Abstract
A new enynyl-benzenoid, antrocamphin O (1,4,7-dimethoxy-5-methyl-6-(3′-methylbut-3-en-1-ynyl)benzo[d][1,3]dioxide), and the known benzenoids antrocamphin A and 7-dimethoxy-5-methyl-1,3-benzodioxole, were isolated from the fruiting bodies of Antrodia camphorata (Taiwanofungus camphoratus). The structure of antrocamphin O was unambiguously assigned by the analysis of spectral data (including 1D and 2D NMR, high-resolution MS, IR, and UV) and total synthesis. Compound 1 was prepared through the Sonogashira reaction of 5-iodo-4,7-dimethoxy-6-methylbenzene and 2-methylbut-1-en-3-yne as the key step. The benzenoids were tested for cytotoxicity against the HT29, HTC15, DLD-1, and COLO 205 colon cancer cell lines, andactivities are reported herein.
Keywords: Antrodia camphorata, enynyl-benzenoid, antrocamphin O, synthesis, cytotoxic activity
1. Introduction
Antrodia camphorata (Taiwanofungus camphoratus), also known as “Niu-chang-chih,” belongs to the familyPolyporaceae in the Basidiomycetes. Designated an endangered species in Taiwan, the fungus is a rare and unique mushroom used in folk medicine and as a health food. To date, over 200 constituent compounds, consisting of small molecules (terpenoids, benzenoids, lignans, benzoquinone derivatives, and succinic and maleic derivatives) and macromolecules (polysaccharides, nucleic acids, and proteins), have been reported [1,2,3]. Preparations from the fruiting bodies have been used for the prevention or treatment of numerous maladies, including liver diseases, food and drug intoxication, diarrhea, abdominal pain, hypertension, itchy skin, and tumorigenic diseases [4]. Previous research has shown the anti-cancer and anti-inflammatory effects of the triterpenoids derived from A. camphorate [3,5]. The major chemical constituents, the enynyl-benzenoids, are the key components responsible for anti-inflammatory activity [6,7]. Because the growth rate of A.camphorata in both the wild and under cultivation is very slow, the fruiting bodies are rare and expensive. Therefore, synthesis of the active compounds is very important. In this paper, we isolated and elucidated the structure of a new active compound, the enynyl-benzenoid antrocamphin O (1), and the known benzenoids, antrocamphin A (2) [8] and 4,7-dimethoxy-5-methyl-1,3-benzodioxole (3) [9], from the fruiting bodies of A. camphorata. We also describe the total synthesis of 1. The benzenoids were tested for cytotoxicity against the HT29, HTC15, DLD-1, and COLO 205 colon cancer cell lines, and their activities are reported herein.
2. Results and Discussion
2.1. Isolation and Identification
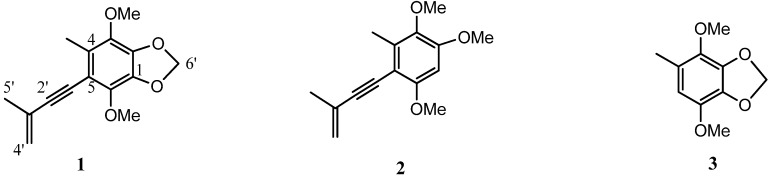
The EtOH extract of the fruiting bodies of A. camphorata (48 g, dried) was subjected to liquid-liquid partitioning with EtOAc, BuOH, and H2O. The bioactive EtOAc-soluble fraction was chromatographed over silica gel, followed by HPLC separation using refractive index detection. This procedure afforded three compounds 1−3. The structural elucidation of compound 1 was carried out using spectroscopic analysis and 2D NMR techniques, including 1H-1H COSY, heteronuclear multiple quantum correlation (HMQC), and heteronuclear multiple bond correlation (HMBC) experiments. Compound 1 was obtained as a colorless amorphous powder. The molecular formula was established as C15H16O4 by high-resolution electron spray ionization MS (HRESIMS, m/z 261.1123 [M+H]+, calcd for C15H17O4, 261.1127). The UV spectrum of 1 displayed absorption maxima at 258 and 288 nm, and the IR spectrum exhibited characteristic absorption peaks for alkynes (2183 cm−1) and alkenes (1605 cm−1). The 1H-NMR (CDCl3) data for 1 (Table 1) showed terminal methylene protons at δ 5.35 (1H, br s, H-4'a) and δ 5.24 (1H, br s, H-4′b), two methoxy protons at δ 3.85 (3H, H-3) and 3.96 (3H, H-6), and two methyl singlets at δ 2.25 (3H, s, 4-Me) and δ 1.98 (3H, br s, H-5'). The 13C-NMR data for 1 (Table 1) indicated 15 carbon resonance signals, in agreement with the HRESIMS data, including olefinic peaks at δ 121.0 (C-4′) and δ 127.2 (C-3′), two methoxy groups at δ 60.0 (3-OMe) and δ 60.3 (6-OMe), two methyl groups at δ 13.8 (4-Me) and δ 23.6 (C-5′), an sp3 methylene at δ 101.4, and other eight quaternary carbons (83.5, 97.5, 109.9, 127.8, 136.3, 137.2, 139.5, and 139.8). The structure of 1 was assigned by 2D NMR data (COSY, HSQC, and HMBC). A 1H-1H COSY correlation for H-5′/H-4′ and HMBC cross-peaks of H-5′ to C-2′, C-3′, and C-4′ suggested the presence of an enynyl moiety. From 3-OCH3 (δ 3.85) to C-3, 4-CH3 (δ 2.25) to C-3/C-4/C-5, and 6-OCH3 (δ 3.96) to C-6 by HMBC experiment, the chemical structure of 1 was identified to be as shown in Figure 1. This is the first report from natural sources of this compound, which was given the trivial name antrocamphin O.
Table 1.
NMR Spectroscopic Data for 1 in CDCl3 (500 MHz).
| Position | Antrocamphin O (1) | ||
|---|---|---|---|
| δC | δH (J in Hz) | HMBC | |
| 1 | 136.3 | ||
| 2 | 139.5 | ||
| 3 | 137.2 | ||
| 4 | 127.8 | ||
| 5 | 109.9 | ||
| 6 | 139.8 | ||
| 1′ | 83.5 | ||
| 2′ | 97.5 | ||
| 3′ | 127.2 | ||
| 4′ | 121.0 | 5.24 (br s), 5.35 (br s) | 2′, 3′, 5′ |
| 5′ | 23.6 | 1.98 (br s) | 2′,3′, 4′ |
| 6′ | 101.4 | 5.91 (s) | 1, 2 |
| 3-OMe | 60.0 | 3.85 (s) | 3 |
| 6-OMe | 60.3 | 3.96 (s) | 6 |
| 4-Me | 13.8 | 2.25 (s) | 3, 4, 5 |
Figure 1.
Structures of 1–3 from the fruiting bodies of A. camphorate.
2.2. Synthesis and Structural Characterization of Antrocamphin O (1)
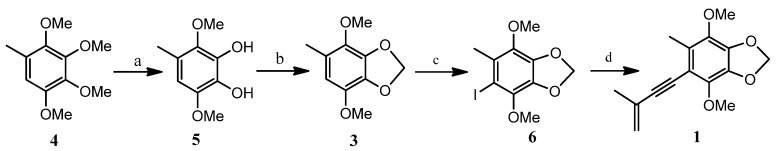
To establish the proposed structure of 1 unambiguously, a sample of reference material was needed, and this prompted us to prepare the authentic compound. The reaction sequence outlined in Scheme 1 was followed. Treatment of the readily available 2,3,4,5-tetramethoxytoluene (4) with AlCl3 followed by demethylation afforded 5 and its isomers [10]. Purification of the isomeric mixture by column chromatography gave 5 in an overall yield of 34%. The NMR data analysis (1H, 13C-NMR, and nOe) revealed the exact structure of 5. After protection with bromochloromethane in the presence Cs2CO3 at 110 °C [11], the 1,3-benzodioxole 3 was obtained in 70% yield. Iodination of 3 with N-iodosuccinimide [12] produced 5-iodo-4,7-dimethoxy-6-methylbenzo[d][1,3]dioxole 6. The Sonogashira reaction [13] was utilized to couple iodide 6 with 2-methyl-1-buten-3-yne, using the catalysts Pd(PPh3)4 and CuI. This key reaction led to the desired compound, antrocamphin O (1).
Scheme 1.
The synthetic route to compound 1.
Reagents and conditions: a, AlCl3, 40 °C, 34%; b, CH2ClBr, Cs2CO3, 110 °C, 70%; c, N-iodosuccinimide, 37 °C, 88%; d, Pd(Ph3)4, CuI, Et3N/THF, 90 °C, 16%.
2.3. Cytotoxicity Assay
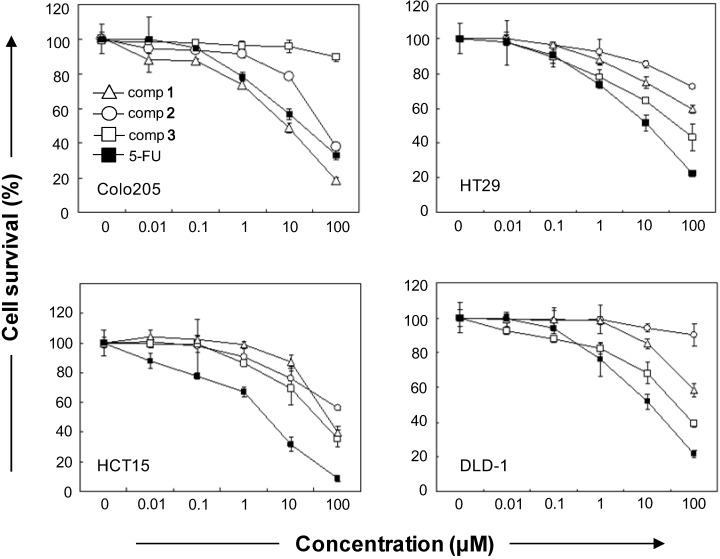
The cytotoxicity evaluation of compounds 1–3 against four human cancerous cell lines (HT29, HTC15, DLD-1, and COLO 205) showed that 1–3 were moderately or less active than 5-fluorouracil (5-FU), except against the COLO 205 cell line (Figure 2). Antrocamphin O (1) was a potent inhibitor of COLO 205 colon cancer, with higher activity than the reference compound 5-FU (Table 2).
Figure 2.
Effects of AC1-derived compounds on cell survival inhibition. Colon cancer cells were either treated with compound 1–3, or 5-FU for 48 h. MTT test was used to exam the relative cell number. 1–3, and 5-FU concentration-dependently inhibited the cell viability in 4 clones of colon cancer cells. Values represent the means ± S.E.M. (n = 3). Significance was accepted at p < 0.05.
Table 2.
The IC50 cytotoxicity of compounds 1–3 and 5-FU toward different colon cancer cells.
| Compound | IC50 (µM) | |||
|---|---|---|---|---|
| HT29 | HTC15 | DLD-1 | Colo205 | |
| 1 | >100 | 70 | >100 | 9.3 |
| 2 | >100 | >100 | >100 | 43.4 |
| 3 | 14 | 39 | 40 | >100 |
| 5-FU | 11.2 | 3.1 | 9.5 | 17 |
3. Experimental
3.1. General Methods
IR spectra were recorded on a JASCO 4100 FT-IR spectrometer. NMR data were recorded on a Bruker DRX-500 SB 500 MHz instrument (Bruker, Rheinstetten, Germany) with CDCl3 as solvent and TMS as internal standard. ESIMS and HRESIMS data were acquired using an ABI API 4000Q Trap and ABI XL Q-TOF mass spectrometer (Applied Biosystems, Foster City, CA, USA), respectively. Semi-preparative HPLC was performed on a Hitachi L-7110 HPLC with a refractive index detector (Thermo Separation Products, Sunnyvale, CA, USA). A Phenomenex Luna silica column (5 μm, 10 × 250 mm, 3 mL·min−1 flow rate) was used for normal-phase separations. Silica gel (Merck, Geduran® Si 60 0.063–0.2 mm) was used for column chromatography. All solvents were either ACS or HPLC grade and were obtained from JT Baker (Phillipsburg, NJ, USA). 5-FU was purchased from Sigma-Aldrich (St. Louis, MO, USA).
3.2. Plant Material
The fruiting bodies of Antrodia camphorata were collected in Taoyuan, Taiwan, in the summer of 2009, and kindly provided to our laboratory by the Cosmos Biotech. Company (Longtan, Taiwan). The sample was authenticated by Tien-Wang Chiu (general manager of Cosmos Biotech. Company), and a voucher specimen (AC2009-1) was deposited at the School of Pharmacy, Taipei Medical University, Taipei, Taiwan.
3.3. Extraction and Isolation
A. camphorata (48 g) were cut into small pieces and extracted with 4 L ethanol at room temperature for seven days. After removal of the solvent under reduced pressure, the ethanolic extract residue was separated by silica gel column chromatography with n-hexane, ethyl acetate, and methanol. Fractions of 100 mL each were collected and monitored by TLC using n-hexane/ethyl acetate (4:1 and 3:1, v/v) or n-hexane/acetone (3:1, v/v) and observed at 254 nm. Fractions with similar components were pooled into 25 portions. Silica gel column chromatography of portions 1–4 (1.5 g) comprised stepwise elution with n-hexane/ethyl acetate (99:1, 98:2, and 95:5, v/v), and finally, with pure ethyl acetate. Subfractions 12–18 were separated by normal-phase HPLC on a semipreparative normal phase column (Phenomenex Luna silica, 5 μm, 10 × 250 mm) at a flow rate of 3 mL·min−1, with a mixture of n-hexane/ethyl acetate (49:1, v/v), to afford compounds 3 (320 mg, tR = 23.9 min) and 1 (12 mg, tR = 26.5 min). Compound 2 (39 mg, tR = 11.9 min) was obtained from subfractions 26–28 by HPLC with an isocratic mixture of n-hexane/acetone (30:1, v/v), and then, with n-hexane/ethyl acetate/acetone (60:1:1, v/v/v).
Antrocamphin O (1): colorless amorphous powder; UV (MeOH) λmax(log ε) 213 (4.52), 258 (3.61), 288 (3.96) nm; IR (neat) νmax 2942, 2183, 1605, 1469, 1449, 1276, 1051 cm−1; 1H- and 13C-NMR data, see Table 1; ESIMS (positive) m/z 261 [M+H]+; HRESIMS (positive) m/z 261.1123 [M+H]+ (calcd. for C15H16O4 261.1127).
Antrocamphin A (2): ESIMS (positive) m/z 247 [M+H]+; 1H and 13C-NMR data, see reference [8].
4,7-Dimethoxy-5-methyl-1,3-benzodioxole (3): ESIMS (positive) m/z 197 [M+H]+; 1H and 13C-NMR data, see reference [9].
3.4. Synthesis
Synthesis of 3,4-dihydroxy-2,5-dimethoxytoluene (5). To a solution of 2,3,4,5-tetramethoxytoluene 4 (0.94 g, 4.43 mmol) in anhydrous dichloromethane (12 mL) was added AlCl3 (1.30 g, 9.77 mmol) under nitrogen. The mixture was heated to 40 °C for 16 h. After cooling to room temperature, the mixture was poured into iced water (60 mL) and extracted with dichloromethane. The organic layer was washed with saturated NaCl solution, and was then purified by column chromatography (eluent: ethyl acetate/hexane = 1:4 to 1:1) to afford pure 5 as a yellowish oil (0.28 g, yield: 34%). TLC Rf: 0.28 (EtOAc:hexane 1:1); 1H-NMR (CDCl3) δ 2.22 (s, 3H), 3.78 (s, 3H), 3.82 (s, 3H), 5.48 (br s, 1H), 5.66 (br s, 1H), 6.24 (s, 1H); 13C-NMR (CDCl3) δ 15.5, 56.2, 60.6, 104.0, 120.9, 131.5, 137.1, 140.3, 143.4.
Synthesis of 4,7-dimethoxy-5-methyl-1,3-benzodioxole (3). To a solution of 5 (240 mg, 1.35 mmol) dissolved in dimethyl sulfoxide (3 mL) were added bromochloromethane (227 mg, 1.76 mmol) and Cs2CO3 (442 mg, 1.35 mmol). The mixture was then heated to 110 °C under nitrogen for 16 h. The mixture was cooled and then diluted by the addition of water (15 mL). The mixture was extracted with isopropanol (15 mL), and the extract was washed with saturated NaCl solution. A dark brown raw product (250 mg) was obtained, which was purified by column chromatography (eluent: ethyl acetate/hexane = 1:5), affording 3 as a colorless liquid (85 mg, yield: 70%). TLC Rf: 0.52 (EtOAc:hexane 1:2); 1H-NMR (CDCl3) δ 2.17 (s, 3H), 3.84 (s, 3H), 3.88 (s, 3H), 5.93 (s, 2H), 6.30 (s, 1H); 13C-NMR (CDCl3) δ 15.8, 56.8, 59.8, 101.3, 108.8, 123.5, 134.6, 136.5, 138.6, 138.8; ESIMS (positive) m/z: 197 [M+H]+.
Synthesisof 5-iodo-4,7-dimethoxy-6-methyl-1,3-benzodioxole (6). To a solution of 3 (240 mg, 1.35 mmol) dissolved in bromochloromethane (2 mL) was added N-iodosuccinimide (217 mg, 0.97 mmol). The reaction was heated to 37 °C and allowed to proceed overnight. The mixture was cooled and then diluted with water (5 mL). The mixture was extracted with CH2Cl2 (6 mL), then washed with 10% Na2S2O3 solution (10 mL). The product, 6, was obtained as a light-orange oil (248 mg, yield: 88%). 1H-NMR (CDCl3) δ 2.34 (s, 3H, -CH3), 3.87 (s, 3H, -OCH3), 3.92 (s, 3H, -OCH3), 5.96 (s, 2H, -OCH2O-); 13C-NMR (CDCl3) δ 21.4, 60.1, 60.2, 87.9, 101.6, 127.7, 136.2, 137.3, 138.3, 139.6.
Synthesis of 4,7-dimethoxy-5-methyl-6-(3-methylbut-3-en-1-ynyl)benzo[d][1,3]dioxole (1). N,N-Dimethylacetamide (DMA, 1.0 mL) was placed in a 10 mL flask and heated to 60 °C for 20 min while purging with argon gas. To the flask was added 6 (230 mg, 0.71 mmol), copper iodide (130 mg, 0.71 mmol), Pd(PPh3)4 (130 mg, 0.07 mmol), and triethylamine (0.15 mL), and the reaction was heated to 80 °C. A solution of 2-methylbut-1-en-3-yne (192 mg, 2.5 mmol) was dissolved in DMA (0.5 mL) and added to the reaction mixture. The mixture was heated at 90 °C for 16 h. The mixture was cooled, and then, it was filtered after adding 15 ml of EtOAc. The filtrate was washed with H2O (6 mL). A brown product was obtained after concentration in vacuo. The raw product was purified by column chromatography (eluent: ethyl acetate/hexane = 1:6) to afford 1 as a colorless liquid (30 mg, yield: 16%). 1H-NMR (CDCl3) δ 2.01 (s, 3H, -CH3), 2.27 (s, 3H, Ar-CH3), 3.87 (s, 3H, -OCH3), 3.98 (s, 3H, -OCH3), 5.26 (s, 1H), 5.37 (s, 1H), 5.93 (s, 2H, -OCH2O-); 13C NMR (CDCl3) δ 13.8, 23.5, 60.0, 60.3, 83.5, 97.5, 101.4, 109.9, 121.0, 127.2, 127.8, 136.3, 137.2, 139.5, 139.8.
3.5. In Vitro Cytotoxicity Assay
3.5.1. Cell Culture
Human colon cancer cells (HCT15, HT29, DLD-1, and COLO 205) were cultured in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum and antibiotics (100 U/mL penicillin and 100 mg/mL streptomycin) at 37 °C in a CO2 incubator. Cells grew to 90% confluence, were harvested with trypsin/EDTA, and sub-cultured in a new tissue culture flask after removing trypsin and EDTA. The cancer cells used in this study had passage numbers of less than ten.
3.5.2. Cytotoxicity Assay
Colon cancer cells (1 × 105 cells) were seeded into 96-well plates and cultured overnight, allowing cell attachment. This was followed by treatment with compounds 1, 2, and 3 for 48 h at various concentrations (from 0.001 to 100 μM), as indicated, and 5-FU as a positive control. At the end of the experiment, the cytotoxicities of the compounds were examined by the MTT ((3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) test. MTT solution (400 mg/mL) was added into the cultured wells and incubated for 1 h. The medium was then aspirated, and the dark blue crystal product was extracted with DMSO. Colorimetric changes were read on a microtiter plate reader with a 570 nm filter and a reference wavelength of 430 nm.
3.5.3. Statistical Analysis
All data were expressed as the mean value ± S.E.M. Comparisons were subjected to the Student’s two-tailed t test. Significance was accepted at p < 0.05.
4. Conclusions
In conclusion, we have achieved the first total synthesis of the rare new natural product, antrocamphin O (1,), using the Sonogashira reaction as the key step. The intermediate 3, from the fruiting bodies of A. camphorata, was also successfully synthesized during the process (Scheme 1). Whereas enynyl-benzenoids are considered the key components responsible for the anti-inflammatory activities demonstrated by these fungi, this paper offers the first report of the inhibition of COLO 205 cancer cells by such compounds too [13].
Acknowledgments
This work was supported by the National Science Council Grants NSC-97-2320-B-038-018-MY3 (WBZ) and NSC 98-2320-B-038-015-MY3 (CKL). We are grateful to S.-H. Wang for the NMR data acquisition in the Instrumentation Center of Taipei Medical University.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Geethangili M., Tzeng Y.M. Review of Pharmacological Effects of Antrodia camphorata and Its Bioactive Compounds. Evid. Based Complement. Alternat. Med. 2011:212641. doi: 10.1093/ecam/nep108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang H.C., Liaw C.C., Yang H.L., Hseu Y.C., Kuo H.T., Tsai Y.C., Chien S.C., Amagaya S., Chen Y.C., Kuo Y.H. Lanostane triterpenoids and sterols from Antrodia camphorata. Phytochemistry. 2012;84:177–183. doi: 10.1016/j.phytochem.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Shi L.S., Chao C.H., Shen D.Y., Chan H.H., Chen C.H., Liao Y.R., Wu S.J., Leu Y.L., Shen Y.C., Kuo Y.H., et al. Biologically active constituents from the fruiting body of Taiwanofungus camphorates. Bioorg. Med. Chem. 2011;19:677–683. doi: 10.1016/j.bmc.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai Z.T., Liaw S.L. The Use and the Effect of Ganoderma. Sang-Yun Press; Taichung, Taiwan: 1982. p. 116. [Google Scholar]
- 5.Lee Y.P., Tsai W.C., Ko C.J., Rao Y.K., Yang C.R., Chen D.R., Yang M.H., Yang C.C., Tzeng Y.M. Anticancer effects of eleven triterpenoids derived fromantrodia camphorate. Anticancer Res. 2012;32:2727–2734. [PubMed] [Google Scholar]
- 6.Liao Y.R., Kuo P.C., Huang S.C., Liang J.W., Wu T.S. An efficient total synthesis of Benzocamphorin H and its anti-inflammatory activity. Tetrahedron Lett. 2012;53:6202–6204. doi: 10.1016/j.tetlet.2012.08.138. [DOI] [Google Scholar]
- 7.Lee C.L., Huang C.H., Wang H.C., Chuang D.W., Wu M.J., Wang S.Y., Hwang T.L., Wu C.C., Chen Y.L., Chang F.R., et al. First total synthesis of antrocamphin A and its analogs as anti-inflammatory and anti-platelet aggregation agents. Org. Biomol. Chem. 2011;9:70–73. doi: 10.1039/c0ob00616e. [DOI] [PubMed] [Google Scholar]
- 8.Chen J.J., Lin W.J., Liao C.H., Shieh P.C. Anti-inflammatory benzenoids from Antrodia camphorate. J. Nat. Prod. 2007;70:989–992. doi: 10.1021/np070045e. [DOI] [PubMed] [Google Scholar]
- 9.Chiang H.C., Wu D.P., Cherng I.W., Ueng C.H. A sesquiterpene lactone, phenyl and biphenyl compounds from Antrodia cinnamomea. Phytochemistry. 1995;39:613–616. doi: 10.1016/0031-9422(95)00025-3. [DOI] [Google Scholar]
- 10.Piggott M.J., Wege D. The synthesis of ventilone A. Aus. J. Chem. 2000;53:749–754. doi: 10.1071/CH00057. [DOI] [Google Scholar]
- 11.Rudyanto M., Kobayashi K., Honda T. Synthetic studies on natural isocoumarins and isocarbostyril derivatives having an alkyl substituent at the 3-position: Total synthesis of scoparines A and B, and ruprechstyril. Heterocycles. 2009;79:753–764. doi: 10.3987/COM-08-S(D)40. [DOI] [Google Scholar]
- 12.Smith L.R., Mahoney N., Molyneux R.J. Synthesis and structure-phytotoxicity relationships of acetylenic phenols and chromene metabolites, and their analogues, from the grapevine pathogen Eutypa lata. J. Nat. Prod. 2003;66:169–176. doi: 10.1021/np020415t. [DOI] [PubMed] [Google Scholar]
- 13.Lee C.K., Chen P.Y., Lo J.M., Chung W.B., Chiu T.W., Yu C.C. Compound extracted from Antrodia camphorate, medical composition containing the same and their application in producing the producing the medical formulations for preventing and treating rectal camcer. CN 103012358 A 20130403. 2013;(Faming Zhuanli Shenqing)