Abstract
A methanolic crude extract of Parkia biglobosa was prepared and later partitioned in succession with different solvents of increasing polarity ranging from n-hexane, chloroform and ethyl acetate to butanol. Phytochemical screening of the extract revealed the presence of alkaloids, tannins, saponins, flavonoids, steroids, glycoside and sugars. The inhibition zones exhibited by the extract against the tested bacteria ranged between 14 ± 0.00 mm (against Escherichia coli) and 28 ± 0.71 mm (against Pseudomonas aeruginosa). The MIC of the methanolic extract of P. biglobosa against isolates ranged between 0.63 mg/mL and 5 mg/mL, while the MIC values exhibited by the n-hexane and aqueous fractions ranged between 0.63 mg/mL and 10 mg/mL. Overall the extract and fractions of P. biglobosa used in this work were found to possess antimicrobial properties which compared favourably with those of streptomycin. These observations make this plant a potential source of bioactive compounds that can be used in management of bacterial infections. The use of this plant as herbal medicaments in African countries and the reports on the toxicity of the plant further show that the plant is non-toxic to humans.
Keywords: minimum inhibitory concentration (MIC), stem bark extract, fraction, phytochemistry, resistance, infections, bioactive, antibacterial, antibiotics
1. Introduction
The problem of infectious microorganisms which were thought to have been controlled by antibiotics has led to re-emergence of more virulent microorganisms in a new form of resistant strains [1,2]. The problem thus makes it mandatory for mankind to seek new antimicrobial agents and/or new effective ways of treating infectious diseases caused by microorganisms such as the drug resistant bacteria [3]. One of the possible basic approaches to cure and control infections caused by multiple drug resistant (MDR) strains of bacteria is to explore the medicinal properties of herbs and higher plants.
The use of medicinal plants as treatments against microbial invasion can be traced back to early civilizations in China, India and the Near East, thus making phytomedicine doubtlessly one of mankind’s oldest professions [4]. Medicinal plants have been used for centuries as remedies for human diseases and have provided new sources of chemical compounds with biological activity as antimicrobial agents [5].
The World Health Organisation (WHO) has pointed it out that medicinal plants could be the best source to obtain a variety of drugs [6]. Therefore, there has been a global resurgence in the use of herbal preparations in disease management in all continents of the World and most developing countries are now integrating phytomedicine into their health care systems [7].
Parkia biglobosa belongs to the family Fabacea and sub-family Mimosaceae. The plant is popularly called the African locust bean tree, and it is known to occur in a diversity of agro-ecological zones ranging from the tropical rain forest to arid zones [8]. It is a perennial deciduous plant that typically grows to a height ranging from 7–20 m but can sometimes reach 30 m under exceptional conditions [9,10].
Parkia species have traditionally found usefulness as foods and folklore remedies for some ailments [7,11]. The roots and leaves are used in Gambia to prepare lotions to treat sore eyes [11], for the treatment of dental disorders in Cote d’Ivoire [12] and as a remedy for diarrhoea in the northern parts of Nigeria. It has been reported to have anti-hypertensive properties [13] and the plant has been used by many tribes as an anti-diabetic, anti-hyperlipidaemic and as anti-snake venom agent [14].
Parkia biglobosa is thus a plant that has shown potential as a source of chemotherapeutic compounds [13,15], while many folkloric and ethnobotanical applications of this plant have been reported. This study therefore investigated the phytochemical composition, and preliminary antibacterial potential of the stem bark of the plant.
2. Results and Discussion
2.1. Results
2.1.1. Phytochemical Screening of the Plant
Phytochemical screening of the methanolic extract of P. biglobosa stem bark showed the presence of alkaloids, tannins, saponins, flavonoids, steroids, glycoside and sugars (Table 1).
Table 1.
Phytochemical screening of stem bark extract of Parkia biglobosa.
| Chemical Test | Result |
|---|---|
| Alkaloids | ± |
| Anthraquinones | ‒ |
| Flavonoids | +++ |
| Cardiac glycosides | +++ |
| Reducing sugars | +++ |
| Saponins | +++ |
| Steroids | ± |
| Tannins | +++ |
Key: ± = Trace, +++ = Abundant, ‒ = Absent.
2.1.2. Antimicrobial Activity of P. biglobosa Methanolic Stems Bark Extract
Table 2 shows the sensitivity patterns of the bacterial isolates to the methanolic stem bark extract of P. biglobosa. All the isolates tested were susceptible to the activity of the extract at a concentration of 20 mg/mL. The zones of inhibition exhibited by the extract against tested bacterial isolates ranged between 14 ± 0.00 mm and 28 ± 0.71 mm. The smallest zone of inhibition (14 ± 0.00 mm) was observed against E. coli and the highest zone of inhibition (28 ± 0.71 mm) was observed against P. aeruginosa. All the bacterial isolates used were resistant to 5% methanol used as the control.
Table 2.
The sensitivity patterns of Parkia biglobosa methanolic extract against the test bacterial isolates.
| Bacterial Isolates | Zones of Inhibition (mm) ** | ||
|---|---|---|---|
| P. biglobosa (20 mg/mL) | Streptomycin (1 mg/mL) | Methanol (5%) | |
| Bacillus anthracis (LIO) | 19 ± 1.00 | 19 ± 0.71 | 0 ± 0.00 |
| Pseudomonas aeruginosa (NCIB 950) | 28 ± 0.71 | 0 ± 0.00 | 0 ± 0.00 |
| Bacillus stearothermophillus (NCIB 8222) | 19 ± 2.50 | 21 ± 1.58 | 0 ± 0.00 |
| Bacillus cereus (NCIB 6349) | 23 ± 1.50 | 21 ± 1.22 | 0 ± 0.00 |
| Bacillus polymyxa (LIO) | 16 ± 0.84 | 18 ± 0.00 | 0 ± 0.00 |
| Corynebacterium pyogenes (LIO) | 16 ± 0.71 | 20 ± 0.00 | 0 ± 0.00 |
| Pseudomonas fluorescence (NCIB 3756) | 20 ± 0.71 | 20 ± 0.00 | 0 ± 0.00 |
| Clostridium sporogenes (NCIB 532) | 17 ± 0.00 | 24 ± 0.71 | 0 ± 0.00 |
| Enterococcus faecalis (NCIB 775) | 15 ± 0.71 | 20 ± 0.00 | 0 ± 0.00 |
| Staphylococcus aureus (NCIB 8588) | 18 ± 0.71 | 20 ± 1.41 | 0 ± 0.00 |
| Bacillus subtilis (NCIB 3610) | 22 ± 1.10 | 19 ± 0.89 | 0 ± 0.00 |
| Klebsiella pneumoniae (NCIB 418) | 18 ± 1.58 | 0 ± 0.00 | 0 ± 0.00 |
| Escherichia coli (NCIB 86) | 14 ± 0.00 | 11 ± 0.71 | 0 ± 0.00 |
| Proteus vulgaris (LIO) | 17 ± 0.71 | 16 ± 1.41 | 0 ± 0.00 |
Key: LIO; Locally Isolated Organism, NCIB; National Collection of Industrial Bacteriology, (mm) **; Mean of five replicates, 0 ± 0.00 = No activity.
2.1.3. Antimicrobial Activity of Fractions Obtained from the Methanolic Extract of P. biglobosa
Table 3 shows the antimicrobial activity results of various solvent fractions of stem bark extract of P. biglobosa on the bacterial isolates at 10 mg/mL each. Aqueous and n-hexane fractions were active against all the bacterial isolates investigated, while the ethyl acetate and butanol fractions were active against only two and three test organisms, respectively, and the chloroform fraction was not active against any of the test organisms. The zone of inhibition observed for n-hexane ranges between 12 ± 0.71 mm and 22 ± 0.71 mm, while that of the aqueous fraction ranges between 11 ± 0.00 mm and 18 ± 0.71 mm. The lowest zones of inhibition were exhibited by the n-hexane fraction against E. coli and E. faecalis, while P. aeruginosa showed the highest zone of inhibition. The lowest zone of inhibition exhibited by the aqueous fraction against C. sporogenes was 11 ± 0.00 mm while the highest zone exhibited against M. luteus was 18 ± 0.71 mm. All the bacterial isolates used were resistant to 5% methanol used as the control.
Table 3.
Sensitivity testing of partitioned fractions of Parkia biglobosa stem bark extract on bacterial isolates.
| Bacterial Isolates | Zones of Inhibition (mm) at 10 mg/mL ** | |||||
|---|---|---|---|---|---|---|
| n-HEX | CHLORO | ETHYL | BUT | AQU | METH (5%) | |
| B. anthracis (LIO) | 17 ± 0.71 | 0 ± 0.00 | 10 ± 0.71 | 0 ± 0.00 | 14 ± 0.71 | 0 ± 0.00 |
| Ps. aeruginosa (NCIB 950) | 22 ± 0.71 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 12 ± 0.71 | 0 ± 0.00 |
| B. stearothermophillus (NCIB 8222) | 14 ± 0.71 | 0 ± 0.00 | 0 ± 0.00 | 9 ± 0.00 | 14 ± 1.22 | 0 ± 0.00 |
| B. cereus (NCIB 6349) | 14 ± 0.71 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 13 ± 0.71 | 0 ± 0.00 |
| B. polymyxa (LIO) | 14 ± 0.71 | 0 ± 0.00 | 11 ± 0.00 | 13 ± 0.89 | 15 ± 0.71 | 0 ± 0.00 |
| C. pyogenes (LIO) | 13 ± 1.48 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 14 ± 0.00 | 0 ± 0.00 |
| Ps. Fluorescence (NCIB 3756) | 14 ± 1.00 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 14 ± 1.00 | 0 ± 0.00 |
| C. sporogenes (NCIB 532) | 15 ± 1.48 | 0 ± 0.00 | 0 ± 0.00 | 10 ± 0.94 | 11 ± 0.00 | 0 ± 0.00 |
| M. luteus (NCIB 196) | 14 ± 0.71 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 18 ± 0.71 | 0 ± 0.00 |
| E. faecalis (NCIB 775) | 12 ± 0.71 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 12 ± 0.84 | 0 ± 0.00 |
| Staph. aureus (NCIB 8588) | 14 ± 0.71 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 14 ± 0.71 | 0 ± 0.00 |
| B. subtilis (NCIB 3610) | 15 ± 1.00 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 14 ± 0.71 | 0 ± 0.00 |
| K. pneumoniae (NCIB 418) | 14 ± 0.71 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 14 ± 0.71 | 0 ± 0.00 |
| E. coli (NCIB 86) | 12 ± 0.71 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 12 ± 0.71 | 0 ± 0.00 |
| P. vulgaris (LIO) | 17 ± 1.00 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 13 ± 0.71 | 0 ± 0.00 |
Key: LIO = Locally Isolated Organism, NCIB = National Collection of Industrial Bacteriology, N-HEX = n-hexane fraction, CHLORO = Chloroform fraction, ETHYL = Ethyl acetate fraction, BUT = Butanol fraction, AQU = Aqueous fraction, METH = 5% methanol as control, (mm) ** = Mean of five replicates.
2.1.4. The Minimum Inhibitory Concentrations of the Extract and Standard Antibiotics against Susceptible Bacterial Isolates
The minimum inhibitory concentrations (MIC) of the methanolic stem bark extract of P. biglobosa as well as those of standard antibiotics are shown in Table 4. The MIC of methanolic extract of P. biglobosa against bacterial isolates ranged between 0.63 mg/mL and 5.00 mg/mL while the ranges of MIC exhibited by the antibiotics against the bacterial isolates were as follows: tetracycline 0.13 mg/mL and >1 mg/mL, penicillin G 0.50 mg/mL and >1 mg/mL, streptomycin 0.03 mg/mL and >1 mg/mL and ampicillin 0.002 mg/mL and >0.128 mg/mL.
Table 4.
The minimum inhibitory concentrations (mg/mL) of the methanolic extract, n-hexane and aqueous fractions and standard antibiotics against bacterial isolates.
| Bacterial Isolates | PBM | N-HEX | ACQ | TET | PEN | STREP | AMP |
|---|---|---|---|---|---|---|---|
| B. anthracis (LIO) | 1.25 | 2.50 | 2.50 | 1.00 | >1 | 0.25 | 0.128 |
| P. aeruginosa (NCIB 950) | 2.50 | 2.50 | 10.00 | >1.00 | >1 | >1 | >0.128 |
| B. stearothermophillus (NCIB 8222) | 1.25 | 1.25 | 2.50 | 0.13 | 1.00 | 0.06 | 0.008 |
| B. cereus (NCIB 6349) | 2.50 | 1.25 | 2.50 | 0.25 | 0.50 | 0.06 | 0.064 |
| B. polymyxa (LIO) | 1.25 | 2.50 | 2.50 | 0.13 | 0.50 | 0.13 | 0.064 |
| C. pyogenes (LIO) | 1.25 | 2.50 | 2.50 | 0.13 | >1 | 0.13 | 0.016 |
| P. fluorescence (NCIB 3756) | 0.63 | 2.50 | 2.50 | >1 | >1 | 0.03 | >0.128 |
| C. sporogenes (NCIB 532) | 1.25 | 2.50 | 2.50 | 0.25 | >1 | 0.06 | 0.016 |
| M. luteus (NCIB 196) | 0.63 | 0.63 | 0.63 | 0.13 | >1 | 0.03 | 0.002 |
| E. faecalis (NCIB 775) | 5.00 | 5.00 | 10.00 | 0.13 | 0.06 | 0.03 | 0.004 |
| S. aureus (NCIB 8588) | 2.50 | 5.00 | 10.00 | >1 | 0.50 | 0.13 | >0.128 |
| B. subtilis (NCIB 3610) | 5.00 | 10.00 | 10.00 | 0.25 | 0.50 | 0.25 | 0.064 |
| K. pneumoniae (NCIB 418) | 5.00 | 10.00 | 10.00 | 0.50 | >1 | >1 | 0.032 |
| E. coli (NCIB 86) | 2.50 | 10.00 | 10.00 | 0.13 | >1 | 0.25 | 0.128 |
| P. vulgaris (LIO) | 0.63 | 1.25 | 2.50 | 0.50 | >1 | 0.50 | 0.016 |
Key: LIO = Locally Isolated Organism, NCIB = National Collection of Industrial Bacteriology, PBM = P. biglobosa extract, N-HEX = n-hexane fraction, ACQ = aqueous fraction, TET = Tetracycline; PEN = Penicillin G, STREP = Streptomycin, AMP = Ampicillin.
2.2. Discussion
The phytochemical screening of P. biglobosa revealed the presence of flavonoids, tannins, saponins, cardiac glycosides, reducing sugars, steroids and alkaloids (Table 1). Several other studies have reported similar phytocompounds from this plant and or closely related species [16,17,18,19,20]; all these corroborate our reported data. These compounds are known to be biologically active [21] and thus may contribute to the antimicrobial activities of P. biglobosa.
Phytochemicals exert antimicrobial activity through different mechanisms. For example, flavonoids exhibit a wide range of biological activities which include antimicrobial, anti-inflammatory, anti-angionic, analgesic, anti-allergic effects, cytostatic and antioxidant properties [22]. Flavonoids’ ability to scavenge hydroxyl radicals, superoxide anion radicals and lipid peroxyl radicals highlights many of the health-promoting functions of flavonoids in organisms which are important for prevention of diseases associated with oxidative damage of membranes, proteins and DNA [23]. Flavonoids in the human diet may reduce the risk of various cancers, as well as preventing menopausal symptoms [23]. The antibacterial activity of flavonoids had been reported to be a result of their ability to form complexes with bacterial cell walls, extracellular and soluble proteins [23]. All these facts support the usefulness of P. biglobosa in folklore remedies and one of the reasons why this plant is widely used for the treatment of many diseases among tribes in Africa.
Tannins act by iron deprivation, hydrogen bonding or specific interaction with proteins such as enzymes, cell envelopes and complex formation with polysaccharides [23,24,25]. Herbs that have tannins as their component are astringent in nature and are used for treating intestinal disorders such as diarrhoea and dysentery [25] thus exhibiting antimicrobial activity. P. biglobosa extract inhibited the growth of E. coli and thus supports the usefulness of this plant in treating diarrhea and dysentery among Yoruba tribe of Southwestern Nigeria.
Saponins are considered a key ingredient in Traditional Chinese Medicine and are responsible for most of the observed biological effects [26]. Saponins are known to produce inhibitory effects on inflammation [27]. Saponins have also been reported to possess antibacterial property with their mode of action attributed to their ability to cause leakage of proteins and certain enzymes from bacterial cells [28,29,30].
Alkaloids are another kind of phytochemicals observed in the stem bark extract of P. biglobosa. Alkaloids have been associated with medicinal uses for centuries and other possible roles have not been examined. One of the most common biological properties of alkaloids is their toxicity against cells of foreign organisms. These activities have been widely studied for their potential use in the elimination and reduction of human cancer cell lines [31]. In addition, alkaloids possess anti-inflammatory, anti-asthmatic and anti-anaphylactic activities with consequences of altered immunological status in vivo [31,32]. The antibacterial properties of alkaloids have been reported to be as a result of their ability to intercalate with DNA [32].
Cardiac glycosides are an important class of naturally occurring drugs whose actions help in the treatment of congestive health failure [33]. This class of phytochemical compound was detected in P. biglobosa extract and thus supports the usefulness of this plant for the treatment of cardiac infections along with other ailments such as dental caries and cough among the Yoruba tribe of southwestern Nigeria. Steroid compounds also present in P. biglobosa bark extract are of importance and interest due to their relationship with such compounds as the sex hormones [34]. Some kinds of steroids have been reported to have immune-enhancing benefits [35,36]. Taken together all these facts support the utilization of P. biglobosa in various countries such as Nigeria, Mali and Cote d’Ivoire where it has been used to prepare local medications for the treatment of diseases.
The antimicrobial activities of P. biglobosa bark extract was investigated against some microbial isolates. The extract at a concentration of 20 mg/mL was found to inhibit the growth of all the fifteen test bacterial isolates comprising of both Gram-positive and Gram-negative organisms (Table 2). The zones of inhibition exhibited by the extract ranged between 14 ± 0.00 mm for E. coli and 28 ± 0.71 mm for P. aeruginosa.
The bacteria isolates used in this study include pathogens such as E. coli known to cause urinary tract infections [37]; E. faecalis which is associated with endocarditis and polymicrobial bacteremia [38]; P. fluorescence which usually affects patients with compromised immune systems [39,40] and K. pneumoniae known to be the causative agent of pneumonia. All these pathogens were susceptible to the plant extract used in this study, thus supporting the use of P. biglobosa in folklore remedies in the treatment of diseases caused by these pathogens. The extract was observed to inhibit the growth of both Gram-negative and Gram-positive bacteria, thus showing it to possess broad spectrum activity.
The antimicrobial activities of the partitioned fractions against test isolates show different degrees of activity at 10 mg/mL. Out of the five fractions derived from the extract of the plant stem bark, only the n-hexane and aqueous fractions show strong activity, while the chloroform, ethyl acetate and butanol fractions showed little or no activity against the test isolates used (Table 3). This suggests that n-hexane and/or water will be good solvents for the extraction of the active principle present in the stem bark of P. biglobosa.
The MIC results in Table 4 reflect a trend that tends to show different interactions among bioactive components of the stem bark extract of P. biglobosa. The lowest MIC exhibited by the stem bark extract against P. vulgaris, P. fluorescence and M. luteus was 0.63 mg/mL. On the other hand, the highest MIC of 5 mg/mL was exhibited against K. pneumoniae, E. faecalis and B. subtilis. The lowest MIC observed for the n-hexane fraction and aqueous fraction against M. luteus was 0.63 mg/mL. The highest MIC observed for aqueous fraction against P. aeruginosa, E. coli, K. pneumoniae, E. faecalis B. subtilis and Staph. aureus was 10 mg/mL while the highest MIC (10 mg/mL) for n-hexane fraction was observed against E. coli, K. pneumoniae, E. faecalis and B. subtilis. Furthermore, simultaneous comparison of the MIC values exhibited by stem bark extract, aqueous fraction and n-hexane fraction against each test bacterium showed that MIC values of crude back extract against test bacteria were smaller than they were for n-hexane fraction and aqueous fraction. An exception was observed for M. luteus which has the same MIC values for crude bark extract, n-hexane fraction and aqueous fraction (Table 4). This shows that there might be synergistic antibacterial-enhancing interactions between different bioactive components of the stem bark extract. Antibacterial-enhancing interactions among bioactive components of plant extracts have been reported earlier [41]. An example of the possibility of a synergistic interaction between the bioactive components of the stem bark extract of P. biglobosa can be seen by comparing the MIC values exhibited by the extract, n-hexane fraction and aqueous fraction against P. fluorescence. The MIC value (0.63 mg/mL) exhibited by crude bark extract against P. fluorescence was more than two-fold lower than the 2.50 mg/mL MIC value exhibited by the n-hexane fraction and aqueous fraction against this test organism (Table 4).
Table 5 shows the minimum bactericidal concentrations exhibited by the extract, fractions and the standard antibiotics used in this study against the susceptible test isolates. The MBC exhibited by the extract against the test isolates ranged between 1.25 mg/mL and 10.0 mg/mL, while the n-hexane fraction showed a MBC ranging between 1.25 mg/mL and 10.0 mg/mL. Thus, the MBC exhibited by both extract and n-hexane fraction followed the same pattern, while that of the aqueous fraction showed a different pattern. Penicillin showed a weak MBC against the test isolates when compared with those observed for streptomycin and ampicillin.
Table 5.
The minimum bactericidal concentrations (mg/mL) of the extract, n-hexane and aqueous fractions and standard antibiotics against bacterial isolates.
| Bacterial Isolates | PBM | N-HEX | ACQ | TET | PEN | STREP | AMP |
|---|---|---|---|---|---|---|---|
| B. anthracis (LIO) | 2.50 | 5.00 | 5.00 | ND | ND | 0.50 | ND |
| P. aeruginosa (NCIB 950) | 5.00 | 5.00 | ND | ND | ND | ND | ND |
| B. stearothermophillus (NCIB8222) | 2.50 | 2.25 | 5.00 | 0.25 | ND | 0.13 | 0.016 |
| B. cereus (NCIB 6349) | 5.00 | 2.50 | 5.00 | 0.50 | 1.00 | 0.13 | 0.128 |
| B. polymyxa (LIO) | 5.00 | 5.00 | 5.00 | 0.25 | 1.00 | 0.02 | 0.128 |
| C. pyogenes (LIO) | 2.50 | 5.00 | 5.00 | 0.25 | ND | 0.02 | 0.128 |
| P. fluorescence (NCIB 3756) | 2.50 | 5.00 | 5.00 | ND | ND | 0.02 | ND |
| C. sporogenes (NCIB 532) | 2.50 | 5.00 | 5.00 | 0.50 | ND | 0.03 | 0.032 |
| M. luteus (NCIB 196) | 1.25 | 1.25 | 1.25 | 0.25 | ND | 0.02 | 0.004 |
| E. faecalis (NCIB 775) | 10.00 | 10.0 | ND | 0.25 | 0.13 | 0.02 | 0.008 |
| Staph. aureus (NCIB 8588) | 5.00 | 10.0 | ND | 0.50 | 1.00 | 0.02 | ND |
| B. subtilis (NCIB 3610) | 10.00 | ND | ND | 0.50 | 1.00 | 0.50 | 0.128 |
| K. pneumoniae (NCIB 418) | 10.00 | ND | ND | 1.00 | ND | ND | 0.064 |
| E. coli (NCIB 86) | 5.00 | ND | ND | 0.25 | ND | 0.50 | ND |
| P. vulgaris (LIO) | 2.50 | 2.50 | ND | 1.00 | ND | 1.00 | 0.032 |
Key: LIO = Locally Isolated Organism, NCIB = National Collection of Industrial Bacteriology, PBM = P. biglobosa extract, N-HEX = n-hexane fraction, ACQ = aqueous fraction, TET = Tetracycline; PEN = Penicillin G, STREP = Streptomycin, AMP = Ampicillin, ND = not determined.
3. Experimental
3.1. Plant Materials
The stem bark of Parkia biglobosa was used for the study. The plant materials were collected from a location in Oshogbo, Osun State, Nigeria and were identified at the Herbarium of Botany Department, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria. Voucher specimens were prepared and deposited for reference purposes under herbarium specimen number Ife 16721.
3.2. Extraction of the Plant Stems Bark
The method of Edeoga [42] with slight modifications was adopted for extraction and for phytochemical screening tests. Fresh plant materials were oven-dried at 40 °C to a constant weight. The dried plant materials were ground into fine powder and about 1.5 kg of the powder was soaked in water and methanol mixture in ratio 2:3 (v/v) for three days at 40 °C. The liquid extract of the plant sample was evaporated to dryness in vacuo to give a methanolic crude extract (422 g); a small quantity of the dry extract was used for phytochemical screening test.
3.3. Phytochemistry of the Plant Stems Bark
A small portion of the dry extract was subjected to the phytochemical test using Trease and Evans [43] and Harbourne [44] methods to test for alkaloids, tannins, flavonoids, steroids, saponins, reducing sugars and cardiac glycoside and anthraquinones.
3.3.1. Test for Alkaloids
One half of a gram of the plant extract was dissolved in 1% HCl (5 mL) on a steam bath. The filtrate (1 mL) was treated with few drops of Dragendorff’s reagent (potassium iodide 0.11 M, bismuth nitrate 0.6 M in acetic acid 3.5 M). Turbidity or precipitation was taken as indicative of the presence of alkaloids.
3.3.2. Test for Tannin
Plant extract (about 1.0 g) was stirred with sterile distilled water (10 mL) and filtered (using Whatman number 1 filter paper). A blue colouration resulting from the addition of 2 drops of 10% FeCl3 reagent to the filtrate indicated the presence of tannins.
3.3.3. Test for Flavonoids
An aliquot of the extract (0.2 g) was dissolved in methanol (2 mL) and heated. A chip of magnesium metal strip was added to the mixture followed by the addition of a few drops of concentrated HCl. The occurrence of a red or orange colouration was indicative of the presence of flavonoids.
3.3.4. Test for Saponins
Powdered sample (about 2 g) was boiled in distilled water (20 mL) in a water bath and then filtered. The filtrate (10 mL) was mixed with distilled water (5 mL) and shaken vigorously to form a stable persistent froth. The froth was mixed with 3 drops of olive oil, shaken vigorously, and then observed for the formation of emulsions.
3.3.5. Test for Steroids
The extract (about 0.5 g) was dissolved in CHCl3 (3 mL) and then filtered. To the filtrate was added concentrated H2SO4 to form a lower layer. A reddish brown colour was taken as positive for steroids.
3.3.6. Test for Cardiac Glycosides
The extract (about 0.5 g) was dissolved in glacial acetic acid (2 mL) containing 1 drop of 1% FeCl3. This was underlaid with concentrated H2SO4. A brown ring at the interface indicated the presence of a deoxy sugar, characteristic of cardiac glycosides. A violet ring may form just above ring and gradually spreads through this layer.
3.3.7. Test for Reducing Sugars
One millilitre each of Fehling’s solutions I and II was added to an aqueous solution of the extract (2 mL). The mixture was heated in a boiling water bath for about 2–5 min. The production of a brick red precipitate indicated the presence of reducing sugars.
3.3.8. Test for Anthraquinones
A sample of plant extract (0.5 g) was boiled with 0.01M HCl and filtered while still hot. The filtrate was shaken with benzene (10 mL). The benzene layer was removed, and ammonium hydroxide (5 mL, 10%) was added. A violet, red, or pink colouration in the ammonia phase is positive for anthraquinones.
3.4. Solvent Partitioning of the Methanolic Extract
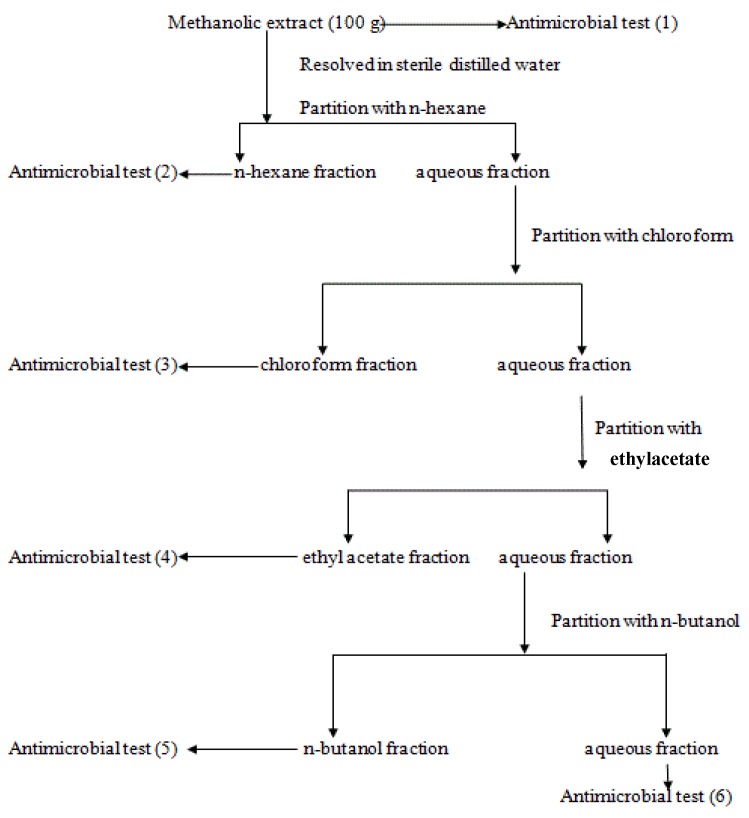
Exactly 100 g of the stem bark methanolic extract was resolved in sterile distilled water (200 mL) in a separatory funnel and extracted with n-hexane (4 × 200 mL). The resulting n-hexane phase was concentrated to dryness in vacuo and the resulting powder (28.0 g) was kept in a freezer in an air-tight container. The resulting aqueous phase was further extracted with chloroform (4 × 200 mL). The chloroform fraction obtained was also concentrated in vacuo to dryness and the recovered powder (24.0 g) was kept in freezer for further use. The ethyl acetate (9.8 g) and n-butanol (10.6 g) fractions were also obtained using the above procedure. The remaining aqueous fraction (about 28.0 g) was also freeze-dried to powder; this was also kept for further use in the freezer in an air-tight container. The procedure is summarized in the flowchart shown in Figure 1.
Figure 1.
Extraction and Fractionation Scheme of the stem bark methanolic extract of P. biglobosa.
3.5. Antibacterial Activity
3.5.1. Sensitivity Testing of the Methanolic Extract and Fractions of P. biglobosa and Standard Antibiotics on Bacterial Isolates
Susceptibility of bacterial strains to plant extract and fractions and that of standard antibiotics were carried out following a modified bioassay method of Betoni [45]. Extracts and fractions were all reconstituted in 5% methanol which was also used as a control. Mueller Hinton sterile agar plates was seeded with indicator bacterial strains (106 cfu/mL) and allowed to stand at room temperature for 3 h. Using a sterile cork borer, wells were made on the seeded plates and these were filled separately with plant extract, fractions and antibiotics of known concentrations (1 mL). The set of plates were incubated at 37 °C for 24 h and the zones of inhibition were measured.
3.5.2. Determination of Minimum Inhibitory Concentrations (MICs)
The minimum inhibitory concentrations (MICs) of the plant extract, fractions and standard antibiotics were determined by modifications to the agar dilution method of Betoni [45]. The extract and fractions were diluted in 5% methanol to give concentrations ranging between 0.025 mg/mL and 1.0 mg/mL in nutrient broth. With the aid of a standard pipette, about 1 mL of 18 hour old bacterial broth (106 cfu/mL) culture was introduced into appropriately labelled test tubes. A set of tubes containing only growth medium plus each of the test bacteria was set up separately to serve as controls. All the tubes were incubated at 37 °C for 24 h. The minimum inhibitory concentration was taken as lowest concentration that will prevent growth of the bacterial strains. The same test was repeated for standard antibiotics at different concentrations.
3.6. Data Analyses
Data was analysed for a 4 × 4 Latin square design with the statistical program using the GLM model (Statistical Analysis Systems, SAS Institute, Cary, NC, USA, 2001). Results were contrasted with negative and a positive control. The means of the values was compared using independent t test of significance (p < 0.05).
4. Conclusions
It was concluded from this study that the stem bark extract of P. biglobosa is rich in phytochemicals such as flavonoids, tannins, cardiac glycosides, alkaloids, saponins and steroids. These phytochemicals have been reported to be of pharmaceutical importance. This supports the use of this plant in folklore medicine for the herbal treatment of infections such as dental caries, pneumonia, bronchitis, diarrhoea and cough. The extract and fractions of P. biglobosa used in this work were also found to possess antimicrobial properties, making this plant a potential source of bioactive compounds that can be used in the management of bacterial infections. The widespread use of this plant in herbal medicaments in countries such as Mali, Cote d’Ivoire and Nigeria and the report of Gernah on the toxicity of the plant [46] show that the plant is non-toxic to humans, hence different formulations could be prepared for further studies. Work is still ongoing to further purify the fractions from the stem bark extract of P. biglobosa to isolate pure antimicrobial compounds. It is hoped these compounds will lead to the formulation of new and more potent antimicrobial drugs that will prove useful in the treatment of infections caused by microorganisms that have developed multiple resistance to currently available synthetic antimicrobial compounds.
Acknowledgments
The authors are grateful to the Medunsa National Pharmacovigilance Centre, University of Limpopo, South Africa for supporting this research and to HC Illoh for the plant identification.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the extracts are available from the authors.
References
- 1.Levy S.B., Marshal B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 2.Udobi C.E., Onaolapo J.A., Agunu A. Antibacterial activities and bioactive components of the aqueous fraction of the stem bark of Parkia biglobosa (Jacq) (Mimosaceae) Nigerian J. Pharm. Sc. 2008;7:49–55. [Google Scholar]
- 3.Salvat A., Antonacci L., Fortunato R.H., Suarez E.Y., Godoy H.M. Screening of some plants from Northern Argentina for their antimicrobial activity. Lett. Applied Microbiol. 2001;32:293–297. doi: 10.1046/j.1472-765X.2001.00923.x. [DOI] [PubMed] [Google Scholar]
- 4.Mahesh B., Satish S. Antimicrobial activity of some important medical plant against plant and human pathogen. World J. Agric. Sc. 2008;4:839–843. [Google Scholar]
- 5.Das K., Tiwari R.K.S., Shrivastava D.K. Techniques for evaluation of medicinal plant products as antimicrobial agent: current methods and future trends. J. Med. Plt. Res. 2010;4:104–111. [Google Scholar]
- 6.Gislene G.F.N., Juliana L., Paulo C.F., Giuliana L.S. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Brazilian J. Microbiol. 2000;31:247–256. [Google Scholar]
- 7.El-Mahmood A.M., Ameh J.M. In vitro antibacterial activity of Parkia biglobosa (Jacq.) root bark extract against some microorganisms associated with urinary tract infections. Afr. J. Biotech. 2007;6:1272–1275. [Google Scholar]
- 8.Udobi C.E., Onaolapo J.A., Agunu A. Antibacterial potential of the methanolic extract and aqueous fraction of the stem bark of the African locust bean (Parkia biglobosa) Eur. J.Sci. Res. 2010;43:596–602. [Google Scholar]
- 9.Sabiti E.N., Cobbian J. Parkia biglobosa: A potential multipurpose fodder tree legume in West Africa. Int. Tr. Cr. J. 1992;7:113–139. doi: 10.1080/01435698.1992.9752911. [DOI] [Google Scholar]
- 10.Hopkins H.C. The taxonomy, reproductive biology and economic potential of Parkia (Leguminosae: Mimosideae) in Africa and Madagascar. Bot. J. Lin. Soc. 1983;87:135–167. doi: 10.1111/j.1095-8339.1983.tb00987.x. [DOI] [Google Scholar]
- 11.Ajaiyeoba E.O. Phytochemical and antibacterial properties of Parkia biglobosa and Parkia bicolor leaf extracts. Afr. J. Biomed. Res. 2002;5:125–129. [Google Scholar]
- 12.Kouadio F., Kanko C., Juge M., Grimaud N., Jean A., N’Guessan Y.T., Petit J.Y. Analgesic and anti-inflammatory activities of an extract from Parkia biglobosa used in traditional medicine in the Ivory Coast. Phyto. Res. 2000;14:635–637. doi: 10.1002/1099-1573(200012)14:8<635::AID-PTR427>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 13.Millogo-Kone H., Guissou I.P., Nacoulma O., Traore A.S. Antimicrobial effects of the stem Bark extracts of Parkia biglobosa (Jacq.) on Shigellae. Afr. J. Trad. Compl. Alt. Med. 2007;4:392–396. doi: 10.4314/ajtcam.v4i4.31234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odetola A.A., Akinloye O., Egunjobi C., Adekunle W.A., Ayoola A.O. Possible antidiabetic and antihyperlipidaemic effect of fermented Parkia biglobosa (JACQ) extract in alloxan-induced diabetic rats. Clin. Exp. Pharmacol. Physiol. 2006;33:808–812. doi: 10.1111/j.1440-1681.2006.04444.x. [DOI] [PubMed] [Google Scholar]
- 15.Udobi C.E., Onaolapo J.A. Phytochemical analysis and antibacterial evaluation of the leaf stem bark and root of the African locust bean (Parkia biglobosa) J. Med. Pl. Res. 2009;3:338–344. [Google Scholar]
- 16.Agro Forestry Database: A tree species reference and selection guide. [(accessed on 24 May 2013)]. Available online: http://www.worldagroforestrycentre.org/sea/products/afdbases/af/asp/SpeciesInfo.asp?SpID=1255.
- 17.Builders M., Wannang N., Aguiyi J. Antiplasmodial activities of Parkia biglobosa leaves: In vivo and in vitro studies. An. Biol. Res. 2011;2:8–20. [Google Scholar]
- 18.Millogo-Kone H., Guissou I.P., Nacoulma O., Traore A. Comparative Study of Leaf and Stem Bark Extracts of Parkia biglobosa against Enterobacteria. Afr. J. Trad. Com. 2008;5:238–243. doi: 10.4314/ajtcam.v5i3.31279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odeyemi O.O., Owoade R.A., Akinjurolere R. Toxicity and Population supressions effects (Parkia clappertonium) and dried fish pests (Dermestes maculated) and Necrobia rufipes. Global J. Pure Appl. Sci. 2000;6:191–195. [Google Scholar]
- 20.Ferguson L.R. Role of plant polyphenols in genomic stability. Mut. Res. J. 2001;475:89–111. doi: 10.1016/S0027-5107(01)00073-2. [DOI] [PubMed] [Google Scholar]
- 21.Tsuchiya H., Sato M., Miyazaki T., Fujiwara S., Tanigaki S., Ohyama M., Tanaka T., Iinuma M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 1996;50:27–34. doi: 10.1016/0378-8741(96)85514-0. [DOI] [PubMed] [Google Scholar]
- 22.Maikai V.A., Maikai B.V., Kobo P.I. Antimicrobial properties of stem bark extract of Ximenia americana. J. Agric. Sci. 2009;1:30–34. [Google Scholar]
- 23.Scalbert A. Antimicrobial properties of tannins. Phytochemistry. 1991;30:3875–3883. doi: 10.1016/0031-9422(91)83426-L. [DOI] [Google Scholar]
- 24.Hisanori A., Kazuyasu F., Osamu Y., Takashi O., Keiji I. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 2001;48:487–491. doi: 10.1093/jac/48.4.487. [DOI] [PubMed] [Google Scholar]
- 25.Dharmananda S. Gallnuts and the uses of tannins in Chinese medicine; Proceedings of institute for Traditional Medicine; Portland, OR, USA. 2003. [Google Scholar]
- 26.Liu J., Henkel T. Traditional Chinese medicine (TCM): Are polyphenols and saponins the key ingredients triggering biological activities? Cur. Med. Chem. 2002;9:1483–1485. doi: 10.2174/0929867023369709. [DOI] [PubMed] [Google Scholar]
- 27.Just M.J., Recio M.C., Giner R.M., Cuellar M.J., Manez S., Bilia A.R., Rios J.L. Anti-inflammatory activity of unusual lupine saponins from Bupleurum fruticescens. Pl. Med. J. 1998;64:404–407. doi: 10.1055/s-2006-957469. [DOI] [PubMed] [Google Scholar]
- 28.Tamil S.A., Dinesh M.G., Satyan R.S., Chandrasekaran B., Rose C. Leaf and seed extracts of Bixa orellana L. exerts anti-microbial activity against bacterial pathogens. J. Appl. Pharm. Sci. 2011;1:116–120. [Google Scholar]
- 29.Nobori T., Miura K., Wu D.J., Lois A., Takabayashi K., Carson D.A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994;368:753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- 30.Ganguly T., Sainis K.B. Inhibition of cellular immune responses by Tylophoraindica in experimental models. Phytomedicine. 2001;8:348–355. doi: 10.1078/0944-7113-00055. [DOI] [PubMed] [Google Scholar]
- 31.Staerk D., Christensen J., Lemmich E., Duus J.O., Olsen C.E., Jaroszewski J.W. Cytotoxic activity of some phenanthroindolizidine N-oxide alkaloids from Cynanchum vincetoxicum. J. Nat.Prod. 2000;63:1584–1586. doi: 10.1021/np0003443. [DOI] [PubMed] [Google Scholar]
- 32.Phillipson J.D., O’Neill M.J. New leads to the treatment of protozoal infections based on natural product molecules. Acta Pharm. Nord. 1987;1:131–144. [Google Scholar]
- 33.Ikeda Y., Fujii Y., Nakaya I., Yamazaki M. Quantitative HPLC analysis of cardiac glycosides in Digitalis purpurea leaves. J. Nat. Prod. 1995;58:897–901. doi: 10.1021/np50120a012. [DOI] [PubMed] [Google Scholar]
- 34.Okwu D.E. Evaluation of the chemical composition of medicinal plants belonging to Euphorbiaceae in Pakistan. Pakistan Vet. J. 2001;14:160–162. [Google Scholar]
- 35.Donald P.R., Lamprecht J.H., Freestone M., Albrecht C.F., Bouic P.J.D., Kotze D., Jaarsveld P.P. A randomized placebo-controlled trial of the efficacy of beta-sitosterol and its glucoside as adjuvants in the treatment of pulmonary tuberculosis. Int. J. Tuber Lung Dis. 1997;1:518–522. [PubMed] [Google Scholar]
- 36.Berges R.R., Windeler J., Trampisch H.J., Senge T.H. The B-sitosterol study group: randomized, placebo-controlled, double-blind clinical trial of B-sitosterol in patients with benign prostatic hyperplasia. Lancet. 1995;345:1529–1532. doi: 10.1016/S0140-6736(95)91085-9. [DOI] [PubMed] [Google Scholar]
- 37.Dromigny J.A., Nabeth P., Juergens-Behr A., Perrier-Gros-Claude J.D. Risk factors for antibiotic resistant Escherichia coli isolated from community-acquired urinary tract infections in Dakar, Senegal. J. Antimicrob. Chemoth. 2005;56:236–239. doi: 10.1093/jac/dki158. [DOI] [PubMed] [Google Scholar]
- 38.Zervos M.J., Dembinski S., Mikesell T., Schaberg D. High-level resistance to gentamicin in Streptococcus faecalis: risk factors and evidence for exogenous acquisition of infection. J. Infect. Dis. 1986;153:1075–1083. doi: 10.1093/infdis/153.6.1075. [DOI] [PubMed] [Google Scholar]
- 39.Hye J.C., Chan H.S., Seong H.P., Hyun Yang K.H.D., Juil K., Hyung-Kab K., Duk-Hwa C., Jung H.A., Yuseok M. Involvement of epidermal growth factor receptor-linked signaling responses in Pseudomonas fluorescens infected alveolar epithelial cells. Infect. Immunity. 2011;79:1998–2005. doi: 10.1128/IAI.01232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gershman M.D., Kennedy D.J., Noble-Wang J. Multistate outbreak of Pseudomonas fluorescens blood stream infection after exposure to contaminated heparinized saline flush prepared by a compounding pharmacy. Clin. Infect. Dis. 2008;47:1372–1379. doi: 10.1086/592968. [DOI] [PubMed] [Google Scholar]
- 41.El-Mahmood A.M., Doughari J.H., Chanji F.J. In vitro antibacterial activities of extracts of Nauclea latifolia and Daniella oliveri. Sci. Res. Essay. 2008;3:102–105. [Google Scholar]
- 42.Edeoga H.O., Okwu D.E., Mbaebe B.O. Phytochemical constituent of some nigerian medicinal plants. Afr. J. Biotechnol. 2005;4:685–688. [Google Scholar]
- 43.Trease G.E., Evans W.C. Pharmacognosy. 12th ed. Bailliers Tindal; London, UK: 1983. [Google Scholar]
- 44.Harbourne J.B. Chapman and Hall; London, UK: 1983. Phytochemical Methods-A Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- 45.Betoni J.E.C., Mantovani R.P., Barbosa L.N., Di Stasi L.C., Fernandes A., Jr. Synergism between plant extract and antimicrobial drugs used on Staphylococcus aureus diseases. Mem. Inst. Oswaldo Cruz. 2006;101:387–390. doi: 10.1590/S0074-02762006000400007. [DOI] [PubMed] [Google Scholar]
- 46.Abagale S.A., Twumasi S.K., Awudza J.A.M. Chemical analyses of aqueous extract of Parkia biglobosa fruit husk collected from Northern Ghana. Sc. Res. Ess. 2013;8:589–595. [Google Scholar]