Abstract
Chalcone derivatives have attracted increasing attention due to their numerous pharmacological activities. Changes in their structures have displayed high degree of diversity that has proven to result in a broad spectrum of biological activities. The present study highlights the synthesis of some halogen substituted chalcones 3(a–i) containing the 5-chlorothiophene moiety, their X-ray crystal structures and the evaluation of possible biological activities such as antibacterial, antifungal and reducing power abilities. The results indicate the tested compounds show a varied range of inhibition values against all the tested microbial strains. Compound 3c with a p-fluoro substituent on the phenyl ring exhibits elevated antimicrobial activity, whereas the compounds 3e and 3f displayed the least antimicrobial activities. The compounds 3d, 3e, 3f and 3i showed good ferric and cupric reducing abilities, and the compounds 3b and 3c showed the weakest reducing power in the series.
Keywords: heteroaryl, antimicrobial, reducing, substituent, overlay
1. Introduction
Chalcones are important constituents of many natural products. They are abundant in edible plants where they are considered to be the precursors of flavonoids and isoflavonoids. There is growing interest in the pharmacological potential of chalcones which constitute an important group of natural and synthetic products that have been screened for a wide range of pharmacological activities such as antibacterial [1,2], antitumor [3,4,5], anti-inflammatory [6,7,8,9], antifungal [10] and antioxidant properties [11,12,13,14,15]. Chalcones are also well known intermediates for synthesizing various heterocyclic compounds. Several methods have been reported for the synthesis of chalcones, among them aldol condensation and Claisen-Schmidt condensation between aryl ketones and aromatic aldehydes in acidic or basic media still occupy prominent positions. Chalcones are characterized by possessing an enone moiety between two aromatic rings. Elemental sulfur has been well known to act as an antifungal agent for a long time. Several naturally occurring antifungal agents are also known to contain sulfur [16]. Many researchers have reported chalcones containing sulfur either as a part of heteroaryl ring (thiophene) or as a side chain (thiomethyl group). Tomar et al. have reported the synthesis and antimicrobial activity of chalcones containing the 2,5-dichlorothiophene moiety [17]. Seema et al. have reported the synthesis and biological evaluation of α,β-unsaturated ketones as potential antifungal agents [18]. Tran et al. have reported the synthesis and antibacterial activity of some heterocyclic chalcone analogues alone and in combination with antibiotics [19]. Ranganathan et al. have reported the synthesis and antimicrobial studies of some of the 5-chloro-2-acetylthiophene chalcones [20]. In continuation of our research work on the X-ray crystal structure studies of 5-chlorothiophene chalcone analogues [21], in which an effort has been made to diversify the pharmacological activities by exploring the structural diversity of conventional chalcones, a series of halogen(s)-substituted chalcone analogues containing electron-rich thiophene heterocycles were synthesized. This was done with the expectation that the derivatives with halogen functionalities at different positions on the phenyl ring would be even more potent as antimicrobial agents than the starting compounds. Herein we report the synthesis of some novel halogen substituted chalcone analogues with 5-chlorothiophene moiety using the conventional base-catalyzed Claisen-Schmidt condensation, their crystal structures and the evaluation of their antimicrobial, ferric ion and cupric ion reducing power abilities.
2. Results and Discussion
Chalcones, a versatile class of natural and synthetic compounds, have attracted the interest of researchers for their wide range of biological activities. Chalcones containing the thiophene moiety are supposed to further enhance these activities. The best known method for the synthesis of chalcones is the Claisen-Schmidt condensation between acetophenone and benzaldehyde in basic media. New chalcones have been designed and synthesized by the reaction of 2-acetyl-5-chlorothiophene with halogen-substituted benzaldehydes using a catalytic amount of NaOH in methanol as shown in Scheme 1.
Scheme 1.
Synthesis of heterocyclic chalcone analogues.
2.1. X-Ray Crystal Structure Description for Compounds 3a–3i
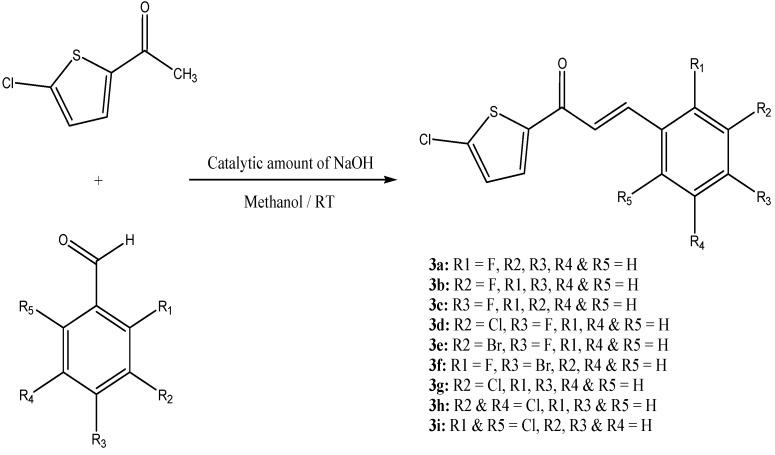
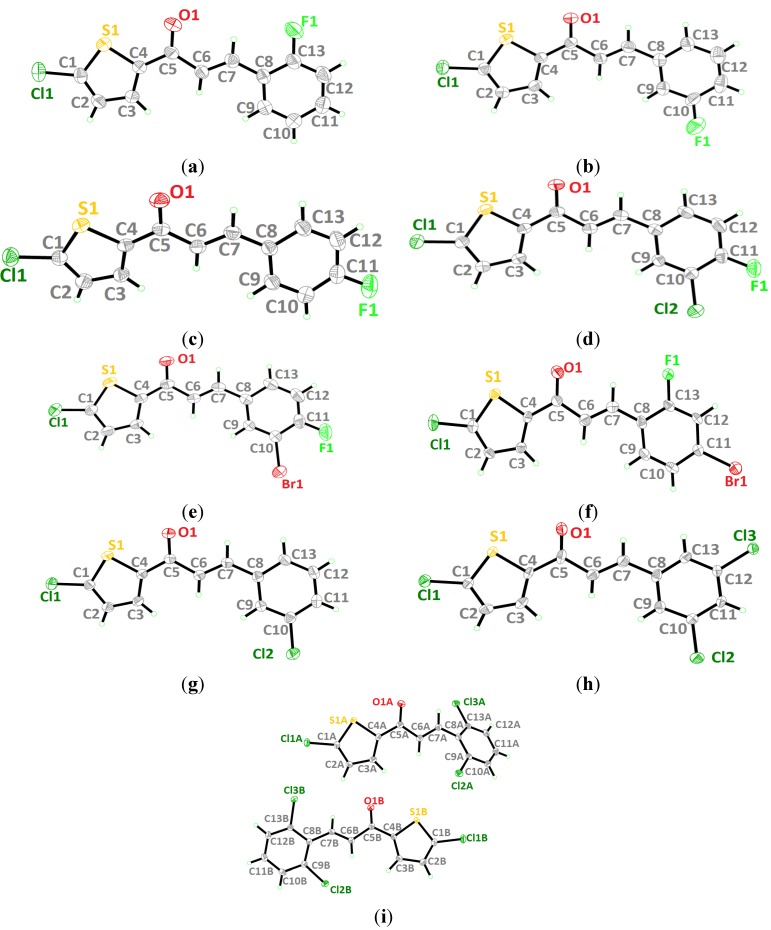
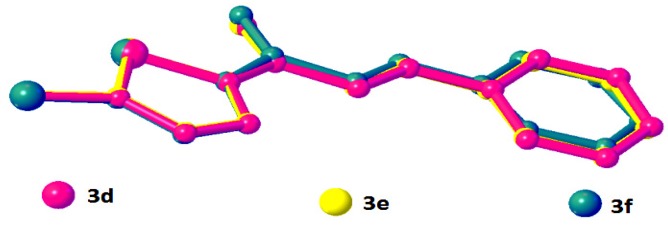
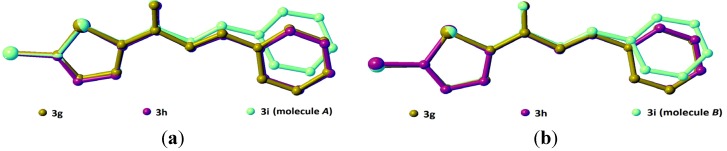
The crystal structures of 3(a–i) are as shown in Figure 1. Table 1 lists the crystallographic data for all the nine compounds. Four out of nine compounds were crystallized in orthorhombic system, where 3a in space group Pca21, 3c, 3d and 3e in space group Pbca. The remaining compounds (3b, 3f, 3g, 3h and 3i) crystallized in monoclinic system with space group P21/c. The overall conformation of the molecules (3a–i) can be described by the dihedral angles formed between the thiophene (S1/C1—C4) and the benzene (C8—C13) rings. The respective parameters are listed in Table 2. As seen from the table, the conformation of the molecules is almost planar except for compound 3i and it can be related to the presence of substituent in the -ortho position of the benzene ring. Compound 3i contains two crystallographically independent molecules (molecules A & B). The thiophene and benzene rings in compounds 3i are relatively far from planarity as the values of dihedral angles between them are 45.68 (11)° in molecule A and 24.00 (11)° in molecule B. The overlay of both molecules over all atoms is shown in Figure 2, with the r.m.s value of 0.339 Å.
Figure 1.
(a)–(i). Ortep diagram of compounds 3a to 3i (3h and 3i are drawn at 50% ellipsoids for non-hydrogen atoms and the remaining compounds are drawn at 30% ellipsoids for non-hydrogen atoms).
Table 1.
Crystal data and parameters for structure refinement of 3a to 3i.
| Compound | 3a | 3b | 3c | 3d | 3e | 3f | 3g | 3h | 3i |
|---|---|---|---|---|---|---|---|---|---|
| CCDC deposition number | 939875 | 939876 | 939877 | 942741 | 942742 | 948856 | 944072 | 946445 | 948855 |
| Molecular formula | C13H8ClFOS | C13H8ClFOS | C13H8ClFOS | C13H7Cl2FOS | C14H7BrClFOS | C13H7BrClFOS | C13H8Cl2OS | C13H7Cl3OS | C13H7Cl3OS |
| Molecular weight | 266.70 | 266.70 | 266.70 | 301.15 | 345.61 | 345.61 | 283.15 | 317.60 | 317.60 |
| Crystal system | Orthorhombic | Monoclinic | Orthorhombic | Orthorhombic | Orthorhombic | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | Pca21 | P21/c | Pbca | Pbca | Pbca | P21/c | P21/c | P21/c | P21/c |
| a (Å) | 27.963 (9) | 14.282 (6) | 7.9809 (8) | 7.4870 (9) | 7.476 (2) | 3.8704 (7) | 14.5437 (10) | 14.9864(10) | 30.3418 (16) |
| b (Å) | 3.9233 (15) | 7.698 (4) | 11.0207 (11) | 11.6186 (14) | 11.724 (3) | 31.953 (6) | 7.5227 (6) | 3.8085 (3) | 3.7985 (2) |
| c (Å) | 11.146 (4) | 11.376 (5) | 27.518 (3) | 29.239 (4) | 30.009 (8) | 10.273 (2) | 11.7604 (9) | 24.6297 (13) | 23.4311 (12) |
| α (°) | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 |
| β (°) | 90 | 104.430 | 90 | 90 | 90 | 95.243 (3) | 104.721 (1) | 115.264 (3) | 109.297 (1) |
| γ (°) | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 |
| V (Å3) | 1222.8 (7) | 1211.3 (9) | 2420.3 (4) | 2543.4 (5) | 2630.4 (13) | 1265.2 (4) | 1244.44 (16) | 1271.30 (15) | 2548.8 (2) |
| Z | 4 | 4 | 8 | 8 | 8 | 4 | 4 | 4 | 8 |
| Dcalc (g cm−3) | 1.449 | 1.462 | 1.464 | 1.573 | 1.745 | 1.814 | 1.511 | 1.659 | 1.655 |
| Crystal dimensions (mm) | 0.93 × 0.18 × 0.05 | 0.25 × 0.25 × 0.05 | 0.79 × 0.37 × 0.04 | 0.75 × 0.52 × 0.10 | 0.58 × 0.28 × 0.09 | 0.89 × 0.12 × 0.09 | 0.50 × 0.23 × 0.08 | 0.51 × 0.10 × 0.05 | 0.60 × 0.10 × 0.10 |
| Colour | Colourless | Colourless | Colourless | Colourless | Colourless | Colourless | Colourless | Colourless | Colourless |
| μ(mm−1) | 0.47 | 0.48 | 0.48 | 0.67 | 3.48 | 3.62 | 0.67 | 0.87 | 0.86 |
| Radiation λ (Å) | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Tmin/Tmax | 0.668/0.975 | 0.889/0.975 | 0.704/0.980 | 0.633/0.935 | 0.237/0.752 | 0.141/0.744 | 0.733/0.946 | 0.665/0.955 | 0.624/0.922 |
| Reflections measured | 8618 | 9071 | 24679 | 16026 | 16116 | 2855 | 11650 | 11375 | 20108 |
| Ranges/indices (h, k, l) | −37, 39; −5, 5; −15, 15 | −16, 16; −9, 9; −13, 13 | −11, 11; −15, 15; −38, 37 | −10, 10; −14, 16; −41, 37 | −10, 9; −12, 16; −42, 31 | −5, 5; −41, 41; −1, 13 | −18, 18; −9, 9; −15, 15 | −19, 19; −4, 4; −31, 31 | −39, 39; −4, 4; −30, 30 |
| θ limit (°) | 2.3–22.4 | 3.0–19.5 | 3.2–25.3 | 2.8–26.4 | 2.7–25.3 | 2.8–29.8 | 2.9–29.0 | 2.8–30.1 | 2.9–29.9 |
| Unique reflections | 3368 | 2126 | 3540 | 3738 | 3816 | 2855 | 2829 | 2894 | 5762 |
| Observed reflections (I > 2σ(I)) | 1899 | 1133 | 2016 | 2486 | 1935 | 2624 | 2312 | 2607 | 4784 |
| Parameters | 154 | 154 | 154 | 163 | 163 | 164 | 154 | 163 | 325 |
| Goodness of fit on F2 | 0.99 | 1.00 | 1.03 | 1.03 | 1.01 | 1.09 | 1.04 | 1.05 | 1.03 |
| R1, wR2 [I ≥ 2σ(I)] | 0.049, 0.136 | 0.059, 0.191 | 0.047, 0.135 | 0.041, 0.135 | 0.044, 0.136 | 0.051, 0.127 | 0.030, 0.101 | 0.046, 0.120 | 0.033, 0.091 |
Table 2.
The dihedral angles formed between the chlorothiophene and benzene rings.
| Compound | Dihedral angle between two rings (°) |
|---|---|
| 3a | 5.5 (2) |
| 3b | 15.1 (3) |
| 3c | 14.98 (13) |
| 3d | 9.45 (9) |
| 3e | 6.58 (16) |
| 3f | 1.2 (2) |
| 3g | 16.40 (8) |
| 3h | 2.07 (15) |
| 3i |
Molecule
A-45.68 (11) Molecule B-24.00 (11) |
Figure 2.
Overlay of molecules A and B in compound 3i, calculated using the chlorothiophene moiety.
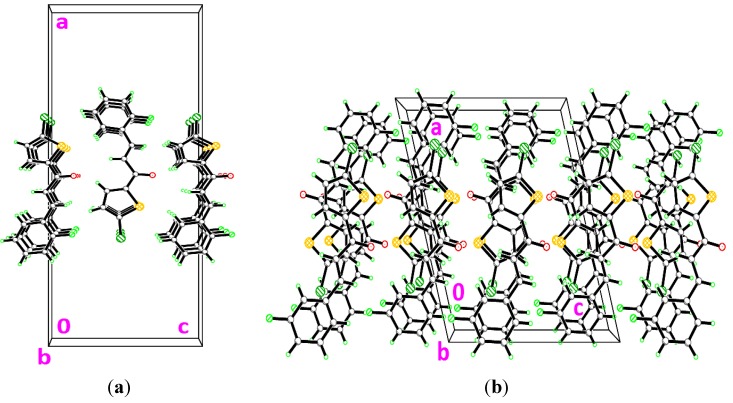
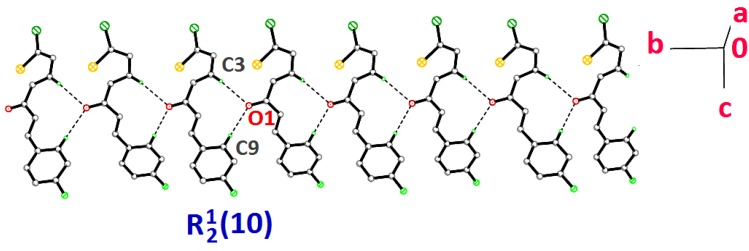
Compounds 3a, 3b and 3c differ structurally from each other by having the fluoro-substituent at -ortho, -meta and -para positions of the benzene rings, respectively. The planarity of the molecules are indicated by the dihedral angles as shown in Table 2, ranging from 5.5 (2) to 15.1 (3)°. Figure 3 shows the overlaid molecules over all the non-H atoms, calculated using the chlorothiophene moiety with the r.m.s values of 0.103 Å for 3a/3b and 3a/3c, and 0.051 Å for 3b/3c. There is no classical hydrogen bond found in compounds 3a and 3b. The formation of short intra-molecular S···O contacts of 2.926(3) and 2.933(4) Å which is 0.39 Å shorter than the sum of van der Waals radii of the sulfur and oxygen atoms, in both 3a and 3b, could be due to the charge delocalization into the carbonyl group from the thiophene ring. This helps to stabilize the molecular structure. In the crystal structure (Figure 4a), molecules in 3a are stacked along the b-axis, with alternative stacks forming an inverted head-to-tail arrangement. In 3b, the molecules stacked on each other in an inverse fashion along the b-axis (Figure 4b), whereas in compound 3c, the molecules are linked into chains along b-axis as illustrated in Figure 5, via intermolecular C3—H3A···O1 and C9—H9A···O1 hydrogen bonds (Table 3), forming 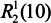 ring motifs [22].
ring motifs [22].
Figure 3.
Overlay of all non-H atoms in compounds 3a, 3b and 3c, calculated using the chlorothiophene moiety.
Figure 4.
Crystal structures of (a) 3a and (b) 3b. The molecules are stacked along the b-axis.
Figure 5.
Crystal structure of 3c forming chains along the b-axis. Dashed lines indicate the intermolecular hydrogen bonds.
Table 3.
Hydrogen bond geometries for the compounds 3c, 3f, 3h and 3i.
| D–H···A | d(D–H) (Å) | d(H···A)(Å) | d(D···A)(Å) | Angle(D–H···A)(°) |
|---|---|---|---|---|
| 3c | ||||
| C3—H3A···O1 i | 0.93 | 2.56 | 3.438(3) | 157 |
| C9—H9A···O1 i | 0.93 | 2.54 | 3.474(3) | 179 |
| 3f | ||||
| C2—H2A···F1 ii | 0.95 | 2.40 | 3.295 (5) | 156 |
| C3—H3A···O1 ii | 0.95 | 2.48 | 3.381 (5) | 158 |
| 3h | ||||
| C7—H7A···O1 iii | 0.93 | 2.49 | 3.337(4) | 151 |
| C13—H13A···O1 iii | 0.93 | 2.48 | 3.300(4) | 148 |
| 3i | ||||
| C2B—H2BA···O1A iv | 0.95 | 2.50 | 3.209(3) | 131 |
Symmetry code: (i) −x+1/2, y−1/2, z; (ii) x−1, −y+1/2, z−1/2; (iii) −x+1, −y+2, −z; (iv) x, −y+5/2, z+1/2.
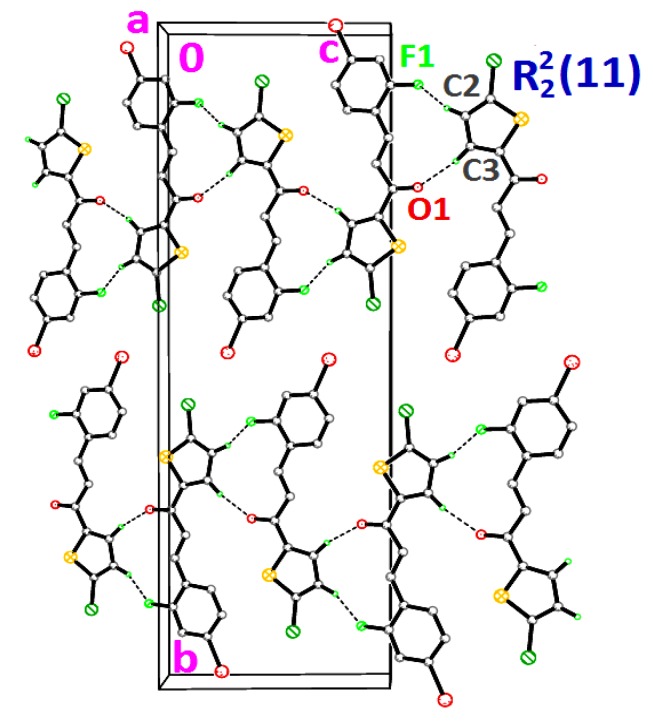
Compounds 3d, 3e and 3f differ from 3a–3c by the additional halogen substituent at the benzene rings. The chlorothiophene and the benzene rings of 3d–3f are approximately planar, with dihedral angles ranging from 1.2 (2) to 9.45 (9)°. The conformations of these three molecules are very analogous as indicated by the overlay, excluding H atoms and the halogen substituents on the benzene rings (Figure 6) with r.m.s values of 0.033 Å for 3d/3e, 0.111 Å for 3d/3e and 0.088 Å for 3e/3f. Compounds 3d and 3e were observed to possess no significant hydrogen bonds. They are stabilized by the formation of short intramolecular S···O contacts of 2.933 (4) and 2.929 (3) Å, respectively, which is 0.39 Å shorter than the sum of van der Waals radii of the sulfur and oxygen atoms. In the crystal structure, molecules in 3d and 3e stacked along the a-axis, as depicted in Figure 7a,b, respectively. In the crystal packing of 3f (Figure 8), intermolecular C2—H2A···F1 and C3—H3A···O1 hydrogen bonds (Table 3) link the molecules into chains along the c-axis, generating 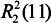 ring motifs [22].
ring motifs [22].
Figure 6.
Overlay of compounds 3d, 3e and 3f excluding halogen substituents at benzene rings and H-atoms.
Figure 7.
Crystal structures of (a) 3d and (b) 3e, showing the stacking along the a-axis.
Figure 8.
Crystal structure of 3f, showing the chains along the c-axis. Dashed lines indicate the intermolecular hydrogen bonds.
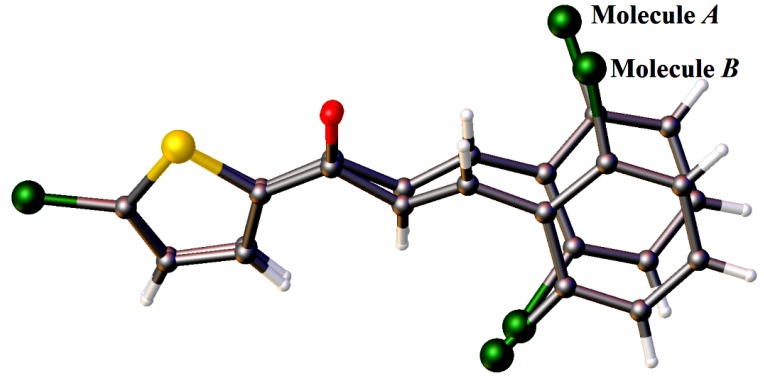
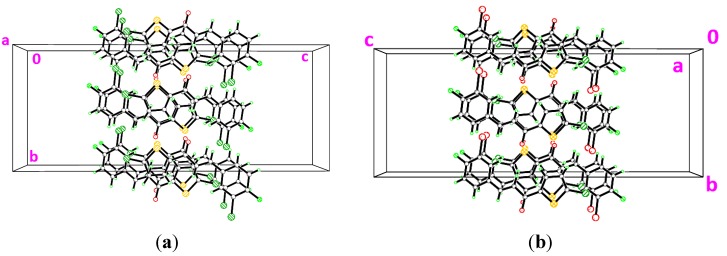
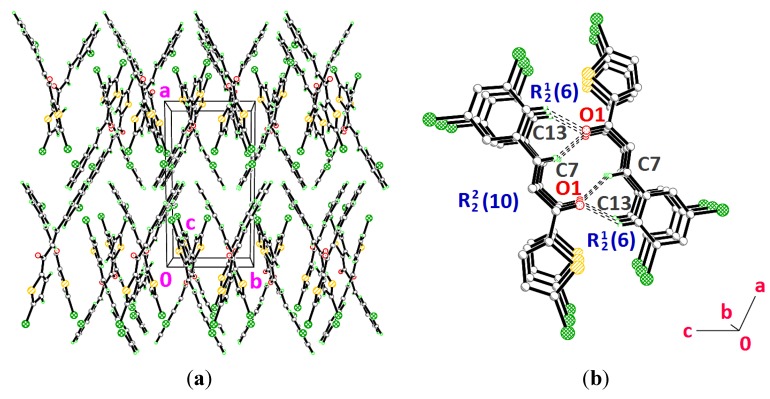
Compounds 3g, 3h and 3i present one or two chloro-substituents at the benzene rings. Compounds 3g and 3h are approximately planar as indicated in Table 2. Figure 9a,b show the overlays of all non-H atoms of 3g/3h, 3g/3i and 3h/3i, calculated using the chlorothiophene moiety excluding their chloro-substituents. The r.m.s values given by the overlays of 3g/3h, 3g/3i (molecule A) and 3h/3i (molecule A) are 0.167, 0.323 and 0.447 Å, respectively. These values are compared with another overlay (Figure 9b), taking only molecule B into the calculation and the r.m.s values given by the overlays of 3g/3i (molecule B) and 3h/3i (molecule B) are 0.205 and 0.248 Å, respectively. In general, due to intermolecular hydrogen bonding of molecules A and B in compound 3i, they are very much deviated from the molecules of compounds 3g and 3h. Crystal packing diagram of 3g with no significant hydrogen bonds is depicted in Figure 10a. However, the molecules in 3g are stabilized by the short intra-molecular S···O contacts of 2.9348(14) Å, which is 0.39 Å shorter than the sum of van der Waals radii of the sulfur and oxygen atoms. The molecules in compound 3h (Figure 10b) are linked to form dimers with 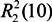 ring motifs [22] via intermolecular C7—H7A···O1 hydrogen bonds (Table 3). These sets of dimers are further connected by intermolecular C13—H13A···O1 hydrogen bonds (Table 3) into another two
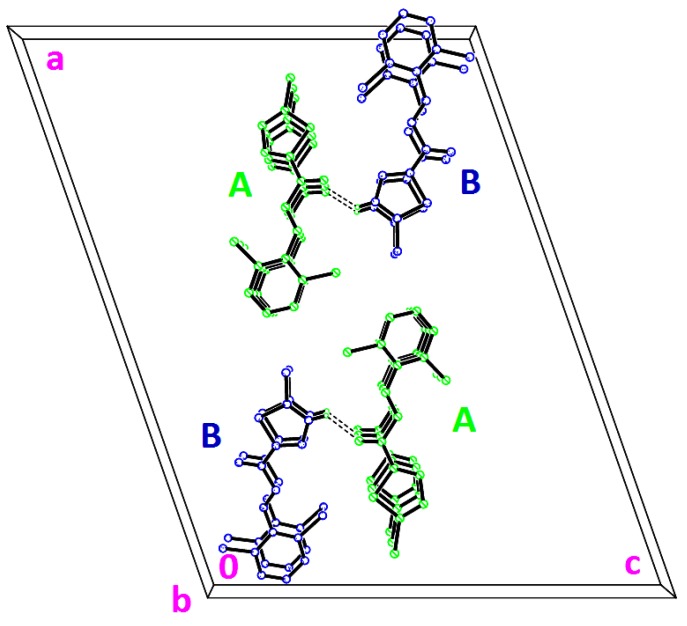
ring motifs [22] via intermolecular C7—H7A···O1 hydrogen bonds (Table 3). These sets of dimers are further connected by intermolecular C13—H13A···O1 hydrogen bonds (Table 3) into another two 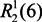 ring motifs. The molecules stacked along the b-axis. In the crystal structure of 3i (Figure 11), molecules A highlighted in green are joined with the adjacent molecules B highlighted in blue through intermolecular C2B—H2BA···O1A hydrogen bonds (Table 3).
ring motifs. The molecules stacked along the b-axis. In the crystal structure of 3i (Figure 11), molecules A highlighted in green are joined with the adjacent molecules B highlighted in blue through intermolecular C2B—H2BA···O1A hydrogen bonds (Table 3).
Figure 9.
Overlay of compounds 3g, 3h and (a) 3i with only molecule A and (b) 3i with only molecule B where their chloro-substituents were excluded.
Figure 10.
Crystal structures of (a) 3g viewed along the c-axis (b) 3h viewed along the b-axis. Dashed lines indicate the intermolecular hydrogen bonds.
Figure 11.
Crystal packing of 3i, viewed along the b-axis. Dashed lines indicate the intermolecular hydrogen bonds.
2.2. In Vitro Antimicrobial Activity
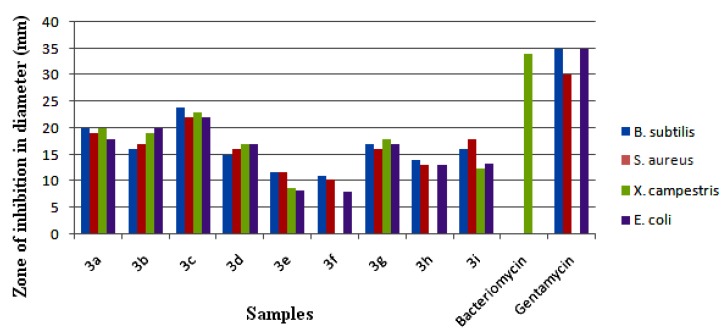
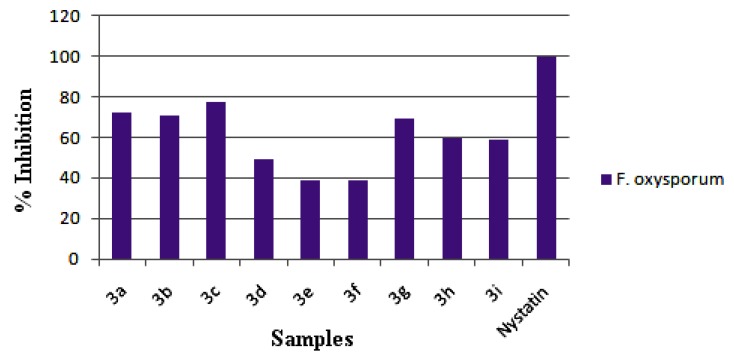
The synthesized compounds 3(a–i) were evaluated for their in vitro antibacterial activity and the results are compared with bacteriomycin and gentamycin used as standard drugs (Figure 12). All the tested compounds displayed varied antibacterial activity against four pathogenic bacterial strains: Bacillus subtilis MTCC 121, Staphylococcus aureus MTCC, Xanthomonas campestris MTCC 7908 and Escherichia coli MTCC 7410. Among the series 3(a–i), compound 3c showed an elevated antibacterial activity against the tested bacterial strains and the compounds 3a, 3b and 3g showed good antibacterial activity against all the tested organisms. The compounds 3d, 3h and 3i displayed moderate activity, while the compounds 3e and 3f showed the least inhibitory activity in the series. Further, all the synthesized compounds 3(a–i) were evaluated for their in vitro antifungal activity against Fusarium oxysporum. The results are shown and compared with the standard drug nystatin in Figure 13. Compounds 3a, 3b and 3c showed good antifungal activity against F. oxysporum when compared to other compounds in the series. Compounds 3d, 3g, 3h and 3i were found to be moderately active against the tested fungal strain. The compounds 3e and 3f were less active.
Figure 12.
In vitro antibacterial activities of 3(a–i).
Figure 13.
In vitro antifungal activity of 3(a–i).
A close survey of the antimicrobial results indicates the compounds under test show a varied range of inhibition values against all the tested bacterial and fungal strains. The elevated activity of the compound 3c may be due to the p-fluoro substituent on the phenyl ring. Moreover the good activity of compounds 3a, 3b and 3g can be attributed to the o-fluoro, m-fluoro and m-chloro substitution, respectively. The least activity of compounds 3e and 3f against the tested fungal strain may be due to the presence of bromo substituents. An increase in the number of halogen substituents on the phenyl ring results in a decrease in the antimicrobial activity of the tested compounds. The activities of the reported compounds against the tested bacterial strains were in the order 3c > 3a > 3b > 3g > 3h > 3i > 3d > 3e > 3f.
2.3. Reducing Power Ability
The synthesized compounds were further tested for their reducing power ability. Ferric ion reducing antioxidant power (FRAP) assay and cupric ion reducing antioxidant capacity (CUPRAC) were measured using butylated hydroxytoluene as the standard.
2.3.1. Ferric Reducing Antioxidant Power (FRAP) Assay
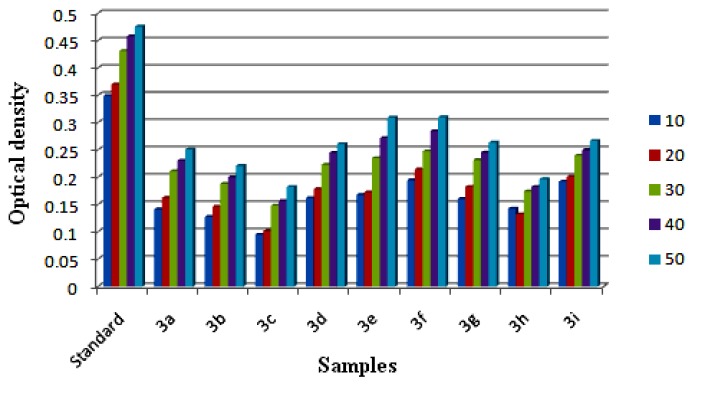
In practice, the antioxidant activity of a substance is directly correlated to its reducing ability. Standard assays like FRAP provide a reliable method to verify the antioxidant ability of a substance. Substances having reduction potential react with potassium ferricyanide forming potassium ferrocyanide. The formed potassium ferrocyanide further reacts with FeCl3 to form an intense Prussian blue complex which has a maximum absorbance at 700 nm. The complex formed is directly proportional to the reducing capacity of the test sample. An increase in absorbance is equal to the reducing power of the sample. Results are depicted in Figure 14 and from the analysis it is clear that the compounds 3d, 3e, 3f and 3i showed good ferric reducing ability, whereas 3a, 3g and 3h were moderate, and the compounds 3b and 3c showed the least reducing ability among the series.
Figure 14.
Ferric ion reducing power ability of samples 3(a–i) at different concentration (10–50 μg/mL) measured at 700 nm. Values are expressed as absorbance; high absorbance indicates high reducing power.
2.3.2. Cupric Ion Reducing Antioxidant Capacity (CUPRAC) Assay
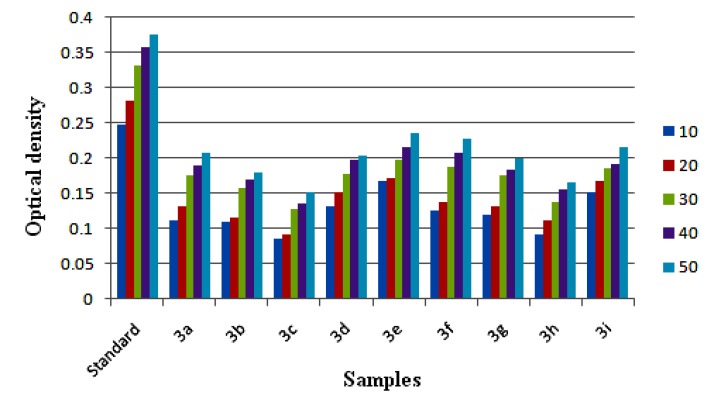
In this assay a sample under evaluation effectively reduces Cu2+ to Cu+, changing the characteristic ion absorption. The reduced Cu+ ion combines with the chromogenic reagent neocuproine forming a stable 2:1 complex which has a maximum absorption at 450 nm. This method operates at pH 7. Results, shown in Figure 15, indicate that the majority of these test compounds have good reducing ability. These compounds displayed 40% less reducing power compared to the standard. Compounds 3d, 3e, 3f and 3i showed good cupric reducing ability, whereas compounds 3a, 3g and 3h were moderate, and the compounds 3b and 3c showed the least reducing ability among the series.
Figure 15.
Cupric ion reducing power ability of samples 3(a–i) at different concentration (10–50 μg/mL) measured at 700 nm. Values are expressed as absorbance; high absorbance indicates high reducing power.
3. Experimental
3.1. Materials and Method
Melting points were determined on a Stuart Scientific (UK) apparatus. The purity of each compound was confirmed by thin layer chromatography using Merck silica gel 60 F254-coated aluminium plates. The mass spectra were recorded on a Jeol JMS-D 300 mass spectrometer operating at 70 eV. Elemental analyses (CHN) were carried out on a Perkin Elmer Series II, 2400 analyzer. X-ray analysis was done using a Bruker SMART Apex II or Apex II Duo CCDC diffractometer. The data were processed with SAINT and absorption correction was done using SADABS [23]. The structures were solved by direct method using the program SHELXTL [24], and were refined by full-matrix least squares technique on F2 using anisotropic displacement parameters. The non-hydrogen atoms were refined anisotropically. In these compounds, all the H atoms were calculated geometrically with isotropic displacement parameters set to 1.2 (1.5 for methyl groups) times the equivalent isotropic U values of the parent carbon atoms. The overlay structures were drawn using Olex2 software [25]. Crystallographic data for compounds 3(a–i) have been deposited at the Cambridge Crystallographic Data Centre with CCDC No: 939875, 939876, 939877, 942741, 942742, 948856, 944072, 946445 and 948855, respectively. Copies of the data can be obtained free of charge on application to the CCDC, 12 Union Road, Cambridge CB2 IEZ, UK. Fax: +44-(0)1223-336033 or E-Mail: deposit@ccdc.cam.ac.uk.
3.2. General Procedure for the Synthesis of Chalcones 3(a–i)
A mixture of 2-acetyl-5-chlorothiophene (0.01 mol) and a halogen-substituted benzaldehyde (0.01 mol) was dissolved in methanol (20 mL). Catalytic amount of NaOH was added to the solution drop-wise with vigorous stirring. The reaction mixture was stirred for about 5–6 h at room temperature. The resultant crude products were filtered, washed successively with distilled water and recrystallized from ethanol to get corresponding chalcones. Crystals suitable for X-ray diffraction studies were obtained by the slow evaporation technique using a suitable solvent.
(2E)-1-(5-Chlorothiophen-2-yl)-3-(2-fluorophenyl)prop-2-en-1-one (3a): Solvent for growing crystals: Mixture of acetone and ethanol (1:1 v/v); Yield: 64%; M.P. 112–114 °C; LCMS: m/z = 267 (M++1); Elemental analysis: Calculated for C13H8ClFOS: C, 58.54%; H, 3.02%; Found: C, 58.51%; H, 3.11%. CCDC No.: 939875.
(2E)-1-(5-Chlorothiophen-2-yl)-3-(3-fluorophenyl)prop-2-en-1-one (3b): Solvent for growing crystals: Mixture of acetone and ethanol (1:1 v/v); Yield: 61%; M.P. 118–120 °C; LCMS: m/z = 267 (M++1); Elemental analysis: Calculated for C13H8ClFOS: C, 58.54%; H, 3.02%; Found: C, 58.49%; H, 3.17%. CCDC No.: 939876.
(2E)-1-(5-Chlorothiophen-2-yl)-3-(4-fluorophenyl)prop-2-en-1-one (3c): Solvent for growing crystals: Mixture of acetone and ethanol (1:1 v/v); Yield: 68%; M.P. 128–130 °C; LCMS: m/z = 267 (M++1); Elemental analysis: Calculated for C13H8ClFOS: C, 58.54%; H, 3.02%; Found: C, 58.51%; H, 3.13%. CCDC No.: 939877.
(2E)-1-(5-Chlorothiophen-2-yl)-3-(3-chloro-4-fluorophenyl)prop-2-en-1-one (3d): Solvent for growing crystals: Mixture of acetone, ethanol and acetonitrile (1:1:1 v/v); Yield: 63%; M.P. 167–169 °C; LCMS: m/z = 302 (M++1); Elemental analysis: Calculated for C13H7Cl2FOS: C, 51.85%; H, 2.34%; Found: C, 51.79%; H, 2.48%. CCDC No.: 942741.
(2E)-1-(5-Chlorothiophen-2-yl)-3-(3-bromo-4-fluorophenyl)prop-2-en-1-one (3e): Solvent for growing crystals: Mixture of acetone, ethanol and acetonitrile (1:1:1 v/v); Yield: 60%; M.P. 144–146 °C; LCMS: m/z = 346 (M++1); Elemental analysis: Calculated for C12H7BrClFOS: C, 45.18%; H, 2.04%; Found: C, 45.13%; H, 2.11%. CCDC No.: 942742.
(2E)-1-(5-Chlorothiophen-2-yl)-3-(4-bromo-2-fluorophenyl)prop-2-en-1-one (3f): Solvent for growing crystals: N,N-dimethylformamide; Yield: 62%; M.P. 133–134 °C; LCMS: m/z = 346 (M++1); Elemental analysis: Calculated for C12H7BrClFOS: C, 45.18%; H, 2.04%; Found: C, 45.15%; H, 2.09%. CCDC No.: 948856.
(2E)-3-(3-Chlorophenyl)-1-(5-chlorothiophen-2-yl)prop-2-en-1-one (3g): Solvent for growing crystals: Mixture of acetone and ethanol (1:1 v/v); Yield: 70%; M.P. 120–122 °C; LCMS: m/z = 284 (M++1); Elemental analysis: Calculated for C13H8Cl2OS: C, 55.14%; H, 2.85%; Found: C, 55.11%; H, 2.92%. CCDC No.: 944072.
(2E)-1-(5-Chlorothiophen-2-yl)-3-(3,5-dichlorophenyl)prop-2-en-1-one (3h): Solvent for growing crystals: Mixture of acetone, ethanol and ethyl acetate (1:1:1 v/v); Yield: 67%; M.P. 129–131 °C; LCMS: m/z = 318 (M++1); Elemental analysis: Calculated for C13H7Cl3OS: C, 49.16%; H, 2.22%; Found: C, 49.13%; H, 2.29%. CCDC No.: 946445.
(2E)-1-(5-Chlorothiophen-2-yl)-3-(2,6-dichlorophenyl)prop-2-en-1-one (3i): Solvent for growing crystals: N,N-dimethylformamide; Yield: 64%; M.P. 170–172 °C; LCMS: m/z = 318 (M++1); Elemental analysis: Calculated for C13H7Cl3OS: C, 49.16%; H, 2.22%; Found: C, 49.12%; H, 2.27%. CCDC No.: 948855.
3.3. In Vitro Antimicrobial Activities
3.3.1. Antibacterial Activity
All the synthesized compounds were screened for their antibacterial activity against Gram-positive bacteria such as Bacillus subtilis MTCC 121 and Staphylococcus aureus MTCC 7443 and Gram-negative bacteria such as Xanthomonas campestris MTCC 7908 and Escherichia coli MTCC 7410 in DMF by disc diffusion method on nutrient agar medium [26]. Each Petri plate was filled with sterile medium (nutrient agar medium, 15 mL) uniformly and smeared with cultures of Gram-positive bacteria to which 50 µL (1 mg/mL: i.e., 50 µg/disc) of the test compounds was added. The treatments also include 50 µL of DMF as negative control and bacteriomycin and gentamycin as positive control for comparisons. Three replicates were maintained for each treatment. The plates were incubated at 37 ± 2 °C for 24 h and the zone of inhibition was determined.
3.3.2. Antifungal Activity
Antifungal activity of the synthesized compounds was screened against Fusarium oxysporum MTCC 2480 in DMF by the poisoned food technique [27]. Potato dextrose agar (PDA) medium was prepared; to each Petri plate PDA (15 mL) was added and allowed to solidify. A 5 mm disc of 7 day-old culture of the test fungi was placed at the center of the Petri plate and incubated at 26 °C for 7 days. After the incubation period, the percentage inhibition was measured and three replicates were maintained for each treatment. Nystatin was used as the standard. Synthesized compounds were tested at the dosage of 500 µL of the novel compounds/Petri plate, where the concentration was 0.1 mg/mL by poisoned food technique.
3.3.3. Ferric Ion Reducing Antioxidant Power (FRAP) Assay
The synthesized compounds were screened for ferric reducing power antioxidant ability by the method reported by Oyaizu [28]. The method is based on the reduction of ferric (Fe3+) to ferrous (Fe2+), which is accomplished in presence of antioxidants. Samples of 3(a–i) having a concentration of 10–50 μg/mL were mixed with an equal volume of 0.2 M phosphate buffer (pH 6.6) and 1% potassium ferricyanide and the mixture were incubated for 20 min at 50 °C. The mixture was acidified with 2.5 mL of 10% trichloroacetic acid and then centrifuged at 3,000 rpm for about 15 min. The upper supernatant liquid was diluted with distilled water and 0.1% ferric chloride was added. The absorbance was measured at 700 nm. The increase in absorbance is directly proportional to the reducing ability of the compound. The control was prepared as above without the sample.
3.3.4. Cupric Ion Reducing Antioxidant Capacity (CUPRAC) Assay
The synthesized compounds were also evaluated for their cupric ion reducing power by a reported method [29]. CUPRAC is a widely applicable method for evaluating the antioxidant properties of a substance. A mixture of CuCl2 (1 mL, 0.01 M) solution, ethanolic neocuproine (1 mL, 0.0075 M) and ammonium acetate (1 mL, 1 M) were dissolved and 1 mL of test samples (10–50 μg/mL) was added along with 0.1 mL of distilled water. The mixture was incubated for about 30 min and the absorbance was measured at 450 nm against the blank solution. Control is prepared as above without the sample.
3.3.5. Statistical Analysis
All the assay measurement were performed in triplicate (n = 3) and are expressed as mean of the three determinations.
4. Conclusions
Halogen-substituted chalcone derivatives 3(a–i) bearing the 5-chlorothiophene moiety were synthesized in good yield and the structures of these compounds were determined by single crystal X-ray diffraction analysis. The crystal structure diversities of these compounds and various interactions responsible for their crystal stability are described. In addition, in vitro antimicrobial and reducing power ability of these compounds are evaluated. The reported compounds produced a varied range of inhibition results against the tested microbial strains, which is due to the presence of electron negative halogen(s) substituents at different positions on the phenyl ring. The antimicrobial activity of the tested compounds follows the order 3c > 3a > 3b > 3g > 3h > 3i > 3d > 3e > 3f. These compounds displayed about 40% less reducing ability for ferric and cupric ions when compared to the standards. The present study demonstrated the relationship of the halogen linkage on the aromatic ring to the biological activities exhibited by these compounds.
Acknowledgments
CSC thanks Universiti Sains Malaysia for a postdoctoral research fellowship. CKQ thanks USM for APEX DE2012 Grant (No. 1002/PFIZIK/910323). WSL thanks Malaysian Government for MyBrain15 (MyPhD) scholarship. The authors extend their appreciation to The Deanship of Scientific Research at King Saud University for the funding the work through the research group project No. RGP VPP-207.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/18/10/12707/s1.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Alcaraz L.E., Blanco S.E., Puig O.N., Tomas F., Ferretti F.H. Antibacterial activity of flavonoids against methicillin-resistant Staphylococcus aureus strains. J. Theor. Biol. 2000;205:231–240. doi: 10.1006/jtbi.2000.2062. [DOI] [PubMed] [Google Scholar]
- 2.Baviskar B.A., Baviskar B., Shiradkar M.R., Deokate U.A., Khadabadi S.S. Synthesis and antimicrobial activity of some novel benzimidazolyl chalcones. J. Chem. 2009;6:196–200. [Google Scholar]
- 3.Echeverria C., Santibañez J.F., Donoso-Tauda O., Escobar C.A., Ramirez-Tagle R. Structural antitumoral activity relationships of synthetic chalcones. Int. J. Mol. Sci. 2009;10:221–231. doi: 10.3390/ijms10010221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modzelewska A., Pettit C., Achanta G., Davidson N.E., Huang P., Khan S.R. Anticancer activities of novel chalcone and bis-chalcone derivatives. Bioorg. Med. Chem. 2006;14:3491–3495. doi: 10.1016/j.bmc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Vogel S., Heilmann J. Synthesis, cytotoxicity, and antioxidative activity of minor prenylated chalcones from Humulus lupulus. J. Nat. Prod. 2008;71:1237–1241. doi: 10.1021/np800188b. [DOI] [PubMed] [Google Scholar]
- 6.Babasaheb P.B., Sachin A.P., Rajesh N.G. Synthesis and biological evaluation of nitrogen containing chalcones as possible anti-inflammatory and antioxidant agents. Bioorg. Med. Chem.Lett. 2010;20:730–733. doi: 10.1016/j.bmcl.2009.11.068. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y.H., Kim J., Park H., Kim H.P. Anti-inflammatory activity of the synthetic chalcone derivatives: Inhibition of inducible nitric oxide synthase-catalyzed nitric oxide production from lipopolysaccharide-treated RAW 264.7 cells. Biol. Pharm Bull. 2007;30:1450–1455. doi: 10.1248/bpb.30.1450. [DOI] [PubMed] [Google Scholar]
- 8.Ballesteros J.F., Sanz M.J., Ubeda A., Miranda M.A., Iborra S., Paya M., Alcaraz M.J. Synthesis and pharmacological evaluation of 2'-hydroxychalcones and flavones as inhibitors of inflammatory mediators generation. J. Med. Chem. 1995;38:2794–2797. doi: 10.1021/jm00014a032. [DOI] [PubMed] [Google Scholar]
- 9.Vogel S., Barbic M., Jürgenliemk G., Heilmann J. Synthesis, cytotoxicity, anti-oxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur. J. Med. Chem. 2010;45:2206–2213. doi: 10.1016/j.ejmech.2010.01.060. [DOI] [PubMed] [Google Scholar]
- 10.Lopez S.N., Castelli M.V., Zacchino S.A., Domnguez J.N., Lobo G., Charris-Charris J., Cortes J.C.G., Ribas J.C., Devia C., Rodrguez A.M., et al. In vitro antifungal evaluation and structure-activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorg. Med. Chem. 2001;9:1999–2013. doi: 10.1016/S0968-0896(01)00116-X. [DOI] [PubMed] [Google Scholar]
- 11.Beom-Tae K., Kwang-Joong O., Jae-Chul C., Ki-Jun H. Synthesis of dihydroxylated chalcone derivatives with diverse substitution patterns and their radical scavenging ability toward DPPH free radicals. Bull. Korean Chem. Soc. 2008;29:1125–1130. doi: 10.5012/bkcs.2008.29.6.1125. [DOI] [Google Scholar]
- 12.Doan T.N., Tran T.-D. Synthesis, antioxidant and antimicrobial activities of a novel series of chalcones, pyrazolic chalcones, and allylic chalcones. Pharmacol. Pharm. 2011;2:282–288. doi: 10.4236/pp.2011.24036. [DOI] [Google Scholar]
- 13.Go M.L., Wu X., Liu X.L. Chalcones: An update on cytotoxic and chemoprotective properties. Curr. Med. Chem. 2005;12:481–499. doi: 10.2174/0929867053363153. [DOI] [PubMed] [Google Scholar]
- 14.Sivakumar P.M., Prabhakar P.K., Doble M. Synthesis, antioxidant evaluation, and quantitative structure-activity relationship studies of chalcones. Med. Chem. Res. 2011;20:482–492. doi: 10.1007/s00044-010-9342-1. [DOI] [Google Scholar]
- 15.Vogel S., Ohmayer S., Brunner G., Heilmann J. Natural and non-natural prenylated chalcones: Synthesis, cytotoxicity and anti-oxidative activity. Bioorg. Med. Chem. 2008;16:4286–4293. doi: 10.1016/j.bmc.2008.02.079. [DOI] [PubMed] [Google Scholar]
- 16.Lemar K.M., Turner M.P., Lloyd D. Garlic (Allium sativum) as an anti-Candida agent: A comparison of the efficacy of fresh garlic and freeze-dried extracts. J. Appl. Microbiol. 2002;93:398–405. doi: 10.1046/j.1365-2672.2002.01707.x. [DOI] [PubMed] [Google Scholar]
- 17.Tomar V., Bhattacharjee G., Kamaluddina K. Synthesis and antimicrobial evaluation of newchalcones containing piperazine or 2,5-dichlorothiophene moiety. Bioorg. Med. Chem. Lett. 2007;17:5321–5324. doi: 10.1016/j.bmcl.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Bag D., Ramar S., Degani M.S. Synthesis and biological evaluation of a, b-unsaturated ketone as potential antifungal agents. Med. Chem. Res. 2009;18:309–316. [Google Scholar]
- 19.Tran T.D., Nguyen T.T., Do T.H., Huynh T.N., Tran C.D., Thai K.M. Synthesis and antibacterial activity of some heterocyclic chalcone analogues alone and in combination with antibiotics. Molecules. 2012;17:6684–6696. doi: 10.3390/molecules17066684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranganathan K., Arulkumaran R., Kamalakkannan D., Sundararajan R., Sakthinathan S.P., Vijayakumar S., Suresh R., Vanangamudi G., Thirumurthy K., Mayavel P., et al. Silica-H2SO4 catalyzed environmentally benign crossed aldol condensation: Synthesis, spectral studies and biological activities of some 5-chloro-2-thienyl chalcones. Int. J. Pharm. Med. Biol. Sci. 2012;1:62–85. [Google Scholar]
- 21.Kumar C.S.C., Loh W.-S., Ooi C.W., Quah C.K., Fun H.-K. Sructural correlation of some heterocyclic chalcone analogues and evaluation of their antioxidant potential. Molecules. 2013;18:11996–12011. doi: 10.3390/molecules181011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernstein J., Davis R.E., Shimoni L., Chang N.L. Patterns in hydrogen bonding: Functionality and graph set analysis in crystals. Angew. Chem. Int. Edit. Engl. 1995;34:1555–1573. doi: 10.1002/anie.199515551. [DOI] [Google Scholar]
- 23.Bruker . Bruker AXS Inc.; Madison, WI, USA: 2009. APEX2, SAINT and SADABS. [Google Scholar]
- 24.Sheldrick G.M. A short history of SHELX. Acta Cryst. 2008;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 25.Dolomanov O.V., Bourhis L.J., Gildea R.J., Howard J.A.K., Puschmann H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009;42:339–341. doi: 10.1107/S0021889808042726. [DOI] [Google Scholar]
- 26.Bauer W.M., Kirby J.C., Sherris, Truck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- 27.Satish S., Mohana D.C., Raghavendra M.P., Raveesha K.A. Antifungal activity of some plant extracts against important seed borne pathogens of Aspergillus sp. J. Agric. Technol. 2007;3:109–119. [Google Scholar]
- 28.Oyaizu M. Studies on products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- 29.Apak R., Guclu K., Ozyurek M., Celik S.E. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta. 2008;160:413–419. doi: 10.1007/s00604-007-0777-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.