Abstract
Ganoderma triterpenes (GTs) are the major secondary metabolites of Ganoderma lucidum, a traditional Chinese medicine, popularly used for complementary cancer therapy. GTs are lanostane-tetracyclic triterpenes. They have been reported to possess anti-tumor, anti-inflammation, antioxidant, antimicrobial and blood fat reducing effects. To date, 316 GTs have been found and their similar chemical structures have proved difficult to elucidate. This paper compiles 316 naturally occurring triterpenes from Ganoderma based on the literature published through January 2013 along with their structures, physiological activities and 13C-NMR spectral data.
Keywords: Ganoderma, triterpenes, chemical structure, 13C-NMR data, bioactivity
1. Introduction
Ganoderma lucidum (Leyss. ex Fr.) Karst, a medicinal fungus called “Lingzhi” in China, is one of the most highly regarded medicinal fungi in the world. It is ranked as rare and precious in the ancient Chinese medical encyclopedias “Shen Nong’s Ben Cao Jing” and “Ben Cao Gang Mu”. The main Lingzhi-producing regions are East China, Southwest China and the provinces of Hebei, and Guangxi. It can be used in the prevention and treatment of various types of disease, such as cancer, hepatopathy, arthritis, hypertension, neurasthenia, debility, etc. Its the most attractive characteristics are its immunomodulatory and antitumor activities [1,2,3,4,5,6,7,8]. Ganoderma contains many bioactive natural components, including triterpenes (GTs), polysaccharides, proteins, and unsaturated fatty acids. The triterpenes and polysaccharides are deemed to be the primary bioactive compounds of Ganoderma.
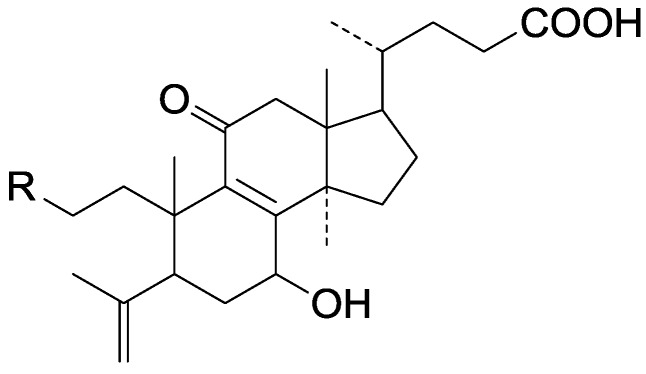
Kubota isolated ganoderic acid A and ganoderic acid B from Ganoderma lucidum (FR.) KARST in 1982 [9]. Since then, more than 316 triterpenes have been isolated from the fruiting bodies, spores, gills, and mycelia of many Ganoderma mushrooms. This total was derived from our investigation of the references. As reported, the majority of GTs exhibit a wide range of biological activities, including antitumor, anti-HIV-1, antihypertensive, antiangiogenic, immunomodulatory, antiandrogenic, antihepatitis B, antioxidant, anticomplement, and antimicrobial activities [10,11,12,13]. All GTs are tetracyclic triterpenes. Their chemical structures are more complex than those of other lanostanes, owing to their highly oxidized state. Generally, GTs contain 30 or 27 carbon atoms, and some have 24. The numbers of substituents as well as the positions increase the structural complexity. In this paper, all 316 triterpenes are listed. In accordance with the number of carbon atoms and their molecular features, they can be divided into different structural groups. The 13C-NMR data of those triterpenes, elucidation of the compounds’ structures and their bioactivities are discussed. We aim at providing a useful and fast way for identifying GTs. Finally, possible trends and perspectives for future investigation of these mushrooms are also included.
2. Ganoderma Triterpenes
Triterpenes are widely distributed in traditional Chinese medicines. Their structures are considered to be derived from the acyclic precursor squalene. More than 20,000 triterpenes have been isolated and identified from Nature, including squalene, lanostane, dammarane, lupine, oleanane, ursane, and hopane structure types [14,15]. The Ganoderma triterpenes belong to the lanostane triterpenes (Figure 1).
Figure 1.
A prototypical lanostane triterpenoid skeleton.
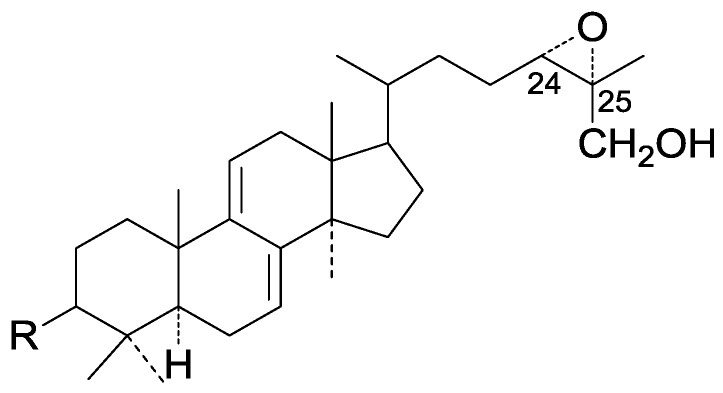
Most of them contain 30 or 27 carbon atoms. A few have 24 carbon atoms. These compounds possesses the same skeleton, namely a trans configuration of rings A/B, B/C, C/D and and 10β, 13β, 14α, 17β substituents. Moreover, substituents are always found at the C-3, 7, 11, 12, 15, 22, 23, 24 and 25 positions of the parent nucleus.
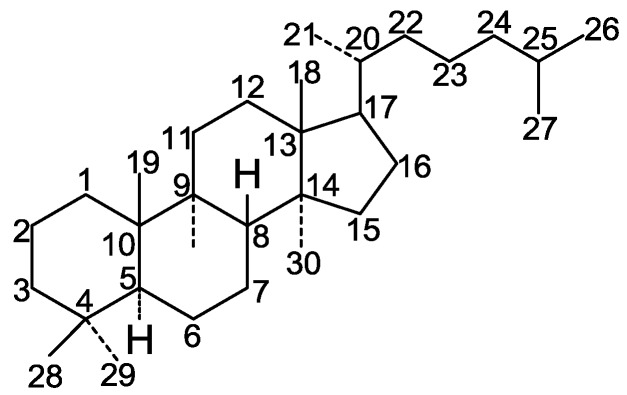
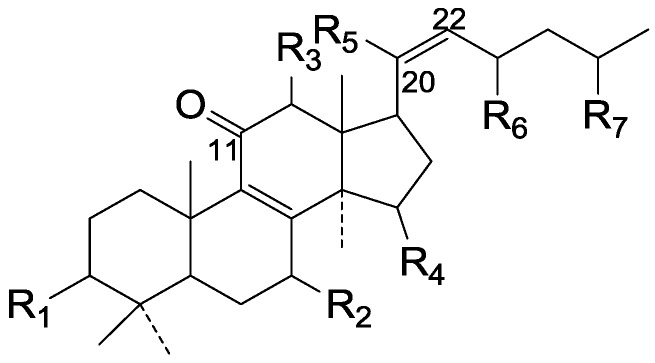
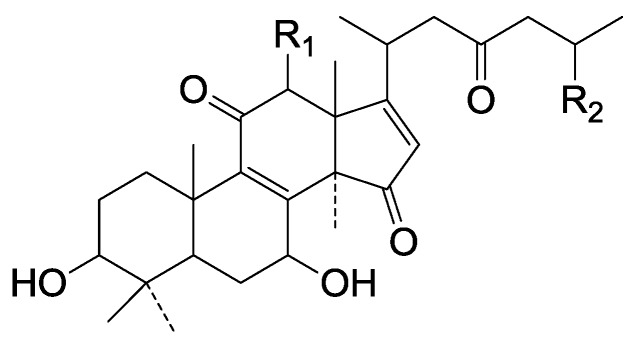
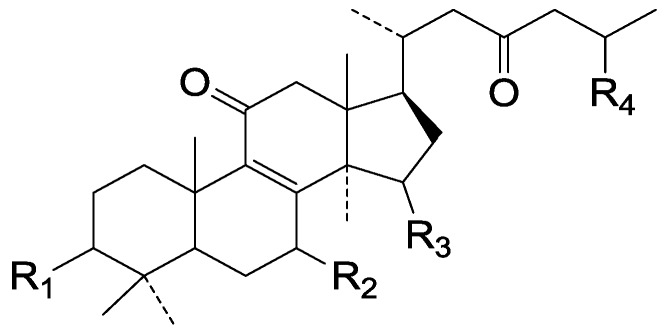
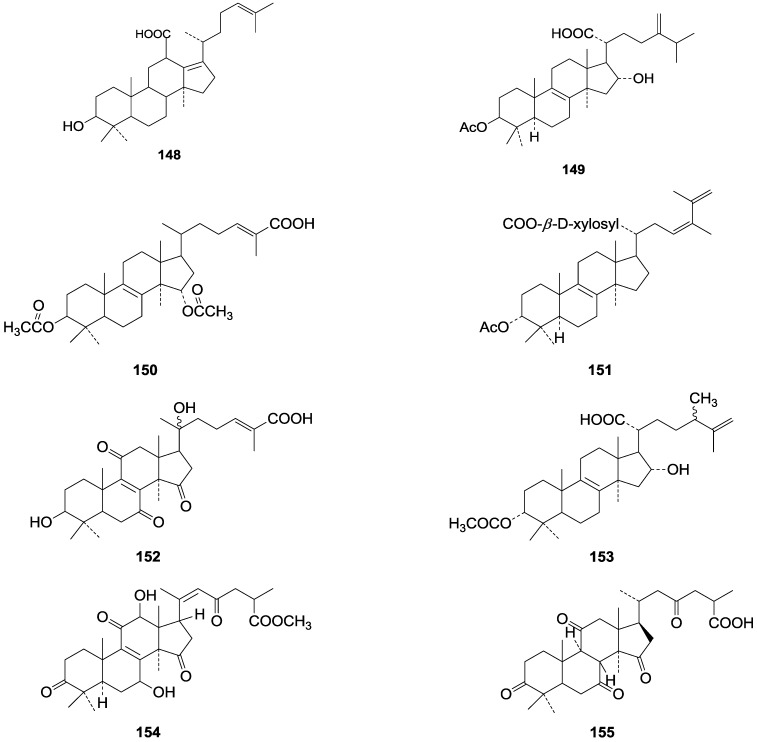
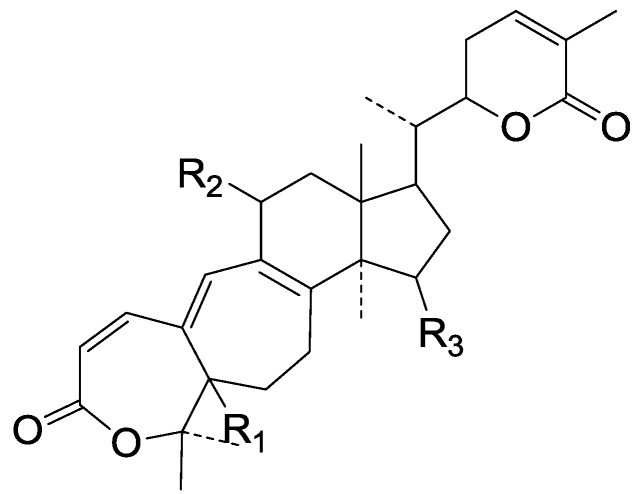
On the basis of the substituent groups and double bonds in the same position, they are classified into different types. Compounds 1–221 (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15, Figure 16, Figure 17, Figure 18, Figure 19, Figure 20 and Figure 21) possess 30 carbon atoms. Among them, 1–37 (Figure 2) contain double bonds between C-8 and C-9, a keto group at C-23, and substituent groups at C-3, 7, 11, 12, 15, 25. In this figure, compounds 1, 3, 4, 7, 8, 11–14, 17, 18, 20, 25, 26, 28, 31, 32, 34, and 35 possess β-hydroxy groups at C-3, and the others possess a keto group, except 3β-oxo-formyl-7β, 12β-dihydroxy-5α-lanost-11,15,23-trioxo-8-en(E)-26-oic acid (21) with a formyl located at the C-3 position. Compounds 2, 3, 9–17, 19–23, 25, 27, 31, 34–36 have hydroxy groups at C-7, and furthermore, 19, 20, 22 have α-configurations. What’s more, compounds 1, 4, 18, 24, 26, 28–30, 32, 33, and 37 have a carbonyl at C-7. In this group, C-11 mainly has a carbonyl substituent except in ganoderic acid Df (27) with a β-hydroxyl at this position. The majority of these compounds do not have any substituents at C-12, while compounds 1, 4, 24, 25, 28, 29, 31 possess β-acetyloxy and compounds 21, 35–37 possess β-hydroxyls. All of these compounds display carbonyls or β-hydroxyls at C-15. As to other configurations, both α- and β-C-21, 17β (compounds 5–16, 21, 28–30, 35) and 20 α-configurations can be found in this group. Carboxyl, formyl, ethanoyl or butyryl moieties can be found at C-25, most commonly carboxyl. These compounds have extensive biological activities.
Figure 2.
Structures of compounds 1–37.
| Cpd | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|
| 1 | β-OH | =O | =O | β-O-Ac | =O | α-CH3 | COOBu |
| 2 | =O | β-OH | =O | H | α-OH | α-CH3 | COOBu |
| 3 | β-OH | β-OH | =O | H | =O | α-CH3 | COOBu |
| 4 | β-OH | =O | =O | β-O-Ac | α-OH | α-CH3 | COOH |
| 5 | =O | H | =O | H | α-OH | β-CH3 | COOH |
| 6 | =O | H | =O | H | α-OH | β-CH3 | COOCH3 |
| 7 | β-OH | H | =O | H | α-OH | β-CH3 | COOH |
| 8 | β-OH | H | =O | H | α-OH | β-CH3 | COOCH3 |
| 9 | =O | β-OH | =O | H | α-OH | β-CH3 | COOH |
| 10 | =O | β-OH | =O | H | α-OH | β-CH3 | COOCH3 |
| 11 | β-OH | β-OH | =O | H | =O | β-CH3 | COOH |
| 12 | β-OH | β-OH | =O | H | =O | β-CH3 | COOCH3 |
| 13 | β-OH | β-OH | =O | H | α-OH | β-CH3 | COOH |
| 14 | β-OH | β-OH | =O | H | α-OH | β-CH3 | COOCH3 |
| 15 | =O | β-OH | =O | H | =O | β-CH3 | COOH |
| 16 | =O | β-OH | =O | H | =O | β-CH3 | COOCH3 |
| 17 | β-OH | β-OH | =O | H | α-OH | α-CH3 | COOCH3 |
| 18 | β-OH | =O | =O | H | α-OH | α-CH3 | COOCH3 |
| 19 | =O | α-OH | =O | H | α-OH | α-CH3 | COOCH3 |
| 20 | β-OH | α-OH | =O | H | α-OH | α-CH3 | COOCH3 |
| 21 | O-CHO | β-OH | =O | β-OH | =O | β-CH3 | COOH |
| 22 | =O | α-OH | =O | H | α-OH | α-CH3 | COOH |
| 23 | =O | β-OH | =O | H | =O | α-CH3 | COOH |
| 24 | =O | =O | =O | β-O-COCH3 | =O | α-CH3 | COOEt |
| 25 | β-OH | β-OH | =O | β-O-COCH3 | =O | α-CH3 | COOCH3 |
| 26 | β-OH | =O | =O | =O | =O | α-CH3 | COOH |
| 27 | =O | β-OH | β-OH | H | =O | α-CH3 | COOH |
| 28 | β-OH | =O | =O | β-O-Ac | =O | β-CH3 | COOH |
| 29 | =O | =O | =O | β-O-Ac | =O | β-CH3 | COOH |
| 30 | =O | =O | =O | H | =O | β-CH3 | COOH |
| 31 | β-OH | β-OH | =O | β-O-Ac | =O | α-CH3 | COOH |
| 32 | β-OH | =O | =O | H | =O | α-CH3 | COOH |
| 33 | =O | =O | =O | H | α-OH | α-CH3 | COOH |
| 34 | β-OH | β-OH | =O | H | α-OH | α-CH3 | COOH |
| 35 | β-OH | β-OH | =O | β-OH | =O | β-CH3 | COOH |
| 36 | =O | β-OH | =O | β-OH | =O | α-CH3 | COOH |
| 37 | =O | =O | =O | β-OH | =O | α-CH3 | COOH |
Figure 3.
Structures of compounds 38–70.
| Cpd | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 |
|---|---|---|---|---|---|---|---|---|
| 38 | =O | β-OH | =O | H | =O | α-CH3 | H | COOH |
| 39 | =O | β-OH | =O | H | α-OH | α-CH3 | H | COOH |
| 40 | β-OH | β-OH | =O | β-O-Ac | =O | α-CH3 | H | COOH |
| 41 | β-OH | H | =O | β-O-Ac | α-O-Ac | α-CH3 | H | COOH |
| 42 | =O | =O | =O | H | =O | α-CH3 | β-OH | COOH |
| 43 | β-OH | =O | H | H | H | β-CH3 | H | COOH |
| 44 | =O | OH | =O | H | =O | α-CH3 | OH | COOH |
| 45 | =O | =O | H | H | H | α-CH3 | H | CHO |
| 46 | β-OH | =O | H | H | H | α-CH3 | H | CHO |
| 47 | =O | β-OH | =O | H | α-OH | α-CH3 | β-OH | COOH |
| 48 | =O | α-OH | =O | H | α-OH | α-CH3 | β-OH | COOH |
| 49 | β-OH | β-OH | =O | H | =O | α-CH3 | β-OH | COOH |
| 50 | β-OH | =O | =O | H | =O | α-CH3 | β-OH | COOH |
| 51 | β-OH | β-OH | =O | β-OH | =O | α-CH3 | β-OH | COOH |
| 52 | β-OH | =O | =O | β-OH | =O | α-CH3 | β-OH | COOH |
| 53 | β-OH | β-OH | =O | H | =O | α-CH3 | H | COOH |
| 54 | =O | H | =O | H | α-OH | β-CH3 | H | COOH |
| 55 | =O | α-OH | H | H | H | β-CH3 | H | CHO |
| 56 | α-O-Ac | α-O-Ac | H | H | α-OH | β-CH3 | H | COOH |
| 57 | =O | =O | =O | H | H | α-CH3 | H | CHO |
| 58 | =O | =O | H | H | H | α-CH3 | H | CH2OH |
| 59 | α-O-Ac | α-O-CH3 | H | H | α-OH | β-CH3 | H | COOH |
| 60 | =O | =O | α-OH | H | H | α-CH3 | H | COOH |
| 61 | =O | =O | β-OH | H | H | α-CH3 | H | COOH |
| 62 | β-OH | =O | H | H | H | α-CH3 | H | CH2OH |
| 63 | β-OH | =O | H | H | H | α-CH3 | H | CHO |
| 64 | =O | =O | H | H | H | α-CH3 | H | COOH |
| 65 | =O | α-OH | H | H | α-O-Ac | α-CH3 | H | COOH |
| 66 | β-OH | β-OH | =O | H | α-OH | α-CH3 | β-OH | COOH |
| 67 | β-OH | H | =O | H | α-OH | α-CH3 | β-OH | COOH |
| 68 | =O | β-OH | =O | H | α-OH | α-CH3 | H | CHO |
| 69 | =O | H | =O | H | α-OH | α-CH3 | β-OH | COOH |
| 70 | α-O-Ac | α-OH | H | H | α-O-Ac | β-CH3 | H | COOH |
Figure 4.
Structures of compounds 71–84.
| Cpd | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| 71 | α-O-Ac | α-OH | α-O-Ac | β-CH3 | ξ-O-Ac |
| 72 | α-O-Ac | α-O-Ac | α-OH | β-CH3 | ξ-O-Ac |
| 73 | α-O-Ac | α-O-CH3 | H | β-CH3 | ξ-O-Ac |
| 74 | α-O-Ac | α-O-CH3 | α-OH | β-CH3 | ξ-O-Ac |
| 75 | α-O-Ac | α-OH | α-OH | β-CH3 | ξ-O-Ac |
| 76 | α-OH | α-O-CH3 | H | β-CH3 | ξ-O-Ac |
| 77 | α-O-Ac | α-OH | H | α-CH3 | β-O-Ac |
| 78 | β-O-Ac | =O | H | α-CH3 | β-O-Ac |
| 79 | β-OH | =O | H | α-CH3 | β-O-Ac |
| 80 | =O | α-OH | α-O-Ac | α-CH3 | β-O-Ac |
| 81 | =O | α-OH | H | α-CH3 | β-O-Ac |
| 82 | =O | α-O-CH3 | H | α-CH3 | β-O-Ac |
| 83 | α-O-Ac | α-OH | α-O-Ac | α-CH3 | β-O-Ac |
| 84 | α-O-Ac | α-O-CH3 | α-O-Ac | α-CH3 | β-O-Ac |
Figure 5.
Structures of compounds 85–98.
| Cpd | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|
| 85 | β-OH | =O | β-O-Ac | =O | β-CH3 | =O | COOH |
| 86 | =O | β-OH | H | =O | β-CH3 | ξ-OH | ξ-COOH |
| 87 | =O | β-OH | H | =O | β-CH3 | =O | ξ-COOCH3 |
| 88 | =O | β-OH | H | α-OH | β-CH3 | =O | COOH |
| 89 | β-OH | β-OH | H | =O | β-CH3 | =O | COOH |
| 90 | β-OH | β-OH | H | α-OH | β-CH3 | =O | COOH |
| 91 | =O | β-OH | H | =O | β-CH3 | =O | COOH |
| 92 | =O | β-OH | β-O-Ac | =O | β-CH3 | =O | COOH |
| 93 | =O | =O | H | =O | β-CH3 | =O | COOH |
| 94 | =O | =O | H | α-OH | β-CH3 | =O | COOH |
| 95 | β-OH | =O | H | =O | β-CH3 | =O | COCH3 |
| 96 | β-OH | =O | H | α-OH | β-CH3 | =O | COCH3 |
| 97 | β-OH | =O | H | =O | β-CH3 | =O | COOH |
| 98 | β-OH | β-OH | β-O-Ac | =O | α-CH3 | =O | COOH |
Figure 6.
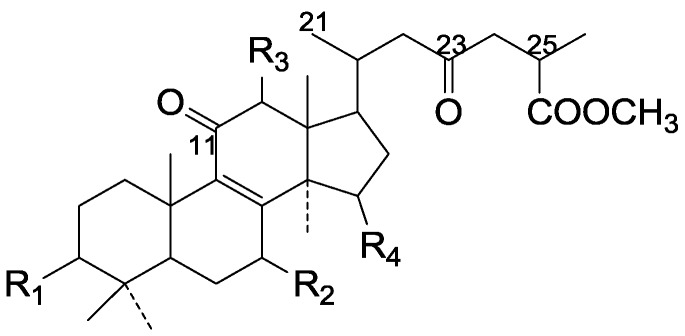
Structures of compounds 99–105.
| Cpd | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 99 | β-OH | β-OH | H | α-OH |
| 100 | =O | =O | H | =O |
| 101 | =O | =O | β-O-Ac | =O |
| 102 | β-OH | =O | β-O-Ac | =O |
| 103 | β-OH | β-OH | β-OH | =O |
| 104 | =O | β-OH | OH | =O |
| 105 | β-OH | =O | OH | =O |
Figure 7.
Structures of compounds 106‒110

| Cpd | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 |
|---|---|---|---|---|---|---|---|---|
| 106 | =O | =O | H | α-OH | β-CH3 | ξ-OH | =O | COOH |
| 107 | β-OH | β-OH | H | =O | α-CH3 | β-OH | ξ-OH | ξ-COOH |
| 108 | =O | β-OH | H | =O | α-CH3 | β-OH | ξ-OH | ξ-COOH |
| 109 | β-OH | β-OH | β-OH | =O | β-CH3 | β-OH | =O | COOH |
| 110 | β-OH | β-OH | =O | =O | α-CH3 | ξ-OH | =O | COOH |
Figure 8.
Structures of compounds 111‒116
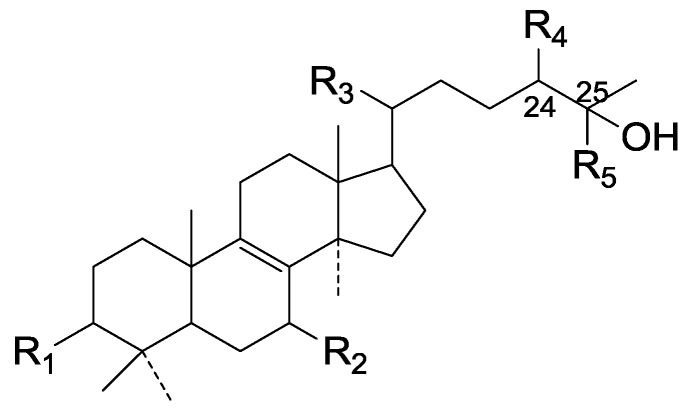
| Cpd | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| 111 | =O | =O | α-CH3 | α-OH | CH3 |
| 112 | =O | α-O-Et | β-CH3 | ξ-OH | CH2OH |
| 113 | =O | =O | β-CH3 | ξ-OH | CH2OH |
| 114 | =O | α-O-CH3 | β-CH3 | ξ-OH | CH2OH |
| 115 | β-OH | =O | β-CH3 | ξ-OH | CH2OH |
| 116 | β-OH | =O | α-CH3 | α-OH | CH3 |
Figure 9.
Structures of compounds 117‒123.
| Cpd | R1 | R2 | R3 |
|---|---|---|---|
| 117 | β-OH | α-COOH | CH2OH |
| 118 | α-O-Ac | CH2O-β-D-xylosyl | CH3 |
| 119 | α-O-COCH3 | α-COOH | CH3 |
| 120 | =O | α-COO-β-D-glucopyranosyl | CH3 |
| 121 | α-O-Ac | α-COOH | CH3 |
| 122 | =O | α-COOH | CH3 |
| 123 | β-OH | α-COOH | CH3 |
Figure 10.
Structures of compounds 124–126.
| Cpd | R1 | R2 |
|---|---|---|
| 124 | H | COOH |
| 125 | H | COOCH3 |
| 126 | β-O-Ac | COOH |
Figure 11.
Structures of compounds 127–130.
| Cpd | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 127 | β-OH | β-OH | H | =O |
| 128 | =O | =O | β-OH | α-OH |
| 129 | =O | β-OH | H | =O |
| 130 | =O | β-OH | α-OH | =O |
Figure 12.
Structures of compounds 131‒133.
| Cpd | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 131 | β-OH | =O | H | β-CH3 |
| 132 | =O | β-CH3 | α-OH | β-CH3 |
| 133 | =O | =O | H | α-CH3 |
Figure 13.
Structures of compounds 134 and 135.
| Cpd | R1 | R2 |
|---|---|---|
| 134 | α-OH | β-CH3 |
| 135 | =O | α-CH3 |
Figure 14.
Structures of compounds 136‒139.
| Cpd | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 136 | β-O-Ac | β-OH | =O | COOH |
| 137 | β-O-Ac | =O | α-OH | COOH |
| 138 | β-O-Ac | β-OH | =O | COOEt |
| 139 | =O | =O | α-OH | COOEt |
Figure 15.
Structures of compounds 140‒141.
| Cpd | R1 | R2 | R3 |
|---|---|---|---|
| 140 | =O | =O | β-OH |
| 141 | β-OH | α-OH | =O |
Figure 16.
Structures of compounds 142‒145.
| Cpd | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 |
|---|---|---|---|---|---|---|---|---|---|
| 142 | =O | =O | =O | H | α-OH | β-CH3 | =O | H | COOH |
| 143 | =O | =O | =O | H | α-OH | β-CH3 | =O | H | COOCH3 |
| 144 | =O | =O | =O | =O | =O | β-CH3 | =O | H | COOH |
| 145 | H | H | H | H | H | α-CH3 | H | ξ-CH3 | =O |
Figure 17.
Structures of compounds 146‒147.
| Cpd | R |
|---|---|
| 146 | CH2 |
| 147 | α-CH3 |
Figure 18.
Structures of compounds 148‒155.
Table 6.
Ganoderma triterpenes 156–196 in Figure 19.
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 156 | 15-Hydroxy-ganoderic acid S (15α-hydroxy-3-oxo-5α-lanosta-7,9(11),24(E)-trien-26-oic acid) |
G. lucidum (fruit bodies) | [35] |
| 157 | 3α, 16α-Dihydroxylanosta-7,9(11),24-trien-21-oic acid | G. applanatum (fruit bodies) | [69] |
| 158 | 3α, 16α, 26-Trihydroxylanosta-7,9(11),24-trien-21-oic acid | G. applanatum (fruit bodies) | [69] |
| 159 | Ganoderic acid S1 | G. lucidum (fruit bodies) | [73] |
| 160 | Ganoderic acid SZ (3-oxo-lanosta-7,9(11),24(Z)-trien-26-oic acid) |
G. lucidum (fruit bodies) | [74] |
| 161 | 5α-Lanosta-7,9(11),24-triene-15α-26-dihydroxy-3-one | G. concinna | [75] |
| 162 | Ganoderic acid Me (3α, 15α-diacetoxy-5α-lanost-7,9(11),24E-trien-26-oic acid) | G. lucidum (cultured mycelial mat) | [41] |
| 163 | Ganoderic acid Mf (3α-acetoxy-15α-hydroxy-5α-lanost-7,9(11),24E-trien-26-oic acid) | G. lucidum (cultured mycelial mat) | [41] |
| 164 | Ganodermenonol (26-hydroxy-5α-lanosta-7,9(11),24-trien-3-one) | G. lucidum (dried fruit bodies) | [76] |
| 165 | Ganodermadiol (5α-lanosta-7,9(11),24-triene-3β, 26-diol) | G. lucidum (dried fruit bodies) | [76] |
| 166 | Ganodermatriol (5α-lanosta-7,9(11),24-triene-3β, 26,27-triol) | G. lucidum (fruit bodies) | [77] |
| 167 | Ganodermic acid S (lanosta-7,9(11),24-trien-3β, 15α-diacetoxy-26-oic acid) | G. lucidum | [78] |
| 168 | Carnosodione (26,27-dihydroxylanosta-7,9(11),24-trien-3,16-dione) | G. carnosum (fruit bodies) | [79] |
| 169 | Canoderol B ((24E)-5α-lanosta-7,9(11),24-trien-3,26-diol) | G. lucidum | [23] |
| 170 | Ganoderic acid Mk (3α, 22-diacetoxy-15α-hydroxy-5α-lanost-7,9(11),24E-trien-26-oic acid) | G. lucidum (mycelia mat) | [43] |
| 171 | Ganoderiol B (15α, 26,27-trihydroxy-5α-lanosta-7,9(11),24-trien-3-one) | G. lucidum (fruit bodies) | [77] |
| 172 | Ganoderic acid T ((22S, 24E)-3α, 15α, 22-triacetoxy-5α-lanosta-7,9,(11),24-trien-26-oic acid) | G. lucidum (cultured mycelia) | [80] |
| 173 | Ganoderic acid S ((22S, 24E)-22-acetoxy-3α-hydroxy-5α-lanosta-7,9(11),24-trien-26-oic acid) | G. lucidum (cultured mycelia) | [23,80] |
| 174 | Ganoderic acid R ((22S, 24E)-3α, 22-diacetoxy-5α-lanosta-7,9,(11),24-trien-26-oic acid) | G. lucidum (cultured mycelia) | [80] |
| 175 | Ganorbiformin G | G. orbiforme | [46] |
| 176 | Lanosta-7,9(11),24-trien-3β, 15α, 22β-triacetoxy-26-oic acid | G. lucidum | [81] |
| 177 | Lanosta-7,9(11),24-trien-15α-acetoxy-3α-hydroxy-23-oxo-26-oic acid | G. lucidum | [81] |
| 178 | Lanosta-7,9(11),24-trien-3α, l5α-diacetoxy-23-oxo-26-oic acid | G. lucidum | [81] |
| 179 | Lanosta-7,9(11),24-trien-3α, 15α-hydroxy-23-oxo-26-oic acid | G. lucidum | [81] |
| 180 | Lanosta-7,9(11),24-trien-3α-acetoxy-15α, 22β-dihydroxy-26-oic acid | G. lucidum | [81] |
| 181 | Ganodermic acid T-N (3β-hydroxy-15α-acetoxy-lanosta-7,9(11),24-trien-26-oic acid) | G. lucidum (mycelia) | [82] |
| 182 | Ganodermic acid T-O (3β-acetoxy-15α-hydroxy-lanosta-7,9(11),24-trien-26-oic acid) | G. lucidum (mycelia) | [82] |
| 183 | Ganodermic acid T-Q (3β-oxo-15α-acetoxy-lanosta-7,9(11),24-trien-26-oic acid) | G. lucidum (mycelia) | [82] |
| 184 | Compound 10 | G. orbiforme | [46] |
| 185 | Ganoderic acid P ((22S, 24E)-15α, 22-diacetoxy-3α-hydroxy-5α-lanosta-7,9(11),24-trien-26-oic acid) | G. lucidum (cultured mycelium) | [50] |
| 186 | Ganoderic acid Q ((22S, 24E)-3α, 22-diacetoxy-15α-hydroxy-5α-lanosta-7,9(11),24-trien-26-oic acid) | G. lucidum (cultured mycelium) | [50] |
| 187 | Ganoderic acid Jc (15α, 23-dihydroxy-3-oxo-5α-lanosta-7,9(11),24-trien-26-oic acid) | G. sinense (fruit bodies) | [47] |
| 188 | Ganodermatetraol (3β, 15α, 26,27-tetrahydroxy-5α-lanosta-7,9(11),24-triene) | G. sinense (fruit bodies) | [47] |
| 189 | 5α-Lanosta-7,9(11),24-triene-3β-hydroxy-26-al | G. concinna | [75] |
| 190 | Ganoderiol F (26,27-dihydroxy-5α-lanosta-7,9(11),24-trien-3-one) | G. lucidum (fruit bodies) | [39] |
| 191 | 26,27-Dihydroxy-5α-lanosta-7,9(11),24-triene-3,22-dione | G. lucidum (basidiocarp) | [83] |
| 192 | 26-Hydroxy-5α-lanosta-7,9(11),24-triene-3,22-dione | G. lucidum (basidiocarp) | [83] |
| 193 | Ganodermic acid P1 (lanosta-7,9(11),24-trien-3α, 22β-diacetoxy-15α-hydroxy-26-oic acid) | G. lucidum (mycelia) | [84] |
| 194 | Ganodermic acid P2 (lanosta-7,9(11),24-trien-15α, 22β-diacetoxy-3β-hydroxy-26-oic acid) | G. lucidum (mycelia) | [84] |
| 195 | Lanosta-7,9(11),24-trien-3β, 15α, 22-triacetoxy-26-oic acid | G. amboinense (fruit bodies) | [85] |
| 196 | 16α-Hydroxy-3-oxolanosta-7,9(11),24-trien-21-oic acid | G. applanatum (fruit bodies) | [69] |
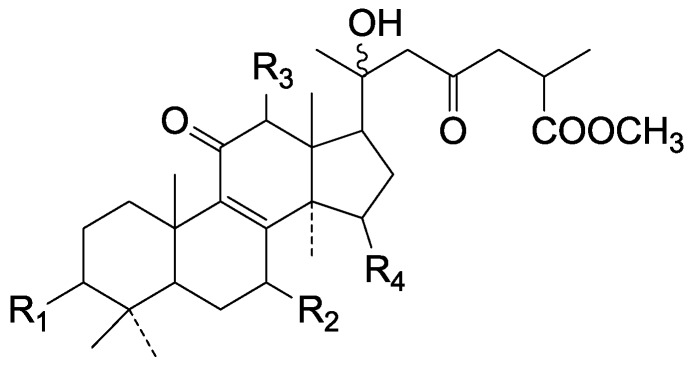
Figure 19.
Structures of compounds 156–196.
| Cpd | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | |
|---|---|---|---|---|---|---|---|---|---|
| 156 | =O | α-OH | H | β-CH3 | H | H | COOH | CH3 | |
| 157 | α-OH | H | α-OH | α-COOH | H | H | CH3 | CH3 | |
| 158 | α-OH | H | α-OH | α-COOH | H | H | CH2OH | CH3 | |
| 159 | =O | H | H | α-CH3 | H | H | COOH | CH3 | |
| 160 | =O | H | H | β-CH3 | H | H | COOH | CH3 | |
| 161 | =O | α-OH | H | α-CH3 | H | H | CH2OH | CH3 | |
| 162 | α-O-Ac | α-O-Ac | H | β-CH3 | ξ-H2 | H | COOH | CH3 | |
| 163 | β-O-Ac | α-OH | H | β-CH3 | ξ-H2 | H | COOH | CH3 | |
| 164 | =O | H | H | α-CH3 | H | H | CH2OH | CH3 | |
| 165 | β-OH | H | H | α-CH3 | H | H | CH2OH | CH3 | |
| 166 | β-OH | H | H | α-CH3 | H | H | CH2OH | CH2OH | |
| 167 | β-O-Ac | α-O-Ac | H | β-CH3 | H | H | COOH | CH3 | |
| 168 | =O | H | O | β-CH3 | H | H | CH2OH | CH2OH | |
| 169 | β-OH | H | H | β-CH3 | H | H | CH2OH | CH3 | |
| 170 | α-O-Ac | α-OH | H | β-CH3 | ξ-O-Ac | H | COOH | CH3 | |
| 171 | =O | α-OH | H | α-CH3 | H | H | CH2OH | CH2OH | |
| 172 | α-O-Ac | α-O-Ac | H | α-CH3 | β-O-Ac | H | COOH | CH3 | |
| 173 | α-OH | H | H | α-CH3 | β-O-Ac | H | COOH | CH3 | |
| 174 | α-O-Ac | H | H | α-CH3 | β-O-Ac | H | COOH | CH3 | |
| 175 | =O | H | H | α-CH3 | β-O-Ac | H | COOH | CH3 | |
| 176 | β-O-Ac | α-O-Ac | H | β-CH3 | β-O-Ac | H | COOH | CH3 | |
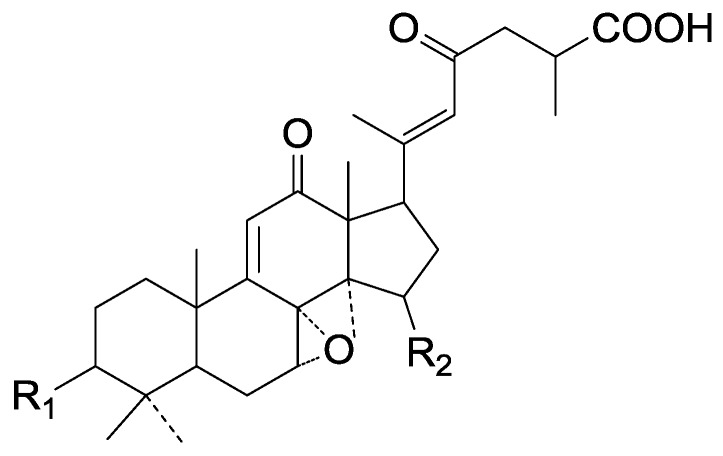
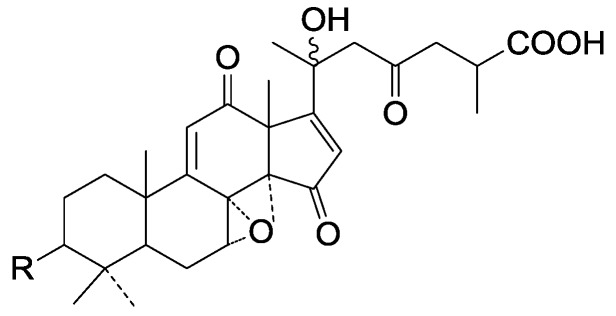
| 177 | α-OH | α-O-Ac | H | β-CH3 | H | =O | COOH | CH3 | |
| 178 | α-O-Ac | α-O-Ac | H | β-CH3 | H | =O | COOH | CH3 | |
| 179 | α-O-Ac | α-OH | H | β-CH3 | H | =O | COOH | CH3 | |
| 180 | α-O-Ac | α-OH | H | β-CH3 | H | H | COOH | CH3 | |
| 181 | β-OH | α-O-Ac | H | β-CH3 | H | H | COOH | CH3 | |
| 182 | β-O-Ac | α-OH | H | β-CH3 | H | H | COOH | CH3 | |
| 183 | =O | α-O-Ac | H | β-CH3 | H | H | COOH | CH3 | |
| 184 | =O | α-O-Ac | H | α-CH3 | β-O-Ac | H | COOH | CH3 | |
| 185 | α-OH | α-O-Ac | H | α-CH3 | β-O-Ac | H | COOH | CH3 | |
| 186 | α-O-Ac | α-OH | H | α-CH3 | β-O-Ac | H | COOH | CH3 | |
| 187 | =O | α-OH | H | α-CH3 | H | OH | COOH | CH3 | |
| 188 | β-OH | α-OH | H | α-CH3 | H | H | CH2OH | CH2OH | |
| 189 | β-OH | H | H | α-CH3 | H | H | CHO | CH3 | |
| 190 | =O | H | H | β-CH3 | H | H | CH2OH | CH2OH | |
| 191 | =O | H | H | α-CH3 | =O | H | CH2OH | CH2OH | |
| 192 | =O | H | H | α-CH3 | =O | H | CH2OH | CH3 | |
| 193 | α-O-Ac | α-OH | H | β-CH3 | O-Ac | H | COOH | CH3 | |
| 194 | β-OH | α-O-Ac | H | β-CH3 | O-Ac | H | COOH | CH3 | |
| 195 | β-O-Ac | α-O-Ac | H | α-CH3 | β-O-Ac | H | COOH | CH3 | |
| 196 | =O | H | α-OH | α-COOH | H | H | CH3 | CH3 |
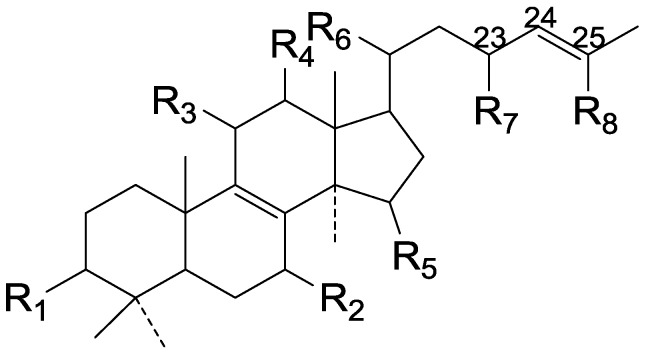
Figure 20.
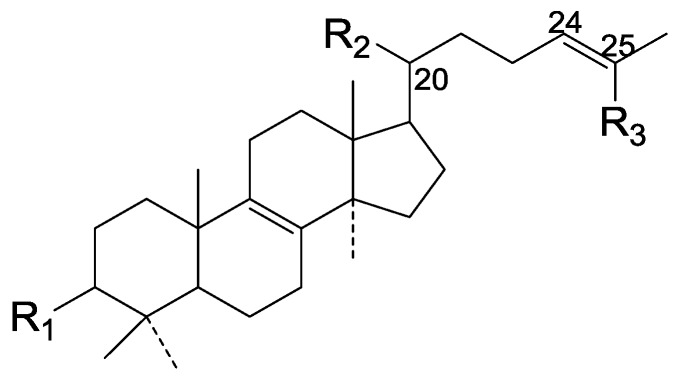
Structures of compounds 197–213.
| Cpd | R1 | R2 | R3 | R4 | R5 | R6 |
|---|---|---|---|---|---|---|
| 197 | β-OH | H | α-CH3 | △24,25 | △24,25 | CHO |
| 198 | β-O-Ac | H | α-CH3 | OAc | OH | CH2-O-Ac |
| 199 | =O | H | β-CH3 | △24,25 | △24,25 | CHO |
| 200 | =O | H | β-CH3 | △24,25 | △24,25 | CH2OH |
| 201 | β-OH | H | α-CH3 | α-OH | OH | CH3 |
| 202 | =O | H | β-CH3 | α-OH | H | CH2OH |
| 203 | β-OH | H | α-CH3 | OH | OH | CH2OH |
| 204 | =O | H | α-CH3 | OH | OH | CH3 |
| 205 | β-OH | α-O-Ac | β-CH3 | H | H | COOH |
| 206 | =O | α-OH | α-CH3 | △24,25 | △24,25 | COOH |
| 207 | α-OH | α-OH | β-CH3 | △24,25 | △24,25 | COOH |
| 208 | β-OH | α-OH | β-CH3 | △24,25 | △24,25 | COOH |
| 209 | α-O-Ac | α-O-Ac | β-CH3 | △24,25 | △24,25 | COOH |
| 210 | =O | H | α-CH3 | OH | OH | CH2OH |
| 211 | =O | α-OH | α-CH3 | △24,25 | △24,25 | COOH |
| 212 | =O | α-OH | α-CH3 | △24,25 | △24,25 | CH2OH |
| 213 | β-OH | H | α-CH3 | △24,25 | △24,25 | COOH |
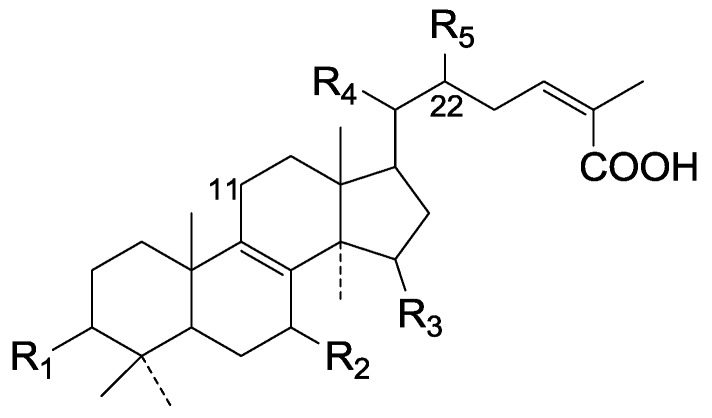
Figure 21.
Structures of compounds 214–219.
| Cpd | R1 | R2 | R3 |
|---|---|---|---|
| 214 | α-OH | α-OH | α-OH |
| 215 | β-OH | α-OH | β-OH |
| 216 | α-O-Ac | α-O-Ac | α-OH |
| 217 | β-O-Ac | α-O-Ac | α-OH |
| 218 | α-OH | α-OH | β-O-Ac |
| 219 | β-OH | α-OH | β-O-Ac |
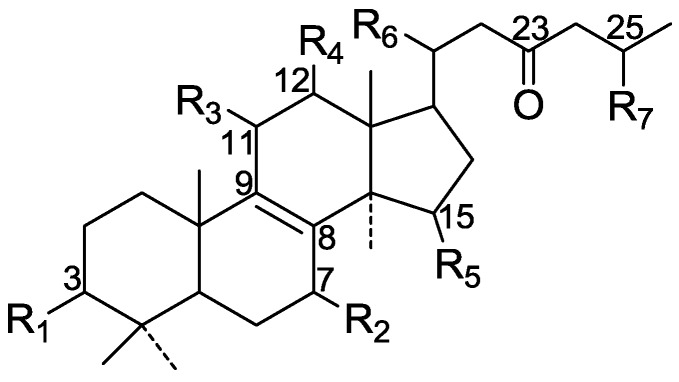
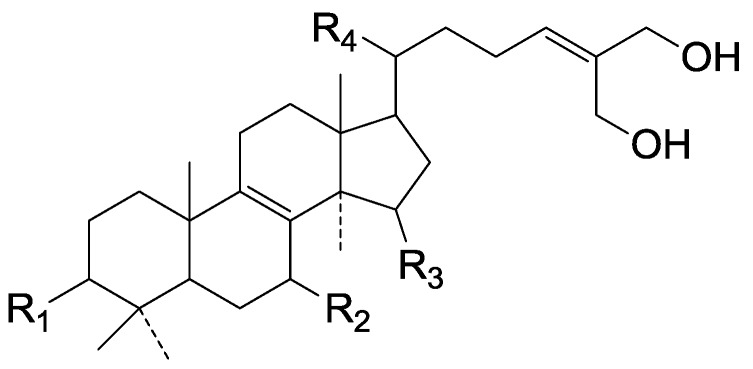
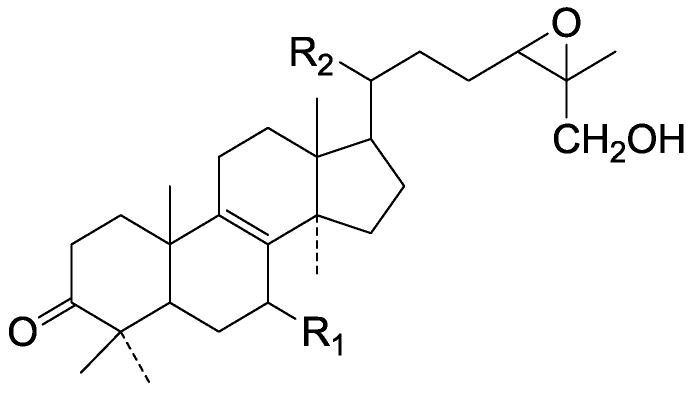
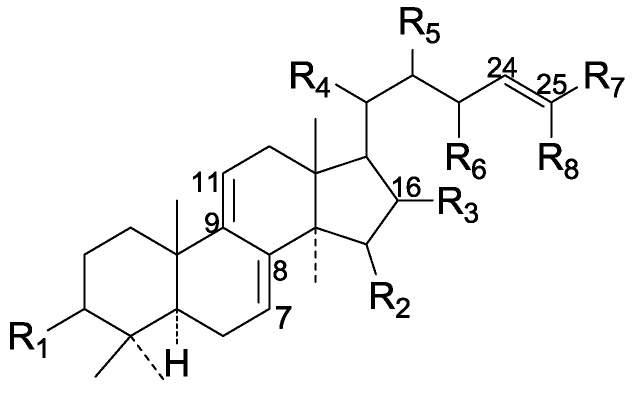
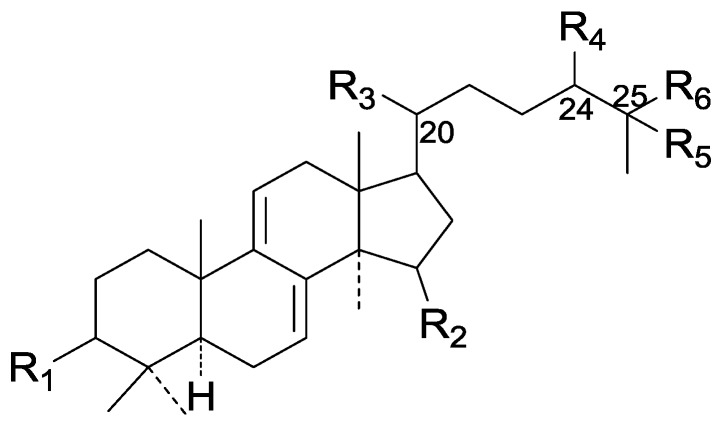
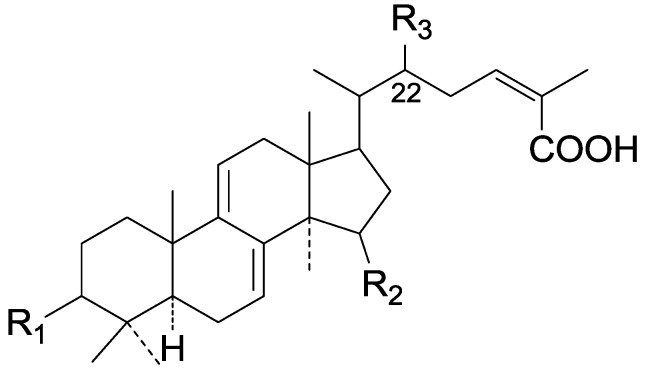
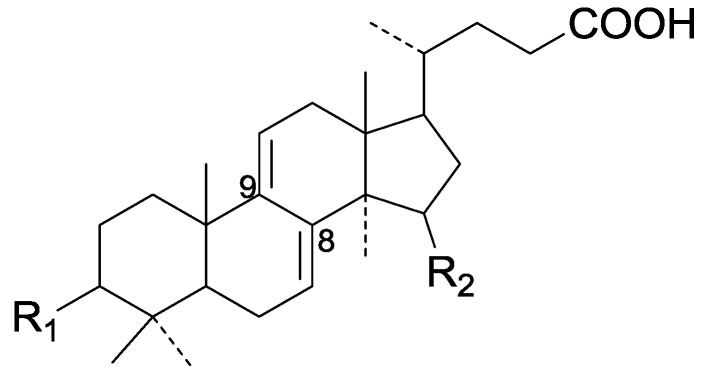
Compared with the compounds in Figure 2, compounds 38–70 in Figure 3 possess double bonds between C-24 and C-25, and have a hydroxy or no substituent at C-23, instead of a carbonyl. Some other substituents are also found at C-3, 7, 11, 12, 15, 23, 25. In this group, lucialdehyde C (46) displays strong antitumor activity and ganoderic acid β (53) reveals great anti-HIV-1 protease activity. Compounds 71–84 (Figure 4) get an acetate substituent at C-22 and no substituent at C-11. Meanwhile, compounds 85–98 (Figure 5) have double bonds at C-20(22) and keto groups at C-11. From all the listed structures, we can clearly identify compounds 99–105 in Figure 6 by the carboxymethyl substitution at C-25, carbonyl substituent at C-11, a keto group at C-23, and β-configuration of C-21. Compounds 106–110 are assigned to the same group owing to the methyl at C-20, carbonyl substituent at C-11, and carboxyl at C-25. Lucidumol A (111), ganoderiol C-H (112–115), and ganoderitriol M (116) differ from the others on account of the hydroxy at C-24 and C-25. As is shown in Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15, Figure 16 and Figure 17, compounds 117–123, 124–126, 127–130, 131–133, 134–135, 136–139, 140–141, 142–145 and 146–147 possess extremely similar skeletons. Because of their distinctive skeletons, 148–155 are listed independently. There are no double bonds between C-8 and C-9 in compounds 156–221, and two double bonds at C-7(8) and C-9(11), respectively. Among the compounds above, ganoderic acid Jc (187), ganoderiol F (190), and 15α,26-dihydroxy-5α-lanosta-7,9,24(E)-trien-3-one (212) showed remarkable antitumor activity. Significant anti-HIV-1 protease activity has been expressed in ganoderic acid S1 (159) and ganodermic acid T-Q (183). Compounds 156–196 (Figure 19) have the same skeleton with substituents at C-3, 15, 16, 20, 22, 23, 25 and double bonds at C-24(25). In this group, 3α, 16α-dihydroxylanosta-7,9(11),24-trien-21-oic acid (157), 3α, 16α, 26-trihydroxylanosta-7,9(11), 24-trien-21-oic acid (158) and 16α-hydroxy-3-oxolanosta-7,9(11),24-trien-21-oic acid (196) possess a hydroxyl at C-16 and carboxyl at C-20. Compounds 197–213 (Figure 20) have the same position of substituents. They possess an α- or β-configuration at C-21. The majority have double bonds between C-24 and C-25, except some with hydroxyl, acetoxyl or no substituents at C-24 and C-25. Compounds 214–219 (Figure 21) have a hydroxy or acetoxyl at C-22, while epoxyganoderiol B (220) and C (221) (Figure 22) possess an epoxy at C-24(25). Compounds 222–266 have the basic skeleton of 27 carbon atoms. Furthermore, they are also subdivided into different groups due to the difference of substituents and position of double bonds. The C-8(9) double bonds are the same in compounds 222–260 (Figure 23). 4,4,14α-Trimethyl-5α-chol-7,9(11)-dien-3-oxo-24-oic acid (261) and ganoderic acid Jd (262) (Figure 24) get two double bonds at C-7(8) and C-9(11), respectively. Compared with the compounds in Figure 22, compounds 263–266 in Figure 25 have hydroxy substituents at C-29. Compounds 267–287 are divided into different groups on account of their characteristic skeletons. We list the structures of compounds 288–307 successively, in consideration of the number of substituents and the substituents’ complicated positions. Fornicatin B(308), G(309), A(310), H(311) and australic acid (312) are 3,4-seco-trinorlanostane triterpenoids. In addition, compounds 313–316 only have 24 carbon atoms. The names, corresponding plant resources and references of the compounds are compiled in Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10 and Table 11.
Figure 22.
Structure of compounds 220–221.
| Cpd | R1 |
|---|---|
| 220 | β-OH |
| 221 | =O |
Figure 23.
Structures of compounds 222–260.
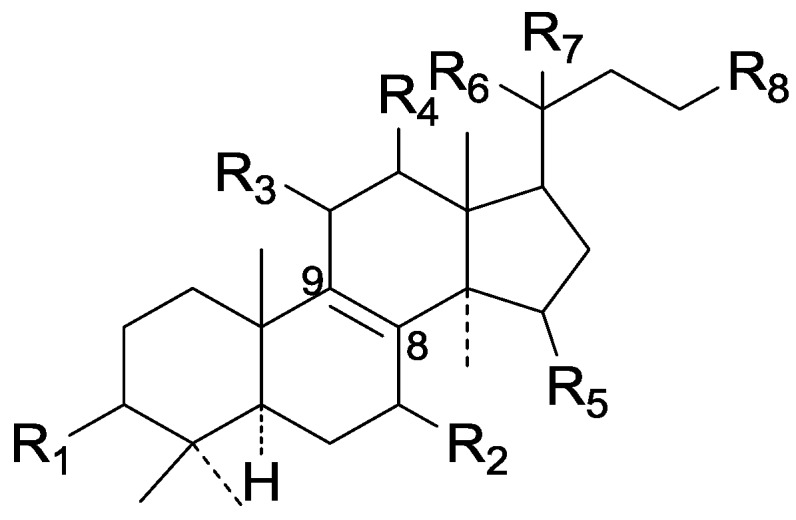
| Cpd | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 |
|---|---|---|---|---|---|---|---|---|
| 222 | β-OH | β-OH | =O | H | =O | α-CH3 | H | COOBu |
| 223 | =O | β-OH | =O | H | =O | α-CH3 | H | COOBu |
| 224 | β-OH | β-OH | =O | H | =O | CH2 | △20,21 | COOH |
| 225 | =O | β-OH | =O | H | =O | β-CH3 | ξ-OH | COOH |
| 226 | =O | =O | =O | β-O-Ac | =O | β-CH3 | H | COOCH3 |
| 227 | =O | β-OH | =O | H | =O | CH2 | △20,21 | COOH |
| 228 | =O | β-OH | =O | H | =O | CH2 | △20,21 | COOCH3 |
| 229 | β-OH | β-OH | =O | H | =O | α-CH3 | H | COOH |
| 230 | =O | =O | =O | β-O-Ac | =O | β-CH3 | H | COOH |
| 231 | β-OH | =O | =O | β-O-Ac | =O | β-CH3 | H | COOCH3 |
| 232 | =O | =O | =O | H | =O | β-CH3 | H | COOCH3 |
| 233 | =O | OH | =O | H | =O | α-CH3 | H | COOEt |
| 234 | β-O-CHO | β-OH | =O | OH | =O | β-CH3 | H | COOH |
| 235 | =O | β-OH | =O | H | =O | β-CH3 | H | COOH |
| 236 | =O | β-OH | =O | β-OH | =O | β-CH3 | H | COOH |
| 237 | β-OH | β-OH | =O | β-OH | =O | β-CH3 | H | COOH |
| 238 | =O | =O | H | H | H | α-CH3 | H | COOH |
| 239 | β-OH | β-OH | =O | β-O-Ac | =O | α-CH3 | H | COOH |
| 240 | β-OH | β-OH | =O | β-O-Ac | =O | α-CH3 | H | COOCH3 |
| 241 | =O | β-OH | =O | H | α-OH | α-CH3 | H | COOCH3 |
| 242 | β-OH | =O | =O | H | =O | α-CH3 | H | COOH |
| 243 | =O | =O | =O | O-Ac | =O | β-CH3 | H | COOCH3 |
| 244 | β-OH | =O | =O | O-Ac | =O | β-CH3 | H | COOCH3 |
| 245 | =O | =O | =O | α-OH | =O | β-CH3 | H | COOCH3 |
| 246 | β-OH | =O | =O | β-OH | =O | β-CH3 | H | COOCH3 |
| 247 | β-OH | α-OH | =O | H | α-OH | β-CH3 | H | COOCH3 |
| 248 | β-OH | β-OH | =O | H | =O | α-CH3 | H | COOCH3 |
| 249 | =O | β-OH | =O | β-OH | =O | α-CH3 | H | COOBu |
| 250 | =O | β-OH | =O | H | =O | α-CH3 | H | COOCH3 |
| 251 | =O | =O | =O | β-O-Ac | =O | α-CH3 | H | COOH |
| 252 | =O | =O | =O | β-O-Ac | =O | β-CH3 | ξ-OH | COOH |
| 253 | =O | =O | =O | H | =O | β-CH3 | ξ-OH | COOH |
| 254 | β-OH | =O | =O | β-O-Ac | =O | β-CH3 | ξ-OH | COOH |
| 255 | β-OH | β-OH | =O | H | =O | β-CH3 | ξ-OH | COOH |
| 256 | β-OH | β-OH | =O | β-O-Ac | =O | β-CH3 | ξ-OH | COOH |
| 257 | =O | =O | =O | H | =O | α-CH3 | H | COOH |
| 258 | β-OH | β-OH | =O | H | =O | α-CH3 | H | COOCH3 |
| 259 | β-OH | =O | =O | β-O-Ac | =O | α-CH3 | H | COOH |
| 260 | =O | β-OH | =O | H | =O | α-CH3 | H | COOH |
Figure 24.
Structures of compounds 261–262.
| Cpd | R1 | R2 |
|---|---|---|
| 261 | =O | H |
| 262 | =O | α-OH |
Figure 25.
Structures of compounds 263–266.
| Cpd | R1 | R2 | R3 |
|---|---|---|---|
| 263 | β-OH | H | β-CH3 |
| 264 | =O | H | β-CH3 |
| 265 | =O | β-OH | β-CH3 |
| 266 | β-OH | H | α-CH3 |
Table 1.
Ganoderma triterpenes 1–37 in Figure 2.
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 1 | n-Butyl ganoderate H (n-butyl 12β-acetoxy-3β-hydroxy-7,11,15,23-tetraoxo-5α-lanost-8-en-26-oate) | G. lucidum (fruit bodies) | [16] |
| 2 | Butyl ganoderate A | G. lucidum (fruit bodies) | [17] |
| 3 | Butyl ganoderate B | G. lucidum (fruit bodies) | [17] |
| 4 | Ganoderic acid α (12β-acetoxy-3β, 15β-dihydroxy-7,11,23-trioxo-5α-lanosta-8-en-26-oic acid) | G. lucidum (fruit bodies) | [18] |
| 5 | Ganolucidic acid A | G. lucidum (gill surface) | [19] |
| 6 | Methyl ganolucidate A (methyl 15α-hydroxy-3,11,23-trioxo-5α-lanost-8-en-26-oate) | G. lucidum (gill surface) | [19,20] |
| 7 | Ganolucidic acid B | G. lucidum (gill surface) | [19] |
| 8 | Methyl ganolucidate B | G. lucidum (gill surface) | [19,20] |
| 9 | Ganoderic acid A (7β, 15α-dihydroxy-3,11,23-trioxo-5α-lanost-8-en-26-oic acid) | G. lucidum | [9,21] |
| 10 | Methyl ganoderate A (methyl 7β, 15α-dihydroxy-3,11,23-trioxo-5α-lanost-8-en-26-oate) | G. lucidum | [21] |
| 11 | Ganoderic acid B (3β, 7β-dihydroxy-11,15,23-trioxo-5α-lanost-8-en-26-oic acid) | G. lucidum | [21] |
| 12 | Methyl ganoderate B (methyl 3β, 7β-dihydroxy-11,15,23-trioxo-5α-lanost-8-en-26-oate) | G. lucidum | [9,21] |
| 13 | Ganoderic acid C (3β, 7β, 15α-trihydroxy-11,23-dioxo-5α-lanost-8-en-26-oic acid) | G. lucidum | [21] |
| 14 | Methyl ganoderate C | G. lucidum | [21] |
| 15 | Ganoderic acid D (7β-hydroxy-3,11,15,23-tetraoxo-5α-lanost-8-en-26-oic acid) | G. lucidum | [21] |
| 16 | Methyl ganoderate D | G. lucidum | [21] |
| 17 | Methyl ganoderate C2 (methyl 3β, 7β, 15α-trihydroxy-11,23-dioxo-5α-lanost-8-en-26-oate) | G. lucidum (gills) | [22] |
| 18 | Methyl ganoderate K | G. lucidum (gills) | [22,23] |
| 19 | Compound B8 | G. lucidum (gills) | [22] |
| 20 | Compound B9 | G. lucidum (gills) | [22] |
| 21 | 3β-Oxo-formyl-7β, 12β-dihydroxy-5α-lanost-11,15,23-trioxo-8-en(E)-26-oic acid | G. lucidum (fruit bodies) | [24] |
| 22 | Ganoderic acid B8 | G. lucidum (fruit bodies) | [25] |
| 23 | Ganoderic acid C1 | G. lucidum (fruit bodies) | [25] |
| 24 | 12β-Acetoxy-3,7,11,15,23-pentaoxo-5α-lanosta-8-en-26-oic acid ethyl ester | G. lucidum | [26] |
| 25 | 3β, 7β-Dihydroxy-12β-acetoxy-11,15,23-trioxo-5α-lanosta-8-en-26-oic acid methyl ester | G. lucidum | [27] |
| 26 | 3β-Hydroxy-7,11,12,15,23-pentaoxolanost-8-en-26-oic acid | G. lucidum (fruit bodies) | [28] |
| 27 | Ganoderic acid Df (7β, 11β-dihydroxy-3,15,23-trioxo-5α-lanosta-8-en-26-oic acid) | G. lucidum | [29] |
| 28 | Ganoderic acid H | G. lucidum (gill surface) | [30] |
| 29 | Ganoderic acid F (12β-acetoxy-3,7,11,15-pentaoxo-5α-lanost-8-en-26-oic acid) | G. lucidum (dried fruit bodies) | [31] |
| 30 | Ganoderic acid E (3,7,11,15,23-pentaoxo-5α-lanost-8-en-26-oic acid) | G. lucidum (dried fruit bodies) | [31] |
| 31 | Ganoderic acid K | G. lucidum (fruit bodies) | [32] |
| 32 | Ganoderic acid AM1 | G. lucidum (fruit bodies) | [32] |
| 33 | Ganoderic acid J | G. lucidum (fruit bodies) | [32] |
| 34 | Ganoderic acid C2 (3β, 7β, 15α-trihydroxy-11,23-dioxo-5α-lanosta-8-en-26-oic acid) | G. lucidum (gills) | [22] |
| 35 | Ganoderic acid G (3β, 7β, 15β-trihydroxy-11,15,23-trioxo-5α-lanosta-8-en-26-oic acid) | G. lucidum (dried fruit bodies) | [31] |
| 36 | 7β, 12β-Dihydroxy-3,11,15,23-tetraoxo-5α-lanosta-8-en-26-oic acid | G. lucidum | [26] |
| 37 | 12β-Hydroxy-3,7,11,15,23-pentaoxo-5α-lanosta-8-en-26-oic acid | G. lucidum | [26] |
Table 2.
Ganoderma triterpenes (38–70) in Figure 3.
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 38 | Ganoderic acid GS-1 (7β-hydroxy-3,11,15-trioxolanosta-8,24(E)-dien-26-oic acid) | G. sinense (fruit bodies) | [33] |
| 39 | Ganoderic acid GS-2 (7β, 15α-dihydroxy-3,11-dioxolanosta-8,24(E)-dien-26-oic acid) | G. sinense (fruit bodies) | [33] |
| 40 | Ganoderic acid GS-3 (12β-acetoxy-3β, 7β-dihydroxy-11,15-dioxo-lanosta-8,24(E)-dien-26-oic acid) | G. sinense (fruit bodies) | [33] |
| 41 | Ganoderic acid AP2 (12β, 15α-diacetoxy-3β-hydroxy-11-oxolanost-8,24(E)-dien-26-oic acid) | G. applanatum (fruit bodies) | [34] |
| 42 | 23S-Hydroxy-3,7,11,15-tetraoxolanost-8,24E-diene-26-oic acid | G. lucidum (fruit bodies) | [32] |
| 43 | 7-Oxoganoderic acid Z (3β-hydroxy-7-oxo-5α-lanosta-8,24(E)-dien-26-oic acid) | G. lucidum (fruit bodies) | [35] |
| 44 | Ganoderic acid LM2 ((23S) 7β,-dihydroxy-3,11,15-trioxo-5α-lanosta-8,24-dien-26-oic acid) | G. lucidum (fruit bodies) | [36] |
| 45 | Lucialdehyde B ((24E)-3,7-dioxo-5α-lanosta-8,24-dien-26-al) | G. lucidum (fruit bodies) | [25] |
| 46 | Lucialdehyde C ((24E)-3β-hydroxy-7-oxo-5α-lanosta-8,24-dien-26-al) | G. lucidum (fruit bodies) | [25] |
| 47 | Ganoderic acid γ ((23S)-7β, 15α, 23-trihydroxy-3,11-dioxolanosta-8,24(E)-diene-26-oic acid) | G. lucidum (spores) | [37] |
| 48 | Ganoderic acid δ ((23S)-7α, 15α, 23-trihydroxy-3,11-dioxolanosta-8,24(E)-diene-26-oic acid) | G. lucidum (spores) | [37] |
| 49 | Ganoderic acid ε ((23S)-3β, 7β, 23-trihydroxy-11,15-dioxolanosta-8,24(E)-diene-26-oic acid) | G. lucidum (spores) | [37] |
| 50 | Ganoderic acid ζ ((23S)-3β, 23-dihydroxy-7,11,15-trioxolanosta-8,24(E)-diene-26-oic acid) | G. lucidum (spores) | [37] |
| 51 | Ganoderic acid η ((23S)-3β, 7β, 12β, 23-tetrahydroxy-11,15-dioxolanosta-8,24(E)-diene-26-oic acid) | G. lucidum (spores) | [37] |
| 52 | Ganoderic acid θ ((23S)-3β, 12β, 23-trihydroxy-7,11,15-trioxolanosta-8,24(E)-diene-26-oic acid) | G. lucidum (spores) | [37] |
| 53 | Ganoderic acid β (3β, 7β-dihydroxy-11,15-dioxolanosta-8,24(E)-dien-26-oic acid) | G. lucidum (spores) | [38] |
| 54 | Ganolucidic acid E (15α-hydroxy-3,11-dioxo-5α-lanosta-8,24E-dien-26-oic acid) | G. lucidum (fruit bodies) | [39] |
| 55 | Ganoderal B (7α-hydroxy-3-oxo-5α-lanosta-8,24E-dien-26-al) | G. lucidum | [40] |
| 56 | Ganoderic acid Ma (3α, 7α-diacetoxy-15α-hydroxy-5α-lanost-8,24E-dien-26-oic acid) | G. lucidum (fruit bodies) | [41] |
| 57 | Lucialdehyde D (3,7,11-trioxo-5α-lanosta-8,24-diene-26-al) | G. pfeifferi (fruit bodies) | [42] |
| 58 | Ganoderone A (5α-lanosta-8,24-diene-26-hydroxy-3,7-dione) | G. pfeifferi (fruit bodies) | [42] |
| 59 | ganoderic acid Mi (3α-acetoxy-15α-hydroxy-7α-methoxy-5α-lanost-8,24E-dien-26-oic acid) | G. lucidum (mycelial mat) | [43] |
| 60 | 11α-Hydroxy-3,7-dioxo-5α-lanosta-8,24(E)-dien-26-oic acid | G. lucidum | [26] |
| 61 | 11β-Hydroxy-3,7-dioxo-5α-lanosta-8,24(E)-dien-26-oic acid | G. lucidum | [26] |
| 62 | Lucidadiol (5α-lanosta-8,24-dien-3β, 26-dihydroxy-7-one) | G. lucidum | [44] |
| 63 | Lucidal (5α-lanosta-8,24E-dien-3β-hydroxy-7-on-26-al) | G. lucidum | [44] |
| 64 | Ganoderic acid DM (3,7-dioxo-8,24(E)-dien-lanosta-26-oic acid) | G. lucidum (cultured fruit bodies) | [45] |
| 65 | Ganoderic acid V | G. orbiforme | [46] |
| 66 | Ganolucidic acid γa (3β, 7β, 15α, 23-tetrahydroxy-11-oxo-5α-lanosta-8,24-dien-26-oic acid) | G. sinense (fruit bodies) | [47] |
| 67 | Ganolucidate F (3β, 15α, 23-trihydroxy-11-oxo-5α-lanosta-8,24-dien-26-oic acid) | G. sinense (fruit bodies) | [47] |
| 68 | Lucialdehyde E (7β, 15α-dihydroxy-3,11-dioxo-5α-lanosta-8,24-dien-26-al) | G. lucidum (spores) | [48] |
| 69 | Ganolucidic acid D | G. lucidum (spores) | [37] |
| 70 | Ganoderic acid W | G. lucidum (fruit bodies) | [41] |
Table 3.
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 71 | Ganoderic acid Mb (3α, 15α, 22-triacetoxy-7α-hydroxy-5α-lanost-8,24E-dien-26-oic acid) | G. lucidum (fruit bodies) | [41] |
| 72 | Ganoderic acid Mc (3α, 7α, 22-triacetoxy-15α-hydroxy-5α-lanost-8,24E-dien-26-oic acid) | G. lucidum (fruit bodies) | [41] |
| 73 | Ganoderic acid Md (3α, 22-diacetoxy-7α-methoxy-5α-lanost-8,24E-dien-26-oic acid) | G. lucidum (fruit bodies) | [41] |
| 74 | Ganoderic acid Mg (3α, 22-diacetoxy-15α-hydroxy-7α-methoxy-5α-lanost-8,24E-dien-26-oic acid) | G. lucidum (mycelial mat) | [43] |
| 75 | Ganoderic acid Mh (3α, 22-diacetoxy-7α, 15α-dihydroxy-5α-lanost-8,24E-dien-26-oic acid) | G. lucidum (mycelial mat) | [43] |
| 76 | Ganoderic acid Mj (22-acetoxy-3α-hydroxy-7α-methoxy-5α-lanost-8,24E-dien-26-oic acid) | G. lucidum (mycelial mat) | [43] |
| 77 | 3α, 22β-Diacetoxy-7α-hydroxyl-5α-lanost-8,24E-dien-26-oic acid | G. lucidum (mycelial mat) | [49] |
| 78 | Ganorbiformin B | G. orbiforme | [46] |
| 79 | Ganorbiformin C | G. orbiforme | [46] |
| 80 | Ganorbiformin D | G. orbiforme | [46] |
| 81 | Ganorbiformin E | G. orbiforme | [46] |
| 82 | Ganorbiformin F | G. orbiforme | [46] |
| 83 | Ganoderic acid O ((22S, 24E)-3α, l5α, 22-triacetoxy-7α-hydroxy-5α-lanosta-7,24-dien-26-oic acid) | G. lucidum (cultured mycelium) | [50] |
| 84 | 7-O-Methylganoderic acid O ((22S, 24E)-3α, l5α, 22-triacetoxy-7α-methoxy-5α-lanosta-8,24-dien-26-oic acid) | G. Lucidum (cultured mycelium) | [50] |
| 85 | 12β-Acetoxy-3β-hydroxy-7,11,15,23-tetraoxo-lanost-8,20E-diene-26-oic acid | G. lucidum (fruit bodies) | [32] |
| 86 | 23-Dihydroganoderenic acid D (7β, 23ξ-dihydroxy-3,11,15-trioxolanosta-8,20E(22)-dien-26-oic acid) | G. applanatum (fruit bodies) | [51] |
| 87 | Methyl ganoderenate D (7β-hydroxy-3,11,15,23-tetraoxolanosta-8,20E(22)-dien-26-oic acid methyl ester) | G. applanatum (fruit bodies) | [51] |
| 88 | Ganoderenic acid A ((20E)-7β, 15α-dihydroxy-3,11,23-trioxo-5α-lanost-8,20-dien-26-oic acid) | G. lucidum (dried fruit bodies) | [31] |
| 89 | Ganoderenic acid B ((20E)-3β, 7β-dihydroxy-11,15,23-trioxo-5α-lanost-8,20-dien-26-oic acid) | G. lucidum (dried fruit bodies) | [31] |
| 90 | Ganoderenic acid C ((20E)-3β, 7β, 15α-trihydroxy-11,23-dioxo-5α-lanost-8,20-dien-26-oic acid) | G. lucidum (dried fruit bodies) | [31] |
| 91 | Ganoderenic acid D ((20E)-7β-hydroxy-3,11,15,23-tetraoxo-5α-lanost-8,20-dien-26-oic acid) | G. lucidum (dried fruit bodies) | [31] |
| 92 | 12β-Acetoxy-7β-hydroxy-3,11,15,23-tetraoxo-5α-lanosta-8,20-dien-26-oic acid | G. lucidum | [26] |
| 93 | Ganoderenic acid F (3,7,11,15,23-pentaoxo-5α-lanosta-8,20E-dien-26-oic acid) | G. applanatum (fruit bodies) | [52] |
| 94 | Ganoderenic acid G (15α-hydroxy-3,7,11,23-tetraoxo-5α-lanosta-8,20E-dien-26-oic acid) | G. applanatum (fruit bodies) | [52] |
| 95 | Methy ganoderenate H (methyl 3β-hydroxy-7,11,15,23-tetraoxo-5α-lanosta-8,20E-dien-26-oate) | G. applanatum (fruit bodies) | [52] |
| 96 | Methyl ganoderenate I (3β, 15α-dihydroxy-7,11,23-trioxo-5α-lanosta-8,20E-dien-26-oate) | G. applanatum (fruit bodies) | [52] |
| 97 | Ganoderenic acid H | G. lucidum (fruit bodies) | [32] |
| 98 | 12β-Acetoxy-3β, 7β-dihydroxy-11,15,23-trioxo-5α-lanosta-8,20-dien-26-oic acid | G. lucidum | [26] |
Table 4.
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 99 | Methyl ganoderate D (methyl 3β, 7β, 15α-trihydroxy-11,23-dioxo-5α-lanost-8-en-26-oate) | G. lucidum (fruit bodies) | [53,54] |
| 100 | Methyl ganoderate E (methyl 3β, 7β, 15α-trihydroxy-11,23-dioxo-5α-lanost-8-en-26-oate) | G. lucidum (gills) | [54,55] |
| 101 | Methyl ganoderate F (methyl 12β-acetoxy-3,7,11,15,23-pentaoxo-5α-lanost-8-en-26-oate) | G. lucidum (gills) | [56] |
| 102 | Methyl ganoderate H (methyl 3β-hydroxy-12β-acetoxy-7,11,15,23-tetraoxo-5α-lanost-8-en-26-oate) | G. lucidum (gills) | [30,56] |
| 103 | Methyl ganoderate G (methyl 3β, 7β, 12β-trihydroxy-11,15,23-trioxo-5α-lanost-8-en-26-oate) | G. lucidum | [20] |
| 104 | Compound C5 | G. lucidum (gill surface) | [30] |
| 105 | Compound C6 | G. lucidum (gill surface) | [30] |
| 106 | Ganoderic acid AP3 (15α, 20ξ-dihydroxy-3,7,11,23-tetraoxolanost-8-en-26-oic acid) | G. applanatum (fruit bodies) | [34] |
| 107 | 23-Dihydroganoderic acid I (3β, 7β, 20,23ξ-tetrahydroxy-11,15-dioxolanosta-8-en-26-oic acid) | G. applanatum (fruit bodies) | [51] |
| 108 | 23-Dihydroganoderic acid N (7β, 20,23ξ-trihydroxy-3,11,15-trioxolanosta-8-en-26-oic acid) | G. applanatum (fruit bodies) | [51] |
| 109 | 20-Hydroxylganoderic acid G | G. lucidum (fruit bodies) | [57] |
| 110 | Ganoderic acid I | G. lucidum (gills) | [22] |
| 111 | Lucidumol A ((24S)-24,25-dihydroxylanost-8-ene-3,7-dione) | G. lucidum (spores) | [38] |
| 112 | Ganoderiol C (7α-ethoxy-24,25,26-trihydroxy-5α-lanost-8-en-3-one) | G. lucidum (fruit bodies) | [39] |
| 113 | Ganoderiol D (24,25,26-trihydroxy-5α-lanost-8-en-3,7-dione) | G. lucidum (fruit bodies) | [39] |
| 114 | Ganoderiol G (24,25,26-trihydroxy-7α-methoxy-5α-lanost-8-en-3-one) | G. lucidum (fruit bodies) | [39] |
| 115 | Ganoderiol H (3β, 24,25,26-tetrahydroxy-5α-lanost-8-en-7-one) | G. lucidum (fruit bodies) | [39] |
| 116 | Ganoderitriol M ((24S)-lanosta-7-oxo-8-en-3β, 24,25-triol) | G. lucidum (fruit bodies) | [58] |
| 117 | Sinensoic acid (3,26-dihydroxy-5-lanosta-8,24E-dien-21-oic acid) | G. sinense (fruit bodies) | [59] |
| 118 | Tsugarioside B (3α-acetoxy-5α-lanosta-8,24-diene-21-O-β-d-xyloside) | G. tsugae (fruit bodies) | [60] |
| 119 | Tsugaric acid A (3α-acetoxy-5α-lanosta-8,24-dien-21-oic acid) | G. tsugae | [61] |
| 120 | Ganosinoside A (3-oxo-5α-lanosta-8,24-dien-21-oic acid ester β-d-glucoside) | G. sinense (fruit bodies) | [47] |
| 121 | Tsugarioside A (3α-acetoxy-5α-lanosta-8,24-dien-21-oic acid ester β-d-glucoside) | G. tsugae (fruit bodies) | [60] |
| 122 | 3-Oxo-5α-lanosta-8,24-dien-21-oic acid | G. resinaceum (fruit bodies) | [62] |
| 123 | 3β-Hydroxy-5α-lanosta-8,24-dien-21-oic acid | G. tsugae (fruit bodies) | [60] |
Table 5.
Ganoderma triterpenes 124–147 in Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15, Figure 16 and Figure 17 and Ganoderma triterpenes 148–155 in Figure 18.
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 124 | 3β, 7β-Dihydroxy-11,15,23-trioxolanost-8,16-dien-26-oic acid | G. lucidum (fruit bodies) | [63] |
| 125 | 3β, 7β-Dihydroxy-11,15,23-trioxolanost-8,16-dien-26-oic acid methyl ester | G. lucidum (fruit bodies) | [63] |
| 126 | 12β-Acetoxy-3β, 7β-dihydroxy-11,15,23-trioxolanost-8,16-dien-26-oic acid | G. lucidum (fruit bodies) | [63] |
| 127 | Methyl ganoderate I | G. lucidum | [20,22] |
| 128 | Methyl ganoderate AP (methyl 12β, l5α, 20-trihydroxy-3,7,11,23- tetraoxo-5α-lanost-8-en-26-oate) | G. applanatum (fruit bodies) | [52] |
| 129 | Methyl ganoderate N (Methyl 7β, 20-dihydroxy-3,11,15,23-tetraoxo-5α-lanost-8-en-26-oate) | G. lucidum (fruit bodies) | [64] |
| 130 | Methyl ganoderate M (methyl 7β, 12α-dihydroxy-3,11,15,23-tetraoxo-5α-lanost-8-en-26-oate) | G. lucidum (fruit bodies) | [64] |
| 131 | Ganoderiol E (3β, 26,27-trihydroxy-5α-lanosta-8,24-dien-7-one) | G. lucidum (fruit bodies) | [39] |
| 132 | Ganoderiol I (15α, 26,27-trihydroxy-5α-lanosta-8,24-dien-3-one) | G. lucidum (fruit bodies) | [39] |
| 133 | Ganoderiol J (26,27-dihydroxy-5α-lanosta-8,24-dien-3,7-dione) | G. sinense (fruit bodies) | [47] |
| 134 | Epoxyganoderiol A (24S, 25S-epoxy-7α, 26-dihydroxy-5α-lanost-8-en-3-one) | G. lucidum | [40] |
| 135 | Ganoderone C (5α-lanosta-8-ene-24,25-epoxy-26-hydroxy-3,7-dione) | G. pfeifferi (fruit bodies) | [42] |
| 136 | 3-O-Acetylganoderic acid B (3β-acetoxy-7β-hydroxy-11,15,23-trioxolanost-8-en-26-oic acid) | G. lucidum (mycelia) | [65] |
| 137 | 3-O-Acetylganoderic acid K (3β-acetyloxy-15α-hydroxy-7,11,23-trioxolanost-8-en-26-oic acid) | G. lucidum (mycelia) | [65] |
| 138 | Ethyl 3-O-acetylganoderate B | G. lucidum (mycelia) | [65] |
| 139 | Ethyl ganoderate J | G. lucidum (mycelia) | [65] |
| 140 | Applanoxidic acid G (15β, 20-dihydroxy-7α, 8α-epoxy-3,12,23-trioxo-5α-lanosta-9(11),16-dien-26-oic acid) | G. applanatum | [66] |
| 141 | Applanoxidic acid H (3β, 12α, 20-trihydroxy-7α, 8α-epoxydioxo-5α-lanosta-9(11),16-dien-26-oic acid) | G. applanatum | [66] |
| 142 | 8β, 9α-Dihydroganoderic acid J | G. lucidum (fruit bodies) | [57] |
| 143 | Methyl 8β, 9α-dihydroganoderate J | G. lucidum (fruit bodies) | [57] |
| 144 | Ganosporeric acid A (3,7,11,12,15,23-hexaoxo-5α-lanosta-8-en-26-oic acid) | G. lucidum (spores) | [67] |
| 145 | 24ξ-Methyl-5α-lanosta-25-one | G. applanatum (fruit bodies) | [68] |
| 146 | 3α-Carboxyacetoxy-24-methylene-23-oxolanost-8-en-26-oic acid | G. applanatum (fruit bodies) | [69] |
| 147 | 3α-Carboxyacetoxy-24-methyl-23-oxolanost-8-en-26-oic acid | G. applanatum (fruit bodies) | [69] |
| 148 | Fornicatin C ((3β)-3-hydroxy-18(13→12β)-abeo-lanosta-13(17),24-dien-18-oic acid) | G. fornicatum (fruit bodies) | [70] |
| 149 | 3-Epipachymic acid (3α-acetoxy-16α-hydroxy-24-methylene-5α-lanost-8-en-21-oic acid) | G. resinaceum (fruit bodies) | [62] |
| 150 | 3β, 15α-Diacetoxylanosta-8,24-dien-26-oic acid | G. lucidum (mycelia) | [71] |
| 151 | Tsugaric acid C ((24R,S)-3α-acetoxy-24-hydroxy-5α-lanosta-8,25-dien-21-oic acid) | G. tsugae (fruit bodies) | [60] |
| 152 | Ganoderic acid V1 ((24E)-3β, 20ξ-dihydroxy-7,11,15-trioxo-5α-lanosta-8,24-dien-26-oic acid) | G. lucidum | [72] |
| 153 | Tsugaric acid B (3α-acetoxy-16α-hydroxy-24ξ-methyl-5α-lanosta-8,25-dien-21-oic acid) | G. tsugae | [61] |
| 154 | Methyl ganoderenate E (7β, 12β-dihydroxy-3,11,15,23-tetraoxo-5α-lanosta-8,20E-dien-26-oate) | G. lucidum (fruit bodies) | [64] |
| 155 | 8β, 9α-Dihydroganoderic acid C | G. lucidum (mycelia) | [65] |
Table 7.
Ganoderma triterpenes (197–213) in Figure 20.
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 197 | Lucialdehyde A ((24E)-3β-hydroxy-5α-lanosta-7,9(11),24-trien-26-al) | G. lucidum (fruit bodies) | [25] |
| 198 | Ganoderiol a triacetate (3β, 24,26-triacetoxy-5α-lanosta-7, 9(11)-dien-25-ol) | G. sinense (fruit bodies) | [86] |
| 199 | Ganoderal A | G. lucidum | [23] |
| 200 | Ganoderol A | G. lucidum | [23] |
| 201 | Lucidumol B ((24S)-lanosta-7,9(11)-diene-3β, 24,25-triol) | G. lucidum (spores) | [38] |
| 202 | Ganodermanontiol (24,25,26-trihydroxy-5α-lanosta-7,9(11)-dien-3-one) | G. lucidum (spores) | [67] |
| 203 | Ganoderiol A (5α-lanosta-7,9(11)-dien-3β, 24,25,26-tetraol) | G. lucidum (fruit bodies) | [77] |
| 204 | Ganodermanondiol | G. lucidum (fruit bodies) | [87] |
| 205 | Ganoderic acid X (3α-hydroxy-15α-acetoxy-lanosta-7,9(11),24-trien-26-oic acid) | G. amboinense | [88] |
| 206 | Ganoderic acid TR | G. lucidum | [89] |
| 207 | Ganodermic acid Ja (lanosta-7,9(11),24-trien-3α, 15α-dihydroxy-26-oic acid) | G. lucidum (mycelia) | [84] |
| 208 | Ganodermic acid Jb (lanosta-7,9(11),24-trien-3β, 15α-dihydroxy-26-oic acid) | G. lucidum (mycelia) | [84] |
| 209 | Ganodermic acid R (lanosta-7,9(11),24-trien-3α, 15α-diacetoxy-26-oic acid) | G. lucidum | [78] |
| 211 | 15α-Hydroxy-3-oxo-5α-lanosta-7,9,24(E)-triene-26-oic acid | G. lucidum | [26] |
| 212 | 15α, 26-Dihydroxy-5α-lanosta-7,9,24(E)-trien-3-one | G. lucidum | [26] |
| 213 | 3β-Hydroxy-5α-lanosta-7,9,24(E)-trien-26-oic acid | G. lucidum | [26] |
Table 8.
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 214 | 3α, 15α, 22α-Trihydroxylanosta-7,9(11),24-trien-26-oic acid | G. lucidum (mycelia) | [71] |
| 215 | 3β, 15α, 22β-Trihydroxylanosta-7,9(11),24-trien-26-oic acid | G. lucidum (mycelia) | [71] |
| 216 | 3α, 15α-Diacetoxy-22α-hydroxylanosta-7,9(11),24-trien-26-oic acid | G. lucidum (mycelia) | [71] |
| 217 | 3β, 15α-Diacetoxy-22α-hydroxylanosta-7,9(11),24-trien-26-oic acid | G. lucidum (mycelia) | [71] |
| 218 | 22β-Acetoxy-3α, 15α-dihydroxylanosta-7,9(11),24-trien-26-oic acid | G. lucidum (mycelia) | [71] |
| 219 | 22β-Acetoxy-3β, 15α-dihydroxylanosta-7,9(11),24-trien-26-oic acid | G. lucidum (mycelia) | [71] |
| 220 | Epoxyganoderiol B (24S, 25S-epoxy-26-hydroxy-5α-lanosta-7,9(11)-diene-3-one) | G. lucidum | [40] |
| 221 | Epoxyganoderiol C (24S, 25S-epoxy-5α-lanosta-7,9(11)-diene-3β, 26-diol) | G. lucidum | [40] |
Table 9.
Ganoderma triterpenes (222–260) in Figure 23.
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 222 | Butyl lucidenate N | G. lucidum (fruit bodies) | [17] |
| 223 | Butyl lucidenate A | G. lucidum (fruit bodies) | [17] |
| 224 | 20(21)-Dehydrolucidenic acid N (3β, 7β-dihydroxy-11,15-dioxo-25,26,27- trinorlanosta-8,20-dien-24-oic acid) | G. sinense (fruit bodies) | [33] |
| 225 | 20-Hydroxylucidenic acid A (7β, 20ξ-dihydroxy-3,11,15-trioxo-25,26,27-trinorlanost-8-en-24-oic acid) | G. sinense (fruit bodies) | [33] |
| 226 | Methyl lucidenate D (methyl 12β-acetoxy-3,7,11,15-tetraoxo-5α-lanost-8-en-24-oate) | G. lucidum (fruit bodies) | [53,54] |
| 227 | 20(21)-Dehydrolucidenic acid A (7β-Hydroxy-3,11,15-trioxo-25,26,27-trisnorlanosta-8,20(21)-dien-24-oic acid) | G. lucidum (fruit bodies) | [90] |
| 228 | Methyl 20(21)-dehydrolucidenate A (methyl 7β-hydroxy-3,11,15-trioxo-25,26,27-trisnorlanosta-8,20(21)-dien-24-oate) | G. lucidum (fruit bodies) | [90] |
| 229 | Lucidenic acid N (3,7-dihydroxy-4,4,14-trimethyl-11,15-dioxo-5-chol-8-en-24-oic acid) | G. lucidum (dried fruit bodies) | [91,92] |
| 230 | Lucidenic acid D (12β-acetoxy-4,4,14α-trimethyl-3,7,11,15-tetraoxo-5α-chol-8-en-24-oic acid) | G. lucidum (dried fruit bodies) | [31] |
| 231 | Methyl lucidenate E | G. lucidum (gills) | [54] |
| 232 | Methyl lucidenate F | G. lucidum (gills) | [23,54] |
| 233 | Ethyl lucidenates A (ethyl 7β-hydroxy-4,4,14α-trimethyl-3,11,15-trioxo-5α-chol-8-en-24-oate) | G. lucidum (fruit bodies) | [93] |
| 234 | 3β-Oxo-formyl-7β, 12β-dihydroxy-4,4,14α-trimethyl-5α-chol-11,15-dioxo-8-en(E)-24-oic acid | G. lucidum | [24] |
| 235 | Lucidenic acid A (7β-hydroxy-4,4,14α-trimethyl-3,11,15-trioxo-5α-chol-8-en-24-oic acid) | G. lucidum (dried fruit bodies) | [94] |
| 236 | Lucidenic acid B (7β, 12-dihydroxy-4,4,14α-trimethyl-3,11,15-trioxo-5α-chol-8-en-24-oic acid) | G. lucidum (dried fruit bodies) | [94] |
| 237 | Lucidenic acid C (3β, 7β, 12-trihydroxy-4,4,14α-trimethyl-11,15-dioxo-5α-chol-8-en-24-oic acid) | G. lucidum (dried fruit bodies) | [94] |
| 238 | 4,4,14α-Trimethyl-3,7-dioxo-5α-chol-8-en-24-oic acid | G. lucidum | [26] |
| 239 | Lucidenic acid P (3β, 7β-dihydroxy-12β-acetoxy-25,26,27-trinor-11,15-dioxo dioxolanost-8-en-24-oic acid) | G. lucidum (fruit bodies) | [95] |
| 240 | Methyl lucidenate P | G. lucidum (fruit bodies) | [95] |
| 241 | Methyl lucidenate Q (methyl-7β, 15α-dihydroxy-25,26,27-trinor-3,11-dioxolanost-8-en-24-oate) | G. lucidum (fruit bodies) | [95] |
| 242 | 3β-Hydroxy-4,4,14-trimethyl-7,11,15-trioxochol-8-en-24-oic acid | G. lucidum (fruit bodies) | [28] |
| 243 | Methyl lucidenate D2 | G. lucidum (gill surface) | [30] |
| 244 | Methyl lucidenate E2 | G. lucidum (gill surface) | [30] |
| 248 | Methyl lucidenate N (methyl 3β, 7β-dihydroxy-4,4,14α-trimethyl-11,15-dioxo-5α-chol-8-en-24-oate) | G. lucidum (fruit bodies) | [96] |
| 249 | t-Butyl lucidenate B (t-butyl 7β, 12β-dihydroxy-4,4,14α-trimethyl-3,11,15-trioxo-5α-chol-8-en-24-oate) | G.lucidum (fruit bodies) | [96] |
| 250 | Methyl lucidenate A | G. lucidum (fruit bodies) | [93] |
| 251 | Lucidenic acid D2 | G. lucidum (fruit bodies) | [95] |
| 252 | 20-Hydroxylucidenic acid D2 ((20ξ)-12β-acetoxy-20-hydroxy-3,7,11,15-tet-raoxo-25,26,27-trisnorlanost-8-en-24-oic acid) | G. lucidum (fruit bodies) | [90] |
| 253 | 20-Hydroxylucidenic acid F ((20ξ)-20-hydroxy-3,7,11,15-tetraoxo-25,26,27-trisnorlanost-8-en-24-oic acid) | G. lucidum (fruit bodies) | [90] |
| 254 | 20-Hydroxylucidenic acid E2 (12β-acetoxy-3β-hydroxy-7,11,15-trioxo-25,26,27-trisnorlanost-8-en-24-oic acid) | G. lucidum (fruit bodies) | [90] |
| 255 | 20-Hydroxylucidenic acid N ((20ξ)-3β, 7β, 20-trihydroxy-11,15-dioxo-25,26,27-trisnorlanost-8-en-24-oic acid) | G. lucidum (fruit bodies) | [90] |
| 256 | 20-Hydroxylucidenic acid P ((20ξ)-12β-acetoxy-3β, 7β, 20-trihydroxy-11,15-dioxo-25,26,27-trisnorlanost-8-en-24-oic acid) | G. lucidum (fruit bodies) | [90] |
| 257 | Lucidenic acid F | G. lucidum (gills) | [22] |
| 258 | Methyl lucidenate C | G. lucidum | [26] |
| 259 | Lucidenic acid E2 | G. lucidum (fruit bodies) | [95] |
| 260 | Lucideric acid A | G. lucidum | [26] |
Table 10.
Ganoderma triterpenes 261–280 in Figure 24, Figure 25, Figure 26, Figure 27, Figure 28, Figure 29, Figure 30 and Figure 31.
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 261 | 4,4,14α-Trimethyl-5α-chol-7,9(11)-dien-3-oxo-24-oic acid | G. lucidum (dried fruit bodies) | [73] |
| 262 | Ganoderic acid Jd (15α-hydroxy-3-oxo-5α-lano-sta-7,9(11)-dien-24-oic acid) | G. sinense (fruit bodies) | [47] |
| 263 | Methyl lucidenate H (methyl 3β, 7β-dihydroxy-4α-hydroxymethyl-4β, 14α-dimethyl-11,15-dioxo-5α-chol-8-en-24-oate) | G. lucidum (fruit bodies) | [64] |
| 264 | Methyl lucidenate I (3β-hydroxy-4α-hydroxymethyl-4β, 14α-dimethyl-7,11,15-trioxo-5α-chol-8-en-24-oate) | G. lucidum (fruit bodies) | [64] |
| 265 | Methyl lucidenate J (3β, 12β-dihydroxy-4α-hydroxymethyl-4β, 14α-dimethyl-7,11,15-trioxo-5α-chol-8-en-24-oate) | G. lucidum (fruit bodies) | [64] |
| 266 | Methyl lucidenate Ha | G. sinense (fruit bodies) | [47] |
| 267 | Colossolactone I ((22S)-3-β-hydroxylanosta-8,24-dien-26,22-olide) | G. colossum | [97] |
| 268 | Colossolactone II ((22S)-1,3-β-dihydroxylanosta-8,24-dien-26,22-olide) | G. colossum | [97] |
| 269 | Colossolactone D | G. colossum (fruit bodies) | [98] |
| 270 | Colossolactone E | G. colossum (fruit bodies) | [98] |
| 271 | Colossolactone F | G. colossum (fruit bodies) | [98] |
| 272 | Colossolactone G | G. colossum (fruit bodies) | [98] |
| 273 | Ganosporelactone A | G. lucidum (spores) | [99] |
| 274 | Ganosporelactone B | G. lucidum (spores) | [99] |
| 275 | Ganosinensin B (ganodermanontriol 24-O-{(2Z, 5E, 9E)-2-[2-(2,5-dihydroxyphenyl)-2-oxo-ethylidene]-11-hydroxy-6,10-dimethylundeca-5,9-dienate) | G. sinense (fruit bodies) | [100] |
| 276 | Ganosinensin C (ganodermanontriol 24-O-{(2Z, 5E, 9E)-2-[2-(2,5-dihydroxyphenyl)ethylidene]-11-hydroxy-6,10-dimethylundeca-5,9- dien-ate) | G. sinense (fruit bodies) | [100] |
| 277 | Ganodermacetal (methyl 7β, 15α-isopropylide-nedioxy-3β-hydroxy-11,23-dioxo-5α-lanost-8-en-26-oate) | G. amboinense (fruit bodies) | [85] |
| 278 | Methyl ganoderate A acetonide (methyl 7β, 15α-isopropylidenedioxy-3,11,23-trioxo-5α-lanost-8-en-26-oate) | G. lucidum (fruit bodies) | [16] |
| 279 | Applanoxidic acid A (15α-hydroxy-7α, 8α-epoxy-3,12,23-trioxo-5α-lanosta-9(11),20E-dien-26-oic acid) | G. applanatum | [101] |
| 280 | Applanoxidic acid B (3β-hydroxy-7α, 8α-epoxy-12,15,23-trioxo-5α-lanosta-9(11),20E-dien-26-oic acid) | G. applanatum | [101] |
Table 11.
Ganoderma triterpenes 281–287, 288–307, 308–311, 312, 313–315, 316 in Figure 32, Figure 33, Figure 34, Figure 35, Figure 36, Figure 37, Figure 38, Figure 39 and Figure 40.
| No. | Compound Name | Source | Ref. |
|---|---|---|---|
| 281 | Applanoxidic acid C (20-hydroxy-7α, 8α-epoxy-3,12,15,23-tetraoxo-5α-lanosta-9(11),16-dien-26-oic acid) | G. applanatum | [101] |
| 282 | Applanoxidic acid D (3β, 20-dihydroxy-7α, 8α-epoxy-12,15,23-trioxo-5α-lanosta-9(11),16-dien-26-oic acid) | G. applanatum | [101] |
| 283 | Lanosta-7,9(11),24-trien-3-one15,26-dihydroxy | G. zonatum Murill. | [27] |
| 284 | Lanosta-7,9(11),24-trien-26-oic,3-hydroxy | G. zonatum Murill. | [27] |
| 285 | Ganoderic acid Y ((24E)-3-ol-5α-lanosta-7,9(11),24-trien-26-oic acid) | G. zonatum Murill. | [27] |
| 286 | Applanoxidic acid E (15β-hydroxy-7α, 8α-epoxy-3,12,23-trioxo-5α-lanosta-9(11),20E-dien-26-oic acid) | G. applanatum | [66] |
| 287 | Applanoxidic acid F (7α, 8α-epoxy-3,12,15,23-tetraoxo-5α-lanosta-9(11),20E-dien-26-oic acid) | G. applanatum | [66] |
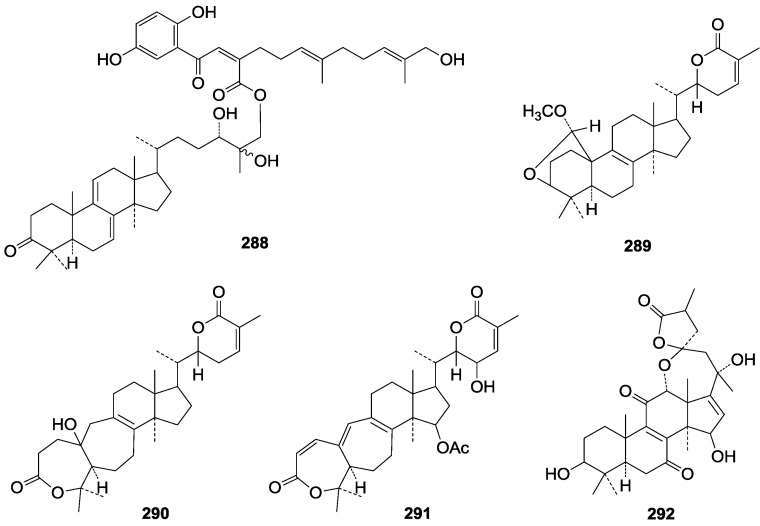
| 288 | Ganosinensin A (ganodermanontriol 26-O-{(2Z, 5E, 9E)-2-[2-(2,5-dihydroxyphenyl)-2-oxo-ethylidene]-11-hydroxy-6,10-dimethylundeca-5,9-dienate}) | G. sinense (fruit bodies) | [100] |
| 289 | Colossolactone III ((22S)-3β, 19-epoxy-lanosta-8,24-dien-26,22-olide) | G. colossum | [97] |
| 290 | Colossolactone IV ((22S)-A,B-dihomo-19-nor-4-oxalanosta-8,24-dien-26,22-olide) | G. colossum | [97] |
| 291 | Colossolactone VIII ((22S, 23R)-A,B-dihomo-19-nor-15-β-acetoxy-23-hydroxy-4-oxa-3-oxolanosta-1,8,19,24-tetraen-26,22-olide) | G. colossum | [102] |
| 292 | Austrolactone ((23S, 25S)-12α, 23-epoxy-3β, 15β, 20α-trihydroxy-7,11-dioxo-5α-lanosta-8,16-dien-23,26-olide) | G. australe | [103] |
| 293 | Ganolactone B (3β, 7β-dihydroxy-11,15-dioxolanosta-8-en-24→20 lactone) | G. sinense (fruit bodies) | [86] |
| 294 | Ganolactone (7β-hydroxy-3,11,15-trioxo-lanosta-8-en-24→20s lactone) | G. lucidum (fruit bodies) | [104] |
| 295 | Colossolactone B | G. colossum (fruit bodies) | [98] |
| 296 | Colossolactone C | G. colossum (fruit bodies) | [98] |
| 297 | 3α-(3-Hydroxy-5-methoxy-3-methyl-1,5-dioxopentyloxy)-24-methylene-5α-lanost-8-en-21-oic acid | G. resinaceum (fruit bodies) | [62] |
| 298 | Colossolactone A | G. colossum (fruit bodies) | [98] |
| 299 | Methyl ganosinensate A | G. sinense (fruit bodies) | [56] |
| 300 | Ganosinensic acid A | G. sinense (fruit bodies) | [56] |
| 301 | Ganosinensic acid B | G. sinense (fruit bodies) | [56] |
| 302 | Tsugarioside C (3α-acetoxy-(Z)-24-methyl-5α-lanosta-8,23,25-trien-21-oic acid ester β-D-xyloside) | G. tsugae (fruit bodies) | [60] |
| 303 | Ganorbiformin A | G. colossum (fruit bodies) | [46] |
| 304 | Colossolactone V ((22R)-3,4-seco-19,22-diacetoxy-4-hydroxylanosta-8,24(Z)-dien-3,26-dioic acid 3-methyl-ester) | G. colossum (fruit bodies) | [102] |
| 305 | Colossolactone VI ((22R)-3,4-seco-19,22-diacetoxy-4-hydroxylanosta-7,9(11),24(Z)-trien-3,26-dioic acid 3-methyl ester) | G. colossum (fruit bodies) | [102] |
| 306 | Colossolactone VII ((22S)-3,4-seco-19-acetoxy-4-hydroxylanosta-8,24-dien-26,22-olide 3-methyl ester) | G. colossum (fruit bodies) | [102] |
| 307 | Furanoganoderic acid (21,23-epoxy-15α-hydroxy-3,7,1l-trioxo-5α-lanosta-8,20(21),22-trien-26-oic acid) | G. applanatum (fruit bodies) | [52] |
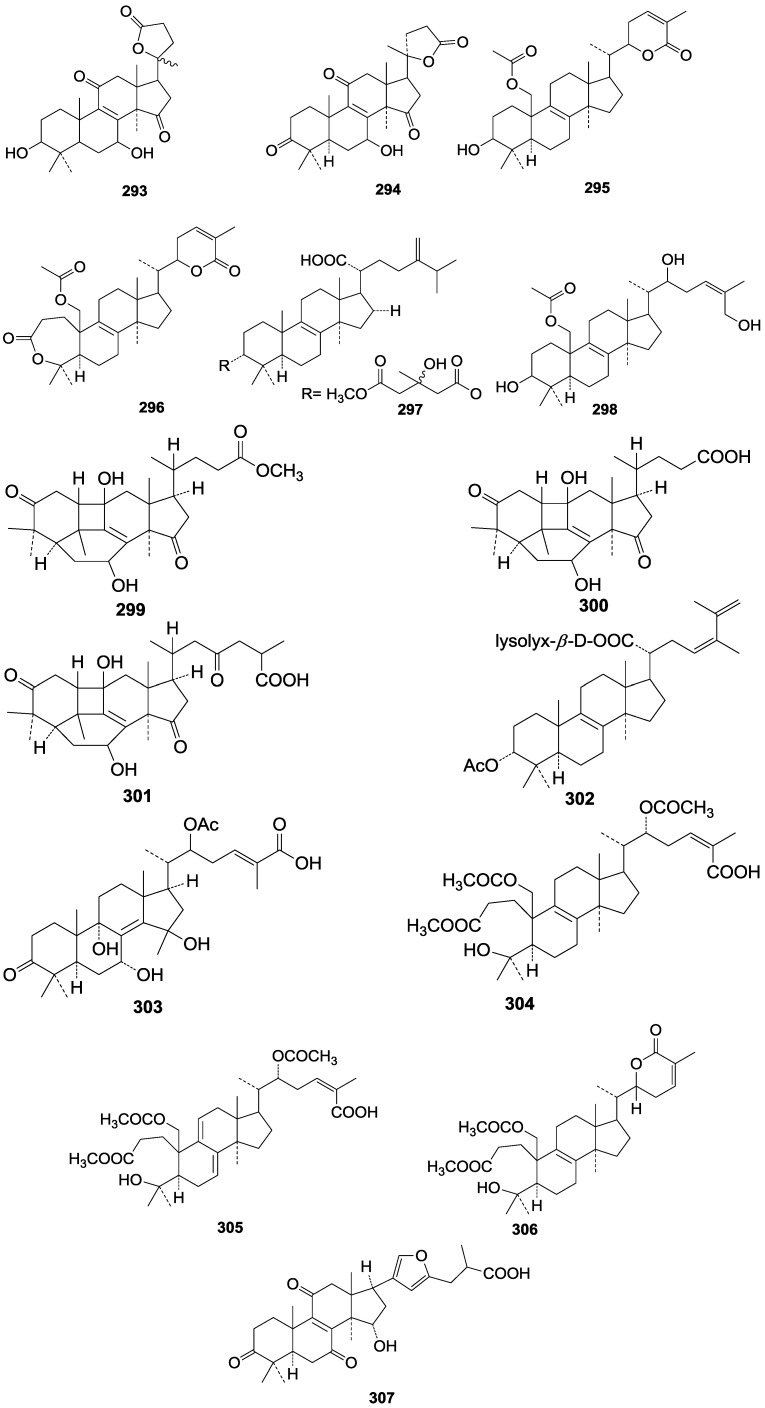
| 308 | Fornicatin B (7β-hydroxy-11-oxo-3,4-seco-25,26,27-trinorlanosta-4(28),8-dien-3,24-dioic acid) | G. fornicatum (fruit bodies) | [105] |
| 309 | Fornicatin G (7β-hydroxy-11-oxo-3,4-seco-25,26,27-trinorlanosta-4(28),8-dien-24-oic-3-acetyl ester) | G. cochlear (sporophore) | [106] |
| 310 | Fornicatin A (4, 7β-epoxy-28-hydroxy-11-oxo-3,4-seco-25,26,27-trinorlanosta-8-en-3,24-dioic acid) | G. fornicatum (fruit bodies) | [105] |
| 311 | Fornicatin H (4, 7β-epoxy-28-hydroxy-11-oxo-3,4-seco-25,26,27-trinorlansta-8-en-3,24-diester) | G. cochlear (sporophore) | [106] |
| 312 | Australic acid ((20Z, 23R, 25R)-15α-acetyl-7α, 8α-epoxy-12-oxo-3,4-seco-5α-lanosta-4(28),9,20(22)-trien-23,26-olid-3-oic acid) | G. australe | [103] |
| 313 | Lucidone A | G. tsugae | [107] |
| 314 | Lucidenol | G. tsugae | [107] |
| 315 | Ganosineniol A | G. sinense (fruit bodies) | [47] |
| 316 | 8α, 9α-Epoxy-4,4,14α-trimethyl-3,7,11,15,20-pentaoxo-5α-pregnane | G. concinna | [75] |
Figure 27.
Structures of compounds 269–272.
| Cpd | R1 | R2 | R3 |
|---|---|---|---|
| 269 | α-H | β-H | β-OH |
| 270 | α-H | β-H | β-O-Ac |
| 271 | α-H | β-OH | β-O-Ac |
| 272 | ξ-OH | H | β-O-COCH3 |
Figure 28.
Structures of compounds 273–274.
| Cpd | R |
|---|---|
| 273 | =O |
| 274 | β-OH |
Figure 30.
Structures of compounds 277–278.
| Cpd | R1 | R2 |
|---|---|---|
| 277 | β-OH | COOH |
| 278 | =O | COOCH3 |
Figure 31.
Structures of compounds 279–280.
| Cpd | R1 | R2 |
|---|---|---|
| 279 | =O | α-OH |
| 280 | β-OH | =O |
Figure 32.
Structures of compounds 281–282.
| Cpd | R |
|---|---|
| 281 | =O |
| 282 | β-OH |
Figure 33.
Structures of compounds 283–285.
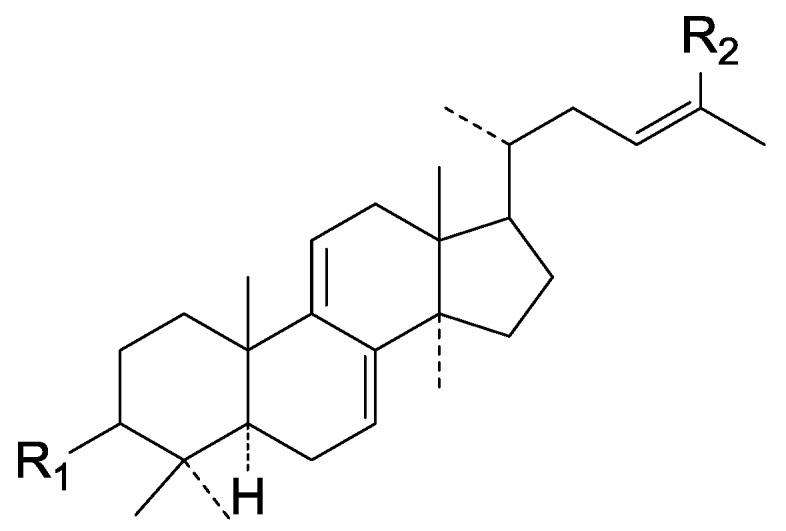
| Cpd | R | R2 |
|---|---|---|
| 283 | α-OH | COOH |
| 284 | =O | CH2OH |
| 285 | β-OH | COOH |
Figure 34.
Structures of compounds 286–287.
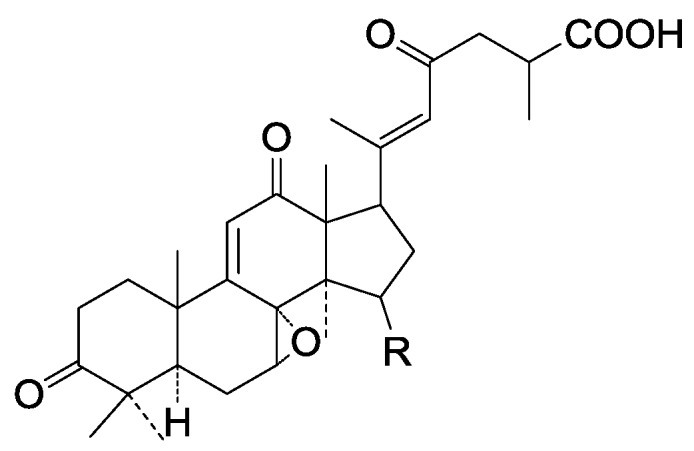
| Cpd | R |
|---|---|
| 286 | α-OH |
| 287 | =O |
Figure 35.
Structures of compounds 288–307.
Figure 36.
Structures of compounds 308–309.
| Cpd | R |
|---|---|
| 308 | COOH |
| 309 | COOCH2CH3 |
Figure 37.
Structure of compounds 310–311.
| Cpd | R1 | R2 |
|---|---|---|
| 310 | COOH | COOH |
| 311 | COOCH3 | COOCH3 |
Figure 38.
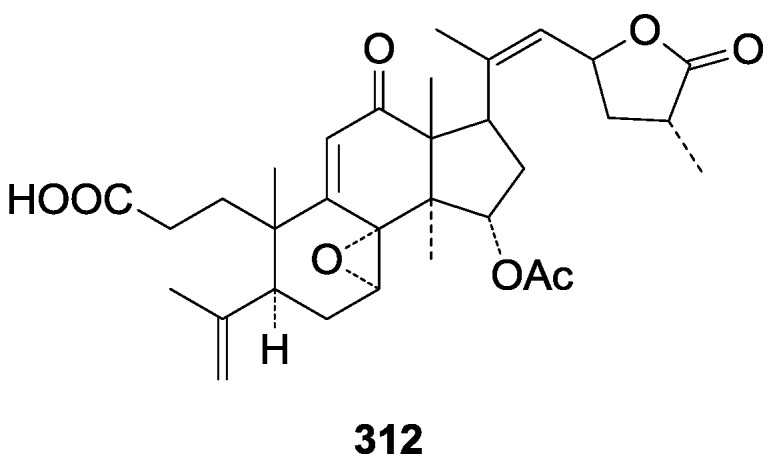
Structure of compound 312.
Figure 39.
Structures of compounds 313–315.
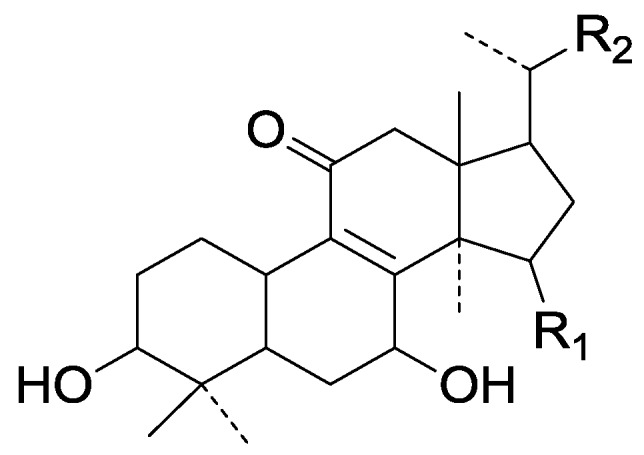
| Cpd | R1 | R2 |
|---|---|---|
| 313 | =O | =O |
| 314 | =O | OH |
| 315 | α-OH | CH2OH |
Figure 40.
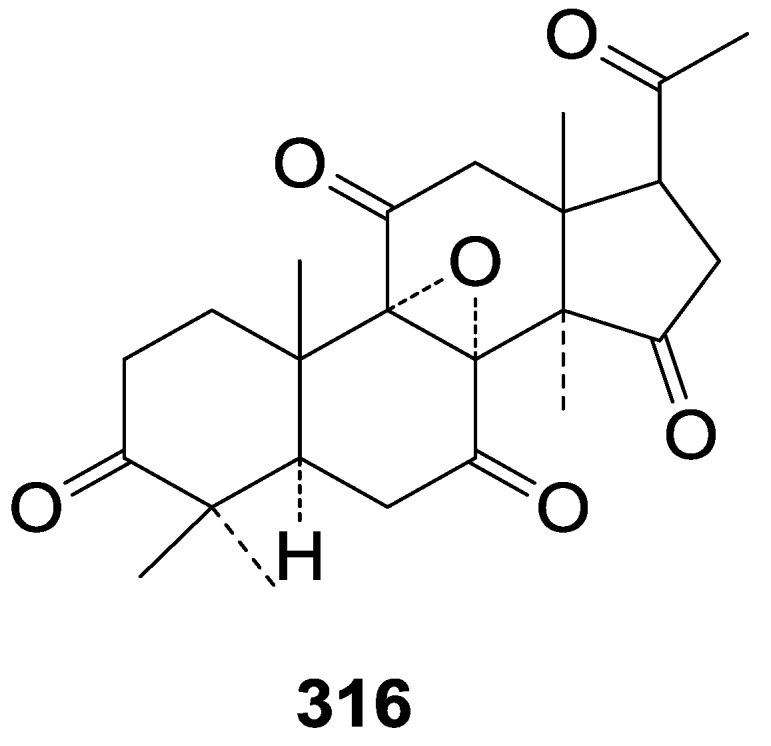
Structure of compound 316.
3. 13C-NMR Data of Ganoderma Triterpenes
The reported GTs 13C-NMR data are shown in Table 12. For compounds 5, 7, 22, 23, 28, 31–34, 36, 37, 54, 55, 59, 89, 95, 97, 98, 110, 112, 114, 121–123, 130–132, 146, 147, 154, 159, 169, 195, 199, 200, 206, 211–213, 235–237, 240, 245–247, 250, 251, 257–260, 265, 283–285, 313 and 314 have no 13C-NMR data reported or cannot be researched.
Table 12.
The 13C-NMR spectural data of compounds 1–316 except those which have no reported 13C-NMR data.
| NO. | 1b) | 2b) | 3b) | 4b) | 6b) | 8b) | 9b) | 10b) | 11b) | 12b) | 13b) | 14b) | 15b) | 16b) | 17b) | 18b) | 19b) | 20b) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 33.4 | 35.7 | 35.0 | 33.1 | 35.1 | 34.4 | 35.7 | 35.6 | 34.8 | 34.8 | 35.0 | 34.7 | 35.7 | 35.7 | 34.6 | 34.3 | 34.8 | 34.2 |
| C2 | 27.5 | 34.5 | 27.9 | 27.2 | 34.2 | 28.0 | 34.4 | 34.4 | 27.6 | 28.3 | 28.0 | 28.2 | 34.3 | 34.3 | 27.8 | 27.7 | 34.1 | 27.3 |
| C3 | 77.5 | 217.1 | 78.5 | 77.3 | 217.7 | 78.7 | 208.4 | 208.7 | 78.3 | 78.5 | 77.5 | 78.2 | 217.5 | 217.5 | 78.2 | 77.5 | 217.8 | 78.5 |
| C4 | 40.6 | 46.8 | 39.0 | 39.0 | 47.0 | 39.0 | 47.0 | 46.7 | 38.8 | 39.0 | 38.9 | 38.6 | 46.8 | 46.8 | 38.6 | 40.2 | 47.2 | 39.1 |
| C5 | 51.5 | 49.0 | 49.3 | 51.2 | 51.7 | 51.8 | 49.2 | 48.8 | 49.2 | 45.7 | 49.5 | 49.1 | 49.0 | 49.0 | 49.1 | 49.8 | 45.2 | 47.7 |
| C6 | 36.8 | 29.3 | 26.7 | 36.6 | 18.7 | 17.4 | 29.2 | 29.1 | 26.6 | 27.8 | 27.6 | 27.8 | 27.7 | 27.8 | 28.2 | 36.5 | 27.9 | 28.0 |
| C7 | 199.1 | 69.1 | 67.1 | 199.0 | 29.6 | 30.4 | 69.1 | 68.9 | 66.9 | 67.0 | 69.5 | 69.5 | 66.4 | 66.3 | 69.5 | 205.3 | 66.7 | 68.0 |
| C8 | 151.9 | 159.1 | 157.1 | 145.6 | 163.2 | 162.9 | 159.6 | 159.5 | 156.8 | 155.0 | 159.6 | 158.0 | 157.8 | 157.8 | 158.1 | 154.6 | 159.3 | 158.8 |
| C9 | 145.9 | 140.5 | 142.9 | 151.7 | 138.6 | 140.0 | 140.6 | 140.1 | 142.7 | 142.9 | 142.2 | 142.0 | 141.3 | 141.3 | 141.9 | 149.8 | 140.0 | 141.6 |
| C10 | 39.3 | 38.2 | 38.8 | 40.3 | 37.1 | 37.8 | 46.8 | 46.8 | 38.6 | 45.4 | 38.9 | 38.6 | 38.3 | 38.3 | 38.5 | 38.9 | 38.0 | 38.6 |
| C11 | 194.2 | 199.6 | 198.0 | 193.9 | 198.1 | 198.3 | 200.0 | 199.7 | 197.9 | 200.0 | 201.2 | 199.8 | 197.6 | 197.6 | 199.9 | 201.3 | 199.1 | 199.4 |
| C12 | 79.3 | 51.9 | 50.5 | 79.1 | 51.7 | 52.1 | 51.9 | 51.8 | 50.3 | 51.0 | 52.3 | 52.0 | 50.2 | 50.2 | 51.9 | 52.3 | 51.8 | 52.2 |
| C13 | 48.1 | 47.0 | 45.5 | 47.9 | 46.8 | 47.2 | 38.2 | 38.0 | 45.4 | 38.8 | 47.4 | 47.1 | 45.0 | 45.0 | 47.1 | 48.0 | 46.4 | 46.1 |
| C14 | 58.7 | 54.1 | 59.6 | 58.5 | 53.6 | 53.5 | 54.2 | 54.1 | 59.4 | 58.5 | 54.4 | 54.0 | 59.4 | 59.4 | 54.0 | 52.8 | 53.4 | 53.5 |
| C15 | 205.9 | 72.7 | 217.7 | 66.2 | 72.9 | 73.0 | 72.6 | 72.4 | 217.5 | 207.5 | 72.4 | 72.4 | 216.6 | 216.4 | 72.5 | 72.1 | 72.4 | 72.3 |
| C16 | 38.1 | 36.7 | 41.1 | 38.0 | 38.6 | 38.7 | 36.2 | 36.3 | 40.9 | 41.0 | 35.9 | 36.2 | 41.0 | 41.0 | 36.1 | 36.3 | 37.8 | 37.8 |
| C17 | 44.9 | 48.4 | 45.8 | 44.6 | 48.7 | 48.7 | 48.3 | 48.2 | 45.6 | 49.2 | 48.5 | 48.1 | 45.7 | 45.8 | 48.1 | 48.2 | 49.0 | 49.0 |
| C18 | 12.3 | 17.5 | 17.6 | 12.1 | 17.2 | 17.1 | 17.4 | 17.3 | 17.4 | 17.2 | 17.2 | 17.1 | 17.7 | 17.7 | 17.1 | 17.4 | 17.5 | 17.3 |
| C19 | 18.1 | 19.5 | 18.7 | 17.9 | 19.0 | 19.0 | 19.6 | 19.4 | 18.5 | 17.6 | 19.6 | 19.5 | 18.2 | 18.2 | 19.6 | 17.6 | 17.5 | 17.3 |
| C20 | 29.6 | 32.8 | 32.1 | 29.4 | 32.6 | 32.5 | 32.8 | 32.8 | 32.0 | 32.1 | 33.0 | 32.7 | 32.0 | 32.0 | 32.7 | 32.4 | 32.5 | 32.5 |
| C21 | 21.8 | 19.7 | 19.8 | 21.5 | 19.4 | 19.4 | 19.4 | 19.7 | 19.6 | 19.8 | 19.7 | 19.6 | 19.6 | 19.7 | 19.6 | 19.5 | 19.3 | 19.3 |
| C22 | 48.6 | 49.9 | 49.4 | 48.5 | 49.6 | 49.7 | 49.8 | 49.7 | 49.0 | 49.3 | 50.0 | 49.8 | 49.0 | 49.1 | 49.7 | 49.5 | 49.6 | 49.6 |
| C23 | 207.7 | 208.6 | 207.9 | 206.1 | 208.3 | 208.3 | 217.3 | 217.4 | 207.6 | 215.9 | 210.0 | 208.5 | 207.5 | 207.6 | 208.7 | 208.2 | 208.3 | 208.3 |
| C24 | 46.8 | 46.9 | 46.8 | 46.6 | 46.8 | 46.8 | 46.7 | 46.8 | 46.6 | 46.9 | 46.9 | 46.7 | 46.6 | 46.8 | 46.7 | 46.8 | 46.9 | 46.9 |
| C25 | 34.9 | 35.0 | 35.1 | 35.1 | 34.7 | 34.6 | 34.8 | 34.8 | 34.6 | 34.9 | 35.0 | 34.7 | 34.5 | 34.7 | 34.6 | 34.7 | 34.7 | 34.7 |
| C26 | 175.9 | 176.0 | 175.9 | 181.0 | 176.2 | 176.1 | 27.4 | 27.5 | 180.3 | 26.8 | 178.5 | 176.2 | 180.3 | 176.1 | 176.3 | 176.1 | 176.2 | 176.4 |
| C27 | 17.3 | 17.3 | 17.3 | 17.1 | 17.1 | 17.1 | 180.1 | 176.3 | 16.9 | 176.2 | 17.2 | 17.1 | 16.9 | 17.1 | 17.1 | 17.1 | 17.1 | 17.1 |
| C28 | 28.1 | 27.5 | 28.4 | 27.8 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| C29 | 15.7 | 20.9 | 15.6 | 15.5 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| C30 | 21.5 | 19.8 | 24.6 | 21.2 | 27.8 | 28.3 | 17.0 | 17.2 | 28.2 | 15.5 | 28.3 | 28.2 | 27.0 | 27.0 | 28.2 | 27.8 | 27.6 | 28.2 |
| OCOCH3 | Bu1' | Bu1' | CH3CO | C31 | C31 | C31 | C31 | C31 | C31 | C31 | C31 | C31 | C31 | C31 | C31 | C31 | C31 | |
| 170.4 | 64.8 | 64.8 | 170.2 | 20.6 | 15.7 | 20.8 | 20.8 | 15.5 | 24.5 | 15.9 | 15.7 | 20.8 | 20.8 | 15.7 | 15.4 | 20.5 | 15.8 | |
| OCOCH3 | Bu 2' | Bu 2' | CH3CO | C32 | C32 | C32 | C32 | C32 | C32 | C32 | C32 | C32 | C32 | C32 | C32 | C32 | C32 | |
| 21.0 | 30.9 | 30.9 | 20.9 | 18.8 | 18.8 | 19.8 | 19.7 | 24.4 | 18.7 | 19.5 | 19.4 | 24.7 | 24.7 | 19.4 | 20.3 | 21.1 | 21.1 | |
| N-BU1' | Bu 3' | Bu3' | OCH3 | OCH3 | COOCH3 | COOCH3 | COOCH3 | COOCH3 | OCH3 | OCH3 | OCH3 | OCH3 | ||||||
| 64.8 | 19.3 | 19.3 | 51.9 | 51.8 | 52.0 | 51.7 | 51.9 | 51.9 | 51.9 | 51.9 | 51.9 | 51.9 | ||||||
| NO. | 21b) | 24b) | 25b) | 26b) | 27c) | 29b) | 30b) | 35c) | 38b) | 39b) | 40b) | 41b) | 42d) | 43b) | 44a) | 45b) | 46b) | 47c) |
| C1 | 34.5 | 33.9 | 34.4 | 34.5 | 35.4 | 37.4 | 37.2 | 35.2 | 35.6 | 35.7 | 34.4 | 33.8 | 34.0 | 34.8 | 36.0 | 35.4 | 34.8 | 35.2 |
| C2 | 24.0 | 33.6 | 27.4 | 28.4 | 34.7 | 34.1 | 34.6 | 27.6 | 34.4 | 34.5 | 27.4 | 27.5 | 33.5 | 27.4 | 34.5 | 34.3 | 28.8 | 33.9 |
| C3 | 79.9 | 215.1 | 78.2 | 78.4 | 221.0 | 214.9 | 215.4 | 78.0 | 216.8 | 218.0 | 78.2 | 78.5 | 215.5 | 78.0 | 215.9 | 214.6 | 78.0 | 218.6 |
| C4 | 38.0 | 46.8 | 38.6 | 40.7 | 47.2 | 46.9 | 47.0 | 39.1 | 46.2 | 47.0 | 38.5 | 38.8 | 38.7 | 39.0 | 47.0 | 47.2 | 39.8 | 46.4 |
| C5 | 49.2 | 50.8 | 49.1 | 53.0 | 45.7 | 51.0 | 50.9 | 49.8 | 48.8 | 49.0 | 49.1 | 51.3 | 49.7 | 49.9 | 48.7 | 50.4 | 50.7 | 48.3 |
| C6 | 26.6 | 37.4 | 36.7 | 37.8 | 28.1 | 33.7 | 33.8 | 27.4 | 27.6 | 29.2 | 26.6 | 17.3 | 36.8 | 36.6 | 28.8 | 37.1 | 36.7 | 28.1 |
| C7 | 66.1 | 198.6 | 66.2 | 201.1 | 67.1 | 198.6 | 199.4 | 67.0 | 66.3 | 69.2 | 66.2 | 29.3 | 199.6 | 199.1 | 66.0 | 198.0 | 199.0 | 68.2 |
| C8 | 157.4 | 149.8 | 155.8 | 151.9 | 161.8 | 149.9 | 149.8 | 157.3 | 157.8 | 159.2 | 155.9 | 161.5 | 146.1 | 138.9 | 159.7 | 139.8 | 138.9 | 160.4 |
| C9 | 141.6 | 145.9 | 142.9 | 153.6 | 140.2 | 146.1 | 146.8 | 143.1 | 141.2 | 140.6 | 142.0 | 140.1 | 149.0 | 164.9 | 140.9 | 162.6 | 164.7 | 139.6 |
| C10 | 37.5 | 39.2 | 38.5 | 42.4 | 38.4 | 39.4 | 39.3 | 38.9 | 38.2 | 38.2 | 38.5 | 37.5 | 46.4 | 39.8 | 38.4 | 39.4 | 38.9 | 37.6 |
| C11 | 199.3 | 194.1 | 192.0 | 195.0 | 72.0 | 194.1 | 199.4 | 200.3 | 197.7 | 200.1 | 192.1 | 191.6 | 199.8 | 23.7 | 198.2 | 23.8 | 23.6 | 200.3 |
| C12 | 77.9 | 78.9 | 79.5 | 199.8 | 52.4 | 79.0 | 48.9 | 78.4 | 50.1 | 52.0 | 79.8 | 80.1 | 48.5 | 30.2 | 50.9 | 30.1 | 30.2 | 51.5 |
| C13 | 51.9 | 47.6 | 49.6 | 62.2 | 47.6 | 47.7 | 43.9 | 52.4 | 44.9 | 46.9 | 49.9 | 51.5 | 56.8 | 45.0 | 45.2 | 45.0 | 45.0 | 46.3 |
| C14 | 60.3 | 58.7 | 60.6 | 61.3 | 54.1 | 58.7 | 57.2 | 60.9 | 59.3 | 54.1 | 60.5 | 53.9 | 43.5 | 47.8 | 59.0 | 47.8 | 49.0 | 53.7 |
| C15 | 216.8 | 205.6 | 216.2 | 207.5 | 201.0 | 205.5 | 207.0 | 217.8 | 218.1 | 72.9 | 216.4 | 74.6 | 207.8 | 32.0 | 217.0 | 28.7 | 27.5 | 71.6 |
| C16 | 38.3 | 37.8 | 37.9 | 40.8 | 35.3 | 37.8 | 39.8 | 38.6 | 41.2 | 36.7 | 37.3 | 33.6 | 39.9 | 28.8 | 42.0 | 31.8 | 32.0 | 35.6 |
| C17 | 45.7 | 44.3 | 45.2 | 40.7 | 49.5 | 44.5 | 44.3 | 46.6 | 46.7 | 48.7 | 46.1 | 48.6 | 44.4 | 49.0 | 46.8 | 49.0 | 49.9 | 48.5 |
| C18 | 12.0 | 12.0 | 12.0 | 13.5 | 17.8 | 12.1 | 16.0 | 12.3 | 17.7 | 17.4 | 13.4 | 12.3 | 15.4 | 15.8 | 17.8 | 15.9 | 15.3 | 16.6 |
| C19 | 18.8 | 18.7 | 18.6 | 18.6 | 17.9 | 18.7 | 18.6 | 19.1 | 18.2 | 19.6 | 18.6 | 19.0 | 18.4 | 18.4 | 18.2 | 17.9 | 18.4 | 18.9 |
| C20 | 28.7 | 29.4 | 28.2 | 33.8 | 33.2 | 29.5 | 32.1 | 29.1 | 35.5 | 36.2 | 35.5 | 34.1 | 32.9 | 36.2 | 33.9 | 36.2 | 36.3 | 33.1 |
| C21 | 21.3 | 21.6 | 21.9 | 20.4 | 19.5 | 21.6 | 19.8 | 21.8 | 18.2 | 18.5 | 20.8 | 19.7 | 19.7 | 18.6 | 19.8 | 18.6 | 18.6 | 19.0 |
| C22 | 48.3 | 48.4 | 47.9 | 50.2 | 50.0 | 48.4 | 48.8 | 48.7 | 34.5 | 34.8 | 33.1 | 33.7 | 42.8 | 34.8 | 43.8 | 34.7 | 34.8 | 42.9 |
| C23 | 208.1 | 207.6 | 207.4 | 211.1 | 210.7 | 207.3 | 207.6 | 210.3 | 25.6 | 25.9 | 26.5 | 26.3 | 65.4 | 25.9 | 66.5 | 26.0 | 26.0 | 65.9 |
| C24 | 46.1 | 46.6 | 46.6 | 48.0 | 47.2 | 46.4 | 46.5 | 46.9 | 144.1 | 145.3 | 143.2 | 144.2 | 144.4 | 145.6 | 145.0 | 155.2 | 155.3 | 143.2 |
| C25 | 34.2 | 34.7 | 34.6 | 36.6 | 37.4 | 34.6 | 34.6 | 35.4 | 127.0 | 127.2 | 127.1 | 127.2 | 126.9 | 126.6 | 128.8 | 139.2 | 139.2 | 128.4 |
| C26 | 180.5 | 175.6 | 176.1 | 180.7 | 179.0 | 180.8 | 180.9 | 178.8 | 171.2 | 172.0 | 171.0 | 172.7 | 169.0 | 172.4 | 170.7 | 195.3 | 195.3 | 170.2 |
| C27 | 16.9 | 17.1 | 17.1 | 18.0 | 17.4 | 16.9 | 16.9 | 17.3 | 12.1 | 12.3 | 12.1 | 12.0 | 12.7 | 12.0 | 13.4 | 9.2 | 9.2 | 12.3 |
| C28 | 27.9 | 27.6 | 28.0 | 28.7 | 27.8 | -- | -- | -- | 24.7 | 19.7 | 24.1 | 28.2 | 27.0 | 25.0 | 27.0 | 25.4 | 27.5 | 27.0 |
| C29 | 16.4 | 20.3 | 15.4 | 16.6 | 20.7 | -- | -- | -- | 26.9 | 27.6 | 28.1 | 15.7 | 19.9 | 27.5 | 20.8 | 21.4 | 15.8 | 20.1 |
| C30 | 23.0 | 20.7 | 24.0 | 24.9 | 21.4 | 27.6 | 27.6 | 28.4 | 20.7 | 20.9 | 15.4 | 19.8 | 20.0 | 15.3 | 25.1 | 24.9 | 25.0 | 19.0 |
| C31 | CH3CO | CH3CO | C31 | C31 | C31 | CH3CO | 12-COCH3 | |||||||||||
| 161.0 | 170.2 | 170.4 | 20.4 | 20.3 | 15.8 | 170.1 | 170.5 | |||||||||||
| CH3CO | CH3CO | C32 | C32 | C32 | CH3CO | 12-COCH3 | ||||||||||||
| 20.9 | 20.9 | 20.8 | 21.0 | 23.5 | 20.7 | 21.0 | ||||||||||||
| OCH2CH3 | OCH3 | OCOCH3 | 15-COCH3 | |||||||||||||||
| 60.7 | 51.9 | 170.2 | 170.6 | |||||||||||||||
| NO | 48c) | 49c) | 50c) | 51c) | 52c) | 53b) | 56b) | 57b) | 58b) | 60b) | 61b) | 62b) | 63b) | 64b) | 65b) | 66a) | 67a) | 68a) |
| C1 | 34.9 | 35.7 | 33.6 | 34.4 | 33.2 | 34.7 | 30.1 | 35.0 | 35.3 | 34.8 | 34.5 | 34.7 | 34.6 | 35.4 | 35.2 | 35.5 | 35.2 | 35.2 |
| C2 | 34.3 | 28.0 | 26.9 | 27.1 | 26.7 | 27.6 | 23.3 | 34.1 | 34.3 | 34.6 | 34.4 | 27.3 | 27.3 | 34.4 | 34.3 | 29.0 | 28.9 | 34.1 |
| C3 | 219.5 | 78.7 | 77.2 | 78.0 | 76.8 | 78.3 | 77.3 | 215.5 | 214.6 | 214.4 | 214.9 | 77.8 | 77.8 | 214.6 | 217.2 | 77.6 | 77.9 | 218.4 |
| C4 | 46.6 | 39.7 | 39.0 | 38.4 | 40.0 | 38.8 | 36.2 | 46.9 | 47.2 | 47.5 | 47.4 | 38.8 | 38.8 | 47.2 | 46.6 | 39.3 | 39.7 | 45.9 |
| C5 | 45.3 | 50.0 | 50.8 | 49.0 | 51.2 | 49.1 | 40.5 | 49.8 | 50.4 | 50.8 | 51.6 | 49.7 | 49.8 | 49.0 | 44.8 | 49.9 | 52.4 | 48.6 |
| C6 | 27.7 | 27.6 | 36.2 | 26.5 | 36.4 | 26.6 | 26.1 | 37.1 | 37.1 | 37.5 | 37.2 | 36.5 | 36.5 | 37.2 | 28.4 | 28.8 | 30.9 | 28.8 |
| C7 | 66.6 | 67.7 | 199.9 | 66.2 | 199.5 | 66.8 | 70.0 | 201.3 | 198.1 | 199.4 | 199.8 | 199.0 | 198.9 | 198.0 | 66.2 | 69.4 | 17.9 | 68.6 |
| C8 | 160.9 | 158.4 | 148.6 | 156.6 | 146.2 | 156.9 | 131.1 | 151.5 | 139.6 | 142.1 | 139.7 | 138.9 | 138.8 | 139.6 | 134.8 | 160.5 | 164.8 | 159.8 |
| C9 | 139.7 | 143.9 | 151.8 | 142.0 | 151.0 | 142.7 | 145.1 | 149.8 | 162.7 | 158.7 | 160.6 | 164.8 | 164.6 | 162.8 | 140.1 | 141.3 | 139.8 | 140.3 |
| C10 | 37.9 | 39.4 | 40.4 | 38.1 | 38..8 | 38.6 | 38.3 | 38.8 | 39.4 | 40.1 | 39.7 | 39.7 | 39.7 | 39.4 | 38.1 | 39.1 | 38.3 | 37.5 |
| C11 | 200.1 | 200.1 | 200.1 | 199.5 | 201.4 | 198.0 | 21.4 | 201.8 | 23.8 | 65.8 | 67.2 | 23.6 | 23.5 | 23.8 | 20.8 | 200.1 | 198.6 | 199.8 |
| C12 | 52.0 | 51.2 | 49.6 | 78.2 | 77.6 | 50.3 | 31.2 | 51.2 | 30.1 | 44.6 | 42.7 | 30.0 | 30.1 | 30.2 | 31.2 | 52.9 | 52.9 | 51.6 |
| C13 | 47.0 | 46.4 | 44.2 | 51.7 | 49.5 | 45.3 | 45.8 | 46.7 | 44.9 | 47.6 | 43.4 | 44.8 | 44.9 | 45.0 | 45.3 | 47.6 | 47.4 | 46.2 |
| C14 | 53.4 | 60.3 | 57.0 | 60.1 | 57.5 | 59.3 | 52.1 | 48.7 | 47.8 | 48.1 | 48.9 | 47.7 | 47.7 | 47.8 | 51.2 | 54.7 | 54.0 | 53.7 |
| C15 | 71.7 | 218.5 | 209.0 | 217.4 | 207.6 | 217.9 | 72.4 | 31.9 | 31.9 | 32.6 | 31.9 | 31.9 | 31.9 | 28.7 | 76.4 | 72.4 | 72.1 | 72.1 |
| C16 | 37.5 | 42.0 | 45.6 | 36.8 | 36.7 | 41.1 | 39.7 | 27.43 | 28.7 | 27.9 | 28.7 | 28.7 | 28.7 | 31.9 | 36.4 | 37.5 | 39.6 | 36.2 |
| C17 | 49.7 | 47.2 | 42.7 | 46.2 | 45.5 | 46.1 | 48.8 | 49.1 | 49.0 | 49.7 | 48.1 | 48.9 | 48.9 | 50.5 | 49.3 | 49.5 | 49.9 | 47.5 |
| C18 | 17.3 | 17.6 | 15.9 | 11.9 | 10.6 | 17.4 | 16.3 | 16.9 | 15.9 | 16.9 | 17.3 | 15.7 | 15.7 | 15.9 | 16.6 | 17.4 | 17.0 | 17.0 |
| C19 | 17.5 | 18.8 | 17.6 | 18.6 | 17.6 | 18.4 | 17.7 | 17.9 | 17.9 | 19.2 | 19.5 | 18.2 | 18.2 | 17.9 | 17.3 | 19.9 | 19.4 | 19.3 |
| C20 | 33.5 | 34.0 | 33.1 | 28.5 | 29.4 | 35.5 | 36.2 | 36.1 | 36.2 | 36.0 | 36.2 | 36.1 | 36.1 | 36.2 | 36.2 | 34.6 | 34.4 | 35.9 |
| C21 | 19.2 | 20.0 | 19.3 | 22.0 | 21.3 | 18.2 | 18.4 | 18.3 | 18.7 | 18.4 | 18.6 | 18.6 | 18.5 | 18.4 | 18.2 | 20.2 | 19.9 | 18.4 |
| C22 | 43.3 | 43.7 | 40.4 | 41.3 | 41.8 | 37.4 | 34.7 | 34.5 | 35.9 | 34.6 | 34.6 | 35.8 | 34.7 | 34.7 | 34.6 | 44.5 | 44.4 | 34.4 |
| C23 | 66.4 | 66.8 | 65.9 | 67.0 | 66.5 | 25.6 | 25.8 | 25.92 | 24.5 | 25.8 | 25.8 | 24.4 | 25.9 | 25.9 | 25.9 | 67.0 | 66.9 | 26.0 |
| C24 | 142.4 | 144.2 | 142.8 | 142.7 | 142.2 | 143.9 | 145.2 | 154.6 | 126.8 | 145.3 | 145.3 | 126.8 | 155.1 | 145.5 | 144.9 | 145.4 | 145.4 | 156.3 |
| C25 | 130.2 | 129.6 | 128.8 | 129.3 | 129.0 | 127.5 | 126.8 | 139.4 | 134.4 | 126.8 | 126.7 | 134.3 | 139.1 | 126.5 | 126.7 | 128.7 | 128.7 | 138.6 |
| C26 | 172.0 | 171.2 | 170.8 | 170.8 | 175.0 | 172.3 | 172.4 | 195.2 | 69.0 | 172.7 | 172.7 | 69.0 | 195.3 | 172.5 | 171.2 | 170.8 | 170.8 | 194.4 |
| C27 | 13.1 | 13.1 | 12.6 | 12.8 | 12.6 | 12.1 | 12.0 | 9.2 | 13.6 | 11.9 | 11.9 | 13.5 | 9.0 | 12.0 | 12.1 | 13.5 | 13.5 | 9.2 |
| C28 | 27.5 | 28.6 | 27.5 | 27.9 | 27.4 | 28.1 | 27.2 | 27.44 | 25.4 | 25.1 | 25.1 | 24.9 | 24.9 | 25.0 | 26.5 | 28.8 | 28.9 | 20.8 |
| C29 | 20.5 | 16.1 | 15.3 | 15.2 | 15.2 | 15.4 | 22.7 | 20.3 | 21.4 | 21.6 | 21.8 | 27.3 | 27.3 | 25.9 | 21.2 | 16.7 | 16.7 | 27.5 |
| C30 | 21.0 | 24.9 | 21.6 | 22.9 | 20.1 | 24.4 | 17.9 | 25.87 | 24.9 | 25.3 | 24.9 | 15.2 | 15.2 | 21.4 | 20.1 | 20.2 | 19.8 | 19.1 |
| COCH3 | ||||||||||||||||||
| 171.9 | ||||||||||||||||||
| COCH3 | ||||||||||||||||||
| 21.9 | ||||||||||||||||||
| NO. | 69c) | 70b) | 71b) | 72b) | 73b) | 74b) | 75b) | 77b) | 78b) | 79b) | 80b) | 81b) | 82b) | 83h) | 84b) | 85b) | 86b) | 87b) |
| C1 | 34.9 | 30.3 | 30.3 | 30.1 | 30.2 | 30.3 | 30.3 | 30.3 | 34.5 | 34.8 | 35.2 | 35.3 | 35.3 | 31.3 | 30.0 | 33.3 | 35.9 | 35.6 |
| C2 | 34.0 | 23.3 | 23.3 | 23.3 | 23.4 | 23.2 | 23.3 | 23.3 | 23.8 | 27.4 | 34.2 | 34.3 | 34.3 | 24.3 | 23.3 | 27.3 | 34.5 | 34.2 |
| C3 | 219.6 | 77.5 | 77.5 | 77.3 | 77.6 | 77.3 | 77.5 | 77.6 | 79.6 | 77.9 | 217.2 | 217.4 | 217.4 | 77.7 | 77.2 | 77.4 | 216.7 | 216.4 |
| C4 | 46.8 | 36.3 | 36.3 | 36.2 | 36.4 | 36.5 | 36.4 | 36.3 | 37.8 | 38.9 | 46.6 | 46.7 | 46.7 | 37.2 | 36.3 | 39.1 | 47.0 | 46.7 |
| C5 | 51.3 | 40.1 | 40.0 | 40.5 | 40.4 | 40.1 | 40.5 | 40.2 | 49.9 | 49.8 | 44.7 | 45.0 | 45.0 | 40.8 | 39.6 | 51.2 | 49.2 | 48.8 |
| C6 | 18.4 | 27.4 | 27.4 | 26.0 | 22.4 | 21.3 | 28.1 | 28.9 | 36.4 | 36.6 | 28.4 | 30.0 | 23.3 | 28.5 | 21.3 | 36.5 | 27.9 | 27.6 |
| C7 | 29.3 | 66.5 | 66.5 | 69.9 | 76.5 | 76.3 | 66.8 | 67.0 | 198.6 | 198.9 | 66.1 | 66.7 | 76.1 | 67.1 | 75.9 | 198.5 | 66.5 | 66.2 |
| C8 | 165.1 | 133.9 | 133.8 | 131.1 | 134.3 | 132.8 | 134.5 | 135.6 | 138.9 | 138.8 | 134.6 | 136.4 | 135.3 | 141.7 | 142.9 | 145.8 | 157.8 | 157.3 |
| C9 | 137.9 | 141.8 | 141.8 | 145.0 | 141.3 | 143.6 | 141.7 | 141.3 | 164.4 | 164.6 | 140.1 | 139.4 | 139.5 | 135.1 | 132.7 | 151.9 | 141.4 | 141.2 |
| C10 | 36.8 | 38.4 | 38.4 | 38.3 | 38.1 | 38.4 | 38.1 | 38.2 | 39.6 | 39.8 | 38.1 | 37.9 | 37.8 | 39.2 | 38.7 | 40.5 | 38.6 | 38.3 |
| C11 | 199.1 | 20.7 | 20.7 | 21.2 | 21.0 | 20.9 | 20.6 | 21.0 | 23.6 | 23.6 | 20.7 | 21.3 | 21.0 | 21.5 | 21.1 | 193.5 | 197.5 | 196.8 |
| C12 | 51.6 | 31.2 | 31.2 | 31.2 | 31.1 | 31.4 | 31.7 | 31.0 | 30.1 | 30.1 | 31.2 | 31.0 | 31.1 | 32.1 | 30.7 | 78.5 | 49.2 | 48.9 |
| C13 | 46.4 | 45.5 | 45.3 | 45.6 | 45.0 | 46.2 | 45.7 | 45.0 | 44.9 | 44.9 | 45.1 | 45.0 | 44.9 | 46.0 | 45.6 | 57.8 | 45.9 | 45.9 |
| C14 | 53.3 | 51.2 | 51.3 | 52.1 | 50.0 | 52.8 | 52.4 | 49.7 | 47.8 | 47.8 | 51.2 | 49.7 | 49.9 | 52.4 | 51.7 | 48.7 | 58.8 | 58.6 |
| C15 | 72.0 | 76.5 | 76.0 | 72.2 | 30.2 | 72.0 | 72.4 | 29.8 | 31.9 | 31.9 | 75.9 | 29.9 | 30.1 | 76.0 | 75.2 | 204.6 | 217.3 | 216.6 |
| C16 | 38.1 | 36.6 | 36.3 | 39.3 | 27.9 | 37.3 | 38.1 | 27.9 | 28.5 | 28.5 | 36.1 | 27.9 | 27.8 | 37.3 | 37.1 | 37.6 | 38.1 | 37.8 |
| C17 | 49.0 | 49.3 | 45.9 | 45.5 | 47.2 | 46.4 | 46.4 | 47.1 | 45.6 | 45.7 | 45.9 | 47.1 | 47.2 | 46.9 | 45.2 | 48.9 | 48.3 | 49.7 |
| C18 | 16.7 | 16.5 | 16.3 | 16.2 | 15.9 | 16.6 | 16.4 | 15.9 | 15.6 | 15.6 | 16.4 | 16.0 | 16.0 | 17.0 | 16.1 | 13.3 | 19.2 | 19.0 |
| C19 | 18.6 | 17.5 | 17.5 | 17.7 | 17.5 | 17.2 | 17.3 | 17.3 | 18.5 | 18.4 | 17.3 | 17.3 | 17.4 | 18.0 | 17.6 | 17.8 | 18.4 | 18.1 |
| C20 | 33.1 | 36.3 | 39.9 | 39.5 | 39.8 | 39.3 | 39.5 | 39.7 | 39.5 | 39.5 | 39.9 | 39.7 | 39.7 | 41.0 | 39.9 | 154.7 | 138.5 | 153.3 |
| C21 | 18.8 | 18.2 | 12.7 | 12.9 | 12.9 | 13.0 | 12.9 | 12.8 | 13.1 | 13.1 | 12.6 | 12.8 | 12.8 | 13.6 | 12.7 | 21.1 | 18.3 | 21.0 |
| C22 | 42.9 | 34.7 | 74.4 | 74.6 | 74.8 | 74.9 | 74.7 | 74.7 | 74.8 | 74.8 | 74.3 | 74.7 | 74.7 | 74.8 | 74.3 | 126.0 | 126.9 | 124.7 |
| C23 | 66.0 | 25.9 | 31.9 | 31.7 | 31.9 | 31.9 | 31.9 | 31.8 | 31.8 | 31.8 | 31.9 | 31.8 | 31.8 | 32.8 | 31.8 | 197.8 | 74.5 | 197.9 |
| C24 | 142.4 | 145.0 | 138.9 | 139.1 | 139.6 | 139.1 | 139.1 | 139.4 | 139.4 | 139.4 | 138.8 | 139.6 | 139.5 | 140.0 | 139.1 | 47.5 | 37.2 | 47.7 |
| C25 | 129.5 | 126.8 | 129.5 | 129.3 | 129.1 | 129.3 | 129.3 | 129.2 | 129.0 | 129.0 | 129.5 | 129.2 | 129.0 | 130.2 | 129.3 | 34.4 | 34.5 | 34.8 |
| C26 | 171.5 | 172.2 | 172.0 | 171.7 | 172.1 | 172.0 | 171.4 | 172.4 | 171.3 | 171.2 | 171.9 | 172.0 | 171.7 | 172.9 | 172.2 | 180.2 | 179.8 | 176.3 |
| C27 | 12.7 | 12.0 | 12.3 | 12.3 | 12.3 | 12.3 | 12.4 | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 | 13.1 | 12.3 | 17.0 | 16.0 | 17.2 |
| C28 | 27.5 | 27.4 | 27.4 | 27.2 | 27.3 | 27.3 | 27.4 | 27.4 | 27.4 | 27.4 | 26.5 | 26.5 | 26.5 | -- | -- | 27.9 | 27.3 | 27.0 |
| C29 | 20.2 | 21.9 | 22.0 | 21.9 | 22.2 | 22.0 | 21.9 | 22.0 | 16.3 | 15.3 | 21.2 | 21.3 | 21.3 | -- | -- | 15.5 | 21.0 | 20.8 |
| C30 | 18.7 | 20.2 | 20.2 | 18.0 | 25.6 | 18.5 | 19.2 | 26.3 | 25.1 | 25.0 | 26.1 | 26.1 | 25.4 | 28.2 | 27.3 | 21.3 | 24.8 | 24.7 |
| OCOCH3 | COCH3 | COCH3 | COCH3 | COCH3 | COCH3 | CO | 3-OC- | 22-OC- | 15-OC- | 22-OC- | 7-OCH3 | C31 | C31 | C31 | OCH3 | |||
| 170.9 | 170.9 | 171.9 | 171.1 | 170.9 | 170.9 | 170.9 | OCH3 | OCH3 | OCH3 | OCH3 | 55.8 | 22.6 | 22.2 | 170.3 | 51.9 | |||
| OCOCH3 | COCH3 | COCH3 | COCH3 | COCH3 | COCH3 | CO | 170.8 | 170.6 | 170.5 | 170.7 | C32 | C32 | C32 | |||||
| 21.4 | 21.4 | 21.7 | 21.6 | 21.5 | 21.4 | 170.7 | 21.1 | 19.0 | 20.5 | |||||||||
| NO. | 88c) | 89c) | 90b) | 91b) | 92b) | 93h) | 94h) | 96h) | 99b) | 100b) | 101b) | 102b) | 103b) | 104b) | 105b) | 106b) | 107b) | 108b) |
| C1 | 35.5 | 34.8 | 34.8 | 35.6 | 35.1 | 34.9 | 35.2 | 34.5 | 35.7 | 37.3 | 37.5 | 36.6 | 34.6 | 35.2 | 33.4 | 35.0 | 35.1 | 35.9 |
| C2 | 34.3 | 27.7 | 27.8 | 34.2 | 34.1 | 34.7 | 33.9 | 27.9 | 34.3 | 34.7 | 34.1 | 27.3 | 27.6 | 34.3 | 27.4 | 33.9 | 28.0 | 34.5 |
| C3 | 217.4 | 78.3 | 78.1 | 216.5 | 215.9 | 213.3 | 213.0 | 77.0 | 217.5 | 217.2 | 214.8 | 77.3 | 78.3 | 216.8 | 77.5 | 215.4 | 78.6 | 216.8 |
| C4 | 46.7 | 39.0 | 38.8 | 46.8 | 46.8 | 46.8 | 46.3 | 40.4 | 46.8 | 43.9 | 46.9 | 40.4 | 38.6 | 47.0 | 40.3 | 46.4 | 39.1 | 47.0 |
| C5 | 48.8 | 49.2 | 50.0 | 48.9 | 49.2 | 48.5 | 49.0 | 50.1 | 49.0 | 50.9 | 51.0 | 51.4 | 49.2 | 49.5 | 51.5 | 49.0 | 49.4 | 49.2 |
| C6 | 29.0 | 26.7 | 27.6 | 27.7 | 27.6 | 36.1 | 36.8 | 36.5 | 27.8 | 33.8 | 33.7 | 33.2 | 26.9 | 27.8 | 36.8 | 36.8 | 26.9 | 27.9 |
| C7 | 68.9 | 66.9 | 69.4 | 66.3 | 65.8 | 198.4 | 204.5 | 205.0 | 66.3 | 199.3 | 198.7 | 198.7 | 66.2 | 65.8 | 198.8 | 204.6 | 67.1 | 66.5 |
| C8 | 159.1 | 156.6 | 158.8 | 157.4 | 156.7 | 146.9 | 150.3 | 149.6 | 157.8 | 149.7 | 149.9 | 151.6 | 157.4 | 158.3 | 150.5 | 150.4 | 156.9 | 157.9 |
| C9 | 140.3 | 142.8 | 142.0 | 141.4 | 141.6 | 149.5 | 152.6 | 154.6 | 141.3 | 146.8 | 146.1 | 145.7 | 141.9 | 140.5 | 147.1 | 152.3 | 142.7 | 141.3 |
| C10 | 38.1 | 38.7 | 38.8 | 38.4 | 38.2 | 39.5 | 39.2 | 38.8 | 38.3 | 39.4 | 39.3 | 39.1 | 38.4 | 37.9 | 39.2 | 39.1 | 38.9 | 38.5 |
| C11 | 199.1 | 198.0 | 200.0 | 197.8 | 191.8 | 198.1 | 199.8 | 200.1 | 197.6 | 199.3 | 194.1 | 193.9 | 199.3 | 199.5 | 201.6 | 201.2 | 198.0 | 197.8 |
| C12 | 50.5 | 49.1 | 50.8 | 48.9 | 78.5 | 47.8 | 50.5 | 51.0 | 50.2 | 48.9 | 79.0 | 79.1 | 77.9 | 78.1 | 77.5 | 52.1 | 51.1 | 50.9 |
| C13 | 48.1 | 46.3 | 49.3 | 46.0 | 50.3 | 44.8 | 48.6 | 49.2 | 45.0 | 47.0 | 47.7 | 47.9 | 51.9 | 51.7 | 49.8 | 47.8 | 46.0 | 45.7 |
| C14 | 53.4 | 58.7 | 53.5 | 58.7 | 59.9 | 56.8 | 52.4 | 52.5 | 59.4 | 57.2 | 58.6 | 58.4 | 60.3 | 60.4 | 57.9 | 52.8 | 59.8 | 59.8 |
| C15 | 72.7 | 216.4 | 72.5 | 216.4 | 215.1 | 204.9 | 72.9 | 72.9 | 216.4 | 206.8 | 205.4 | 205.5 | 216.8 | 215.5 | 206.0 | 71.8 | 217.9 | 218.0 |
| C16 | 31.8 | 37.7 | 31.6 | 37.9 | 38.4 | 37.1 | 32.3 | 32.5 | 41.0 | 39.8 | 37.8 | 37.8 | 38.4 | 38.5 | 37.9 | 30.2 | 36.2 | 36.4 |
| C17 | 52.2 | 49.7 | 52.4 | 49.7 | 50.1 | 50.8 | 52.0 | 52.1 | 45.8 | 44.5 | 44.5 | 44.7 | 45.8 | 45.8 | 45.3 | 50.9 | 50.2 | 50.4 |
| C18 | 19.0 | 18.8 | 18.8 | 19.0 | 14.4 | 17.5 | 16.7 | 15.5 | 17.7 | 16.1 | 12.1 | 12.1 | 12.0 | 12.1 | 10.9 | 18.9 | 19.1 | 19.4 |
| C19 | 19.9 | 18.4 | 19.7 | 18.1 | 18.1 | 18.4 | 17.4 | 17.3 | 18.2 | 18.6 | 18.7 | 17.9 | 18.8 | 18.3 | 18.1 | 17.5 | 18.6 | 18.4 |
| C20 | 157.0 | 153.8 | 157.3 | 153.6 | 154.1 | 153.6 | 155.6 | 155.7 | 32.0 | 32.0 | 29.4 | 29.3 | 28.7 | 28.7 | 29.5 | 73.3 | 73.4 | 73.3 |
| C21 | 21.3 | 21.0 | 21.3 | 20.9 | 20.3 | 21.5 | 21.1 | 21.1 | 19.7 | 19.8 | 21.6 | 21.6 | 21.4 | 21.4 | 21.3 | 26.6 | 26.4 | 26.3 |
| C22 | 124.3 | 124.7 | 124.5 | 124.7 | 126.1 | 124.6 | 124.5 | 124.6 | 49.1 | 49.1 | 48.5 | 48.4 | 48.4 | 48.5 | 48.9 | 52.5 | 48.5 | 48.5 |
| C23 | 198.6 | 197.2 | 199.6 | 196.9 | 197.8 | 197.2 | 197.4 | 197.3 | 207.6 | 207.6 | 207.4 | 207.4 | 208.1 | 208.1 | 208.1 | 211.2 | 74.8 | 74.8 |
| C24 | 47.5 | 47.6 | 48.1 | 47.5 | 47.5 | 47.6 | 47.6 | 47.9 | 46.8 | 46.7 | 46.7 | 46.6 | 46.4 | 46.4 | 46.6 | 47.7 | 36.8 | 36.8 |
| C25 | 35.1 | 34.8 | 34.8 | 34.8 | 34.4 | 35.1 | 35.2 | 35.2 | 34.7 | 34.6 | 34.7 | 34.6 | 34.6 | 34.7 | 34.7 | 34.5 | 33.8 | 33.7 |
| C26 | 180.2 | 180.0 | 179.0 | 180.6 | 180.0 | 181.2 | 181.2 | 175.9 | 176.1 | 176.1 | 176.0 | 176.0 | 176.1 | 176.1 | 176.1 | 177.8 | 178.8 | 178.8 |
| C27 | 17.1 | 17.1 | 17.3 | 17.0 | 17.0 | 17.0 | 17.0 | 17.3 | 17.1 | 17.1 | 17.1 | 17.0 | 17.1 | 17.1 | 17.1 | 17.0 | 16.1 | 16.1 |
| C28 | -- | -- | -- | -- | 26.6 | 27.2 | 27.0 | 27.7 | -- | -- | -- | -- | -- | -- | -- | 27.2 | 28.4 | 27.2 |
| C29 | -- | -- | -- | -- | 21.0 | 20.3 | 20.3 | 18.7 | -- | -- | -- | -- | -- | -- | -- | 20.2 | 15.7 | 21.0 |
| C30 | 27.5 | 28.2 | 28.2 | 27.0 | 24.0 | 20.9 | 20.7 | 20.7 | 27.0 | 27.6 | 27.6 | 27.9 | 28.1 | 26.3 | 28.0 | 20.6 | 25.1 | 25.3 |
| C31 | C31 | C31 | C31 | CH3CO | CO- | C31 | C31 | C31 | C31 | C31 | C31 | C31 | ||||||
| 20.7 | 15.5 | 15.8 | 20.8 | 170.6 | OCH3 | 20.8 | 20.9 | 20.8 | 15.5 | 15.4 | 21.3 | 15.6 | ||||||
| C32 | C32 | C32 | C32 | CH3CO | 51.4 | C32 | C32 | C32 | C32 | C32 | C32 | C32 | ||||||
| 19.4 | 24.4 | 19.5 | 24.7 | 20.6 | 24.7 | 20.3 | 20.4 | 21.2 | 23.1 | 23.3 | 20.3 | |||||||
| NO. | 109f) | 111b) | 113b) | 115a) | 116b) | 117a) | 118b) | 119b) | 120b) | 124b) | 125b) | 126b) | 127b) | 128h) | 129b) | 133a) | 134b) | 135b) |
| C1 | 35.9 | 35.3 | 35.4 | 35.2 | 34.9 | 35.9 | 30.2 | 30.4 | 36.1 | 34.6 | 34.6 | 34.2 | 34.9 | 34.5 | 35.7 | 35.2 | 35.5 | 35.4 |
| C2 | 28.4 | 34.4 | 34.4 | 28.9 | 27.5 | 28.5 | 23.3 | 23.4 | 34.7 | 27.5 | 27.7 | 27.3 | 27.8 | 33.9 | 34.3 | 34.3 | 34.4 | 34.3 |
| C3 | 79.2 | 214.8 | 214.6 | 77.1 | 78.0 | 77.8 | 78.1 | 78.0 | 216.3 | 78.2 | 78.2 | 78.2 | 78.4 | 208.9 | 217.8 | 214.9 | 217.3 | 214.6 |
| C4 | 39.9 | 47.2 | 47.3 | 40.1 | 38.8 | 39.3 | 36.7 | 36.8 | 47.3 | 38.6 | 38.7 | 38.5 | 38.9 | 46.2 | 45.3 | 47.1 | 46.7 | 47.2 |
| C5 | 50.5 | 50.3 | 49.1 | 49.6 | 49.9 | 50.7 | 45.3 | 45.3 | 51.3 | 49.3 | 49.3 | 49.2 | 49.2 | 49.1 | 49.4 | 50.2 | 50.7 | 50.4 |
| C6 | 28.4 | 37.1 | 37.2 | 37.2 | 36.5 | 18.5 | 18.0 | 18.0 | 19.6 | 26.0 | 26.1 | 26.0 | 26.7 | 36.8 | 27.7 | 37.0 | 28.2 | 37.1 |
| C7 | 67.7 | 198.2 | 198.1 | 198.7 | 198.8 | 26.6 | 26.0 | 26.0 | 26.5 | 67.1 | 67.1 | 66.6 | 66.9 | 203.5 | 66.3 | 198.2 | 66.8 | 198.0 |
| C8 | 157.3 | 139.5 | 139.6 | 138.9 | 138.8 | 134.1 | 134.0 | 133.8 | 135.0 | 158.0 | 158.1 | 158.3 | 156.6 | 151.1 | 157.6 | 139.4 | 136.7 | 139.5 |
| C9 | 144.2 | 162.8 | 162.8 | 164.9 | 164.6 | 134.1 | 134.6 | 134.6 | 133.9 | 142.2 | 142.1 | 142.6 | 142.3 | 152.6 | 141.0 | 163.0 | 139.6 | 162.6 |
| C10 | 39.8 | 39.4 | 39.5 | 39.5 | 39.7 | 37.2 | 36.9 | 36.9 | 37.1 | 39.1 | 39.2 | 38.7 | 38.7 | 38.9 | 38.3 | 39.3 | 38.0 | 39.4 |
| C11 | 199.4 | 23.8 | 23.9 | 23.8 | 23.7 | 21.0 | 21.0 | 20.8 | 21.4 | 197.4 | 197.3 | 192.2 | 197.8 | 200.9 | 197.6 | 23.8 | 21.1 | 23.8 |
| C12 | 78.7 | 30.1 | 30.2 | 30.5 | 30.2 | 30.6 | 30.8 | 30.8 | 29.0 | 44.3 | 44.4 | 75.8 | 50.7 | 78.5 | 50.5 | 30.0 | 31.0 | 30.1 |
| C13 | 53.3 | 44.9 | 45.0 | 45.2 | 45.0 | 44.7 | 44.3 | 44.3 | 45.0 | 51.4 | 51.4 | 54.0 | 45.7 | 54.4 | 46.8 | 44.8 | 45.1 | 44.9 |
| C14 | 62.7 | 47.4 | 47.8 | 48.2 | 47.8 | 49.6 | 49.9 | 49.6 | 49.9 | 58.3 | 58.3 | 59.4 | 59.7 | 55.0 | 59.7 | 47.7 | 49.7 | 47.8 |
| C15 | 217.4 | 28.6 | 28.7 | 28.3 | 32.0 | 27.3 | 27.5 | 27.0 | 31.0 | 210.9 | 210.8 | 209.9 | 217.7 | 72.1 | 216.8 | 31.8 | 30.0 | 31.9 |
| C16 | 38.6 | 31.8 | 31.9 | 32.7 | 28.7 | 29.2 | 29.7 | 29.0 | 27.3 | 122.4 | 122.3 | 122.5 | 36.1 | 33.3 | 36.3 | 28.6 | 29.9 | 28.7 |
| C17 | 53.9 | 49.0 | 50.5 | 50.6 | 49.0 | 47.5 | 40.6 | 47.2 | 47.6 | 187.6 | 187.6 | 187.1 | 49.3 | 55.3 | 49.0 | 48.8 | 45.1 | 49.0 |
| C18 | 13.8 | 15.9 | 16.0 | 16.1 | 15.9 | 16.1 | 16.1 | 16.0 | 16.5 | 30.9 | 30.9 | 26.3 | 19.0 | 13.1 | 19.3 | 15.8 | 16.2 | 15.9 |
| C19 | 19.5 | 24.9 | 17.9 | 18.3 | 18.4 | 19.2 | 19.0 | 18.9 | 18.6 | 18.5 | 18.5 | 18.4 | 18.4 | 17.3 | 18.1 | 17.8 | 17.2 | 17.9 |
| C20 | 73.8 | 36.6 | 36.6 | 37.3 | 36.7 | 48.9 | 44.9 | 47.5 | 48.5 | 28.6 | 28.6 | 29.1 | 73.0 | 72.9 | 72.9 | 36.0 | 36.4 | 36.2 |
| C21 | 28.3 | 18.9 | 18.9 | 19.3 | 19.0 | 178.4 | 70.2 | 182.0 | 175.8 | 19.4 | 19.4 | 18.5 | 26.7 | 26.4 | 26.7 | 18.5 | 18.5 | 18.7 |
| C22 | 52.3 | 33.4 | 33.6 | 34.5 | 33.5 | 33.0 | 30.7 | 32.5 | 33.4 | 47.6 | 47.6 | 47.4 | 52.7 | 51.2 | 52.7 | 36.0 | 32.8 | 32.6 |
| C23 | 211.5 | 28.6 | 28.8 | 29.1 | 28.6 | 26.1 | 24.7 | 25.9 | 26.4 | 205.8 | 205.9 | 206.1 | 210.4 | 208.9 | 210.4 | 24.2 | 25.3 | 25.0 |
| C24 | 49.4 | 79.5 | 79.1 | 77.1 | 79.6 | 124.0 | 124.8 | 123.6 | 124.7 | 46.1 | 46.3 | 46.0 | 47.7 | 48.3 | 47.7 | 131.4 | 60.6 | 60.3 |
| C25 | 36.2 | 73.2 | 73.9 | 74.8 | 73.2 | 136.7 | 131.4 | 132.2 | 131.9 | 34.4 | 34.6 | 34.2 | 34.5 | 35.0 | 34.5 | 136.7 | 60.7 | 60.7 |
| C26 | 180.2 | 23.2 | 67.7 | 69.3 | 23.3 | 67.8 | 17.7 | 17.6 | 25.8 | 179.9 | 176.0 | 180.3 | 175.9 | 176.3 | 175.9 | 67.4 | 65.4 | 65.5 |
| C27 | 17.8 | 26.5 | 20.9 | 20.1 | 26.6 | 13.7 | 25.7 | 25.7 | 17.8 | 16.9 | 17.1 | 16.7 | 17.0 | 17.2 | 17.0 | 59.8 | 14.2 | 14.2 |
| C28 | 28.9 | 25.3 | 25.4 | 27.9 | 25.0 | -- | 27.6 | 27.6 | 26.4 | 28.2 | 28.2 | 28.0 | -- | 27.9 | 27.0 | 25.2 | 26.5 | 25.4 |
| C29 | 16.4 | 21.4 | 21.4 | 16.0 | 27.5 | -- | 21.8 | 21.8 | 21.3 | 15.5 | 15.5 | 15.3 | -- | 19.6 | 20.8 | 21.3 | 21.3 | 21.4 |
| C30 | 23.3 | 17.9 | 25.0 | 25.2 | 15.3 | 28.4 | 24.4 | 24.3 | 24.4 | 33.2 | 33.2 | 33.0 | 28.2 | 20.7 | 25.1 | 24.8 | 26.1 | 24.9 |
| C31 | C1' | Glc | C31 | C31 | C31 | CO- | CO- | |||||||||||
| 16.2 | 102.7 | C1' | 51.9 | 170.7 | 15.5 | OCH3 | OCH3 | |||||||||||
| C32 | C2' | 95.8 | C32 | C32 | 51.4 | 52.0 | ||||||||||||
| 24.3 | 71.9 | 20.7 | 24.8 | |||||||||||||||
| NO. | 136b) | 137b) | 138b) | 139b) | 140 | 141 | 142c) | 143b) | 144b) | 145b) | 148a) | 149e) | 150b) | 151g) | 152b) | 153c) | 155b) | |
| C1 | 34.7 | 34.8 | 34.7 | 34.7 | 35.9 | 35.6 | 37.2 | 36.3 | 37.2 | 39.2 | 34.1 | 31.1 | 35.2 | 30.5 | 33.7 | 30.5 | 35.9 | |
| C2 | 24.2 | 24.1 | 24.2 | 24.2 | 33.6 | 27.6 | 35.2 | 34.0 | 34.5 | 18.2 | 28.5 | 23.6 | 24.1 | 24.6 | 27.4 | 23.0 | 33.8 | |
| C3 | 80.2 | 79.3 | 80.2 | 80.2 | 216.4 | 78.2 | 217.5 | 214.2 | 214.5 | 41.3 | 78.3 | 77.8 | 80.8 | 78.8 | 77.6 | 78.2 | 215.3 | |
| C4 | 37.8 | 37.9 | 37.7 | 37.8 | 46.0 | 41.6 | 49.0 | 47.8 | 47.4 | 38.3 | 39.4 | 37.0 | 37.8 | 38.1 | 40.5 | 36.5 | 47.7 | |
| C5 | 49.2 | 49.9 | 49.5 | 49.2 | 40.5 | 39.1 | 53.6 | 52.8 | 50.9 | 59.5 | 52.4 | 45.8 | 50.3 | 46.9 | 50.8 | 45.1 | 54.4 | |
| C6 | 26.7 | 37.0 | 26.7 | 26.7 | 23.1 | 22.2 | 40.9 | 40.0 | 33.4 | 22.3 | 19.1 | 18.3 | 26.3 | 19.4 | 36.2 | 17.7 | 40.3 | |
| C7 | 66.9 | 202.8 | 66.9 | 66.9 | 62.4 | 62.6 | 215.6 | 212.8 | 198.2 | 32.4 | 22.5 | 26.4 | 18.0 | 27.3 | 199.3 | 25.8 | 204.8 | |
| C8 | 157.0 | 154.7 | 157.0 | 157.0 | 63.6 | 62.8 | 55.0 | 54.0 | 150.0 | 42.8 | 51.2 | 134.7 | 132.9 | 135.6 | 146.8 | 133.9 | 46.2 | |
| C9 | 142.8 | 149.9 | 142.8 | 142.8 | 167.7 | 158.2 | 60.4 | 59.5 | 149.5 | 53.1 | 44.9 | 134.9 | 135.4 | 136.3 | 151.4 | 134.3 | 59.1 | |
| C10 | 38.9 | 40.1 | 38.9 | 38.9 | 40.5 | 37.9 | 38.0 | 36.5 | 39.2 | 37.5 | 35.7 | 37.2 | 37.0 | 38.4 | 39.1 | 36.6 | 37.5 | |
| C11 | 197.9 | 199.5 | 197.9 | 197.5 | 130.0 | 128.7 | 210.1 | 207.6 | 197.0 | 22.3 | 27.1 | 21.0 | 20.8 | 22.2 | 199.8 | 21.0 | 207.7 | |
| C12 | 50.4 | 52.3 | 50.4 | 50.4 | 203.6 | 78.1 | 53.7 | 52.6 | 192.6 | 41.5 | 49.0 | 29.6 | 31.1 | 30.8 | 49.9 | 28.6 | 50.6 | |
| C13 | 45.6 | 48.1 | 45.6 | 45.6 | 50.2 | 50.2 | 51.0 | 50.0 | 59.0 | 42.1 | 145.2 | 46.2 | 44.7 | 45.8 | 44.9 | 45.6 | 46.2 | |
| C14 | 59.6 | 53.0 | 59.6 | 59.6 | 64.8 | 64.4 | 51.3 | 49.7 | 61.0 | 59.5 | 47.9 | 48.8 | 51.0 | 51.0 | 57.2 | 49.3 | 54.7 | |
| C15 | 217.6 | 72.3 | 217.6 | 217.6 | 78.4 | 204.0 | 75.5 | 74.1 | 203.8 | 35.6 | 33.8 | 43.6 | 76.0 | 28.3 | 207.7 | 42.1 | 210.4 | |
| C16 | 41.1 | 36.5 | 41.1 | 41.1 | 127.2 | 127.2 | 39.0 | 38.4 | 38.9 | 30.5 | 31.3 | 76.6 | 36.5 | 28.7 | 35.0 | 76.6 | 40.2 | |
| C17 | 45.7 | 48.3 | 45.8 | 45.7 | 167.7 | 168.9 | 49.0 | 47.7 | 38.3 | 53.1 | 137.3 | 57.3 | 49.0 | 48.6 | 47.8 | 56.1 | 44.5 | |
| C18 | 17.6 | 17.5 | 17.6 | 17.6 | 30.9 | 31.0 | 17.1 | 16.6 | 12.4 | 14.6 | 179.2 | 17.8 | 16.1 | 17.0 | 17.8 | 17.0 | 15.8 | |
| C19 | 18.7 | 18.0 | 18.7 | 18.7 | 24.7 | 25.7 | 13.7 | 13.1 | 18.5 | 18.6 | 19.7 | 19.0 | 19.1 | 20.0 | 17.8 | 21.4 | 13.0 | |
| C20 | 32.2 | 32.6 | 32.2 | 32.2 | 72.2 | 72.2 | 33.5 | 32.0 | 32.2 | 36.0 | 35.4 | 48.7 | 36.2 | 49.0 | 73.9 | 47.9 | 32.1 | |
| C21 | 19.9 | 19.6 | 19.9 | 19.9 | 28.6 | 28.7 | 20.0 | 19.4 | 23.3 | 17.9 | 18.6 | 178.8 | 18.2 | 178.1 | 26.5 | 179.7 | 19.4 | |
| C22 | 49.5 | 49.5 | 49.5 | 49.5 | 53.9 | 53.9 | 50.5 | 49.6 | 48.6 | 35.3 | 35.1 | 31.6 | 34.6 | 34.3 | 42.1 | 32.0 | 49.1 | |
| C23 | 207.8 | 208.5 | 207.8 | 207.8 | 208.0 | 208.7 | 211.2 | 208.3 | 207.3 | 22.3 | 23.2 | 33.2 | 25.9 | 32.2(24R) | 23.4 | 24.9 | 207.7 | |
| C24 | 46.8 | 46.8 | 46.9 | 46.8 | 48.0 | 48.2 | 47.8 | 46.8 | 46.5 | 58.2 | 125.6 | 156.1 | 145.2 | 76.5(24R) | 143.6 | 33.6 | 47.1 | |
| C25 | 34.6 | 34.8 | 35.0 | 34.6 | 34.1 | 34.5 | 36.1 | 34.6 | 33.6 | 213.2 | 131.5 | 34.1 | 126.7 | 150.0(24R) | 127.6 | 155.1 | 34.5 | |
| C26 | 179.9 | 179.3 | 175.8 | 179.9 | 180.1 | 179.6 | 179.6 | 176.2 | 180.3 | 32.1 | 25.8 | 21.9 | 172.6 | 110.8(24R) | 173.0 | 106.4 | 176.2 | |
| C27 | 17.1 | 17.1 | 17.4 | 17.1 | 16.8 | 16.9 | 17.8 | 17.1 | 16.8 | 31.8 | 17.7 | 21.9 | 12.0 | 18.7(24R) | 12.1 | 18.6 | 16.7 | |
| C28 | 28.3 | 17.3 | 28.3 | 28.3 | 24.0 | 24.6 | 25.9 | 25.2 | -- | 20.3 | 26.7 | 27.8 | 18.2 | 28.7 | 27.9 | 27.3 | 25.4 | |
| C29 | 16.7 | 20.3 | 16.7 | 16.7 | 21.7 | 22.2 | 21.9 | 21.2 | -- | 35.0 | 16.4 | 22.0 | 27.9 | 22.9 | 15.5 | 21.5 | 20.7 | |
| C30 | 24.5 | 28.3 | 24.6 | 24.5 | 26.9 | 27.0 | 12.8 | 12.5 | 27.4 | 28.7 | 25.7 | 16.5 | 25.3 | 22.4 | 24.8 | 12.7 | ||
| CO | CO | OCH3 | C31 | C31 | CH3CO | COCH3 | ||||||||||||
| 171.1 | 170.9 | 51.9 | 20.3 | 107.0 | 171.1 | 171.3 | ||||||||||||
| CH3 | CH3 | C32 | CH3CO | CH3CO | COCH3 | |||||||||||||
| 21.5 | 21.4 | 19.3 | 21.1 | 171.1 | 21.8 | |||||||||||||
| NO. | 156b) | 157d) | 158d) | 160b) | 161d) | 162b) | 163b) | 164 | 165 | 166 | 167 | 168b) | 170b) | 171 | 172b) | 173b) | 174b) | 175b) |
| C1 | 36.6 | 29.8 | 29.7 | 36.6 | 35.8 | 30.9 | 30.7 | 36.6 | 35.8 | 35.4 | 35.3 | 36.4 | 30.6 | 36.9 | 30.8 | 29.8 | 30.5 | 36.6 |
| C2 | 34.8 | 25.7 | 25.6 | 34.9 | 34.8 | 23.2 | 23.2 | 34.8 | 28.2 | 28.1 | 24.1 | 34.7 | 23.1 | 35.0 | 23.3 | 25.5 | 23.0 | 34.8 |
| C3 | 216.6 | 73.8 | 73.8 | 216.9 | 216.6 | 78.1 | 78.1 | 217.0 | 79.0 | 80.8 | 80.6 | 216.4 | 78.1 | 215.2 | 78.2 | 76.0 | 78.0 | 216.9 |
| C4 | 47.4 | 37.2 | 37.0 | 47.5 | 47.3 | 36.6 | 36.6 | 47.4 | 38.7 | 38.0 | 37.4 | 47.4 | 36.5 | 47.5 | 36.8 | 37.1 | 36.4 | 47.5 |
| C5 | 50.5 | 42.8 | 42.8 | 50.7 | 50.4 | 44.0 | 44.1 | 50.7 | 50.4 | 50.3 | 48.8 | 50.6 | 44.0 | 51.0 | 44.1 | 43.1 | 44.0 | 50.7 |
| C6 | 23.6 | 22.6 | 22.6 | 23.7 | 23.6 | 22.9 | 22.8 | 23.6 | 23.1 | 24.3 | 22.7 | 23.6 | 22.8 | 23.9 | 22.7 | 27.5 | 27.5 | 23.7 |
| C7 | 121.2 | 120.6 | 120.6 | 119.9 | 121.0 | 121.2 | 121.3 | 119.8 | 120.3 | 120.0 | 120.9 | 121.7 | 121.6 | 121.6 | 121.6 | 120.3 | 120.2 | 120.3 |
| C8 | 141.0 | 142.0 | 141.9 | 142.9 | 141.0 | 140.3 | 140.8 | 142.8 | 142.7 | 142.6 | 140.0 | 139.9 | 140.5 | 142.1 | 140.2 | 142.2 | 142.2 | 142.5 |
| C9 | 144.8 | 146.0 | 145.9 | 144.5 | 144.7 | 145.9 | 146.2 | 144.5 | 145.9 | 145.6 | 145.5 | 145.0 | 146.2 | 145.4 | 146.2 | 145.9 | 145.8 | 144.6 |
| C10 | 37.3 | 37.1 | 37.1 | 37.2 | 37.2 | 37.4 | 37.4 | 37.2 | 37.4 | 37.2 | 37.2 | 37.4 | 37.3 | 37.6 | 37.5 | 37.3 | 37.1 | 37.2 |
| C11 | 116.9 | 115.1 | 115.1 | 117.3 | 117.0 | 115.7 | 115.7 | 117.2 | 116.3 | 116.5 | 116.0 | 116.8 | 115.3 | 117.3 | 115.5 | 115.5 | 115.5 | 116.9 |
| C12 | 38.5 | 35.3 | 35.3 | 37.8 | 38.5 | 38.0 | 38.5 | 37.8 | 37.9 | 37.8 | 37.9 | 36.6 | 38.4 | 38.9 | 38.1 | 37.6 | 37.6 | 37.8 |
| C13 | 44.4 | 43.9 | 43.9 | 43.8 | 44.3 | 44.2 | 44.5 | 43.7 | 43.8 | 43.8 | 43.9 | 44.3 | 44.3 | 44.6 | 44.1 | 43.6 | 43.6 | 43.7 |
| C14 | 52.0 | 48.5 | 48.4 | 50.3 | 51.9 | 51.5 | 52.1 | 50.3 | 49.2 | 49.3 | 51.2 | 42.9 | 52.1 | 52.6 | 51.6 | 50.3 | 50.3 | 50.3 |
| C15 | 74.6 | 43.4 | 43.3 | 31.5 | 74.6 | 77.5 | 74.7 | 27.9 | 28.0 | 27.9 | 77.2 | 47.0 | 74.5 | 73.7 | 77.4 | 31.2 | 31.3 | 31.4 |
| C16 | 40.0 | 75.1 | 75.0 | 27.9 | 40.1 | 37.0 | 40.1 | 31.4 | 31.5 | 31.5 | 36.8 | 219.2 | 39.6 | 40.5 | 36.7 | 22.9 | 22.8 | 27.6 |
| C17 | 48.9 | 56.2 | 56.2 | 50.8 | 48.8 | 48.9 | 48.9 | 50.9 | 50.9 | 50.8 | 48.7 | 60.5 | 45.5 | 49.4 | 45.6 | 47.3 | 47.3 | 47.4 |
| C18 | 16.0 | 16.9 | 16.9 | 15.7 | 15.9 | 16.0 | 16.0 | 15.7 | 15.7 | 15.7 | 15.8 | 16.8 | 15.8 | 16.5 | 15.9 | 15.4 | 15.4 | 15.5 |
| C19 | 22.2 | 22.7 | 22.7 | 22.1 | 22.1 | 22.7 | 22.5 | 22.4 | 22.8 | 22.9 | 22.7 | 22.1 | 22.7 | 22.2 | 23.0 | 22.7 | 22.4 | 21.1 |
| C20 | 35.9 | 46.9 | 46.9 | 36.2 | 35.8 | 36.0 | 36.0 | 36.0 | 36.1 | 36.1 | 35.8 | 31.5 | 39.3 | 36.3 | 39.8 | 39.3 | 39.3 | 39.4 |
| C21 | 18.3 | 177.2 | 177.2 | 18.3 | 18.3 | 18.5 | 18.3 | 18.4 | 18.4 | 18.4 | 18.0 | 18.6 | 12.8 | 18.7 | 12.8 | 12.6 | 12.6 | 12.7 |
| C22 | 34.7 | 31.9 | 31.7 | 35.8 | 36.6 | 34.7 | 34.8 | 35.9 | 36.0 | 35.8 | 34.5 | 35.7 | 74.7 | 30.0 | 74.6 | 74.6 | 74.6 | 74.7 |
| C23 | 25.8 | 26.0 | 25.4 | 26.9 | 25.4 | 26.0 | 25.9 | 24.5 | 24.6 | 24.8 | 25.8 | 24.8 | 31.7 | 24.7 | 32.1 | 31.7 | 31.8 | 31.9 |
| C24 | 145.1 | 124.3 | 123.1 | 147.1 | 126.6 | 144.9 | 145.2 | 126.9 | 127.0 | 128.6 | 144.9 | 131.2 | 139.1 | 127.6 | 139.2 | 139.5 | 139.6 | 139.5 |
| C25 | 126.8 | 131.2 | 135.7 | 125.7 | 134.6 | 126.8 | 126.8 | 134.3 | 134.3 | 137.4 | 126.7 | 137.3 | 129.4 | 140.8 | 129.4 | 129.0 | 129.0 | 129.1 |
| C26 | 172.2 | 25.7 | 66.5 | 172.1 | 69.0 | 172.0 | 172.1 | 69.0 | 69.1 | 66.8 | 173.0 | 67.7 | 172.2 | 65.5 | 172.0 | 172.2 | 172.3 | 171.9 |
| C27 | 12.0 | 17.7 | 13.6 | 20.6 | 13.5 | 12.1 | 12.1 | 13.6 | 13.7 | 59.8 | 11.8 | 60.5 | 12.3 | 58.6 | 12.4 | 12.2 | 12.2 | 12.3 |
| C28 | 17.0 | 28.7 | 28.7 | 25.4 | 16.9 | 27.8 | 27.8 | 25.3 | 25.6 | 25.6 | 18.2 | 22.4 | 27.8 | 25.7 | -- | -- | -- | 25.3 |
| C29 | 25.4 | 22.8 | 22.8 | 25.3 | 25.4 | 22.5 | 22.7 | 25.4 | 27.8 | 28.2 | 28.0 | 25.3 | 22.5 | 22.4 | -- | -- | -- | 22.5 |
| C30 | 22.5 | 26.2 | 26.1 | 22.5 | 22.1 | 18.2 | 17.2 | 22.0 | 15.8 | 17.0 | 16.8 | 25.7 | 17.3 | 18.0 | 27.9 | 28.1 | 27.7 | 25.5 |
| COCH3 | COCH3 | AcCH3 | COCH3 | C31 | C31 | C31 | 22-O-COCH3 | |||||||||||
| 171.2 | 170.8 | 21.1 | 170.8 | 22.6 | 22.5 | 22.5 | 170.7 | |||||||||||
| COCH3 | COCH3 | AcCH3 | COCH3 | C32 | C32 | C32 | 22-O-COCH3 | |||||||||||
| 21.4 | 21.3 | 21.2 | 21.3 | 18.6 | 25.7 | 25.6 | 21.1 | |||||||||||
| NO. | 176b) | 177b) | 178b) | 179b) | 180b) | 181b) | 182b) | 183b) | 184b) | 185b) | 186b) | 187a) | 188a) | 189b) | 190b) | 191b) | 192b) | 193b) |
| C1 | 35.4 | 29.9 | 30.5 | 30.6 | 30.5 | 35.6 | 35.3 | 36.6 | 36.6 | 29.9 | 30.6 | 36.8 | 36.8 | 35.6 | 36.1 | 35.8 | 35.4 | 30.5 |
| C2 | 24.2 | 25.5 | 23.0 | 23.1 | 23.1 | 27.6 | 24.1 | 37.5 | 34.8 | 25.5 | 23.1 | 35.0 | 29.2 | 27.8 | 34.9 | 36.5 | 36.5 | 23.1 |
| C3 | 80.7 | 76.7 | 78.0 | 78.1 | 78.0 | 78.8 | 80.7 | 216.6 | 216.4 | 76.1 | 78.1 | 215.3 | 78.4 | 78.8 | 216.8 | 216.5 | 216.5 | 78.0 |
| C4 | 37.6 | 37.3 | 36.4 | 36.5 | 36.4 | 38.5 | 37.4 | 47.4 | 47.4 | 37.0 | 36.5 | 47.5 | 39.8 | 38.6 | 47.5 | 47.6 | 47.6 | 36.4 |
| C5 | 48.9 | 42.9 | 43.8 | 44.0 | 43.9 | 48.7 | 48.9 | 50.4 | 50.4 | 42.9 | 45.0 | 50.9 | 50.1 | 49.0 | 50.8 | 60.7 | 60.8 | 44.2 |
| C6 | 22.8 | 23.0 | 22.7 | 22.8 | 22.7 | 22.9 | 22.6 | 23.6 | 23.7 | 23.0 | 22.8 | 23.8 | 23.9 | 22.9 | 23.7 | 23.8 | 23.8 | 22.7 |
| C7 | 121.3 | 121.5 | 121.3 | 121.4 | 121.2 | 121.3 | 121.0 | 121.0 | 121.3 | 121.5 | 121.6 | 121.6 | 122.5 | 120.3 | 120.0 | 121.8 | 121.8 | 121.5 |
| C8 | 140.0 | 140.0 | 140.0 | 140.6 | 140.6 | 140.0 | 140.6 | 140.4 | 140.2 | 140.0 | 140.5 | 142.0 | 142.4 | 142.4 | 142.9 | 140.1 | 140.2 | 140.4 |
| C9 | 145.8 | 146.0 | 145.8 | 146.1 | 146.1 | 145.8 | 145.7 | 145.0 | 144.7 | 146.0 | 146.2 | 145.3 | 147.4 | 145.9 | 144.6 | 145.1 | 145.1 | 146.1 |
| C10 | 37.3 | 37.3 | 37.3 | 37.3 | 37.2 | 37.3 | 37.2 | 37.3 | 37.3 | 37.3 | 37.3 | 37.5 | 38.3 | 37.3 | 37.3 | 37.6 | 37.6 | 37.2 |
| C11 | 115.9 | 115.5 | 115.4 | 115.5 | 115.4 | 115.8 | 116.0 | 116.9 | 116.7 | 115.3 | 115.3 | 117.2 | 116.6 | 116.0 | 117.3 | 117.0 | 117.0 | 115.2 |
| C12 | 38.0 | 37.9 | 37.8 | 38.3 | 38.3 | 37.9 | 38.3 | 38.0 | 38.0 | 37.9 | 38.4 | 38.8 | 39.2 | 37.7 | 37.9 | 36.8 | 36.8 | 38.4 |
| C13 | 43.9 | 44.1 | 44.1 | 44.5 | 44.3 | 44.0 | 44.2 | 44.1 | 43.9 | 43.9 | 44.2 | 44.5 | 45.0 | 43.7 | 43.8 | 44.5 | 44.5 | 43.9 |
| C14 | 51.3 | 51.7 | 51.4 | 52.2 | 52.0 | 51.2 | 51.8 | 51.3 | 51.4 | 51.4 | 52.0 | 52.6 | 53.0 | 50.2 | 50.4 | 43.1 | 43.1 | 52.0 |
| C15 | 77.0 | 77.0 | 77.2 | 74.6 | 74.5 | 77.3 | 74.5 | 77.2 | 76.7 | 76.9 | 74.5 | 73.6 | 74.2 | 31.4 | 27.9 | 34.9 | 34.9 | 74.4 |
| C16 | 36.6 | 37.2 | 37.1 | 40.1 | 40.2 | 36.9 | 39.8 | 37.0 | 36.6 | 36.7 | 39.8 | 40.9 | 41.0 | 27.7 | 31.5 | 25.0 | 25.3 | 39.6 |
| C17 | 45.4 | 48.7 | 48.6 | 48.8 | 49.2 | 48.7 | 48.7 | 48.9 | 45.5 | 45.4 | 45.0 | 50.1 | 49.8 | 50.7 | 50.9 | 31.6 | 31.7 | 45.4 |
| C18 | 15.7 | 16.0 | 15.9 | 16.0 | 15.7 | 15.9 | 15.8 | 16.0 | 15.8 | 15.8 | 15.8 | 16.3 | 16.8 | 15.7 | 15.8 | 17.0 | 17.1 | 15.7 |
| C19 | 22.8 | 22.6 | 22.5 | 22.7 | 22.5 | 22.7 | 22.7 | 22.4 | 22.1 | 22.7 | 22.6 | 22.1 | 23.6 | 22.6 | 22.5 | 22.3 | 22.3 | 22.6 |
| C20 | 39.6 | 32.8 | 32.8 | 33.0 | 33.4 | 35.8 | 35.8 | 35.9 | 39.6 | 39.6 | 39.3 | 34.4 | 36.8 | 36.0 | 36.1 | 50.8 | 50.8 | 39.2 |
| C21 | 12.6 | 19.4 | 19.3 | 19.6 | 19.4 | 18.1 | 18.1 | 18.2 | 12.7 | 12.7 | 12.8 | 20.3 | 19.0 | 18.2 | 18.4 | 18.7 | 18.8 | 12.7 |
| C22 | 74.4 | 51.5 | 51.5 | 51.9 | 67.0 | 34.5 | 34.7 | 34.6 | 74.4 | 74.4 | 74.6 | 44.7 | 37.3 | 34.6 | 36.7 | 219.3 | 219.0 | 74.5 |
| C23 | 31.9 | 201.6 | 201.4 | 201.8 | 43.6 | 25.8 | 25.7 | 25.9 | 31.9 | 31.9 | 31.7 | 67.1 | 25.1 | 25.9 | 24.4 | 47.1 | 47.1 | 31.7 |
| C24 | 139.0 | 133.8 | 133.9 | 134.1 | 144.8 | 144.9 | 145.0 | 144.5 | 139.0 | 139.1 | 139.2 | 145.5 | 127.9 | 155.4 | 131.7 | 131.4 | 126.8 | 139.2 |
| C25 | 129.2 | 139.5 | 139.4 | 139.3 | 128.3 | 126.7 | 126.8 | 126.8 | 129.2 | 129.2 | 129.4 | 128.7 | 141.3 | 139.1 | 136.8 | 137.4 | 134.8 | 129.2 |
| C26 | 171.3 | 171.2 | 171.8 | 171.0 | 172.0 | 172.9 | 172.9 | 172.1 | 171.3 | 171.6 | 172.0 | 170.3 | 65.8 | 195.3 | 67.7 | 60.2 | 69.3 | 172.1 |
| C27 | 12.3 | 14.1 | 13.9 | 14.1 | 12.6 | 11.9 | 11.9 | 12.0 | 12.3 | 12.3 | 12.3 | 13.5 | 58.8 | 9.0 | 60.2 | 67.9 | 13.8 | 12.2 |
| C28 | 18.4 | 18.5 | 18.3 | 17.2 | 17.1 | 18.3 | 17.0 | 18.2 | 25.4 | -- | -- | 25.6 | 29.3 | 25.4 | 25.5 | 25.5 | 25.5 | 17.2 |
| C29 | 28.1 | 28.2 | 27.7 | 27.8 | 27.7 | 28.1 | 28.0 | 25.4 | 22.4 | -- | -- | 22.3 | 17.1 | 28.0 | 22.1 | 22.6 | 22.6 | 27.6 |
| C30 | 16.9 | 22.6 | 22.3 | 22.5 | 22.3 | 15.7 | 16.8 | 22.1 | 18.3 | 28.2 | 27.7 | 18.0 | 18.6 | 15.5 | 25.4 | 25.8 | 25.8 | 22.4 |
| AcCO | AcCO | AcCO | AcCO | AcCO | AcCO | AcCO | AcCO | 15-O- | C31 | C31 | C1' | |||||||
| 171.1 | 171.0 | 171.1 | 170.9 | 170.7 | 171.1 | 170.9 | 171.2 | COCH3 | 22.8 | 22.5 | 170.8 | |||||||
| AcCO | AcCH3 | AcCO | AcCH3 | AcCH3 | AcCO | AcCO | AcCO | 171.1 | C32 | C32 | C2' | |||||||
| 170.6 | 21.4 | 170.7 | 21.3 | 21.2 | 21.3 | 21.2 | 21.4 | 18.5 | 17.3 | 170.5 | ||||||||
| AcCH3 | AcCH3 | C3' | ||||||||||||||||
| 21.0 | 21.6 | 21.2 | ||||||||||||||||
| NO. | 194b) | 196d) | 197b) | 198b) | 201b) | 202b) | 203 | 204b) | 205b) | 207b) | 208b) | 209 | 210b) | 214b) | 215b) | 216b) | 217b) | 218b) |
| C1 | 35.6 | 36.1 | 35.7 | 35.4 | 35.7 | 36.6 | 36.6 | 36.5 | 29.9 | 30.0 | 35.5 | 30.5 | 36.7 | 29.8 | 35.6 | 30.6 | 35.4 | 29.9 |
| C2 | 27.7 | 34.4 | 28.0 | 22.8 | 27.9 | 34.8 | 28.7 | 34.9 | 25.6 | 25.6 | 27.0 | 23.0 | 34.8 | 25.4 | 27.4 | 23.1 | 24.2 | 25.6 |
| C3 | 78.8 | 215.3 | 78.9 | 80.8 | 78.9 | 216.7 | 78.1 | 216.9 | 76.1 | 76.3 | 78.3 | 78.0 | 217.0 | 75.8 | 78.6 | 78.0 | 80.7 | 76.1 |
| C4 | 38.6 | 46.9 | 38.7 | 37.6 | 38.7 | 47.4 | 39.4 | 47.5 | 37.3 | 37.3 | 38.3 | 36.4 | 47.5 | 37.2 | 38.5 | 36.5 | 37.6 | 37.4 |
| C5 | 48.7 | 50.5 | 49.1 | 49.3 | 49.1 | 50.7 | 49.8 | 50.3 | 42.9 | 43.1 | 48.5 | 43.8 | 50.3 | 42.9 | 49.0 | 43.9 | 49.0 | 43.0 |
| C6 | 22.9 | 23.2 | 23.0 | 24.3 | 23.0 | 23.6 | 23.5 | 23.7 | 23.0 | 23.0 | 22.6 | 22.7 | 23.6 | 22.8 | 22.8 | 22.8 | 22.9 | 22.9 |
| C7 | 121.6 | 120.1 | 120.4 | 120.0 | 120.2 | 119.9 | 121.0 | 119.9 | 121.3 | 121.4 | 121.0 | 121.0 | 119.9 | 121.2 | 121.2 | 121.2 | 121.2 | 121.7 |
| C8 | 139.9 | 142.1 | 142.5 | 142.7 | 142.6 | 142.8 | 143.0 | 142.8 | 140.2 | 140.9 | 140.5 | 140.1 | 142.8 | 140.6 | 140.7 | 140.1 | 140.2 | 140.5 |
| C9 | 146.0 | 144.2 | 146.0 | 146.7 | 145.9 | 144.5 | 146.6 | 144.5 | 146.0 | 146.3 | 145.9 | 145.4 | 144.4 | 146.1 | 146.0 | 145.9 | 145.7 | 146.3 |
| C10 | 37.4 | 37.0 | 37.4 | 37.8 | 37.3 | 37.2 | 37.8 | 37.8 | 37.3 | 37.3 | 37.1 | 37.2 | 37.8 | 37.2 | 37.3 | 37.3 | 37.3 | 37.4 |
| C11 | 115.5 | 116.7 | 116.1 | 116.5 | 116.2 | 117.2 | 116.6 | 117.2 | 115.6 | 115.7 | 115.5 | 115.5 | 117.2 | 115.4 | 115.8 | 115.5 | 116.1 | 115.3 |
| C12 | 37.9 | 35.3 | 37.8 | 37.2 | 37.8 | 37.8 | 38.1 | 37.2 | 38.0 | 38.5 | 38.2 | 37.9 | 37.2 | 38.3 | 38.4 | 37.9 | 38.0 | 38.5 |
| C13 | 43.9 | 43.8 | 43.8 | 43.7 | 43.7 | 43.7 | 44.1 | 43.8 | 44.1 | 44.4 | 44.0 | 44.0 | 43.7 | 44.2 | 44.1 | 44.1 | 44.1 | 44.2 |
| C14 | 51.3 | 48.3 | 50.3 | 50.3 | 50.3 | 50.3 | 50.7 | 50.7 | 51.4 | 52.2 | 51.6 | 51.3 | 50.7 | 51.9 | 51.9 | 51.4 | 51.4 | 52.1 |
| C15 | 76.9 | 43.3 | 31.5 | 26.0 | 31.5 | 27.6 | 28.1 | 27.9 | 77.4 | 74.8 | 73.9 | 77.3 | 27.9 | 74.2 | 74.3 | 77.3 | 77.1 | 74.6 |
| C16 | 36.6 | 75.0 | 27.8 | 31.5 | 27.8 | 28.8 | 31.9 | 28.7 | 37.0 | 40.0 | 39.2 | 36.9 | 28.8 | 39.8 | 38.7 | 37.2 | 37.2 | 39.8 |
| C17 | 45.4 | 56.2 | 50.9 | 50.8 | 50.9 | 51.0 | 51.5 | 50.9 | 48.9 | 48.8 | 48.7 | 48.8 | 51.0 | 49.2 | 45.0 | 49.4 | 49.4 | 45.4 |
| C18 | 15.7 | 16.9 | 15.7 | 16.9 | 15.7 | 15.7 | 16.6 | 15.7 | 16.0 | 15.9 | 15.5 | 15.9 | 15.3 | 15.7 | 15.7 | 15.9 | 15.9 | 15.8 |
| C19 | 22.7 | 21.8 | 22.7 | 22.8 | 22.7 | 22.4 | 23.1 | 22.5 | 22.7 | 22.7 | 22.4 | 22.5 | 22.4 | 22.6 | 22.7 | 22.6 | 22.9 | 22.7 |
| C20 | 39.5 | 46.9 | 36.2 | 36.5 | 36.5 | 36.5 | 37.1 | 36.6 | 36.0 | 35.9 | 35.6 | 35.9 | 36.6 | 33.3 | 40.7 | 33.6 | 33.6 | 39.3 |
| C21 | 12.6 | 177.2 | 18.3 | 18.6 | 18.6 | 18.6 | 19.0 | 18.6 | 18.2 | 18.3 | 17.9 | 18.1 | 18.6 | 19.3 | 12.4 | 19.3 | 19.3 | 12.8 |
| C22 | 74.4 | 31.8 | 34.7 | 32.6 | 33.5 | 31.4 | 34.4 | 31.5 | 34.7 | 34.8 | 34.5 | 34.5 | 31.5 | 66.5 | 72.1 | 67.2 | 67.2 | 74.6 |
| C23 | 31.8 | 26.0 | 26.1 | 27.8 | 28.7 | 33.5 | 28.9 | 33.5 | 25.9 | 25.7 | 25.4 | 25.8 | 33.5 | 43.4 | 34.8 | 43.3 | 43.4 | 31.7 |
| C24 | 138.9 | 124.2 | 155.4 | 76.6 | 79.6 | 79.2 | 77.2 | 79.6 | 145.1 | 145.2 | 143.3 | 145.0 | 79.1 | 143.6 | 139.9 | 144.7 | 144.7 | 139.2 |
| C25 | 129.2 | 131.1 | 139.1 | 73.3 | 73.2 | 73.9 | 74.8 | 73.3 | 126.6 | 127.0 | 127.0 | 126.7 | 74.1 | 128.6 | 129.0 | 128.1 | 128.1 | 129.1 |
| C26 | 171.5 | 25.6 | 195.4 | 68.5 | 23.6 | 67.6 | 69.3 | 25.5 | 171.9 | 172.8 | 170.5 | 172.9 | 67.6 | 170.3 | 170.4 | 171.2 | 171.2 | 171.0 |
| C27 | 12.2 | 17.6 | 9.2 | 20.2 | 26.5 | 22.0 | 20.1 | 25.3 | 12.0 | 12.0 | 11.7 | 11.8 | 22.0 | 12.7 | 11.4 | 12.8 | 12.8 | 12.3 |
| C28 | 18.3 | 25.4 | 28.1 | 28.1 | 28.1 | -- | 28.8 | 23.2 | 18.5 | 17.4 | 16.8 | 18.3 | 25.4 | 17.0 | 17.1 | 18.4 | 18.4 | 17.3 |
| C29 | 28.1 | 22.1 | 15.8 | 15.7 | 15.8 | -- | 16.0 | 26.6 | 28.2 | 28.2 | 27.7 | 27.7 | 25.4 | 28.0 | 28.0 | 27.8 | 28.1 | 28.1 |
| C30 | 15.7 | 25.8 | 25.6 | 25.5 | 25.6 | 25.3 | 25.9 | 22.1 | 22.8 | 22.8 | 15.4 | 22.3 | 20.9 | 22.7 | 15.7 | 22.4 | 16.9 | 22.8 |
| C1' | OCOCH3 | C31 | C31 | AcCH3 | CH3CO | CH3CO | CH3CO | |||||||||||
| 171.0 | 170.64 | 20.9 | 171.2 | 21.2 | 170.8 | 170.5 | 170.6 | |||||||||||
| C2' | 170.93 | C32 | C32 | AcCH3 | CH3CO | CH3CO | CH3CO | |||||||||||
| 170.5 | 171.11 | 25.4 | 21.4 | 21.3 | 170.6 | 171.0 | 21.03 | |||||||||||
| C3' | OCOCH3 | AcCO | CH3CO | CH3CO | ||||||||||||||
| 21.3 | 20.83 | 170.7 | 21.4 | 21.3 | ||||||||||||||
| C4' | 20.99 | AcCO | CH3CO | CH3CO | ||||||||||||||
| 20.9 | 21.29 | 171.0 | 21.3 | 21.4 | ||||||||||||||
| NO. | 219b) | 220b) | 221b) | 222b) | 223b) | 224b) | 225b) | 226b) | 227b) | 228b) | 229b) | 230b) | 231b) | 232b) | 233b) | 234b) | 238b) | 239b) |
| C1 | 35.6 | 36.6 | 35.7 | 35.0 | 35.8 | 34.7 | 35.8 | 37.4 | 35.7 | 36.0 | 34.8 | 37.4 | 36.7 | 37.3 | 35.6 | 34.2 | 35.3 | 34.4 |
| C2 | 27.3 | 34.8 | 27.8 | 27.9 | 34.5 | 27.5 | 34.1 | 34.0 | 34.3 | 34.5 | 27.6 | 34.0 | 27.4 | 34.6 | 34.2 | 23.9 | 34.3 | 27.2 |
| C3 | 78.6 | 216.8 | 78.9 | 78.5 | 216.8 | 78.3 | 216.6 | 214.8 | 216.5 | 215.9 | 78.3 | 215.2 | 77.5 | 215.3 | 218.1 | 79.9 | 214.7 | 78.0 |
| C4 | 38.5 | 47.5 | 38.7 | 38.8 | 47.0 | 38.6 | 46.7 | 46.9 | 46.8 | 46.8 | 38.6 | 46.9 | 40.5 | 43.9 | 46.7 | 37.5 | 47.2 | 38.5 |
| C5 | 48.9 | 50.7 | 49.1 | 49.3 | 49.1 | 49.1 | 48.8 | 51.0 | 49.0 | 48.9 | 49.1 | 50.9 | 51.4 | 51.0 | 48.7 | 49.2 | 50.4 | 49.1 |
| C6 | 22.8 | 23.7 | 23.0 | 26.8 | 27.8 | 26.5 | 27.6 | 33.8 | 27.7 | 29.1 | 26.6 | 33.7 | 33.3 | 33.9 | 27.6 | 26.6 | 37.1 | 26.6 |
| C7 | 121.6 | 120.0 | 120.3 | 67.0 | 66.5 | 66.9 | 66.2 | 198.5 | 66.3 | 65.7 | 66.9 | 198.7 | 198.8 | 199.5 | 66.2 | 66.1 | 198.1 | 66.1 |
| C8 | 140.4 | 142.8 | 142.6 | 157.2 | 158.1 | 156.8 | 157.4 | 149.7 | 157.8 | 159.8 | 156.8 | 149.8 | 151.6 | 149.7 | 157.9 | 157.4 | 139.5 | 155.9 |
| C9 | 146.2 | 144.5 | 146.0 | 142.9 | 141.4 | 142.6 | 141.1 | 146.2 | 141.3 | 140.9 | 142.7 | 146.2 | 146.0 | 146.9 | 141.1 | 141.6 | 162.7 | 142.9 |
| C10 | 37.3 | 37.2 | 37.4 | 38.8 | 38.4 | 38.8 | 38.2 | 39.3 | 38.3 | 38.5 | 38.8 | 39.3 | 39.2 | 39.4 | 38.2 | 37.5 | 39.4 | 38.5 |
| C11 | 115.4 | 117.2 | 116.2 | 198.2 | 197.9 | 197.8 | 196.9 | 194.1 | 197.5 | 198.0 | 198.0 | 194.1 | 194.1 | 199.4 | 197.8 | 199.5 | 23.8 | 192.3 |
| C12 | 38.3 | 37.8 | 37.8 | 50.6 | 50.4 | 49.1 | 49.9 | 79.1 | 49.1 | 49.8 | 50.3 | 79.1 | 79.4 | 49.0 | 50.2 | 78.2 | 30.1 | 79.8 |
| C13 | 44.0 | 43.8 | 43.8 | 45.5 | 45.1 | 46.0 | 45.0 | 47.6 | 45.3 | 45.5 | 45.3 | 47.6 | 48.0 | 47.0 | 44.9 | 51.9 | 44.9 | 50.4 |
| C14 | 51.8 | 50.3 | 50.3 | 59.6 | 59.5 | 58.8 | 59.2 | 58.6 | 58.8 | 58.3 | 59.4 | 58.6 | 58.5 | 57.2 | 59.3 | 60.2 | 47.8 | 60.6 |
| C15 | 74.0 | 31.5 | 31.5 | 218.1 | 218.2 | 217.7 | 215.8 | 205.8 | 217.7 | 215.5 | 217.5 | 205.9 | 206.0 | 207.3 | 216.8 | 217.1 | 28.5 | 216.7 |
| C16 | 39.1 | 27.9 | 27.9 | 41.2 | 41.3 | 38.4 | 35.6 | 37.4 | 38.7 | 39.5 | 41.0 | 37.6 | 37.6 | 39.9 | 41.1 | 38.0 | 31.8 | 37.4 |
| C17 | 45.3 | 50.9 | 50.9 | 46.4 | 46.5 | 46.1 | 49.5 | 45.2 | 46.3 | 46.7 | 46.1 | 45.2 | 45.5 | 45.2 | 46.2 | 46.5 | 48.9 | 46.0 |
| C18 | 15.6 | 15.7 | 15.7 | 17.6 | 17.9 | 18.4 | 19.0 | 12.0 | 18.8 | 19.3 | 17.4 | 12.0 | 12.1 | 16.1 | 17.6 | 12.0 | 15.9 | 13.1 |
| C19 | 22.7 | 22.5 | 22.7 | 18.6 | 18.3 | 18.5 | 18.1 | 18.6 | 18.2 | 18.6 | 18.4 | 18.7 | 18.0 | 18.6 | 18.2 | 18.8 | 17.9 | 18.6 |
| C20 | 39.1 | 36.1 | 36.0 | 35.4 | 35.4 | 143.9 | 85.9 | 33.0 | 143.9 | 145.8 | 35.1 | 33.1 | 33.0 | 35.4 | 35.2 | 31.7 | 35.9 | 31.8 |
| C21 | 12.5 | 18.4 | 18.4 | 18.3 | 18.2 | 112.2 | 25.9 | 20.1 | 112.3 | 111.6 | 18.0 | 20.0 | 20.2 | 18.3 | 18.0 | 20.5 | 18.3 | 20.4 |
| C22 | 74.9 | 32.7 | 32.7 | 30.8 | 30.9 | 31.3 | 34.2 | 30.1 | 31.3 | 31.8 | 30.4 | 29.9 | 30.2 | 30.8 | 30.6 | 29.4 | 30.8 | 29.5 |
| C23 | 31.5 | 25.3 | 25.3 | 31.3 | 31.3 | 32.3 | 27.4 | 31.6 | 31.9 | 32.6 | 30.7 | 31.6 | 31.8 | 31.0 | 31.1 | 31.5 | 30.9 | 30.0 |
| C24 | 137.2 | 60.6 | 60.6 | 173.8 | 173.8 | 177.3 | 175 | 173.6 | 175.1 | 173.2 | 178.2 | 178.7 | 173.7 | 173.8 | 173.5 | 177.8 | 178.1 | 178.2 |
| C25 | 129.9 | 60.7 | 60.7 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| C26 | 169.9 | 65.4 | 65.4 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| C27 | 12.3 | 14.2 | 14.2 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| C28 | 17.1 | 25.4 | 28.1 | 28.4 | 27.2 | 24.2 | 25.0 | -- | 27.0 | 27.0 | 24.4 | -- | -- | -- | -- | 27.9 | 25.3 | 28.0 |
| C29 | 27.9 | 22.1 | 15.8 | 15.6 | 20.9 | 28.1 | 26.9 | -- | 20.8 | 20.9 | 28.1 | -- | -- | -- | -- | 16.4 | 21.4 | 15.3 |
| C30 | 15.6 | 25.4 | 25.6 | 24.6 | 24.9 | 15.4 | 20.7 | 27.6 | 24.6 | 25.2 | 15.4 | 27.6 | 27.9 | 27.7 | 27.0 | 23.1 | 24.9 | 24.0 |
| CH3CO | Bu1' | Bu1' | C31 | COOCH3 | C31 | C31 | C31 | C31 | C31 | COCH3 | ||||||||
| 171.1 | 64.6 | 64.6 | 20.8 | 51.5 | 20.4 | 15.6 | 20.9 | 20.7 | 161.0 | 170.5 | ||||||||
| CH3CO | Bu2' | Bu2' | C32 | C32 | C32 | C32 | C32 | COCH3 | ||||||||||
| 20.87 | 30.9 | 30.9 | 20.4 | 20.8 | 21.4 | 20.3 | 24.6 | 20.7 | ||||||||||
| NO. | 241b) | 242b) | 243b) | 244b) | 248b) | 249b) | 252b) | 253b) | 254b) | 255b) | 256b) | 261b) | 262a) | 263a) | 264a) | 266a) | 267b) | 268a) |
| C1 | 35.6 | 33.6 | 34.0 | 33.3 | 35.0 | 35.4 | 34.0 | 34.6 | 33.2 | 34.8 | 34.4 | 36.6 | 36.8 | 35.4 | 36.7 | 35.3 | 35.4 | 73.8 |
| C2 | 34.3 | 27.4 | 33.8 | 27.4 | 27.9 | 34.5 | 33.6 | 33.8 | 27.3 | 27.6 | 27.4 | 34.8 | 35.0 | 28.3 | 27.9 | 28.9 | 27.7 | 39.8 |
| C3 | 216.9 | 77.6 | 214.8 | 77.5 | 78.5 | 215.9 | 214.9 | 215.1 | 77.4 | 78.2 | 78.1 | 216.5 | 215.3 | 72.0 | 70.7 | 79.3 | 78.8 | 75.5 |
| C4 | 46.8 | 39.1 | 46.9 | 40.5 | 38.8 | 47.2 | 46.9 | 47.0 | 40.5 | 38.6 | 38.6 | 47.4 | 47.4 | 43.0 | 43.6 | 43.1 | 38.8 | 40.2 |
| C5 | 48.8 | 50.8 | 51.0 | 51.4 | 49.3 | 49.6 | 50.9 | 50.9 | 51.4 | 49.1 | 49.1 | 50.7 | 50.9 | 42.5 | 44.5 | 50.2 | 50.2 | 49.1 |
| C6 | 29.0 | 36.3 | 37.4 | 36.7 | 26.9 | 28.0 | 37.4 | 37.2 | 36.6 | 26.6 | 26.7 | 23.7 | 23.8 | 27.8 | 34.1 | 28.5 | 18.2 | 17.6 |
| C7 | 68.8 | 199.4 | 198.5 | 198.8 | 67.0 | 66.0 | 198.4 | 199.1 | 198.0 | 66.0 | 66.1 | 119.9 | 121.6 | 66.7 | 200.4 | 67.0 | 26.4 | 26.0 |
| C8 | 159.2 | 151.6 | 149.7 | 151.6 | 157.2 | 158.6 | 145.6 | 146.2 | 151.4 | 156.5 | 155.9 | 142.6 | 142.0 | 158.8 | 152.2 | 158.0 | 134.1 | 134.1 |
| C9 | 140.3 | 147.0 | 146.2 | 156.0 | 142.9 | 140.6 | 149.6 | 149.6 | 145.4 | 142.6 | 142.9 | 144.4 | 145.3 | 142.9 | 147.4 | 142.6 | 134.4 | 137.0 |
| C10 | 38.0 | 40.4 | 39.3 | 39.2 | 38.9 | 38.1 | 39.3 | 34.6 | 39.1 | 38.9 | 38.6 | 37.2 | 37.5 | 39.2 | 40.8 | 39.1 | 36.9 | 44.1 |
| C11 | 199.6 | 199.9 | 194.1 | 194.1 | 198.1 | 199.9 | 193.3 | 198.0 | 193.0 | 197.0 | 191.0 | 117.0 | 117.2 | 198.3 | 199.8 | 198.4 | 20.9 | 25.1 |
| C12 | 51.8 | 49.6 | 79.1 | 79.4 | 50.6 | 78.5 | 78.6 | 48.8 | 78.9 | 50.2 | 79.1 | 37.8 | 38.7 | 51.1 | 49.9 | 51.1 | 30.7 | 32.0 |
| C13 | 46.6 | 44.3 | 47.6 | 48.0 | 45.5 | 51.9 | 47.9 | 44.3 | 48.2 | 45.4 | 49.8 | 43.7 | 44.5 | 45.6 | 44.5 | 45.6 | 44.4 | 44.3 |
| C14 | 53.9 | 57.0 | 58.6 | 58.5 | 59.6 | 60.5 | 58.9 | 57.1 | 58.9 | 59.3 | 61.0 | 50.2 | 52.6 | 59.2 | 57.6 | 59.0 | 49.8 | 50.4 |
| C15 | 72.6 | 208.1 | 205.8 | 206.0 | 218.1 | 217.5 | 203.8 | 204.9 | 203.9 | 215.7 | 214.5 | 27.9 | 73.6 | 216.8 | 208.0 | 216.7 | 30.7 | 31.6 |
| C16 | 36.6 | 40.2 | 37.4 | 37.6 | 41.2 | 37.9 | 35.4 | 34.3 | 35.7 | 35.7 | 37.1 | 31.5 | 40.4 | 41.4 | 40.5 | 41.5 | 27.7 | 28.7 |
| C17 | 48.5 | 45.6 | 45.2 | 45.5 | 46.4 | 46.8 | 48.8 | 48.1 | 49.2 | 49.5 | 50.2 | 50.8 | 49.3 | 46.2 | 45.5 | 46.3 | 45.7 | 46.6 |
| C18 | 17.3 | 16.2 | 12.0 | 12.1 | 17.6 | 12.3 | 13.0 | 17.4 | 13.2 | 18.8 | 14.2 | 15.7 | 16.4 | 17.8 | 16.4 | 17.9 | 15.5 | 16.2 |
| C19 | 19.4 | 17.8 | 18.6 | 18.0 | 18.6 | 18.5 | 18.7 | 18.6 | 17.9 | 18.3 | 18.5 | 22.0 | 22.1 | 19.2 | 18.6 | 19.2 | 19.1 | 15.5 |
| C20 | 35.7 | 35.3 | 33.0 | 33.0 | 35.4 | 31.8 | 86.4 | 86.0 | 86.6 | 85.9 | 86.7 | 36.2 | 36.1 | 35.4 | 35.5 | 35.3 | 40.4 | 40.7 |
| C21 | 18.1 | 18.2 | 20.1 | 20.2 | 18.2 | 20.7 | 26.1 | 26.3 | 26.1 | 25.9 | 25.2 | 18.3 | 18.4 | 18.1 | 18.2 | 18.0 | 13.3 | 13.8 |
| C22 | 30.0 | 30.5 | 30.1 | 30.2 | 30.9 | 30.0 | 34.5 | 34.2 | 34.5 | 34.2 | 34.6 | 29.7 | 32.0 | 31.0 | 31.0 | 31.0 | 80.2 | 80.5 |
| C23 | 31.0 | 30.8 | 31.6 | 31.8 | 31.1 | 32.4 | 28.0 | 27.3 | 28.1 | 27.5 | 28.3 | 30.1 | 31.9 | 31.0 | 31.1 | 30.9 | 27.7 | 28.7 |
| C24 | 174.3 | 178.0 | 173.6 | 173.7 | 174.1 | 173.9 | 175.6 | 175.8 | 175.6 | 175.9 | 175.5 | 178.2 | 176.4 | 174.0 | 174.0 | 174.1 | 139.7 | 140.5 |
| C25 | -- | 27.8 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 66.6 | 65.1 | -- | 128.1 | 127.8 |
| C26 | -- | 15.5 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 13.2 | 13.1 | -- | 166.6 | 166.2 |
| C27 | -- | 21.8 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 24.8 | 21.6 | -- | 17.1 | 18.7 |
| C28 | 27.4 | -- | -- | -- | 28.4 | 26.5 | 27.6 | 27.6 | 27.9 | 28.1 | 28.1 | 25.4 | 25.6 | -- | -- | 23.7 | 27.9 | 28.2 |
| C29 | 20.7 | -- | -- | -- | 15.6 | 21.4 | 20.4 | 20.3 | 15.5 | 15.4 | 15.4 | 22.4 | 22.3 | -- | -- | 64.2 | 15.4 | 15.4 |
| C30 | 19.4 | -- | 27.6 | 27.9 | 24.6 | 23.5 | 21.1 | 21.3 | 21.6 | 24.7 | 24.5 | 25.3 | 17.9 | -- | -- | 24.9 | 24.3 | 24.9 |
| CO- | C31 | C31 | C1' | C1' | COCH3 | COCH3 | COCH3 | CO- | CO- | CO- | ||||||||
| OCH3 | 20.4 | 15.6 | 51.9 | 64.6 | 170.1 | 170.1 | 170.3 | OCH3 | OCH3 | OCH3 | ||||||||
| 51.6 | C32 | C32 | C2' | COCH3 | COCH3 | COCH3 | 51.4 | 51.4 | 51.4 | |||||||||
| 20.8 | 21.4 | 30.9 | 21.0 | 21.0 | 21.2 | |||||||||||||
| NO. | 269b) | 270b) | 271b) | 272b) | 273b) | 274b) | 275b) | 276b) | 277a) | 278b) | 279b) | 280b) | 281b) | 282b) | 286 | 287 | 288b) | 289b) |
| C1 | 143.7 | 143.6 | 144.2 | 147.9 | 35.2 | 35.0 | 36.6 | 36.6 | 35.5 | 35.2 | 36.0 | 35.7 | 35.7 | 35.4 | 36.2 | 36.1 | 36.7 | 29.7 |
| C2 | 118.0 | 118.1 | 118.5 | 116.7 | 34.3 | 28.3 | 34.8 | 34.8 | 29.3 | 34.3 | 33.6 | 27.7 | 35.4 | 27.5 | 33.7 | 33.6 | 34.9 | 22.8 |
| C3 | 167.1 | 167.0 | 166.8 | 163.9 | 213.2 | 77.0 | 217.0 | 217.0 | 78.1 | 217.2 | 216.2 | 78.0 | 215.7 | 77.9 | 216.5 | 216.0 | 216.9 | 77.5 |
| C4 | 80.5 | 80.5 | 80.6 | 77.5 | 47.0 | 38.6 | 47.5 | 47.5 | 40.1 | 44.8 | 45.9 | 41.5 | 46.0 | 41.8 | 46.0 | 45.9 | 47.5 | 36.4 |
| C5 | 49.0 | 49.0 | 49.2 | 92.6 | 49.8 | 49.6 | 50.8 | 50.8 | 50.5 | 49.4 | 40.8 | 39.3 | 40.7 | 39.4 | 39.4 | 40.7 | 50.7 | 47.7 |
| C6 | 38.7 | 38.4 | 37.3 | 44.1 | 27.9 | 27.9 | 23.6 | 23.6 | 27.2 | 27.2 | 23.2 | 21.8 | 22.9 | 21.8 | 23.2 | 22.7 | 23.7 | 20.4 |
| C7 | 27.6 | 27.2 | 27.2 | 26.8 | 65.8 | 66.6 | 119.4 | 119.4 | 71.7 | 70.1 | 60.5 | 58.7 | 59.1 | 59.0 | 62.9 | 62.6 | 119.4 | 25.7 |
| C8 | 147.6 | 147.0 | 149.3 | 149.5 | 157.8 | 156.8 | 142.8 | 142.7 | 158.3 | 158.3 | 66.0 | 71.4 | 62.1 | 62.9 | 64.3 | 57.6 | 142.7 | 137.6 |
| C9 | 130.5 | 129.9 | 131.8 | 127.6 | 140.9 | 143.0 | 144.4 | 144.4 | 140.7 | 138.7 | 163.4 | 165.6 | 167.6 | 168.9 | 164.8 | 164.8 | 144.3 | 128.3 |
| C10 | 139.1 | 139.3 | 140.1 | 132.9 | 37.8 | 39.2 | 37.2 | 37.1 | 39.3 | 37.5 | 40.2 | 37.9 | 40.8 | 38.0 | 40.3 | 40.7 | 37.2 | 39.4 |
| C11 | 26.9 | 26.8 | 67.7 | 28.0 | 199.4 | 200.5 | 117.3 | 117.2 | 200.0 | 199.7 | 130.8 | 128.7 | 129.8 | 128.5 | 130.1 | 129.7 | 117.2 | 22.5 |
| C12 | 31.4 | 31.1 | 42.5 | 31.1 | 78.1 | 79.0 | 37.7 | 37.7 | 53.3 | 52.1 | 201.2 | 200.9 | 199.8 | 200.2 | 202.8 | 200.5 | 37.7 | 31.0 |
| C13 | 43.7 | 44.3 | 43.2 | 44.3 | 49.9 | 50.5 | 43.7 | 43.7 | 40.1 | 46.9 | 50.3 | 54.9 | 54.5 | 54.3 | 53.3 | 55.0 | 43.8 | 44.3 |
| C14 | 56.4 | 54.9 | 55.6 | 55.1 | 63.3 | 63.0 | 50.3 | 50.2 | 53.7 | 52.9 | 59.3 | 62.0 | 62.8 | 61.5 | 60.2 | 58.8 | 50.3 | 50.4 |
| C15 | 76.2 | 78.0 | 77.6 | 78.4 | 215.5 | 215.0 | 27.8 | 27.8 | 71.9 | 70.8 | 71.7 | 209.6 | 202.8 | 203.1 | 76.6 | 209.5 | 27.8 | 31.1 |
| C16 | 40.6 | 38.6 | 38.5 | 38.4 | 60.4 | 60.6 | 31.5 | 31.4 | 33.3 | 32.5 | 33.6 | 38.5 | 125.2 | 125.2 | 39.5 | 38.3 | 31.5 | 27.6 |
| C17 | 45.6 | 45.3 | 45.1 | 45.7 | 54.2 | 54.0 | 50.8 | 50.7 | 49.6 | 48.9 | 45.8 | 43.0 | 181.6 | 181.9 | 48.7 | 42.9 | 50.9 | 45.7 |
| C18 | 17.3 | 16.7 | 18.9 | 16.8 | 12.8 | 13.3 | 15.7 | 15.7 | 18.1 | 17.6 | 18.1 | 18.1 | 30.3 | 29.8 | 19.6 | 18.1 | 15.8 | 15.7 |
| C19 | 143.0 | 142.8 | 141.0 | 139.4 | 18.3 | 19.2 | 22.0 | 22.0 | 20.7 | 19.6 | 21.6 | 18.3 | 24.9 | 25.9 | 21.3 | 21.6 | 22.1 | 104.0 |
| C20 | 40.0 | 39.9 | 39.9 | 39.8 | 33.0 | 33.3 | 36.4 | 36.5 | 33.6 | 32.7 | 157.3 | 154.9 | 73.0 | 72.9 | 158.6 | 158.6 | 36.5 | 40.4 |
| C21 | 13.4 | 13.3 | 13.2 | 13.3 | 19.2 | 19.6 | 18.5 | 18.5 | 20.3 | 19.8 | 20.6 | 20.4 | 29.5 | 29.4 | 20.9 | 20.4 | 18.7 | 13.3 |
| C22 | 80.0 | 79.8 | 79.6 | 79.7 | 52.5 | 52.3 | 33.1 | 33.1 | 50.6 | 49.9 | 126.0 | 126.7 | 52.9 | 52.9 | 126.3 | 126.8 | 33.5 | 80.1 |
| C23 | 27.8 | 27.8 | 27.7 | 27.7 | 87.0 | 87.5 | 25.8 | 25.7 | 209.3 | 208.5 | 198.8 | 198.7 | 206.2 | 206.3 | 199.2 | 198.5 | 29.8 | 27.9 |
| C24 | 139.6 | 139.4 | 139.4 | 139.3 | 40.6 | 40.4 | 78.8 | 76.8 | 47.8 | 46.9 | 47.6 | 47.7 | 47.8 | 47.8 | 47.6 | 47.6 | 77.2 | 139.0 |
| C25 | 128.3 | 128.4 | 128.4 | 128.4 | 34.3 | 34.8 | 73.6 | 73.5 | 36.3 | 34.8 | 34.8 | 34.9 | 34.5 | 33.1 | 33.7 | 34.9 | 73.9 | 128.0 |
| C26 | 166.4 | 166.3 | 166.2 | 166.2 | 178.6 | 179.0 | 66.5 | 68.7 | 178.6 | 176.4 | 179.6 | 179.7 | 179.9 | 179.9 | 180.1 | 180.2 | 69.6 | 166.5 |
| C27 | 17.1 | 17.1 | 17.1 | 17.1 | 15.6 | 15.6 | 18.1 | 18.3 | 18.3 | 17.3 | 17.0 | 17.0 | 16.9 | 16.9 | 17.0 | 17.0 | 20.3 | 17.2 |
| C28 | 29.0 | 28.8 | 28.1 | 24.9 | -- | -- | 25.3 | 25.3 | 29.3 | 27.8 | 14.6 | 17.1 | 24.8 | 17.1 | 21.7 | 24.8 | 25.4 | 23.8 |
| C29 | 26.3 | 26.4 | 26.7 | 24.8 | -- | -- | 22.5 | 22.4 | 17.4 | 20.8 | 24.6 | 25.8 | 21.6 | 25.7 | 24.8 | 21.7 | 22.5 | 25.7 |
| C30 | 26.6 | 26.4 | 25.4 | 24.4 | 25.8 | 28.5 | 25.4 | 25.4 | 20.2 | 19.8 | 28.7 | 28.7 | 28.7 | 28.7 | 28.6 | 28.6 | 25.5 | 23.2 |
| C1' | C1' | C1' | C31 | C31 | C1' | C1' | C1' | COCH3 | C1' | OCH3 | ||||||||
| 170.4 | 170.3 | 170.1 | 21.3 | 16.0 | 195.5 | 31.8 | 102.7 | 52.1 | 195.3 | 55.2 | ||||||||
| C2' | C2' | C2' | C32 | C32 | C2' | C2' | C2' | C(CH3)2 | C2' | |||||||||
| 21.4 | 21.4 | 21.3 | 25.2 | 24.6 | 127.8 | 140.6 | 24.1 | 102.3 | 126.6 | |||||||||
| NO. | 290b) | 291b) | 292b) | 293a) | 294b) | 295b) | 296b) | 297e) | 298b) | 299a) | 300a) | 301a) | 302b) | 303b) | 304b) | 305b) | 306b) | 307b) |
| C1 | 27.5 | 143.8 | 34.1 | 35.5 | 35.7 | 31.2 | 28.7 | 31.3 | 32.6 | 57.0 | 57.0 | 57.0 | 30.4 | 31.0 | 28.8 | 28.8 | 29.7 | 35.2 |
| C2 | 27.1 | 118.0 | 27.4 | 28.6 | 34.2 | 27.7 | 28.4 | 23.6 | 28.0 | 36.5 | 36.5 | 36.5 | 23.3 | 34.6 | 27.7 | 25.5 | 28.3 | 34.0 |
| C3 | 177.3 | 167.0 | 76.5 | 77.6 | 216.4 | 78.7 | 179.0 | 78.3 | 78.9 | 216.5 | 216.5 | 216.4 | 78.1 | 216.6 | 175.6 | 174.5 | 175.5 | 214.9 |
| C4 | 74.5 | 77.8 | 39.2 | 49.7 | 46.8 | 38.9 | 75.3 | 37.0 | 39.0 | 47.0 | 47.0 | 47.1 | 36.7 | 47.1 | 75.2 | 75.0 | 74.8 | 46.6 |
| C5 | 55.1 | 48.9 | 50.3 | 49.7 | 48.9 | 50.2 | 47.9 | 46.0 | 50.1 | 55.4 | 55.4 | 55.5 | 45.3 | 39.7 | 47.9 | 48.0 | 47.8 | 49.3 |
| C6 | 33.8 | 35.8 | 36.9 | 28.1 | 27.6 | 17.6 | 23.9 | 18.4 | 17.8 | 29.4 | 29.4 | 29.4 | 18.0 | 28.8 | 24.3 | 21.5 | 24.8 | 36.9 |
| C7 | 27.1 | 27.1 | 203.8 | 66.7 | 66.2 | 25.7 | 26.7 | 26.3 | 26.2 | 68.3 | 68.4 | 68.3 | 25.9 | 66.2 | 24.3 | 117.9 | 26.0 | 204.5 |
| C8 | 139.2 | 146.0 | 146.2 | 158.7 | 157.5 | 137.1 | 143.3 | 134.4 | 137.9 | 136.4 | 136.3 | 136.3 | 133.7 | 132.9 | 143.1 | 134.1 | 139.6 | 150.9 |
| C9 | 121.7 | 147.0 | 155.9 | 142.7 | 141.2 | 131.7 | 126.1 | 135.1 | 130.4 | 154.7 | 154.7 | 154.8 | 134.7 | 76.3 | 126.1 | 141.1 | 126.1 | 153.2 |
| C10 | 91.5 | 142.8 | 40.4 | 39.3 | 38.3 | 39.6 | 45.4 | 37.3 | 42.2 | 48.6 | 48.6 | 48.6 | 36.9 | 41.4 | 45.4 | 42.2 | 45.7 | 39.4 |
| C11 | 33.0 | 26.3 | 198.5 | 198.0 | 196.8 | 23.0 | 20.8 | 21.2 | 22.0 | 83.5 | 83.6 | 83.5 | 20.8 | 26.2 | 22.6 | 120.4 | 22.5 | 200.9 |
| C12 | 30.7 | 31.1 | 79.3 | 50.9 | 50.1 | 31.0 | 31.0 | 30.8 | 31.0 | 37.4 | 37.4 | 37.4 | 30.8 | 34.2 | 31.2 | 39.4 | 31.2 | 49.6 |
| C13 | 44.5 | 44.3 | 54.1 | 45.8 | 45.1 | 44.4 | 44.3 | 45.0 | 44.4 | 45.6 | 45.6 | 45.7 | 44.3 | 45.2 | 51.4 | 43.7 | 44.1 | 48.2 |
| C14 | 50.5 | 54.9 | 50.6 | 59.0 | 59.3 | 50.2 | 51.5 | 49.9 | 50.4 | 62.3 | 62.4 | 62.4 | 49.5 | 155.0 | 51.8 | 49.9 | 51.4 | 51.8 |
| C15 | 30.1 | 77.8 | 80.1 | 215.0 | 215.8 | 30.7 | 31.3 | 27.5 | 30.7 | 215.0 | 215.3 | 214.8 | 27.0 | 77.5 | 30.5 | 31.5 | 30.8 | 72.9 |
| C16 | 27.1 | 38.5 | 128.1 | 36.5 | 35.8 | 27.6 | 27.4 | 29.4 | 27.5 | 41.0 | 41.1 | 41.0 | 28.9 | 47.9 | 28.5 | 26.0 | 26.6 | 31.7 |
| C17 | 45.5 | 44.8 | 157.7 | 49.7 | 49.0 | 45.8 | 45.9 | 47.7 | 47.1 | 46.8 | 46.9 | 46.6 | 47.0 | 49.7 | 46.9 | 47.5 | 47.8 | 39.2 |
| C18 | 15.5 | 16.7 | 26.0 | 18.6 | 18.1 | 15.6 | 15.9 | 16.4 | 16.6 | 17.7 | 17.7 | 17.7 | 16.3 | 17.2 | 15.7 | 16.0 | 15.8 | 16.6 |
| C19 | 41.5 | 142.8 | 20.8 | 19.4 | 19.1 | 67.8 | 67.3 | 19.1 | 65.8 | 18.8 | 18.8 | 18.8 | 19.0 | 17.0 | 67.2 | 61.6 | 67.2 | 17.6 |
| C20 | 40.3 | 35.8 | 71.2 | 86.5 | 85.5 | 40.4 | 40.3 | 49.1 | 41.6 | 35.7 | 35.9 | 32.6 | 47.8 | 37.8 | 40.1 | 40.0 | 40.2 | 124.4 |
| C21 | 13.3 | 12.8 | 32.8 | 25.8 | 26.0 | 13.3 | 13.4 | 178.5 | 12.0 | 18.6 | 18.7 | 20.3 | 175.2 | 13.3 | 12.9 | 12.6 | 13.3 | 138.4 |
| C22 | 80.1 | 84.0 | 48.6 | 27.8 | 27.5 | 80.2 | 80.3 | 31.9 | 72.7 | 31.4 | 31.9 | 49.8 | 32.9 | 74.6 | 75.6 | 75.5 | 80.1 | 107.7 |
| C23 | 27.9 | 63.6 | 106.8 | 34.2 | 34.2 | 27.9 | 27.8 | 32.8 | 34.0 | 31.1 | 31.8 | 208.9 | 123.5 | 31.6 | 33.1 | 33.1 | 27.3 | 153.6 |
| C24 | 139.7 | 143.8 | 44.6 | 176.7 | 175.9 | 139.6 | 139.7 | 156.0 | 125.4 | 174.1 | 176.1 | 46.9 | 132.3 | 138.5 | 141.0 | 140.9 | 142.9 | 34.3 |
| C25 | 128.0 | 127.7 | 34.1 | -- | -- | 128.2 | 128.2 | 34.3 | 137.9 | -- | -- | 35.7 | 155.4 | 129.8 | 128.2 | 128.1 | 128.0 | 38.5 |
| C26 | 166.5 | 164.0 | 178.7 | -- | -- | 166.6 | 166.7 | 22.0 | 61.4 | -- | -- | 178.4 | 106.7 | 172.0 | 172.4 | 171.7 | 166.3 | 180.0 |
| C27 | 17.1 | 16.8 | 14.5 | -- | -- | 17.1 | 17.1 | 21.9 | 22.4 | -- | -- | 17.7 | 17.7 | 12.5 | 20.6 | 20.5 | 17.0 | 18.9 |
| C28 | 32.0 | 28.5 | 29.6 | 25.4 | 25.0 | 28.1 | 33.7 | 28.0 | 28.4 | 27.1 | 27.1 | 27.1 | 27.6 | 25.8 | 33.7 | 33.5 | 28.7 | 27.3 |
| C29 | 25.2 | 26.6 | 15.1 | 16.5 | 27.0 | 15.5 | 26.1 | 21.9 | 15.5 | 18.9 | 18.9 | 18.9 | 21.8 | 22.0 | 26.1 | 25.8 | 23.8 | 20.4 |
| C30 | 24.5 | 26.6 | 29.7 | 28.8 | 20.7 | 24.2 | 24.9 | 24.5 | 24.7 | 20.6 | 20.7 | 20.6 | 24.4 | 31.4 | 24.3 | 24.3 | 22.5 | 20.5 |
| CO | C1' | C1' | C1' | C1' | OCH3 | C1' | C1' | C1' | C1' | |||||||||
| 170.4 | 171.1 | 170.7 | 171.2 | 170.5 | 51.4 | 94.5 | 170.6 | 170.5 | 170.4 | |||||||||
| NO. | 308a) | 309b) | 310a) | 311b) | 312a) | 315a) | 316b) | NO. | 308a) | 309b) | 310a) | 311b) | 312a) | 315a) | 316b) | |||
| C1 | 32.7 | 31.8 | 38.0 | 36.6 | 37.1 | 35.5 | 32.4 | C18 | 17.7 | 17.7 | 18.0 | 17.8 | 17.7 | 17.7 | 19.6 | |||
| C2 | 30.8 | 30.4 | 29.6 | 29.9 | 30.1 | 28.8 | 32.2 | C19 | 22.4 | 22.4 | 25.5 | 24.9 | 23.0 | 20.0 | 16.3 | |||
| C3 | 176.5 | 173.9 | 176.4 | 174.7 | 175.6 | 77.6 | 214.1 | C20 | 36.2 | 36.2 | 36.2 | 36.0 | 139.9 | 40.1 | 205.2 | |||
| C4 | 146.5 | 146.4 | 87.3 | 86.1 | 145.0 | 39.4 | 47.0 | C21 | 18.5 | 18.5 | 18.1 | 18.1 | 19.0 | 17.5 | 31.2 | |||
| C5 | 45.0 | 45.0 | 48.0 | 48.4 | 44.8 | 49.9 | 43.5 | C22 | 31.9 | 27.7 | 31.9 | 31.3 | 126.3 | 66.9 | 26.9 | |||
| C6 | 35.5 | 35.4 | 32.1 | 32.5 | 27.0 | 29.0 | 36.7 | C23 | 31.9 | 32.4 | 31.6 | 31.0 | 75.2 | -- | 20.3 | |||
| C7 | 67.9 | 67.8 | 72.9 | 73.1 | 61.3 | 69.5 | 198.3 | C24 | 176.5 | 176.5 | 176.4 | 174.5 | 36.8 | -- | 17.5 | |||
| C8 | 165.3 | 165.7 | 161.4 | 161.2 | 66.5 | 160.5 | 66.8 | C25 | -- | -- | -- | -- | 34.3 | -- | -- | |||
| C9 | 137.4 | 137.1 | 135.4 | 135.1 | 163.0 | 141.9 | 68.1 | C26 | -- | -- | -- | -- | 179.0 | -- | -- | |||
| C10 | 41.3 | 41.2 | 41.6 | 41.1 | 43.8 | 39.1 | 37.4 | C27 | -- | -- | -- | -- | 15.5 | -- | -- | |||
| C11 | 200.4 | 200.4 | 199.8 | 200.2 | 130.0 | 200.3 | 200.6 | C28 | 115.6 | 115.7 | 71.3 | 71.3 | 115.2 | 28.8 | -- | |||
| C12 | 52.1 | 52.0 | 50.9 | 50.8 | 201.1 | 52.8 | 46.0 | C29 | 23.4 | 23.2 | 25.0 | 25.3 | 23.0 | 16.7 | ||||
| C13 | 47.3 | 41.2 | 45.3 | 45.2 | 59.1 | 47.7 | 45.5 | C30 | 27.6 | 27.7 | 24.5 | 23.8 | 15.1 | 20.2 | ||||
| C14 | 53.4 | 53.3 | 50.9 | 50.6 | 51.8 | 54.5 | 54.8 | CH2O | 3-OCH3 | OAc | ||||||||
| C15 | 32.4 | 31.9 | 30.1 | 29.3 | 71.8 | 72.6 | 205.9 | 60.4 | 51.9 | 20.7 | ||||||||
| C16 | 27.8 | 32.4 | 27.2 | 29.5 | 31.9 | 36.6 | 36.4 | CH3CH2O | 24-OCH3 | 169.9 | ||||||||
| C17 | 50.1 | 50.1 | 50.2 | 50.0 | 44.3 | 45.4 | 52.4 | 14.4 | 51.8 |
Notes: NO. 1 N-BU 2' 30.8, N-BU 3' 19.3, N-BU 4' 13.9; NO. 2 Bu 4' 13.9; NO. 3 Bu 4' 13.9; NO. 24 OCH2CH3 14.1; NO. 29 OCOCH3 20.9; NO. 41 15-COCH3 21.3; NO.65 15-OCOCH3 21.1; NO. 73 7-OCH3 55.6; NO. 74 7-OCH3 54.5; NO.77 AcCH3 21.4, AcCH3 21.0; NO. 78 3-OCOCH3 21.2, 22-OCOCH3 170.6, 22-OCOCH3 21.0; NO. 79 22-OCOCH3 21.0; NO. 80 15-OCOCH3 21.1, 22-OCOCH3, 170.6, 22-OCOCH3 21.0; NO. 81 22-OCOCH3 21.1; NO. 82 22-OCOCH3 170.6, 22-OCOCH3 21.0; NO. 83 AcCH3 21.1, AcCH3 21.3, AcCH3 21.3, CO 170.3, CO 170.3, CO 170.5; NO. 84 AcCH3 21.0, AcCH3 21.6, AcCH3 21.7, CO 170.6, CO 170.1, CO 170.2, OCH3 55.2; NO. 99 COOCH3 51.9; NO. 100 OCH3 51.9; NO. 101 OCH3 51.9, COCH3 20.9, COCH3 170.2; NO. 102 OCH3 51.8, COCH3 20.8, COCH3 170.1; NO. 103 OCH3 51.9; NO. 104 OCH3 51.9; NO. 105 OCH3 51.9; NO. 118 C3' 73.7, C4' 69.7, C5' 63.7, COCH3 170.9, COCH3 21.4; NO. 120 C2' 74.0, C3' 79.2, C4' 71.4, C5' 79.0, C6' 62.5; NO. 127 OCH3 52.0; NO. 138 COO-CH2CH3 60.9, COOCH2CH3 14.4, CO of AcO-C(3) 171.1, CH3 of AcO-C(3) 21.5; NO. 139 COOCH2CH3 60.8, COOCH2CH3 14.4; NO. 145 C24' 6.8; NO. 149 CH3CO 170.4; NO. 150 CH3CO 21.4, CH3CO 21.3; NO. 151 C23 31.8(24S), C24 75.6(24S), C25 149.8(24S), C26 111.5(24S), C27 18.1(24S); NO. 153 C24' 21.5; NO. 167 AcCO 170.8, AcCO 171.0.; NO. 172 AcCH3 21.2, AcCH3 21.4, AcCH3 21.5, CO 170.7, CO 170.9, CO 170.2; NO. 173 AcCH3 21.0, CO 170.6; NO. 174 AcCH3 21.0, AcCH3 21.3, CO 170.6, CO 170.6; NO. 176 AcCO 170.0, AcCH3 21.4, AcCH3 21.3, AcCH3 21.0; NO. 178 AcCH3 21.2, AcCH3 21.2; NO. 184 15-OCOCH3 21.4, 22-OCOCH3 170.6, 22-OCOCH3 21.0; NO. 185 AcCH3 21.5, CO 170.6, CO 171.2; NO. 186 AcCH3 21.7, CO 170.7, CO 170.9; NO. 193 C4' 20.9; NO. 222 Bu3' 19.3, Bu4' 13.9; NO. 223 Bu3' 19.3, Bu4' 13.9; NO. 226 COOCH3 20.8, OCH3 51.4, COCH3 170.0; NO. 230 COCH3 20.8, COCH3 170.1; NO. 231 OCH3 51.6, COCH3 20.9, COCH3 170.1; NO. 232 OCH3 51.7; NO. 233 C33 60.5, C34 14.2; NO. 243 OCH3 51.6, COCH3 20.8, COCH3 170.0; NO. 244 OCH3 51.6, COCH3 20.9, COCH3 170.1; NO. 249 C3' 19.3, C4' 13.9; NO. 275 C3' 145.2, C4' 34.5 C5' 25.6, C6' 122.1, C7' 136.9, C8' 39.0, C9' 25.6, C10' 125.5, C11' 134.5, C12' 38.7, C13' 13.8, C14' 16.2, C15', 169.0, C1'' 157.1, C2'' 112.7, C3'' 117.3, C4'' 148.2, C5'' 126.0, C6'' 119.8; NO. 276 C3' 133.3, C4' 35.9 C5' 28.5, C6' 124.6, C7' 136.7, C8' 38.9, C9' 27.3, C10' 126.8, C11' 135.8, C12' 69.0, C13' 13.7, C14' 16.2, C15' 172.3, C1'' 128.0, C2'' 149.3, C3'' 114.8, C4'' 151.2, C5'' 116.9, C6'' 117.8; NO. 277 C3' 27.0; NO. 278 CH3 26.4, CH3 23.5; NO. 288 C3' 146.3, C4' 34.6C5' 25.5, C6' 122.1, C7' 136.6, C8' 39.1, C9' 25.5, C10' 125.3, C11' 134.4, C12' 68.6, C13' 13.9, C14' 16.3, C15' 168.3, C1'' 157.0, C2'' 114.9, C3'' 117.2, C4'' 148.1, C5'' 126.1, C6'' 119.8; NO. 291 CH3 21.4; NO. 295 C2' 21.1; NO. 296 C2' 21.1; NO. 297 C2' 46.4, C3' 69.9 C4' 46.1, C5' 171. 9,3'-CH3 28.4, OCH3 51.2, C31 107.1; NO. 298 C2' 21.1; NO. 302 C2' 72.3, C3' 75.9, C4' 69.5, C5' 65.8, COCH3 170.9, COCH3 21.4, C241 25.7; NO. 303 22-OCOCH3 171.3, 22-OCOCH3, 21.2; NO. 304 C2' 20.7, C1'' 170.9, C2'' 21.1, OCH3 51.8; NO. 305 C2' 21.1, C1'' 170.8, C2'' 21.2, OCH3 51.8; NO. 306 C2' 20.7, OCH3 51.4. (a) Measured in C5D5N; (b) Measured in CDCl3; (c) Measured in CD3OD(50%) and CDCl3(50%); (d) Measured in DMSO-d6; (e) Measured in pyridine-d5; (f) Measured in CD3OD; (g) Measured in (CD3)2CO; (h) Measured in C6D6.
As summarized above, a large number of triterpenes together with their potential pharmacological activities are described from Ganoderma. Being inclined to complement the prior reviews, we summarized the triterpenes from Ganoderma. They contain 30 or 27 carbon atoms, including some with 24 carbon atoms. The great majority of the triterpenes possess double bonds on the ring, at C-8 (9), with hydroxy and carbonyl substituted at C-3, C-7, C-11, and C-15 generally. For this type, the carbon atoms mentioned above are usually a characteristic for its structural determination. The 13C-NMR data of hydroxy substituted C-3 appear from 77–80 ppm, while the data of carbonyl substituted increase to 208–218 ppm. As to the double bonds, the resonance of C-8 arises at 131–165 ppm and the C-9 signal arises at 134–165 ppm, fluctuated for the neighbouring substituent groups.
In the other type, with double bonds located at C-7(8) and C-9(11), the resonance of C-7 appears from 119 ppm to 121 ppm, while the C-8 signal increases to 140–143 ppm. The C-9 signal appears at 144–147 ppm, while the C-11 signal moves to 115–118 ppm. C-23 tends to be oxidized to a carbonyl with 13C-NMR signals appearing at 206 ppm to 217 ppm, or to hydroxy with signals in the 65–67 ppm range. When double bonds appear between C-20 and C-22, the C-23 signal will move to 197–200 ppm. Moreover, C-24 and C-25 are sometimes linked by double bonds in some Ganoderma triterpenes. In this case, the 13C-NMR peaks of C-24 appear at 144–156 ppm and those of C-25 appear at 126–140 ppm. According to the compiled 13C-NMR data, this review should provide a useful and fast way for the identification of GTs.
4. The Bioactivities of Ganoderma Triterpenes
4.1. Anti-Tumor Activity
Cancer has been acknowledged as a huge threat to human health and most governments are committed to diminish this threat. The urgent task of finding anti-tumor drugs with high efficiency and low toxicity have drawn countless researchers’ efforts directed to the discovery of lead compounds or bioactive ingredients from nature resources such as Ganoderma. The GTs were extensively evaluated for cytotoxic activities against a series of tumor cell lines. Compounds 45, 46, 164 and 204 showed cytotoxic effects against the tested tumor cell lines. Compound 46 exhibited the most potent cytotoxicity against LLC, T-47D, Sarcoma 180 and Meth-A tumor cells [25]. Compounds 62, 190 and 212 showed strong cytotoxic activities against human Hela cervical cancer cells [26]. According to Cheng’s report, the ganoderic alcohols showed stronger activities than ganoderic acids which implies that a hydroxy group substituted at 26 may be a very important structural feature for cytotoxic activity, however, the more hydroxyl groups there are, the lower the inhibitory activity will be [26]. Compounds 42 and 85 showed cytotoxicity against p388, Hela, BEL-7402, and SGC-7901 human cancer cell lines, with IC50 values in the 8–25 μM range [32]. Compounds 47–52 were studied in vitro against Meth-A and LLC tumor cell lines [37]. Compound 187 displayed selective inhibitory activity against HL-60 cells, and compound 131 exhibited selective cytotoxic activity against MCF-7 cells. Compounds 7, 67 and 188 showed the ability to induce hPXR-mediated CYP3A4 expression [47]. Compounds 9, 23, 57 and 68 showed significant cytotoxic activity, with IC50 values of 18.7, 21.4, 16.2 and 20.1 μg/mL, respectively [48]. Compounds 77, 163, 170 and 173 were tested in vitro for their cytotoxic activities against 95D and Hela tumor cell lines with IC50 values ranging from 14.7 to 38.5 μM [49]. Compound 121 showed significant activity against T-24 cells, while compounds 119, 123, showed significant activity against T-24, HT-3, and CaSKi cells, respectively [60]. Compound 297 showed significant cytotoxic activity with an IC50 value of 2.5 μg/mL in the Hep-2 cell line [62]. Treatment of human hepatoma HuH-7 cells with compound 205 caused immediate inhibition of DNA synthesis as well as activation of ERK and JNK mitogen-activated protein kinases, and cell apoptosis. Molecular events of apoptosis including degradation of chromosomal DNA, decrease in the level of Bcl-xL, the disruption of mitochondrial membrane, cytosolic release of cytocherome c and activation of caspase-3 were elucidated. The ability of compound 205 to inhibit topoisomerases and to sensitize cancer cells towards apoptosis meets the criteria of a potential anticancer drug [88]. Compounds 30, 229 and 235 showed significant cytotoxic activities against Hep G2, Hep G2,2,15, and P-388 cell lines [91]. Compound 233 showed cytotoxicity against HL-60 and CA46 cancer cell lines [93]. Biological activity as an anti-tumor promoter was observed for compounds 279–282 [101]. Compound 285 showed moderate cytotoxicity against liver cancer and lung cancer cell lines [27]. Compounds 140, 279, 281, 287, 292 and 312 inhibited the viability and growth of the HL-60 cell lines [103].
4.2. Anti-HIV and Anti-HIV-1 Protease Activity
It was reported that compounds 270, 272, 291 and 304–306 were inhibitory against HIV-1 protease, with IC50 values for the most potent compounds ranging from 5 μg∙mL−1 to 13 μg∙mL−1 [102]. Moreover, compounds 190 and 210 were found to be active as anti-HIV-1 agents with an inhibitory concentration of 7.8 μg∙mL−1 for both, and compounds 4, 11, 23, 28, 171 and 203 were moderately active inhibitors against HIV-1 protease with a 50% inhibitory concentration of 0.17–0.23 mM [18]. While compounds 5, 53, 201 and 204 showed significant anti-HIV-1 protease activity with IC50 values of 20–90 μM [38]. In addition, compounds 39, 224 and 255 inhibited human immunodeficiency virus-1 protease with IC50 values of 20–24 μM.
4.3. Neurotrophic Activity
A series of reports has shown that Ganoderma triterpenes exhibit neurotrophic activity. Bioassay results revealed that compounds 12 and 261 have nerve growth factor-like neuronal survival-promoting effects, whereas the two compounds mentioned above and compounds 10, 159 and 183 showed brain-derived neurotrophic factor-like neuronal survival-promoting activities [73]. Compounds 1 and 278, exhibiting specific anti-acetylc-holinesterase activity, are being examined as possible drug candidates for the treatment of Alzheimer’s and related neurodegenerative diseases. Compounds 62, 204, 210 and some other Ganoderma triterpenes exhibited moderate acetylcholinesterase-inhibitory activity, with IC50 values ranging from 9.40 to 31.03 μM. These results indicated that these lanostane triterpenes are preferential inhibitors of acetylcholinesterase and may be suitable as drug candidates [16].
4.4. Hepatoprotection
It is also reported that compound 11 showed significant hepatoprotective activity. However, increased doses of compound 11 (up to 10 times) did not further reduce GOT/GPT levels in the serum of the mice [107]. Compound 144 has an activity of lowering the levels GPT in mice with liver injury by CCl4 and GaNI and exhibits hepatoprotective effects [67].
4.5. Antiobesity Activity
In 2010, the inhibitory effect of triterpenes isolated from G. lucidum on adipocyte differentiation in 3T3-L1 cells was reported for the first time [17]. According to a report on the subsequent research, compound 249 reduced the triglyceride accumulation significantly by 72% at 80 μM and it effectively suppressed the glycerol-3-phosphate dehydrogenase activity in the cells. It suppressed the gene expressed of PPARγ, C/EBPα, and SREBP-1c in a dose-dependent manner during differentiation. These findings demonstrate that compound 249 contributes to the inhibitory effect on adipocyte differentiation in 3T3-L1 cells [96].
4.6. Hypoglycemic Activity
The inhibitory effect on aldose reductase was examined for compound 27 and its methyl ester. The results indicated that the IC50 of 27 is 22.8 μM, whereas that of its methyl ester is more than 200 μM, which suggested that a carboxyl group of side chain of compound 27 is essential for potent inhibitory activity because of much lower level of inhibitory activity of its methyl ester. However, the exact reason for the difference in inhibition between compound 27 and its methyl ester remains unclear [29]. Compound 169 was also found to have high α-glucosidase inhibition, with IC50 of 119.8 μM [108].
4.7. Other Bioactivities
Ganoderma has been investigated for other bioactivities. Compounds 45 and 58 were found to exhibit potent inhibitory activity against herpes simplex virus [42]. Compounds 13 and 15 were shown to inhibit histamine release from rat mast cells [21]. In the study on compounds 3 and 156, it was found they both exhibited inhibitory activities against the HMG-CoA reductase and acyl CoA acyltransferase [35]. Another study demonstrated that compounds 44 and 49 exhibited potent enhancement of ConA-induced mice splenocytes proliferation in vitro [36]. It was found that compounds 161, 189 and 316 possess the bioactivity to induce apoptosis in human promyelocytic leukemia HL-60 cells [75]. An investigation on the ability of some Ganoderma triterpenes to inhibit 5α-reductase in rat liver microsomes revealed that compounds 64, 161 and 206 showed the inhibitory activity. Further study suggested that a carboxyl group of the 17β-side chain of compound 206 was essential to elicit the inhibitory activity [89]. The in vitro tests showed that compounds 308 and 310 exhibited modest inhibitory activity against rabbit platelet aggregation induced by platelet activating factor (PAF), and compound 310 also displayed weak inhibition against platelet aggregation induced by adenosine diphosphate (ADP) [105]. The C-3 epimer of compound 172 also exhibited significant antimycobacterial activity against mycobacterium tuberculosis H37Ra [46].
5. Conclusions
Ganoderma triterpenes (GTs) are a class of compounds with various chemical structures and a diverse range of biological activities. Biomedical analysis has shown that triterpenes possess important pharmacological activities and are thought to be potential candidates for drug discovery, but their low abundance, complex procedures of extraction and purification, the difficult preparation of high purity triterpenoids from G .lucidum is currently limited at the laboratory scale. Thus, how to enhance the content of triterpenoids and improve the technology of the extraction and purification of triterpenoids from Ganoderma lucidum is a problem that needs to be solved. We can expect to enhance GT production through the regulation of GA biosynthesis, thus promoting the industrial development of G. lucidum and provide an important resource for the development and application of new antineoplastic, anti-HIV, and other drugs.
Based on the above analysis of structural complexity and functional group variety, it is especially important to prove the structure-function relationships to make up for the inadequacy of this aspect. Although extensive research has been done on this herb, there is still a lot of scope for further research, especially on the mechanisms of biological activity of GTs with emphasis on agents with anti-tumor, anti-HIV, neurotrophic properties. G. lucidum and G. sinense that are recorded in the pharmacopoeia of China in 2010 have been widely applied in China [8]. Their long-standing medicinal history indicates their irreplaceable functions. In further study, researchers may need to pay more attention to the two species, and focus on the active substances such as the triterpenes summarized above. To achieve better quality control, the studies on other species are also important, so that the differences between species can be illustrated clearly. Additionally, more important bioactive constituents should be integrated into the quality control system of Ganoderma. Further experiments including in vitro, in vivo and clinical studies should be encouraged to identify any potential side effects.
Acknowledgments
This work was supported by the National Science-technology Support Plan Project (NO. 2012BAI29B00).
Author Contributions
Qing Xia, Huazheng Zhang, Gaimei She, and Lanzhen Zhang have all been involved in drafting this review. Qing Xia and Huazheng Zhang contributed equally to this work. Xuefei Sun, Haijuan Zhao, Lingfang Wu and Xin Mao discussed the results and commented on the manuscript. Dan Zhu, Guanghui Yang, Yanyan Shao and Xiaoxue Zhang corrected the 13C-NMR data. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Xie J.T., Wang C.Z., Wicks S., Yin J.J., Kong J., Li J., Li Y.C., Yuan C.S. Ganoderma lucidum extract inhibits proliferation of SW 480 human colorectal cancer cells. Exp. Oncol. 2006;28:25–29. [PubMed] [Google Scholar]
- 2.Liu G.Q., Zhang K.C. Mechanisms of the anticancer action of Ganoderma lucidum (Leyss. ex Fr.) Karst: A new understanding. J. Integr. Plant Biol. 2005;47:129–135. doi: 10.1111/j.1744-7909.2005.00037.x. [DOI] [Google Scholar]
- 3.Yuen J.W.M., Gohel M.D.I. Anticancer effects of Ganoderma lucidum: A review of scientific evidence. Nutr. Cancer. 2005;53:11–17. doi: 10.1207/s15327914nc5301_2. [DOI] [PubMed] [Google Scholar]
- 4.Petrova R.D., Wasser S.P., Mahajna J.A., Denchev C.M., Nevo E. Potential role of medicinal mushrooms in breast cancer treatment: Current knowledge and future perspectives. Int. J. Med. Mushrooms. 2005;7:141–155. doi: 10.1615/IntJMedMushr.v7.i12.140. [DOI] [Google Scholar]
- 5.Lin Z.B., Zhang H.N. Antitumor and immunoregulatory activities of Ganoderma lucidum and its possible mechanisms. Acta Pharmacol. Sin. 2004;25:1387–1395. [PubMed] [Google Scholar]
- 6.Gao Y.H., Zhou S.F. Chemopreventive and tumoricidal properties of lingzhi mushroom Ganoderma lucidum (W.Curt.:Fr.) Lloyd (Aphyllophoromy cetideae). Part II. mechanism considerations (review) Int. J. Med. Mushrooms. 2004;6:219–230. doi: 10.1615/IntJMedMushr.v6.i3.20. [DOI] [Google Scholar]
- 7.Lee S.Y., Rhee H.M. Cardiovascular effects of mycelium extract of Ganoderma lucidum: Inhibition of sympathetic outflow as a mechanism of its hypotensive action. Chem. Pharm. Bull. 1990;38:1359–1364. doi: 10.1248/cpb.38.1359. [DOI] [PubMed] [Google Scholar]
- 8.National Pharmacopoeia Committee . Chinese Pharmacopoeia. 2010 ed. Chemical Industry Press; Beijing, China: 2010. pp. 174–175. Part 1. [Google Scholar]
- 9.Kubota T., Asaka Y., Miura I., Mori H. Structure of Ganoderic acid A and B, two new lanostane type bitter triterpenes from Ganoderma lucidum (FR.) Karst. Helv. Chim. Acta. 1982;65:611–619. doi: 10.1002/hlca.19820650221. [DOI] [Google Scholar]
- 10.Luo J., Lin Z.B. Advances of pharmacological effects of triterpenes from Ganoderma lucidum. Acta Pharmacol. Sin. 2002;37:574–578. [PubMed] [Google Scholar]
- 11.Xie J.T., Wang C.Z., Wicks S., Yin J.J., Kong J., Li J., Li Y.C., Yuan C.S. Anti-inflammatory and anti-tumor-promoting effects of triterpene acids and sterols from the fungus Ganoderma lucidum. Chem. Biodivers. 2007;4:224–231. doi: 10.1002/cbdv.200790027. [DOI] [PubMed] [Google Scholar]
- 12.Boh B., Berovic M., Zhang J., Lin Z.B. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007;13:265–301. doi: 10.1016/S1387-2656(07)13010-6. [DOI] [PubMed] [Google Scholar]
- 13.Xu Z., Chen X., Zhong Z., Chen L., Wang Y. Ganoderma lucidum polysaccharides: Immunomodulation and potential anti-tumor activities. Am. J. Chin. Med. 2011;39:15–27. doi: 10.1142/S0192415X11008610. [DOI] [PubMed] [Google Scholar]
- 14.Liby K.T., Yore M.M., Sporn M.B. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Rev. Cancer. 2007;7:357–369. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- 15.Hill R.A., Connolly J.D. Triterpenoids. Nat. Prod. Rep. 2012;29:780–818. doi: 10.1039/c2np20027a. [DOI] [PubMed] [Google Scholar]
- 16.Lee I., Ahn B., Choi J., Hattori M., Min B., Bae K. Selective cholinesterase inhibition by lanostane triterpenes from fruiting bodies of Ganoderma lucidum. Bioorg. Med. Chem. Lett. 2011;21:6603–6607. doi: 10.1016/j.bmcl.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 17.Lee I., Seo J., Kim J., Kim H., Youn U., Lee J., Jung H., Na M., Hattori M., Min B., et al. Lanostane triterpenes from the fruiting bodies of Ganoderma lucidum and their inhibitory effects on adipocyte differentiation in 3T3-L1 cells. J. Nat. Prod. 2010;73:172–176. doi: 10.1021/np900578h. [DOI] [PubMed] [Google Scholar]
- 18.El-Mekkawy S., Meselhy M.R., Nakamura N., Tezuka Y., Hattori M., Kakiuchi N., Shimotohno K., Kawahata T., Otake T. Anti-HIV-1 and anti-HIV-1-protease substances from Ganoderma lucidum. Phytochemistry. 1998;49:1651–1657. doi: 10.1016/S0031-9422(98)00254-4. [DOI] [PubMed] [Google Scholar]
- 19.Kikuchi T., Kanomi S., Murai Y., Kadota S., Tsubono K., Ogita Z. Constituents of the fungus Ganoderma lucidum (FR.) Karst. III. Structure of Ganolucidic acids A and B, new lanostane-type triterpenoids. Chem. Pharm. Bull. 1986;34:4030–4036. doi: 10.1248/cpb.34.4030. [DOI] [Google Scholar]
- 20.Kikuchi T., Matsuda S., Murai Y., Ogita Z. Ganoderic acid G and I and Ganolucidic acid A and B, new triterpenoids from Ganoderma lucidum. Chem. Pharm. Bull. 1985;33:2628–2631. doi: 10.1248/cpb.33.2628. [DOI] [Google Scholar]
- 21.Kohda H., Tokumoto W., Sakamoto K., Fujii M., Hirai Y., Yamasaki K., Komoda Y., Nakamura H., Ishihara S. The biologically active constituents of Ganoderma lucidum (FR.) Karst. histamine release-inhibitory triterpenes. Chem. Pharm. Bull. 1985;33:1367–1374. doi: 10.1248/cpb.33.1367. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi T., Matsuda S., Kadota S., Murai Y., Tsubono K., Ogita Z. Constituents of the fungus Ganoderma lucidum (FR.) Karst. I. structure of Ganoderic acids of C2, E, I, and K, Lucidenic acid F and related compounds. Chem. Pharm. Bull. 1986;34:3695–3712. doi: 10.1248/cpb.34.3695. [DOI] [Google Scholar]
- 23.Morigiwa A., Kitabatake K., Fujimoto Y., Ikekawa N. Angiotensin converting enzyme-inhibitory triterpenes from Ganoderma lucidum. Chem. Pharm. Bull. 1986;34:3025–3028. doi: 10.1248/cpb.34.3025. [DOI] [PubMed] [Google Scholar]
- 24.Luo J., Lin Z.B. Srncture identification of triterpenes from fruiting bodies of Ganoderma lucidum by NMR spectra and X-ray diffraction analysis. Chin. Tradit. Herb. Drugs. 2002;33:197–200. [Google Scholar]
- 25.Gao J.J., Min B.S., Ahn E.M., Nakamura N., Lee H.K., Hattori M. New triterpene aldehydes, lucialdehydes A-C, from Ganoderma lucidum and their cytotoxicity against murine and human tumor cells. Chem. Pharm. Bull. 2002;50:837–840. doi: 10.1248/cpb.50.837. [DOI] [PubMed] [Google Scholar]
- 26.Cheng C.R., Yue Q.X., Wu Z.Y., Song X.Y., Tao S.J., Wu X.H., Xu P.P., Liu X., Guan S.H., Guo D.A. Cytotoxic triterpenoids from Ganoderma lucidum. Phytochemistry. 2010;71:1579–1585. doi: 10.1016/j.phytochem.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Kinge T.R., Mih A.M. Secondary metabolites of oil palm isolates of Ganoderma zonatum Murill. from Cameroon and their cytotoxicity against five human tumour cell lines. Afr. J Biotechnol. 2011;10:8440–8447. [Google Scholar]
- 28.Guan S.H., Yang M., Liu X., Xia J.M., Wang X.M., Jin H., Guo D.A. Two new lanostanoid triterpenes from fruit body of Ganoderma lucidum—The major componet of Sunrecome. Nat. Prod. Commun. 2006;1:177–181. [Google Scholar]
- 29.Fatmawati S., Shimizu K., Kondo R. Ganoderic acid Df, a new triterpenoid with aldose reductase inhibirory activity from the fruiting body of Ganoderma lucidum. Fitoterapia. 2010;86:1033–1036. doi: 10.1016/j.fitote.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 30.Kikuchi T., Kanomi S., Murai Y., Kadota S., Tsubono K., Ogita Z.I. Constituents of the fungus Ganoderma lucidum (FR.) Karst. II.: Structure of Ganoderic acids F, G, and H, Lucidenic acids D2 and E2, and related compounds. Chem. Pharm. Bull. 1986;34:4018–4029. doi: 10.1248/cpb.34.4018. [DOI] [Google Scholar]
- 31.Komoda Y., Nakamura H., Ishihara S., Uchida M., Kohda H., Yamasaki K. Structure of new terpenoid constituents of Ganoderma lucidum (FR.) Karst (polyporaceae) Chem. Pharm. Bull. 1985;33:4829–4835. doi: 10.1248/cpb.33.4829. [DOI] [PubMed] [Google Scholar]
- 32.Guan S.H., Xia J.M., Yang M., Wang X.M., Liu X., Guo D.A. Cytotoxic lanostanoid triterpenes from Ganoderma lucidum. J. Asian Nat. Prod. Res. 2008;10:695–700. doi: 10.1080/10286020802016297. [DOI] [PubMed] [Google Scholar]
- 33.Sato N., Zhang Q., Ma C.M., Hattori M. Anti-human immunodeficiency virus-1 protease activity of new lanos-tane-type triterpenoids from Ganoderma sinense. Chem. Pharm. Bull. 2009;57:1076–1080. doi: 10.1248/cpb.57.1076. [DOI] [PubMed] [Google Scholar]
- 34.Wang F., Liu J.K. Highly oxygenated lanostane triterpenoids from the fungus Ganoderma applanatum. Chem. Pharm. Bull. 2008;56:1035–1037. doi: 10.1248/cpb.56.1035. [DOI] [PubMed] [Google Scholar]
- 35.Li C.J., Li Y.M., Sun H.H. New ganoderic acids, bioactive triterpenoid metabolites from the mushroom Ganoder-ma lucidum. Nat. Prod. Res. 2006;20:985–991. doi: 10.1080/14786410600921466. [DOI] [PubMed] [Google Scholar]
- 36.Luo J., Zhao Y.Y., Lin Z.B. A new lanostane-type triterpene from the fruiting bodies of Ganoderma lucidum. J. Asian Nat. Prod. Res. 2002;4:129–134. doi: 10.1080/10286020290027416. [DOI] [PubMed] [Google Scholar]
- 37.Min B.S., Gao J.J., Nakamura N., Hattori M. Triterpenes from the spores of Ganoderma lucidum and their cytotoxicity against Meth-A and LLC tumor cells. Chem. Pharm. Bull. 2000;48:1026–1033. doi: 10.1248/cpb.48.1026. [DOI] [PubMed] [Google Scholar]
- 38.Min B.S., Nakamura N., Miyashiro H., Bae KW., Hattori M. Triterpenes from the spores of Ganoderma lucidum and their inhibitory activity against HIV-1 protease. Chem. Pharm. Bull. 1998;46:1607–1612. doi: 10.1248/cpb.46.1607. [DOI] [PubMed] [Google Scholar]
- 39.Nishitoba T., Oda K., Sato H., Sakamura S. Novel triterpeniods from the fungus Ganoderma lucidum. Agric. Biol. Chem. 1988;52:367–372. doi: 10.1271/bbb1961.52.367. [DOI] [Google Scholar]
- 40.Nishitoba T., Sato H., Oda K., Sakamura S. Novel triterpeniods and a steroid from the fungus Ganoderma luci-dum. Agric. Biol. Chem. 1988;52:211–216. doi: 10.1271/bbb1961.52.211. [DOI] [Google Scholar]
- 41.Nishitoba T., Sato H., Shirasu S., Sakamura S. Novel triterpeniods from the mycelial mat at the previous stage of fruiting of Ganoderma lucidum. Agric. Biol. Chem. 1987;51:619–622. doi: 10.1271/bbb1961.51.619. [DOI] [Google Scholar]
- 42.Niedermeyer T.H.J., Lindequist U., Renate M., Gordes D., Schmidt E., Thurow K., Lalk M. Antiviral terpenoid constituents of Ganoderma pfeifferi. J. Nat. Prod. 2005;68:1728–1731. doi: 10.1021/np0501886. [DOI] [PubMed] [Google Scholar]
- 43.Nishitoba T., Sato H., Sakamura S. Novel mycelial components, Ganoderic acid Mg, Mh, Mi, Mj, and Mk, from the fungus Ganoderma lucidum. Agric. Biol. Chem. 1987;51:1149–1153. doi: 10.1271/bbb1961.51.1149. [DOI] [Google Scholar]
- 44.González A.G., León F., Rivera A., Muñoz C.M., Bermejo J. Lanostanoid triterpenes from Ganoderma lucidum. J. Nat. Prod. 1999;62:1770–1701. [Google Scholar]
- 45.Cai H., Wang F.S., Yang J.S., Zhang Y.M., Hu K., Zhao Y.J., Bai Y., Zhang Z.X. Studies on the triterpenoid constituents from the fruiting body Ganoderma lucidum (FR) Karst. Chin. J. Vet. Sci. 1997;17:511–513. [Google Scholar]
- 46.Isaka M., Chinthanom P., Kongthong S., Srichomthong K., Choeklin R. Lanostane triterpenes from cultures of the basidiomycete Ganoderma orbiforme BCC 22324. Phytochemistry. 2013;87:133–139. doi: 10.1016/j.phytochem.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 47.Liu J.Q., Wang C.F., Li Y., Luo H.R., Qiu M.H. Isolation and bioactivity evaluation of terpenoids from the medicinal fungus Ganoderma sinense. Planta Med. 2012;78:368–376. doi: 10.1055/s-0031-1280441. [DOI] [PubMed] [Google Scholar]
- 48.Ma B.J., Zhou Y., Ruan Y., Ma J.C., Ren W., Wen C.N. Lanostane-type triterpenes from the sporoderm-broken spor-es of Ganoderma lucidum. J. Antibiot. 2012;65:165–167. doi: 10.1038/ja.2011.135. [DOI] [PubMed] [Google Scholar]
- 49.Li Y.B., Liu R.M., Zhong J.J. A new ganoderic acid from Ganoderma lucidum mycelia and its stability. Fitoterapia. 2013;84:115–122. doi: 10.1016/j.fitote.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Hirotani M., Asaka I., Ino C., Furuya T., Shiro M. Ganoderic acid derivatives and ergosta-4,7,22-triene-3,6-dione from Ganoderma lucidum. Phytochemistry. 1987;26:2797–2803. doi: 10.1016/S0031-9422(00)83593-1. [DOI] [Google Scholar]
- 51.Shim S.H., Ryu J., Kim J.S., Kang S.S., Xu Y., Jung S.H., Lee Y.S., Lee S., Shin K.H. New lanostane-type triterpenoids from Ganoderma applanatum. J. Nat. Prod. 2004;67:1110–1113. doi: 10.1021/np030383p. [DOI] [PubMed] [Google Scholar]
- 52.Nishitoba T., Goto S., Sato H., Sakamura S. Bitter triterpenoids from the fungus Ganoderma applanatum. Phytochemistry. 1989;28:193–197. doi: 10.1016/0031-9422(89)85036-8. [DOI] [Google Scholar]
- 53.Hao R.X., Zhang J.S., Tang Q.J., Hu L.H., Zheng Z.P., Pan Y.J. Isolation, purification and identification of two new triterpenoid constituents from the fruiting bodies of Ganoderma lucidum. Mycosystema. 2006;25:599–602. [Google Scholar]
- 54.Kikuchi T., Matsuda S., Kadota S., Murai Y., Ogita Z. Ganoderic acid D, E, F, and H and Lucidenic acid D, E, and F, new triterpenoids from Ganoderma lucidum. Chem. Pharm. Bull. 1985;33:2624–2627. doi: 10.1248/cpb.33.2624. [DOI] [Google Scholar]
- 55.Hirotani M., Furuya T. Ganoderic acid derivatives, highly oxygenated lanostane-type triterpenoids, from Ganoderma lucidum. Phytochemistry. 1986;25:1189–1193. doi: 10.1016/S0031-9422(00)81578-2. [DOI] [Google Scholar]
- 56.Wang C.F., Liu J.Q., Yan Y.X., Chen J.C., Lu Y., Guo Y.H., Qiu M.H. Three new triterpenoids containing four-membered ring from the fruiting body of Ganoderma sinense. Org. Lett. 2010;12:1656–1659. doi: 10.1021/ol100062b. [DOI] [PubMed] [Google Scholar]
- 57.Ma J.Y., Ye Q., Hua Y.J., Zhang D.C., Cooper R., Chang M.N., Chang J.Y., Sun H.H. New lanostanoids from the mushroom Ganoderma lucidum. J. Nat. Prod. 2002;65:72–75. doi: 10.1021/np010385e. [DOI] [PubMed] [Google Scholar]
- 58.Chen M., Zhang M., Sun S., Xia B., Zhang H.Q. A new triterpene from the fruiting bodies of Ganoderma lucidum. Acta Pharm. Sin. 2009;44:768–770. [PubMed] [Google Scholar]
- 59.Liu C., Chen R.Y. A new triterpene from fungal fruiting bodies of Ganoderma sinense. Chin. Tradit. Herb. Drugs. 2010;41:8–11. [Google Scholar]
- 60.Su H.J., Fann Y.F., Chung M.I., Won S.J., Lin C.N. New lanostanoids of Ganoderma tsugae. J. Nat. Prod. 2000;63:514–516. doi: 10.1021/np990367l. [DOI] [PubMed] [Google Scholar]
- 61.Lin C.N., Fann Y.F., Chung M.I. Steroids of formosan Ganoderma Tsugae. Phytochemistry. 1997;46:1143–1146. doi: 10.1016/S0031-9422(97)00387-7. [DOI] [Google Scholar]
- 62.Niu X.M., Li S.H., Xiao W.L., Sun H.D., Che C.T. Two new lanostanoids from Ganoderma resinaceum. J. Asian Nat. Prod. Res. 2007;9:659–664. doi: 10.1080/10286020600979910. [DOI] [PubMed] [Google Scholar]
- 63.Guan S.H., Yang M., Wang X.M., Xia J.M., Zhang Z.M., Liu X., Guo D.A. Structure elucidation and complete NMR spectral assignments of three new lanostanoid triterpenes with unprecedented Δ16,17 double bond from Ganoderma lucidum. Magn. Reson. Chem. 2007;45:789–791. doi: 10.1002/mrc.2046. [DOI] [PubMed] [Google Scholar]
- 64.Nishitoba T., Sato H., Sakamura S. Triterpenoids from the fungus Ganoderma lucidum. Phytochemistry. 1987;26:1777–1784. doi: 10.1016/S0031-9422(00)82287-6. [DOI] [Google Scholar]
- 65.Li Y.Y., Mi Z.Y., Tang Y., Wang G., Li D.S., Tang Y.J. Lanostanoids isolated from Ganoderma lucidum mycelium cultured by submerged fermentation. Helv. Chim. Acta. 2009;92:1586–1593. doi: 10.1002/hlca.200900028. [DOI] [Google Scholar]
- 66.Chairul S.M., Hayashi Y. Lanostanoid triterpenes from Ganoderma applanatum. Phytochemistry. 1994;35:1305–1308. doi: 10.1016/S0031-9422(00)94843-X. [DOI] [Google Scholar]
- 67.Chen R.Y., Yu D.Q. Studies on the triterpenoid constituents of the spores from Ganoderma lucidum karst. J. Chin. Pharm. Sci. 1993;2:91–96. [PubMed] [Google Scholar]
- 68.Gan K.H., Kuo S.H., Lin C.N. Steroidal constituents of Ganoderma applanatum and Ganderma neo-japonicum. J. Nat. Prod. 1998;61:1421–1422. doi: 10.1021/np980184j. [DOI] [PubMed] [Google Scholar]
- 69.De Silva E.D., van der Sar S.A., Santha R.L., Wijesundera R.L., Cole A.L., Blunt J.W., Munro M.H. Lanostane triterpenoids from the Sri Lankan Basidiomycete Ganoderma applanatum. J. Nat. Prod. 2006;69:1245–1248. doi: 10.1021/np0602214. [DOI] [PubMed] [Google Scholar]
- 70.Qiao Y., Zhang X.M., Dong X.C., Qiu M.H. A new 18(13→12β)-abeo-lanostadiene triterpenoid from Ganoderma fornicatum. Helv. Chim. Acta. 2006;89:1038–1041. doi: 10.1002/hlca.200690081. [DOI] [Google Scholar]
- 71.Lin L.J., Shiao M.S. Seven new triterpenes from Ganoderma lucidum. J. Nat. Prod. 1988;51:918–924. doi: 10.1021/np50059a017. [DOI] [PubMed] [Google Scholar]
- 72.Hirotani M., Ino C., Furuya T. Comparative study on the strain-specific triterpenoid components of Ganoderma lucidum. Phytochemistry. 1993;33:379–382. doi: 10.1016/0031-9422(93)85523-T. [DOI] [Google Scholar]
- 73.Zhang X.Q., Ip F.C.F., Zhang D.M., Chen L.X., Zhang W., Li Y.L., Ip N.Y., Ye W.C. Triterpenoids with neurotrophic activity from Ganoderma lucidum. Nat. Prod. Res. 2011;25:1607–1613. doi: 10.1080/14786419.2010.496367. [DOI] [PubMed] [Google Scholar]
- 74.Li C.J., Yin J.H., Guo F.J., Zhang D.C., Sun H.H. Ganoderic acid Sz, a new lanostanoid from the mushroom Ganoderma lucidum. Nat. Prod. Res. 2005;19:461–465. doi: 10.1080/14786410412331272077. [DOI] [PubMed] [Google Scholar]
- 75.Gonzalez A.G., Leon F., Rivera A., Padron J.I., Ganzalez-Plata J., Zuluaga J.C., Quintana J., Estevez F., Bermejo J. New lanostanoids from the Fungus Ganoderma concinna. J. Nat. Prod. 2002;65:417–421. doi: 10.1021/np010143e. [DOI] [PubMed] [Google Scholar]
- 76.Arisawa M., Fujita A., Saga M., Fukumura H., Hayashi T., Shimizu M., Morita N. Three new lanostanoids from Ganoderma lucidum. J. Nat. Prod. 1986;49:621–625. doi: 10.1021/np50046a010. [DOI] [PubMed] [Google Scholar]
- 77.Sato H., Nishitoba T., Shirasu S., Oda K., Sakamura S. Ganoderiol A and B, new triteroenoids from the fungus Ganoderma lucidum (Reishi) Afr. Biol. Chem. 1986;50:2887–2890. [Google Scholar]
- 78.Shiao M.S., Lin L.J. Two new triterpenes of the fungus Ganoderma lucidum. J. Nat. Prod. 1987;50:886–890. doi: 10.1021/np50053a019. [DOI] [Google Scholar]
- 79.Keller A.C., Keller J., Maillard M.P., Hostettmann K. A lanostane-type steroid from the fungus Ganoderma carnosum. Phytochemistry. 1997;46:963–965. doi: 10.1016/S0031-9422(97)00381-6. [DOI] [Google Scholar]
- 80.Hirotani M., Ino C., Furuya T., Shiro M. Ganoderic acids T, S and R, new triterpenoids from the cultured mycelia of Ganoderma lucidum. Chem. Pharm. Bull. 1986;34:2282–2285. doi: 10.1248/cpb.34.2282. [DOI] [Google Scholar]
- 81.Shiao M.S., Lin L.J., Yeh S.F. Triterpenes from Ganoderma lucidum. Phytochemistry. 1988;27:2911–2914. doi: 10.1016/0031-9422(88)80687-3. [DOI] [PubMed] [Google Scholar]
- 82.Lin L.J., Shiao M.S., Yea S.F. Triterpenes from Ganoderma lucidum. Phytochemistry. 1988;27:2269–2271. doi: 10.1016/0031-9422(88)80140-7. [DOI] [Google Scholar]
- 83.Gerhäuser C., Zhang W.D., Ho-Chong-Line N., Fourasté I. New lanostanoids from Ganoderma lucidum that induce NAD(P)H: Quinine oxidoreductase in cultured hepalclc 7 murine hepatoma cells. Planta Med. 2000;66:681–684. doi: 10.1055/s-2000-8647. [DOI] [PubMed] [Google Scholar]
- 84.Shiao M.S., Lin L.J., Yea S.F. Triterpenes in Ganoderma lucidum. Phytochemistry. 1988;27:873–875. doi: 10.1016/0031-9422(88)84110-4. [DOI] [Google Scholar]
- 85.Yang S.X., Yu Z.C., Lu Q.Q., Shi W.Q., Laatsch H., Gao J.M. Toxic lanostane triterpenes from the basidiomycete Ganoderma amboinense. Phytochem. Lett. 2012;5:576–580. doi: 10.1016/j.phytol.2012.05.017. [DOI] [Google Scholar]
- 86.Qiao Y., Zhang X.M., Qiu M.H. Two novel lanostane triterpenoids from Ganoderma Sinense. Molecules. 2007;12:2038–2046. doi: 10.3390/12082038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fujita A., Arisawa M., Saga M., Hayashi T., Fukumura H., Morita N. Two new lanostanoids from Ganoderma lucidum. J. Nat. Prod. 1986;49:1122–1125. doi: 10.1021/np50048a029. [DOI] [PubMed] [Google Scholar]
- 88.Li C.H., Chen P.Y., Chang U.M., Kan L.S., Fang W.H., Tsai K.S., Lin S.B. Ganoderic acid X, a lanostanoid triterpene, inhibits topoisomerases and induces apoptosis of cancer cells. Life Sci. 2005;77:252–265. doi: 10.1016/j.lfs.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 89.Liu J., Kurashiki K., Shimizu K., Kondo R. Structure–activity relationship for inhibition of 5α-reductase by triterpenoids isolated from Ganoderma lucidum. Bioorg. Med. Chem. 2006;14:8654–8660. doi: 10.1016/j.bmc.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 90.Akihisa T., Tagata M., Ukiya M., Tokuda H., Suzuki T., Kimura Y. Oxygenated lanostane-type triterpenoids from the fungus Ganoderma lucidum. J. Nat. Prod. 2005;68:559–563. doi: 10.1021/np040230h. [DOI] [PubMed] [Google Scholar]
- 91.Wu T.S., Shi L.S., Kuo S.C. Cytotoxicity of Ganoderma lucidum Triterpenes. J. Nat. Prod. 2001;64:1121–1122. doi: 10.1021/np010115w. [DOI] [PubMed] [Google Scholar]
- 92.Weng C.J., Chau C.F., Chen K.D., Chen D.H., Yen G.C. The anti-invasive effect of lucidenic acids isolated from a new Ganoderma lucidum strain. Mol. Nutr. Food Res. 2007;51:1472–1477. doi: 10.1002/mnfr.200700155. [DOI] [PubMed] [Google Scholar]
- 93.Li P., Deng Y.P., Wei X.X., Xu J.H. Triterpenoids from Ganoderma lucidum and their cytotoxic activities. Nat. Prod. Res. 2013;27:17–22. doi: 10.1080/14786419.2011.652961. [DOI] [PubMed] [Google Scholar]
- 94.Nishitoba T., Sato H., Kasai T., Kawagishi H., Sakamura S. New bitter C27 and C30 terpenoids from the fungus Ganodema lucidum(Reishi) Agric. Biol. Chem. 1984;48:2905–2907. doi: 10.1271/bbb1961.48.2905. [DOI] [Google Scholar]
- 95.Iwatsuki K., Akihisa T., Tokuda H., Ukiya M., Oshikubo M., Kimura Y., Asano T., Nomura A., Nishino H. Lucidenic acids P and Q, methyl lucidenate P, and other triterpenoids from the fungus Ganoderma lucidum and their inhibitory effects on epstein-barr virus activation. J. Nat. Prod. 2003;66:1582–1585. doi: 10.1021/np0302293. [DOI] [PubMed] [Google Scholar]
- 96.Lee I., Kim H., Youn U., Kim J., Min B., Jung H., Na M., Hattori M., Bae K. Effect of lanostane triterpenes from the fruiting bodies of Ganoderma lucidum on adipocyte differentiation in 3T3-L1 cells. Planta Med. 2010;76:1558–1563. doi: 10.1055/s-0030-1249827. [DOI] [PubMed] [Google Scholar]
- 97.El Dine R.S., el Halawany A.M., Nakamura N., Ma C.M., Hattori M. New lanostane triterpene lactones from the vietnamese mushroom Ganoderma colossum (FR.) C. F. BAKER. Chem. Pharm. Bull. 2008;56:642–646. doi: 10.1248/cpb.56.642. [DOI] [PubMed] [Google Scholar]
- 98.Kleinwächter P., Anh N., Kiet T.T., Schlegel B., Dahse H.M., Härtl A., Gräfe U. Colossolactones, new triterpenoid Metabolites from a Vietnamese mushroom Ganoderma colossum. J. Nat. Prod. 2001;64:236–239. doi: 10.1021/np000437k. [DOI] [PubMed] [Google Scholar]
- 99.Chen R.Y., Yu D.Q. Application of 2D NMR techniques in the structure determination of Ganosporelactone A and B. Acta Pharm. Sin. 1991;26:430–436. [PubMed] [Google Scholar]
- 100.Sato N., Ma C.M., Komatsu K., Hattori M. Triterpene-farnesyl hydroquinone conjugates from Ganoderma sinense. J. Nat. Prod. 2009;72:958–961. doi: 10.1021/np800687t. [DOI] [PubMed] [Google Scholar]
- 101.Tokuyama T., Hayashi Y., Nishizawa M., Tokuda H., Chairul S.M., Hayashi Y. Applanoxidic acids A, B, C, and D, biologically active tetracyclic triterpenes from Ganoderma applanatum. Phytochemistry. 1991;30:4105–4109. doi: 10.1016/0031-9422(91)83476-2. [DOI] [Google Scholar]
- 102.El Dine R.S., el Halawany A.M., Ma C.M., Hattori M. Anti-HIV-1 protease activity of lanostane triterpenes from the vietnamese mushroom Ganoderma colossum. J. Nat. Prod. 2008;71:1022–1026. doi: 10.1021/np8001139. [DOI] [PubMed] [Google Scholar]
- 103.Leon F., Valenica M., Rivera A., Nieto I., Quintana J., Estevez F., Bermejo J. Novel cytostatic lanostanoid triterpenes from Ganoderma australe. Helv. Chim. Acta. 2003;86:3088–3095. doi: 10.1002/hlca.200390251. [DOI] [Google Scholar]
- 104.Wang F.S., Cai H., Yang J.S., Zhang Y.M., Zhao Y.J. Triterpenoids from the fruiting body of Ganoderma lucidum. J. Chin. Pharm. Sci. 1997;6:192–197. [Google Scholar]
- 105.Niu X.M., Qiu M.H., Lu Z.G., Lu Y., Cao P., Zheng Q. Two novel 3,4-seco-trinorlanostane triterpenoids isolated from Ganoderma fornicatum. Tetrahedron Lett. 2004;45:2989–2993. doi: 10.1016/j.tetlet.2004.02.056. [DOI] [Google Scholar]
- 106.Peng X.R., Liu J.Q., Xia J.J., Yang Y.H., Qiu M.H. Two new triterpenoids from Ganoderma cochlear. Chin. Tradit. Herb. Drugs. 2012;43:1045–1049. [Google Scholar]
- 107.Su C.H., Lai M.N., Chan M.H. Hepato-protective triterpenoids from Ganoderma tsugae Murrill. In: Chang S., Buswell J.A., Chiu S., editors. Mushroom Biology and Mushroom Products. The Chinese University Press; Hong Kong, China: 1993. pp. 275–283. [Google Scholar]
- 108.Fatmawati S., Shimizu K., Kondo R., Ganoderol B. A potent α-glucosidase inhibitor isolated from the fruiting body of Ganoderma lucidum. Phytomedicine. 2011;18:1053–1055. doi: 10.1016/j.phymed.2011.03.011. [DOI] [PubMed] [Google Scholar]