Abstract
A series of artemisinin-indoloquinoline hybrids were designed and synthesized in an attempt to develop potent and selective anti-tumor agents. Compounds 7a–7f, 8 and 9 were prepared and characterized. Their antiproliferative activities against MV4-11, HCT-116, A549, and BALB/3T3 cell lines in vitro were tested. Nearly all of the tested compounds (7–9, except for compounds 7d and 7e against HCT-116) showed an increased antitumor activity against HCT-116 and A549 cell lines when compared to the dihydroartemisinin control. Especially for the artemisinin-indoloquinoline hybrid 8, with an 11-aminopropylamino-10H-indolo[3,2-b]quinoline substituent, the antiproliferative activity against the A549 cell line had improved more than ten times. The IC50 value of hybrid 8 against A549 cell lines was decreased to 1.328 ± 0.586 μM, while dihydroartemisin showed IC50 value of >20 µM in the same cell line. Thus, these results have proven that the strategy of introducing a planar basic fused aromatic moiety, such as the indoloquinoline skeleton, could improve the antiproliferative activity and selectivity towards cancer cell lines.
Keywords: artemisinin, indoloquinoline, hybrid, antiproliferative activity, cytotoxicity
1. Introduction
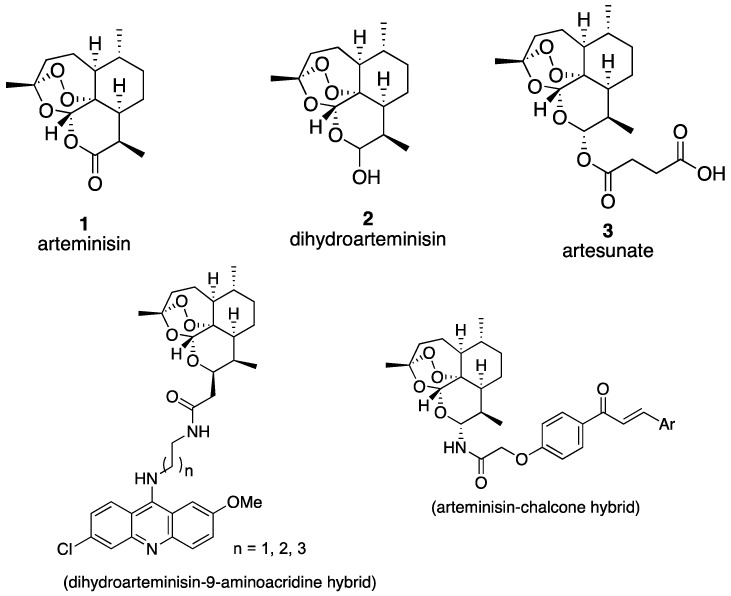
Artemisinin (1), a sesquiterpene lactone from Artemisia annua, was isolated as a result of an extensive survey for antimalarial agents in Chinese traditional herb medicines by Chinese scientists since the early 1970s [1]. Today, 1 and its derivatives, dihydroarteminisin (DHA, 2) and artesunate (3), are used in the first-line treatment for multidrug-resistant malaria [2,3]. Besides the antimalarial activity, artemisinin and its semisynthetic analogs are endowed with the potential of anti-tumor [4,5,6,7], antiangiogenic [8,9], anti-inflammatory [10], anti-metastasis [11], and growth inhibition effects [12]. The most unique and important structural feature installed in artemisinin is a peroxide group in the 1,2,4-trioxane moiety, which can react with an iron complex to produce cytotoxic free radicals and selectively induces apoptosis in many high free iron level cell lines, such as cancer cells [13]. This biological sequence makes artemisinin and its analogs potent anticancer lead compounds.
However, compared with many traditional cancer chemotherapeutic medicines of natural origin, such as camptothecin [14], doxorubicin [15], etc., simple artemisinin analogs are still less potent [4,16]. High dosage and frequent administration would be required in order to achieve the same effectiveness in the anticancer treatment due to their short half-lives.
Many more potent artemisinin-derived antitumor agents are being developed [17,18,19]. One of the important strategies for an improved action is the generation of hybrid molecules, which involves the covalent linking of the artemisinin or its analogs with some more potent and target-selective moieties [20]. For instance, the 9-aminoacridine moiety, which is a key structure in Amsacrine, an antineoplastic agent for acute lymphoblastic leukemia, was linked with dihydroartemisinin (2) to form the hybrid. The antiproliferative activity of the hybrid compounds has been increased [21]. This multiple target strategy led to the design of various hybrids like artemisinin-chalcone, in which the synergistic effect of chalcone on artemisinin was demonstrated [7,22], as shown in Figure 1.
Figure 1.
The structure of artemisinin and its analogues, and subsequent hybrid compounds.
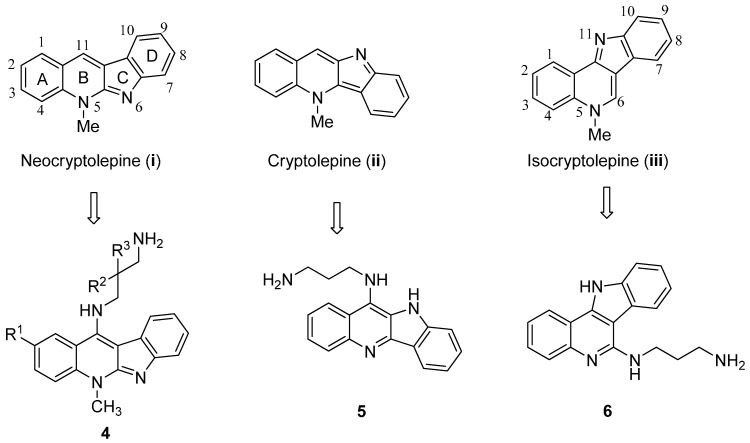
The indoloquinoline skeleton has been widely found in many alkaloids [23,24,25]. In particular, alkaloids from plants are promising candidates of new lead compounds in the search for new drugs against infectious diseases and cancers [26,27]. Anticancer potential of indoloquinoline alkaloids are comprehensively documented in the recent review articles [28,29,30]. Indeed, the indoloquinoline alkaloids like neocryptolepine (i), cryptolepine (ii), and isocryptolepine (iii), isolated from Cryptolepis sanguinolenta (Lindl.) Schltr. have been used as scaffolds for drug discovery, since this plant is used as a traditional herbal medicine in West and Central Africa (Figure 2) [31,32]. Its planar fused ring system can intercalate into the DNA of tumor cells [33,34]. Besides double-helical DNA, indoloquinoline derivatives have also been found to bind DNA triplexes as well as G-quadruplexes with high affinity [35]. Thus, many indoloquinoline-featured compounds have been studied as antitumor agents [36,37,38,39,40,41,42,43]. Neocryptolepine i can intercalate into DNA and inhibit topoisomerase II [36]. On the other hand, cryptolepine ii, a regioisomer of i, is also a DNA intercalating agent and inhibits topoisomerase II, showing a high level of cytotoxicity [42]. A series of 5,11-dimethylindolo[2,3-b]quinolines or 6,11-dimethylindolo[2,3-b]quinolines derivatives have been tested for their chemotherapeutic activities, showing a cytotoxicity against several human cancer cell lines, with IC50 value ranging from 0.6 to 9 μM [44,45,46]. In these studies, the substituents, such as the Br, MeO, or Me groups, attached on the indoloquinoline core were shown to have highly infectious activities. Metal ions, such as ruthenium(II), osmium(II) or copper(II), have been introduced to the indoloquinolines scaffold for assaying their antiproliferavitve activity. Anticancer activity in vitro and in vivo have shown that the osmium(II) or copper(II) complex displayed significant growth-inhibitory activity [47,48,49]. 5H-indolo[2,3-b]quinoline (DiMIQ) derivatives containing an amino acid or a dipeptide at the C-9 position also shown impressive antitumor activity. The attachment of the hydrophilic amino acid or the peptide has increased its hydrophilic properties and made the modified DiMIQ less in vivo toxic and promising anticancer agent [36,43].
Figure 2.
Structures of indoloquinolines from Cryptolepis sanguinolenta and their modifications.
In our previous study, we also developed a series of indoloquinoline-derived antitumor agents like 4 and 6, by modification at the C-11 position of i and at the C-6 position of iii using diverse ω-aminoalkylamino substituents (Figure 2) [43,44,45]. The target selectivity of the indoloquinoline moiety towards cancer cell lines has been well documented in these studies. Based on these facts, we are considering whether the potency of artemisinin analogues against cancer cell lines could be improved by linking to the indoloquinoline moiety. Thus, in this work, a series of dihydroartemisinin-indoloquinoline hybrids 7–9 were designed and synthesized. Their potencies as an antitumor agent were evaluated by antiproliferative screening.
2. Results and Discussion
2.1. Chemistry
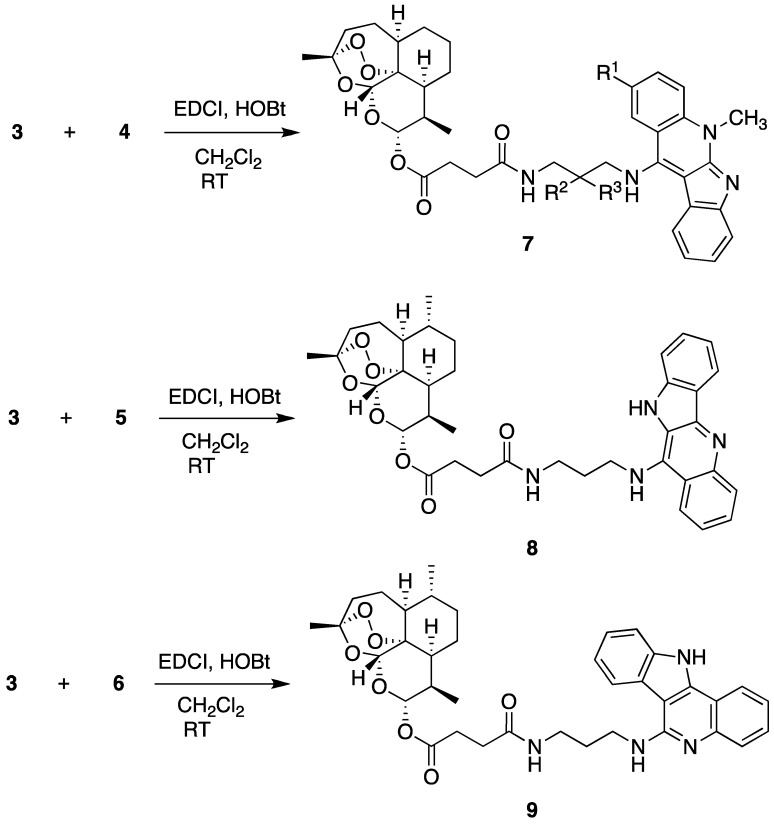
The artesunate-indoloquinoline hybrids 7, 8 and 9 were obtained using the synthetic process shown in Scheme 1. Artesunate (1), which was also an artemisinin derived antimalarial agent with a better hydrophilicity, was employed as the starting material. The carboxylic acid group of the artesunate underwent condensation with ω-aminoalkylamino-indoloquinoline intermediates in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCl) and 1-hydroxybenzotriazole (HOBt) at room temperature to form the target compounds 7, 8 and 9. The preparations of the intermediate 11-(ω-aminoalkylamino)-5-methyl-5H-indolo[2,3-b]quinolines 4 and the 6-(ω-aminoalkylamino)-11H-indolo[3,2-c]quinolines 6 were carried out according to the method that we previously described in the literature [50,51,52]. The synthesis of 11-(ω-aminoalkylamino)-10H-indolo[3,2-b]quinolin 5 was carried out according to the method reported by Lavrado et al. [53]. After the appropriate purification, these hybrids were evaluated by the biological activity screening.
Scheme 1.
Synthetic schemes for the artesunate-indoloquinoline hybrids.
2.2. Biological Evaluation
The results of the antiproliferative activity screening for compounds 7–8 are summarized in Table 1 and Table 2. Biological activities of the compound 9 obtained from the isocryptolepine derivative 6 and artesunate 3 are cited in Reference [54] due to its low purity.
Table 1.
Antiproliferative activity of the artemisinin-indoloquinoline hybrids against human leukemia MV4-11 cell line.
| Compound | R1 | R2 | R3 | MV4-11 a IC50 (μM) |
|---|---|---|---|---|
| cisplatin | 2.820 ± 0.450 | |||
| doxorubicin HCl | 0.006 ± 0.002 | |||
| 7a | H | H | H | 0.286 ± 0.079 |
| 7b | Cl | CH3 | CH3 | 0.072 ± 0.022 |
| 7c | Br | CH3 | CH3 | 0.242 ± 0.031 |
| 7d | CO2Me | H | H | 0.148 ± 0.015 |
| 7e | CO2Me | CH3 | CH3 | 0.075 ± 0.001 |
| 7f | CO2Me | H | OH | 0.226 ± 0.019 |
| 8 | 0.286 ± 0.127 | |||
| 4a b | H | H | H | 0.066 ± 0.023 |
| 4d c | CO2Me | H | H | 0.086 ± 0.020 |
| 6 d | 0.124 ± 0.010 |
Table 2.
Antiproliferative activity against cancer cell lines of A549 and HCT116, and cytotoxicity against normal mice fibroblast BALB/3T3.
| Compound | BALB/3T3 a IC50 (μM) | A549 b IC50 (μM) | HCT116 c IC50 (μM) |
|---|---|---|---|
| cisplatin | 9.498 ± 0.500 | 8.965 ± 3.333 | 9.465 ± 1.300 |
| 7b | 6.423 ± 0.996 | 4.555 ± 2.086 | 0.893 ± 0.397 |
| 7d | 4.953 ± 0.220 | 3.663 ± 0.535 | 1.756 ± 0.329 |
| 7e | 5.945 ± 1.163 | 5.060 ± 0.911 | 2.206 ± 0.687 |
| 7f | 4.914 ± 0.430 | 4.444 ± 0.685 | 0.832 ± 0.216 |
| 8 | 2.725 ± 0.731 | 1.328 ± 0.586 | 0.557 ± 0.085 |
| 4d | 0.768 ± 0.155 | 0.649 ± 0.080 | 0.130 ± 0.014 |
| 6 d | 1.047 ± 0.127 | 0.172 ± 0.052 | 0.258 ± 0.107 |
| DHA (2) | - | >20 e | 1.34 ± 1.06 e |
First, their antiproliferative activities against human leukemia cell line MV4-11 were evaluated (Table 1). All tested compounds have shown an antitumor activity with an IC50 in the microM range. Compound 7b turned out to be the most active hybrids (IC50 about 0.072 μM). Compared to the corresponding amino-modified indoloquinoline intermediate 4a, the antiproliferative activity of the artesunate-indoloquinoline hybrid 7a was weakened, with an increased IC50 value from 0.066 ± 0.023 μM to 0.286 ± 0.079 μM. We also observed the same trend for the intermediate 4d and the hybrid 7d. Agent 9, which is a hybrid from artesunate and 6-(3-aminopropylamino)-11H-indolo[3,2-c]quinoline (6), showed a better potency than agent 7a, which reflects the difference of the counterpart in the artesunate hybrids. Compounds 7b, 7d, 7e, 7f, 8 and 9 were then selected for the next evaluation.
In order to test the selectivity of these hybrids between the normal cells and cancer cells, the normal mice fibroblast BALB/3T3 cells, human lung cancer cells A549 and human colon cancer cells HCT116 were employed in the evaluation of the second step. The clinical chemotherapy drug cisplatin and antimalarial drug dihydroartemisinin were used as positive control. Generally, these tested compounds inhibited cell proliferation of these three cell lines, though the selectivity towards the HCT116 cells was better than the others. As shown in Table 2, all the tested agents (7–9) have an enhanced antiproliferative activity against the lung cancer cells A549 with IC50 values ranging from 1.33 to 5.06 µM, which are more active in comparison to DHA (2) due to the introduced planar fused ring system. Especially compound 8, with an IC50 value of 1.328 ± 0.586 μM better than the both positive control, produced the best result in the screening. On the contrary, DHA (2) showed a poor activity against A549, with an IC50 of more than 20 µM. All the tested agents exhibited an antiproliferative activity superior to the positive control cisplatin. When against the HCT116 cell lines, the best result was provided by compound 8. It inhibited the HCT116 cells proliferation with an IC50 of 0.557 ± 0.085 μM.
3. Experimental Section
3.1. General Methods
The commercially obtained reagents were directly used without further purification. The 1H-NMR and 13C-NMR spectra were measured by a Varian INOVA-600 spectrometer with CDCl3 as the solvent unless otherwise indicated. High-resolution mass spectra were obtained on a Bruker micrOTOF II-SKA spectrometer. The intermediate 4 and 6 were synthesized according to the method we previously reported [50,51,52].
3.1.1. General Procedure for the Synthesis of Artesunate-Indoloquinoline Hybrids 7
Artesunate 3 (102 mg), EDTI (39 mg) and HOBt (27.6 mg) were dissolved in CH2Cl2 with stirring for 1 h, then appropriate substituted 5-methyl-5H-indolo[2,3-b]quinoline was added with stirring together at room temperature for 6 h. TLC checked the completion of the reaction. The reaction mixture was washed by brine, dried over anhydrous MgSO4. After concentrated under vacuum, the crude products were purified by flash chromatography using AcOEt-MeOH (1:10 V/V) as the eluent to yield pure 7 as solids.
3.1.2. Physical Data for Compounds 7
Artesunate-[N1-(5-methyl-5H-indolo[2,3-b]quinolin-11-yl)propane-1,3-diamine] hybrid (7a). Yield: 55%. 1H-NMR (CDCl3) δ 8.37 (d, J = 8.3 Hz, 1H), 7.91 (d, J = 7.7 Hz, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.67 (d, J = 7.3 Hz, 1H), 7.61 (d, J = 8.6 Hz, 1H), 7.37 (t, J = 7.6 Hz, 2H), 7.15 (t, J = 7.5 Hz, 1H), 6.51 (dd, J = 14.4, 8.0 Hz, 2H), 5.68 (d, J = 9.8 Hz, 1H), 5.24 (s, 1H), 4.20 (s, 3H), 3.94–3.87 (m, 1H), 3.85–3.78 (m, 1H), 3.57–3.50 (m, 1H), 3.28 (dd, J = 14.1, 5.7 Hz, 1H), 2.82 (dd, J = 9.0, 5.5 Hz, 1H), 2.74–2.69 (m, 1H), 2.55 (dd, J = 9.2, 5.3 Hz, 1H), 2.51–2.40 (m, 2H), 2.27 (dd, J = 13.9, 3.8 Hz, 1H), 1.96–1.91 (m, 1H), 1.82–1.78 (m, 1H), 1.74–1.67 (m, 2H), 1.48 (ddd, J = 14.7, 9.9, 3.7 Hz, 3H), 1.37–1.27 (m, 4H), 1.15 (dd, J = 11.4, 6.5 Hz, 1H), 1.11–0.99 (m, 2H), 0.86 (dd, J = 15.8, 10.0 Hz, 4H), 0.69 (d, J = 7.1 Hz, 3H); 13C-NMR (CDCl3) δ 172.8, 171.9, 156.9, 152.2, 148.4, 137.7, 130.4, 125.5, 124.0, 123.8, 121.9, 121.0, 118.7, 116.9, 116.5, 114.5, 106.6, 104.4, 92.3, 91.4, 80.1, 51.4, 45.0, 43.67, 37.1, 36.1, 36.0, 33.9, 32.8, 31.7, 31.3, 30.8, 29.9, 25.9, 24.4, 21.7, 20.1, 11.9. HRMS (ESI) calcd for C38H47N4O7 [M+H]+ Exact Mass: 671.3445, found 671.3442.
Artesunate-[N1-(2-chloro-5-methyl-5H-indolo[2,3-b]quinolin-11-yl)-2,2-dimethjylpropane-1,3-diamine] hybrid (7b). Yield: 47%. 1H NMR (CDCl3) δ 8.59 (d, J = 2.0 Hz, 1H), 7.93 (s, 1H), 7.88 (s, 1H), 7.81 (s, 1H), 7.73 (dd, J = 6.9, 2.3 Hz, 2H), 7.38–7.35 (m, 1H), 7.22 (ddd, J = 7.9, 6.8, 1.1 Hz, 1H), 7.06 (s, 1H), 5.63 (d, J = 9.9 Hz, 1H), 4.90 (s, 1H), 4.40 (s, 3H), 3.73–3.66 (m, 2H), 3.41–3.35 (m, 1H), 3.15–3.08 (m, 1H), 2.89 (ddd, J = 11.9, 7.6, 5.0 Hz, 2H), 2.72 (ddd, J = 13.5, 8.7, 4.8 Hz, 1H), 2.66–2.60 (m, 1H), 2.38 (ddd, J = 9.9, 7.1, 4.6 Hz, 1H), 2.23 (td, J = 14.3, 3.9 Hz, 1H), 1.92–1.85 (m, 1H), 1.74–1.67 (m, 1H), 1.39–1.33 (m, 1H), 1.31–1.27 (m, 1H), 1.23–1.13 (m, 5H), 1.05 (dd, J = 10.5, 4.1 Hz, 1H), 0.78 (s, 4H), 0.71 (s, 5H), 0.62 (s, 3H), 0.58 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3) δ 174.0, 172.2, 145.0, 143.6, 135.5, 131.7, 128.2, 126.1, 124.3, 123.5, 123.4, 122.6, 118.6, 118.1, 116.9, 114.8, 111.0, 104.2, 92.3, 91.3, 79.9, 54.3, 51.2, 46.5, 44.8, 38.8, 36.8, 36.0, 35.2, 33.8, 31.5, 30.7, 29.7, 25.7, 24.4, 24.0, 23.9, 21.5, 19.9, 11.7. HRMS (ESI) calcd for C40H50ClN4O7 [M+H]+ Exact Mass: 733.3368, found 733.3364.
Artesunate-[N1-(2-bromo-5-methyl-5H-indolo[2,3-b]quinolin-11-yl)-2,2-dimethylpropane-1,3-diamine] hybrid (7c). Yield: 43%. 1H-NMR (CDCl3) δ 8.62 (d, J = 7.9 Hz, 1H), 7.90 (d, J = 7.9 Hz, 1H), 7.79–7.72 (m, 3H), 7.55 (t, J = 7.6 Hz, 1H), 7.32 (t, J = 7.6 Hz, 1H), 7.25 (t, J = 7.6 Hz, 1H), 7.21 (m, 1H), 5.63 (d, J = 9.9 Hz, 1H), 4.92 (s, 1H), 4.38 (s, 3H), 3.73–3.67 (m, 2H), 3.32 (dd, J = 13.8, 6.8 Hz, 1H), 3.11 (dd, J = 13.9, 5.7 Hz,1H), 2.87–2.77 (m, 3H), 2.73–2.68 (m, 1H), 2.38–1.33 (m, 1H), 2.20 (td, J = 14.0, 3.7 Hz, 1H), 1.86–1.83 (m, 1H), 1.71–1.68 (m, 1H), 1.34 (dt, J = 13.1, 4.3 Hz, 1H), 1.27 (d, J = 11.2 Hz,1H), 1.23–1.13 (m, 5H), 1.04 (m, 1H), 0.74–0.65 (m, 6H), 0.58–0.56 (m, 6H); 13C NMR (CDCl3) δ 174.2, 172.1, 151.9, 143.6, 136.8, 132.1, 128.2, 125.8, 124.0, 123.5, 121.6, 121.3, 118.4, 117.2, 115.4, 113.9, 111.2, 104.2, 92.1, 91.2, 79.9, 60.4, 54.4, 51.2, 46.3, 44.8, 38.9, 36.8, 36.1, 35.3, 33.8, 31.5, 30.4, 29.6, 25.7, 24.4, 24.0, 23.9, 19.9, 11.7.; HRMS (ESI) calcd for C40H50BrN4O7 [M+H]+ Exact Mass: 777.2863, found 777.2858.
Artesunate-[methyl 11-(3-aminopropylamino)-5-methyl-5H-indolo[2,3-b]quinoline-2-carboxylate] hybride (7d). Yield: 46%. 1H-NMR (CDCl3) δ 9.09 (d, J = 1.7 Hz, 1H), 8.30 (dd, J = 8.9, 1.8 Hz, 1H), 8.01 (d, J = 7.7 Hz, 1H), 7.73 (d, J = 7.9 Hz, 1H), 7.65 (d, J = 9.0 Hz, 1H), 7.39 (t, J = 7.5 Hz, 1H), 7.21 (t, J = 7.2 Hz, 1H), 6.66 (s, 1H), 6.35 (s, 1H), 5.70 (d, J = 9.9 Hz, 1H), 5.27 (s, 1H), 4.26 (s, 3H), 4.00 (d, J = 3.2 Hz, 3H), 3.94 (d, J = 6.5 Hz, 1H), 3.88 (d, J = 7.0 Hz, 1H), 3.61 (d, J = 7.2 Hz, 1H), 3.41 (dd, J = 14.2, 5.8 Hz, 1H), 2.90–2.84 (m, 1H), 2.79–2.73 (m, 1H), 2.58 (dd, J = 8.9, 5.4 Hz, 1H), 2.54–2.50 (m, 1H), 2.50–2.45 (m, 1H), 2.35–2.28 (m, 1H ), 1.99–1.94 (m, 1H), 1.90 (dd, J = 10.8, 5.7 Hz, 2H), 1.82 (s, 1H), 1.61–1.55 (m, 2H), 1.52 (dd, J = 13.6, 4.4 Hz, 1H), 1.35 (m, 4H), 1.22–1.11 (m, 3H), 0.91 (m 4H), 0.73 (d, J = 7.1 Hz, 3H); 13C NMR (CDCl3) δ 172.8, 171.97, 166.7, 166.3, 140.6, 130.8, 127.4, 126.0(2C), 123.7, 122.5, 121.7(2C), 119.9, 117.3, 116.7, 115.8, 114.7, 104.5, 92.4, 91.5, 80.2, 52.6, 51.5, 45.2, 44.6, 37.3, 36.3, 36.2, 34.1, 33.4, 31.8, 31.6, 31.1, 30.1, 26.0, 24.6, 21.9, 20.3, 12.0. HRMS (ESI) calcd for C40H49N4O9 [M+H]+ Exact Mass: 729.3500, found 729.3502.
Artesunate-[methyl 11-(3-amino-2,2-dimethylpropylamino)-5-methyl-5H-indolo[2,3-b]quinoline-2-carboxylate] hybrid (7e). Yield: 51%. 1H-NMR (CDCl3) δ 9.27 (d, J = 1.7 Hz, 1H), 8.32–8.28 (m, 1H), 8.02 (d, J = 7.7 Hz, 1H), 7.74 (d, J = 7.9 Hz, 1H), 7.68–7.63 (m, 1H), 7.43–7.39 (m, 1H), 7.24–7.21 (m, 1H), 7.15 (s, 1H), 6.31–6.26 (m, 1H), 5.65 (d, J = 9.9 Hz, 1H), 5.09 (s, 1H), 4.26 (d, J = 5.1 Hz, 3H), 3.99 (s, 3H), 3.74 (ddd, J = 21.1, 13.5, 6.1 Hz, 2H), 3.60 (dd, J = 14.4, 8.0 Hz, 1H), 3.03 (dd, J = 14.4, 5.7 Hz, 1H), 2.97 (ddd, J = 17.7, 9.7, 4.8 Hz, 1H), 2.79 (ddd, J = 17.7, 6.2, 4.7 Hz, 1H), 2.67 (ddd, J = 14.5, 9.7, 4.6 Hz, 1H), 2.61–2.55 (m, 1H), 2.39 (ddd, J = 9.9, 7.1, 4.6 Hz, 1H), 2.28 (td, J = 14.3, 3.9 Hz, 1H), 1.97–1.91 (m, 1H), 1.81–1.76 (m, 1H), 1.39–1.32 (m, 3H), 1.30 (s, 3H), 1.22–1.18 (m, 1H), 1.10 (td, J = 11.3, 6.7 Hz, 1H), 0.93–0.88 (m, 1H), 0.86 (d, J = 6.2 Hz, 3H), 0.82 (s, 3H), 0.74 (t, J = 10.9 Hz, 2H), 0.66 (s, 3H), 0.58 (d, J = 7.1 Hz, 3H); 13C-NMR (CDCl3) δ 173.2, 172.1, 166.5, 164.8, 148.5, 140.4, 131.8, 130.5, 126.6, 125.3, 124.1, 122.4(2C) 122.3, 119.3, 117.2, 116.1, 114.3, 104.2, 92.3, 91.3, 80.0, 60.3, 54.2, 52.3, 51.3, 46.6, 44.8, 38.6, 36.8, 36.1, 33.8, 31.5, 30.9, 29.7, 25.8, 24.4, 24.0, 23.7, 21.4, 20.0, 11.6. HRMS (ESI) calcd for C42H53N4O9 [M+H]+ Exact Mass: 757.3813, found 757.3809.
3.1.3. Procedure for the Synthesis of Artesunate-Indoloquinoline Hybrid 8
Artesunate 3 (102 mg), EDTI (39 mg) and HOBt (27.6 mg) were dissolved in CH2Cl2 with stirring for 1 h, then N1-(10H-indolo[3,2-b]quinolin-11-yl)propane-1,3-diamine 5 was added to the mixture with stirring together at room temperature for 6 h. TLC checked the completion of the reaction. The reaction mixture was washed by brine, dried over anhydrous MgSO4. After concentrated under vacuum, the crude products were purified by flash chromatography using AcOEt-MeOH (1:10 V/V) as the eluent to yield pure 8 as solid.
3.1.4. Physical Data for Compound 8
Artesunate-[11-(3-aminopropylamino)-10H-indolo[3,2-b]quinoline] hybrid (8). Yield: 40%. 1H-NMR (CDCl3) δ11.18 (br. s., 1 H), 8.04 (d, J = 7.63 Hz, 1 H), 8.00 (d, J = 8.51 Hz, 1 H), 7.91 (d, J = 7.92 Hz, 1 H), 7.87 (d, J = 8.22 Hz, 1 H), 7.78 (d, J = 7.92 Hz, 1 H), 7.30 - 7.39 (m, 2 H), 7.24 (m, 1 H), 7.13 (d, J = 7.63 Hz, 1 H), 7.07 (m, 1 H), 6.87 (t, J = 6.75 Hz, 1 H), 6.56 (t, J = 6.75 Hz, 1 H), 5.65 (d, J = 9.98 Hz, 1 H), 5.15 (s, 1 H), 4.02 (br. s., 1 H), 3.38–3.33 (m, 2 H), 2.78–2.69 (m, 2 H), 2.66–2.57 (m, 2 H), 2.41–2.50 (m, 1 H), 2.28–2.25 (m, 1 H), 2.01–1.97 (m, 4 H), 1.87 (d, J = 14.09 Hz, 1 H), 1.77–1.75 (m, 1 H), 1.56–1.51 (m, 2 H), 1.47 (dt, J = 13.50, 4.25 Hz, 1 H), 1.32–1.25 (m, 4 H), 1.18–1.10 (m, 1 H), 1.10–1.01 (m, 2 H), 0.92–0.80 (m, 4 H), 0.77–0.67 (d, J = 7.04 Hz, 3 H); 13C-NMR (CDCl3) δ 172.7, 172.0, 143.7, 142.6, 142.1, 136.3, 134.1, 129.8, 128.2, 124.4, 123.3, 121.8, 119.9, 116.1, 114.1 114.0, 112.4, 104.3, 92.2, 91.3, 80.0, 60.4, 51.3, 45.0, 37.1, 36.1, 33.9, 31.7, 30.6, 29.7, 25.8, 24.4, 21.8, 21.0, 20.1, 14.2, 12.0. HRMS (ESI) calcd for C37H45N4O7 [M+H]+. Exact Mass: 657.3288, found 657.3289.
3.2. Cell Line
MV4-11 cells were cultured in the RPMI 1640 medium (IIET, Wroclaw) supplemented with 2 mM L-glutamine and 1.0 mM sodium pyruvate, 10% fetal bovine serum (all from Sigma Aldrich, Germany). HCT116 and A549 cells were cultured in the RPMI1640 + OptiMEM (50:50) medium (IIET, Wroclaw) supplemented with 2 mM L-glutamine and 5% fetal bovine serum (all from Sigma Aldrich, Germany), BALB/3T3 cells were cultured in Dulbecco medium (IIET, Wroclaw) supplemented with 2 mM L-glutamine and 1.0 mM sodium pyruvate, 10% fetal bovine serum (all from Sigma Aldrich, Germany). All culture medium was supplemented with 100 units/mL penicillin and 100 mg/mL streptomycin (both from Polfa, Tarchomin S.A., Warsaw, Poland). All cell lines were grown at 37 °C with 5% CO2 humidified atmosphere.
3.3. Antiproliferative Assay in Vitro
Test solutions of the compounds tested (1 mg/mL) were prepared by dissolving the substances in 100 μL of DMSO completed with 900 μL of tissue culture medium. Afterward, the tested compounds were diluted in culture medium to reach the final concentrations of 10, 1, 0.1, 0.01 μg/mL. Twenty four hours prior to the addition of the tested compounds, the cells were plated in 96-well plates (Sarstedt, Germany) at a density of 1 × 104 or 0.5× 104 (HCT116) cells per well. The assay was performed after 72 h of exposure to varying concentrations of the tested agents. The in vitro cytotoxic effect of all agents was examined using the MTT (for MV4-11 cell line) or SRB (for A549, HCT116 and BALB/3T3 cell lines) assay [55]. The results were calculated as an IC50 (inhibitory concentration 50)–the dose of tested agent that inhibits proliferation of 50% of the cell population. IC50 values were calculated for each experiment separately and mean values with SD are presented in Table 1 and Table 2. Each compound in each concentration was tested in triplicate in a single experiment, which was repeated 3–5 times.
4. Conclusions
In this study, we have synthesized and characterized a series of new artesunate-indoloquinoline hybrids, including compounds 7, 8 and 9. Their anticancer activities were evaluated by antiproliferative screening in vitro against the MV4-11, HCT-116, A549, and BALB/3T3 cell lines. Results have shown that nearly all of the tested compounds displayed an antiproliferative activity compared to the dihydroartemisinin. It was proved that the introduction of the indoloquinoline skeleton improved the antiproliferative activity and selectivity towards cancer cell lines of artemisinin. Further research on the modifications of indoloquinoline and use of different artemisinin analogues is still ongoing.
Acknowledgments
We are grateful to Okayama University for its support and to the Advanced Science Research Center for the NMR experiments. We are thankful to X.-Q. Yu, Sichuan University, for the HRMS analyses. This study was partially supported by the Adaptable and Seamless Technology Transfer Program of JST, No. AS242Z02199Q.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/11/19021/s1.
Supplementary Files
Author Contributions
TI, JW and JN designed research; LW, NW, YF, NKD, RK, MŚ and ZJD performed research and analyzed the data; TI, JW and LW wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 7a–7f are available from the authors.
References and Notes
- 1.Klayman D.L. Qinghaosu (artemisinin): An antimalarial drug from China. Science. 1985;228:1049–1055. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]
- 2.Poespoprodjo J.R., Fobia W., Kenangalem E., Lampah D.A., Sugiarto P., Tjitra E., Anstey N.M., Price R.N. Dihydroartemisinin-piperaquine treatment of multidrug resistant falciparum and vivax malaria in pregnancy. PLoS One. 2014;9:e84976. doi: 10.1371/journal.pone.0084976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo X.D., Shen C.C. The chemistry, pharmacology, and clinical applications of qinghaosu (Artemisinin) and its derivatives. Med. Res. Rev. 1987;7:29–52. doi: 10.1002/med.2610070103. [DOI] [PubMed] [Google Scholar]
- 4.Morrissey C., Gallis B., Solazzi J.W., Kim B.J., Gulati R., Vakar-Lopez F., Goodlett D.R., Vessella R.L., Sasaki T. Effect of artemisinin derivatives on apoptosis and cell cycle in prostate cancer cells. Anticancer Drugs. 2010;21:423–432. doi: 10.1097/CAD.0b013e328336f57b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Efferth T. Molecular pharmacology and pharmacogenomics of artemisinin and its derivatives in cancer cells. Curr. Drug Targets. 2006;7:407–421. doi: 10.2174/138945006776359412. [DOI] [PubMed] [Google Scholar]
- 6.Chen T., Li M., Zhang R.W., Wang H. Dihydroartemisinin induces apoptosis and sensitizes human ovarian cancer cells to carboplatin therapy. J. Cell. Mol. Med. 2009;13:1358–1370. doi: 10.1111/j.1582-4934.2008.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie L.J., Zhai X., Liu C., Li P., Li Y.X., Guo G.X., Gong P. Anti-tumor activity of new artemisinin–chalcone hybrids. Arch. Pharm. 2011;344:639–647. doi: 10.1002/ardp.201000391. [DOI] [PubMed] [Google Scholar]
- 8.Lee S. Artemisinin, promising lead natural product for various drug developments. Mini Rev. Med. Chem. 2007;7:411–422. doi: 10.2174/138955707780363837. [DOI] [PubMed] [Google Scholar]
- 9.Soomro S., Langenberg T., Mahringer A., Konkimalla V.B., Horwedel C., Holenya P., Brand A., Cetin C., Fricker G., Dewerchin M., et al. Design of novel artemisinin-like derivatives with cytotoxic and anti-angiogenic properties. J. Cell. Mol. Med. 2011;15:1122–1135. doi: 10.1111/j.1582-4934.2010.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Z., Ding J., Yang C., Gao Y., Li X., Chen X., Peng Y., Fang J., Xiao S. Immunomodulatory and anti-inflammatory properties of artesunate in experimental colitis. Curr. Med. Chem. 2012;19:4541–4551. doi: 10.2174/092986712803251575. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z.H., Yu Y., Ma J., Zhang H.R., Zhang H., Wang X.Q., Wang J.C., Zhang X., Zhang Q. LyP-1 modification to enhance delivery of artemisinin or fluorescent probe loaded polymeric micelles to highly metastatic tumor and its lymphatics. Mol. Pharm. 2012;9:2646–2657. doi: 10.1021/mp3002107. [DOI] [PubMed] [Google Scholar]
- 12.Chen H., Sun B., Wang S., Pan S., Gao Y., Bai X., Xue D. Growth inhibitory effects of dihydroartemisinin on pancreatic cancer cells: Involvement of cell cycle arrest and inactivation of nuclear factor-kappaB. J. Cancer Res. Clin. Oncol. 2010;136:897–903. doi: 10.1007/s00432-009-0731-0. [DOI] [PubMed] [Google Scholar]
- 13.Marques O., da Silva B.M., Porto G., Lopes C. Iron homeostasis in breast cancer. Cancer Lett. 2014;28:1–14. doi: 10.1016/j.canlet.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Rahier N.J. Camptothecin and Its Analogs. In: Cragg G.M., Kingston D.G.I., Newman D.J., editors. Anticancer Agents from Natural Products. 2nd ed. CRC press; Boca Raton, FL, USA: 2012. pp. 5–25. [Google Scholar]
- 15.Arcamone F. Doxorubicin. In: De Stevens G., editor. Anticancer Antibiotics. Academic Press; New York, NY, USA: 1981. pp. 1–369. [Google Scholar]
- 16.Lai H.C., Singh N.P., Sasaki T. Development of artemisinin compounds for cancer treatment. Invest. New Drugs. 2013;31:230–246. doi: 10.1007/s10637-012-9873-z. [DOI] [PubMed] [Google Scholar]
- 17.Buragohain P., Saikia B., Surinenia N., Barua N.C., Saxena A.K., Suri N. Synthesis of a novel series of artemisinin dimers with potent anticancer activity involving Sonogashira cross-coupling reaction. Bioorg. Med. Chem. Lett. 2014;24:237–239. doi: 10.1016/j.bmcl.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y., Zhang X.M., Wang X.F., Zhao X.M., Ren T.R., Wang F., Yu B. Enhanced delivery of artemisinin and its analogues to cancer cells by their adducts with human serum transferrin. Int. J. Pharm. 2014;467:113–122. doi: 10.1016/j.ijpharm.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 19.Blazquez A.G., Fernandez-Dolon M., Sanchez-Vicente L., Maestre A.D., Gomez-San Miguel A.B., Alvarez M., Serrano M.A., Jansenc H., Efferth T., Marin J.J.G., et al. Novel artemisinin derivatives with potential usefulness against liver/colon cancer and viral hepatitis. Bioorg. Med. Chem. 2013;21:4432–4441. doi: 10.1016/j.bmc.2013.04.059. [DOI] [PubMed] [Google Scholar]
- 20.Xie L.J., Zhai X., Ren L.X., Meng H.Y., Liu C., Zhu W.F., Zhao Y.F. Design, synthesis and antitumor activity of novel artemisinin derivatives using hybrid approach. Chem. Pharm. Bull. 2011;59:984–990. doi: 10.1248/cpb.59.984. [DOI] [PubMed] [Google Scholar]
- 21.Jones M., Mercer A.E., Stocks P.A., la Pensée L.J.I., Cosstick R., Park B.K., Kennedy M.E., Piantanida I., Ward S.A., Davies J., et al. Antitumour and antimalarial activity of artemisinin-acridine hybrids. Bioorg. Med. Chem. Lett. 2009;19:2033–2037. doi: 10.1016/j.bmcl.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 22.Yang X., Wang W., Tan J., Song D., Li M., Liu D., Jing Y., Zhao L. Synthesis of a series of novel dihydroartemisinin derivatives containing a substituted chalcone with greater cytotoxic effects in leukemia cells. Bioorg. Med. Chem. Lett. 2009;19:4385–4388. doi: 10.1016/j.bmcl.2009.05.076. [DOI] [PubMed] [Google Scholar]
- 23.Cimanga K., de Bruyne T., Pieters L., Claeys M., Vlietinck A. New alkaloids from Cryptolepis. sanguinolenta. Tetrahedron Lett. 1996;37:1703–1706. doi: 10.1016/0040-4039(96)00112-8. [DOI] [Google Scholar]
- 24.Paulo A., Gomes E.T., Steele J., Warhurst D.C., Houghton P.J. Antiplasmodial activity of Cryptolepis sanguinolenta alkaloids from leaves and roots. Planta Med. 2000;66:30–34. doi: 10.1055/s-2000-11106. [DOI] [PubMed] [Google Scholar]
- 25.Cimanga K., Bruyne T.D., Pieters L., Vlietinck A.J., Turger C.A. In vitro and in vivo antiplasmodial activity of cryptolepine and related alkaloids from Cryptolepis sanguinolenta. J. Nat. Prod. 1997;60:688–691. doi: 10.1021/np9605246. [DOI] [PubMed] [Google Scholar]
- 26.Parvatkar P.T., Parameswaran P.S., Tilve S.G. Isolation, biological activities, and synthesis of indoloquinoline alkaloids: Cryptolepine, isocryptolepine, and neocryptolepine. Curr. Org. Chem. 2011;15:1036–1057. [Google Scholar]
- 27.Alexandra P., Elsa T.G., Jonathan S., Dave C.W., Peter J.H. Antiplasmodial activity of cryptolepis sanguinolenta alkaloids from leaves and roots. Planta Medica. 2000;66:30–34. doi: 10.1055/s-2000-11106. [DOI] [PubMed] [Google Scholar]
- 28.Kumar E.V.K.S., Etukala J.R., Ablordeppey S.Y. Indolo[3,2-b]quinolines: Synthesis, biological evaluation and structure activity-relationships. Mini-Rev. Med. Chem. 2008;8:538–554. doi: 10.2174/138955708784534418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavrado J., Moreira R., Paulo A. Indoloquinolines as scaffolds for drug discovery. Curr. Med. Chem. 2010;17:2348–2370. doi: 10.2174/092986710791698521. [DOI] [PubMed] [Google Scholar]
- 30.Afzal O., Kumar S., Haider Md R., Ali M.R., Kumar R., Jaggi M., Bawa S. A review on anticancer potential of bioactive heterocycle quinolone. Eur. J. Med. Chem. 2014 doi: 10.1016/j.ejmech.2014.07.044. in press. [DOI] [PubMed] [Google Scholar]
- 31.Cimanga K., de Bruyne T., Lasure A., van Poel B., Pieters L., Claeys M., Vanden B.D., Kambu K., Tona L., Vlietinck A.J. In vitro biological activities of alkaloids from cryptolepis sanguinolenta. Planta Med. 1996;62:22–27. doi: 10.1055/s-2006-957789. [DOI] [PubMed] [Google Scholar]
- 32.Cimanga K.T., de Bruyne T., Pieters L., Totte J., Tona L., Kambu K., Vanden Berghe D., Vlietinck A.J. Antibacterial and antifungal activities of neocryptolepine, biscryptolepine, and cryptoquindoline, alkaloids isolated from cryptolepis sanguinolenta. Phytomedicine. 1998;5:209–221. doi: 10.1016/S0944-7113(98)80030-5. [DOI] [PubMed] [Google Scholar]
- 33.Guittat L., Alberti P., Rosu F., van Miert S., Thetiot E., Pieters L., Gabelica V., de Pauw E., Ottaviani A., Riou J.F., et al. Interactions of cryptolepine and neocryptolepine with unusual DNA structures. Biochimie. 2003;85:535–547. doi: 10.1016/S0300-9084(03)00035-X. [DOI] [PubMed] [Google Scholar]
- 34.Li W., Ji Y.Y., Wang J.W., Zhu Y.M. Cytotoxic Activities and DNA Binding Properties of 1-Methyl-7H-indeno[1,2-b]Quinolinium-7-(4-dimethylamino) Benzylidene Triflate. DNA Cell. Biol. 2012;31:1046–1053. doi: 10.1089/dna.2011.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riechert-Krause F., Autenrieth K., Eick A., Weisz K. Spectroscopic and calorimetric studies on the binding of an indoloquinoline drug to parallel and antiparallel DNA triplexes. Biochemistry. 2013;52:41–52. doi: 10.1021/bi301381h. [DOI] [PubMed] [Google Scholar]
- 36.Bailly C., Laine W., Baldeyrou B., de Pauw-Gillet M.C., Colson P., Houssier C., Cimanga K., van Miert S., Vlietinck A.J., Pieters L. DNA intercalation, topoisomerase II inhibition and cytotoxic activity of the plant alkaloid neocryptolepine. Anti. Cancer Drug Design. 2000;15:191–201. [PubMed] [Google Scholar]
- 37.Sidoryk K., Świtalska M., Wietrzyk J., Jaromin A., Pietka-Ottlik M., Cmoch P., Zagrodzka J., Szczepek W., Kaczmarek Ł., Peczyńska-Czoch W. Synthesis and biological evaluation of new amino acid and dipeptide derivatives of neocryptolepine as anticancer agents. J. Med. Chem. 2012;55:5077–5087. doi: 10.1021/jm300468t. [DOI] [PubMed] [Google Scholar]
- 38.Seville S., Phillips R.M., Shnyder S.D., Wright C.W. Synthesis of cryptolepine analogues as potential bioreducible anticancer agents. Bioorg. Med. Chem. 2007;15:6353–6360. doi: 10.1016/j.bmc.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 39.Dhanabal T., Sangeetha R., Mohan P.S. Structure–activity relationship of antiparasitic and cytotoxic indoloquinoline alkaloids, and their tricyclic and bicyclic analogues. Bioorg. Med. Chem. 2009;17:7209–7217. doi: 10.1016/j.bmc.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 40.Chien C.M., Yang S.H., Lin K.L., Chen Y.L., Chang L.S., Lin S.R. Novel indoloquinoline derivative, IQDMA, suppresses STAT5 phosphorylation and induces apoptosis in HL-60 cells. Chem. Biol. Interact. 2008;176:40–47. doi: 10.1016/j.cbi.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Beauchard A., Jaunet A., Murillo L., Baldeyrou B., Lansiaux A., Chérouvrier J.R., Domon L., Picot L., Bailly C., Besson T., et al. Synthesis and antitumoral activity of novel thiazolobenzotriazole, thiazoloindolo[3,2-c]quinoline and quinolinoquinoline derivatives. Eur. J. Med. Chem. 2009;44:3858–3865. doi: 10.1016/j.ejmech.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Dassonneville L., Lansiaux A., Wattelet A., Wattez N., Mahieu C., van Miert S., Pieters L., Bailly C. Cytotoxicity and cell cycle effects of the plant alkaloids cryptolepine and neocryptolepine: Relation to drug-induced apoptosis. Eur. J. Pharm. 2000;409:9–18. doi: 10.1016/S0014-2999(00)00805-0. [DOI] [PubMed] [Google Scholar]
- 43.Sidoryk K., Jaromin A., Edward J.A., Świtalska M., Stefańska J., Cmoch P., Zagrodzka J., Szczepek W., Peczyńska-Czoch W., Wietrzyk J., et al. Searching for new derivatives of neocryptolepine: Synthesis, antiproliferative, antimicrobial and antifungal activities. Eur. J. Med. Chem. 2014;78:304–313. doi: 10.1016/j.ejmech.2014.03.060. [DOI] [PubMed] [Google Scholar]
- 44.Kaczmarek L., Peczynska-Czoch W., Osiadacz J., Mordarski M., Sokalski W.A., Marcinkowska E., Glazman-Kusnierczyk H., Radzikowski C. Synthesis, and cytotoxic activity of some novel indolo[2,3-b]quinoline derivatives: DNA topoisomerase II inhibitors. Bioorg. Med. Chem. 1999;7:2457–2464. doi: 10.1016/s0968-0896(99)00200-x. [DOI] [PubMed] [Google Scholar]
- 45.Osiadacz J., Majka J., Czarnecki K., Kaczmarek L., Sokalski W.A. Sequence-selectivity of 5,11-dimethyl-5H-indolo[2,3-b]quinoline binding to DNA. Footprinting and molecular modeling studies. Bioorg. Med. Chem. 2000;8:937–943. doi: 10.1016/s0968-0896(00)00031-6. [DOI] [PubMed] [Google Scholar]
- 46.Godlewska J., Luniewski W., Zagrodzki B., Kaczmarek L., Bielawska-Pohl A., Dus D., Wietrzyk J., Opolski A., Siwko M., Jaromin A., et al. Biological evaluation of ω-(dialkylamino)alkyl derivatives of 6H-indolo[2,3-b]quinoline-novel cytotoxic DNA topoisomerase II inhibitors. Anticancer Res. 2005;25:2857–2868. [PubMed] [Google Scholar]
- 47.Filak L.K., Goschl S., Heffeter P., Samper K.G., Egger A.E., Jakupec M.A., Keppler B.K., Berger W., Arion V.B. Metal-arene complexes with indolo[3,2-c]-quinolines: Effects of ruthenium vs. osmium and modifications of the lactam unit on intermolecular interactions, anticancer activity, cell cycle, and cellular accumulation. Organometallics. 2013;32:903–914. doi: 10.1021/om3012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Filak L.K., Goschl S., Hackl S., Jakupec M.A., Arion V.B. Ruthenium- and osmium-arene complexes of 8-substituted indolo[3,2-c]quinolines: Synthesis, X-ray diffraction structures, spectroscopic properties, and antiproliferative activity. Inorg. Chim. Acta. 2012;393:252–260. doi: 10.1016/j.ica.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Primik M.F., Goschl S., Jakupec M.A., Roller A., Keppler B.K., Arion V.B. Structure-activity relationships of highly cytotoxic copper(II) complexes with modified indolo[3,2-c]quinoline ligands. Inorg. Chem. 2010;49:11084–11095. doi: 10.1021/ic101633z. [DOI] [PubMed] [Google Scholar]
- 50.Wang L., Świtalska M., Mei Z.W., Lu W.J., Takahara Y., Feng X.W., El-Sayed I.E., Wietrzyk J., Inokuchi T. Synthesis and in vitro antiproliferative activity of new 11-aminoalkylamino-substituted 5H- and 6H-indolo[2,3-b]quinolines; structure-activity relationships of neocryptolepines and 6-methyl congeners. Bioorg. Med. Chem. 2012;20:4820–4829. doi: 10.1016/j.bmc.2012.05.054. [DOI] [PubMed] [Google Scholar]
- 51.Lu W.J., Świtalska M., Wang L., Yonezawa M., El-Sayed I.E., Wietrzyk J., Inokuchi T. In vitro antiproliferative activity of 11-aminoalkylaminosubstituted 5H-indolo[2,3-b]quinolines; improving activity of neocryptolepines by installation of ester substituent. Med. Chem. Res. 2013;22:4492–4504. doi: 10.1007/s00044-012-0443-x. [DOI] [Google Scholar]
- 52.Wang N., Świtalska M., Wu M.Y., Imai K., Ngoc T.A., Pang C.Q., Wang L., Wietrzyk J., Inokuchi T. Synthesis and in vitro cytotoxic effect of 6-amino-substituted 11H- and 11Me-indolo[3,2-c]quinolones. Eur. J. Med. Chem. 2014;78:314–323. doi: 10.1016/j.ejmech.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 53.Lavrado J., Cabal G.G., Prudencio M., Mota M.M., Gut J., Rosenthal P.J., Diaz C., Guedes R.C., dos Santos D.J.V.A., Bichenkova E., et al. Incorporation of basic side chains into cryptolepine scaffold: Structure-antimalarial activity relationships and mechanistic studies. J. Med. Chem. 2011;54:734–750. doi: 10.1021/jm101383f. [DOI] [PubMed] [Google Scholar]
- 54.IC50 values of the compound 9 against the MV4–11 cell line: 0.128 ± 0.036 μM), BALB/3T3 cell line: 4.979 ± 0.471μM, A549 cell line: 1.806 ± 0.516 μM, HCT116 cell line: 0.855 ± 0.235 μM
- 55.Lu J.J., Meng L.H., Cai Y.J., Chen Q., Tong L.J., Lin L.P., Ding J. Dihydroartemisinin induces apoptosis in HL-60 leukemia cells dependent of iron and p38 mitogen-activated protein kinase activation but independent of reactive oxygen species. Cancer Biol. Ther. 2008;7:1017–1023. doi: 10.4161/cbt.7.7.6035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.