Abstract
Ibrutinib and acalabrutinib are irreversible inhibitors of Bruton tyrosine kinase used in the treatment of B-cell malignancies. They bind irreversibly to cysteine 481 of Bruton tyrosine kinase, blocking autophosphorylation on tyrosine 223 and phosphorylation of downstream substrates including phospholipase C-γ2. In the present study, we demonstrate that concentrations of ibrutinib and acalabrutinib that block Bruton tyrosine kinase activity, as shown by loss of phosphorylation at tyrosine 223 and phospholipase C-γ2, delay but do not block aggregation in response to a maximally-effective concentration of collagen-related peptide or collagen. In contrast, 10- to 20-fold higher concentrations of ibrutinib or acalabrutinib block platelet aggregation in response to glycoprotein VI agonists. Ex vivo studies on patients treated with ibrutinib, but not acalabrutinib, showed a reduction of platelet aggregation in response to collagen-related peptide indicating that the clinical dose of ibrutinib but not acalabrutinib is supramaximal for Bruton tyrosine kinase blockade. Unexpectedly, low concentrations of ibrutinib inhibited aggregation in response to collagen-related peptide in patients deficient in Bruton tyrosine kinase. The increased bleeding seen with ibrutinib over acalabrutinib is due to off-target actions of ibrutinib that occur because of unfavorable pharmacodynamics.
Introduction
The major physiological ligands that activate platelets in hemostasis and thrombosis signal through G protein-coupled and tyrosine kinase-linked receptors. The former includes receptors for thrombin (PAR1, PAR4), thromboxane A2 (TP) and ADP (P2Y1, P2Y12), and the latter receptors for collagen/fibrin (glycoprotein VI: GPVI), podoplanin (CLEC-2), von Willebrand factor (GPIb-IX-V) and fibrinogen (integrin αIIbβ3).1,2
GPVI is a receptor for collagen and fibrin which forms a complex with the Fc receptor γ-chain (FcRg).2–4 GPVI triggers powerful platelet activation through Src, Syk and Tec family tyrosine kinases leading to activation of phospholipase C- γ2 (PLC γ2).5 GPVI is expressed exclusively on platelets and the platelet precursor cell, the megakaryocyte.6 Mice deficient in GPVI have a minor increase in tail bleeding times but fail to form occlusive thrombi in a FeCl3 injury arterial thrombosis assay.7 Patients homozygous for an insertion that introduces a stop codon and prevents expression of the immunoglobulin receptor on the platelet surface have a relatively mild bleeding diathesis,8 although there are too few of such individuals to determine whether they are protected from thrombosis.
Bruton tyrosine kinase (Btk) is a member of the Tec family of tyrosine kinases and mediates phosphorylation and activation of PLC γ2 downstream of GPVI and the B-cell antigen receptor. The irreversible Btk inhibitor ibrutinib has been introduced into the clinic for treatment of B-cell malignancies but has been reported to increase rates of major hemorrhage in a subgroup of patients.9,10 The increase in bleeding has been attributed to a loss of platelet activation by GPVI11–13 and GPIb,11 with the inhibition of the two receptors having been shown to correlate.14
In contrast to ibrutinib-treated subjects, patients with X-linked agammaglobulinemia (XLA) do not bleed excessively.15 XLA is caused by mutations in the BTK gene which result in a loss or reduction of Btk expression, or expression of a non-functional protein. A potential explanation for this difference in bleeding propensity is that ibrutinib blocks activation of platelets by both Btk and the closely related kinase Tec. Tec is expressed in human and mouse platelets, and has been shown to support PLC γ2 activation in mouse platelets.16 Interestingly, major hemorrhage is not seen in patients treated with the structurally related Btk inhibitor, acalabrutinib, despite this also inhibiting Btk by covalent modification of C481.9,17 It has been postulated that this is due to its greater selectivity for Btk over Tec in comparison to ibrutinib.17,18
In the present study we compared the inhibitory effects of ibrutinib and acalabrutinib on platelet activation and protein phosphorylation by GPVI alongside ex vivo studies on patients prescribed the two inhibitors, as well as on XLA patients.
Methods
Reagents
Details on the source of reagents and chemical analyses can be found in the Online Supplementary Information.
Light transmission aggregometry
Aggregation was measured in siliconized glass vials at 37°C in a Model 700 aggregometer (ChronoLog, Havertown, PA, USA) with stirring at 1200 rpm. Platelets were warmed to 37°C for 5 min before the experiments. Platelets were pre-incubated with ibrutinib, acalabrutinib or dimethyl sulfoxide (DMSO) vehicle for 5 min prior to agonist addition unless otherwise stated. Results were averaged and the half maximal inhibitory concentration (IC50) values were calculated from these data.
Protein phosphorylation
Washed platelets were pre-treated with 9 μM eptifibatide to block integrin αIIbβ3 activation. Agonists were added while stirring at 1200 rpm in an aggregometer at 37°C for 180 s unless otherwise stated. The platelets were stimulated in the presence of ibrutinib (17 nM - 7 μM), acalabrutinib (50 nM – 200 μM) or vehicle (DMSO). For whole cell lysate experiments, activation was terminated with 5X SDS reducing sample buffer. For immunoprecipitation, 8x108/mL platelets were used and reactions were terminated by addition of 2X ice-cold Nonidet P-40 lysis buffer containing the protease inhibitors sodium orthovanadate (5 μM), leupeptin (10 μg/mL), AEBSF (200 μg/mL), aprotinin (10 μg/mL) and pepstatin (1 μg/mL). Platelet lysates were precleared, and detergent-insoluble debris was discarded. An aliquot was dissolved with SDS sample buffer for detection of total tyrosine phosphorylation. Lysates were incubated with either the indicated antibodies and protein A- or protein G-Sepharose. Lysates were separated by sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE), electro-transferred, and western blotted. Western blots were imaged using ECL autoradiography film. In order to analyze levels of phosphorylation, western blot films were scanned and band intensity measured using ImageJ 1.5 with values normalized to basal levels. Results were averaged and IC50 values were calculated from these data.
Other
Details on the methods for blood sampling, platelet preparation, granule release, [Ca2+]i mobilization, measurement of platelet adhesion under flow, cell lines, plasmids, transfections and the luciferase assay can be found in the Online Supplementary Information.
Statistical analysis
All data are presented as mean ± standard error of the mean (SEM) with statistical significance taken as P<0.05 unless otherwise stated. Statistical analyses, unless otherwise specified, were performed using one-way analysis of variance (ANOVA) with a Bonferroni post-test. Ex vivo platelet aggregation was determined by optical densities, which were compared using a one-way ANOVA with the Tukey multiple comparison test. Correlations of aggregation with tyrosine phosphorylation were assessed using the Pearson correlation coefficient. IC50 values were analyzed using the Welch t-test. All statistical analyses were performed using GraphPad Prism 7.
Ethical approval
Ethical approval for collecting blood from patients and healthy volunteers was granted by the National Research Ethics Service (10/H1206/58) and Birmingham University Internal Ethical Review (ERN_11-0175), respectively. Work on HLA patients has ethical approval via the University of Birmingham HBRC 16-251 Amendment 1.
Results
Inhibition of GPVI-induced platelet aggregation by high concentrations of ibrutinib is reversible
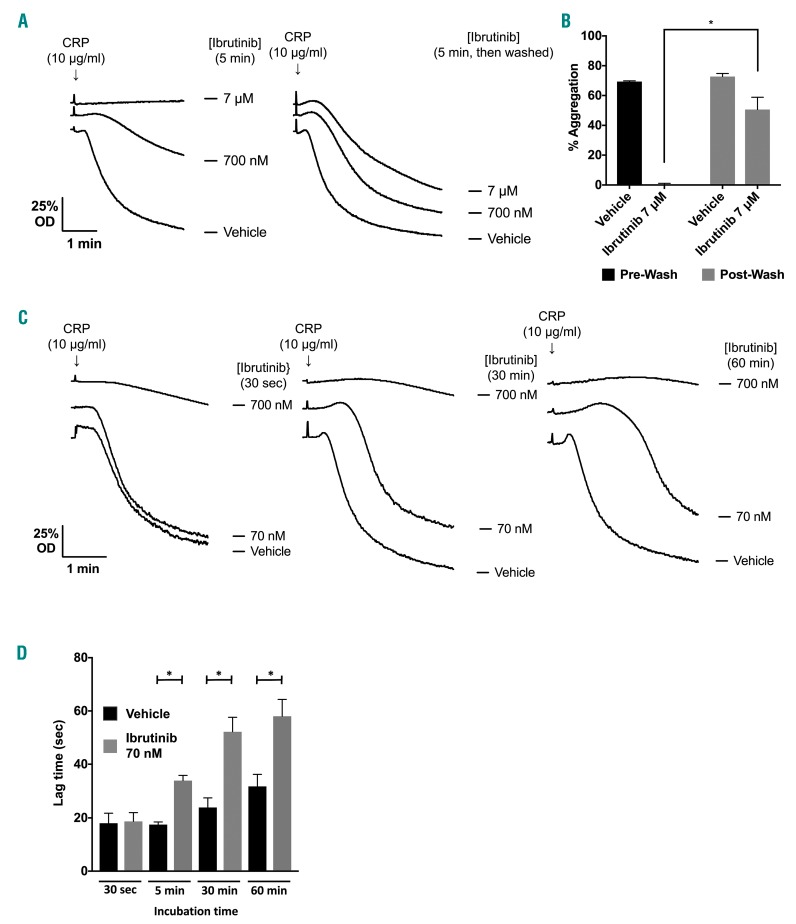
Ibrutinib is 97% bound to plasma proteins and unbound levels reach approximately 0.5 μM in patients.11 At this concentration, ibrutinib has been shown to block GPVI-induced platelet aggregation.12,19 If this is due to inhibition of Btk and other Tec kinases then the inhibition should be irreversible and time-dependent (i.e. inhibition should increase with time). To test this, platelets were treated with a concentration of ibrutinib that causes complete inhibition of GPVI-mediated aggregation in washed platelets before washout of ibrutinib and stimulation with the GPVI-specific agonist collagen-related peptide (CRP). Platelets showed almost full recovery on washout demonstrating that the inhibitory effect is not due solely to covalent modification of Btk or Tec (Figure 1A,B). In support of this, incubation of washed platelets with a high concentration of ibrutinib (700 nM) for ≥30 s was sufficient to block aggregation in response to a high dose of CRP (Figure 1C). However, at a lower concentration, ibrutinib (70 nM) caused a time-dependent delay in aggregation in response to a high concentration of CRP, which was apparent at incubation times of ≥5 min (Figures 1D and 2Ai). This concentration of ibrutinib also caused a reduced response to a sub-maximal concentration of CRP (Online Supplementary Figure S2A). The time-dependent delay is consistent with an irreversible action and contrasts with the rapid onset of inhibition observed at the high concentration of ibrutinib. A similar set of observations was seen in washed platelets stimulated by collagen (Online Supplementary Figure S1A). Similar results were also seen in the presence of 0.3% bovine serum albumin, which was used in case the results were influenced by adsorption of ibrutinib to the surface of the aggregometer tube (Online Supplementary Figure S1B).
Figure 1.
Increasing ibrutinib incubation time has no effect on degree of inhibition of platelet aggregation and this inhibition is reversed by washing. (A) Representative traces of washed platelets at 4x108/mL stimulated with CRP (10 μg/mL for 180 s). Prior to addition of the agonist, platelets were pre-incubated with either ibrutinib or vehicle (DMSO) for 5 min. Alongside, washed platelets at 4x108/mL identically treated with either ibrutinib or vehicle were washed twice in Tyrode buffer and platelets resuspended to 4x108/mL; platelets were then stimulated with CRP (10 μg/mL for 180 s). The data shown are representative of three identical experiments. (B) Mean data for (A) (n=3) analyzed with one-way ANOVA. Results are shown as mean ± SEM. *P<0.05. (C) Washed platelets (4x108/mL) were incubated with ibrutinib or vehicle (DMSO) for 30 s - 60 min before being stimulated with CRP (10 μg/mL). The optical density (OD) of platelet suspensions was measured in a ChronoLog Model 700 aggregometer with stirring at 1200 rpm. Traces representative of three similar experiments are shown. (D) Delay in aggregation seen with ibrutinib-treated washed platelets (n=3) analyzed with one-way ANOVA. Results are shown as mean ± SEM. *P<0.05. aggregation or Ca2+ mobilization.
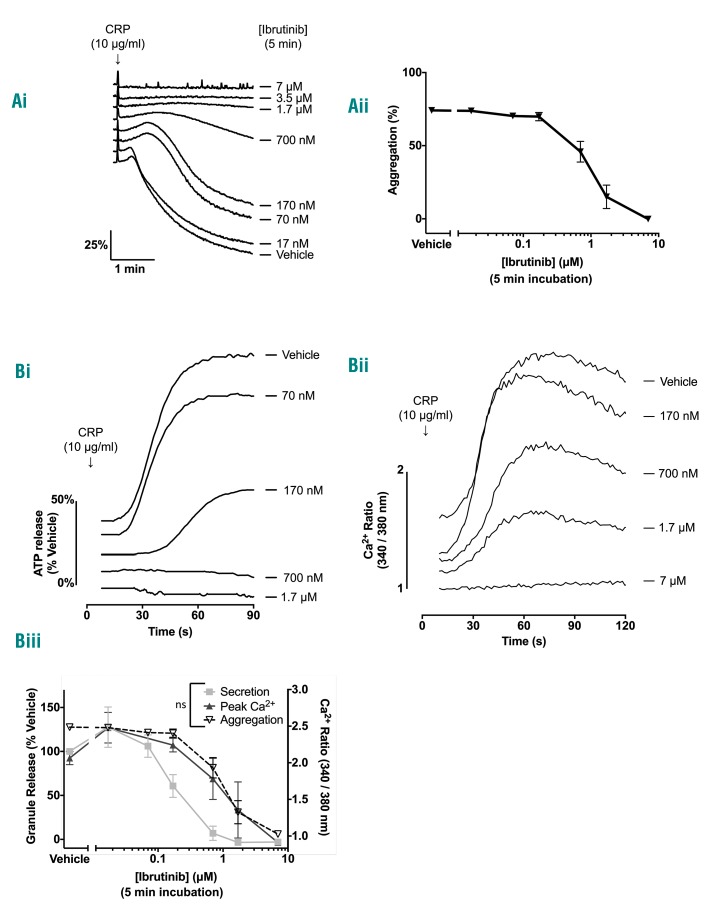
Figure 2.
Ibrutinib dose-dependently inhibits glycoprotein VI-mediated platelet aggregation, ATP secretion and Ca2+ mobilization. (A) (i) Representative traces showing the effect of increasing doses of in vitro ibrutinib incubated for 5 min with washed platelets at 4x108/mL. (ii) Ibrutinib dose-response curves in washed platelets (n=7). (B) Representative traces showing the effect of increasing doses of in vitro ibrutinib incubated with washed platelets at 4x108/mL for 5 min on (i) ATP secretion and (ii) Ca2+ mobilization in response to stimulation with CRP (10 μg/mL) for 180 s. (iii) Ibrutinib dose-response curves in washed platelets on ATP secretion (n=3) and Ca2+ mobilization (n=3). The dose-response curve for inhibition of washed platelet aggregation from (Aiii) is shown as a dotted line to enable comparison. Results are shown as mean ± SEM. All experiments were stimulated with CRP (10 μg/mL). For comparison of IC50: ns = non-significant.
Platelet secretion and Ca2+ mobilization play key roles in platelet activation. Consistent with the results for aggregation, low (70 nM) and high (700 nM) concentrations of ibrutinib had, respectively, no effect or blocked ATP secretion in response to a high concentration of CRP (Figure 2Bi, 2Biii). Similarly, the peak Ca2+ concentration following administration of a high concentration of CRP was not altered in the presence of a low concentration of ibrutinib (70 nM) but was markedly reduced by a high concentration (700 nM). The dose-response curve for inhibition of aggregation was similar to that for loss of Ca2+ mobilization (Figure 2Bii, 2Biii) and was not affected by the presence of the cyclooxygenase inhibitor indomethacin (Online Supplementary Figure S1D). There was no statistical difference between the IC50 of ibrutinib for secretion,
Taken together these results show that ibrutinib has two distinct effects on platelet activation by CRP. At a low concentration (70 nM), ibrutinib delays but does not inhibit activation, whereas at a 10-fold higher concentration of ibrutinib (700 nM) activation is blocked. The latter action is reversible indicating that it is not mediated by covalent modification of Btk or other Tec kinases.
Low-dose ibrutinib blocks Btk but not Tec
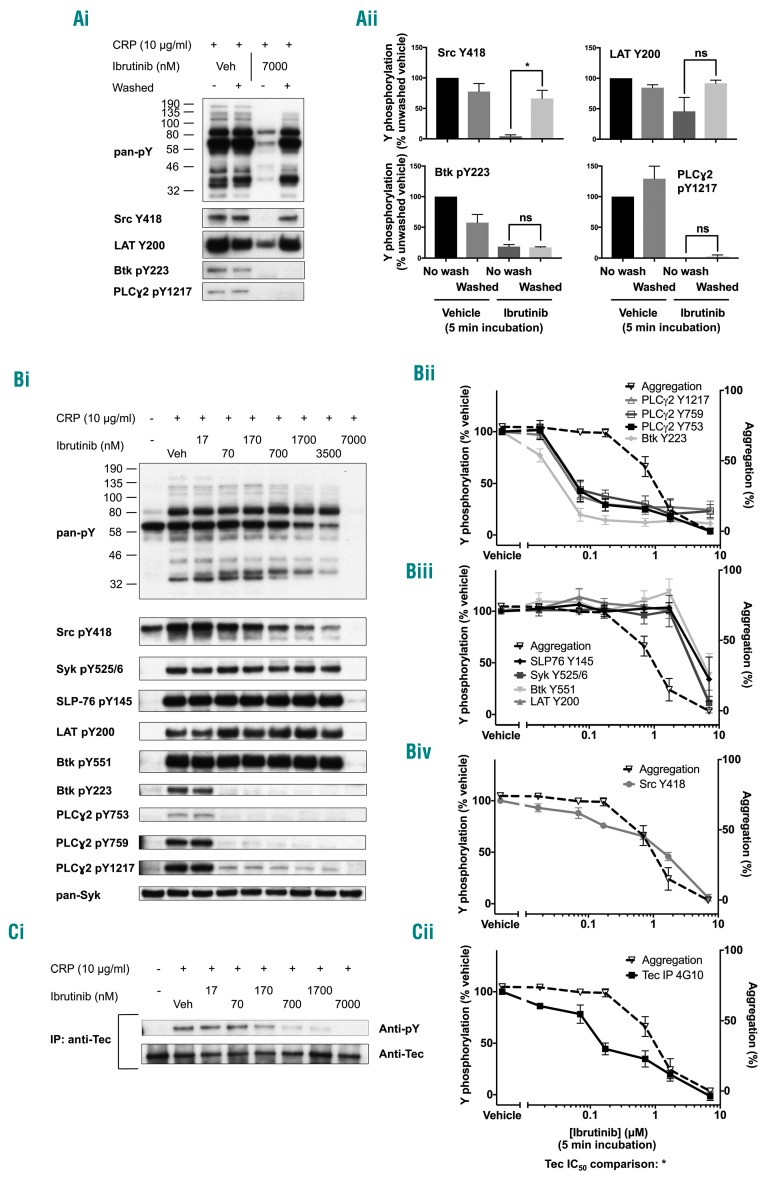
The concentration-response curve to ibrutinib on tyrosine phosphorylation was investigated in washed platelets in the same conditions as for the platelet function studies above. CRP induced robust tyrosine phosphorylation in whole cell lysates which was dose-dependently inhibited by ibrutinib. Correspondingly with the results for aggregation, this inhibitory effect of ibrutinib on global tyrosine phosphorylation was also reversible on washout (Figure 3Ai). Using phosphospecific antibodies, we were able to see that the inhibition of Src pY418 (which lies upstream of Btk) was also reversible but that autophosphorylation of Btk at pY223 and Btk substrates PLCγ2 pY753 and pY121711,20 was irreversible (Figure 3Ai-ii).
Figure 3.
Ibrutinib dose-dependently inhibits glycoprotein VI-mediated signaling. (A) Eptifibatide (9 μM)-treated washed human platelets (4×108/mL) were stimulated with CRP (10 μg/mL for 180 s) followed by lysis with 5X SDS reducing sample buffer. Prior to addition of agonist, platelets were pre-incubated with either ibrutinib or vehicle (DMSO). Some platelets underwent two further washing steps prior to addition of agonist. (i) Whole cell lysates were then separated by SDS-PAGE and western blotted with the stated antibodies for whole cell phosphorylation, kinases and proteins downstream of GPVI. Blots are representative of three experiments. (ii) Percentage tyrosine phosphorylation as compared to that of non-washed vehicle platelets was measured and is represented as the mean ± SEM of three identical experiments. (Bi) Washed platelets were treated as in (A) but with a wider range of ibrutinib doses. (ii - iv) The percentage of tyrosine phosphorylation as compared to that of vehicle-treated platelets was measured and is represented as the mean ± SEM of four identical experiments. The dose-response curve for inhibition of washed platelet aggregation from Figure 2Aii is shown as a dotted line to enable comparison. (C) Eptifibatide (9 μM)-treated washed human platelets (8×108/mL) were stimulated with CRP (10 μg/mL for 180 s) followed by lysis with 2X ice cold lysis sample buffer. Lysates were pre-cleared and Tec was immunoprecipitated before addition of SDS reducing sample buffer and separation by SDS-PAGE and western blotted with the anti-pY antibody 4G10. Membranes were stripped and then reprobed with the pan-Tec antibody. (i) The trace is representative of three identical experiments. (ii) The percentage of tyrosine phosphorylation as compared to that of vehicle-treated platelets was measured and is represented as the mean ± SEM of three identical experiments. *P<0.05. ns = non-significant.
A detailed analysis of the dose response to ibrutinib on a wider range of proteins in the GPVI signaling cascade was investigated using further phosphospecific antibodies (Figure 3Bi). Autophosphorylation of Btk and downstream PLCγ2 was reduced to basal levels by a low dose of ibrutinib (70 nM) (Figure 3Bii). In contrast, phosphorylation of Btk on Y551, which is mediated by Src family kinases,21 and proteins that lie upstream of Btk, namely Src Y418, Syk Y525/6, SLP-76 Y145 and LAT Y200, was not altered (Figure 3Biii-iv). Inhibition of phosphorylation of Src on its activation site, Y418, was observed at a 10-fold higher concentration of ibrutinib (Figure 3Biv) and was shown to correlate with inhibition of aggregation (Pearson correlation coefficient 0.959). Inhibition of whole cell phosphorylation and phosphorylation of Syk Y525/6, SLP-76 Y145, Btk Y551 and LAT Y200 was seen at a 7 μM dose of ibrutinib which is 10-fold higher than the maximal concentration in patients (Figure 3Bii, 3Biii). The IC50 values for each phosphorylation event, when they could be calculated, are included in Online Supplementary Table S1. Blockade of Btk pY223 and PLCγ2 phosphorylation by 70 nM ibrutinib was also observed with lower concentrations of CRP or in the absence of the integrin αIIbβ3 blocker, eptifibatide (Online Supplementary Figures S2Aiv and S2B). There was no significant increase in phosphorylation of PLCγ2 up to 180 s in response to CRP in the presence of 70 nM ibrutinib (Online Supplementary Figure S2Ci-iii).
Due to the absence of phosphospecific antibodies for the Btk-related Tec family kinase, Tec, the effect of ibrutinib on Tec phosphorylation was investigated following immunoprecipitation and re-probing with the antiphosphotyrosine monoclonal antibody 4G10. The effect of ibrutinib was biphasic with partial blockade at 170 nM and full blockade observed at 7 μM (Figure 3C).
These results demonstrate that a concentration of 70 nM ibrutinib is sufficient to block Btk at its autophosphorylation site and on PLCγ2 on Y753, Y759 and Y1217 and that this effect is irreversible. At this concentration of ibrutinib, aggregation in response to a high concentration of CRP is delayed but is not blocked. At a 10- to 20-fold higher concentration, ibrutinib reversibly blocks aggregation in parallel with reversible loss of phosphorylation of Src on Y418. Reversible inhibition of tyrosine phosphorylation of other proteins is seen at a 100-fold higher concentration than that required for blockade of Btk. Ibrutinib causes biphasic inhibition of phosphorylation of Tec, with inhibition occurring at 3- to 5-fold higher concentrations than those required to block phosphorylation of Btk on Y223, and full blockade at a 100-fold higher concentration. These results are consistent with loss of Tec autophosphorylation at 170 nM of ibrutinib and loss of phosphorylation on the activation site by higher concentrations.
Low-dose ibrutinib has no effect on platelet adhesion and aggregation in response to collagen under flow conditions
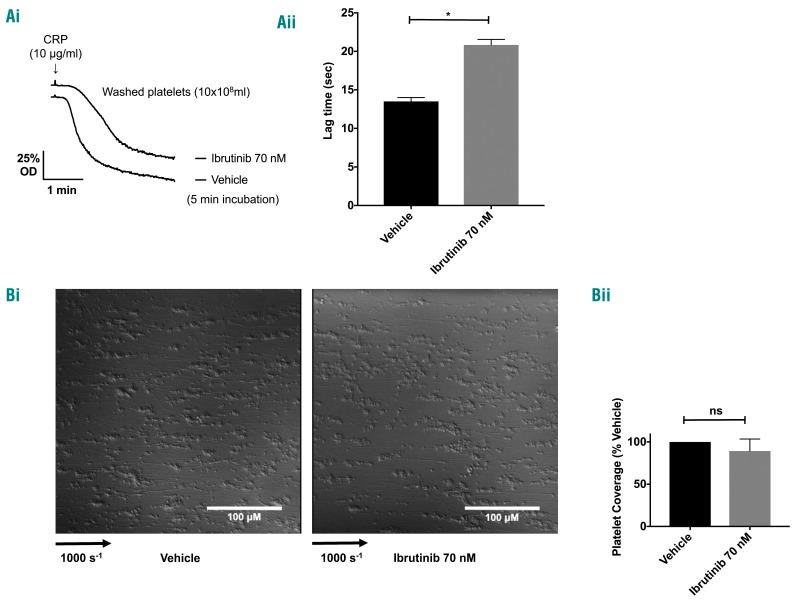
The relevance of the observation that aggregation is delayed but not blocked in response to high concentrations of CRP and collagen was addressed using flow studies in which GPVI functions in conjunction with other tyrosine kinase-linked receptors that also signal via Btk, namely GPIb and integrin αIIbβ3. To ensure that a known degree of Btk blockade was achieved, washed platelets were incubated with ibrutinib at a concentration sufficient to fully and irreversibly inhibit Btk kinase activity (70 nM). Inhibition of Btk autophosphorylation was confirmed by a delay in aggregation in response to CRP (Figure 4A) and by measurement of phosphorylation (data not shown). Following incubation, platelets were reconstituted with autologous red blood cells and platelet-poor plasma and flowed over collagen at arterial shear rates. Adhesion of ibrutinib-treated platelets was unchanged when compared to that of vehicle-treated platelets (Figure 4B).
Figure 4.
A low dose of ibrutinib has no effect on platelet adhesion to collagen under flow. (A) Washed platelets (10x108/mL) were incubated with ibrutinib or vehicle (DMSO) for 5 min and stimulated with CRP (10 μg/mL). (i) Representative trace and (ii) mean data from three identical experiments show a characteristic delay associated with inhibition of Btk autophosphorylation. (B) Platelets were reconstituted with autologous red blood cells and platelet-poor plasma and flowed at arterial shear over collagen-coated microcapillaries for 3 min before being fixed and imaged. (i) Representative differential interference contrast images are shown. (ii) Platelet coverage as a percentage of values for vehicle-treated platelets was calculated and is shown as mean ± SEM from three identical experiments. *P<0.05, ns: non-significant.
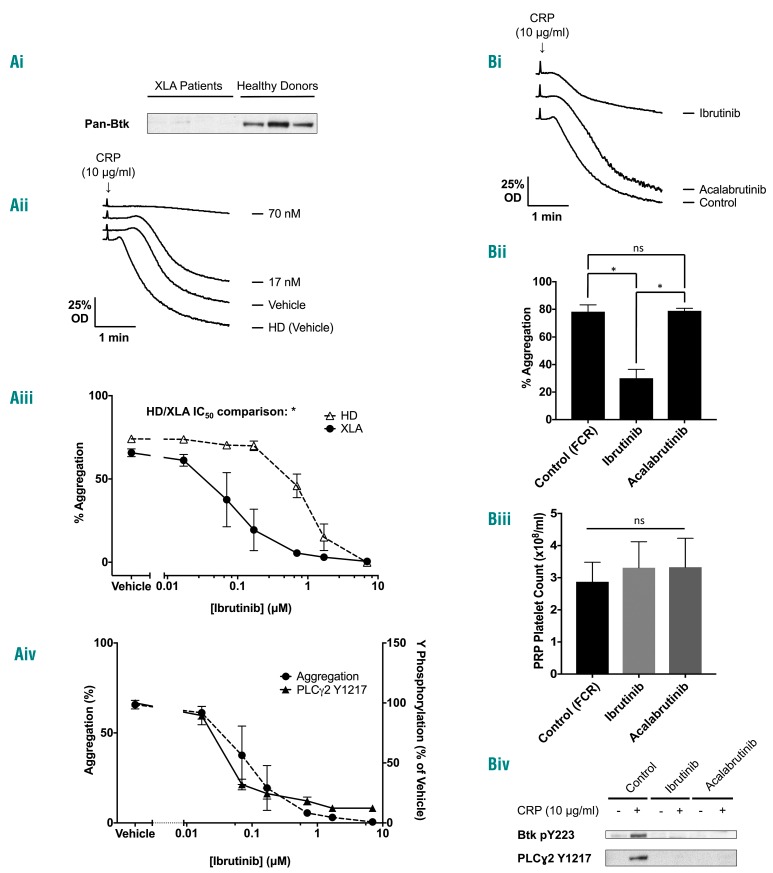
Btk-specific concentrations of ibrutinib block GPVI mediated aggregation in patients with X-linked agammaglobulinemia
The B-cell immunodeficiency XLA is caused by mutations in the BTK gene. Using knowledge of patients’ mutations (Online Supplementary Table S2), and an antibody to the N-terminus of Btk, we selected unrelated patients lacking Btk protein to test for off-target effects of ibrutinib (Figure 5Ai). Strikingly, the concentration-response curve for inhibition of CRP-induced aggregation by ibrutinib was shifted to the left in the XLA patients when compared to that of the healthy donors (Figure 5Aii-iii), whereas the curve for inhibition of PLCγ2 phosphorylation was unchanged (Figure 5Aiv). Since the only known difference between XLA patients and controls is the absence of Btk, this demonstrates an off-target effect of ibrutinib that was unmasked in the absence of Btk protein. This off-target action occurred over a similar concentration range to that required for inhibition of Btk. The GPCR agonists ADP and PAR1 peptide stimulated robust aggregation in XLA patients, as previously demonstrated16 (data not shown).
Figure 5.
Patients with X-linked agammaglobulinemia, who lack Btk expression are more sensitive than healthy donors to ibrutinib inhibition of glycoprotein VI-mediated platelet aggregation. Ibrutinib, but not acalabrutinib, blocks glycoprotein VI-mediated platelet aggregation ex vivo. (A) Citrated blood was taken from XLA patients. (i) Whole cell lysates were then separated by SDS-PAGE and western blot with the polyclonal N-terminal Btk antibody. Platelet-rich plasma (PRP) from XLA patients was stimulated with CRP (10 μg/mL) for 180 s. (ii) A representative aggregation trace of XLA patients or healthy donor (HD). (iii) Ibrutinib dose-response curves in washed platelets of XLA patients (n=4). Healthy donor responses from Figure 2Aiii are shown as a dotted line for comparison. (iv) Whole cell lysates were then separated by SDS-PAGE and western blotted with the phosphospecific antibody to PLCγ2 pY1217 (n=3). The aggregation curve for XLA patients is shown as a dotted line for comparison. (B) Patients taking ibrutinib 420 mg once daily, acalabrutinib 100 mg twice daily or a control chemotherapy regime of fludarabine (25 μg/m2 IV days 1-3), cyclophosphamide (250 μg/m2 IV days 1-3) and rituximab (375 μg/m2 IV day 1) (FCR) had citrated blood taken on day 28 of the treatment cycle (2-3 h after the dose of Btk inhibitor). PRP from this blood was then stimulated with CRP (10 μg/mL) for 180 s. (i) A representative trace. (ii) Mean and SEM from five, nine and three patients for FCR, ibrutinib and acalabrutinib respectively. (iii) Comparison of platelet counts in PRP from all groups of patients. Statistical analysis was performed using a one-way ANOVA with the Tukey multiple comparisons test, *P<0.05, ns=not significant. (iv) A representative western blot from eptifibatide (9 μM)-treated washed platelets (4×108/mL) from the same patients stimulated with CRP (10 μg/mL for 180 s) followed by lysis with 5X SDS sample buffer and probed with Btk pY223 and PLCγ2 pY1217 phosphospecific antibody.
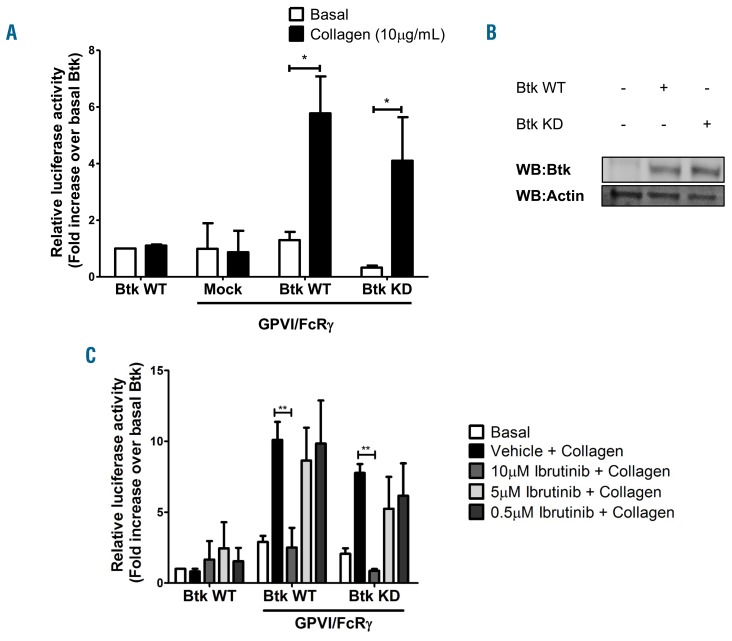
One possible explanation for the increased sensitivity of XLA patients to ibrutinib relative to controls is that Btk also functions as an adapter protein in the GPVI signaling pathway (as the only known difference in these two groups is Btk protein). To investigate this, we transfected Btk-deficient DT40 chicken B cells with GPVI and its signaling partner, FcRg, in the presence of wild-type (WT) or kinase-dead (KD) Btk. Importantly, these cells express PLCγ2 but do not express other Tec family kinases.22,23 The K430E mutant of Btk has been previously reported to lack kinase activity.24 Cells lacking Btk or GPVI were unresponsive to collagen. Cells transfected with WT or KD Btk reconstituted NFAT signaling to a similar degree (Figure 6A,B), demonstrating that Btk also functions as an adapter protein in the GPVI signaling pathway. A low dose of ibrutinib had no effect on cells transfected with WT or KD Btk whereas a high dose of ibrutinib blocked NFAT signaling in both WT and KD transfected cells (Figure 6C).
Figure 6.
Kinase-dead Btk is sufficient for glycoprotein VI signaling. Btk-deficient DT40 cells were transfected with either wild-type (WT) or kinase-dead (KD) Btk with or without GPVI/FcRg. All cells were transfected with a NFAT-luciferase reporter plasmid. Cells were stimulated with collagen (10 μg/mL) in the presence of serum. (A) Luciferase activity was measured and is shown as mean ± SEM of five identical experiments. (B) Representative western blot showing equal Btk expression in WT and KD-transfected cells. (C) Cells were stimulated with collagen (10 μg/mL) in the presence or absence of ibrutinib (0.5 μM – 10 μM). Serum was excluded during stimulation to avoid plasma binding of the drugs. Luciferase activity between vehicle and drug-treated samples was measured and is shown as the mean ± SEM of three independent experiments. *P<0.05, **P<0.01.
Together these results demonstrate that Btk functions as an adapter protein, as well as a kinase, in XLA platelets and in transfected DT40 cells.
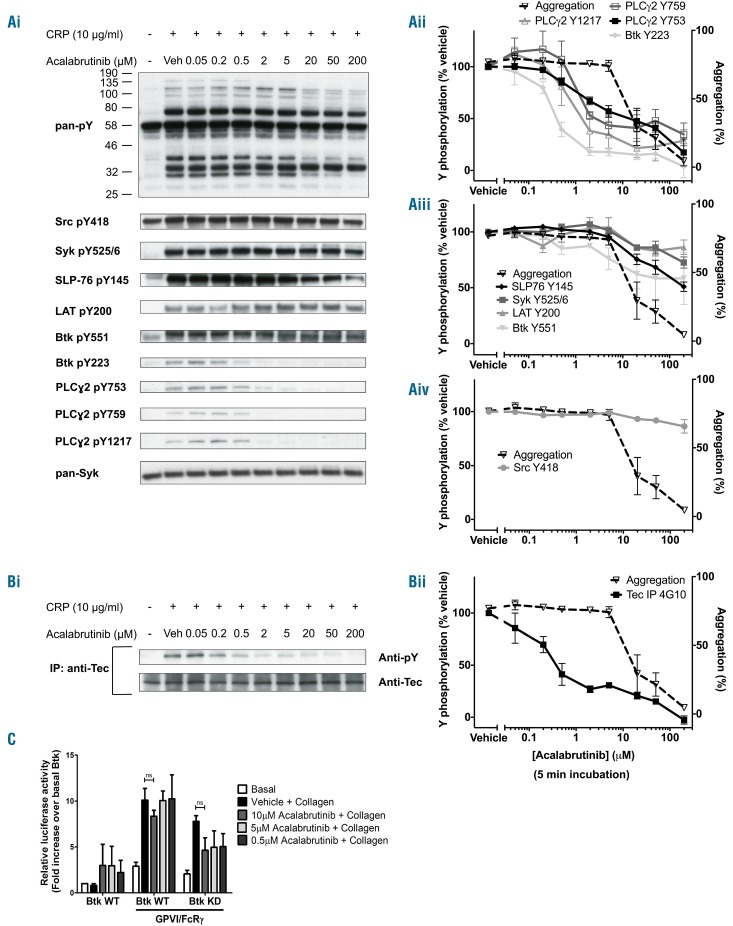
Acalabrutinib inhibits Btk Y223 phosphorylation and platelet aggregation, secretion and Ca2+ mobilization by glycoprotein VI
Studies were extended to a second-generation Btk inhibitor, acalabrutinib, which, like ibrutinib, irreversibly binds to Btk at C481 and is highly plasma protein-bound. Acalabrutinib has a higher selectivity over other tyrosine kinases relative to ibrutinib, including Src, Syk and Tec,25 but a 5-fold lower IC50 for Btk.17 In patients the mean peak free drug concentration of acalabrutinib is 1.3 μM.17
Acalabrutinib has a similar, dose-dependent effect on platelet aggregation to that of ibrutinib. At a concentration of 2 μM in washed platelets, acalabrutinib induced a slight delay in aggregation (Online Supplementary Figure S3A) but had no effect on the overall magnitude of response (Figure 7Aii). The difference in the dose-dependency relative to ibrutinib is consistent with the lower IC50 of acalabrutinib for Btk. Similar to ibrutinib, inhibition of platelet aggregation by CRP occurs at acalabrutinib concentrations that are one order of magnitude higher than those which cause a delay in aggregation; and the curves for inhibition of ATP secretion and Ca2+ mobilization lie slightly to the left of that for aggregation (Online Supplementary Figure S3A-E). As with ibrutinib, acalabrutinib blocked tyrosine phosphorylation of Btk on Y223 and PLCγ2 on Y759 and Y1217 at a concentration (2 μM) that caused a delay in onset but no reduction in aggregation (Figure 7Ai,ii). Higher concentrations of acalabrutinib (up to 200 μM) had no effect on phosphorylation of Src Y418, Syk Y525/6 and LAT Y200 but caused a small reduction in phosphorylation of Btk Y551 and SLP-76 Y145 (Figure 7Ai,iii-iv). Interestingly, acalabrutinib also caused a biphasic inhibition of Tec phosphorylation with partial inhibition observed at approximately 1 μM and full blockade at 200 μM (Figure 7B). The IC50 values for each phosphorylation event are included in Online Supplementary Table S1. Concentrations of acalabrutinib that blocked phosphorylation of Btk in platelets had no effect on NFAT activation by CRP in DT40 cells transfected with WT or KD Btk (Figure 7C).
Figure 7.
Acalabrutinib dose-dependently inhibits glycoprotein VI-mediated signaling. (A) Eptifibatide (9 μM)-treated washed human platelets (4×108/mL) were stimulated with CRP (10 μg/mL for 180 s) followed by lysis with 5X SDS reducing sample buffer. Prior to addition of agonist, platelets were pre-incubated with either acalabrutinib or vehicle (DMSO). (i) Whole cell lysates were then separated by SDS-PAGE and western blot with the stated antibodies for whole cell phosphorylation, kinases and proteins downstream of GPVI. Blots are representative of three experiments. (ii - iv) The percentage of tyrosine phosphorylation as compared to that of vehicle-treated platelets was measured and is represented as the mean ± SEM of three identical experiments. The dose-response curve for inhibition of aggregation from Online Supplementary Figure S3D is shown as a dotted line to enable comparison. (B) Eptifibatide (9 μM)-treated washed human platelets (8×108/mL) were stimulated with CRP (10 μg/mL for 180 s) followed by lysis with 2X ice cold lysis sample buffer. Lysates were precleared and Tec was immunoprecipitated before addition of SDS reducing sample buffer and separation by SDS-PAGE and western blot with the anti-pY antibody 4G10. Membranes were stripped and reprobed with the pan-Tec antibody. The trace is representative of three identical experiments. (C) Btk-deficient DT40 cells were transfected with either wild-type (WT) or kinase-dead (KD) Btk with or without GPVI/FcRg. All cells were transfected with a NFAT-luciferase reporter plasmid. Cells were stimulated with collagen (10 μg/mL) in the presence or absence of acalabrutinib (0.5-10 μM). Serum was excluded during stimulation to avoid plasma binding of the drugs. Luciferase activity between vehicle and drug-treated samples was measured and is shown as the mean ± SEM of three independent experiments. ns = non-significant.
Allowing for the fact that acalabrutinib has a 5-fold lower potency for Btk, these results are in line with those for ibrutinib.
Glycoprotein VI-mediated platelet aggregation is blocked ex vivo in patients taking ibrutinib, but not acalabrutinib
We investigated the effect of ibrutinib and acalabrutinib in patients with chronic lymphoid leukemia (CLL) taking ibrutinib 420 mg once daily, acalabrutinib 100 mg twice daily or a non-Btk targeting control chemotherapy regimen. GPVI-induced platelet aggregation was blocked in the platelet-rich plasma of patients taking ibrutinib but was not blocked in patients taking acalabrutinib or in the control group despite complete inhibition of autophosphorylation of Btk pY223 and its downstream substrate PLCγ2 at pY1217 by both inhibitors (Figure 5Bi-iv). Platelet aggregation induced by the GPCR agonists, ADP and PAR1 peptide, was not altered in the patients taking either inhibitor (data not shown).
Discussion
In this study we show that (i) irreversible blockade of Btk by ibrutinib and acalabrutinib delays but does not block the platelet aggregation induced by high concentrations of GPVI agonists; (ii) blockade of GPVI-mediated aggregation by ibrutinib and acalabrutinib occurs at a concentration one to two orders of magnitude higher than is required to block Btk due to an off-target action which is reversible; (iii) the ratio between inhibition of Btk kinase activity and platelet aggregation induced by GPVI is the same for ibrutinib and acalabrutinib; (iv) clinically relevant concentrations of ibrutinib but not acalabrutinib block activation of platelets by GPVI; (v) platelet adhesion and aggregation under flow conditions is maintained following inhibition of Btk; (vi) Btk supports platelet activation by GPVI by acting as an adapter protein and as a tyrosine kinase; and (vii) ibrutinib blocks platelet aggregation in XLA patients at concentrations that block Btk.
These results show that platelets, in which Btk kinase function and downstream PLCγ2 phosphorylation have been blocked, have a slight delay in aggregation in response to high concentrations of GPVI ligands, while platelet adhesion and aggregation under arterial flow con ditions are unaltered. These observations, together with reports that patients with XLA or those treated with acalabrutinib do not experience major bleeding,15,17 provide powerful evidence that inhibition of Btk does not give rise to major bleeding. The major bleeding observed in patients treated with ibrutinib relative to acalabrutinib is due to the differential dosing regimens of the two Btk inhibitors, with the clinical dose of ibrutinib blocking activation of platelets by GPVI due to one or more off-target effects.
The conclusion that inhibition of Btk does not give rise to major bleeding on treatment with ibrutinib contrasts with the conclusion of the studies by Levade et al.11 and Bye et al.13. Levade et al.11 demonstrated a close correlation between inhibition of autophosphorylation of Btk at Y223 and aggregation in GPVI-activated platelets. While we are unable to explain this in the light of the present observations, we note that Levade et al.11 also reported that phosphorylation of PLCγ2 at Y753, which is mediated by Btk, was inhibited at a 10-fold lower concentration of ibrutinib as is seen in the present study. Bye et al.13 used a single, supramaximal concentration of ibrutinib which also blocked Src phosphorylation for their biochemical and flow-based assays. The determination of full concentration-response curves in the present study has highlighted the mismatch between inhibition of Btk and loss of platelet aggregation, and has provided evidence that the bleeding diathesis that is seen in some ibrutinib-treated patients is due to off-target effects.
An unexpected observation in the present study was that platelets are able to aggregate in response to a high dose of CRP despite the absence of detectable PLCγ2 phosphorylation. One explanation for this is that Btk also supports activation of PLCγ2 as an adapter protein as shown by the observation that transfection of KD Btk restores GPVI signaling in DT40 cells. A similar result has been previously shown for Btk in B-cell receptor signaling.24,26 This is in keeping with previous studies showing that phosphorylation of PLCγ2 at Y1217 is not required for its enzymatic activity in Ramos cells.27
We were surprised to find that platelets from XLA patients, who lack Btk protein, have increased susceptibility to ibrutinib relative to platelets from controls. The only known difference between the XLA patients and controls in the presence of ibrutinib is the absence of Btk protein, although this could also change the balance of activatory and inhibitory phosphorylation within the GPVI signaling cascade. Furthermore, the absence of Btk renders aggregation of these platelets critically dependent on PLCγ2 phosphorylation in contrast to controls. The target for ibrutinib which gives rise to inhibition of aggregation in the XLA patients is not known. There are several kinases that are inhibited by ibrutinib over a similar range of concentrations to that for inhibition of Btk.18 Within this group only Csk is known to be expressed in platelets.28
We have shown that blockade of GPVI-mediated platelet aggregation by ibrutinib is reversible, which contrasts with the irreversible blockade of Btk and Tec.18 The reversibility provides evidence that blockade is not mediated by inhibition of Tec family kinases, as was previously postulated,9,11,17 because Tec also has a cysteine residue in its ATP binding domain analogous to C481 on Btk. This is further supported by the observation that ibrutinib-mediated blockade of NFAT signaling in DT40 cells, which lack Tec, follows a similar pattern as that for platelet aggrega tion; namely no effect at low doses with blockade at high doses. For ibrutinib, we have shown that inhibition of aggregation correlates strongly with loss of phosphorylation at Src Y418. However, this is not altered by acalabrutinib demonstrating an as yet unidentified off-target action. Bye et al.13 also showed that ibrutinib dose-dependently inhibits phosphorylation of Src Y418. However, in a different study they found that both low-dose ibrutinib and acalabrutinib potentiated Src Y418 phosphorylation.29 We were not able to replicate this latter finding.
We have shown that, despite acalabrutinib’s more favorable selectivity to Btk over other Src, Syk and Tec kinases in in vitro kinase assays, the window between Btk inhibition and blockade of GPVI-induced aggregation in vitro is similar to that of ibrutinib. Despite this, acalabrutinib, but not ibrutinib, fails to block GPVI-mediated platelet activation ex vivo. We propose that this is because of the differential dosing and pharmacodynamics of the two Btk inhibitors. Acalabrutinib is used at a dose of 1.5 mg/kg twice daily17,30 and ibrutinib at a single daily dose of 6 mg/kg in CLL or 8 mg/kg in mantle cell lymphoma. Pharmacokinetic studies have shown that ibrutinib achieves Btk occupancy of >95% at doses of 2.5 mg/kg but that doses of 6 mg/kg are required to maintain this over 24 h.31 Acalabrutinib at 1.5 mg/kg twice daily also achieves full Btk occupancy over 24 h.17 The peak unbound plasma concentration of ibrutinib in patients is 0.5 μM11 and that of acalabrutinib 1.3 μM.17 The initial and terminal half-lives of ibrutinib are 2-3 h and 4-8 h, respectively.31 The half-life of acalabrutinib is 1 h.17 Despite the peak concentration of acalabrutinib being approximately 2-fold higher than the concentration of ibrutinib, the 5-fold lower potency of acalabrutinib as an inhibitor of Btk30 means that, in potency terms, it is dosed at a lower level consistent with the lack of inhibition of GPVI. This implies that ibrutinib could be used at a lower concentration to achieve Btk blockade. Indeed, there is retrospective clinical evidence that doses less than 6 mg/kg are as effective as 6 mg/kg for treating CLL32 and a prospective clinical trial using doses as low as 2.5 mg/kg is being undertaken.33
It is important to consider the incidence of minor and major bleeding in patients taking ibrutinib for CLL or at the higher dose for mantle cell lymphoma. In reported studies involving patients treated with ibrutinib for mantle cell lymphoma, minor and major bleeding was seen in 9-15% and 1-5% of patients, respectively.34–36 In the study with the largest cohort of patients with mantle cell lymphoma, the rate of major hemorrhages was 5%. This is comparable to the 4-8% major hemorrhage rate seen in patients taking ibrutinib for CLL.9 Thus, there is no increase in bleeding rates with higher doses of ibrutinib. This implies that the inhibitory effect of 420 mg ibrutinib on platelets is at a physiological maximum.
During the writing of this manuscript, Bye et al. reported thrombus instability on collagen in a flow adhesion assay in blood treated in vitro with high doses of ibrutinib and ex vivo in patients treated with ibrutinib.29 This is consistent with our findings that Btk kinase function is not required for platelet adhesion to collagen under flow, but that off-target effects of ibrutinib seen with higher doses mediate this inhibition. They also reported complete blockade of platelet aggregation in response to supramaximal concentrations of collagen in patients receiving ibrutinib or acal- abrutinib29 in contrast to the findings of this study. Bye et al. used the Optimul 96-well microtiter assay to measure aggregation rather than the widely used light transmission aggregometry. We have shown that Optimul is a more sensitive assay than light transmission aggregometry.37,38 We suggest that the delay in onset of aggregation observed using light transmission aggregometry with concentrations of ibrutinib or acalabrutinib that just block Btk manifest as complete blockade in the Optimul assay. Bye et al. also concluded that the increased bleeding observed with ibrutinib is due to blockade of Src family kinases (SFK).29 We agree that bleeding caused by ibrutinib is due to off-target actions, and that acalabrutinib has a greater selectively to Btk over SFK relative to ibrutinib. However, our results show that a similar fold increase in the concentration of ibrutinib and acalabrutinib causes inhibition of platelet aggregation in response to CRP but without concomitant SFK blockade in the acalabrutinib-treated platelets. Thus, the off-target action of ibrutinib and acalabrutinib that inhibits aggregation cannot be explained solely by differential blockade of SFK.
The results of our study explain the lack of major bleeding side effects experienced by patients taking acalabrutinib and suggest that the bleeding side effect of ibrutinib can potentially be abolished by reducing the dose. Furthermore, this study also shows that the bleeding caused by ibrutinib is not due to an irreversible action. This predicts that the GPVI blockade wears off over a period of 24 h as the drug is cleared.31 We hypothesize that, in the event of a major bleed, there may be no need to use expensive and potentially harmful platelet transfusions to correct the signaling deficit. Nevertheless, each clinical scenario should be judged on its own merits and individual clinicians’ discretion is crucial.
In conclusion, the present study shows that inhibition of Btk kinase activity causes only partial inhibition of GPVI signaling in platelets and provides evidence that Btk supports GPVI signaling by functioning as an adapter protein as well as a kinase. The excessive bleeding induced by ibrutinib relative to acalabrutinib is likely to reflect a non-Tec family kinase off-target inhibitory effect of ibrutinib, probably on Src.
Supplementary Material
Acknowledgments
This work was supported by British Haeart Foundation (BHF) Programme grants (RG/13/18/30563), a BHF clinical fellowship to PLRN (FS/17/20/32738), an AMS springboard grant to AYP (SBF002\1099) and BHF studentship to ATH, the University of Birmingham’s Institute of Translation Medicine and Institute of Cardiovascular Sciences; SPW holds a BHF Chair (CH03/003).
We would like to thank Alex Bye and Jon Gibbins for their expertise on the Ca2+ mobilization assay. We would also like to thank Vicky Simms, Natalie Poulter and Steve Thomas for their help with the flow adhesion assay and Mark Crowther, Nick Pemberton, Salim Shafeek, Kate Arthur, Gaynor Pemberton, Lesley Candlin and Rebekah Hart at Worcestershire Royal Hospital, Shankara Paneesha, Alison Hardy and Melanie Kelly at Birmingham Heartlands Hospital and Tina McSkeane, Gillian Marshall and Michelle Harry at the Queen Elizabeth Hospital for provision of patients’ samples. Finally, we would like to thank Andrew Wilkinson and Robert Neely from the School of Chemistry at the University of Birmingham for their help with the chemical analysis of ibrutinib and acalabrutinib.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/12/2097
References
- 1.Li Z, Delaney MK, O’Brien KA, Du X. Signalling during platelet adhesion and activation. Arterioscler. Thromb Vasc Biol. 2010;30(12):2341–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alshehri OM, Hughes CE, Montague S, et al. Fibrin activates GPVI in human and mouse platelets. Blood. 2015;126(13):1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieswandt B. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102(2):449–461. [DOI] [PubMed] [Google Scholar]
- 4.Mammadova-Bach E, Ollivier V, Loyau S, et al. Platelet glycoprotein VI binds to polymerized fibrin and promotes thrombin generation. Blood. 2015;126(5):683–691. [DOI] [PubMed] [Google Scholar]
- 5.Watson SP, Herbert JMJ, Pollitt AY. GPVI and CLEC-2 in hemostasis and vascular integrity. J Thromb Haemost. 2010;8(7):1456–1467. [DOI] [PubMed] [Google Scholar]
- 6.Jandrot-Perrus M, Busfield S, Lagrue AH, et al. Cloning, characterization, and functional studies of human and mouse glycoprotein VI: a platelet-specific collagen receptor from the immunoglobulin superfamily. Blood. 2000;96(1):1798–1807. [PubMed] [Google Scholar]
- 7.Bender M, Hagedorn I, Nieswandt B. Genetic and antibody-induced glycoprotein VI deficiency equally protects mice from mechanically and FeCl3-induced thrombosis. J Thromb Haemost. 2011;9(7):1423–1426. [DOI] [PubMed] [Google Scholar]
- 8.Nurden AT, Nurden P. Congenital platelet disorders and understanding of platelet function. Br J Haematol. 2013;165(2):165–178. [DOI] [PubMed] [Google Scholar]
- 9.Shatzel JJ, Olson SR, Tao DL, et al. Ibrutinib-associated bleeding: pathogenesis, management, and risk reduction strategies. J Thromb Haemost. 2015;38(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caron F, Leong DP, Hillis C, Fraser G, Siegal D. Current understanding of bleeding with ibrutinib use: a systematic review and meta-analysis. Blood Adv. 2017;1(12):772–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levade M, David E, Garcia C, Laurent P, Payrastre B. Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood. 2014;124(26): 3991–3995. [DOI] [PubMed] [Google Scholar]
- 12.Kamel S, Horton L, Ysebaert L, et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia. 2015;29(4):783–787. [DOI] [PubMed] [Google Scholar]
- 13.Bye AP, Unsworth AJ, Vaiyapuri S, Stainer AR, Fry MJ, Gibbins JM. Ibrutinib inhibits platelet integrin αIIbβ3 outside-in signalling and thrombus stability but not adhesion to collagen. Arterioscler Thromb Vasc Biol. 2015;35(11):2326–2335. [DOI] [PubMed] [Google Scholar]
- 14.Kazianka L, Drucker C, Skrabs C, et al. Ristocetin-induced platelet aggregation for monitoring of bleeding tendency in CLL treated with ibrutinib. Leukemia. 2017;31(5):1117–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quek LS, Bolen J, Watson SP. A role for Bruton’s tyrosine kinase (Btk) in platelet activation by collagen. Curr Biol. 1998;8(20): 1137–1140. [DOI] [PubMed] [Google Scholar]
- 16.Atkinson BT, Ellmeier W, Watson SP. Tec regulates platelet activation by GPVI in the absence of Btk. Blood. 2003;102(10):3592–3599. [DOI] [PubMed] [Google Scholar]
- 17.Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honigberg L, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA. 2010;107(29):13075–13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rushworth SA, MacEwan DJ, Bowles KM. Ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(13): 1277–1279. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe D, Hashimoto S, Ishiai M, et al. Four tyrosine residues in phospholipase C-gamma 2, identified as Btk-dependent phosphorylation sites, are required for B cell antigen receptor-coupled calcium signalling. J Biol Chem. 2001;276(42):38595–38601. [DOI] [PubMed] [Google Scholar]
- 21.Wahl MI, Fluckiger AC, Kato RM, et al. Phosphorylation of two regulatory tyrosine residues in the activation of Bruton’s tyrosine kinase via alternative receptors. Proc Natl Acad Sci USA. 1997;94(21):11526–11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomlinson MG, Kurosaki T, Berson AE, Fujii GH, Bolen JB. Reconstitution of Btk signalling by the atypical tec family tyrosine kinases Bmx and Txk. J Biol Chem. 1999;274(19):13577–13585. [DOI] [PubMed] [Google Scholar]
- 23.Takata M, Homma Y, Kurosaki T. Requirement of phospholipase C-γ2 activation in surface immunoglobulin M-induced B cell apoptosis. J Exp Med. 1995;182(4): 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomlinson MG, Woods DB, McMahon M, et al. A conditional form of Bruton’s tyrosine kinase is sufficient to activate multiple downstream signalling pathways via PLC gamma 2 in B cells. BMC Immunol. 2001;2(4):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu J, Zhang M, Liu D. Acalabrutinib (ACP-196): a selective second- generation BTK inhibitor. J Hematol Oncol. 2016;9(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salto K, Tolias KF, Abdelhafid S, et al. Btk regulates PtdIns-4,5-P2 synthesis: importance for calcium signalling and PI3K activity. Immunity. 2003;19:669–678. [DOI] [PubMed] [Google Scholar]
- 27.Kim YJ, Sekiya F, Poulin B, Bae YS, Rhee SG. Mechanism of B-cell receptor-induced phosphorylation and activation of phospholipase C-γ2. Mol Cell Biol. 2004;24(22):9986–9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burkhart JM, Vaudel M, Gambaryan S, et al. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood. 2012;120(15):e73–e82. [DOI] [PubMed] [Google Scholar]
- 29.Bye AP, Unsworth AJ, Desborough MJ, et al. Severe platelet dysfunction in NHL patients receiving ibrutinib is absent in patients receiving acalabrutinib. Blood Adv. 2017;1(26):2610–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Covey T, Barf T, Gulrajani M, et al. Abstract 2596: ACP-196: a novel covalent Bruton’s tyrosine kinase (Btk) inhibitor with improved selectivity and in vivo target coverage in chronic lymphocytic Leukemia (CLL) patients. Cancer Res. 2015;75(15 Suppl):2596–2596. [Google Scholar]
- 31.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2012;31(1):88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banerjee R, Timlin C, Fitzpatrick D, et al. Comparable outcomes in chronic lymphocytic leukeemia patients treated with reduced dose ibrutinib: results from a multi-center study. Haematologica. 2016;101(s1): 56–57. [DOI] [PubMed] [Google Scholar]
- 33.Bose P, Gandhi VV, Keating MJ. Pharmacokinetic and pharmacodynamic evaluation of ibrutinib for the treatment of chronic lymphocytic leukeemia: rationale for lower doses. Expert Opin Drug Metab Toxicol. 2016;11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dreyling M, Jurczak W, Silva RS, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387(10020):770–779. [DOI] [PubMed] [Google Scholar]
- 35.Rule S, Dreyling M, Goy A, et al. Outcomes in 370 patients with mantle cell lymphoma treated with ibrutinib: a pooled analysis from three open-label studies. Br J Haematol. 2017;179(3):430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Eng J Med. 2013;369(6):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lordkipanidzé M, Lowe GC, Kirkby NS, et al. Characterization of multiple platelet activation pathways in patients with bleeding as a high-throughput screening option: use of 96-well Optimul assay. Blood. 2014;123(8):e11–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan MV, Leadbeater PD, Watson SP, Warner TD. Not all light transmission aggregation assays are created equal: qualitative differences between light tramsission and 96-well plate aggregometry. Platelets. 2018. May 1:1–4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes CE, Pollitt AY, Mori J, et al. CLEC-2 activates Syk through dimerization. Blood. 2010;115(14):2947–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fuorescence properties. J Biol Chem. 2001;260(6):3440–3450. [PubMed] [Google Scholar]
- 41.Takata M, Kurosaki T. A role for Bruton’s tyrosine kinase in B cell antigen receptor-mediated activation of phospholipase C-γ2. J Exp Med. 1996;184(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes CE, Sinha U, Pandey A, et al. Critical role for an acidic amino acid region in platelet signalling by the HemITAM (Hemi-immunoreceptor Tyrosine-based Activation Motif) containing receptor CLEC-2 (C-type lectin receptor-2). J Biol Chem. 2013;288(7):5127–5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.