Abstract
This review covers important anticancer and antifungal compounds reported from filamentous fungi and in particular from Aspergillus, Penicillium and Talaromyces. The taxonomy of these fungi is not trivial, so a focus of this review has been to report the correct identity of the producing organisms based on substantial previous in-house chemotaxonomic studies.
Keywords: anticancer, polyketides, non-ribosomal peptides, terpenoids, fungi, natural products, taxonomy
1. Introduction
Filamentous fungi such as Aspergillus, Penicillium and Talaromyces are some of the most incredible chemical factories known today. Accordingly, numerous bioactives such as mycotoxins, antifungal and anticancer agents have been reported in the literature within the last more than 100 years [1]. Despite this many new compounds revealing remarkable new bioactivities are still being discovered, including well-known metabolites such as griseofulvin [2,3,4,5]. This, combined with the fact that large combinatorial libraries have not provided the anticipated number of new chemical entities explains why the field of natural products is currently assuming new prominence. Thus, natural products are still used as scaffolds for synthetic organic chemistry, although nowadays primarily for designing smaller and more focused diversity oriented synthesis-derived libraries [6].
It has been estimated that approximately 1.5 million or likely as many as 3 million fungal species exist on Earth, of which only around 100,000 species have been described so far [7]. A multitude of new species are likely to be discovered from diverse habitats, such as tropical forest plants and soils, associated to insects and in the marine environment. In addition to untapped biodiversity recent sequencing of complete fungal genomes has revealed that many gene clusters are silent, suggesting the possibility for many more compounds [8]. Despite several efforts to stimulate such pathways using epigenetic modifiers [9,10], it is evident that we still do not know the full biosynthetic potential even of well studied model organisms such as Aspergillus nidulans, altogether strongly indicating Nature’s potential as source of new promising bioactive small molecule scaffolds.
The renewed interest in natural product discovery is further enhanced with the new strategies and methodologies for fast dereplication that have been developed within the last decade. Thus chromatographic and spectroscopic methods are combined with database searching in for example Antibase, which is a comprehensive database for natural products from microorganisms [11]. The performance of mass spectrometers is continuously improving, including easy access to both positive and negative ionization spectra even during fast ultra-high-performance liquid chromatography (UHPLC). Altogether the use of accurate mass measurements for dereplication of unknown compounds reduces the number of predicted elemental compositions, ensuring that database searches are conducted with the fewest possible candidates [12]. The use of UV spectral information is often important in the dereplication process for prioritizing between the MS-generated candidates, as well as for UV-guided discovery of novel compounds [13,14].
This article reviews anticancer and often also antifungal natural products primarily produced by Aspergillus, Penicillium and Talaromyces. For practical reasons the compounds have been grouped into major biosynthetic classes, according to the biosynthetic origin of the core part of the structures, despite that many compounds are actually of mixed biosynthetic origin (e.g., prenylated). This review is broader in scope than other recent reviews only focusing on endophytes [15,16], and a strong focus has been on giving the correct identity of the species reported in the literature, where many may previously have been misidentified or names have been changed according to the 2011 Amsterdam Declaration on Fungal Nomenclature [17].
2. Polyketide-Derived Anticancer Compounds
Polyketides represents one of the major classes of natural products of which many are biological active [1]. Today much is known about the enzymes involved in the biosynthesis of a huge diversity of both non-reduced (aromatic), partly reduced and highly reduced polyketides [8].
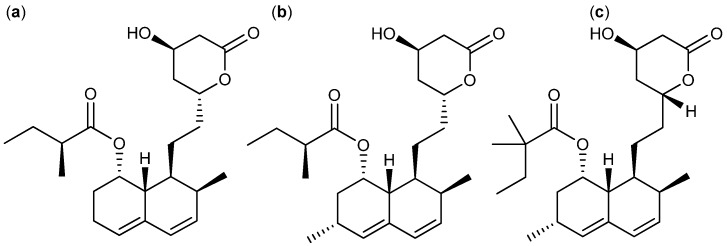
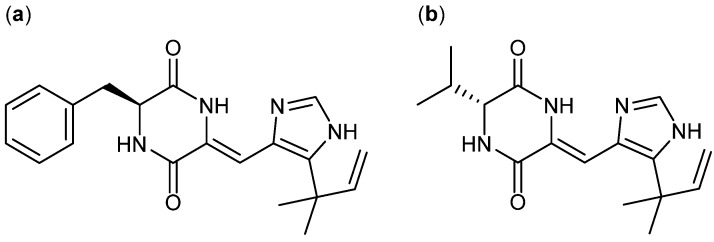
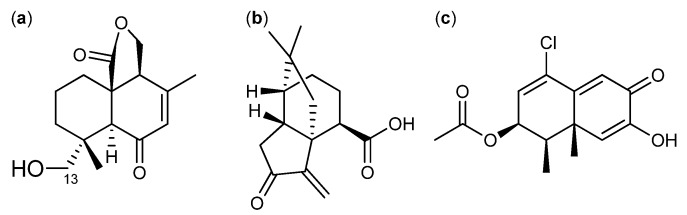
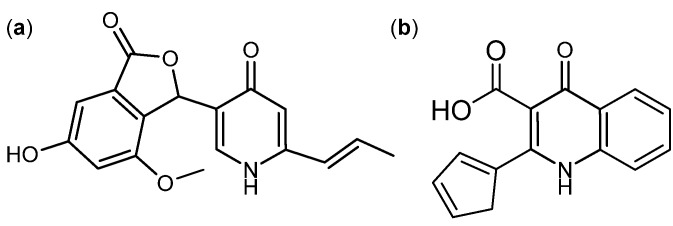
Some famous fungal polyketides with anticancer activity belong to the statin family. The statins are well known cholesterol synthesis inhibitors that are used in clinical treatment of hypercholesterolemia and cardiovascular diseases [18,19,20,21]. Moreover, members the statin family are known to have antifungal properties against Aspergillus spp. and Candida spp. [18,22,23,24]. The statin family includes a long list of both natural and synthetic compounds, for example the naturally derived compactin, lovastatin and pravastatin. The statin structure is based on a dicyclohexene ring system connected to a dicyclohexene ring system connected to a side chain with a closed lactone ring or an open acid form [21]. The compactins are primarily produced by P. solitum and P. hirsutum [20,25] (first misidentified as P. brevicompactum [18] and a fungus identified as P. citrinum [19]). Another group of statins that has an extra methyl group attached on the dicyclohexene ring system are produced by A. terreus [26] and Monascus spp. [27]. Several in vitro activities of the statins have been published throughout the years. Reports showed that compactin (Figure 1a) inhibited acute myeloid leukemia (AML) cells with a full inhibitory concentration (IC100) of 2.6 µM [28]. The analogs lovastatin (Figure 1b) and simvastatin (Figure 1c) have been shown to be even more potent.
Figure 1.
Statins: (a) Compactin, (b) Lovastatin and (c) Simvastatin.
Lovastatin and the synthetic simvastatin selectively inhibited colony growth of primary AML cells with 75%–95% effectiveness. No effect was seen on normal bone marrow [29]. The more recent reported activities includes reduction of proliferation by lovastatin in four lung cancer cell lines with median inhibitory concentration (IC50) values between 1.5 and 30 µM [30]. In 2010 it was shown that lovastatin induced apoptosis in ten ovarian cancer cell lines tested, with IC50 values between 2 and 39 µM [31], and recently lovastatin was found to inhibit breast cancer MCF-7, liver cancer HepG2, and cervical cancer HeLa cell lines with IC50 values of 0.7, 1.1 and 0.6 µg/mL, respectively [32]. Simvastatin inhibited two lung cancer, three melanoma, and four breast cancer cell lines with IC50 values between 0.8 and 5.4 µM and induced apoptosis with reduced tumor growth in hepatic cancer cells [33,34]. Encouraged by these results simvastatin has entered clinical trials as an anticancer drug [35].
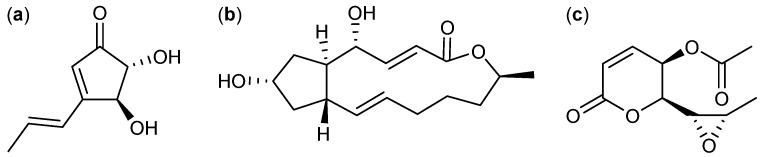
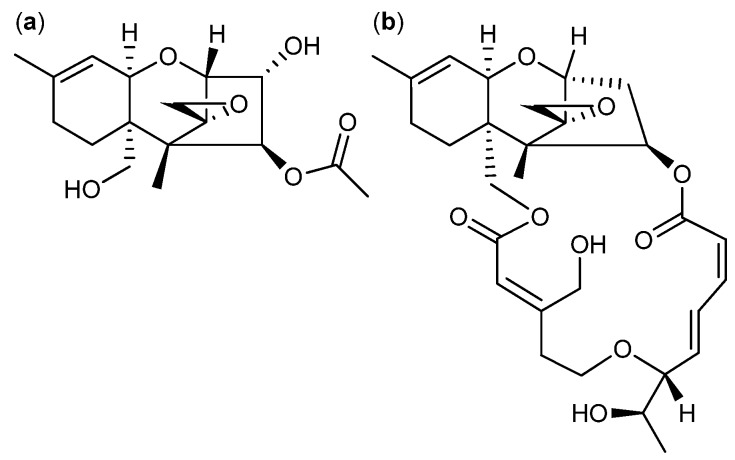
The three small polyketides terrein, brefeldin A, and asperlin are examples of well-known metabolites that a couple of decades after they were discovered were shown to exhibit novel anticancer activities. The small antifungal [36] polyketide terrein (Figure 2a) produced by A. terreus has been known since 1935 [37]. Almost 80 years later it was found that terrein inhibits breast cancer by induction of apoptosis with an IC50 value of 1.1 nM in MCF-7 cell line. That makes terrein 100-fold more potent than taxol against this cell line. Additionally terrein was found active against pancreatic and liver cancer cell lines PANC-1 (IC50 9.8 µM) and HepG2 (IC50 66.8 µM) [38].
Figure 2.
Small polyketides: (a) Terrein, (b) Brefeldin A, and (c) Asperlin.
Brefeldin A (Figure 2b), another small antifungal [39,40] polyketide isolated in 1958 from P. brefeldianum [41] was identified almost 40 years later as inducer of apoptosis in leukemia (HL-60 and K-582), colon (HT-29), prostate (DU-145), cervical (KB and HeLa), breast (MCF-7 and BC-1), and lung (SPC-A-1 and NCI-H187) cancer cell lines [42,43,44,45]. The inhibiting effect of brefeldin A was demonstrated with IC50 values 35.7 nM (HL-60), 32 nM (KB), 6.4 nM (HeLa), 7.1 nM (MCF-7), 40 nM (BC-1), 6.3 nM (SPC-A-1), and 110 nM (NCI-H187) [44,45].
Asperlin (Figure 2c) was isolated from A. nidulans in 1966 [46]. In 2011 it was discovered that asperlin reduces cell proliferation and induce G2/M cell cycle arrest in the human cervical carcinoma HeLa cell line [47].
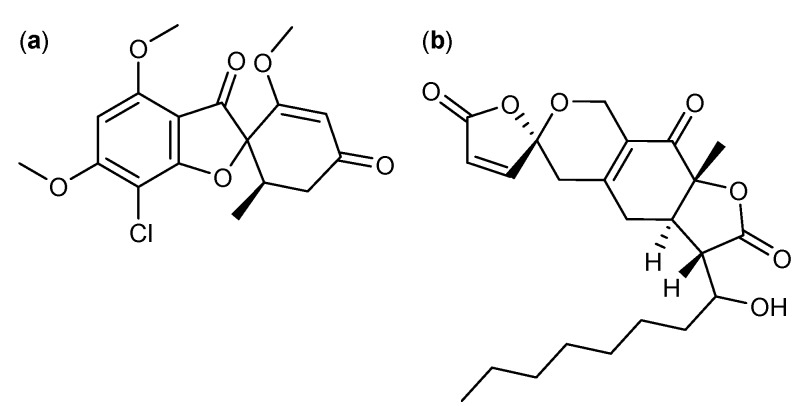
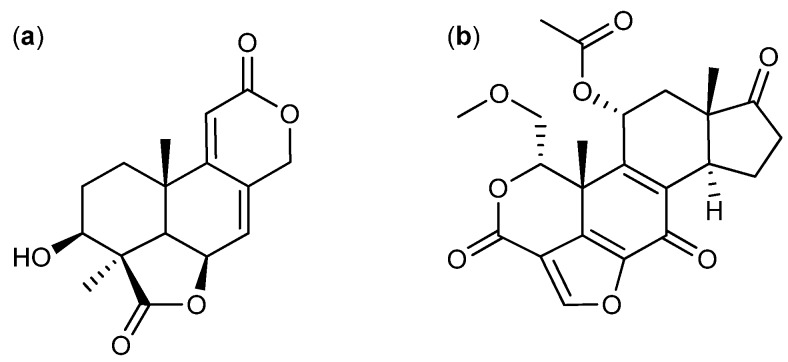
Filamentous fungi are known to produce anticancer polyketides with different spiro ring structures. One of the more well-known is the antifungal [48,49] compound griseofulvin (Figure 3a) from P. griseofulvum [50]. Griseofulvin was introduced commercially in 1965 and first considered for cancer treatment in 1973 [49,51]. Later it was shown to induce cell proliferation and mitosis in the human cervical cancer cell line HeLa with an IC50 value of 20 µM, as well as it inhibits centrosomal clustering in human squamous cancer SCC-114 cell line with an IC50 value of 35 µM [2,3]. The synthetic analog GF-15 increased the inhibitory effect of centrosomal clustering in SCC-114 cells 25-fold with an IC50 value of 0.9 µM [5]. It was further shown that combined treatment of griseofulvin and the cancer chemotherapeutic agent nocodazole in vivo improved the effect of nocodazole and arrested tumor growth in mice infected with COLO 205 tumors [4].
Figure 3.
Spiro compounds: (a) Griseofulvin, and (b) Sequoiamonascin A.
Other examples of fungal anticancer polyketides with spiro ring structures are sequoiamonascin A (Figure 3b) and B from A. parasiticus [52]. Sequoiamonascin A demonstrated selective cytotoxicity against six leukemia cell lines and two melanoma cell lines with a median growth inhibitory (GI50) log10 value of -6.00 [52]. Furthermore, sequoiamonascin A shows cytotoxic activity against breast cancer MCF-7, lung cancer NCI-H460 and central nervous system (CNS) cancer SF-268 cell lines where cell growth can be reduced to 1%–2% when treated with 10 µM sequoiamonascin A [52].
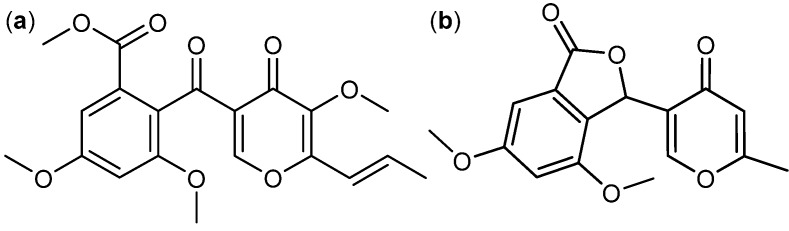
A group of fungal γ-pyrones were found to have activity against cancer. An example of these is the antifungal [48,49] 3-O-methylfunicone (Figure 4a) produced by T. pinophilus (originally published as P. pinophlium) [1,48]. It induced apoptosis and affects cell proliferation in various cancer cell lines including HeLa, MCF-7, A-375P and A-375M [50,51,52,53]. Another structurally related compound is penisimplicissin (Figure 4b) isolated from T. pinophilus (originally published as P. simplicissimum) [54]. It displayed selective cytotoxicity towards leukemia HL-60 and CCRF-CEM cell lines with log10 GI50 −6.7 and −5.8, respectively [55].
Figure 4.
γ-Pyrones: (a) 3-O-Methylfunicone and (b) Penisimplicissin.
A group of more than 20 closely related azaphilones, namely the antifungal [56] chaetomugilin family, has been isolated within the last five years from marine fish-derived Chaetomium globosum [57,58,59,60,61,62,63,64]. The chaetomugilins C, I, P and 11-epichaetomugilin I (Figure 5) showed significant inhibition of two human leukemia P-388, HL-60 and one murine leukemia L-1210, as well as human cervical cancer cell line, KB with 11-epichaetomugilin I as the more potent with IC50 values of 0.7 µM (P-388), 1.0 µM (HL-60), 1.6 µM (L-1210), and 1.2 µM (KB) [62].
Figure 5.
11-Epichaetomugilin I.
The fungal metabolite norsolorinic acid (Figure 6a) with a tricyclic structure is produced by A. parasiticus and A. nidulans [65,66,67,68]. Norsolorinic acid selectively induced cell cycle arrest in G0/G1 phase of the cell cycle and consequently induced apoptosis in human bladder cancer T-24 and human breast cancer MCF-7 with IC50 values of 10.5 and 12.7 µM, respectively [67,68].
Figure 6.
(a) Norsolorinic acid and (b) Austocystin D.
Austocystin D (Figure 6b) was isolated from A. pseudoustus [69] in 1974 (originally misidentified as A. ustus [70]). Austocystin D was shown to selectively inhibit growth of human colon carcinoma LS174T cells in mice and tumor cell lines that overexpress the multidrug resistance-associated protein [71]. Additionally, austocystin D inhibited a number of cancer cell lines: SR (leukemia, GI50 16 nM), U-87 (brain, GI50 4946 nM), MCF-7 (breast, GI50 < 1 nM), MDA-MB-231 (breast, GI50 549 nM), PC-3 (prostate, GI50 3 nM), SW-620 (colon, GI50 27 nM), HCT-15 (colon, GI50 42 nM), and MX-2 (uterine, GI50 3358 nM) [72]. Although, displaying diverse activities towards many types of cancer lines, austocystin D never entered clinical trials due to a low safety window [71].
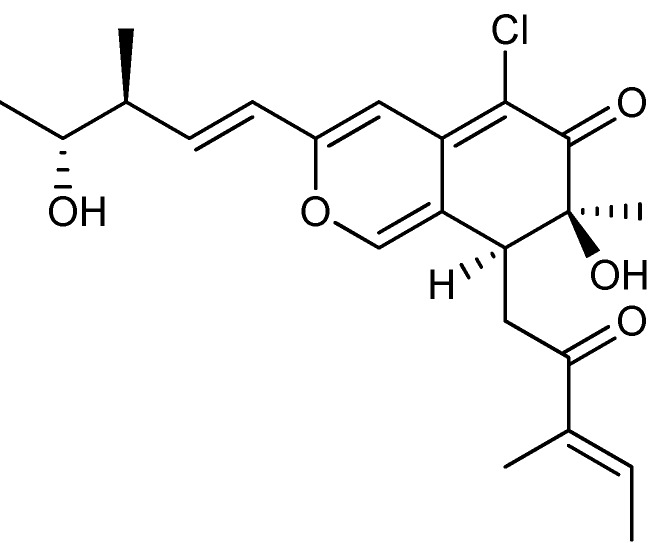
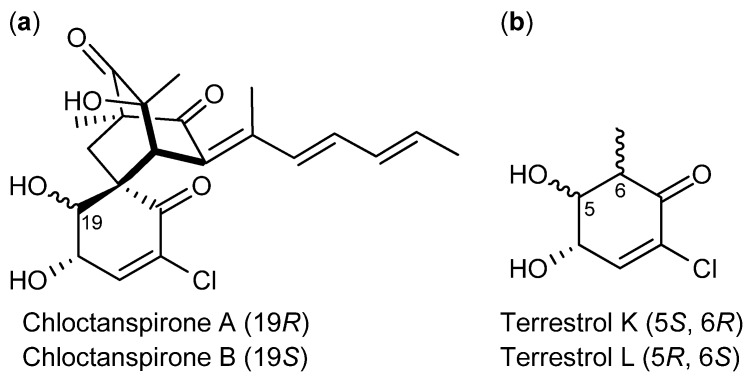
The two epimers chloctanspirone A and B (Figure 7a) are probably produced by P. chrysogenum or P. rubens [73,74] (originally published incorrectly as P. terrestre [75]). Chloctanspirone A and B were the first chlorinated sorbicillinoids isolated from a natural source and the first to be identified with their very unique ring structure. Chloctanspirone A was the more active analog and inhibited human leukemia HL-60 and lung cancer cell line A-549 cell lines with IC50 values of 9.2 and 39.7 µM, respectively [76]. Chloctanspirone B however, only showed moderately or no activity against the same cell lines. Interestingly, the two precursors terrestrols K and L (Figure 7b) that contain the epicenter, which dissociates chloctanspirone A from B were inactive. This points to the conclusion that the cyclohexenone moiety has an impact on the activity though it is not the pharmacophore [76].
Figure 7.
(a) Chloctanspirone A, B and (b) Precursors terrestrols K, L.
A group of around 20 p-terphenyls were isolated from A. candidus and found to be cytotoxic against the human cervical cancer cell line HeLa [77]. Thirty years later a new group of prenylated-p-terphenyls, called prenylterphenyllins (Figure 8a), terprenins (Figure 8b), and prenylcandidusins (Figure 8c) were isolated from A. candidus and A. taichungensis [78,79]. The activity of these compounds were studied against lung cancer cell line A-549, human leukemia cell lines HL-60 and P-388, and human epidermoid cancer cell lines KB3-1 [78,79]. Prenylterphenyllin A was found to be the more active against A-549 and HL-60 with IC50 values of 8.3 and 1.5 µM, respectively [79], whereas 4′′-deoxyisoterprenin displayed higher activity towards KB3-1 with an IC50 value of 6.2 µM [78], and finally prenylcandidusin B showed higher activity against P-388 with an IC50 value of 1.6 µM [79].
Figure 8.
Prenylterphenyllins: (a) Prenylterphenyllin A, (b) 4′′-Deoxyisoterprenin, and (c) Prenylcandidusin B.
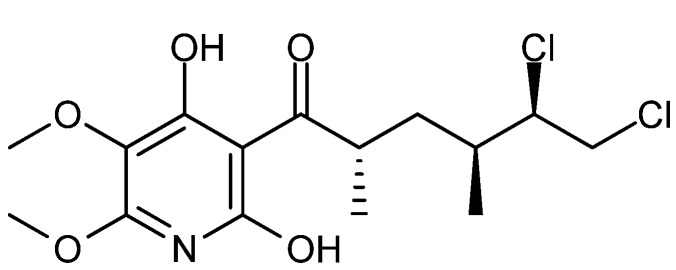
In 2011 five new di- and tricitrinols were added to the known citrinin family from P. citrinum [80]. All five of them showed cytotoxic activity against human leukemia HL-60, colon cancer HCT-116, and cervical cancer KB cell lines. The more potent was tricitrinol B (Figure 9), with IC50 values of 3.2, 4.8 and 3.9 µM, respectively [80].
Figure 9.
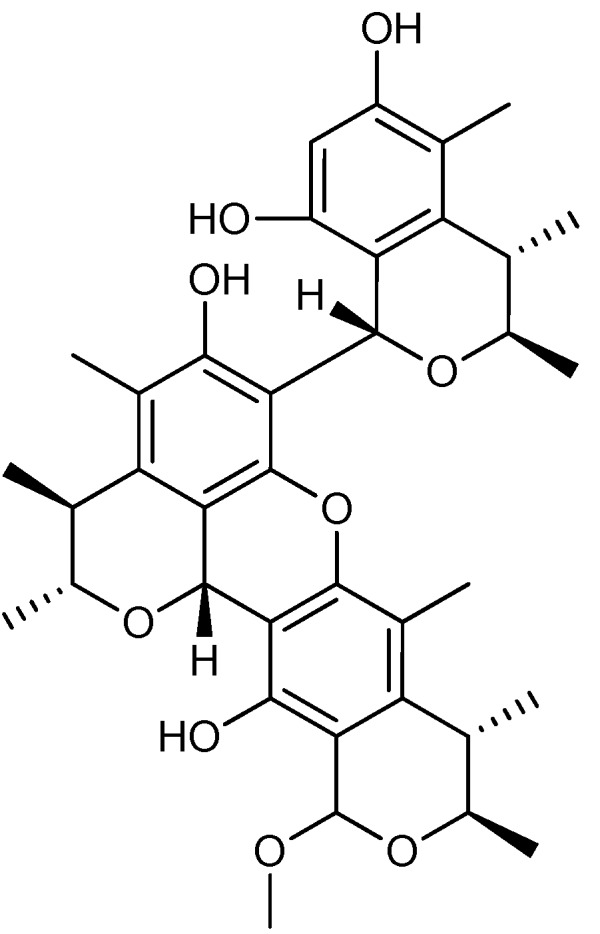
Tricitrinol B.
The class of perylenequinones has been used for centuries in Chinese herbal medicine, but fungal production of several perylenequinones has likewise been reported [81]. The calphostins were isolated from Cladosporium cladosporioides and shown to inhibit cervical cancer HeLa-S3 and breast cancer MCF-7 cell lines. Most potent was calphostin C (Figure 10a) with IC50 values of 0.23 and 0.18 µM, respectively [82]. Furthermore, calphostin C induced apoptosis in acute lymphoblastic leukemia [83]. The hypocrellins, another branch, of the perylenequinone family was isolated from Hypocrella bambusae, originally published incorrectly as Shira bambusicola [84,85]. Hypocrellin D was one of the more potent analogs and inhibited liver (Bel-7721) and lung (A-549 and Anip-973) cancer cell lines, with IC50 values, 1.8, 8.8, 38.4 µg/ml, respectively [85]. Additionally, the hypocrellins had a photodynamic effect on a wide range of tumor cell lines. Due to their insolubility in water several analogs have been synthesized to improve the pharmacokinetics and drug delivery. Two of the more successful analogs were carboxylate salt derivatives of hypocrellin B (Figure 10b) with increased water solubility and activity against human breast cancer MCF-7 when photodynamic therapy was used [81,86]. In 2012 a new group of perylenequinones called the phaeosphaerins were isolated from Phaeosphaeria sp. with phaeosphaerin B (Figure 10c) as the more potent against prostate PC-3, DU-145, LNCaP cancer cell lines with IC50 values of 2.4 µM, 9.5 µM and 2.7 µM, respectively [87].
Figure 10.
Perylenequinones: (a) Calphostin C, (b) Hypocrellin B and (c) Phaeosphaerin B.
3. Nitrogen-Containing Anticancer Compounds
Fungal nitrogen containing compounds represent another large group of natural products, of which many have famous bioactivities. In general these types of compounds incorporate amino acid building blocks into often complex heteroaromatic compounds such as diketopiperazines, quinazolines and benzodiazepines [1]. Often these compounds are referred to as alkaloids, due to their basic nature, when containing primary, secondary or tertiary amine functionalities. However, compounds only containing amide bonds, that are essentially neutral, are often also referred to as alkaloids. In recent years it has become clear that many nitrogen containing compounds are biosynthesized by multifunctional enzymes (non-ribosomal peptide synthases, NRPS) with modular arrangements comparable to that seen for some polyketide synthases. In such contexts these compounds are usually referred to as non-ribosomal peptides (NRPs) even though they are also called alkaloids [88]. In the following section no attempt has been made to differentiate between the different naming of the numerous amino acid-derived metabolites.
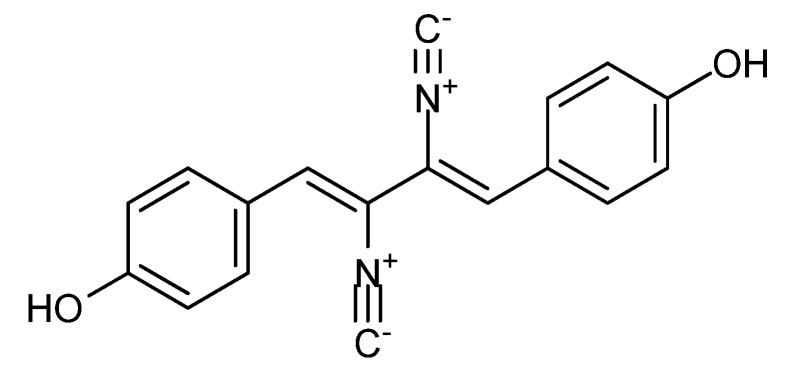
The antifungal [89] xanthocillin X (Figure 11) was first isolated from Dichotomomyces albus ascribed to D. cejpii [90], but is produced by P. chrysogenum as well [1]. In 1968 xanthocillin X was found active against a Ehrlich ascites carcinoma-mouse strain with median lethal dose (LD50) of 40 mg/kg [91]. Approximately 40 years later it was shown that xanthocillin X inhibited leukemia K-562, human cervical cancer HeLa, breast cancer MCF-7 and MDA-MB-231, liver cancer HepG2, lung cancer NCI-H460, and prostate cancer DU-145 cell lines with IC50 values between 0.4 and 12 µg/ml [89,92]. Two structural analogs named BU-4704 and xanthocillin X di-methoxy were found more potent than xanthocillin X. Instead of the two hydroxy groups BU-4704 contains a methoxy and a sulfonic acid group and xanthocillin X di-methoxy contains two methoxy groups. BU-4704 inhibited human colon HCT-116 and murine melanoma B16-F10 with IC50 values of 0.6 and 4.3 µg/mL, respectively [93]. Xanthocillin X di-methoxy inhibited HepG2, MCF-7 and KB cancer cell lines with IC50 values of 0.18, 0.38 and 0.44 µg/mL, respectively [94].
Figure 11.
Xanthocillin X.
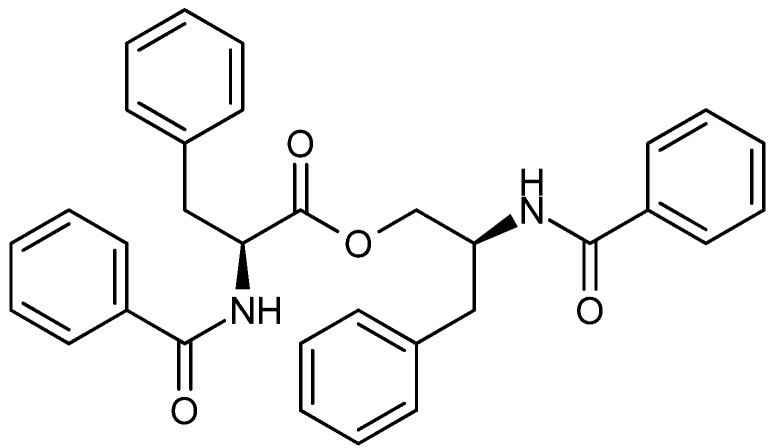
Asperphenamate (Figure 12) was first isolated from A. flavipes [95] and later found to be produced by several of Penicillium spp. as well [96]. Asperphenamate displayed moderate cytotoxic activity against several cancer cell lines [97]. Due to the low activity and a low water solubility asperphenamate was used as a lead for synthetic structurally isomers. It was found that the (R,S) stereoisomers were more potent than the (S,R) stereoisomers [98]. The more active asperphenamate derivate was N-benzoyl-O-(N′-(1-benzyloxycarbonyl-4-piperidiylcarbonyl)-D-phenylalanyl)-D-phenyl- alaninol (BBP). BBP was approx. 20-fold more active than asperphenamate against breast cancer (MCF-7, T47D and MDA-MB231), hepatic (BEL-4702), lung (A-549), and cervical (HeLa) cancer as well as leukemia (HL-60) cell lines, with IC50 values between 3.0 and 18.3 µM [99].
Figure 12.
Asperphenamate.
Cyclic di-peptides called diketopiperazines are in general known as cell cycle inhibitors of the G2/M phase [100]. Tryptophan/proline diketopiperazines covers the compound groups fumitremorgins, stephacidins, notoamides, tryprostatins, etc. This group of compounds is produced by a long range of Aspergilli [101,102,103,104,105,106,107,108,109,110]. The fumitremorgins are produced by A. fumigatus and A. fischeri [101,102,103,111]. Fumitremorgin C (Figure 13a) was found active against human leukemia P-388 with an ED50 value of 3.9 µg/mL and the analog 12,13-dihydroxyfumitremorgin C has antiproliferative effects on human leukemia U-937 and human prostate cancer PC-3, with IC50 values of 1.8 and 6.6 µM, respectively [111,112]. Furthermore fumitremorgin C was specifically and potently cytotoxic against multi-drug resistant breast- and colon cancer [113,114].
Figure 13.
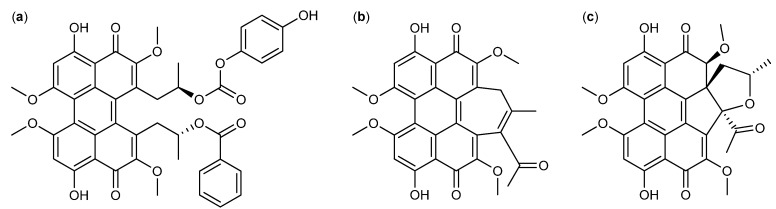
Tryptophan/proline diketopiperazines: (a) Fumitremorgin C, (b) Stephacidin A, (c) Notoamide A, (d) 18-Oxotryprostatin A and (e) Spirotryprostatin E.
Two other tryptophan/proline diketopiperazines are stephacidin A (Figure 13b) and B produced by A. ochraceus and A. westerdijkiae [106,107]. Stephacidin B is the dimeric form of stephacidin A and was approximate 10-fold more potent. Stephacidin B exhibited inhibitory effect on prostate, ovarian, colon, breast and lung cancer cell lines with IC50 values between 0.06 and 0.4 µM [106].
The notoamides (Figure 13c) produced by A. amoenus are close analogs to the stephacidins, but with a spiro ring structure [115]. Contrary to the stephacidins the notoamides only showed low to moderate inhibition of two leukemic cell lines with IC50 values 22–52 µg/mL [116].
Finally, the tryprostatins (Figure 13d) and spirotryprostatins (Figure 13e) isolated from A. fumigatus where found active against lung (NCI-H-522 and A-549), breast (MCF-7) and prostate (PC-3) cancer as well as leukemia (HL-60 and MOLT-4). The more potent analogs were Ds1-tryprostatin B with GI50 values of 15.8 µM (NCI-H-522), 15.9 µM (MCF-7), and 11.9 µM (PC-3) and 18-oxotryprostatin A with an IC50 value of 1.3 µM (A-549) [108,110]. Spirotryprostatin E was the more potent of the spirotryprostatins, with IC50 values of 3.1 µM (MOLT-4), 2.3 µM (HL-60) and 3.1 µM (A-549) [109].
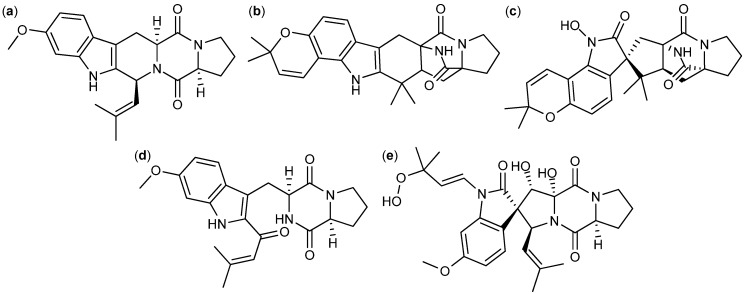
Other diketopiperazines originating from histidine are phenylahistin (Figure 14a) and aurantiamine (Figure 14b). Phenylahistin was first isolated from A. ustus in 1997 as a mixture of enantiomers [117]. (−)-Phenylahistin was more active than (+)-phenylahistin against dermal (A-431), lung (A-549), ovary (HeLa), leukemia (K-562 and P-388), breast (MCF-7), CNS (TE-671) and colon (WiDr) cancer cell lines with IC50 between 0.18 and 3.7 µM [118]. A synthetic analog plinabulin (NPI-2358) displays very potent activity against human prostate carcinoma cell line DU-145 and has now entered phase II clinical trials [119]. Recently, more than 60 synthetic analogs of plinabulin have been designed and synthesized. The more active analog with a benzoyl group coupled on the phenylalanine unit was 10-fold more active than plinabulin with an IC50 value of 1.4 nM [119]. The activity of phenylahistin was also compared to the diketopiperazine aurantiamine and other synthetic analogues [120], originally isolated by Larsen et al. [121].
Figure 14.
(a) Phenylahistin and (b) Aurantiamine.
Another group of diketopiperazines with anticancer activity contain a di-sulfide bridge in the diketopiperazine ring. One of them the antifungal, immunosuppressive and antimicrobial compound gliotoxin (Figure 15a) that was isolated from A. fumigatus, and D. cejpii [122,123,124]. Already in 1947 the anticancer activity of gliotoxin was suggested and in 2004 it was found that gliotoxin was a very potent inhibitor of six breast cancer cell lines with IC50 values between 38 and 985 nM [125,126]. In 2012, gliotoxin was found active against human leukemia U-937 and human prostate cancer PC-3 cell lines with IC50 values of 0.2 and 0.4 µM, respectively [112].
Figure 15.
Diketopiperazines with a di-sulfide bridge: (a) Gliotoxin, (b) Emestrin A and (c) Acetylapoaranotin.
Another diketopiperazine with a di-sulfide bridge is the antifungal [127] compound emestrin A (Figure 15b) that was isolated from Emericella striata, now called A. striatus [128]. Emestrin A inhibits human leukemia (HL-60) with an IC50 value of 83.5 nM [129]. Eight structural analogs of emestrin A were isolated from Cladorrhinum sp. and found to have strong antiproliferative effects on the human prostate DU-145 cancer cell line with an IC50 value of 9.3 nM for the more potent emestrin C. Furthermore, it was proven that the activity decreased when the macro cyclic ring was opened and the polysulfide bridge in the diketopiperazine was absent [130].
Recently, a new diketopiperazine with a di-sulfide bridge named acetylapoaranotin (Figure 15c) produced by a marine Aspergillus sp. was discovered and found active against colon (HCT-116), gastric (AGS), lung (A-549), and breast (MCF-7) cancer cell lines with IC50 values of 13.8, 12, 2 and 10 µM, respectively [131].
The peptide leucinostatin A (Figure 16) was first isolated in 1973 from Purpureocillium lilacinum (originally published as Penicillium lilacinum or Paecilomyces lilacinus) [132,133]. It was found active against a number of fungi and Gram-positive bacteria, as well as Ehrlich solid carcinoma of mice with an ED50 value of 1.6 mg/kg [132,134]. Furthermore leucinostatin A inhibited a long range of breast, melanoma, lung, ovary, colon and laryngeal cancers as well as eight leukemia cell lines with IC50 values between 4 nM and 12 µM [132,135,136]. Leucinostatin A inhibited growth of prostate cancer DU-145 cells in vitro and in vivo [137]. A natural analog leucinostatin A β di-O-glycoside displayed active against breast cancer BT-20 cell line, though not as potent as leucinostatin A [138].
Figure 16.
Leucinostatin A.
4. Terpenoid-Derived Anticancer Compounds
Terpenoids form a third large and structurally diverse biosynthetic family of natural products derived from C5 isoprene units. Until now active anticancer terpenoids mostly belong to the sesqui- or diterpenoids [15].
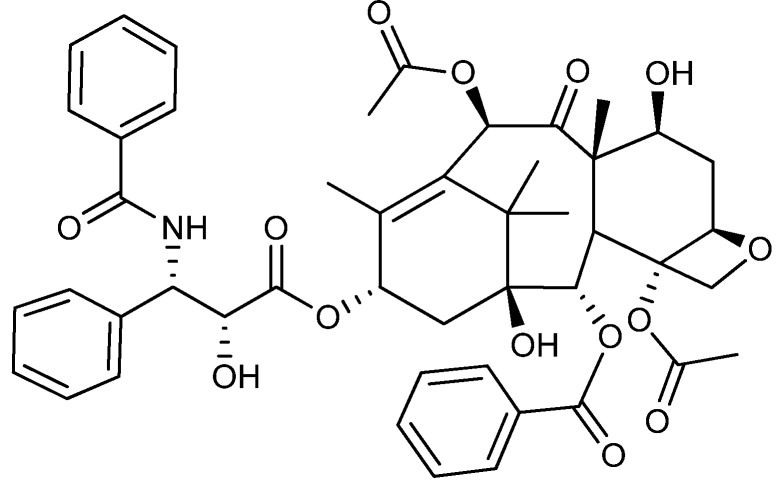
Taxol, also known as paclitaxel (Figure 17), is one of the best known anticancer drugs produced by fungi, though it originally was isolated from the bark of yew tree Taxus brevifolia [139]. Clinical development of taxol was delayed due to problems with production of large enough quantities of the compound. This problem was solved 20 years later when it was demonstrated that taxol was also produced by the fungus Taxomyces andreanea [140] and later including P. raistrickii [141].
Figure 17.
Taxol.
Taxol was approved as anticancer drug against a wide range of tumors in the 1990s and is the first billion dollar drug against cancer [142,143]. Taxol functions by inducing cell cycle arrest in G2/M phase as well as apoptosis through a unique mode-of-action by promotion and stabilization of tubulin polymerization [144]. Today, taxol is routinely used to treat ovarian, breast and lung tumors as well as Kaposi’s sarcoma [145]. Besides its anticancer activity taxol displays antifungal activity as well [146].
Many sesquiterpenes have been found active against cancer. Two of these are the drimane sesquiterpenes fudecadione A and B (Figure 18a) that were isolated in 2011 from Penicillium sp. BCC 17468. Fudecadione A was found active against human lung cancer NCI-H187, human breast cancer MCF-7, and human oral epidermoid carcinoma KB cell lines, with IC50 values of 24.9, 12.6 and 22.6 µM, respectively. Fudecadione B, on the other hand, was inactive against all three cancer call lines [147]. The activity of the two compounds suggests that the pharmacophore is located around the carbon at position 13, where fudecadione B is more branched [147].
Figure 18.
(a) Fudecadione A, (b) Terrecyclic acid A and (c) a novel unnamed chloro-trinoreremophilane sesquiterpene.
Another active sesquiterpene is the antifungal terrecyclic acid A (Figure 18b), which was isolated from A. terreus in 1986 [148]. Terrecyclic acid A exhibit cytotoxic activity against human lung cancer NCI-H460, human breast cancer MCF-7, and human CNS cancer SF-268 cell lines with IC50 values of 10.6, 24.1 and 14.7 µM, respectively, and against leukemia in mice P-388 with LD50 values of 63-125 mg/kg [148,149].
In 2013 a novel and as yet unnamed chlorotrinoreremophilane sesquiterpene (Figure 18c) was isolated from Penicillium sp. PR19N-1 [150]. This novel sesquiterpene exhibited cytotoxic activity against human leukemia HL-60 and lung cancer A-549 cell lines with IC50 values of 11.8 and 12.2 µM, respectively [150].
A large group of mycotoxins called the trichothecenes cover more than 150 analogs and are mainly produced by a number of Fusarium spp. [151]. All the trichothecenes contain a sesquiterpenoid ring structure with an epoxide. The epoxide is often responsible for the cytotoxic activity by binding to the 60S ribosomal subunit in eukaryote cells thereby inhibiting protein synthesis [152,153]. Many of the trichothecenes exhibit cytotoxic activity against both fungi and cancer cell lines [154,155,156]. One of the more potent is AETD (Figure 19a) that inhibits HL-60, U-937, HeLa, MCF-7 and Hep-G2 cell lines with IC50 values of 10, 22, 45, 53 and 170 nM [157]. Other bioactive groups are the roridins where a macrocyclic ring is connected to the sesquiterpenoid unit. One of the more active compounds of this group is 12′-hydroxyroridin E (Figure 19b), which inhibits leukemia L-1210 with an IC50 value of 0.2 µM [158]. Another trichothecene called anguidine even entered clinical trials against cancer, but did not progress beyond phase II due to a lack of therapeutic efficacy [152,159].
Figure 19.
Trichothecenes (a) AETD and (b) 12′-Hydroxyroridin E.
Wentilactone A and B (Figure 20a) were isolated from A. wentii in 1980 [160]. In 2012 it was reported that wentilactone B induced apoptosis and inhibited proliferation in human hepatoma cancer cell line SMMC-7721, with an IC50 value of 19 µM [161].
Figure 20.
(a) Wentilactone B and (b) Wortmannin.
The antifungal compound wortmannin (Figure 20b) is produced by T. wortmannii originally published as P. wortmannii [162] or a related species. Wortmannin inhibit the activity of leukemia HL-60 and K-562 cell lines with IC50 values of 30 and 25 nM, respectively [163,164], including the breast cancer MCF-7 cell line that was inhibited by 51.3% after 48 h with a concentration of 25 nM [165]. In human pancreatic cancer cells lines PK1 and PK8 wortmannin induces apoptosis both in vitro and in vivo [166,167]. Finally, wortmannin has been shown to inhibit proliferation in lung cancer cell lines KNS-62 and Colo-699 both in vitro and in vivo, with IC50 values between 100 and 200 nM [168].
The ophiobolins are sesterterpenes mainly isolated from the fungal genus Bipolaris [169,170], but are also found in Aspergillus [171], Sarocladium [172,173], and Drechslera [174]. Isolation of ophiobolins from Aspergillus spp. misidentified as A. ustus were later ascribed to the three different species: A. calidoustus, A. insuetus, and A. keveii from the Aspergillus section Usti [69]. Ophiobolin A (Figure 21) exhibit inhibitory activity against cancer cell lines includes lung cancer A-549, colon cancer HT-29, melanoma Mel-20, leukemia P-388, and ovarian cancer OVCAR-3 with IC50 values of 0.1, 0.1, 0.1, 0.06 and 0.3 µM, respectively [175,176]. In 2012 the novel ophiobolin O was discovered and it was found to inhibit breast cancer MCF-7 and leukemia P-388 cell lines with IC50 values of 17.9 and 4.7 µM, respectively [177,178]. Most recently in 2013 the novel 3-anhydro-6-hydroxyophiobolin A was isolated and found active against lung cancer (HepG2) and leukemia (K-562) cell lines with IC50 values of 6.5 and 4.1 µM, respectively [179]. Besides anticancer activity the ophiobolin family show antifungal activity against a wide range of fungi [180,181].
Figure 21.
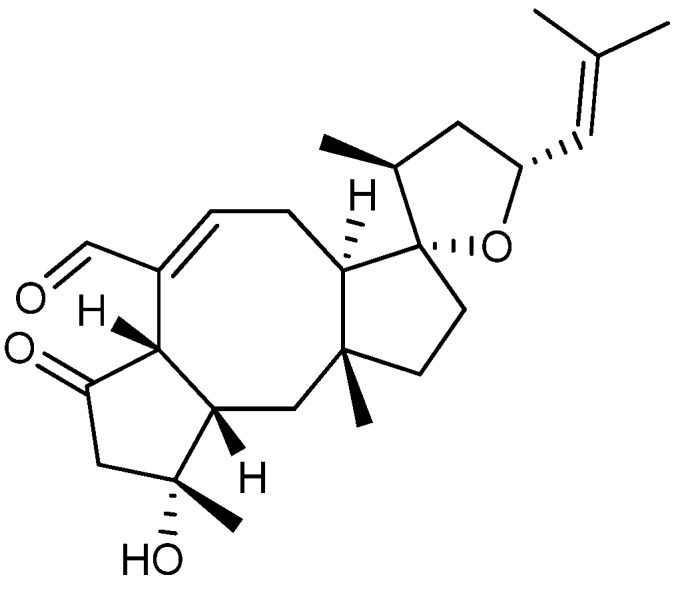
Ophiobolin A.
5. Anticancer Natural Products of Mixed or Unresolved Biosynthetic Origin
Filamentous fungi are capable of producing secondary metabolites of mixed biosynthetic origin. This includes both compounds such as meroterpenoids that comprise a huge class of compounds that integrate a polyketide part with a terpenoid part [182], in addition to the cytochalasins [183] and chaetoglobosins that are biosynthesized by incorporation of amino acids into a core polyketide part. The cytochalasins contains a phenylalanine coupled to the polyketide chain where the chaetoglobosins have an tryptophan moiety [183,184]. Both the cytochalasins and the chaetoglobosins exhibit antifungal activities against a broad range fungal species [56,185,186]. The cytochalasins are produced by many fungal genera including Aspergillus, Hypoxylon, Metarrhizium, Zygosporium, Hypocrella and Phoma [90,187,188,189,190]. Many of the cytochalasins have shown inhibitory activities towards lung cancer A-549. One of the more potent is cytochalasin E (Figure 22a) which inhibited human ovarian A-2780S, human colon HCT-116 and SW-620, and lung A-549 cancer as well as human leukemia P-388 with IC100 values of 0.02, 1.0, and 0.2 µg/ml and IC50 values of 0.006 and 0.09 µM, respectively [191,192].
Figure 22.
(a) Cytochalasin E and (b) Chaetoglobosin B.
The chaetoglobosins were originally isolated from Chaetomium globosum in 1973 [184]. Several chaetoglobosins have been isolated over the years from P. discolor and P. expansum among others [1] and many of them showed activity against cancer cell lines. Of the more potent ones was chaetoglobosin B (Figure 22b) that inhibited human breast BC cancer cell line with an IC50 value of 3.0 µM [193] and chaetoglobosin D that inhibited adenocarcinoma KKU-100 and KKU-OCA17 cancer cell lines with IC50 values of 3.4 and 12.2 µM, respectively [193]. Chaetoglobosin U showed activity as well against KB tumor cell line with an IC50 value of 16.0 µM [194], and chaetoglobosin X with activity against murine hepatic cancer H-22 cell line with an IC50 value of 7.5 µM [195].
Another group of compounds with mixed a biosynthetic pathway are the pseurotins (Figure 23) containing phenylalanine coupled to a polyketide and with a spiro ring structure. The pseurotins were first isolated from the bacteria Pseudeurotium ovalis and later from the fungus A. fumigatus [196,197]. In 2012 four pseurotin analogs were proven active against a human breast cancer cell line MCF-7 with the more active pseurotin D inhibiting MCF-7 with an IC50 value of 15.6 µM [198].
Figure 23.
Pseurotin A.
The γ-pyridone alkaloids, penicidone A–C (Figure 24a) were isolated from an endophytic Talaromyces sp. The biosynthesis of penicidones probably happens through the polyketide pathway similar to penisimplicissin (Figure 24b), but with a unique introduction of nitrogen [199]. The penicidones were showed to be active against human colon cancer SW1116, human AML (K-562), and human cervical cancer (KB and HeLa) cell lines with penicidone B being the more potent analog. Penicidone B inhibited the four cancer cell lines with IC50 values of 54.2 µM (SW1116), 21.1 µM (K-562), 29.6 µM (KB), and 35.2 µM (HeLa) [199]. Two other γ-pyridone alkaloids called penicinoline (Figure 24b) and methylpenicinoline were isolated from an endophytic Penicillium sp. and Auxarthron reticulatum in 2010 and 2011, respectively [200,201]. Penicinoline and methylpenicinoline were shown to inhibit the lung cancer HepG2 cell line with IC50 values of 13.2 and 11.3 µM, respectively [202].
Figure 24.
(a) Penicidone B and (b) Penicinoline.
Communesins are fungal metabolites produced in P. marinum, P. expansum, P. buchwaldii and P. rivulorum [96,203,204,205]. The group is of highly mixed biosynthetic origin with structures combining a nitrogen-containing part, a polyketide chain and isoprene. Communesin B (Figure 25) displays the highest activity of them all against leukemia P-338, U-937, THP-1, NAMALWA, MOLT-3 and SUP-B15 cell lines with ED50 values of 0.5, 10.4, 11.4, 9.9, 8.1 and 7.2 µg/mL, respectively [206,207].
Figure 25.
Communesin B.
The antibiotic compound fumagillin (Figure 26a) has been one of the more potent and interesting compounds associated with anticancer activity produced by fungi. Fumagillin, classified as a meroterpenoid, was first isolated from A. fumigatus in 1951 [208] and further produced by P. scabrosum [209]. The antitumor activity of fumagillin is caused by its potent angiogenesis-inhibiting effect. Unfortunately, fumagillin had some unpleasant side effects and consequentially investigations into structural analogs have been conducted [210]. The synthetic fumagillin analog TNP-470 (Figure 26b) contains an amine and chlorine in the side chain, which led to a highly increased angiogenesis—inhibiting effect. Furthermore, TNP-470 is less toxic to normal cells compared to fumagillin [210,211]. In vitro and in vivo tests showed that TNP-470 inhibited tumor growth in prostate (PC-3) and breast cancer (MDA-MB-231). TNP-470 entered preclinical trails in 1992 and phase I and II in 2000 but did not progress into phase III, due to a very short systemic half-live and observed neurotoxicity in patients [212,213,214]. Recently, in 2013, a novel natural fumagillin analog namely ligerin (Figure 26c) was isolated from a marine-derived Penicillium sp. [215]. Ligerin inhibited lung cancer POS1 cell line with an IC50 value of 117 nM [215].
Figure 26.
(a) Fumagillin, (b) Synthetic analog TNP-470 and (c) Ligerin.
A strain so far identified as Talaromyces purpurogenus (earlier P. purpurogenum) is known to produce another group of meroterpenoids namely purpurogemutantin (Figure 27a), purpurogemutantidin (Figure 27b), and the known antifungal macrophorin A (Figure 27c) [216,217]. All three compounds inhibit human leukemia K-562 and HL-60, human cervical cancer HeLa, human gastric adenocarcinoma BGC-823 and human breast cancer MCF-7 cell lines. Purpurogemutantidin (Figure 27a) was the more potent, with IC50 values of 0.9, 2.4, 16.6, 31.0, and 26.3 µM, respectively. The only exception is macrophorin A that displayed higher activity towards the HL-60 cell line with an IC50 value of 0.9 µM [216].
Figure 27.
(a) Purpurogemutantin, (b) Purpurogemutantidin and (c) Macrophorin A.
Botryodiplodin (PSX-1), a small antifungal [218] compound of unresolved biosynthetic origin (Figure 28), was isolated from T. stipitatus (originally named P. stipitatum) [219] and exhibits activity against Ehrlich ascites carcinoma, leukemia L-5178, Sarcoma 37, and cervical cancer Hela cell lines with a median effective dose (ED50) values of approximately 1 µg/mL [219,220].
Figure 28.
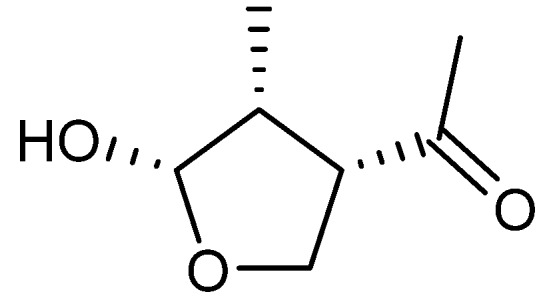
Botryodiplodin (PSX-1).
The atpenins A4, A5 and B (Figure 29) of unresolved biosynthetic pathway were first isolated from P. atramentosum in 1988 and shown to possess antifungal activities [221]. Twenty years later the first three atpenins were isolated again from the same fungi along with two new analogs, NBRI23477 A and B [222]. The activities of the five atpenins were examined individually against human prostate cancer cell line DU-145 and in a stromal co-cultured assay. Growth of the DU-145 cells was inhibited more effectively in the co-cultured setup, with atpenin A5 being the more potent analog with an IC50 value of 0.02 µg/mL [222]. All the reviewed compounds are summarized in Table 1 with their individual organisms of origin, biosynthetic pathways, target cancer cells, IC50 (if available), and whether the compounds exhibit antifungal activity too.
Figure 29.
Atpenin A5.
Table 1.
List of fungal anticancer natural products primarily produced by Aspergillus, Penicillium and Talaromyces summarizing producing organisms, biosynthetic origin, target cancer cells, IC50 (if available), and if the compounds exhibit antifungal activity.
| Species (original published) | Compound | Type | Target cell | IC50 | Anti-fungal (+/−) |
|---|---|---|---|---|---|
|
T. pinophilus [1] (P. pinophilum [48]) |
3-O-methylfunicone [50,51,52,53] | Polyketide | HeLa | - | + [48,49] |
| MCF-7 | - | ||||
| A-375P | - | ||||
| A-375M | - | ||||
| A. nidulans [46] | Asperlin [47] | Polyketide | HeLa | - | − |
|
A. flavus [95] Penicillium spp. [96] |
Asperphenamate | NPR | - | - | − |
|
A. flavus [95] Penicillium spp. [96] |
BBP [99] | NPR | MCF-7 | 3.0 µM | |
| T47D | 4.7 µM | ||||
| MDA-MB231 | 5.2 µM | ||||
| BEL-4702 | 12.7 µM | ||||
| A-549 | 15.1 µM | ||||
| HeLa | 17.0 µM | ||||
| HL-60 | 18.3 µM | ||||
| Aspergillus sp. [131] | Acetylapoaranotin [131] | NPR | HCT-116 | 13.8 µM | − |
| AGS | 12 µM | ||||
| A-549 | 2 µM | ||||
| MCF-7 | 10 µM | ||||
| P. atramentosum [222] | Atpenin A5 [222] | Unresolved biosynthetic origin | DU-145 (co-culture) | 0.02 µg/mL | + [221] |
|
A. puniceus [69] A. turkensis [69] A. pseudoustus [69] A. ustus [70] |
Austocystin D [71,72] | Polyketide | SR | 16 nM (GI50) | − |
| U-87 | 4946 nM (GI50) | ||||
| MCF-7 | <1 nM (GI50) | ||||
| MDA-MB-231 | 549 nM (GI50) | ||||
| PC-3 | 3 nM (GI50) | ||||
| SW-620 | 27 nM (GI50) | ||||
| HCT-15 | 42 nM (GI50) | ||||
| MX-2 | 3358 nM (GI50) | ||||
|
P. brevicompactum [20] P. paneum [223] T. stipitatus [1] (P. stipitatum [220]) |
Botryodiplodin [219,220] | Unresolved biosynthetic origin | EAC | 0.6 µg/mL (ED50) | + [218] |
| L-5178 | 0.8 µg/mL (ED50) | ||||
| Sarcoma 37 | 1.5 µg/mL (ED50) | ||||
| HeLa | 2.0 µg/mL (ED50) | ||||
| P. brefeldianum [39] | Brefeldin A [42,43,44,45] | Polyketide | HL-60 | 35.7 nM | + [39,40] |
| KB | 32 nM | ||||
| HeLa | 6.4 nM | ||||
| MCF-7 | 7.1 nM | ||||
| SPC-A-1 | 3.6 nM | ||||
| BC-1 | 40 nM | ||||
| NCI-H187 | 110 nM | ||||
| Cladosporium cladosporioides [82] | Calphostin C [82,83] | Polyketide | HeLa S3 | 0.2–8.5 µM | − |
| MCF-7 | 0.2–2.7 µM | ||||
| ALL | - | ||||
|
Chaetomium globosum [184] P. discolor [1] P. expansum [1] |
Chaetoglobosin B [193] | Mixed biosynthetic origin | BC | 3.0 µM | + [56,186] |
|
Chaetomium globosum [184] P. discolor [1] P. expansum [1] |
Chaetoglobosin D [193] | Mixed biosynthetic origin | KKU-100 | 3.4 µM | |
| KKU-OCA17 | 12.2 µM | ||||
|
Chaetomium globosum [184] P. discolor [1] P. expansum [1] |
Chaetoglobosin U [194] | Mixed biosynthetic origin | KB | 16.0 µM | |
|
Chaetomium globosum [184] P. discolor [1] P. expansum [1] |
Chaetoglobosin X [195] | Mixed biosynthetic origin | H-22 | 7.5 µM | |
|
Chaetomium globosum [57,58,59,60,61,63,64] T. pinophilus [1] |
11-epichaetomugilin I [62] | Polyketide | P-388 | 0.7 pM | + [56] |
| HL-60 | 1.0 pM | ||||
| L-1210 | 1.6 pM | ||||
| KB | 1.2 pM | ||||
|
P. chrysogenum [74] P. rubens [73] (P. terrestre [76]) |
Chloctanspirone A [76] | Polyketide | HL-60 | 9.2 µM | − |
| A-549 | 39.7 µM | ||||
|
P. buchwaldii [96] P. marinum [1] P. expansum [204] P. rivulorum [205] |
Communesin B [206,207] | Mixed biosynthetic origin | P-388 | 0.5 µg/mL (ED50) | − |
| U-937 | 10.4 µg/mL (ED50) | ||||
| THP-1 | 11.4 µg/mL (ED50) | ||||
| NAMALWA | 9.9 µg/mL (ED50) | ||||
| MOLT-3 | 8.1 µg/mL (ED50) | ||||
| SUP-B15 | 7.2 µg/mL (ED50) | ||||
|
Mariannaea elegans (Spicaria elegans [192]) A. clavatus [190] A. rhizopodus [90] Hypoxylon terricola [187] |
Cytochalasin E [191,192] | Mixed biosynthetic origin | A-2780 | 0.02 µg/mL (IC100) | + [185] |
| HCT-116 | 1.0 µg/mL (IC100) | ||||
| SW-620 | 0.2 µg/mL (IC100) | ||||
| A-549 | 0.006 µM | ||||
| P-388 | 0.09 µM | ||||
|
A. striatus (Emericella striata [128]) Cladorrhinum sp. |
Emestrin A [129] | NPR | HL-60 | 83.5 nM | + [127] |
|
A. striatus (Emericella striata [128]) Cladorrhinum sp. |
Emestrin C [130] | NPR | DU-145 | 9.3 nM | |
| Penicillium sp. BCC 17468 [147] | Fudecadione A [147] | Terpene | MCF-7 | 12.6 µg/mL | − |
| KB | 22.3 µg/mL | ||||
| NCI-H187 | 24.9 µg/mL | ||||
|
A. fumigatus [208] P. scabrosum [209] |
Fumagillin [210] | Mixed biosynthetic origin | Angiogenesis inhibitor | - | − |
| TNP-470 [210,211,212,213,-214] | Synthetic | PC-3 | - | ||
| MDA-MB-231 | - | ||||
| Penicillium sp. [215] | Linerin [215] | Mixed biosynthetic origin | POS1 | 117 nM | |
|
A. fumigatus [101,102] A. fischeri [103] |
Fumitremorgin C [111,113,114] | NPR | P-388 | 3.9 µg/mL (ED50) | + [224] |
|
A. fumigatus [101,102] A. fischeri [103] |
12,13-dehydroxyfumitremorgin C [112] | NPR | U-937 | 1.8 µM | |
| PC-3 | 6.6 µM | ||||
|
A. fumigatus [122] D. cejpii [123] |
Gliotoxin [112,125,126] | NPR | MCF-7 | 985 nM | + [124] |
| T47D | 365 nM | ||||
| BT-474 | 102 nM | ||||
| ZR75-1 | 158 nM | ||||
| MDA MB231 | 38 nM | ||||
| MDA MB435 | 87 nM | ||||
| U-937 | 0.2 µM | ||||
| PC-3 | 0.4 µM | ||||
| P. griseofulvum [225] | Griseofulvin [2,3,4,226,227] | Polyketide | HeLa | 20 µM | + [226,228] |
| SCC-114 | 35 µM | ||||
| GF-15 [5] | Synthetic | SCC-114 | 0.9 µM | ||
| Hypocrella bambusae(Shiraia bambusicola [84,85]) | Hypocrellin D [85] | Polyketide | Bel-7721 | 1.8 µg/mL | + [229] |
| A-549 | 8.8 µg/mL | ||||
| Anip-973 | 38.4 µg/mL | ||||
| Synthetic analog [86] | Synthetic | MCF-7 (PDT) | - | ||
|
Purpureocillium lilacinum [133] (P. lilacinum [132]) |
Leucinostatin A [132,135,136,137,138] | NPR | Ehrlich | 1.6 mg/kg (LD50) | + [132,134] |
| L-1210 | 410.3 nM (IC100) | ||||
| BT-20 | 2.3 nM | ||||
| MCF-7 | 4 nM | ||||
| G-361 | 7 nM | ||||
| HT-144 | 6 nM | ||||
| A-549 | 16 nM | ||||
| A-427 | 59 nM | ||||
| SK-MES-1 | 12 µM | ||||
| Caov-3 | 17 nM | ||||
| Caov-4 | 53 nM | ||||
| SKOV-3 | 1,236 nM | ||||
| HT-29 | 119 nM | ||||
| LoVo | 114 nM | ||||
| HEp-2 | 40 nM | ||||
| HL-60 | 12 nM | ||||
| HSB-2 | 5 nM | ||||
| K-562 | 6 nM | ||||
| KG-1 | 46 nM | ||||
| RPMI-1788 | 158 nM | ||||
| SKW-6.4 | 6 nM | ||||
| U-937 | 319 nM | ||||
| Raji | 11 nM | ||||
|
A. parasiticus [65,66] A. nidulans [67,68] |
Norsolorinic acid [67,68] | Polyketide | T-24 | 10.5 µM | − |
| MCF-7 | 12.7 µM | ||||
| A. amoenus [115] | Notoamides [116,230] | NPR | HeLa/L-1210 | 22–52 µg/mL | − |
|
Bipolaris sp. [169,170] A. calidoustus [69] A. insuetus [69] A. keveii [69] (A. ustus [171]) Sarocladium oryzae [173] (Cephalosporium caerulens [172] (D. maydis [174] D. sorghicola [174] |
Ophiobolin A [175,176] | Terpene | A-549 | 0.1 µM | + [180,181] |
| HT-29 | 0.1 µM | ||||
| Mel-20 | 0.1 µM | ||||
| P-388 | 0.06 µM | ||||
| OVCAR-3 | 0.3 µM | ||||
| Bipolaris oryzae [179] | 3-anhydro-6-hydroxyophiobolin A [179] | Terpene | HepG2 | 6.5 µM | |
| K-562 | 4.1 µM | ||||
|
Aspergillus section Usti [69] (A. ustus [177]) |
Ophiobolin O [177,178] | Terpene | MFC-7 | 17.9 µM | |
| P-388 | 4.7 µM | ||||
| Talaromyces sp. (Penicillium sp.) [199] | Penicidone B [199] | Mixed biosynthetic origin | SW1116 | 54.2 µM | − |
| K-562 | 21.1 µM | ||||
| KB | 29.6 µM | ||||
| HeLa | 35.2 µM | ||||
|
Penicillium sp. [200] Auxarthron reticulatum [201] |
Penicinoline [202] | Mixed biosynthetic origin | HepG2 | 13.2 µM | − |
|
Penicilliumsp. [200] Auxarthron reticulatum [201] |
Methyl-penicinoline [202] | Mixed biosynthetic origin | HepG2 | 11.3 µM | |
|
T. pinophilus [1] (P. simplicissimum [54]) |
Penisimplicissin [55] | Polyketide | HL-60 | −6.7 (log10 GI50) | − |
| CCRF-CEM | −5.8 (log10 GI50) | ||||
| Phaeosphaeria sp. [87] | Phaeosphaerin B [87] | Polyketide | PC-3 | 2.4 µM | − |
| DU-145 | 9.5 µM | ||||
| LNCaP | 2.7 µM | ||||
| A. ustus [117] | Phenylahistin [118] | NPR | A-431 | 0.22 µM | − |
| A-549 | 0.30 µM | ||||
| HeLa | 0.20 µM | ||||
| K-562 | 0.19 µM | ||||
| P-388 | 0.33 µM | ||||
| MCF-7 | 3.7 µM | ||||
| TE-671 | 0.18 µM | ||||
| A. taichungensis [79] | Prenylterphenyllin A [79] | Polyketide | A-549 | 8.3 µM | − |
| HL-60 | 1.5 µM | ||||
| A. candidus [77,78] | 4”-deoxyisoterprenin [78] | Polyketide | KB3-1 | 6.2 µM | |
| A. taichungensis [79] | Prenylcandidusin B [79] | Polyketide | P-388 | 1.6 µM | |
|
Pseudeurotium ovalis [196] A. fumigatus [197] |
Pseurotin D [198] | Mixed biosynthetic origin | MFC-7 | 15.6 µM | − |
|
T. purpurogenus mutant BD-1-6 [1] (P. purpurogenum [216]) |
Purpurogemutantidin [216] | Mixed biosynthetic origin | K-562 | 0.9 µM | + [217] |
| HeLa | 16.6 µM | ||||
| BGC-823 | 31.0 µM | ||||
| MCF-7 | 26.3 µM | ||||
|
T. purpurogenus mutant BD-1-6 [1] (P. purpurogenum [216]) |
Macrophorin A [216] | Mixed biosynthetic origin | HL-60 | 0.9 µM | |
| A. parasiticus [231] | Sequoiamonascin A [231] | Polyketide | MCF-7 | 1% | − |
| NCI-H460 | 1% | ||||
| SF-268 | 2% | ||||
| Percent cell growth compared to untreated cells at 10 µM | |||||
|
P. solitum [20,25] (P. brevicompactum [18]) P. hirsutum [20] (P. citrinum [19]) |
Compactin [28] | Polyketide | AML | 2.6 µM (IC100) | + [18] |
|
A. terreus [26] Monascus sp. [27] |
Lovastatin [29,30,31,32] | Polyketide | OVHS-1 | 39 µM | + [22,23,24] |
| Calu-1 | 3 µM | ||||
| H-460 | 3 µM | ||||
| A-549 | 10 µM | ||||
| H-441 | 30 µM | ||||
| Ovca-432 | 2 µM | ||||
| A-2780 | 3 µM | ||||
| Hey | 3 µM | ||||
| Ovca-429 | 5 µM | ||||
| HOC-7 | 10 µM | ||||
| DOV-13 | 11 µM | ||||
| Skov-3 | 21 µM | ||||
| A-2780-ADR | 21 µM | ||||
| A-2780-CIS | 22 µM | ||||
| MCF-7 | 1.7 µM | ||||
| HepG2 | 2.7 µM | ||||
| HeLa | 1.5 µM | ||||
| AML | - | ||||
| Simvastatin [1,20,29,33,34] | Synthetic | DLRP | 0.9 µM | + [23,24] | |
| H-1299 | 1.3 µM | ||||
| HT-144 | 1.0 µM | ||||
| MI-14 | 0.8 µM | ||||
| SK-MEL-28 | 0.8 µM | ||||
| BT-474A | 4.2 µM | ||||
| SKBR-3 | 2.2 µM | ||||
| MDA-MB-453 | 5.4 µM | ||||
| BT-20 | 1.7 µM | ||||
| AML | - | ||||
|
A. ochraceus [106] A. westerdijkiae [107] |
Stephacidin B [106] | NPR | PC-3 | 0.4 µM | − |
| LNCaP | 0.06 µM | ||||
| A-2780 | 0.3 µM | ||||
| A-2780/DDP | 0.4 µM | ||||
| A-2780/Tax | 0.3 µM | ||||
| HCT-116 | 0.5 µM | ||||
| HCT-116/mdr+ | 0.5 µM | ||||
| HCT-116/topo | 0.4 µM | ||||
| MCF-7 | 0.3 µM | ||||
| SKBR-3 | 0.3 µM | ||||
| LX-1 | 0.4 µM | ||||
|
Taxomyces andreanea [140] P. raistrickii [141] |
Taxol [139,142,143,144,145] | Terpene | Clinical use: Ovarian tumors Breast tumors Lung tumors Kaposi’s sarcoma |
- | + [146] |
| A. terreus [148] | Terrecyclic acid A [148,149,232] | Terpene | NCI-H460 | 10.6 µM | + [148] |
| MCF-7 | 24.1 µM | ||||
| SF-268 | 14.7 µM | ||||
| P-388 | 63-125 mg/kg (LD50) | ||||
| A. terreus [37] | Terrein [38] | Polyketide | MCF-7 | 1.1 nM | + [36] |
| PANC-1 | 9.8 µM | ||||
| HepG2 | 66.8 µM | ||||
|
Fusarium spp. [151] Isaria japonica [157] |
4-Acetyl-12,13-epoxy-9-trichothecene- 3,15-diol (AETD) [157] | Terpene | HL-60 | 10 nM | + [154] |
| U-937 | 22 nM | ||||
| HeLa | 45 nM | ||||
| MCF-7 | 53 nM | ||||
| Hep-G2 | 170 nM | ||||
|
Fusarium spp. [151] Myrothecium roridum [158] |
12’hydroxyroridin E [158] | Terpene | L-1210 | 0.2 µM | |
| BC-1 | 0.9 µM | ||||
| NCI-H187 | 1.5 µM | ||||
| P. citrinum [80] | Tricitrinol B [80] | Polyketide | HL-60 | 3.2 µM | − |
| HCT-116 | 4.8 µM | ||||
| KB | 3.9 µM | ||||
| A. fumigatus [104,105,108,109] | Ds2-tryprostatin B [108] | NPR | NCI-H-522 | 15.8 µM (GI50) | − |
| MCF-7 | 15.9 µM (GI50) | ||||
| PC-3 | 11.9 µM (GI50) | ||||
| A. fumigatus [104,105,108,109] | 18-oxotryprostatin A [110] | NPR | A-549 | 1.3 µM | |
| A. fumigatus [104,105,108,109] | Spirotryprostatin E [109] | NPR | MOLT-4 | 3.1 µM | |
| HL-60 | 2.3 µM | ||||
| A-549 | 3.1 µM | ||||
| Penicillium sp. [150] | Unnamed chloro-trinoreremophilane sesquiterpene [150] | Terpene | HL-60 | 11.8 µM | − |
| A-549 | 12.2 µM | ||||
| A. wentii [160] | Wentilactones [161] | Terpene | SMMC-7721 | 19 µM | + [233] |
|
T. wortmannii [1] (P. wortmannii [162]) Or similar species. |
Wortmannin [163,164,165,166,167,168] | Terpene | HL-60 | 30 nM | + [162] |
| K-562 | 25 nM | ||||
| KNS-62 | 100–200 nM | ||||
| Colo-699 | 100–200 nM | ||||
|
P. chrysogenum [1] D. cejpii [90] (D. albus [90]) |
Xanthocillin X [89,91,92] | NPR | Ehrlich ascites carcinoma-mouse strain | 40 mg/kg (LD50) | + [89] |
| K-562 | 0.4 µg/mL | ||||
| HeLa | 1.2 µg/mL | ||||
| MCF-7 | 12 µg/mL | ||||
| HepG2 | 7 µg/mL | ||||
| NCI-H460 | 10 µg/mL | ||||
| Du-145 | 8 µg/mL | ||||
| MDA-MB-231 | 8 µg/mL | ||||
| Aspergillus sp. [93] | BU-4704 [93] | NPR | HCT-116 | 0.6 µg/mL | |
| B16-F10 | 4.3 µg/mL | ||||
| P. chrysogenum [1] | Xanthocillin X di-methoxy [94] | NPR | HepG2 | 0.2 µg/mL | |
| MCF-7 | 0.4 µg/mL | ||||
| KB | 0.4 µg/mL |
6. Conclusions
In this article we have reviewed 50 compounds or compound families with anticancer and often also antifungal activities, primarily produced by Aspergillus, Penicillium and Talaromyces. Mycologists predict that less than 10% of all fungal species have been isolated so far, indicating a huge potential for further discovery of novel bioactive chemical scaffolds, if these fungi can be cultured in the laboratory. New strategies such as the application of epigenetic modifiers may help to uncover the full biosynthetic potential of fungi and other microorganisms. Development and improvement of screening methods and assays will further assist revealing new bioactivities of already known compounds. Additionally, ongoing progress in fast dereplication, including improvement of the performance of mass spectrometers and high resolution of UHPLC chromatograms, will ensure that database searches will lead to fewer possible candidates thereby advancing the drug discovery process. Altogether the future seems promising for discovery of many more bioactive small molecules to be used either as scaffolds for: (i) diversity oriented synthesis, or (ii) as a starting point for cloning and engineering of whole biosynthetic gene clusters towards novel engineered bioactive natural products.
Acknowledgments
We thank the Danish Cancer Society (grant number R-20-A1157-10-52) for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Frisvad J.C., Smedsgaard J., Larsen T.O., Samson R.A. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud. Mycol. 2004;49:201–241. [Google Scholar]
- 2.Panda D., Rathinasamy K., Santra M.K., Wilson L. Kinetic suppression of microtubule dynamic instability by griseofulvin: implications for its possible use in the treatment of cancer. Proc. Natl. Acad. Sci. USA. 2005;102:9878–9883. doi: 10.1073/pnas.0501821102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rebacz B., Larsen T.O., Clausen M.H., Rønnest M.H., Löffler H., Ho A.D., Krämer A. Identification of griseofulvin as an inhibitor of centrosomal clustering in a phenotype-based screen. Cancer Res. 2007;67:6342–6350. doi: 10.1158/0008-5472.CAN-07-0663. [DOI] [PubMed] [Google Scholar]
- 4.Ho Y.-S., Duh J.-S., Jeng J.-H., Wang Y.-J., Liang Y.-C., Lin C.-H., Tseng C.-J., Yu C.-F., Chen R.-J., Lin J.-K. Griseofulvin potentiates antitumorigenesis effects of nocodazole through induction of apoptosis and G2/M cell cycle arrest in human colorectal cancer cells. Int. J. Cancer. 2001;91:393–401. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1070>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Rønnest M.H., Rebacz B., Markworth L., Terp A.H., Larsen T.O., Krämer A., Clausen M.H. Synthesis and structure-activity relationship of griseofulvin analogues as inhibitors of centrosomal clustering in cancer cells. J. Med. Chem. 2009;52:3342–3347. doi: 10.1021/jm801517j. [DOI] [PubMed] [Google Scholar]
- 6.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawksworth D.L. Global species numbers of fungi: are tropical studies and molecular approaches contributing to a more robust estimate? Biodivers. Conserv. 2012;21:2425–2433. doi: 10.1007/s10531-012-0335-x. [DOI] [Google Scholar]
- 8.Klejnstrup M.L., Frandsen R.J.N., Holm D.K., Nielsen M.T., Mortensen U.H., Larsen T.O., Nielsen J.B. Genetics of polyketide metabolism in Aspergillus nidulans. Metabolites. 2012;2:100–133. doi: 10.3390/metabo2010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams R.B., Henrikson J.C., Hoover A.R., Lee A.E., Cichewicz R.H. Epigenetic remodeling of the fungal secondary metabolome. Org. Biomol. Chem. 2008;6:1895–1897. doi: 10.1039/b804701d. [DOI] [PubMed] [Google Scholar]
- 10.Henrikson J.C., Hoover A.R., Joyner P.M., Cichewicz R.H. A chemical epigenetics approach for engineering the in situ biosynthesis of a cryptic natural product from Aspergillus niger. Org. Biomol. Chem. 2009;7:435–438. doi: 10.1039/b819208a. [DOI] [PubMed] [Google Scholar]
- 11.Laatsch H. AntiBase 2012. [(accessed on 5 September 2013)]. Available online: http://eu.wiley.com/WileyCDA/WileyTitle/productCd-3527334068.html/
- 12.Nielsen K.F., Månsson M., Rank C., Frisvad J.C., Larsen T.O. Dereplication of microbial natural products by LC-DAD-TOFMS. J. Nat. Prod. 2011;74:2338–2348. doi: 10.1021/np200254t. [DOI] [PubMed] [Google Scholar]
- 13.Hansen M.E., Smedsgaard J., Larsen T.O. X-hitting: an algorithm for novelty detection and dereplication by UV spectra of complex mixtures of natural products. Anal. Chem. 2005;77:6805–6817. doi: 10.1021/ac040191e. [DOI] [PubMed] [Google Scholar]
- 14.Larsen T.O., Petersen B.O., Duus J.Ø., Sørensen D., Frisvad J.C., Hansen M.E. Discovery of new natural products by application of X-hitting, a novel algorithm for automated comparison of full UV spectra, combined with structural determination by NMR spectroscopy. J. Nat. Prod. 2005;68:871–874. doi: 10.1021/np040248s. [DOI] [PubMed] [Google Scholar]
- 15.Wang L.-W., Zhang Y.-L., Lin F.-C., Hu Y.-Z., Zhang C.-L. Natural products with antitumor activity from endophytic fungi. Mini-Rev. Med. Chem. 2011;11:1056–1074. doi: 10.2174/138955711797247716. [DOI] [PubMed] [Google Scholar]
- 16.Kharwar R.N., Mishra A., Gond S.K., Stierle A., Stierle D. Anticancer compounds derived from fungal endophytes: Their importance and future challenges. Nat. Prod. Rep. 2011;28:1208–1228. doi: 10.1039/c1np00008j. [DOI] [PubMed] [Google Scholar]
- 17.Hawksworth D.L., Crous P.W., Redhead S.A., Reynolds D.R., Samson R.A., Seifert K.A., Taylor J.W., Wingfield M.J., Abaci O., Aime C., et al. The Amsterdam declaration on fungal nomenclature. IMA Fungus. 2011;2:105–112. doi: 10.5598/imafungus.2011.02.01.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown A.G., Smale T.C., King T.J., Hasenkamp R., Thompson R.H. Crystal and molecular structure of compactin, a new antifungal metabolite from Penicillium brevicompactum. J. Chem. Soc. Perk. T. 1. 1976;1976:1165–1173. [PubMed] [Google Scholar]
- 19.Endo A., Kuroda M., Tsujita Y. ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinum. J. Antibiot. 1976;29:1346–1348. doi: 10.7164/antibiotics.29.1346. [DOI] [PubMed] [Google Scholar]
- 20.Frisvad J.C., Filtenborg O. Terverticillate penicillia: Chemotaxonomy and mycotoxin production. Mycologia. 1989;81:837–861. doi: 10.2307/3760103. [DOI] [Google Scholar]
- 21.Wong W.-L., Dimitroulakos J., Minden M., Penn L. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16:508–519. doi: 10.1038/sj.leu.2402476. [DOI] [PubMed] [Google Scholar]
- 22.Qiao J., Kontoyiannis D.P., Wan Z., Li R., Liu W. Antifungal activity of statins against Aspergillus species. Med. Mycol. 2007;45:589–593. doi: 10.1080/13693780701397673. [DOI] [PubMed] [Google Scholar]
- 23.Chamilos G., Lewis R.E., Dimitrios P., Kontoyiannis D.P. Lovastatin has significant activity against zygomycetes and interacts synergistically with voriconazole. Antimicrob. Agents Chemother. 2006;50:96–103. doi: 10.1128/AAC.50.1.96-103.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macreadie I.G., Johnson G., Schlosser T., Macreadie P.I. Growth inhibition of Candida species and Aspergillus fumigatus by statins. FEMS Microbiol. Lett. 2006;262:9–13. doi: 10.1111/j.1574-6968.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 25.Larsen T.O., Lange L., Schnorr K., Stender S., Frisvad J.C. Solistatinol, a novel phenolic compactin analogue from Penicillium solitum. Tetrahedron Lett. 2007;48:1261–1264. doi: 10.1016/j.tetlet.2006.12.038. [DOI] [Google Scholar]
- 26.Alberts A.W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., Rothrock J., Lopez M., Joshua H., Harris E., et al. Mevinolin: A highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc. Natl. Acad. Sci. USA. 1980;77:3957–61. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Endo A., Monacolin K. A new hypocholesterolemic agent produced by a Monascus speicies. J. Antibiot. 1979;32:852–854. doi: 10.7164/antibiotics.32.852. [DOI] [PubMed] [Google Scholar]
- 28.Sharma S., Hulcher F., Dodge W. Effects of cholesterol synthesis inhibitor on the proliferation of avian granulocytic and monocytic progenitor cells and primary acute monocytic leukemia cells. J. Nutr. 1984;2:65–69. [Google Scholar]
- 29.Newman A., Clutterbuck R.D., Powles R.L., Catovsky D., Millar J.L. A comparison of the effect of the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors simvastatin, lovastatin and pravastatin on leukaemic and normal bone marrow progenitors. Leukemia Lymphoma. 1997;24:533–537. doi: 10.3109/10428199709055590. [DOI] [PubMed] [Google Scholar]
- 30.Maksimova E., Yie T.-A., Rom W.N. In vitro mechanisms of lovastatin on lung cancer cell lines as a potential chemopreventive agent. Lung. 2008;186:45–54. doi: 10.1007/s00408-007-9053-7. [DOI] [PubMed] [Google Scholar]
- 31.Martirosyan A., Clendening J.W., Goard C.A., Penn L.Z. Lovastatin induces apoptosis of ovarian cancer cells and synergizes with doxorubicin: potential therapeutic relevance. BMC Cancer. 2010;10:103. doi: 10.1186/1471-2407-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahmoud A.M., Al-Abd A.M., Lightfoot D.A., El-Shemy H.A. Anti-cancer characteristics of mevinolin against three different solid tumor cell lines was not solely p53-dependent. J. Enzym. Inhib. Med. Ch. 2012;27:673–679. doi: 10.3109/14756366.2011.607446. [DOI] [PubMed] [Google Scholar]
- 33.Relja B., Meder F., Wilhelm K., Henrich D., Marzi I., Lehnert M. Simvastatin inhibits cell growth and induces apoptosis and G0/G1 cell cycle arrest in hepatic cancer cells. Int. J. Mol. Med. 2010;26:735–741. doi: 10.3892/ijmm_00000520. [DOI] [PubMed] [Google Scholar]
- 34.Glynn S.A., O’Sullivan D., Eustace A.J., Clynes M., O’Donovan N. The 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, simvastatin, lovastatin and mevastatin inhibit proliferation and invasion of melanoma cells. BMC Cancer. 2008;8:9. doi: 10.1186/1471-2407-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osmak M. Statins and cancer: Current and future prospects. Cancer Lett. 2012;324:1–12. doi: 10.1016/j.canlet.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Ghisalberti E.L., Narbey M.J., Rowland C.Y. Metabolites of Aspergillus terreus antagonistic towards the take-all fungus. J. Nat. Prod. 1990;53:520–522. doi: 10.1021/np50068a043. [DOI] [Google Scholar]
- 37.Raistrick H., Smith G. LXXI. Studies in the biochemistry of micro-organisms. XLII. The metabolic products of Aspergillus terreus Thom. A new mould metabolic product-terrein. Biochem. J. 1935;29:606–611. doi: 10.1042/bj0290606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao W.-Y., Shen C.-N., Lin L.-H., Yang Y.-L., Han H.-Y., Chen J.-W., Kuo S.-C., Wu S.-H., Liaw C.-C. Asperjinone, a nor-neolignan, and terrein, a suppressor of ABCG2-expressing breast cancer cells, from thermophilic Aspergillus terreus. J. Nat. Prod. 2012;75:630–635. doi: 10.1021/np200866z. [DOI] [PubMed] [Google Scholar]
- 39.Härri E., Loeffler W., Sigg H.P., Stähelin H., Tamm C. Über die isolierung neuer stoffwechselprodukte aus Penicillium brefeldianum. Helv. Chim. Acta. 1963;46:1235–1246. doi: 10.1002/hlca.19630460419. [DOI] [Google Scholar]
- 40.Betina V., Nemec P., Dobias J., Barath Z. Cyanein, a new antibiotic from Penicillium cyaneum. Folia Biol. 1962;7:353–357. doi: 10.1007/BF02928123. [DOI] [PubMed] [Google Scholar]
- 41.Singleton V., Bohonos N., Ullstrup A. Decumbin, a new compound from a species of Penicillium. Nature. 1958;181:1072–1073. doi: 10.1038/1811072a0. [DOI] [PubMed] [Google Scholar]
- 42.Shao R.G., Shimizu T., Pommier Y. Brefeldin A is a potent inducer of apoptosis in human cancer cells independently of p53. Exp. Cell Res. 1996;227:190–196. doi: 10.1006/excr.1996.0266. [DOI] [PubMed] [Google Scholar]
- 43.Chapman J.R., Tazaki H., Mallouh C., Konno S. Brefeldin A-induced apoptosis in prostatic cancer DU-145 cells: A possible p53-independent death pathway. BJU Int. 1999;83:703–708. doi: 10.1046/j.1464-410x.1999.00973.x. [DOI] [PubMed] [Google Scholar]
- 44.Wang J., Huang Y., Fang M., Zhang Y., Zheng Z., Zhao Y., Su W. Brefeldin A, a cytotoxin produced by Paecilomyces sp. and Aspergillus clavatus isolated from Taxus mairei and Torreya grandis. FEMS Imunol. Med. Mic. 2002;34:51–57. doi: 10.1111/j.1574-695X.2002.tb00602.x. [DOI] [PubMed] [Google Scholar]
- 45.Chinworrungsee M., Wiyakrutta S., Sriubolmas N., Chuailua P., Suksamrarn A. Cytotoxic activities of trichothecenes isolated from an endophytic fungus belonging to order Hypocreales. Archi. Pharmacal Res. 2008;31:611–616. doi: 10.1007/s12272-001-1201-x. [DOI] [PubMed] [Google Scholar]
- 46.Argoudelia A.D., Zieserl J.F. The structure of U-13,933, a new antibiotic. Tetrahedron Lett. 1966;7:1969–1973. doi: 10.1016/S0040-4039(00)76280-0. [DOI] [PubMed] [Google Scholar]
- 47.He L., Nan M.-H., Oh H.C., Kim Y.H., Jang J.H., Erikson R.L., Ahn J.S., Kim B.Y. Asperlin induces G2/M arrest through ROS generation and ATM pathway in human cervical carcinoma cells. Biochem. Bioph. Res. Co. 2011;409:489–493. doi: 10.1016/j.bbrc.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 48.Brian P.W. Studies on the biological activity of griseofulvin. Ann. Bot. 1949;13:59–77. [Google Scholar]
- 49.Hector R.F. An overview of antifungal drugs and their use for treatment of deep and superficial mycoses in animals. Clin. Tech. Small Anim. Pract. 2005;20:240–249. doi: 10.1053/j.ctsap.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Oxford A.E., Raistrick H., Simonart P. XXIX. Studies in the biochemistry of microorganisms. LX. Griseofulvin, C17H17O6Cl, a metabolic product of Penicillium griseofulvum Dierckx. Bioch. J. 1939;33:240–248. doi: 10.1042/bj0330240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grisham L.M., Wilson L., Bensch K.G. Antimitotic action of griseofulvin does not involve disruption of microtubules. Nature. 1973;224:294–296. doi: 10.1038/244294a0. [DOI] [PubMed] [Google Scholar]
- 52.Stierle D.B., Stierle A.A., Bugni T. Sequoiamonascins A–D: Novel anticancer metabolites isolated from a redwood endophyte. J. Org. Chem. 2003;68:4966–4969. doi: 10.1021/jo0340253. [DOI] [PubMed] [Google Scholar]
- 53.De Stefano S., Nicoletti R., Milone A., Zambardino S. 3-O-Methylfunicone, a fungitoxic metabolite produced by the fungus Penicillium pinophilum. Phytochemistry. 1999;52:1399–1401. doi: 10.1016/S0031-9422(99)00320-9. [DOI] [Google Scholar]
- 54.Stammati A., Nicoletti R., De Stefano S., Zampaglioni F., Zucco F. Cytostatic properties of a novel compound derived from Penicillium pinophilum: An in vitro study. Altern. Lab. Anim. 2002;30:69–75. doi: 10.1177/026119290203000107. [DOI] [PubMed] [Google Scholar]
- 55.Buommino E., Nicoletti R., Gaeta G.M., Orlando M., Ciavatta M.L., Baroni A., Tufano M.A. 3-O-methylfunicone, a secondary metabolite produced by Penicillium pinophilum, induces growth arrest and apoptosis in HeLa cells. Cell Proliferat. 2004;37:413–426. doi: 10.1111/j.1365-2184.2004.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buommino E., Boccellino M., de Filippis A., Petrazzuolo M., Cozza V., Nicoletti R., Ciavatta M.L., Quagliuolo L., Tufano M.A. 3-O-methylfunicone produced by Penicillium pinophilum affects cell motility of breast cancer cells, downregulating avb5 integrin and inhibiting metalloproteinase-9 secretion. Mol. Carcinogen. 2007;46:930–940. doi: 10.1002/mc.20322. [DOI] [PubMed] [Google Scholar]
- 57.Baroni A., de Luca A., de Filippis A., Petrazzuolo M., Manente L., Nicoletti R., Tufano M.A., Buommino E. 3-O-methylfunicone, a metabolite of Penicillium pinophilum, inhibits proliferation of human melanoma cells by causing G(2)+M arrest and inducing apoptosis. Cell Proliferat. 2009;42:541–553. doi: 10.1111/j.1365-2184.2009.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buommino E., Tirino V., de Filippis A., Silvestri F., Nicoletti R., Ciavatta M.L., Pirozzi G., Tufano M. A 3-O-methylfunicone, from Penicillium pinophilum, is a selective inhibitor of breast cancer stem cells. Cell Proliferat. 2011;44:401–409. doi: 10.1111/j.1365-2184.2011.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Komai S.-I., Hosoe T., Itabashi T., Nozawa K., Yaguchi T., Fukushima K., Kawai K.-I. New vermistatin derivatives isolated from Penicillium simplicissimum. Heterocycles. 2005;65:2771–2776. doi: 10.3987/COM-05-10523. [DOI] [Google Scholar]
- 60.Stierle A.A., Stierle D.B., Girtsman T. Caspase-1 inhibitors from an extremophilic fungus that target specific leukemia cell lines. J. Nat. Prod. 2012;75:344–350. doi: 10.1021/np200414c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qin J.-C., Zhang Y.-M., Gao J.-M., Bai M.-S., Yang S.-X., Laatsch H., Zhang A.-L. Bioactive metabolites produced by Chaetomium globosum, an endophytic fungus isolated from Ginkgo biloba. Bioorgan. Med. Chem. Lett. 2009;19:1572–1574. doi: 10.1016/j.bmcl.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 62.Yamada T., Doi M., Shigeta H., Muroga Y., Hosoe S., Numata A., Tanaka R. Absolute stereostructures of cytotoxic metabolites, chaetomugilins A–C, produced by a Chaetomium species separated from a marine fish. Tetrahedron Lett. 2008;49:4192–4195. doi: 10.1016/j.tetlet.2008.04.060. [DOI] [Google Scholar]
- 63.Yasuhide M., Yamada T., Numata A., Tanaka R. Chaetomugilins, new selectively cytotoxic metabolites, produced by a marine fish-derived Chaetomium species. J. Antibiot. 2008;61:615–622. doi: 10.1038/ja.2008.81. [DOI] [PubMed] [Google Scholar]
- 64.Yamada T., Yasuhide M., Shigeta H., Numata A., Tanaka R. Absolute stereostructures of chaetomugilins G and H produced by a marine-fish-derived Chaetomium species. J. Antibiot. 2009;62:353–357. doi: 10.1038/ja.2009.39. [DOI] [PubMed] [Google Scholar]
- 65.Muroga Y., Yamada T., Numata A., Tanaka R. Chaetomugilins I–O, new potent cytotoxic metabolites from a marine-fish-derived Chaetomium species. Stereochemistry and biological activities. Tetrahedron. 2009;65:7580–7586. doi: 10.1016/j.tet.2009.06.125. [DOI] [Google Scholar]
- 66.Muroga Y., Yamada T., Numata A., Tanaka R. 11- and 4’-epimers of chaetomugilin A, novel cytostatic metabolites from marine fish-derived fungus Chaetomium globosum. Helv. Chim. Acta. 2010;93:542–549. doi: 10.1002/hlca.200900272. [DOI] [Google Scholar]
- 67.Yamada T., Muroga Y., Jinno M., Kajimoto T., Usami Y., Numata A., Tanaka R. New class azaphilone produced by a marine fish-derived Chaetomium globosum. The stereochemistry and biological activities. Bioorgan. Med. Chem. 2011;19:4106–4113. doi: 10.1016/j.bmc.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 68.Yamada T., Jinno M., Kikuchi T., Kajimoto T., Numata A., Tanaka R. Three new azaphilones produced by a marine fish-derived Chaetomium globosum. J. Antibiot. 2012;65:413–417. doi: 10.1038/ja.2012.40. [DOI] [PubMed] [Google Scholar]
- 69.Chen G.-D., Li Y.-J., Gao H., Chen Y., Li X.-X., Li J., Guo L.-D., Cen Y.-Z., Yao X.-S. New azaphilones and chlorinated phenolic glycosides from Chaetomium elatum with caspase-3 inhibitory activity. Planta Med. 2012;78:1683–1689. doi: 10.1055/s-0032-1315211. [DOI] [PubMed] [Google Scholar]
- 70.Lee L., Bennett J.W., Goldblatt L.A., Lundin R.E. Norsolorinic acid from a mutant strain of Aspergillus parasiticus. J. Am. Oil Chemi. Soc. 1971;48:93–94. doi: 10.1007/BF02635696. [DOI] [PubMed] [Google Scholar]
- 71.Bennett J.W., Lee L.S., Vinnett C. The correlation of aflatoxin and norsolorinic acid production. J. Am. Oil Chem. Soc. 1971;48:368–370. doi: 10.1007/BF02890764. [DOI] [PubMed] [Google Scholar]
- 72.Wang C.C.C., Chiang Y.-M., Kuo P.-L., Chang J.-K., Hsu Y.-L. Norsolorinic acid inhibits proliferation of T24 human bladder cancer cells by arresting the cell cycle at the G0/G1 phase and inducing a Fas/membrane-bound Fas ligand-mediated apoptotic pathway. Clini. Exp. Pharmacol. P. 2008;35:1301–1308. doi: 10.1111/j.1440-1681.2008.05007.x. [DOI] [PubMed] [Google Scholar]
- 73.Wang C.C.C., Chiang Y.-M., Kuo P.-L., Chang J.-K., Hsu Y.-L. Norsolorinic acid from Aspergillus nidulans inhibits the proliferation of human breast adenocarcinoma MCF-7 cells via Fas-mediated pathway. Basic Clin. Pharmacol. 2008;102:491–497. doi: 10.1111/j.1742-7843.2008.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Samson R.A., Varga J., Meijer M., Frisvad J.C. New taxa in Aspergillus section. Usti. Stud. Mycol. 2011;69:81–97. doi: 10.3114/sim.2011.69.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steyn P.S., Vleggaar R. Austocystins. Six novel dihydrofuro (3',2':4,5)furo(3,2-b)xanthenones from Aspergillus ustus. J. Chem. Soc. Perk. T. 1. 1974;1974:2250–2256. [PubMed] [Google Scholar]
- 76.Ireland C., Aalbersberg W., Andersen R., Ayral-Kaloustian S., Berlinck R., Bernan V., Carter G., Churchill A., Clardy J., Concepcion G., et al. Anticancer agents from unique natural products sources. Pharm. Biol. 2003;41:15–38. doi: 10.1080/1388020039051742. [DOI] [Google Scholar]
- 77.Marks K.M., Park E.S., Arefolov A., Russo K., Ishihara K., Ring J.E., Clardy J., Clarke A.S., Pelish H.E. The selectivity of austocystin D arises from cell-line-specific drug activation by cytochrome P450 enzymes. J. Nat. Prod. 2011;74:567–573. doi: 10.1021/np100429s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Houbraken J., Frisvad J.C., Samson R. A. Fleming’s penicillin producing strain is not Penicillium chrysogenum but P. rubens. IMA Fungus. 2011;2:87–95. doi: 10.5598/imafungus.2011.02.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Houbraken J., Frisvad J.C., Seifert K.A., Overy D.P., Tuthill D.M., Valdez J.G., Samson R.A. New penicillin-producing Penicillium species and an overview of section Chrysogena. Persoonia. 2012;29:78–100. doi: 10.3767/003158512X660571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu W., Gu Q., Zhu W., Cui C., Fan G., Zhu T., Liu H., Fang Y. Chloctanspirones A and B, novel chlorinated polyketides with an unprecedented skeleton, from marine sediment derived fungus Penicillium terrestre. Tetrahedron Lett. 2005;46:4993–4996. [Google Scholar]
- 81.Li D., Chen L., Zhu T., Kurtán T., Mándi A., Zhao Z., Li J., Gu Q. Chloctanspirones A and B, novel chlorinated polyketides with an unprecedented skeleton, from marine sediment derived fungus Penicillium terrestre. Tetrahedron. 2011;67:7913–7918. doi: 10.1016/j.tet.2011.08.037. [DOI] [Google Scholar]
- 82.Takahashi C., Yoshihira K., Natori S., Umeda M. The structures of toxic metabolites of Aspergillus candidus. I. The compounds A and E, cytotoxic p-terphenyls. Chem. Pharm. Bull. 1976;24:613–620. doi: 10.1248/cpb.24.613. [DOI] [PubMed] [Google Scholar]
- 83.Wei H., Inada H., Hayashi A., Higashimoto K., Pruksakorn P., Kamada S., Arai M., Ishida S. Prenylterphenyllin and its dehydroxyl analogs, new cytotoxic substances from a marine-derived fungus Aspergillus candidus IF10. J. Antibiot. 2007;60:586–590. doi: 10.1038/ja.2007.75. [DOI] [PubMed] [Google Scholar]
- 84.Cai S., Sun S., Zhou H., Kong X., Zhu T., Li D., Gu Q. Prenylated polyhydroxy-p-terphenyls from Aspergillus taichungensis ZHN-7-07. J. Nat. Prod. 2011;75:1106–1110. doi: 10.1021/np2000478. [DOI] [PubMed] [Google Scholar]
- 85.Du L., Liu H.-C., Fu W., Li D.-H., Pan Q.-M., Zhu T.-J., Geng M.-Y., Gu Q.-Q. Unprecedented citrinin trimer tricitinol B functions as a novel topoisomerase IIα inhibitor. J. Med. Chem. 2011;54:5796–5810. doi: 10.1021/jm200511x. [DOI] [PubMed] [Google Scholar]
- 86.Mulrooney C.A., O’Brien E.M., Morgan B.J., Kozlowski M.C. Perylenequinones: Isolation, synthesis, and biological activity. Eur. J. Org. Chem. 2012;2012:3887–3904. doi: 10.1002/ejoc.201200184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kobayashi E., Ando K., Nakano H., Iida T., Ohno H., Morimoto M., Tamaoki T. Calphostins (UCN-1028), novel and specific inhibitors of protein kinase C. I. Fermentation, isolation, physico-chemical properties and biological activities. J. Antibiot. 1989;42:1470–1474. doi: 10.7164/antibiotics.42.1470. [DOI] [PubMed] [Google Scholar]
- 88.Zhu D.M., Narla R.K., Fang W.H., Chia N.C., Uckun F.M. Calphostin C triggers calcium-dependent apoptosis in human acute lymphoblastic leukemia cells. Clini. Cancer Res. 1998;4:2967–2976. [PubMed] [Google Scholar]
- 89.Wu H., Lao X.-F., Wang Q.-W., Lu R.-R. The shiraiachromes: novel fungal perylenequinone pigments from Shiraia bambusicola. J. Nat. Prod. 1989;52:948–951. doi: 10.1021/np50065a006. [DOI] [Google Scholar]
- 90.Fang L., Qing C., Shao H., Yang Y., Dong Z., Wang F., Zhao W., Yang W., Liu J. Hypocrellin D, a cytotoxic fungal pigment from fruiting bodies of the ascomycete Shiraia bambusicola. J. Antibiot. 2006;59:351–354. doi: 10.1038/ja.2006.49. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y., Song L., Xie J., Qiu H., Gu Y., Zhao J. Novel surfactant-like hypocrellin derivatives to achieve simultaneous drug delivery in blood plasma and cell uptake. Photochem. Photobiol. 2010;86:667–672. doi: 10.1111/j.1751-1097.2010.00711.x. [DOI] [PubMed] [Google Scholar]
- 92.Li G., Wang H., Zhu R., Sun L., Wang L., Li M., Li Y., Liu Y., Zhao Z., Lou H. Phaeosphaerins A−F, cytotoxic perylenequinones from an endolichenic fungus, Phaeosphaeria sp. J. Nat. Prod. 2012;75:142–147. doi: 10.1021/np200614h. [DOI] [PubMed] [Google Scholar]
- 93.Gao X., Chooi Y.-H., Ames B.D., Wang P., Walsh C.T., Tang Y. Fungal indole alkaloid biosynthesis: Genetic and biochemical investigation of the tryptoquialanine pathway in Penicillium aethiopicum. J. Am. Chem. Soc. 2011;133:2729–2741. doi: 10.1021/ja1101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shang Z., Li X., Meng L., Li C., Gao S., Huang C., Wang B. Chemical profile of the secondary metabolites produced by a deep-sea sediment-derived fungus Penicillium commune SD-118. Chin. J. Oceanol. Limn. 2012;30:305–314. doi: 10.1007/s00343-012-1075-1. [DOI] [Google Scholar]
- 95.Varga J., Due M., Frisvad J.C., Samson R.A. Taxonomic revision of Aspergillus section Clavati based on molecular, morphological and physiological data. Stud. Mycol. 2007;59:89–106. doi: 10.3114/sim.2007.59.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ando K., Suzuki S., Takatsuki A., Arima K., Tamura G. A new antibiotic, 1-(p-hydroxyphenyl)-2,3-diisocyano-4-(p-methoxyphenyl)-buta-1,3-diene. I. Isolation and biological properties. J. Antibiot. 1968;21:582–586. doi: 10.7164/antibiotics.21.582. [DOI] [PubMed] [Google Scholar]
- 97.Kozlovskiĭ A.G., Zhelifonova V.P., Antipova T.V, Adanin V.M., Novikova N.D., Deshevaia E.A., Schlegel B., Dahse H.M., Gollmick F.A., Grafe U. Penicillium expansum, a resident fungal strain of the orbital complex Mir, producing xanthocillin X and questiomycin A. Prikl. Biokhim. Mikrobiol. 2004;40:344–349. [PubMed] [Google Scholar]
- 98.Tsunkawa M., Ohkusa N., Kobaru S., Narita Y., Mirate S., Sawada Y., Oki T. BU-4704, a new member of the xanthocillin class. J. Antibiot. 1993;46:687–688. doi: 10.7164/antibiotics.46.687. [DOI] [PubMed] [Google Scholar]
- 99.Zhan X., Zhao H., Guan Y., Xiao C., He Q. Mechanism of inhibiting proliferation by xanthocillin X dimethyl in tumor cells. Zhongguo Xinyao Zazhi. 2010;19:832–836. [Google Scholar]
- 100.Clark A.M., Hufford C.D., Robertson L.W. Two metabolites from Aspergillus flavipes. Lloydia. 1977;40:146–151. [PubMed] [Google Scholar]
- 101.Frisvad J.C., Houbraken J., Popma S., Samson R.A. Two new Penicillium species Penicillium buchwaldii and Penicillium spathulatum, producing the anticancer compound asperphenamate. FEMS microbiol. Lett. 2013;339:77–92. doi: 10.1111/1574-6968.12054. [DOI] [PubMed] [Google Scholar]
- 102.Wu P.-L., Lin F.-W., Wu T.-S., Kuoh C.-S., Lee K.-H., Lee S.-J. Cytotoxic and anti-HIV principles from the rhizomes of Begonia nantoensis. Chem. Pharm. Bull. 2004;52:345–349. doi: 10.1248/cpb.52.345. [DOI] [PubMed] [Google Scholar]
- 103.Yuan L., Wang J.H., Sun T.M. Total synthesis and anticancer activity studies of the stereoisomers of asperphenamate and patriscabratine. Chin. Chem. Lett. 2010;21:155–158. doi: 10.1016/j.cclet.2009.10.004. [DOI] [Google Scholar]
- 104.Li Y., Luo Q., Yuan L., Miao C., Mu X., Xiao W., Li J., Sun T., Ma E. JNK-dependent Atg4 upregulation mediates asperphenamate derivative BBP-induced autophagy in MCF-7 cells. Toxicol. Appl. Pharm. 2012;263:21–31. doi: 10.1016/j.taap.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 105.Borthwick A.D. 2,5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012;112:3641–3716. doi: 10.1021/cr200398y. [DOI] [PubMed] [Google Scholar]
- 106.Yamazaki M., Suzuki S., Miyaki K. Tremorgenic toxins from Aspergillus fumigatus Fres. Chem. Pharm. Bull. 1971;19:1739–1740. doi: 10.1248/cpb.19.1739. [DOI] [PubMed] [Google Scholar]
- 107.Abraham W.-R., Arfmann H. 12,13-Dihydroxy-fumitremorgin C from Aspergillus fumigatus. Phytochemistry. 1990;29:1025–1026. doi: 10.1016/0031-9422(90)80080-Z. [DOI] [Google Scholar]
- 108.Samson R.A., Hong S., Peterson S.W., Frisvad J.C., Varga J. Polyphasic taxonomy of Aspergillus section Fumigati and its teleomorph Neosartorya. Stud. Mycol. 2007;59:147–203. doi: 10.3114/sim.2007.59.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cui C.-B., Kakeya H., Okada G., Onose R., Ubukata M., Takahashi I., Isono K., Osada H. Tryprostatins A and B, novel mammalian cell cycle inhibitors produced by Aspergillus fumigatus. J. Antibiot. 1995;48:1382–1384. doi: 10.7164/antibiotics.48.1382. [DOI] [PubMed] [Google Scholar]
- 110.Cui C., Kakeya H., Osada H. Novel mammalian cell cycle inhibitors, spirotryprostatins A and B, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron. 1996;52:12651–12666. doi: 10.1016/0040-4020(96)00737-5. [DOI] [Google Scholar]
- 111.Qian-Cutrone J., Huang S., Shu Y.-Z., Vyas D., Fairchild C., Menendez A., Krampitz K., Dalterio R., Klohr S.E., Gao Q. Stephacidin A and B: two structurally novel, selective inhibitors of the testosterone-dependent prostate LNCaP cells. J. Am. Chem. Soc. 2002;124:14556–14557. doi: 10.1021/ja028538n. [DOI] [PubMed] [Google Scholar]
- 112.Finefield J.M., Frisvad J.C., Sherman D.H., Williams R.M. Fungal origins of the bicyclo[2.2.2]diazaoctane ring system of prenylated indole alkaloids. J. Nat. Prod. 2012;75:812–833. doi: 10.1021/np200954v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao S., Smith K.S., Deveau A.M., Dieckhaus C.M., Johnson M.A., Macdonald T.L., Cook J.M. Biological activity of the tryprostatins and their diastereomers on human carcinoma cell lines. J. Med. Chem. 2002;45:1559–1562. doi: 10.1021/jm0155953. [DOI] [PubMed] [Google Scholar]
- 114.Wang F., Fang Y., Zhu T., Zhang M., Lin A., Gu Q., Zhu W. Seven new prenylated indole diketopiperazine alkaloids from holothurian-derived fungus Aspergillus fumigatus. Tetrahedron. 2008;64:7986–7991. doi: 10.1016/j.tet.2008.06.013. [DOI] [Google Scholar]
- 115.Zhang M., Wang W.-L., Fang Y.-C., Zhu T.-J., Gu Q.-Q., Zhu W.-M. Cytotoxic alkaloids and antibiotic nordammarane triterpenoids from the marine-derived fungus Aspergillus sydowi. J. Nat. Prod. 2008;71:985–989. doi: 10.1021/np700737g. [DOI] [PubMed] [Google Scholar]
- 116.Numata A., Takahashi C., Miyamoto T., Matsushita T., Kawai K., Usami Y., Matsumura E., Inoue M., Ohishi H., Shingu T. Structures of cytotoxic substances and new quinazoline derivatives produced by a fungus from a saltwater fish. Tennen Yuki Kagobutsu Toronkai Koen Yoshishu. 1991;33:723–730. [Google Scholar]
- 117.Wang Y., Li Z.-L., Bai J., Zhang L.-M., Wu X., Zhang L., Pei Y.-H., Jing Y.-K., Hua H.-M. 2,5-diketopiperazines from the marine-derived fungus Aspergillus fumigatus YK-7. Chem. Biodivers. 2012;9:385–393. doi: 10.1002/cbdv.201100061. [DOI] [PubMed] [Google Scholar]
- 118.Rabindran S.K., He H., Singh M., Brown E., Collins K.I., Annable T., Greenberger L.M. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res. 1998;1998:5850–5858. [PubMed] [Google Scholar]
- 119.Rho M.C., Hayashi M., Fukami A., Obata R., Sunazuka T., Tomoda H., Komiyama K., Omura S. Reversal of multidrug resistance by 7-O-benzoylpyripyropene A in multidrug-resistant tumor cells. J. Antibiot. 2000;53:1201–1206. doi: 10.7164/antibiotics.53.1201. [DOI] [PubMed] [Google Scholar]
- 120.Jurjevic Z., Peterson S.W., Horn B.W. Aspergillus section Versicolores: nine new species and multilocus DNA sequence based phylogeny. IMA Fungus. 2012;3:59–79. doi: 10.5598/imafungus.2012.03.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kato H., Yoshida T., Tokue T., Nojiri Y., Hirota H., Ohta T., Williams R.M., Tsukamoto S. Notoamides A–D: Prenylated indole alkaloids isolated from a marine-derived fungus, Aspergillus sp. Angew. Chem. Int. Edit. 2007;46:2254–2256. doi: 10.1002/anie.200604381. [DOI] [PubMed] [Google Scholar]
- 122.Kanoh K., Kohno S., Asari T., Harada T., Katada J., Muramatsu M., Kawashima H., Sekiya H., Uno I. (−)-phenylahistin: A new mammalian cell cycle inhibitor produced by Aspergillus ustus. Bioorg. Med. Chem. Lett. 1997;7:2847–2852. doi: 10.1016/S0960-894X(97)10104-4. [DOI] [Google Scholar]
- 123.Kanoh K., Kohno S., Katada J., Hayashi Y., Muramatsu M., Uno I. Antitumor activity of phenylahistin in vitro and in vivo. Biosci. 1999;63:1130–1133. doi: 10.1271/bbb.63.1130. [DOI] [PubMed] [Google Scholar]
- 124.Yamazaki Y., Tanaka K., Nicholson B., Deyanat-Yazdi G., Potts B., Yoshida T., Oda A., Kitagawa T., Orikasa S., Kiso Y., et al. Synthesis and structure−Activity relationship study of antimicrotubule agents phenylahistin derivatives with a didehydropiperazine-2,5-dione structure. J. Med. Chem. 2012;55:1056–1071. doi: 10.1021/jm2009088. [DOI] [PubMed] [Google Scholar]
- 125.Hayashi Y., Orikasa S., Tanaka K., Kanoh K., Kiso Y. Total synthesis of anti-microtubule diketopiperazine derivatives: phenylahistin and aurantiamine. J. Org. Chem. 2000;65:8402–8405. doi: 10.1021/jo0012905. [DOI] [PubMed] [Google Scholar]
- 126.Larsen T.O., Frisvad J.C., Jensen S.R. Aurantiamine, a diketopiperazine from two varieties of Penicillium aurantiogriseum. Phytochemistry. 1992;31:1613–1615. doi: 10.1016/0031-9422(92)83116-G. [DOI] [Google Scholar]
- 127.Menzel A.O., Wintersteiner O., Hoogerheide J.O. The isolation of gliotoxin and fumigacin from culture filtrates of Aspergillus fumigatus. J. Biol. Chem. 1944;152:419–429. [Google Scholar]
- 128.Kaouadji M. Gliotoxin: Uncommon 1H couplings and revised 1H- and 13C-NMR assignments. J. Nat. Prod. 1990;53:717–719. doi: 10.1021/np50069a032. [DOI] [Google Scholar]
- 129.Carberry S., Molloy E., Hammel S., O’Keeffe G., Jones G.W., Kavanagh K., Doyle S. Gliotoxin effects on fungal growth: Mechanisms and exploitation. Fungal Genet. Biol. 2012;49:302–312. doi: 10.1016/j.fgb.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 130.Kidd J.G. Effects of an antibiotic from Aspergillus fumigatus Fresenius on tumor cells in vitro, and its possible identity with gliotoxin. Science. 1947;105:511–513. doi: 10.1126/science.105.2733.511. [DOI] [PubMed] [Google Scholar]
- 131.Vigushin D.M., Mirsaidi N., Brooke G., Sun C., Pace P., Inman L., Moody C.J., Coombes R.C. Gliotoxin is a dual inhibitor of farnesyltransferase and geranylgeranyltransferase I with antitumor activity against breast cancer in vivo. Med. Mycol. 2004;21:21–30. doi: 10.1385/MO:21:1:21. [DOI] [PubMed] [Google Scholar]
- 132.Seya H., Nozawa K., Nakajima S., Kawai K.-I., Udagawa S.-I. Studies on fungal products. Part 8. Isolation and structure of emestrin, a novel antifungal macrocyclic epidithiodioxopiperazine from Emericeella striata. X-Ray molecular structure of emestrin. J. Chem. Soc. Perk. T. 1. 1986;1986:109–116. [Google Scholar]
- 133.Seya H., Nakajima S., Kawai K.-I., Udagawa S.-I. Structure and absolute configuration of emestrin, a new macrocyclic epidithiodioxopiperazine from Emericella striata. J. Chem. Soc. Chem. Comm. 1985;739:657–658. [Google Scholar]
- 134.Ueno Y., Umemori K., Nilmi E., Tanuma S., Nagata S., Sugamata M., Ihara T., Sekljlma, M., Kawai K.-I., Ueno I., Takhiro F. Induction of apoptosis by T-2 toxin and other natural toxins in HL-60 human promyelotic leukemia cells. Nat. Toxins. 1995;3:129–137. doi: 10.1002/nt.2620030303. [DOI] [PubMed] [Google Scholar]
- 135.Onodera H., Hasegawa A., Tsumagari N., Nakai R., Ogawa T., Kanda Y. MPC1001 and its analogues: New antitumor agents from the fungus Cladorrhinum species. Org. Lett. 2004;6:4101–4104. doi: 10.1021/ol048202d. [DOI] [PubMed] [Google Scholar]
- 136.Choi E.J., Park J.-S., Kim Y.-J., Jung J.-H., Lee J.K., Kwon H.C., Yang H.O. Apoptosis-inducing effect of diketopiperazine disulfides produced by Aspergillus sp. KMD 901 isolated from marine sediment on HCT116 colon cancer cell lines. J. Appl. Microbiol. 2011;110:304–313. doi: 10.1111/j.1365-2672.2010.04885.x. [DOI] [PubMed] [Google Scholar]
- 137.Arai T., Mikami Y., Fukushima K., Utsumi T., Yazawa K. A new antibiotic, leucinostatin, derived from Penicillium lilacinum. J. Antibiot. 1973;26:157–161. doi: 10.7164/antibiotics.26.157. [DOI] [PubMed] [Google Scholar]
- 138.Luangsa-Ard J., Houbraken J., van Doorn T., Hong S.-B., Borman A.M., Hywel-Jones N.L., Samson R.A. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS microbiol. Lett. 2011;321:141–149. doi: 10.1111/j.1574-6968.2011.02322.x. [DOI] [PubMed] [Google Scholar]
- 139.Fukushima K., Arai T., Mori Y., Tsuboi M., Suzuki M. Studies on peptide antibiotics, leucinostatins. I. Separation, physico- chemical properties and biological activities of leucinostatins A and B. J. Antibiot. 1983;36:1606–1612. doi: 10.7164/antibiotics.36.1606. [DOI] [PubMed] [Google Scholar]
- 140.Strobel G.A., Torczynski R., Bollon A. Acremonium sp.—A leucinostatin A producing endophyte of European yew (Taxus baccata) Plant Sci. 1997;128:97–108. doi: 10.1016/S0168-9452(97)00131-3. [DOI] [Google Scholar]
- 141.Ishiguro K., Arai T. Action of the peptide antibiotic leucinostatin. Antimicrobi. Agents Chemither. 1976;9:893–898. doi: 10.1128/AAC.9.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kawada M., Inoue H., Ohba S.-I., Masuda T., Momose I., Ikeda D. Leucinostatin A inhibits prostate cancer growth through reduction of insulin-like growth factor-I expression in prostate stromal cells. Int. J. Cancer. 2010;126:810–818. doi: 10.1002/ijc.24915. [DOI] [PubMed] [Google Scholar]
- 143.Strobel G.A., Hess W.M. Glucosylation of the peptide leucinostatin A, produced by an endophytic fungus of European yew, may protect the host from leucinostatin toxicity. Chem. Biol. 1997;4:529–536. doi: 10.1016/S1074-5521(97)90325-2. [DOI] [PubMed] [Google Scholar]
- 144.Wani M.C., Taylor L.H., Wall M.E., Coggon P., McPhail A.T. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 145.Stierle A., Strobel G., Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science. 1993;260:214–216. doi: 10.1126/science.8097061. [DOI] [PubMed] [Google Scholar]
- 146.Niedens B.R., Parker S.R., Stierle D.B., Stierle A.A. First fungal aromatic L-amino acid decarboxylase from a paclitaxel-producing Penicillium raistrickii. Mycologia. 2013;91:619–626. [Google Scholar]
- 147.Kohler D.R., Goldspiel B.R. Paclitaxel (taxol) Pharmacotherapy. 1994;14:3–34. doi: 10.1002/j.1875-9114.1994.tb02785.x. [DOI] [PubMed] [Google Scholar]
- 148.Visalakchi S., Muthumary J. Taxol (anticancer drug) producing endophytic fungi: An overview. Int. J. Pharm. Bio Sci. 2010;1:1–9. [Google Scholar]
- 149.Schiff P.B., Fant J., Horwitz S.B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 150.Jordan M.A., Wilson L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 151.Young D.H., Michelotti E.L., Swindell C.S., Krauss N.E. Antifungal properties of taxol and various analogues. Experientia. 1992;48:882–885. doi: 10.1007/BF02118425. [DOI] [PubMed] [Google Scholar]
- 152.Pittayakhajonwut P., Dramae A., Intaraudom C., Boonyuen N., Nithithanasilp S., Rachtawee P., Laksanacharoen P. Two new drimane sesquiterpenes, fudecadiones A and B, from the soil fungus Penicillium sp. BCC 17468. Planta Med. 2011;77:74–76. doi: 10.1055/s-0030-1250057. [DOI] [PubMed] [Google Scholar]
- 153.Hirota A., Nakagawa M., Hirota H., Takahashi T. Terrecylic acid A, a new antibiotic from Aspergillus terreus IV. Absolute stereochemistry of terrecyclic acid A. J. Antibiot. 1986;39:1–4. doi: 10.7164/antibiotics.39.1. [DOI] [PubMed] [Google Scholar]
- 154.Wijeratne E.M.K., Turbyville T.J., Zhang Z., Bigelow D., Pierson L.S., VanEtten H.D., Whitesell L., Canfield L.M., Gunatilaka A.A.L. Cytotoxic constituents of Aspergillus terreus from the rhizosphere of Opuntia versicolor of the Sonoran desert. J. Nat. Prod. 2003;66:1567–1573. doi: 10.1021/np030266u. [DOI] [PubMed] [Google Scholar]
- 155.Wu G., Lin A., Gu Q., Zhu T., Li D. Four new chloro-eremophilane sesquiterpenes from an antarctic deep-sea derived fungus, Penicillium sp. PR19N-1. Mar. Drugs. 2013;11:1399–1408. doi: 10.3390/md11041399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Woloshuk C.P., Shim W.-B. Aflatoxins, fumonisins, and trichothecenes: a convergence of knowledge. FEMS Microbiol. Rev. 2013;37:94–109. doi: 10.1111/1574-6976.12009. [DOI] [PubMed] [Google Scholar]
- 157.Sudakin D. Trichothecenes in the environment: relevance to human health. Toxicol. Lett. 2003;143:97–107. doi: 10.1016/S0378-4274(03)00116-4. [DOI] [PubMed] [Google Scholar]
- 158.Shifrin V.I., Anderson P. Trichothecene mycotoxins trigger a ribotoxic stress response that activates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase and induces apoptosis. J. Biol. Chem. 1999;274:13985–13992. doi: 10.1074/jbc.274.20.13985. [DOI] [PubMed] [Google Scholar]
- 159.Campos F.F., Johann S., Cota B.B., Alves T.M.A., Rosa L.H., Caligiorne R.B., Cisalpino P.S., Rosa C.A., Zani C.L. Antifungal activity of trichothecenes from Fusarium sp. against clinical isolates of Paracoccidioides brasiliensis. Mycoses. 2011;54:E122–E129. doi: 10.1111/j.1439-0507.2009.01854.x. [DOI] [PubMed] [Google Scholar]
- 160.Sun T., Zheng W., Peng H., Zhang A., Chen Y., Tan R., Shen P. A small molecule IFB07188 inhibits proliferation of human cancer cells by inducing G2/M cell cycle arrest and apoptosis. Biomedi. Pharmacother. 2012;66:512–518. doi: 10.1016/j.biopha.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 161.Pae H.O., Oh G.S., Choi B.M., Seo E.A., Oh H., Shin M.K., Kim T.H., Kwon T.O., Chung H.T. Induction of apoptosis by 4-acetyl-12,13-epoxyl-9-trichothecene-3,15-diol from Isaria japonica Yasuda through intracellular reactive oxygen species formation and caspase-3 activation in human leukemia HL-60 cells. Toxicol. in Vitro. 2003;17:49–57. doi: 10.1016/S0887-2333(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 162.OH G.-S., Hong K.-H., OH H., Pae H.-O., Kim I.-K., Kwon T.-O., Shin M.-K., Chung H.-T. 4-Acetyl-12,13-epoxyl-9-trichothecene-3 ,15-diol isolated from the fruiting bodies of Iaria japonica YASUDA induces apoptosis of human leukemia cells (HL-60) Biol. Pharm. Bull. 2001;24:785–789. doi: 10.1248/bpb.24.785. [DOI] [PubMed] [Google Scholar]
- 163.Xu J., Takasaki A., Kobayashi H., Oda T., Yamada J., Mangindaan R.E.P., Ukai K., Nagai H., Namikoshi M. Four new macrocyclic trichothecenes from two strains of marine-derived fungi of the genus Myrothecium. J. Antibiot. 2006;59:451–455. doi: 10.1038/ja.2006.63. [DOI] [PubMed] [Google Scholar]
- 164.Goodwin W., Haas C.D., Fabian C., Heller-Bettinger I., Hoogstraten B. Phase I clinical evaluation of anguidine (diacetoxyscirpenol, NSC-141537) Cancer. 1977;42:23–26. doi: 10.1002/1097-0142(197807)42:1<23::aid-cncr2820420104>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 165.Dornera J.W., Colea R.J., Springerb J.P., Coxc R.H., Cutlerd H., Wicklow D.T. Isolation and identification of two new biologically active norditerpene dilactones from Aspergillus wentii. Phytochemistry. 1980;19:1157–1161. doi: 10.1016/0031-9422(80)83075-5. [DOI] [Google Scholar]
- 166.Zhang Z., Miao L., Sun W., Jiao B., Wang B., Yao L., Huang C. Wentilactone B from Aspergillus wentii induces apoptosis and inhibits proliferation and migration of human hepatoma SMMC-7721 cells. Bio. Pharm. Bull. 2012;35:1964–1971. doi: 10.1248/bpb.b12-00368. [DOI] [PubMed] [Google Scholar]
- 167.Brian P.W., Curtis P.J., Hemming H.G., Norris G.L.F. Wortmannin, an antibiotic produced by Penicillium wortmanni. Trans. Br. Mycol. Soc. 1957;40:365–368. doi: 10.1016/S0007-1536(57)80033-3. [DOI] [Google Scholar]
- 168.Nakamura M., Nakashima S., Katagiri Y., Ajozawa Y. Effect of wortmannin and 2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) on N-formyl-methionyl-leucyl-phenylalanine-induced phospholipase D activation in differentiated HL60 Cells. Biochem. Pharmacol. 1997;53:1929–1936. doi: 10.1016/S0006-2952(97)00169-X. [DOI] [PubMed] [Google Scholar]
- 169.Wang X., Wu Q., Zhang L., Wu Y., Shu Y. Wortmannin induced apoptosis of leukemia cells by reducing PI3K/Akt. Chin. German J. Clin. Oncol. 2010;9:734–738. doi: 10.1007/s10330-010-0715-1. [DOI] [Google Scholar]
- 170.Yun J., Lv Y.G., Yao Q., Wang L., Li Y.P., Yi J. Wortmannin inhibits proliferation and induces apoptosis of MCF-7 breast cancer cells. Eur. J. Gynaecol. Oncol. 2012;33:367–369. [PubMed] [Google Scholar]
- 171.Ng S.S.W., Tsao M., Chow S., Hedley D.W. Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine-induced apoptosis in human pancreatic cancer cells. Cancer Res. 2000;60:5451–5455. [PubMed] [Google Scholar]
- 172.Ng S.S.W., Tsao M., Nicklee T., Hedley D.W. Wortmannin inhibits PKB/Akt phosphorylation and promotes gemcitabine antitumor activity in orthotopic human pancreatic cancer xenografts in immunodeficient mice. Clini. Cancer Res. 2001;7:3269–3275. [PubMed] [Google Scholar]
- 173.Boehle A.S., Kurdow R., Boenicke L., Schniewind B., Faendrich F., Dohrmann P., Kalthoff H. Wortmannin inhibits growth of human non-small-cell lung cancer in vitro and in vivo. Langenbeck Arch. Surg. 2002;387:234–239. doi: 10.1007/s00423-002-0314-x. [DOI] [PubMed] [Google Scholar]
- 174.Au T.K., Chick W.S., Leung P.C. The biology of ophiobolins. Life Sci. 2000;67:733–742. doi: 10.1016/S0024-3205(00)00668-8. [DOI] [PubMed] [Google Scholar]
- 175.Ahn J.-W., Lee M.-K., Choi S.-U., Lee C.-O., Kim B.-S. Cytotoxic ophiobolins produced by Bipolaris sp. J. Microbiol. Biotechn. 1998;8:406–408. [Google Scholar]
- 176.Singh S.B., Smith J.L., Sabnis G.S., Dombrowski A.W., Schaeffer J.M., Goetz M.A., Bills G.F. Structure and conformation of ophiobolin K and 6- epiophiobolin K from Aspergillus ustus as a nematocidal agent. Tetrahedron. 1991;47:6931–6938. doi: 10.1016/S0040-4020(01)96148-4. [DOI] [Google Scholar]
- 177.Nozoe S., Itai A., Tsuda K., Okuda S. The chemical transformation of cephalonic acid. Tetrahedron Lett. 1967;42:4113–4117. doi: 10.1016/S0040-4039(01)89702-1. [DOI] [PubMed] [Google Scholar]
- 178.Bills G.F., Platas G., Gams W. Conspecificity of the cerulenin and helvolic acid producing “Cephalosporium caerulens”, and the hypocrealean fungus Sarocladium oryzae. Mycolo. Res. 2004;108:1291–1300. doi: 10.1017/S0953756204001297. [DOI] [PubMed] [Google Scholar]
- 179.Sugawara F., Strobel G., Strange R.N., Siedow J.N., Van Duyne G.D., Clardy J. Phytotoxins from the pathogenic fungi Drechslera maydis and Drechslera sorghicola. Proc. Natl. Acad. Sci. USA. 1987;84:3081–3085. doi: 10.1073/pnas.84.10.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.De Vries-van Leeuwen I.J., Kortekaas-Thijssen C., Mandouckou J.A.N., Kas S., Evidente A., de Boer A.H. Fusicoccin—A selectively induces apoptosis in tumor cells after interferon-alpha priming. Cancer Lett. 2010;293:198–206. doi: 10.1016/j.canlet.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 181.Shen X., Krasnoff S.B., Lu S.W., Dunbar C.D., O’Neal J., Turgeon B.G., Yoder O.C., Gibson D.M., Hamann M.T. Characterization of 6-epi-3-anhydroophiobolin B from Cochliobolus heterostrophus. J. Nat. Prod. 1999;62:895–897. doi: 10.1021/np980462e. [DOI] [PubMed] [Google Scholar]
- 182.Yang T., Lu Z., Meng L., Wei S., Hong K., Zhu W., Huang C. The novel agent ophiobolin O induces apoptosis and cell cycle arrest of MCF-7 cells through activation of MAPK signaling pathways. Bioorgan. Med. Chem. 2012;22:579–585. doi: 10.1016/j.bmcl.2011.10.079. [DOI] [PubMed] [Google Scholar]
- 183.Zhang D., Fukuzawa S., Satake M., Li X., Kuranaga T., Niitsu A., Yoshizawa K., Tachibana K. Ophiobolin O and 6-epi-ophiobolin O, two new cytotoxic sesterterpenes from the marine derived fungus Aspergillus sp. Nat. Prod. Commun. 2012;7:1411–1414. [PubMed] [Google Scholar]
- 184.Wang Q.-X., Yang J.-L., Qi Q.-Y., Bao L., Yang X.-L., Liu M.-M., Huang P., Zhang L.-X., Chen J.-L., Cai L., et al. 3-Anhydro-6-hydroxy-ophiobolin A, a new sesterterpene inhibiting the growth of methicillin-resistant Staphylococcus aureus and inducing the cell death by apoptosis on K562, from the phytopathogenic fungus Bipolaris oryzae. Bioorgan. Med. Chem. Lett. 2013;23:3547–3550. doi: 10.1016/j.bmcl.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 185.Li E., Clark A.M., Rotella D.P., Hufford C.D. Microbial metabolites of ophiobolin A and antimicrobial evaluation of ophiobolins. J. Nat. Prod. 1995;58:74–81. doi: 10.1021/np50115a009. [DOI] [PubMed] [Google Scholar]
- 186.Krizsán K., Bencsik O., Nyilasi I., Galgóczy L., Vágvölgyi C., Papp T. Effect of the sesterterpene-type metabolites, ophiobolins A and B, on zygomycetes fungi. FEMS Microbiol. Lett. 2010;313:135–140. doi: 10.1111/j.1574-6968.2010.02138.x. [DOI] [PubMed] [Google Scholar]
- 187.Geris R., Simpson T.J. Meroterpenoids produced by fungi. Nat. Prod. Rep. 2009;26:1063–1094. doi: 10.1039/b820413f. [DOI] [PubMed] [Google Scholar]
- 188.Schümann J., Hertweck C. Molecular basis of cytochalasan biosynthesis in fungi: gene cluster analysis and evidence for the involvement of a PKS-NRPS hybrid synthase by RNA silencing. J. Am. Chem. Soc. 2007;129:9564–9565. doi: 10.1021/ja072884t. [DOI] [PubMed] [Google Scholar]
- 189.Sekita S., Yoshihira K., Natori S., Kuwano H. Structures of chaetoglobosin A and B, cytotoxic metabolites of Chaetomium globosum. Tetrahedron Lett. 1973;14:2109–2112. doi: 10.1016/S0040-4039(01)86820-9. [DOI] [Google Scholar]
- 190.Fu J., Zhou Y., Li H., Ye Y., Guo J. Antifungal metabolites from Phomopsis sp By254, an endophytic fungus in Gossypium hirsutum. Afr. J. Microbiol. Res. 2011;5:1231–1236. [Google Scholar]
- 191.Wicklow D.T., Rogers K.D., Dowd P.F., Gloer J.B. Bioactive metabolites from Stenocarpella maydis, a stalk and ear rot pathogen of maize. Fungal Biol. 2011;115:133–142. doi: 10.1016/j.funbio.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 192.Frisvad J.C., Andersen B., Thrane U. The use of secondary metabolite profiling in chemotaxonomy of filamentous fungi. Mycol. Res. 2008;112:231–240. doi: 10.1016/j.mycres.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 193.Cimmino A., Andolfi A., Berestetskiy A., Evidente A. Production of phytotoxins by Phoma exigua var. exigua, a potential mycoherbicide against perennial thistles. J. Agr. Food Chem. 2008;56:6304–6309. doi: 10.1021/jf8004178. [DOI] [PubMed] [Google Scholar]
- 194.Xu H., Fang W.-S., Chen X.-G., He W.-Y., Cheng K.-D. Cytochalasin D from Hypocrella Bambusae. J. Asian Nat. Prod. Res. 2001;3:151–155. doi: 10.1080/10286020108041383. [DOI] [PubMed] [Google Scholar]
- 195.Demain A.L., Hunt N.A., Malik V., Kobbe B., Hawkins H., Matsuo K., Wogan G.N. Improved procedure for production of cytochalasin E and tremorgenic mycotoxins by Aspergillus clavatus. Appl. Environ. Microbiol. 1976;31:138–140. doi: 10.1128/aem.31.1.138-140.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wagenaar M.M., Corwin J., Strobel G., Clardy J. Three new cytochalasins produced by an endophytic fungus in the genus Rhinocladiella. J. Nat. Prod. 2000;63:1692–1695. doi: 10.1021/np0002942. [DOI] [PubMed] [Google Scholar]
- 197.Liu R., Gu Q., Zhu W., Cui C., Fan G., Fang Y., Zhu T., Liu H. 10-Phenyl-[12]-cytochalasins Z7, Z8, and Z9 from the marine-derived fungus Spicaria elegans. J. Nat. Prod. 2006;69:871–875. doi: 10.1021/np050201m. [DOI] [PubMed] [Google Scholar]
- 198.Thohinung S., Kanokmedhakul S., Kanokmedhakul K., Kukongviriyapan V., Tusskorn O., Soytong K. Cytotoxic 10-(indol-3-yl)-[13]cytochalasans from the fungus Chaetomium elatum ChE01. Arch. Pharm. Res. 2010;33:1135–1141. doi: 10.1007/s12272-010-0801-5. [DOI] [PubMed] [Google Scholar]
- 199.Ding G., Song Y.C., Chen J.R., Xu C., Ge H.M., Wang X.T., Tan R.X. Chaetoglobosin U, a cytochalasan alkaloid from endophytic Chaetomium globosum IFB-E019. J. Nat. Prod. 2006;69:302–304. doi: 10.1021/np050515+. [DOI] [PubMed] [Google Scholar]
- 200.Wang Y., Xu L., Ren W., Zhao D., Zhu Y., Wu X. Bioactive metabolites from Chaetomium globosum L18, an endophytic fungus in the medicinal plant Curcuma wenyujin. Phytomedicine. 2012;19:364–368. doi: 10.1016/j.phymed.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 201.Bloch P., Tamm C., Bollinger P., Petcher T.J., Weber H.P. 13. Pseurotin, a new metabolite of Pseudeurotium ovalis Stolk having an unusual hetero-spirocyclic system. Helv. Chim. Acta. 1976;59:133–137. doi: 10.1002/hlca.19760590114. [DOI] [PubMed] [Google Scholar]
- 202.Wenke J., Anke H., Sterner O. Pseurotin A and 8-O-demethylp-seurotin A from Aspergillus fumigatus and their inhibitory activities on chitin synthase. Biosci. Biotechnol. Biochem. 1993;57:961–964. doi: 10.1271/bbb.57.961. [DOI] [Google Scholar]
- 203.Martinez-Luis S., Cherigo L., Arnold E., Spadafore C., Gerwick W.H., Cubilla-Rios L. Antiparasitic and anticancer constituentd of the endophytic fungus Aspergillus sp. strain F1544. Nat. Prod. Commun. 2012;7:165–168. [PubMed] [Google Scholar]
- 204.Ge H.M., Shen Y., Zhu C.H., Tan S.H., Ding H., Song Y.C., Tan R.X. Penicidones A–C, three cytotoxic alkaloidal metabolites of an endophytic Penicillium sp. Phytochemistry. 2008;69:571–576. doi: 10.1016/j.phytochem.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 205.Shao C.-L., Wang C.-Y., Gu Y.-C., Wei M.-Y., Pan J.-H., Deng D.-S., She Z.-G., Lin Y.-C. Penicinoline, a new pyrrolyl 4-quinolinone alkaloid with an unprecedented ring system from an endophytic fungus Penicillium sp. Bioorg. Med. Chem. Lett. 2010;20:3284–3286. doi: 10.1016/j.bmcl.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 206.Elsebai M.F., Rempel V., Schnakenburg G., Kehraus S., Christa E.M., Gabriele M.K. Identification of a potent and selective cannabinoid CB1 receptor antagonist from Auxarthron reticulatum. ACS Med. Chem. Lett. 2011;2:866–869. doi: 10.1021/ml200183z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gao H., Zhang L., Zhu T., Gu Q., Li D. Unusual pyrrolyl 4-quinolinone alkaloids from the marine-derived fungus Penicillium sp. ghq208. Chem. Pharm. Bull. 2012;60:1458–1460. doi: 10.1248/cpb.c12-00487. [DOI] [PubMed] [Google Scholar]
- 208.Frisvad J.C., Samson R.A. Polyphasic taxonomy of Penicillium subgenus Penicillium A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud. Mycol. 2004;49:1–173. [Google Scholar]
- 209.Larsen T.O., Frisvad J.C., Ravn G., Skaaning T. Mycotoxin production by Penicillium expansum on blackcurrant and cherry juice. Food Addit. Contam. 1998;15:671–675. doi: 10.1080/02652039809374696. [DOI] [PubMed] [Google Scholar]
- 210.Dalsgaard P.W., Blunt J.W., Munro M.H.G., Frisvad J.C., Christophersen C. Communesins G and H, new alkaloids from the psychrotolerant fungus Penicillium rivulum. J. Nat. Prod. 2005;68:258–261. doi: 10.1021/np049646l. [DOI] [PubMed] [Google Scholar]
- 211.Numata A., Takahashi C., Ito Y., Takada T., Kawai K., Usami Y., Matsumura E., Imachia M., Ito T., Hasegawa T. Communesins, cytotoxic metabolites of a fungus isolated from a marine alga. Tetrahedron. 1993;34:2355–2358. doi: 10.1016/S0040-4039(00)77612-X. [DOI] [Google Scholar]
- 212.Jadulco R., Edrada R.A., Ebel R., Berg A., Schaumann K., Wray V., Steube K., Proksch P. New communesin derivatives from the fungus Penicillium sp. derived from the Mediterranean sponge Axinella verrucosa. J. Nat. Prod. 2004;67:78–81. doi: 10.1021/np030271y. [DOI] [PubMed] [Google Scholar]
- 213.Eble T.E., Hanson F.R. Fumagillin, an antibiotic from Aspergillus fumigatus H-3. Antibiot. Chemother. 1951;1:54–58. [PubMed] [Google Scholar]
- 214.Frisvad J.C., Samson R.A., Stolk A.C. A new species of Penicillium, P. scabrosum. Persoonia. 1990;14:177–182. [Google Scholar]
- 215.Ingber D., Fujita T., Kishimoto S., Sudo K., Kanamaru T., Brem H., Folkman J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature. 1990;348:555–557. doi: 10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- 216.Kusaka M., Sudo K., Fujita T., Marui S., Itoh F., Ingber D., Folkman J. Potent anti-angiogenic action of AGM-1470: comparison to the fumagillin parent. Biochem. Bioph. Res. Co. 1991;174:1070–1076. doi: 10.1016/0006-291X(91)91529-L. [DOI] [PubMed] [Google Scholar]
- 217.Yamaoka M., Yamamoto T., Ikeyama S., Sudo K., Fujita T. Angiogenesis inhibitor TNP-470 (AGM-1470) potently inhibits the tumor growth of hormone-independent human breast and prostate carcinoma cell lines. Cancer Res. 1993;51:5233–5236. [PubMed] [Google Scholar]
- 218.Kruger E.A., Figg W.D. TNP-470: An angiogenesis inhibitor in clinical development for cancer. Expert Opin. Inv. Drug. 2000;9:1383–1396. doi: 10.1517/13543784.9.6.1383. [DOI] [PubMed] [Google Scholar]
- 219.Bhargava P., Marshall J.L., Rizvi N., Dahut W., Yoe J., Figuera M., Phipps K., Ong V.S., Kato A., Hawkins M.J. A Phase I and pharmacokinetic study of TNP-470 administered weekly to patients with advanced cancer. Clin. Cancer Res. 1999;5:1989–1995. [PubMed] [Google Scholar]
- 220.Vansteelandt M., Blanchet E., Egorov M., Petit F., Toupet L., Bondon A., Monteau F., le Bizec B., Thomas O.P., Pouchus Y.F., et al. Ligerin, an antiproliferative chlorinated sesquiterpenoid from a marine-derived Penicillium strain. J. Nat. Prod. 2013;76:297–301. doi: 10.1021/np3007364. [DOI] [PubMed] [Google Scholar]
- 221.Fang S.-M., Cui C.-B., Li C.-W., Wu C.-J., Zhang Z.-J., Li L., Huang X.-J., Ye W.-C. Purpurogemutantin and purpurogemutantidin, new drimenyl cyclohexenone derivatives produced by a mutant obtained by diethyl sulfate mutagenesis of a marine-derived Penicillium purpurogenum G59. Mar. Drugs. 2012;10:1266–1287. doi: 10.3390/md10061266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sassa T., Ishizaki A., Nukina M., Ikeda M., Sugiyama T. Isolation and identification of new antifungal macrophorins E, F and G as malonyl meroterpenes from Botryosphaeria berengeriana. Biosci. Biotechnol. Biochem. 1998;62:2260–2262. doi: 10.1271/bbb.62.2260. [DOI] [PubMed] [Google Scholar]
- 223.Cabedo N., López-Gresa M.P., Primo J., Ciavatta M.L., González-Mas M.C. Isolation and structural elucidation of eight new related analogues of the mycotoxin (−)-botryodiplodin from Penicillium coalescens. J. Agr. Food Chem. 2007;55:6977–6983. doi: 10.1021/jf071568v. [DOI] [PubMed] [Google Scholar]
- 224.Fuska J., Proksa B., Uhrín D. The antibiotic PSX-1 produced by Penicillium stipitatum is identical with botryodiplodin. Folia Microbiol. 1988;33:238–240. doi: 10.1007/BF02925912. [DOI] [PubMed] [Google Scholar]
- 225.Fuska J., Kuhr I., Nemec P., Fuskova A. Antitumor antibiotics produced by Penicillium stipitatum THOM. J. Antibiot. 1973;27:123–127. doi: 10.7164/antibiotics.27.123. [DOI] [PubMed] [Google Scholar]
- 226.Omura S., Tomoda H., Kimura K., Zhen D.-Z., Kumagai H., Igarashi K., Imamura N., Takahashi Y., Tanaka Y., Iwai Y. Atpenins, new antifungal antibiotics produced by Penicillium sp. J. Antibiot. 1988;41:1769–1773. doi: 10.7164/antibiotics.41.1769. [DOI] [PubMed] [Google Scholar]
- 227.Kawada M., Momose I., Someno T., Tsujiuchi G., Ikeda D. New atpenins, NBRI23477 A and B, inhibit the growth of human prostate cancer cells. J. Antibiot. 2009;62:243–246. doi: 10.1038/ja.2009.20. [DOI] [PubMed] [Google Scholar]
- 228.Boysen M., Skouboe P., Frisvad J., Rossenl L. Reclassification of the Penicillium roqueforti group into three species on the basis of molecular genetic and biochemical profiles. Microbiology. 1996;142:541–549. doi: 10.1099/13500872-142-3-541. [DOI] [PubMed] [Google Scholar]
- 229.Li X.-J., Zhang Q., Zhang A.-L., Gao J.-M. Metabolites from Aspergillus fumigatus, an endophytic fungus associated with Melia azedarach, and their antifungal, antifeedant, and toxic activities. J. Agr. Food Chem. 2012;60:3424–3431. doi: 10.1021/jf300146n. [DOI] [PubMed] [Google Scholar]
- 230.Ma G., Khan S.I., Jacob M.R., Babu L., Li Z., Pasco D.S., Walker L.A., Khan I.A., Tekwani B.L. Antimicrobial and antileishmanial activities of hypocrellins A and B. Antimicrob. Agents Ch. 2004;48:4450–4452. doi: 10.1128/AAC.48.11.4450-4452.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tsukamoto S., Kato H., Samizo M., Nojiri Y., Onuki H., Hirota H., Ohta T. Notoamides F-K, prenylated indole alkaloids isolated from a marine-derived Aspergillus sp. J. Nat. Prod. 2008;71:2064–2067. doi: 10.1021/np800471y. [DOI] [PubMed] [Google Scholar]
- 232.Nakagawa M., Hirota A., Sakai H. Terrecyclic acid A, a new antibiotic from Aspergillus terreus. I. Taxonomy, production, and chemical and biological properties. J. Antibiot. 1982;35:778–782. doi: 10.7164/antibiotics.35.778. [DOI] [PubMed] [Google Scholar]
- 233.Sun H.-F., Li X.-M., Meng L., Cui C.-M., Gao S.-S., Li C.-S., Huang C.-G., Wang B.-G. Asperolides A–C, tetranorlabdane diterpenoids from the marine alga-derived endophytic fungus Aspergillus wentii EN-48. J. Nat. Prod. 2012;75:148–152. doi: 10.1021/np2006742. [DOI] [PubMed] [Google Scholar]