs. CIE naïve rats, #p<0.05 vs. Valcyte+ CIE rats. (c) Mean (±SE) freezing (change in freezing from baseline) in males did not differ between groups during the CS 1 and trace 1 period, during the CS 5 and trace 5 period and ITI 1 and ITI 5 periods. Freezing increased significantly from the CS 1 to CS 5 and from trace 1 to trace 5. *p<0.05 vs. tone and trace 1. (d) Mean (±SE) freezing (change in freezing from baseline) in females did not differ between groups during the CS 1 and trace 1 period, during the CS 5 and trace 5 period and ITI 1 and ITI 5 periods. Freezing increased significantly from the CS 1 to CS 5 and from trace 1 to trace 5. *p<0.05 vs. tone and trace 1. n = 16 CIE naïve males, n = 11 Valcyte− CIE males, n = 14 Valcyte+ CIE males; n = 13 CIE naïve females, n = 15 Valcyte− CIE females, n = 10 Valcyte+ CIE females.
Abstinence from CIE does not alter freezing during training in male and female rats
In males, repeated measures two-way ANOVA did not detect a significant training × treatment group interaction (F[6,148] = 1.558, p = 0.16) and CIE treatment (F[2,148] = 0.7563, p = 0.43), however, detected a significant effect of training (F[3,148]=218.4, p < 0.001; Figure 2c). In females, repeated measures two-way ANOVA did not detect a significant training × treatment group interaction (F[6,140]=1.5, p = 0.17) and CIE treatment (F[2,140]=0.7, p = 0.49), however, detected a significant effect of training (F[3,140]=292.6, p < 0.001; Figure 2d). All animals learned the CS-US association and demonstrated significant freezing behavior by trial 2 of the 5 trials. In order to determine whether the rats encoded the timing of CS and US freezing behavior was measured during the inter-trial interval (ITI). In males and females, freezing behavior did not significantly differ between the first and the last ITI (no interaction, no effect of treatment or training). However, in males and females, repeated measures indicated a significant reduction in freezing behavior during ITI 5 compared with tone 5 and trace 5, indicating that freezing behavior was maximal during CS and US and declined during the ITI (males: F[3,140]=292.6, p < 0.001; Figure 2c; females (F[3,140]=292.6, p < 0.001; Figure 2d)).
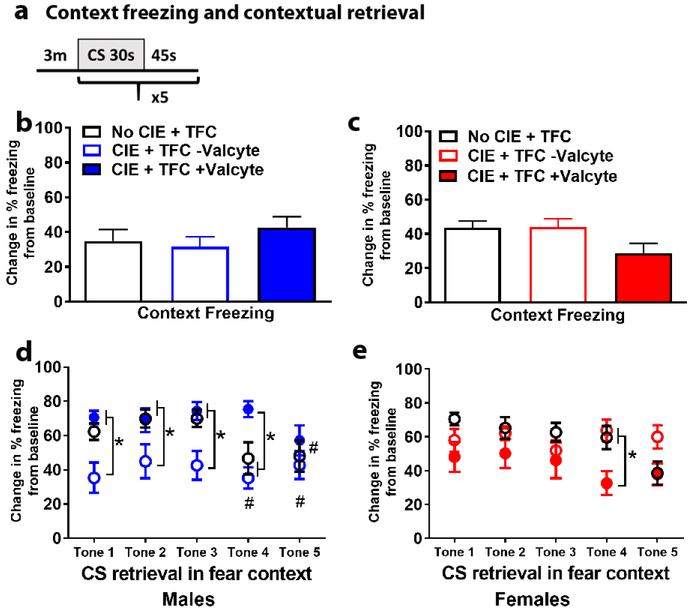
Abstinence from CIE does not alter context freezing in male and female rats
Twenty-four hours following training, rats were returned to the training context for 3 min with no tones or footshocks presented (Figure 3a). There were no significant differences in context freezing in males F[2,37] = 0.75, p = 0.4 (Figure 3b) or females F[2,35] = 2.7, p = 0.07 (Figure 3c) by one-way ANOVA.
Figure 3:
Male and female TK rats after CIE have distinct responses during CS retrieval. (a) Schematic of CS retrieval protocol. (b-c) Mean (±SE) freezing (change in freezing from baseline) did not differ between treatment groups in males (b) and females (c) during the 3 min context retrieval. (d) Mean (±SE) freezing (change in freezing from baseline) in males show that Valcyte− CIE rats had reduced freezing during CS retrieval during tones 1-4 compared with CIE naïve rats and Valcyte+ CIE rats. *p<0.05 vs. Valcyte− CIE rats; #p<0.05 vs. tone 1. (e) Mean (±SE) freezing (change in freezing from baseline) in females show no significant effect of treatment on CS retrieval during tones 1-3. During tone 4, Valcyte+ CIE rats show reduced freezing compared with CIE naïve rats and Valcyte− CIE rats. *p<0.05 vs. Valcyte+ CIE rats.
Abstinence from CIE reduces CS retrieval in male rats without effecting CS retrieval in female rats
On testing day, after context freezing testing, male and female rats experienced CS-only trials. Freezing was measured during CS. In males, repeated measures two-way ANOVA demonstrated a session × treatment interaction (F[8,132] = 2.0, p = 0.05), main effect of treatment (F[2,33] = 6.4, p = 0.004) and main effect of testing (F[4,132] = 3.3, p = 0.012) during CS. Posthoc analysis revealed that CIE rats with intact NPCs showed reduced freezing during CS during the first three CS trials (p<0.05). Valcyte prevented this effect during CS, and reduced latency to freezing behavior during CS periods. By the fifth trial all male rats showed similar freezing during CS (Figure 3d).
In females, repeated measures two-way ANOVA demonstrated a session × treatment interaction (F[8,140] = 2.3, p = 0.02), main effect of treatment (F[2,35] = 3.7, p = 0.03) and main effect of testing (F[4,140] = 3.1, p = 0.017) during CS. Posthoc analysis revealed that CIE rats with Valcyte showed reduced freezing during CS during the fourth CS trials (p<0.05). Valcyte enhanced latency to freezing behavior during CS trials (Figure 3e).
Abstinence from CIE and TFC produce distinct effects on NPCs and immature neurons in male and female rats
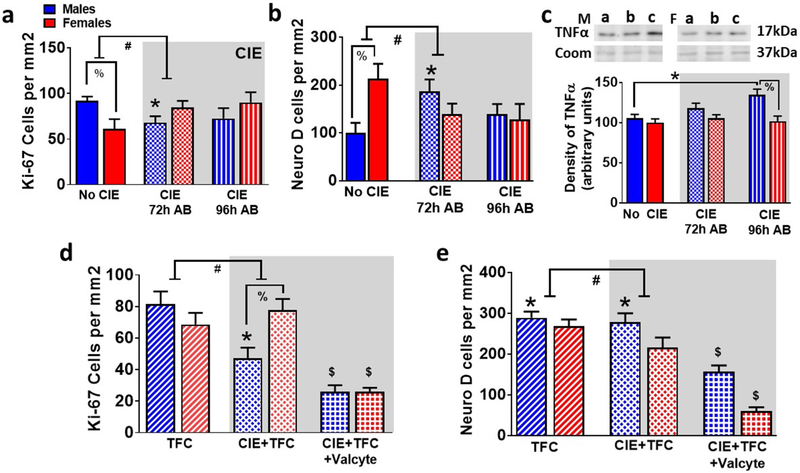
The effects of abstinence from CIE on NPCs (Ki-67 and NeuroD cells; (Galinato et al., 2018)) in males and females was investigated. We first evaluated whether 72h of abstinence would produce an increase in NPCs in TK rats (Hansson et al., 2010; Somkuwar et al., 2016). Next, in order to determine whether the alterations in Ki-67 cells and NeuroD cells seen at the 72h abstinence time point were transient or long lasting, we examined the number of Ki-67 and NeuroD cells at 72h and 96h after CIE was terminated. This allowed us to evaluate whether the rebound burst in NPCs was specific to the 72h time point. For Ki-67 cells, two-way ANOVA showed a significant sex × CIE interaction (F[2,37] = 4.2, p = 0.02), and did not show a main effect of CIE or sex. Posthoc analysis revealed lower number of Ki-67 cells in no CIE females compared to males and lower number of cells in 72h males compared to no CIE males (p<0.05; Figure 4a). For NeuroD cells, two-way ANOVA showed a significant sex × CIE interaction (F[2,37] = 4.2, p = 0.02), and did not show a main effect of CIE or sex. Posthoc analysis revealed higher number of NeuroD cells in no CIE females compared to males and higher number of cells in 72h males compared to no CIE males (p<0.05; Figure 4b). In addition to the immunohistochemical analysis of the proliferation/immature neuron markers, we evaluated changes in a marker of neuroimmune response, TNFα, as previous findings in adult rats indicate that the burst in neuronal progenitors that occurs during abstinence from chronic alcohol experience is followed by an increase in expression of neuroimmune markers (Nixon & Crews, 2004; Nixon et al., 2008; Marshall et al., 2016; Peng et al., 2017). Two-way ANOVA did not detect a significant sex × CIE interaction (F[2,37] = 2.04, p = 0.14), however, detected a main effect of sex (F[1,37] = 10.3, p = 0.002). Posthoc analysis revealed higher expression of TNFα at 96h time point compared with no CIE animals and higher expression of TNFα in 96h males compared to 96h females (p<0.05; Figure 4c).
Figure 4:
Valcyte reduces proliferation and differentiation of NPCs in male and female TK rats. (a-b) Quantitative analysis of the total number of Ki-67 (a) and NeuroD (b) labeled cells in no CIE and CIE rats. AB, abstinence. (c) Density of TNFα by Western blotting analysis. Representative immunoblots of TNFα and coomassie from males (M, left panel) and females (F, right panel). a, no CIE; b, 72h AB; c, 96h AB. (d-e) Quantitative analysis of Ki-67 (d) and NeuroD (e) cells from TFC and CIE + TFC groups. Data shown are represented as mean +/− SE. #p<0.05 indicating significant interactions; *p<0.05 compared with drug/behavior naïve controls; %p<0.05 vs males; $p<0.05 vs. CIE+TFC group by three-way ANOVA followed by posthoc tests. n = 6 behavior naïve males, n = 9 CIE males, n = 16 CIE naïve TFC males, n = 11 Valcyte− CIE TFC males, n = 14 Valcyte+ CIE TFC males; n = 6 behavior naïve females, n = 9 CIE females n = 13 CIE naïve TFC females, n = 15 Valcyte− CIE TFC females, n = 10 Valcyte+ CIE TFC females.
We next determined the effects of TFC on NPCs in male and female rats. Immunohistochemical analysis of Ki-67 cells did not show a sex × CIE × TFC interaction (F[5,108] = 0.264, p = 0.608), did not show a significant sex × TFC interaction (F[3,108] = 0.99, p = 0.30), however, showed a significant sex × CIE interaction (F[3,108] = 11.6, p = 0.001) and a significant CIE × TFC interaction (F[3,108] = 5.2, p = 0.02). Posthoc analysis revealed lower number of Ki-67 cells in females vs. males (p<0.05) and reduced the number of Ki-67 cells in CIE males compared to naïve controls (p<0.05). CIE did not alter the number of Ki-67 cells in females. Posthoc analysis revealed a lower number of Ki-67 cells in males vs. females that experienced TFC during abstinence (p<0.05) and reduced number of Ki-67 cells in Valcyte treated males and females that experienced TFC during abstinence compared with vehicle treated rats (p<0.05). TFC did not alter the number of Ki-67 cells in males and females that were CIE naïve (Figure 4d).
The effects of abstinence from CIE on immature neurons in males and females were investigated. Immunohistochemical analysis of NeuroD cells did not show a sex × CIE × TFC interaction (F[5,93] = 1.9, p = 0.16), however, showed a significant sex × TFC interaction (F[3,93] = 4.04, p = 0.04), a significant sex × CIE interaction (F[3,93] = 5.7, p = 0.019) and a significant CIE × TFC interaction (F[3,93] = 5.5, p = 0.02). Posthoc analysis revealed higher number of NeuroD cells in females vs. males (p<0.05) and increased number of NeuroD cells in CIE males compared to naïve controls (p<0.05). CIE did not alter the number of NeuroD cells in females. Posthoc analysis showed that TFC increased the number of NeuroD cells in males that were CIE naïve and CIE experienced (p<0.05). TFC did not alter the number of NeuroD cells in females that were CIE naïve and abstinent from CIE. Valcyte treated males and females that experienced TFC during abstinence showed reduced number of NeuroD cells compared with vehicle treated males and females that experienced TFC during abstinence (p<0.05; Figure 4e).
NPCs generated during abstinence prevent TFC-induced plasticity in glutamatergic receptor expression in male rats
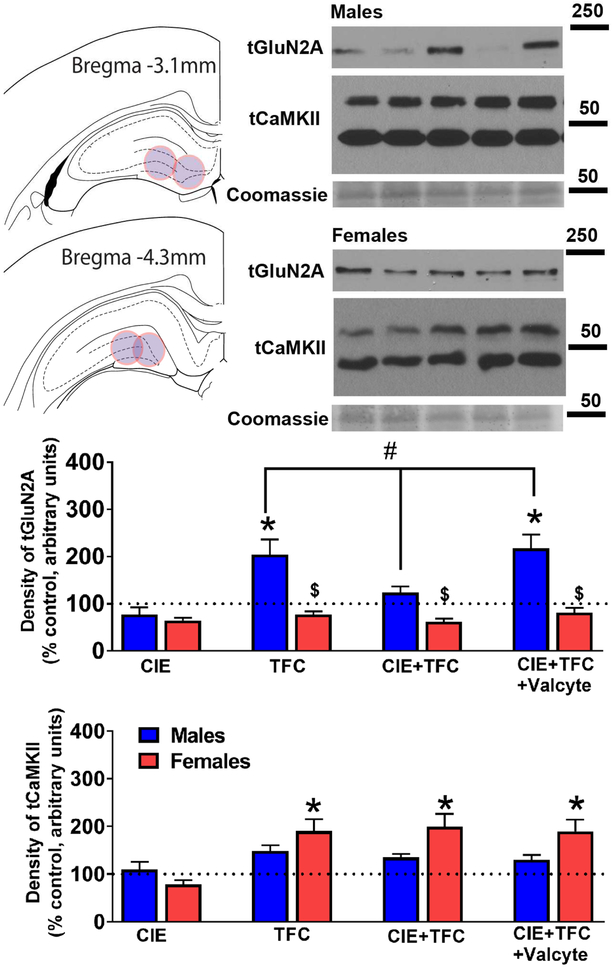
The effects of abstinence from CIE on density of tGluN2A and tCaMKII receptor expression in males and females were investigated. Immunoblotting analysis for tGluN2A did not show a sex × CIE × TFC interaction (F[5,111] = 0.24, p = 0.63) and a significant sex × CIE interaction (F[3,111] = 0.7, p = 0.40), however, showed a significant sex × TFC interaction (F[3,111] = 12.4, p = 0.001) and a significant CIE × TFC interaction (F[3,111] = 7.8, p = 0.006). Posthoc analysis revealed that in females, TFC in CIE naïve and CIE rats did not alter the density of tGluN2A and Valcyte treatment did not affect the density of tGluN2A. In males, TFC in CIE naïve animals increased the density of tGluN2A (p<0.05); this effect was not evident with TFC in CIE males. Importantly, Valcyte treatment in TFC-CIE males effected the density of tGluN2A and increased it to the levels in TFC-CIE naïve animals (p<0.05; Figure 5).
Figure 5:
GluN2A and CaMKII expression is distinctly effected by TFC and CIE in male and female rats. (a) Schematic showing location of tissue punches taken in the dorsal DG of the hippocampus. (b-c) Representative Western blots for protein expression in male (b) and female (c) DG enriched tissue. Lane 1- Naïve control; 2- CIE; 3- TFC; 4- CIE+TFC; 5- CIE+TFC+Valcyte. Molecular weight of ladder in kDa is indicated to the right of the blots. (d-e) Density of protein expression for total GluN2A and CaMKII in dorsal DG from male and female rats. #p<0.05 indicating significant interactions; *p<0.05 compared to drug and sucrose naïve age matched controls; &p<0.05 vs. CIE+TFC-Valcyte; $p<0.05 vs. males by three-way ANOVA followed by posthoc tests. Data shown are represented as mean +/− SEM. n = 6 behavior naïve males, n = 9 CIE males, n = 16 CIE naïve TFC males, n = 11 Valcyte− CIE TFC males, n = 14 Valcyte+ CIE TFC males; n = 6 behavior naïve females, n = 9 CIE females n = 13 CIE naïve TFC females, n = 15 Valcyte− CIE TFC females, n = 10 Valcyte+ CIE TFC females.
Immunoblotting analysis for tCaMKII did not show a sex × CIE × TFC interaction (F[5,111] = 0.09, p = 0.74), a significant sex × CIE interaction (F[3,111] = 0.09, p = 0.75), and a significant CIE × TFC interaction (F[3,111] = 0.01, p = 0.92); however, showed a significant sex × TFC interaction (F[3,111] = 5.3, p = 0.023). Posthoc analysis revealed that in females, TFC in CIE naïve and CIE rats increased the density of tCaMKII (p<0.05), and Valcyte treatment did not have any effects. In males, TFC in CIE naïve and CIE animals did not alter the density of tCaMKII (Figure 5).
NPCs generated during abstinence prevent TFC-induced plasticity in mossy fiber projections in male rats
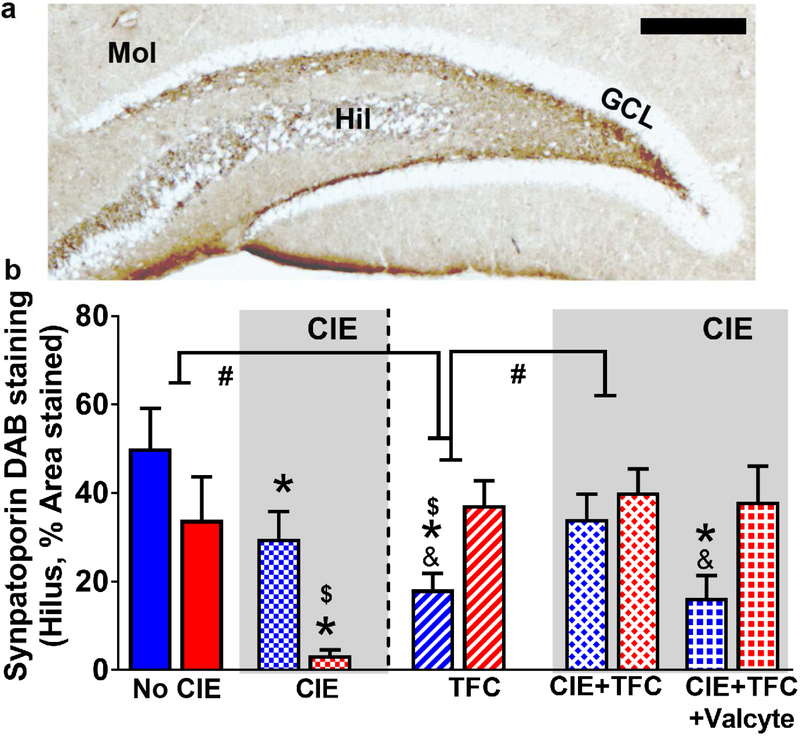
The effects of abstinence from CIE on density of mossy fiber projections in males and females were investigated. Immunohistochemical analysis of synaptoporin did not show a sex × CIE × TFC interaction (F[5,93] = 0.3, p = 0.84) and a significant sex × CIE interaction (F[3,93] = 0.8, p = 0.36), however, showed a significant sex × TFC interaction (F[3,93] = 16.04, p < 0.001) and a significant CIE × TFC interaction (F[3,93] = 10.5, p = 0.002). Posthoc analysis revealed lower expression of synaptoporin in CIE males and females compared with controls, and lower expression in CIE females vs. CIE males (ps<0.05). In females, TFC in CIE naïve and CIE rats did not alter the density of synaptoporin and Valcyte treatment did not affect the density of synaptoporin. In males, TFC in CIE naïve animals reduced the density of synaptoporin (p<0.05); this effect was not evident with TFC in CIE males. Importantly, Valcyte treatment in TFC-CIE males effected the density of synaptoporin and reduced it to the levels in TFC-CIE naïve animals (p<0.05; Figure 6).
Figure 6:
CIE and TFC differently alter mossy fiber tracts in the DG in male and female rats. (a) Photomicrograph of a section from one control male rat through the anterior dorsal hippocampus stained with synaptoporin. Staining revealed mossy fiber tracts and terminal fields in the hilus (Hil), and the CA3 pyramidal projections. Molecular layer (Mol), granule cell layer (GCL). Scale bar in (a) is 500 um. (b) Quantitative analysis of the density measures in the hilus. Data shown are represented as mean +/− SEM. #p<0.05 indicating significant interactions; *p<0.05 compared to drug and sucrose naïve age matched controls; &p<0.05 vs. CIE+TFC-Valcyte; $p<0.05 vs. males by three-way ANOVA followed by posthoc tests. n = 6 behavior naïve males, n = 9 CIE males, n = 16 CIE naïve TFC males, n = 11 Valcyte− CIE TFC males, n = 14 Valcyte+ CIE TFC males; n = 6 behavior naïve females, n = 9 CIE females n = 13 CIE naïve TFC females, n = 15 Valcyte− CIE TFC females, n = 10 Valcyte+ CIE TFC females.
Discussion
The present results are the first to show that NPCs born during abstinence from chronic ethanol experience play a direct role in reduced expression of tone conditioning (CS retrieval) when trained using a TFC procedure, and that these effects are sex specific. Mechanisms underlying NPC-induced reduction in CS retrieval include reduced expression of GluN2A receptors and enhanced expression of synaptoporin in the DG of male rats. These findings help demonstrate that the rebound proliferation of NPCs in the DG in ethanol withdrawn male rats is aberrant and contributes to deficits in hippocampal dependent behaviors in adult subjects.
Formation and expression of fear memories in a time-limited manner depend on a functional and intact hippocampus (Frankland & Bontempi, 2005; Beeman et al., 2013; Doron & Goshen, 2017; Woods & Kheirbek, 2017). Mechanistic studies undisputedly demonstrate that the dorsal hippocampus and specifically the DG are involved in the acquisition, consolidation, and expression of TFC (McEchron et al., 2000; Quinn et al., 2002; Yoon & Otto, 2007; Quinn et al., 2008; Beeman et al., 2013; Pierson et al., 2015). However, mechanistic studies show that the role of adult hippocampal neurogenesis in TFC is controversial, with studies in adult rats indicating that neurogenesis is needed for formation of trace memories involving fearful stimuli (Shors et al., 2001; Shors et al., 2002; Achanta et al., 2009) and others in adult mice demonstrating that neurogenesis is not necessary for formation of trace memories, however, buffers against nonassociative, anxiogenic effects of a fearful stimuli (Seo et al., 2015). In addition, it has been demonstrated that increasing neurogenesis by wheel running or systemic injections of a pro-neurogenesis drug memantine in mice after formation of fear memories reduces CS retrieval or produces amnesic effects in response to the CS in a novel context (Ishikawa et al., 2016; Gao et al., 2018). Therefore, it appears that mature and newly born granule cell neurons in the DG play distinct roles in formation of fearful memories, and time-limited retrieval of fear memories (Beeman et al., 2013; Seo et al., 2015; Gao et al., 2018).
In the context of alcohol use disorder (AUD), short-term and prolonged ethanol exposure does not alter acquisition of fear responses in delay fear conditioning and TFC (Melia et al., 1996; Gould, 2003; Weitemier & Ryabinin, 2003; Holmes et al., 2012; Broadwater & Spear, 2013; Hunt & Barnet, 2016; Goodfellow et al., 2018), however, produces deficits in CS retrieval, seen as reduced freezing or amnesic effects in response to the CS in a novel context (Gould, 2003; Weitemier & Ryabinin, 2003; Hunt & Barnet, 2016; Goodfellow et al., 2018). The impaired and amnesic hippocampal functioning in ethanol experienced animals could be resulting from neuroplastic and neuroadaptive changes in the DG (Gilmartin & McEchron, 2005; Czerniawski et al., 2012; Pierson et al., 2015). For example, several neuroplastic and neuroadaptive changes are seen in the hippocampus after chronic ethanol experience (reviewed in (Nixon, 2006; Mandyam, 2013; Zorumski et al., 2014; Kutlu & Gould, 2016; Montesinos et al., 2016)). Such neuroplastic changes could involve the proliferative burst in the NPCs in the DG 72 hours into withdrawal from CIE, a timeframe associated with negative affect (Hansson et al., 2010; Somkuwar et al., 2016). The effect this proliferative burst has on hippocampal functioning, particularly emotional memories dependent on the hippocampus, remained unclear and were determined in the current study.
We used transgenic rats in which neural progenitors can be selectively and inducibly ablated (Snyder et al., 2016; Galinato et al., 2018). Male and female rats experienced CIE for 6 weeks and were withdrawn from CIE. Female rats showed greater BALs compared with males when maintained at similar drip-rate/body weight ratio in the vapor chambers. This is consistent with the previous literature in humans and rodents that demonstrate sex differences in alcohol drinking, pharmacokinetics, peak BAL and alcohol elimination rate (Rivier et al., 1992; Rivier, 1993; Thomasson, 1995; Priddy et al., 2017). Male and female rats that received Valcyte during withdrawal and early abstinence showed a reduction in Ki67+ cells and NeuroD+ cells indicating that Valcyte abolished the rebound burst in NPCs. Valcyte− CIE female rats with intact NPCs showed enhanced freezing responses compared to CIE naïve rats and Valcyte+ CIE rats during the baseline session that occurred at 72 hours of abstinence. Enhanced freezing during the baseline session is suggested to indicate anxiety-like behavior (Brandao et al., 2008; Pettersson et al., 2015), a phenotype that is demonstrated in female rats after chronic ethanol experience (Getachew et al., 2008). Valcyte prevented the rebound burst in NPCs in CIE female rats and reduced freezing responses during baseline session, demonstrating a role for NPCs generated during early abstinence in anxiety-like behavior. Enhanced freezing during baseline session in Valcyte− CIE females did not affect freezing behavior during acquisition of fear responses in TFC and during CS retrieval, indicating that these behaviors were not predicted by the anxiety-like response or high degree of context fear generalization in Valcyte− CIE female rats (Seo et al., 2015). Unlike Valcyte− CIE females, Valcyte− CIE male rats did not show altered freezing behavior during baseline session compared with no CIE and Valcyte+ CIE rats. Valcyte− CIE male rats did not differ in acquiring TFC compared to no CIE and Valcyte+ CIE male rats. These results in CIE female and male rats show that CIE did not alter learning of TFC, a finding in line with previous reports conducted with single dose of ethanol experience (Weitemier & Ryabinin, 2003; Hunt & Barnet, 2016; Goodfellow et al., 2018). Valcyte− CIE males showed normal freezing during context retrieval and reduced freezing during CS retrieval when compared with no CIE and Valcyte+ CIE males. In CIE males, Valcyte treatment recovered the freezing response during CS retrieval without altering context retrieval. This is an important finding, as a previous report shows that Valcyte treatment enhanced context retrieval in CIE naïve mice, suggesting that loss of NPCs in normal mice induced impaired associative conditioning to the trace CS (Seo et al., 2015). These findings also indicate that NPCs in normal rodents supports conditioned fear to the trace CS, and this relationship is abolished in CIE rats. Furthermore, it is tempting to consider that the suppression of freezing in Valcyte− CIE males may reflect a preferential disruption by CIE in the temporal component of TFC. These findings suggest that chronic prolonged ethanol experience via CIE may have selectively disrupted the hippocampus-dependent ability of timing, but may not have impaired the ability to acquire a conditioned freezing response per se. Taken together, these data suggests that exposure to CIE may create a higher vulnerability for hippocampal impairment in male subjects when compared with female subjects. Additionally, the proliferative burst in NPCs that is seen 72 hours into withdrawal from CIE in males plays a mechanistic role in disrupted consolidation of TFC.
Recent studies have demonstrated a mechanistic role of GluNs in acquisition and CS retrieval, when subjects were trained in a TFC paradigm (Huerta et al., 2000; Quinn et al., 2005; Jarome et al., 2012; Holehonnur et al., 2016). For example, blockade of GluN receptors prior to training disrupts the consolidation of fear memories (Stiedl et al., 2000) and similarly, genetic deletion of hippocampal GluNs disrupts TFC (Huerta et al., 2000). The GluN2A and GluN2B subunits mark the principal GluN subtypes, and expression of the GluN2A determines qualitative and functional properties of hippocampal neurons (Monyer et al., 1994). Our findings demonstrate that TFC enhances GluN2A expression in no CIE males and Valcyte+ CIE males and this increase correlated with intact consolidation of fear memories. We also show that reduced GluN2A subunits in Valcyte− CIE male rats correlated with reduced consolidation or amnesic effects, indicating that rebound effect of NPCs during early abstinence prevented neuroadaptations in GluNs in the DG. Whether newly born NPCs born during the proliferative burst differentially express GluNs to modulate neuroplasticity and behavior is yet to be determined and is an interesting future pursuit (Hagihara et al., 2011). In female rats, TFC enhanced tCaMKII expression and this effect was evident in all TFC groups, supporting previously reported role of tCaMKII in fear memory consolidation (Jarome et al., 2016). Taken together, these findings demonstrate sex-specific alterations in plasticity-related proteins in the DG, whose expression correlates with consolidation of fear memories in subjects trained on TFC.
In the DG, NPCs and newly born GCNs contribute to the density of mossy fiber tracts (Romer et al., 2011). Given the inverse correlation between mossy fiber density and GluN2 expression in the DG (Ni et al., 2009), we determined the density of mossy fiber projections in male and female rats. Our findings demonstrate that during early abstinence, mossy fiber density in the hilus was significantly reduced in both genders. These findings support the previously indicated reduced mossy fiber density in ethanol experienced subjects (Cadete-Leite et al., 1989; Feller et al., 1991; Brandao et al., 1996). Notably, mossy fiber density was significantly enhanced by TFC in females and this correlated with enhanced expression of CaMKII, indicating a cellular mechanism for enhanced learning and memory behaviors in females. In no CIE and Valcyte+ CIE males, TFC reduced mossy fiber density and this correlated with enhanced expression of GluN2A. The inverse relationship between mossy fiber density, GluNs and learning and memory function dependent on the hippocampus has been previously reported (Ni et al., 2009), suggesting distinct neurobiological mechanisms underlying fear consolidation in female versus male subjects (Bouchet et al., 2017; Keiser et al., 2017).
In conclusion, our findings demonstrate a direct role of NPCs that were born during early abstinence from prolonged exposure to CIE in males in reduced CS retrieval after TFC. We show plasticity-related adaptations in the DG that correlate with rebound burst of NPCs. Additional neuroimmune responses in the DG in concert with aberrant NPC proliferation could contribute to the memory deficits in CIE male rats, and exploring these mechanisms could be critical in linking microglial proliferation in the DG in ethanol-induced disruption in fear consolidation (Klaus et al., 2016; Marshall et al., 2016; Peng et al., 2017; Goodfellow et al., 2018).
Supplementary Material
Withdrawal from chronic ethanol increases neural progenitor cells in the dentate gyrus in male rats
Increase in neural progenitor cells is mechanistically linked to chronic ethanol-induced amnesic effects
Aberrant burst in neural progenitor cells regulates plasticity related proteins in the dentate gyrus
Acknowledgements
We thank Dr. Heather Cameron and Michelle Brewer from the National Institutes of Mental Health, National Institutes of Health (NIH), USA for providing the GFAP-TK rats. We thank Alekhya Bommireddipalli, Leyda Villagrasa and Jacque Quigley from the independent study program at UCSD, SURF program at Scripps Research Institute and the Summer Research program from National Institutes on Drug Abuse, NIH, USA for excellent technical assistance with tissue processing and immunohistochemistry. We thank Lani Francis from the Scripps Research Institute vivarium for her assistance with breeding and genotyping GFAP-TK rats. This work was supported by grants from the National Institute on Drug Abuse, USA (DA034140 to C.D.M.) and National Institute of Alcoholism and Alcohol Abuse, USA (AA020098, AA06420 to C.D.M. and T32AA00747 and F32AA023690 to M.C.S.) and start-up funds from Veterans Medical Research Foundation (C.D.M.). A significant proportion of this work was submitted in part as Master’s Thesis by K.K.M. to the Division of Biological Sciences, University of California, San Diego.
Footnotes
Conflict of Interest
The authors declare no competing financial interests in relation to the work described.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Achanta P, Fuss M & Martinez JL Jr. (2009) Ionizing radiation impairs the formation of trace fear memories and reduces hippocampal neurogenesis. Behav Neurosci, 123, 1036–1045. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang WN & Feldon J (2003) Dorsal hippocampus and classical fear conditioning to tone and context in rats: effects of local NMDA-receptor blockade and stimulation. Hippocampus, 13, 657–675. [DOI] [PubMed] [Google Scholar]
- Beeman CL, Bauer PS, Pierson JL & Quinn JJ (2013) Hippocampus and medial prefrontal cortex contributions to trace and contextual fear memory expression over time. Learn Mem, 20, 336–343. [DOI] [PubMed] [Google Scholar]
- Bengochea O & Gonzalo LM (1990) Effect of chronic alcoholism on the human hippocampus. Histol Histopathol, 5, 349–357. [PubMed] [Google Scholar]
- Bouchet CA, Lloyd BA, Loetz EC, Farmer CE, Ostrovskyy M, Haddad N, Foright RM & Greenwood BN (2017) Acute exercise enhances the consolidation of fear extinction memory and reduces conditioned fear relapse in a sex-dependent manner. Learn Mem, 24, 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandao F, Cadete-Leite A, Andrade JP, Madeira MD & Paula-Barbosa MM (1996) Piracetam promotes mossy fiber synaptic reorganization in rats withdrawn from alcohol. Alcohol, 13, 239–249. [DOI] [PubMed] [Google Scholar]
- Brandao ML, Zanoveli JM, Ruiz-Martinez RC, Oliveira LC & Landeira-Fernandez J (2008) Different patterns of freezing behavior organized in the periaqueductal gray of rats: association with different types of anxiety. Behav Brain Res, 188, 1–13. [DOI] [PubMed] [Google Scholar]
- Brandt J, Butters N, Ryan C & Bayog R (1983) Cognitive loss and recovery in long-term alcohol abusers. Arch Gen Psychiatry, 40, 435–442. [DOI] [PubMed] [Google Scholar]
- Broadwater M & Spear LP (2013) Age differences in fear retention and extinction in male Sprague-Dawley rats: effects of ethanol challenge during conditioning. Behav Brain Res, 252, 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadete-Leite A, Tavares MA, Pacheco MM, Volk B & Paula-Barbosa MM (1989) Hippocampal mossy fiber-CA3 synapses after chronic alcohol consumption and withdrawal. Alcohol, 6, 303–310. [DOI] [PubMed] [Google Scholar]
- Chowdhury N, Quinn JJ & Fanselow MS (2005) Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behav Neurosci, 119, 1396–1402. [DOI] [PubMed] [Google Scholar]
- Cohen A, Soleiman MT, Talia R, Koob GF, George O & Mandyam CD (2015) Extended access nicotine self-administration with periodic deprivation increases immature neurons in the hippocampus. Psychopharmacology (Berl), 232, 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cora MC, Kooistra L & Travlos G (2015) Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicologic pathology, 43, 776–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE & Polich J (2009) Binge drinking in young adults: Data, definitions, and determinants. Psychol Bull, 135, 142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniawski J, Ree F, Chia C & Otto T (2012) Dorsal versus ventral hippocampal contributions to trace and contextual conditioning: differential effects of regionally selective NMDA receptor antagonism on acquisition and expression. Hippocampus, 22, 1528–1539. [DOI] [PubMed] [Google Scholar]
- Doron A & Goshen I (2017) Investigating the transition from recent to remote memory using advanced tools. Brain Res Bull. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL & Meyerhoff DJ (2011) Cortical Thickness, Surface Area, and Volume of the Brain Reward System in Alcohol Dependence: Relationships to Relapse and Extended Abstinence. Alcohol Clin Exp Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enikolopov G, Overstreet-Wadiche L & Ge S (2015) Viral and transgenic reporters and genetic analysis of adult neurogenesis. Cold Spring Harbor perspectives in biology, 7, a018804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst AJ, Dempster JP, Yee R, Dennis C & Nakano L (1976) Alcohol toxicity, blood alcohol concentration and body water in young and adult rats. J Stud Alcohol, 37, 347–356. [DOI] [PubMed] [Google Scholar]
- Feller DJ, Tso-Olivas DY & Savage DD (1991) Hippocampal mossy fiber zinc deficit in mice genetically selected for ethanol withdrawal seizure susceptibility. Brain Res, 545, 73–79. [DOI] [PubMed] [Google Scholar]
- Frankland PW & Bontempi B (2005) The organization of recent and remote memories. Nat Rev Neurosci, 6, 119–130. [DOI] [PubMed] [Google Scholar]
- Frankland PW & Josselyn SA (2016) Hippocampal Neurogenesis and Memory Clearance. Neuropsychopharmacology, 41, 382–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinato MH, Takashima Y, Fannon MJ, Quach LW, Morales Silva RJ, Mysore KK, Terranova MJ, Dutta RR, Ostrom RW, Somkuwar SS & Mandyam CD (2018) Neurogenesis during Abstinence Is Necessary for Context-Driven Methamphetamine-Related Memory. J Neurosci, 38, 2029–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao A, Xia F, Guskjolen AJ, Ramsaran AI, Santoro A, Josselyn SA & Frankland PW (2018) Elevation of Hippocampal Neurogenesis Induces a Temporally Graded Pattern of Forgetting of Contextual Fear Memories. J Neurosci, 38, 3190–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew B, Hauser SR, Taylor RE & Tizabi Y (2008) Desipramine blocks alcohol-induced anxiety- and depressive-like behaviors in two rat strains. Pharmacol Biochem Behav, 91, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC & Davis M (1997) Second-order fear conditioning prevented by blocking NMDA receptors in amygdala. Nature, 388, 471–474. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR & McEchron MD (2005) Single neurons in the dentate gyrus and CA1 of the hippocampus exhibit inverse patterns of encoding during trace fear conditioning. Behav Neurosci, 119, 164–179. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M & Koob GF (2008a) Vapor inhalation of alcohol in rats. Curr Protoc Neurosci, Chapter 9, Unit 9 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Lumeng L & Koob GF (2008b) Dependence-induced alcohol drinking by alcohol-preferring (P) rats and outbred Wistar rats. Alcohol Clin Exp Res, 32, 1688–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn SW & Parsons OA (1991) Impaired efficiency in female alcoholics' neuropsychological performance. J Clin Exp Neuropsychol, 13, 895–908. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Luthi A & Herry C (2009) Perineuronal nets protect fear memories from erasure. Science, 325, 1258–1261. [DOI] [PubMed] [Google Scholar]
- Goncalves JT, Schafer ST & Gage FH (2016) Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell, 167, 897–914. [DOI] [PubMed] [Google Scholar]
- Goodfellow MJ, Abdulla KA & Lindquist DH (2016) Neonatal Ethanol Exposure Impairs Trace Fear Conditioning and Alters NMDA Receptor Subunit Expression in Adult Male and Female Rats. Alcohol Clin Exp Res, 40, 309–318. [DOI] [PubMed] [Google Scholar]
- Goodfellow MJ, Shin YJ & Lindquist DH (2018) Mitigation of postnatal ethanol-induced neuroinflammation ameliorates trace fear memory deficits in juvenile rats. Behav Brain Res, 338, 28–31. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A & Shors TJ (1999) Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci, 2, 260–265. [DOI] [PubMed] [Google Scholar]
- Gould TJ (2003) Ethanol disrupts fear conditioning in C57BL/6 mice. J Psychopharmacol, 17, 77–81. [DOI] [PubMed] [Google Scholar]
- Hagihara H, Ohira K, Toyama K & Miyakawa T (2011) Expression of the AMPA Receptor Subunits GluR1 and GluR2 is Associated with Granule Cell Maturation in the Dentate Gyrus. Front Neurosci, 5, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ & Josselyn SA (2007) Neuronal competition and selection during memory formation. Science, 316, 457–460. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Nixon K, Rimondini R, Damadzic R, Sommer WH, Eskay R, Crews FT & Heilig M (2010) Long-term suppression of forebrain neurogenesis and loss of neuronal progenitor cells following prolonged alcohol dependence in rats. Int J Neuropsychopharmacol, 13, 583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holehonnur R, Phensy AJ, Kim LJ, Milivojevic M, Vuong D, Daison DK, Alex S, Tiner M, Jones LE, Kroener S & Ploski JE (2016) Increasing the GluN2A/GluN2B Ratio in Neurons of the Mouse Basal and Lateral Amygdala Inhibits the Modification of an Existing Fear Memory Trace. J Neurosci, 36, 9490–9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O & Camp M (2012) Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci, 15, 1359–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta PT, Sun LD, Wilson MA & Tonegawa S (2000) Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron, 25, 473–480. [DOI] [PubMed] [Google Scholar]
- Hunt PS & Barnet RC (2016) Adolescent and adult rats differ in the amnesic effects of acute ethanol in two hippocampus-dependent tasks: Trace and contextual fear conditioning. Behav Brain Res, 298, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa R, Fukushima H, Frankland PW & Kida S (2016) Hippocampal neurogenesis enhancers promote forgetting of remote fear memory after hippocampal reactivation by retrieval. eLife, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs NS, Cushman JD & Fanselow MS (2010) The accurate measurement of fear memory in Pavlovian conditioning: Resolving the baseline issue. J Neurosci Methods, 190, 235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Ferrara NC, Kwapis JL & Helmstetter FJ (2016) CaMKII regulates proteasome phosphorylation and activity and promotes memory destabilization following retrieval. Neurobiol Learn Mem, 128, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Kwapis JL, Werner CT, Parsons RG, Gafford GM & Helmstetter FJ (2012) The timing of multiple retrieval events can alter GluR1 phosphorylation and the requirement for protein synthesis in fear memory reconsolidation. Learn Mem, 19, 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser AA, Turnbull LM, Darian MA, Feldman DE, Song I & Tronson NC (2017) Sex Differences in Context Fear Generalization and Recruitment of Hippocampus and Amygdala during Retrieval. Neuropsychopharmacology, 42, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Zamora-Martinez ER, Edwards S & Mandyam CD (2015) Structural reorganization of pyramidal neurons in the medial prefrontal cortex of alcohol dependent rats is associated with altered glial plasticity. Brain Struct Funct, 220, 1705–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella S, Konig HG & Prehn JH (2016) Bid Promotes K63-Linked Polyubiquitination of Tumor Necrosis Factor Receptor Associated Factor 6 (TRAF6) and Sensitizes to Mutant SOD1-Induced Proinflammatory Signaling in Microglia. eNeuro, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus F, Paterna JC, Marzorati E, Sigrist H, Gotze L, Schwendener S, Bergamini G, Jehli E, Azzinnari D, Fuertig R, Fontana A, Seifritz E & Pryce CR (2016) Differential effects of peripheral and brain tumor necrosis factor on inflammation, sickness, emotional behavior and memory in mice. Brain Behav Immun, 58, 310–326. [DOI] [PubMed] [Google Scholar]
- Kutlu MG & Gould TJ (2016) Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: contributions to development and maintenance of addiction. Learn Mem, 23, 515–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron-Milad K, Tsareva A, Ahmed N & Milad MR (2013) Sex differences and estrous cycle in female rats interact with the effects of fluoxetine treatment on fear extinction. Behav Brain Res, 253, 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD (2013) The Interplay between the Hippocampus and Amygdala in Regulating Aberrant Hippocampal Neurogenesis during Protracted Abstinence from Alcohol Dependence. Frontiers in psychiatry, 4, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD & Koob GF (2012) The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends Neurosci, 35, 250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, Geil CR & Nixon K (2016) Prior Binge Ethanol Exposure Potentiates the Microglial Response in a Model of Alcohol-Induced Neurodegeneration. Brain Sci, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V & Lutz B (2002) The endogenous cannabinoid system controls extinction of aversive memories. Nature, 418, 530–534. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Tseng W & Disterhoft JF (2000) Neurotoxic lesions of the dorsal hippocampus disrupt auditory-cued trace heart rate (fear) conditioning in rabbits. Hippocampus, 10, 739–751. [DOI] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Corodimas KP, Wilson MC & Ledoux JE (1996) Hippocampal-dependent learning and experience-dependent activation of the hippocampus are preferentially disrupted by ethanol. Neuroscience, 74, 313–322. [DOI] [PubMed] [Google Scholar]
- Ming GL & Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron, 70, 687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E & LeDoux JE (2009) Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science, 324, 951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos J, Alfonso-Loeches S & Guerri C (2016) Impact of the Innate Immune Response in the Actions of Ethanol on the Central Nervous System. Alcohol Clin Exp Res, 40, 2260–2270. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B & Seeburg PH (1994) Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron, 12, 529–540. [DOI] [PubMed] [Google Scholar]
- Ni H, Jiang YW, Tao LY, Cen JN & Wu XR (2009) Effects of penicillin-induced developmental epilepticus on hippocampal regenerative sprouting, related gene expression and cognitive deficits in rats. Toxicol Lett, 188, 161–166. [DOI] [PubMed] [Google Scholar]
- Nixon K (2006) Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus, 16, 287–295. [DOI] [PubMed] [Google Scholar]
- Nixon K & Crews FT (2004) Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci, 24, 9714–9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Kim DH, Potts EN, He J & Crews FT (2008) Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiol Dis, 31, 218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT & Koob GF (2004) Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res, 28, 1676–1682. [DOI] [PubMed] [Google Scholar]
- Peng H, Geil Nickell CR, Chen KY, McClain JA & Nixon K (2017) Increased expression of M1 and M2 phenotypic markers in isolated microglia after four-day binge alcohol exposure in male rats. Alcohol, 62, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson R, Naslund J, Nilsson S, Eriksson E & Hagsater SM (2015) Acute escitalopram but not contextual conditioning exerts a stronger "anxiogenic" effect in rats with high baseline "anxiety" in the acoustic startle paradigm. Psychopharmacology (Berl), 232, 1461–1469. [DOI] [PubMed] [Google Scholar]
- Pierson JL, Pullins SE & Quinn JJ (2015) Dorsal hippocampus infusions of CNQX into the dentate gyrus disrupt expression of trace fear conditioning. Hippocampus, 25, 779–785. [DOI] [PubMed] [Google Scholar]
- Priddy BM, Carmack SA, Thomas LC, Vendruscolo JC, Koob GF & Vendruscolo LF (2017) Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol Biochem Behav, 152, 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Loya F, Ma QD & Fanselow MS (2005) Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus, 15, 665–674. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Ma QD, Tinsley MR, Koch C & Fanselow MS (2008) Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learn Mem, 15, 368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Oommen SS, Morrison GE & Fanselow MS (2002) Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus, 12, 495–504. [DOI] [PubMed] [Google Scholar]
- Reijmers LG, Perkins BL, Matsuo N & Mayford M (2007) Localization of a stable neural correlate of associative memory. Science, 317, 1230–1233. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Chan SH, Crawford EF, Lee YK, Funk CK, Koob GF & Mandyam CD (2009) Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiol Dis, 36, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C (1993) Female rats release more corticosterone than males in response to alcohol: influence of circulating sex steroids and possible consequences for blood alcohol levels. Alcohol Clin Exp Res, 17, 854–859. [DOI] [PubMed] [Google Scholar]
- Rivier C, Rivest S & Vale W (1992) Alcohol-induced inhibition of LH secretion in intact and gonadectomized male and female rats: possible mechanisms. Alcohol Clin Exp Res, 16, 935–941. [DOI] [PubMed] [Google Scholar]
- Romer B, Krebs J, Overall RW, Fabel K, Babu H, Overstreet-Wadiche L, Brandt MD, Williams RW, Jessberger S & Kempermann G (2011) Adult hippocampal neurogenesis and plasticity in the infrapyramidal bundle of the mossy fiber projection: I. Co-regulation by activity. Front Neurosci, 5, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A & Hen R (2011) Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature, 472, 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Bauer EP, Rosis S, Farb CR, Rodrigues SM & LeDoux JE (2005) Memory consolidation of Pavlovian fear conditioning requires nitric oxide signaling in the lateral amygdala. Eur J Neurosci, 22, 201–211. [DOI] [PubMed] [Google Scholar]
- Seo DO, Carillo MA, Chih-Hsiung Lim S, Tanaka KF & Drew MR (2015) Adult Hippocampal Neurogenesis Modulates Fear Learning through Associative and Nonassociative Mechanisms. J Neurosci, 35, 11330–11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T & Gould E (2001) Neurogenesis in the adult is involved in the formation of trace memories. Nature, 410, 372–376. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y & Gould E (2002) Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus, 12, 578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Grigereit L, Russo A, Seib DR, Brewer M, Pickel J & Cameron HA (2016) A Transgenic Rat for Specifically Inhibiting Adult Neurogenesis. eNeuro, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Fannon MJ, Staples MC, Zamora-Martinez ER, Navarro AI, Kim A, Quigley JA, Edwards S & Mandyam CD (2016) Alcohol dependence-induced regulation of the proliferation and survival of adult brain progenitors is associated with altered BDNF-TrkB signaling. Brain Struct Funct, 221, 4319–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H & Frisen J (2013) Dynamics of hippocampal neurogenesis in adult humans. Cell, 153, 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples MC, Fannon MJ, Mysore KK, Dutta RR, Ongjoco AT, Quach LW, Kharidia KM, Somkuwar SS & Mandyam CD (2017) Dietary restriction reduces hippocampal neurogenesis and granule cell neuron density without affecting the density of mossy fibers. Brain Res, 1663, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples MC & Mandyam CD (2016) Thinking after Drinking: Impaired Hippocampal-Dependent Cognition in Human Alcoholics and Animal Models of Alcohol Dependence. Frontiers in psychiatry, 7, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiedl O, Birkenfeld K, Palve M & Spiess J (2000) Impairment of conditioned contextual fear of C57BL/6J mice by intracerebral injections of the NMDA receptor antagonist APV. Behav Brain Res, 116, 157–168. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO & Pfefferbaum A (1995) Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res, 19, 110–122. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Lim KO & Pfefferbaum A (2000) Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: relationships to changes in brain structure. Neuropsychology, 14, 178–188. [PubMed] [Google Scholar]
- Szapiro G, Vianna MR, McGaugh JL, Medina JH & Izquierdo I (2003) The role of NMDA glutamate receptors, PKA, MAPK, and CAMKII in the hippocampus in extinction of conditioned fear. Hippocampus, 13, 53–58. [DOI] [PubMed] [Google Scholar]
- Thomasson HR (1995) Gender differences in alcohol metabolism. Physiological responses to ethanol. Recent Dev Alcohol, 12, 163–179. [DOI] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH & Schinder AF (2008) Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci, 11, 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP & Koob GF (2002) Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res, 26, 1494–1501. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ & Ryabinin AE (2003) Alcohol-induced memory impairment in trace fear conditioning: a hippocampus-specific effect. Hippocampus, 13, 305–315. [DOI] [PubMed] [Google Scholar]
- Woods NI & Kheirbek MA (2017) The Small World of a Fear Memory. Neuron, 94, 226–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T & Otto T (2007) Differential contributions of dorsal vs. ventral hippocampus to auditory trace fear conditioning. Neurobiol Learn Mem, 87, 464–475. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Mennerick S & Izumi Y (2014) Acute and chronic effects of ethanol on learning-related synaptic plasticity. Alcohol, 48, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.