Abstract
Diffuse alveolar hemorrhage after hematopoietic stem cell transplantation is a frequently fatal complication with no standard therapy. Although significant changes in supportive and intensive care measures for patients undergoing hematopoietic stem cell transplantation have been made over the past decades, the impact of these changes on the incidence and outcome of patients with diffuse alveolar hemorrhage has not been examined. We analyzed 1228 patients who underwent allogeneic hematopoietic stem cell transplantation between 2008-2015 at the University of Minnesota to study the incidence, risk factors, and outcomes of diffuse alveolar hemorrhage. Diffuse alveolar hemorrhage developed in 5% of allogeneic hematopoietic stem cell transplant recipients, at a median of 30 days (range +3 to +168 days) after transplantation. The incidence of diffuse alveolar hemorrhage was significantly greater in recipients of umbilical cord blood than peripheral blood or bone marrow grafts (HR: 2.08, 95% CI: 1.16-3.74; P=0.01). In multivariate analysis, delayed neutrophil engraftment or primary graft failure was a risk factor for diffuse alveolar hemorrhage following peripheral blood or bone marrow hematopoietic stem cell transplants (HR: 5.51, 95% CI: 1.26-24; P=0.02) and delayed platelet engraftment was associated with significantly increased diffuse alveolar hemorrhage in umbilical cord blood transplant recipients (HR: 6.96, 95% CI: 2.39-20.29; P<0.05). Myeloablative regimens including total body irradiation were also risk factors for diffuse alveolar hemorrhage (HR: 1.8, 95% CI: 1.03-3.13, P=0.05) in both peripheral blood or bone marrow and umbilical cord blood hematopoietic stem cell transplants (HR: 1.87, 95% CI: 0.95-3.71). Patients with diffuse alveolar hemorrhage had an inferior 6-month treatment-related mortality (HR: 6.09, 95% CI: 4.33-8.56, P<0.01) and 2-year overall survival (HR: 4.16, 95% CI: 3.06-5.64; P<0.01) using either graft source. The etiology of diffuse alveolar hemorrhage is multifactorial, involving lung injury influenced by high-dose total body irradiation, graft source, and delayed engraftment or graft failure. The survival of patients with diffuse alveolar hemorrhage after hematopoietic stem cell transplantation remains poor. Clinical interventions or experimental studies (e.g., cell expansion for umbilical cord blood transplants or thrombopoietin use) that modulate these risk factors may limit the incidence and improve the outcomes of diffuse alveolar hemorrhage.
Introduction
Pulmonary complications occur frequently in patients after hematopoietic stem cell transplantation (HCT). Diffuse alveolar hemorrhage (DAH) is a serious pulmonary complication with a high mortality rate after HCT.1,2 The incidence of DAH varies between 3% and 10% after allogeneic HCT.3,4
The clinical symptoms of DAH include cough, hypoxemia, fever, and rarely hemoptysis.5 Chest radiography shows non-specific bilateral areas of ground-glass attenuation and patchy areas of consolidation.6 Bronchoalveolar lavage findings are characteristic for the diagnosis of DAH with progressively bloodier lavage returns. The etiology of DAH remains unknown, but lung tissue injury, inflammation and cytokine release have all been implicated in the pathogenesis of DAH.5 Some may require that no microorganism is isolated from the bronchoalveolar lavage fluid; however, DAH syndromes after allogeneic HCT have been shown to have similar clinical characteristics and presentation with or without an associated infection.3,7
The reported outcomes of patients with DAH after allogeneic HCT have been dismal. Generally, patients with DAH require intensive care unit support and often mechanical ventilation because of severe hypoxemia. The mortality rate can reach 70-100% in patients with DAH because of multiorgan failure and/or sepsis.5,8–10
Recent changes in allogeneic HCT and supportive care practices include: (i) more frequent use of alternative donor allografts [e.g., umbilical cord blood (UCB) and haploidentical donors]; (ii) use of reduced intensity conditioning for older patients or those with comorbidities; (iii) advances in the use of antibiotics targeting fungal and viral infection; and (iv) improvements in intensive care unit support. We therefore evaluated the impact of these changes on the contemporary incidence, risks, and outcomes of DAH after allogeneic HCT.
Methods
All patients who received peripheral blood stem cells (PB), bone marrow (BM) or UCB HCT between 2008 and 2015 at the University of Minnesota were included. The data were prospectively collected in our institutional Blood and Marrow Transplant Database on patients who had provided written informed consent to Institutional Research Board-approved studies, supplemented by individual medical record review as needed.
Definitions
Patients received myeloablative or reduced intensity conditioning regimens (based on age ≥55 years or significant comorbidities, as previously described).11 The dose of total body irradiation (TBI) differed, being 1320 cGy in fractionated doses for myeloablative conditioning and 200 cGy for reduced intensity conditioning regimens. UCB HCT grafts were matched at four to six loci of six HLA-A, -B (antigen level), and -DRB1 (allele level) to the recipient and in patients receiving two UCB units were generally similarly matched to each other.12 HLA-matched was defined as 6/6 (or 4-5/6 + 5-6/6 for double UCB) and 8/8 for PB and BM graft sources with graft nucleated cell doses and CD34 content as previously described.13,14 Standard risk was defined as patients with leukemia, lymphoma or other malignancy in first or second complete remission, chronic myelogenous leukemia in first chronic phase, myelodysplastic syndrome without excess blasts, or nonmalignant diseases. All other patients were considered high risk.
DAH was diagnosed by strict clinical criteria and alveolar lavage in all patients during fiberoptic bronchoscopy. The criteria included acute onset of hypoxemia with presence of diffuse pulmonary infiltrates on a chest X-ray or computed tomography scan and the presence of progressively bloodier return with each subsequent bronchoalveolar lavage.2,15 Aliquots of saline were successively instilled and aspirated and then visually examined to detect the presence of hemorrhage. The bronchoalveolar lavage fluid was submitted for cytological and microbiological examination. Cytological evaluation included specimen review using potassium hydroxide, Giemsa, and Papanicolaou stains. Appropriate microbiological and cytological examinations for bacteria, fungi, mycobacteria, Pneumocystis jiroveci, and viruses were also performed. For this study, we classified all patients as having DAH regardless of documented infection from bronchoalveolar lavage given our previous analysis demonstrating that infection-associated alveolar hemorrhage and DAH were related clinical syndromes with similar clinical presentation and risks.3
Supportive measures for the management of alveolar hemorrhage included correction of platelet and coagulation abnormalities, careful maintenance of fluid and electrolyte balance, and aggressive ventilator and oxygen support. All patients received prophylactic and empiric antimicrobial agents as clinically indicated. Corticosteroids, administered in most patients (>90%), consisted of a standard regimen of high-dose methylprednisolone, 500 mg twice a day for 3 days, followed by 250 mg twice a day for 3 days, 125 mg twice a day for 3 days, 60 mg twice a day for 3 days, and then 60 mg once a day tapered off over a 2-month period. Pediatric patients also received a similar dose and schedule of methylprednisolone with the dose of methylprednisolone adjusted per body surface area (i.e., starting dose 250 mg/m2/dose intravenously, twice a day for 3 days).
Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count ≥0.5×109/L. Event times for neutrophil and platelet recovery were measured from the date of transplantation and were censored for death or disease progression before day 21 without neutrophil recovery. Platelet engraftment was defined as the first day when the platelet count was >20×109/L and subsequently remained so without transfusions for 7 days. Neutrophil counts that never decreased below 0.5×109/L and platelet counts that never decreased below 20×109/L (without transfusion support) were defined as indicating engraftment at day +1 for either cell lineage. Primary graft failure was defined as a lack of neutrophil recovery at day 42 or less than 10% marrow reconstitution of donor origin, even with neutrophil recovery.
Statistical analysis
The baseline characteristics of the patients and their transplants and information on post-transplantation complications and outcomes were prospectively collected by the Biostatistical Support Group at the University of Minnesota using standardized collection procedures. Demographic and transplant characteristics were summarized by standard descriptive statistical methods. The statistical comparison of categorical variables was performed using a chi-square test, while the Kruskal-Wallis (Wilcoxon) rank-sum test was used for comparisons of continuous variables between patients with and without DAH.
A cumulative incidence estimator was used to calculate the probabilities of neutrophil engraftment, DAH and infection, reflecting non-event deaths as a competing risk. The cumulative incidence of treatment-related mortality was also calculated, reflecting relapse as a competing risk.16 Fine and Gray regression analysis was used to compare the differences between cumulative incidence curves for the endpoints of neutrophil engraftment, treatment-related mortality, DAH and infection.17 The Kaplan-Meier method was used to estimate the probabilities of disease-free survival and overall survival through 2 years after allogeneic HCT and the log-rank test was used for univariate comparisons.18 A Cox proportional hazard regression model was used to estimate differences between the adjusted survival curves,19 with DAH being treated as a time-dependent variable.
Factors that were considered in regression models included gender (male versus female), age at transplant (0-35 years versus ≥35 years), recipient’s cytomegalovirus serostatus (positive versus negative), intensity of the transplant conditioning regimens (myeloablative versus reduced intensity), graft source (PB/BM versus UCB), TBI use (yes versus no), composite factor of TBI and conditioning intensity, diagnosis (malignant versus nonmalignant), neutrophil engraftment (treated as a time-dependent variable), platelet engraftment (treated as a time-dependent variable), disease risk (standard risk versus high risk) and greatest mismatch for HLA disparity considering the worst matched of the two UCB units (matched versus mismatched). Factors with a univariate P-value <0.15 or those with established potential clinical importance were included in the multivariate analysis. Prognostic factor models for all clinical outcomes were built using a backward selection method (P<0.05 was considered significant for remaining in the model). All statistical analyses were implemented using Statistical Analysis System statistical software version 9.3 (SAS Institute Inc., Cary, NC, USA). The cut-off significance level for all P values was 0.05.
Results
A total of 1228 patients undergoing allogenic HCT were included in the study: 658 received PB/BM grafts and 570 received UCB grafts. There were significant differences between PB/BM recipients and UCB graft recipients regarding patient- and disease-characteristics (Online Supplementary Table S1). The median total nucleated cell count was higher and the median time-to-engraftment was shorter in PB/BM graft recipients than in UCB graft recipients.
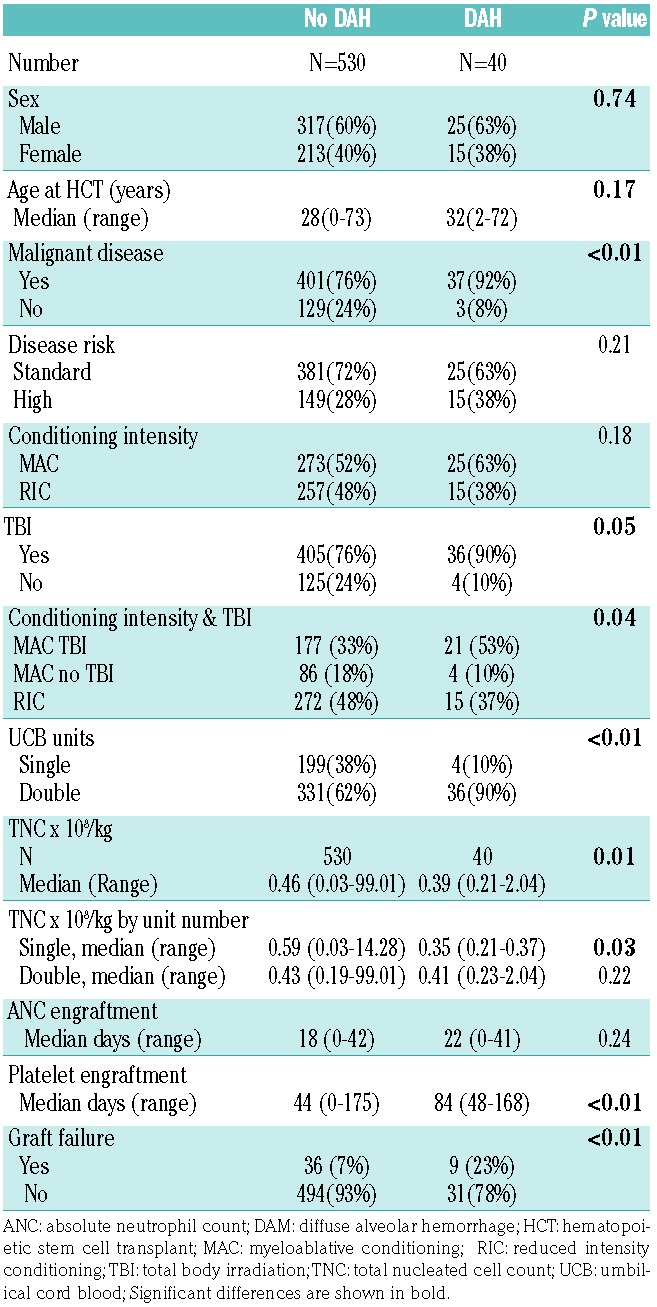
DAH was diagnosed in 59 patients (5%) (Table 1) at a median of 30 days (26 days for BM/PB and 33 days for UCB grafts) after HCT. The median time from HCT to neutrophil engraftment was 19 days (14 days for BM/PB and 22 days for UCB) while that for platelet engraftment was 63 days (20 days for BM/PB and 84 days for UCB grafts). Fifty-one percent of patients had DAH within the 2 weeks preceding or after neutrophil engraftment. Seventy-nine percent of patients with DAH had no platelet engraftment at the time of DAH.
Table 1.
Patient and transplant characteristics.

The patients with DAH had a median age of 32 years, 66% were male, 85% had a malignant disease, 42% had a history of smoking, 5.1% had a history of pre-HCT lung disease, 54% were seropositive for cytomegalovirus, 59% received myeloablative conditioning, 86% received TBI, 64% were HLA-matched and 20% had a sibling donor. At the time of DAH, the median platelet count was 24×109/L (range, 1.0-114), 34% had renal dysfunction (creatinine >1.3 mg/dL), 34% had abnormal liver function (alanine transaminase >35 U/L), 8% had an elevated international normalized ratio >1.5, 25% had a prolonged partial thromboplastin time (≥37 s, although in most cases it was <40 s), and no tested patient (n=37) had a low fibrinogen level <180 mg/dL. Forty-six percent of patients had fever, 27% had a documented systemic infection, and in 19% of cases an infectious organism was isolated from the bronchoalveolar lavage fluid along with the findings of DAH. In patients with DAH, the incidence of grade II-IV acute graft-versus-host disease (GvHD) by day +100 was 36%, with most cases of grade II-IV GvHD (69.5%) occurring prior to DAH. Most patients (92%) received high-dose steroids for the treatment of DAH and seven patients (12%) additionally received an anti-tumor necrotizing factor: etanercept in six cases and infliximab in one. In three patients, anti-tumor necrotizing factor was mainly used for the treatment of concurrent severe gut GvHD. Seventy-five percent of the patients required mechanical ventilator support.
Risk factors
DAH occurred more often in patients who received UCB grafts, TBI at a myeloablative dose, and HLA-mismatched donor grafts: it was also more common among those who had a malignant disease (Table 1). The median time to platelet engraftment (63 days versus 30 days) and to neutrophil engraftment (19 days versus 14 days) was significantly delayed in the group with DAH compared with the group that did not develop DAH (P<0.01 for each). Primary graft failure was significantly more frequent in the DAH group (Table 1). Only 1.6% of patients who never experienced severe thrombocytopenia had DAH compared with 8.2% of patients with severe thrombocytopenia (P=0.04). The median graft cell dose was significantly lower in the group that had DAH (3.1×108/kg) than in the group that did not develop DAH (5.22 × 108/kg) (P<0.01) among PB/BM HCT recipients, whereas among UCB HCT recipients it was lower in those with DAH than in those without (0.39×108/kg versus 0.46×108/kg, respectively; P<0.01) (Table 1). Sex, age, disease risk, recipient cytomegalovirus serology, and the incidence of acute GvHD were all similar between the groups with and without DAH.
DAH was observed in 7% (40/570) of the UCB graft recipients (Table 2). These UCB graft recipients received myeloablative TBI more often than UCB graft recipients without DAH did (90% versus 76%; P=0.05), more often had double UCB grafts (90% versus 62%; P<0.01) and received fewer cells/kg recipient weight [0.39×108/kg (range, 0.21-2.04×108/kg) versus 0.46×1088/kg (range, 0.03-99.01×107/kg; P<0.01]. Neutrophil and platelet engraftment failure (by the time of death or DAH) was strongly associated with DAH (30% versus 9%, and 70% versus 21%, respectively, in the groups with and without DAH) (Table 2).
Table 2.
Characteristics of patients receiving umbilical cord blood hematopoietic stem cell transplantation.
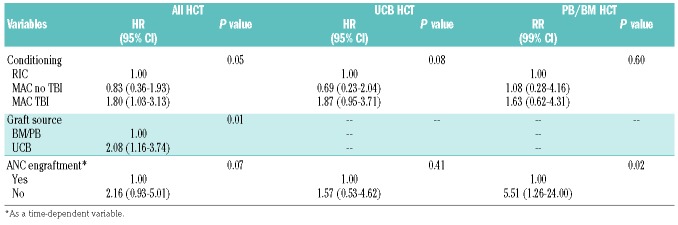
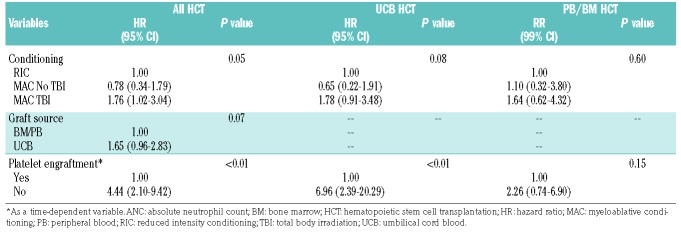
Multivariate analysis showed that UCB HCT recipients had a 2-fold higher incidence of DAH than PB/BM HCT recipients (HR: 2.08, 95% CI: 1.16-3.74; P=0.01). Delayed neutrophil engraftment or graft failure was a risk factor for DAH in PB/BM HCT recipients (HR: 5.51, 95% CI: 1.26-24; P=0.02) whereas delayed platelet engraftment was associated with significantly increased DAH in UCB HCT recipients (HR: 6.96, 95% CI: 2.39-20.29; P<0.05) (Table 3A,B). Two different engraftment models were tested because neutrophil engraftment was strongly correlated with platelet engraftment. TBI at a myeloablative dose was also a risk factor for DAH (HR: 1.8, 95% CI: 1.03-3.13; P=0.05), an effect that was more pronounced in UCB HCT recipients (HR: 1.87, 95% CI: 0.95-3.71; P=0.08) than in BM/PB HCT recipients (HR: 1.63, 95% CI: 0.62-4.31; P=0.60) (Table 3A,B).
Table 3A.
Multivariate analysis of risks for diffuse alveolar hemorrhage: neutrophil engraftment.
Table 3B.
Multivariate analysis for diffuse alveolar hemorrhage: platelet engraftment.
Treatment-related mortality and survival
DAH was associated with higher treatment-related mortality at 6 months (HR: 6.09, 95% CI: 4.33-8.56; P<0.01) and a lower overall survival at 2 years (HR: 4.16, 95% CI: 3.06-5.64; P<0.01) following HCT with either graft source (PB/BM or UCB). UCB was also a risk factor for poorer treatment-related mortality at 6 months (HR: 1.43, 95% CI: 1.08-1.9; P=0.01) and overall survival (HR: 1.22, 95% CI: 1.02-1.48; P=0.03).
A total of 44 patients with DAH (74.5%) required intubation. Of the DAH patients requiring intubation, 54% and 66% died 30 and 60 days after intubation while only 13% and 26% of DAH patients not requiring intubation died by 30 and 60 days after the diagnosis of DAH (P=0.01). Among the intubated patients, UCB HCT recipients had a higher mortality at 6 months compared with the BM/PB graft recipients (84% versus 56%; P=0.05) (Online Supplementary Figure S1). Of seven patients who received an anti-tumor necrotizing factor drug, only one (14%) survived.
Discussion
We observed that even in this recent era, the overall incidence of DAH was 5% for all patients, similar to the incidence recorded in earlier studies.5,20,21 We found that myeloablative TBI, UCB HCT, and delayed engraftment or graft failure were significant risk factors for DAH.
Thrombocytopenia is important in hemorrhagic complications after allogeneic HCT;22,23 however, its importance in DAH is controversial.15,24,25 The relation of platelet recovery with DAH is not direct. In our study, both the severity and duration of thrombocytopenia were significantly associated with DAH. However, the risks of DAH cannot be fully explained solely by low platelet counts given that the median platelet count was >20×109/L in our patients with DAH. Robbins et al. also showed that platelet transfusions did not prevent the development and/or progression of DAH.15 Patients with DAH more often had malignant disease and myeloablative conditioning, but reduced intensity conditioning was not associated with DAH.
We also found that myeloablative conditioning containing TBI was a risk factor for DAH. These findings strongly suggest that direct lung injury at the time of conditioning by myeloablative dose TBI predisposes to DAH after HCT. Others have suggested that irradiation induces cellular damage and plays an important direct role in lung injury26 and the association with high-dose TBI during conditioning contributes to the risk of DAH.15,26–28 Moreover, TBI-induced lung injury may prolong or deepen thrombocytopenia given that in human and some animal studies the lung can contribute to platelet production.29–31 TBI may also damage the vascular and rheological microenvironments of the pulmonary capillaries which may be more hemostatic than the microenvironments of pulmonary epithelial tissues.32
A longer duration of thrombocytopenia was associated with increased DAH in our study. It is well-known that UCB HCT is associated with delayed engraftment/graft failure compared with other related or unrelated grafts.12,15,33–35 Moreover, UCB HCT recipients also received more myeloablative conditioning with TBI, another risk factor for DAH. Overall, we found that UCB grafts were associated with more DAH than PB/BM grafts. In multivariate analysis, UCB HCT was confirmed as an independent risk factor for DAH.
Delayed neutrophil engraftment was another risk factor for DAH, particularly for patients receiving PB/BM grafts. It is known that a higher number of stem cells/total nucleated cells expedites engraftment.36 Patients with DAH received fewer total nucleated cells and more often had delayed engraftment than patients without DAH in both the UCB and PB/BM HCT recipients. Among the patients undergoing single unit UCB HCT, the association between lower infused cell dose and DAH was even more evident.
Most cases of DAH occurred within 2 weeks of neutrophil engraftment. This suggests that a sudden neutrophil influx may contribute to lung injury.1,7,15,37–39 This phenomenon can even occur in a periengraftment period when patients still have neutropenia.15,38,40
Older age (>40 years),3,5,15 severe acute GvHD,3,7,41 and compromised renal function15,41 have each been reported as risk factors for DAH. In our study, neither age nor acute GvHD was recognized as a risk factor. Kidney dysfunction was observed in one-third of the patients with DAH. Clinically apparent coagulopathy, as determined by standard tests, was uncommon in our DAH patients, as in earlier studies.42 Pretransplant respiratory infections have also been described as risk factors for DAH after HCT.10 Although there is no study specifically evaluating the association between smoking and pulmonary complications among HCT recipients, tobacco use and prior lung disease have not been reported as risk factors for DAH.43 Likewise, no correlation was found between smoking status, bronchiolitis index determined by bronchoscopy, or inflammatory cell fractions, and the likelihood of developing DAH in autologous HCT recipients.44 We observed that almost half of the patients with DAH had a history of smoking but were rarely diagnosed with a specific lung disease prior to their allogeneic HCT.
The therapy of DAH remains empirical and thus inadequate, due in part to the unknown pathogenesis of the condition. Because the immune response and inflammation are suggested to contribute to the pathogenesis of DAH, steroid treatment and mechanical ventilator support for acute respiratory failure are used commonly, although often unsuccessfully, for therapy.21,25,38,45 Etanercept or other anti-cytokine agents,46 drugs targeting coagulopathy such as aminocaproic acid and recombinant factor VIIa,47–49 and prophylactic use of an interleukin-1 receptor antagonist to prevent GvHD50 have also been used. All led to poor outcomes in patients with DAH and the mortality rates for this syndrome remain very high3,9,37,38,51–53 due to respiratory failure, sepsis and multi-organ failure.38,54
Our study confirms that the pathogenesis of DAH is complex, affected by conditioning regimen, graft source, and engraftment kinetics and that the outcome remains poor, particularly for patients requiring intubation/mechanical ventilation. Improved management of DAH awaits better understanding of the complex relationship of these multiple risk factors and the definition of the best strategy to expedite engraftment and limit lung injury. Formal testing in comparative trials of therapeutic strategies is needed to validate approaches and limit the high mortality of this devastating syndrome.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/12/2109
References
- 1.Wah TM, Moss HA, Robertson RJ, Barnard DL. Pulmonary complications following bone marrow transplantation. Br J Radiol. 2003;76(906):373–379. [DOI] [PubMed] [Google Scholar]
- 2.Yen KT, Lee AS, Krowka MJ, Burger CD. Pulmonary complications in bone marrow transplantation: a practical approach to diagnosis and treatment. Clin Chest Med. 2004;25(1):189–201. [DOI] [PubMed] [Google Scholar]
- 3.Majhail NS, Parks K, Defor TE, Weisdorf DJ. Diffuse alveolar hemorrhage and infection-associated alveolar hemorrhage following hematopoietic stem cell transplantation: related and high-risk clinical syndromes. Biol Blood Marrow Transplant. 2006;12(10):1038–1046. [DOI] [PubMed] [Google Scholar]
- 4.Gupta S, Jain A, Warneke CL, et al. Outcome of alveolar hemorrhage in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2007;40(1):71–78. [DOI] [PubMed] [Google Scholar]
- 5.Afessa B, Tefferi A, Litzow MR, Peters SG. Outcome of diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med. 2002;166(10):1364–1368. [DOI] [PubMed] [Google Scholar]
- 6.Franquet T, Muller NL, Lee KS, Gimenez A, Flint JD. High-resolution CT and pathologic findings of noninfectious pulmonary complications after hematopoietic stem cell transplantation. AJR Am J Roentgenol. 2005;184(2):629–637. [DOI] [PubMed] [Google Scholar]
- 7.Roychowdhury M, Pambuccian SE, Aslan DL, et al. Pulmonary complications after bone marrow transplantation: an autopsy study from a large transplantation center. Arch Pathol Lab Med. 2005;129(3):366–371. [DOI] [PubMed] [Google Scholar]
- 8.Baker MS, Diab KJ, Carlos WG, Mathur P. Intrapulmonary recombinant factor VII as an effective treatment for diffuse alveolar hemorrhage: a case series. J Bronchol Interv Pulmonol. 2016;23(3):255–258. [DOI] [PubMed] [Google Scholar]
- 9.Agusti C, Ramirez J, Picado C, et al. Diffuse alveolar hemorrhage in allogeneic bone marrow transplantation. A postmortem study. Am J Respir Crit Care Med. 1995;151(4):1006–1010. [DOI] [PubMed] [Google Scholar]
- 10.Diab M, ZazaDitYafawi J, Soubani AO. Major pulmonary complications after hematopoietic stem cell transplant. Exp Clin Transplant. 2016;14(3):259–270. [DOI] [PubMed] [Google Scholar]
- 11.Ustun C, Courville EL, DeFor T, et al. Myeloablative, but not reduced-intensity, conditioning overcomes the negative effect of flow-cytometric evidence of leukemia in acute myeloid leukemia. Biol Blood Marrow Transplant. 2016;22(4):669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116(22):4693–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102(5):1915–1919. [DOI] [PubMed] [Google Scholar]
- 14.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110(8):3064–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins RA, Linder J, Stahl MG, et al. Diffuse alveolar hemorrhage in autologous bone marrow transplant recipients. Am J Med. 1989;87(5):511–518. [DOI] [PubMed] [Google Scholar]
- 16.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16(8):901–910. [DOI] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 19.Cox DR. Regression models and life-tables. J Roy Stat Soc Ser B. 1972;34(2): 187–220. [Google Scholar]
- 20.Feinstein MB, Mokhtari M, Ferreiro R, Stover DE, Jakubowski A. Fiberoptic bronchoscopy in allogeneic bone marrow transplantation: findings in the era of serum cytomegalovirus antigen surveillance. Chest. 2001;120(4):1094–1100. [DOI] [PubMed] [Google Scholar]
- 21.Heggen J, West C, Olson E, et al. Diffuse alveolar hemorrhage in pediatric hematopoietic cell transplant patients. Pediatrics. 2002;109(5):965–971. [DOI] [PubMed] [Google Scholar]
- 22.Lunde LE, Dasaraju S, Cao Q, et al. Hemorrhagic cystitis after allogeneic hematopoietic cell transplantation: risk factors, graft source and survival. Bone Marrow Transplant. 2015;50(11):1432–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nevo S, Enger C, Hartley E, et al. Acute bleeding and thrombocytopenia after bone marrow transplantation. Bone Marrow Transplant. 2001;27(1):65–72. [DOI] [PubMed] [Google Scholar]
- 24.Jules-Elysee K, Stover DE, Yahalom J, White DA, Gulati SC. Pulmonary complications in lymphoma patients treated with high-dose therapy autologous bone marrow transplantation. Am Rev Respir Dis. 1992;146(2):485–491. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Abraham R, Paret G, Cohen R, et al. Diffuse alveolar hemorrhage following allogeneic bone marrow transplantation in children. Chest. 2003;124(2):660–664. [DOI] [PubMed] [Google Scholar]
- 26.Nusair S, Breuer R, Shapira MY, Berkman N, Or R. Low incidence of pulmonary complications following nonmyeloablative stem cell transplantation. Eur Respir J. 2004;23(3):440–445. [DOI] [PubMed] [Google Scholar]
- 27.Shankar G, Scott Bryson J, Darrell Jennings C, Kaplan AM, Cohen DA. Idiopathic pneumonia syndrome after allogeneic bone marrow transplantation in mice. Role of pretransplant radiation conditioning. Am J Respir Cell Mol Biol. 1999;20(6):1116–1124. [DOI] [PubMed] [Google Scholar]
- 28.Escuissato DL, Warszawiak D, Marchiori E. Differential diagnosis of diffuse alveolar haemorrhage in immunocompromised patients. Curr Opin Infect Dis. 2015;28(4):337–342. [DOI] [PubMed] [Google Scholar]
- 29.Lefrancais E, Ortiz-Munoz G, Caudrillier A, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544(7648):105–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zucker-Franklin D, Philipp CS. Platelet production in the pulmonary capillary bed: new ultrastructural evidence for an old concept. Am J Pathol. 2000;157(1):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufman RM, Airo R, Pollack S, Crosby WH. Circulating megakaryocytes and platelet release in the lung. Blood. 1965;26(6):720–731. [PubMed] [Google Scholar]
- 32.Kroll MH, Afshar-Kharghan V. Platelets in pulmonary vascular physiology and pathology. Pulm Circ. 2012;2(3):291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisdorf D, Eapen M, Ruggeri A, et al. Alternative donor transplantation for older patients with acute myeloid leukemia in first complete remission: a center for international blood and marrow transplant research-eurocord analysis. Biol Blood Marrow Transplant. 2014;20(6):816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodrigues CA, Rocha V, Dreger P, et al. Alternative donor hematopoietic stem cell transplantation for mature lymphoid malignancies after reduced-intensity conditioning regimen: similar outcomes with umbilical cord blood and unrelated donor peripheral blood. Haematologica. 2014;99(2):370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jillella AP, Ustun C. What is the optimum number of CD34+ peripheral blood stem cells for an autologous transplant? Stem Cells Dev. 2004;13(6):598–606. [DOI] [PubMed] [Google Scholar]
- 37.Weisdorf DJ. Diffuse alveolar hemorrhage: an evolving problem? Leukemia. 2003;17(6):1049–1050. [DOI] [PubMed] [Google Scholar]
- 38.Metcalf JP, Rennard SI, Reed EC, et al. Corticosteroids as adjunctive therapy for diffuse alveolar hemorrhage associated with bone marrow transplantation. University of Nebraska Medical Center Bone Marrow Transplant Group. Am J Med. 1994;96(4):327–334. [DOI] [PubMed] [Google Scholar]
- 39.Chan CK, Hyland RH, Hutcheon MA. Pulmonary complications following bone marrow transplantation. Clin Chest Med. 1990;11(2):323–332. [PubMed] [Google Scholar]
- 40.Capizzi SA, Kumar S, Huneke NE, et al. Periengraftment respiratory distress syndrome during autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27(12):1299–1303. [DOI] [PubMed] [Google Scholar]
- 41.Pena E, Souza CA, Escuissato DL, et al. Noninfectious pulmonary complications after hematopoietic stem cell transplantation: practical approach to imaging diagnosis. Radiographics. 2014;34(3):663–683. [DOI] [PubMed] [Google Scholar]
- 42.Wanko SO, Broadwater G, Folz RJ, Chao NJ. Diffuse alveolar hemorrhage: retrospective review of clinical outcome in allogeneic transplant recipients treated with aminocaproic acid. Biol Blood Marrow Transplant. 2006;12(9):949–953. [DOI] [PubMed] [Google Scholar]
- 43.Ho VT, Weller E, Lee SJ, Alyea EP, Antin JH, Soiffer RJ. Prognostic factors for early severe pulmonary complications after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7(4):223–229. [DOI] [PubMed] [Google Scholar]
- 44.Sisson JH, Thompson AB, Anderson JR, et al. Airway inflammation predicts diffuse alveolar hemorrhage during bone marrow transplantation in patients with Hodgkin disease. Am Rev Respir Dis. 1992;146(2):439–443. [DOI] [PubMed] [Google Scholar]
- 45.Graf L, Stern M. Acute phase after haematopoietic stem cell transplantation: bleeding and thrombotic complications. Hamostaseologie. 2012;32(1):56–62. [DOI] [PubMed] [Google Scholar]
- 46.Yanik GA, Horowitz MM, Weisdorf DJ, Logan BR, Ho VT, Soiffer RJ, et al. Randomized, double-blind, placebocon trolled trial of soluble tumor necrosis factor receptor: enbrel (etanercept) for the treatment of idiopathic pneumonia syndrome after allogeneic stem cell transplantation: blood and marrow transplant clinical trials network protocol. Biol Blood Marrow Transplant. 2014;20(6):858–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rathi NK, Tanner AR, Dinh A, et al. Low-, medium- and high-dose steroids with or without aminocaproic acid in adult hematopoietic SCT patients with diffuse alveolar hemorrhage. Bone Marrow Transplant. 2015;50(3):420–426. [DOI] [PubMed] [Google Scholar]
- 48.Heslet L, Nielsen JD, Levi M, Sengelov H, Johansson PI. Successful pulmonary administration of activated recombinant factor VII in diffuse alveolar hemorrhage. Crit Care. 2006;10(6):R177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Estella A, Jareno A, Perez-Bello Fontaina L. Intrapulmonary administration of recombinant activated factor VII in diffuse alveolar haemorrhage: a report of two case stories. Cases J. 2008;1(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Antin JH, Weisdorf D, Neuberg D, et al. Interleukin-1 blockade does not prevent acute graft-versus-host disease: results of a randomized, double-blind, placebo-controlled trial of interleukin-1 receptor antagonist in allogeneic bone marrow transplantation. Blood. 2002;100(10):3479–3482. [DOI] [PubMed] [Google Scholar]
- 51.Raptis A, Mavroudis D, Suffredini A, Molldrem J, Rhee FV, Childs R, et al. High-dose corticosteroid therapy for diffuse alveolar hemorrhage in allogeneic bone marrow stem cell transplant recipients. Bone Marrow Transplant. 1999;24(8):879–883. [DOI] [PubMed] [Google Scholar]
- 52.Srivastava A, Gottlieb D, Bradstock KF. Diffuse alveolar haemorrhage associated with microangiopathy after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1995;15(6):863–867. [PubMed] [Google Scholar]
- 53.Huaringa AJ, Leyva FJ, Giralt SA, Blanco J, Signes-Costa J, Velarde H, et al. Outcome of bone marrow transplantation patients requiring mechanical ventilation. Crit Care Med. 2000;28(4):1014–1017. [DOI] [PubMed] [Google Scholar]
- 54.Lewis ID, DeFor T, Weisdorf DJ. Increasing incidence of diffuse alveolar hemorrhage following allogeneic bone marrow transplantation: cryptic etiology and uncertain therapy. Bone Marrow Transplant. 2000;26(5):539–543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.