Abstract
Phytochemical investigation of the 70% EtOH extract of the leaves of Alstonia scholaris afforded seven new monoterpenoid indole alkaloids: scholarisins I-VII (1-7), and three known compounds: (3R,5S,7R,15R,16R,19E)-scholarisine F (8), 3-epi-dihydro- corymine (9), and (E)-16-formyl-5α-methoxystrictamine (10). Structural elucidation of all the compounds was accomplished by spectral methods such as 1D- and 2D-NMR, IR, UV, and HRESIMS. The isolated compounds were tested in vitro for cytotoxicity against seven tumor cell lines, anti-inflammatory activities against Cox-1 and Cox-2, and antifungal potential against five species of fungi. Compounds 1, 6, and 10 exhibited significant cytotoxicities against all the tested tumor cell lines with IC50 values of less than 30 μM and selective inhibition of Cox-2 comparable with the standard drug NS-398 (>90%). Additionally, 1, 2, 3 and 8 showed antifungal activity against two fungal strains (G. pulicaris and C. nicotianae).
Keywords: Alstonia rupestris, Apocynaceae, monoterpenoid indole alkaloids, cytotoxicity, anti-inflammatory, antifungal
1. Introduction
The genus Alstonia, which belongs to the family Apocynaceae, is widely distributed throughout the tropical areas of the World, including Central America, Africa, Indo-Malaya, Australia and Asia [1,2,3]. The genus Alstonia comprises about 60 species, eight of which grow in China [4]. Several of these species are used in Traditional Chinese Medicine, for example in the treatment of malaria, dysentery, defervescence, antitussive, and to arrest hemorrhages [5,6,7,8,9,10]. Monoterpenoid indole alkaloids occur abundantly in the family Apocynaceae [11,12,13,14,15,16,17], and to date, more than 300 such monoterpenoid indole alkaloids have been reported from the plants of this genus [18,19,20,21,22]. This type of alkaloids originates from the condensation of tryptophan with secologanin to give strictosidine and then further elaboration gives an impressive array of structural variants [23]. Monoterpenoid indole alkaloids were reported to have anticancer, antibacterial, antifertility, and anti-tussive activities [24,25,26,27,28]. Alstonia rupestris Kerr. is usually endemic in the western part of Guangxi Province of China. To the best of our knowledge, the phytochemistry of the A. rupestris has been rarely reported previously, which prompted the present study. Present investigation on chemical constituents of the EtOH extract of the leaves of A. rupestris led to seven new monoterpenoid indole alkaloids: scholarisin I-VII (1–7) together with three known compounds: (3R,5S,7R,15R,16R,19E)-scholarisine F (8), 3-epi-dihydrocorymine (9), and (E)-16- formyl-5α-methoxystrictamine (10) (Figure 1). The structures of these compounds were elucidated mainly by NMR spectroscopic and mass spectroscopic methods. Furthermore, all the alkaloids were in vitro evaluated for their cytotoxic, anti-inflammatory and antifungal activities.
Figure 1.
The structures of compounds 1–10.
2. Results and Discussion
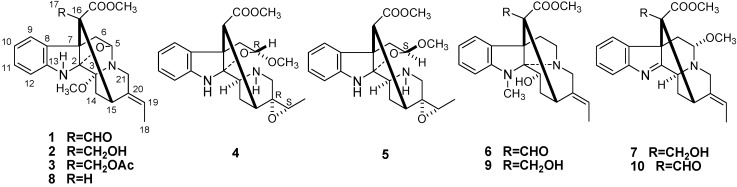
Compound 1 was obtained as a white amorphous powder. The positive HRESIMS spectrum displayed a pseudomolecular ion at m/z 419.1585 [M+Na]+ (calcd for C22H24N2O5Na, 419.1583) consistent with a molecular formula of C22H24N2O5, corresponding to 12 degrees of unsaturation. Its UV characteristic absorption peaks at 285, 240, and 228 nm was for a indole chromophore. The IR spectrum exhibited absorptions at 3,425 and 1,725 cm−1 for NH and C=O functions, respectively. Its 13C-NMR spectrum showed 22 carbon signals [OCH3 × 2, CH3 × 1, CH2 (sp3) × 3, CH (sp3) × 2, C (sp3) × 4, CH (sp2) × 6 and C (sp2) × 4, Table 1]. The 1H-NMR spectrum exhibited four aromatic proton signals [δH 7.31 and 6.68 (each, 1H, dd, J = 7.8, 1.8 Hz), 6.84 and 7.08 (each, 1H, dt, J = 7.8, 1.8 Hz)] ascribed to an ortho-disubstituted benzene ring, an ethylidene side chain [1.51 (d, J = 7.0, H-18) and 5.47 (q, J = 7.0, H-19)], a NH signal at δH 4.96, a downfield proton signal at δH 8.52 due to a formyl group and a singlet peak at δH 3.50 assigned to one methoxy group. The HMBCs of the proton signal at δH 3.50 (OCH3) with the carbon signals at δC 85.4 (C-3) indicated the methoxy group substitution at C-3. In the NOE spectrum, the correlation of the methoxy group at C-3 with H-21α (δH 2.26) indicated the α orientation of the methoxy group (Figure 2). The NOE correlation of H-5/H-6β and H-15/H-17 evidenced the β and α orientation of H-5 and H-15, respectively. The E-form of the double bond of 19/20 was determined on the basis of the NOE correlations of H-19/21 and H-18/15. These data suggested that the structure of 1 was almost identical with (3R,5S,7R,15R,16R,17R,19E)-scholarisine F (8) [28]. The distinct difference was the presence of one more formyl group at C-16 in 1, which was supported by the observation of the HMBC correlations of the proton signal at δH 8.52 (H-17) with the carbon signals of C-7, C-15, and carbonyl group of carbomethoxy and the downfield chemical shift of C-16 from δC 51.5 in 8 to δC 65.6 in 1. Thus, 1 was named as scholarisin I and the structure was showed in Figure 1.
Table 1.
13C-NMR data of compounds 1–7 in CDCl3.
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 2 | 107.5 | 105.6 | 107.0 | 103.5 | 104.9 | 102.0 | 186.7 |
| 3 | 85.4 | 85.1 | 85.3 | 51.9 | 51.1 | 70.6 | 50.8 |
| 5 | 86.1 | 85.6 | 85.9 | 105.4 | 106.9 | 63.5 | 89.7 |
| 6 | 42.5 | 44.4 | 42.9 | 42.8 | 40.9 | 42.5 | 43.6 |
| 7 | 55.1 | 53.1 | 55.5 | 52.3 | 52.4 | 62.4 | 53.2 |
| 8 | 131.5 | 131.6 | 133.1 | 138.0 | 136.8 | 131.3 | 142.4 |
| 9 | 126.0 | 126.0 | 126.1 | 122.7 | 123.4 | 121.6 | 121.6 |
| 10 | 121.5 | 122.1 | 121.9 | 119.9 | 120.0 | 127.5 | 124.9 |
| 11 | 128.9 | 128.6 | 128.9 | 128.1 | 128.5 | 130.6 | 125.4 |
| 12 | 110.7 | 110.4 | 110.3 | 109.1 | 110.2 | 112.5 | 120.8 |
| 13 | 147.9 | 148.3 | 147.8 | 144.6 | 145.9 | 148.7 | 156.1 |
| 14 | 25.0 | 28.5 | 23.6 | 26.2 | 27.0 | 34.2 | 35.8 |
| 15 | 34.1 | 33.5 | 37.3 | 27.7 | 28.3 | 34.2 | 33.4 |
| 16 | 65.6 | 54.4 | 55.0 | 52.8 | 52.1 | 65.6 | 58.8 |
| 17 | 197.4 | 65.9 | 66.2 | - | - | 194.5 | 63.0 |
| 18 | 13.4 | 13.7 | 13.6 | 12.8 | 12.9 | 15.1 | 13.5 |
| 19 | 120.7 | 119.7 | 119.8 | 58.4 | 58.5 | 133.2 | 127.8 |
| 20 | 130.5 | 131.3 | 131.3 | 61.5 | 61.6 | 129.9 | 137.7 |
| 21 | 47.9 | 48.1 | 48.2 | 44.7 | 44.5 | 66.6 | 50.5 |
| CO2CH3 | 168.0 | 169.3 | 169.4 | 172.1 | 172.6 | 171.9 | 169.1 |
| CO2CH3 | 52.7 | 51.5 | 51.7 | 51.8 | 51.6 | 53.9 | 51.4 |
| N1-CH3 | - | - | - | - | - | 50.5 | - |
| OCH3 | 48.6 | 48.5 | 48.4 | 54.8 | 57.0 | - | 54.7 |
| COCH3 | - | - | 171.3 | - | - | - | - |
| COCH3 | - | - | 20.1 | - | - | - | - |
Figure 2.
Key HMBC ( ) and NOESY (
) and NOESY ( ) correlations of of compound 1.
) correlations of of compound 1.
Compound 2 was isolated as a white amorphous powder. Its positive HRESIMS spectrum showed a quasimolecular ion peak at m/z 399.1924 [M+H]+, consistent with the molecular formula C22H26N2O5. Comparing the 1H- and 13C-NMR data of 2 with those of compound 1, the data were almost identical. The only significant difference was that the formyl group at C-16 was replaced by a hydroxymethyl group in compound 2, which was confirmed by the HMBC correlations of the proton signal of the hydroxymethyl group [δH 3.22, 3.46 (each, 1H, d, J = 13.8)] with the carbonyl group of carbomethoxy at δC 169.3. On the basis of the observation of NOESY data similar to those of 1, the stereochemistry of 2 was expected to be the same. Accordingly, the structure of 2 was established as scholarisin II and the structure was showed in Figure 1.
Compound 3 was obtained as a white amorphous powder. The EIMS afforded a molecular weight of m/z 440, and its HRESIMS revealed the [M+H]+ peak at m/z 441.2025 (calcd. for C24H29N2O6. 441.2026), corresponding to the molecular formula C24H28N2O6. The general features of its IR and NMR spectra closely resembled those of 2, except for the presence of one more Ac group. The OAc group were positioned at C-17 based on HMBC correlations of H-17 [δ 3.87 and 4.01 (each, 1H, d, J = 13.8)] with the acyl carbon (δ 171.3) of the acetyl group. The stereochemistry of 3 was expected to be the same as 2 on the basis of the NOESY data. Thus, compound 3 was elucidated as scholarisin III and the structure was as shown in Figure 1.
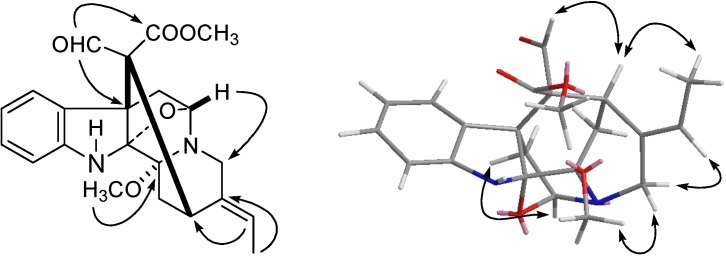
Compound 4, a white amorphous powder, gave one quasimolecular ion peak at m/z 409.1735 [M+Na]+ in its HRESIMS, accounting for a molecular formula of C21H26N2O5. The IR spectrum showed absorption peaks at 3430 (NH) and 1740 (C=O) cm−1. In the 1H-NMR spectrum, four aromatic proton signals at δH 7.12 and 6.56 (each, 1H, dd, J = 8.2, 1.8 Hz), 6.78 and 7.06 (each, 1H, dt, J = 8.2, 1.8 Hz)] showed an ortho-disubstituted benzene ring, two singlet peaks at δH 3.10 and 3.70 were assigned to the protons of a methoxy and a carbomethoxy group, respectively. Its 13C-NMR displayed a pattern similar to that of scholarisine C [28], except that the double bond of 19/20 was substituted by a methine at δC 58.4 (d, C-19) and a quaternary carbon at δC 61.5 (s, C-20). The molecular formula C21H26N2O5 displayed 10 unsaturation degrees, which indicated the presence of 19,20-epoxide combined with the appropriate NMR data. The NOE correlation of H-3/H-14α indicated the α configuration of C-3 (Figure 3). On the basis of the NOE correlations of H-15/H-19 and H-18/21, the configurations of C-19 and C-20 was elucidated as S and R, respectively. The R configuration of C-5 was determined by the coupling constant of H-5 (d, J = 5.2 Hz) compared with that of scholarisine C (d, J = 5.4 Hz) [28]. Therefore, compound 4 was determined as scholarisin IV, with the structure as shown in Figure 1.
Figure 3.
Key HMBC ( ) and NOESY (
) and NOESY ( ) correlations of of compound 4.
) correlations of of compound 4.
Compound 5, a white amorphous powder, exhibited a molecular formula of C21H26N2O5, based on the HRESIMS spectrum which showed a pseudomolecular ion at m/z 387.1923 [M+H]+ (calcd. 387.1920). The general features of NMR spectra closely resembled those of 4, except for the configuration of C-5. H-5 was observed as a doublet of doublets at δH 4.90 (1H, dd, J = 7.2, 5.6 Hz) in the 1H-NMR spectrum, which indicated the S configuration of C-5 [28]. This evidence indicated that compound 5 was an isomer of 4, and 5 was identified as scholarisin V, with the structure shown in Figure 1.
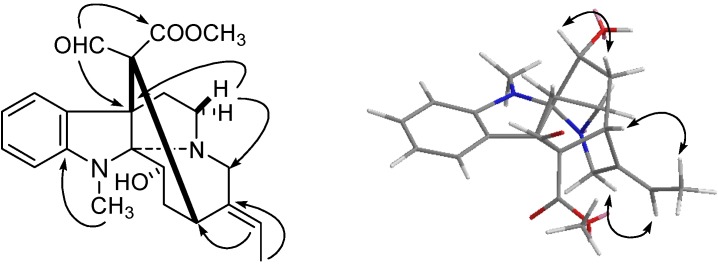
Compound 6, a white amorphous powder, exhibited a molecular formula of C22H26N2O4, based on the HRESIMS spectrum which showed a pseudomolecular ion at m/z 383.1974 [M+H]+ (calcd. 383.1971). The UV absorptions at 286, 241 and 220 nm showed the presence of an indole chromophore. The IR spectrum indicated the presence of formyl group (1720 cm−1), and benzene ring (1650 cm−1). Its 13C-NMR spectrum showed 22 carbon signals [CH3 × 2, OCH3 × 1, CH2 (sp3) × 4, CH (sp3) × 3, C (sp3) × 2, CH (sp2) × 6 and C (sp2) × 4, Table 1]. The 1H-NMR spectrum exhibited four ortho-disubstituted aromatic proton signals [δH 7.76 and 6.79 (each, 1H, dd, J = 7.8, 2.0 Hz), 6.92 and 7.24 (each, 1H, dt, J = 7.8, 2.0 Hz)], an ethylidene side chain [1.83 (d, J = 7.2, H-18) and 5.86 (q, J = 7.2, H-19)], a N-CH3 (δH 2.03), a formyl group (δH 8.55), one methoxy group (δH 3.80). These data showed similarities to those of 3-epi-dihydrocorymine (9) [29]. Comparing the 1H- and 13C-NMR data of 6 with those of 3-epi-dihydrocorymine, the data were almost identical. The only significant difference was that the signals of one hydroxymethyl group was replaced by those of the formyl group (δC 194.5; δH 8.55), which was supported by the observation of the HMBC correlations of the proton signal at δH 8.55 (H-17) with the carbon signals of C-7, C-15 and carbonyl group of carbomethoxy (Figure 4). In the NOE experiment, the correlation of H-3/H-21β indicated the β orientation of C-3. The E-form of the double bond of 19/20 was determined on the basis of the NOE correlations of H-19/21 and H-18/15. On the basis of the observation of NOESY data similar to those of 9, the stereochemistry of 6 was expected to be the same. Accordingly, the structure of 6 was established as scholarisin VI (Figure 1).
Figure 4.
Key HMBC ( ) and NOESY (
) and NOESY ( ) correlations of of compound 6.
) correlations of of compound 6.
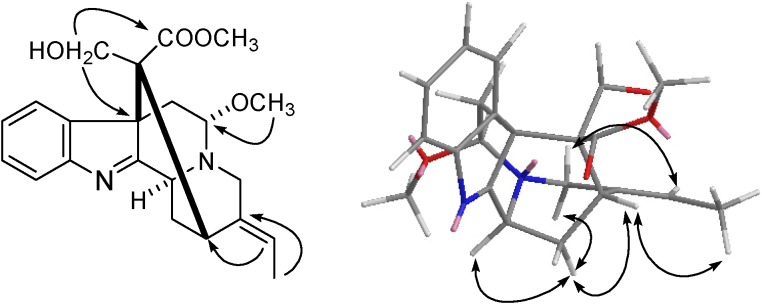
The molecular formula of compound 7 was assigned as C22H26N2O4 on the basis of the quasi- molecular ion peak [M+Na]+ at m/z 405.1787 in the HRESIMS. The 13C-NMR and DEPT spectra displayed signals of three Me, four CH2, and nine CH groups, together with six quaternary C-atoms. The NMR signals at δH 7.79 (dd, J = 8.0, 2.0 Hz, H-9), 7.32 (dt, J = 8.0, 2.0 Hz, H-10), 7.14 (dt, J = 8.0, 2.0 Hz, H-11), 7.39 (dd, , J = 8.0, 2.0 Hz, H-12), and those at δC 142.4 (C-8), 121.6 (C-9), 124.9 (C-10), 125.4 (C-11) , 120.8 (C-12), and 156.1 (C-13) were characteristic for the presence of an indole moiety. The NMR data of 7 was almost identical with those of (E)-16-formyl-5α-methoxystrictamine (10) [30]. The only significant difference was that a hydroxymethyl group [δH 3.68, 3.91 (each, 1H, d, J = 13.2)] in 7 instead of the formyl group at C-16 in 10, was confirmed by HMBC correlations of H-17 with C-7, C-15 and carbonyl group of carbomethoxy (Figure 5). The relative configuration of compound 7 was determined by the NOESY experiment. Based on the similarity of NOE spectrum with that of 10, the NOE interactions of H-3/H-14α, H-15/H-14α, H-5/H-21, and H-15/H-18 indicated the H-3α, H-15α, and 19 E configuration compared with that of (E)-16-formyl-5α-methoxystrictamine. From these data, 7 was named scholarisin VII.
Figure 5.
Key HMBC ( ) and NOESY (
) and NOESY ( ) correlations of of compound 7.
) correlations of of compound 7.
The in vitro cytotoxic activities of the isolated alkaloids were evaluated against seven tumor cell lines by using the revised MTT method as described in the Experimental. The results are summarized in Table 2. Alkaloids 1, 6, and 10 exhibited significant cytotoxicity (IC50 < 30 μM) while 2, 3, and 7–9 showed weak cytotoxic activites (IC50 > 40 μM) against all the tested tumor cell lines. Furthermore, alkaloids 4 and 5 without the linkage between C-5 and N-4 were non-cytotoxic (IC50 > 80 μM). The results indicated that the linkage between C-5 and N-4 was essential for cytotoxic properties, while the formyl group on C-16 might strengthen the cytotoxic activities for this type of alkaloids.
Table 2.
Cytotoxicity of compounds 1–10 against seven human tumor cell lines (IC50, μM) a.
| Compound | Cell lines | ||||||
|---|---|---|---|---|---|---|---|
| A-549 | BGC-823 | HepG2 | HL-60 | MCF-7 | SMMC-7721 | W480 | |
| 1 | 10.3 | 11.3 | 9.2 | 12.0 | 10.7 | 23.7 | 28.0 |
| 2 | 52.7 | 61.8 | 49.0 | 59.4 | 54.3 | 59.7 | 59.5 |
| 3 | 44.1 | 40.8 | 30.4 | 39.6 | 36.8 | 47.0 | 40.1 |
| 4 | - | - | - | - | - | - | 94.9 |
| 5 | 97.4 | - | - | - | - | 92.1 | - |
| 6 | 13.0 | 12.9 | 10.8 | 12.3 | 11.3 | 24.9 | 29.9 |
| 7 | 49.1 | 53.2 | 43.6 | 48.2 | 46.7 | 49.4 | 52.7 |
| 8 | 47.8 | 51.5 | 44.8 | 50.7 | 48.9 | 53.2 | 51.4 |
| 9 | 61.3 | 67.1 | 58.3 | 71.8 | 64.2 | 66.2 | 62.1 |
| 10 | 16.1 | 15.7 | 14.8 | 17.2 | 14.7 | 31.2 | 35.5 |
| Doxorubicin | 18.3 | 14.7 | 22.0 | 31.7 | 24.9 | 35.4 | 15.9 |
a Doxorubicin activities are expressed as IC50 values in nM, and those of compounds 1–8 are expressed as IC50 values in μM. (-) IC50 > 100 μM.
The compounds 1–10 were tested in vitro for their anti-inflammatory activities. The results of the anti-inflammatory assay were summarized in Table 3. Among the assayed compounds, only alkaloids 1, 6 and 10 with formyl group at C-16 displayed selective inhibition of Cox-2 (> 90%). Alkaloids 2–5 and 7–9 had no anti-inflammatory activities or selective inhibition of Cox-2 comparable to those of 1, 6 and 10 although they possess the same monoterpene indole skeleton. The observations indicated that the formyl group at C-16 should be essential for this type of alkaloids to possess the anti-inflammatory activity.
Table 3.
Evaluation of Anti-Inflammatory Activity of Compounds 1–10 a.
| Compound | COX-1 | COX-2 |
|---|---|---|
| 1 | 45.2 | 96.4 |
| 2 | <0 | 14.9 |
| 3 | 12.9 | 50.4 |
| 4 | <0 | 21.1 |
| 5 | <0 | 24.3 |
| 6 | 36.9 | 95.5 |
| 7 | <0 | 17.6 |
| 8 | <0 | <0 |
| 9 | 13.6 | 46.8 |
| 10 | 38.5 | 92.0 |
| SC-560 | 63.2 | |
| NS-398 | 97.1 |
a Percent inhibition (all compounds and reference drugs concentration: 100 μM).
All compounds were tested for their antifungal activities by the disc diffusion method by measuring the inhibition zones and for the most active compounds, minimum inhibitory concentration (MIC) values were also determined. Antifungal properties (Table 4) showed that alkaloids 1, 2, 3 and 8 exhibited antifungal activity against two fungi (G. pulicaris and C. nicotianae), with MIC values of 0.64–0.69 mM, 1.37–1.44 mM, 1.80–1.91 mM and 1.55–1.71 mM, respectively. Alkaloids 1 possessed rather higher antifungal potent with lower MIC value. The other alkaloids had no a antifungal activities. These result suggested that the structure skeleton of 1 may be essential and the N-carbamate group could strengthen the antifungal activities of this type of alkaloids.
Table 4.
Antifungal activities (zones of inhibition/and MIC mM, n = 3) of compounds 1–10.
| Compound | G. pulicaris | A. alternata | C. nicotianae | P. capsici. | G. amomi |
|---|---|---|---|---|---|
| 1 | 20/0.69 | - | 19/0.64 | - | - |
| 2 | 18/1.37 | - | 17/1.44 | - | - |
| 3 | 15/1.91 | - | 16/1.80 | - | - |
| 4 | - | - | - | - | - |
| 5 | - | - | - | - | - |
| 6 | - | - | - | - | - |
| 7 | - | - | - | - | - |
| 8 | 15/1.71 | - | 17/1.55 | - | - |
| 9 | - | - | - | - | - |
| 10 | - | - | - | - | - |
| Nystatin | 21/0.007 | 19/0.006 | 19/0.006 | 20/0.010 | 19/0.009 |
-: No activity.
3. Experimental
3.1. General
Optical rotations were determined with a JASCO P2000 digital polarimeter (Tokyo, Japan). Ultraviolet (UV) and infrared (IR) spectra were obtained on JASCO V-650 and JASCO FT/IR-4100 spectrophotometers (Tokyo, Japan), respectively. NMR spectra were measured on a Bruker AM-600 spectrometer (1H-NMR) and Bruker AM-400 spectrometer (13C-NMR). EIMS and HREIMS (70 eV) were carried out on a Finnigan MAT 95 mass spectrometer. All solvents used were of analytical grade (Shanghai Chemical Reagents Company Ltd., Shanghai, China). Silica gel (200–300 mesh), silica gel H (Qingdao Haiyang Chemical Co. Ltd., Qingdao, China), C18 reversed-phase silica gel (150–200 mesh, Merck), and MCI gel (CHP20P, 75–150 lm, Mitsubishi Chemical Industries Ltd., Tokyo, Japan) were used for column chromatography. HPLC separation was performed on an instrument consisting of a Waters 600 controller, a Waters 600 pump, and a Waters 2487 dual λ absorbance detector, with a Prevail (250 × 10 mm i.d.) preparative column packed with C18 silica (5 μm).
3.2. Plant Material
The leaves of A. scholaris were collected in Yongning, Guangxi Province, China, in September 2011. The sample was identified by one of the authors (G. B. Shi). A specimen (201109001AS) was deposited in the Herbarium of Shengyang Medicine College, Shengyang, China.
3.3. Extraction and Isolation
The dried leaves of A. scholaris (16 kg) were powdered and extracted thrice with 70% ethanol (25 L) at room temperature and then concentrated under reduced pressure to give a crude extract (198.5 g). The crude extract was partitioned between equal volumes of ethyl acetate and water to provide an EtOAc-soluble (77.5 g) and an aqueous layer. The EtOAc-soluble fraction was subjected to silica gel column chromatography eluted with CHCl3/MeOH (from 100:1 to 1:1) to yield seven fractions (F1-F7). F2 (4.3 g) was further subjected to silica gel column chromatography eluted with CHCl3/MeOH (from 10:1 to 1:1) to give three subfractions F2a (276 mg), F2b (253 mg), and F2c (226 mg). Subfraction F2a was separated by repeated column chromatography over Sephadex LH-20 (CHCl3/MeOH, 1:1, and MeOH), then puried on silica gel column chromatography eluted with n-hexane/EtOAc (7:3) to yield 8 (99.1 mg). Subfraction F2b was further subjected to reverse phase high performance liquid chromatography (RP-HPLC) eluted with methanol/water (70:30) to furnish compounds 4 (56.1 mg) and 5 (56.1 mg). F2c was subjected to a normal phase high performance liquid chromatography (NP-HPLC) eluted with n-hexane/ethyl acetate (8:1) to afford compound 3 (65.3 mg) and 1 (64.3 mg). F3 (4.9 g) was subjected to a silica gel column chromatography eluted with n-hexane/EtOAc (from 100% n-hexane to 100% EtOAc) to furnish four subfractions (F3a-F3d). F3b (588 mg) was separated on a reverse-phase HPLC eluted with methanol/water (65:35) to yield three compounds 2 (59.3 mg) and 10 (49.5 mg). F3c (406 mg) was chromatographed on a reverse phase HPLC column eluted with methanol/water (55:45) to yield 7 (78.9 mg). F4 (2.3 g) was separated using a silica gel column and eluted with n-hexane/ CH2Cl2/MeOH (30:70:1.5) to give two subfractions F4a (380 mg) and F4b (260 mg). F4a was further subjected to a reverse phase HPLC column eluted with methanol/water (65:35) to provide two compounds 6 (69.3 mg) and 9 (49.5 mg).
Scholarisin I (1). White amorphous powder. : −38.8 (c = 0.80, MeOH). UV (CHCl3) λmax (log ε) 285 (2.81), 240 (3.40), 228 (2.83) nm. IR (KBr) νmax 3425, 1725, 1465, 1175, 1090, 1062, 870, 753 cm−1. 1H-NMR (CDCl3, 600 MHz) and 13C-NMR (CDCl3, 125 MHz) data see Table 5 and Table 1 respectively. EI-MS m/z: 396 ([M]+). HRESIMS (pos.) m/z: 419.1585 ([M+Na]+, C22H24N2O5Na. calc. 419.1583).
Table 5.
1H-NMR data of compounds 1–7 in CDCl3 (δ in ppm and J in Hz).
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| N1-H | 4.96 (s) | 4.98 (s) | 5.00 (s) | 5.04 (s) | 4.43 (s) | - | - |
| 3 | - | - | - | 3.96 (m) | 3.36 (m) | 4.73 (dd, 14.0,4.0) | 4.48 (dd, 14.0,4.0) |
| 5α | - | - | - | - | - | 2.18 (m) | - |
| 5β | 4.88 (dd, 4.2,3.6) | 4.86 (d, 5.2) | 4.92 (dd, 4.0,3.6) | 5.28 (dd, 4.0,3.8) | 4.92 (dd, 7.2,5.6) | 2.32 (m) | 3.84 (dd, 4.0,3.8) |
| 6α | 2.25 (dd, 13.6,4.2) | 2.26 (d, 13.8) | 2.29 (d, 14.0) | 2.75 (dd, 14.0,4.0) | 2.76 (dd, 14.4,5.6) | 2.06 (m) | 2.20 (dd, 14.0,4.0) |
| 6β | 3.41 (dd, 13.6,3.6) | 3.48 (dd, 13.8,5.2) | 3.52 (dd, 14.0,5.2) | 3.08 (dd, 14.0,3.8) | 3.10 (dd, 14.4,7.2) | 2.33 (m) | 3.75 (dd, 14.0,3.8) |
| 9 | 7.31 (dd, 7.8,1.8) | 7.72 (dd, 8.2,2.0) | 7.76 (dd, 7.8,2.0) | 7.12 (dd, 8.2,1.8) | 7.14 (dd, 8.0,2.0) | 7.76 (dd, 7.8,2.0) | 7.79 (dd, 8.0,2.0) |
| 10 | 6.84 (dt, 7.8,1.8) | 6.70 (dt, 8.2,2.0) | 6.82 (dt, 7.8,2.0) | 6.78 (dt, 8.2,1.8) | 6.77 (dt, 8.0,2.0) | 6.92 (dt, 7.8,2.0) | 7.39 (dt, 8.0,2.0) |
| 11 | 7.08 (dt, 7.8,1.8) | 6.98 (dt, 8.2,2.0) | 7.14 (dt, 7.8,2.0) | 7.06 (dt, 8.2,1.8) | 7.10 (dt, 8.0,2.0) | 7.24 (dt, 7.8,2.0) | 7.14 (dt, 8.0,2.0) |
| 12 | 6.68 (dd, 7.8,1.8) | 6.66 (dd, 8.2,2.0) | 6.78 (dd, 7.8,2.0) | 6.56 (dd, 8.2,1.8) | 6.66 (dd, 8.0,2.0) | 6.79 (dd, 7.8,2.0) | 7.32 (dd, 8.0,2.0) |
| 14α | 2.26 (dd, 14.0,3.8) | 2.30 (dd, 14.0,3.8) | 2.33 (dd, 13.8,3.8) | 2.23 (m) | 2.25 (m) | 2.27 (m) | 2.88 (m) |
| 14β | 2.18 (dd, 14.0,4.0) | 2.27 (dd, 14.0,4.0) | 2.29 (dd, 13.8,4.0) | 1.91 (m) | 1.93 (m) | 2.24 (m) | 1.96 (m) |
| 15 | 3.63 (dd, 4.0,3.8) | 3.69 (dd, 4.0,3.8) | 3.72 (dd, 4.0,3.8) | 2.95 (dd, 4.0,3.8) | 2.97 (dd, 4.0,3.8) | 3.62 (dd, 4.0,3.8) | 3.66 (dd, 4.0,3.8) |
| 16 | - | - | - | 2.92 (d, 4.8) | 2.59 (d, 4.2) | - | - |
| 17a | 8.52 (s) | 3.32 (d, 13.8) | 3.87 (d, 13.6) | - | - | 8.55 (s) | 3.68 (d, 13.2) |
| 17b | - | 3.46 (d, 13.8) | 4.01 (d, 13.6) | - | - | - | 3.91 (d, 13.2) |
| 18 | 1.51 (d, 7.0) | 1.55 (d, 7.2) | 1.58 (d, 7.0) | 1.40 (d, 7.0) | 1.41 (d, 6.8) | 1.83 (d, 7.2) | 1.55(d, 7.0) |
| 19 | 5.47 (q, 7.0) | 5.48 (q, 7.2) | 5.50 (q, 7.0) | 2.93 (q, 7.0) | 2.95 (q, 6.8) | 5.86 (d, 7.2) | 5.54(d, 7.0) |
| 21α | 3.85 (d, 13.8) | 3.87 (d, 14.2) | 3.89 (d, 13.8) | 3.36 (d, 14.0) | 3.39 (d, 14.0) | 3.88 (d, 13.8) | 4.07 (d, 13.6) |
| 21β | 3.31 (d, 13.8) | 3.33 (d, 14.2) | 3.35 (d, 13.8) | 3.08 (d, 14.0) | 3.11 (d, 14.0) | 3.35 (d, 13.8) | 3.08 (d, 13.6) |
| CO2CH3 | 3.69 (s) | 3.78 (s) | 3.82 (s) | 3.70 (s) | 3.67 (s) | 3.80 (s) | 3.71 (s) |
| N1-CH3 | - | - | - | - | 2.03 (s) | - | |
| OCH3 | 3.50 (s) | 3.52 (s) | 3.53 (s) | 3.10 (s) | 3.41 (s) | - | 3.25 (s) |
| COCH3 | - | - | 1.53 (s) | - | - | - |
Scholarisin II (2). White amorphous powder. : −30.9 (c = 0.62, MeOH). UV (CHCl3) λmax(logε) 286 (2.85), 240 (3.46), 228 (2.78), 228 (2.82) nm. IR (KBr) νmax 3445, 3420, 1730, 1464, 1170, 1065, 875 cm−1. 1H-NMR (CDCl3, 600 MHz) and 13C-NMR (CDCl3, 125 MHz) data see Table 5 and Table 1 respectively. EI-MS m/z: 398 ([M]+). HRESIMS (pos.) m/z: calc. 399.1924 [M+H]+, C22H27N2O5. calc. 399.1920).
Scholarisin III (3). White amorphous powder. : −35.4 (c = 0.76, MeOH). UV (CHCl3) λmax(logε) 286 (2.80), 242 (3.30), 227 (2.89) nm. IR (KBr) νmax 3425, 1735, 1460, 1172, 1092, 1062, 871 cm−1. 1H-NMR (CDCl3, 600 MHz) and 13C-NMR (CDCl3, 125 MHz) data see Table 5 and Table 1 respectively. EI-MS m/z: 440 ([M]+). HRESIMS (pos.) m/z: 441.2025 ([M+H]+, C24H29N2O6. calc. 441.2026).
Scholarisin IV (4). White amorphous powder. : −38.2 (c = 0.30, MeOH). UV (CHCl3) λmax(logε) 284 (3.45), 241 (3.62), 220 (3.32) nm. IR (KBr) νmax 3430, 2950, 1740, 1628, 1465, 1102, 750 cm−1. 1H-NMR (CDCl3, 600 MHz) and 13C-NMR (CDCl3, 125 MHz) data see Table 5 and Table 1 respectively. EI-MS: 386 ([M]+). HRESIMS (pos.) m/z: 409.1735 ([M+Na]+, C21H26N2O5Na. calc. 409.1739).
Scholarisin V (5). White amorphous powder. : −18.5 (c = 0.23, MeOH). UV (CHCl3) λmax(logε) 285 (3.15), 240 (3.68), 228 (3.01) nm. IR (KBr) νmax 3423, 1735, 1635, 1465, 1447, 1170, 1035 cm−1. 1H-NMR (CDCl3, 600 MHz) and13C-NMR (CDCl3, 125 MHz) data see Table 5 and Table 1 respectively. EI-MS m/z: 386 ([M]+). HRESIMS (pos.) m/z: 387.1923 ([M+H]+, C21H27N2O5. calc. 387.1920).
Scholarisin VI (6). White amorphous powder. : −38.5 (c = 0.35, MeOH). UV (CHCl3) λmax(logε) 286 (3.30), 241 (3.81), 220 (3.31) nm. IR (KBr) νmax 3428, 1720, 1650, 1605, 1465, 1215, 1165, 1035 cm−1. 1H-NMR (CDCl3, 600 MHz) and 13C-NMR (CDCl3, 125 MHz) data see Table 5 and Table 1 respectively. EI-MS m/z: 382 ([M]+). HRESIMS (pos.) m/z: 383.1974 ([M+H]+, C22H27N2O4. calc. 383.1971).
Scholarisin VII (7). White amorphous powder. : −56.5 (c = 0.19, MeOH). UV (CHCl3) λmax(logε) 285 (3.41), 239 (3.73), 218 (3.27), 195 (3.83) nm. IR (KBr) νmax 3448, 1735, 1635, 1455, 1442, 1166, 1015 cm−1. 1H-NMR (CDCl3, 600 MHz) and 13C-NMR (CDCl3, 125 MHz) data see Table 2 and Table 1 respectively. EI-MS m/z: 382 ([M]+). HRESIMS (pos.) m/z: 405.1787 ([M+Na]+, C22H26N2O4Na. calc. 405.1790).
3.4. Cytotoxicity Assay in Vitro
The isolated compounds 1–10 were subjected to cytotoxic evaluation against A-549 cells (lung cancer), BGC-823 cells (human gastric carcinoma), HepG2 cells (human hepatocellular carcinoma), HL-60 (human myeloid leukemia), MCF-7 cells (human breast cancer), SMMC-7721 (hepatocellular carcinoma), and W480 (colon cancer) by employing the revised MTT method as described in the literature [31]. Doxorubicin was used as the positive control. All tumor cell lines were cultured on RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 U mL−1 penicillin and 100 μg/mL streptomycin in 25 cm3 culture flasks at 37 °C in humidified atmosphere with 5% CO2. For the cytotoxicity tests, cells in exponential growth stage were harvested from culture by trypsin digestion and centrifuging at 180 ×g for 3 min, then resuspended in fresh medium at a cell density of 5 × 104 cells per mL. The cell suspension was dispensed into a 96-well microplate at 100 μL per well, and incubated in humidified atmosphere with 5% CO2 at 37 °C for 24 h, and then treated with the compounds at various concentrations (0, 1, 10, 100 μM). After 48 h of treatment, 50 μL of 1 mg/mL MTT solution was added to each well, and further incubated for 4 h. The cells in each well were then solubilized with DMSO (100 μL for each well) and the optical density (OD) was recorded at 570 nm. All drug doses were tested in triplicate and the IC50 values were derived from the mean OD values of the triplicate tests versus drug concentration curves. The 50% inhibition concentration (IC50 value) was determined by curve fitting and was used as criteria to judge the cytotoxicity (active: IC50 ≤ 20 μM; moderately active: 20 μM < IC50 ≤ 80 μM; not active: IC50 > 80 μM). All cell lines were purchased from Cell Bank of Shanghai Institute of Biochemistry & Cell Biology, Chinese Academy of Sciences. Other reagents were purchased from Shanghai Sangon Biological Engineering Technology & Services Co., Ltd. (Shanghai, China).
3.5. Anti-Inflammatory Assay in Vitro
The anti-inflammatory activities were determined according to a literature method with minor modifications [32]. The reaction system was incubated at 25 °C for 5 min, by sequential addition of the buffer, heme, test compounds, and Cox-1 or Cox-2 into the system followed by mixing with TMPD and arachidonic acid. The absorbance value was recorded at a wavelength of 590 nm after another 15 min of incubation at 25 °C. SC-560 and NS-398 were used as positive controls, which gave the inhibition of Cox-1 (63.20%) and Cox-2 (97.13%) respectively (Table 3). All cell lines were purchased from the Cell Bank of Shanghai Institute of Biochemistry & Cell Biology, Chinese Academy of Sciences. (Shanghai, China).
3.6. Antifungal Activity Bioassay
All compounds (purity > 90%) were screened for their antifungal activity in vitro using the disk-diffusion method as described in the literature with minor modifications [33]. Strains including five species of fungi [Gibberella pulicaris (KZN 4207), Alternaria alternata (TX-8025), Colletotrichum nicotianae (SACC-1922), Phytophthora capsici (KACC-40157), Gonatopyricularia amomi (MB-9671)] were used. Nystatin were used as positive controls for antifungal activity. A disk containing only DMSO was used as the negative control. Agar medium was used in the antifungal activity. To each agar plate, an inoculum containing 107 bacteria/mL or a 0.5 optical density of the McFarland Scale was incorporated. The plates were solidified and sterile filter paper disks (6-mm diameter) were done on each one. Solution of each compound (5 mM) in DMSO, antifungal agents (nystatin 10 μM/mL), and control vehicles (DMSO) were added into too. The plates were aerobically incubated at 37 °C for the five species of fungi during 24 h. The diameter of the inhibition zone was measured for testing of antifungal activities. Experiments were performed in triplicate, and the results are presented as the mean values of the diameters of the inhibitory zones from three runs. The MIC values of the most active compounds, in the previous experiment, were determined using the dilution method in 96-hole plates. The diameters of the inhibitory zones and the MIC value were used as criteria to judge the antimicrobial activity (active: the diameters of the inhibitory zones ≥ 16 mm, MIC ≤ 5 mM; moderately active: the diameters of the inhibitory zones are visible, MIC > 5 mM; not active: the diameters of the inhibitory zones are invisible). All fungal were purchased from the Shanghai Institute of Biochemistry & Cell Biology, Chinese Academy of Sciences (Shanghai, China).
4. Conclusions
A chemical investigation of the 70% EtOH extract of the dried leaves of A. scholaris resulted in the isolation of seven new monoterpenoid indole alkaloids: scholarisin I-VII (1-7), and three known compounds: (3R,5S,7R,15R,16R,19E)-scholarisine F (8), 3-epi-dihydrocorymine (9), and (E)-16- formyl-5α-methoxystrictamine (10). All the isolated compounds 1-10 were evaluated for their cytotoxic activities against seven tumor cell lines and alkaloids 1, 6 and 10 possessed significant cytotoxicities against all the tested tumor cell lines with low IC50 values (<30 μM). In screening in vitro of cytotoxic activities of all the alkaloids anti-inflammatory properties against Cox-1 and Cox-2, 1, 6 and 10 showed selective inhibition of Cox-2 (>90%) comparable with the standard drug NS-398. Additionally, 1, 2, 3 and 8 had antifungal activity against two fungal spp. (G. pulicaris and C. nicotianae).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1–10 are available from the authors.
References
- 1.Narine L.L., Maxwell A.R. Monoterpenoid indole alkaloids from Palicourea crocea. Phytochem. Lett. 2009;2:34–36. [Google Scholar]
- 2.Tan S.J., Lim K.H., Subramaniam G., Kam T.S. Macroline-sarpagine and macroline-pleiocarpamine bisindole alkaloids from Alstonia angustifolia. Phytochemistry. 2012 doi: 10.1016/j.phytochem.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Ku W.F., Tan S.J., Low Y.Y., Komiyama K., Kam T.S. Angustilobine and andranginine type indole alkaloids and an uleine-secovallesamine bisindole alkaloid from Alstonia angustiloba. Phytochemistry. 2011;72:2212–2218. doi: 10.1016/j.phytochem.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Li P.T., Leeuwenberg A.J.M., Middleton D.J. Flora of China. Volume 16. Science Press; Beijing, China and Missouri Botanical Garden, St. Louis, MO, USA: 1995. p. 154. [Google Scholar]
- 5.Channa S., Dar A., Atta-ur-Rahman A.S. Evaluation of Alstonia scholaris leaves for broncho-vasodilatory activity. J. Ethnopharmacol. 2005;97:469–476. doi: 10.1016/j.jep.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Sexton J.E. Alkaloids of the Alstonia Species. In: Manske R.H.F., editor. The Alkaloids: Chemistry and Physiology. Volume 8. Academic Press; New York, NY, USA: 1965. pp. 159–202. [Google Scholar]
- 7.Gandhi M., Vinayak V.K.J. Preliminary evaluation of extracts of Alstonia scholaris bark for in vivo antimalarial activity in mice. J. Ethnopharmacol. 1990;29:51–57. doi: 10.1016/0378-8741(90)90097-d. [DOI] [PubMed] [Google Scholar]
- 8.Wright C.W., Allen D., Phillipson J.D., Kirby G.C., Warhurst D.C., Massiot G., Le Men-Olivier L. Alstonia species: Are they effective in malaria treatment? J. Ethnopharmacol. 1993;40:41–45. doi: 10.1016/0378-8741(93)90087-l. [DOI] [PubMed] [Google Scholar]
- 9.Leaman D.J., Arnason J.T., Yusuf R., Roemantyo H.S., Soedjito H., Angerhofer C.K., Pezzuto J.M. Malaria remedies of the Kenyah of the Apo Kayan, East Kalimantan, Indonesian Borneo: A quantitative assessment of local consensus as an indicator of biological efficac. J. Ethnopharmacol. 1995;49:1–16. doi: 10.1016/0378-8741(95)01289-3. [DOI] [PubMed] [Google Scholar]
- 10.Kam T.S., Nyedh K.T., Sim K.M., Yoganathan K. Alkaloids from Alstonia Scholaris. Phytochemistry. 1997;45:1303–1305. [Google Scholar]
- 11.Lim S.H., Low Y.Y., Tan S.J., Lim K.H., Thomas N.F., Kam T.S. Perhentidines A-C: Macroline-macroline bisindoles from Alstonia and the absolute configuration of perhentinine and macralstonine. J. Nat. Prod. 2012;75:942–950. doi: 10.1021/np300120p. [DOI] [PubMed] [Google Scholar]
- 12.Lim S.H., Tan S.J., Low Y.Y., Kam T.S. Lumutinines A-D, linearly fused macroline-macroline and macroline-sarpagine bisindoles from Alstonia macrophylla. J. Nat. Prod. 2011;74:2556–2562. doi: 10.1021/np200730j. [DOI] [PubMed] [Google Scholar]
- 13.Tan S.J., Choo Y.M., Thomas N.F., Robinson W.T., Komiyama K., Kam T.S. Unusual indole alkaloid-pyrrole, -pyrone, and -carbamic acid adducts from Alstonia angustifoli. Tetrahedron. 2010;66:7799–7806. [Google Scholar]
- 14.Arai H., Hirasawa Y., Rahman A., Kusumawati I., Zaini N.C., Sato S., Aoyama C., Takeo J., Morita H. Alstiphyllanines E-H, picraline and ajmaline-type alkaloids from Alstonia macrophylla inhibiting sodium glucose cotransporter. Bioorg. Med. Chem. 2010;18:2152–2158. doi: 10.1016/j.bmc.2010.01.077. [DOI] [PubMed] [Google Scholar]
- 15.Hirasawa Y., Arai H., Zaima K., Oktarina R., Rahman A., Ekasari W., Widyawaruyanti A., Indrayanto G., Zaini N.C., Morita H. Alstiphyllanines A–D, Indole alkaloids from Alstonia macrophylla. J. Nat. Prod. 2009;72:304–307. doi: 10.1021/np8007107. [DOI] [PubMed] [Google Scholar]
- 16.Hirasawa Y., Miyama S., Kawahara N., Goda Y., Rahman A., Ekasari W., Widyawaruyanti A., Indrayanto G., Zaini N.C., Morita H. Indole alkaloids from the leaves of Alstonia scholaris. Heterocycles. 2009;79:1107–1112. [Google Scholar]
- 17.Kam T.S., Choo Y.M. New indole alkaloids from Alstonia macrophylla. J. Nat. Prod. 2004;67:547–552. doi: 10.1021/np034041v. [DOI] [PubMed] [Google Scholar]
- 18.Koyama K., Hirasawa Y., Hosoya T., Hoe T.C., Chan K.L., Morita H. Alpneumines A-H, new anti-melanogenic indole alkaloids from Alstonia pneumatophora. Bioorg. Med. Chem. 2010;18:4415–4421. doi: 10.1016/j.bmc.2010.04.086. [DOI] [PubMed] [Google Scholar]
- 19.Koyama K., Hirasawa Y., Nugroho A.E., Hosoya T., Hoe T.C., Chan K.L., Morita H. Alsmaphorazines A and B, novel indole alkaloids from Alstonia pneumatophora. Org. Lett. 2010;12:4188–4191. doi: 10.1021/ol101825f. [DOI] [PubMed] [Google Scholar]
- 20.Macabeo A.P.G., Krohn K., Gehle D., Read R.W., Brophy J.J., Cordell G.A., Franzblau S.G., Aguinaldo A.M. Indole alkaloids from the leaves of Philippine Alstonia scholaris. Phytochemistry. 2005;66:1158–1162. doi: 10.1016/j.phytochem.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Salim A.A., Garson M.J., Craikc David J. New Indole Alkaloids from the Bark of Alstonia scholaris. J. Nat. Prod. 2004;67:1591–1594. doi: 10.1021/np0498612. [DOI] [PubMed] [Google Scholar]
- 22.Tan S.J., Low K., Gehle Y.Y., Choo Y.M., Abdullah Z., Etoh T., Hayashi M., Komiyama K., Kam T.S. Strychnan and secoangustilobine A type alkaloids from Alstonia spatulata. Revision of the C-20 configuration of scholaricine. J. Nat. Prod. 2010;73:1891–1897. doi: 10.1021/np100552b. [DOI] [PubMed] [Google Scholar]
- 23.Hutchinson C.R. Tetrahedron report number 105: Camptothecin: Chemistry, biogenesis and medicinal chemistry. Tetrahedron. 1981;37:1047–1065. [Google Scholar]
- 24.Kam T.S., Choo Y.M., Komiyama K. Unusual spirocyclic macroline alkaloids, nitrogenous derivatives, and a cytotoxic bisindole from Alstoni. Tetrahedron. 2004;60:3957–3966. [Google Scholar]
- 25.Kam T.S., Tan S.J., Ng S.W., Komiyama K. Bipleiophylline, an unprecedented cytotoxic bisindole alkaloid constituted from the bridging of two indole moieties by an aromatic spacer unit. Org. Lett. 2008;10:3749–3752. doi: 10.1021/ol801354s. [DOI] [PubMed] [Google Scholar]
- 26.Jagetia G.C., Baliga M.S. Evaluation of anticancer activity of the alkaloid fraction of Alstonia scholaris (Sapthaparna) in vitro and in vivo. Phytother. Res. 2006;20:103–109. doi: 10.1002/ptr.1810. [DOI] [PubMed] [Google Scholar]
- 27.Khan M.R., Omoloso A.D., Kihara M. Antibacterial activity of Alstonia scholaris and Leea tetramera. Fitoterapia. 2003;74:736–740. doi: 10.1016/s0367-326x(03)00192-8. [DOI] [PubMed] [Google Scholar]
- 28.Feng T., Cai X.H., Zhao P.J., Du Z.Z., Li W.Q., Luo X.D. Monoterpenoid indole alkaloids from the bark of Alstonia scholaris. Planta. Med. 2009;75:1537–1541. doi: 10.1055/s-0029-1185900. [DOI] [PubMed] [Google Scholar]
- 29.Zhou H., He H.P., Luo X.D., Wang Y.H., Yang X.W., Di Y.T., Hao X.J. Three New Indole Alkaloids from the Leaves of Alstonia scholaris. Helv. Chim. Acta. 2005;88:2508–2512. [Google Scholar]
- 30.Lim K.H., Hiraku O., Komiyama K., Kam T.S. Jerantinines A-G, cytotoxic Aspidosperma alkaloids from Tabernaemontana corymbosa. J. Nat. Prod. 2008;71:1591–1594. doi: 10.1021/np800435c. [DOI] [PubMed] [Google Scholar]
- 31.Yu J.O., Liao Z.X., Lei J.C., Hu X.M. Antioxidant and cytotoxic activities of various fractions of ethanol extract of Dianthus superbus. Food. Chem. 2007;104:1215–1219. [Google Scholar]
- 32.Duan W.G., Zhang L.Y. Prostaglandins, Cyclooxygenase inhibitors not inhibit resting lung cancer A549 cell proliferation. Prostaglandins Leukot. Essent. Fatty. Acids. 2006;74:317–321. doi: 10.1016/j.plefa.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Espine-Ingroff A., White T., Pfaller M.A. Manual of Clinical Microbiology. 7th ed. American Society for Microbiology; Washington, DC, USA: 1999. pp. 1640–1652. [Google Scholar]