Abstract
Eleven new 2,6-di-tert-butyl-4-(5-aryl-1,3,4-oxadiazol-2-yl)phenols 5a–k were synthesized by reacting aryl hydrazides with 3,5-di-tert butyl 4-hydroxybenzoic acid in the presence of phosphorus oxychloride. The resulting compounds were characterized based on their IR, 1H-NMR, 13C-NMR, and HRMS data. 2,2-Diphenyl-1-picrylhydrazide (DPPH) and ferric reducing antioxidant power (FRAP) assays were used to test the antioxidant properties of the compounds. Compounds 5f and 5j exhibited significant free-radical scavenging ability in both assays.
Keywords: 2,6-di-tert-butylphenol; hindered phenol; antioxidant; 1,3,4-oxadiazole; FRAP; DPPH
1. Introduction
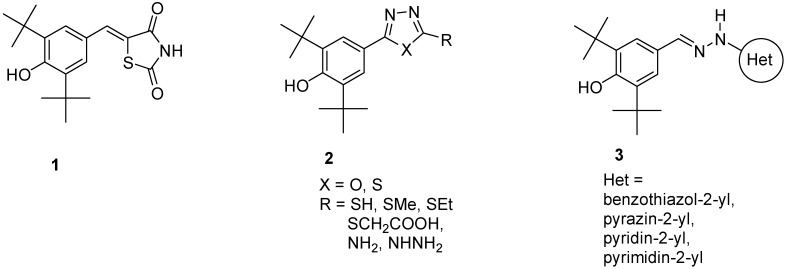
Phenolic antioxidants inhibit or prevent oxidative stress in biological systems. Free radicals are one of the main causes of many pathological conditions such as those that cause several degenerative [1] and chronic diseases [2]. Furthermore, numerous heterocyclic compounds containing di-tert-butyl phenol exhibit various types of biological activity in addition to their antioxidant ability [3,4]. Cyclo-oxygenase and 5-lipoxygenase [5,6] exhibit anti-inflammatory [3,7] and anticancer [8,9] activities. Many synthesized compounds contain long-chain resonance and exhibit high antioxidant activity, such as 1, 2, and 3 [10,11,12] (Figure 1).
Figure 1.
Di-tert-butyl-phenols with long-chain resonance as antioxidants.
Antioxidants donate protons to become a stable free radicals. This stability increases with the extent of delocalisation [13] and enhances antioxidant ability [14]. A number of 1,3,4-oxadiazole derivatives have exhibited various types of biological activity [15,16,17] and antioxidant ability [18,19].
This paper describes the synthesis of eleven 2,6-di-tert-butyl-4-(5-aryl-1,3,4-oxadiazole-2-yl)phenols 5a–k (Scheme 1) and evaluates their antioxidant activity. These new oxadiazoles are designed to be effective antioxidants owing to their long-chain resonance. This study also investigates the effects of different substituents on the phenyl ring C at position 6 of the 1,3,4-oxadiazole. This structure is likely to possess superior antioxidant activity compared to the 2,6-di-tert-butyl phenol given the enhanced stability of the free radical of the 1,3,4-oxadiazole as a result of resonance. The inductive and resonance effects could have a major role in enhancing the scavenging ability of the compound. The antioxidant ability of these compounds were measured by means of FRAP and DPPH assays.
Scheme 1.
Synthesis of 2,6-di-tert-butyl-4-(5-aryl-1,3,4-oxadiazol-2-yl)phenols.
2. Results and Discussion
Chemistry
Eleven aromatic acids were converted to their corresponding aryl hydrazides 4a–k by reacting the corresponding aromatic acids with thionyl chloride and then with hydrazine hydrate in dry benzene at 0 °C. The aryl hydrazides were then reacted with 3,5-di-tert butyl 4-hydroxybenzoic acid in the presence of POCl3 as dehydrating agent to obtain new 2,6-di-tert-butyl-4-(5-aryl-1,3,4-oxadiazol-2-yl)phenols, as demonstrated in Scheme 1. Table 1 shows the aryl groups with the corresponding yields and HREIM data.
Table 1.
Synthesized compounds, yields, molecular formulas (MFs) and HRMS data.
| No. | Compounds | Yield % | MF | HREIMS found | HREIMS calc. |
|---|---|---|---|---|---|
| 5a | 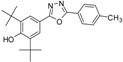 |
76.1 | C23H28N2O2 | 364.2147 | 364.2151 |
| 5b | 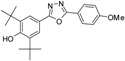 |
76 | C23H28N2O3 | 380.2095 | 380.2100 |
| 5c | 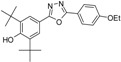 |
84.2 | C24H30N2O3 | 394.2249 | 394.2256 |
| 5d | 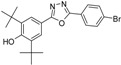 |
73.6 | C22H25BrN2O2 | 428.1093 | 428.1099 |
| 5e | 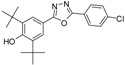 |
83.1 | C22H25ClN2O2 | 384.1597 | 384.1605 |
| 5f | 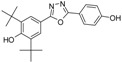 |
70 | C22H26N2O3 | 366.1938 | 366.1943 |
| 5g | 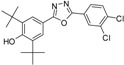 |
80.3 | C22H24Cl2N2O2 | 418.1219 | 418.1215 |
| 5h | 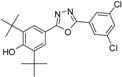 |
74.5 | C22H24Cl2N2O2 | 418.1210 | 418.1215 |
| 5i | 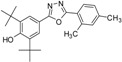 |
59.9 | C24H30N2O2 | 378.2301 | 378.2304 |
| 5j | 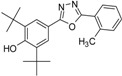 |
68.4 | C23H28N2O2 | 364.2144 | 364.2151 |
| 5k | 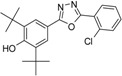 |
81 | C22H25ClN2O2 | 384.1600 | 384.1605 |
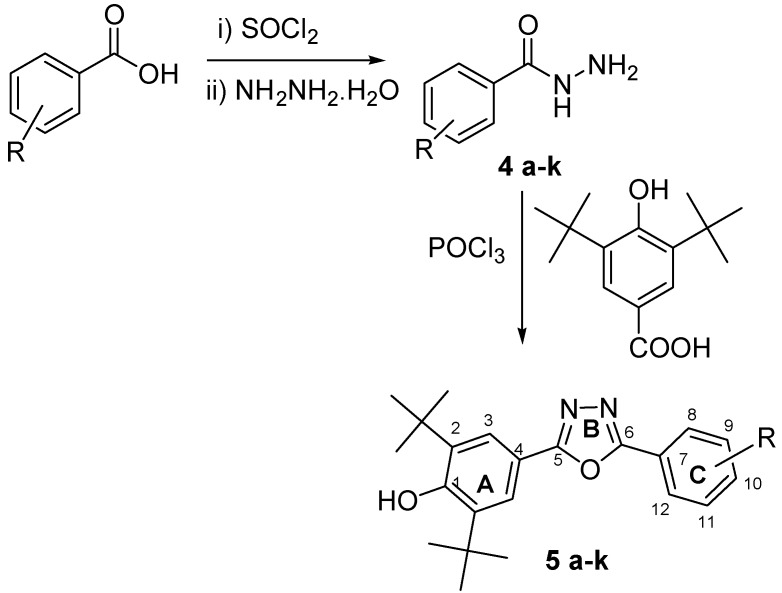
The structures of the compounds were established on the basis of their spectral data. The IR exhibited all the expected peaks, with the OH of the hindered phenol showing medium to strong peaks due to nonhydrogen bonding [20] at 3658–3525, CHaliphatic ones at 2963–2947, and the C=N of the oxadiazole ring at 1624 cm−1 to 1608 cm−1. The 1H-NMR spectra displayed the di-tert butyl group with integration equal to 18H, whose chemical shift ranged between 1.44 ppm and 1.52 ppm. The OH of the hindered phenol appeared at δ5.63 to δ5.69. All aryl protons and their substituents appeared in the expected regions. The 13C-NMR spectra were consistent with the IR and 1H-NMR spectra and our expectations. The carbons of the oxadiazole ring appeared at 161 ppm to 166 ppm, which represented 2(C=N). HMBC was employed to distinguish between C5 and C6 through the long-range coupling J3. The weak coupling J2 was also determined (Figure 2), and it exhibited the most significant correlations for the aromatic area.
Figure 2.
HMBC expansion region of 5d.
H3 exhibited correlations with C5 and C1 and weak correlations with C2 and C4. H8 exhibited a correlation with C6 and C10 owing to J3, and with C7 and C9 owing to J2. This spectrum confirmed that the C5 of the oxadiazole appeared at a lower field than C6. The HREIM values for all synthesized compounds were consistent with the calculated mass and the molecular formula. For more details, see the Experimental section.
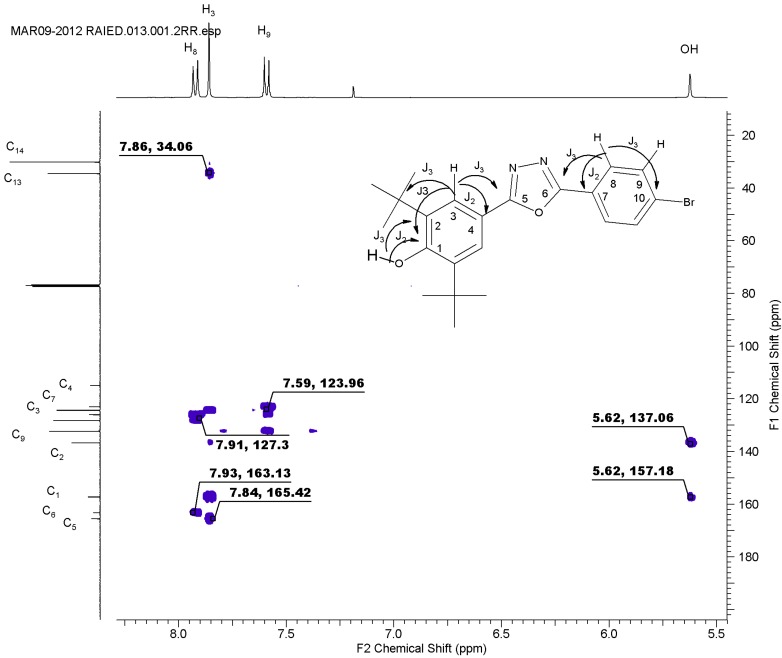
The EIMs show the molecular ion M•+ for all compounds and the base peak (100%) were either the same value of the molecular ion or the molecular ion minus methyl radical [M•+−•CH3]. The fragmentations in EIMs confirm the proposed structures and the HREIMs confirmed the accurate mass and the molecular formula. The interesting fragmentation observed in the mass spectrum was the loss of isocyanic acid (HNCO). This fragmentation which strated from [M•++H] was reported in literature [21,22,23]. The oxadiazoles loose HNCO but not phenol. However, in our case, losing HNCO started from molecular ion (M•+) which was subsequently protonated. The loss of HNCO can be explained through the rearrangement of the molecular ion and migration. Scheme 2 describes the proposed mechanism of the elimination of isocyanic acid.
Scheme 2.
Proposed pathways of HNCO loss.
Two pathways were suggested for the radical migration, path (B) and (C). Both pathways, (B) and (C) have intermediate (A) as their starting point which is formed by intra fragmentation of the molecular ion and migration of 2,6-di-tert-butylphenol. Path B is similar to the mass fragmentation pathway proposed by Frański et al. [22]. Table 2 summarizes the value of the molecular ion, the base peak and value after losing HNCO.
Table 2.
Molecular ion found, calculated, base peak and the m/z after losing HNCO.
| 5 | M•+ Found | M•+ calculated | m/z of base peak 100% | M•+-HNCO |
|---|---|---|---|---|
| 5a | 364.2 | 364.21 | 349.2 | 321.1 |
| 5b | 380.2 | 380.21 | 380.2 | 337.1 |
| 5c | 394.3 | 394.22 | 394.3 | 351.1 |
| 5d | 428.2 | 428.10 | 413.1 | 385.1 |
| 5e | 384.2 | 384.16 | 369.1 | 341.1 |
| 5f | 366.2 | 366.19 | 366.2 | 323.1 |
| 5g | 418.2 | 418.12 | 403.1 | 375.1 |
| 5h | 418.2 | 418.12 | 403.1 | 375.0 |
| 5i | 378.3 | 378.23 | 378.3 | 335.1 |
| 5j | 364.3 | 364.21 | 349.2 | 321.1 |
| 5k | 384.2 | 384.16 | 369.2 | 341.1 |
3. Antioxidant Assays
3.1. FRAP Assay
The FRAP assay was performed according to the Benzie and Strain [24] method. The FRAP reagent was prepared by combining 300 mM acetate buffer and 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) solution in 40 mM HCl and 20 mM FeCl3·6H2O, in a ratio of 10:1:1. The FRAP reagent was incubated at 37 °C prior to use. Ten microliters of the sample was reconstituted in the carrier (solvent or ultrapure water) and mixed with 300 μL of FRAP reagent. The mixture was incubated at 37 °C for 4 min in a microplate reader. The absorbance of the complex was 593 nm. The FRAP value can be calculated using the following equation [25]:
| FRAP = [(0–4 min ∆A593 nm of test sample)/(0–4 min ∆A593 nm of standard)] × [standard] (µM) × Y × 1000 |
where Y is absorbance of the spectrophotometer.
3.2. DPPH Assay
The assay was performed as reported by Gerhauser et al. [26]. Five microliters of the sample (dissolved in ethanol) was added into 195 μL of 100 μM DPPH reagent in ethanol (96%) and mixed in a 96-well plate. The intensity of the color was measured for 3 h at an interval of 20 min at 515 nm. Ascorbic acid and BHT were used as reference.
3.3. Antioxidant Activity
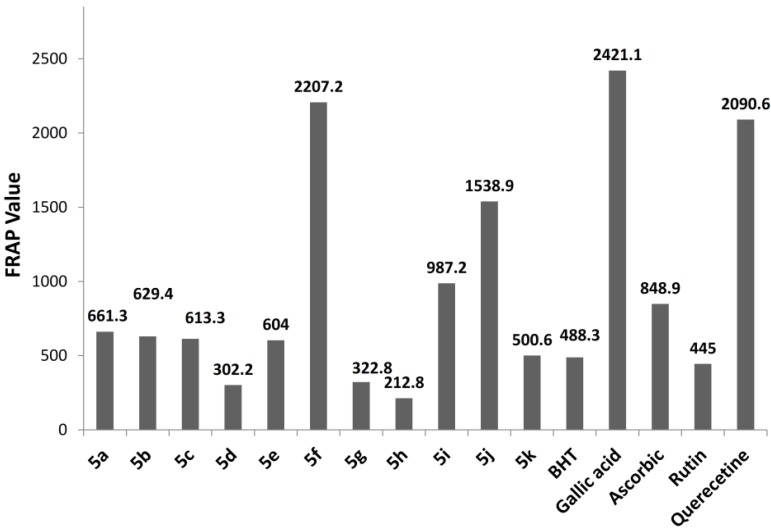
Differences occurred between the structures of the synthesized compound in ring C owing to different substituent and positions, whereas rings A and B were the same. Various antioxidant abilities were exhibited in both assays based on the type of substituent and their position, which have important roles in enhancing or negating antioxidant ability. The inductive effects of the electron-donating group +I and electron-withdrawing group (EWG) −I, the mesomeric effect (electron-releasing group +M or electron-withdrawing group −M), and the resonance effect directly affected antioxidant ability. Compound 5f exhibited higher antioxidant capacity, with a FRAP value of 2207.25 (see Table 3). This result is consistent with the concept that the hydroxyl group enhances antioxidant ability [27,28,29,30]. The +M of the hydroxyl at the para position is more important than the −I. Compound 5j exhibited excellent antioxidant ability (1538.9).
Table 3.
Antioxidant activity of the synthesized oxadiazoles.
| Compound | FRAP a | DPPH Inhibition % ±SD | IC50 ± SEM b (100 µg/mL) |
|---|---|---|---|
| 5a | 648.3 | 76.02 ± 0.059 | 41.76 ± 0.042 |
| 5b | 629.4 | 62.03 ± 0.327 | 50.69 ± 0.181 |
| 5c | 613.3 | 56.04 ± 0.187 | 54.60 ± 0.469 |
| 5d | 302.2 | 30.85 ± 0.166 | >100 |
| 5e | 640.0 | 50.44 ± 0.045 | 99.2 ± 0.032 |
| 5f | 2207.2 | 89.05 ± 0.024 | 15.79 ± 0.017 |
| 5g | 322.8 | 30.35 ± 0.038 | >100 |
| 5h | 212.8 | 29.26 ± 0.041 | >100 |
| 5i | 987.2 | 79.22 ± 0.037 | 41.27 ± 0.027 |
| 5j | 1538.9 | 87.21 ± 0.084 | 15.9 ± 0.054 |
| 5k | 500.6 | 42.14 ± 0.078 | >100 |
| BHT | 488.3 | 66.03 ± 0.051 | 79.84 ± 0.036 |
| Gallic acid | 2421.1 | - | - |
| Ascorbic acid | 848.9 | 90.65 ± 0.122 | 22.71 ± 0.086 |
| Rutin | 445.0 | - | - |
| Quercetin | 2090.6 | - | - |
| Trolox | 779.4 | - | - |
a Standard deviation (SD) value in FRAP was between 0.01–0.16; b SED standard mean error and IC50: 50% effective concentration.
The FRAP value and the substituent followed the following sequence: 4-OH > 2-Me > 2,4-di-Me > 4-Me > 4-OMe ≈ 4-OEt > 4-Cl> 2-Cl > 4-Br ≈ 3,4-di Cl ≈ 3,5-di Cl. This sequence demonstrates that the electron-releasing group, which exerts mesomeric and inductive effects, enhances antioxidant ability, whereas the inductive-withdrawing group decreases antioxidant ability. The results show that the position of substituent also affects antioxidant ability, as illustrated in Figure 3.
Figure 3.
FRAP assay for the synthesized 2,6-di-tert-butyl-4-(5-aryl-1,3,4-oxadiazol-2-yl)phenols.
The methyl group at position 2 enhances the antioxidant ability more than that at position 4, and exhibits higher antioxidant ability than 4-methoxy. However, the differences between 4-methoxy and 4-ethoxy were too small. The analogue with EWG in positions 2, 3, and 4, i.e., 4-Br, 4-Cl, 3,4-diCl, 3,5-diCl reduced or negated antioxidant activity. The results may be explained by the fact that previously described analogues with EWG’s, increase the bond dissociation energy. Another possibility is that they exhibit decreased antioxidant ability [31,32]. The DPPH results (Table 3) were compatible with, and possessed the same sequence, as the FRAP assay. However, all values for DPPH are on the whole lower than the values for the FRAP assay in comparison to ascorbic acid. For instance, in FRAP assay, 5f showed about twice the antioxidant ability of ascorbic acid, whereas in the DPPH assay, 5f exhibited similar antioxidant ability to ascorbic acid. This difference could be attributed to the different mechanisms for FRAP and DPPH. FRAP involves the single electron transfer mechanism, whereas DPPH assay depends on the H-atom transfer mechanism [33]. The steric hindrance between the synthesized compound and DPPH may account for the difference [34].
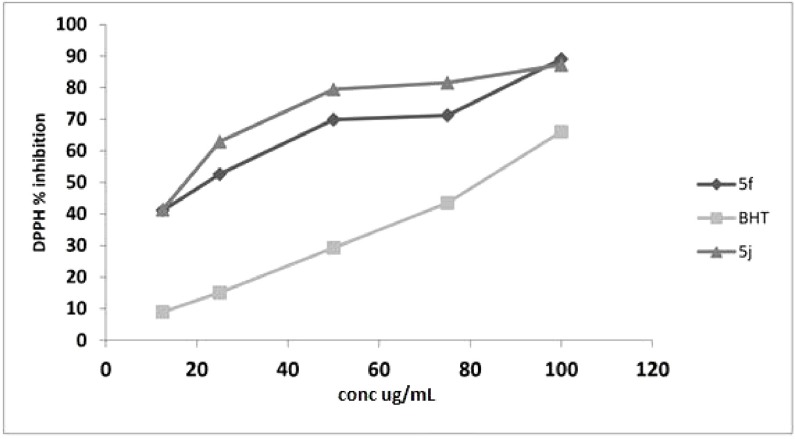
Compounds 5f and 5j exhibited lower IC50 values of 15.79 and 15.9 µg/mL, respectively, compared to ascorbic acid. The IC50 of para substituted analogues with methyl, methoxy, and ethoxy were lower than that of BHT. By contrast, for electron withdrawing group at para, meta, and ortho, the analogues showed reduced antioxidant activity. All compounds were screened in terms of their free radical scavenging properties using five concentrations: 12.5, 25, 50, 75, and 100 µg/mL. Both 5f and 5j exhibited significant antioxidant capability at low concentrations, as depicted in Figure 4.
Figure 4.
DPPH inhibitions of 5f and 5j at different concentration.
4. Experimental
General Information
The chemicals used for the synthesis were supplied by Sigma-Aldrich (Petaling Jaya, Selangor, Malaysia), Fisher (Shah Alam, Selangor, Malaysia), and Merck (Petaling Jaya, Selangor, Malaysia). The melting point was determined by open capillary tube method using an MEL-TEMP II apparatus and was uncorrected. The purity of the compounds was checked through thin layer chromatography (silica gel TLC) using Merck plates. The plates were visualized by mean of iodine vapors and UV light. The IR spectra were obtained using a PerkinElmer 400 Fourier transform infrared spectrometer. All NMR spectra were recorded on either a JEOL-ECA 400 MHz or JEOL-Lambda 400 MHz spectrometer. CDCl3 and DMSO-d6 were used as solvents with TMS as the internal standard. Mass spectra were recorded using a TSQ7000 for HREI/MS (NUS Singapore). For UV spectroscopy, a Power Wave X340 (BIO-TEK Instruments, Inc., Winooski, VT, USA) was used to record the FRAP and DPPH assays.
General Synthesis of 2,6-Di-tert-butyl-4-(5-aryl-1,3,4-oxadiazol-2-yl)phenols
To a mixture of (0.31 g, 1.24 mmol) of 3,5-di-tert-butyl-4-hydroxybenzoic acid and 1.24 mmole aryl acid hydrazide in a 50 mL round bottom flask, 5 mL of phosphorus oxychloride was added in a few portions at room temperature. The mixture was refluxed for 3 h with stirring on water bath 80–90 °C. After cooling, the mixture poured onto 100 mL crushed ice and stirred for 15 min. Sodium bicarbonate was added in a few portions until the pH was around to 7–8. The precipitate was filtered, washed with water and dried then purified either by column chromatography or by crystallization from suitable solvent.
2,6-Di-tert-butyl-4-[5-(4-methylphenyl)-1,3,4-oxadiazol-2-yl]phenol (5a). The product was recrystallized from chloroform-ethanol (1-1) to obtain white crystals. Yield 0.343 g (76.0%), m.p. 196–197 °C, IR (KBr, νmax/cm−1): 3658 (OH), 3011 (CHaromatic), 2962–2947 (CHaliphatic), 1610 (C=N), 1585, 1498 (C=C), 1219 (C-O), 1H-NMR (CDCl3, 400 MHz, ppm): 1.51 (s, 18H, H14, 2 × t-Bu), 2.42 (s, 3H, p-CH3-ph), 5.67 (s, 1H, OH), 7.31 (d, 2H, J = 8.28 Hz, H9, H11), 7.94 (s, 2H, H3), 8.01 (d, 2H, J = 8.28 Hz, H8, H12), 13C-NMR (CDCl3, 100 MHz, ppm): 21.73 (p-CH3Ph), 30.25 (6C, C14, 2 × C(CH3)3), 34.56 (2C, C13, 2 × C(CH3)3), 115.43 (C4), 121.52 (C7), 124.35 (C3), 126.87 (2C, C8 & C12), 129.77 (2C, C9 & C11), 136.81 (C2), 141.99 (C10), 157.12 (C1), 164.22 & 165.21 (C5 & C6). HREIMs, m/z = 364.2147 [M•+] (calc. for C23H28O2N2, 364.2151).
2,6-Di-tert-butyl-4-[5-(4-methox yphenyl)-1,3,4-oxadiazol-2-yl]phenol (5b). The solid product was recrystallized from ethyl acetate to yield white crystals. Yield 0.346 g (73%), m.p. 179–181 °C, IR (KBr, νmax/cm−1): 3625 (OHphenol), 3006 (CHaromatic), 2955 (CHaliphatic), 1611 (C=N), 1585, 1495 (C=C), 1219 (C-O), 1020 (O-CH3), 1H-NMR (CDCl3, 400 MHz, ppm): 1.51 (s, 18H, H14, 2 × t-Bu), 3.89 (s, 3H, OCH3), 5.63 (s, 1H, OH), 7.03 (d, 2H, J = 9.04 Hz, H9, H11), 7.92 (s, 2H, H3), 8.06 (d, 2H, J = 8.56 Hz, H8, H12), 13C-NMR (CDCl3, 100 MHz, ppm): 30.13 (6C, C14, 2 × C(CH3)3), 34.45 (2C, C13, 2 × C(CH3)3), 55.44 (OCH3), 114.40 (2C, C8 & C12), 115.37 (C4), 116.72 (C7), 124.19 (C3), 128.58 (2C, C9 & C11), 136.68 (C2), 156.95 (C1), 162.11 (C10), 163.89 & 164.75 (C6 & C5). HREIMs, m/z = 380.2095 [M•+] (calc. for C23H28O3N2, 380.2100).
2,6-Di-tert-butyl-4-[5-(4-ethoxyphenyl)-1,3,4-oxadiazol-2-yl]phenol (5c). The crude solid was recrystallized from ethyl acetate-methanol (1:1) to give a white amorphous solid. Yield 0.371 g (76%), m.p. 176–178 °C, IR (KBr, νmax/cm−1), 3628 (OHphenol), 3009 (CHaromatic), 2957 (CHaliphatic), 1611 (C=N), 1543, 1495 (C=C), 1221 (C-O), 1111 (O-CH2), 1H-NMR (CDCl3 ,400 MHz, ppm): 1.46 (t, 3H, J = 7.32 Hz, OCH2CH3), 1.51 (s, 18H, H14, 2 × t-Bu), 4.11 (q, 2H, J = 8 Hz, OCH2), 5.64 (s, 1H, OH), 7.1 (d, 2H, J = 8.8 Hz, H9 & H11), 7.92 (s, 1H, H3), 8.05 (d, 2H, J = 8.04 Hz, H8 & H12), 13C-NMR (CDCl3, 100 MHz, ppm): 14.71 (OCH2CH3), 30.17 (6C, C14, 2 × C(CH3)3), 34.48 (2C, C13, 2 × C(CH3)3), 63.73 (OCH2), 114.91 (2C, C8 & C12), 115.50 (C4), 116.60 (C7), 124.18 (C3), 128.57 (2C, C9 & C11), 136.69 (C2), 156.93 (C1), 161.52 (C10), 164.00 & 164.90 (C6 & C5). HREIMs, m/z = 394.2249 [M•+] (calc. for C24H30O3N2, 394.2256).
4-(5-(4-Bromophenyl)-1,3,4-oxadiazol-2-yl)-2,6-di-tert-butylphenol (5d). The crude product was recrystallized from ethyl acetate-methanol (1-1) to give white crystals. Yield 0.447 g (84%), m.p. 168–170 °C, IR (KBr, νmax/cm−1): 3525 (OHphenol), 3005 (CHaromatic), 2953 (CHaliphatic), 1602 (C=N), 1543, 1495 (C=C), 1244 (C-O), 1H-NMR (CDCl3, 400 MHz, ppm): 1.51 (s, 18H, H14, 2 × t-Bu), 5.66 (s, 1H, OH), 7.67 (d, 2H, J = 8.42 Hz, H9 & H11), 7.92 (s, 2H, H3), 7.9 (d, 2H, J = 8.54 Hz, H8 & H12), 13C-NMR (CDCl3, 100 MHz, ppm): 30.23 (6C, C14, 2 × C(CH3)3), 34.56 (2C, C13, 2 × C(CH3)3), 115.12 (C4), 123.22 (C7), 124.43 (C3), 126.1 (2C, C8 & C12), 128.31 (2C, C9 & C11), 132.41 (C10), 136.87 (C2), 157.33 (C1), 163.37 & 165.66 (C5 & C6) ppm. HREIMs m/z = 428.1093 [M•+] (calc. for C22H25O2N2Br, 428.1099).
4-(5-(4-Chlorophenyl)-1,3,4-oxadiazol-2-yl)-2,6-di-tert-butylphenol (5e). The crude material was recrystallized from ethyl acetate-methanol (1:1) to obtain a white solid. Yield 0.396 g (83%), m.p. 162–164 °C, IR (KBr, νmax/cm−1): 3583 (OHphenol), 3004 (CHaromatic), 2959 (CHaliphatic), 1607 (C=N), 1571, 1540 (C=C), 1239 (C-O). 1H-NMR (CDCl3-400 MHz, ppm): 1.51 (s, 18H, H14, 2 × t-Bu), 5.67 (s, 1H, OH), 7.51 (d, 2H, J = 8.52 Hz, H9 & H11), 7.93 (s, 2H, H3), 8.07 (d, 2H, J = 8.52 Hz, H8 & H12), 13C-NMR (CDCl3,100 MHz, ppm): 30.23 (6C, C14, 2 × C(CH3)3), 34.56 (2C, C13, 2 × C(CH3)3), 115 (C4), 122.78 (C7), 124.27 (C3), 129.46 (2C, C9 & C11) 128.18 (2C, C8 & C12), 136.87 (C2), 137.71 (C10), 157.32 (C1), 163.29 & 165.64 (C5 & C6). HREIMs m/z = 384.1597 [M•+] (calc. for C22H25O2N2Cl, 384.1605).
2,6-Di-tert-butyl-4-(5-(4-hydroxyphenyl)-1,3,4-oxadiazol-2-yl)phenol (5f). The crude mixture was purified by column chromatography using (6:1) hexane ethyl acetate as eluent to give a white amorphous solid. Yield 0.318 g (70%), m.p. 144–146 °C, IR (KBr, νmax/cm−1): 3617 (OHphenol), 2958 (CHaliphatic), 1609 (C=N), 1546, 1506 (C=C), 1250 (C-O), 1H-NMR (CDCl3, 400 MHz, ppm): 1.51 (s, 18H, H14, 2 × t-Bu), 5.65 (s, 1H, OH), 6.70 (bs, 1H, OH), 7.01 (d, 2H, J = 8.76 Hz, H9 & H11), 7.92 (s, 1H, H3), 8.01 (d, 2H, J = 8.8 Hz, H8 & H12), 13C-NMR (CDCl3, 100 MHz, ppm): 31.06 (6C, C14, 2 × C(CH3)3), 34.56 (2C, C13, 2 × C(CH3)3), 115.27 (C4), 115.58 (C7), 116.40 (2C, C8 & C12), 124.37 (C3), 128.97 (2C, C9 & C11), 136.85 (C2), 157.24 (C1) 159.83 (C10), 164.33 & 165.05 (C5 & C6). HREIMs m/z = 366.1938 [M•+] (calc. for C22H26O3N2, 366.1943).
2,6-Di-tert-butyl-4-(5-(3,4-dichlorophenyl)-1,3,4-oxadiazol-2-yl)phenol (5g). The crude product was recrystallized from benzene to give a white solid. Yield 0.416 g (80%), m.p. 222–224 °C, IR (KBr, νmax/cm−1): 3580 (OHphenol), 3003(CHaromatic), 2952 (CHaliphatic), 1606 (C=N), 1546, 1462 (C=C), 1239 (C-O). 1H-NMR (CDCl3, 400 MHz, ppm): 1.44 (s, 18H, H14, 2 × t-Bu), 5.67 (s, 1H, OH), 7.59 (d, 2H, J = 8.52 Hz, H11), 7.91–7.95 (m, 3H, H12 & H3'), 8.19 (d, J = 2.2 Hz, 1H, H8), 13C-NMR (CDCl3, 100 MHz, ppm): 30.23 (6C, C14, 2 × C(CH3)3), 34.57 (2C, C13, 2 × C(CH3)3), 114.89 (C4), 124.11 (C7), 124.51 (C3), 125.95 (C12), 128.53 (C11), 131.28 (C8), 133.68 (C9), 135.89 (C10), 136.94 (C2), 157.50 (C1), 162.3 & 165.94 (C5 & C6). HREIMs m/z = 418.1219[M•+] (calc. for C22H24O2N2Cl2, 418.1215).
2,6-Di-tert-butyl-4-(5-(3,5-dichlorophenyl)-1,3,4-oxadiazol-2-yl)phenol (5h). The crude product was purified by recrystallized from acetonitrile to afford a white amorphous solid. Yield 0.386 g (74%), m.p. 195–197 °C, IR (KBr, νmax/cm−1): 3600 (OHphenol), 3007 (CHaromatic), 2961 (CHaliphatic), 1606 (C=N), 1574, 1550 (C=C), 1240 (C-O), 1H-NMR (CDCl3, 400 MHz, ppm): 1.52 (s, 18H, H14, 2 × t-Bu), 5.69 (s, 1H, OH), 7.51 (t, 1H, J = 1.24 Hz, H10), 7.93 (s, 2H, H3), 8.01 (t, 2H, J = 1.72 Hz, H8 & H12), 13C-NMR (CDCl3, 100 MHz, ppm): 30.39 (6C, C14, 2 × C(CH3)3), 34.50 (2C, C13, 2 × C(CH3)3), 114.73 (C4), 124.48 (C3), 125.02 (2C, C8 & C12), 126.87 (C7), 131.24 (C10), 135.92 (C9 & C11), 136.93 (C2), 157.50 (C1), 161.877 (C5), 166.05 (C6). HREIMs m/z = 418.1210 [M•+] (calc. for C22H24O2N2Cl2, 418.1215).
2,6-Di-tert-butyl-4-(5-(2,4-dimethylphenyl)-1,3,4-oxadiazol-2-yl)phenol (5i). The crude product was purified by recrystallized from toluene to give white crystalline needles. Yield 0.28 g (60%), m.p. 170–172 °C. IR (KBr, νmax/cm−1): 3587 (OHphenol), 2957 (CHaliphatic), 1614 (C=N), 1550, 1536 (C=C), 1238 (C-O), 1H-NMR (CDCl3, 400 MHz, ppm): 1.50 (s, 18H, H14, 2 × t-Bu), 2.39 (s, 3H, H15), 2.70 (s, 3H, H16), 5.64 (s, 1H, OH), 7.17–7.14 (m, 2H, H9 & H11), 7.95–7.90 (m, 3H, H12, H3'), 13C-NMR (CDCl3, 100 MHz, ppm): 21.50 (C16, o-CH3), 22.04 (C15, p-CH3), 30.22 (6C, C14, 2 × C(CH3)3), 34.55 (2C, C13, 2 × C(CH3)3), 115.40 (C4), 12.60 (C7), 124.38 (C3), 126.98 (C11), 128.96 (C12), 132.59 (C9), 136.79 (C2), 138.19 (C8), 141.43 (C10), 157.11 (C1), 164.49 & 164.81 (C5 & C6). HREIMs m/z =378.2301 [M•+] (calc. for C24H30O2N2, 378.2307).
2,6-Di-tert-butyl-4-[5-(2-methylphenyl)-1,3,4-oxadiazol-2-yl] phenol (5j). The product was recrystallized from ethyl acetate to afford white crystals, 0.308 g (68.5%), m.p. 132–134 °C, IR (KBr, νmax/cm−1): 3588 (OHphenol), 3008 (CHaromatic), 2963 (CHaliphatic), 1607 (C=N), 1592, 1537 (C=C), 1238 (C-O), 1H-NMR (CDCl3,400 MHz, ppm): 1.52 (s, 18H, H14, 2 × t-Bu), 5.66 (s, 1H, OH), 7.44–7.34 (m, 3H, H9, H10, H11), 7.96 (s, 2H, H3, H3'), 8.02 (d, 1H, J = 7.32 Hz, H12), 13C-NMR (CDCl3, 100 MHz, ppm): 2 2.11 (o-CH3), 30.22 (6C, C14, 2 × C(CH3)3), 34.52 (2C, C13, 2 × C(CH3)3), 115.53 (C4), 123.40 (C7), 124.37 (C3), 126.21 (C11), 128.96 (C12), 131.03 (C10), 131.80 (C9), 136.80 (C2), 138.35 (C8), 157.14 (C1), 164.32 & 165.04 (C5 & C6), HREIMs m/z = 364.2144 [M•+] (calc. for C23H28O2N2, 364.2151).
2,6-Di-tert-butyl-4-(5-(2-chlorophenyl)-1,3,4-oxadiazol-2-yl)phenol (5k). The crude product was recrystallized from ethyl acetate to obtain white crystals. Yield 0.386 g (81%), m.p. 113–115 °C, IR (KBr, νmax/cm−1): 3584 (OHphenol), 3004 (CHaromatic), 2959 (CHaliphatic), 1607 (C=N), 1570, 1539 (C=C), 1239 (C-O), 1H-NMR (CDCl3, 400 MHz, ppm): 1.48(s, 18H, H14, 2 × t-Bu), 5.66 (s, 1H, OH), 7.38–7.46 (m, 2H, H10, H11), 7.53 (d, 1H, J = 8.04 Hz, H12), 7.95 (s, 2H, H3, H3'), 8.05 (d, 1H, J = 6.32 Hz, H9), 13C-NMR (CDCl3, 100 MHz, ppm): 30.27 (6C, C14, 2 × C(CH3)3), 34.61 (2C, C13, 2 × C(CH3)3), 115.18 (C4), 123.65 (C7), 124.61 (C3), 127.21 (C11), 131.33 (C12), 132.32 (C9), 133.14 (C8), 136.79 (C9), 136.94 (C2), 157.28 (C1), 162.55 & 166.04 (C5 & C6). HREIMs m/z = 384.1600 [M•+] (calc. for C22H25O2N2Cl, 384.1605).
5. Conclusions
A series of new 1,3,4-oxadiazole compound incorporating hindered phenol moities were successfully synthesized and characterized. All of the new compounds were screened for antioxidant activity using the FRAP and DPPH assays. The substituents on ring C demonstrated a significant role in improving or negating the antioxidant activity of the compounds. The analogues incorporating electron releasing substituents exhibited high antioxidant activity, whereas those with electron withdrawing substituent demonstrated reduced antioxidant activity.
Acknowledgments
We would like to thank the University of Malaya for a UMRG grant (RG149-11AFR) and a PPP grant (PS345-2009B) and the Ministry of Higher Education of Malaysia for supporting this study. We are also grateful to Amir Atto for his contributions and advice and to Noel F. Thomas for proofreading the manuscript.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/3/3436/s1.
Author Contributions
All authors contributed equally for the manuscript. Raied M. Shakir (PhD candidates), Mahmood Ameen Abdulla (DPPH and FRAP assays).
Conflictts of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Footnotes
Sample Availability: Samples of the compounds 5a–k are available from the authors.
References
- 1.Kar S., Subbaram S., Carrico P.M., Melendez J.A. Redox-control of matrix metalloproteinase-1: A critical link between free radicals, matrix remodeling and degenerative disease. Respir. Physiol. Neurobiol. 2010;174:299–306. doi: 10.1016/j.resp.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalil Z., Khodr B. A role for free radicals and nitric oxide in delayed recovery in aged rats with chronic constriction nerve injury. Free Radic. Biol. Med. 2001;31:430–439. doi: 10.1016/S0891-5849(01)00597-4. [DOI] [PubMed] [Google Scholar]
- 3.Ziakas G.N., Rekka E.A., Gavalas A.M., Eleftheriou P.T., Kourounakis P.N. New analogues of butylated hydroxytoluene as anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. 2006;14:5616–5624. doi: 10.1016/j.bmc.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 4.Yehye W.A., Abdul Rahman N., Alhadi A.A., Khaledi H., Ng S.W., Ariffin A. Butylated hydroxytoluene analogs: Synthesis and evaluation of their multipotent antioxidant activities. Molecules. 2012;17:7645–7665. doi: 10.3390/molecules17077645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruiz J., Pẻrez C., Pouplana R. QSAR study of dual cyclooxygenase and 5-lipoxygenase inhibitors 2,6-di-tert-butylphenol derivatives. Bioorg. Med. Chem. 2003;11:4207–4216. doi: 10.1016/S0968-0896(03)00449-8. [DOI] [PubMed] [Google Scholar]
- 6.Mullican M.D., Wilson M.W., Connor D.T., Kostlan C.R., Schrier D.J., Dyer R.D. Design of 5-(3,5-di-tert-butyl-4-hydroxyphenyl)-l,3,4-thiadiazo-l 1,3,4 -oxadiazoles and -1,2,4-triazoles as orally-active, nonulcerogenic antiinflammatory agents. J. Med. Chem. 1993;36:1090–1099. doi: 10.1021/jm00060a017. [DOI] [PubMed] [Google Scholar]
- 7.Moore G.G., Swingle K.F. 2,6-Di-tert-butyl-4-(2'-thenoyl)phenol(R-830): A novel nonsteroidal anti-inflammatory agent with antioxidant properties. Agents Actions. 1982;12:674–683. doi: 10.1007/BF01965078. [DOI] [PubMed] [Google Scholar]
- 8.Ye X., Zhou W., Li Y., Sun Y., Zhang Y., Ji H., Lai Y. Darbufelone, a novel anti-inflammatory drug, induces growth inhibition of lung cancer cells both in vitro and in vivo. Cancer Chemother. Pharmacol. 2010;66:277–285. doi: 10.1007/s00280-009-1161-z. [DOI] [PubMed] [Google Scholar]
- 9.Sagar B.H., Stephen M. Antioxidants during anticancer therapy. Focus Altern. Complement. Ther. 2004;9:96–106. [Google Scholar]
- 10.Jeong T.-S., Kim K.S., Kim J.-R., Cho K.-H., Lee S., Lee W.S. Inhibitory efects of multi-substituted benzylidenethiazolidine-2,4-diones on LDL oxidation. Bioorg. Med. Chem. 2004;12:4017–4023. doi: 10.1016/j.bmc.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Song Y., Connor D.T., Doubleday R., Sorenson R.J., Sercel A.D., Unangst P.C., Roth B.D., Gilbertsen R.B., Chan K., Schrier D.J., et al. Synthesis, structure-activity relationships, and in vivo evaluations of substituted di-tert-butylphenols as a novel class of potent, selective, and orally active cyclooxygenase-2 inhibitors. 1. Thiazolone and oxazolone series. J. Med. Chem. 1999;42:1151–1160. doi: 10.1021/jm9805081. [DOI] [PubMed] [Google Scholar]
- 12.Cuadro A.M., Valenciano J.S., Vaquero J.J., Alvarez-Builla J., Sunkel C., de Casa-Juana M.F., Ortega M.P. Synthesis and biological evaluation of 2,6-di-tert-butylphenol hydrazones as 5-lipoxygenase inhibitors. Bioorg. Med. Chem. 1998;6:173–180. doi: 10.1016/S0968-0896(97)10018-9. [DOI] [PubMed] [Google Scholar]
- 13.Nonhebel D.C., Walton J.C. Free-Radical Chemistry, Structure and Mechanism. Cambridge University Press; Cambridge, UK: 1974. p. 477. [Google Scholar]
- 14.Hung C.-Y., Yen G.-C. Antioxidant activity of phenolic compounds isolated from mesona procumbens hemsl. J. Agric. Food Chem. 2002;50:2993–2997. doi: 10.1021/jf011454y. [DOI] [PubMed] [Google Scholar]
- 15.De Oliveira C.S., Lira B.F., Barbosa-Filho J.M., Lorenzo J.G.F., de Athayde-Filho P.F. Synthetic approaches and pharmacological activity of 1,3,4-oxadiazoles: A review of the literature from 2000–2012. Molecules. 2012;17:10192–10231. doi: 10.3390/molecules170910192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Oliveira C.S., Lira B.F., dos Santos Falcão-Silva V., Siqueira-Junior J.P., Barbosa-Filho J.M., de Athayde-Filho P.F. Synthesis, molecular properties prediction, and anti-staphylococcal activity of N-acylhydrazones and new 1,3,4-oxadiazole derivatives. Molecules. 2012;17:5095–5107. doi: 10.3390/molecules17055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Sadek M., Hassan S., Abdelwahab H., Yacout G. Synthesis and bioassay of a new class of furanyl-1,3,4-oxadiazole derivatives. Molecules. 2013;18:8550–8562. doi: 10.3390/molecules18078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musad E.A., Mohamed R., Ali Saeed B., Vishwanath B.S., Lokanatha Rai K.M. Synthesis and evaluation of antioxidant and antibacterial activities of new substituted bis(1,3,4-oxadiazoles), 3,5-bis(substituted) pyrazoles and isoxazoles. Bioorg. Med. Chem. Lett. 2011;21:3536–3540. doi: 10.1016/j.bmcl.2011.04.142. [DOI] [PubMed] [Google Scholar]
- 19.Maheshwari R., Chawla P., Saraf S. Comparison between antioxidant activity of 2,5-disubstituted 1,3,4-oxadiazoles containing heteroaromatic ring and aromatic ring at 2nd position. Med. Chem. Res. 2011;20:1650–1655. doi: 10.1007/s00044-010-9480-5. [DOI] [Google Scholar]
- 20.Silverstein R., Webster F. Spectromeetric Identification Of Organic Compounds. John Wiley & Sons Inc.; New York, NY, USA: 1998. pp. 90–91. [Google Scholar]
- 21.Frański R., Eitner K., Schroeder G. Mass spectrometric study of some protonated and lithiated 2,5-disubstituted-1,3,4-oxadiazoles. J. Am. Soc. Mass Spectrom. 2003;14:289–294. doi: 10.1016/S1044-0305(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 22.Frański R., Schroeder G., Rybachenko V., Szwajka O.P. Loss of isocyanic acid from the internal oxadiazole ring of protonated molecules of some 2,5-diaryl-1,3,4-oxadiazoles. Rapid Commun. Mass Spectrom. 2002;16:390–395. doi: 10.1002/rcm.575. [DOI] [PubMed] [Google Scholar]
- 23.Frański R., Gierczyk B., Schroeder G. Mass spectrometric fragmentation pathways of isotope labeled 2,5-disubstituted-1,3,4-oxadiazoles and thiadiazoles. Int. J. Mass Spectrom. 2004;231:47–49. doi: 10.1016/j.ijms.2003.09.014. [DOI] [Google Scholar]
- 24.Benzie I.F., Strain J.J. The Ferric Reducing Ability of Plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 25.Rustaiyan A., Javidnia K., Farjam M.H., Aboee-Mehrizi F., Ezzatzadeh E. Antimicrobial and antioxidant activity of the Ephedra sarcocarpa growing in Iran. J. Med. Plants Res. 2011;5:4251–4255. [Google Scholar]
- 26.Gerhäuser C., Klimo K., Heiss E., Neumann I., Gamal-Eldeen A., Knauft J., Liu G.-Y., Sitthimonchai S., Frank N. Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2003;523–524:163–172. doi: 10.1016/S0027-5107(02)00332-9. [DOI] [PubMed] [Google Scholar]
- 27.Shahidi F. Natural Antioxidants: Chemistry, Health Effects, and Applications. AOCS Press; Champaign, IL, USA: 1997. p. 18. [Google Scholar]
- 28.Simić A., Manojlović D., Šegan D., Todorović M. Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules. 2007;12:2327–2340. doi: 10.3390/12102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moalin M., Strijdonck G.P.F., Beckers M., Hagemen G.J., Borm P.J., Bast A., Haenen G.R. A planar conformation and the hydroxyl groups in the B and C rings play a pivotal role in the antioxidant capacity of quercetin and quercetin derivatives. Molecules. 2011;16:9636–9650. doi: 10.3390/molecules16119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torres R., Urbina F., Morales C., Modak B., Monache F.D. Antioxidant properties of lignans and ferulic acid from the resinous exudate of larrea nitida. J. Chil. Chem. Soc. 2003 doi: 10.4067/S0717-97072003000300012. [DOI] [Google Scholar]
- 31.Lucarini M., Pedrielli P., Pedulli G.F., Cabiddu S., Fattuoni C. Bond dissociation energies of O-H bonds in substituted phenols from equilibration studies. J. Org. Chem. 1996;61:9259–9263. doi: 10.1021/jo961039i. [DOI] [Google Scholar]
- 32.Inami K., Iizuka Y., Furukawa M., Nakanishi I., Ohkubo K., Fukuhara K., Fukuzumi S., Mochizuki M. Chlorine atom substitution influences radical scavenging activity of 6-chromanol. Bioorg. Med. Chem. 2012;20:4049–4055. doi: 10.1016/j.bmc.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Huang D., Ou B., Prior R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 34.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995;28:25–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.