Abstract
Delphinidin is an anthocyanidin commonly found in various fruits and vegetables. Delphinidin has been known to possess many functions, such as an antioxidant, and anti-inflammatory, anti-cancer and anti-muscular atrophy agent. In this study, we attempted to evaluate the effects of delphinidin on lipid accumulation in hepatocytes. The results showed that palmitic acid (PA)-induced cellular senescence in HepG2 cells and reduced the expression of SMARCD1, which is known to regulate senescence-associated lipid accumulation in hepatocytes. However, delphinidin-3-glucoside (D3 g) suppressed PA-induced senescence and reversed the expression of SMARCD1 to the level of untreated HepG2 cells. Consequently, D3 g inhibited PA-induced lipid accumulation through the restoration of the expression of SMARCD1 and fatty acid oxidation genes. Taken together, our results suggest that D3 g suppresses the lipid accumulation induced by hepatocyte senescence.
Electronic supplementary material
The online version of this article (10.1007/s10616-018-0246-0) contains supplementary material, which is available to authorized users.
Keywords: Delphinidin, Senescence, Lipid, HepG2, SMARCD1
Introduction
Delphinidin is an anthocyanidin commonly found in pigmented fruits and vegetables and is present in the pericarp, testa, and bark of various plants (Wang et al. 2017). Delphinidin possesses many biological functions, such as antioxidant, and has anti-inflammatory, anti-cancer, and anti-muscular atrophy effects (Murata et al. 2017; Wang et al. 2017; Kang et al. 2018; Chen et al. 2018). Recently, delphinidin has been shown to possess beneficial effects on obesity. Rahman et al. (2016) showed the effects of delphinidin on adipogenesis and the related underlying molecular mechanisms. In this study, we evaluated the effects of delphinidin on the lipid accumulation in hepatocytes and the underlying molecular mechanisms.
SMARCD1 is a member of the SWI/SNF chromatin remodeling complex family, which regulates the transcription of target genes (Zhang et al. 2016). Previously, we identified SMARCD1 as a senescence-associated gene (Udono et al. 2015), and demonstrated that PGC-1α-mediated SMARCD1 has roles in the fatty acid oxidation and palmitic acid (PA)-induced hepatic senescence (Inoue et al. 2017; Kang et al. 2018). In the present study, we investigated whether SMARCD1 is a target molecule of delphinidin to suppress lipid accumulation in hepatocytes.
Materials and methods
Cell culture and reagents
HepG2 cells (a human cell line derived from hepatocyte carcinoma, RIKEN, BRC, Tsukuba, Japan) were cultured in DMEM medium (Nissui, Tokyo, Japan) supplemented with 10% fetal bovine serum (FBS, Life Technologies, Gaithersburg, MD, USA) at 37 °C in a 95% air/5% CO2 atmosphere. Palmitic acid (PA) was purchased from Chem Service (West Chester, PA, USA). PA complexed with bovine serum albumin (BSA) was prepared as described previously (Zhang et al. 2016). Cells were treated with 0.1 mM PA. Delphinidin-3-glucoside (D3 g) and resveratrol were purchased from Tokiwa Phytochemical (Chiba, Japan) Wako (Osaka, Japan), respectively. D3 g and resveratrol were dissolved in dimethyl sulfoxide (DMSO) and added to the cells at a concentration of 10 μM.
Quantitative reverse transcriptase PCR (qRT-PCR)
RNA was extracted using the High Pure RNA Isolation kit (Roche Diagnostics GmbH, Mannheim, Germany), and cDNA was prepared using ReverTra Ace (Toyobo, Osaka, Japan). The qPCR was performed using the KAPA SYBR Fast qPCR kit (KAPA Biosystems, Woburn, MA, USA) and a Thermal Cycler Dice Real Time System TP-800 (TaKaRa, Shiga, Japan) as described previously (Inoue et al. 2017). The samples were analyzed in triplicates and target gene levels were normalized to the expression levels of β-actin (reference gene). PCR primers used in the study are described in the Supplementary Table 1.
Fluorescence senescence-associated β-galactosidase (SA-β-Gal) assay
Fluorescence SA-β-Gal assay was performed as described previously (Udono et al. 2012). The images were acquired using the IN Cell Analyzer 1000 (GE Healthcare, Little Chalfont, UK), and analyzed using the IN Cell Developer Toolbox 1.9 (GE Healthcare). The imaging data were reported as SA-β-Gal intensity (mean fluorescence intensity per cell) and the number of SA-β-Gal positive/negative cells. The threshold of SA-β-Gal intensity was set to a value so that approximately 75% of the control cells were negative.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde for 15 min at room temperature, and blocked with a blocking solution (5% goat serum, 0.3% Triton-X 100) for 1 h. Next, cells were incubated overnight at 4 °C with anti-phospho-p38 (#4511, Cell Signaling, Beverly, MA, USA); anti-p16 (ab81278; Abcam, Cambridge, MA, USA); anti-phospho-histone H2A.X (#9718, Cell Signaling) antibodies. Cells were further incubated with the secondary antibody (Alexa Fluor 555 F(ab’) fragment of goat anti-rabbit IgG; Life Technologies) at room temperature for 1 h. Finally, cells were incubated with 1 μg/mL of Hoechst 33342 solution (Dojindo, Kumamoto, Japan) at room temperature for 10 min. The images from the immunofluorescence were analyzed using the IN Cell Developer Toolbox 1.9, and the results were depicted using SpotFire DecisionSite Client 8.2 software (PerkinElmer, Waltham, MA, USA).
Quantification of intracellular lipid
HepG2 cells were fixed with 10% formaldehyde for 10 min at room temperature, and stained with 0.06 μg/mL BODIPY 493/503 (Life Technologies) and 1 μg/mL Hoechst 33342 solution. Fluorescence images were obtained using the IN Cell Analyzer 1000, fluorescence intensity and area of lipid droplets were quantitatively determined using the IN Cell Developer Toolbox 1.9 (Inoue et al. 2017).
Statistical analysis
All experiments were performed at least 3 times. The results are presented as mean ± standard deviation. Statistical significance was determined using a two-sided Student’s t test. Multiple comparisons between groups were made by one-way ANOVA with Turkey’s post hoc test. Statistical significance was defined as P < 0.05.
Results and discussion
Delphinidin-3-glucoside (D3 g) suppressed the palmitic acid (PA)-induced cellular senescence in HepG2 cells.
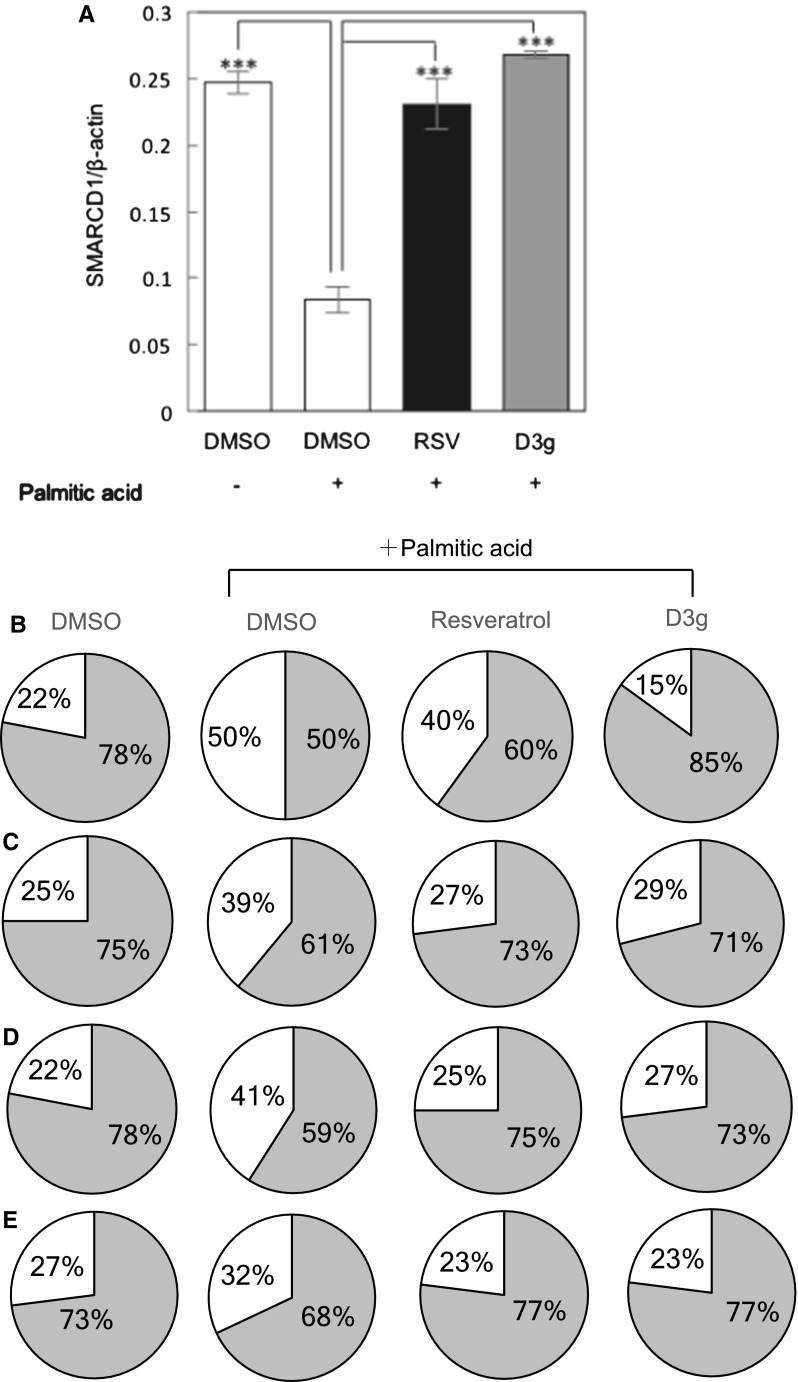
As reported previously (Inoue et al. 2017), PA induced cellular senescence in HepG2 cells, as evidenced by the augmented SA-β-Gal activity, increased expression of phospho-p38, p16 and γH2AX (Fig. 1b–e). Concurrently, PA also reduced the expression of SMARCD1 possibly through the induction of cellular senescence, which is known to be a member of the SWI/SNF chromatin remodeling complex family and regulates the senescence-associated lipid accumulation in hepatocytes (Inoue et al. 2017) (Fig. 1a). Resveratrol is known to suppress cellular senescence, then we used it as a positive control (Ido et al. 2015). Actually, resveratrol and D3 g, an anthocyanidin present in many fruits including pomegranates, suppressed the PA-induced cellular senescence in HepG2 cells. In the present study, we used D3 g, because Wang et al. reported that the glycoside form of anthocyanin showed a high inhibitory activity on triglyceride accumulation in HepG2 cells (Wang et al. 2016). We should confirm whether D3 g directly affects hepatic cells without metabolizing to its aglycon form in a future study. On the other hand, activity and expression of cellular senescence markers were reversed to the state similar to non-treated cells upon treatment with D3 g as well as resveratrol (Fig. 1b–e). Furthermore, D3 g as well as resveratrol also reversed the expression of SMARCD1 to the level of non-treated cells possibly through the suppression of cellular senescence. All these results suggest that D3 g as well as resveratrol suppressed the PA-induced cellular senescence in HepG2 cells.
Fig. 1.
Delphinidin-3-glucoside (D3 g) suppresses the palmitic acid (PA)-induced cellular senescence in HepG2 cells. HepG2 cells were treated with 0.1 mM PA in the presence of 10 μM RSV or D3 g for 24 h. a Relative expression of SMARCD1 in HepG2 cells was analyzed by qRT-PCR. b Activity and expression of senescence markers (b SA-β-Gal, c phospho p38, d p16, e γH2AX) were measured in HepG2 cells. The pie charts show the ratio of senescence marker-positive (white)/negative cells (gray)
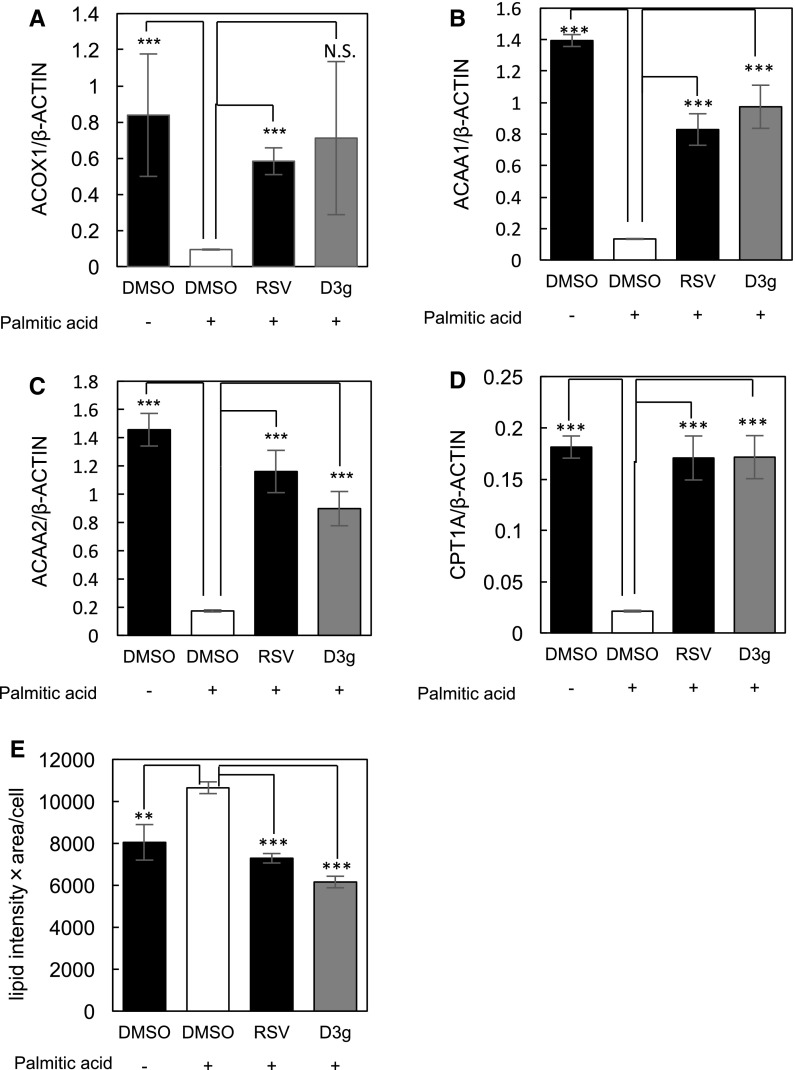
D3 g suppressed the PA-induced accumulation of lipid droplets in HepG2 cells.
We previously reported that cellular senescence induction triggers lipid accumulation in hepatocytes (Inoue et al. 2017), which suggests that D3 g and resveratrol inhibit PA-induced lipid accumulation in HepG2 cells through suppressing the induction of cellular senescence. As shown in Fig. 2a–d, PA suppressed the expression of fatty acid oxidation (FAO) genes, including acyl-CoA oxidase 1 (ACOX1), acetyl-CoA acyltransferase 1 and 2 (ACAA1 and ACAA2), and carnitine palmitoyltransferase 1A (CPT1A) (Fig. 2a–d). SMARCD1 is involved in the physiological regulation of hepatic fat oxidation (Li et al. 2008) and plasma cholesterol levels (Meng et al. 2015). Furthermore, we demonstrated a PGC-1α-mediated SMARCD1 plays a role in the fatty acid oxidation and PA-induced hepatic senescence. In our previous study, inhibition of SMARCD1 expression induced the accumulation of lipid droplets through the inactivation of PGC-1α-dependent transcription and suppressing the FAO gene expression (Inoue et al. 2017). In the present study, PA suppressed the expression of SMARCD1 in HepG2 cells, which result in the reduction of the FOA gene expression (Fig. 2a–d). Concurrently, D3 g as well as resveratrol reversed the expression of SMARCD1, resulting in a reversal of FAO gene expression in the PA-treated HepG2 cells.
Fig. 2.
D3 g suppresses the PA-induced accumulation of lipid droplets in HepG2 cells. HepG2 cells were treated with 0.1 mM PA in the presence of 10 μM RSV or D3 g for 24 h. a–d, Relative expression of FAO genes in HepG2 cells were analyzed by qRT-PCR. e The number of lipid droplets and lipid area in HepG2 cells were analyzed by using the IN Cell Analyzer 1000. Statistical significance was determined using a two-sided Student’s t-test. Multiple comparisons between groups were made by one-way ANOVA with Turkey’s post hoc test. Statistical significance was defined as P < 0.05 (**P < 0.01; ***P < 0.001). N.S. means not significant
Finally, we tested for the effects of D3 g and resveratrol on the PA-induced accumulation of lipid droplets. As shown in Fig. 2e, PA induced the accumulation of lipid droplets in HepG2 cells, while D3 g and resveratrol suppressed the PA-induced accumulation of lipid droplets. All these results suggest that D3 g as well as resveratrol suppresses the lipid accumulation induced by hepatocyte senescence.
To date, delphinidin has been reported to reduce intracellular lipid accumulation and promote lipolysis through inhibiting the pre-adipocyte differentiation (Rahman et al. 2016). Rahman et al. reported that delphinidin suppressed the expression of early adipogenic transcription factors and effectively inhibited adipogenesis followed by the stabilization of β-catenin. In this study, we present a novel mechanism of delphinidin to suppress lipid accumulation. Delphinidin alleviated the PA-induced reduction of SMARCD1 expression, leading to the avoidance of PA-induced cellular senescence and concurrent suppression of PA-induced accumulation of lipid droplets in HepG2 cells. Thus, we demonstrated that SMARCD1 might be a promising candidate for the suppression of accumulation of lipid droplets, and therefore, the prevention of metabolic diseases, including obesity and hepatic steatosis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
References
- Chen J, Zhu Y, Zhang W, Peng X, Xhou J, Li F, Han B, Liu X, Ou Y, Yu X. Delphinidin induced protective autophagy via mTOR pathway suppression and AMPK pathway activation in HER-2 positive breast cancer cells. BMC Cancer. 2018;18:342. doi: 10.1186/s12885-018-4231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ido Y, Duranton A, Lan F, Weikel K-A, Breton L, Ruderman N-B. Resveratrol prevents oxidative stress-induced senescence and proliferative dysfunction by activating the AMPK-FOXO3 cascade in cultured primary human keratinocytes. PLoS One. 2015;10(2):e0115341. doi: 10.1371/journal.pone.0115341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue C, Zhao C, Tsuduki Y, Udono M, Wang L, Nomura M, Katakura Y. SMARCD1 regulates senescence-associated lipid accumulation in hepatocytes. NPJ Aging Mech Dis. 2017;3:11. doi: 10.1038/s41514-017-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H-M, Park B-S, Kang H-K, Park H-R, Yu S-B, Kim I-R. Delphinidin induces apoptosis and inhibits epithelial-to-mesenchymal transition via the ERK/p38 MAPK-signaling pathway in human osteosarcoma cell lines. Environ Toxicol. 2018;29:379–649. doi: 10.1002/tox.22548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Liu C, Li N, Hao T, Hill D-E, Vidal M, Lin J-D. Genome-wide coactivation analysis of PGC-1α identifies BAF60a as a regulator of hepatic lipid metabolism. Cell Metab. 2008;8:105–117. doi: 10.1016/j.cmet.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z-X, Wang L, Chang L, Sun J, Bao J, Li Y, Chen E, Lin J-D. A diet-sensitive BAF60a-mediated pathway links hepatic bile acid metabolism to cholesterol absorption and atherosclerosis. Cell Rep. 2015;13:1658–1669. doi: 10.1016/j.celrep.2015.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M, Nonaka H, Komatsu S, Goto M, Morozumi M, Yamada S, Lin I-C, Yamashita S, Tachibana H. Delphinidin prevents muscle atrophy and upregulates miR-23a expression. J Agric Food Chem. 2017;65:45–50. doi: 10.1021/acs.jafc.6b03661. [DOI] [PubMed] [Google Scholar]
- Rahman N, Jeon M, Kim Y-S. Delphinidin, a major anthocyanin, inhibits 3T3-L1 pre-adipocyte differentiation through activation of Wnt/β-catenin signaling. BioFactors. 2016;42:49–59. doi: 10.1002/biof.1251. [DOI] [PubMed] [Google Scholar]
- Udono M, Kadooka K, Yamashita S, Katakura Y. Quantitative analysis of cellular senescence phenotypes using an imaging cytometer. Methods. 2012;56:383–388. doi: 10.1016/j.ymeth.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Udono M, Fujii K, Harada G, Tsuzuki Y, Kadooka K, Zhang P, Fujii H, Amano M, Nishimura S, Tashiro K, Kuhara S, Katakura Y. Impaired ATP6V0A2 expression contributes to Golgi dispersion and glycosylation changes in senescent cells. Sci Rep. 2015;5:17342. doi: 10.1038/srep17342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao L, Wang D, Huo Y, Ji B. Anthocyanin-rich extracts from blackberry, wild blueberry, strawberry, and chokeberry: antioxidant activity and inhibitory effect on oleic acid-induced hepatic steatosis in vitro. J Sci Food Agric. 2016;96:2494–2503. doi: 10.1002/jsfa.7370. [DOI] [PubMed] [Google Scholar]
- Wang CH, Zhu LL, Ju KF, Liu J-L, Li K-P. Anti-inflammatory effect of delphinidin on intramedullary spinal pressure in a spinal cord injury rat model. Exp Ther Med. 2017;14:5583–5588. doi: 10.3892/etm.2017.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Li L, Bao Z, Huang F. Role of BAF60a/BAF60c in chromatin remodeling and hepatic lipid metabolism. Nutr Metab (Lond) 2016;13:30. doi: 10.1186/s12986-016-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.